Abstract
A substantial body of research now implicates the circadian clock in the regulation of an array of diverse biological processes including glial function, metabolism, peripheral immune responses, and redox homeostasis. Sleep abnormalities and other forms of circadian disruption are common symptoms of aging and neurodegeneration. Circadian clock disruption may also influence the aging processes and the pathogenesis of neurodegenerative diseases. The specific mechanisms governing the interaction between circadian systems, aging, and the immune system are still being uncovered. Here, we will review the evidence supporting a bidirectional relationship between aging and the circadian system. Further, we explore the hypothesis that age-related circadian deterioration may exacerbate multiple pathogenic processes, priming the brain for neurodegeneration.
Keywords: circadian clock, Bmal1, neurodegeneration, neuroinflammation, aging, oxidative stress
Introduction
The myriad correlations between aging, aging-related disease, and circadian rhythms1–3 provide ample justification for investigation into potential causative relationships between these phenomena. The progressively increasing prevalence of circadian dysfunction with increasing age suggests that aging drives circadian dysfunction. However, disruption of the circadian clock – either behaviorally or through genetic manipulation - can also drive aging-like phenotypes, suggesting that the relationship between aging and circadian rhythm dysfunction is bidirectional1. More recently, evidence has accumulated documenting changes in circadian systems preceding or being predictive of the development of neurodegenerativo diseases, suggesting that circadian dysfunction could increase dementia risk3. However, this possibility as well as the implication that aging and circadian dysfunction could represent concomitant, positively reinforcing cycles of deterioration remain active areas of investigation. Additionally, while the mechanisms by which these cycles may lead to increased risk for dementia remain unknown, immune dysregulation and oxidative stress have been identified as prime candidates3. Initial studies suggest the circadian clock as a potentially viable therapeutic target for the treatment of both neurodegeneration and other age-related diseases. In connecting these concepts, it is helpful to contextualize newer investigations exploring circadian clock regulation of the immune system by considering what is known linking circadian dysfunction with aging (Also see Hood et al., 20171).
Overview of the mammalian circadian system
Circadian rhythms are a fundamental part of biology, as most organisms have a circadian clock that allows behavioral and physiological adaptation to the 24-hour light-dark cycle of earth. In mammals, the “master clock” of the body resides in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN receives synaptic input from the retina and the cellular clocks within neurons of the SCN are thus entrained to the external light-dark cycle. These cellular clocks then keep 24-hour time and the SCN has specific neural circuitry to ensure timekeeping that is both robust and flexible4. The SCN provides synchronizing cues through regulation of endocrine and autonomic nervous system function to cellular clocks throughout the body, including in neurons and glia in the brain5,6. The core molecular clock found in each cell is comprised of a positive transcriptional limb and negative feedback limb. The positive limb is composed of the bHLH-PAS transcription factor BMAL1 (aka Arntl), which forms hetereodimers with CLOCK or NPAS to drive circadian transcription via binding to Ebox motifs. The negative limb consists of the PERIOD and CRYPTOMCHROME families of proteins, which are direct transcriptional targets of BMAL1 and which in turn inhibit BMAL1 function5. The ROR and REV-ERB proteins, positive and negative regulators of Bmal1 transcription, respectively, are also transcriptional targets of BMAL1 and further modulate clock timing. This core clock is tuned to a 24-hour period through the concerted actions of numerous post-translational mechanisms carried out by a network of secondary clock proteins7. These core clock genes are expressed in nearly every cell in the body and can generate circadian rhythms in transcription and cellular function in the absence of any external cues. The circadian clock regulates between 10–50% of all transcripts in a cell, depending on tissue type, and influences critical processes such as cell cycle, redox homeostasis, inflammation, and metabolism8. This breadth of clock-controlled genes may partially explain the wide ranging consequences of circadian clock disruption for aging as well as in the pathogenesis of many chronic diseases9.
Behavioral circadian disruptions in aging
On a behavioral level, circadian disruption is a widely-studied characteristic of both aging10–13 and neurodegeneration14–19. Specifically, age-associated sleep changes, including sleep fragmentation, represent perhaps the most consistent and clear evidence linking behavioral circadian disruption to aging1. Sleep disturbances such as difficulties with falling and staying asleep12, increased sleep to wake transitions (sleep fragmentation)10,17, and increased daytime drowsiness and napping10,12 are all characteristic of elderly populations. Sleep structure is also altered20 with a particularly prominent age-associated decrease in slow wave sleep11,21–24, which is deemed important for protein clearance25–28, maintaining metabolic health29, and potentially in memory consolidation30. Interestingly, a recent report details dampening of rhythms in cortical excitability with age, which correlates with sleep changes and potentially contributes to age-related cognitive decline31. Older populations tend to display earlier chronotypes20,32–34 while, at least in men, an individual’s chronotype shifts earlier as age increases35. Somewhat paradoxically, in a Dutch population aged 18–65, a later sleep onset was correlated with shorter telomere length36, a feature associated with cellular aging and senescence37. While the robustness of an individual’s sleep rhythm declines with age, their ability to adapt to an environmentally imposed phase shift, as with jet-lag, also declines with age in humans38,39 and in mice40,41. Increasing fragmentation of circadian activity rhythms is also specifically noted in aging men and is independent of preclinical Alzheimer Disease pathology17. However, further research is required to disentangle whether these changes reflect alterations to the circadian system itself, independent from aberrant regulation of sleep homeostasis. The incorporation of other circadian readouts in addition to sleep may help facilitate this endeavor.
Other systemic circadian changes with aging
Outside of sleep, alterations in several other systemic circadian processes have been shown with age. For instance, body temperature normally exhibits peak temperature in the evening while the trough occurs in the early morning before waking42. In aged humans there is a phase advance in body temperature rhythm such that the nadir occurs earlier. The relationship between sleep and body temperature rhythms may also be altered, with age being associated with a later body temperature nadir relative to time of awakening32,43. At least in men44,45, there may also be a reduction in amplitude43 and variability46 of the temperature rhythm in aged adults (60s or older).
Melatonin, a hormone regulated by the SCN and secreted by the pineal gland, normally induces sleep, possibly by acting on BMAL147 and regulates body temperature48. A potential decrease in melatonin secretion with age53–55 has been inconsistently documented56,57 and may be specific to women54. Additionally, it is possible that a decrease in melatonin secretion could be indicative of pathological instead of healthy aging57,58. In the SCN, the expression of melatonin receptor declines with age, which may contribute to the dispersion of behavioral rhythms59,60. This decrease may be at least partially responsible for the loss of both sleep and body temperature rhythm robustness in advanced age.
Glucocorticoids, steroid hormones of which cortisol is the primary form in humans, have a complex relationship to stress, the immune system, and the regulation of plasma glucose homeostasis61. Glucocorticoids are regulated by the SCN, follow a circadian pattern of secretion, and are potent synchronizers of a number of peripheral molecular clocks61–63. Van Cauter et al. as well as several others64–66 found that the rhythm in circulating cortisol is dampened in aging, enabled by a progressive rising of the nadir and accompanied by an overall increase in levels67. However, others found a phase shift in elderly subjects, but no change in amplitude68, while still others found no major changes in cortisol with age69. In mice, an age-related decline in glucocorticoid signaling in the hippocampus, potentially due to a decrease in glucocorticoid receptor expression, may play a role in the depletion of the neural stem cell pool70. Despite disagreement on specific cortisol rhythm abnormalities (possibly due to high variability or vastly different sample sizes), a remaining diurnal rhythm in circulating cortisol in aged humans is consistent between studies64–69. This persistence of a cortisol rhythm in aging, which in contrast to the case of melatonin is retained during pathological aging15,58, adds another level of complexity to the series of interconnected feedback loops that experience age-associated alterations or losses in robustness.
Age-related changes in the SCN
In addition to the impairments and alterations observed in SCN-regulated rhythms, age-associated changes in the SCN itself have been documented. An impairment in the rhythm of neuronal firing in mice71,72 and flies73, as well as decreases or altered rhythms74 in the expression of neuropeptides arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) in the SCN have been observed in humans, especially in men75, and in rodents76,77 with advanced age. A loss in GABAergic synapses in the SCN has also been reported in aged mice78. GABA-mediated neuronal activity, as well as the expression of VIP are critically important for the cohesiveness of SCN neuronal firing rhythms79,80 and the maintenance of behavioral rhythms depends on the coordination of SCN neuron firing81,82. In a small sample of elderly people and Alzheimer patients, fragmented sleep-wake rhythms during life were associated with loss of VIP-ergic neurons in the SCN on post-mortem examination83. Thus, current data support the hypothesis that changes in SCN neurons could contribute to age-associated behavioral rhythm desynchrony. However, this possibility requires more thorough evaluation to show that these changes in neuronal populations directly influence organismal rhythms.
Aging and circadian clock gene expression
Reports documenting age-induced alterations in the molecular clock have been more controversial. Some have shown dampening or dispersion in the SCN expression rhythms of Bmal1, Clock84,85, and Per286,87 while others report normal Per1 and Per2 rhythms88,89 with advanced age. Altered molecular rhythms, including an impaired ability to phase reset, have also been observed in the mouse liver90 (although to a lesser extent in some reports91,92), heart93, kidney, lung88, thymus38, and pancreas92 among others. However, intact molecular rhythms have been observed in muscle and epidermal stem cells of aged mice94. Interestingly, the induction of replicative cellular senescence has been found to impair entrainment of the molecular clock95, suggesting that perhaps the accumulation of senescent cells in a given tissue with age could play a role in the dispersion of circadian phases between cells. Outside of genes directly involved in the core molecular clock, a large number (more than 1000) of clock-controlled genes display altered rhythmicity, some even gaining rhythms with age in the human prefrontal cortex96. The liver91 as well as muscle and epidermal stem cells94 also undergo substantial circadian reprogramming in aged mice. However, more data is needed to solidify the physiological relevance of altered molecular rhythms, especially in the aged brain.
The circadian system, healthspan, and lifespan
Changes in the circadian system can be predictive of, while inducing circadian disruption can reduce, healthspan and lifespan. For instance, the degree of deviation from a 24-hour circadian period was found to be negatively correlated with lifespan in both rodent and primate species97,98. Conversely, implantation of young SCN tissue improved the molecular99 and behavioral rhythms100 of rats and the longevity of aged hamsters after surgery compared with cortex- and mock-implanted controls101. Additionally, inducing weekly phase shifts, especially phase advances, can shorten the lifespan of aged, but not young, mice40 while phase shifting can also increase the vulnerability of mice to an lipopolysaccharide (LPS) challenge102,103. Genetically, ablating the clock via a global knockout of Bmal1 shortens lifespan and induces a number of other “aging-like” pathologies, such as cataracts and sarcopenia in mice104. Moreover, deficiencies in either Per2105 or Clock/Bmal1106 mediated transcription has been shown to exacerbate cancer or drive age-dependent insulin dysfunction and diabetes, respectively. The pathologies in the Bmal1 KO model have since been partially attributed to loss of Bmal1 during development/early life107 and exhibit tissue specificity108. However, these and further studies utilizing macrophage/monocyte102,109–112, muscle113, liver114, brain-specific (sparing the SCN)115, and other tissue-specific circadian mutants have recapitulated components of aging-like phenotypes and vulnerabilities, including insulin resistance (recently reviewed116). Interestingly, a contingent of these metabolic abnormalities, including lipid accumulation and glucose intolerance, can be mitigated by time restricted feeding, highlighting the importance of the clock in maintaining metabolic homeostasis117–119. These studies also suggest time restricted feeding as a potentially viable behavioral intervention for age-related metabolic dysregulation.
Maintaining the integrity of circadian rhythms is crucial for optimizing a large host of physiological outputs including, but not limited to long-term potentiation120,121 and associated cognition122,123, metabolic health124, reaction time125,126, and muscle performance113,127–129, age-associated deteriorations of which have been extensively documented. Accordingly, the perturbation of rhythms, for instance with nighttime light exposure130, circadian misalignment (shift work)131, or jet-lag132 can impair these functions. Circadian disruption also negatively impacts insulin sensitivity as well as increases risk factors133–136 and worsens outcomes137 for acute neurological and cardiovascular events, which already display daily rhythms in occurrence138–140. These data suggest that at the very least, disruption of the circadian clock is detrimental in the context of aging. The intriguing possibility that such disruptions could be driving the aging process itself, negatively impacting healthspan and lifespan should be of particular interest to future studies. Additionally, recent studies suggest that the efficacy and toxicity of drug therapies for age-related diseases such as cancer can be dramatically affected by the circadian phase in which they are administered141–143. Circadian regulation of treatment efficacy may become even more complicated and warrants further investigation in the context of aging, given the age-related alterations in phase and dispersion of rhythms discussed here. The intimate interaction between the immune system and the circadian clock, discussed in the next section, adds yet another layer of complexity to be considered, and perhaps leveraged, in the development of therapeutics to treat age-related disease.
Mechanistically, the circadian clock is linked to the mammalian target of rapamycin (mTOR) and Sirtuin 1 (SIRT1)87,144–147. These factors are closely tied to the regulation of aging with mTOR negatively and SIRT1 positively impacting healthspan and lifespan148–152. SIRT1 interacts with the BMAL1/CLOCK complex and may impact circadian transcription directly by deacetylating BMAL1153, PER2154, and histone H3, acting counter to the histone acetyltransferase functions of the CLOCK protein itself153. Additionally, levels of NAD+, an essential metabolite and necessary substrate for SIRT1 deacetylase activity151,155, as well as the expression of NAMPT, the rate-limiting enzyme in the NAD+ salvage pathway156, have been shown to oscillate in the mouse liver157,158 and human red blood cells (NADH)159. This circadian clock regulation of NAD+ through NAMPT is important for maintaining homeostatic levels of mitochondrial oxidative phosphorylation160 and for feeding back into SIRTT153,157,158 (as well as mitochondrial SIRT3160) activity. Although through a modestly different mechanism, modulation of the circadian clock by SIRT1 is also present in the SON87. In concordance with decreased expression of Sirt1 in aged animals, this control of the clock by SIRT1 wanes with age87. An age-associated systemic decline in NAD+ (recently reviewed161–163), possibly due to decreased levels of clock-regulated NAMPT in several tissues164–166 has been thoroughly documented. Taken together, these data suggest that a deficit in the interaction between SIRT1 and circadian signaling could bear some responsibility for the connection between circadian dysfunction and the aging process1,87. In support of this idea, aged mice experience a substantial dampening of the protein acetylation rhythms under dual regulation by NAD+/SIRT1 and the circadian clock in the liver91. These rhythms are restored by caloric restriction91, currently the most robust lifespan extension intervention167. Caloric restriction can also induce circadian reprogramming in both young168 and old animals as well as greatly enhance NAD+ levels and SIRT1 activity91.
Circadian physiology is also inextricably linked with a number of other metabolic pathways169, including the insulin signaling116 and mTOR pathways144–146,170, the suppression of which have both been shown to extend lifespan and healthspan148,149,152,171–173. Insulin induces phosphorylation of BMAL1 via AKT, thereby inhibiting BMAL1 transcriptional activity174. On the other hand, downstream insulin signaling target mTOR can also induce BMAL1 phosphorylation via S6K1, a modification that enables BMAL1 to play a critical role in mTOR-regulated translation175. Additionally, activation or inhibition of mTOR results in acceleration or dampening of the circadian clock, respectively144,145. Calorie restriction, which extends lifespan, impairs insulin signaling, and inhibits mTOR, also increases Bmal1 expression and BMAL1 mediated transcription176. Finally, loss of Bmal1 has been found to increase mTOR activity (although not in all reports47), while inhibition of mTOR extends the lifespan of Bmal1 KO mice by 50%146. Taken together, these data suggest a bidirectional relationship whereby maintaining a metabolic equilibrium that favors longevity also promotes robustness of the circadian clock, while maintaining the integrity of the clock may promote longevity by sustaining metabolic homeostasis.
Glial clocks and aging
In addition to in the SCN and throughout the body177, oscillating molecular clocks have been documented in a variety of extra-SCN brain regions178 as well as in astrocytes6 and microglia179,180. In the SCN, astrocytic clocks are synchronized by VIP181 and can be altered by immune factors such as TNFα182. Astrocytic extracellular ATP release183, which has potential implications for allodynia184, gliotransmission185, and glutamate uptake186 are regulated by the clock. Additionally, astrocytes play a substantial role in maintaining behavioral circadian rhythms. For instance, under certain conditions, glial clock dysfunction can cause behavioral arrhythmicity in flies187. Several recent studies have independently documented an even more impressive role for the clock in SCN astrocytes in determining the phase and period of mouse circadian rhythms188–190. Surprisingly, it was also shown that SCN astrocytes are capable of generating population-wide circadian clock oscillations and mouse activity rhythms in the absence of intact neuronal clocks191. Despite the prominence of the astrocyte clock in the SCN, relatively little is known about its function elsewhere in the brain and outside of behavioral rhythm maintenance. However, recent evidence suggests that glial clocks may play a substantial role in regulating the neuroimmune system – discussed in more detail in the next section - with potential implications for neurodegeneration192. Notably, glia regulate blood-brain barrier permeability, which has been shown to exhibit circadian oscillation in flies193. Additionally, multiple groups have reported marked aging-induced changes to the astrocytic194,195 and microglial196 transcriptomes that may substantially overshadow those in neuronal populations197. Together, these data suggest that glial clocks may represent a fresh perspective from which to consider the ballooning interest in the role of both astrocytes and microglia in the pathogenesis of neurodegenerative diseases.
The clock and the immune system
Recent studies have convincingly demonstrated circadian regulation of the immune system in the periphery198, while emerging evidence links the clock to regulation of the immune response in the CNS3,192. Indeed, the circadian clock regulates inflammatory and oxidative stress responses. For example, both lesions of the SCN199 and light induced rhythm disruption200 can exacerbate release of cytokines TNFα199 and IL-6199,200 in response to LPS, while LPS can differentially activate SCN neurons based on time of day199. Chronic circadian phase shifts (chronic jet-lag)201 or merely varying the time of day102,103 can heighten both inflammation and LPS-induced endotoxemic death in mice. In addition to the aging-related pathologies previously discussed, global and brain-specific Bmal1 KO as well as global Clock/Npas2 double KO mice have age-dependent increases in ROS damage, chronic inflammation104,115 including increased Tnfa, microglia and astrocyte activation, and synapse degeneration115. In monkeys, Bmal1 KO can also induce immune system activation and depression-like symptoms202.
Importantly, clock genes including Clock, Per2203, Bmal1, and the BMAL1 target Nr1d1 oscillate in peripheral macrophages112,203 and lymphocytes204. In humans, the LPS-induced blood levels of cytokines Interferon-γ (IFN-γ), Interleukin-10 (IL-10)205, and TNFα vary consistently based on time of day while IL-6 levels vary inconsistently206,207. In mice, lymphocyte trafficking204, LPS-induced monocyte recruitment, cytokine levels including TNFα, IL-6203, IL-12111, inducible nitric oxide synthase (iNOS - reactive NO-producing enzyme)112, chemokines including CCL5111, and mortality208 exhibit time of day dependence with a reduction during late wake/early rest periods112,203. This reduction can be abolished upon monocyte Bmal1112 or Nr1d1111 KO indicating an immune-suppressive role for these proteins. Accordingly, Bmal1 KO in monocytes reduces survival in response to infection and exacerbates chronic inflammation and glucose intolerance in a mouse model of diet-induced obesity112. Deletion of Bmal1 also induces an Nrf2-dependent increase in ROS and IL-1β in macrophages110. Disruption of BMAL1-regulated neutrophil aging can impair immune defense and vascular protection in mice109.
In vitro, Bmal1 KO can cause increased neuronal degeneration, death, and susceptibility to oxidative damage115. Additionally, it was found that the BMAL1/CLOCK complex binds chemokine Ccl2 and Ccl8 promoters112 while BMAL1 binds the promoters of genes protective against oxidative stress, which are also downregulated in global Bmal1 KO mice115. Macrophages from global Nr1d1 KO increase IL-6 secretion while REV-ERBα (Nr1d1) agonist GSK4112111,209 and Nr1d1 overexpression in culture209 suppresses IL-6 release. In further support of BMAL1-mediated immune suppression, global KO of two repressors of BMAL1 activity, Per2210 and microRNA miR-155, can reduce TNFα102, IL-1β, and IFN-γ110 secretion upon LPS treatment. Taken together, these and similar studies make a strong case for the circadian clock as an important immune regulator, providing a limiting check on immune over activation in the periphery.
The neuroimmune system, primarily under the purview of glia, may also be subject to regulation by the molecular clock. In addition to the astrocytic clock discussed previously, a few recent reports have documented oscillating clock gene expression including Bmal1, Per2, and Nr1d1 in microglia179,180,211. Cytokine levels including Il-6, Tnfa, and the critical inflammasome component Nlrp3 (only measured after LPS), among others, show circadian variation in unstimulated and LPS-stimulated whole hippocampus and microglia179. Aging abolishes these differences, clamping the microglial inflammatory response to LPS at its highest level in younger mice212. Little is known about astrocyte clock function in the immune system. However, we have shown that astrocyte clock dysfunction induces astrogliosis and can impair neuronal survival213. Additionally, BDNF and Nrf2-dependent oxidative stress protection provided by astrocytes to neurons214 and NF-κB-mediated inflammation may both be regulated by the astrocytic clock215. Nr1d1 KO induces microgliosis and astrogliosis in vivo and exacerbates the neuroinflammatory response to LPS treatment, including NF-κB signaling, in vivo as well as in cultured microglia216. However, one study demonstrated a surprising depression of Il-6 expression in microglia and a mitigation of stroke damage in vivo after deleting microglial Bmal1217. The varied results from glial clock manipulations suggest a more nuanced clock regulation of the glial immune response and underscore the need for further investigation. Such efforts may be especially relevant in the context of neurodegeneration where glial cells play an increasingly appreciated and crucial role in disease progression.
The general finding of a more active immune system at the rest to wake transition111,112,179 is likely preemptive, preparing the body for increased possibilities of infection exposure during “morning” foraging and conservationist, minimizing both energy expenditure at unneeded times and collateral damage induced by a constitutively active immune system218. These studies in the peripheral and central immune systems as well as the pro-inflammatory, pro-ROS phenotypes of Bmal1 mutants support the possibility that the circadian clock could regulate the CNS immune response in microglia and astrocytes. In total, these data suggest that alterations to circadian systems documented both in the SCN and in tissue-specific clocks could play a substantial role in immune hyperactivation with aging. Thus, these alterations may generate tissue environments susceptible to the overproduction of oxidative stress, and prime the body for the development of neurodegenerative disease.
Circadian clocks and oxidative stress
Considerable evidence supports a bidirectional relationship between the circadian clock and oxidative stress, as changes in redox status can influence core clock function, while clock proteins themselves regulate redox homeostasis of cells219. Binding of BMAL1/CLOCK to DNA is dependent on the NAD(H)/NADP(H) ratio, a barometer of cellular redox status, with increased binding occurring under reducing conditions220. Circadian rhythms in hydrogen peroxide levels are observed in cultured cells221 and mouse liver, and can directly regulate rhythms in CLOCK function via cysteine oxidation222. Deletion of the redox-responsive protein p66shc, which is itself rhythmic, disrupts these oxidation rhythms in CLOCK and alters mouse behavioral and transcriptional rhythms222. Inhibition of pentose phosphate pathway (PPP), which is critical for generation of NADPH to fuel ROS generation by the NADPH oxidase enzymes, as well as for glutathione production, can also alter clock function223. PPP inhibition with resultant loss of NADPH leads to oxidative stress, activation of the NRF2 redox response pathway, increased BMAL1/CLOCK DNA binding, altered clock gene expression, and lengthened circadian period223. NRF2 itself appears to regulate clock function, as Nrf2−/− cells have diminished clock gene expression and blunted circadian rhythms in Per2 expression224. In the SCN, circadian rhythms in redox tone regulate rhythmic neuronal activity via regulation of potassium currents225. Thus, oxidative stress can regulate clock function by multiple pathways.
Conversely, the core clock controls expression of redox response genes and dictates cellular responses to oxidative stress. Drosophila exhibit circadian rhythms in ROS sensitivity which are lost in arrhythmic Per01 mutant flies. Per01 flies have shortened lifespans, increased oxidative damage, and age-related neuronal degeneration226,227. Glutathione, a critical small molecule antioxidant present in all cells, is regulated by the clock at several levels. Glutathione levels, glutathione-producing enzymes, and glutathione transferase enzymes all show circadian oscillation in drosophila and mice228,229. NRF2, which strongly regulates glutathione synthesis, is a transcriptional target of BMAL1110. BMAL1 controls Nrf2 in pancreatic beta cells230, macrophages110, and lung cells231, with loss of BMAL1 causing blunted NRF2-mediated antioxidant responses and enhancing ROS levels. Accordingly, deletion of BMAL1 leads to increased oxidative damage in multiple organs, including the brain104,115. The clock protein REV-ERBα can be induced by oxidative stress and can in turn regulate expression of the antioxidant transcription factor F0X01 as well as stimulate autophagy and mitochondrial biogenesis232–234. Overexpression of REV-ERBα provides protection against oxidative stressors and improves mitochondrial function232–234. Oxidative stress in flies can even reprogram genome-wide circadian transcription toward a redox stress response235. Taken together, these data suggest that the circadian clock responds to changes in cellular redox tone and regulates expression of redox response pathways. As oxidative stress is strongly implicated in many aspects of aging, age-related changes in clock function could promote oxidative damage.
Circadian dysfunction in Alzheimer Disease
As detailed above, the circadian clock has a panoply of effects on cellular aging, inflammation, and oxidative stress and is impacted by all of these processes. Thus, it is perhaps not surprising that circadian disruption is a common symptom of multiple neurodegenerative diseases (as reviewed in detail elsewhere3,18,236). Among these, Alzheimer Disease (AD) is the most common age-related neurodegenerative condition and is associated with considerable circadian dysfunction237. The sequence of pathogenic events in AD is thought to begin with the accumulation of amyloid plaques, composed of aggregated amyloid-beta (Aβ) peptide, followed by the formation of hyperphosphorylated tau protein aggregates (neurofibrillary tangles) within neurons. Plaques appear 10–20 years before disease onset, while tau pathology is apparent within 5 years of first symptom and is closely associated with inflammation and neurodegeneration238. Several human studies using actigraphy show that circadian and sleep fragmentation occur during the presymptomatic phase of the disease and worsens with disease progression17,239–241. This pattern is similar to that seen in normal aging, but more severe. Degeneration of the SCN, with subsequent blunting of rhythmic melatonin release, may provide a mechanistic explanation for this exacerbation83,242–244. However, alterations in BMAL1 methylation245 or direct effects of Aβ on BMAL1 degradation have also been proposed246. In the mouse brain, interstitial fluid Aβ levels exhibit a clear circadian rhythm which is driven by the sleep–wake cycle247. Deletion of Bmal1 causes severe circadian fragmentation, significantly blunts Aβ rhythms, and increases amyloid plaque deposition in a transgenic mouse model of AD248. The exact mechanisms underlying this effect of clock disruption on plaques is unclear. One potential mechanism is through dysregulation of sleep, as sleep deprivation can increase Aβ plaque deposition in mice 247, perhaps by increasing neuronal activity-dependent Aβ production249 or by impairing Aβ clearance by the glymphatic system250. Humans also have diurnal rhythms in cerebrospinal fluid (CSF) Aβ levels251. Moreover, sleep deprivation in healthy adults acutely increases CSF Aβ252 and may increase amyloid deposition27. However, kinetic labeling studies suggest this effect occurs via increased Aβ production, rather than impaired clearance252. Extracellular levels of tau also increase during wakefulness and are exacerbated by sleep deprivation in both mice and humans25, while sleep deprivation in mice increases tau pathology25,253,254. Thus, the clock may influence amyloid deposition and tau pathology in part through effects on sleep.
Aside from sleep regulation, circadian disruption could potentially influence AD or other neurodegenerative disease by any of the previously mentioned mechanisms, including alterations in inflammation, glial function, NAD+/SIRT1 signaling, mitochondrial function, or redox homeostasis. Circadian regulation of protein misfolding and proteostasis in the brain is also relatively unexplored, though the clock has been linked to regulation of autophagy and the proteasome234,255,256. Accordingly, the core clock could potentially be leveraged as a therapeutic mechanism to optimize these factors in the aging brain and prevent degeneration. Attempts at improving circadian function indirectly through light and/or melatonin supplementation have yielded modest or mixed results on sleep integrity and cognition257–259, but may offer increased benefit when used in combination260,261. A variety of drugs which directly target the circadian system are currently being developed and tested262–264, suggesting that a circadian therapy may be an option in the future.
Conclusions
Impaired circadian function and immune dysfunction, including altered redox homeostasis, coexist consistently across aging and pathological conditions, including neurodegenerative disease1,3,198. Despite documented regulatory overlap between these areas, the idea that the age-worn circadian system could represent a common link between these phenomena has not been thoroughly explored. Circadian rhythm integrity, including sleep and metabolic cycle competency, is crucial for maintaining brain homeostasis, but breaks down in aging and during neurodegeneration (Fig. 1). At the same time, the circadian clock is vital in optimizing immune function, which is also compromised in aging and neurodegeneration. Elucidation of unifying threads that directly link these observations has the potential to address unanswered questions in several fields simultaneously. These efforts may reveal both innovative therapeutic strategies for tempering the ravages of time and age-related disease while also establishing intriguing avenues for future study.
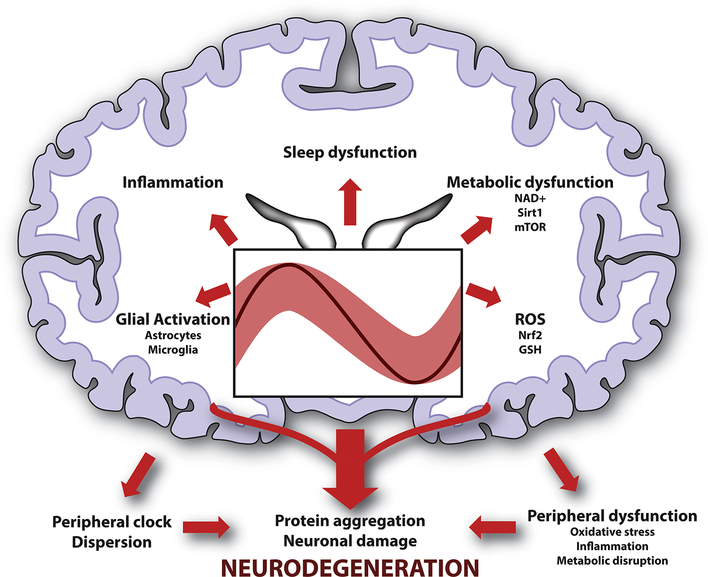
Figure 1.
Interaction of aging, circadian rhythms, and neurodegeneration. Age-related dampening and dispersion of circadian rhythms (imprecise light red oscillation depicting aged vs robust and precise dark red oscillation depicting young), can promote various pathogenic changes in the brain, including oxidative stress, inflammation, glial activation, and metabolic dysfunction. Disruption of normal sleep-wake patterns can also contribute to these pathologies. Loss of peripheral circadian synchronization can also promote systemic inflammation and impact the immune system, potentially contributing to brain dysfunction. Thus, the circadian system orchestrates brain homeostasis through multiple emerging mechanisms disruption of which may prime the brain for neurodegeneration.
Acknowledgements:
This work was funded by NIH grant R01AG054517 (ESM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hood S & Amir S The aging clock: circadian rhythms and later life. Journal of Clinical Investigation 127, 437–446, doi: 10.1172/jci90328 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kondratova AA & Kondratov RV The circadian clock and pathology of the ageing brain. Nat Rev Neurosci 13, 325–335, doi: 10.1038/nrn3208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leng Y, Musiek ES, Hu K, Cappuccio FP & Yaffe K Association between circadian rhythms and neurodegenerative diseases. The Lancet Neurology 18, 307–318, doi: 10.1016/s1474-4422(18)30461-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver DR The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms 13, 100–112 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Mohawk JA, Green CB & Takahashi JS Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35, 445–462, doi: 10.1146/annurev-neuro-060909-153128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prolo LM, Takahashi JS & Herzog ED Circadian rhythm generation and entrainment in astrocytes. J Neurosci 25, 404–408, doi: 10.1523/JNEUROSCI.4133-04.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J et al. Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver. Proc Natl Acad Sci U S A 115, E1916–E1925, doi: 10.1073/pnas.1715225115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang R, Lahens NF, Ballance HI, Hughes ME & Hogenesch JB A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A 111, 16219–16224, doi: 10.1073/pnas.1408886111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans JA & Davidson AJ Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci 119, 283–323, doi: 10.1016/B978-0-12-396971-2.00010-5 [DOI] [PubMed] [Google Scholar]
- 10.Carskadon MA, Brown ED & Dement WC Sleep fragmentation in the elderly: Relationship to daytime sleep tendency. 3, 321–327, doi: 10.1016/0197-4580(82)90020-3 (1982). [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y & Endo S All-night sleep polygraphic recordings of healthy aged persons: REM and slow-wave sleep. Sleep 5, 277–283, doi: 10.1093/sleep/5.3.277 (1982). [DOI] [PubMed] [Google Scholar]
- 12.Foley DJ et al. Sleep Complaints Among Elderly Persons: An Epidemiologic Study of Three Communities. Sleep 18, 425–432, doi: 10.1093/sleep/18.6.425 (1995). [DOI] [PubMed] [Google Scholar]
- 13.Van Someren EJ Circadian rhythms and sleep in human aging. Chronobiol Int 17, 233–243, doi: 10.1081/cbi-100101046 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Okawa M et al. Circadian rhythm disorders in sleep-waking and body temperature in elderly patients with dementia and their treatment. Sleep 14, 478–485, doi: 10.1093/sleep/14.6.478 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Hatfield CF, Herbert J, van Someren EJ, Hodges JR & Hastings MH Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain 127, 1061–1074, doi: 10.1093/brain/awh129 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Morton AJ et al. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci 25, 157–163, doi: 10.1523/JNEUROSCI.3842-04.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musiek ES et al. Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol 75, 582–590, doi: 10.1001/jamaneurol.2017.4719 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musiek ES & Holtzman DM Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008, doi: 10.1126/science.aah4968 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breen DP et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 71, 589–595, doi: 10.1001/jamaneurol.2014.65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrier J, Land S, Buysse DJ, Kupfer DJ & Monk TH The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). 38, 232–242, doi: 10.1111/1469-8986.3820232 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Dijk DJ, Duffy JF & Czeisler CA Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int 17, 285–311, doi: 10.1081/cbi-100101049 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Dijk D & Czeisler C Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. The Journal of Neuroscience 15, 3526–3538, doi: 10.1523/jneurosci.15-05-03526.1995 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohayon MM, Carskadon MA, Guilleminault C & Vitiello MV Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifespan. Sleep 27, 1255–1273, doi: 10.1093/sleep/27.7.1255 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Landolt H-P, Dijk D-J, Achermann P & Borbély AA Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Research 738, 205–212, doi: 10.1016/s0006-8993(96)00770-6 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Holth JK et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884, doi: 10.1126/science.aav2546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iliff JJ et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra111, doi: 10.1126/scitranslmed.3003748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shokri-Kojori E et al. beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A 115, 4483–4488, doi: 10.1073/pnas.1721694115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ju Y-ES et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 140, 2104–2111, doi: 10.1093/brain/awx148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tasali E, Leproult R, Ehrmann DA & Van Cauter E Slow-wave sleep and the risk of type 2 diabetes in humans. Proceedings of the National Academy of Sciences 105, 1044–1049, doi: 10.1073/pnas.0706446105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasch B & Born J About Sleep’s Role in Memory. Physiological Reviews 93, 681–766, doi: 10.1152/physrev.00032.2012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaggioni G et al. Age-related decrease in cortical excitability circadian variations during sleep loss and its links with cognition. Neurobiol Aging 78, 52–63, doi: 10.1016/j.neurobiolaging.2019.02.004 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Duffy JF, Dijk D-J, Klerman EB & Czeisler CA Later endogenous circadian temperature nadir relative to an earlier wake time in older people. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 275, R1478–R1487, doi: 10.1152/ajpregu.1998.275.5.r1478 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Roenneberg T et al. Epidemiology of the human circadian clock. Sleep Medicine Reviews 11, 429–438, doi: 10.1016/j.smrv.2007.07.005 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Zhdanova IV et al. Aging of intrinsic circadian rhythms and sleep in a diurnal nonhuman primate, Macaca mulatta. J Biol Rhythms 26, 149–159, doi: 10.1177/0748730410395849 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Broms U et al. Long-term consistency of diurnal-type preferences among men. 31, 182–188, doi: 10.3109/07420528.2013.836534 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Wynchank D et al. Delayed sleep-onset and biological age: late sleep-onset is associated with shorter telomere length. Sleep 42, doi: 10.1093/sleep/zsz139 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Blackburn EH, Epel ES & Lin J Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198, doi: 10.1126/science.aab3389 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Sellix MT et al. Aging Differentially Affects the Re-entrainment Response of Central and Peripheral Circadian Oscillators. 32, 16193–16202, doi: 10.1523/jneurosci.3559-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monk TH, Buysse DJ, Carrier J & Kupfer DJ Inducing jet-lag in older people: Directional asymmetry. 9, 101–116, doi: 10.1046/j.1365-2869.2000.00184.x (2000). [DOI] [PubMed] [Google Scholar]
- 40.Davidson AJ et al. Chronic jet-lag increases mortality in aged mice. Current Biology 16, R914–R916, doi: 10.1016/j.cub.2006.09.058 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentinuzzi VS, Scarbrough K, Takahashi JS & Turek FW Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 273, R1957–R1964, doi: 10.1152/ajpregu.1997.273.6.r1957 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Refinetti R & Menaker M The circadian rhythm of body temperature. Physiology & Behavior 51, 613–637, doi: 10.1016/0031-9384(92)90188-8 (1992). [DOI] [PubMed] [Google Scholar]
- 43.Czeisler CA et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. 340, 933–936, doi: 10.1016/0140-6736(92)92817-y (1992). [DOI] [PubMed] [Google Scholar]
- 44.Monk TH, Buysse DJ, Reynolds CF, Kupfer DJ & Houck PR Circadian temperature rhythms of older people. 30, 455–474, doi: 10.1016/0531-5565(95)00007-4 (1995). [DOI] [PubMed] [Google Scholar]
- 45.Vitiello MV et al. Circadian temperature rhythms in young adult and aged men. 7, 97–100, doi: 10.1016/0197-4580(86)90146-6 (1986). [DOI] [PubMed] [Google Scholar]
- 46.Gubin DG, Gubin GD, Waterhouse J & Weinert D The Circadian Body Temperature Rhythm in the Elderly: Effect of Single Daily Melatonin Dosing. 23, 639–658, doi: 10.1080/07420520600650612 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Beker MC et al. Interaction of melatonin and Bmal1 in the regulation of PI3K/AKT pathway components and cellular survival. Sci Rep 9, 19082, doi: 10.1038/s41598-019-55663-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brzezinski A Melatonin in humans. N Engl J Med 336, 186–195, doi: 10.1056/NEJM199701163360306 (1997). [DOI] [PubMed] [Google Scholar]
- 49.Cagnacci A et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxf) 54, 339–346, doi: 10.1046/j.1365-2265.2001.01232.x (2001). [DOI] [PubMed] [Google Scholar]
- 50.Lyssenko V et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41, 82–88, doi: 10.1038/ng.288 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA & Garaulet M Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 37, 1715–1719, doi: 10.5665/sleep.4088 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardeland R et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 93, 350–384, doi: 10.1016/j.pneurobio.2010.12.004 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Skene DJ et al. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer’s disease. Brain Res 528, 170–174, doi: 10.1016/0006-8993(90)90214-v (1990). [DOI] [PubMed] [Google Scholar]
- 54.Kin NM, Nair NP, Schwartz G, Thavundayil JX & Annable L Secretion of melatonin in healthy elderly subjects: a longitudinal study. Ann N Y Acad Sci 1019, 326–329, doi: 10.1196/annals.1297.055 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Zhao ZY, Xie Y, Fu YR, Bogdan A & Touitou Y Aging and the circadian rhythm of melatonin: a cross-sectional study of Chinese subjects 30–110 yr of age. Chronobiol Int 19, 1171–1182, doi: 10.1081/cbi-120015958 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Duffy JF et al. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab 282, E297–303, doi: 10.1152/ajpendo.00268.2001 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Zeitzer JM et al. Do plasma melatonin concentrations decline with age? The American Journal of Medicine 107, 432–436, doi: 10.1016/s0002-9343(99)00266-1 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Waller KL et al. Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition. Nat Sci Sleep 8, 47–53, doi: 10.2147/NSS.S75946 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von Gall C & Weaver DR Loss of responsiveness to melatonin in the aging mouse suprachiasmatic nucleus. Neurobiol Aging 29, 464–470, doi: 10.1016/j.neurobiolaging.2006.10.015 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Wu YH, Zhou JN, Van Heerikhuize J, Jockers R & Swaab DF Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging 28, 1239–1247, doi: 10.1016/j.neurobiolaging.2006.06.002 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Oster H et al. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr Rev 38, 3–45, doi: 10.1210/er.2015-1080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balsalobre A et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347, doi: 10.1126/science.289.5488.2344 (2000). [DOI] [PubMed] [Google Scholar]
- 63.Oster H et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4, 163–173, doi: 10.1016/j.cmet.2006.07.002 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Van Cauter E, Leproult R & Plat L Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 284, 861–868, doi: 10.1001/jama.284.7.861 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Vgontzas AN et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab 88, 2087–2095, doi: 10.1210/jc.2002-021176 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Kumari M et al. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology 35, 1091–1099, doi: 10.1016/j.psyneuen.2010.01.010 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Van Cauter E, Leproult R & Kupfer DJ Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81, 2468–2473, doi: 10.1210/jcem.81.7.8675562 (1996). [DOI] [PubMed] [Google Scholar]
- 68.Sherman B, Wysham C & Pfohl B Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab 61, 439–443, doi: 10.1210/jcem-61-3-439 (1985). [DOI] [PubMed] [Google Scholar]
- 69.Touitou Y et al. Adrenal circadian system in young and elderly human subjects: a comparative study. J Endocrinol 93, 201–210, doi: 10.1677/joe.0.0930201 (1982). [DOI] [PubMed] [Google Scholar]
- 70.Schouten M et al. Circadian glucocorticoid oscillations preserve a population of adult hippocampal neural stem cells in the aging brain. Molecular Psychiatry, doi: 10.1038/s41380-019-0440-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamura TJ et al. Age-related decline in circadian output. J Neurosci 31, 10201–10205, doi: 10.1523/JNEUROSCI.0451-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farajnia S et al. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci 32, 5891–5899, doi: 10.1523/JNEUROSCI.0469-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Curran JA, Buhl E, Tsaneva-Atanasova K & Hodge JJL Age-dependent changes in clock neuron structural plasticity and excitability are associated with a decrease in circadian output behavior and sleep. Neurobiology of Aging 77, 158–168, doi: 10.1016/j.neurobiolaging.2019.01.025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofman MA & Swaab DF Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res 651, 134–142, doi: 10.1016/0006-8993(94)90689-0 (1994). [DOI] [PubMed] [Google Scholar]
- 75.Zhou JN, Hofman MA & Swaab DF VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging 16, 571–576, doi: 10.1016/0197-4580(95)00043-e (1995). [DOI] [PubMed] [Google Scholar]
- 76.Chee CA, Roozendaal B, Swaab DF, Goudsmit E & Mirmiran M Vasoactive intestinal polypeptide neuron changes in the senile rat suprachiasmatic nucleus. Neurobiol Aging 9, 307–312, doi: 10.1016/s0197-4580(88)80070-8 (1988). [DOI] [PubMed] [Google Scholar]
- 77.Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE & Mirmiran M Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res 409, 259–264, doi: 10.1016/0006-8993(87)90710-4 (1987). [DOI] [PubMed] [Google Scholar]
- 78.Palomba M et al. Decline of the presynaptic network, including GABAergic terminals, in the aging suprachiasmatic nucleus of the mouse. J Biol Rhythms 23, 220–231, doi: 10.1177/0748730408316998 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Aton SJ, Colwell CS, Harmar AJ, Waschek J & Herzog ED Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8, 476–483, doi: 10.1038/nn1419 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aton SJ, Huettner JE, Straume M & Herzog ED GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci U S A 103, 19188–19193, doi: 10.1073/pnas.0607466103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herzog ED, Aton SJ, Numano R, Sakaki Y & Tei H Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms 19, 35–46, doi: 10.1177/0748730403260776 (2004). [DOI] [PubMed] [Google Scholar]
- 82.Vasalou C, Herzog ED & Henson MA Small-World Network Models of Intercellular Coupling Predict Enhanced Synchronization in the Suprachiasmatic Nucleus. 24, 243–254, doi: 10.1177/0748730409333220 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang JL et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol 78, 317–322, doi: 10.1002/ana.24432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wyse CA & Coogan AN Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res 1337, 21–31, doi: 10.1016/j.brainres.2010.03.113 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Kolker DE et al. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms 18, 159–169, doi: 10.1177/0748730403251802 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Nakamura TJ et al. Age-Related Changes in the Circadian System Unmasked by Constant Conditions. eNeuro 2, doi: 10.1523/ENEURO.0064-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang HC & Guarente L SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153, 1448–1460, doi: 10.1016/j.cell.2013.05.027 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamazaki S et al. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A 99, 10801–10806, doi: 10.1073/pnas.152318499 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asai M et al. Circadian profile ofPer gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. 66, 1133–1139, doi: 10.1002/jnr.10010 (2001). [DOI] [PubMed] [Google Scholar]
- 90.Davidson AJ, Yamazaki S, Arble DM, Menaker M & Block GD Resetting of central and peripheral circadian oscillators in aged rats. Neurobiology of Aging 29, 471–477, doi: 10.1016/j.neurobiolaging.2006.10.018 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato S et al. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell 170, 664–677.e611, doi: 10.1016/j.cell.2017.07.042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Novosadova Z, Polidarova L, Sladek M & Sumova A Alteration in glucose homeostasis and persistence of the pancreatic clock in aged mPer2(Luc) mice. Sci Rep 8, 11668, doi: 10.1038/s41598-018-30225-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bonaconsa M et al. Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Exp Gerontol 55, 70–79, doi: 10.1016/j.exger.2014.03.011 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Solanas G et al. Aged Stem Cells Reprogram Their Daily Rhythmic Functions to Adapt to Stress. Cell 170, 678–692.e620, doi: 10.1016/j.cell.2017.07.035 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Kunieda T et al. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ Res 98, 532–539, doi: 10.1161/01.RES.0000204504.25798.a8 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Chen CY et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A 113, 206–211, doi: 10.1073/pnas.1508249112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wyse CA, Coogan AN, Selman C, Hazlerigg DG & Speakman JR Association between mammalian lifespan and circadian free-running period: the circadian resonance hypothesis revisited. Biology Letters 6, 696–698, doi: 10.1098/rsbl.2010.0152 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Libert S, Bonkowski MS, Pointer K, Pletcher SD & Guarente L Deviation of innate circadian period from 24 h reduces longevity in mice. Aging Cell 11, 794–800, doi: 10.1111/j.1474-9726.2012.00846.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cai A, Scarbrough K, Hinkle DA & Wise PM Fetal grafts containing suprachiasmatic nuclei restore the diurnal rhythm of CRH and POMC mRNA in aging rats. Am J Physiol 273, R1764–1770, doi: 10.1152/ajpregu.1997.273.5.R1764 (1997). [DOI] [PubMed] [Google Scholar]
- 100.Li H & Satinoff E Fetal tissue containing the suprachiasmatic nucleus restores multiple circadian rhythms in old rats. Am J Physiol 275, R1735–1744, doi: 10.1152/ajpregu.1998.275.6.R1735 (1998). [DOI] [PubMed] [Google Scholar]
- 101.Hurd MW & Ralph MR The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms 13, 430–436, doi: 10.1177/074873098129000255 (1998). [DOI] [PubMed] [Google Scholar]
- 102.Curtis AM et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci U S A 112, 7231–7236, doi: 10.1073/pnas.1501327112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marpegan L et al. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int 26, 1430–1442, doi: 10.3109/07420520903408358 (2009). [DOI] [PubMed] [Google Scholar]
- 104.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV & Antoch MP Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20, 1868–1873, doi: 10.1101/gad.1432206 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fu L, Pelicano H, Liu J, Huang P & Lee CC The Circadian Gene Period2 Plays an Important Role in Tumor Suppression and DNA Damage Response In Vivo. Cell 111, 41–50, doi: 10.1016/s0092-8674(02)00961-3 (2002). [DOI] [PubMed] [Google Scholar]
- 106.Marcheva B et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631, doi: 10.1038/nature09253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang G et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Science Translational Medicine 8, 324ra316–324ra316, doi: 10.1126/scitranslmed.aad3305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McDearmon EL et al. Dissecting the Functions of the Mammalian Clock Protein BMAL1 by Tissue-Specific Rescue in Mice. Science 314, 1304–1308, doi: 10.1126/science.1132430 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adrover JM et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 50, 390–402 e310, doi: 10.1016/j.immuni.2019.01.002 (2019). [DOI] [PubMed] [Google Scholar]
- 110.Early JO et al. Circadian clock protein BMAL1 regulates IL-1beta in macrophages via NRF2. Proc Natl Acad Sci U S A 115, E8460–E8468, doi: 10.1073/pnas.1800431115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gibbs JE et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A 109, 582–587, doi: 10.1073/pnas.1106750109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nguyen KD et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341, 1483–1488, doi: 10.1126/science.1240636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dyar KA et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab 3, 29–41, doi: 10.1016/j.molmet.2013.10.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jacobi D et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab 22, 709–720, doi: 10.1016/j.cmet.2015.08.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Musiek ES et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. Journal of Clinical Investigation 123, 5389–5400, doi: 10.1172/jci70317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stenvers DJ, Scheer FAJL, Schrauwen P, La Fleur SE & Kalsbeek A Circadian clocks and insulin resistance. Nature Reviews Endocrinology 15, 75–89, doi: 10.1038/s41574-018-0122-1 (2019). [DOI] [PubMed] [Google Scholar]
- 117.Chaix A, Lin T, Le HD, Chang MW & Panda S Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab 29, 303–319 e304, doi: 10.1016/j.cmet.2018.08.004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Villanueva JE et al. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nature Communications 10, doi: 10.1038/s41467-019-10563-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jamshed H et al. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 11, 1234, doi: 10.3390/nu11061234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barnes CA, McNaughton BL, Goddard GV, Douglas RM & Adamec R Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science 197, 91–92, doi: 10.1126/science.194313 (1977). [DOI] [PubMed] [Google Scholar]
- 121.Chaudhury D, Wang LM & Colwell CS Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms 20, 225–236, doi: 10.1177/0748730405276352 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smarr BL, Jennings KJ, Driscoll JR & Kriegsfeld LJ A time to remember: the role of circadian clocks in learning and memory. Behav Neurosci 128, 283–303, doi: 10.1037/a0035963 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eckel-Mahan KL et al. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nature Neuroscience 11, 1074–1082, doi: 10.1038/nn.2174 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Paschos GK et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med 18, 1768–1777, doi: 10.1038/nm.2979 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Graw P, Krauchi K, Knoblauch V, Wirz-Justice A & Cajochen C Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav 80, 695–701, doi: 10.1016/j.physbeh.2003.12.004 (2004). [DOI] [PubMed] [Google Scholar]
- 126.Scott JP, McNaughton LR & Polman RC Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiol Behav 87, 396–408, doi: 10.1016/j.physbeh.2005.11.009 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Peek CB et al. Circadian Clock Interaction with HIF1alpha Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab 25, 86–92, doi: 10.1016/j.cmet.2016.09.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sato S et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab 30, 92–110 e114, doi: 10.1016/j.cmet.2019.03.013 (2019). [DOI] [PubMed] [Google Scholar]
- 129.Ezagouri S et al. Physiological and Molecular Dissection of Daily Variance in Exercise Capacity. Cell Metab 30, 78–91 e74, doi: 10.1016/j.cmet.2019.03.012 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Fonken LK, Kitsmiller E, Smale L & Nelson RJ Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J Biol Rhythms 27, 319–327, doi: 10.1177/0748730412448324 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Wefers J et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proceedings of the National Academy of Sciences 115, 7789–7794, doi: 10.1073/pnas.1722295115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH & McEwen BS Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A 108, 1657–1662, doi: 10.1073/pnas.1018375108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lieu SJ, Curhan GC, Schernhammer ES & Forman JP Rotating night shift work and disparate hypertension risk in African-Americans. J Hypertens 30, 61–66, doi: 10.1097/HJH.0b013e32834e1ea3 (2012). [DOI] [PubMed] [Google Scholar]
- 134.Suwazono Y et al. Shift work is a risk factor for increased blood pressure in Japanese men: a 14-year historical cohort study. Hypertension 52, 581–586, doi: 10.1161/HYPERTENSIONAHA.108.114553 (2008). [DOI] [PubMed] [Google Scholar]
- 135.Morris CJ, Purvis TE, Hu K & Scheer FA Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A 113, E1402–1411, doi: 10.1073/pnas.1516953113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Curtis AM et al. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A 104, 3450–3455, doi: 10.1073/pnas.0611680104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beker MC et al. Time-of-Day Dependent Neuronal Injury After Ischemic Stroke: Implication of Circadian Clock Transcriptional Factor Bmal1 and Survival Kinase AKT. Mol Neurobiol 55, 2565–2576, doi: 10.1007/s12035-017-0524-4 (2018). [DOI] [PubMed] [Google Scholar]
- 138.Thosar SS, Butler MP & Shea SA Role of the circadian system in cardiovascular disease. J Clin Invest 128, 2157–2167, doi: 10.1172/JCI80590 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muller JE et al. Circadian Variation in the Frequency of Onset of Acute Myocardial Infarction. New England Journal of Medicine 313, 1315–1322, doi: 10.1056/nejm198511213132103 (1985). [DOI] [PubMed] [Google Scholar]
- 140.Panza JA, Epstein SE & Quyyumi AA Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med 325, 986–990, doi: 10.1056/NEJM199110033251402 (1991). [DOI] [PubMed] [Google Scholar]
- 141.Slat EA et al. Cell-intrinsic, Bmal1-dependent Circadian Regulation of Temozolomide Sensitivity in Glioblastoma. J Biol Rhythms 32, 121–129, doi: 10.1177/0748730417696788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paschos GK, Baggs JE, Hogenesch JB & FitzGerald GA The role of clock genes in pharmacology. Annu Rev Pharmacol Toxicol 50, 187–214, doi: 10.1146/annurev.pharmtox.010909.105621 (2010). [DOI] [PubMed] [Google Scholar]
- 143.Borniger JC et al. Time-of-Day Dictates Transcriptional Inflammatory Responses to Cytotoxic Chemotherapy. Sci Rep 7, 41220, doi: 10.1038/srep41220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ramanathan C et al. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet 14, e1007369, doi: 10.1371/journal.pgen.1007369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cao R et al. Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling. Neuron 79, 712–724, doi: 10.1016/j.neuron.2013.06.026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khapre RV et al. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging (Albany NY) 6, 48–57, doi: 10.18632/aging.100633 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Imai S “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim Biophys Acta 1804, 1584–1590, doi: 10.1016/j.bbapap.2009.10.024 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu JJ et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell Rep 4, 913–920, doi: 10.1016/j.celrep.2013.07.030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lamming DW et al. Rapamycin-Induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science 335, 1638–1643, doi: 10.1126/science.1215135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Satoh A et al. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metabolism 18, 416–430, doi: 10.1016/j.cmet.2013.07.013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Imai S.-i. & Guarente L NAD+ and sirtuins in aging and disease. Trends in Cell Biology 24, 464–471, doi: 10.1016/j.tcb.2014.04.002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harrison DE et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395, doi: 10.1038/nature08221 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nakahata Y et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340, doi: 10.1016/j.cell.2008.07.002 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Asher G et al. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 134, 317–328, doi: 10.1016/j.cell.2008.06.050 (2008). [DOI] [PubMed] [Google Scholar]
- 155.Vaziri H et al. hSIR2SIRT1 Functions as an NAD-Dependent p53 Deacetylase. Cell 107, 149–159, doi: 10.1016/s0092-8674(01)00527-x (2001). [DOI] [PubMed] [Google Scholar]
- 156.Revollo JR, Grimm AA & Imai S The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279, 50754–50763, doi: 10.1074/jbc.M408388200 (2004). [DOI] [PubMed] [Google Scholar]
- 157.Ramsey KM et al. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science 324, 651–654, doi: 10.1126/science.1171641 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakahata Y, Sahar S, Astarita G, Kaluzova M & Sassone-Corsi P Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657, doi: 10.1126/science.1170803 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O’Neill JS & Reddy AB Circadian clocks in human red blood cells. Nature 469, 498–503, doi: 10.1038/nature09702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peek CB et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science (New York, N.Y 342, 1243417, doi: 10.1126/science.1243417 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yoshino J, Baur JA & Imai S.-i. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metabolism 27, 513–528, doi: 10.1016/j.cmet.2017.11.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fang EF et al. NAD(+) in Aging: Molecular Mechanisms and Translational Implications. Trends Mol Med 23, 899–916, doi: 10.1016/j.molmed.2017.08.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lautrup S, Sinclair DA, Mattson MP & Fang EF NAD(+) in Brain Aging and Neurodegenerative Disorders. Cell Metab 30, 630–655, doi: 10.1016/j.cmet.2019.09.001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stein LR & Imai S. i. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. The EMBO Journal, doi: 10.1002/embj.201386917 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoshida M et al. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metabolism, S1550413119302554, doi: 10.1016/j.cmet.2019.05.015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yoshino J, Mills, Kathryn F, Yoon, Myeong J & Imai S.-i. Nicotinamide Mononucleotide, a Key NAD+ Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metabolism 14, 528–536, doi: 10.1016/j.cmet.2011.08.014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mitchell SJ et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metabolism 23, 1093–1112, doi: 10.1016/j.cmet.2016.05.027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Makwana K, Gosai N, Poe A & Kondratov RV Calorie restriction reprograms diurnal rhythms in protein translation to regulate metabolism. FASEB J 33, 4473–4489, doi: 10.1096/fj.201802167R (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Panda S Circadian physiology of metabolism. Science 354, 1008–1015, doi: 10.1126/science.aah4967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang D et al. Liver clock protein BMAL1 promotes de novo lipogenesis through insulin-mTORC2-AKT signaling. J Biol Chem 289, 25925–25935, doi: 10.1074/jbc.M114.567628 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kenyon C, Chang J, Gensch E, Rudner A & Tabtiang RAC. elegans mutant that lives twice as long as wild type. Nature 366, 461–464, doi: 10.1038/366461a0 (1993). [DOI] [PubMed] [Google Scholar]
- 172.Tatar M A Mutant Drosophila Insulin Receptor Homolog That Extends Life-Span and Impairs Neuroendocrine Function. Science 292, 107–110, doi: 10.1126/science.1057987 (2001). [DOI] [PubMed] [Google Scholar]
- 173.Selman C et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J 22, 807–818, doi: 10.1096/fj.07-9261com (2008). [DOI] [PubMed] [Google Scholar]
- 174.Dang F et al. Insulin post-transcriptionally modulates Bmal1 protein to affect the hepatic circadian clock. Nat Commun 7, 12696, doi: 10.1038/ncomms12696 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lipton JO et al. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell 161, 1138–1151, doi: 10.1016/j.cell.2015.04.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Patel SA, Chaudhari A, Gupta R, Velingkaar N & Kondratov RV Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J 30, 1634–1642, doi: 10.1096/fj.15-282475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yoo SH et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 101, 5339–5346, doi: 10.1073/pnas.0308709101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Abe M et al. Circadian rhythms in isolated brain regions. J Neurosci 22, 350–356 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fonken LK et al. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 45, 171–179, doi: 10.1016/j.bbi.2014.11.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hayashi Y et al. The intrinsic microglial molecular clock controls synaptic strength via the circadian expression of cathepsin S. Sci Rep 3, 2744, doi: 10.1038/srep02744 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marpegan L, Krall TJ & Herzog ED Vasoactive intestinal polypeptide entrains circadian rhythms in astrocytes. J Biol Rhythms 24, 135–143, doi: 10.1177/0748730409332042 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Duhart JM et al. Suprachiasmatic astrocytes modulate the circadian clock in response to TNF-alpha. J Immunol 191, 4656–4664, doi: 10.4049/jimmunol.1300450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marpegan L et al. Circadian regulation of ATP release in astrocytes. J Neurosci 31, 8342–8350, doi: 10.1523/JNEUROSCI.6537-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Koyanagi S et al. Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat Commun 7, 13102, doi: 10.1038/ncomms13102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Womac AD, Burkeen JF, Neuendorff N, Earnest DJ & Zoran MJ Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci 30, 869–876, doi: 10.1111/j.1460-9568.2009.06874.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Beaule C, Swanstrom A, Leone MJ & Herzog ED Circadian modulation of gene expression, but not glutamate uptake, in mouse and rat cortical astrocytes. PLoS One 4, e7476, doi: 10.1371/journal.pone.0007476 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ng FS, Tangredi MM & Jackson FR Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol 21, 625–634, doi: 10.1016/j.cub.2011.03.027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Barca-Mayo O et al. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun 8, 14336, doi: 10.1038/ncomms14336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tso CF et al. Astrocytes Regulate Daily Rhythms in the Suprachiasmatic Nucleus and Behavior. Curr Biol 27, 1055–1061, doi: 10.1016/j.cub.2017.02.037 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brancaccio M, Patton AP, Chesham JE, Maywood ES & Hastings MH Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 93, 1420–1435 e1425, doi: 10.1016/j.neuron.2017.02.030 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brancaccio M et al. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 363, 187–192, doi: 10.1126/science.aat4104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McKee CA, Lananna BV & Musiek ES Circadian regulation of astrocyte function: implications for Alzheimer’s disease. Cell Mol Life Sci, doi: 10.1007/s00018-019-03314-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cuddapah VA, Zhang SL & Sehgal A Regulation of the Blood-Brain Barrier by Circadian Rhythms and Sleep. Trends Neurosci 42, 500–510, doi: 10.1016/j.tins.2019.05.001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Clarke LE et al. Normal aging induces A1-like astrocyte reactivity. Proceedings of the National Academy of Sciences 115, E1896–E1905, doi: 10.1073/pnas.1800165115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Boisvert MM, Erikson GA, Shokhirev MN & Allen NJ The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep 22, 269–285, doi: 10.1016/j.celrep.2017.12.039 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Grabert K et al. Microglial brain region−dependent diversity and selective regional sensitivities to aging. Nature Neuroscience 19, 504–516, doi: 10.1038/nn.4222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Soreq L et al. Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Reports 18, 557–570, doi: 10.1016/j.celrep.2016.12.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Scheiermann C, Gibbs J, Ince L & Loudon A Clocking in to immunity. Nat Rev Immunol 18, 423–437, doi: 10.1038/s41577-018-0008-4 (2018). [DOI] [PubMed] [Google Scholar]
- 199.Guerrero-Vargas NN et al. Reciprocal interaction between the suprachiasmatic nucleus and the immune system tunes down the inflammatory response to lipopolysaccharide. J Neuroimmunol 273, 22–30, doi: 10.1016/j.jneuroim.2014.05.012 (2014). [DOI] [PubMed] [Google Scholar]
- 200.Adams KL, Castanon-Cervantes O, Evans JA & Davidson AJ Environmental circadian disruption elevates the IL-6 response to lipopolysaccharide in blood. J Biol Rhythms 28, 272–277, doi: 10.1177/0748730413494561 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Castanon-Cervantes O et al. Dysregulation of Inflammatory Responses by Chronic Circadian Disruption. 185, 5796–5805, doi: 10.4049/jimmunol.1001026 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Qiu P et al. BMAL1 knockout macaque monkeys display reduced sleep and psychiatric disorders. National Science Review 6, 87–100, doi: 10.1093/nsr/nwz002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Keller M et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A 106, 21407–21412, doi: 10.1073/pnas.0906361106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Druzd D et al. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 46, 120–132, doi: 10.1016/j.immuni.2016.12.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Petrovsky N & Harrison LC Diurnal rhythmicity of human cytokine production: a dynamic disequilibrium in T helper cell type 1/T helper cell type 2 balance? J Immunol 158, 5163–5168 (1997). [PubMed] [Google Scholar]
- 206.Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J & Gogenur I Pronounced inflammatory response to endotoxaemia during nighttime: a randomised cross-over trial. PLoS One 9, e87413, doi: 10.1371/journal.pone.0087413 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hermann C et al. Endogenous cortisol determines the circadian rhythm of lipopolysaccharide-- but not lipoteichoic acid--inducible cytokine release. Eur J Immunol 36, 371–379, doi: 10.1002/eji.200535470 (2006). [DOI] [PubMed] [Google Scholar]
- 208.Spengler ML et al. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc Natl Acad Sci U S A 109, E2457–2465, doi: 10.1073/pnas.1206274109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sato S et al. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol 192, 407–417, doi: 10.4049/jimmunol.1301982 (2014). [DOI] [PubMed] [Google Scholar]
- 210.Liu J et al. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun 74, 4750–4756, doi: 10.1128/IAI.00287-06 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Nakazato R et al. Selective Upregulation of Per1 mRNA Expression by ATP Through Activation of P2X7 Purinergic Receptors Expressed in Microglial Cells. Journal of Pharmacological Sciences 116, 350–361, doi: 10.1254/jphs.11069FP (2011). [DOI] [PubMed] [Google Scholar]
- 212.Fonken LK et al. Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol Aging 47, 102–112, doi: 10.1016/j.neurobiolaging.2016.07.019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lananna BV et al. Cell-Autonomous Regulation of Astrocyte Activation by the Circadian Clock Protein BMAL1. Cell Rep 25, 1–9 e5, doi: 10.1016/j.celrep.2018.09.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ishii T, Warabi E & Mann GE Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic Biol Med 133, 169–178, doi: 10.1016/j.freeradbiomed.2018.09.002 (2019). [DOI] [PubMed] [Google Scholar]
- 215.Sugimoto T et al. Clock gene Per1 regulates the production of CCL2 and interleukin-6 through p38, JNK1 and NF-kappaB activation in spinal astrocytes. Mol Cell Neurosci 59, 37–46, doi: 10.1016/j.mcn.2014.01.003 (2014). [DOI] [PubMed] [Google Scholar]
- 216.Griffin P et al. Circadian clock protein Rev-erbalpha regulates neuroinflammation. Proc Natl Acad Sci U S A 116, 5102–5107, doi: 10.1073/pnas.1812405116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nakazato R et al. The intrinsic microglial clock system regulates interleukin-6 expression. Glia 65, 198–208, doi: 10.1002/glia.23087 (2017). [DOI] [PubMed] [Google Scholar]
- 218.Curtis AM, Bellet MM, Sassone-Corsi P & O’Neill LA Circadian clock proteins and immunity. Immunity 40, 178–186, doi: 10.1016/j.immuni.2014.02.002 (2014). [DOI] [PubMed] [Google Scholar]
- 219.Stangherlin A & Reddy AB Regulation of circadian clocks by redox homeostasis. The Journal of biological chemistry 288, 26505–26511, doi: 10.1074/jbc.R113.457564 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rutter J, Reick M, Wu LC & McKnight SL Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293, 510–514, doi: 10.1126/science.1060698 [DOI] [PubMed] [Google Scholar]
- 221.Khapre RV, Kondratova AA, Susova O & Kondratov RV Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle 10, 4162–4169 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pei JF et al. Diurnal oscillations of endogenous H2O2 sustained by p66(Shc) regulate circadian clocks. Nat Cell Biol 21, 1553–1564, doi: 10.1038/s41556-019-0420-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rey G et al. The Pentose Phosphate Pathway Regulates the Circadian Clock. Cell Metab, doi: 10.1016/j.cmet.2016.07.024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wible RS et al. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife 7, doi: 10.7554/eLife.31656 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wang TA et al. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science (New York, N.Y 337, 839–842 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Krishnan N, Kretzschmar D, Rakshit K, Chow E & Giebultowicz JM The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging (Albany NY) 1, 937–948 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Krishnan N et al. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol Dis 45, 1129–1135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Beaver LM et al. Circadian regulation of glutathione levels and biosynthesis in Drosophila melanogaster. PloS one 7, e50454, doi: 10.1371/journal.pone.0050454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xu YQ et al. Diurnal variation of hepatic antioxidant gene expression in mice. PLoS One 7, e44237, doi: 10.1371/journal.pone.0044237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lee J et al. Bmal1 and beta-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced beta-cell failure in mice. Mol Cell Biol 33, 2327–2338, doi: 10.1128/MCB.01421-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pekovic-Vaughan V et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev 28, 548–560, doi: 10.1101/gad.237081.113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sengupta S et al. The circadian gene Rev-erbalpha improves cellular bioenergetics and provides preconditioning for protection against oxidative stress. Free Radic Biol Med 93, 177–189, doi: 10.1016/j.freeradbiomed.2016.02.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yang G et al. Oxidative stress and inflammation modulate Rev-erbalpha signaling in the neonatal lung and affect circadian rhythmicity. Antioxid Redox Signal 21, 17–32, doi: 10.1089/ars.2013.5539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Woldt E et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 19, 1039–1046, doi: 10.1038/nm.3213 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kuintzle RC et al. Circadian deep sequencing reveals stress-response genes that adopt robust rhythmic expression during aging. Nat Commun 8, 14529, doi: 10.1038/ncomms14529 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Videnovic A, Lazar AS, Barker RA & Overeem S ‘The clocks that time us’--circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 10, 683–693, doi: 10.1038/nrneurol.2014.206 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Musiek ES, Xiong DD & Holtzman DM Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med 47, e148, doi: 10.1038/emm.2014.121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jack CR Jr. et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562, doi: 10.1016/j.jalz.2018.02.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ju YE et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol 70, 587–593, doi: 10.1001/jamaneurol.2013.2334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lim AS, Kowgier M, Yu L, Buchman AS & Bennett DA Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 36, 1027–1032, doi: 10.5665/sleep.2802 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tranah GJ et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 70, 722–732 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Swaab DF, Fliers E & Partiman TS The Suprachiasmatic Nucleus of the Human-Brain in Relation to Sex, Age and Senile Dementia. Brain Research 342, 37–44, doi:Doi 10.1016/0006-8993(85)91350-2 (1985). [DOI] [PubMed] [Google Scholar]
- 243.Skene DJ & Swaab DF Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol 38, 199–206 (2003). [DOI] [PubMed] [Google Scholar]
- 244.Uchida K, Okamoto N, Ohara K & Morita Y Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res 717, 154–159 (1996). [DOI] [PubMed] [Google Scholar]
- 245.Cronin P et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement 13, 689–700, doi: 10.1016/j.jalz.2016.10.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Song H et al. Abeta-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol Neurodegener 10, 13, doi: 10.1186/s13024-015-0007-x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kang JE et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kress GJ et al. Regulation of amyloid-beta dynamics and pathology by the circadian clock. J Exp Med 215, 1059–1068, doi: 10.1084/jem.20172347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bero AW et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci 14, 750–756, doi: 10.1038/nn.2801 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Xie L et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377, doi: 10.1126/science.1241224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huang Y et al. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol 69, 51–58, doi: 10.1001/archneurol.2011.235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lucey BP et al. Effect of sleep on overnight CSF amyloid-beta kinetics. Ann Neurol, doi: 10.1002/ana.25117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhu Y et al. Chronic Sleep Disruption Advances the Temporal Progression of Tauopathy in P301S Mutant Mice. J Neurosci 38, 10255–10270, doi: 10.1523/JNEUROSCI.0275-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Di Meco A, Joshi YB & Pratico D Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging 35, 1813–1820, doi: 10.1016/j.neurobiolaging.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 255.Ma D, Panda S & Lin JD Temporal orchestration of circadian autophagy rhythm by C/EBPbeta. EMBO J 30, 4642–4651, doi: 10.1038/emboj.2011.322 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Desvergne A, Ugarte N, Petropoulos I & Friguet B Circadian modulation of proteasome activities and removal of carbonylated proteins. Free Radic Biol Med 75 Suppl 1, S18, doi: 10.1016/j.freeradbiomed.2014.10.631 (2014). [DOI] [PubMed] [Google Scholar]
- 257.Singer C et al. A Multicenter, Placebo-controlled Trial of Melatonin for Sleep Disturbance in Alzheimer’s Disease. Sleep 26, 893–901, doi: 10.1093/sleep/26.7.893 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gehrman PR et al. Melatonin Fails to Improve Sleep or Agitation in Double-Blind Randomized Placebo-Controlled Trial of Institutionalized Patients With Alzheimer Disease. The American Journal of Geriatric Psychiatry 17, 166–169, doi: 10.1097/jgp.0b013e318187de18 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Xu J et al. Melatonin for sleep disorders and cognition in dementia: a meta-analysis of randomized controlled trials. Am J Alzheimers Dis Other Demen 30, 439–447, doi: 10.1177/1533317514568005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dowling GA et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. J Am Geriatr Soc 56, 239–246, doi: 10.1111/j.1532-5415.2007.01543.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Riemersma-Van Der Lek RF Effect of Bright Light and Melatonin on Cognitive and Noncognitive Function in Elderly Residents of Group Care Facilities. JAMA 299, 2642, doi: 10.1001/jama.299.22.2642 (2008). [DOI] [PubMed] [Google Scholar]
- 262.Solt LA et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68, doi: 10.1038/nature11030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hirota T et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol 8, e1000559, doi: 10.1371/journal.pbio.1000559 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Oshima T et al. Cell-based screen identifies a new potent and highly selective CK2 inhibitor for modulation of circadian rhythms and cancer cell growth. Sci Adv 5, eaau9060, doi: 10.1126/sciadv.aau9060 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]