Graphical abstract
Keywords: Hydrogen sulfide, L-cysteine, L-cysteine desulfhydrase, Plant defence, Plant signalling, Sulfur metabolism
Abstract
Background
Sulfur and diverse sulfur-containing compounds constitute important components of plant defences against a wide array of microbial pathogens. Among them, hydrogen sulfide (H2S) occupies a prominent position as a gaseous signalling molecule that plays multiple roles in regulation of plant growth, development and plant responses to stress conditions. Although the production of H2S in plant cells has been discovered several decades ago, the underlying pathways of H2S biosynthesis, metabolism and signalling were only recently uncovered.
Aim of the review
Here we review the current knowledge on the biosynthesis of H2S in plant cells, with special attention to L-cysteine desulfhydrase (DES) as the key enzyme controlling H2S levels biosynthesis in the cytosol of plant cells during plant growth, development and diverse abiotic and biotic stress conditions.
Key Scientific Concepts of Review
Recent advances have revealed molecular mechanisms of DES properties, functions and regulation involved in modulations of H2S production during plant responses to abiotic and biotic stress stimuli. Studies on des mutants of the model plant Arabidopsis thaliana uncovered molecular mechanisms of H2S action as a signalling and defence molecule in plant-pathogen interactions. Signalling pathways of H2S include S-persulfidation of protein cysteines, a redox-based post-translational modification leading to activation of downstream components of H2S signalling. Accumulated evidence shows DES and H2S implementation into salicylic acid signalling and activation of pathogenesis-related proteins and autophagy within plant immunity. Obtained knowledge on molecular mechanisms of H2S action in plant defence responses opens new prospects in the search for crop varieties with increased resistance to bacterial and fungal pathogens.
Introduction
Hydrogen sulfide (H2S) has been recognized as a multifaceted gasotransmitter involved in a diverse array of biological processes across all kingdoms including plants [1], [2], [3]. Since the first observation of H2S emissions from leaves of several plant species by Wilson et al. 1978 [4], H2S has emerged as a vital signalling molecule involved in the regulation of multiple mechanisms in plant growth and development and responses to external stimuli. H2S was identified as an important component of signalling pathways regulating stomatal closure [5], [6], [7], root organogenesis [8] and photosynthesis [9]. Hydrogen sulfide also plays important roles in fruit biology and freshness and regulation of postharvest senescence of horticultural products. Application of H2S in the form of aqueous solutions of sodium hydrosulfide (NaHS) or sodium sulfide (Na2S) can decelerate fruit ripening and senescence in numerous fruits, partially by inhibiting the growth of fungal spores [10], [11].
Under stress conditions, H2S can shape plants ability of adaptation to diverse environmental stimuli by alleviating stress-induced injuries and activation of defence mechanisms [12], [13], [14]. Hydrogen sulfide can mediate enhancement of plant tolerance to salinity, drought, heavy metal and high-temperature stress, based on priming effect of H2S on plant redox signalling, antioxidant capacity and specific components of cellular defence. [15], [16], [17]. Exogenous application of H2S induces plant cross-adaptation to multiple abiotic stresses [18].
Signalling functions of H2S in animal cells are known to be mediated namely by protein persulfidation of protein cysteines, interactions with metal centres in the protein active sites and reactions with S-nitrosothiols and electrophilic compounds [3]. Importantly, the biological functions of H2S in plants involve interactions and cross-talk with signalling pathways of other plant gasotransmitters and reactive nitrogen and oxygen species [19], [20], [21], [22], [23], [24], [25]. Complex interactions of signalling pathways of H2S and nitric oxide (NO) were revealed to regulate stomatal movement in plant leaves [19] where 8-mercapto-cGMP was shown as the active component of H2S-mediated guard cell signalling [26]. Furthermore, biological functions of H2S in plant growth, development and responses to abiotic stresses are determined by a dual role of H2S in interactions with phytohormones. Endogenous H2S levels are regulated by phytohormones, whereas H2S can influence the production, transport, and signalling pathways of diverse plant hormones in plant physiological responses [27].
The role of H2S in plant signalling and responses to abiotic stresses has attracted considerable attention and has been extensively reviewed elsewhere [14], [18], [21], [28]. Within this special issue, Corpas and Palma [29] provide an excellent overview of the current state of knowledge on H2S signalling in plants and potential application to increased plant performance under conditions of diverse environmental stresses.
It has become evident that similarly to reactive nitrogen and oxygen species, H2S can perform a dual role in plant pathogenesis, i.e. signalling functions and direct inhibitory or toxic actions towards penetrating pathogens [30]. Crop fertilization with sulfur has been known for long to stimulate plant resistance, which is actually known to be mediated by H2S. Pathogen resistance can be also potentiated by H2S by induced expression of salicylic acid-dependent pathogen-related genes [30]. A substantial part of available published reports comprises rather descriptive studies performed by plant or fruit treatments with exogenous sulfide that has not provided deeper mechanistic insights into H2S biological roles. L-cysteine desulfhydrase (DES1) has been shown to act as the key enzyme in the control of H2S production and signalling in physiological conditions during plant growth and development as well as during plant-pathogen interactions. Moreover, H2S has been recently reported to play a role in the regulation of plant autophagy, a key mechanism of plant innate immunity [31], [32]. However, compared to H2S role in plant responses to abiotic stresses, the sources, targets and mechanisms of H2S action in diverse processes during plant-pathogen interactions are only partially uncovered. Major advances in this field have been achieved using des1 mutant of Arabidopsis thaliana and need to be replicated and extended also in plant crop species and their relevant pathogens.
Up to our best knowledge, the actual state of the art in the field of H2S functions in plant biotic interactions has not been previously reviewed. In this review, we focus namely to H2S role in plant-pathogen interactions and we summarize in more detail the specific involvement of H2S in plant responses to pathogen infection, with special attention dedicated to H2S-producing enzyme L-cysteine desulfhydrase and its connections to plant sulfur metabolism.
L-cysteine and sulfide: A central role in plant sulfur metabolism and defence responses
In a central position within the plant primary metabolism and plant responses to stress conditions, amino acid L-cysteine (L-Cys) serves as a precursor of essential biomolecules and defence sulfur-containing metabolites [33], [34]. L-Cys incorporated into peptide and protein molecules plays an outstanding role in redox-based signalling in various plant cell compartments. In a prominent place, the cysteine-containing tripeptide glutathione (GSH, γ–glutamyl-cysteinyl glycine) plays a crucial role in the maintenance of redox homeostasis and cellular protection to oxidative stress [35]. Protein cysteine thiols are targets of diverse post-translational modifications, which can strongly affect protein structure, activity, functions, and localization [36]. Oxidative modifications of thiol groups (-SH) in protein cysteines include a reversible formation of disulfides (-S-S-), sulfenic (-SOH) or sulfinic (-SO2H) groups, whereas modifications caused by the action of signalling molecules NO and H2S are represented by S-nitrosation and S-persulfidation, respectively [37], [38], [39]. Other examples of plant metabolites derived from L-Cys include amino acid methionine, enzyme cofactors like biotin and Fe-S clusters and S-adenosyl methionine, which provides methyl groups for methylation reactions in the biosynthesis of polyamines, phytosiderophores and phytohormone ethylene.
In the last decades, the involvement of diverse types of sulfur-containing compounds in plant defences and resistance to microbial pathogens has been widely uncovered. Besides the well-established role of GSH and GSH-dependent enzymes [40], [41], L-Cys and its metabolites sulfide and carbonyl sulfide have been recognized within plant resistance mechanisms [42], [43], [44]. Sulfur-rich proteins, such as thionins, contribute to the disintegration of pathogen cell walls and induce the formation of ion channels in pathogen membranes [45], [46]. Plant tissue challenge with microbial pathogens induces phytoalexins de novo [47], [48], [49]. Isocyanates as degradation products of glucosinolates represent another important group of antimicrobial compounds [50]. Elemental sulfur (S0) is known to accumulate in vascular tissue upon fungal infections and to inhibit pathogen germination, respiration, and metabolism, possibly through interaction with protein thiol groups [51], [52], [53].
Collectively, a chemically diverse group of sulfur-containing metabolites, including elemental sulfur, glutathione, glucosinolates, phytoalexins and gaseous H2S are involved in pathogen resistance. Their occurrence is species-specific and in a large extent influenced by the sulphur nutritional status of the plant.
Synthesis and catabolism of L-cysteine as H2S precursor in the plant cytosol
L-Cysteine, as a potential donor molecule of reduced sulfur, is produced in the last step of sulfate assimilation in plants by incorporation of sulfide into O-acetylserine catalysed by O-acetylserine (thiol)lyase (OAS-TL), which is found in various isoforms in the cytosol, mitochondria and chloroplasts [33], [54], [55], [56]. The cytosol of plant cells is thus the main cellular compartment of L-Cys biosynthesis. As a result of very high activity of cytosolic OAS-TL isoform OAS-A1, usual cytosolic L-Cys concentrations range around 300 µM, whereas in other compartments L-Cys is found at levels lower than 10 µM [57]. Due to its high reactivity, increasing concentrations of L-Cys potentially cause toxic effects to plant cells. L-Cys is an effective reductant of iron (III) to iron (II) ions, which participate in Fenton-type reactions with reactive oxygen species (ROS) causing oxidative damage of cellular components [58], [59]. For this reason, the maintenance of L-Cys homeostasis by the coordinate action of key enzymes of its biosynthesis and catabolism, i.e. OAS-A1 and L-cysteine desulfhydrase (DES), is of utmost importance for the proper functioning of plant metabolism under physiological and stress conditions.
The most abundant cytosolic OAS-TL isoform OAS-A1 is involved in plant responses to abiotic stress, namely to heavy metals exposure, through metal-chelating activity of phytochelatins, synthesized from L-Cys with GSH as an intermediate [35]. A major contribution to understanding the role of the key enzymes in L-Cys biosynthesis and catabolism was provided by studies of Arabidopsis oas-a1 and des1 mutants, respectively [33], [60], [61]. The knockout oas-a1.1 and oas-a1.2 mutants were characterized by decreased intracellular L-Cys and glutathione levels. Compromised antioxidant capacity results in perturbation of H2O2 homeostasis, as documented by spontaneous cell death lesions occurring in leaves of oas mutants [60]. Mutation of the DES1 gene results in elevated total Cys content caused by reduced total Cys desulfuration activity in leaves. Arabidopsis des1 mutants show premature leaf senescence, whereas enhanced antioxidant defences and tolerance to oxidative stress [61].
A 25% decrease of L-Cys concentration was observed in the oas-a1 mutant, in contrast to a 25% increase of L-Cys levels in a des1 mutant which did not cause any toxic effects to the mutant plants. OAS-A1 down-regulation resulted in higher ROS levels, likely associated with defective homeostasis of H2S, whereas the DES1 deficit was accompanied with decreased ROS. In consequence, decreased GSH levels in OAS-A1 deficient plants were associated with an increased ratio of oxidized glutathione. Subsequently, oas-a1 mutants showed compromised tolerance to cadmium exposure, in comparison to wild-type plants. The overall oxidative stress induced by OAS-A1 deficiency was evident even under control growth conditions when plants showed the spontaneous formation of leaf cell death lesions [33], [60], [61].
The chloroplastic OAS-TL isoform in Arabidopsis thaliana has been described as an S-sulfocysteine synthase (SSCS) enzyme which has a crucial role in the proper photosynthetic performance of the chloroplast under long-day growth conditions. SSCS is located in the thylakoid lumen and it was suggested to function as a sensor to detect accumulated thiosulfate caused by ineffective removal of ROS under conditions of excess light [62], [63], [64].
Together with plant OAS-TL, mitochondrial β-cyanoalanine synthases (CAS-C1) belong to the large superfamily of pyridoxal 5′-phosphate-dependent enzymes together with OAS-TL. As a mechanism of cyanide detoxification, CAS catalyses the biosynthesis of the nonprotein amino acid β-cyano-Ala from L-Cys and cyanide, producing sulfide as a product [65], [66]; however, the contribution of CAS to H2S production is highly variable among plant species. Nine CAS genomic sequences were reported in A. thaliana [67]. Using T-DNA insertion mutants, cytosolic Bsas1.1, plastidic Bsas2.1, and mitochondrial Bsas2.2 were found to play important roles in L-Cys biosynthesis, with a major contribution of cytosolic Bsas1.1 in leaves and root, and mitochondrial Bsas2.2 in the root.
Addition of O-acetylserine inhibits emissions of gaseous H2S from plant tissues and increased L-Cys levels [68], [69]. On the other hand, compounds known to inhibit GSH biosynthesis induce H2S emission, suggesting that under conditions when biosynthetic pathways consuming L-Cys are inhibited, sulfides are emitted in the form of gaseous H2S [70]. A correlation between enzymatically-produced H2S and the total amount of sulfur was observed in Brassica napus [71]. With increased sulfur content, DES1 activity was observed to decrease whereas OAS-TL activity decreased. However, later reports found that under sulfur deficiency, plants showed up-regulation of both OAS-TL and DES1 [72].
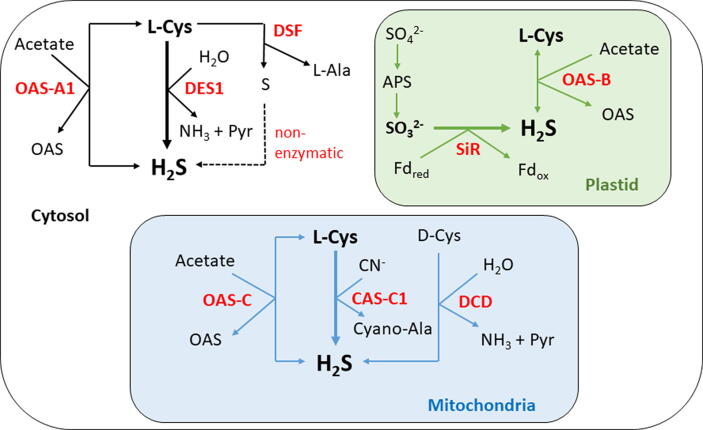
The schematic overview of known biochemical pathways of H2S biosynthesis and conversion in plant cells highlight the central role of DES1 in cytosolic H2S productions (Fig. 1). Other enzymes such as CAS-C1, D-cysteine desulfhydrase (β-DCD), L-cysteine desulfurase (DSF) and ferredoxin are capable to contribute to H2S production in other plant cell compartments, but to which extent this might occur in different plant species under specific growth or stress conditions is largely unknown. Current knowledge thus demonstrates that L-Cys occupies a central position in plant sulphur metabolism as a reduced sulfur donor in the biosynthesis of defence compounds. Besides the involvement of L-Cys and its metabolites in redox signalling within plant cell compartments, cytosolic and mitochondrial L-Cys play crucial roles in plant immunity and cyanide detoxification, respectively. The functions of L-Cys in plant responses to pathogen challenge are in a large extent mediated by L-Cys-derived H2S, as discussed in the next sections of this review.
Fig. 1.
Overview of H2S production in plant cells. APS, adenosine phosphosulfate; CAS-C1, β-cyanoalanine synthase (EC 4.4.1.9); Cyano-Ala, cyanoalanine; DCD, D-cysteine desulfhydrase (EC 4.4.1.15.); DES1, L-cysteine desulfhydrase (EC 4.4.1.2.); DSF, L-cysteine desulfurase (EC 2.8.1.7); Fd, ferredoxin; OAS, O-acetylserine; OAS-A1, cytosolic O-acetylserine (thiol)lyase; OAS-B, plastidial O-acetylserine (thiol)lyase; OAS-C, mitochondrial O-acetylserine (thiol)lyase; Pyr, pyruvate; SiR, sulfite reductase (EC 1.8.7.1.).
L-cysteine desulfhydrase: A key enzyme of H2S production in plants
L-Cysteine degradation in plant cytosol is known to proceed in a reaction catalysed by L-cysteine desulfhydrase (DES), leading to H2S, pyruvate and ammonia. Cytosolic levels of L-Cys and also of H2S are therefore controlled by activities of OAS-TL and DES [72], [73]. Detailed characterization of des1 mutants of A. thaliana, together with pharmacological approaches using DES1 inhibitors as well as H2S donors and scavengers, have recently provided valuable insights into the role of DES1 and H2S in signalling pathways of plant responses to biotic and abiotic stress stimuli [44], [74], [75], [76].
L-cysteine desulfhydrase (DES1, EC 4.4.1.1.)) is the main enzyme of L-Cys catabolism in plant cytosol, which catalyses L-Cys decomposition to pyruvate, ammonia and H2S in a stoichiometric ratio 1:1:1. DES1 regulates cytosolic L-Cys levels together with major cytosolic OAS-TL, highly active OAS-A1 involved in L-Cys biosynthesis [33], [54]. Plant DES1 belongs to the family of O-acetylserin (thiol)lyases (OAS-TL), which in A. thaliana comprises 8 described members involved in sulfur metabolism (Table 1).
Table 1.
Overview of the OAS-TL gene family in A. thaliana. CAS, β–cyanoalanine synthase; DES, L–cysteine desulfhydrase; OAS-TL, O–acetylserine (thiol)lyase; SSCS, S–sulfocysteine synthase.
| Gene | Locus | Localization | Enzyme activity |
|---|---|---|---|
| OAS-A1 | At4g14880 | Cytosol | OAS-TL |
| OAS-B | At2g43750 | Chloroplasts | OAS-TL |
| OAS-C | At3g59760 | Mitochondria | OAS-TL |
| CYS-D1 | At3g04940 | Cytosol | OAS-TL |
| CYS-D2 | At5g28020 | Cytosol | OAS-TL |
| CAS-C1 | At3g61440 | Mitochondria | CAS |
| SCS | At3g03630 | Chloroplasts | SSCS |
| DES1 | At5g28030 | Cytosol | DES |
This enzyme was described for the first time based on sequence homology to other members of OAS-TL family during Arabidopsis genome sequencing. Originally, the enzyme was termed as CS-LIKE according to its cysteine synthase-like activity. Alvarez et al. [61] achieved production and purification of recombinant Arabidopsis DES1 in E. coli and performed detailed in vitro characterization of enzyme molecular properties. Similarly to other members of OAS-TL family, DES1 requires pyridoxal phosphate (PLP) as a cofactor and contains all conserved amino acid residues involved in PLP binding. On the other hand, DES1 differs from other members of OAS-TL family in the sequence of β8A-β9A loop, which is otherwise highly conserved in OAS-TLs due to its role in protein interaction with serine O-acetyltransferases [55], [77].
Purification of recombinant DES1 by affinity chromatography results in increased cysteine desulfhydrase activity of the protein but decreased O-acetylserine lyase activity, in agreement with enzyme primary role in cysteine degradation. This is also supported by enzyme higher activity towards L-Cys, measured by the value of Michaelis constant KM, which is 14-times lower for L-Cys in DES reaction compared to KM value for O-acetylserine in OAS-TL reaction. However, the limit reaction rate is quite low, determined as 0.04 µmol.min−1.mg−1 for purified Arabidopsis DES1. Purified AtDES1 also possesses D-cysteine desulfhydrase activity, but one order of magnitude lower compared to L-Cys desulfhydrase activity [61].
A homologous DES1 gene was isolated from rapeseed (Brassica napus) and sequenced, showing 85% homology to Arabidopsis gene and coding a 323 amino acid polypeptide of 34.5 kDa molecular weight [78]. BnDES1 was found highly expressed in rapeseed flowers, whereas the expression levels in vegetative tissues were much lower. Similarly to AtDES1, BnDES12 also shows a minor O-acetylserine (thiol)lyase activity. A recent report revealed OAS-TL family in Solanum lycopersicum, where measurable DES activity was found in some isoforms, namely SlOAS4 and SlOAS6 [79]. Reported pH optimum values for plant DES range from 8.0 in tobacco [80], 9.0 in Brassica [78] to 10.0 in A. thaliana [81]; however, the physiological relevance of these differences in DES pH optima is not known. Based on the published reports, DES1 enzyme catalysing L-Cys desulfhydration has been established as the main sources of the endogenous production of H2S in the plant cytosol, at least in A. thaliana. Moreover, it has been shown that DES1 is regulated by plant hormones on the transcriptional level, enabling DES1 and H2S level regulation in response to stress stimuli.
L-cysteine desulfhydrase activity has not been detected in animals, where H2S production has been demonstrated to be catalysed by cystathionine–γ–lyase and cystathionine-β-synthase [82] or 3-mercaptopyruvate sulfur transferase [83]. However, L-cysteine desulfhydrase was discovered to operate in some bacteria, e.g. Escherichia coli [84], [85].
It should be noted that D-cysteine desulfhydrase (EC 4.4.1.15), which catalyses the conversion of D-cysteine to the same products as DES1, including H2S, represent a completely different enzyme both in protein structure and biochemical properties [86]. D-cysteine desulfhydrase activity has been observed in multiple plant species including Arabidopsis, where two genes At3g26115 and At1g48420 coding proteins with D-cysteine desulfhydrase activity were identified [87], [88]. Interestingly, the biological function of D-cysteine as well as of D-enantiomers of other amino acid is still not known. One of the suggested functions of D-cysteine desulfhydrase might be degradation of malformin, a phytotoxic peptide produced by Aspergillus niger containing D-cysteine [89].
Production of H2S can be catalysed also by cytosolic L-cysteine desulfurase (EC 2.8.1.7) when using L-cysteine methyl ester as a substrate [90]. The main role of L-cysteine desulfurases is to catalyse L-Cys desulfuration to give L-alanine and elemental sulfur. Proteins showing L-cysteine desulfurase have been identified in the cytosol, chloroplast and mitochondria, where it provides elemental sulfur for the biosynthesis of biotin, thiamine and Fe-S clusters [91], [92].
DES1 regulates H2S production and signalling during plant growth and abiotic stresses
As already mentioned, DES1 is the specific source for the production of cytosolic H2S, involved in signalling pathways of vital plant processes like autophagy and stomatal regulation. During the development of Arabidopsis plants, the highest DES1 gene expression was found in leaves of 14-days old seedlings and 35-old plants just after the termination of flowering, whereas the lowest expression levels were observed in rosettes of 20-days old plants before the appearance of visible buds [93]. At the tissue level, DES1 transcripts were abundant in mesophyll and epidermal cells, including guard cells, in cells surrounding hydathode pores, in trichomes and flowers.
It is known that mutations in AtDES1 induce leaf senescence, accompanied by higher expression of genes involved in plant ageing and increase levels of related transcription factors. Absence of DES1 activity leads to substantially decreased overall desulfurase activity in leaves, associated with increased L-Cys levels [61]. Furthermore, DES1 deficiency in Arabidopsis results in an accumulation of various isoforms of autophagy-related proteins 8 (ATG8), as a sign of activated autophagy processes [31]. The DES1 reaction product, H2S, is likely involved in autophagy regulation, as suggested by the inhibitory effect of exogenous H2S to the accumulation of ATG8 proteins in des1 mutants.
Metabolic rates of L-Cys and H2S production are closely related to the plant nitrogen uptake. Plants under high nitrogen nutrition conditions contain higher activities of OAS-TL and DES1, increased level of sulfur-containing compounds and decreased sulfate levels compared to plants grown in nitrogen-deficient conditions. These findings suggest that sufficient nitrogen uptake enables a higher rate of sulfur incorporation into proteins. The observed higher DES1 activity can serve as a protective factor to avoid excessive L-Cys accumulation [72].
Interestingly, decreased levels of nitric oxide (NO) were observed in des1 mutant, suggesting DES1 is involved by an unknown mechanism in production this gaseous signalling compound in stomata [94]. Currently, it has been recognized that H2S regulates multiple developmental processes and stress responses in interaction with signalling pathways plant hormones [27] including signalling gasotransmitters NO [95] and carbon monoxide [96] or reactive oxygen species like hydrogen peroxide [28].
The role of H2S in stomata closure has been extensively studied [5], [6], [7], [94]. DES1 is involved in the signalling pathway of abscisic acid (ABA) in the leaf stomata, where it participates in the regulation of guard cell movements in stomata closure and opening. ABA is known to induce DES1 expression in wild-type plants, whereas in the ABA-nonresponsive des1 mutant stomata closure can be induced by exogenous H2S [97].
Treatment with H2S, provided as an aqueous solution of NaHS, promotes lateral roots in tomato seedlings with increased auxin levels, suggesting H2S produced by DES1 is involved in the auxin signalling pathway regulating lateral roots formation [98]. A role for DES1 has been proposed in phytohormone-induced programmed cell death in the aleurone layer of wheat [99]. This was evidenced by observations that gibberellins cause decreased DES activity in wheat aleurone and that H2S could prevent the gibberellin-triggered programmed cell death of aleurone cells.
Expression and activity of DES1 in plants were revealed to be modulated by diverse external conditions and stress stimuli [33]. The function of DES1 in response to abiotic stress-mediated by H2S production has been reported in increased tolerance to drought [100], osmotic stress [95] and heat stress [96]. Conversely, des1 mutants showed increased resistance to cadmium exposure, likely mediated by increased levels of L-Cys used to synthesize cadmium-chelating phytochelatins [33], [61]. DES1 transcript levels and activity are induced in Arabidopsis by cold and salinity stress, hydrogen peroxide, and ABA [75]. Treatment of maize seedlings by salicylic acid induces DES1 activity, leading to H2S accumulation involved in increased tolerance to high temperatures [101]. SA-induced tolerance to high temperatures was found potentiated by H2S, although it did not affect SA levels or enzymes of SA biosynthesis. The role of H₂S in plant tolerance to dehydration stress was demonstrated with H₂S-mediated activation of carbonic anhydrase and OAS-TL activity, whereas both dehydration stress and an exogenous application of NaHS induced DES1 activity increasing plant H₂S levels produced from accumulated Cys [102]. In salt-stressed tobacco, high NaCl treatment stimulated CAS and CS activities leading to in H2S accumulation in tobacco leaves, whereas sulphite reductase activity was decreased [103].It has been recognized that the complex biological connections between H2S and other phytohormones and plant regulators include diverse pathways depending on the plant species and tissue. The observed cross-talk of H2S and plant hormones suggests that H2S can serve as an integral molecule of plant hormone signalling. H2S is known to control the expression of genes involved in phytohormone biosynthesis, which might alter actual proportions of hormone levels controlling multiple processes during plant growth and stress responses [27]. Furthermore, similarly to reactive nitrogen and oxygen species, H2S-dependent post-translational modification of proteins and enzyme such as cysteine persulfidation can affect the distribution and signalling of plant hormones. Current evidence in suggest that NO and H2S act in plants synergistically or antagonistically, depending on their actual levels, as signalling compounds or damage effectors. An important part of their signalling effects proceeds via reversible redox-based modifications of protein cysteines, which include S-nitrosation and persulfidation for NO and H2S, respectively [23].
Taken together, results of experimental studies indicate that DES1 and its product H2S contribute to the establishment of plant abiotic stress tolerance, likely mediated by regulation of stress gene transcription, metabolism of reactive oxygen species and auxin signalling pathways.
DES1-mediated H2S production in plant defences
Current accumulated knowledge support a functional implementation of DES1 into signalling pathways of salicylic acid (SA), the key plant hormones in responses to microbial pathogens as well as in plant growth and development [104], [105]. WRKY transcription factors are key regulators of specific plant developmental processes, including seed dormancy, seed germination, and senescence and also plant responses to biotic and abiotic stresses [106]. Expression levels of multiple WRKY members were previously found to be modulated by pathogen infection or SA treatment [107] and WRKY transcription factors are known to down-regulate the expression of DES in A. thaliana [108]. Transcript levels of WRKY54, which serves as a transcription factor regulating gene expression of PR proteins were increased in des1 mutants and decreased in oas-a1 mutants [60]. Simultaneously, des1 mutants showed a lower degree of L-glutathione oxidation compared to oas-a1 mutants. Higher levels of L-Cys in des1 mutants, resulting in lower intracellular redox potential, thus can contribute to increased plant resistance to invading pathogens. This has been further confirmed in a subsequent study of Alvarez et al. [42], which characterized Arabidopsis des1 mutants as more resistant to both biotrophic and necrotrophic pathogens, whereas oas-a1 mutants showed compromised pathogen resistance. In parallel, higher levels of SA, putatively involved in long-distance plant signalling, were observed in des1 mutants. Transcriptomic analysis showed induction of four PR proteins including defensins PDF1-2a and PDF1-2b. Collectively, high Cys-associated decreased intracellular redox potential might play an important role within plant defence to pathogens; however, this mechanism requires further experimental investigations in Arabidopsis and other plant species.
Arabidopsis cad2-1 mutants show approx. 70% decreased levels of L-glutathione but unchanged levels of L-Cys, as compared to wild-type plants, unlike oas-a1 mutants where both L-Cys and glutathione levels are reduced. As the repression of WRKY54 was not found in cad2-1 mutants, suggesting the inhibition of PR expression in oas-a1 mutants was caused by decreased L-Cys levels. Interestingly, members of the WRKY family were recently identified to pose binding capacity to the promotor of DES1 gene and regulate its expression [108]. Furthermore, oas-a1 mutant plants lack the capacity of the hypersensitive reaction, which can be restored by addition of L-Cys but not glutathione, in agreement with a specific requirement for L-Cys in incompatible interactions in plant pathogenesis [33], [42]. The involvement of L-Cys in plant immunity was tested on oas-a1 plants exposed to a virulent bacterial pathogen Pseudomonas syringae pv. tomato DC3000, which produces effectors suppressing plant immunity induced by pathogen molecular patterns. Arabidopsis oas-a1 mutant plants showed increased susceptibility to this pathogen [109].
Development of Arabidopsis mutants in two genes of cysteine desulfhydrases enabled to study the function of DES1 and H2S in plant tolerance biotic and abiotic stress stimuli [75]. Transcript levels of DES1 and D-cysteine desulfhydrase (EC 4.4.1.15) were increased by bacterial pathogen as well as diverse abiotic stress conditions including cold, dehydration, salt and hydrogen peroxide treatment; however, with different timing. The highest increase in DES1 expression and activity was detected from 1 to 3 h after pathogen infection, whereas increased DES1 mRNA continued several hours after the abiotic stress treatment. Compared to wild-type plants, DES1 overexpressors showed lower counts of bacterial cells in infected tissues, in contrast to increased counts of pathogen cells in DES1 knock-outs. Plant defence responses were induced and suppressed by NaHS or H2S scavenger hypotaurine, respectively. Moreover, expression of PR protein genes was induced in DES overexpressors by NaHS treatment, while it was found increased in DES1 knock-down mutants and by H2S scavenger. Collectively, these data support the functional role of DES1 enzyme in H2S production involved in the regulation of plant resistance mechanisms, putatively mediated by activation of salicylic acid-dependent signalling and defence genes [75].
Interestingly, plants under sulfur deficient conditions are able to uptake gaseous H2S or carbonyl sulfide (OCS) from the environment, whereas H2S and OCS are released on pathogen infection, resulting in an overall decrease of sulfur content in infected plants [110]. During the flowering period, plant responses to infection were slower with decreased and decelerated emission of H2S and OCS, suggesting differential regulation of sulfur metabolism in the vegetative and reproductive stage of plant development. Moreover, emitted gaseous molecules can serve as long-distance signals to alert closely located plants about ongoing infection in their proximity. In agreement with this hypothesis, increased amount of thiol-containing compounds was detected in plant growing close to infected plants, compared to control plants grown separately [111].
In summary, the use of transgenic plants with modulated DES1 activity and endogenous H2S level, in combination with exogenous treatments with H2S donors or scavengers, confirm proposed protective role of H2S in biotic stress resistance.
Mechanisms of H2S action in plant-pathogen interactions
As evident, major advances in the detailed understanding of H2S sources and functions in plant metabolism and stress responses have been obtained in the model plant A. thaliana, with available mutants of key enzymes of sulphur metabolism. In contrast, the understanding of the role and mechanisms of H2S action in defence responses of crop plants is quite limited (Table 2).
Table 2.
Summary of published studies on the role of H2S in plant defences to pathogens.
| Plant species | Pathogen | Treatment | Observed effects | Source |
|---|---|---|---|---|
| Vitis vinifera (grapes) | Uncinula necator | Elemental sulfur | Fungicidal effects of sulfur-derived H2S | [112] |
| n.a. | Botrytis cinerea | Saturated solution of H2S | Inhibition of spore germination | [113] |
| n.a. | Botrytis cinerea, Cladosporium herbarumFusicladium dendriticumMonilia cinereaMonilia fructigena Pencillium verdicatum Physalospora miyabeana | Fumigation with H2S | Fungicidal effect | [114] |
| Brassica napus | Pyrenopeziza brassicae | n.a. | Increased DES1 activity | [72] |
| Vitis vinifera | Uncinula necator | n.a. | Increased H2S release in the early phase of infection | [43] |
| Vitis vinifera (leaves) | Uncinula necator | Elemental sulfur applied in the early phase of pathogenesis | Uptake of 10 µM/h of H2S by the pathogen provides fungicidal effect | [115] |
| Brassica napus | Sclerotinia sclerotiorum | n.a. | Increased H2S release | [111] |
| Actinidia deliciosaCitrus sinensisCitrus reticulataMalus domesticaPyrus bretschneideri Solanum lycopersicum | Aspergillus nigerPenicillium italicum | Fumigation with H2S released from NaHS solution | Reduced postharvest decay of fruits induced by fungal pathogensInhibition of spore germination, germ tube elongation and mycelial growth | [118] |
| Ipomoea batatas | Rhizopus nigricans, Mucor rouxianus, Geotrichum candidum | Fumigation with H2S released from NaHS solution | Inhibition of fungal growth | [119] |
| Pyrus pyrifolia | Aspergillus niger, Penicillium expansum | H2S fumigation | Inhibition of fungal growth | [117] |
| Fragaria ananassa (strawberry) | n.a. | Fruit immersion in NaHS solution alone or in combination with a NO donor | Accumulation of antifungal enzymes chitinase and beta-glucanase | [120] |
| Arabidopsis thaliana | Pseudomonas syringae pv. tomato DC3000 | DES1 and DCD overexpression, H2S donor NaHSDES1 and DCD knock-down, H2S scavenger hypotaurine | Decreased bacteria count in infected tissuesIncreased bacteria count in infected tissues | [75] |
| n.a. | Pseudomonas syringae pv. phaseolicola (Pph) 1302A | H2S donors (NaHS, Na2S, AP39 – mitochondria-targeted H2S donor) | Inhibition of cell growth, increased virulence | [121] |
n.a., not applicable.
Early studies found killing grape mildews by H2S fumigation in a close jar [112] and completely inhibited germination of Botrytis cinerea spores sowed in a saturated solution of H2S [113]. This was confirmed by a more extensive study on a set of fungal phytopathogens (including B. cinerea, Cladosporium herbarum, Fusicladium dendriticum, Monilia cinerea and M. fructigena, Pencillium verdicatum, Physalospora miyabeana) which showed H2S acting as a general poison toxic at a low concentration to all used fungi [114]. Importantly, this study noted the conversion of sulphur to volatile H2S, which mediates the toxic effects previously attributed to the sulphur treatments.
The enzyme DES1 was identified as the H2S source in rapeseed plants infected by a fungal pathogen Pyrenopeziza brassicae, which resulted in a 50% increase of DES1 activity [72]. Fungal infection of the grapevine by grape powdery mildew (Uncinula necator) induced an increased release of H2S, namely during the early phase of the infection; however, it strongly decreased 10 days after infection [43]. An application of elemental sulfur to powdery mildew-infected grape leaves showed the highest efficiency when applied in the early phase of pathogenesis prior to the formation of fungal appressoria in penetrated leaf cells [115]. It was estimated that uptake of 10 µM/h of H2S by the pathogen would provide a fungicidal effect. Role of H2S in plant defence against fungal pathogen was evidenced by significant increase of H2S emissions from crops challenged by fungal infection [72], [111]. Collectively, in a similar manner to model plants, crops have been demonstrated to exert capabilities to respond to fungal infection by modulations of L-Cys metabolism, H2S emissions and increased levels of GSH and phytoalexins [116].
Recently, it was found that H2S could extend postharvest storage of fresh-cut pears and inhibit the growth of fungal pathogens Aspergillus niger and Penicillium expansum [117]. The inhibitory effects of H2S to these fungal pathogens both on inoculated fruits and in vitro culture was confirmed for several fruits including apple, lemon, kiwi and tomato [118]. Fumigation with H2S released from NaHS solution inhibited the growth of fungal pathogens Rhizopus nigricans, Mucor rouxianus and Geotrichum candidum on slices of sweet potato (Ipomoea batatas); however, the molecular mechanism of the antifungal H2S action has not been elucidated [119]. Similarly, strawberry (Fragaria ananassa) fruits treated with NaHS solution, or with a combination of NaHS and a NO donor, resulted in increased activities of potentially antifungal enzymes chitinase and beta-1,3-glucanase; however, if this effect can contribute to fruit resistance to fungal contamination and decay has not been tested [120].
Recently, the antimicrobial effect of H2S was corroborated also for a microbial pathogen using Arabidopsis plants infected with P. syringae pv. tomato DC3000 [75]. Plants overexpressing LCD and DCD1 had lower bacterial counts compared to WT plants, unlike LCD and DCD1 knockdown plants exhibiting higher bacterial infection. Furthermore, both LCD and DCD1 overexpressors and plants treated with an NaHS solution showed higher levels of transcription of pathogenesis-related genes. In vitro, P. syringae pv. phaseolicola were found to be resistant to low levels of H2S, whereas high doses of NaHS, Na2S and a mitochondria-targeted H2S donor AP39 inhibited cell growth, which was mediated by excision of a genomic island from the bacterial genome [121]. It has been suggested that H2S emitted from the plants in response to bacterial challenges can modify the genomic structure of invading bacteria and thus affect their virulence, which might be exploited to increase crops resistance.
It would be not surprising to found that pathogens have evolved mechanisms for efficient H2S removal and detoxification. H2S is known to block cell respiration as a strong inhibitor of cytochrome c oxidase [122], so its elimination is vital to enable the growth of the microbial pathogen in a low oxygen environment, as in bacterial biofilms, plant xylem vessels or root tissues. In plant pathogens Xylella fastidiosa and Agrobacterium tumefaciens, the biofilm growth-associated repressor (BigR) regulates transcription of the bigR operon, which is important for H2S detoxification through the action of a sulfur dioxygenase in conjunction with a sulfite exporter [123]. It was shown that the respiratory oxidase cytochrome bd in the model microorganism Escherichia coli is resistant to H2S inhibition [124]; however, it seems that this mechanism of respiration resistance to H2S inhibition is present only in enterobacteria.
Hydrogen sulfide in biological systems occurs as diprotonated gaseous H2S as well as HS- anion, which co-exist in a chemical equilibrium [1], [37], [94]. Gaseous H2S can diffuse freely across the cell membranes and migrate outside of the plant tissues, which will result in HS- protonation and formation of H2S to re-establish the equilibrium. Some previous studies concluded that amounts of H2S produced by plants were not sufficient to exert its toxic effect to plant pathogens [25]. H2S fumigation experiments showed that even relatively high 20 µl·L-1concentrations of H2S (i.e. two orders of magnitude higher than levels known to decrease plant growth) reduced the growth of fungal pathogen Rhizoctonia solani only by 17%; moreover, prolonged fumigation resulted in increased growth of bacterial colonies [125]. In contrast, other results demonstrated that plant were capable to reduce fungal pathogen growth through localized high H2S production at the site of infection and on the leaf surface. In A. thaliana, H2S concentrations in leaf mesophyll were reported within the range of 4–10 µM [126]. Still, it has not been decisively demonstrated if pathogen destruction is the primary role of H2S emission or whether it is just its side effect.
Another unresolved issue concerns the capability of plant-produced H2S to enter pathogen cells. H2S can be transported from chloroplasts to the cytosol by directed transport enabled by specific transporter proteins. It is supposed that cytosolic HS-, representing at pH 7.4 approx. 75% of hydrogen sulfide, can be transported to the apoplastic space, although the HS- transporters in plant membranes have not been characterized yet [44]. Apoplastic pH is known to increase during plant-pathogen interactions, which can ensure that the equilibrium is shifted toward HS- anion, thus avoiding diffusion of H2S back into the plant cells [127]. Nevertheless, the molecular mechanism of how H2S enters pathogen cells remains unresolved.
Locally increased H2S concentrations in the site of pathogen attack were suggested to inhibit spore germination or to decrease the growth rate of fungal hyphae. H2S can be oxidized in presence of electron acceptor or by the catalytic action of superoxide dismutases to elemental sulfur, which is known to be toxic in significantly lower levels compared to H2S itself [41], [88]. Besides its direct toxic effect to plant pathogens, H2S is involved in the activation of signalling pathways regulating plant responses to pathogen recognition and penetration. Among these mechanisms, protein persulfidation (previously termed also as S-sulfhydration) as a post-translational protein modification can strongly affect protein biological activity [29], [39], [97]. On reaction with H2S, persulfidated proteins have cysteine thiol groups modified to –SSH group. In Arabidopsis des1 mutants, persulfidation levels were changed in important proteins involved in intracellular signalling processes, e.g. ASNF1-related protein kinase 2.2 or the ABA receptor [97].
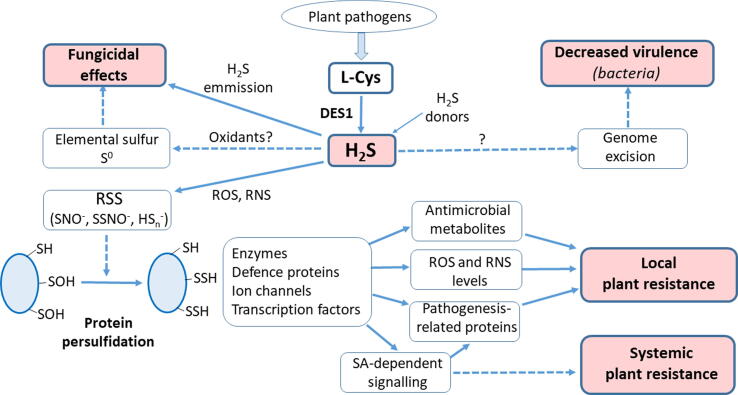
It can be hypothesized that besides direct toxic effects to microbial cells, molecular mechanisms of H2S effects in plant-pathogen interaction include numerous chemical reactions with RNS and ROS leading to reactive sulfur species, which mediate protein post-translational modifications like persulfidation (Fig. 2). Persulfidation might be an efficient regulatory mechanism to activate defence responses, including activation of enzymes, ion channels or transcription factors, ultimately leading to activation of defence phytohormone signalling, production of antimicrobial metabolites and establishment of local or systemic resistance.
Fig. 2.
Schematic overview of known molecular mechanisms of H2S involvement in increased plant resistance to microbial pathogens. DES1, L-cysteine desulfurase; PR, pathogenesis-related; RNS, reactive nitrogen species; ROS, reactive oxygen species; RSS, reactive sulfur species; SA, salicylic acid; SNO-, thionitrite; SSNO-, perthionitrite; HSn-, polysulfides.
Conclusions and future perspectives
Precise regulation of L-Cys homeostasis in the cytosol of plant cells by OAS-A1 and DES1 is necessary for plant sulfur metabolism and plant responses to stress conditions. Modulations of enzyme activities of OAS-A1 and DES during plant development and in reaction to environmental conditions regulate the levels of L-cysteine and H2S [128]. It should be noted that many published reports describing the effects of H2S in biological systems including plants were obtained using solutions of NaHS or Na2S as “H2S donors”. In solution, these inorganic sulfides are H2S equivalents, but their dissolution results in a fast formation of high H2S levels, unlike in case of synthetic H2S releasing compounds that can mimic low and steady H2S levels occurring in living tissues. Moreover, in an aerobic environment, NaHS and Na2S solutions are known to contain numerous sulfur species, including polysulfides, S0 as well as thiyl radicals as products of sulfide autoxidation [129]. Introduction and validation of reliable methods for the quantitative analysis of H2S and its metabolites, already widely used within animal H2S research, is required to solve controversies on biological effects of H2S in plants under physiological and stress conditions.
In plant systems, rigorous studies focused to the identification of the active agent and analysis of reaction mechanisms have been lacking, including the proposed insertion of sulfur atom(s) into sulfhydryl groups [130], [131]. The first report on the Arabidopsis persulfidome established the prominent role of this post-translational modification in plant H2S signalling [39], [97], [132]. The recently developed dimedone switch method provided deeper insights into the mechanistic details of protein persulfidation, which occurs on sulfenylated cysteine residues and thus protects proteins from over-oxidation under stress conditions [133]. This mechanism is evolutionary conserved from the bacteria to humans and represent a putative interconnection of signalling pathways of H2S, ROS and NO through diverse cysteine post-translational modifications; however, if this persulfidation mechanism operates also in plants has not been tested yet. Significant gaps exist in the knowledge of the regulation of endogenous H2S levels, the sources and their modulation for H2S signalling as well as the molecular targets of H2S both in plant and pathogen cells. So to fully understand the regulatory and signalling roles of DES1 and its reaction product H2S, further systematic studies are required on the sulfur chemistry in plant cell compartments varying in pH values and levels of ROS, thiols and other reaction partners [28], [134].
The major part of our actual knowledge on the role of L-Cys and H2S in plant resistance to phytopathogens has been obtained on model plant species A. thaliana using specific mutants with down- or up-regulated enzymes of L-Cys metabolic pathways. Thus further experiments on agriculturally relevant crops, ideally in the field conditions, can contribute to transfer the knowledge on molecular mechanisms of the involvement of sulfur-containing compounds in plant biotic interaction into their practical application towards increased crop resistance. In this regard, new technologies available for direct plant genome editing [135] can be considered a promising tools to further understand plant pathosystems by modulations of genes coding plant proteins and enzymes involved in H2S metabolism, signalling and defence mechanisms activated upon pathogen challenge.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
This research was funded by Palacký University in Olomouc (IGA_PrF_2020_013).
Biographies

Daniel Vojtovič graduated in 2019 in Master studies of Biochemistry in Department of Biochemistry, Faculty of Science of Palacky University in Olomouc, Czech Republic. His thesis was dedicated to the role of L-cysteine desulfhydrase in plant responses to biotic stresses. Since 2019 he has been enrolled in the PhD program in Biochemistry in the Department of Biochemistry, with research projects focused to signalling and regulatory functions of redox modification of protein cysteines in plant cells.

Lenka Luhová graduated in Biochemistry in 1984 from Masaryk University in Brno and then obtained her PhD in Biochemistry in Faculty of Medicine at Palacky University in Olomouc, Czech Republic. Since 1992 she has joined the Department of Biochemistry, where she was involved in research projects on the metabolism of plant polyamines. Her actual research interests include plant stress biochemistry, with a special focus on the role of reactive nitrogen and oxygen species in plant signalling and stress responses. She has published 76 publications in international journals (h-index 17), receiving more than 700 citations.

Marek Petřivalský obtained PhD title in Ecology in 1995 from Masaryk University in Brno, Czech Republic. His initial research career in Veterinary Research Institute was devoted to cytochromes P450 and other animal detoxification systems as biomarkers of environmental pollution. Since 1999, he has joined the Department of Biochemistry in Palacký University in Olomouc, where he switched his research interests to plant biochemistry, namely to plant responses to abiotic and biotic stresses. His current research has been focused on reactive nitrogen, oxygen and sulphur species in plant signalling. He is author of 58 publications (h-index 15), with more than 670 citations.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Li Q., Lancaster J.R., Jr. Chemical foundations of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kabil O., Vitvitsky V., Banerjee R. Sulfur as a signaling nutrient through hydrogen sulfide. Annu Rev Nutr. 2014;34:171–205. doi: 10.1146/annurev-nutr-071813-105654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bian J.S., Olson K.R., Zhu Y.C. Hydrogen Sulfide: Biogenesis, Physiology, and Pathology. Oxid Med Cell Longev. 2016;2016:6549625. doi: 10.1155/2016/6549625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson L.G., Bressan R.A., Filner P. Light-dependent emission of hydrogen sulfide from plants. Plant Physiol. 1978;61:184–189. doi: 10.1104/pp.61.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Mata C., Lamattina L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010;188:977–984. doi: 10.1111/j.1469-8137.2010.03465.x. [DOI] [PubMed] [Google Scholar]
- 6.García-Mata C., Lamattina L. Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci. 2013;201–202:66–73. doi: 10.1016/j.plantsci.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Lisjak M., Teklic T., Wilson I.D., Whiteman M., Hancock J.T. Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ. 2013;36:1607–1616. doi: 10.1111/pce.12073. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y.T., Li M.Y., Cui W.T., Lu W., Shen W. Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J Plant Growth Regul. 2012;2012(31):519–528. [Google Scholar]
- 9.Chen J., Wu F.H., Wang W.H., Zheng C.J., Lin G.H., Dong X.J. Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. J Exp Bot. 2011;62:4481–4493. doi: 10.1093/jxb/err145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huo J., Huang D., Zhang J., Fang H., Wang B., Wang C. Hydrogen Sulfide: A Gaseous Molecule in Postharvest Freshness. Front Plant Sci. 2018;9:1172. doi: 10.3389/fpls.2018.01172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziogas V., Molassiotis A., Fotopoulos V., Tanou G. Hydrogen Sulfide: A Potent Tool in Postharvest Fruit Biology and Possible Mechanism of Action. Front Plant Sci. 2018;9:1375. doi: 10.3389/fpls.2018.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo H., Xiao T., Zhou H., Xie Y., Shen W. Hydrogen sulfide: a versatile regulator of environmental stress in plants. Acta Physiol Plant. 2016;38:16. [Google Scholar]
- 13.Hancock J.T. Hydrogen sulfide and environmental stresses. Environ Exp Bot. 2019;161:50–56. [Google Scholar]
- 14.Corpas F.J. Hydrogen Sulfide: A New Warrior against Abiotic Stress. Trends Plant Sci. 2019;24:983–988. doi: 10.1016/j.tplants.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Fotopoulos V., Christou A., Manganaris G.A. Hydrogen sulfide as a potent regulator of plant responses to abiotic stress factors. In: Gaur R.K., Sharma P., editors. Molecular Approaches in Plant Abiotic Stress. CRC Press; Boca Raton: 2013. pp. 353–373. [Google Scholar]
- 16.Da-Silva C.J., Modolo L.V. Hydrogen sulfide: a new endogenous player in an old mechanism of plant tolerance to high salinity. Acta Bot Brasil. 2018;32:150–160. [Google Scholar]
- 17.Li J., Yu Z., Choo S., Zhao J., Wang Z., Xie R. Chemico-proteomics reveal the enhancement of salt tolerance in an invasive plant species via H2S signaling. ACS Omega. 2020;5:14575–14585. doi: 10.1021/acsomega.0c01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z.G., Min X., Zhou Z.H. Hydrogen sulfide: a signal molecule in plant cross-adaptation. Front Plant Sci. 2016;7:1621. doi: 10.3389/fpls.2016.01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scuffi D., Lamattina L., García-Mata C. Gasotransmitters and Stomatal Closure: Is There Redundancy, Concerted Action, or Both? Front Plant Sci. 2016;7:277. doi: 10.3389/fpls.2016.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock J.T., Whiteman M. Hydrogen sulfide signaling: interactions with nitric oxide and reactive oxygen species. Ann N Y Acad Sci. 2016;1365:5–14. doi: 10.1111/nyas.12733. [DOI] [PubMed] [Google Scholar]
- 21.Hasanuzzaman M., Bhuyan M., Mahmud J.A., Nahar K., Mohsin S.M., Parvin K. Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal Behav. 2018;13 doi: 10.1080/15592324.2018.1477905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corpas F.J., González-Gordo S., Cañas A., Palma J.M. Nitric oxide and hydrogen sulfide in plants: which comes first? J Exp Bot. 2019;70:4391–4404. doi: 10.1093/jxb/erz031. [DOI] [PubMed] [Google Scholar]
- 24.Bhuyan M.H.M.B., Hasanuzzaman M., Parvin K., Mohsin S.M., Al Mahmud J., Nahar K. Nitric oxide and hydrogen sulfide: two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 2020;90:409–424. [Google Scholar]
- 25.Xuan L., Li J., Wang X., Wang C. Crosstalk between Hydrogen Sulfide and Other Signal Molecules Regulates Plant Growth and Development. Int J Mol Sci. 2020;21:4593. doi: 10.3390/ijms21134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petřivalský M., Luhová L. Nitrated Nucleotides: New Players in Signaling Pathways of Reactive Nitrogen and Oxygen Species in Plants. Front Plant Sci. 2020;11:598. doi: 10.3389/fpls.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He H., Garcia-Mata C., He L.F. Interaction between hydrogen sulfide and hormones in plant physiological responses. Plant Growth Regul. 2019;87:175. [Google Scholar]
- 28.Hancock J.T., Whiteman M. Hydrogen sulfide and cell signalling: team player or referee? Plant Physiol Biochem. 2014;78:37–42. doi: 10.1016/j.plaphy.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Corpas F.J., Palma J.M. H2S signaling in plants and applications in agriculture. J Adv Res. 2020;24:131–137. doi: 10.1016/j.jare.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aroca A., Gotor C., Bassham D.C., Romero L.C. Hydrogen Sulfide: From a Toxic Molecule to a Key Molecule of Cell Life. Antioxidants (Basel) 2020;9:621. doi: 10.3390/antiox9070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gotor C., Garcia I., Crespo J.L., Romero L.C. Sulfide as a signaling molecule in autophagy. Autophagy. 2013;9:609–611. doi: 10.4161/auto.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laureano-Marín A.M., Moreno I., Romero L.C., Gotor C. Negative Regulation of Autophagy by Sulfide Is Independent of Reactive Oxygen Species. Plant Physiol. 2016;171:1378–1391. doi: 10.1104/pp.16.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero L.C., Aroca M.A., Laureano-Marin A.M., Morena I., García I., Gotor C. Cysteine and Cysteine-Related Signaling Pathways in Arabidopsis thaliana. Mol Plant. 2014;7:264–276. doi: 10.1093/mp/sst168. [DOI] [PubMed] [Google Scholar]
- 34.Gotor C., Laureano-Marín A.M., Moreno I., Aroca A., García I., Romero L.C. Signaling in the plant cytosol: cysteine or sulfide? Amino Acids. 2015;47:2155. doi: 10.1007/s00726-014-1786-z. [DOI] [PubMed] [Google Scholar]
- 35.Hasanuzzaman M., Nahar K., Anee T.I., Fujita M. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plant. 2017;23:249–326. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J., Carroll K.S., Liebler D.C. The Expanding Landscape of the Thiol Redox Proteome. Mol Cell Proteom. 2016;15:1–11. doi: 10.1074/mcp.O115.056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filipovic M.R., Zivanovic J., Alvarez B., Banerjee R. Chemical Biology of H2S Signaling through Persulfidation. Chem Rev. 2018;118:1253–1337. doi: 10.1021/acs.chemrev.7b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Couturier J., Chibani K., Jacquot J.P., Rouhier N. Cysteine-based redox regulation and signaling in plants. Front Plant Sci. 2013;4:1–7. doi: 10.3389/fpls.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aroca A., Gotor C., Romero L.C. Hydrogen Sulfide Signaling in Plants: Emerging Roles of Protein Persulfidation. Front Plant Sci. 2018;9:1369. doi: 10.3389/fpls.2018.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rausch T., Wachter A. Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci. 2005;10:503–509. doi: 10.1016/j.tplants.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Bloem E., Haneklaus S., Schnug E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front Plant Sci. 2015;5:1–12. doi: 10.3389/fpls.2014.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarez C., Bermúdez M.A., Romero L.C., Gotor C., García I. Cysteine homeostasis plays an essential role in plant immunity. New Phytol. 2012;193:165–177. doi: 10.1111/j.1469-8137.2011.03889.x. [DOI] [PubMed] [Google Scholar]
- 43.Bloem E., Haneklaus S., Salac I., Wickenhauser P., Schnug E. Facts and fiction about sulfur metabolism in relation to plant-pathogen interactions. Plant Biol. 2007;9:596–607. doi: 10.1055/s-2007-965420. [DOI] [PubMed] [Google Scholar]
- 44.Calderwood A., Kopriva S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric Oxide. 2014;41:72–78. doi: 10.1016/j.niox.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Hughes P., Dennis E., Whitecross M., Llewellyn D., Gage P. The cytotoxic plant protein, beta-purothionin, forms ion channels in lipid membranes. J Biol Chem. 2000;275:823–827. doi: 10.1074/jbc.275.2.823. [DOI] [PubMed] [Google Scholar]
- 46.Tam J.P., Wang S., Wong K.H., Tan W.L. Antimicrobial Peptides from Plants. Pharmaceuticals (Basel) 2015;8:711–757. doi: 10.3390/ph8040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuc J. Relevance of Phytoalexins – A Critical Review. Acta Horticult. 1994;381:526–539. [Google Scholar]
- 48.Zook M., Hammerschmidt R. Origin of the thiazole ring of camalexin, a phytoalexin from Arabidopsis thaliana. Plant Physiol. 1997;113:463–468. doi: 10.1104/pp.113.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bednarek P. Sulfur-Containing Secondary Metabolites from Arabidopsis thaliana and other Brassicaceae with Function in Plant Immunity. ChemBioChem. 2012;13:1846–1859. doi: 10.1002/cbic.201200086. [DOI] [PubMed] [Google Scholar]
- 50.Dufour V., Stahl M., Baysse C. The antibacterial properties of isothiocyanates. Microbiol. 2015;161:229–243. doi: 10.1099/mic.0.082362-0. [DOI] [PubMed] [Google Scholar]
- 51.Beffa T. Inhibitory action of elemental sulphur (S0)on fungal spores. Can J Microbiol. 1993;39:731–735. [Google Scholar]
- 52.Williams J.S., Hall S.A., Hawkesford M.J., Beale M.H., Cooper R.M. Elemental sulfur and thiol accumulation in tomato and defense against a fungal vascular pathogen. Plant Physiol. 2002;128:150–159. [PMC free article] [PubMed] [Google Scholar]
- 53.Nwachukwu I.D., Slusarenko A.J., Gruhlke M.C. Sulfur and sulfur compounds in plant defence. Nat Prod Commun. 2012;7:395–400. [PubMed] [Google Scholar]
- 54.Wirtz M., Droux M., Hell R. O-acetylserine (thiol)lyase: an enigmatic enzyme of plant cysteine biosynthesis revisited in Arabidopsis thaliana. J Exp Bot. 2004;55:1785–1798. doi: 10.1093/jxb/erh201. [DOI] [PubMed] [Google Scholar]
- 55.Wirtz M., Hell R. Functional analysis of the cysteine synthase protein complex from plants: structural, biochemical and regulatory properties. J Plant Physiol. 2006;163:273–296. doi: 10.1016/j.jplph.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi H., Kopriva S., Giordano M., Saito K., Hell R. Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol. 2011;62:157–184. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- 57.Krueger S., Niehl A., Lopez-Martin M.C., Steinhauser D., Donath A., Hildebrandt T. Analysis of cytosolic and plastidic serine acetyltransferase mutants and subcellular metabolite distributions suggests interplay of the cellular compartments for cysteine biosynthesis in Arabidopsis. Plant, Cell Environ. 2009;32:349–367. doi: 10.1111/j.1365-3040.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 58.Jacob C., Giles G.I., Giles N.M., Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- 59.Park S., Imlay J.A. High levels of intracellular cysteine promote oxidative DNA damage by driving the fenton reaction. J Bacteriol. 2003;185:1942–1950. doi: 10.1128/JB.185.6.1942-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Martin M.C., Becana M., Romero L.C., Gotor C. Knocking Out Cytosolic Cysteine Synthesis Compromises the Antioxidant Capacity of the Cytosol to Maintain Discrete Concentrations of Hydrogen Peroxide in Arabidopsis. Plant Physiol. 2008;147:562–572. doi: 10.1104/pp.108.117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvarez C., Calo L., Romero L.C., García I., Gotor C. An O-Acetylserine(thiol)lyase Homolog with L-Cysteine Desulfhydrase Activity Regulates Cysteine Homeostasis in Arabidopsis. Plant Physiol. 2010;152:656–669. doi: 10.1104/pp.109.147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bermúdez M.A., Páez-Ochoa M.A., Gotor C., Romero L.C. Arabidopsis S-sulfocysteine synthase activity is essential for chloroplast function and long-day light-dependent redox control. Plant Cell. 2010;22:403–416. doi: 10.1105/tpc.109.071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bermúdez M.A., Galméz J., Moreno I., Mullineaux P.M., Gotor C., Romero L.C. Photosynthetic adaptation to length of day is dependent on S-sulfocysteine synthase activity in the thylakoid lumen. Plant Physiol. 2012;160:274–288. doi: 10.1104/pp.112.201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotor C., Romero L.C. S-sulfocysteine synthase function in sensing chloroplast redox status. Plant Signal Behav. 2013;8 doi: 10.4161/psb.23313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blumenthal S.G., Hendrickson H.R., Abrol Y.P., Conn E.E. Cyanide metabolism in higher plants. 3. The biosynthesis of beta-cyanolanine. J Biol Chem. 1968;243:5302–5307. [PubMed] [Google Scholar]
- 66.García I., Rosas T., Bejarano E.R., Gotor C., Romero L.C. Transient Transcriptional Regulation of the CYS-C1 Gene and Cyanide Accumulation upon Pathogen Infection in the Plant Immune Response. Plant Physiol. 2013;162:2015–2027. doi: 10.1104/pp.113.219436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe M., Kusano M., Oikawa A., Fukushima A., Noji M., Saito K. Physiological roles of the beta-substituted alanine synthase gene family in Arabidopsis. Plant Physiol. 2008;146:310–320. doi: 10.1104/pp.107.106831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rennenberg H., Sekiya J., Wilson L.G., Filner P. Evidence for a futile sulfur cycle in leaves. Plant Physiol. 1981;67:S723. [Google Scholar]
- 69.Rennenberg H. Role of O-acetylserine in hydrogen sulfide emissions from pumpkin leaves in response to sulfate. Plant Physiol. 1983;73:560–565. doi: 10.1104/pp.73.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rennenberg H., Filner P. Stimulation of H2S emission from pumpkin leaves by inhibition of glutathione synthesis. Plant Physiol. 1982;69:766–770. doi: 10.1104/pp.69.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burandt P., Papenbrock J., Schmidt A., Bloem E., Haneklaus S., Schnug E. Genotypical differences in total sulfur contents and cysteine desulfhydrase activities in Brassica napus L. Phyton. 2001;41:75–86. [Google Scholar]
- 72.Bloem E., Riemenschneider A., Volker J., Papenbrock J., Schmidt A., Salac I. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J Exp Bot. 2004;55:2305–2312. doi: 10.1093/jxb/erh236. [DOI] [PubMed] [Google Scholar]
- 73.Riemenschneider A., Nikiforova V., Hoefgen R., De Kok L.J., Papenbrock J. Impact of elevated H2S on metabolite levels, activity of enzymes and expression of genes involved in cysteine metabolism. Plant Physiol Biochem. 2005;43:473–483. doi: 10.1016/j.plaphy.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Alvarez C., Garcia I., Moreno I., Pérez-Pérez M.E., Crespo J.L., Romero L.C. Cysteine-generated sulfide in the cytosol negatively regulates autophagy and modulates the transcriptional profile in arabidopsis. Plant Cell. 2012;24:4621–4634. doi: 10.1105/tpc.112.105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi H., Ye T., Han N., Bian H., Liu X., Chan Z. Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Integr Plant Biol. 2015;57:628–640. doi: 10.1111/jipb.12302. [DOI] [PubMed] [Google Scholar]
- 76.Fotopoulos V., Christou A., Antoniou C., Manganaris G.A. Hydrogen sulphide: a versatile tool for the regulation of growth and defence responses in horticultural crops. J Horticult Sci Biotech. 2015;90:227–234. [Google Scholar]
- 77.Droux M., Ruffet M.L., Douce R., Job D. Interactions between serine acetyltransferase and O-acetylserine (thiol)lyase in higher plants – structural and kinetic properties of the free and bound enzymes. Eur J Biochem. 1998;255:235–245. doi: 10.1046/j.1432-1327.1998.2550235.x. [DOI] [PubMed] [Google Scholar]
- 78.Xie Y., Lai D., Mao Y., Zhang W., Shen W., Guan R. Molecular Cloning, Characterization, and Expression Analysis of a Novel Gene Encoding L-Cysteine Desulfhydrase from Brassica napus. Mol Biotechnol. 2013;54:737–746. doi: 10.1007/s12033-012-9621-9. [DOI] [PubMed] [Google Scholar]
- 79.Liu D., Lu J., Li H., Wang J., Pei Y. Characterization of the O-acetylserine(thiol)lyase gene family in Solanum lycopersicum L. Plant Mol Biol. 2019;99:123–134. doi: 10.1007/s11103-018-0807-9. [DOI] [PubMed] [Google Scholar]
- 80.Harrington H.M., Smith I.K. Cysteine Metabolism in Cultured Tobacco Cells. Plant Physiol. 1980;65:151–155. doi: 10.1104/pp.65.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burandt P., Schmidt A., Papenbrock J. Three O-acetyl-L-serine(thiol)lyase isoenzymes from Arabidopsis catalyse cysteine synthesis and cysteine desulfuration at different pH values. J Plant Physiol. 2002;159:111–119. [Google Scholar]
- 82.Kabil O., Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yadav P.K., Vitvitsky V., Carballal S., Seravalli J., Banerjee R. Thioredoxin regulates human mercaptopyruvate sulfurtransferase at physiologically-relevant concentrations. J Biol Chem. 2020;295:6299–6311. doi: 10.1074/jbc.RA120.012616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metaxas M.A., Delwiche E.A. The L-cystein desulfhydrase of Escherichia coli. J Bacter. 1955;70:735–737. doi: 10.1128/jb.70.6.735-737.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takagi H., Ohtsu I. L-Cysteine Metabolism and Fermentation in Microorganisms. Adv Biochem Eng Biotechnol. 2017;159:129–151. doi: 10.1007/10_2016_29. [DOI] [PubMed] [Google Scholar]
- 86.Li Z.G. Analysis of some enzymatic activities of hydrogen sulfide metabolism in plants. Meth Enzymol. 2015;555:253–269. doi: 10.1016/bs.mie.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 87.Rennenberg H., Arabatzis N., Grundel I. Cysteine desulphydrase activity in higher plants: Evidence for the action of L- and D-cysteine specific enzymes. Phytochemistry. 1987;26:1583–1589. [Google Scholar]
- 88.Papenbrock J., Riemenschneider A., Kamp A., Schulz-Vogt H.N., Schmidt A. Characterization of Cysteine-Degrading and H2S-Releasing Enzymes of Higher Plants – From the Field to the Test Tube and Back. Plant Biol. 2007;9:582–588. doi: 10.1055/s-2007-965424. [DOI] [PubMed] [Google Scholar]
- 89.Riemenschneider A., Wegele R., Schmidt A., Papenbrock J. Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J. 2005;272:1291–1304. doi: 10.1111/j.1742-4658.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- 90.Heidenreich T., Wollers S., Mendel R.R., Bittner F. Characterization of the NifS-like Domain of ABA3 from Arabidopsis thaliana Provides Insight into the Mechanism of Molybdenum Cofactor Sulfuration. J Biol Chem. 2005;280:4213–4218. doi: 10.1074/jbc.M411195200. [DOI] [PubMed] [Google Scholar]
- 91.Van Hoewyk D., Pilon M., Pilon-Smits E.H.H. The functions of NifS-like proteins in plant sulfur and selenium metabolism. Plant Sci. 2008;174:117–123. [Google Scholar]
- 92.Turowski V.R., Busi M.V., Gomez-Casati D.F. Structural and functional studies of the mitochondrial cysteine desulfurase from Arabidopsis thaliana. Mol Plant. 2012;5:1001–1010. doi: 10.1093/mp/sss037. [DOI] [PubMed] [Google Scholar]
- 93.Laureano-Marin A.M., Garcia I., Romero L.C., Gotor C. Assessing the transcriptional regulation of L-cysteine desulfhydrase 1 in Arabidopsis thaliana. Front Plant Sci. 2014;5:683. doi: 10.3389/fpls.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scuffi D., Alvarez C., Laspina N., Gotor C., Lamattina L., Garcia-Mata C. Hydrogen Sulfide Generated by L-Cysteine Desulfhydrase Acts Upstream of Nitric Oxide to Modulate Abscisic Acid-Dependent Stomatal Closure. Plant Physiol. 2014;166:2065–2076. doi: 10.1104/pp.114.245373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khan M.N., Mobin M., Abbas Z.K., Siddiqui M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide. 2017;68:91–102. doi: 10.1016/j.niox.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 96.Li Z.G., Gu S.P. Hydrogen sulfide as a signal molecule in hematin-induced heat tolerance of tobacco cell suspension. Biol Plantarum. 2016;60:595–600. [Google Scholar]
- 97.Aroca A., Benito J.M., Gotor C., Romero L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J Exp Bot. 2017;68:4915–4927. doi: 10.1093/jxb/erx294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fang T., Cao Z., Li J., Shen W., Huang L. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem. 2014;76:44–51. doi: 10.1016/j.plaphy.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 99.Xie Y., Zhang C.h., Lai D., Sun Y., Samma M.K., Zhang J. Hydrogen sulfide delays GA-triggered programmed cell death in wheat aleurone layers by the modulation of glutathione homeostasis and heme oxygenase-1 expression. J Plant Physiol. 2014;171:53–62. doi: 10.1016/j.jplph.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 100.Jin Z., Xue S., Luo Y., Tian B., Fang H., Li H. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem. 2013;62:41–46. doi: 10.1016/j.plaphy.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 101.Li ZG. Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal Behav 2015;10:e1051278. [DOI] [PMC free article] [PubMed]
- 102.Khan M.N., AlZuaibr F.M., Al-Huqail A.A., Siddiqui M.H., Ali H.M., Al-Muwayhi M.A. Hydrogen Sulfide-Mediated Activation of O-Acetylserine (Thiol) Lyase and L/D-Cysteine Desulfhydrase Enhance Dehydration Tolerance in Eruca sativa Mill. Int J Mol Sci. 2018;19:1–18. doi: 10.3390/ijms19123981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Da-Silva C.J., Fontes E.P.B., Modolo L.V. Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana. Plant Sci. 2017;256:148–159. doi: 10.1016/j.plantsci.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 104.Kumar D. Salicylic acid signaling in disease resistance. Plant Sci. 2014;228:127–134. doi: 10.1016/j.plantsci.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 105.Liu X., Rockett K.S., Kørner C.J., Pajerowska-Mukhtar K.M. Salicylic acid signalling: new insights and prospects at a quarter-century milestone. Essays Biochem. 2015;58:101–113. doi: 10.1042/bse0580101. [DOI] [PubMed] [Google Scholar]
- 106.Rushton P.J., Somssich I.E., Ringler P., Shen Q.J. WRKY transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 107.Dong J., Chen C., Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51(21):37. doi: 10.1023/a:1020780022549. [DOI] [PubMed] [Google Scholar]
- 108.Liu Z., Fang H., Pei Y., Jin Z., Zhang L., Liu D. WRKY transcription factors down-regulate the expression of H2S-generating genes, LCD and DES in Arabidopsis thaliana. Sci Bull. 2015;60:995–1001. [Google Scholar]
- 109.Tahir J., Wanatabe M., Jing H.C., Hunter D.A., Tohge T., Nunes-Nesi A. Activation of R-mediated innate immunity and disease susceptibility is affected by mutations in a cytosolic O-acetylserine (thiol) lyase in Arabidopsis. Plant J. 2013;73:118–130. doi: 10.1111/tpj.12021. [DOI] [PubMed] [Google Scholar]
- 110.Ausma T., De Kok L.J. Atmospheric H2S: Impact on Plant Functioning. Front Plant Sci. 2019;10:743. doi: 10.3389/fpls.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bloem E., Haneklaus S., Kesselmeier J., Schnug E. Sulfur Fertilization and Fungal Infections Affect the Exchange of H2S and COS from Agricultural Crops. J Agric Food Chem. 2012;60:7588–7596. doi: 10.1021/jf301912h. [DOI] [PubMed] [Google Scholar]
- 112.Pollacci E. Della ragione per cui il sulfo uceide l'oidio della vite, e sulla emissione d'idrogeno libero dalle piante. Gazz Chim Ital. 1875;5:451–460. [Google Scholar]
- 113.Foreman F.W. The Fungicidal Properties of Liver of Sulphur. J Agric Sci. 1910;3:400–416. [Google Scholar]
- 114.Marsh R.W. Investigations on the fungicidal action of sulphur. III. Studies on the toxicity of sulphuretted hydrogen and on the interaction of sulphur with fungi. J Hortic Sci. 1929;7:237–250. [Google Scholar]
- 115.Haneklaus S, Bloem E, Schnug E. Plant disease control by nutrient management: sulphur. In: Walters D (ed) Disease Control in Crops: Biological and Environmentally Friendly Approaches. Wiley-Blackwell, Oxford 2009; 263 pp.
- 116.Kruse C., Jost R., Lipschis M., Kopp B., Hartmann M., Hell R. Sulfur-enhanced defence: effects of sulfur metabolism, nitrogen supply, and pathogen lifestyle. Plant Biol. 2007;9:608–619. doi: 10.1055/s-2007-965432. [DOI] [PubMed] [Google Scholar]
- 117.Hu K.D., Wang Q., Hu L.Y., Gao S.P., Wu J., Li Y.H. Hydrogen sulfide prolongs postharvest storage of fresh-cut pears (Pyrus pyrifolia) by alleviation of oxidative damage and inhibition of fungal growth. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0085524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu L.H., Hu K.D., Hu L.Y., Li Y.H., Hu L.B., Yan H. An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang J., Hu K., Hu L., Li Y., Liu Y., Zhang H. Hydrogen Sulfide Acts as a Fungicide to Alleviate Senescence and Decay in Fresh-cut Sweetpotato. Hort Sci. 2014;49:938–943. [Google Scholar]
- 120.Zhang C., Shi J.Y., Zhu L.Q., Li C.L., Wang Q.G. Cooperative effects of hydrogen sulfide and nitric oxide on delaying softening and decay of strawberry. Int J Agric Biol Eng. 2014;7:114–122. [Google Scholar]
- 121.Neale H., Deshappriya N., Arnold D., Wood M.E., Whiteman M., Hancock J.T. Hydrogen sulfide causes excision of a genomic island in Pseudomonas syringae pv. phaseolicola. Eur J Plant Pathol. 2017;149:911–921. [Google Scholar]
- 122.Nicholls P., Marshall D.C., Cooper C.E., Wilson M.T. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem Soc Trans. 2013;41:1312–1316. doi: 10.1042/BST20130070. [DOI] [PubMed] [Google Scholar]
- 123.Guimarães B.G., Barbosa R.L., Soprano A.S., Campos B.M., de Souza T.A., Tonoli C.C. Plant pathogenic bacteria utilize biofilm growth-associated repressor (BigR), a novel winged-helix redox switch, to control hydrogen sulfide detoxification under hypoxia. J Biol Chem. 2011;286:26148–26157. doi: 10.1074/jbc.M111.234039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Forte E., Giuffrè A. How bacteria breathe in hydrogen sulfide-rich environments. Biochem (Lond) 2016;38:8–11. [Google Scholar]
- 125.Yang Z., Haneklaus S., De Kok L.J., Schnug E., Singh B.R. Effect of H2S and dimethyl sulfide (DMS) on growth and enzymatic activities of Rhizoctonia solani and its implications for sulfur-induced resistance (SIR) of agricultural crops. Phyton. 2006;46:55–70. [Google Scholar]
- 126.Riemenschneider A. Isolation and characterization of cysteine- degrading and H2S-releasing proteins in higher plants. PhD Thesis, University of Hanover 2006, Germany. [DOI] [PubMed]
- 127.Geilfus C.M. The pH of the Apoplast: Dynamic Factor with Functional Impact Under Stress. Mol Plant. 2017;10:1371–1386. doi: 10.1016/j.molp.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 128.Romero L., García I., Gotor C. L-Cysteine Desulfhydrase 1 modulates the generation of the signaling molecule sulfide in plant cytosol. Plant Signal Behav. 2013;8 doi: 10.4161/psb.24007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao Y., Biggs T.D., Xian M. Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem Commun (Camb) 2014;50:11788–11805. doi: 10.1039/c4cc00968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Toohey J.I. Sulfur signaling: Is the agent sulfide or sulfane? Anal Biochem. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 131.Toohey J.I., Cooper A.J.L. Thiosulfoxide (Sulfane) Sulfur: New Chemistry and New Regulatory Roles in Biology. Molecules. 2014;19:12789–12813. doi: 10.3390/molecules190812789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Filipovic M.R., Jovanovic V.M. More than just an intermediate: hydrogen sulfide signalling in plants. J Exp Bot. 2017;68:4733–4736. doi: 10.1093/jxb/erx352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zivanovic J., Kouroussis E., Kohl J.B., Adhikari B., Bursac B., Schott-Roux S. Selective Persulfide Detection Reveals Evolutionarily Conserved Antiaging Effects of S-Sulfhydration. Cell Metab. 2019;30:1152–1170. doi: 10.1016/j.cmet.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jin Z, Pei Y. Physiological Implications of Hydrogen Sulfide in Plants: Pleasant Exploration behind Its Unpleasant Odour. Oxid Med Cell Longev 2015; Article ID 397502. [DOI] [PMC free article] [PubMed]
- 135.Sameeullah M., Khan F.A., Özer G., Aslam N., Gurel E., Waheed M.T. CRISPR/Cas9-Mediated Immunity in Plants Against Pathogens. Curr Issue Mol Biol. 2018;26:55–64. doi: 10.21775/cimb.026.055. [DOI] [PubMed] [Google Scholar]