Abstract
Early atherosclerosis features functional and structural changes in the endothelial barrier function that affect the traffic of molecules and solutes between the vessel lumen and the vascular wall. Such changes are mechanistically related to the development of atherosclerosis. Proatherogenic stimuli and cardiovascular risk factors, such as dyslipidaemias, diabetes, obesity, and smoking, all increase endothelial permeability sharing a common signalling denominator: an imbalance in the production/disposal of reactive oxygen species (ROS), broadly termed oxidative stress. Mostly as a consequence of the activation of enzymatic systems leading to ROS overproduction, proatherogenic factors lead to a pro-inflammatory status that translates in changes in gene expression and functional rearrangements, including changes in the transendothelial transport of molecules, leading to the deposition of low-density lipoproteins (LDL) and the subsequent infiltration of circulating leucocytes in the intima. In this review, we focus on such early changes in atherogenesis and on the concept that proatherogenic stimuli and risk factors for cardiovascular disease, by altering the endothelial barrier properties, co-ordinately trigger the accumulation of LDL in the intima and ultimately plaque formation.
Keywords: Atherosclerosis, Endothelium, Vascular permeability, Cardiovascular risk factors
1. Introduction
The vascular endothelium is the main regulator of the selective exchanges of solutes and cells between the flowing blood and the surrounding tissues. Small molecules may cross this inner vascular layer gaining access to the tunica intima according to concentration gradients, whereas the passage of larger molecules and cells can only occur via vesicles and receptors, or when the endothelial junctions are impaired.1 Although the term ‘increased vascular permeability’ broadly refers to a compilation of structural and functional changes in the entire vessel wall, the passage of macromolecules, fluids, and cells into the intima, occurring at the inception of atherosclerosis, is primarily due to changes in endothelial barrier function. Endothelial dysfunction(s)2 initiate a dysregulated transendothelial flux, which lead(s) to abnormal deposition of molecules and cells in the intima. This results in intimal enlargement and local inflammation, both featuring in early atherosclerosis.
Low-density lipoproteins (LDL) are the main vehicle of blood cholesterol transport, and their accumulation in the intima characterizes the formation of the fatty streak,3 the earliest morphological change occurring in atherosclerosis.4 Understanding the control of endothelial permeability to LDL in the broader context of changes in endothelial function is therefore crucial to an understanding of atherosclerosis.
We have here compiled the most relevant information on mechanisms by which the vascular endothelium allows molecules or cells to permeate the intima. This process involves changes in three compartments: (i) the ‘glycocalyx’, which is the surface layer of glycoproteins, proteoglycans, and glycosaminoglycans that together create a scaffold on the endothelial surface5; (ii) the energy-dependent vesicular trafficking, referred to as the ‘transcellular pathway’6; and (iii) the opening or rearrangements of cell-to-cell junctions, also known as the ‘paracellular pathway’.7,8 An enhanced vesicular trafficking and/or widened intercellular spaces in the presence of a disarrayed or overtly disintegrated glycocalyx may all facilitate the transendothelial flux of LDL, paving the way to their subendothelial retention.9,10
We also herein review the molecular changes involved in endothelial permeability to LDL caused by cardiovascular risk factors; specifically dyslipidaemia, diabetes, hypertension, obesity, and smoking. All these factors have been reported to promote transendothelial uptake of LDL through mechanisms likely related to increased oxidative stress, highlighting the concept that all such risk factors promote atherosclerosis—at least in part—by inducing changes in endothelial permeability to LDL.
2. Literature search methods
For the literature search, we have used the following terms: ‘Dyslipidaemia AND atherosclerosis AND endothelial permeability’, with 56 output articles; ‘Diabetes AND atherosclerosis AND endothelial permeability’, with 97 output articles; ‘Hypertension AND atherosclerosis AND endothelial permeability’, with 99 output articles; ‘Obesity AND atherosclerosis AND endothelial permeability’, with 8 output articles; and ‘Oxidative stress AND atherosclerosis AND endothelial permeability’, with 51 output articles; ‘smoking AND atherosclerosis AND endothelial permeability’ with 21 output articles. Based on the title or abstract, articles were chosen for text review, and cited when appropriate. Additional records were identified and cited from the analysis of references cited in each article and from the authors’ own expertise.
3. Increased endothelial permeability and intimal LDL accumulation
In the late 1970s, Minick et al.11,12 tested the hypothesis that persistent absence of the endothelium favours intimal thickening, lipid accumulation, and atherosclerosis. Their results showed that, in rabbit aortas, the greatest accumulation of lipids and the largest intimal thickening was present in areas covered by a regenerated endothelium, and not in adjacent denuded areas, i.e. intimal areas lacking an endothelial lining. These experiments apparently supported the hypothesis that in areas of regenerated endothelium severe endothelial hyperpermeability is a major factor favouring intimal lipid uptake, more so than in areas with the complete absence of endothelium. Subsequent studies in moderately hypercholesterolaemic rabbits13 showed that the influx of labelled LDL into the aortic tunica intima mainly proceeds through focal sites, where inter-endothelial junctions are impaired. In Sprague-Dawley rats, impaired junctional function may occur as the consequence of cell damage and/or cell division14,15 and is found for months in the regenerated endothelium.14,15
An important reason why an endothelium with impaired barrier function may cause lipid accumulation more than in its complete absence is potentially linked to the mechanisms by which LDL accumulate in the intima. The relatively small fenestrae in an intact intimal elastic lamina (IEL) limit LDL flux into the media as compared to that of fluids.16 A sieving of the IEL with intimal accumulation of LDL was hypothesized to occur in the presence of what we would now call ‘dysfunctional’ endothelium,2 particularly in areas of recent endothelial regeneration. Such sieving of the IEL was actually found in specimens of human aortas, where concentration of unbound LDL in the intima markedly exceeded that of plasma.17,18 In an elegant physical model, Fry19 explained the transport and accumulation of atherogenic agents in elastic and muscular arteries ignoring at that time chemical reactions occurring in the intima. He demonstrated that both ‘mild’ increases in endothelial permeability and subjacent interstitial sieving were required for the accumulation of LDL in conditions known to promote atherosclerosis, such as hypercholesterolaemia and hypertension. Figure 1 summarizes the transport of LDL to and from the arterial intima, including binding of LDL to glycosaminoglycans, by which LDL can become more vulnerable to oxidation, favouring their uptake by phagocytes.20 These monocyte/macrophages see bound and modified LDL as foreign particles, initiating an immune response. In addition, oxidised LDL (ox-LDL) also promote endothelial activation with the release of soluble and membrane-bound mediators that further fuel the inflammatory response.21
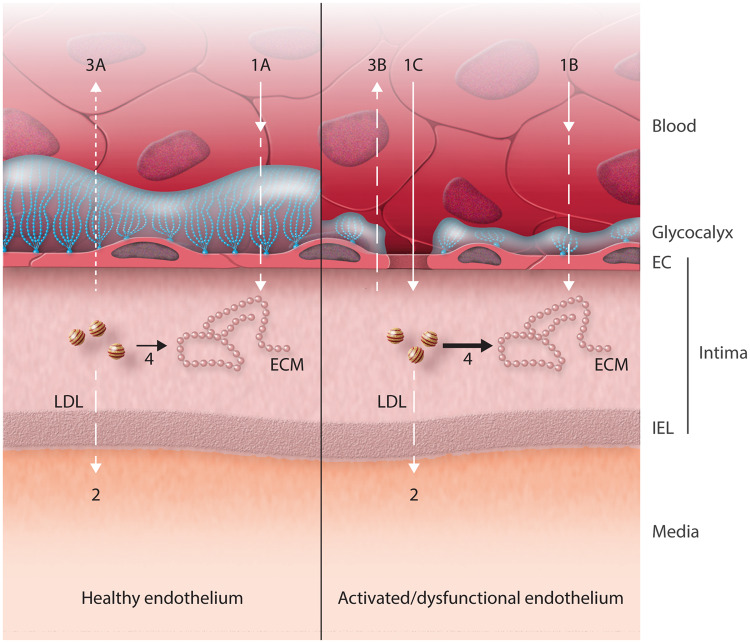
Figure 1.
The entrance of LDL in the arterial intima of healthy vessels is determined by the glycocalyx and endothelial vesicles (1A). In vessels where the glycocalyx is compromised, the entrance of LDL in the arterial intima is determined by endothelial vesicles (1B) and open endothelial junctions (1C). The efflux occurs through the IEL (2) and the backward diffusion (3A and 3B), which may be of minor relevance as it goes against hydraulic conductivity. LDL can be trapped by binding to the extracellular matrix (ECM) (4). Adapted from Fry.19
Subsequent studies further underpinned the role of endothelial permeability in atherosclerosis. In reviewing these data, Nielsen22 summarized a great number of findings as follows: ‘In laboratory animals, the regional variation in the arterial wall permeability predicts the pattern of subsequent dietary induced atherosclerosis. Moreover, mechanical or immunological injury of the arterial wall increases the LDL permeability and is accompanied by accelerated development of experimental atherosclerosis’. Based on these statements, it is still unclear whether the increase in permeability is a cause or a consequence of the inflammatory activation that overtakes the tunica intima.
4. The intimal uptake of LDL—methods of investigation
In vitro studies on LDL transport using endothelial monolayers consist in growing such cell monolayers on filters and placing them in chemotactic chambers. Under these conditions, the fluid above and below the monolayer can be readily sampled and evaluated for lipoprotein transport.23,24 The extent of LDL transcytosis can be measured using a non-radioactive ‘in vitro’ method, by which confluent endothelial cells (ECs) grown in culture in the upper compartment of a chemotaxis chamber are exposed to a solution of labelled LDL.25,26 The passage of labelled LDL from the upper to the lower compartment can be evaluated by measuring the concentration of LDL in the lower compartment by fluorimetry. To distinguish whether the passage of LDL is mediated by transcytosis or by impaired cell junctions allowing for paracellular transport phenomena, the permeation rate can also be evaluated after treatment of cells with inhibitors of transcytosis. It is possible to carry-out the analysis of LDL uptake in EC incubating EC with labelled LDL under appropriate culture conditions before the uptake assessment by fluorescence microscopy.27 These methods ignore the contribution of the glycocalyx, as this is poorly developed in culture.
Vascular wall permeability to LDL can now also be measured using in vivo/ex vivo methods. In vivo/ex vivo measurements of LDL uptake in carotid and aorta can be assessed by administering radiolabelled LDL, followed by autoradiography.28,29 This method consists in obtaining LDL from animal or human plasma in vivo, labelling the lipoprotein fraction, and then injecting both LDL and serum albumin into patients then undergoing surgery. Subsequently, surgical or endarterectomy specimens are fixed and sliced transversely, and processed by autoradiography.29 It is also possible to calculate LDL transport from the blood into the arterial wall after obtaining in vivo anatomy data from computed tomography images.30 Alternatively, local changes in arterial wall permeability to LDL,13,31,32 as well as the contribution of the glycocalyx,5 can be assessed by electron microscopy.
5. Structures involved in endothelial permeability to LDL
The first regulator of LDL transendothelial passage is the glycocalyx,33 a thick and negatively charged matrix layer, that lines the inner wall of healthy blood vessels34 (Figure 1). Once through the glycocalyx, LDL can cross the endothelium via transcytosis, a process that occurs through vesicles, which transport lipoproteins from the apical to the basolateral aspect of ECs.31 In Sprague-Dawley rats under pathological conditions, LDL may also, however, cross the endothelium through junctions with a wider inter-junctional space, the so-called ‘leaky junctions’, associated with dying or dividing cells.35,36
5.1 The glycocalyx
The glycocalyx is a thick layer of glycoproteins, proteoglycans, and hyaluronan (HA) that lines the endothelial surface. It is firmly attached to the luminal surface, as many of these molecules are membrane-bound and extend ∼0.05 to 0.4 µm from the endothelium towards the lumen.37 In addition, a second layer, partly intertwined within the former, extends by an additional ∼0.3 to 0.5 µm, and is composed mostly by HA. This second layer of the glycocalyx traps plasma- and endothelium-derived proteins and is in direct contact with the bloodstream.34 The glycocalyx plays a role in the regulation of vascular permeability, in the transmission of shear stress to the endothelial surface, provides a barrier to pathogens, and prevents blood cell margination towards the vessel wall.
In murine models, there is evidence that composition and thickness of the glycocalyx depend on local haemodynamic shear forces from the flowing blood.37–39 Moreover, in a pig model, laminar flow, likely through mechanical distortions of the glycocalyx, enhances nitric oxide (NO) production by the underlying ECs, a process that may become insufficient when shear forces are altered.40 As shown in male Syrian golden hamsters, the glycocalyx, as a selective barrier to solutes, allows anionic and protein molecules to gain access to the membrane bilayer, depending on size, charge, and structure,41 while water and electrolytes can pass freely. The binding and intercalation of plasma constituents within the structural elements of the glycocalyx create the so-called endothelial surface layer.42 The glycocalyx also plays a critical role in regulating LDL transport into the arterial wall, although the exact nature of the interactions between LDL and the glycocalyx is still under investigation.
Initially in coronary arteries of cholesterol-fed White Carneau pigeons, and subsequently in carotid arteries of hypercholesterolaemic mice, it was shown that the thickness of the glycocalyx was reduced in plaque regions of the arteries (45–64% and 71%, respectively).37,43,44 In addition, the glycocalyx coverage was reduced by 29% in atherosclerotic areas.44 These changes are dependent both on the plaque-inducing diet and location in the vessel. Reducing the thickness of the glycocalyx, or decreasing its barrier function would both favour the access of LDL to the endothelial surface, expose endothelial receptors and enhance binding of monocytes,45–47 which lead to enhanced accumulation of LDL into the intima5,10 (Figure 1). In specific conditions such as hypertension this process benefits from convective transmural fluxes, causing concentration and polarization of LDL at the luminal surface of arteries.48
The reduction in glycocalyx in atherosclerotic regions is probably due to both decreased synthesis and increase breakdown. Exposure to laminar flow is required for the continued production of proteoglycans, and when laminar flow ceases, rapid shedding of proteoglycans occurs.38 Furthermore, enhanced breakdown of the glycocalyx can also occur by enhanced generation of reactive oxygen species (ROS) and inflammatory activation of the endothelium, which both can lead to increased activity of proteoglycan-shedding metalloproteinases. It is known that the glycocalyx is severely impaired in diabetes, a disease accompanied by enhanced ROS production. Furthermore, ox-LDL reduce the effective thickness of the glycocalyx.49 Finally, Son et al.50 showed that disturbed flow targets and downregulates the inhibitor of metalloproteinases TIMP3 via induction of mouse-specific miR-712 (and human miR-205). Under laminar flow, TIMP3 limits the proteolytic activity of and the shedding by several metalloproteinases.51 Thus, laminar flow protects the glycocalyx by several mechanisms: it enhances NO production, which in turn counteracts ROS and inflammatory activation; and limits the activity of metalloproteinases in shedding the glycocalyx by maintaining TIMP3 synthesis.
5.2 LDL transcytosis and the role of caveolae
To reach the subendothelial space, after crossing the glycocalyx, LDL also have to pass the EC barrier (Figure 1). The passage of LDL through normal endothelial junctions is not possible for a variety of reasons, including the mere steric hindrance, being LDL diameter (20–30 nm) larger than the inter-junctional space (3–6 nm). Most of LDL transport is mediated by clathrin-coated and caveolin vesicles, both originated from invaginations of the plasma membrane and playing a major role in the continuous exchange of molecules, including LDL.52 Under physiological conditions, open caveolae and clathrin-coated vesicles at the luminal aspect of the endothelium bind LDL through LDL receptors, specifically scavenger receptor class B member 1 (SBR1), and more recently also activin receptor-like kinase 1 receptors.53,54 Once bound, LDL become internalized, shuttled through the endothelium, and delivered at the abluminal membrane.31 This process is called transcytosis.55 Pathological stimuli can upregulate endothelial transcytosis, and caveolin vesicles are key regulators of the endothelial barrier function.55 Particularly, silencing caveolin-1 (Cav-1) in ECs prevents the endocytosis of LDL, suggesting that Cav-1 function is critical for the endocytosis of LDL and potentially their transcytosis across EC. Compared with mice with the Cav-1+/+ background, mice silenced for Cav-1 displayed increased endothelial vascular cell adhesion molecule (VCAM)-1 expression, suggesting an important role for Cav-1 in vascular inflammation, and explaining its involvement in the inception of atherosclerosis.56
Transcytosis is a two-step process: first, receptor-mediated endocytosis into specific vesicles must take place, followed by exocytosis, an event that requires their recognition, docking and binding into the correct plasma membrane. This second step is facilitated by the soluble N-ethylmaleimide-sensitive factor (NSF) Attachment Protein (SNAP) receptors.55 In cultured EC, the transport of LDL cholesterol is largely controlled by protein kinase C (PKC) and the tyrosine kinase Src (Src)26 (Figure 2). Exocytosis requires integral membrane proteins members of the SNAp REceptor complex (SNARE), which comprises soluble SNAP receptors,61 and mediates the docking and fusion of vesicles with the cell membrane in order for vesicles to release their contents (Figure 2). The capacity of vesicle-mediated exchange of LDL over the endothelium is, however, quite limited.
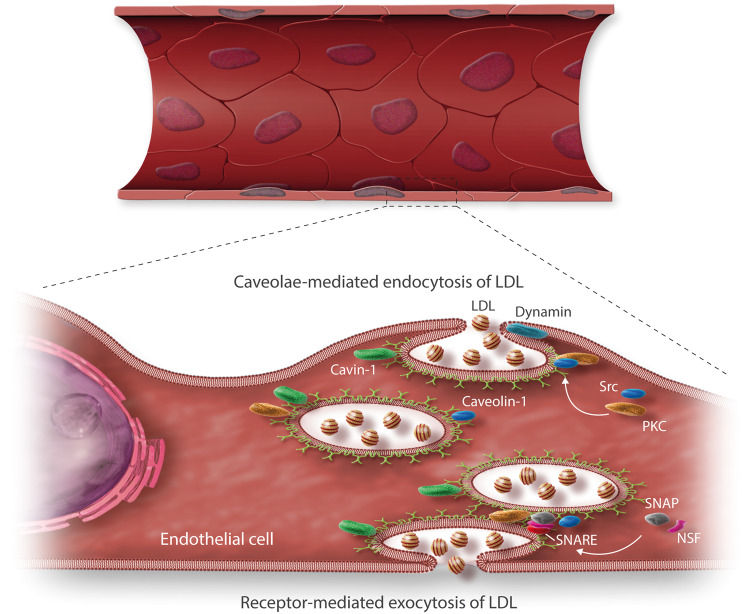
Figure 2.
Caveolae-/Cav-1-/cavin-1-mediated endocytosis and exocytosis of LDL particles in endothelial cells. The vesicular transport of LDL mediated by the caveolae/Cav-1/cavin-1 complex is triggered by PKC or Src,26 with the contribution of dynamin, a target protein for Src57 and PKC.58 Dynamin acts as a guanosine triphosphatase in the fixing of caveolae,59 and enables signals for the vesicle internalization.60 LDL endocytosis is further influenced by signalling pathways that further regulate LDL uptake. In turn, exocytosis is mediated by an integral membrane protein family, called SNARE, which comprises SNAP receptors61 and promotes the docking and fusion of vesicles with the cell membrane in order to release their contents through its interaction with N-Ethyl Maleimide (NEM)-Sensitive Factor,55 which is activated after the recruitment by its SNAP receptor.62
Once released from the basolateral aspect of the cell, LDL are trapped in the subendothelium, where they may undergo oxidative modifications, becoming strongly pro-atherogenic and able to induce the expression of adhesion molecules on the endothelial surface.63 The enhanced vesicular trafficking induced by atherogenic risk factors facilitates the transendothelial flux of LDL, enhancing their sub-endothelial retention, an early step in atherogenesis9 (Figure 2).
Importantly, the transport of LDL by low density lipoprotein receptor (LDLR) and SBR1 has been shown also to mediate increased transcytosis of high molecular weight compounds.64 This has been shown by exposing endothelial monolayers to LDL and visualizing the incorporation and transcytosis of high molecular weight dextrans.64 These findings clarify the effect of high levels of LDL and cholesterol on the endothelial barrier function, and highlight the possible implications of high level cholesterol to disease.
5.3 Endothelial cell–cell junctions
In animal model, LDL may also cross the endothelium through the paracellular pathway, when the barrier ‘leaks’.35,36 The paracellular pathway relies on structural changes in cell-cell junctions. Early electron microscopy studies65 revealed numerous and highly ordered junctional complexes between EC. These structures vary between distinct vessels, being more developed in arteries and capillaries and less so in post-capillary venules, where cell extravasation and exchange of plasma constituents are particularly prominent.65 The main groups of junctional complexes that control permeability are adherens junctions (AJ) and tight junctions (TJ),66 composed of membrane proteins connected to the cytoskeleton through transmembrane and cytosolic proteins.67 Their role is to impede the diffusion of molecules between the apical and the basolateral membrane of EC through tight contact of juxtaposed membranes68 (Figure 3).
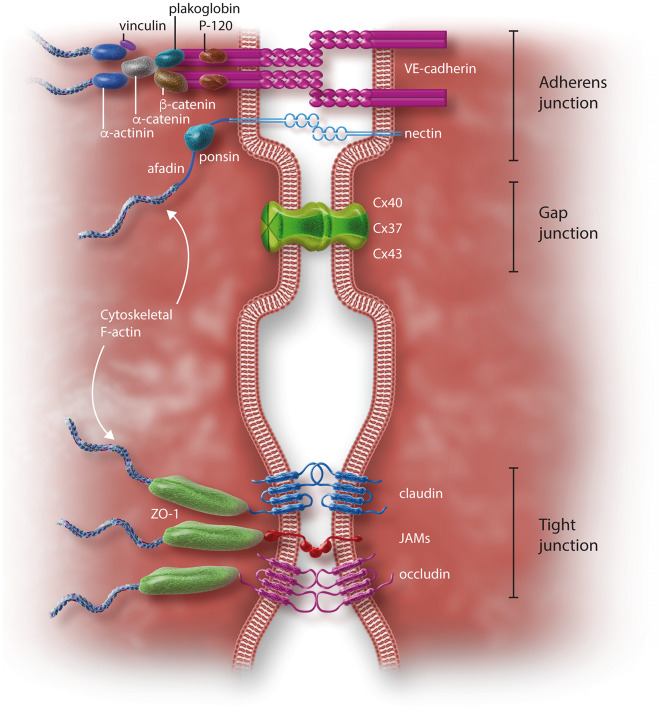
Figure 3.
Proteins involved in endothelial junctions. AJ are represented by the cadherin–catenin complex and the nectin–afadin complex. VE-cadherin binds to β-catenin and plakoglobin (probably these two different molecules both connect to the actin cytoskeleton by a still uncertain mechanism that probably involves alpha-actinin and eplin, as it does for E-cadherin), and p120 through its cytoplasmic tail.69 β-Catenin and plakoglobin in turn link α-catenin, a filamentous (F) actin-binding protein, which is the primary link between the AJ and the actin cytoskeleton.70 α-Catenin can bind vinculin and α-actinin, stabilizing the anchorage to F-actin microfilaments (for a review see71). When α-catenin is stretched (upon tension on the junctions), a latent vinculin binding site becomes available and initiates a second interaction of the cadherin–catenin complex with the F-actin cytoskeleton.72 Nectin binds afadin, which in turn binds ponsin, connecting nectin to the F-actin cytoskeleton.73 TJ are formed by occludin, claudin, JAMs,66 and by many intracellular components, such as ZO-1, which assemble molecular complexes and connect junctional structures to the cytoskeleton.67 GJ are formed by three Cx: Cx43, Cx40, and Cx3771 (see also Table 1).
TJ bring plasma membrane from adjacent cells into very close proximity, and were first incorrectly thought to be a fusion between the outer leaflets of plasma membranes.74 Ultrastructurally, TJ appear as networks of linear fibrils circumscribing the cell, intersected by short transversal fibrils,74,75 with plasma membranes in tight contact with each other. TJ are formed by occludins, claudins, and junctional adhesion molecules (JAMs)73 and contain many intracellular components, such as the zonula occludens-1 (ZO-1), which assembles molecular complexes and connects junctional structures with the cytoskeleton.71Table 1 lists the most important molecules and the resulting structures involved in TJ regulation of permeability.
Table 1.
Structural and functional factors involved in the control of endothelial permeability: tight junctions
| Name | Acronim/short name | Role and molecular relationship | References |
|---|---|---|---|
| Integral membrane proteins | |||
| Occludin | OCLN | Occludin is a 65 kDa protein containing two extracellular loops and four membrane-spanning regions, localizes at the TJ of epithelial and endothelial cells and might contribute to intercellular adhesion. Its endothelial expression correlates with the permeability of vascular tree | 71 |
| Claudin | CLDN | Claudin is a 22 kDa protein containing four membrane-spanning regions, two extracellular loops, and two cytoplasmic termini, and has central role in TJ formation in various tissues. Claudin-5 is typical of endothelium | 76 , 77 |
| Junctional adhesion molecule-A | JAM-A | JAMA-A is a 32-kDa glycoprotein composed of an extracellular region, a transmembrane segment, and a short cytoplasmic tail. JAM-A interacts with ZO-1, AF-6/Afadin, PAR-3/ASIP, CASK/Lin-2, and MUPP-1. Togeter with occludin and claudin, JAM-A binds cytoplasmic and cytoskeletal proteins involved in cell signalling, cell polarity, transcriptional regulation, and membrane trafficking | 78–80 |
| Endothelial cell selective adhesion molecule | ESAM | ESAM is a protein of 55 kDa localized at the TJ trough its colocalization with ZO-1 in brain and muscle capillaries. It participates in TJ assembly and in the regulation of paracellular permeability | 81 |
| Coxsackie and adenovirus receptor | CAR | CAR also participates in TJ assembly and in the regulation of paracellular permeability | 82 |
| Intracellular proteins | |||
| Zonula occludens-1 | ZO-1 | A 220–225 kDa protein belonging to the family of membrane-associated guanylate | 83 |
| Calcium/calmodulin-dependent serine protein kinase | CASK | CASK is a MAGUK protein, associating indirectly JAM-A to the cytoskeleton, via the actin/spectrin binding protein | 84 , 85 |
| Membrane-associated guanylate kinase inverted-1 | MAGI-1 | MAGI-1 is a MAGUK protein and has been recently shown to bind α-actinin-4 and associated to TJ | 86 |
| Afadin | AF6 | Afadin is a multi-domain protein involved in signalling and organization of cell junctions during embryogenesis. It contains PDZ domains and has been reported either at the TJ or at the AJ, also found in association with TJ proteins, such as JAM-A | 79 |
| Proteinase-activated receptor 3/Agouti-signalling protein | PAR-3/ASIP | PAR-3/ASIP has been localized at the TJ of enterocytes and MDCK cells | 87 |
| Cingulin | CGN | Cingulin is a 140- to 160-kDa component of endothelial and epithelial TJ and does not contain PDZ domain associated to TJ | 88 |
| Tight junction-associated antigen | 7H6 | 7H6 is a 155-kDa antigen localized at the TJ which likely plays a role in the barrier function of TJ in epithelial and endothelial cells | 89 |
| Tight junction associated protein 1 provided | PILT | PILT is a 61-kDa protein only recruited to TJ following the formation of claudin-based strands, not directly interacting with claudin | 90 |
| Serine–threonine kinase | WNK-4 | WNK-4 is the localization at the TJ and its mutations cause a form of secondary hypertension in humans | 91 |
| Junction-enriched and -associated protein | Jeap | JEAP is a 98-kDa protein that contains a carboxy-terminal consensus motif for binding PDZ domains. It is specifically expressed in epithelia of exocrine glands | 92 |
| Ras-related protein Rab3b | Rab3b | Monomeric G protein localized to the TJ with a role in membrane and vesicle trafficking | 93 |
| Ras-related protein Rab13 | Rab13 | Monomeric G protein localized to the TJ with a role in membrane and vesicle trafficking | 94 |
| Vesicle-associated membrane protein | VAP-33 | Monomeric G protein localized to the TJ with a role in membrane and vesicle trafficking | 95 |
| Exocyst complex component 3/4 | Sec6/Sec8 | Monomeric G proteins localized to the TJ with a role in membrane and vesicle trafficking | 96 |
Many additional factors forming complexes with TJs and implicated in their regulation could not be here summarized.
MUPP-1, multi-PDZ domain protein 1; MDCK, Madin–Darby canine kidney epithelial cells.
Serial-section electron microscopy studies revealed that—with the exception of the blood–brain barrier—TJ in the capillary endothelium of the rat heart are mosaic structures, organized as irregular networks between neighbouring cells, and provided with discontinuities about 4 nm wide.97 This supports the notion that, in most healthy continuous endothelia, the paracellular pathway is only viable for small solutes, and is responsible for the relatively low electrical resistances when compared to epithelia and the brain endothelium, which are sealed by a TJ belt.98,99 Although macromolecules may also pass these TJ mosaic discontinuities, a subsequent belt of closed AJs only allows the passage of small solutes, but not of macromolecules.
AJ are primarily composed of clusters of cadherin–catenin complexes typical of all epithelial and endothelial layers. Within the many types of cadherins, vascular-endothelial (VE)-cadherin or cadherin-5100 is specific to ECs. Cadherins in general are transmembrane proteins that extend into the extracellular domain and, in the case of VE-cadherin, form homodimeric interactions via their C-terminal domains. After such engagement, the cytosolic regions of these proteins become connected with the actin cytoskeleton.69 Such interactions are reinforced by a group of molecules that include, on the cadherin side, β-catenin, plakoglobin, and p120.69 β-Catenin and plakoglobin, in turn, link α-catenin, which further connects with actin via α-actinin and perhaps another still uncertain protein—eplin—mediating the anchorage of actin microfilaments to AJ (for reviews see101,102).
When α-catenin is stretched, a new domain is exposed, binding vinculin and enabling the formation of a second bridge to the actin cytoskeleton. An additional group of proteins have been recently acknowledged to contribute to AJ, including the nectin–afadin protein complexes, which itself includes nectin, afadin, and ponsin.71 Nectin binds to afadin, which in turn binds ponsin, connecting nectin to the actin cytoskeleton103 (Figure 3). For a more comprehensive list of the molecules and structures involved in the regulation of permeability by AJ, see Table 2. The expression of VE-cadherin, which is essential for maintenance of the endothelial barrier function,115 is altered in early atherosclerosis and specifically in ECs of the human carotid artery and aorta that are involved in intra-plaque neovascularization.116 It is worth noting that an increased density of ‘leaky’ intercellular clefts have a much more limited effect on transendothelial LDL transport when the glycocalyx is present.10 Thus, a joint perturbation of several structural components of the endothelial barrier is necessary for a substantial subendothelial accumulation of LDL.
Table 2.
Structural and functional factors involved in the control of endothelial permeability: adherens junctions
| Name | Acronym/short name | Role and molecular relationship | References |
|---|---|---|---|
| The cadherin–catenin complex | |||
| Vascular endothelial-cadherin | VE-cadherin | VE-cadherin is a calcium-dependent protein involved in membrane permeability which binds with its cytoplasmic domain the β-catenin, plakoglobin, and catenin-p120. This protein complex interacts with receptors of VEGFR2 growth factors, FGF-R1, and TGFb-R | 100 , 101 , 104 |
| Catenin (cadherin-associated protein), beta 1 | β-Catenin | β-Catenin binds α-catenin, homologous to vinculin, which binds to α-actinin. This protein is involved in the canonical Wnt signalling pathway | 105 |
| Plakoglobin | JUP | β-Catenin binds α-catenin, homologous to vinculin, which can binds to α-actinin | 105 |
| Catenin-P120 | P120 | Catenin-P120 is an additional VE-cadherin partner which is a Src substrate and is homologous to β-catenin and plakoglobin | 106 |
| Alfa-catenin | α-Catenin | α-Catenin can be bounded by plakoglobin and β-catenin, which is homologous to vinculin and anchors the complex to actin | 71 , 105 |
| Vinculin | VCL | vinculin is bounded by α-catenin (340), and may further stabilize AJ anchorage to actin | 107 |
| Alfa-actinin | α-Actinin | α-Actinin can be bounded by α-catenin and further stabilize AJ anchorage to actin | 108 |
| Rac guanosine exchange factor | Tiam | Tiam codistributes with VE-cadherin at AJ | 109 |
| Density-Enhanced Phosphatase 1 | DEP-1 | VE-cadherin binds VEGFR2 inducing its dephosphorylation through the action of DEP-1 | 110 |
| Vascular endothelial-protein tyrosine phosphatase | VE-PTP | VE-PTP interacts with VE-cadherin modulating the cadherin/catenin complex and its phosphorylation and/or its intracellular partners. VE-PTP also associates with Tie-2, dampening the tyrosine kinase activity of this receptor that can support stabilization of endothelial junctions | 111 , 112 |
| Protein Tyrosine Phosphatase | PTPN11 or SHP2 | SHP2 could be associated with VE-cadherin and decrease its phosphorylation, enhancing endothelial barrier function | 113 |
| Beta-arrestin 2 | β-arrestin 2 | The β-arrestin-dependent endocytosis of VE-cadherin is promoted by VEGF and it controls endothelial-cell permeability | 114 |
| Neural cadherin | N-cadherin | N-cadherin is a member of the classical cadherin family of transmembrane glycoproteins that mediate cell-to-cell adhesion via a homophilic binding mechanism. It's interaction with actin-based cytoskeleton, which is important for adhesion, is mediated by the catenins | 114 |
| Heart cadherin | T-cadherin | T-cadherin is a glycosyl phosphatidylinositol anchored cell-surface glycoprotein and it promotes intercellular adhesion. It's expression in the human vasculature may be relevant to control of the normal vascular architecture | 87 |
| Nectin–afadin complex | |||
| Nectin cell adhesion molecule 1, adherens junction formation factor, SH3-DOMAIN Protein 5 | Nectin, afadin, ponsin | The nectin–afadin complex is a family of cell calcium-independent adhesion molecules. The nectin binds afadin that connects the nectin to the cytoskeleton. The ponsin binds afadin and vinculin and α-catenin. Nectin and afadin are localized at the cadherins and can interact with each other through the catenin | 100 |
Many additional factors forming complexes with TJs and implicated in their regulation, could not be summarized.
VEGFR2, vascular endothelial growth factors receptor 2; FGF-R1, fibroblast growth factor receptor 1; TGFβ-R , transforming growth factor beta receptor.
Other junctional contacts include gap junctions (GJ), formed by connexins (Cx), which are members of a large family of transmembrane proteins. In contrast to AJ and TJ, GJ allow ECs to communicate through the exchange of small molecular weight solutes between neighbouring cells117 (Figure 3). Endothelial GJ are formed by three Cx: Cx43, Cx40, and Cx37.100 GJ have also been proven to play an important role in atherogenesis, but have no direct effect on endothelial permeability. In fact, significant changes in the expression of vascular Cx have been described during the formation of atherosclerotic plaques in murine and human atherosclerotic plaques,118 and this expression is influenced by atherosclerotic risk factors, modifying the GJ channel or the hemichannel-mediated communication between cells, and influencing the progression of atherosclerosis 119 (Figure 3).
6. Atherosclerotic risk factors and endothelial permeability
The impairment of endothelial function(s) is widely recognized as the first clinical correlate of atherosclerosis and the first step in its inception.2,120 In fact, atherosclerotic risk factors are known to promote endothelial dysfunction(s) in the earliest phase of the process,2 affecting structure and function of the glycocalyx and NO production, as well as promoting the underlying critical changes that lead to the atheroma.33 Conditions of enhanced cardiovascular risk, such as dyslipidaemias, diabetes, hypertension, obesity, and smoking, all increase vascular permeability to LDL. Furthermore, micro-mechanical forces, such as fluid shear stress or cyclic strain, also influence endothelial permeability.121 We here therefore highlight the effect of cardiovascular risk factors on endothelial permeability. Importantly, all of these conditions affect cholesterol transfer across the endothelium, having increased oxidative stress as a common denominator.2
6.1 Dyslipidaemias
Dyslipidaemias, in their various forms, lead to EC dysfunction, thus increasing cardiovascular risk.120,122,123 The most common types of dyslipidaemia associated with increased cardiovascular risk are those characterized by high levels of LDL cholesterol.124 High levels of LDL generate a prominent increase in lipoprotein transcytosis125 that, together with changes in aortic endothelial permeability, as seen in cholesterol-fed rabbits,22 are the two major determinants of the LDL flux into the arterial wall and their deposition in the intima.126 Sprague-Dawley rats fed a high-cholesterol diet or normal diet for 12 months featured significant differences in the intercellular cleft morphology and the associated junctional complexes compared with rats fed a normal diet for 1 month.127 In such conditions, the density of GJ decreases, while the density of TJ and the other junctional complexes increase after 12 months compared with 1 month of normal diet.127 Although it is unlikely that LDL molecules can pass through GJ, it is recognized that the GJ assembly is modulated by exposure to LDL and apolipoprotein B.128 Hypercholesterolaemia may weaken the endothelial barrier function by activating Ras Homolog Family Member (Rho)A, while statins decrease permeability by suppressing Rho function in cultured human ECs.129 Hypercholesterolaemic serum may increase endothelial permeability of cultured ECs through the activation of phosphatidylinositol (PI)3-kinase.130 This latter is a major intracellular step regulating cell functions, such as cell growth, survival, and intracellular trafficking. Moreover, the thickness and function of the endothelial glycocalyx are profoundly reduced in patients with heterozygous familial hypercholesterolaemia, and such changes may contribute to increased vulnerability to atherosclerosis. Such perturbations are partially reversed by an even short-term statin therapy.131
6.2 Diabetes
Diabetic subjects have a 2- to 4-fold higher risk of cardiovascular events,132 and cardiovascular disease causes nearly 80% of diabetes-associated deaths.133 Mechanisms by which diabetes mellitus contributes to atherosclerosis are multiple and are still only partly understood.134,135
A damage to the vascular glycocalyx and the hyperglycaemia-related loss of glycocalyx function, followed by glycocalyx thinning and subsequent disease of the underlying vascular tissue, might be of particular importance in diabetes or in the metabolic syndrome and in situations of insulin resistance.33 Systemic hyperglycaemia has been shown to cause a generalized glycocalyx thinning in human subjects,136 probably because of the interaction of glucose with the glycoproteinaceous constituents of the glycocalyx.33 In type 1 diabetic patients, plasma HA, a main glycocalyx constituent, and hyaluronidase are both increased, resulting in increased synthesis and shedding of HA.136,137 This has led to the conclusion that type 1 diabetes is characterized by a damage to the endothelial glycocalyx.137 Moreover, hyperglycaemia attenuates the shear stress-dependent dilatation in distal pig arteries, consistent with the loss of mechano-transducing properties of the endothelial glycocalyx by hyperglycaemia.138 All these changes might represent one of the first steps in atherogenesis in the presence of diabetes.33
Several in vitro and in vivo studies have demonstrated that hyperglycaemia alters endothelial functions,139 activates PKC chronically,140 and markedly increases vascular permeability, monocyte adhesion, the expression of cell adhesion molecules, the generation of ROS, and the activation of nuclear factor (NF)-κB.141 High glucose in vitro, simulating hyperglycaemia, increases endothelial permeability of human umbilical vein ECs (HUVECs) by activating the RhoA-Rho Associated Coiled-Coil Containing Protein Kinase (ROCK) signalling pathway,142 a crucial set of mediators of endothelial barrier function.143 Treatment of ECs with high glucose also leads to tyrosine phosphorylation of VE-cadherin, with a consequent dissociation from β-catenin, barrier disruption, and increased trans-endothelial migration of monocytes.139
Vascular endothelial growth factor (VEGF) is regarded as the ‘master regulator of angiogenesis’.144 It typically induces migration and proliferation of ECs, modulates thrombogenicity and, most of all, enhances vascular permeability145 through the activation of VEGF receptor (VEGFR)-2 and the following calcium influx, activation of phospholipase C (PLC) and of NO synthase, all converging in the guanylyl cyclase-mediated activation of the Rho–Ras-Related C3 Botulinum Toxin Substrate (Rac) pathway and in the subsequent functional alteration of the junctional proteins cadherins, ZOs and occludins linked to the actin cytoskeleton.145 In the arteries of diabetic patients with atherosclerosis, VEGF is overexpressed, and treatment of endothelial monolayers with VEGF downregulates ZO-1 expression, thus favouring the formation of intercellular gaps and the increase in permeability to LDL.146 Exposure to high glucose concentrations has also been associated with increased expression of toll-like receptor (TLR)2 and 4 in ECs. It is likely that such activation contributes to increase endothelial permeability because of the downregulation of junctional proteins through an extracellular signal-regulated kinase (ERK) 1/2 dependent mechanism.147
6.3 Hypertension
Arterial hypertension is the most prevalent risk factor associated with increased cardiovascular morbidity and mortality.148,149 Hypertension accelerates the development, progression and complication of atherosclerosis, through mechanisms involving endothelial dysfunction, vascular oxidative stress, inflammation and remodelling.150,151 Hypertension is primarily characterized by morphological changes in the arterial endothelium and by hypertrophy of the smooth muscle layer in the tunica media.152 Many studies on hypertensive animals have reported increased transendothelial LDL permeability in arteries, and indicated that hypertension may lead to enhanced LDL entry into the intima by altering the permeability of the endothelium rather than by increasing the filtration rate.153,154 Mechanisms for the enhancement of endothelial permeability under hypertension in the rat aorta might consist of increased EC mitosis and apoptosis, and the associated transient formation of leaky junctions, which increase endothelial permeability to macromolecules.154 A quantitative model revealed important details about the influence of hypertension on LDL transport and its accumulation in the subendothelial space: LDL fluxes across the leaky junction, the intima, the IEL, and the media are all highly affected by the transmural pressure, which, in turns, affects EC turnover and compaction of the intima.155 Another mathematical model has shown that a seriously damaged glycocalyx augments the flux of plasma solvent and solutes, and both fluxes are further increased in the presence of hypertension.156 Furthermore, angiotensin II, the principal effector molecule of the renin angiotensin system (RAS), the activation of which is a key regulator of blood pressure and cardiovascular function, has been recently shown to increase LDL transcytosis across ECs and accelerate LDL retention in the subendothelial space of human umbilical venous walls. Mechanistically, proteins involved in caveolae-mediated transcytosis, including LDLR, Cav-1, and cavin-1, were found to be tightly associated with angiotensin II-induced LDL transcytosis across ECs.157
The local chemically-active interstitial concentration of a particular atherogenic molecule is a fundamental driving force in a system of reactions that produces local atherogenic changes. Such concentration of specific molecules was used as a measure of the potential for lesion development—an expression of local ‘risk’—in another model whereby transport processes interact with a tissue barrier. Such model explained that sites along the arterial tree that were characterized by ‘mild’ increases in endothelial permeability to macromolecules make the arterial intima particularly prone to increased accumulation of larger macromolecules, including LDL, thus developing high chemical activities. These sites were at high risk even in the absence of other risk factors, and this risk was dramatically increased with hypertension and/or elevated serum concentration of atherogenic soluble factors and/or pre-existing intimal thickening.19
A further possible mechanism explaining changes in endothelial permeability caused by hypertension might involve the role of calcium. Indeed, a mathematical model has been developed, predicting a sigmoidal dependence of calcium influx from shear stress.158 In cultured ECs, it has been shown that these changes in cytosolic calcium concentration might lead to calcium-dependent activation of myosin light chain (MLC) kinase, as well as RhoA/Rho kinase-dependent inhibition of the myosin phosphatase facilitating MLC phosphorylation, resulting in contraction of the cells and, finally, in endothelial barrier disruption.143
6.4 Obesity
The imbalance in energy homeostasis resulting in increased adipose tissue mass favours the initiation of cardiovascular and other diseases.159 This occurs because the adipose tissue acts as an endocrine organ, secreting molecules with profound local and systemic effects,160 including increased endothelial permeability.161 Abdominal obesity results in the enhanced expression of systemic circulating proinflammatory cytokines and growth factors, including, but not limited to, tumour necrosis factor (TNF)-α, interleukin (IL)-6, resistin, leptin, VEGF, and free fatty acids (FFA), which predispose to the onset of endothelial dysfunction(s).162 Obesity also results in the reduced expression of an anti-inflammatory cytokine, adiponectin,163 which normally prevents endothelial dysfunction(s).164
TNFα was among the first cytokines recognized to be expressed by the adipose tissue.165 Its levels correlate with the degree of adiposity and the associated insulin resistance,166 as well as with the risk of coronary artery disease.167 Many experimental data have accumulated regarding how TNFα contributes to boosting atherogenesis (for an exhaustive review see168). However, its regulatory effect on endothelial permeability is a quite novel acquisition. In addition to stimulating proinflammatory canonical pathways leading to deep changes in vascular cell gene expressions,168 TNFα activates the Rho A/ROCK axis. ROCK phosphorylates and inhibits MLC phosphatase, which promotes MLC phosphorylation, which in turn triggers the acto-myosin contraction,169 thus changing the endothelium from a pavement-like monolayer, with ECs containing a belt of peripheral actin, into a monolayer formed by elongated cells enriched in actin stress fibres.170
More recently TNFα was also demonstrated to increase the transcytosis of LDL across the undamaged endothelial barrier, and promote LDL retention in the subendothelial space through a mechanism involving the activation of NF-κB and of peroxisome proliferator activated receptor (PPAR)γ, which, upon a co-ordinated activation by TNFα, in concert increase the gene expression of Cav-1 and -2 and of the LDLR.26
Although the binding of TNFα to its membrane receptor activates many intracellular signalling pathways,168 the activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex and the following signalling sequelae leading to the oxidative modification of junctional proteins are considered the pathway most impacting the endothelial barrier properties.171
In more recent times, also the adipocyte-secreted cytokine resistin has been linked to obesity, insulin resistance, and atherosclerosis172 and has been specifically shown to increase endothelial permeability in cardiovascular patients.161 Mechanistic in vitro data suggest for this cytokine the ability to downregulate the endothelial expression of the junction proteins ZO-1 and occluding, through the stimulation of a signalling pathway enhancing the production of ROS and the activation of the mitogen activated protein kinases (MAPK) p38.173 Interestingly adiponectin suppresses multiple cellular effects known to be associated with TNFα-mediated endothelial hyperpermeability including actin stress fibre development, intercellular gap formation, and tubulin disassembly, through the activation of PKA and the increase of cAMP,174 known downregulators of RhoA GTPases activity.175
Other direct or indirect links between adipokines and cell junctional proteins that could alter endothelial permeability are plausible, and should be further investigated as well. Adiponectin, for example, is an important mediator of vascular disease, as it activates AMP-activated protein kinase and the subsequent increases in NO production in ECs. Because of its markedly reduced production in obesity, adiponectin can be responsible of the loss of the NO-mediated protection in vitro and in vivo.176
The body mass index and visceral fat accumulation in human subjects associates, among others, with elevated serum levels of VEGF.177 Although all human adipose depots may express VEGF178 the omentum was shown to express the highest levels,178 to the point that it has been hypothesized that the omental production of VEGF mostly determines serum levels of VEGF in obese patients.179 In addition and possible synergism with other adipokines, including resistin and leptin,180 VEGF contributes to increase endothelial permeability again by upregulating the intracellular production of ROS.180 However, it is also interesting to report that VEGF may mediate LDL transport across the endothelial barrier through an ‘uncanonical signalling pathway’, as recently demonstrated.181 It was indeed observed that LDL, by binding to LDLR, induce the autophosphorylation of VEGFR1 and its internalization together with both LDL and LDLR,181 thus providing an additional mechanistic route for LDL transport.182
An alternative pathway for the destabilization of endothelial permeability is also effected by saturated FFA such as palmitate.183 Plasma levels of saturated FFA are often elevated in obese patients184 and are considered critical contributors for obesity-induced pathological conditions, including diabetes and accelerated atherosclerosis. It has been recently shown that palmitate affects endothelial barrier functionality by activating the Nucleotide-Binding Oligomerization Domain, Leucine Rich Repeat And Pyrin Domain Containing (Nlrp)3 inflammasome complex183 through a mechanism involving the increased release of mitochondrial ROS, leading to reduced expression of the inter-endothelial junction proteins ZO-1 and -2.183
Overall, these data provide important mechanistic links between the adipose tissue secretome and impairment of endothelial barrier permeability.
6.5 Smoking
Cigarette smoking has been strongly associated with subclinical atherosclerosis in multiple vascular beds.185 Smoking injures the blood vessel wall by damaging ECs, thus potentially increasing permeability to lipids and other blood components,186 and affects all phases of atherosclerosis, from endothelial dysfunction(s) to acute occlusive clinical events.187
It has been reported that tobacco smoke cooperates with IL-1β to alter β-catenin trafficking in the vascular endothelium, eventually increasing permeability and inducing the expression of cyclooxygenase-2 in vitro and in vivo through a mechanism involving the overproduction of intracellular ROS.187 It has indeed been shown that the unsaturated aldehydes acrolein and crotonaldehyde contained in the gas phase of cigarette smoke are able to activate the endothelial activity of NADPH oxidase and the subsequent production of superoxide anion (O2−).188 More recently, it has been shown that tobacco smoke interacts with IL-1β determining suppression in the activity of protein deleted on chromosome 10 phosphatase and tensin homolog (PTEN), leading to increased VE-cadherin and β-catenin phosphotyrosine and to a disassembly of VE-cadherin/β-catenin membrane complexes. These events lead to the accumulation of β-catenin within the nucleus.189 Moreover, cigarette smoke contains metals that catalyse the oxidation of cellular proteins, causing a loss of microtubule function in turn culminating in microtubule depolymerization, the proteasome-dependent degradation of cytoskeletal α-tubulin, and eventually a contraction of vascular ECs and endothelial leakiness.190
The ‘cell turnover-leaky junction’ theory states that dying or dead ECs enhance endothelial permeability and provide a site for the localization of atherosclerosis.35 In fact, the long-term exposure to nicotine increases aortic EC death and promotes the transendothelial transport of macromolecules in rats.191 These findings are consistent with the observation that, although open junctions occupy less than 10−5% of the en face area of the endothelium, endothelial permeability in larger arteries can increase by 50–100% due to the experimentally observed regional variations in cell turnover.36
6.6 Haemodynamic forces
Although atherosclerosis is associated with systemic risk factors, it is a focal disease preferentially developing in predisposed, athero-prone regions,192 such as in the vicinity of branch points, the outer wall of bifurcations, and the inner wall of curvatures, generally characterized by low, disturbed, or oscillating blood flow. Local factors, such as haemodynamic forces, indeed play a major role in the regional localization of atherosclerosis,193 being able of modulating, through complex mechanoreception and mechanotransduction processes, endothelial functions and phenotype.194 These haemodynamic forces include flow-generated endothelial shear stress—a tangential frictional force, which plays the most fundamental role in atherosclerosis—and blood pressure-derived circumferential tensile/cyclic stress, acting perpendicularly to the vessel wall. The current consensus is that physiologic laminar flow and high shear stress (>15 dyn/cm2) suppress several endothelial proatherogenic genes and favour the expression of atheroprotective genes and bioactive products, including vasodilators (NO and prostacyclin) and antithrombotic agents (thrombomodulin). Conversely, disturbed flow and low shear stress (<4 dyn/cm2), occurring at arterial branching points and curvatures, stimulate EC turnover and apoptosis, and the expression of genes and gene products that promote atherogenesis, including cytokines and growth factors, adhesion molecules, ROS, and prothrombotic factors.195–197
An important underlying mechanism for the effects of local haemodynamic forces on atherogenesis is through the flow pattern-mediated regulation of endothelial permeability. Atheroprone areas, where blood flow is disturbed, feature increased permeability to macromolecules, including fluorescent, radiolabelled and chromogenic tracer-labelled particles, such as albumin and LDL. In porcine iliac arteries in vivo, sites exposed to low wall shear stress were found more likely to exhibit elevated permeability to albumin.198 Besides inducing SREBPs-dependent LDL uptake and synthesis, disturbed flow and low shear stress increase endothelial permeability to LDL, as demonstrated in different areas of the vasculature for both rabbits and pigs, as well as in a computational model of LDL transport in human coronary arteries.199,200 Conversely, laminar flow and high shear stress appear to limit endothelial permeability to LDL.201
In addition to the influence on glycocalix turnover discussed above, altered expression and discontinuous distribution of intercellular junction proteins, such as VE-cadherin,202 and induction of VEGF203 in ECs by disturbed flow and low shear stress provide possible molecular mechanisms for the increased endothelial permeability in regions of disturbed flow or low shear stress. The pronounced EC mitosis and apoptosis demonstrated both in the rabbit thoracic aorta in vivo and in cultured ECs,204,205 as well as the morphological changes of ECs from elongated to more rounded shape associated with low shear stress compared with high shear stress, might also be responsible for the greater permeability of ECs in segments exposed to disturbed flow.204,206,207 In these regions, the increased residence time of LDL due to flow stagnation also contributes to the infiltration LDL across the arterial wall.208
Another emerging mechanism related to local differences in shear stress profiles with impact on endothelial permeability is the generation of mesenchymal cells from the endothelium, known as endothelial-mesenchymal transition (EndoMT). This transition process, during which ECs can achieve a fibro-proliferative phenotype, is characterized on the one hand by the loss of EC markers, including VE-cadherin, PECAM-1; and by the increased expression of mesenchymal cell markers, including α-smooth muscle actin (α-SMA) and vimentin, on the other. This is accompanied by a loss of cell–cell adhesions and cell polarity, with consequent damage of endothelial junction stability and increased vascular permeability. Several lines of evidence indicate that EndoMT contributes to atherosclerotic pathobiology, from its inception to plaque destabilization,209 through mechanisms including endothelial barrier dysfunction. Pulmonary artery EC monolayers undergoing endoMT indeed failed to form integral biological barriers and featured enhanced leakage.210 The presence of ECs with mesenchymal characteristics has also been identified as overlying human and animal atherosclerotic plaques211,212 in relation to atherosclerosis severity. EndoMT in atherosclerosis may be driven by inflammatory stimuli, including an imbalance in transforming growth factor (TGF)-β and fibroblast growth factor receptor 1 (FGFR1) signalling.211 Furthermore, compared with LDL, ox-LDL increased radiation-induced EndoMT in human aortic ECs and in atherosclerotic tissues of irradiated ApoE−/− mice.213 Accordingly, EndoMT was induced by ox-LDL-induced foam cells via the CCL-4/CCR5/TGF-β axis.214 EndoMT is also sensitive to haemodynamic forces, as demonstrated both in cultured ECs exposed to flow and in animal models with varying levels of flow. Disturbed flow promotes EndoMT via the GATA4-TWIST1 signalling and the transcription factor Snail,215 while laminar uniform shear stress prevents EndoMT via activation of MEK5/ERK5 signalling.212 This shear stress-modulating effect on EndoMT might therefore contribute to explain the focal nature of atherosclerosis.
7. Oxidative stress: a common feature of atherogenic stimuli affecting endothelial permeability
Increased ROS production and dysregulated redox balance, also known as ‘oxidative stress’, contribute to many of the molecular events underlying atherogenesis and, correspondently, to the loss of endothelial barrier function.
Low levels of ROS, derived from various enzymatic sources, are the product of the normal cellular metabolism. In the vascular endothelium, sources of ROS include the xanthine oxido-reductase system, the mitochondrial respiratory chain, and the NADPH oxidase complex.216
Originally NADPH oxidase was considered an exclusive expression of phagocytic cells. It is now evident that a homologous of phagocytic NADPH oxidases is also functionally active in the vascular endothelium. The prototypical gp91phox-containing phagocytic NADPH oxidase is in the endothelium termed Nox2, and comprises 5 subunits: p47phox p67phox, p40phox, p22phox, and the catalytic subunit gp91phox.217 In basal conditions, p47phox, p67phox, and p40phox are in the cytosol, while p22phox and gp91phox are in the membrane in a heterodimeric flavoprotein conformation known as cytochrome b558. Upon proinflammatory and proatherogenic conditions, p47phox and p67phox, supported by the activation of the small Rho GTPase Rac-1, forms a complex that translocate to the plasma membrane, where it associates with cytochrome b558 constituting the active enzymatic complex able to transfers electrons to O2, thus producing both superoxide anion (O2−) and hydrogen peroxide217 (Figure 4).
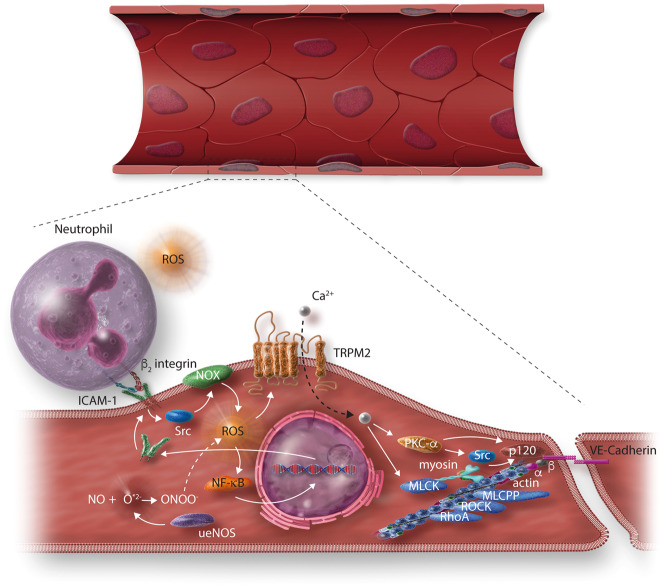
Figure 4.
Increased oxidative stress as the final common pathway of the effects of cardiovascular risk factors, and its effects on the endothelial permeability in endothelial cells. ROS stimulate a NF-κB-dependent upregulation of intercellular adhesion molecule (ICAM)-1 expression. ICAM-1 binds the β2 integrins of neutrophils, inducing activation of endothelial NADPH oxidase (NOX) via Src, and enhancing ROS production.218 ROS release from neutrophils and NOX activates the redox sensitive Ca2+ permeability transient receptor potential cation channel subfamily M member 2 (TRPM2), leading to Ca2+ entry into endothelial cells (ECs) and promoting PKC-α activation, which, in turn, may regulate EC permeability via the phosphorylation of p120 and its dissociation from VE-cadherin,219 and the activation of Src,220 leading to tyrosine phosphorylation of VE-cadherin and β-catenin, eventually resulting in AJ destabilization.218 Ca2+ entry (bound to calmodulin) activates MLC kinase, which phosphorylates MLC, mechanism through which myosin starts to move along the actin fibres221 and eliciting a co-ordinated spatial activation of RhoA and a global reorganization of the actin cytoskeleton, resulting in endothelial barrier dysfunction.102 RhoA, via activation of Rho kinase (ROCK), inhibits MLC phosphatase, making the effect of MLC kinase activity longer lasting.222 A loss of endothelial nitric oxide (NO) bioavailability is caused by its reaction with , produced by uncoupled endothelial nitric oxide synthase (eNOS) (ueNOS), yielding ONOO–production, which contributes to vary the cellular redox state.223
As revised above, despite differences existing in upstream signalling among the various proatherogenic risk factors, most of them share the ability to activate NADPH oxidase and increase the production of ROS as a common denominator, directly linking such factors to endothelial injury in terms of altered endothelial permeability.
Mechanistically, ROS regulate vascular permeability by directly attacking cellular cytosolic and membrane components, such as increasing the tyrosine phosphorylation of VE-cadherin,224 which prevents the association of β-catenin and p120-catenin and results in the inhibition of endothelial barrier function.225 On the other hand, ROS may also contribute to increased endothelial permeability by inducing the activation of NF-κB. It has been shown that the activation of NF-κB alters the distribution of vascular junctional proteins ZO-1 and -2, and of occludins, thus increasing endothelial permeability independently from the induction of proinflammatory gene expression.226
Moreover, enhanced NO degradation by ROS has been found in animals227–229 and humans230–232 in the presence of hypertension, diabetes, cigarette smoking, and heart failure. Among ROS, superoxide (O2*–) can be produced by endothelial NO synthase (eNOS), under conditions of substrate or cofactor deficiency (referred to as an ‘uncoupled’ state).233 Because O2*– and NO* are both radicals and contain unpaired electrons in their outer orbitals, they undergo a radical–radical reaction, leading to the formation of peroxynitrite (ONOO–), which can be protonated to peroxynitrous acid—the cleavage products of which are among the most abundant ROS in biological systems223 and contribute to alter the cellular redox state. As tyrosine phosphatases contain a reactive cysteine group in their active centre, oxidant species can shift the balance between tyrosine kinases and phosphatases,234 and hence influence VE-cadherin phosphorylation and function (Figure 4).
Oxidative stress also affects the abundance and function of junctional molecules: in cultured ECs, hydrogen peroxide promotes a reduced expression of cadherins and occludin in intercellular junctions, suggesting a destabilizing role of ROS-mediated signalling and oxidative stress on vascular integrity235,236 (Figure 4).
Oxygen-derived free radicals also mediate the disruption of the glycocalyx surface layer and increase vascular wall adhesiveness by ox-LDL, as shown in the hamster cremaster muscle preparation.49 In the same model, ox-LDL decrease the effective glycocalyx dimensions by removal of proteoglycans or adsorbed proteins from the endothelial surface; and increase capillary volume accessible to red blood cells in the absence of changes in anatomic capillary diameter.237 These effects may be mediated by the action of ox-LDL on NO bioavailability, which further disturbs the balance between oxygen radical production and NO at the endothelial surface, as demonstrated by the protective effect of the administration of superoxide dismutase and catalase237.
The endothelial redox state is also orchestrated by different flow patterns and shear stresses. While several papers in the literature report increased and sustained endothelial NADPH oxidase activation and ROS production under conditions of disturbed flow and low shear stress, steady laminar shear stress is commonly associated with an induction of antioxidant defences (superoxide dismutase, haeme oxygenase-1, NF erythroid 2-like 2, etc.) and a suppression of ROS generation.238–240 The modulation of ROS production by mechanical forces may mediate their downstream effects on the vasculature. Accordingly, the ‘physiologic’ shear stress-mediated suppression of the cellular redox stress may contribute in part to the attenuation of endothelial barrier dysfunction, as demonstrated in human microvascular ECs challenged by proinflammatory cytokines.241
Recently, it has been shown that ROS are induced by low shear stress via the angiotensin II type 1 receptor/eNOS/NO pathway,242,243 and their effects are suppressed by the bradycardic agent ivabradine in cultured HUVECs.244 Cyclic strain245 or endothelin-1-induced ROS in animal cultured ECs,246 modulated gene expression in HUVECs. These findings highlight that the relationship between the micromechanical environment and the development of atherosclerosis242,247 are likely due to a direct influence of haemodynamic forces on EC morphology, metabolism, and inflammatory phenotype through various signal transduction mechanisms altering specific expressions, with ROS as a likely common downstream effector,248 In micro- and macrovascular ECs, ROS have been implicated in the EndoMT induced by inflammatory stimuli, paving the ground for more detailed studies aimed at identifying the relationship between mechanical endothelial ROS production, EndoMT and atherosclerosis.209–213
8. Conclusions and clinical perspectives
On the basis of the evidence summarized above, atherosclerosis may indeed be promoted, at its early stages, by changes in the transendothelial permeability to LDL. The ‘response to retention hypothesis’ model states that a subendothelial retention of lipoproteins is in fact an early step in atherogenesis. The practical consequence of such a reconstruction of pathogenetic events is therefore that a primary therapeutic focus might be to prevent the entry and subsequent subendothelial retention of LDL.249 This strategy would be complementary to the much more widespread current approaches aimed at reducing LDL since, as discussed above, all the main recognized modifiable atherosclerosis risk factors have the ability to alter endothelial permeability to these molecules.
Great breakthroughs have been made in our understanding of signalling mechanisms and mediators regulating endothelial permeability in normal and diseased states, as well as in elucidating the dynamic interactions between EC barrier dysfunction and atherosclerotic risk factors. These results on the one hand can help the development of new methods to distinguish dysfunctional or activated ECs from healthy or quiescent ECs; and, on the other hand, they can foster the development of novel therapeutic targets and drugs against atherosclerosis.
The in vivo assessment of endothelial barrier function/dysfunction through molecular imaging or monitoring circulating components of the pertinent regulating systems may hold promise to discover biomarkers early detecting and/or monitoring the progression of atherosclerotic vascular disease. Appropriate criteria for the appraisal of these potential biomarkers need to be fulfilled, including reproducible measurement, as well as early—and specific—detection of otherwise subclinical vascular disease. Monitoring in vivo endothelial permeability through molecular imaging of nanoparticle deposition within plaque has been recently demonstrated to specifically identify atheromas susceptible to thrombosis in an experimental atherothrombosis model in rabbits, as well as in human coronary artery atheroma in vivo.250
Since glycocalyx degradation is strongly correlated with vascular disease progression, a clinical monitoring of glycocalyx integrity/thickness, as well as the assessment of the glycocalyx fragments, such as syndecan-1 and/or HA, are being examined as reliable diagnostic or prognostic indicators of vascular endothelial damage in various pathological conditions,251 including atherosclerosis. Circulating components of the endothelial glycocalyx have been demonstrated in humans undergoing vascular surgery associated with ischaemia/reperfusion injury, and are proposed as sensitive markers of early EC distress.252
The direct targeting of endothelial hyperpermeability can be considered as a promising approach to prevent vascular damage during inflammation and atherogenesis. Pharmacological interventions to prevent glycocalyx degradation have been considered, including the use of HA to repair the glycocalyx, although further studies are here certainly needed. Hydroxyethyl starch has been reported to prevent capillary leakage in the early stages of the acute respiratory distress syndrome,253 either by acting on endothelial surface layer pores caused by glycocalyx degradation,254 or by specific interaction with the glycocalyx.255 RhoA, through its downstream kinase ROCK, plays a central role in the loss of endothelial barrier integrity. Therefore fasudil, a derivative of isoquinoline, has been proposed as a safe and clinically approved inhibitor of ROCK,256 and—due to its acceptable safety profile257,258—may be a candidate for reversing endothelial barrier dysfunction, although in some animal studies it also appears to increase blood flow-mediated vasodilation, with demonstrated therapeutic benefits in the treatment of hypertension.259,260
Previous studies have shown that platelet-endothelial cell adhesion molecule-1 (PECAM-1) plays a key role in maintaining EC junctional integrity (for a review see261). Thus, antibody-driven affinity modulation of PECAM-1 has been proposed as a strategy to regulate EC barrier function, suggesting a novel approach for controlling EC migration and changes in barrier function in a variety of vascular permeability disorders.262
The extravasation of 125I-LDL through rabbit aortic ECs was significantly increased by VEGF and decreased by salvianolic acid B (SalB): VEGF here reduced TJ-associated proteins occludin and claudin-5, and increased expression of caveolar structural proteins CAV-1 and -2, an effect abolished by SalB.
Incubation of bovine coronary ECs with VEGF significantly increased their permeability to 164I-ox-LDL, an effect significantly inhibited by extract of Ginkgo biloba leaves, suggesting that such extracts may have clinical applications in the treatment of vascular disease.263,264 Cryptotanshinone, a major compound derived from the Chinese herb Salvia miltiorrhiza, also attenuates the increased endothelial permeability, likely due to the restoration of NO bioavailability in ECs.265
Despite all these many attempts at exploiting current knowledge to design and develop new pharmacological compounds active on the pathways described above, important gaps of knowledge still remain, and need to be filled to best develop endothelial barrier-targeted treatments for atherosclerosis. First, the improvements in endothelial barrier function demonstrated in experimental models need to translate into clinically meaningful anti-atherogenic effects in humans. In this context, a major challenge is to conduct interdisciplinary research that combines bioengineering, physiology, and clinical medicine; and possibly using a systems biology approach, integrating information at the molecular, cellular, and organ levels. Second, as in the case of the inhibition of key players of endothelial permeability such as ROCK or VEGF, the ability of a therapeutic to prevent EC barrier dysfunction without interfering with basic barrier formation and functions in response to physiologic stimuli should be specifically shown. Third, although ROS overproduction is recognized as a key common switch in EC hyperpermeability and cardiovascular disease, it seems extremely difficult to finely and specifically modulate pathological ROS overproduction while leaving the physiological role of ROS as second messengers unaffected.266 Fourth, although novel potential therapeutic targets are increasingly recognized, including EndoMT reversal, the hypothesis to treat atherosclerosis via these targets should be addressed in future and eventually human studies.
Therefore, information related to mechanisms that promote changes in endothelial permeability holds the promise to lead to new diagnostic markers, and the development of new therapeutic strategies—including drugs—targeting such processes. More research on such strategies is therefore certainly warranted.
Conflict of interest: none declared.
References
- 1. Egawa G, Nakamizo S, Natsuaki Y, Doi H, Miyachi Y, Kabashima K. Intravital analysis of vascular permeability in mice using two-photon microscopy. Sci Rep 2013;3:1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Caterina R, Libby P. Endothelial Dysfunctions in Vascular Disease. Oxford, UK: Blackwell Publishing, 2007. [Google Scholar]
- 3. Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 2002;417:750–754. [DOI] [PubMed] [Google Scholar]
- 4. Joris I, Zand T, Nunnari JJ, Krolikowski FJ, Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol 1983;113:341–358. [PMC free article] [PubMed] [Google Scholar]
- 5. van den Berg BM, Spaan JA, Vink H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch 2009;457:1199–1206. 10.1007/s00424-008-0590-6 [DOI] [PubMed] [Google Scholar]
- 6. Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol 2003;23:1161–1168. 10.1161/01.ATV.0000070546.16946.3A [DOI] [PubMed] [Google Scholar]
- 7. Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukoc Biol 1996;59:100–115. [PubMed] [Google Scholar]
- 8. Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol 2000;279:L419–L422. [DOI] [PubMed] [Google Scholar]
- 9. Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995;15:551–561. 10.1161/01.ATV.15.5.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X, Fan Y, Deng X. Effect of the endothelial glycocalyx layer on arterial LDL transport under normal and high pressure. J Theor Biol 2011;283:71–81. 10.1016/j.jtbi.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 11. Minick CR, Stemerman MG, Insull W Jr. Effect of regenerated endothelium on lipid accumulation in the arterial wall. Proc Natl Acad Sci U S A 1977;74:1724–1728. 10.1073/pnas.74.4.1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Minick CR, Stemerman MB, Insull W Jr. Role of endothelium and hypercholesterolemia in intimal thickening and lipid accumulation. Am J Pathol 1979;95:131–158. [PMC free article] [PubMed] [Google Scholar]
- 13. Stemerman MB. Effects of moderate hypercholesterolemia on rabbit endothelium. Arteriosclerosis 1981;1:25–32. 10.1161/01.ATV.1.1.25 [DOI] [PubMed] [Google Scholar]
- 14. Chuang PT, Cheng HJ, Lin SJ, Jan KM, Lee MM, Chien S. Macromolecular transport across arterial and venous endothelium in rats. Studies with Evans blue-albumin and horseradish peroxidase. Arteriosclerosis 1990;10:188–197. [DOI] [PubMed] [Google Scholar]
- 15. Huang AL, Jan KM, Chien S. Role of intercellular junctions in the passage of horseradish peroxidase across aortic endothelium. Lab Invest 1992;67:201–209. [PubMed] [Google Scholar]
- 16. Fry DL, Cornhill JF, Sharma H, Pap JM, Mitschelen J. Uptake of low density lipoprotein, albumin, and water by deendothelialized in vitro minipig aorta. Arteriosclerosis 1986;6:475–490. [DOI] [PubMed] [Google Scholar]
- 17. Smith EB, Staples EM. Plasma protein concentrations in interstitial fluid from human aortas. Proc R Soc Lond B Biol Sci 1982;217:59–75. 10.1098/rspb.1982.0094 [DOI] [PubMed] [Google Scholar]
- 18. Smith EB, Ashall C. Low-density lipoprotein concentration in interstitial fluid from human atherosclerotic lesions. Relation to theories of endothelial damage and lipoprotein binding. Biochim Biophys Acta 1983;754:249–257. [DOI] [PubMed] [Google Scholar]
- 19. Fry DL. Mass transport, atherogenesis, and risk. Arteriosclerosis 1987;7:88–100. 10.1161/01.ATV.7.1.88 [DOI] [PubMed] [Google Scholar]
- 20. Hurt-Camejo E, Camejo G, Rosengren B, Lopez F, Ahlstrom C, Fager G, Bondjers G. Effect of arterial proteoglycans and glycosaminoglycans on low density lipoprotein oxidation and its uptake by human macrophages and arterial smooth muscle cells. Arterioscler Thromb 1992;12:569–583. [DOI] [PubMed] [Google Scholar]
- 21. Osterud B, Bjorklid E. Role of monocytes in atherogenesis. Physiol Rev 2003;83:1069–1112. 10.1152/physrev.00005.2003 [DOI] [PubMed] [Google Scholar]
- 22. Nielsen LB. Transfer of low density lipoprotein into the arterial wall and risk of atherosclerosis. Atherosclerosis 1996;123:1–15. 10.1016/0021-9150(96)05802-9 [DOI] [PubMed] [Google Scholar]
- 23. Territo M, Berliner JA, Fogelman AM. Effect of monocyte migration on low density lipoprotein transport across aortic endothelial cell monolayers. J Clin Invest 1984;74:2279–2284. 10.1172/JCI111655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Langeler EG, Snelting-Havinga I, van Hinsbergh VW. Passage of low density lipoproteins through monolayers of human arterial endothelial cells. Effects of vasoactive substances in an in vitro model. Arteriosclerosis 1989;9:550–559. [DOI] [PubMed] [Google Scholar]
- 25. Schmitz G, Wulf G, Bruning T, Assmann G. Flow-cytometric determination of high-density-lipoprotein binding sites on human leukocytes. Clin Chem 1987;33:2195–2203. [PubMed] [Google Scholar]
- 26. Bian F, Yang X, Zhou F, Wu PH, Xing S, Xu G, Li W, Chi J, Ouyang C, Zhang Y, Xiong B, Li Y, Zheng T, Wu D, Chen X, Jin S. C-reactive protein promotes atherosclerosis by increasing LDL transcytosis across endothelial cells. Br J Pharmacol 2014;171:2671–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu G, Li W, Chi J, Ouyang C, Zheng T, Wu D, Zhang Y, Li Y, Jin S. TNF-alpha promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-kappaB and PPAR-gamma. J Mol Cell Cardiol 2014;72:85–94. [DOI] [PubMed] [Google Scholar]
- 28. Bratzler RL, Chisolm GM, Colton CK, Smith KA, Lees RS. The distribution of labeled low-density lipoproteins across the rabbit thoracic aorta in vivo. Atherosclerosis 1977;28:289–307. [DOI] [PubMed] [Google Scholar]
- 29. Iuliano L, Mauriello A, Sbarigia E, Spagnoli LG, Violi F. Radiolabeled native low-density lipoprotein injected into patients with carotid stenosis accumulates in macrophages of atherosclerotic plaque: effect of vitamin E supplementation. Circulation 2000;101:1249–1254. [DOI] [PubMed] [Google Scholar]
- 30. Olgac U, Poulikakos D, Saur SC, Alkadhi H, Kurtcuoglu V. Patient-specific three-dimensional simulation of LDL accumulation in a human left coronary artery in its healthy and atherosclerotic states. Am J Physiol Heart Circ Physiol 2009;296:H1969–H1982. [DOI] [PubMed] [Google Scholar]
- 31. Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol 1983;96:1677–1689. 10.1083/jcb.96.6.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vasile E, Antohe F, Simionescu M, Simionescu N. Transport pathways of beta-VLDL by aortic endothelium of normal and hypercholesterolemic rabbits. Atherosclerosis 1989;75:195–210. [DOI] [PubMed] [Google Scholar]
- 33. Noble MI, Drake-Holland AJ, Vink H. Hypothesis: arterial glycocalyx dysfunction is the first step in the atherothrombotic process. QJM 2008;101:513–518. 10.1093/qjmed/hcn024 [DOI] [PubMed] [Google Scholar]
- 34. Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 1996;79:581–589. 10.1161/01.RES.79.3.581 [DOI] [PubMed] [Google Scholar]
- 35. Lin SJ, Jan KM, Chien S. Role of dying endothelial cells in transendothelial macromolecular transport. Arteriosclerosis 1990;10:703–709. 10.1161/01.ATV.10.5.703 [DOI] [PubMed] [Google Scholar]
- 36. Weinbaum S, Tzeghai G, Ganatos P, Pfeffer R, Chien S. Effect of cell turnover and leaky junctions on arterial macromolecular transport. Am J Physiol 1985;248:H945–H960. [DOI] [PubMed] [Google Scholar]
- 37. van den Berg BM, Spaan JA, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol 2006;290:H915–H920. [DOI] [PubMed] [Google Scholar]
- 38. Keller R, Pratt BM, Furthmayr H, Madri JA. Aortic endothelial cell proteoheparan sulfate. II. Modulation by extracellular matrix. Am J Pathol 1987;128:299–306. [PMC free article] [PubMed] [Google Scholar]
- 39. Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A 2003;100:7988–7995. 10.1073/pnas.1332808100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kelly RF, Snow HM. Characteristics of the response of the iliac artery to wall shear stress in the anaesthetized pig. J Physiol 2007;582:731–743. 10.1113/jphysiol.2007.128736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol 2000;278:H285–H289. [DOI] [PubMed] [Google Scholar]
- 42. Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol 2010;105:687–701. 10.1007/s00395-010-0118-z [DOI] [PubMed] [Google Scholar]
- 43. Lewis JC, Taylor RG, Jones ND, St Clair RW, Cornhill JF. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest 1982;46:123–138. [PubMed] [Google Scholar]
- 44. Cancel LM, Ebong EE, Mensah S, Hirschberg C, Tarbell JM. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis 2016;252:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Devaraj S, Yun JM, Adamson G, Galvez J, Jialal I. C-reactive protein impairs the endothelial glycocalyx resulting in endothelial dysfunction. Cardiovasc Res 2009;84:479–484. 10.1093/cvr/cvp249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grundmann S, Schirmer SH, Hekking LH, Post JA, Ionita MG, de Groot D, van Royen N, van den Berg B, Vink H, Moser M, Bode C, de Kleijn D, Pasterkamp G, Piek JJ, Hoefer IE. Endothelial glycocalyx dimensions are reduced in growing collateral arteries and modulate leucocyte adhesion in arteriogenesis. J Cell Mol Med 2009;13:3463–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schierke F, Wyrwoll MJ, Wisdorf M, Niedzielski L, Maase M, Ruck T, Meuth SG, Kusche-Vihrog K. Nanomechanics of the endothelial glycocalyx contribute to Na+-induced vascular inflammation. Sci Rep 2017;7:46476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vincent PE, Weinberg PD. Flow-dependent concentration polarization and the endothelial glycocalyx layer: multi-scale aspects of arterial mass transport and their implications for atherosclerosis. Biomech Model Mechanobiol 2014;13:313–326. 10.1007/s10237-013-0512-1 [DOI] [PubMed] [Google Scholar]
- 49. Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation 2000;101:1500–1502. 10.1161/01.CIR.101.13.1500 [DOI] [PubMed] [Google Scholar]
- 50. Son DJ, Kumar S, Takabe W, Kim CW, Ni CW, Alberts-Grill N, Jang IH, Kim S, Kim W, Won Kang S, Baker AH, Woong Seo J, Ferrara KW, Jo H. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun 2013;4:3000.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cooper S, McDonald K, Burkat D, Leask RL. Stenosis hemodynamics disrupt the endothelial cell glycocalyx by MMP activity creating a proinflammatory environment. Ann Biomed Eng 2017;45:2234–2243. [DOI] [PubMed] [Google Scholar]
- 52. Sorrentino V, Nelson JK, Maspero E, Marques AR, Scheer L, Polo S, Zelcer N. The LXR-IDOL axis defines a clathrin-, caveolae-, and dynamin-independent endocytic route for LDLR internalization and lysosomal degradation. J Lipid Res 2013;54:2174–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol 2009;29:431–438. 10.1161/ATVBAHA.108.179564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kraehling JR, Chidlow JH, Rajagopal C, Sugiyama MG, Fowler JW, Lee MY, Zhang X, Ramírez CM, Park EJ, Tao B, Chen K, Kuruvilla L, Larriveé B, Folta-Stogniew E, Ola R, Rotllan N, Zhou W, Nagle MW, Herz J, Williams KJ, Eichmann A, Lee WL, Fernández-Hernando C, Sessa WC. Genome-wide RNAi screen reveals ALK1 mediates LDL uptake and transcytosis in endothelial cells. Nat Commun 2016;7:13516.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 2007;293:L823–L842. [DOI] [PubMed] [Google Scholar]
- 56. Pavlides S, Gutierrez-Pajares JL, Iturrieta J, Lisanti MP, Frank PG. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res 2014;356:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ahn S, Kim J, Lucaveche CL, Reedy MC, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J Biol Chem 2002;277:26642–26651. [DOI] [PubMed] [Google Scholar]
- 58. Powell KA, Valova VA, Malladi CS, Jensen ON, Larsen MR, Robinson PJ. Phosphorylation of dynamin I on Ser-795 by protein kinase C blocks its association with phospholipids. J Biol Chem 2000;275:11610–11617. [DOI] [PubMed] [Google Scholar]
- 59. Schnitzer JE. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev 2001;49:265–280. 10.1016/S0169-409X(01)00141-7 [DOI] [PubMed] [Google Scholar]
- 60. Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol 1998;141:101–114. 10.1083/jcb.141.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clary DO, Rothman JE. Purification of three related peripheral membrane proteins needed for vesicular transport. J Biol Chem 1990;265:10109–10117. [PubMed] [Google Scholar]
- 62. Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 1993;75:409–418. [DOI] [PubMed] [Google Scholar]
- 63. Maiolino G, Rossitto G, Caielli P, Bisogni V, Rossi GP, Calo LA. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediators Inflamm 2013;2013:714653.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Magalhaes A, Matias I, Palmela I, Brito MA, Dias S, Deli MA. LDL-cholesterol increases the transcytosis of molecules through endothelial monolayers. PLoS One 2016;11:e0163988.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Simionescu M, Simionescu N. Endothelial transport of macromolecules: transcytosis and endocytosis. A look from cell biology. Cell Biol Rev 1991;25:1–78. [PubMed] [Google Scholar]
- 66. Lampugnani MG. Endothelial cell-to-cell junctions: adhesion and signaling in physiology and pathology. Cold Spring Harb Perspect Med 2012;2:a006528.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Simionescu M, Antohe F. Functional ultrastructure of the vascular endothelium: changes in various pathologies. Handb Exp Pharmacol 2006;(176 pt. 1):41–69. [DOI] [PubMed] [Google Scholar]
- 68. Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol 2003;24:327–334. 10.1016/S1471-4906(03)00117-0 [DOI] [PubMed] [Google Scholar]
- 69. Vleminckx K, Kemler R. Cadherins and tissue formation: integrating adhesion and signaling. Bioessays 1999;21:211–220. [DOI] [PubMed] [Google Scholar]
- 70. Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005;123:903–915. 10.1016/j.cell.2005.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 2004;84:869–901. 10.1152/physrev.00035.2003 [DOI] [PubMed] [Google Scholar]
- 72. Han MK, de Rooij J. Resolving the cadherin-F-actin connection. Nat Cell Biol 2016;19:14–16. 10.1038/ncb3457 [DOI] [PubMed] [Google Scholar]
- 73. Ebnet K. Organization of multiprotein complexes at cell-cell junctions. Histochem Cell Biol 2008;130:1–20. 10.1007/s00418-008-0418-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gumbiner BM. Breaking through the tight junction barrier. J Cell Biol 1993;123:1631–1633. 10.1083/jcb.123.6.1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anderson JM, Balda MS, Fanning AS. The structure and regulation of tight junctions. Curr Opin Cell Biol 1993;5:772–778. 10.1016/0955-0674(93)90024-K [DOI] [PubMed] [Google Scholar]
- 76. Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A 1999;96:511–516. 10.1073/pnas.96.2.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 1999;147:185–194. 10.1083/jcb.147.1.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu BMK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J 2001;20:3738–3748. 10.1093/emboj/20.14.3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem 2000;275:27979–27988. [DOI] [PubMed] [Google Scholar]
- 80. Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem 2002;277:455–461. [DOI] [PubMed] [Google Scholar]
- 81. Nasdala I, Wolburg-Buchholz K, Wolburg H, Kuhn A, Ebnet K, Brachtendorf G, Samulowitz U, Kuster B, Engelhardt B, Vestweber D, Butz S. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J Biol Chem 2002;277:16294–16303. [DOI] [PubMed] [Google Scholar]
- 82. Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A 2001;98:15191–15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fanning AS, Anderson JM. Protein modules as organizers of membrane structure. Curr Opin Cell Biol 1999;11:432–439. 10.1016/S0955-0674(99)80062-3 [DOI] [PubMed] [Google Scholar]
- 84. Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem 2001;276:9291–9296. [DOI] [PubMed] [Google Scholar]
- 85. Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM, Wood DF. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol 1998;142:129–138. 10.1083/jcb.142.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Patrie KM, Drescher AJ, Welihinda A, Mundel P, Margolis B. Interaction of two actin-binding proteins, synaptopodin and alpha-actinin-4, with the tight junction protein MAGI-1. J Biol Chem 2002;277:30183–30190. [DOI] [PubMed] [Google Scholar]
- 87. Ivanov D, Philippova M, Antropova J, Gubaeva F, Iljinskaya O, Tararak E, Bochkov V, Erne P, Resink T, Tkachuk V. Expression of cell adhesion molecule T-cadherin in the human vasculature. Histochem Cell Biol 2001;115:231–242. [DOI] [PubMed] [Google Scholar]
- 88. Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature 1988;333:272–276. [DOI] [PubMed] [Google Scholar]
- 89. Satoh H, Zhong Y, Isomura H, Saitoh M, Enomoto K, Sawada N, Mori M. Localization of 7H6 tight junction-associated antigen along the cell border of vascular endothelial cells correlates with paracellular barrier function against ions, large molecules, and cancer cells. Exp Cell Res 1996;222:269–274. [DOI] [PubMed] [Google Scholar]
- 90. Kawabe H, Nakanishi H, Asada M, Fukuhara A, Morimoto K, Takeuchi M, Takai Y. Pilt, a novel peripheral membrane protein at tight junctions in epithelial cells. J Biol Chem 2001;276:48350–48355. [DOI] [PubMed] [Google Scholar]
- 91. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 2001;293:1107–1112. [DOI] [PubMed] [Google Scholar]
- 92. Nishimura M, Kakizaki M, Ono Y, Morimoto K, Takeuchi M, Inoue Y, Imai T, Takai Y. JEAP, a novel component of tight junctions in exocrine cells. J Biol Chem 2002;277:5583–5587. [DOI] [PubMed] [Google Scholar]
- 93. Weber E, Berta G, Tousson A, St John P, Green MW, Gopalokrishnan U, Jilling T, Sorscher EJ, Elton TS, Abrahamson DR. Expression and polarized targeting of a rab3 isoform in epithelial cells. J Cell Biol 1994;125:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zahraoui A, Joberty G, Arpin M, Fontaine JJ, Hellio R, Tavitian A, Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in non polarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J Cell Biol 1994;124:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lapierre LA, Tuma PL, Navarre J, Goldenring JR, Anderson JM. VAP-33 localizes to both an intracellular vesicle population and with occludin at the tight junction. J Cell Sci 1999;112(pt. 21): 3723–3732. [DOI] [PubMed] [Google Scholar]
- 96. Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 1998;93:731–740. [DOI] [PubMed] [Google Scholar]
- 97. Bundgaard M. The three-dimensional organization of tight junctions in a capillary endothelium revealed by serial-section electron microscopy. J Ultrastruct Res 1984;88:1–17. 10.1016/S0022-5320(84)90177-1 [DOI] [PubMed] [Google Scholar]
- 98. O'Donnell MP, Vargas FF. Electrical conductivity and its use in estimating an equivalent pore size for arterial endothelium. Am J Physiol 1986;250:H16–H21. [DOI] [PubMed] [Google Scholar]
- 99. Crone CBM, Olesen SP. Endothelial cells In: Ryan US. (ed). Endothelial Properties Assessed from Single-Capillary Methods. Boca Raton, FL: CRC Press Inc; 1988. p91–101. [Google Scholar]
- 100. Dejana E, Corada M, Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J 1995;9:910–918. [PubMed] [Google Scholar]
- 101. Dejana E, Vestweber D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog Mol Biol Transl Sci 2013;116:119–144. [DOI] [PubMed] [Google Scholar]
- 102. Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 2010;72:463–493. 10.1146/annurev-physiol-021909-135833 [DOI] [PubMed] [Google Scholar]
- 103. Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol 1999;145:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Herren B, Levkau B, Raines EW, Ross R. Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol Biol Cell 1998;9:1589–1601. 10.1091/mbc.9.6.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ben-Ze'ev A, Geiger B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol 1998;10:629–639. 10.1016/S0955-0674(98)80039-2 [DOI] [PubMed] [Google Scholar]
- 106. Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci 2000;113(pt. 8):1319–1334. [DOI] [PubMed] [Google Scholar]
- 107. Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol 1998;142:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol 1995;130:67–77. 10.1083/jcb.130.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 2002;13:1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lampugnani MG, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol 2003;161:793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J 2002;21:4885–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Frye M, Dierkes M, Kuppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, Nottebaum AF, Vestweber D. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med 2015;212:2267–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ukropec JA, Hollinger MK, Salva SM, Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J Biol Chem 2000;275:5983–5986. [DOI] [PubMed] [Google Scholar]
- 114. Williams EJ, Williams G, Howell FV, Skaper SD, Walsh FS, Doherty P. Identification of an N-cadherin motif that can interact with the fibroblast growth factor receptor and is required for axonal growth. J Biol Chem 2001;276:43879–43886. [DOI] [PubMed] [Google Scholar]
- 115. Miyazaki T, Taketomi Y, Takimoto M, Lei XF, Arita S, Kim-Kaneyama JR, Arata S, Ohata H, Ota H, Murakami M, Miyazaki A. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation 2011;124:2522–2532. [DOI] [PubMed] [Google Scholar]
- 116. Bobryshev YV, Cherian SM, Inder SJ, Lord RS. Neovascular expression of VE-cadherin in human atherosclerotic arteries and its relation to intimal inflammation. Cardiovasc Res 1999;43:1003–1017. 10.1016/S0008-6363(99)00125-X [DOI] [PubMed] [Google Scholar]
- 117. Beyer EC. Gap junctions. Int Rev Cytol 1993;137C:1–37. [PubMed] [Google Scholar]
- 118. Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2002;22:225–230. 10.1161/hq0102.104125 [DOI] [PubMed] [Google Scholar]
- 119. Morel S, Burnier L, Kwak BR. Connexins participate in the initiation and progression of atherosclerosis. Semin Immunopathol 2009;31:49–61. 10.1007/s00281-009-0147-6 [DOI] [PubMed] [Google Scholar]
- 120. Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol 2015;6:365.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 2010;87:320–330. 10.1093/cvr/cvq146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Vogel RA. Cholesterol lowering and endothelial function. Am J Med 1999;107:479–487. 10.1016/S0002-9343(99)00261-2 [DOI] [PubMed] [Google Scholar]
- 123. Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res 2009;335:191–203. 10.1007/s00441-008-0678-5 [DOI] [PubMed] [Google Scholar]
- 124. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615–1622. [DOI] [PubMed] [Google Scholar]
- 125. Nielsen LB, Nordestgaard BG, Stender S, Kjeldsen K. Aortic permeability to LDL as a predictor of aortic cholesterol accumulation in cholesterol-fed rabbits. Arterioscler Thromb 1992;12:1402–1409. [DOI] [PubMed] [Google Scholar]
- 126. Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W Jr., Richardson M, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb 1992;12:120–134. [DOI] [PubMed] [Google Scholar]
- 127. Lee WC, Chao WT, Yang VC. Effects of high-cholesterol diet on the interendothelial clefts and the associated junctional complexes in rat aorta. Atherosclerosis 2001;155:307–312. 10.1016/S0021-9150(00)00578-5 [DOI] [PubMed] [Google Scholar]
- 128. Meyer RA, Lampe PD, Malewicz B, Baumann WJ, Johnson RG. Enhanced gap junction formation with LDL and apolipoprotein B. Exp Cell Res 1991;196:72–81. [DOI] [PubMed] [Google Scholar]
- 129. van Nieuw Amerongen GP, Vermeer MA, Negre-Aminou P, Lankelma J, Emeis JJ, van Hinsbergh VW. Simvastatin improves disturbed endothelial barrier function. Circulation 2000;102:2803–2809. [DOI] [PubMed] [Google Scholar]
- 130. Bian C, Xu G, Wang J, Ma J, Xiang M, Chen P. Hypercholesterolaemic serum increases the permeability of endothelial cells through zonula occludens-1 with phosphatidylinositol 3-kinase signaling pathway. J Biomed Biotechnol 2009;2009:814979.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Meuwese MC, Mooij HL, Nieuwdorp M, van Lith B, Marck R, Vink H, Kastelein JJ, Stroes ES. Partial recovery of the endothelial glycocalyx upon rosuvastatin therapy in patients with heterozygous familial hypercholesterolemia. J Lipid Res 2009;50:148–153. 10.1194/jlr.P800025-JLR200 [DOI] [PubMed] [Google Scholar]
- 132. Ding H, Triggle CR. Endothelial cell dysfunction and the vascular complications associated with type 2 diabetes: assessing the health of the endotheliu. Vasc Health Risk Manag 2005;1:55–71. 10.2147/vhrm.1.1.55.58939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Winer N, Sowers JR. Epidemiology of diabetes. J Clin Pharmacol 2004;44:397–405. 10.1177/0091270004263017 [DOI] [PubMed] [Google Scholar]
- 134. Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes–part I: pathways of vascular disease in diabetes. Vascul Pharmacol 2011;54:68–74. 10.1016/j.vph.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 135. Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes–part II: cellular mechanisms and therapeutic targets. Vascul Pharmacol 2011;54:75–79. 10.1016/j.vph.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 136. Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006;55:480–486. [DOI] [PubMed] [Google Scholar]
- 137. Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006;55:1127–1132. [DOI] [PubMed] [Google Scholar]
- 138. Kelly R, Ruane-O'hora T, Noble MI, Drake-Holland AJ, Snow HM. Differential inhibition by hyperglycaemia of shear stress- but not acetylcholine-mediated dilatation in the iliac artery of the anaesthetized pig. J Physiol 2006;573:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Haidari M, Zhang W, Willerson JT, Dixon RA. Disruption of endothelial adherens junctions by high glucose is mediated by protein kinase C-beta-dependent vascular endothelial cadherin tyrosine phosphorylation. Cardiovasc Diabetol 2014;13:105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 2010;106:1319–1331. 10.1161/CIRCRESAHA.110.217117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ku SK, Bae JS. Vicenin-2 and scolymoside inhibit high-glucose-induced vascular inflammation in vitro and in vivo. Can J Physiol Pharmacol 2016;94:287–295. 10.1139/cjpp-2015-0215 [DOI] [PubMed] [Google Scholar]
- 142. Zhao XY, Wang XF, Li L, Zhang L, Shen DL, Li DH, Jin QS, Zhang JY. Effects of high glucose on human umbilical vein endothelial cell permeability and myosin light chain phosphorylation. Diabetol Metab Syndr 2015;7:98.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 2000;87:335–340. [DOI] [PubMed] [Google Scholar]
- 144. Nieves BJ, D’Amore PA, Bryan BA. The function of vascular endothelial growth factor. Biofactors 2009;35:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 2010;87:262–271. 10.1093/cvr/cvq105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wong BW, Wong D, Luo HL, McManus BM. Vascular endothelial growth factor-D is overexpressed in human cardiac allograft vasculopathy and diabetic atherosclerosis and induces endothelial permeability to low-density lipoproteins in vitro. J Heart Lung Transpl 2011;30:955–962. [DOI] [PubMed] [Google Scholar]
- 147. Nagyoszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Hasko J, Krizbai IA. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int 2010;57:556–564. [DOI] [PubMed] [Google Scholar]
- 148. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, Investigators IS. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 149. Nakanishi R, Baskaran L, Gransar H, Budoff MJ, Achenbach S, Al-Mallah M, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJW, DeLago A, Hadamitzky M, Hausleiter J, Cury R, Feuchtner G, Kim YJ, Leipsic J, Kaufmann PA, Maffei E, Raff G, Shaw LJ, Villines TC, Dunning A, Marques H, Pontone G, Andreini D, Rubinshtein R, Bax J, Jones E, Hindoyan N, Gomez M, Lin FY, Min JK, Berman DS. Relationship of hypertension to coronary atherosclerosis and cardiac events in patients with coronary computed tomographic angiography. Hypertension 2017;70:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep 2010;12:448–455. 10.1007/s11906-010-0150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Dinh QN, Drummond GR, Sobey CG, Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014;2014:406960.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Rafflenbeul W. Hypertension treatment and prevention of new atherosclerotic plaque formation. Drugs 1994;48:11–15. 10.2165/00003495-199400481-00005 [DOI] [PubMed] [Google Scholar]
- 153. Bretherton KN, Day AJ, Skinner SL. Effect of hypertension on the entry of 125 I-labelled low density lipoprotein into the aortic intima in normal-fed rabbits. Atherosclerosis 1976;24:99–106. 10.1016/0021-9150(76)90067-8 [DOI] [PubMed] [Google Scholar]
- 154. Wu CH, Chi JC, Jerng JS, Lin SJ, Jan KM, Wang DL, Chien S. Transendothelial macromolecular transport in the aorta of spontaneously hypertensive rats. Hypertension 1990;16:154–161. [DOI] [PubMed] [Google Scholar]
- 155. Dabagh M, Jalali P, Tarbell JM. The transport of LDL across the deformable arterial wall: the effect of endothelial cell turnover and intimal deformation under hypertension. Am J Physiol Heart Circ Physiol 2009;297:H983–H996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Facchini L, Bellin A, Toro EF. Modeling loss of microvascular wall homeostasis during glycocalyx deterioration and hypertension that impacts plasma filtration and solute exchange. Curr Neurovasc Res 2016;13:147–155. 10.2174/1567202613666160223121415 [DOI] [PubMed] [Google Scholar]
- 157. Bian F, Cui J, Zheng T, Jin S. Reactive oxygen species mediate angiotensin II-induced transcytosis of low-density lipoprotein across endothelial cells. Int J Mol Med 2017;39:629–635. 10.3892/ijmm.2017.2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wiesner TF, Berk BC, Nerem RM. A mathematical model of the cytosolic-free calcium response in endothelial cells to fluid shear stress. Proc Natl Acad Sci U S A 1997;94:3726–3731. 10.1073/pnas.94.8.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 2007;117:2362–2368. 10.1172/JCI32239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548–2556. 10.1210/jc.2004-0395 [DOI] [PubMed] [Google Scholar]
- 161. Langheim S, Dreas L, Veschini L, Maisano F, Foglieni C, Ferrarello S, Sinagra G, Zingone B, Alfieri O, Ferrero E, Maseri A, Ruotolo G. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol 2010;298:H746–H753. [DOI] [PubMed] [Google Scholar]
- 162. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013;9:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–880. 10.1038/nature05487 [DOI] [PubMed] [Google Scholar]
- 164. Molica F, Morel S, Kwak BR, Rohner-Jeanrenaud F, Steffens S. Adipokines at the crossroad between obesity and cardiovascular disease. Thromb Haemost 2014;113:553–566. [DOI] [PubMed] [Google Scholar]
- 165. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995;95:2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun 2010;11:145–156. [DOI] [PubMed] [Google Scholar]
- 167. Bilgic GS, Akan G, Atalar F, Erten G. PAI-1 and TNF-alpha profiles of adipose tissue in obese cardiovascular disease patients. Int J Clin Exp Pathol 2015;8:15919–15925. [PMC free article] [PubMed] [Google Scholar]
- 168. McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol 2009;6:410–417. 10.1038/nrcardio.2009.57 [DOI] [PubMed] [Google Scholar]
- 169. Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 2002;39:187–199. 10.1016/S1537-1891(03)00008-9 [DOI] [PubMed] [Google Scholar]
- 170. Kant S, Swat W, Zhang S, Zhang ZY, Neel BG, Flavell RA, Davis RJ. TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway. Gene Dev 2011;25:2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Marcos-Ramiro B, García-Weber D, Millán J. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost 2014;112:1088–1102. [DOI] [PubMed] [Google Scholar]
- 172. Piestrzeniewicz K, Łuczak K, Komorowski J, Maciejewski M, Jankiewicz Wika J, Goch JH. Resistin increases with obesity and atherosclerotic risk factors in patients with myocardial infarction. Metab Clin Exp 2008;57:488–493. [DOI] [PubMed] [Google Scholar]
- 173. Jamaluddin MS, Yan S, Lü J, Liang Z, Yao Q, Chen C, Mohanraj R. Resistin increases monolayer permeability of human coronary artery endothelial cells. PLoS One 2013;8:e84576.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Xu SQ, Mahadev K, Wu X, Fuchsel L, Donnelly S, Scalia RG, Goldstein BJ. Adiponectin protects against angiotensin II or tumor necrosis factor alpha-induced endothelial cell monolayer hyperpermeability: role of cAMP/PKA signaling. Arterioscler Thromb Vasc Biol 2008;28:899–905. [DOI] [PubMed] [Google Scholar]
- 175. Dong JM, Leung T, Manser E, Lim L. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKalpha. J Biol Chem 1998;273:22554–22562. 10.1074/jbc.273.35.22554 [DOI] [PubMed] [Google Scholar]
- 176. Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 2007;56:1387–1394. [DOI] [PubMed] [Google Scholar]
- 177. Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia 2003;46:1483–1488. [DOI] [PubMed] [Google Scholar]
- 178. Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res 1997;67:147–154. [DOI] [PubMed] [Google Scholar]
- 179. Mazidi M, Rezaie P, Kengne AP, Stathopoulou MG, Azimi-Nezhad M, Siest S. VEGF, the underlying factor for metabolic syndrome; fact or fiction? Diabetes Metab Syndr 2016;(Suppl. 1):S61–S64. [DOI] [PubMed] [Google Scholar]
- 180. Cao RH, Brakenhielm E, Wahlestedt C, Thyberg J, Cao YH. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A 2001;98:6390–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Usui R, Shibuya M, Ishibashi S, Maru Y. Ligand-independent activation of vascular endothelial growth factor receptor 1 by low-density lipoprotein. Embo Rep 2007;8:1155–1161. 10.1038/sj.embor.7401103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Yla-Herttuala S, Alitalo K. On the relationship of LDL and VEGFR1: not just a family affair. Embo Rep 2007;8:1127–1128. 10.1038/sj.embor.7401124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wang L, Chen Y, Li X, Zhang YZ, Gulbins E, Zhang Y. Enhancement of endothelial permeability by free fatty acid through lysosomal cathepsin B-mediated Nlrp3 inflammasome activation. Oncotarget 2016;7:73229–73241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes 2011;18:139–143. 10.1097/MED.0b013e3283444b09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hisamatsu T, Miura K, Arima H, Kadota A, Kadowaki S, Torii S, Suzuki S, Miyagawa N, Sato A, Yamazoe M, Fujiyoshi A, Ohkubo T, Yamamoto T, Murata K, Abbott RD, Sekikawa A, Horie M, Ueshima H. Smoking, smoking cessation, and measures of subclinical atherosclerosis in multiple vascular beds in Japanese men. J Am Heart Assoc 2016;5:e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Krupski WC. The peripheral vascular consequences of smoking. Ann Vasc Surg 1991;5:291–304. 10.1007/BF02329389 [DOI] [PubMed] [Google Scholar]
- 187. Barbieri SS, Weksler BB. Tobacco smoke cooperates with interleukin-1beta to alter beta-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivo. FASEB J 2007;21:1831–1843. 10.1096/fj.06-7557com [DOI] [PubMed] [Google Scholar]
- 188. Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscl Throm Vasc Biol 2004;24:1031–1036. 10.1161/01.ATV.0000127083.88549.58 [DOI] [PubMed] [Google Scholar]
- 189. Barbieri SS, Ruggiero L, Tremoli E, Weksler BB. Suppressing PTEN activity by tobacco smoke plus interleukin-1beta modulates dissociation of VE-cadherin/beta-catenin complexes in endothelium. Arterioscl Throm Vasc Biol 2008;28:732–738. [DOI] [PubMed] [Google Scholar]
- 190. Bernhard D, Csordas A, Henderson B, Rossmann A, Kind M, Wick G. Cigarette smoke metal-catalyzed protein oxidation leads to vascular endothelial cell contraction by depolymerization of microtubules. FASEB J 2005;19:1096–1107. [DOI] [PubMed] [Google Scholar]
- 191. Lin SJ, Hong CY, Chang MS, Chiang BN, Chien S. Long-term nicotine exposure increases aortic endothelial cell death and enhances transendothelial macromolecular transport in rats. Arterioscler Thromb 1992;12:1305–1312. 10.1161/01.ATV.12.11.1305 [DOI] [PubMed] [Google Scholar]
- 192. Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007;49:2379–2393. [DOI] [PubMed] [Google Scholar]
- 193. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999;282:2035–2042. 10.1001/jama.282.21.2035 [DOI] [PubMed] [Google Scholar]
- 194. Hsieh HJ, Liu CA, Huang B, Tseng AH, Wang DL. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J Biomed Sci 2014;21:3.. 10.1186/1423-0127-21-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics 2002;9:27–41. 10.1152/physiolgenomics.00075.2001 [DOI] [PubMed] [Google Scholar]
- 196. Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation 2003;108:438–444. [DOI] [PubMed] [Google Scholar]
- 197. Dunn J, Thabet S, Jo H. Flow-dependent epigenetic DNA methylation in endothelial gene expression and atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35:1562–1569. 10.1161/ATVBAHA.115.305042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. LaMack JA, Himburg HA, Li XM, Friedman MH. Interaction of wall shear stress magnitude and gradient in the prediction of arterial macromolecular permeability. Ann Biomed Eng 2005;33:457–464. 10.1007/s10439-005-2500-9 [DOI] [PubMed] [Google Scholar]
- 199. Fry DL, Herderick EE, Johnson DK. Local intimal-medial uptakes of 125I-albumin, 125I-LDL, and parenteral Evans blue dye protein complex along the aortas of normocholesterolemic minipigs as predictors of subsequent hypercholesterolemic atherogenesis. Arterioscler Thromb 1993;13:1193–1204. 10.1161/01.ATV.13.8.1193 [DOI] [PubMed] [Google Scholar]
- 200. Soulis JV, Fytanidis DK, Papaioannou VC, Giannoglou GD. Wall shear stress on LDL accumulation in human RCAs. Med Eng Phys 2010;32:867–877. [DOI] [PubMed] [Google Scholar]
- 201. Berk BC, Min W, Yan C, Surapisitchat J, Liu Y, Hoefen R. Atheroprotective mechanisms activated by fluid shear stress in endothelial cells. Drug News Perspect 2002;15:133–139. [DOI] [PubMed] [Google Scholar]
- 202. Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, Wang Y, Jin G, Usami S, Chien S. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res 2005;42:77–89. [DOI] [PubMed] [Google Scholar]
- 203. Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res 2002;102:13–21. 10.1006/jsre.2001.6295 [DOI] [PubMed] [Google Scholar]
- 204. Davies PF, Remuzzi A, Gordon EJ, Dewey CF Jr, Gimbrone MA Jr, Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A 1986;83:2114–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, Joseph L, Griem ML, Wernick MN, Jacobs E, Polacek DC, dePaola N, Barakat AI. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol 1997;59:527–549. [DOI] [PubMed] [Google Scholar]
- 206. Chen YL, Jan KM, Lin HS, Chien S. Ultrastructural studies on macromolecular permeability in relation to endothelial cell turnover. Atherosclerosis 1995;118:89–104. 10.1016/0021-9150(95)05596-O [DOI] [PubMed] [Google Scholar]
- 207. Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1). Circ Res 2000;86:185–190. 10.1161/01.RES.86.2.185 [DOI] [PubMed] [Google Scholar]
- 208. Gimbrone MA Jr, Topper JN, Nagel T, Anderson KR, Garcia CG. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci 2006;902:230–239. discussion 239-240. [DOI] [PubMed] [Google Scholar]
- 209. Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman K-R, D’escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, Boehm M, Kovacic JC. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun 2016;7:11853.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Good RB, Gilbane AJ, Trinder SL, Denton CP, Coghlan G, Abraham DJ, Holmes AM. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol 2015;185:1850–1858. [DOI] [PubMed] [Google Scholar]
- 211. Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 2015;125:4514–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Moonen JR, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJ, Zeebregts CJ, Krenning G, Harmsen MC. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res 2015;108:377–386. [DOI] [PubMed] [Google Scholar]
- 213. Kim M, Choi SH, Jin YB, Lee HJ, Ji YH, Kim J, Lee YS, Lee YJ. The effect of oxidized low-density lipoprotein (ox-LDL) on radiation-induced endothelial-to-mesenchymal transition. Int J Radiat Biol 2013;89:356–363. [DOI] [PubMed] [Google Scholar]
- 214. Yang Y, Luo NS, Ying R, Xie Y, Chen JY, Wang XQ, Gu ZJ, Mai JT, Liu WH, Wu MX, Chen ZT, Fang YB, Zhang HF, Zuo ZY, Wang JF, Chen YX. Macrophage-derived foam cells impair endothelial barrier function by inducing endothelial-mesenchymal transition via CCL-4. Int J Radiat Biol 2017;40:558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Mahmoud MM, Serbanovic-Canic J, Feng S, Souilhol C, Xing R, Hsiao S, Mammoto A, Chen J, Ariaans M, Francis SE, Van der Heiden K, Ridger V, Evans PC. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci Rep 2017;7:3375.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Forstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med 2008;5:338–349. 10.1038/ncpcardio1211 [DOI] [PubMed] [Google Scholar]
- 217. Touyz RM, Briones AM, Sedeek M, Burger D, Montezano AC. NOX isoforms and reactive oxygen species in vascular health. Mol Interv 2011;11:27–35. [DOI] [PubMed] [Google Scholar]
- 218. Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 2007;179:4053–4064. 10.4049/jimmunol.179.6.4053 [DOI] [PubMed] [Google Scholar]
- 219. Konstantoulaki M, Kouklis P, Malik AB. Protein kinase C modifications of VE-cadherin, p120, and beta-catenin contribute to endothelial barrier dysregulation induced by thrombin. Am J Physiol Lung Cell Mol Physiol 2003;285:L434–L442. [DOI] [PubMed] [Google Scholar]
- 220. Tatin F, Varon C, Genot E, Moreau V. A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci 2006;119:769–781. 10.1242/jcs.02787 [DOI] [PubMed] [Google Scholar]
- 221. Hu G, Place AT, Minshall RD. Regulation of endothelial permeability by Src kinase signaling: vascular leakage versus transcellular transport of drugs and macromolecules. Chem Biol Interact 2008;171:177–189. 10.1016/j.cbi.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Barandier C, Ming XF, Rusconi S, Yang Z. PKC is required for activation of ROCK by RhoA in human endothelial cells. Biochem Biophys Res Commun 2003;304:714–719. 10.1016/S0006-291X(03)00668-5 [DOI] [PubMed] [Google Scholar]
- 223. Kalinowski L, Malinski T. Endothelial NADH/NADPH-dependent enzymatic sources of superoxide production: relationship to endothelial dysfunction. Acta Biochim Pol 2004;51:459–469. [PubMed] [Google Scholar]
- 224. Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires rac and reactive oxygen species. J Biol Chem 2009;284:25602–25611. 10.1074/jbc.M109.009894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem 2005;280:31906–31912. 10.1074/jbc.M505568200 [DOI] [PubMed] [Google Scholar]
- 226. Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, Schindler C. NF-kappa B regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest 2006;116:2955–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Winquist RJ, Bunting PB, Baskin EP, Wallace AA. Decreased endothelium-dependent relaxation in New Zealand genetic hypertensive rats. J Hypertens 1984;2:541–545. [DOI] [PubMed] [Google Scholar]
- 228. Lockette W, Otsuka Y, Carretero O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension 1986;8:II61–II66. [DOI] [PubMed] [Google Scholar]
- 229. Durante W, Sen AK, Sunahara FA. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol 1988;94:463–468. 10.1111/j.1476-5381.1988.tb11548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 1996;93:1107–1113. [DOI] [PubMed] [Google Scholar]
- 231. Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 1996;97:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Solzbach U, Hornig B, Jeserich M, Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation 1997;96:1513–1519. 10.1161/01.CIR.96.5.1513 [DOI] [PubMed] [Google Scholar]
- 233. Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA Jr, Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 1998;95:9220–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Staal FJ, Anderson MT, Staal GE, Herzenberg LA, Gitler C, Herzenberg LA. Redox regulation of signal transduction: tyrosine phosphorylation and calcium influx. Proc Natl Acad Sci U S A 1994;91:3619–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H(2)O(2)-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol 2000;279:C21–C30. [DOI] [PubMed] [Google Scholar]
- 236. Salvayre R, Auge N, Benoist H, Negre-Salvayre A. Oxidized low-density lipoprotein-induced apoptosis. Biochim Biophys Acta 2002;1585:213–221. [DOI] [PubMed] [Google Scholar]
- 237. Constantinescu AA, Vink H, Spaan JA. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol 2001;280:H1051–H1057. [DOI] [PubMed] [Google Scholar]
- 238. De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res 1998;82:1094–1101. [DOI] [PubMed] [Google Scholar]
- 239. Takabe W, Warabi E, Noguchi N. Anti-atherogenic effect of laminar shear stress via Nrf2 activation. Antioxid Redox Signal 2011;15:1415–1426. 10.1089/ars.2010.3433 [DOI] [PubMed] [Google Scholar]
- 240. Chatterjee S, Browning EA, Hong N, DeBolt K, Sorokina EM, Liu W, Birnbaum MJ, Fisher AB. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol 2012;302:H105–H114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Rochfort KD, Collins LE, McLoughlin A, Cummins PM. Shear-dependent attenuation of cellular ROS levels can suppress proinflammatory cytokine injury to human brain microvascular endothelial barrier properties. J Cereb Blood Flow Metab 2015;35:1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Rezvan A, Ni CW, Alberts-Grill N, Jo H. Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: role of oxidative stress. Antioxid Redox Signal 2011;15:1433–1448. 10.1089/ars.2010.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Chao Y, Ye P, Zhu L, Kong X, Qu X, Zhang J, Luo J, Yang H, Chen S. Low shear stress induces endothelial reactive oxygen species via the AT1R/eNOS/NO pathway. J Cell Physiol 2018;233:1384–1395. [DOI] [PubMed] [Google Scholar]
- 244. Li B, Zhang J, Wang Z, Chen S, Huang Y. Ivabradine Prevents Low Shear Stress Induced Endothelial Inflammation and Oxidative Stress via mTOR/eNOS Pathway. PLoS One 2016;11:e0149694.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Howard AB, Alexander RW, Nerem RM, Griendling KK, Taylor WR. Cyclic strain induces an oxidative stress in endothelial cells. Am J Physiol 1997;272:C421–C427. [DOI] [PubMed] [Google Scholar]
- 246. Cheng TH, Shih NL, Chen SY, Loh SH, Cheng PY, Tsai CS, Liu SH, Wang DL, Chen JJ. Reactive oxygen species mediate cyclic strain-induced endothelin-1 gene expression via Ras/Raf/extracellular signal-regulated kinase pathway in endothelial cells. J Mol Cell Cardiol 2001;33:1805–1814. [DOI] [PubMed] [Google Scholar]
- 247. Heo KS, Fujiwara K, Abe J. Shear stress and atherosclerosis. Mol Cells 2014;37:435–440. 10.14348/molcells.2014.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Pan S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid Redox Signal 2009;11:1669–1682. 10.1089/ars.2009.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832–1844. 10.1161/CIRCULATIONAHA.106.676890 [DOI] [PubMed] [Google Scholar]
- 250. Stein-Merlob AF, Hara T, McCarthy JR, Mauskapf A, Hamilton JA, Ntziachristos V, Libby P, Jaffer FA. Atheroma susceptible to thrombosis exhibit impaired endothelial permeability in vivo as assessed by nanoparticle-based fluorescence molecular imaging. Circ Cardiovasc Imaging 2017;10:e005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Ushiyama A, Kataoka H, Iijima T. Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care 2016;4:59.. 10.1186/s40560-016-0182-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007;116:1896–1906. [DOI] [PubMed] [Google Scholar]
- 253. Huang CC, Kao KC, Hsu KH, Ko HW, Li LF, Hsieh MJ, Tsai YH. Effects of hydroxyethyl starch resuscitation on extravascular lung water and pulmonary permeability in sepsis-related acute respiratory distress syndrome. Crit Care Med 2009;37:1948–1955. [DOI] [PubMed] [Google Scholar]
- 254. Vincent JL. Plugging the leaks? New insights into synthetic colloids. Crit Care Med 1991;19:316–318. 10.1097/00003246-199103000-00003 [DOI] [PubMed] [Google Scholar]
- 255. Tatara T, Itani M, Sugi T, Fujita K. Physical plugging does not account for attenuation of capillary leakage by hydroxyethyl starch 130/0.4: a synthetic gel layer model. J Biomed Mater Res Part B Appl Biomater 2013;101:85–90. [DOI] [PubMed] [Google Scholar]
- 256. Yamaguchi H, Kasa M, Amano M, Kaibuchi K, Hakoshima T. Molecular mechanism for the regulation of rho-kinase by dimerization and its inhibition by fasudil. Structure 2006;14:589–600. [DOI] [PubMed] [Google Scholar]
- 257. Suzuki Y, Shibuya M, Satoh S, Sugiyama H, Seto M, Takakura K. Safety and efficacy of fasudil monotherapy and fasudil-ozagrel combination therapy in patients with subarachnoid hemorrhage: sub-analysis of the post-marketing surveillance study. Neurol Med Chir (Tokyo) 2008;48:241–247. discussion 247–248. [DOI] [PubMed] [Google Scholar]
- 258. Zhao J, Zhou D, Guo J, Ren Z, Zhou L, Wang S, Zhang Y, Xu B, Zhao K, Wang R, Mao Y, Xu B, Zhang X, Fasudil Aneurysmal Subarachnoid Hemorrhage Study G. Efficacy and safety of fasudil in patients with subarachnoid hemorrhage: final results of a randomized trial of fasudil versus nimodipine. Neurol Med Chir (Tokyo) 2011;51:679–683. [DOI] [PubMed] [Google Scholar]
- 259. Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart 2005;91:391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res 2006;99:1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Privratsky JR, Newman PJ. PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res 2014;355:607–619. 10.1007/s00441-013-1779-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Mei H, Campbell JM, Paddock CM, Lertkiatmongkol P, Mosesson MW, Albrecht R, Newman PJ. Regulation of endothelial cell barrier function by antibody-driven affinity modulation of platelet endothelial cell adhesion molecule-1 (PECAM-1). J Biol Chem 2014;289:20836–20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Qiu Y, Rui YC, Li TJ, Zhang L, Yao PY. Inhibitory effect of extracts of Ginkgo biloba leaves on VEGF-induced hyperpermeability of bovine coronary endothelial cells in vitro. Acta Pharmacol Sin 2004;25:1306–1311. [PubMed] [Google Scholar]
- 264. Ba J, Peng H, Chen Y, Gao Y. Effects and mechanism analysis of vascular endothelial growth factor and salvianolic acid B on 125I-low density lipoprotein permeability of the rabbit aortary endothelial cells. Cell Biochem Biophys 2014;70:1533–1538. 10.1007/s12013-014-0089-z [DOI] [PubMed] [Google Scholar]
- 265. Ang KP, Tan HK, Selvaraja M, Kadir AA, Somchit MN, Akim AM, Zakaria ZA, Ahmad Z. Cryptotanshinone attenuates in vitro oxLDL-induced pre-lesional atherosclerotic events. Planta Med 2011;77:1782–1787. [DOI] [PubMed] [Google Scholar]
- 266. Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol 2015;71:40–56. 10.1016/j.vph.2015.03.005 [DOI] [PubMed] [Google Scholar]