Abstract
Eugenol, the generic name of 4-allyl-2-methoxyphenol, is the major component of clove essential oil, and has demonstrated relevant biological potential with well-known antimicrobial and antioxidant actions. New O-alkylated eugenol derivatives, bearing a propyl chain with terminals like hydrogen, hydroxyl, ester, chlorine, and carboxylic acid, were synthesized in the present work. These compounds were later subjected to epoxidation conditions to give the corresponding oxiranes. All derivatives were evaluated against their effect upon the viability of insect cell line Sf9 (Spodoptera frugiperda), demonstrating that structural changes elicit marked effects in terms of potency. In addition, the most promising molecules were evaluated for their impact in cell morphology, caspase-like activity, and potential toxicity towards human cells. Some molecules stood out in terms of toxicity towards insect cells, with morphological assessment of treated cells showing chromatin condensation and fragmentation, which are compatible with the occurrence of programmed cell death, later confirmed by evaluation of caspase-like activity. These findings point out the potential use of eugenol derivatives as semisynthetic insecticides from plant natural products.
Keywords: eugenol derivatives, semisynthetic insecticides, phenylpropanoids, Spodoptera frugiperda, natural product-derived insecticides
1. Introduction
Due to the exponential increase in population, it is necessary to ensure that agricultural production follows the resulting food needs. The need for the prevention and control of plant diseases, as well as insect pests, is a crucial issue facing crop protection. To date, the most common strategy for controlling these issues has depended on the use of conventional pesticides, most of which are synthetic pesticides, including insecticides [1,2,3].
The intensive use of synthetic pesticides has resulted in damage to the environment, health hazards, and loss of biodiversity [4,5], so it is necessary to adopt less harmful strategies that can include the use of natural-based pesticides, which will result in a healthy environment and sustainable agriculture [6,7]. The concomitant use of natural pesticides/semisynthetic pesticides and synthetic pesticides could also take place as a changeover alternative to circumvent several negative effects of the exclusive use of synthetic compounds [8,9,10]. Owing to the structural diversity and biological activities of natural products, they could be rich sources of inspiration for the design optimization of active principles in formulation development [11,12].
Plants offer an extraordinary diversity of secondary metabolites, with proven efficacy against mosquito species of medical and veterinary importance, as well as against other noxious arthropod pests and vectors [13,14]. In recent years, the application of essential oils (EOs) and their bioactive compounds is gearing up rapidly as biopesticides, in order to limit the use of hazardous synthetic products, and these EOs are well-established as an alternative for the control of pre- and postharvest pests affecting agriculture-based food commodities [13,15,16]. These compounds have revealed great promise in applications like mosquito ovicide oils, given that they have shown particular potential as insecticides in organic agriculture [2,17].
In fact, EOs extracted by steam distillation of many aromatic plants have recently received a lot of attention due to their broad spectrum of action. These natural ingredients, composed of complex mixtures of monoterpenes, biogenetically related phenols, and sesquiterpenes [17,18,19], display antibacterial, antiviral, and antifungal activities, in addition to insecticide properties, as already mentioned [19,20,21,22,23,24]. Owing to their wide spectrum of activity, these compounds are nowadays considered as an alternative to chemicals in many applications, such as food preservation, pharmaceuticals, alternative medicine, and natural therapies [25,26,27]. EOs are typically characterized by a low melting point; most of them are liquid at room temperature, and their application in plant protection has some limitations, due to their poor solubility in water and high volatility. However, they display efficacy, biodegradability, various modes of action, and low toxicity, as well as an availability of source materials [6].
Eugenol, the major component of Syzygium aromaticum (clove) oil, has been used as a starting material and building block molecule for the manufacturing of bioactive compounds, on account of its particular structure and ready availability, in addition to numerous applications found in pharmaceutical, food, agricultural, and cosmetics industries [28,29]. Eugenol has also demonstrated antimicrobial and antioxidant activities [30], being also a powerful insecticide, effective on a wide variety of domestic arthropod pests [31,32,33]. A structural modification of EOs has been shown to enhance the biocidal effect of these phytochemicals by increasing their activity [34,35].
Considering all the above facts, in the present work semisynthetic eugenol derivatives—namely, O-alkylated bearing the propyl chain with hydrogen, hydroxyl, ester, chlorine and carboxylic acid as terminals, as well as the corresponding O-alkylated oxiranes—were synthesized. The main objective behind obtaining these eugenol derivatives was their evaluation as possible semisynthetic insecticides. Therefore, the biological activity of all compounds compared to a commercial synthetic insecticide was tested against Sf9 (Spodoptera frugiperda) insect cell line. The results turned out to be very promising for future applications as active ingredients in formulations, with structural changes eliciting marked effects in terms of potency and, equally important, low toxicity towards human cells.
2. Results
Eugenol 1 is easily obtained by hydrodistillation from clove, and is known for its various biological activities, as mentioned above, namely insecticidal. As an attempt to find semisynthetic alternatives with improved insecticidal activity, eugenol derivatives 2a–f and 3a–e were prepared; two of these have been shown to be highly and selectively toxic to insects, but not to human cells.
2.1. Synthesis of Eugenol Derivatives 2a–f and 3a–e
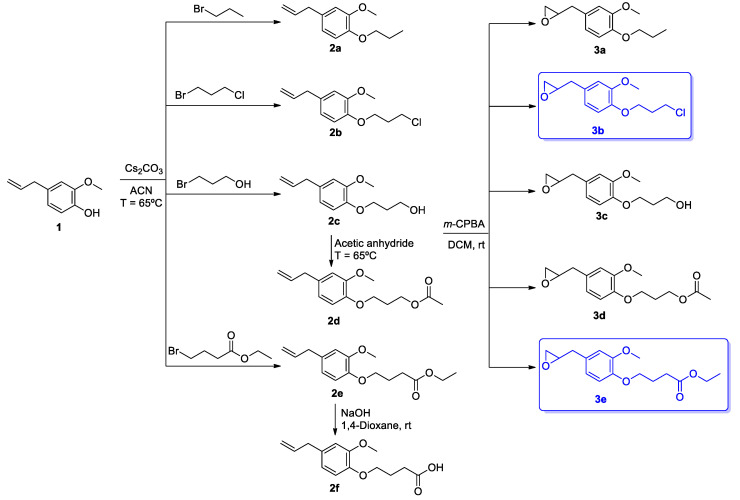
4-Allyl-2-methoxyphenol, eugenol 1 was obtained by hydrodistillation from clove to a high degree of purity (≥95%), as confirmed by its 1H NMR spectrum, and used in the synthesis of six O-alkylated derivatives (2a–f) and five oxiranes (3a–e), as shown in Scheme 1. The structures of all the compounds were confirmed by 1H and 13C NMR spectroscopy and high-resolution mass spectrometry (HRMS), and the corresponding analytical data are shown below.
Scheme 1.
Synthesis of eugenol derivatives 2a–f and 3a–e.
2.1.1. 4-Allyl-2-Methoxyphenol 1
Compound 1 was an off-white oil (14% yield of extraction).
1H NMR (CDCl3, 400 MHz): δH = 3.34 (2H, d, J = 6.8 Hz, CH2Ph), 3.89 (3H, s, OCH3), 5.07–5.12 (2H, m, CH=CH2), 5.81 (1H, broad s, OH), 5.93–6.03 (1H, m, CH=CH2), 6.70–6.88 (2H, m, H-3 and H-5), 6.87 (1H, d, J = 8.4 Hz, H-6) ppm.
2.1.2. 4-Allyl-2-Methoxy-1-Propoxybenzene 2a
Starting from compound 1 (0.200 g, 1.22× 10−3 mol) and using 1-bromopropane (0.12 mL, 1.34 × 10−3 mol), compound 2a was obtained as a light yellow oil (0.224 g, 89% yield). Rf = 0.67 (ethyl acetate/light petroleum = 1:10). 1H NMR (CDCl3, 400 MHz): δH = 1.06 (3H, t, J = 7.6 Hz, OCH2CH2CH3), 1.88 (2H, sext, J = 7.2 Hz, OCH2CH2CH3), 3.35 (2H, d, J = 6.8 Hz, CH2Ph), 3.87 (3H, s, OCH3), 3.97 (2H, t, J = 7.2 Hz, OCH2CH2CH3), 5.07–5.14 (2H, m, CH=CH2), 5.94–6.04 (1H, m, CH=CH2), 6.72–6.74 (2H, m, H-3 and H-5), 6.83 (1H, d, J = 8.0 Hz, H-6) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 10.23 (OCH2CH2CH3), 22.36 (OCH2CH2CH3), 39.60 (CH2Ph), 55.69 (OCH3), 70.44 (OCH2CH2CH3), 112.24 (C-3), 113.06 (C-6), 115.30 (CH=CH2), 120.25 (C-5), 132.44 (C-4), 137.51 (CH=CH2), 146.76 (C-1), 149.23 (C-2) ppm. HRMS: m/z (ESI) calculated for C13H19O2 [M+1]+ = 207.1380; found = 207.1381.
2.1.3. 4-Allyl-1-(3-Chloropropoxy)-2-Methoxybenzene 2b
Starting from compound 1 (0.200 g, 1.22 × 10−3 mol) and using 1-bromo-3-chloro-propane (0.93 mL, 9.44 × 10−3 mol), compound 2b was obtained as a light yellow oil (0.223 g, 72% yield). Rf = 0.67 (ethyl acetate/light petroleum = 1:10). 1H NMR (CDCl3, 400 MHz): δH = 2.28 (2H, quint, J = 6.0 Hz, OCH2CH2CH2Cl), 3.35 (2H, d, J = 6.8 Hz, CH2Ph), 3.78 (2H, t, J = 6.4 Hz, OCH2CH2CH2Cl), 3.86 (3H, s, OCH3), 4.15 (2H, t, J = 5.6 Hz, OCH2CH2CH2Cl), 5.06–5.13 (2H, m, CH=CH2), 5.93–6.03 (1H, m, CH=CH2), 6.72–6.74 (2H, m, H-3 and H-5), 6.86 (1H, d, J = 6.8 Hz, H-6) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 32.31 (OCH2CH2CH2Cl), 39.72 (CH2Ph), 41.64 (OCH2CH2CH2Cl), 55.85 (OCH3), 65.88 (OCH2CH2CH2Cl), 112.45 (C-6), 113.91 (C-3), 115.60 (CH=CH2), 120.48 (C-5), 133.37 (C-4), 137.55 (CH=CH2), 146.48 (C-1), 149.52 (C-2) ppm. HRMS: m/z (ESI) calculated for C13H1735ClNaO2 [M+Na = 263.0809; found = 263.0805; calculated for C13H1737ClNaO2 [M+Na]+ = 265.0783; found = 265.0780.
2.1.4. 3-(4-Allyl-2-Methoxyphenoxy)Propan-1-ol 2c
Starting from compound 1 (0.250 g, 1.52 × 10−3 mol) and using 3-bromopropan-1-ol (0.06 mL, 1.67 × 10−3 mol), compound 2c was obtained as a light yellow oil (0.180 g, 53% yield). Rf = 0.23 (ethyl acetate/light petroleum 1:3). 1H NMR (CDCl3, 400 MHz): δH = 2.07 (2H, quint, J = 5.6 Hz, OCH2CH2CH2OH), 3.34 (2H, d, J = 6.4 Hz, CH2Ph), 3.85 (3H, s, OCH3), 3.88 (2H, t, J = 6.4 Hz, OCH2CH2CH2OH), 4.18 (2H, t, J = 5.6 Hz, OCH2CH2CH2OH), 5.05–5.12 (2H, m, CH=CH2), 5.91–6.01 (1H, m, CH=CH2), 6.71–6.73 (2H, m, H-3 and H-5), 6.84 (1H, d, J = 8.4, H-6) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 31.75 (OCH2CH2CH2OH), 39.82 (CH2Ph), 55.75 (OCH3), 61.56 (OCH2CH2CH2OH), 68.69 (OCH2CH2CH2OH), 112.06 (C-3), 113.48 (C-6), 115.64 (CH = CH2), 120.44 (C-5), 133.41 (C-4), 137.57 (CH=CH2), 146.46 (C-1), 149.37 (C-2) ppm. HRMS: m/z (ESI) calculated for C13H19O3 [M+1]+ = 223.1329; found = 223.1329.
2.1.5. Ethyl 4-(4-Allyl-2-Methoxyphenoxy)Butanoate 2e
Starting from compound 1 (0.200 g, 1.22 × 10−3 mol) and using ethyl 4-bromobutyrate (0.19 mL, 1.34 × 10−3 mol), compound 2e was obtained as a colorless oil (0.249 g, 73% yield). Rf = 0.44 (ethyl acetate/light petroleum 1:10). 1H NMR (CDCl3, 400 MHz): δH = 1.24 (3H, t, J = 8.4 Hz, CO2CH2CH3), 2.14 (2H, quint, J = 6.4 Hz, OCH2CH2CH2CO2CH2CH3), 2.53 (2H, t, J = 7.2 Hz, OCH2CH2CH2CO2CH2CH3), 3.33 (2H, d, J = 6.8 Hz, CH2Ph), 3.85 (3H, s, OCH3), 4.04 (2H, t, J = 6.4 Hz, OCH2CH2CH2CO2CH2CH3), 4.14 (2H, q, J = 7.2 Hz, OCH2CH2CH2CO2CH2CH3), 5.04–5.11 (2H, m, CH=CH2), 5.91–6.01 (1H, m, CH=CH2) 6.69–6.72 (2H, H-3 and H-5), 6.82 (1H, d, J = 7.2, H-6) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 14.14 (OCH2CH2CH2CO2CH2CH3), 24.57 (OCH2CH2CH2CO2CH2CH3), 30.73 (OCH2CH2CH2CO2CH2CH3), 39.74 (CH2Ph), 55.85 (OCH3), 60.31 (OCH2CH2CH2CO2CH2CH3), 68.09 (COCH2CH2CH2CO2CH2CH3), 112.41 (C-3) 113.60 (C-6), 115.54 (CH = CH2), 120.44 (C-5), 133.06 (C-4), 137.60 (CH = CH2), 146.58 (C-1), 149.46 (C-2), 173.20 (CO2CH2CH3) ppm. HRMS: m/z (ESI) calculated for C16H23O4 [M+1]+ = 279.1591; found = 279.1592.
2.1.6. Methyl 4-(4-Allyl-2-Methoxyphenoxy)Butanoate 2d
Starting from compound 2c (0.248 g, 1.12 × 10−3 mol) and using acetic anhydride (0.16 mL, 1.68 mmol), compound 2d was obtained as a light yellow oil (0.248 g, 84% yield). Rf = 0.29 (ethyl acetate/light petroleum 1:10). 1H NMR (CDCl3, 400 MHz): δH = 2.02 (3H, s, CO2CH3), 2.11 (2H, quint, J = 6.8 Hz, OCH2CH2CH2CO2CH3), 3.30 (2H, d, J = 6.8 Hz, CH2Ph), 3.81 (3H, s, OCH3), 4.05 (2H, t, J = 6.4 Hz, OCH2CH2CH2CO2CH3), 4.25 (2H, t, J = 6.4 Hz, OCH2CH2CH2CO2CH3), 5.01–5.08 (2H, m, CH=CH2), 5.88–5.98 (1H, m, CH=CH2), 6.67–6.69 (2H, m, H-3 and H-5), 6.80 (1H, d, J = 8.0 Hz, H-6) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 20.66 (CO2CH3), 28.43 (OCH2CH2CH2CO2CH3), 39.57 (CH2Ph), 55.64 (OCH3), 61.18 (OCH2CH2CH2CO2CH3), 65.60 (OCH2CH2CH2CO2CH3), 112.30 (C-3), 113.53 (C-6), 115.38 (CH=CH2), 120.26 (C-5), 133.03 (C-4), 137.40 (CH=CH2), 146.38 (C-1), 149.35 (C-2), 170.76 (CO2CH3) ppm. HRMS: m/z (ESI) calculated for C15H20NaO4 [M+Na]+ = 287.1254; found = 287.1253.
2.1.7. 4-(4-Allyl-2-Methoxyphenoxy)Butanoic Acid 2f
To a suspension of compound 2e (0.200 g, 7.19 × 10−4 mol) in 1,4-dioxane (3.0 mL), 1 M aqueous sodium hydroxide (2.16 mL, 2.16× 10−3 mol) was added, and compound 2f was obtained as a white solid (0.158 g, 88 % yield). Rf = 0.78 (ethyl acetate/light petroleum 1:10). 1H NMR (CDCl3, 400 MHz): δH = 2.15 (2H, quint, J = 7.2 Hz, OCH2CH2CH2CO2H), 2.62 (2H, t, J = 6.8 Hz, OCH2CH2CH2CO2H), 3.34 (2H, d, J = 6.4 Hz, CH2Ph), 3.85 (3H, s, OCH3), 4.06 (2H, t, J = 6.0 Hz, OCH2CH2CH2CO2H), 5.05 – 5.12 (2H, m, CH=CH2), 5.92–6.02 (1H, m, CH=CH2), 6.70–6.83 (2H, m, H-3 and H-5), 6.83 (1H, d, J = 8.4 Hz, H-6) ppm.13C NMR (CDCl3, 100.6 MHz): δC = 24.31 (OCH2CH2CH2CO2H), 30.55 (OCH2CH2CH2CO2H), 39.77 (CH2Ph), 55.85 (OCH3), 67.99 (OCH2CH2CH2CO2H), 112.45 (C-3), 113.73 (C-6), 115.60 (CH=CH2), 120.48 (C-5), 133.26 (C-4), 137.60 (CH=CH2), 146.49 (C-1), 149.50 (C-2), 179.15 (CO2H) ppm. HRMS: m/z (ESI) calculated for C14H19O4 [M+1]+ = 251.1278; found = 251.1274.
2.1.8. 2-(3-Methoxy-4-propoxybenzyl)oxirane 3a
Starting compound 2a (0.218 g, 9.70 × 10−4 mol) and using m-chloroperbenzoic acid (0.434 g, 2.51 × 10−3 mol), compound 3a was obtained as a yellow oil (0.016 g, 7% yield). Rf = 0.30 (ethyl acetate/light petroleum 1:10). 1H NMR (CDCl3, 400 MHz): δH = 1.04 (3H, t, J = 7.6 Hz, OCH2CH2CH3), 1.82–1.91 (2H, m, OCH2CH2CH3), 2.55 (1H, q, J = 2.8 Hz CH2 oxirane), 2.76–2.87 (3H, m, CH2Ph and CH2 oxirane), 3.12–3.17 (1H, m, CH oxirane), 3.86 (3H, s, OCH3), 3.97 (2H, t, J = 6.8 Hz, OCH2CH2CH3), 6.76–6.84 (2H, m, H-2 and H-6), 6.83 (1H, d, J = 8.0 Hz, H-5) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 10.39 (OCH2CH2CH3), 22.47 (OCH2CH2CH3), 38.27 (CH2Ph), 46.78 (CH2 oxirane), 52.57 (CH oxirane) 55.99 (OCH3), 70.59 (OCH2CH2CH3), 112.79 (C-2), 113.13 (C-5), 120.94 (C-6), 129.72 (C-1), 147.36 (C-4), 149.36 (C-3) ppm. HRMS: m/z (ESI) calculated for C13H18NaO3 [M+Na]+ = 0245.1148; found = 245.1148.
2.1.9. 2-(4-(3-Chloropropoxy)-3-Methoxybenzyl)Oxirane 3b
Starting from compound 2b (0.213 g, 8.85 × 10−4 mol) and using m-chloroperbenzoic acid (0.198 g, 1.15 × 10−3 mol), compound 3b was obtained as a yellow oil (0.151 g, 67% yield). Rf = 0.52 (ethyl acetate/light petroleum 1:3). 1H NMR (CDCl3, 400 MHz): δH = 2.28 (2H, quint, J = 6.0 Hz, OCH2CH2CH2), 2.55 (1H, q, J = 2.8 Hz, CH2 oxirane), 2.79–2.83 (3H, m, CH2Ph and CH2 oxirane), 3.12–3.16 (1H, m, CH oxirane), 3.77 (2H, t, J = 6.4 Hz, OCH2CH2CH2), 3.86 (3H, s, OCH3), 4.15 (2H, t, J = 6.0 Hz, OCH2CH2CH2), 6.77–6.79 (2H, m, H-2 and H-6), 6.86 (1H, d, J = 7.6 Hz, H-5) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 32.27 (OCH2CH2CH2Cl), 38.26 (CH2Ph), 41.63 (OCH2CH2CH2Cl), 46.76 (CH2 oxirane), 52.53 (CH oxirane), 55.93 (OCH3), 65.82 (OCH2CH2CH2Cl), 112.88 (C-6), 113.85 (C-5), 121.01 (C-2), 130.47 (C-1), 146.96 (C-4), 149.54 (C-3) ppm. HRMS: m/z (ESI) calculated for C13H1735ClO3 [M+Na]+ = 279.0758; found = 279.0754; calculated for C13H1737ClO3 [M+Na]+ = 281.0733; found = 281.0722.
2.1.10. 3-(2-Methoxy-4-(Oxiran-2-Ylmethyl)Phenoxy)Propan-1-ol 3c
Starting compound 2c (0.156g, 7.03 × 10−4 mol) and using m-chloroperbenzoic acid (0.346 g, 2.0 × 10−3 mol), compound 3c was obtained as a yellow oil (0.096 g, 57% yield). Rf = 0.58 (ethyl acetate). 1H NMR (CDCl3, 400 MHz): δH = 2.08 (2H, quint, J = 6.0 Hz, OCH2CH2CH2OH), 2.55 (1H, q, J = 2.8 Hz, CH2 oxirane), 2.80–2.85 (3H, m, CH2Ph and CH2 oxirane), 3.13–3.17 (1H, m, CH oxirane), 3.86 (3H, s, OCH3), 3.89 (2H, t, J = 5.6 Hz, OCH2CH2CH2OH), 4.19 (2H, t, J = 5.6 Hz, OCH2CH2CH2OH), 6.71–6.73 (2H, m, H-3 and H-5), 6.84 (1H, d, J = 8.4, H-6) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 31.75 (OCH2CH2CH2OH), 38.34 (CH2Ph), 46.78 (CH2 oxirane), 52.56 (CH oxirane), 55.83 (OCH3), 61.57 (OCH2CH2CH2OH), 68.64 (OCH2CH2CH2OH), 112.50 (C-3), 113.45 (C-6), 120.96 (C-5), 130.52 (C-4), 146.95 (C-1), 149.41 (C-2) ppm. HRMS: m/z (ESI) calculated for C13H18NaO4 [M+Na]+ = 261.1097; found = 261.1098.
2.1.11. 3-(2-Methoxy-4-(Oxiran-2-Ylmethyl)Phenoxy)Propyl Acetate 3d
Starting from compound 2d (0.1039 g, 3.93 × 10−4 mol) and using m-chloroperbenzoic acid (0.194 g, 1.12 × 10−3 mol), compound 3c was obtained as light yellow oil (0.031g; 28% yield). Rf = 0.32 (ethyl acetate/light petroleum 1:3). 1H NMR (CDCl3, 400 MHz): δH = 2.05 (3H, s, CO2CH3), 2.15 (2H, quint, J = 6.4 Hz, OCH2CH2CH2CO2CH3), 2.55 (1H, q J = 2.8 Hz,CH2 oxirane), 2.79–2.82 (3H, m, CH2Ph and CH2 oxirane), 3.12–3.16 (1H, m, CH oxirane), 3.86 (3H, s, OCH3), 4.09 (2H, t, J = 6.4 Hz, OCH2CH2CH2CO2CH3), 4.28 (2H, t, J = 6.4 Hz, OCH2CH2CH2CO2CH3), 6.77–6.79 (2H, m, H-3 and H-5), 6.84 (1H, d, J = 8.0 Hz, H-6) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 20.90 (CO2CH3), 23.79 (OCH2CH2CH2CO2CH3), 38.26 (CH2Ph), 46.77 (CH2 oxirane), 52.55 (CH oxirane), 55.93 (OCH3), 61.37 (OCH2CH2CH2CO2CH3), 65.73 (OCH2CH2CH2CO2CH3), 112.88 (C-3), 113.59 (C-6), 120.96 (C-5), 130.33 (C-4), 147.02 (C-1), 149.51 (C-2), 170.07 (CO2CH3) ppm. HRMS: m/z (ESI) calculated for C15H20NaO5 [M+Na]+ = 303.1203; found = 303.1202.
2.1.12. Ethyl 4-(2-Methoxy-4-(Oxiran-2-Ylmethyl)Phenoxy)Butanoate 3e
Starting from compound 2e (0.173 g, 6.22 × 10−4 mol) and m-chloroperbenzoic acid (0.278 g, 1.61 × 10−3 mol), compound 3e was obtained as a yellow oil (0.022 g, 13% yield). Rf = 0.31 (ethyl acetate/light petroleum 1:3). 1H NMR (CDCl3, 400 MHz): δH = 1.26 (3H, t, J = 7.2 Hz, CO2CH2CH3), 2.14 (2H, quint, J = 7.2 Hz, OCH2CH2CH2CO2CH2CH3), 2.51–2.56 (3H, m, CH2 oxirane and OCH2CH2CH2CO2CH2CH3), 2.76–2.86 (3H, m, CH2Ph and CH2 oxirane), 3.12–3.16 (1H, m, CH oxirane), 3.86 (3H, s, OCH3), 4.05 (2H, t, J = 6.0 Hz, OCH2CH2CH2CO2CH2CH3), 4.14 (2H, q, J = 7.2 Hz CO2CH2CH3), 6.75–6.79 (2H, m, H-3 and H-5), 6.84 (1H, d, J = 8.0 Hz, H-6) ppm. 13C NMR (CDCl3, 100.6 MHz): δC = 14.19 (CO2CH2CH3), 24.57 (OCH2CH2CH2CO2CH2CH3), 30.74 (OCH2CH2CH2CO2CH2CH3), 38.29 (CH2Ph), 46.78 (CH2 oxirane), 52.55 (CH oxirane), 55.95 (OCH3), 60.36 (CO2CH2CH3), 68.07 (OCH2CH2CH2CO2CH2CH3), 112.87 (C-3), 113.58 (C-6), 120.98 (C-5), 130.18 (C-4), 147.09 (C-1), 149.50 (C-2), 173.20 (CO2CH2CH3) ppm. HRMS: m/z (ESI) alculated for C15H22NaO5 [M+Na]+ = 317.1359; found = 317.1359.
2.2. Screening of Toxicity Towards Insect Cells
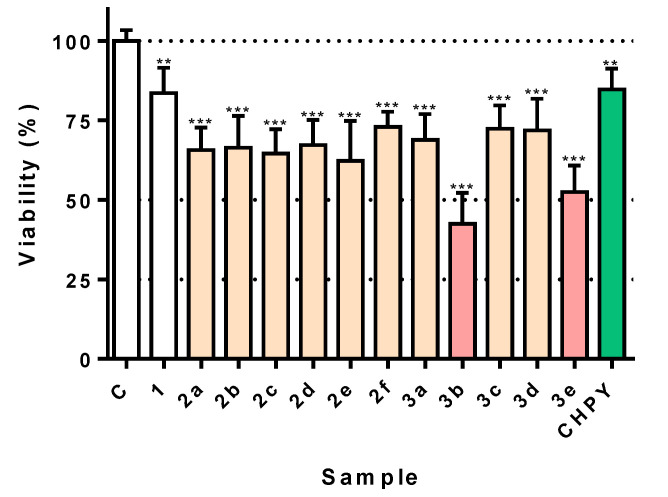
As a model for insecticide activity, two-dimensional (2D) cultures of Sf9 cells, which derive from the ovary cells of Spodoptera frugiperda (a common pest) were used. All molecules were assayed at the same concentration (100 µg/mL) in order to allow direct comparison of their potency. The starting material, eugenol 1, was nearly devoid of any toxicity, causing a marginal decrease of viability (Figure 1). All eugenol derivatives arising from alkylation reactions of the hydroxyl group, and possessing a propyl chain with hydrogen, hydroxyl, ester, chlorine, and carboxylic acid as terminals (2a–f) displayed higher toxicity than the starting eugenol 1, with cells showing around 55–65% of viability (Figure 1). When analyzing the results of the oxiranes 3a–e series, which results from the epoxidation of the compound 2 series, a distinct trend was found. In a general way, all members of the 3 series displayed enhanced toxicity when compared to 1. Among all derivatives, 3b and 3e were clearly the most potent, with the latter eliciting ca. 50% viability loss, while the former reached nearly 60% viability loss, nearly double the effect of the commercial insecticide chlorpyrifos (Figure 1). For this reason, these two molecules were further characterized for their effect.
Figure 1.
Viability of cells exposed to the molecules under study 2a–f and 3a–e (100 µg/mL), medium (control), or the reference insecticide chlorpyrifos (CHPY; 100 µg/mL). Cells were incubated for 24 h, after which viability was evaluated. ** p < 0.01; *** p < 0.001.
2.3. Impact of Eugenol Derivatives 3b and 3e in Insect Cell Morphology
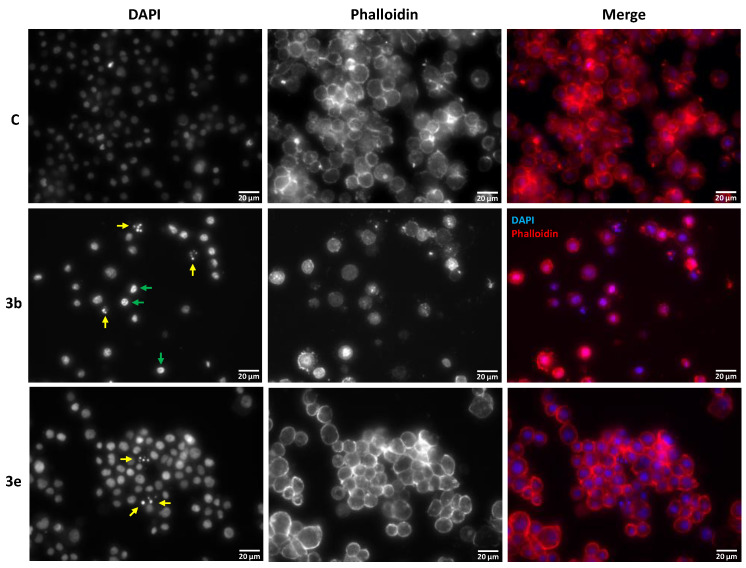
We were interested in assessing the impact that 3b and 3e had in the morphology of the insect cells under study. For this reason, treated cells were imaged to evaluate chromatin status and overall cell morphology. We used both molecules at the same concentration that had been assessed for viability studies (100 µg/mL); however, the effect of 3e was so pronounced that no cells remained. For this reason, in the specific case of this molecule, the concentration of 50 µg/mL was used.
Incubation with either molecules resulted in reduced cell density when compared with control cells, which is in line with the results reported for their impact in cell viability.
When examining the 4’, 6-diamidino-2-phenylindole (DAPI) channel (Figure 2), it was clear that the 3b- and 3e-treated cells exhibited chromatin changes, karyorrhexis (nuclear fragmentation; yellow arrows), and pyknosis (chromatin condensation; green arrows). We have quantified this effect (Supplementary Table S1), results, showing that incubation with 3b resulted in an increase of fragmented and condensed chromatin to 34.8 ± 0.3% and 12.6 ± 4.2%, respectively, while 3e elicited 21.2 ± 0.8% and 5.8 ± 0.5%, respectively.
Figure 2.
Morphology of Sf9 cells exposed to compounds 3b (100 µg/mL) and 3e (50 µg/mL) after 24 h of incubation (S Plan Fluor ELWD 40× DIC N1 objective). Overall cell morphology was evaluated using phalloidin (actin) and DAPI (chromatin status). Yellow arrow: chromatin fragmentation; green arrow: chromatin condensation; C: control. Color blind-friendly image in Supplementary Figure S1.
2.4. Oxiran-Bearing Eugenol Derivatives 3b and 3e Activate Caspase-Like Proteases in Sf9 Cells
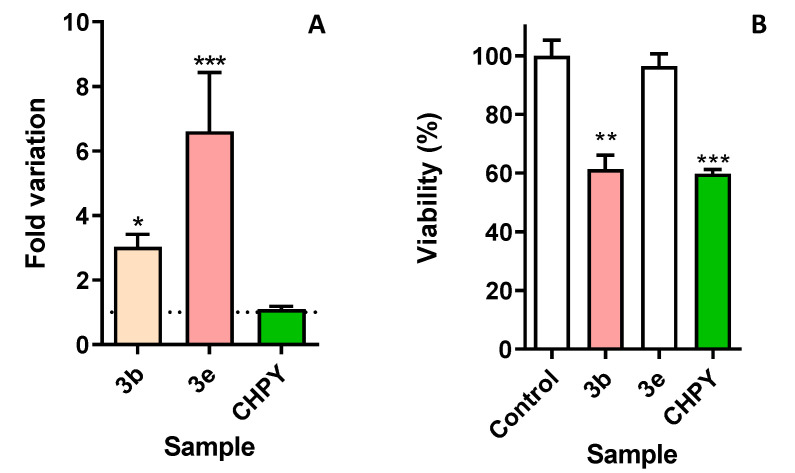
Considering the lack of commercial options for the assessment of insect counterparts of mammalian caspases, it was hypothesized that the degree of homology described between insect and mammalian caspases [36] should be high enough for substrate cross-reactivity. For this reason, a substrate for mammalian executor caspase isoforms 3/7 has been used. As shown in Figure 3A, incubation of cells with 3b resulted in an about three-fold increase in caspase-like activity, reaching over six-fold in the case of 3e. For benchmarking purposes, the commercial insecticide chlorpyrifos was also evaluated, and its effect was not statistically different from control cells.
Figure 3.
(A) Caspase-like activity found in Sf9 cells after incubation with compounds 3b (100 µg/mL) and 3e (50 µg/mL), or the reference insecticide chlorpyrifos (CHPY, 100 µg/mL), for 24 h. (B) Viability of human keratinocytes exposed to compounds 3b and 3e (100 µg/mL), medium (control), or the reference insecticide chlorpyrifos (CHPY, 100 µg/mL). Cells were incubated for 24 h, after which viability was evaluated. * p < 0.05; ** p < 0.01; *** p < 0.001.
2.5. Some Eugenol Derivatives are Selectively Toxic to Insect but Not Human Cells
Bearing in mind the importance of developing new and more potent insecticides that have a safe toxicological profile towards mammalian organisms, the impact of 3b and 3e in human cells has been evaluated. Considering the usual routes of poisoning, specifically skin, human keratinocytes, were chosen. As it can be seen in Figure 3B, 3b caused around 40% loss of cell viability (equivalent to the commercial insecticide chlorpyrifos), which is still less pronounced than the effect elicited in insect cells. In the case of 3e, Figure 3B shows that it had no effect in human cells at the same concentration in which it caused around 50% of cell viability loss in insect cells, thus displaying a selective effect towards the latter.
3. Discussion
3.1. Synthesis of Eugenol Derivatives 2a–f and 3a–e
Structural modifications in the hydroxyl group and double bond of 4-allyl-2-methoxyphenol, eugenol 1, obtained by hydrodistillation of clove, were carried out. Alkylation of the hydroxyl group of 4-allyl-2-methoxyphenol 1 with 1-bromopropane,1-bromo-3-chloropropane, 3-bromopropan-1-ol, and ethyl 4-bromobutanoate, using cesium carbonate as a base and by heating at 65 °C in acetonitrile, gave 4-allyl-2-methoxy-1-propoxybenzene 2a, 4-allyl-1-(3-chloropropoxy)-2-methoxybenzene 2b, 3-(4-allyl-2-methoxyphenoxy)propan-1-ol 2c, and ethyl 4-(4-allyl-2-methoxyphenoxy)butanoate 2e, respectively.
Compound 2c was further reacted with acetic anhydride by heating at 65 °C to obtain 3-(4-allyl-2-methoxyphenoxy)propyl acetate 2d. In addition, compound 2e was subjected to hydrolysis with aqueous 1M NaOH in 1,4-dioxane at room temperature to give 4-(4-allyl-2-methoxyphenoxy)butanoic acid 2f. Compounds 2a–e were obtained as oils or a solid material (2f) in 53% to 88% yields, and were fully characterized by 1H and 13C NMR spectroscopy, as well as HRMS. The 1H NMR spectra of compounds 2a–f showed the different characteristic signals for the aliphatic protons of methylene and methyl groups (δ = 1.03–4.30 ppm), as well as the expected protons for the eugenol’s double bond as multiplets, CH2 (δ = 5.01–5.14 ppm) and CH (δ = 5.90–6.04 ppm). 13C NMR spectra of all compounds showed signals of the aliphatic carbons from the methylene and methyl groups (δ = 10.23–70.44 ppm), and for compounds 2d, 2e, and 2f was visible the presence of signals for the carbonyl groups (δ = 170.76–179.15 ppm).
To perform epoxidation of the double bond of eugenol derivatives, compounds 2a–e reacted with m-chloroperbenzoic acid in dichloromethane at room temperature, and the respective derivatives, namely 2-(3-methoxy-4-propoxybenzyl)oxirane 3a, 2-(4-(3-chloropropoxy)-3-methoxybenzyl)oxirane 3b, 3-(2-methoxy-4-(oxiran-2-ylmethyl)phenoxy)propan-1-ol 3c, 3-(2-methoxy-4-(oxiran-2-ylmethyl)phenoxy)propyl acetate 3d, and ethyl 4-(2-methoxy-4-(oxiran-2-ylmethyl)phenoxy)butanoate 3e were obtained. These compounds 3a–e were isolated as yellow oils in yields up to 67%, and were fully characterized by the usual analytical techniques. It stands out that epoxidation of compounds 2a–e was verified by the presence of the protons signals related the oxirane ring (δ = 2.52–3.17 ppm) and the absence of the signals of protons for the double bond of eugenol skeleton. The presence of carbon signals relative to oxirane ring, CH2 (δ = 46.76–46.78 ppm, and CH = (δ 52.53–52.57 ppm) also confirmed the structure of expected eugenol derivatives 3a–e.
3.2. Differential Effect of Eugenol Derivatives Towards Insect Cells
When evaluating the impact of all molecules obtained, a clear trend could be established. All derivatives arising from alkylation reactions of the hydroxyl group and possessing a propyl chain with hydrogen, hydroxyl, ester, chlorine, and carboxylic acid as terminals (2a–f) displayed higher toxicity than the starting molecule 1 (Figure 1). In a general way, all member of the 3 series displayed enhanced toxicity when compared to eugenol, which suggests a role for the oxirane group in the potency of this family of compounds. Among all derivatives, 3b and 3e were clearly the most potent, eliciting a degree of viability loss higher than the commercial insecticide chlorpyrifos (Figure 1).
3.3. Eugenol Derivatives Trigger a Process of Programmed Cell Death
The results from morphological assessment showed the advent of chromatin changes, such as karyorrhexis and pyknosis (Figure 2). This result, although not conclusive per se, was compatible with a process of programmed cell death. For this reason, we decided to assess the activity of caspase homologues, given the role and relevance of these serine proteases in several types of programmed cell deaths. As shown in Figure 3A, incubation of cells with 3b resulted in an about three-fold increase in caspase-like activity; this value increased to over six-fold in the case of 3e. For benchmarking purposes, the commercial insecticide chlorpyrifos was also evaluated, and its effect was not statistically different from control cells. This result is not completely unexpected, considering that chlorpyrifos belongs to the class of organophosphates pesticides, thus exerting its toxicity by disrupting the nervous system of the target organisms, specifically by irreversibly inhibiting acetylcholinesterase, leading to the build-up of acetylcholine levels [37]. Although death can ultimately occur in vivo, apoptosis is not the primary result of its effect. The fact that the eugenol derivatives described herein exert their effect via a distinct mechanism than organophosphates, which are known for their toxicity, is promising. Furthermore, in view of the chemical structures of the molecules presented here, eugenol derivatives are unlikely to inhibit acetylcholinesterase, which suggests that they may act through distinct mechanisms.
3.4. New Eugenol Derivatives are Not Toxic to Human Cells
Finally, the effect of the most promising molecules, 3b and 3e, was evaluated towards human cells. Considering the most frequent routes of contact with pesticides, we have chosen keratinocytes as a model, given their pivotal role in skin anatomy. As shown in Figure 3B, very distinct selectivity was found. Compound 3b caused around 40% loss of cell viability, which is still less than the effect elicited in insect cells. In the case of 3e, Figure 3B shows that it had no effect in human cells at the same concentration in which it caused around 50% of cell viability loss, thus displaying a selective effect towards insect cells. Even more interesting is that this selective effect outperforms that of the commercial insecticide chlorpyrifos, which was highly toxic to keratinocytes and caused over 40% of viability loss.
4. Materials and Methods
4.1. Chemicals
Dichloromethane, acetonitrile, ethyl acetate, light petroleum, 1,4-dioxane, 1-bromo-3-chloropropane, cesium carbonate, and m-chloroperbenzoic acid were purchased from Fisher Scientific (Geel, Belgium). The 1-Bromopropane, 3-bromopropan-1-ol and ethyl 4-bromobutanoate were from Sigma-Aldrich (St. Louis, MO, United States). The anhydrous magnesium sulphate and acetic anhydride were PanReac Applichem (Barcelona, Spain) products. Chloroform-d was produced by Eurisotop (Cambridge, England). Thin-layer chromatography (TLC) analyses were carried out on 0.25 mm-thick, precoated silica plates (Merck Fertigplatten Kieselgel 60F254, Germany), and spots were visualized under UV light. Chromatography on silica gel was carried out on Merck Kieselgel (230–240 mesh).
4.2. Analytical Instruments
The NMR spectra were obtained on a Bruker Avance III at an operating frequency of 400.0 MHz for 1H NMR and 100.6 MHz for 13C NMR, using the solvent peak as internal reference at 25 °C. All chemical shifts are given in ppm using δ Me4Si = 0 ppm as reference, and J values are given in hertz. Assignments were made by a comparison of chemical shifts, peak multiplicities, and J values, and were supported by spin decoupling–double resonance and bidimensional heteronuclear correlation techniques. High-resolution mass spectrometry analyses were performed at the CACTUS, Unidade de Masas e Proteómica, at the University of Santiago de Compostela, Spain.
4.3. Synthesis of Eugenol Derivatives 2a–f and 3a–e
4.3.1. Extraction of Eugenol 1 from Syzygium Aromaticum
The extraction of 4-allyl-2-methoxyphenol, eugenol 1, was made from Syzygium aromaticum (cloves) in a round-bottom flask containing distilled water (200 mL) and the cloves (21.415 g). Hydrodistillation assembly was performed, and the mixture was refluxed during 2 h. The distillate was extracted with dichloromethane (3 × 150 mL), the organic phase was dried over anhydrous magnesium sulphate, and solvent evaporation under vacuum yielded 4-allyl-2-methoxyphenol, eugenol (1).
4.3.2. General Procedure for Synthesizing Compounds 2a–c and 2e
To a solution of 4-allyl-2-methoxyphenol 1 (1 equiv) in acetonitrile (5 mL), the corresponding alkyl halide (1.1 equiv) and cesium carbonate (5 equiv) were added, and the resulting mixture was heated at 60 °C for 2 h 30 min. The progress of the reaction was monitored by TLC (ethyl acetate/light petroleum = 1:10). The excess of base was filtered, the solvent was evaporated, and the crude mixture was purified by column chromatography on silica gel using ethyl acetate/light petroleum (1:10) as the eluent.
4.3.3. General Procedure for Synthesizing Compound 2d
To compound 2c acetic anhydride was added, and the resulting mixture was stirred at 65 °C for 12 h. The reaction was monitored by TLC (ethyl acetate/light petroleum = 1:10). After completion, the mixture was diluted with ethyl acetate and washed with sodium bicarbonate (2 × 5 mL); the organic phase was dried with anhydrous magnesium sulfate, and the solvent was evaporated.
4.3.4. General Procedure for Synthesizing Compound 2f
To a suspension of compound 2e in 1,4-dioxane, 1 M aqueous sodium hydroxide was added. The solution was stirred at room temperature for 8 h, and acidified to pH 2–3 with 1 M aqueous potassium hydrogen sulfate. The reaction mixture was evaporated and dichloromethane was added, giving a precipitate that was filtered. Then the solvent was evaporated.
4.3.5. General Procedure for Synthesizing Compounds 3a–e
The respective precursor (1 equiv) 2a–e (4 mL) dissolved in dichloromethane was added dropwise to a solution of m-chloroperbenzoic acid (1 equiv) in dichloromethane (6 mL) at 0 °C. After stirring for 1 h, m-chloroperbenzoic acid was again added (1 equiv), and the reaction mixture was stirred for more 12 h. A 10% aqueous solution of sodium sulfate (10 mL) was added, and the resulting solution was washed with 5% aqueous solution of sodium hydrogen carbonate (2 × 10 mL). The organic phase was dried with anhydrous magnesium sulfate, and the solvent was evaporated.
4.4. Preparation Methods
4.4.1. Cell Culture
Sf9 (Spodoptera frugiperda) cells were maintained as a suspension culture and cultivated in Grace’s medium with 10% FBS and 1% penicillin/streptomycin, at 28 °C. Cells were used in experiments while in the exponential phase of growth. On the other hand, HaCaT (human keratinocyte) cells were cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C, in a humidified atmosphere of 5% CO2.
4.4.2. Viability Assessment
For the assessment of viability, a resazurin-based method was used. Sf9 and HaCaT cells were plated at a density of 3.0 × 104 and 1.5 × 104 cells/well, respectively, incubated for 24 h, and then exposed to the molecules under study for 24 h. After this period, a commercial solution of resazurin was added (1:10), and the kinetic reaction of fluorescence increase monitored at 560/590 nm. For HaCaT and Sf9 cells, 30 and 60 min of incubation were used, respectively.
4.4.3. Morphological Assessment
For morphological studies, Sf9 cells were cultured in 96-well plates at the same density used for viability experiments, in the presence of the molecules under study. After incubation, cells were washed with Hanks’ balanced salt solution (HBSS) and fixed in 10% formalin solution for 30 min, at room temperature. CF543 (5 U/mL) and DAPI (0.25 µg/mL) were added, and cells were stained for 25 min at room temperature and washed with HBSS.
Images were acquired in an inverted Eclipse Ts2R-FL (Nikon) equipped with a Retiga R1 camera and an S Plan Fluor ELWD 20x DIC N1 objective. Images were analyzed with Fiji [38]. For quantitative parameters, the Fiji’s Cell Counter plugin was used.
4.4.4. Caspase-Like Activity
Sf9 cells were plated at the same density described for viability studies and exposed to the molecules under study for the designated time. Generally, the same method described by the authors previously for mammalian cells [39] was used; however, it was adapted for insect cells. After the incubation period, caspase-3/7 substrate was added to wells and cells incubated for 20 min at 22 °C. The luminescent signal was measured in a microplate reader (Cytation 3, BioTek, Winooski, VT, USA), and was performed in duplicate in three independent experiments.
4.4.5. Statistical Analysis
For biological assays, the Shapiro–Wilks normality test was performed in the data to ensure that it followed a normal distribution. Comparison between the means of controls and each experimental condition was performed using ANOVA. Outliers were identified by the Grubbs’ test. Data was expressed as the mean ± standard deviation (SD) of at least three independent experiments. GraphPad Prism 7.0 software was used, and values were considered statistically significant when p < 0.05.
5. Conclusions
Overall, with the present work it was demonstrated that medicinal chemistry approaches are valid strategies for obtaining new semisynthetic derivatives of a natural essential oil, which can act as promising alternatives to the available synthetic insecticides. Having started with a natural molecule devoid of activity, eugenol 1, it was possible to obtain several molecules with enhanced activity, some of them highly potent at concentrations as low as 100 μg/mL. The most potent molecules were shown to elicit morphological changes compatible with some processes of programmed cell death, being also capable of increasing the activity of serine proteases pivotal to some of these death pathways, notably caspases.
Finally, it was showed that ethyl 4-(2-methoxy-4-(oxiran-2-ylmethyl)phenoxy)butanoate 3e exhibits a more favorable safety profile towards human cells than that of the commercial insecticide chlorpyrifos, thus paving the way for the design of new alternatives to insecticides currently in use.
Abbreviations
| EOs | Essential oils |
| TLC | Thin-layer chromatography |
| NMR | Nuclear magnetic resonance |
| HRMS | High-resolution mass spectrometry |
| Sf9 | Spodoptera frugiperda |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/23/9257/s1.
Author Contributions
Conceptualization, M.S.T.G., A.G.F., and D.M.P.; methodology, M.J.G.F., M.S.T.G., A.G.F., D.M.P., and R.B.P.; formal analysis, M.J.G.F., M.S.T.G., A.G.F., D.M.P., and R.B.P.; investigation, M.J.G.F., D.M.P., and R.B.P.; supervision: M.S.T.G., A.G.F., and D.M.P.; writing—original draft preparation, M.J.G.F., M.S.T.G., and D.M.P.; writing—review and editing, M.J.G.F., M.S.T.G., D.M.P., A.G.F., E.M.S.C., and R.B.P.; project administration, M.S.T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by COMPETE 2020 program, co-financed by the FEDER and the European Union, PTDC/ASP-AGR/30154/2017 (POCI-01-0145-FEDER-030154). The authors also acknowledge the Foundation for Science and Technology (FCT; Portugal), and FEDER-COMPETE/QREN-EU for financial support to the research centers CQ/UM (UIDB/00686/2020), CF-UM-UP (UIDB/04650/2020) and REQUIMTE (UIDB/50006/2020). The NMR spectrometer Bruker Avance III 400 is part of the National NMR Network and was purchased within the framework of the National Program for Scientific Re-equipment, contract REDE/1517/RMN/2005, with funds from POCI 2010 (FEDER) and the FCT. The authors would also like to thank RIAIDT-USC for the use of their analytical facilities.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pino-Otín M.R., Ballestero D., Navarro E., González-Coloma A., Val J., Mainar A.M. Ecotoxicity of a novel biopesticide from Artemisia absinthium on non-target aquatic organisms. Chemosphere. 2019;216:131–146. doi: 10.1016/j.chemosphere.2018.09.071. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro I.A.T.A., Silva R., Silva A.G., Milet-Pinheiro P., Paiva P.M.G., Navarro D.M.A.F., Silva M.V., Napoleao T.H., Correia M.T.S. Chemical characterization and insecticidal effect against Sitophilus zeamais (maize weevil) of essential oil from Croton rudolphianus leaves. Crop Prot. 2020;129:105043. doi: 10.1016/j.cropro.2019.105043. [DOI] [Google Scholar]
- 3.Sharma A., Shukla A., Attri K., Kumar M., Kumar P., Suttee A., Singh G., Barnwal R.P., Singla N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020;201:110812. doi: 10.1016/j.ecoenv.2020.110812. [DOI] [PubMed] [Google Scholar]
- 4.Peres M.C., de Souza Costa G.C., dos Reis L.E.L., da Silva L.D., Peixoto M.F., Alves C.C.F., Forim M.R., Quintela E.D., Araújo W.L., Cazal C.M. In natura and nanoencapsulated essential oils from Xylopia aromatica reduce oviposition of Bemisia tabaci in Phaseolus vulgaris. J. Pest. Sci. 2020;93:807–821. doi: 10.1007/s10340-019-01186-6. [DOI] [Google Scholar]
- 5.Rong S., Xu H., Li L., Chen R., Gao X., Xu Z. Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic. Biochem. Physiol. 2020;162:69–77. doi: 10.1016/j.pestbp.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Lengai G.M.W., Muthomi J.W., Mbega E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020;7:e00239. doi: 10.1016/j.sciaf.2019.e00239. [DOI] [Google Scholar]
- 7.Leong W.-H., The S.-Y., Hossain M.M., Nadarajaw T., Zabidi-Hussin Z., Chin S.-Y., Lai K.-S., Lim S.-H.E. Application, monitoring and adverse effects in pesticide use: The importance of reinforcement of Good Agricultural Practices (GAPs) J. Environ. Manag. 2020;260:109987. doi: 10.1016/j.jenvman.2019.109987. [DOI] [PubMed] [Google Scholar]
- 8.Mfarrej M.F.B., Rara F.M. Competitive, sustainable natural pesticides. Acta Ecol. Sin. 2019;39:145–151. doi: 10.1016/j.chnaes.2018.08.005. [DOI] [Google Scholar]
- 9.Cantrell C.L., Dayan F.E., Duke S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012;75:1231–1242. doi: 10.1021/np300024u. [DOI] [PubMed] [Google Scholar]
- 10.Zhi X.-Y., Jiang L.-Y., Li T., Song L.-L., Wang Y., Cao H., Yang C. Semisynthesis and insecticidal bioactivities of benzoxazole and benzoxazolone derivatives of honokiol, a naturally occurring neolignan derived from Magnolia officinalis. Bioorg. Med. Chem. Lett. 2020;30:27086. doi: 10.1016/j.bmcl.2020.127086. [DOI] [PubMed] [Google Scholar]
- 11.Yang G.-Z., Zhang J., Peng J.-W., Zhang Z.-J., Zhao W.-B., Wang R.-X., Ma K.-Y., Li J.-C., Liu Y.-Q., Zhao Z.-M., et al. Discovery of luotonin A analogues as potent fungicides and insecticides: Design, synthesis and biological evaluation inspired by natural alkaloid. Eur. J. Med. Chem. 2020;194:112253. doi: 10.1016/j.ejmech.2020.112253. [DOI] [PubMed] [Google Scholar]
- 12.Ntie-Kang F., Njume L.E., Malange Y.I., Günther S., Sippl W., Yong J.N. The Chemistry and biological activities of natural products from Northern African plant families: From Taccaceae to Zygophyllaceae. Nat. Prod. Bioprospect. 2016;6:63–96. doi: 10.1007/s13659-016-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavela R., Maggi F., Iannarelli R., Benelli G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019;193:236–271. doi: 10.1016/j.actatropica.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Salman M., Abbas R.Z., Israr M., Abbas A., Mehmood K., Khan M.K., Sindhu Z.D., Hussaind R., Saleemie M.K., Shaha S. Repellent and acaricidal activity of essential oils and their components against Rhipicephalus ticks in cattle. Vet. Parasitol. 2020;283:109178. doi: 10.1016/j.vetpar.2020.109178. [DOI] [PubMed] [Google Scholar]
- 15.Saroj A., Oriyomi O.V., Nayak A.K., Haider S.Z. Phytochemicals of plant-derived essential oils: A novel green approach against pests. In: Egbuna C., Sawicka B., editors. Natural Remedies for Pest, Disease and Weed Control. Elsevier Science; Amsterdam, The Netherlands: 2020. pp. 65–79. [DOI] [Google Scholar]
- 16.Matos L.F., Barbosa D.R.S., Lima E.C., Dutra K.A., Navarro D.M.A.F., Alves J.L.R., Silva G.N. Chemical composition and insecticidal effect of essential oils from Illicium verum and Eugenia caryophyllus on Callosobruchus maculatus in cowpea. Ind. Crops Prod. 2020;145:112088. doi: 10.1016/j.indcrop.2020.112088. [DOI] [Google Scholar]
- 17.Vargas-Méndez L.Y., Sanabria-Flórez P.L., Saavedra-Reyes L.M., Merchan-Arenas D.R., Kouznetsov V.V. Bioactivity of semisynthetic eugenol derivatives against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae infesting maize in Colombia. Saudi J. Biol. Sci. 2019;26:1613–1620. doi: 10.1016/j.sjbs.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akhtar Y., Yeoung Y.-R., Isman M.B. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusia ni and Pseudaletia unipuncta. Phytochem. Rev. 2008;7:77–88. doi: 10.1007/s11101-006-9048-7. [DOI] [Google Scholar]
- 19.Pavela R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015;76:174–187. doi: 10.1016/j.indcrop.2015.06.050. [DOI] [Google Scholar]
- 20.Mazzeo P.P., Carraro C., Monica A., Capucci D., Pelagatti P., Bianchi F., Agazzi S., Careri M., Raio A., Carta M., et al. Designing a palette of cocrystals based on essential oil constituents for agricultural applications. ACS Sustain. Chem. Eng. 2019;7:17929–17940. doi: 10.1021/acssuschemeng.9b04576. [DOI] [Google Scholar]
- 21.Yu Z., Tang J., Khare T., Kumar V. The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue? Fitoterapia. 2020;140:104433. doi: 10.1016/j.fitote.2019.104433. [DOI] [PubMed] [Google Scholar]
- 22.Dianez F., Santos M., Parra C., Navarro M.J., Blanco R., Gea F.J. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018;67:400–410. doi: 10.1111/lam.13053. [DOI] [PubMed] [Google Scholar]
- 23.Hammer K.A., Carson C.F., Riley T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L., Liang X., Ou Z., Ye M., Shi Y., Chen Y., Zhao J., Zheng D., Xiang H. Screening of chemical composition, anti-arthritis, antitumor and antioxidant capacities of essential oils from four Zingiberaceae herbs. Ind. Crops Prod. 2020;149:112342. doi: 10.1016/j.indcrop.2020.112342. [DOI] [Google Scholar]
- 25.Koul O., Walia S., Dhaliwal G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008;4:63–84. [Google Scholar]
- 26.Mossa A.T.H. Green pesticides: Essential oils as biopesticides in insect-pest management. J. Environ. Sci. Technol. 2016;9:354–378. doi: 10.3923/jest.2016.354.378. [DOI] [Google Scholar]
- 27.Bhavaniramya S., Vishnupriya S., Al-Aboody M.S., Vijayakumar R., Baskaran D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019;2:49–55. doi: 10.1016/j.gaost.2019.03.001. [DOI] [Google Scholar]
- 28.Kaufman T.S. The multiple faces of eugenol. A versatile starting material and building block for organic and bio-organic synthesis and a convenient precursor toward bio-based fine chemicals. J. Braz. Chem. Soc. 2015;26:1055–1085. doi: 10.5935/0103-5053.20150086. [DOI] [Google Scholar]
- 29.Teixeira R.R., Gazolla P.A.R., Silva A.M., Borsodi M.P.G., Bergmann B.R., Ferreira R.S., Vaz B.G., Vasconcelos G.A., Lima W.P. Synthesis and leishmanicidal activity of eugenol derivatives bearing 1,2,3-triazole functionalities. Eur. J. Med. Chem. 2018;146:274–286. doi: 10.1016/j.ejmech.2018.01.046. [DOI] [PubMed] [Google Scholar]
- 30.Singh N., Rao A.S., Nandal A., Kumar S., Yadav S.S., Ganaie S.A., Narasimhan B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl—A versatile spice used in food and nutrition. Food Chem. 2020;338:127773. doi: 10.1016/j.foodchem.2020.127773. [DOI] [PubMed] [Google Scholar]
- 31.Wang L., Zhang Y. Eugenol nanoemulsion stabilized with zein and sodium caseinate by self-assembly. J. Agric. Food Chem. 2017;65:2990–2998. doi: 10.1021/acs.jafc.7b00194. [DOI] [PubMed] [Google Scholar]
- 32.Silva F.F.M., Monte F.J.Q., Lemos T.L.G., Nascimento P.G.G., Costa A.K.M., Paiva L.M.M. Eugenol derivatives: Synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem. Cent. J. 2018;12:34. doi: 10.1186/s13065-018-0407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ju J., Xie Y., Yu H., Guo Y., Cheng Y., Qian H., Yao W. Analysis of the synergistic antifungal mechanism of eugenol and citral. LWT Food Sci. Technol. 2020;123:109128. doi: 10.1016/j.lwt.2020.109128. [DOI] [Google Scholar]
- 34.Novato T., Gomes G.A., Zeringóta V., Franco C.T., Oliveira D.R., Melo D., Carvalho M.G., Daemon E., Monteiro C.M.O. In vitro assessment of the acaricidal activity of carvacrol, thymol, eugenol and their acetylated derivatives on Rhipicephalus microplus (Acari: Ixodidae) Vet. Parasitol. 2018;260:1–4. doi: 10.1016/j.vetpar.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Chen C.-H., Tung S.-H., Jeng R.-J., Abu-Omar M.M., Lin C.-H. A facile strategy to achieve fully bio-based epoxy thermosets from eugenol. Green Chem. 2019;21:4475–4488. doi: 10.1039/C9GC01184F. [DOI] [Google Scholar]
- 36.Shu B., Zhang J., Sethuraman V., Cui G., Yi X., Zhong G. Transcriptome analysis of Spodoptera frugiperda Sf9 cells reveals putative apoptosis-related genes and a preliminary apoptosis mechanism induced by azadirachtin. Sci. Rep. 2017;7:13231. doi: 10.1038/s41598-017-12713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidhu G.K., Singh S., Kumar V., Dhanjal D.S., Datta S., Singh J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019;49:1135–1187. doi: 10.1080/10643389.2019.1565554. [DOI] [Google Scholar]
- 38.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chouiter M.I., Boulebd H., Pereira D.M., Valentão P., Andrade P.B., Belfaitah A., Silva A.M.S. New chalcone-type compounds and 2-pyrazoline derivatives: Synthesis and caspase-dependent anticancer activity. Future Med. Chem. 2020;12:493–509. doi: 10.4155/fmc-2019-0342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.