Neuromedin U promotes immune cell activation and cytokine production, and contributes to several inflammatory disorders. The inflammatory roles of NmU have been indicated to be neurogenic or neuron‐independent. Understanding the potential roles of NmU in immunity and inflammation could be beneficial for the development of novel therapies for NmU‐involving diseases.
Keywords: immunity, inflammation, neuromedin U, NmUR1, NmUR2
Summary
Since the discovery of neuromedin U (NmU) from porcine spinal cord in 1985, this neuropeptide has been subsequently identified in many other species with multiple physiological and pathophysiological roles detected, ranging from smooth muscle contraction, feeding, energy balance to tumorigenesis. Intriguingly, NmU is also emerging to play pro‐inflammatory roles involving immune cell activation and cytokine release in a neuron‐dependent or neuron‐independent manner. The NmU‐mediated inflammatory responses have already been observed in worm infection, sepsis, autoimmune arthritis and allergic animal models. In this review, we focus on the roles of NmU in immunity and inflammation by highlighting the interactions between NmU and immune cells, summarizing the signalling mechanism involved in their reactions and discussing its potential contributions to inflammatory diseases.
Abbreviations
- CFA
complete Freund's adjuvant
- CGRP
calcitonin gene‐related peptide
- CNS
central nervous system
- CTMCs
connective tissue‐type mast cells
- ERK
extracellular signal‐regulated kinases
- GHSR
growth hormone secretagogue receptor
- GIT
gastrointestinal tract
- hsMCs
human skin‐derived mast cells
- ILC2
group 2 innate lymphoid cell
- LPS
lipopolysaccharide
- MAPK
mitogen‐activated protein kinase
- MEK
mitogen‐activated protein kinase kinase
- MRGPR
Mas‐related G protein‐coupled receptor
- NFAT
nuclear factor of activated T cells
- NmU
neuromedin U
- NmUR1
neuromedin U receptor 1
- NmUR2
neuromedin U receptor 2
- NTSR
neurotensin receptor
- PI3 K
phosphoinositide 3‐kinase
- PLC
phospholipase C
- PMC
peritoneal mast cell
- PSGL‐1
P‐selectin glycoprotein ligand‐1
- SP
substance P
- TLR
Toll‐like receptor
- TRPV3
transient receptor potential vanilloid 3
Introduction
Neuromedin U (NmU) is a group of structurally conserved neuropeptides, belonging to neuromedin superfamily. 1 Four groups of neuromedins have been identified based on their structures and functions, including the bombesin‐like (NmB and NmC), the kassinin‐like (NmK and NmL) and the neurotensin‐like (NmN) peptides and the neuromedin U group (NmU and NmS). 2 Different from other neuromedins, all NmU members, except in carp and goldfish, contain an identical C‐terminal pentapeptide (‐Phe‐Arg‐Pro‐Arg‐Asn‐NH2). The discovery of NmU commenced from the identification of two different versions of this peptide, NmU‐8 and NmU‐25, from porcine spinal cord, with the suffix U denoting their potent contractile effect on rat uterus. 3 Meanwhile, the hypertensive effect of NmU in rats was also observed. 3 , 4 Following the further investigations, multiple forms of NmU in various species, including fish, birds, amphibians and other mammals, have been discovered. 1 In humans, NmU peptide consists of 25 amino acid residues with the same C‐terminal octapeptide (‐Tyr‐Phe‐Leu‐Phe‐Arg‐Pro‐Arg‐Asn‐NH2) to that in porcine. 5 Two G protein‐coupled receptors have been recognized as the major receptors for NmU, designated as neuromedin U receptor 1 (NmUR1) and neuromedin U receptor 2 (NmUR2). 6 NmUR1 is mainly expressed in peripheral tissues, while NmUR2 is primarily detected in the central nervous system (CNS). 6 In addition to inducing smooth muscle contraction, pleiotropic roles of NmU have been detected including control of blood pressure, stress response, food intake, metabolic homeostasis, hormone release, circadian rhythm and tumorigenesis. 2 , 7 Recently, NmU peptides have also been shown to contribute to inflammation with roles in both innate and adaptive immune responses. 8 , 9 , 10 , 11
Neurogenic inflammation is commonly defined as an inflammatory phenomenon initiated by neuropeptides and/or neurotransmitters derived from sensory neurons. 12 NmU‐producing neurons have been located anatomically in the vicinity of immune cells such as group 2 innate lymphoid cells (ILC2s) and T cells in some tissues such as the lung and gut, and NmU peptides released from afferent neurons have been demonstrated to activate immune cells directly, indicating a role of NmU in neurogenic inflammation. 11 However, NmU can also be produced by non‐neuronal cells, including epidermal keratinocytes and immune cells, suggesting a neuron‐independent inflammatory responses in an autocrine or paracrine manner. 8 , 9 , 13 So far, several papers have discussed the general structure, distribution and functions of NmU. This review will focus on the role of NmU in immunity and inflammation, elucidating the effects of NmU on immune cells and the potential signalling mechanisms mediated by NmU in inflammatory responses and diseases.
The structure of NmU
Sequence analysis confirmed that the NmU precursor is a 174‐amino acid polypeptide in human being and rat, with 74% homology between the species. 5 , 14 Both precursors include a characteristic secretory signal sequence and several paired dibasic amino acids, which serve as putative enzymatic cleavage sites. 5 , 14 , 15 The sequence between the last two cleavage sites near the C‐terminus contains the NmU peptide, 23 residues in rat and 25 in human being. 5 , 14
After first discovery of NmU from porcine spinal cord in 1985, 3 the peptides have been identified in other species, including goldfish, 16 zebrafish, 17 pufferfish, 18 carp, 19 frog, 20 tree frog, 21 toad, 22 Japanese quail, 23 chicken, 24 , 25 rat, 26 , 27 guinea‐pig, 28 rabbit, 29 canine, 30 and human being. 5 Based on the length of the peptides, NmU can be generally divided into two forms: the longer form (NmU‐17 to 40) and the short or truncated form (NmU‐8 to 9) (Fig. 1). The short form is considered as the cleaved products of their longer NmU analogues except in guinea‐pig where no longer isoforms have been found. 3 , 25 , 28 , 30 The amino acid sequences of NmU are significantly conserved through evolution, indicating critical functional roles for the peptides. The highly homologous and asparagine amidated C‐terminal region is vital for receptor binding and function, whereas the variable N‐terminus controls the potency of the protein. 3 These structure–activity relationships are supported by the evidence that point mutations or modifications to the porcine NmU‐8 can cause drastic reduction in smooth muscle contraction activity or receptor binding affinity. 31 , 32 Furthermore, porcine des‐amido‐NmU‐8 lost its activity on smooth muscle and blood pressure, 3 while, non‐amidated NmU‐8 was unable to activate the receptor. 8 , 32 Compared with NmU‐8, porcine NmU‐25 induced more reinforced and repeated effect on rat uterus contractile activity. 3 Some residues such as Tyr‐Gln‐Gly‐Pro and Ser‐Gly‐Gly at the N‐terminus in rat have been proved to be especially important for the enhancement of the biological activity. 33 , 34
Figure 1.
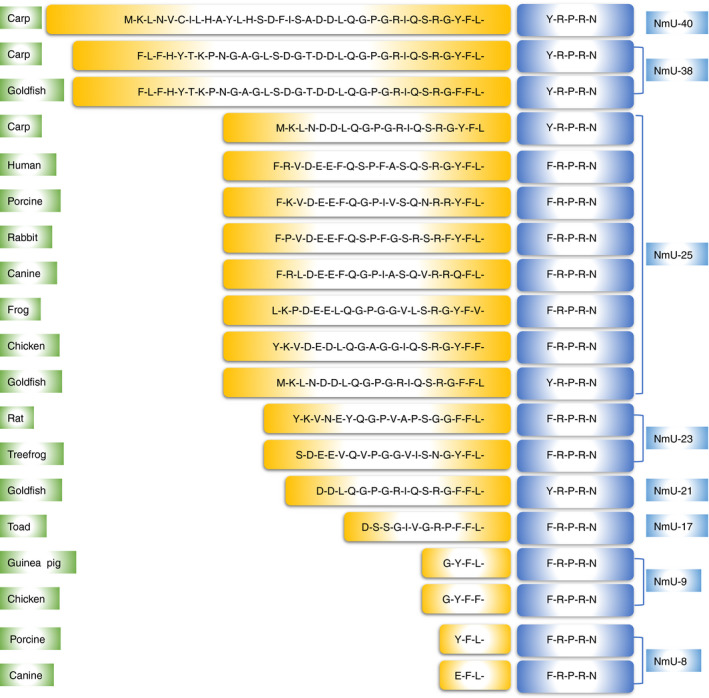
Amino acid sequences of NmU among different species. The highly conserved C‐terminus is highlighted in blue, and other residues are labelled in yellow.
The receptors of NmU
Two major NmU receptors have been established, although some other putative receptors may exist. NmUR1 (also designated as GPR66 or FM‐3) was first cloned based on the high (46–47%) DNA sequence homology to human growth hormone secretagogue receptor (GHSR) and neurotensin receptor (NTSR). 35 , 36 Subsequently, NmUR2 (also known as TGR‐1 or FM‐4) was discovered on the basis of its approximately 50% amino acid similarity with NmUR1. 6 , 37 , 38 , 39 These receptors have been identified in human being, murine, chicken and goldfish, 40 , 41 , 42 but not in all species studied so far.
Several G protein subtypes have been reported to get involved in the signal transduction by NmURs. NmUR1 and NmUR2 from human being and mouse are coupled to Gαq/11 when the receptors were overexpressed in several cell lines. 40 Binding of NmU to either receptors caused influx of Ca2+. 6 , 8 , 38 , 39 , 43 , 44 However, Gαi‐coupling was also reported in the studies with NmUR1/NmUR2‐overexpressed CHO or HEK293 cells where cAMP level was slightly decreased after the activation of the receptors. 38 , 45 Furthermore, a chimeric G protein study demonstrated that NmUR1 was preferentially coupled to Gαq, but NmUR2 mainly to Gαi. 46 Therefore, NmUR1/NmUR2 signalling could be potentially transducted through either Gαq or Gαi depending on certain physiological conditions.
Besides NmUR1/2, NmU could also interact with other potential receptors. In a complete Freund’s adjuvant (CFA)‐induced inflammatory model and an autoantibody‐induced arthritis study, the effect of NmU was independent of NmUR1 and NmUR2. 47 , 48 , 49 In a non‐small‐cell lung cancer study, the interaction of human NmU‐25 with GHSR1b/NTSR1 via a Gαs signalling was suggested. 36 NmU has also been reported to induce degranulation of human skin‐derived mast cells (hsMCs) through Mas‐related G protein‐coupled receptor X2 (MRGPRX2), as well as mouse connective tissue‐type mast cells (CTMCs) through Mrgprb2, a mouse analogue of MRGPRX2. 50 However, whether NmU binds to these putative receptors directly and with what binding affinity remain unclear. With further studies on NmU, additional receptors are likely to be discovered in the future.
The distribution of NmU and its major receptors
In general, NmU peptides appear to be widely produced in many tissues, organs and cells, with the highest levels detected in gastrointestinal tract (GIT) and CNS, 5 , 58 although the expression patterns vary between species (Table 1). The expression pattern of NmURs shows overlap with that of NmU, but NmUR1 is mainly expressed in peripheral tissues while NmUR2 is predominant in the CNS 6 , 8 , 37 , 43 , 59 , 60 , 61 , 62 (Table 1). In human being, NmUR1 is broadly expressed with high levels in the pancreas, testis, small intestines, 37 adipose 43 and cardiovascular tissues. 63 NmUR2 is preferentially expressed in specific regions of the brain, particularly in the hypothalamus, medulla oblongata, pituitary and spinal cord. 6 , 38 However, NmUR1 has also been detected in the CNS, for example the cerebellum, dorsal root ganglion and thalamus, although these findings are not consistent between studies. 6 , 32 , 37 , 61 Similarly, the expression of NmUR2 in peripheral tissues such as the gastrointestinal and genitourinary tract, with high levels in the testis, has also been reported. 39 , 62 Currently, the precise cell types that express NmURs in these organs and tissues remain uncertain.
Table 1.
Distribution of NmU, NmUR1 and NmUR2 in CNS and periphery of different species
| Objects | Species | Samples | Methods | Key findings | References |
|---|---|---|---|---|---|
| NmU | Rat, porcine, guinea‐pig, human being | CNS, gut | RIA | NmU occurred in ileum of all species, sacral spinal cord of rat, porcine and guinea‐pig, cerebral cortex of human being | Domin (1986) 51 |
| NmU | Rat | CNS, peripheral tissues | RIA | NmU was high in pituitary anterior lobe, small intestine, nucleus accumbens, septum, hypothalamus, sacral spinal cord, vas deferens, ureter, fallopian tube, urethra | Domin (1987) 52 |
| NmU was confined to submucosal muscular layers of small intestine | |||||
| NmU | Rat, guinea‐pig | Brain, intestine | RIA, IHC | In rats, NmU was highest in ileum and also stained in submucous and myenteric neurons of small intestine and in nerve fibres of these ganglionated plexuses | Augood (1988) 53 |
| NmU was seen in nerve terminals within ileum submucous and myenteric ganglionated plexuses of guinea‐pig | |||||
| NmU | Rat | Brain, GIT | ICC | NmU was restricted to nerves fibres in the submucous and myenteric plexuses and mucosa of all gut areas except stomach, and NmU was also seen in ganglion cells of both ganglionated plexuses | Ballesta (1988) 54 |
| In CNS, NmU was located in fibres of all brain regions except cerebellum and stained cells were confined to rostrocaudal part of arcuate nucleus | |||||
| NmU | Guinea‐pig | Small intestine | IHC | NmU was detected in myenteric and submucous nerve cells and in nerve fibres of these ganglionated plexuses | Furness (1989) 66 |
| NmU was co‐localized in neurons containing VIP, SP, NPY | |||||
| NmU | Pig | Small intestine | ICC | NmU presented coexistence with neurons containing SP and CGRP in the plexus submucous internus of small intestine | Timmermans (1989) 67 |
| NmU | Frog | CNS, peripheral tissues | RIA | NmU showed highest concentration in small intestine | Domin (1989) 20 |
| NmU | Rat | CNS, digestive tract | RIA, IHC | NmU was abundant in small intestine | Honzawa (1990) 55 |
| NmU was confined to enteric nervous system | |||||
| NmU | Pig | Small intestine | ICC | NmU was found in nerve fibres of submucosal ganglionic plexuses and coexisted with SP‐ and VIP‐containing neurons | Timmermans (1990) 68 |
| NmU | Rat | GIT | Northern blot | NmU was detected in all regions of GIT and highest in duodenum and jejunum | Austin (1994) 56 |
| NmU | Human being | GIT | Northern blot, RIA | NmU expression was similar throughout the GIT, and highest level of NmU was revealed in jejunum using RIA | Austin (1995) 5 |
| NmU, NmUR1 | Rat | CNS, peripheral tissues | PCR | NmU was significantly expressed in pituitary and small intestine | Fujii (2000) 59 |
| NmUR1 was obviously expressed in small intestine and lung | |||||
|
NmU, NmUR1 |
Human being | CNS, peripheral tissues | PCR | NmU showed high levels in intestine, pituitary, bone marrow and fetal liver | Szekeres (2000) 43 |
| NmUR1 was highest in adipose tissue, with moderate levels in spleen, intestine, bone marrow, lymphocytes and pancreas | |||||
| NmUR2 | Rat | CNS, peripheral tissues | PCR | NmUR2 was highest in uterus, with high levels in CNS, mainly in hypothalamus, medulla oblongata and spinal cord | Hosoya (2000) 38 |
| NmU, NmUR1, NmUR2 | Human being, rat | CNS, peripheral tissues | Northern blot, ISH | NmU and NmUR1 were high in the gut, NmUR1 was widely detected in periphery but cannot be detected in brain | Howard (2000) 37 |
| NmUR1 showed specific expression in the goblet cells of ileum, NmUR2 was restricted to specific regions of brain | |||||
| NmUR1, NmUR2 | Human being | CNS, peripheral tissues | PCR | NmUR1 was mainly expressed in peripheral tissues, especially in gastrointestinal and urogenital systems, with low levels in CNS such as cerebellum, DRG, hippocampus, spinal cord | Raddatz (2000) 6 |
| NmUR2 was high in CNS, particularly the medulla oblongata, pontine reticular formation, spinal cord and thalamus | |||||
| NmU, NmUR1 | Human being | CNS, peripheral tissues | Dot blot, Northern blot, PCR | NmU and NmUR1 were widely expressed, with highest levels in GIT, NmUR1 was extremely low in CNS | Hedrick (2000) 8 |
| NmU was detected in DCs, monocytes and B cells, NmUR1 was expressed by NK cells and T cells | |||||
| NmUR2 | Human being | CNS, peripheral tissues | Dot blot, Northern blot | NmUR2 was highly observed in testis and CNS, particularly spinal cord, medulla, corpus callosum, thalamus. Low levels of NmUR2 were also detected in GIT | Shan (2000) 39 |
| NmU, NmUR1, NmUR2 | Mouse | CNS, peripheral tissues | PCR | In CNS, NmU and NmUR2 were highly detected in medulla and spinal cord, but no NmUR1 expression | Funes (2002) 32 |
| In periphery, NmUR1 was widely expressed, with high levels in lung and various types of immune cells | |||||
| NmU | Rat | Brain | ISH | NmU was significantly expressed in pituitary pars tuberalis | Ivanov (2002) 58 |
| NmUR1, NmUR2 | Human being | Peripheral tissues | PCR | NmUR1 mRNA was highest in small intestine, and NmUR2 mRNA was highest in testis | Westfall (2002) 62 |
| NmU, NmUR2 | Mouse, rat | CNS, peripheral tissues | ISH | NmU and NMUR2 showed different expression patterns between mouse and rat in hypothalamus | Graham (2003) 60 |
| NmUR1, NmUR2 | Rat | CNS | ISH | NmUR1 was detected in DRG, and NmUR2 was expressed in laminae I and II | Yu (2003) 61 |
| NmUR1, NmUR2 | Rat, human being | CNS, peripheral tissues | PCR | NmUR1 was mainly in peripheral tissues with highest levels in adipose tissues | Garlton (2004) 57 |
| NmUR2 was predominantly in CNS with highest levels in hypothalamus, medulla oblongata, substantia nigra and thalamus | |||||
| NmUR1, NmUR2 | Human being | Cardiovascular tissues | PCR | NmU and NmUR1 were expressed in cardiovascular tissues | Mitchell (2009) 63 |
DRG, dorsal root ganglia; ICC, immunocytochemistry; IHC, immunohistochemistry; ISH, in situ hybridization; RIA, radioimmunoassay.
NmU‐producing sensory neurons could also produce other neuropeptides such as vasoactive intestine peptide, substance P (SP), neuropeptide Y and calcitonin gene‐related peptide (CGRP), or could be co‐localized with other types of neurons producing these neuropeptides. 11 , 68 Cholinergic neurons producing NmU in the GIT and lungs were detected in close proximity to immune cells, indicating the potential role of NmU in neuroimmune crosstalk and neurogenic inflammation. 11 , 64 , 65 , 69 , 70 , 71 Moreover, indirect evidence indicated the expression of NmUR2 in microglia and astrocytes from the mouse hippocampus, 72 which suggested the potential involvement of NmU in the regulation of CNS inflammation.
NmU and NmUR1 are also expressed in non‐nervous system. NmU mRNA has been detected in human bone marrow, spleen, dendritic cells, monocytes and B cells, 8 , 43 and significant levels of NmUR1 mRNA were also found in human bone marrow, spleen, lymphocytes, 11 , 64 , 65 platelets, 73 eosinophils 10 and mast cells. 13 NmUR1 expression has also been reported in mouse ILC2s, 11 eosinophils, 10 mast cells and macrophages, 9 , 13 and in murine Y‐16 cell line (IL‐5‐dependent) and Th2 cell line (D10.G4.1). 10 , 74 These non‐neuronal distributions provide opportunity for NmU to function as a neuron‐independent mediator in inflammation.
Signalling pathways mediated by NmURs
Several signalling pathways downstream of the activation of NmURs have been documented 36 , 38 , 45 , 50 , 59 , 75 and reviewed. 7 Based on existing reports, the potential signalling pathways used by NmU in inflammation are mainly mediated by NmUR1 (Fig. 2) and little is known about that mediated by other NmURs.
Figure 2.
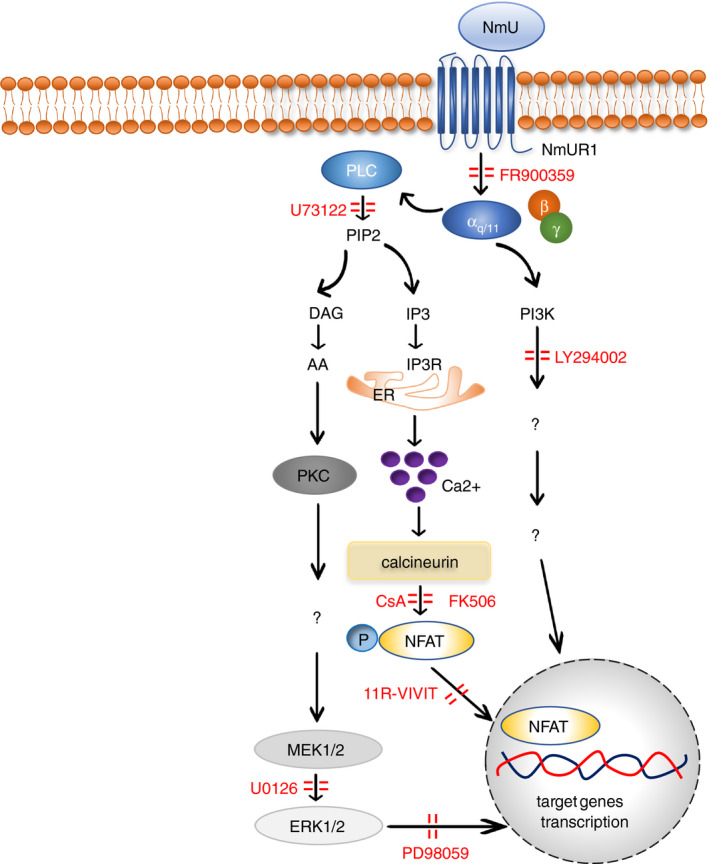
The signalling pathways of NmU in inflammation.
Ca2+/calcineurin/NAFT signalling pathway
It has been demonstrated that the NmU‐NmUR1 pathway mediates Gαq/11 signalling in cells, leading to the activation of phospholipase C (PLC), which catalyses conversion of PIP2 (phosphatidylinositol 4,5‐bisphosphate) into DAG (diacylglycerol) and IP3 (inositol trisphosphate). Subsequently, IP3 binding to its receptor on the endoplasmic reticulum will elicit Ca2+ influx into cytosol. 46 , 76 Liberated Ca2+ binds calmodulin, which activates calcineurin phosphatase. Activated calcineurin in turn dephosphorylates nuclear factor of activated T cells (NFAT), inducing its activation and translocation to the nucleus, whereupon it regulates gene transcription. 77 This calcium‐dependent calcineurin‐NFAT cascade downstream of NmU signalling has been observed in mouse ILC2s and murine Th2 cell line D.10.G4.1. 11 , 74 Preincubation of lymphocytes with selective Gαq/11 inhibitor FR900359 completely abrogated ILC2‐mediated cytokine production induced by NmU in mouse. 65 Preincubation of D10.G4.1 cells with PLC inhibitor U73122 or calcineurin inhibitor cyclosporin A (CsA) also resulted in a dose‐dependent reduction in pro‐inflammatory cytokine production. 74 Pretreatment of ILC2s with calcineurin inhibitor FK506 or CsA, and NFAT inhibitor 11‐VIVIT induced a remarkable diminution of type 2 cytokine release. 11 Taken together, these results suggested that Gαq/11‐mediated PLC/Ca2+/calcineurin/NFAT signalling pathway is critical for NmU‐stimulated cytokine production in immune cells.
Mitogen‐activated protein kinase signalling pathway
NmU‐induced cytokine secretion also depends on the activation of the mitogen‐activated protein kinase (MAPK) signalling pathway as the treatment with U0126, an inhibitor of mitogen‐activated protein kinase kinase (MEK) 1/2, caused significant reduction in the cytokine production in a dose‐dependent manner in D10.G4.1 cells. 74 In mouse ILC2s, administration of PD98059, an inhibitor of extracellular signal‐regulated kinases (ERK) at downstream of MEK, resulted in marked impairment of cytokine production induced by NmU. 11 Crosstalk between Ca2+/calcineurin/NFAT and MAPK signalling cascades in T‐cell activation has been suggested. 78 , 79 Thus, the MAPK signalling pathway may also be used by NmU to control cytokine production in immune cells.
Phosphoinositide 3 kinase signalling pathway
The phosphoinositide 3‐kinase (PI3K) signalling pathway acts as a critical modulator contributing to lymphocyte‐mediated responses. 80 The use of PI3K inhibitor LY294002 caused a concentration‐dependent decline in interleukin release in NmU‐treated D10.G4.1 cells, which demonstrated a pivotal role for PI3K signalling in NmU‐mediated cell activation. 74 NmU has been suggested to promote the secretion of IL‐6 but not TNF‐α from macrophages in lipopolysaccharide (LPS)‐induced endotoxaemia. 9 This macrophage‐derived IL‐6 production was partially dependent on PI3K phosphorylation. 81 Interaction between Ca2+/calcineurin/NFAT and PI3K signalling pathways in T‐cell activation has also been reported in other studies, 82 suggesting that such crosstalk between these pathways could also exist in NmU‐activated immune cells.
The pro‐inflammatory roles of NmU in immune cells
The close proximity between NmU‐producing sensory neurons and different immune cells, and the production of NmU by epidermal keratinocytes, endothelial cells and immune cells suggest potential involvement of this neuropeptide in immune responses. Based on previous reports, the regulatory effects of NmU on the immune systems have been summarized in Fig. 3 and Table 2.
Figure 3.
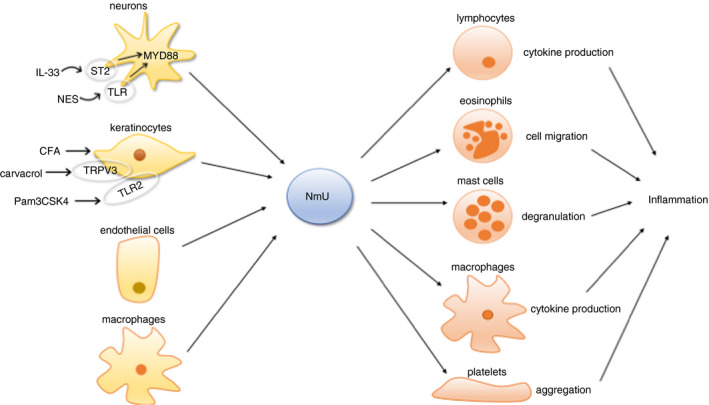
Role of NmU in immunity and inflammation.
Table 2.
Influence of NmU on immune cells and related inflammatory disorders
| Source of NmU | Target cell types | Receptors | Inflammatory mediators | Signalling transduction | Effect of NmU | Related diseases | References |
|---|---|---|---|---|---|---|---|
| – | Mouse Th2 cell line D10.G4.1 | NmUR1 | IL‐4, IL‐5, IL‐6, IL‐10, IL‐13 | Ca2+, PLC, calcineurin, MEK, PI3K | Promote Th2‐induced inflammation | Th2‐mediated disorders such as asthma | Johnson (2004) 74 |
| Keratinocytes | Mast cells | NmUR1 | TNF‐α, IL‐6, MIP‐2, ICAM‐1, β‐hexosaminidase | Ca2+ | Promote mast cell‐mediated inflammation | Hyperalgesia | Moriyama (2005) 13 |
| Macrophages | Macrophages | NmUR1 | IL‐6 | Ca2+, ERK, PI3K | Enhance IL‐6 production from macrophages | Endotoxaemia | Moriyama (2006) 9 |
| Sensory neurons or immune cells | Y‐16 cell line, eosinophils | NmUR1 | – | Ca2+, ERK | Promote eosinophil migration | Allergic disorders | Moriyama (2006) 10 |
| – | – | – | IL‐6 | – | No pro‐inflammatory effect | – | Abbondanzo (2009) 48 |
| Haematopoietic cells | – | – | – | Ca2+ | Promote autoantibody‐mediated inflammation | Arthritis | Rao (2012) 49 |
| Keratinocytes | – | – | IFN‐γ, IL‐4, IL‐23 | – | Negative regulator of allergic skin inflammation | Atopic dermatitis | Mizukawa (2016) 104 |
| Sensory neurons | ILC2s | NmUR1 | IL‐5, IL‐13, Areg, Csf | Ca2+‐calcineurin/NFAT, ERK | Fight against worm infection at early stage | Worm infection, lung inflammation | Cardoso (2017) 11 |
| Enteric cholinergic neurons | ILC2s | NmUR1 | IL‐5, IL‐9, IL‐13 | Gαq ‐dependent signalling | Promote ILC2‐mediated inflammation at mucosal sites | Worm infection, allergic inflammation | Klose (2017) 65 |
| Neurons | ILC2s | NmUR1 | IL‐5, IL‐13 | – | Mediate alarmin or allergen‐driven ILC2‐mediated allergic responses | Mucosal allergic disorders | Wallrapp (2017) 64 |
| Vascular endothelial cells | Platelets | NmUR1 | P‐selectin | Ca2+, P2Y12 signalling | Enhance human platelet activation and aggregation | Platelet‐related diseases | Grippi (2017) 73 |
| Keratinocytes | Mast cells | Mrgprb2, MRGPRX2 | β‐Hexosaminidase | – | Induce mast cell activation | Skin allergic diseases | Matsuo (2018) 50 |
Areg, amphiregulin; Csf2, colony‐stimulating factor 2; ICAM‐1, intracellular cell adhesion molecule 1; MIP‐2, macrophage inflammatory protein 2.
The potential role of NmU in lymphocytes
The expression of NmUR1 has been detected in several types of lymphocytes, including T cells, NK cells and ILC2s. 8 , 11 , 64 Up to now, the functional studies of NmU in these cells were mainly conducted in mice. NmU‐NmUR1 signalling promoted cytokine production, such as IL‐4, IL‐5, IL‐6, IL‐10 and IL‐13, in a murine Th2 cell line (D10.G4.1) and ILC2s. 65 , 74 , 83 In mouse GIT and lungs, NmU‐producing neurons were in close vicinity to NmUR1‐expressing ILC2s. 11 Stimulation of ILC2s with NmU in combination of IL‐25 enhanced rapid secretion of type 2 cytokines both in vivo and in vitro, whereas knockout of NmUR1 impaired the type 2 cytokine release in such stimulation, indicating the critical role of NmU‐NmUR1 axis in neuron/ILC2s crosstalk. 11 , 64 , 65 NmU not only induced pro‐inflammatory type 2 cytokine production, but also promoted ILC2 proliferation, as measured by the upregulation of Ki67, a biomarker of proliferation. 11 , 64 , 65
The potential role of NmU in mast cells
In mouse model, the mRNA levels of NmUR1 in mouse bone marrow‐derived mast cells and peritoneal mast cells (PMCs) were as high as that in the mouse intestine. Administration of NmU resulted in the intracellular Ca2+ mobilization and degranulation of PMCs in a concentration‐dependent manner. 13 The pro‐inflammatory role of NmU through direct activating mast cells was evidenced in the mouse treated with intraplantar injection of CFA where CFA induced intense and rapid production of NmU from keratinocytes, which in turn promoted degranulation and pro‐inflammatory mediator/cytokine production of mast cells, resulting in skin oedema. 13 These immune responses were abolished by mast cell deficiency or NmU deficiency. 13
Compared with bone marrow‐derived mast cells and PMCs, mouse CTMCs expressed relatively lower level of NmUR1 but higher level of Mrgprb2. Similarly, hsMCs were found to express high level of MRGPRX2 but not NmUR1. 50 Although low or no NMUR1 was expressed in mouse CTMCs and hsMCs, NmU‐induced degranulation of these cells was in a dose‐dependent manner, as detected by the release of β‐hexosaminidase. 50 In cultured keratinocytes from human skin, activation of Toll‐like receptor (TLR) 2 with Pam3CSK4, a synthetic triacylated lipopeptide, or activation of transient receptor potential vanilloid 3 (TRPV3) with carvacrol, a plant‐derived phenol, triggered release of NmU. 50 Therefore, keratinocyte/NmU/mast cell axis could play important role in skin inflammation. As several receptors could mediate the effects of NmU in different mast cells, the function of NmU might be heterogeneous in mast cells derived from different tissues and species. 84
The potential role of NmU in eosinophils
Neuromedin U has also been suggested to be an important player in the eosinophilic inflammation. NmUR1 mRNA was detected in a murine eosinophil cell line Y‐16 and human peripheral blood eosinophils, and NmU could directly activate these cells by inducing cell adhesion to the components of extracellular matrix (fibronectin and collagen type I) or cell migration. 10 In allergic mouse model, OVA administration upregulated the level of NmU in the lungs and induced airway eosinophilia. NmU knockout reduced the enrichment of eosinophils without attenuating airway hyperresponsiveness. 10 In contrast, CGRP knockout in the same animal model significantly decreased airway hyperresponsiveness without affecting airway eosinophil infiltration. 85 These observations may ascribe that CGRP stimulates airway smooth muscles and epithelial cells while NmU targets eosinophils. 10 , 85
As human eosinophils have been previously reported to be the source of vasoactive intestine peptide and SP, 86 whether eosinophils could also produce NmU is still yet to be determined.
The potential role of NmU in macrophages
Being populated in tissues, macrophages function as resident phagocytic cells involving in both tissue homeostasis and inflammation. 87 Macrophages express neuropeptide receptors, such as SP receptor NK‐1 and NmU receptor NmUR1, and are believed to be involved in the regulation of neuropeptide‐induced inflammatory responses. 9 , 88 , 89 Macrophages are also able to produce NmU. 9 , 90 Lipopolysaccharide stimulation induced upregulation of NmU but downregulation of NmUR1 in macrophages, whereas in NmU‐knockout macrophages, no downregulation of NmUR1 was detected, indicating an interaction between NmU and NmUR1 via an autocrine or paracrine manner in macrophages. 9 In NmU‐deficient macrophages, the release of IL‐6 but not TNF‐α was obviously decreased after LPS administration. 9 This may be attributed to the fact that two different signalling mechanisms are involved in the release of TNF‐α and IL‐6 in macrophages, and IL‐6 production induced by LPS is regulated by the NmU/NmUR1‐dependent signalling. 9 , 48 , 81
The potential role of NmU in platelets
Platelets are traditionally regarded as anucleate cells that play critical roles in the regulation of immune haemostasis, and currently recognized as a regulator in both innate and adaptive immune responses. 91 , 92 Injured human endothelial cells could produce NmU, which enhanced platelet aggregation induced by ADP. 63 , 73 NmUR1 was found to be expressed in human platelets, and the activation of platelets by the combination of NmU and ADP could be proved by the upregulation of P‐selectin. 73 As one of the main pro‐inflammatory modulators released by platelets, P‐selectin exerts important roles in the platelet–leucocyte aggregation and leucocyte–epithelium adhesion. 91 Several types of immune cells including haematopoietic progenitor cells, monocytes, neutrophils and eosinophils express the receptor for P‐selectin, P‐selectin glycoprotein ligand‐1 (PSGL‐1 or CD162), 93 , 94 further supporting the potential of interaction between platelet and immune cells mediated by P‐selectin. Although, there is no direct evidence so far to confirm the relationship between NmU/ADP‐stimulated platelets and PSGL‐1‐expressing immune cells, it is not difficult to conduct such interaction in vitro, even more, the binding of P‐selectin to platelet‐activated factor‐activated monocytes has already been reported. 95
NmU‐related host defence and inflammatory disorders
NmU in worm infection and atopic inflammation
Type 2 immunity plays critical role in host defence against helminth infection or atopic inflammation such as asthma, dermatitis, rhinitis and other allergic disorders. 96 , 97 Type 2 cytokines produced by type 2 cells such as Th2, Tc2 and ILC2s are considered as important regulators for the recruitment and activation of mast cells, basophils and eosinophils and B‐cell class switching towards IgE production. 96 , 97 NmU can be upregulated after worm infection or allergen exposure, leading to type 2 cell responses and eosinophil activation, 10 , 11 , 65 suggesting a potential bridging role for NmU between these inflammatory challenges and type 2 immunity.
In OVA‐induced asthma model, NmU enhanced the recruitment of eosinophils to the inflammatory areas, and NmU knockout attenuated the OVA‐induced airway eosinophilia. 10 NmU also augmented airway inflammation by promoting the maturation and proliferation of ILC2s, enhancing type 2 cytokine secretion from lung‐resident ILC2s and inducing enrichment of eosinophils in bronchoalveolar lavage and lung tissues. 65 Taken together, NmU could contribute to the pathogenesis of asthma via the interactions with eosinophils and ILC2s.
Peripheral tissues such as skin, muscle, lung and GIT are highly innervated by sensory neurons. 12 NmU released from sensory neurons has been demonstrated to provide protective effects on worm infection through the interactions between cholinergic neurons and immune cells. Helminth infection can stimulate alarmin cytokine IL‐33 production from epithelial cells, macrophage or dendritic cells, 98 and neurons can directly sense the excretory/secretory products from helminth parasite Nippostrongylus brasiliensis through TLR or respond to IL‐33 through its receptor ST2. 99 , 100 , 101 Activation of TLR and ST2 in neurons efficiently induced upregulation of NmU in a myeloid differentiation primary response 88 (the adaptor protein of IL‐33/ST2 and Nippostrongylus brasiliensis excretory/secretory products/TLR signalling pathway)‐dependent manner. 11 , 102 , 103 Deletion of myeloid differentiation primary response 88 abolished NmU production. 11 Upregulation of NmU by parasite infection was also confirmed by using other related nematode parasites Trichutis muris and Heligmosomoides polygyrus. 65 The effect of NmU on ILC2s in response to worm infection was mediated by NmUR1 as NmUR1 knockout not only resulted in the decrease in type 2 cytokine production from ILC2s but also increased worm infection burden. 11
The role of NmU in cutaneous inflammation has been hypothesized to be dual as a pro‐inflammatory mediator at early stage and as an anti‐inflammatory regulator at a later phase. 104 Initially, keratinocyte‐derived NmU has been shown to promote CFA‐induced mouse skin inflammation via NmUR1 in a mast cell‐dependent manner. 13 Subsequently, NmU has been demonstrated to be a negative regulator in skin inflammation at later stage as depletion of NmU from epidermis resulted in dry skin and increased scratching, which would act in concert with repeated hapten treatment to induce IgE‐mediated allergic inflammation. 104 Nevertheless, in another CFA‐induced cutaneous inflammatory model, NmUR1 knockout did not exert any effect on the immune responses, raising the argument on the role of NmU in this skin disorder. 48 Recently, it has been reported that other receptor of NmU besides NmUR1 may contribute to skin inflammation, 50 which was mainly based on the identification of NmU‐induced activation and degranulation of hsMCs through MRGPRX2.
NmU and endotoxaemia
Lipopolysaccharide, the major component of the cell wall in Gram‐negative bacteria, plays an important role in the endotoxin‐induced septic shock 105 . In LPS‐induced endotoxaemia in mice, NmU knockout mitigated degeneration of hepatocytes and formation of thrombosis, which further resulted in the reduction in multiorgan dysfunction and mortality. 9 Additionally, serum level of IL‐6 instead of TNF‐α, IL‐1β and IL‐12p40 was significantly decreased in NmU‐deficient mice after LPS administration, 9 which was considered as an important factor associated with the reduced mortality rate. 9 , 106 Although NmU/NmUR1 signalling was demonstrated to promote IL‐6 production from macrophages in LPS‐induced endotoxaemia, the detailed molecular mechanisms were still undetermined.
NmU and arthritis
NmU released from bone marrow‐derived cells has been shown to enhance the development of autoantibody‐induced arthritis, as the depletion of NmU exhibited a protective influence on the arthritis mouse model. 49 The pro‐inflammatory effect of NmU on arthritis was not attributed to the differentiation of immune cells as the frequency and ratio of leucocyte subsets and platelets remained unchanged after NmU knockout, but more likely to be mediated by the activation of multiple immune cells. 49 However, the exact pro‐inflammatory mechanisms of NmU in arthritis have not been defined. Although NmU knockdown showed benefit, the knockout of NmU receptors such as NmUR1, NmUR2 and NTSR1 did not alleviate autoantibody‐induced arthritis, indicating some unknown receptor might contribute to the role of NmU in arthritis. 49
It has been suggested that SP‐induced neurogenic inflammation was partially NmU‐dependent. 13 NmU‐expressing neurons were found to express SP or be co‐localized with SP‐containing neurons. 66 , 67 Increased levels of SP in synovial fluid and serum were reported to be associated with the patients with rheumatoid arthritis. 107 However, whether NmU and SP can collaborate with each other in the pathogenesis of arthritis remains unknown.
NmU and CNS inflammation
NmU has been demonstrated to promote nociception. 61 , 108 , 109 Intrathecal injection of NmU significantly increased the withdrawal reflex and nociceptive behaviours to noxious thermal stimuli in mice. 108 Meanwhile, mechanical allodynia and hyperalgesia were also observed after administration of NmU in rat spinal cord. 61 Pro‐nociceptive pain was NmUR2‐dependent as NmUR2 but not NmUR1 deficiency in mice led to compromised pain responses to noxious chemical and thermal stimuli. 47 , 110 The NmU‐induced hyperalgesia in the spinal cord was suggested to be caused by the enhanced synaptic transmission by NmU/NmUR2 interaction. 110 However, the mechanism mediating NmU‐associated neuropathic pain is still unclear. Given the crosstalk between neuropathic pain and chronic inflammation, 111 NmU could potentially play a promoting role in this neuropathic pain‐associated inflammation.
NmU has been reported to protect mouse from LPS‐induced memory damage and neuronal cell death. 72 This protective effect of NmU has been ascribed to the upregulation of brain‐derived neurotrophic factor from hippocampus‐derived microglia and astrocytes after NmU treatment. 72 However, the signalling mechanism involved in this process still remains unknown.
Conclusions
Since the discovery of NmU peptides in 1985, various studies have further identified the unique structure and ubiquitous distribution of NmU and their receptors. Meanwhile, the functional roles of NmU have also been investigated. It is worthy to note that, in addition to multiple physiological functions, NmU can promote inflammation through either neurogenic or neuron‐independent mechanisms. At the time of writing, most reports on NmU functions in inflammation have been mainly based on mouse models. We still know little on the role of NmU in the human immune system. A better understanding of NmU‐mediated inflammation may be beneficial for the development of novel therapies for NmU‐involving disorders such as asthma, septic shock, atopic dermatitis and rheumatoid arthritis.
Disclosure
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by grant from the NIHR Biomedical Research Centre Programme to L.X.
Data availability statement
No data were available as no data sets were generated or analysed during this article.
References
- 1. Brighton PJ, Szekeres PG, Willars GB. Neuromedin U and its receptors: structure, function, and physiology roles. Pharmacol Rev 2004; 56:231–48. [DOI] [PubMed] [Google Scholar]
- 2. Martinez VG, O’Driscoll L. Neuromedin U: a multifunctional neuropeptide with pleiotropic roles. Clin Chem 2015; 61:471–82. [DOI] [PubMed] [Google Scholar]
- 3. Minamino N, Kangawa K, Matsuo H. Neuromedin U‐8 and U‐25: novel uterus stimulating and hypertensive peptides identified in porcine spinal cord. Biochem Biophys Res Commun 1985; 130:1078–85. [DOI] [PubMed] [Google Scholar]
- 4. Minamino N, Sudoh T, Kangawa K. Neuromedins: novel smooth‐muscle stimulating peptides identified in porcine spinal cord. Peptides 1985; 6:245–8. [DOI] [PubMed] [Google Scholar]
- 5. Austin C, Lo G, Nandha KA, Meleagros L, Bloom SR. Cloning and characterization of the cDNA encoding the human neuromedin U (NmU) precursor: NmU expression in the human gastrointestinal tract. J Mol Endocrinol 1995; 14:157–69. [DOI] [PubMed] [Google Scholar]
- 6. Raddatz R, Wilson AE, Artymyshyn R, Bonini JA, Borowsky B, Boteju LW, et al Identification and characterization of two neuromedin U receptors differentially expressed in peripheral tissues and the central nervous system. J Biol Chem 2000; 275:32452–9. [DOI] [PubMed] [Google Scholar]
- 7. Przygodzka P, Soboska K, Sochacka E, Boncela J. Neuromedin U: a small peptide in the big world of cancer. Cancers (Basel) 2019; 11:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hedrick JA, Morse K, Shan L, Qiao X, Pang L, Wang S, et al Identification of a human gastrointestinal tract and immune system receptor for the peptide neuromedin U. Mol Pharmacol 2000; 58:870–5. [DOI] [PubMed] [Google Scholar]
- 9. Moriyama M, Matsukawa A, Kudoh S, Takahashi T, Sato T, Kano T, et al The neuropeptide neuromedin U promotes IL‐6 production from macrophages and endotoxin shock. Biochem Biophys Res Commun 2006; 341:1149–54. [DOI] [PubMed] [Google Scholar]
- 10. Moriyama M, Fukuyama S, Inoue H, Matsumoto T, Sato T, Tanaka K, et al The neuropeptide neuromedin U activates eosinophils and is involved in allergen‐induced eosinophilia. Am J Physiol Lung Cell Mol Physiol 2006; 290:L971–977. [DOI] [PubMed] [Google Scholar]
- 11. Cardoso V, Chesné J, Ribeiro H, García‐Cassani B, Carvalho T, Bouchery T, et al Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017; 549:277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001; 413:203–10. [DOI] [PubMed] [Google Scholar]
- 13. Moriyama M, Sato T, Inoue H, Fukuyama S, Teranishi H, Kangawa K, et al The neuropeptide neuromedin U promotes inflammation by direct activation of mast cells. J Exp Med 2005; 202:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lo G, Legon S, Austin C, Wallis S, Wang Z, Bloom SR. Characterization of complementary DNA encoding the rat neuromedin U precursor. Mol Endocrinol 1992; 6:1538–44. [DOI] [PubMed] [Google Scholar]
- 15. Bechtold DA, Ivanov TR, Luckman SM. Appetite‐modifying actions of pro‐neuromedin U‐derived peptides. Am J Physiol Endocrinol Metab 2009; 297:E545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maruyama K, Konno N, Ishiguro K, Wakasugi T, Uchiyama M, Shioda S, et al Isolation and characterisation of four cDNAs encoding neuromedin U (NMU) from the brain and gut of goldfish, and the inhibitory effect of a deduced NMU on food intake and locomotor activity. J Neuroendocrinol 2008; 20:71–8. [DOI] [PubMed] [Google Scholar]
- 17. Chiu CN, Rihel J, Lee DA, Vidal M, Schier AF, Prober DA, et al A zebrafish genetic screen identifies neuromedin U as a regulator of sleep/wake states. Neuron 2016; 89:842–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kono T, Ida T, Kawahara N, Watanabe F, Biswas G, Sato T, et al Identification and immunoregulatory function of neuromedin U (Nmu) in the Japanese pufferfish Takifugu rubripes. Dev Comp Immunol 2017; 73:246–56. [DOI] [PubMed] [Google Scholar]
- 19. Kono T, Hamasuna S, Korenaga H, Iizasa T, Nagamine R, Ida T, et al The role of neuromedin U during inflammatory response in the common carp. Fish Shellfish Immunol 2012; 32:151–60. [DOI] [PubMed] [Google Scholar]
- 20. Domin J, Yiangous Y, Spokes RA, Aitkenll A, Parmar KB, Chrysanthous BJ, et al The distribution, purification, and pharmacological action of an amphibian neuromedin U. J Biol Chem 1989; 264:20881–5. [PubMed] [Google Scholar]
- 21. Salmon AL, Johnsen AH, Bienert M, Mcmurray G, Nandha KA, Bloom SR, et al Isolation, structural characterization, and bioactivity of a novel neuromedin U analog from the defensive skin secretion of the Australasian tree frog, Litoria caerulea . J Biol Chem 2000; 275:4549–54. [DOI] [PubMed] [Google Scholar]
- 22. Lee WH, Liu SB, Shen JH, Jin Y, Lai R, Zhang Y. Identification and molecular cloning of a novel neuromedin U analog from the skin secretions of toad Bombina maxima . Regul Pept 2005; 129:43–7. [DOI] [PubMed] [Google Scholar]
- 23. Shousha S, Nakahara K, Miyazato M. Endogenous neuromedin U has anorectic effects in the Japanese quail. Gen Comp Endocrinol 2005; 140:156–63. [DOI] [PubMed] [Google Scholar]
- 24. O’Harte F, Bockman CS, Zeng W, et al Primary structure and pharmacological activity of a nonapeptide related to neuromedin U isolated from chicken intestine. Peptides 1991; 12:809–12. [DOI] [PubMed] [Google Scholar]
- 25. Domin J, Benito‐orfila MA, Nandha KA, Aitken A, Bloom SR. The purification and sequence analysis of an avian neuromedin U. Regul Pept 1992; 41:1–8. [DOI] [PubMed] [Google Scholar]
- 26. Minamino N, Kangawa K, Honzawa M, Matsuo H. Isolation and structural determination of rat neuromedin U. Biochem Biophys Res Commun 1988; 156:355–60. [DOI] [PubMed] [Google Scholar]
- 27. Conlon JM, Domin J, Dimarzo V, Morris HR, Bloom SR. Primary structure of neuromedin U from the rat. J Neurochem 1988; 51:988–91. [DOI] [PubMed] [Google Scholar]
- 28. Murphy R, Turner CAFJB. Isolation and microsequence analysis of a novel form of neuromedin U from guinea pig small intestine. Peptides 1990; 11:613–7. [DOI] [PubMed] [Google Scholar]
- 29. Kage R, Harte FO, Thim L, Conlon JM. Rabbit neuromedin U‐25: lack of conservation of a posttranslational processing site. Regul Pept 1991; 33:191–8. [DOI] [PubMed] [Google Scholar]
- 30. Oharte F, Bockman CS, Abel PW, Conlon JM. Isolation, structural characterization and pharmacological activity of dog neuromedin U. Peptides 1991; 12:11–5. [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto T, Masui H, Uchida Y, Sakura NOK. Agonistic and antagonistic activities of neuromedin U‐8 analogs substituted with glycine or D‐amino acid on contractile activity of chicken crop smooth muscle preparations. Chem Pharm Bull 1991; 39:2319–22. [DOI] [PubMed] [Google Scholar]
- 32. Funes S, Hedrick JA, Yang S, Shan L, Bayne M, Monsma FJ, et al Cloning and characterization of murine neuromedin U receptors. Peptides 2002; 23:1607–15. [DOI] [PubMed] [Google Scholar]
- 33. Okimura K, Sakura N, Ohta S, et al Contractile activity of porcine neuromedin U‐25 and various neuromedin U‐related peptide fragments on isolated chicken crop smooth muscle. Chem Pharm Bull 1992; 40:1500–3. [DOI] [PubMed] [Google Scholar]
- 34. Sakura N, Ohta S, Uchida Y, Kurosawa K, Okimura KHT. Structure‐activity relationships of rat neuromedin U for smooth muscle contraction. Chem Pharm Bull 1991; 39:2016–20. [DOI] [PubMed] [Google Scholar]
- 35. Tan CP, McKee KK, Liu Q, Palyha OC, Feighner SD, Hreniuk DL, et al Cloning and characterization of a human and murine T‐cell orphan G‐protein‐coupled receptor similar to the growth hormone secretagogue and neurotensin receptors. Genomics 1998; 52:223–9. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi K, Furukawa C, Takano A, Ishikawa N, Kato T, Hayama S, et al The neuromedin U‐growth hormone secretagogue receptor 1b/neurotensin receptor 1 oncogenic signaling pathway as a therapeutic target for lung cancer. Cancer Res 2006; 99:9408–19. [DOI] [PubMed] [Google Scholar]
- 37. Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, et al Identification of receptors for neuromedin U and its role in feeding. Nature 2000; 406:70–4. [DOI] [PubMed] [Google Scholar]
- 38. Hosoya M, Moriya T, Kawamata Y, Ohkubo S, Fujii R, Matsui H, et al Identification and functional characterization of a novel subtype of neuromedin U receptor. J Biol Chem 2000; 275:29528–32. [DOI] [PubMed] [Google Scholar]
- 39. Shan LX, Qiao X, Crona JH, Behan J, Wang S, Laz T, et al Identification of a novel neuromedin U receptor subtype expressed in the central nervous system. J Biol Chem 2000; 275:39482–6. [DOI] [PubMed] [Google Scholar]
- 40. Mitchell J, Maguire J, Davenport A. Emerging pharmacology and physiology of neuromedin U and the structurally related peptide neuromedin S. Br J Pharmacol 2009; 158:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamamoto I, Nakao N, Kaiya H, Miyazato M, Tsushima N. Two chicken neuromedin U receptors: characterization of primary structure, biological activity and tissue distribution. Gen Comp Endocrinol 2011; 174:116–23. [DOI] [PubMed] [Google Scholar]
- 42. Maruyama K, Kaiya H, Miyazato M, Konno N, Wakasugi T, Uchiyama M, et al Isolation and characterisation of two cDNAs encoding the neuromedin U receptor from goldfish brain. J Neuroendocrinol 2011; 23:282–91. [DOI] [PubMed] [Google Scholar]
- 43. Szekeres PG, Muir AI, Spinage LD, Miller JE, Butler SI, Smith A, et al Neuromedin U is a potent agonist at the orphan G protein‐coupled receptor FM3. J Biol Chem 2000; 275:20247–50. [DOI] [PubMed] [Google Scholar]
- 44. Kojima M, Haruno R, Nakazato M, Date Y, Murakami N, Hanada R, et al Purification and identification of neuromedin U as an endogenous ligand for an orphan receptor GPR66 (FM3). Biochem Biophys Res Commun 2000; 276:435–8. [DOI] [PubMed] [Google Scholar]
- 45. Brighton PJ, Szekeres PG, Wise A, Willars GB. Signaling and ligand binding by recombinant neuromedin U receptors/evidence for dual coupling to Gαq/11 and Gαi and an irreversible ligand‐receptor interaction. Mol Pharmacol 2004; 66:1544–56. [DOI] [PubMed] [Google Scholar]
- 46. Hsu S, Luo C. Molecular dissection of G protein preference using Gsα chimeras reveals novel ligand signaling of GPCRs. Am J Physiol Metab 2007; 293:E1021–E1029. [DOI] [PubMed] [Google Scholar]
- 47. Torres R, Croll SD, Vercollone J, Reinhardt J, Griffiths J, Zabski S, et al Mice genetically deficient in neuromedin U receptor 2, but not neuromedin U receptor 1, have impaired nociceptive responses. Pain 2007; 130:267–78. [DOI] [PubMed] [Google Scholar]
- 48. Abbondanzo SJ, Manfra DJ, Chen SC, Pinzon‐Ortiz M, Sun Y, Phillips JE, et al Nmur1‐/‐mice are not protected from cutaneous inflammation. Biochem Biophys Res Commun 2009; 378:777–82. [DOI] [PubMed] [Google Scholar]
- 49. Rao SM, Auger JL, Gaillard P, Weissleder R, Wada E, Torres R, et al The neuropeptide neuromedin U promotes autoantibody‐mediated arthritis. Arthritis Res Ther. 2012; 14:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matsuo Y, Yanase Y, Irifuku R, Takahagi S, Mihara S, Ishii K, et al Neuromedin U directly induces degranulation of skin mast cells, presumably via MRGPRX2. Allergy 2018; 73:2256. [DOI] [PubMed] [Google Scholar]
- 51. Domin J, Ghatei MACP. Characterization of neuromedin U like immunoreactivity in rat, porcine, guinea‐pig and human tissue extracts using a specific radioimmunoassay. Biochem Biophys Res Commun 1986; 140:1127–34. [DOI] [PubMed] [Google Scholar]
- 52. Domin J, Ghatei MA, Chohan P, Bloom SR. Neuromedin U – a study of its distribution in the rat. Peptides 1987; 8:779–84. [DOI] [PubMed] [Google Scholar]
- 53. Augood SJ, Keast JR, Emson PC. Distribution and characterisation of neuromedin U‐like immunoreactivity in rat brain and intestine and in guinea pig intestine. Regul Pept 1988; 20:281–92. [DOI] [PubMed] [Google Scholar]
- 54. Ballesta J, Carlei F, Bishop AE, Steel JH, Gibson SJ, Fahey M, et al Occurrence and developmental pattern of neuromedin U‐immunoreactive nerves in the gastrointestinal tract and brain of the rat. Neuroscience 1988; 25:797–816. [DOI] [PubMed] [Google Scholar]
- 55. Honzawa M, Sudoh T, Minamino N, Kangawa K, Matsuo H. Neuromedin U‐like immunoreactivity in rat intestine: regional distribution and immunohistochemical study. Neuropeptides 1990; 15:1–9. [DOI] [PubMed] [Google Scholar]
- 56. Austin C, Oka M. Distribution and developmental pattern of neuromedin U expression in the rat gastrointestinal tract. J Endocrinol 1994; 12:257–63. [DOI] [PubMed] [Google Scholar]
- 57. Gartlon J, Szekeres P, Pullen M, Sarau HM, Aiyar N, Shabon U, et al Localisation of NMU1R and NMU2R in human and rat central nervous system and effects of neuromedin‐U following central administration in rats. Psychopharmacology 2004; 177:1–14. [DOI] [PubMed] [Google Scholar]
- 58. Ivanov TR, Lawrence CB, Stanley PJ, Luckman SM. Evaluation of neuromedin U actions in energy homeostasis and pituitary function. Endocrinology 2002; 143:3813–21. [DOI] [PubMed] [Google Scholar]
- 59. Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Habata Y, Hinuma S, et al Identification of neuromedin U as the cognate ligand of the orphan G protein‐coupled receptor FM‐3. J Biol Chem. 2000; 275(28):21068–74. [DOI] [PubMed] [Google Scholar]
- 60. Graham ES, Turnbull Y, Fotheringham P, Nilaweera K, Mercer JG, Morgan PJ, et al Neuromedin U and Neuromedin U receptor‐2 expression in the mouse and rat hypothalamus: effects of nutritional status. J Neurochem 2003; 87:1165–73. [DOI] [PubMed] [Google Scholar]
- 61. Yu XH, Cao CQ, Mennicken F, Puma C, Donnell O, Perkins M. Pro‐nociceptive effects of neuromedin U in rat. Neuroscience 2003; 120:467–74. [DOI] [PubMed] [Google Scholar]
- 62. Westfall TD, Mccafferty GP, Pullen M, Gruver S, Sulpizio AC, Aiyar VN, et al Characterization of neuromedin U effects in canine smooth muscle. J Pharmacol Exp Ther 2002; 301:987–92. [DOI] [PubMed] [Google Scholar]
- 63. Mitchell JD, Maguire JJ, Kuc RE, Davenport AP. Expression and vasoconstrictor function of anorexigenic peptides neuromedin U‐25 and S in the human cardiovascular system. Cardiovasc Res 2009; 81:353–61. [DOI] [PubMed] [Google Scholar]
- 64. Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour R‐EE, Nyman J, Dionne D, et al The neuropeptide NMU amplifies ILC2‐driven allergic lung inflammation. Nature 2017; 549:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, et al The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017; 549:282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Furness JB, Pompolo S, Murphy R, Giraud A. Projections of neurons with neuromedin U‐like immunoreactivity in the small intestine of the guinea‐pig. Cell Tissue Res 1989; 257:415–22. [DOI] [PubMed] [Google Scholar]
- 67. Timmermans J, Seheuermann DW, Stach W, Adriaensen D, De G‐L, Polak JM. Neuromedin U‐immunoreactivity in the nervous‐system of the small‐intestine of the pig and its co‐existence with substance‐P and CGRP. Cell Tissue Res 1989; 258:331–7. [DOI] [PubMed] [Google Scholar]
- 68. Timmermans JP, Scheuermann DWSW. Distinct distribution of CGRP‐containing, enkephalin‐containing, galanin‐containing, neuromedin U‐containing, neuropeptide Y‐containing, somatostatin‐containing, substance P‐containing, VIP‐containing and serotonin‐containing neurons in the 2 submucosal. Cell Tissue Res 1990; 260:367–79. [DOI] [PubMed] [Google Scholar]
- 69. Ferry X, Brehin S, Kamel R, Landry Y. G protein‐dependent activation of mast cell by peptides and basic secretagogues. Peptides 2002; 23:1507–15. [DOI] [PubMed] [Google Scholar]
- 70. Numao TADK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol 1992; 149:3309–15. [PubMed] [Google Scholar]
- 71. Wiedermann FJ, Kähler CMRN. Induction of normal human eosinophil migration in vitro by substance P. Acta Haematol 1993; 89:213–5. [DOI] [PubMed] [Google Scholar]
- 72. Iwai T, Iinuma Y, Kodani R, Oka JI. Neuromedin U inhibits inflammation‐mediated memory impairment and neuronal cell‐death in rodents. Neurosci Res. 2008; 61(1):113–9. [DOI] [PubMed] [Google Scholar]
- 73. Grippi C, Izzi B, Gianfagna F, Noro F, Falcinelli E, Di Pardo A, et al Neuromedin U potentiates ADP‐ and epinephrine‐induced human platelet activation. Thromb Res 2017; 159:100–8. [DOI] [PubMed] [Google Scholar]
- 74. Johnson EN, Appelbaum ER, Carptenter DC, Cox RF, Disa J, Foley JJ, et al Neuromedin elicits cytokine release in murine Th2‐type T cell clone D10.G4.1. J Immunol 2004; 173:7230–8. [DOI] [PubMed] [Google Scholar]
- 75. Aiyar N, Disa J, Foley JJ, Buckley PT, Wixted WE, Pullen M, et al Radioligand binding and functional characterization of recombinant human nmU1 and nmU2 receptors stably expressed in clonal human embryonic kidney‐293 cells. Pharmacology 2004; 72:33–41. [DOI] [PubMed] [Google Scholar]
- 76. Dorsam RT, Gutkind JS. G‐protein‐coupled receptors and cancer. Nat Rev Cancer 2007; 7:79–94. [DOI] [PubMed] [Google Scholar]
- 77. Crabtree GR, Olson EN. NFAT signaling: Choreographing the social lives of cells. Cell 2002; 109(2 Suppl. 1):67–79. [DOI] [PubMed] [Google Scholar]
- 78. Genot E, Cleverley S, Henning S, Cantrell D. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J 1996; 15:3923–33. [PMC free article] [PubMed] [Google Scholar]
- 79. Egerton M, Fitzpatrick DR, Catling AD, Kelso A. Differential activation of T cell cytokine production by the extracellular signal‐regulated kinase (ERK) signaling pathway. Eur J Immunol 1996; 26:2279–85. [DOI] [PubMed] [Google Scholar]
- 80. Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol 2003; 3:317–30. [DOI] [PubMed] [Google Scholar]
- 81. Dahle MK, Øverland G, Myhre AE, Stuestøl JF, Hartung T, Krohn CD, et al The phosphatidylinositol 3‐kinase/protein kinase B signaling pathway is activated by lipoteichoic acid and plays a role in Kupffer cell production of interleukin‐6 (IL‐6) and IL‐10. Infect Immun 2004; 72:5704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xue L, Gyles L, Barrow A, Pettipher R. Inhibition of PI3K and calcineurin suppresses chemoattractant receptor‐homologous molecule expressed on Th2 cells (CRTH2)‐dependent responses of Th2 lymphocytes to prostaglandin D2. Biochem Pharmacol 2007; 73:843–53. [DOI] [PubMed] [Google Scholar]
- 83. Bando JK, Gilfillan S, Di LB, Fachi L, Cristiane S. ILC2s are the predominant source of intestinal ILC‐derived IL‐10. J Exp Med 2020; 217:e20191520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zaidi AK, Thangam ERR, Ali H Distinct roles of Ca2+ mobilization and G protein usage on regulation of Toll‐like receptor function in human and murine mast cells. Immunology 2006; 119:412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Aoki‐Nagase T, Nagase T, Oh‐Hashi Y, Shindo T, Kurihara Y, Yamaguchi Y, et al Attenuation of antigen‐induced airway hyperresponsiveness in CGRP‐deficient mice. Am J Physiol Lung Cell Mol Physiol 2002; 283:L963–L970. [DOI] [PubMed] [Google Scholar]
- 86. Aliakbari J, Sreedharan SP, Turek CW, Goetzl EJ. Selective localization of vasoactive intestinal peptide and substance P in human eosinophils. Biochem Biophys Res Commun 1987; 148:1440–5. [DOI] [PubMed] [Google Scholar]
- 87. Geissmann F, Manz MGJS. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327:656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Delgado M, Sun W, Leceta J, Ganea D, Alerts E. VIP and PACAP differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7. 1 and B7. 2 expression. J Immunol 1999; 423:4213–23. [PubMed] [Google Scholar]
- 89. Kincy‐cain T, Bost KL. Substance P‐induced IL‐12 production by murine macrophages. J Immunol 1997; 158:2334–9. [PubMed] [Google Scholar]
- 90. Teranishi H, Hayashi M, Higa R, Mori K, Miyazawa T, Hino J, et al Role of neuromedin U in accelerating of non‐alcoholic steatohepatitis in mice. Peptides 2018; 99:134–41. [DOI] [PubMed] [Google Scholar]
- 91. Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost 2015; 114:449–58. [DOI] [PubMed] [Google Scholar]
- 92. Semple JW, Italiano JEFJ. Platelets and the immune continuum. Nat Rev Immunol 2011; 11:264. [DOI] [PubMed] [Google Scholar]
- 93. Spertini O, Cordey ASMN. P‐selectin glycoprotein ligand 1 is a ligand for L‐selectin on neutrophils, monocytes, and CD34+ hematopoietic progenitor cells. J Cell Biol 1996; 135:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Johansson MW. Activation states of blood eosinophils in asthma. Clin Exp Allergy 2014; 44:482–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weyrich AS, McIntyre TMMRP. Monocyte tethering by P‐selectin regulates monocyte chemotactic protein‐1 and tumor necrosis factor‐alpha secretion. Signal integration and NF‐kappa B translocation. J Clin Invest 1995; 95:2297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol 2018; 3:eaat1604. [DOI] [PubMed] [Google Scholar]
- 97. Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell‐mediated effector immunity. J Allergy Clin Immunol 2015; 135:626–35. [DOI] [PubMed] [Google Scholar]
- 98. Wills‐karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, et al Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med 2012; 209:607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Veiga‐fernandes H, Mucida D, Molecular DM, Moniz E. Neuro‐immune interactions at barrier surfaces. Cell 2016; 165:801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, et al IL‐33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci USA 2016; 113:E7572–E7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jowett GM, Neves JF. Commentary: Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Front Pharmacol 2018; 9:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Deguine J, Barton GM. MyD88: a central player in innate immune signaling. F1000Prime Rep 2014; 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Griesenauer BPS. The ST2/IL‐33 axis in immune cells during inflammatory diseases. Front Immunol 2017; 8:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mizukawa Y, Doi T, Yamazaki Y, Kudo A, Shiohara T. Epidermal neuromedin U attenuates IgE‐mediated allergic skin inflammation. PLoS One 2016; 11:e0160122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today 1999; 5:123–32. [DOI] [PubMed] [Google Scholar]
- 106. Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med 1993; 119:771–8. [DOI] [PubMed] [Google Scholar]
- 107. Connor TMO, Connell JO, Brien DIO, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol 2004; 201:167–80. [DOI] [PubMed] [Google Scholar]
- 108. Cao CQ, Yu XH, Dray A, Filosa A, Perkins MN. A pro‐nociceptive role of neuromedin U in adult mice. Pain 2003; 104:609–16. [DOI] [PubMed] [Google Scholar]
- 109. Nakahara K, Kojima M, Hanada R, et al Neuromedin U is involved in nociceptive reflexes and adaptation to environmental stimuli in mice. Biochem Biophys Res Commun 2004; 323:615–20. [DOI] [PubMed] [Google Scholar]
- 110. Zeng H, Gragerov A, Hohmann JG, Pavlova MN, Schimpf BA, Xu H, et al Neuromedin U receptor 2‐deficient mice display differential responses in sensory perception, stress, and feeding. Mol Cell Biol 2006; 26:9352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pinho‐ribeiro FA Jr, Verri WA, Chiu IM. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol 2017; 38:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were available as no data sets were generated or analysed during this article.


