Abstract
Purpose of review
The purpose of this paper is to both review the available data and also highlight the gaps in knowledge, regarding the link between pediatric NASH and different type of sweeteners including caloric sweeteners (CS) and non-caloric sweeteners (NCS).
Recent findings
Studies have demonstrated that patients with NASH generally have had an unhealthy diet, characterized by on overconsumption of carbohydrates especially fructose. Mechanistically, a high-fructose diet reduces hepatic lipid oxidation, increases proinflammatory response, increases intestinal permeability and decreases microbiome diversity. Consumption and availability of NCS has therefore been increasing dramatically. Most NCS are not considered to be metabolized in the body and therefore thought to be safe for consumption. It was reported that pharmacological properties of rebaudioside, a type of NCS, as a potential hepatoprotector are through anti-inflammatory and antifibrotic mechanisms, associated with enhancing glucose-induced insulin secretion and inducing the difference of microbiome diversity.
Summary
Diet is an important factor in the pathogenesis of NAFLD and popular dietary patterns are contributing to the increased replacement of natural sweeteners with NCS. Screening for NAFLD by pediatricians and counseling on the avoidance of sugar-sweetened beverages are recommended. We feel that the various NCS available to the consumer today merit further investigation, and may potentially have hitherto unknown effects on hepatic metabolic function.
Keywords: nonalcoholic steatohepatitis, caloric sweeteners, non-caloric sweeteners, microbiome
Introduction
It is well known that excess caloric consumption is a major factor for obesity and nonalcoholic fatty liver disease (NAFLD), particularly when coupled with genetic susceptibility and/or a sedentary lifestyle. It is clear that the composition of the diet, independent of caloric content can exert a unique influence on liver function. The average “western” human diet comprises 40–50% carbohydrate, 20% protein and 30–40% fat [1]. Dietary intake of excess carbohydrates is of course well known to be associated with obesity and its related metabolic diseases including nonalcoholic steatohepatitis (NASH). Over the last several decades, increasing consumption of carbohydrates in particular has paralleled the increasing rate of NAFLD and NASH in the United States [2].
Pediatric NAFLD is defined by the presence of excessive hepatic steatosis in the setting of a child being overweight, which is not secondary to genetic or metabolic diseases, infection, toxicity or medications [3]. NAFLD can be further divided into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). NAFL denotes bland steatosis, while NASH is histologically characterized by lobular inflammation and/or fibrosis in the setting of hepatic steatosis [2]. Despite the considerable progress in understanding the complex nature of NASH, the mechanisms involved at the onset and in the progression of liver damage are still unclear. NASH, is now believed to be a multifactorial disorder resulting from a combination of genetic susceptibility, epigenetic influence and superlative environmental factors[4].
Owing to the high prevalence of NAFLD worldwide especially in industrialized countries and the consequent burden of progressive liver disease, NAFLD has rapidly become a leading etiology underlying many cases of hepatocellular carcinoma (HCC). However, the increased HCC risk of patients with NAFLD or NASH is often underdiagnosed and has led to predictions that NASH will be the leading cause for liver transplantation in adults in the United States by 2030 [5]. Given that genetic and epigenetic factors are seldom modifiable, first-line treatment for the vast majority of patients with NASH therefore remains lifestyle modification. Thus, research into this modifiable risk factor for NASH, nutrition, should be an area of extremely high priority.
The increased prevalence of obesity in children has coincided with a large increase in the consumption of sugar-sweetened beverages [6, 7]. Sugar-laden diets are ubiquitous because humans and animals display preferences for sweet taste that starts early in life [8]. Recently a study published in JAMA reported that reduction of sugar-containing beverage consumption reduced weight gain and body fat gain in healthy children [9]. Despite these recent additions for the most part, published guidelines recommend a “better lifestyle”, but do not specify a particular diet sighting “limited available evidence” [3].
The purpose of this paper is to both review the available data and also highlight the gaps in knowledge, regarding the link between pediatric NASH and different type of sweeteners including natural sweeteners (Caloric sweeteners, CS) and artificial sweeteners (Non-caloric sweeteners, NCS).
Caloric sweeteners (CS) and NASH
Carbohydrates are designated as simple or complex based on the number of sugar molecules. Complex carbohydrates are typically polysaccharides with a range of structures that are highly resistant to enzymatic digestion, and less likely to stimulate lipogenesis in the liver [10]. Simple carbohydrates on the other hand, include the dietary sweeteners glucose, fructose and sucrose. Glucose and fructose are monosaccharides, while sucrose is a disaccharide. These molecules are metabolized by the intestine and transported directly via the portal circulation, to the liver wherein via de novo lipogenesis (DNL) there are triglyceride moiety generation and subsequent neutral lipid storage or hepatic steatosis [11]. This is especially true for the fructose molecule as it stimulates more hepatic lipid production due to the lack of feedback regulation [10].
Consumption of sugar-sweetened beverages has been increasingly associated with the negative health outcomes such of obesity, diabetes and the metabolic syndrome. The same holds true for NAFLD and NASH. Forced carbohydrate overfeeding in a pre-clinical study found rapid increase in liver fat content by 27% with only a concurrent 2% increase in body weight [11]. Clinical studies have similarly demonstrated that patients with NASH generally have had an unhealthy diet, characterized by on overconsumption of carbohydrates especially fructose, saturated fat along with a low consumption of fiber and omega-3 containing dietary elements [12]. Studies have further shown that excess fructose consumption alone can lead to insulin resistance through uric acid mediated oxidant stress and inflammatory cytokine production [13].
It is noted that the ability of simple sugars to induce liver injury maybe modulated by the fat component of the diet, specifically saturated fat [10]. The addition of fructose to a diet high in saturated fat led to a greater weight gain, pronounced steatosis and signs of hepatic inflammation rapidly [10, 14]. Our previous pre-clinical work indicated that fructose-sucrose containing drinking water, when provided to mice in the combination with a high saturated-fat diet induced liver inflammation and fibrosis, which was further validated with analysis of a large epidemiological human data set [15].
Decreasing consumption of simple carbohydrates and added sugar play a key role in the treatment and prevention of NASH. Fructose is almost exclusively metabolized by the liver and is a potential determinant of liver fat accumulation. The reverse phenomenon is observed when dietary fructose is restricted and it was associated with a significant reduction in denovo lipogenesis [11]. Mosca et al evaluated the association between fructose assumption and NAFLD in obese children, demonstrating fructose consumption was independently associated with NASH [16], and Schwarz et al studied the effects of a short-term isoenergetic fructose restriction in obese children, which interestingly resulted in decreased overall liver fat tissue as measured by 27%. Another study on adolescent boys with NAFLD revealed 8 weeks of low sugar diet resulted in significant improvement in hepatic steatosis. [9].
Mechanistically, a high-fructose diet reduces hepatic lipid oxidation by blocking the activity of PPAR-α increasing hepatic steatosis. Fructose also acts in the liver at the transcriptional level by increasing the proinflammatory transcription of NF-kb and increasing oxidative stress. Fructose may also promote liver injury by causing bacterial overgrowth and increased intestinal permeability, precipitating endotoxemia and subsequent initiation of inflammation. Jones RB et al found that in human subjects, high dietary fructose is associated with low abundance of Eubacterium and Streptococcus [17]. Finally, a diet high in fat and carbohydrate has also been shown to be associated with microbiome changes consistent with decreased gut bacterial diversity and reductions in beneficial bacteria [18].
Screening for NAFLD using liver enzymes is recommended between the ages 9 and 11 years for children that are obese or children that are overweight with certain risk factors [3]. The liver enzyme, alanine amino transferase (ALT) is minimally invasive and has an acceptable sensitivity, with the median upper limit of normal being 22 mg/dL for girls and 26 mg/dL for boys. Referral to a gastroenterologist is recommended if an elevation of liver enzymes is identified using these screening guidelines.
Noncaloric sweeteners (NCS) and NASH
Given the abundance of data that sugar-sweetened beverages are associated with obesity and NASH, many researchers and healthcare practitioners have proposed that non-caloric, high-intensity sweeteners provide a beneficial alternative in foods and beverages [19]. For the purposes of this review article, the terms high-intensity sweeteners, low-calorie sweeteners, artificial sweeteners, non-caloric sweeteners will be categorized under the umbrella term NCS.
NCS are food additives providing sweetness without calories or glycemic effects [20]. NCS are several magnitudes sweeter than sucrose and are already used ubiquitously in beverages, foodstuffs and medicines. There has been a recent surge in the use of NCS given the popular theme of cutting back sugar from diet. Consumption and availability of artificial sweeteners has therefore been increasing dramatically. Commercially available NCS include sucralose, acesulfame potassium, saccharin, aspartame, stevia (rebaudioside) and sucralose [21].
Saccharin is one of the oldest NCS, and was developed in 1879. Amongst the second-generation sweeteners, aspartame continues to be used extensively and was approved in 1981. More recent NCS on the market include plant-derived products like stevia (rebaudioside) and fermented sugar alcohols like sorbitol which have a laxative effect when consumed in large quantities. Overall there has been exponential increase in the consumption of NCS over the last two decades[22].
Most NCS are not considered to be metabolized in the body and therefore thought to be safe for consumption [21]. Saccharin, cyclamate, acesulfame potassium, aspartame, sucralose and stevia are the NCS approved under the, generally regarded as safe (GRAS) category by FDA. However, there is still a high level of debate about the use of NCS in food supply. We believe that despite FDA approval, there is a need for greater risk assessment to identify possible health problems related to the long-term consumption of NCS. Interestingly, there are data that certain NCS compounds are pharmacologically active and may therefore play a role in the regulation of adipogenesis or its key precursor step of hepatic DNL [23]. Animal studies have reported that intake of NCS can be associated with weight gain and metabolic syndrome [21]. Similarly, Suez et all reported that saccharin, sucralose or aspartame caused marked glucose intolerance [24].
The Stevia leaf’s extract, rebaudioside, perceived as 300 times sweeter than sucrose [25], has been used for centuries as a sweetening food additive in South America, and was recently approved in United States as a type of NCS. The ability to provide sweetness without calories, and the presumption of safety compared to common caloric sweeteners such as high fructose corn syrup (HFCS) [26] has led to the further popularity of NCS compounds. Interestingly, Reynolds TH et al also reported that rebaudioside supplemented water did not change body weight, and promote insulin resistance or obesity [27] while another study observed that rebaudioside may in fact reduce adipogenesis[28] [19], and result in reduction of body weight [29].
It was reported that pharmacological properties of rebaudioside as a potential hepatoprotector are through anti-inflammatory and antifibrotic mechanisms including downregulating NF-kB and proinflammatory cytokines [30]. Casas S et al reported that rebaudioside prevents liver damage by blocking oxidative processes via upregulation of nuclear erythroid factor 2 (Nrf2) and exerts immunomodulatory effects by downregulating the nuclear factor-kB system and acts as an antifibrotic agent by maintaining collagen content [31]. In vitro studies on the metabolism of rebaudioside showed that it is mainly degraded by the microbiome without getting absorbed by gastrointestinal tract, serving as an activator of Nrf2 [32]. A recent article published in the journal Nature Communications, reported that rebaudioside potentiates the perception of sweet taste and enhances glucose-induced insulin secretion to prevent diabetic hyperglycemia in a Trpm5-dependent manner, which in turn is a calcium-activated cation channel expressed in type II taste receptor cells and pancreatic beta-cells [33]. These findings suggested that rebaudioside is a potentially beneficial agent for preventing or managing NASH and should be studied further.
Both synthetic and natural NCS have been shown to exert bacteriostatic effects on a variety of bacteria species [34]. The gut microbiota consists of hundreds of bacterial species and is involved in multiple physiological functions such as carbohydrate and amino acid metabolism. Most recently, several studies on NCS including sucralose, aspartame or saccharin showed that gut microbiota was disrupted in both mice and humans using a number of models and experimental interventions [35], with development of glucose intolerance afterwards. Transplantation of saccharin treated fecal contents into germ-free mice established that glucose intolerance phenotype could be transferred to host animals, thereby implicating the microbiota as causative in metabolic impairment. A recent cross-sectional study revealed that no differences in bacterial abundance and gene function between NCS consumers and non-consumers in adults, but there was significant difference in microbial diversity[36] [37].
Nettleton JE et al found that rebaudioside alter gut microbiota composition and reduce nucleus accumbens tyrosine hydroxylase and dopamine transporter mRNA levels [38]. This small change in diversity maybe a driving force mediating metabolic changes. Interestingly, it was reported that bacteroides are the most efficient group of bacteria at hydrolyzing rebaudioside. Together the potential for NCS driven changes to the microbiome may share responsibility for rescue of pancreatic islet mass and improved glucose intolerance, and again should be studied further.
Conclusion
Screening for liver enzymes between ages 9 and 11 years for children that are obese and for children that are overweight with risk factors by their pediatric primary practitioner is now recommended [3].
There is strong evidence for the avoidance of sugar-sweetened beverages as a strategy to decrease adiposity and help reverse the hepatic consequences of obesity., as fructose is primarily metabolized in the liver mediating hepatic DNL, altered metabolism and visceral adiposity. Popular dietary patterns are also contributing to the increased replacement of natural sweeteners with NCS compounds. We feel that the various NCS available to the consumer today merit further investigation, as each agent is derived from different sources and may potentially have hitherto unknown effects on hepatic metabolic function.
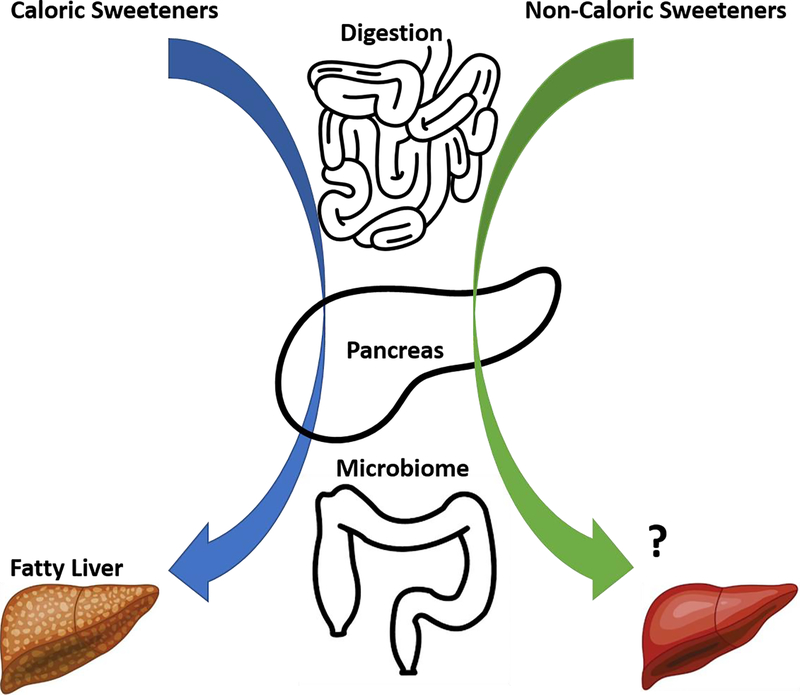
Thus, despite all the sweetness, how our liver responds to caloric and non-caloric sweeteners continues to be an important distinction that remains to be determined (Figure).
Figure.
Schematic diagram illustrated that excessive intake of caloric sweeteners (CS) (e.g. fructose) are metabolized by the intestine, associated with decreasing insulin sensitivity and disrupting microbiome diversity, then further transported directly via portal circulation to the liver to promote de novo lipogenesis (DNL) and subsequently NAFLD and NASH; while non-caloric sweeteners (NCS) which have attracted research attention given the phenomena that sugar-sweetened beverages are associated with metabolic syndrome, are not considered to be metabolized in the body, however be able to enhance insulin sensitivity by acting on pancreas and restore microbiome stability, further induce hepatoprotection therefore serving as a potentially beneficial agent for NASH.
Aberrations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- CS
caloric sweeteners
- NCS
non-caloric sweeteners
- DNL
de novo lipogenesis
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
Papers of particular interest, recently published have been highlighted as: • of importance; •• of major importance.
- 1.Barr SB and Wright JC, Postprandial energy expenditure in whole-food and processed-food meals: implications for daily energy expenditure. Food Nutr Res, 2010. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boursier J, et al. , The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology, 2016. 63(3): p. 764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos MB, et al. , NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr, 2017. 64(2): p. 319–334.• This guideline highlights that the expert NASPGHAN committee on NAFLD reviewed and summarized the available literature, formulating recommendations to guide screening and clinical care of children with NAFLD.
- 4.Brown GT and Kleiner DE, Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism, 2016. 65(8): p. 1080–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anstee QM, et al. , From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol, 2019. 16(7): p. 411–428. [DOI] [PubMed] [Google Scholar]
- 6.Sharp KPH, Schultz M, and Coppell KJ, Is non-alcoholic fatty liver disease a reflection of what we eat or simply how much we eat? JGH Open, 2018. 2(2): p. 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr RM, In NAFLD, You Are What You Eat, Not Simply How Much You Eat. Cell Mol Gastroenterol Hepatol, 2017. 4(2): p. 301–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowlis GH and Kessen W, Human newborns differentiate differing concentrations of sucrose and glucose. Science, 1976. 191(4229): p. 865–6. [DOI] [PubMed] [Google Scholar]
- 9.Schwimmer JB, et al. , Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA, 2019. 321(3): p. 256–265.•• This study highlights that in adolescent boys with NAFLD, 8 weeks of provision of a diet low in free sugar content compared with usual diet resulted in significant improvement in hepatic steatosis.
- 10.Duwaerts CC and Maher JJ, Macronutrients and the Adipose-Liver Axis in Obesity and Fatty Liver. Cell Mol Gastroenterol Hepatol, 2019. 7(4): p. 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Softic S, Cohen DE, and Kahn CR, Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig Dis Sci, 2016. 61(5): p. 1282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eslamparast T, Tandon P, and Raman M, Dietary Composition Independent of Weight Loss in the Management of Non-Alcoholic Fatty Liver Disease. Nutrients, 2017. 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira RM, et al. , Fructose Consumption in the Development of Obesity and the Effects of Different Protocols of Physical Exercise on the Hepatic Metabolism. Nutrients, 2017. 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen T, et al. , Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol, 2018. 68(5): p. 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelmalek MF, et al. , Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology, 2010. 51(6): p. 1961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosca A, et al. , Beverage consumption and paediatric NAFLD. Eat Weight Disord, 2016. 21(4): p. 581–588. [DOI] [PubMed] [Google Scholar]
- 17.Jones RB, et al. , High intake of dietary fructose in overweight/obese teenagers associated with depletion of Eubacterium and Streptococcus in gut microbiome. Gut Microbes, 2019: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canfora EE, et al. , Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol, 2019. 15(5): p. 261–273. [DOI] [PubMed] [Google Scholar]
- 19.Swithers SE, Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab, 2013. 24(9): p. 431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts A, The safety and regulatory process for low calorie sweeteners in the United States. Physiol Behav, 2016. 164(Pt B): p. 439–444. [DOI] [PubMed] [Google Scholar]
- 21.Durán Agüero S, et al. , Noncaloric Sweeteners in Children: A Controversial Theme. Biomed Res Int, 2018. 2018: p. 4806534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattes RD and Popkin BM, Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr, 2009. 89(1): p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepino MY, Metabolic effects of non-nutritive sweeteners. Physiol Behav, 2015. 152(Pt B): p. 450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suez J, et al. , Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature, 2014. 514(7521): p. 181–6.• This article demonstrates that consumption of commonly used non-caloric sweeteners drives the development of glucose intolerance through induction of compositional and functional alterations to the intestinal microbiota, linked to host susceptibility to metabolic disease.
- 25.Samuel P, et al. , Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential. J Nutr, 2018. 148(7): p. 1186S–1205S. [DOI] [PubMed] [Google Scholar]
- 26.Momtazi-Borojeni AA, et al. , A Review on the Pharmacology and Toxicology of Steviol Glycosides Extracted from Stevia rebaudiana. Curr Pharm Des, 2017. 23(11): p. 1616–1622. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds TH, et al. , Long term rebaudioside A treatment does not alter circadian activity rhythms, adiposity, or insulin action in male mice. PLoS One, 2017. 12(5): p. e0177138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do MH, et al. , High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients, 2018. 10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Ruyter JC, et al. , A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med, 2012. 367(15): p. 1397–406.•• This study reported that masked replacement of sugar-containing beverages with noncaloric beverages reduced weight gain and fat accumulation in normal-weight children.
- 30.Molinaro A, et al. , Host-microbiota interaction induces bi-phasic inflammation and glucose intolerance in mice. Mol Metab, 2017. 6(11): p. 1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casas-Grajales S, et al. , Rebaudioside A administration prevents experimental liver fibrosis: an in vivo and in vitro study of the mechanisms of action involved. J Appl Toxicol, 2019. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, et al. , New application of the commercial sweetener rebaudioside a as a hepatoprotective candidate: Induction of the Nrf2 signaling pathway. Eur J Pharmacol, 2018. 822: p. 128–137. [DOI] [PubMed] [Google Scholar]
- 33.Philippaert K, et al. , Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat Commun, 2017. 8: p. 14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang QP, et al. , Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS One, 2018. 13(7): p. e0199080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandić AD and Blaut M, Do we choose control diets wisely? Trends Endocrinol Metab, 2018. 29(7): p. 447–448. [DOI] [PubMed] [Google Scholar]
- 36.Schwenger KJP, et al. , Non-alcoholic fatty liver disease and obesity: the role of the gut bacteria. Eur J Nutr, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Chi L, et al. , Effects of the Artificial Sweetener Neotame on the Gut Microbiome and Fecal Metabolites in Mice. Molecules, 2018. 23(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nettleton JE, et al. , Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients, 2019. 11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]