Abstract
Urological organizations publish detailed evidence-based guidelines to support the urologists in the management of urolithiasis. Our objective was to provide clear guidance on the management of urolithiasis, compare the American Urological Association (AUA) and European Association of Urologists (EAU) guidelines, and present an algorithm for different clinical scenarios. The latest AUA and EAU guidelines on urolithiasis were evaluated for the level of evidence and grade of recommendation. All recommendations on management of urolithiasis (surgical and medical management) were reviewed and included. Both the organizations provide guidance for initial patient assessment, imaging requirements, and therapeutic options, including surgical intervention and medical therapy. In addition, these guidelines provide advice for managing specific patient groups, including pediatric patients and pregnant patients. Although there is a general concordance between both the groups, differences exist particularly for recommended modality of surgical intervention depending on stone location and size. Although both the guidelines were broadly similar, we also highlighted the variations in the level of evidence and grade of recommendation. Although these guidelines provide a valuable evidence-based framework to support the management of urinary tract stones, their implementation must be tailored to individual patient needs and available resources.
Keywords: Bladder stones, guideline, lithotripsy, nephrolithotomy, percutaneous, ureteroscopy, urolithiasis
Introduction
Urinary tract stones are common, with a global prevalence of approximately 14%, which varies depending on age, sex, and ethnicity.[1,2] The majority are found in the upper urinary tract and 5% are found within the bladder.[3] They present a significant clinical and economic workload to the healthcare systems.[4,5] Many professional institutions have developed extensive guidelines to aid the clinicians in the assessment, diagnosis, and management of urolithiasis. The American Urology Association (AUA) and the European Association of Urology (EAU) have both published separate guidelines for the management of stone disease.
The AUA has published separate guidelines for surgical and medical management of the upper tract stone disease, most recently updated in 2016 and 2019, respectively.[6,7] There are separate EAU guidelines for upper tract stones and bladder stones, both of which received a minor update in 2020.[8,9] Both the guidelines evaluate the strength of evidence using different methods (Appendix 1). The AUA assesses the level of evidence alphabetically and uses specific nomenclature to demonstrate the strength of recommendation. The EAU recommendations are classified as “strong” or “weak” using the modified grading of recommendations assessment, development, and evaluation system[10,11] and the key elements considered by the panel to form the basis of the strength of recommendation.
Appendix 1.
Table demonstrating the level of evidence and nomenclature used in the AUA and EAU guidelines
| AUA Grade | Level of evidence | Further clarifications |
|---|---|---|
| A | Well-conducted and highly generalizable RCTs or exceptionally strong observational studies with consistent findings | RCT |
| B | RCTs with some procedural or generalizability weaknesses or moderately strong observational studies with consistent findings. | |
| C | RCTs with serious procedural deficiencies. | |
| AUA nomenclature used in medical management guidelines | Definition and link to level of evidence | |
| Options | Nondirective statement when the balance between benefits and risk/burden is unclear based on Grade A, B, or C evidence. Decision should be made by the clinician with the patient, taking into consideration the patient’s past clinical history, current quality of life, preferences, and values. | |
| Recommendations | Directive statement guiding whether an action should* or should not** be undertaken based on Grade C evidence. | * Should is defined by benefits outweigh risks/burdens ** Should not is defined by risks/burden outweigh |
| Standards | Directive statements guiding whether an action should* or should** not be undertaken based on Grade A or B evidence. | |
| AUA nomenclature used in surgical management guidelines | ||
| Strong recommendations | Directive statement indicating that action should* or should not** be undertaken because there is a significant and substantial change of net benefit or harm. based on Grade A or B evidence. Grade C is rarely used. | Grade A: (high certainty) future research unlikely to change confidence Grade B: (moderate certainty) better evidence could change Grade C: Thisismainly (low certainty) better evidence is likely to change confidence |
| Moderate recommendations | Directive statement indicating an action should* or should not** be undertaken owing to a moderate chance of net benefit or harm. This is can be based on Grade A, B, or C evidence. | |
| Conditional recommendations | Nondirective statements when evidence shows there is no apparent benefit or harm, and the balance between benefits and risks/burden is unclear. This can be based on Grade A, B, or C evidence. | |
| AUA nomenclature used in medical and surgical management guidelines | ||
| Clinical principle | Statement about clinical care which is widely agreed upon among clinicians when there may or may not be supporting evidenc in the medical literature. | |
| Expert opinion | Statement based on the consensus of the panel. This is based on clinical training, experience, knowledge, and judgment when there is no evidence to support. | |
| EAU | Strong or weak recommendations provided according to the following factors | |
|
||
GRADE: grading of recommendations assessment, development, and evaluation; RCT: randomized control trial
Although guidelines are a valuable tool for the clinicians, they are not without issue because they are only periodically updated despite new evidence being published continuously. Therefore, caution should be exercised when interpreting the guidelines, particularly for complex cases.
Primary Assessment
Presentation
Urolithiasis may present with loin pain, fever, nausea, and vomiting or with an incidental finding. Urgent investigation is required in those with features suggestive of infection or solitary kidney (EAU: Strong recommendation). Bladder stones present differently with lower urinary tract symptoms (LUTS) predominantly, but hematuria and suprapubic pain may also be present.
Initial Investigations
Renal and ureteric stones
In the presentation of acute flank pain, noncontrast computed tomography (NCCT) is the most sensitive and specific mode of imaging to confirm the diagnosis of upper urinary tract stones (EAU: Strong recommendation). However, the EAU recommends ultrasound (US) to be used initially, if available, because it is inexpensive and radiation-free. If the urinary tract anatomy needs assessment before stone removal, contrast imaging should be performed (EAU: Strong recommendation).
The AUA recommends NCCT before performing percutaneous nephrolithotripsy (PCNL) (AUA: Strong recommendation), and it could identify the candidate’s suitability for shockwave lithotripsy (SWL) versus ureteroscopy (URS) (AUA: Conditional recommendation). If significant renal function impairment is suspected, functional imaging, e.g. diethylene-triamine-penta-acetate or mercaptoacetyltriglycine could be used to assess the renal function (AUA: Conditional recommendation).
In pregnancy, US is the recommended first-line imaging modality with magnetic resonance imaging being the second-line, and low-dose NCCT as the last resort (EAU: Strong recommendation).
US is also the preferred choice of imaging in children to limit the exposure to ionizing radiation. However, because of the low sensitivity of US,[12,13] a kidney-ureter-bladder (KUB) X-ray or low-dose NCCT may be necessary if the US is inadequate (EAU: Strong recommendation). The AUA recommends obtaining NCCT imaging in children before PCNL (AUA: Strong recommendation).
In addition to imaging, basic biochemical profiling of the blood and urine should be performed on patients presenting as an emergency (Table 1).
Table 1.
Summary of the recommended hematological, biochemical, and urine analysis
| Tests to be performed | |
|---|---|
| Blood | Hematology |
| Red blood cells | |
| White cells | |
| Hemoglobin | |
| Hematocrit | |
| Platelets | |
| Biochemistry | |
| (Ionized) calcium | |
| Creatinine | |
| CRP | |
| Potassium | |
| Sodium | |
| Uric acid | |
| Coagulation | |
| Urine | Urine dip |
| Nitrites | |
| pH | |
| Red cells | |
| White cells | |
| Following urine dip if infection is suspected | |
| Urine microscopy and/or culture |
CRP: C-reactive protein
Bladder stones
US imaging of the bladder is the first-line recommendation in both adults and children presenting with symptoms suggestive of bladder stones. If clinical concern persists despite negative US findings, an NCCT or cystoscopy, which have higher sensitivity than US, should be performed in adults (EAU: Strong recommendation). The KUB X-ray may be useful in adults with confirmed bladder stones to plan treatment and follow-up (EAU: Weak recommendation). No specific second-line investigation for children is advised because of limited evidence. Investigations to determine the underlying cause of the bladder stone should include physical examination, uroflowmetry, urine dip and pH, stone analysis, and serum biochemistry as for upper urinary tract stones.
Management
Ureteric stone
Emergency management
An infected, obstructed renal system is a urological emergency and prompt management is required. Both ureteric stenting and percutaneous nephrostomy are deemed to be equally effective at achieving decompression, and definitive treatment should be postponed until sepsis has resolved (EAU/AUA: Strong recommendation). The EAU strongly recommends immediate initiation of antibiotic therapy, acquiring a urine sample at decompression, and amending antibiotic therapy once sensitivities are available.
Renal colic
The EAU strongly recommends managing the acute renal colic with nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol in the absence of contraindication, with weak evidence supporting the second-line use of opioids. NSAIDs are also beneficial for reducing the recurrent episodes of renal colic in those managed expectantly.[8] If the colic is refractory to medical management, decompression of the renal system or stone removal are indicated (EAU: Strong recommendation).
Uncomplicated ureteric stones may be managed conservatively (AUA/EAU: Strong recommendation). The AUA applies this to stones ≤10 mm, but the EAU is less specific, stating that “small” stones may be observed with a suggestion that small implies a size of <6 mm, because meta-analysis has shown that rates of spontaneous passage of stones reduced with increasing stone size.[14]
The AUA advocates the use of medical expulsive therapy (MET) in the form of alpha-blockers (tamsulosin) for uncomplicated distal ureteric stones ≤10 mm (Strong recommendation). The EAU differs by suggesting that alpha-blockers should be used only in distal ureteric stones >5 mm because a large randomized controlled trial demonstrated no benefit in using alpha-blockers for distal ureteric stones of <5 mm (Strong recommendation).[15]
The AUA guidelines state that definitive stone management is indicated if conservative management, with or without MET, has been unsuccessful after a period of 4–6 weeks (Moderate recommendation). However, it should be noted that the EAU does not place a timeframe on conservative management.
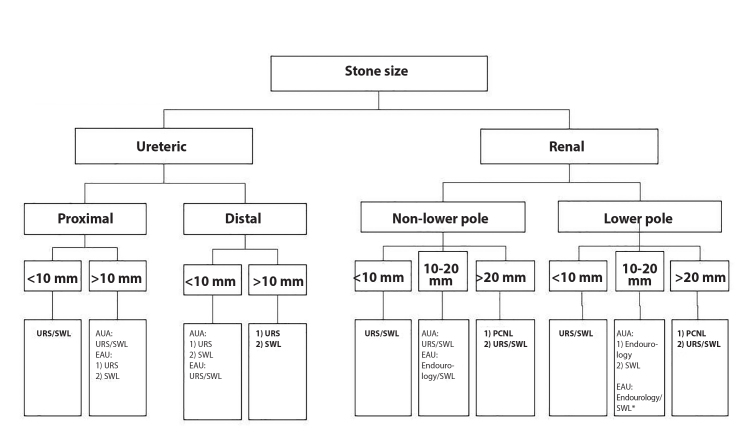
Reimaging is appropriate before treatment if there has been a change in symptoms because this may reflect stone migration or passage and alter management (AUA: Clinical principle). SWL or URS are the most common treatment modalities used for definitive management of ureteric stones. The patients should be informed that SWL is associated with less morbidity and a lower complication rate than URS; however, SWL has a lower stone-free rate (SFR) after a single procedure than URS (AUA/EAU: Strong recommendation), although there is no statistical difference in SFR at 3 months between URS and SWL.[16] URS is recommended as the first-line treatment for ureteric stones >10 mm, with the exception of the AUA stating equivalence between SWL and URS for proximal ureteric stones >10 mm (Figure 1). The EAU guidelines recommend that distal or proximal ureteric stones <10 mm can be managed with either SWL or URS as first-line treatment, whereas the AUA recommend preferential use of URS over SWL for distal/mid ureteric stones (AUA: Strong recommendation). URS is preferred in morbidly obese patients owing to the increased SFR (EAU: Strong recommendation).
Figure 1.
Flowchart demonstrating the recommended management according to the stone size and location
Endourology=URS+PCNL. *=If favourable factors for SWL as discussed in text
Bold Text=denotes recommendation from both AUA and EAU. URS: ureteroscopy; SWL: shockwave lithotripsy; PCNL: percutaneous nephrolithotripsy
Renal stones
Asymptomatic renal stones may be managed conservatively with active surveillance, with both organizations acknowledging a lack of high-quality evidence to support this (AUA: Conditional recommendation). The EAU states that the stones <15 mm may be conservatively managed.[8] If conservative management is chosen, regular surveillance (initially 6 months, then annually) with imaging should be performed to evaluate the symptoms and assess the stone growth (EAU: Strong recommendation). Active management is indicated for new symptoms, increasing stone size for stones >5 mm, infection, or lifestyle reasons, including occupation or patient choice (EAU: Weak recommendation).
Both the institutions recommend PCNL as the preferential treatment modality for all renal stones >20 mm owing to the increased SFR and reduced need for a second procedure (AUA/EAU: Strong recommendation). Staged URS or SWL may be offered as the second-line treatment if PCNL is not appropriate (EAU: Strong recommendation). Both SWL and URS are recommended as first-line treatments for non-lower pole stones ≤20 mm (AUA: Strong recommendation), with the EAU also including PCNL as another option for stones sized 10–20 mm.[8] The guidance on lower pole stones varies slightly with both associations advocating the use of URS or SWL as the first-line treatment for stones <10 mm. However, the AUA states that SWL should not be offered as the first-line therapy for lower pole stones >10 mm, whereas the EAU lists SWL as the first-line treatment alongside URS and PCNL in the absence of unfavorable factors for SWL (AUA/EAU: Strong recommendation).
Unfavorable factors for SWL include long skin-to-stone distance, long calyx, steep infundibular-pelvic angle, narrow infundibulum, or shockwave-resistant stones (calcium oxalate monohydrate, brushite, or cystine).[8] In the rare circumstances that SWL or endourological surgery is unsuccessful or not possible, laparoscopic or open surgery can be offered for stone removal (AUA/EAU: Strong recommendation).
Bladder stones
Transurethral cystolithotripsy offers the same SFR as open suprapubic cystolithotripsy, with shorter length of hospital stay and low rates of major complications or further unplanned procedures.[9] If transurethral cystolithotripsy is not possible, percutaneous cystolithotripsy should be considered because this also has a shorter length of hospital stay than an open procedure (EAU: Strong recommendation). The operation duration is shorter when using a nephroscope rather than a cystoscope (EAU: Weak recommendation). If indicated, surgery for bladder outflow obstruction should be performed simultaneously with bladder stone surgery (EAU: Strong recommendation). SWL is less invasive than other therapeutic options; however, SFR is lower and can be considered alongside laparoscopic or open surgery if endoscopic treatment is not possible (EAU: Weak recommendation).
Treatment specific considerations
Numerous factors need to be considered before selecting the treatment modality, including patient preference and comorbidity, former stone analysis, and imaging findings, including Hounsfield unit on NCCT (EAU: Strong recommendation).
SWL
The AUA strongly recommends against pre-stenting for SWL. Although the risk of steinstrasse is greater in patients without stents, there is no difference in SFR and an increased risk of developing LUTS secondary to the stent.[17] The EAU provides technical advice, which includes advocating the use of ultrasound gel as a coupling agent, incremental increases in power to limit the renal injury, an optimum frequency of 1–1.5 Hz, and careful imaging control to optimize SWL outcomes (EAU: Strong recommendation). Both the groups support the use of antibiotics in the presence of urinary tract infection, with the EAU extending this to those who have been pre-stented (AUA: Clinical principle, EAU: Strong recommendation). The AUA and EAU endorse the off-label use of alpha-blockers after SWL to promote stone fragment expulsion and increase SFR (AUA: Moderate recommendation). However, SWL should not be used in the presence of anatomical or functional obstruction of the collecting system distal to the stone because of the reduced potential for fragment clearance (AUA: Strong recommendation).
Ureteroscopy
The routine placement of a ureteric stent preoperatively is not necessary, although the EAU guidelines note that it improves the outcomes for renal stones in particular (AUA: Strong recommendation).[18] Despite being relatively common practice, both the AUA and EAU advise against postoperative ureteric stenting in uncomplicated cases because this is associated with increased morbidity without an improvement in SFR (AUA/EAU: Strong recommendation).[19] In situations where a stent is placed, MET may facilitate stone fragment expulsion and provide relief from the stent-related symptoms (AUA: Moderate recommendation, EAU: Strong recommendation). URS is the preferred intervention in cases where antithrombotic therapy must be continued because it is associated with less morbidity than SWL or PCNL (EAU: Strong recommendation).
The AUA and EAU recommend using a safety wire whenever possible, although the AUA states that a ureteric access sheath (UAS) can provide the same function if it reaches the renal pelvis (AUA: Expert opinion). There are no formal recommendations regarding the use of UAS in either guidelines, with the EAU stating that their use depends on the urologist’s preference.[8] UAS has advantages in prolonged procedures by reducing the intrarenal pressures, and it can facilitate multiple passages to the renal pelvis in cases of large stone burden; however, it may increase the risk of ureteric mucosal injuries.[20,21] The EAU advocates the use of Holmium-YAG laser lithotripsy for URS because it is effective in all the stone types, and they state that bilateral URS can be considered but may have an increased risk of minor complications.[22] According to both the associations, antibiotic prophylaxis should be used for all the endoscopic stone treatments, including PCNL (AUA: Clinical principle, EAU: Strong recommendation).
PCNL
The EAU discusses patient positioning for PCNL, concluding that both prone and supine positioning are equally safe with no difference in operative time.[8] The AUA recommends preoperative CT before PCNL; the EAU broadens the imaging requirement to include US or intraoperative fluoroscopy (AUA/EAU: Strong recommendation). The AUA recommends flexible nephroscopy as a routine component of standard PCNL because this is associated with increased SFR of 92.5% compared with 70% for rigid nephroscopy alone (AUA: Strong recommendation).[23] The use of smaller instruments (miniaturized PCNL) is associated with reduced blood loss but increased operative time.[24] The EAU guidelines state that nephrostomy placement for uncomplicated PCNL is not required because it is associated with reduced postoperative pain and shorter length of hospital stay; however, it is optional in the AUA guidelines (AUA: Conditional recommendation, EAU: Strong recommendation).
Specific patient groups
Pregnancy
Conservative management of stones during pregnancy is preferred in uncomplicated cases with well-controlled symptoms (AUA/EAU: Strong recommendation). In cases where active management is indicated, there should be multidisciplinary decision making between the urology, radiology, anesthetic, and obstetric teams (AUA: Clinical principle).[25]
Management options include temporizing strategies with nephrostomy or ureteric stenting, which may be poorly tolerated or require regular changes owing to the accelerated encrustation during pregnancy. An alternative would be definitive treatment with URS (AUA: Strong recommendation).
Pediatrics
The management of urolithiasis in children is broadly similar to that in adults, with some important differences. The AUA supports conservative management of uncomplicated ureteric stones ≤10 mm with or without off-label use of MET (AUA: Moderate recommendation). The EAU discusses a lack of evidence regarding conservative management; however, asymptomatic, nonstruvite, noncystine stones <7 mm with no anatomical abnormalities may be managed expectantly.[26] The EAU advises that if treatment is indicated, SWL should be offered as the first-line therapy for ureteric stones <10 mm, with URS as an alternative for stones not amenable to SWL (EAU: Strong recommendation). The AUA suggests that if conservative management fails or is inappropriate, either URS or SWL are the options (AUA: Strong recommendation). Renal stones <20 mm can be managed with SWL or URS (AUA: Moderate recommendation, EAU: Strong recommendation).[27] The EAU advises PCNL for renal stones >20 mm, whereas the AUA includes SWL with a ureteric stent or a nephrostomy tube as an alternative (AUA: Expert opinion, EAU: Strong recommendation). Furthermore, the AUA endorses active surveillance of asymptomatic nonobstructing renal stones, although no size criteria are given (AUA: Expert opinion).
Follow-up imaging
After active stone management with endourology or SWL, imaging is required to assess the stone clearance (EAU: Strong recommendation). NCCT has the highest sensitivity of stone fragment detection but increased ionizing radiation exposure compared with X-ray or US. The EAU suggests 4 weeks as an appropriate time for interval imaging while acknowledging the lack of high-quality data.[8] Consequently, they leave the timing of imaging and decision to treat the stone fragments to the discretion of the clinician.[28] The AUA recommends that if residual fragments are present, the patient should be offered endoscopic intervention (AUA: Moderate recommendation). In addition, they specifically state that if SWL was unsuccessful in the first attempt, the follow-up procedure should be endourological, although cases of partial fragmentation may be considered for further SWL (AUA: Moderate recommendation).
Secondary prevention
All patients with a new diagnosis of urolithiasis should undergo screening to include medical and dietary history, urinalysis, and serum biochemistry with parathyroid hormone if serum calcium level is elevated (AUA: Clinical principle). Once available, the stone should be sent for analysis to determine its composition (AUA: Clinical principle, EAU: Strong recommendation).
Stone analysis and screening results can be used to classify a patient as having a high risk or low risk for stone formation, with only high-risk patients requiring more detailed metabolic assessment (Table 2) (AUA: Standard). Metabolic urine testing of one or two 24-hour urine collections should include total volume, urinary pH, calcium, oxalate, uric acid, citrate, sodium, potassium, and creatinine with additional tests, such as cystine, when necessary (AUA: Expert opinion). The EAU advises that 2 consecutive 24-hour urine collections be performed.[8]
Table 2.
Summary of risk factors for recurrent stone formation
| Examples | |
|---|---|
| General factors | Family history of stone disease |
| Solitary kidney | |
| Obesity | |
| Recurrent UTIs | |
| Medical conditions | Gastrointestinal diseases affecting absorption (e.g. Crohn’s Disease) |
| RTA type 1 | |
| Primary cystinuria (type A, B, and AB) | |
| Cystic fibrosis | |
| Hyperparathyroidism | |
| Gout | |
| PKD | |
| Type 2 diabetes mellitus | |
| Sarcoidosis | |
| Spinal cord injury, neurogenic bladder | |
| Congenital/Anatomical abnormalities | Medullary sponge kidney |
| UPJ obstruction | |
| Calyceal diverticulum, calyceal cyst | |
| Ureteral stricture | |
| Vesico-uretero-renal reflux | |
| Horseshoe kidney | |
| Ureterocele | |
| Drug induced | Acetazolamide |
| Allopurinol | |
| Aluminum magnesium hydroxide | |
| Ascorbic acid | |
| Calcium | |
| Furosemide | |
| Laxatives | |
| Vitamin D | |
| Topiramate |
UTIs: urinary tract infections; RTA: renal tubular acidosis; PKD: polycystic kidney disease; UPJ: ureteropelvic junction
Ensuring an adequate fluid intake to maintain a daily urine output of >2.5 L is emphasized in both the guidelines (AUA: Standard, EAU: Strong recommendation). Both the organizations provide specific pharmacological and dietary guidance depending on the stone composition and metabolic status (Table 3).
Table 3.
Summary of medical management depending on stone composition
| Stone type | Metabolic status | AUA recommended interventions | AUA strength of recommendation | EAU recommended interventions | EAU strength of recommendation |
|---|---|---|---|---|---|
| Calcium oxalate | Hypercaluria | Limit sodium and calcium intake | Standard | Thiazide and alkaline citrates | Strong |
| Thiazide | Standard | ||||
| Hyperoxaluria | Avoid oxalate-rich foods but | Expert opinion | Oxalate intake restriction | Weak | |
| maintain normal calcium intake | Enteric - Alkaline citrates | Weak | |||
| Calcium and magnesium | Weak | ||||
| Primary-Pyridoxine | Strong | ||||
| Hyperuricosuria | Limit non-dairy animal protein | Expert opinion | Avoid excessive intake of animal protein | Strong | |
| Allopurinol | Standard | Allopurinol (first-line) | Strong | ||
| Febuxostat (second-line) | Strong | ||||
| Hypomagnesuria | Magnesium | ||||
| Hypocitraturia | Increase the intake of fruit and vegetables and limit non-dairy animal intake. | Expert opinion | Alkaline citrates and sodium bicarbonate | Strong | |
| Potassium citrate | Standard | ||||
| Hypernaturia | Restricted salt intake | Strong | |||
| Calcium Phosphate | Hypercaluria | Thiazide | Strong | ||
| Acidic urine | L-methionine | Weak | |||
| Uric Acid | Alkaline urine | Potassium citrate | Standard | Alkaline citrates | Strong |
| Hyperuricouria | Allopurinol | Strong | |||
| Cystine | Increase fluid intake | Expert opinion | Increase fluid intake | ||
| Limit sodium and protein intake | Alkaline citrates | ||||
| Potassium citrate | Standard | Tiopronin (added if above treatments are insufficient) | |||
| Tiopronin (second-line, if unresponsive to above) | expert opinion | ||||
| Struvite | Surgical intervention (first-line) | Option | Surgical intervention (first-line) | Strong | |
| AHA (second-line) | AHA (second-line) | ||||
| Acidic urine | Ammonium chloride or methionine | Weak | |||
| Persistent bacteriuria | Antibiotics | Strong | |||
AHA: acetohydroxamic acid; AUA: American Urological Association; EAU: European Association of Urologists
Calcium stones
The AUA suggests that treatment for calcium stones is dependent on urinary levels of calcium, citrate, and uric acid.[29] In the absence of metabolic abnormalities or those who have been appropriately treated, thiazide diuretics and/or potassium citrate are recommended (AUA: Standard).
Uric acid stones
Urinary alkalinization is recommended as the first-line treatment by using alkaline citrates (AUA: Expert opinion). The AUA specifies that allopurinol should not be used as the first-line therapy (AUA: Expert opinion). The EAU recommends allopurinol in the presence of hyperuricosuria (EAU: Strong recommendation).
Cystine stones
Potassium citrate is recommended as the first-line treatment to neutralize the urine alongside increasing fluid intake (AUA: Expert opinion, EAU: Strong recommendation). The AUA specified that in addition to adequate fluid intake, one should limit the sodium and protein intake (AUA: Expert opinion). Refractory cases should be offered cystine-binding thiol drugs, such as tiopronin (AUA: Expert opinion, EAU: Strong recommendation).
Struvite stones
Surgical intervention is the recommended first-line treatment for struvite stones (EAU: Strong recommendation). The AUA recommends the use of acetohydroxamic acid in those who have exhausted all the surgical options (AUA: Option). The EAU strongly recommends the use of antibiotics in the presence of persistent bacteriuria.
Recommendations and areas of future research
The prevalence of urinary tract stones will increase in the future.[30] As more research is carried out and published,[31] the guidelines will help us manage the patients with an evidence-based approach. Although they can help us make these decisions, treatment should be tailored to individual patient needs and available resources. Future studies should adhere to standardized definitions, paying attention to the patient’s quality of life and the cost of stone prevention and treatment.[32]
In conclusion, both AUA and EAU guidelines offer a detailed, evidence-based framework to guide the urologists in the management of stone diseases. Although some discrepancies exist, particularly regarding the choice of surgical management in specific scenarios, there is generally a consensus between both the groups. However, the guidelines are not applicable to every clinical situation and need to be used in conjunction with the most recently published material and tailored to each individual patient.
Main Points.
The American Urology Association (AUA) and the European Association of Urologists (EAU) both publish evidence-based guidelines on the surgical and medical management of upper urinary tract stones. Only the EAU produces guidelines for bladder stones.
Both groups provide recommendations of varying strength according to their assessment of the level of evidence available.
The AUA and EAU guidance is broadly similar with the main differences existing between choice of surgical management depending on upper urinary tract stone size and location.
Footnotes
Peer-review: This manuscript was prepared by the invitation of the Editorial Board and its scientific evaluation was carried out by the Editorial Board.
Author Contributions: Concept – A.P., B.K.S.; Design – T.H., H.C.H., B.K.S.; Supervision – B.K.S.; Data Collection and/or Processing – T.H., H.C.H.; Analysis and/or Interpretation – T.H., H.C.H., A.P., B.K.S.; Literature Search – T.H., H.C.H.; Writing Manuscript – T.H.; Critical Review – A.P., B.K.S.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Curhan GC. Epidemiology of stone disease. Urol Clin North Am. 2007;34:287–93. doi: 10.1016/j.ucl.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rukin NJ, Siddiqui ZA, Chedgy ECP, Somani BK. Trends in upper tract stone disease in England: Evidence from the hospital episodes statistics database. Urol Int. 2017;98:391–6. doi: 10.1159/000449510. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz BF, Stoller ML. The vesical calculus. Urol Clin North Am. 2000;27:333–46. doi: 10.1016/S0094-0143(05)70262-7. [DOI] [PubMed] [Google Scholar]
- 4.Geraghty RM, Jones P, Hermann TRW, Aboumarkzouk O, Somani BK. Ureteroscopy is more cost effective than shock wave lithotripsy for stone treatment: systematic review and meta-analysis. World J Urol. 2018;36:1783–93. doi: 10.1007/s00345-018-2320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geraghty RM, Cook P, Walker V, Somani BK. Evaluation of the economic burden of kidney stone disease in the UK: a retrospective cohort study with a mean follow-up of 19 years. BJU Int. 2020;125:586–94. doi: 10.1111/bju.14991. [DOI] [PubMed] [Google Scholar]
- 6.Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlage MR, et al. Medical Management of Stones: AUA Guideline. J Urol. 2014;192:316–24. doi: 10.1016/j.juro.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical Management of Stones: AUA/Endourology Society Guideline [Internet] AUA; 2016. [cited 2020 May 3]. Available from: https://www.auanet.org/guidelines/kidney-stones-surgical-management-guideline#x3158. [Google Scholar]
- 8.Türk C, Neisius A, Petrik A, Seitz A, Skolarikos A, Thomas K, et al. Urolithiasis [Internet] EAU; 2000. [updated 2020; cited 2020 May 3]. Available from: https://uroweb.org/guideline/urolithiasis/ [Google Scholar]
- 9.Türk C, Donaldson JF, Neisius A, Petrik A, Seitz C, Skolarikos A, et al. Bladder Stones [Internet] EAU; 2019. [updated 2020; cited 2020 May 3]. Available from: https://uroweb.org/guideline/bladder-stones/ [Google Scholar]
- 10.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxford Centre for Evidence-based Medicine Levels of Evidence [Internet] The Centre for Evidence-Based Medicine; 2009. [cited 2020 May 3] Available from: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ [Google Scholar]
- 12.Oner S, Oto A, Tekgul S, Koroglu M, Hascicek M, Sahin A, et al. Comparison of spiral CT and US in the evaluation of pediatric urolithiasis. JBR-TBR. 2004;87:219–23. [PubMed] [Google Scholar]
- 13.Riccabona M, Lindbichler F, Sinzig M. Conventional imaging in paediatric uroradiology. Eur J Radiol. 2002;43:100–9. doi: 10.1016/S0720-048X(02)00113-4. [DOI] [PubMed] [Google Scholar]
- 14.Aboumarzouk OM, Jones P, Amer T, Kotsiris D, Emiliani E, Somani BK, et al. What is the role of alpha blockers for medical expulsive therapy? Results from a meta-analysis of 60 randomised trials and over 9500 patients. Urology. 2018;119:5–16. doi: 10.1016/j.urology.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Ye Z, Zeng G, Yang H, Tang K, Zhang X, Li H, et al. Efficacy and Safety of Tamsulosin in Medical Expulsive Therapy for Distal Ureteral Stones with Renal Colic: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. Eur Urol. 2018;73:385–91. doi: 10.1016/j.eururo.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Drake T, Grivas N, Dabestani S, Knoll T, Lam T, Maclennan S, et al. What are the Benefits and Harms of Ureteroscopy Compared with Shock-wave Lithotripsy in the Treatment of Upper Ureteral Stones? A Systematic Review. Eur Urol. 2017;72:772–86. doi: 10.1016/j.eururo.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Shen P, Jiang M, Yang J, Li X, Li Y, Wei W, et al. Use of ureteral stent in extracorporeal shock wave lithotripsy for upper urinary calculi: a systematic review and meta- analysis. J Urol. 2011;186:1328–35. doi: 10.1016/j.juro.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 18.Jessen JP, Breda A, Brehmer M, Liatsikos EN, Millan Rodriguez F, Osther PJ, et al. International Collaboration in Endourology: Multicenter Evaluation of Prestenting for Ureterorenoscopy. J Endourol. 2016;30:268–73. doi: 10.1089/end.2015.0109. [DOI] [PubMed] [Google Scholar]
- 19.Pengfei S, Yutao L, Jie Y, Wuran W, Yi D, Hao Z, Jia W. The results of ureteral stenting after ureteroscopic lithotripsy for ureteral calculi: a systematic review and meta-analysis. J Urol. 2011;186:1904–9. doi: 10.1016/j.juro.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 20.L’esperance JO, Ekeruo WO, Scales CD, Jr, Marguet CG, Springhart WP, Maloney ME, et al. Effect of ureteral access sheath on stone-free rates in patients undergoing ureteroscopic management of renal calculi. Urology. 2005;66:252–5. doi: 10.1016/j.urology.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Traxer O, Thomas A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J Urol. 2013;189:580–4. doi: 10.1016/j.juro.2012.08.197. [DOI] [PubMed] [Google Scholar]
- 22.Ge H, Zheng X, Na Y, Hou X, Yu C, Ding W, et al. Bilateral Same-Session Ureteroscopy for Treatment of Ureteral Calculi: A Systematic Review and Meta-Analysis. J Endourol. 2016;30:1169–79. doi: 10.1089/end.2016.0472. [DOI] [PubMed] [Google Scholar]
- 23.Gücük A, Kemahlı E, Üyetürk U, Tuygun C, Yıldız M, Metin A. Routine flexible nephroscopy for percutaneous nephrolithotomy for renal stones with low density: a prospective randomized study. J Urol. 2013;190:144–8. doi: 10.1016/j.juro.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Ruhayel Y, Tepeler AS, MacLennan S, Petřík A, Sarica K, et al. Tract Sizes in Miniaturized Percutaneous Nephrolithotomy: A Systematic Review from the European Association of Urology Urolithiasis Guidelines Panel. Eur Urol. 2017;72:220–35. doi: 10.1016/j.eururo.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 25.Somani BK, Dellis A, Liatsikos E, Skolarikos A. Review on diagnosis and management of urolithiasis in pregnancy: an ESUT practical guide for urologists. World J Urol. 2017;35:1637–49. doi: 10.1007/s00345-017-2037-1. [DOI] [PubMed] [Google Scholar]
- 26.Telli O, Hamidi N, Bagci U, Demirbas A, Hascicek AM, Soygur T, et al. What happens to asymptomatic lower pole kidney stones smaller than 10 mm in children during watchful waiting? Pediatr Nephrol. 2017;32:853–7. doi: 10.1007/s00467-016-3570-7. [DOI] [PubMed] [Google Scholar]
- 27.Featherstone NC, Somani BK, Griffin SJ. Ureteroscopy and laser stone fragmentation (URSL) for large (≥1 cm) paediatric stones: Outcomes from a university hospital. J Pediatr Urol. 2017;13:202.e7. doi: 10.1016/j.jpurol.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Somani BK, Desai M, Traxer O, Lahme S. Stone-free rate (SFR): a new proposal for defining levels of SFR. Urolithiasis. 2014;42:95. doi: 10.1007/s00240-013-0630-3. [DOI] [PubMed] [Google Scholar]
- 29.Phillips R, Hanchanale VS, Myatt A, Somani B, Nabi G, Biyani CS. Citrate salts for preventing and treating calcium containing kidney stones in adults. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD010057.pub2.. doi: 10.1002/14651858.CD010057.pub2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geraghty RM, Proietti S, Traxer O, Archer M, Somani BK. Worldwide impact of warmer seasons on the incidence of renal colic and kidney stone disease: evidence from a systemtic review of literature. J Endourol. 2017;31:729–35. doi: 10.1089/end.2017.0123. [DOI] [PubMed] [Google Scholar]
- 31.Pietropaolo A, Proietti S, Geraghty R, Skolarikos A, Papatsoris A, Liatsikos E, et al. Trends of ‘urolithiasis: interventions, simulation, and laser technology’ over the last 16 years (2000–2015) as published in the literature (PubMed): a systematic review from European section of Uro-Technology (ESUT) World J Urol. 2017;25:1651–8. doi: 10.1007/s00345-017-2055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.New F, Somani BK. A complete world literature review of quality of life (QOL) in patients with kidney stone disease (KSD) Curr Urol Rep. 2016;17:88. doi: 10.1007/s11934-016-0647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]