Significance
Like many other animals, including humans, insects frequently move through densely cluttered environments to perform activities critical for their survival, such as foraging. Vertebrates avoid collisions by perceiving their surroundings in relation to their body size and form, but it is unknown whether insects, with much smaller brains, possess such skills. We discovered that flying bumblebees judge the gap between obstacles relative to their wingspan and reorient themselves to fly sideways through tight spaces. Our findings suggest that bees too evaluate the affordance of their surroundings and account for their own size and form to safely navigate through complex environments. This facet of insect flight poses questions about the neural requisites for perception of self-size in animals.
Keywords: affordances, insect flight, self-size perception, navigation, cluttered environments
Abstract
Animals that move through complex habitats must frequently contend with obstacles in their path. Humans and other highly cognitive vertebrates avoid collisions by perceiving the relationship between the layout of their surroundings and the properties of their own body profile and action capacity. It is unknown whether insects, which have much smaller brains, possess such abilities. We used bumblebees, which vary widely in body size and regularly forage in dense vegetation, to investigate whether flying insects consider their own size when interacting with their surroundings. Bumblebees trained to fly in a tunnel were sporadically presented with an obstructing wall containing a gap that varied in width. Bees successfully flew through narrow gaps, even those that were much smaller than their wingspans, by first performing lateral scanning (side-to-side flights) to visually assess the aperture. Bees then reoriented their in-flight posture (i.e., yaw or heading angle) while passing through, minimizing their projected frontal width and mitigating collisions; in extreme cases, bees flew entirely sideways through the gap. Both the time that bees spent scanning during their approach and the extent to which they reoriented themselves to pass through the gap were determined not by the absolute size of the gap, but by the size of the gap relative to each bee’s own wingspan. Our findings suggest that, similar to humans and other vertebrates, flying bumblebees perceive the affordance of their surroundings relative their body size and form to navigate safely through complex environments.
Avoiding collisions with obstacles is a requirement for successful locomotion through most natural habitats, where the physical environment is often cluttered and complex. At the most elemental level, animals moving through their environments need to identify gaps between obstacles and assess their passability. In this context, whether a gap between obstacles “affords” passing is determined by the fit between the spatial layout of the environment and the properties of the organism’s form and action system, as described in classical theses on affordances (1–3). In humans and other highly cognitive vertebrates, the perception of affordances for performing visually guided actions such as grasping, passing through apertures, and climbing is actively shaped throughout ontogeny, as body size, configuration, and experience change (2, 4–7). However, the strategies used by animals with much smaller brains, such as insects, to contend with the challenges of navigating environmental clutter and spatial heterogeneity are unclear.
We used bumblebees to investigate whether flying insects take into account their own size during interactions with their surroundings. Bumblebees and other volant insects that travel long distances (8) and frequently encounter regions of dense clutter can be expected to exhibit strategies to avoid collisions, because damage to sensitive structures such as the wings is irreparable and adversely impacts flight performance and lifespan (9, 10). For an animal attempting to navigate through tight spaces, perceiving the relationship between the layout of the environment and its own size can help inform the animal of its potential for collision-free passage. Bumblebee workers naturally display large variation in body size within a given colony (11, 12), and thus are particularly suitable models for testing the effects of insect body size on aerial navigation and for determining whether insects perceive the external environment in relation to their own spatial dimensions.
To elicit repeatable flight behavior, we trained foraging bumblebees to fly within a 1.6 × 0.3 × 0.3 m (l × w × h) flight tunnel that separated the hive from a foraging arena (Materials and Methods, SI Appendix, Fig. S1, and Movie S1). After bees were habituated to the setup and began foraging normally, we placed an unexpected obstacle within the tunnel, consisting of a thin vertical wall (5-mm thickness) spanning the tunnel’s width and height. The obstructing wall contained a rectangular gap starting midway up and extending to the top of the wall (Materials and Methods, SI Appendix, Fig. S1, and Movie S1). The width of the gap was varied between 20 and 60 mm over different trials, with the presenting order of gap sizes chosen randomly. A high-speed camera placed above the tunnel was used to record bees’ instantaneous positions, heading/yaw orientations (Fig. 1A), and trajectories as they approached the obstructing wall and passed through the gap. To prevent bees from becoming familiar with the experimental paradigm, the obstructing wall was removed after each flight recording. In total, we recorded and analyzed over 400 flights of bees of varying body sizes flying through seven different gap sizes (SI Appendix, Table S1). For the population of bees recorded, wingspan was the longest dimension of the body and it varied linearly by a factor of 1.9 compared to their longitudinal body length while in flight (SI Appendix, Fig. S2A).
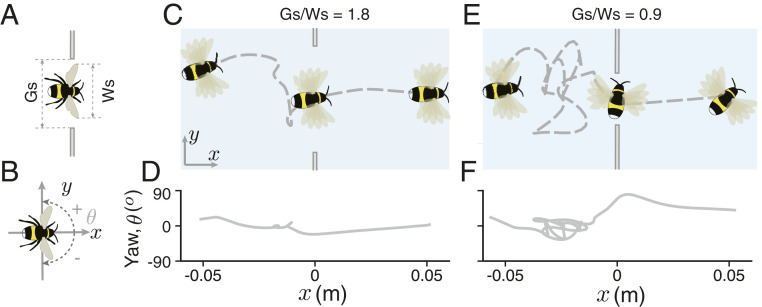
Fig. 1.
Bumblebees can safely fly through gaps that are smaller than their wingspan. (A and B) Illustrations indicating the wingspan of bees (Ws), the size of the gap (Gs), and the positive and negative yaw (heading) angles for bees flying in the tunnel, respectively. (C) Schematic illustration of the flight of a bee flying through a gap that is much wider than its wingspan. (D) The instantaneous yaw angle of bee shown in C. (E) Schematic illustrationof the flightofabeeflying through a gap that is smaller than its wingspan. (F) The instantaneous yaw angle of bee shown in E. Flights, in both cases (C and E), consisted of approach, lateral peering, and—for the smaller gap size (E)—body reorientation (an increase in yaw angle) while passing through the gap. The differences in reorientation behavior can be noted at x = 0 (location of the gap), whereas in F the bee displays a large increase in yaw angle that reorients its body to pass through the small gap, and body reorientation in D is minimal. For the flight shown in C and D, Ws = 27.5 mm and Gs = 50 mm, while for the flight shown in E and F, Ws = 27 mm and Gs = 25 mm.
Results and Discussion
Upon entering the tunnel, all bees flew steadily toward the obstructing wall and began performing lateral scanning maneuvers (flying from side to side), while maintaining a steady gaze directed toward the gap (Fig. 1, SI Appendix, Fig. S3, and Movie S2). Bees scanned the region between the edges of the gap, and mean scanning amplitude was equal to or smaller than gap width in all cases (Fig. 2A). To determine how body size affected the strategies used by bees to fly through gaps of various sizes, we binned bees into three groups based on their wingspan (Materials and Methods). Bees of all sizes performed scanning movements of similar amplitude ahead of each gap, and the amplitude of these maneuvers relative to the gap size consistently increased with decreasing gap size (Fig. 2A).
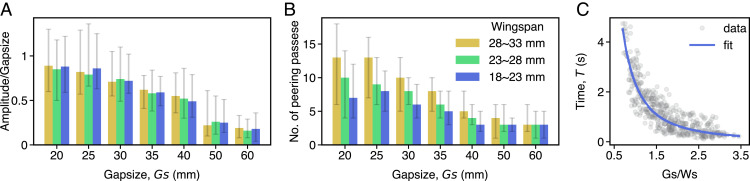
Fig. 2.
Bumblebees spend more time performing repeated peering ahead of relatively narrow gaps. (A) Mean amplitude of lateral peering movements performed by bees of different wingspans approaching gaps of increasing width (see SI Appendix, Tables S2–S4 for statistics). (B) Total number of lateral peering passes performed by bees of different wingspans approaching gaps of increasing width (see SI Appendix, Tables S5–S7 for statistics). For A and B, bees were binned by wingspan as follows: Ws = 28 to 33 mm (yellow), 23 to 28 mm (green), and 18 to 23 mm (blue). (C) Scatterplot (n = 400 flights) showing the time individual bees spent performing peering movements relative to the normalized gap size (gap size divided by wingspan; peering time = 2.344×(Gs/Ws)−1.842, R2 = 0.84). The total number of flights recorded for different gap sizes and bees of different wingspans are given in SI Appendix, Table S1.
What could the purpose of these scanning maneuvers be? Bees are known to use the signals provided by their compound eyes to extract optic flow information and shape their aerial trajectory and gaze strategy, for spatial perception and flight control in their environment (13, 14). The maneuvers performed ahead of the gap (Fig. 1, SI Appendix, Fig. S3, and Movies S2–S4) were similar to peering flights displayed by wasps, honey bees, and bumblebees while memorizing landmarks (15–19) or for depth estimation and spatial localization tasks (20–25). Lateral peering is an active vision strategy in which insects vary the roll angle of their body to redirect a component of the aerodynamic force, creating steady, laterally oscillating flight trajectories (20, 22, 25, 26). Bees scanning in the vicinity of the gap presented all of the characteristics of lateral peering, including maintaining the gap in their frontal visual field (SI Appendix, Fig. S4), where visual acuity is highest (27), and maneuvering sideways by modulating their lateral acceleration (SI Appendix, Fig. S5). By peering between the edges of the gap, bees could combine the time course of their body roll angle, a proxy for lateral acceleration (28), and the angular velocity of the edges of the gap on their retina to discern the spatial dimensions of the gap (see SI Appendix, Fig. S6 for elaboration). This mechanistic explanation could also account for the body-size insensitivity and consistent magnitude of scanning by bees ahead of each gap (Fig. 2A and SI Appendix, Tables S4–S7).
All bees performed more peering passes and engaged in these maneuvers for longer durations when approaching narrower gaps (Fig. 2 B and C). In addition, bigger bees spent more time in the vicinity of the gap before attempting to pass through. Repeated peering passes may help improve bees’ estimation of the gap’s spatial properties, by comparing repeated measurements performed during each pass. This would be particularly important for traversing narrower gaps, where the margin of error is lower during gap traversal. Improving confidence through repeated measurements would also be beneficial if bees do indeed derive estimates of gap size via our proposed mechanism of integrating rate-based metrics of optic flow and flight trajectory (body roll-induced lateral accelerations, see SI Appendix), which may be noisy and imprecise.
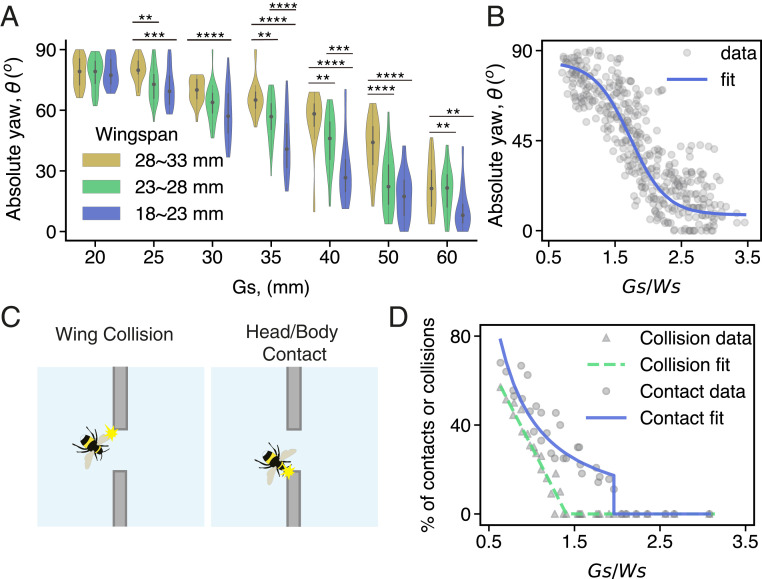
After performing lateral peering maneuvers, all bees were able to successfully fly through the gap, even when traversing the narrowest gap, which was smaller than the wingspan of most of the bees (Figs. 1 and 3A). To accomplish this, bees tended to reorient themselves (i.e., fly sideways, increasing their yaw/heading angle) as they passed through gaps (Fig. 1, SI Appendix, Figs. S1 and S3, and Movies S2 and S5). Bees displayed higher yaw angles (more body reorientation) when passing through smaller gaps, and this trend was consistent across all body sizes (Fig. 3A, compare SI Appendix, Figs. S1 B, i–vi and S3 and Movies S2–S4). By increasing their yaw angle with respect to the flight direction, bees effectively reduced their projected frontal width (i.e., the dimension that they needed to fit between the edges of the gap), because their longitudinal body length was significantly shorter than their wingspan (SI Appendix, Fig. S2B). All bees reoriented themselves maximally (flying completely sideways) when passing through the narrowest gaps (<30 mm), whereas only the larger bees tended to reorient when traversing the widest gap (60 mm; Fig. 3A). We found the largest differences in traversal behavior across bees of different sizes were for flights through intermediate-sized gaps, where larger bees consistently reoriented more compared to smaller bees (Fig. 3A, compare SI Appendix, Figs. S1 B, i–vi and S3). The deliberate modulation of their body orientation while crossing gaps of different sizes provides an indication that bees do indeed factor in their own body size and form in determining how to maneuver through cluttered environments.
Fig. 3.
Bumblebees reorient themselves to pass through narrow gaps. (A) Absolute yaw angle of bees at the midpoint of the gap, observed in bees of different wingspans passing through gaps of increasing width (refer to SI Appendix, Tables S8–S11 for statistics) (B) Scatterplot (n = 400 flights) showing the absolute yaw angle of bees at the midpoint of the gap relative to normalized gap size [gap size divided by wingspan; sigmoidal fit: yaw = 85.22+(−74.88/(1 + 101.7−(Gs/Ws)) × 1.6), R2 = 0.82]. (C) Schematic illustrations of a bee whose wing is colliding with the edge (Left) or who is making contact with the edge (Right) while passing through the gap. Contact was considered to have occurred when any part of the bee’s body (including limbs, head, or antennae, but excluding the wings) touched the edge of the gap. (D) Proportion of flights during which bees’ wings collided with the edges, and during which bees’ bodies made contact with the edges of the gap, as a function of normalized gap size (gap size divided by wingspan). Bees of different wingspans were first segmented into bins of 3 mm between 18 and 33 mm, and the proportion of collisions or contacts was calculated with respect to the number of flights within each wingspan bin and normalized gap size. Power relation between % of flights with contacts CO = 42.61×(Gs/Ws)−1.336, R2 = 0.86, linear relation between % of flights of with collisions CL = −74.76×(Gs/Ws) + 105, R2 = 0.93. See SI Appendix, Table S1 for dimensional representation of collisions and contacts. The number of flights recorded, contacts, and collisions for different gap sizes for bees with different wingspans are given in SI Appendix, Table S1.
When we examined the reorienting response of individual bees with respect to the normalized gap size (gap size divided by each individual’s wingspan), we found a sigmoidal relationship that was consistent across the entire body-size range (Fig. 3B). This suggests that bees’ reactions to the upcoming flight challenge (i.e., the degree to which they reoriented their bodies) was based not on the absolute size of the gap, but rather on the size of the gap relative to their own wingspan. This systematic modulation of body orientation in response to relative gap size bears remarkable similarity to the shoulder rotation response displayed by humans when passing through apertures (7). Adult humans and children initiate a body rotation when walking through apertures that are <1.5 times their shoulder width and maximally rotate their shoulders when apertures are equivalent to their shoulder width (7, 29).
The gap traversal behavior we observed in bees suggests that their strategy for navigating in complex environments includes not only the putative optomotor framework for flight control, but also incorporates properties of the insect’s own form. Although this behavioral performance of bees is similar to that of humans, this finding does not necessarily imply that bees maintain internal embodiments of themselves as has been claimed for humans, apes, dogs, and other vertebrates (4–6) or that they are cognitively aware of their own body dimensions. Nevertheless, at the behavioral level, bees appear to take account of their body size and form in relation to the environment to modulate their flight strategy. The perception of environmental properties in relation to body size to maneuver through narrow gaps presents the hallmarks of affordance analysis (2, 7).
How could bees relate visual information about the environment to their own body form? Experiments on walking locusts and fruit flies suggest that experience specific to the locomotion task facilitates the calibration of visual inputs to body features and such calibrations are maintained in the insect’s memory (30, 31). These studies reported that the insects calibrate optic flow to stride length during development, resulting in body-scaled information about their environment relative to their leg length. Such scaling between visual information and body features is likely to depend on the environmental challenge and to differ for insects when walking vs. flying, due to differences in sensory input and motor action. Furthermore, the potential cost of collisions is higher during flight (wing damage, crash landing, etc.) than when walking, and this could influence the fidelity of such internal calibrations.
Nevertheless, the processes that facilitate the calibration of visual information to body size, including the role of learning and experience (30, 31), could be similar across locomotion modes and quite general across species. In walking humans, the visual angle subtended by the edges of an aperture provides body-scaled information about aperture width, as a ratio of eye height, and is calibrated to body width to perceive passability (7). Further experiments on humans have found that lateral head movements, or heady swaying, during walking also facilitates aperture perception and traversal performance (32). Meanwhile, previous studies on budgerigars have revealed that flying birds modulate their wing beat or tuck in their wings to negotiate gaps and have suggested that birds may calibrate the rate of expansion of the angle subtended by the edges of the gap to gauge its size (33). The scanning maneuvers we observed in bees as they approached a gap would generate optic flow that specifies gap size in the same length unit as the bee’s lateral velocity (SI Appendix, Fig. S6). If the latter is calibrated by experience during development in wingspans per second, then the gap size estimated from peering would be scaled to bees’ own body width.
Learning through trial and error may also play a role in bees’ ability to negotiate narrow gaps, in cases where an individual bee has previously made numerous flights through complex environments, some of which were successful while others resulted in collisions/crashes. Many studies have demonstrated bumblebees’ ability to rapidly learn complex relations and abstract concepts across sensory modalities (34), and since the bees in our study were experienced flyers, it is likely that any trial-and-error learning acquired during previous flights would have played some role in their ability to scale visual information to their own body form. However, for the behaviors observed here—where bees not only judge gaps based on their wingspan, but also reorient to an angle that takes advantage of their body shape and allows them to pass safely through narrow gaps—to be acquired solely through trial-and-error learning would require a relatively complex and lengthy process. A process involving identification of relevant visual variables and their relation to other sensorimotor cues, as well as numerous failed attempts that would result in body and/or wing collisions. Because collisions could lead to irreparable wing damage that impairs flight performance and survival, relying solely on trial-and-error learning to develop complex behavioral strategies for navigating cluttered environments may be an option with limited feasibility.
Although bees were able to successfully fly through gaps under all conditions, they more frequently made contact with the edges of the gap (touching the edges with extended legs or the head/antennae, Movie S6) or experienced wing collisions with the edges as gap size decreased, suggesting that learning could involve such feedback from these body parts. Contact with the edges of the gap occurred in a small portion of flights when gap size was approximately twice the bees’ wingspan, and the frequency of flights in which contact occurred increased sharply with decreasing relative gap size (Fig. 3 C and D). Wing collisions did not occur until gap size was reduced to ∼1.5 times the wingspan, and the proportion of flights where wing collisions occurred also increased with decreasing relative gap size (Fig. 3C). In some extreme cases, bees “head butted” the obstructing wall as they flew sideways through the gap (Movie S7). Such apparently deliberate contacts made between the bee’s head or limbs and the obstacle were likely aimed at protecting the more delicate wings from collisions. The fact that bees did experience frequent wing collisions when flying through smaller gaps (e.g., during ∼40% of flights when flying through gaps equal to the wingspan; Fig. 3B), despite consistently reorienting themselves, highlights the challenge of navigating safely through dense clutter and points to the importance of morphological adaptations in wings that allow them to tolerate collisions (35).
Overall, the bees’ behavior observed here reveals another facet of aerial navigation in insects and highlights the relatively limited neural infrastructure needed to take account of self-form when performing visually guided tasks. This suggests that the capacity to perceive affordances during locomotion through complex environments, a capacity only reported so far for vertebrate animals, could be widespread among insects. The comparison of bees’ performance with that of other animals highlights the various behavioral strategies employed for perceiving the affordance of the environment across different animal taxa (SI Appendix, Fig. S7). From an ecological perspective, the robustness and consistency with which bumblebees in our study were able to navigate through such a challenging environment is likely a key trait that allows these important pollinators to efficiently collect resources in complex, cluttered environments, and one which contributes to their survival.
Materials and Methods
Experiment Setup.
Experiments were performed on individual workers from Bombus terrestris (36) colonies that were maintained within the laboratory. Hives were sourced from a commercial breeder (Koppert Biological Systems) and placed within a 0.4 × 0.4 × 0.3 m mesh enclosure that was covered with dark cloth to simulate the natural underground habitat of the bees. The hive enclosure was connected to a flight tunnel (0.3 × 0.3 × 1.5 m) that led to a 2 × 1.5 × 2 m foraging chamber, which in turn provided access to the outdoor environment (SI Appendix, Fig. S1 and Movie S1). Apart from outdoor foraging, the bees were also provided ad libitum access gravity feeders containing 30% sucrose solution placed within the foraging chamber. Connections between the hive enclosure, flight tunnel, and foraging chamber were made using 30-mm inner diameter and 150-mm-long flexible silicon tubing. Consistent foraging flights by numerous worker bees were observed within 1 d of moving a new hive to the enclosure. Experiments were performed once steady and consistent foraging traffic was noted within the flight tunnel, defined as when >15 flights per minute were observed. The temperature within the hive enclosure, flight tunnel, and foraging chamber was maintained at 23 °C. Ample natural lighting from the windows was available for within the foraging chamber and flight tunnel. The bees were given 1 wk to habituate to the environment before experiments began.
Bumblebee individuals within a hive display natural variation in body size, and our goal was to collect sufficient data from bees covering this entire range of sizes. However, preliminary tests revealed that the majority of bees flying within the flight tunnel were of intermediate size (i.e., smaller and larger bees constituted a smaller portion of individuals that flew within the tunnel). In order to collect a large sample containing bees of widely ranging body sizes flying through gaps of different sizes, we performed experiments on four hives. A total of 400 flights performed by bees from these four hives were recorded and analyzed. The number of flights collected for bees of different body sizes is shown in SI Appendix, Fig. S2.
Testing Procedure.
During experiments, manually controlled gates on either side of the flight tunnel were used to regulate traffic. Only one bee at a time was permitted to enter the flight tunnel, and only bees returning to the hive were considered for analysis as they displayed high motivation to fly through the gap. The sidewalls and floor of the tunnel were lined with a random cloud pattern with spatial frequencies varying by 1/f, similar to that used in ref. 37. An obstacle was created within the flight tunnel by adding a vertical wall containing a rectangular cut-out (aperture) starting from the middle and extending to the top of the tunnel (SI Appendix, Fig. S1) that bees were required to fly through to return to the hive. Seven different experimental conditions were tested, with the width of the rectangular gap set to 20, 25, 30, 35, 40, 50, or 60 mm. Gaps <20 mm were not tested as the bees did not display flight traversal and instead tended to land on the edge of the gap and crawl through. For all conditions, the wall containing the gap was placed 0.75 m from the entrance of the tunnel (SI Appendix, Fig. S1). During experimental sessions, the gap size was varied randomly between each recording, while the bees to be subsequently tested were in the foraging chamber. Once a bee passed through the gap and entered the nest, the obstructing wall was removed to inhibit learning or familiarization with the experimental conditions by bees departing from the hive to forage.
To examine the response of naive bees that had never (or rarely) encountered the obstructing wall and flown through the gap, experimental bouts lasted no longer than 30 min. Russell et al. (38) found that foragers perform on average 4 to 15 foraging trips over the day. Thus, it is unlikely that many bees would have performed multiple trips within an experimental bout. Bees were not individually marked in this study; although this increased the possibility of collecting unequal numbers of flight recordings among different individuals for each condition, the likelihood was greatly reduced by recording consecutive flights from different bees only when bees were returning to the hive. Additionally, because we filmed numerous foragers from different hives and recorded a large number of flight trajectories (n = 400), our dataset is likely to be representative of the population. Finally, because this study was focused on analyzing the flight behavior of bees when passing through gaps (rather than examining individual changes in performance with experience), individual-level monitoring to track learning was not necessary.
Digitization and Analysis.
Flight trajectory, orientation, and morphology.
An Optronis CR6 high-speed camera was placed 1.7 m above the midline of the flight tunnel, aimed directly downward over the gap. Flights were recorded at 200 Hz with a 1/500-s shutter, and a region covering 350 mm of length along the tunnel leading to the gap was kept within the field of view. The ceiling of the flight tunnel consisted of 5-mm UV-transmitting transparent acrylic panels that spanned the width of the tunnel. The majority of bees never collided with the roof while on foraging bouts and flights containing roof collisions were omitted from the analysis. Only flights of individuals that appeared to be returning from foraging trips (i.e., bees that made a steady and direct flight toward the obstructing wall) were used for analysis; at least one such flight was observed every two minutes. Bees carrying pollen were also excluded from this study.
During postprocessing, lens distortion was corrected using standard MATLAB Image Processing Toolbox routines. An object of known dimensions was placed within the field of view at midheight of the tunnel and related to the pixels in the rectified image for two-dimensional (2D) spatial calibration. Custom MATLAB script was written to process each image frame and isolate the bee from the background. An ellipse was fitted to the body of the bee in each frame, and the centroid location, body length and width, and heading (yaw angle) were measured over the entire flight. In order to attenuate digitizing errors, the flight trajectories were passed through a 30-Hz fourth-order Butterworth filter. Yaw/heading orientation was calculated with respect to the flight tunnel using the right-hand rule. As the flights were recorded from a single perspective, body pitch and roll could not be estimated. No systematic bias was noted among recorded flights in the direction to which bees reoriented (i.e., clockwise vs. counterclockwise yaw) while passing through the gap. The body length (estimated as the length of the major axis of the ellipse) varied by <8% over the entire flight sequence, indicating that bees maintained nominally similar altitude as they approached and crossed the gap.
In addition, for 10 frames each at the start and finish of the recorded video segment and during gap crossing, the location of the bee’s head, wing roots, and wing tips were digitized manually. These images also contained shadows indicating the range of wing motion during the flap that were used to estimate the wingspan. A similar method has been used (39) to measure wingspan and estimate wing beat amplitude in bumblebees. We compared measurements from the three sets of manually digitized frames to evaluate consistency in morphological estimates and found <4% variation in the morphological metrics, which was considered acceptable.
The choice of the bins used to separate bees of different wingspans was determined by dividing the entire range of wingspans into three equal segments. For our case, the range represented 18 to 33 mm; therefore, the bees were segmented as either small (18 to 23 mm), medium (23 to 28 mm), or larger (28 to 33 mm).
Body contacts and wing collisions.
Flights during which bees contacted the edges of the gap with their head, body, and/or legs (“body contacts”) were visually noted during digitization. Identifying the wing tip path during the stroke for all instances was challenging due to the high wing beat frequency of bees. Wing collisions were therefore estimated during postprocessing as follows: a circular wedge with radius equal to the wing length for each individual bee and angle equivalent to the mean stroke amplitude (derived from the manually digitized frames) was placed at the root of each wing, for all frames as the bees approached and passed through the gap. The angular position of the wedge with respect to the body was maintained as the bees varied their heading during flight. Wing collisions were considered to have occurred if the arc intersected the edges of the gap during traversal. We tested this method on several recordings made at 3,000 frames per second (where the wings were clearly visible) and found that wing collisions were reliably identified by the postprocessing method.
Peering.
Peering was defined as lateral flight maneuvers performed by the bees while maintaining nominally constant gaze in the direction of the gap. These trajectories were identified during postprocessing using custom routines written in MATLAB; these routines isolated flight segments during which the bees’ dominant velocity was oriented laterally with respect to their body axis and the gap was within 60° of their frontal visual field. In general, the gap remained within 60° of bees’ frontal visual field for nearly the entire time when bees were flying near the gap (SI Appendix, Fig. S4). In the near vicinity of the gap, the number of lateral peering passes was determined by counting segments of the trajectories where the lateral velocity was reversed before the bees initiated a gap-crossing maneuver. Regions very close to the gap (<5 mm) were not considered in peering estimation because traversal maneuvers were initiated here. Only trajectory segments >7 mm were considered to represent genuine peering behavior—but this limit on segment length did not significantly influence overall trends in the results (as compared to including segments of all lengths). Total peering time was estimated by summing the time spent by the bees engaging in the lateral maneuvers.
Statistical Analysis.
Before performing statistical tests, datasets were tested for normality using a Shapiro–Wilk test for the combination of three groups (body-size bins) and seven experimental conditions (gap sizes). We used a two-way ANOVA to test for statistical significance of the variation in quantities between bees of different body size, followed by post hoc tests to determine which groups differed significantly (for example: we used this test to reject the null hypothesis that the reorientation behavior of the bees, across the different gaps presented, was insensitive to the bees’ wingspan [categorized into three bins]) (Fig. 3 and SI Appendix, Table S11). To compare quantities across bees of different wingspans for a particular gap size, t tests were used to assess statistical significance, with an adjusted P value (Bonferroni multiple testing method, P value <0.01). For example, for each gap size we used this test to verify that the peering amplitude of bees was consistent across the different wingspan bins (Fig. 2 and SI Appendix, Table S3). Statistical analyses were performed in R (version 3.5.3), using the tidyverse, ggpubr, and rstatix libraries.
Supplementary Material
Acknowledgments
We would like to thank Lars Chittka, Adrian Dyer, Sapna Sharma, Malen Link, and two anonymous reviewers for their comments and discussion during manuscript preparation. This project was supported by the Alexander von Humboldt Stiftung through a research fellowship awarded to S.R. as well as by the Deutsche Forschungsgemeinschaft (DFG grant number: EG82/19-1) and by the Center of Excellence EX 277 Cognitive Interaction Technology, funded by Deutsche Forschungsgemeinschaft. L.L. acknowledges the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy–EXC 2117-422037984, Zukunftskolleg Independent Research Grant (P82967018 FP 330 670/18), the Max Planck Society, support from Couzin lab, and the support of NVIDIA corporation with the donation of a Titan Xp. W.H.W. acknowledges support from the United States National Institutes of Health, Grant R01EY029745.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016872117/-/DCSupplemental.
Data Availability.
All data are included in the manuscript and supporting information.
References
- 1.Gibson J. J., Visually controlled locomotion and visual orientation in animals. Br. J. Psychol. 49, 182–194 (1958). [DOI] [PubMed] [Google Scholar]
- 2.Gibson J. J., “The theory of affordance” in Perceiving Acting and Knowing, Shaw R., Bransford J., Eds. (Lawrence Erlbaum, 1977), pp. 127–142. [Google Scholar]
- 3.Warren W. H., Jr, Perceiving affordances: Visual guidance of stair climbing. J. Exp. Psychol. Hum. Percept. Perform. 10, 683–703 (1984). [DOI] [PubMed] [Google Scholar]
- 4.Povinelli D. J., Cant J. G. H., Arboreal clambering and the evolution of self-conception. Q. Rev. Biol. 70, 393–421 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Dale R., Plotnik J. M., Elephants know when their bodies are obstacles to success in a novel transfer task. Sci. Rep. 7, 46309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenkei R., Faragó T., Kovács D., Zsilák B., Pongrácz P., That dog won’t fit: Body size awareness in dogs. Anim. Cogn. 23, 337–350 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren W. H. Jr, Whang S., Visual guidance of walking through apertures: Body-scaled information for affordances. J. Exp. Psychol. Hum. Percept. Perform. 13, 371–383 (1987). [DOI] [PubMed] [Google Scholar]
- 8.Osborne J. L., et al. , Bumblebee flight distances in relation to the forage landscape. J. Anim. Ecol. 77, 406–415 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Foster D. J., Cartar R. V., What causes wing wear in foraging bumble bees? J. Exp. Biol. 214, 1896–1901 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Cartar R. V., Morphological senescence and longevity: An experiment relating wing wear and life span in foraging wild bumble bees. J. Anim. Ecol. 61, 225 (1992). [Google Scholar]
- 11.Garófalo C. A., Bionomics of Bombus (Fervidobombus) morio 2. Body size and length of life of workers. J. Apic. Res. 17, 130–136 (1978). [Google Scholar]
- 12.Knee W. J., Medler J. T., The seasonal size increase of bumblebee workers, (Hymenoptera: Bombus). Can. Entomol. 97, 1149–1155 (1965). [Google Scholar]
- 13.Egelhaaf M., Boeddeker N., Kern R., Kurtz R., Lindemann J. P., Spatial vision in insects is facilitated by shaping the dynamics of visual input through behavioral action. Front. Neural Circuits 6, 108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan M. V., Honey bees as a model for vision, perception, and cognition. Annu. Rev. Entomol. 55, 267–284 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Mertes M., Dittmar L., Egelhaaf M., Bumblebee homing: The fine structure of head turning movements. Plos Biol. 10, e0135020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stürzl W., Zeil J., Boeddeker N., Hemmi J. M., How wasps acquire and use views for homing. Curr. Biol. 26, 470–482 (2016). [DOI] [PubMed] [Google Scholar]
- 17.de Ibarra N. H., Philippides A., Riabinina O., Collett T. S., Preferred viewing directions of bumblebees (Bombus terrestris L.) when learning and approaching their nest site. J. Exp. Biol. 212, 3193–3204 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Dittmar L., Stürzl W., Baird E., Boeddeker N., Egelhaaf M., Goal seeking in honeybees: Matching of optic flow snapshots? J. Exp. Biol. 213, 2913–2923 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Braun E., Dittmar L., Boeddeker N., Egelhaaf M., Prototypical components of honeybee homing flight behavior depend on the visual appearance of objects surrounding the goal. Front. Behav. Neurosci. 6, 1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kral K., Poteser M., Motion parallax as a source of distance information in locusts and mantids. J. Insect Behav. 10, 145–163 (1997). [Google Scholar]
- 21.Egelhaaf M., Kern R., Lindemann J. P., Motion as a source of environmental information: A fresh view on biological motion computation by insect brains. Front. Neural Circuits 8, 127 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart F. J., Kinoshita M., Arikawa K., The roles of visual parallax and edge attraction in the foraging behaviour of the butterfly Papilio xuthus. J. Exp. Biol. 218, 1725–1732 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Werner A., Stürzl W., Zanker J., Object recognition in flight: How do bees distinguish between 3D shapes? PLoS One 11, e0147106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecoeur J., Dacke M., Floreano D., Baird E., The role of optic flow pooling in insect flight control in cluttered environments. Sci. Rep. 9, 7707 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravi S., et al. , Gap perception in bumblebees. J. Exp. Biol. 222, jeb184135 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Boeddeker N., Hemmi J. M., Visual gaze control during peering flight manoeuvres in honeybees. Proc. Biol. Sci. 277, 1209–1217 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapustjanskij A., Streinzer M., Paulus H. F., Spaethe J., Bigger is better: Implications of body size for flight ability under different light conditions and the evolution of alloethism in bumblebees. Funct. Ecol. 21, 1130–1136 (2007). [Google Scholar]
- 28.Ravi S., et al. , Bumblebees minimize control challenges by combining active and passive modes in unsteady winds. Sci. Rep. 6, 35043 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilmut K., Barnett A. L., Locomotor behaviour of children while navigating through apertures. Exp. Brain Res. 210, 185–194 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Ben-Nun A., Guershon M., Ayali A., Self body-size perception in an insect. Naturwissenschaften 100, 479–484 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Krause T., Spindler L., Poeck B., Strauss R., Drosophila acquires a long-lasting body-size memory from visual feedback. Curr. Biol. 29, 1833–1841.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Fath A. J., Fajen B. R., Static and dynamic visual information about the size and passability of an aperture. Perception 40, 887–904 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiffner I., Vo H. D., Bhagavatula P. S., Srinivasan M. V., Minding the gap: In-flight body awareness in birds. Front. Zool. 11, 64 (2014). [Google Scholar]
- 34.Chittka L., Bee cognition. Curr. Biol. 27, R1049–R1053 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Mountcastle A. M., Combes S. A., Biomechanical strategies for mitigating collision damage in insect wings: Structural design versus embedded elastic materials. J. Exp. Biol. 217, 1108–1115 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Linnaeus C., Systema Naturae per Regna tria Naturae: Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis (Laurentius Salvius, Stockholm, 10th Ed., 1758). [Google Scholar]
- 37.Monteagudo J., Lindemann J. P., Egelhaaf M., Head orientation of walking blowflies is controlled by visual and mechanical cues. J. Exp. Biol. 220, 4578–4582 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Russell A. L., Morrison S. J., Moschonas E. H., Papaj D. R., Patterns of pollen and nectar foraging specialization by bumblebees over multiple timescales using RFID. Sci. Rep. 7, 42448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillon M. E., Dudley R., Surpassing M., Surpassing Mt. Everest: Extreme flight performance of alpine bumble-bees. Biol. Lett. 10, 20130922 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript and supporting information.