Abstract
Stunting affects 160 million pre-school children globally with adverse life-long consequences. While work within nutritional science suggests that stunting in early childhood is associated with low intakes of animal-sourced foods (ASFs), this topic has received little attention from economists. We attempt to redress this omission through an analysis of 130,432 children aged 6–23 months from 49 countries. We document distinctive patterns of ASF consumption among children in different regions. We find evidence of strong associations between stunting and a generic ASF consumption indicator, as well as dairy, meat/fish, and egg consumption indicators, and evidence that consuming multiple ASFs is more advantageous than any single ASF. We explore why ASF consumption is low but also so variable across countries. Non-tradable ASFs (fresh milk, eggs) are a very expensive source of calories in low-income countries and caloric prices of these foods are strongly associated with children’s consumption patterns. Other demand-side factors are also important, but the strong influence of prices implies an important role for agricultural policies—in production, marketing and trade—to improve the accessibility and affordability of ASFs in poorer countries.
Key words: Animal-sourced foods, dietary diversity, fisheries, livestock, nutrition, stunting
Approximately 160 million children under the age of 5 and living in developing countries are considered chronically undernourished or stunted (de Onis and Branca 2016). That is, their height is at least two standard deviations less than the median height of a well-nourished child of the same age and sex. Nutritional status is a component of health and thus reducing chronic undernutrition has intrinsic value. In addition, chronic undernutrition in early life is causally linked to adverse later-life economic outcomes, including reduced schooling, poorer cognitive skills, lower earnings and a higher likelihood of living in poverty (Hoddinott, Rosegrant, and Torero 2013).
This article was invited by the President of the Agricultural & Applied Economics Association for presentation at the 2018 annual meeting of the Allied Social Sciences Association, after which it was subjected to an expedited peer-review process.
Derek Headey and Kalle Hirvonen are with the International Food Policy Research Institute (IFPRI); John Hoddinott is with Cornell University. The authors thank seminar participants at the Allied Social Science Association (ASSA) meetings in Philadelphia, January 2018, the editor, and three anonymous reviews for helpful comments and suggestions. This research was conducted under the “Advancing Research on Nutrition and Agriculture (AReNA)” project, supported by The Bill & Melinda Gates Foundation (Investment ID: OPP1112307) and the CGIAR Program on Agriculture for Nutrition and Health (A4NH). Funding for this research was received from The Bill and Melinda Gates Foundation through the grant Advancing Research on Nutrition and Agriculture (ARENA) Phase II (Investment ID: OPP1177007). We thank Marie Ruel and two anonymous referees for useful comments. The usual disclaimer applies.
Most stunting manifests in the first 1,000 days of life (Victora et al. 2010). In many countries children are born small, partially protected from infections and low nutrient diets by predominant breastfeeding in the first few months after birth, but then subject to accelerated growth faltering from 6–24 months. Several things need to happen in this 6–24-month period to ensure that growth faltering does not take place. First, since breast milk can no longer provide all nutrients required for healthy growth and development, starting around the age of 6 months, children need to be introduced to complementary foods that have to be provided frequently (because of small gastric capacity) and fed a nutrient-rich diet (or fortified complementary foods) to ensure that they meet their nutrient requirements. Second, because young children’s immune systems are in development, they need to be protected from both acute and chronic infections through preventative measures (e.g., improved water, sanitation, hygiene, food safety, immunization) and optimal use of health services for timely treatment. After 24 months, further growth faltering is less commonplace as immune systems strengthen, but in developing country contexts there is also little evidence of systematic catch-up growth in the first 5 years of life, and stunting tends to be highly persistent at a population level (Leroy et al. 2014).
While there is broad agreement on the important general interplay between diets, health, and care practices, thinking on the importance of specific nutrient dimensions of child undernutrition has evolved over time. In the post-war period, substantial emphasis was placed on protein deficiency as one of the main causes of undernutrition in low-in-come countries (Semba 2016), but an influential article by McLaren (1974) argued that if a child consumed an adequate amount of calories, it was likely that they would also consume adequate amounts of protein. Coinciding with the 1974 global food (calorie) crisis, McLaren’s arguments prompted a much greater emphasis on improving calorie availability. This also suited the emphasis of economists and agronomists on increasing the productivity of calorie-dense staple crops through the Green Revolution. In recent years, however, the 1970s revisionist view has been called into question—there are three strands to this questioning.
The first comes from new work in metabolomics (the study of small molecule chemicals that result from metabolic processes) aimed at understanding cellular growth processes. A relevant example is improvements in understanding mTORs. These are catalytic proteins that signal (or regulate) anabolic processes such as cellular growth and differentiation. One type, mechanistic target of rapamycin complex 1 (mTORC1) regulates growth in chondral plates (i.e., part of the bone where growth takes place) and in skeletal muscle growth. Essential amino acids are needed for the activation of mTORC1 (Semba 2016). These cannot be synthesized from scratch within the human body; they must be obtained via diet; the best sources are animal source foods (ASFs) (meat, poultry, fish, and eggs). Plant sources also contain these, but typically in much lower concentrations. In addition, ASFs are dense in a wide range of micronutrients linked to growth and cognitive development (iron, B12, choline, zinc), and cow’s milk is uniquely rich in calcium and its ability to stimulate the secretion of insulin-like growth factor I (IGF-I), a hormone that stimulates bone and tissue growth (Dror and Allen 2011).
Supporting this view is a smattering of small-scale, somewhat idiosyncratic randomized control trials and observational studies. Walker et al. (1991) provided a high-protein milk-based supplement to stunted children aged 9–24 months in Jamaica, finding that the growth in length was 0.89 cm higher in the treatment group. Likewise, several observational analyses of household surveys show that children from cow-owning households are taller than children whose households do not own cows (Rawlins et al. 2014; Hoddinott, Headey, and Dereje 2015; Choudhury and Headey 2017a) even after controlling for household wealth.1,2 Tang et al. (2014) conducted a cluster-randomized controlled trial in China that provided a daily 60g-portion of minced pork to a treatment group. The growth in length was 0.26 cm higher in the treatment group relative to the control group who received rice. Iannotti et al. (2017) randomly assigned 6–9 month-old children into treatment that provided one egg per day for a 6-month period and a control that followed a usual diet. The effects were large: length-for-age z score in-creased by 0.63 SD and stunting prevalence decreased by 47% relative to the control group.
Finally, economic historians have been examining the links between access to ASFs, primarily meat and dairy, and adult stature. Data from 19th century Bavaria show how statures were greater in regions that had higher levels of per capita milk production (Baten and Murray 2000; Baten 2009).3
Similarly, per capita cattle holdings are found to be an important predictor of within-country differences in female stature in post-colonial Africa (Moradi and Baten 2005). Takahashi (1984) argues that the post-war improvements in child heights in Japan are strongly linked to the introduction of dairy into the Japanese diet.
The common implication of these different strands is that chronic undernutrition in developing countries may reflect low levels of ASF intake. But the small-scale nature of the few randomized control trials of ASFs, together with the idiosyncratic nature of observational work, gives pause. To the best of our knowledge, there are no studies that systematically assesses patterns of ASF consumption in the critical 6–24-month growth window across countries and regions, whether these consumption patterns are associated with stunting, and what economic drivers underpin these patterns of ASF consumption. This paper attempts to redress these knowledge gaps by posing three objectives.
The first is descriptive. Using an unusually large agglomeration of recent Demographic Health Surveys (DHS), encompassing 130,432 children aged 6–23 months living in 49 low-and middle-income countries, we document complex regional patterns of ASF in-take in 24-hour recall data. Second, we use these data to conduct an age-disaggregated multivariate analysis of the associations be-tween children’s food consumption patterns and child height in models saturated with a host of relevant control variables. We assess the predicted decline in stunting due to consumption of any ASF, multiple ASFs, specific ASF groups (dairy, eggs, meat/fish), as well as regional variation in ASF-stunting relationships. Third, we explore why ASF consumption is low, but also so variable across countries and regions, even those at similar levels of income. We link DHS data to national level data on “cereal-relative caloric prices” that tell us how expensive a source of calories each ASF is relative to the cheapest staple cereal in each country. Combined, these data can shed light on why consumption of ASFs vary across countries and house-holds. This analysis provides a potential entry point into this issue for agricultural economists because high relative ASF prices imply an important role for agricultural policies—in production, marketing and trade—to improve the accessibility and affordability of ASFs in poorer countries.
Data and Methods
We use multi-country household survey data from phases 5 and 6 of the Demographic Health Surveys (DHS), covering surveys con-ducted between 2006 and 2014 (ICF International 2015). The DHS are widely used in analyses of child nutrition, including the main dependent variables in our analysis, child stunting, which is defined as a height-for-age Z-score less than 2 standard deviations below the World Health Organization (WHO; 2006) growth standards. Since the mid-2000s (phase 5), the DHS has implemented a highly standardized dietary module in which mothers are asked to recall which of 14 disaggregated food groups their youngest child (0–24 months of age) consumed in the past 24 hours.4 Table 1 lists these aggregated food groups along with the aggregations we use for our analysis. These data are available for 130,432 children aged 6–23 months from 49 countries in five regions, thus ensuring reasonable region-specific sample sizes see supplementary online table 1 for a full list of countries): Latin America & Caribbean (7 countries, 29,387 children); Middle East & North Africa (4 countries, 14,445 children); Central, South & South-East Asia (9 countries, 24,721 children); West & Central Africa (16 countries, 37,474 children); Eastern & Southern Africa (13 countries, 24,405 children).
Table 1. Aggregating Food Groups in Phases 5 and 6 of the Demographic Health Surveys.
| Food Groups Found in DHS Data | Aggregated Food Groups Used in This Analysis | Aggregated Food Groups Used in This Analysis with ASFs Disaggregated |
|---|---|---|
| (1) Grains | Starchy staples: (1) Grains, | Starchy staples: (1) Grains, |
| (2) Roots/tuber | (2) Roots/tuber | |
| (2) Roots/tuber | ||
| (3) Legumes/nuts | Legumes/nuts | Legumes/nuts |
| (4) Vitamin A rich fruits | Any fruit: (4) Vitamin A rich fruits; (6) Other fruits | Any fruit: (4) Vitamin A rich fruits; (6) Other fruits |
| (5) Vitamin A rich vegetables | Any vegetables: (5) Vitamin A rich vegetables; (7) Dark green leafy vegetables; (8) Other vegetables |
Any vegetables: (5) Vitamin A rich vegetables; (7) Dark green leafy vegetables; (8) Other vegetables |
| (6) Other fruits | ||
| (7) Dark green leafy vegetables, | ||
| (8) Other vegetables | ||
| (9) Fresh/powdered cow’s | Any ASF: (9) Fresh/powdered cow’s milk; (10) Infant formula; (11) Eggs; (12) Meat/organs; (13) Fish |
Dairy: (9) Fresh/powdered cow’s milk; (10) Infant formula; |
| (10) Infant formula | ||
| (11) Eggs | Eggs | |
| (12) Meat/organs | Meat/fish: (12) Meat/organs; | |
| (13) Fish | ||
| (13) Fish | ||
| (14) Fortified infant cereals |
While the large sample sizes and broad geographic coverage of these data are considerable strengths, there are limitations. First, we only know whether a child consumed some amount of these food groups in the past 24 hours. We do not have data on quantities, nor do we know the frequency or duration of consumption. Second, where there are multiple children aged less than 24 months in the household, the DHS only collects dietary data for the youngest child. The youngest children within this age range are not relevant to our analysis since: (a) children 0–5 months are (or should be) exclusively breastfed; and (b) one would not expect to see instantaneous growth benefits from a re-cent introduction of complementary foods (e.g., in the 6–11-month range). Instead, the benefits of an improved diet would be expected to accumulate throughout the first two years of life, consistent with the growth-faltering patterns observed in Victora et al. (2010). Indeed, one recent analysis strongly suggests that analyses of the determinants of child growth should typically focus on children who have completed the first two years of life (Alderman and Headey 2018). For this reason, we focus on children aged 6–23 months, but also disaggregate within this age group to focus on 6–11, 12–17 and 18–23-month age brackets. Consistent with Alderman and Headey (2018), our expectation is that the associations between dietary indicators and stunting is strongest for the 18–23-month group.
A bivariate analysis of consumption of ASFs and stunting carries with it the obvious risks associated with the presence of confounding factors. Many factors that might determine ASF consumption might also affect nutritional status through other channels. For this reason, we estimate linear probability models that include the full set of controls listed in table 2—mostly drawn from (Danaei et al. 2016).5 In addition to controlling for child age (through age-in-month dummies) and sex, our selection of control variables is informed by the notion of a nutrition production function (Behrman and Deolalikar 1988) including: nutrients (the food groups described above); illness (diarrhea); maternal knowledge of good nutrition (maternal education); the health environment (the sanitation and water variables); and genetic endowments (maternal height measured in cm).6,7 We control for whether the mother was a teenager (<20 years) when the child was born, which captures aspects of both maternal knowledge of child care and nutrition practices as well as child endowments. We include survey dummies, meaning that the regression coefficients represent within-survey associations with stunting. We further control for subnational fixed effects (states, provinces, et cetera) and whether the child resides in a rural area. The inclusion of geographical fixed effects allows us to capture, to a certain extent, regional patterns in food access, prices, incomes, health, and the health environment. While these control variables capture some aspects of confounding factors, it would be a mistake to assume that they capture all inputs into the nutrition production function and for this reason we refer to our results as associations, not causal estimates. Our regression results are not population-weighted, but the coefficients do reflect implicit weights based on sample size and on within-survey variation in stunting and dietary variables.
Table 2. Summary Statistics for Key Variables.
| Variable | Obs. | Mean | Std. Dev. |
|---|---|---|---|
| Stunting (0/1) | 130,432 | 0.32 | 0.46 |
| Grain/root/tuber (0/1) | 130,432 | 0.76 | 0.43 |
| Legume/nut (0/1) | 130,432 | 0.25 | 0.43 |
| Fruits (0/1) | 130,432 | 0.36 | 0.48 |
| Vegetables (0/1) | 130,432 | 0.32 | 0.47 |
| ASFs (0/1) | 130,432 | 0.62 | 0.49 |
| Dairy (0/1) | 130,432 | 0.35 | 0.48 |
| Eggs ( 0/1) | 130,432 | 0.22 | 0.42 |
| Meat/fish (0/1) | 130,432 | 0.38 | 0.49 |
| Red/white meat (0/1) | 130,432 | 0.24 | 0.43 |
| Fish* (0/1) | 117,626 | 0.20 | 0.40 |
| Fortified infant cereals (0/1) | 130,432 | 0.10 | 0.30 |
| Currently breastfed (0/1) | 130,432 | 0.77 | 0.42 |
| Infant formula (0/1) | 130,432 | 0.10 | 0.30 |
| No food yesterday (0/1) | 130,432 | 0.12 | 0.33 |
| Diarrhea (0/1) | 130,315 | 0.23 | 0.42 |
| Medical facility birth (0/1) | 130,432 | 0.61 | 0.49 |
| Improved latrine (0/1) | 130,432 | 0.30 | 0.46 |
| Unimproved toilet (0/1) | 130,432 | 0.42 | 0.49 |
| Improved water (0/1) | 130,432 | 0.63 | 0.48 |
| Teenage mother (0/1) | 130,432 | 0.18 | 0.39 |
| Mother’s height (cm) | 130,432 | 155.8 | 7.15 |
| Woman 9+ yrs education (0/1) | 130,432 | 0.33 | 0.47 |
| Partner 9+ yrs education (0/1) | 130,432 | 0.38 | 0.48 |
| Partner absent (0/1) | 130,432 | 0.11 | 0.31 |
| Child is boy (0/1) | 130,432 | 0.51 | 0.50 |
| Age (months) | 130,432 | 14.2 | 5.13 |
| Rural (0/1) | 130,432 | 0.65 | 0.48 |
In the final part of the analysis, we supplement DHS data with national level data on cereal-relative calorie prices (CCPs) from Headey et al. (2017). The underlying price data pertain to nationally averaged prices of 200 standardized food products collected by national statistical agencies for 177 countries under the auspices of The International Comparison Program (ICP) coordinated by the World Bank. These weight-based prices were then converted to prices per calorie. Specific foods were then allocated into groups (see table 3), and in each country the cheapest staple cereal was selected as a numeraire. CCPs were then constructed by taking the ratio of the cheapest food in each group to the cheapest staple cereal in a country. As an example, the CCP for eggs in Bangladesh is the ratio of the price of 1 calorie of an egg relative to 1 calorie of rice. Table 3 indicates that most ASF categories involve good coverage of a range of specific food items, although apart from tilapia, there is insufficient coverage of local varieties of freshwater fish. Coverage of fresh and processed dairy products is good (6 products), as is eggs (medium and large).
Table 3. Classification of Cereals and Specific ASF Products in the ICP 2011 Data.
| Food Group | Number of products | Specific Products Used to Construct Minimum Price |
|---|---|---|
| Cereals | 13 | Rice (5 types), bread products (5 types), maize flour, maize, tortilla |
| Cow's milk, | 2 | Pasteurized fresh milk, |
| fresh | unskimmed or low-fat | |
| Cow's milk, | 3 | Condensed milk, |
| long-life | powdered milk, UHT | |
| Meat, fresh | 20 | Whole chicken (2 types), chicken breast, chicken leg; Beef/veal (7 varieties), Lamb/ mutton (4 varieties), Pork (4 varieties), Goat (1 variety); all unprocessed. |
| Chicken eggs, | 2 | Large brown eggs, |
| fresh | medium brown eggs | |
| Fish, fresh | 5 | Fresh Carp, Mackerel or Tilapia; canned Sardines or canned Tuna |
Source: Headey et al. (2017).
We use these national-level price data in linear probability regressions where consumption of individual ASFs is modelled as a function of child and household characteristics from the DHS—including important predictors of ASF consumption such as household wealth and parental education— and national level prices (CCPs). The inclusion of time-invariant prices for 2011 means that these regressions necessarily omit sur-vey/country dummies, but we include a series of additional national-level control variables that might influence ASF prices and simultaneously determine ASF consumption patterns through other mechanisms. These variables are sourced from the World Bank and include GDP per capita, cereal yields, and a conflict dummy variable.
Dietary Patterns among Young Children
Table 4 reports nutrition and dietary indicators for children 6–23 months. Stunting rates are high at 31.5%. The regional disaggregation shows that stunting is much higher in the two sub-Saharan African regions and Asia—where roughly one-third of children in this age range are stunted— than in the two predominantly middle-income regions. Consumption of ASFs follows a similar pattern. While 61.8% of children consumed at least one ASF the previous day, this masks considerable heterogeneity across regions. Latin America and the Caribbean region, with the highest proportion of children consuming an ASF (82.9%), has the lowest prevalence of stunting (22.35%), while the Eastern and Southern Africa region has the lowest proportion of children consuming an ASF (49.3%) and the highest prevalence of stunting (37.4%). Table 4 also shows that consumption rates among different ASFs are broadly similar in the Latin America and Caribbean region, with roughly half of children consuming dairy, meat/fish or eggs in the past 24 hours. Moreover, 36.2% of children in this region consumed two ASFs in the past 24 hours, while 20.6% consumed all three ASFs. In most other regions, one ASF tends to dominate. Dairy dominated in the Middle East and North Africa region (64.0%), although a substantial share of children consumes multiple ASFs there. Dairy also dominates in the South, Central and South-East Asia region (37.7%), although in some countries fish is more important (Bangladesh). Fish is also dominant in the West and Central Africa region (31.3%), but no particular ASF is dominant in the Eastern and Southern Africa region. It is also notable that relatively few children in these poorer regions consume multiple ASFs (<20%).
Table 4. Stunting and Dietary Patterns by Region and Child’s Age.
| All Children (6–23 mo) | Latin America & Caribbean(6–23 mo) | Middle East& North Africa (6–23 mo) | South,Central & SE Asia (6–23 mo) | West & Central Africa (6–23 mo) | Eastern & Southern Africa (6–23 mo) | All Children 6–11 mo | All Children 12–17 mo | All Children 18–23 mo | |
|---|---|---|---|---|---|---|---|---|---|
| Stunted | 31.5% | 22.5% | 25.9% | 37.7% | 32.9% | 37.4% | 21.6% | 32.8% | 41.3% |
| Child consumed: | |||||||||
| Any ASF | 62.0% | 82.9% | 75.5% | 56.8% | 52.0% | 49.3% | 50.7% | 66.4% | 69.8% |
| 1 ASF only | 35.8% | 27.8% | 38.2% | 43.7% | 35.4% | 36.4% | 34.6% | 37.0% | 35.7% |
| 2 ASFs | 20.3% | 36.2% | 30.3% | 13.8% | 13.5% | 12.0% | 15.0% | 22.2% | 24.0% |
| 3 ASFs | 8.5% | 20.6% | 11.0% | 4.3% | 4.4% | 3.2% | 4.8% | 9.4% | 11.8% |
| Dairy | 35.4% | 52.1% | 64.0% | 37.7% | 20.6% | 18.6% | 29.2% | 37.4% | 40.0% |
| Eggs | 22.4% | 45.3% | 30.2% | 15.5% | 12.1% | 13.1% | 16.6% | 24.1% | 27.0% |
| Meat/fish * | 37.9% | 55.9% | 30.1% | 22.7% | 39.4% | 33.7% | 26.4% | 41.8% | 46.3% |
| Red/white meat | 24.3% | 51.3% | 23.6% | 13.2% | 15.4% | 17.4% | 16.5% | 26.8% | 30.4% |
| Fish | 19.6% | N/A | 7.6% | 12.6% | 31.3% | 21.0% | 13.7% | 21.8% | 24.0% |
Note: Data pertain to children 6–23 months of age from DHS surveys from 49 countries. See supplementary online appendix table 1 for a list of countries and sample sizes. Asterisk * indicates that fish consumption data are not available for Peru.
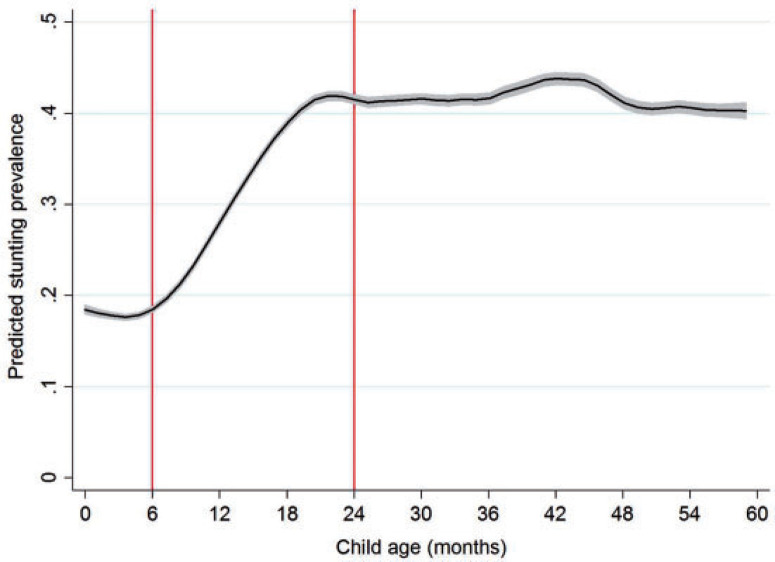
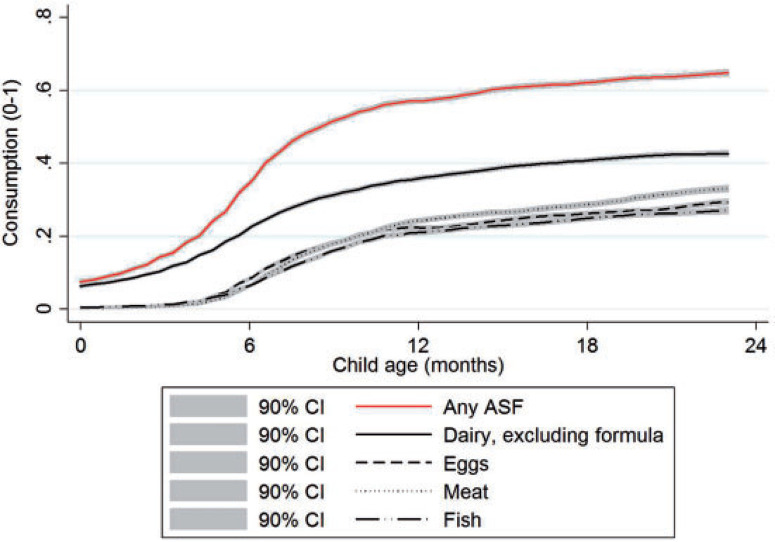
To contextualize age-specific patterns of stunting and consumption of ASFs, figure 1 plots stunting against child age for 49 countries. Just after birth, roughly 18% of the sample is stunted, but this changes little for the first few months of life when most infants are predominantly breastfed. From 4–5 months onwards, stunting rates increase precipitously until about 20 months of age where they level off at just over 40%. Regional data show similar patterns of accelerated growth faltering (supplementary online appendix figure 1). Figure 2 plots consumption of any ASF and some specific ASFs by child age. Dairy is the most commonly consumed ASF in this sample. While the Food and Agriculture Organisation of the United Nations (FAO) recommends dairy products to be introduced only at 12 months because of potentially adverse side effects on fragile infant digestive systems (FAO 2013), in this sample about 20% of children 6 months of age consumed dairy products on the previous day. This rises to 40% by 18 months. The consumption of eggs, meat, and fish are all less common than dairy and are characterized by similar levels of consumption at all ages.
Figure 1. Stunting prevalence by child age for children from 49 countries.
Figure 2. Consumption of any ASF and specific ASFs, by child age.
Associations between Stunting and Animal Sourced Foods
Our parametric results are reported in table 5. The control variables provided above (including the sub-national fixed effects) are included in these regressions but their coefficients are not reported (see supplement table 2 for full results). In the first column, we look at the association between the consumption of any ASF and stunting. The coefficient is statistically significant and negative, and its magnitude implies that, on average, intake of an ASF is associated a 2.3-percentage point reduction in stunting rate. Considering the overall stunting rate of 31.5% in this age-range, a 2.3-per-centage points translates into a 7.2% reduction in stunting. When we disaggregate the data by child age (columns 2, 3, and 4), we see the expected result that the associations tend to strengthen for older age brackets. For children aged 18–23 months—our preferred sample—consumption of an ASF the previous day is associated with a four-percentage point reduction in the likelihood of being stunted. Since the stunting rate among children in this age range is 41.3% (see table 4), this translates into 9.7% reduction in stunting. Note that this association is larger than that observed for other non-staple foods. This includes legumes—an important alternative source of protein, especially in low-income countries (Messina 1999). Moreover, the ASF and legume coefficients are statistically different from zero.8 We also note that the coefficient on fruit is significant and negative in all samples, although it is again statistically smaller than the coefficient on ASFs in the 18–23-month sample.9
Table 5. ASFs Are Negatively Associated with Stunting, Especially in the 18-23-month Age Group.
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
| Sample: | 6-23 Month Children | 6-11 Month Children | 12-17 Month Children | 18-23 Month Children |
| Any ASF | -0.023*** | -0.016*** | -0.011** | -0.040*** |
| (0.003) | (0.005) | (0.006) | (0.006) | |
| Grains/roots/tubers | -0.015*** | -0.023*** | -0.001 | -0.013 |
| (0.004) | (0.006) | (0.008) | (0.009) | |
| Legumes/nuts | -0.006** | -0.007 | -0.006 | -0.003 |
| (0.003) | (0.005) | (0.005) | (0.006) | |
| Any fruit | -0.019*** | -0.015*** | -0.014*** | -0.018*** |
| (0.003) | (0.005) | (0.005) | (0.005) | |
| Any vegetables | 0.003 | -0.006 | -0.001 | 0.004 |
| (0.003) | (0.005) | (0.005) | (0.005) | |
| Child and household level controls? | Yes | Yes | Yes | Yes |
| Rural dummy? | Yes | Yes | Yes | Yes |
| Survey dummies? | Yes | Yes | Yes | Yes |
| Sub-national fixed effects? | Yes | Yes | Yes | Yes |
| Coefficient on ASF is statistically different to the coefficients on other food groups? | Yes | No | No | Yes |
| (except fruit) | ||||
| R-squared | 0.149 | 0.104 | 0.141 | 0.167 |
| Observations | 130,315 | 45,316 | 45,026 | 39,973 |
Note: Unit of observation is a child and the dependent variable obtains a value 1 if the child is stunted, and zero otherwise. Standard errors clustered at the DHS cluster level. Child and household level controls include: Child had a diarrhea (0/1), Medical facilitybirth (0/1), Improved latrine (0/1), Unimproved toilet (0/1), Improved water (0/1), Teenage mother (0/1), Mother’s height (cm), Woman 9+ yrs education (0/1), Partner 9+ yrs education (0/1), Partner absent (0/1), child is boy (0/1), and child’s age (age-in-months dummies). Also included are survey dummies, subnational fixed effects (states, provinces, et cetera) and whether the child resides in a rural area. Statistical significance denoted as
*= p < 0.1.
**= p < 0.05
***= p < 0.01,
In table 6, we re-run column 4 in table 5 on region-disaggregated data. The coefficient on the ASF consumption variable remains negative in all sub-samples and is statistically significant at the 5% level in four out five regions. Only in the Middle East & North Africa (MENA) region is the association be-tween ASF intake and stunting not statistically significant. Elsewhere, consumption of at least one ASF is associated with a 2–9 percentage point reduction in the likelihood of being stunted, with the strongest association pertaining to the Latin America and Caribbean sample, where ASF consumption is both high and diverse (table 4).
Table 6. ASF Consumption Is Associated with Reduced Risks of Stunting in Children 18¬23 Months of Age in All Regions Except the Middle East and North Africa.
| Sample: | (1) Latin America & Caribbean | (2) Middle East & North Africa | (3) South, Central & SE Asia | (4) West & Central Africa | (5) Eastern & Southern Africa |
|---|---|---|---|---|---|
| Any ASF | -0.088*** | -0.018 | -0.042*** | -0.022** | -0.045*** |
| (0.017) | (0.026) | (0.013) | (0.011) | (0.013) | |
| Grains/roots/tubers | -0.039* | 0.046* | -0.015 | -0.024 | -0.004 |
| (0.023) | (0.027) | (0.022) | (0.017) | (0.018) | |
| Legumes/nuts | 0. | -0.010 | -0.032** | 0.012 | -0.007 |
| (0.009) | (0.017) | (0.014) | (0.012) | (0.014) | |
| Any fruit | -0.013 | -0.026 | -0.028** | -0.005 | -0.031** |
| (0.010) | (0.017) | (0.012) | (0.011) | (0.013) | |
| Any vegetables | 0.001 | 0.039** | -0.002 | 0.002 | 0.004 |
| (0.010) | (0.018) | (0.012) | (0.010) | (0.013) | |
| Other food groups? | Yes | Yes | Yes | Yes | Yes |
| Child and household | |||||
| level controls? | Yes | Yes | Yes | Yes | Yes |
| Rural dummy? | Yes | Yes | Yes | Yes | Yes |
| Survey dummies? | Yes | Yes | Yes | Yes | Yes |
| Sub-national | Yes | Yes | Yes | Yes | Yes |
| fixed effects? | |||||
| R-squared | 0.223 | 0.138 | 0.171 | 0.123 | 0.104 |
| Observations | 9,507 | 3,857 | 7,831 | 10,662 | 7,480 |
Note: Unit of observation is a child, 18-23 months and the dependent variable obtains a value 1 if the child is stunted, and zero otherwise. Standard errors clustered at the DHS cluster level. Controls are those listed in table 5. Statistical significance denoted as
*= p < 0.1.
**= p < 0.05,
***= p < 0.01,
Tables 7 and 8 extend this core finding in two ways. Table 7 assesses whether the number of different ASFs consumed is associated with stunting. Biologically, one would expect diversity in ASF consumption to yield larger growth benefits because different ASFs contain different growth-stimulating nutrients. Dairy, for example, is rich in calcium and contains a growth stimulating hormone (IGF-1) but is bereft of iron, whereas meat, fish, and eggs are relatively rich in iron but low in calcium. And as we saw in table 4, there are substantial numbers of children who consume multiple ASFs (over 40% in Latin America and MENA, and less than 20% in other regions). The results in table 7 suggest there are additional benefits to consuming multiple ASFs. In the 6–23-month sample, the implied reduction in stunting to one ASF is just 1.8 points, but this rises to 3.4 points and 4.5 points for 2 ASFs and 3 ASFs, respectively, and all three coefficients are statistically different from one another. The results are much weaker in the 6–11-month and 12–17-month sample, but substantially stronger in the preferred 18–23-month sample. In that sample consumption of one ASF is associated with stunting being reduced by 3.7-percentage points, but consuming one additional ASF is associated with a further 2-percentage point reduction (p < 0.01). Consuming three ASFs also implies a large reduction in stunting (6.1 points), but the implied difference between 2 ASFs and 3 ASFs is not statistically significant.
Table 7. Consuming More than 1 Type of ASFs is Associated with a Significantly Lower Risk of Stunting in the 18-23-Month Age Group.
| (1) 6-23 Months | (2) 6-11 Months | (3) 12-17 Months | (4) 18-23 Months | |
|---|---|---|---|---|
| 1 ASF | -0.018*** | -0.017*** | -0.010* | -0.037*** |
| (0.003) | (0.005) | (0.006) | (0.007) | |
| 2 ASFs | -0.034*** | -0.021*** | -0.009 | -0.057*** |
| (0.004) | (0.007) | (0.007) | (0.008) | |
| 3 ASFs | -0.045*** | -0.009 | -0.014 | -0.061*** |
| (0.005) | (0.010) | (0.009) | (0.010) | |
| Other food groups? | Yes | Yes | Yes | Yes |
| Child and household level controls? | Yes | Yes | Yes | Yes |
| Rural dummy? | Yes | Yes | Yes | Yes |
| Survey dummies? | Yes | Yes | Yes | Yes |
| Sub-national fixed effects? | Yes | Yes | Yes | Yes |
| P-value of H0: 0(1 ASF) = 0(2 ASFs) | 0.*** | 0.416 | 0.852 | 0.002*** |
| P-value of H0: b(1 ASF) = b(3 ASFs) | 0.*** | 0.422 | 0.593 | 0.003*** |
| P-value of H0: b(2 ASF) = b(3 ASFs) | 0.024** | 0.182 | 0.512 | 0.762 |
| R-squared | 0.150 | 0.105 | 0.142 | 0.169 |
| Observations | 130,315 | 45,316 | 45,026 | 39,973 |
*= p < 0.1.
**= p < 0.05
***= p < 0.01
Note: Unit of observation is a child and the dependent variable obtains a value 1 if the child is stunted, and zero otherwise. Standard errors clustered at the DHS cluster level. Controls are those listed in table 5. Statistical significance denoted at
Table 8. All ASF Types Are Associated with Lower Stunting among Children 18-23 months, although Dairy Has the Strongest Association.
| (1) 6-23 months | (2) 6-11 months | (3) 12-17 months | (4) 18-23 months | |
|---|---|---|---|---|
| Dairy | -0.020*** | -0.008 | -0.011* | -0.034*** |
| (0.003) | (0.005) | (0.006) | (0.006) | |
| Eggs | -0.011*** | -0.001 | 0.001 | -0.013** |
| (0.003) | (0.005) | (0.005) | (0.006) | |
| Meat/Fish | -0.017*** | -0.014*** | -0.005 | -0.021*** |
| (0.003) | (0.005) | (0.005) | (0.005) | |
| Other food groups? | yes | yes | Yes | yes |
| Child and household level controls? | yes | yes | Yes | yes |
| Rural dummy? | yes | yes | Yes | yes |
| Survey dummies? | yes | yes | Yes | yes |
| Sub-national fixed effects? | yes | yes | Yes | yes |
| P-value of H0: b(Dairy) > b(Eggs) | 0.034** | 0.184 | 0.076* | 0.008*** |
| P-value of H0: b(Meat) > b(Eggs) | 0.092* | 0.046** | 0.199 | 0.152 |
| P-value of H0: b(Dairy) > b(Meat) | 0.266 | 0.202 | 0.225 | 0.056* |
| R-squared | 0.150 | 0.105 | 0.142 | 0.169 |
| Observations | 130,315 | 45,316 | 45,026 | 39,973 |
= p < 0.1.
= p < 0.05
= p < 0.01
Note: Unit of observation is a child and the dependent variable obtains a value 1 if the child is stunted, and zero otherwise. Standard errors clustered at the DHS cluster level. Controls are those listed in table 5. Statistical significance denoted at and
Table 8 disaggregates by type of ASF; all ASF types are associated with lower stunting among children aged 18–23 months, al-though the coefficient on dairy is significantly larger than the coefficients on eggs and meat/fish. The results in table 8 are consistent with table 7 insofar as the implied benefits of different ASFs are additive. For example, among children aged 18–23 months, the implied reduction in stunting from consuming dairy, eggs, and meat/fish is a 6.6-point reduction (3.4 from dairy, 1.3 points from eggs, and 2.1 points from meat/fish).
table 3also adds a potentially important confounding factor to the model (household wealth) measured via a principal components analysis of household assets (Filmer and Pritchett 2001). Wealth is likely an important determinant of ASF consumption but could also independently influence stunting. However, the results from table 5 are robust to the inclusion of wealth terciles, even though the coefficient on middle-and upper-wealth terciles are themselves highly significant. In the 18–23-month sample, for example, the coefficient on ASF drops from 0.040 to 0.038 and remains statistically significant. Thus, there is little indication that the estimated benefits of ASFs simply represent the greater economic status of ASF-consuming households.
Constraints to ASF Consumption in Developing Countries
The evidence above suggests significant nutritional benefits from consuming ASFs, and yet ASF consumption in poorer countries is generally very low. Why is this? One perceived barrier is a lack of nutritional knowledge. Many nutritional programs have attempted to improve nutritional knowledge, albeit it with mixed success and substantive concerns on sustainability (Dewey and AduAfarwuah 2008; Menon et al. 2015). Consistent with knowledge constraints, parental education has been strongly linked to nutrition out-comes and dietary diversity even when applying methods to reduce endogeneity bias (Alderman and Headey 2017). Gender bias and low levels of maternal empowerment are also widely-cited constraints (Jayachandran and Pandi 2017), as are food taboos (Pacho´n et al. 2007; Zerfu, Umeta, and Baye 2016). Additionally, many studies show that consumers diversify away from starchy staple foods as incomes rise as per Bennett’s law (Choudhury and Headey 2017b), opting for more expensive sources of calories as incomes rise (Subramanian and Deaton 1996), including strong demand for ASFs (Colen et al. 2018). If incomes constrain ASF consumption, this suggests they may be a very expensive source of calories in low-in-come countries. Certainly, many ASFs are imperfectly tradable, especially in environments with underdeveloped value chains (e.g., poor transport, lack of cold storage, and low levels of processing). Highly perishable foods also have associated food safety risks, with semi-commercialized dairy systems being a notable example in both contemporary developing economies (Gizachew et al. 2016) and early twentieth century industrialized societies (Gordon 2016). In turn, this draws attention to the price of ASFs as a factor constraining children’s consumption of these foods.
Table 9 reports data on the price of ASFs and other foods relative to the price to a country-specific numeraire, the cheapest staple cereal (i.e., rice, wheat, and maize products). For example, the value 6.1 for fish in Eastern & Southern Africa tells us that it costs 6.1 times more to consume a calorie from a fish product than it does a calorie from a staple cereal. Across all regions, roots and tubers are very cheap sources of calories. Strikingly, vitamin-A rich fruits and vegetables and “other fruit” are also relatively affordable, partly because many such fruits are highly suitable to tropical conditions, and be-cause some (e.g., mangos, papayas, bananas) are relatively easy to store and transport. By contrast, vegetables, which are highly perish-able and low in calorie density, are an expensive source of calories. Legumes, which are reasonably high in lower-quality protein, are an affordable source of calories in most regions, but less so in Africa.
Table 9. Cereal-relative Calorie Price Ratios for Various Foods, by Region.
| Vegetal sourced food prices | ||||||
|---|---|---|---|---|---|---|
| Roots & Tubers | Vitamin A-rich Fruit and Veg. | Dark Green Leafy Veg. | Other Veg. | Other Fruit | Legumes | |
| High income | 1.6 | 3.0 | 9.0 | 3.3 | 1.7 | 1.2 |
| Latin America & Caribbean | 1.2 | 1.9 | 5.6 | 6.5 | 1.3 | 2.2 |
| Middle East & North Africa | 2.1 | 2.5 | 6.1 | 5.3 | 3.3 | 2.1 |
| South, Central & South-East Asia | 1.5 | 1.9 | 6.2 | 6.0 | 3.1 | 2.0 |
| Western & Central Africa | 1.0 | 2.3 | 11.5 | 11.6 | 3.1 | NA |
| Eastern & Southern Africa | 1.7 | 3.1 | 7.3 | 11.4 | 3.2 | NA |
| Animal sourced foods & fortified baby cereal prices | ||||||
| Cow’s milk, fresh | Cow’s milk, Processed | Chicken eggs | Meat | Fish | Fortified baby cereal | |
| High income | 3.2 | 2.2 | 3.0 | 2.0 | 4.3 | 5.0 |
| Latin America & Caribbean | 3.9 | 3.0 | 4.9 | 3.2 | 3.4 | 9.6 |
| Middle East & North Africa | 10.1 | 3.1 | 6.1 | 6.2 | 6.0 | 16.1 |
| South, Central & South-East Asia | 7.8 | 3.8 | 6.2 | 6.5 | 5.3 | 16.4 |
| Western & Central Africa | 16.5 | 4.0 | 9.9 | 5.3 | 5.0 | 23.4 |
| Eastern & Southern Africa | 13.9 | 5.8 | 9.1 | 5.6 | 6.1 | 18.6 |
Note: Caloric prices are the ratio of the price of 1 calorie of a given food (e.g., eggs) relative to 1 calorie of the cheapest staple cereal in each country (e.g., rice, wheat, and maize products). See Headey et al. (2017) for details. NA refers to not available. ICP data did include some legume prices for sub-Saharan Africa, but not widely consumed local varieties.
Price ratios of ASFs, however, reveal striking differences across income levels and regions. Both perishable fresh milk and highly storable processed milk are relatively cheap sources of calories in high-income countries but also in Latin America and the Caribbean. In North Africa and Middle East region, processed milk is very cheap, but fresh milk is very expensive. It is notable that in both these middle-income regions, consumption of dairy products among infants and young children is widespread, with over half consuming dairy in the past 24 hours (table 4). By contrast, fresh milk is moderately expensive in South, Central and South-East Asia (though relatively cheap in India, where dairy consumption is high), and extremely expensive in sub-Saharan Africa. In Western and Central Africa, for example, fresh milk is 16.5 times as expensive as the cheapest cereals. Processed milk is highly storable, so it is of little surprise that it is more affordable in all regions. Despite this, it would appear that processed milk is not widely consumed in these regions.
Price variation for eggs is even more striking. Eggs are a very cheap source of calories and protein in high-income countries (Iannotti et al. 2014). Yet in the Middle East & North Africa region, they are at least 6 times as expensive as staple cereals, and in Africa they are 9–10 times as expensive. This provides prima facie evidence that the highly perishable nature of eggs and the low productivity of egg production in lower in-come countries would appear to account for the low levels of egg consumption observed earlier.
The price story on flesh foods is quite different. Both red/white meat and fish are relatively tradable, either because of the scope to trade live animals, or the ability to freeze, dry, smoke, or salt flesh foods. Meat and fish are again notably cheap in high-income countries and in Latin America and the Caribbean, but also—compared to other ASFs—relatively affordable in sub-Saharan Africa where meat/fish calories are just 5–6 times as expensive as staple cereals. Notably, fortified baby cereals—a potential substitute for ASFs—are extremely expensive in all regions except high-income countries and Latin America and the Caribbean (see Masters, Nene, and Bell 2017 for a more de-tailed anlaysis).
Table 10 reports linear probability models explaining children’s consumption of four different ASFs. Own prices are always significantly and negatively associated with consumption of each specific ASF. Dairy and egg consumption are especially strongly associated with their relative prices. In the case of dairy, a doubling of the price ratio for fresh milk—for example, the difference between dairy prices in the United States and India— predicts a reduction in dairy consumption of around 10 percentage points. Considering that 35% of the children in our sample consumed dairy (see table 4), this translates into a 29% drop in dairy consumption prevalence. The association for eggs is even stronger, suggesting that the very high prices of eggs in most regions (table 9) provides a strong ex-planation of why egg consumption is not common among infants and young children in poorer countries. Meat and fish consumption share somewhat more modest associations with their own price ratios. In the case of fish this may be the result of some attenuation bias, as the ICP only measures a few fish varieties relevant to lower-income countries.
Table 10. Linear Probability Model Regressions of Consumption of ASFS by Children aged 6-23 months as a Function of Calorie Price Ratios, and Various Child-, Household-, Community-, and National-level Characteristics.
| (1) Dairy consumption | (2) Egg consumption | (3) Meat consumption | (4) Fish consumption | |
|---|---|---|---|---|
| Log own priced | -0.103*** | -0.153*** | -0.066*** | -0.052*** |
| (0.005) | (0.006) | (0.005) | (0.004) | |
| Log cereal yieldsd | 0.066*** | 0.015*** | -0.081*** | -0.064*** |
| Log GDP per capitad | (0.005) | (0.004) | (0.004) | (0.005) |
| 0.075*** | -0.029*** | 0.065*** | -0.084*** | |
| Log urbanization rated | (0.004) | (0.004) | (0.004) | (0.005) |
| -0.002 | 0.204*** | -0.062*** | 0.114*** | |
| Conflict dummyd | (0.007) | (0.006) | (0.007) | (0.009) |
| 0.008 | -0.070*** | -0.053*** | 0.041*** | |
| Wealth, middle tercileb | (0.005) | (0.004) | (0.004) | (0.004) |
| 0.063*** | 0.053*** | 0.024*** | 0.001 | |
| Wealth, upper tercileb | (0.004) | (0.003) | (0.003) | (0.004) |
| 0.162*** | 0.077*** | 0.066*** | -0.040*** | |
| Mother 9+ yrs of schoolb | (0.006) | (0.005) | (0.005) | (0.006) |
| 0.030*** | 0.032*** | 0.044*** | 0.005 | |
| Father 9+ yrs of school0 | (0.004) | (0.004) | (0.004) | (0.004) |
| 0.009** | 0.004 | 0.012*** | 0.023*** | |
| (0.004) | (0.003) | (0.003) | (0.004) | |
| Open defecation, villagec | -0.019*** | -0.061*** | -0.042*** | -0.010* |
| (0.006) | (0.004) | (0.004) | (0.006) | |
| Unimproved water, villagec | -0.049*** | -0.028*** | -0.012*** | +0.028*** |
| Women's autonomyb | (0.006) | (0.004) | (0.004) | (0.005) |
| 0.018*** | 0.012*** | -0.005 | -0.013*** | |
| (0.004) | (0.004) | (0.004) | (0.004) | |
| Currently breastfeda | -0.078*** | -0.008** | -0.050*** | -0.013*** |
| (0.004) | (0.004) | (0.004) | (0.004) | |
| Ruralc | 0. | 0.008** | -0.022*** | -0.027*** |
| Father absentb | (0.004) | (0.004) | (0.004) | (0.004) |
| 0.055*** | -0.033*** | -0.039*** | 0.016*** | |
| Hospital/clinic accessb | (0.007) | (0.005) | (0.005) | (0.006) |
| 0.020*** | 0.034*** | 0.012*** | 0.046*** | |
| (0.004) | (0.003) | (0.003) | (0.004) | |
| R-squared | 0.177 | 0.146 | 0.104 | 0.115 |
| Observations | 98,840 | 100,991 | 91,793 | 89,896 |
*= p < 0.1.
**= p < 0.05
***= p < 0.01
Note: Unit of observation is a child and the dependent variable obtains a value 1 if the child consumed from the food group, and zero otherwise. Asterisks indicate the following:
Superscript a indicates child-level indicators; b= household-level indicators; c= community/ cluster-level indicators; d= national-level indicators. Regressions also control for short birth interval, number of children, teenage motherhood, child gender. These coefficients had small and often insignificant coefficients.
Amongst other national factors there are some interesting associations. Dairy and meat consumption have strong associations with GDP per capita, but in the case of dairy there is also an association with household-level wealth that is much stronger than the corresponding associations for other ASFs. Strikingly, fish consumption is negatively associated with GDP per capita and with household wealth. The negative association with national GDP per capita could partly reflect high levels of consumption in much of sub-Saharan Africa, as well as countries like Cambodia and Bangladesh, but the negative association with wealth likely reflect the fact that in many populations, fish is considered an inferior good relative to red/white meat. Also notable is that the national urbanization rate is very strongly associated with egg and fish consumption, but negatively associated with meat consumption. These results might indicate that more commercialized large-scale low-cost poultry production systems tend to emerge when large urban agglomerations give rise to concentrated centers of demand (Narrod, Pray, and Tiongco 2008).
Conclusions
Despite plausible biological mechanisms linking ASF intake to child growth, there is a surprising dearth of evidence on this relationship in developing countries, and little previous work has systematically documented patterns of ASF consumption among young children or attempted to explain why these patterns exist.
In this paper we document that ASF consumption among young children is relatively low in sub-Saharan Africa and most of Asia, but is also characterized by some distinctive patterns. Most notable are: (a) low levels of dairy consumption in most lowland areas of Africa and much of Asia (Bangladesh, Cambodia), but relatively high levels of fish consumption; (b) low levels of egg and red/ white meat consumption in Africa and Asia; (c) low levels of multiple ASF consumption in Africa and Asia (<20%). We show evidence of strong associations between ASF consumption and child growth, particularly consumption of multiple ASFs and of dairy. Finally, we show that high relative prices of ASF calories are a critical constraint restricting consumption of these foods, especially for highly perishable products such as fresh milk and eggs. While our econometric models are saturated with a wide array of controls and are robust to the inclusion of household wealth, we emphasize that these are associations, not causal relations. That being said, there is also an important reason to suspect that we may be underestimating the association between ASF consumption on stunting: the 24-hour recall is likely to be an imperfect proxy for usual diets (i.e., within-person error) leading to an attenuation bias (Thorne-Lyman, Spiegelman, and Fawzi 2014). This may also be more problematic for some ASFs than others since some (e.g., dairy) may be consumed in larger quantities and more frequently than others (e.g., flesh foods, eggs). All this points to the need for new studies that can illustrate the causal links between quantities of ASF consumption and child nutrition.
For the agriculture and nutrition communities, these findings are of clear importance. Nutritionists have long promoted interventions designed to improve nutritional knowledge on feeding practices, and/or to increase homestead production of ASFs (Leroy and Frongillo 2007; Dewey and AduAfarwuah 2008). The extremely high prices of ASFs suggests these interventions could have limited impacts, however. One reason is that high ASF prices provide strong incentives for producers to sell ASFs rather than feed them to their own children. Behavioral change
interventions are used to encourage house-holds to at least partially resist the financial rewards of selling ASFs, but doing so is costly and potentially unsustainable, and will still fail to increase ASF consumption among non-producers. Potentially a more cost-effective and sustainable strategy is to employ larger-scale investments in the livestock and fisheries sector and its associated value chains, and/or appropriate trade policies, to reduce the prices of ASFs for all consumers.
In the case of eggs—a highly non-tradable product—investments in domestic production are clearly crucial. However, egg production is characterized by huge economies of scale (Narrod, Pray, and Tiongco 2008), suggesting that investments in village production systems are unlikely to be economically efficient. Moreover, a growing body of research also suggests scavenging poultry systems are hazardous to the health of young children (Ngure et al. 2013; Headey and Hirvonen 2016). For dairy, the issue is much more complex since dairy powder is highly tradable but not obviously a close substitute for fresh milk, nor highly desirable in contexts where water quality is a major concern. Many populations in tropical regions also have little tradition of consuming milk, and adults face lactose intolerance issues, suggesting there may be significant cultural issues to be over-come (FAO 2008). Meat and fish are also complex sectors characterized by both intensive and extensive production modalities and some degree of tradability. The importance of fish in many African and Asian diets— even those of young children—makes it a particularly interesting sector for future re-search and investment. One concern is that extensive systems are facing major over-fishing problems in Africa especially, al-though more sustainable fish farming systems have experienced explosive growth in much of Asia and parts of Africa and may offer a pathway to expanding fish consumption (B´en´e et al. 2015).
While ASFs are critical for improving human nutrition, there are significant obstacles to overcome. First, scaling up ASF production comes at a potential environmental cost since livestock and fish production releases methane (CH4) and nitrous oxide (N2O) to the atmosphere, thus contributing to global warming. Depending on the methodology, it is estimated that the livestock sector contributes between 8% and 18% of the total global greenhouse gas (GHG) emissions (IPCC 2006; Steinfeld et al. 2006). The amount of GHG emissions are closely linked to size of the livestock population as well as to the size of the animal, with non-dairy cattle being a major source of methane emissions (O’Mara 2011). Therefore, optimizing the size, productivity and composition of the national live-stock and fisheries sectors to maximize the nutritional benefits whilst minimizing GHG emissions is an important area of future research.
Additionally, a substantial proportion of the world’s undernourished population is vegetarian for cultural reasons. The DHS for India—which accounts for around one-third of the world’s population of stunted children—suggests that around one-third of Indian adults are vegetarian or lacto-vegetarian. Short of radical cultural shifts, these populations will likely continue to rely on legumes as their key source of protein. While legumes were not associated with stunting in our full sample results, we did observe a significant association between legume consumption and child growth in the South Asian sample (table 6), suggesting that legumes play a special role in this region that they do not in other populations. Clearly, in-creasing consumption of ASFs in India and Nepal is challenging, and may require a mix of agricultural policies and tailored behavioral change designed to either increase ASF consumption (dairy particularly among lacto-vegetarians) or appropriately supplement diets with legumes or fortified infant cereals.
The importance of ASFs for child nutrition therefore has important ramifications for future research and for international and national investments in agriculture. We see three lines of attack: (a) as economists, we are used to thinking that large price differentials present opportunities for arbitrage. In the case of ASFs, this is clearly not occurring, suggesting significant barriers to trade. An understanding of the relative roles played by policy decisions (such as tariff levels), technology (addressing perishability issues), or a combination of the two (e.g., phytosanitary regulations) will help inform appropriate pol-icy changes and interventions that should lower these barriers and in turn reduce these price differentials. (b) Understanding whether improvements in ASF value chains can improve diets of pre-school children. Randomized control trials may be difficult to implement but it might be possible to exploit natural experiments such as improved access to these value chains following infrastructure improvements, such as roads. (c) Increased investments in research that will lead to in-creased productivity in ASF sectors and, relatedly, assessing whether such improvements, possibly twinned with demand-side interventions such as nutrition behavior change communication, can in-crease pre-school child ASF consumption and in turn reduce stunting.10 More generally, we see such a research portfolio as part of efforts to shift the international and national agricultural research and policy agendas away from their dominant focus on food quantity towards a more balanced portfolio that pays increased attention to diet quality.
Supplementary Material
Footnotes
1Still other studies review relationships between ASF intake and growth, but not among pre-school children, and only in a mix of high-and low-income countries (Hoppe, Mølgaard, and Michaelsen 2006; de Beer 2012).
2Grasgruber et al. (2016) analyze data on adult male heights from 105 countries, which they link to dietary proxies from the FAO Food Balance Sheets and genetic markers linked to lactose intolerance. These authors show that adult male heights are most highly correlated with animal-sourced protein intake, which is in turn strongly associated with more lactose-tolerant genetic profiles.
3The absence of technology to refrigerate inhibited trade meant that local production and consumption of dairy were highly correlated. In the United States, mechanical refrigeration technology started to spread in the late nineteenth century, facilitating market integration of perishable foods such as dairy (Goodwin, Grennes, and Craig 2002). Craig, Goodwin, and Grennes (2004) estimate that the adoption of refrigeration post 1890s United States increased annual dairy consumption and annual protein intake by 1.7 % and 1.25 %, respectively. The authors further estimate that a large fraction of the improvement in adult statures in 1890s were due to refrigeration.
4We do note one idiosyncrasy: Peruvian DHS do not provide any disaggregated data on flesh foods, with fish and meat/organsaggregated together.
5Our results are robust to using a probit model instead of linear probability model.
6Diarrhea can also be considered as an outcome variable in the sense that ASF consumption could increase or decrease diarrhea risk among young children. However, our results are robust to omitting this variable from the model.
7Assets (or incomes) do not typically directly enter the nutrition production function as they are thought to influence nutrition outcomes indirectly through various inputs. Still, a consistent empirical finding in the literature is that ASFs have high-income elasticities (e.g., Pinstrup-Andersen and Caicedo 1978; Colen et al. 2018), raising a concern that our ASF coefficients are picking up wealth effects. Our main specification does not include household assets, but our results are robust to their inclusion (see table S3 in the supplementary online appendix).
8We can reject the null hypothesis that the coefficients on legumes and ASFs are equal. Consistent with this, adding consumption of legumes to our ASF variable (thus creating a variable capturing the consumption of proteins from animal or plant sources) yields a coefficient slightly smaller than the coefficient on ASFs. Results are available upon request.
9The significant negative coefficient on fruit consumption is biologically plausible. Fruits are soft and highly palatable for young children and require very little preparation time, implying that fruit consumption might be associated with higher calorie intake. Fruits are also rich in a wide array of micronutrients that are beneficial for immune system strengthening. However, while fruit consumption has an association with stunting, the coefficient tends to be smaller than the ASF one (see table 5) and, unlike ASF, is not consistent across regions (see table 6).
References
- Alderman H., and Headey D.. 2018. The Timing of Growth Faltering Has Important Implications for Observational Analyses of the Underlying Determinants of Nutrition Outcomes. PLoS One 13 (4): e0195904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2017. How Important Is Parental Education for Child Nutrition? World Development 94: 448–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baten J. 2009. Protein Supply and Nutritional Status in Nineteenth Century Bavaria, Prussia and France. Economics & Human Biology 7 (2): 165–80. [DOI] [PubMed] [Google Scholar]
- Baten J., and Murray J.E.. 2000. Heights of Men and Women in 19th-Century Bavaria: Economic, Nutritional, and Disease Influences. Explorations in Economic History 37 (4): 351–69. [Google Scholar]
- Behrman J.R., and Deolalikar A.B.. 1988. Health and Nutrition. In Handbook of Development Economics, ed. Chenery Hollis, Srinivasan T. N., 631–711. Amsterdam: North-Holland. [Google Scholar]
- Béné C., Barange M., Subasinghe R., Pinstrup-Andersen P., Merino G., Hemre G.-I., and Williams M.. 2015. Feeding 9 Billion by 2050—Putting Fish Back on the Menu. Food Security 7 (2): 261–74. [Google Scholar]
- Choudhury S., and Headey D.. 2017a. Household dairy production and child growth: Evidence from Bangladesh. Economics & Human Biology (forthcoming). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury, S.. 2017b. What Drives Diversification of National Food Supplies? A Cross-Country Analysis. Global Food Security 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colen L., Melo P., Abdul-Salam Y., Roberts D., Mary S., and Paloma S.G.Y.. 2018. Income Elasticities for Food, Calories and Nutrients across Africa: A Meta-Analysis. Food Policy 77:116–132. [Google Scholar]
- Craig L.A., Goodwin B., and Grennes T.. 2004. The Effect of Mechanical Refrigeration on Nutrition in the United States. Social Science History 28 (2): 325–36. [Google Scholar]
- Danaei G., Andrews K.G., Sudfeld C.R., Fink G., McCoy D.C., Peet E., Sania A., Smith M.C., et al. . 2016. Risk Factors for Childhood Stunting in 137 Developing Countries: A Comparative Risk Assessment Analysis at Global, Regional, and Country Levels. PLoS Medicine 13 (11): e1002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer H. 2012. Dairy Products and Physical Stature: A Systematic Review and Meta-Analysis of Controlled Trials. Economics & Human Biology 10 (3): 299–309. [DOI] [PubMed] [Google Scholar]
- de Onis M., and Branca F.. 2016. Childhood Stunting: A Global Perspective. Maternal & Child Nutrition 12: 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G., and Adu-Afarwuah S.. 2008. Systematic Review of the Efficacy and Effectiveness of Complementary Feeding Interventions in Developing Countries. Maternal & Child Nutrition 4 (S1): 24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror D.K., and Allen L.H.. 2011. The Importance of Milk and Other Animal-Source Foods for Children in Low-Income Countries. Food and Nutrition Bulletin 32 (3): 227–43. [DOI] [PubMed] [Google Scholar]
- IPCC 2006 2006. IPCC Guidelines for National Greenhouse Gas Inventories, ed. Eggleston H.S., Buendia L., Miwa K., Ngara T. and Tanabe K.. Geneva: National Greenhouse Gas Inventories Programme. [Google Scholar]
- Filmer D., and Pritchett L.. 2001. Estimating wealth effects without expenditure data -or tears: an application to education enrollment in states of India. Demography 38:115–32. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organisation of the United Nations. 2013. Milk and Dairy Products in Human Nutrition. Japan: IGES; Rome: Food and Agriculture Organisation. [Google Scholar]
- Gizachew D., Szonyi B., Tegegne A., Hanson J., and Grace D.. 2016. Aflatoxin Contamination of Milk and Dairy Feeds in the Greater Addis Ababa Milk Shed, Ethiopia. Food Control 59: 773–9. [Google Scholar]
- Goodwin B.K., Grennes T.J., and Craig L.A.. 2002. Mechanical Refrigeration and the Integration of Perishable Commodity Markets. Explorations in Economic History 39 (2): 154–82. [Google Scholar]
- Gordon R.J. 2016. The Rise and Fall of American Growth. Princeton, NJ:Princeton University Press [Google Scholar]
- Grasgruber P., Sebera M., Hrazdíra E., Cacek J., and Kalina T.. 2016. Major Correlates of Male Height: A Study of 105 Countries. Economics and Human Biology 21: 172–95. [DOI] [PubMed] [Google Scholar]
- Headey D., Alderman H., Maitra C., and Rao P.. 2017. The Relative Prices of Healthy and Unhealthy Foods in 177 Countries. Paper presented at Agriculture for Nutrition and Health Academy Week. Kathmandhu, Nepal, 10–13th July, 2017. [Google Scholar]
- Headey D., and Hirvonen K.. 2016. Is Exposure to Poultry Harmful to Child Nutrition? An Observational Analysis for Rural Ethiopia. PLoS One 11 (8): e0160590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott J., Headey D., and Dereje M.. 2015. Cows, Missing Milk Markets, and Nutrition in Rural Ethiopia. The Journal of Development Studies 51 (8): 958–75. [Google Scholar]
- Hoddinott J.F., Rosegrant M.W., and Torero M.. 2013. Investments to Reduce Hunger and Undernutrition.In B., Lomborg ed. Global Problems, Smart Solutions. Cambridge: Cambridge University Press. [Google Scholar]
- Hoppe C., Mølgaard C., and Michaelsen K.F.. 2006. Cow’s Milk and Linear Growth in Industrialized and Developing Countries. Annual Review of Nutrition 26: 131–73. [DOI] [PubMed] [Google Scholar]
- Iannotti L.L., Lutter C.K., Bunn D.A., and Stewart C.P.. 2014. Eggs: The Uncracked Potential for Improving Maternal and Young Child Nutrition among the World’s Poor. Nutrition Reviews 72 (6):355–68. [DOI] [PubMed] [Google Scholar]
- Iannotti L.L, Lutter C.K., Stewart C.P., Riofrío C.A.G., Malo C., Reinhart G., Palacios A., et al. . 2017. Eggs in Early Complementary Feeding and Child Growth: A Randomized Controlled Trial. Pediatrics 140 (1): e20163459. [DOI] [PubMed] [Google Scholar]
- ICF International. 2015. The Demographic and Health Surveys Program Available at:. https://dhsprogram.com (accessed November 1, 2017). [Google Scholar]
- Jayachandran S., and Pandi R.. 2017. Why Are Indian Children so Short? The Role of Birth Order and Son Preference. American Economic Review 107 (9): 2600–29. [PubMed] [Google Scholar]
- Leroy J.L., and Frongillo E.A.. 2007. Can Interventions to Promote Animal Production Ameliorate Undernutrition? Journal of Nutrition 137 (10): 2311–6. [DOI] [PubMed] [Google Scholar]
- Leroy J.L., Ruel M., Habicht J.-P., and Frongillo E.A.. 2014. Linear Growth Deficit Continues to Accumulate beyond the First 1 Days in Low-and Middle-Income Countries: Global Evidence from 51 National Surveys. Journal of Nutrition 144 (9): 1460–6. [DOI] [PubMed] [Google Scholar]
- Masters W.A., Nene M.D., and Bell W.. 2017. Nutrient Composition of Premixed and Packaged Complementary Foods for Sale in Low-and Middle-Income Countries: Lack of Standards Threatens Infant Growth. Maternal & Child Nutrition 13 (4): e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D.S. 1974. The Great Protein Fiasco. Lancet 304 (7872): 93–6. [DOI] [PubMed] [Google Scholar]
- Menon P., Nguyen P.H., Saha K.K., Khaled A., Sanghvi T., Baker J., Afsana K., Haque R., Frongillo E.A., Ruel M.T., and Rawat R.. 2016. Combining Intensive Counseling by Frontline Workers with a Nationwide Mass Media Campaign Has Large Differential Impacts on Complementary Feeding Practices but Not on Child Growth: Results of a Cluster-Randomized Program Evaluation in Bangladesh. The Journal of Nutrition 146: 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina M.J. 1999. Legumes and Soybeans: Overview of Their Nutritional Profiles and Health Effects. American Journal of Clinical Nutrition 70 (3): 439s–50s. [DOI] [PubMed] [Google Scholar]
- Moradi A., and Baten J.. 2005. Inequality in Sub-Saharan Africa: New Data and New Insights from Anthropometric Estimates. World Development 33 (8): 1233–65. [Google Scholar]
- Narrod C., Pray K., and Tiongco M.. 2008. Technology Transfer, Policies, and the Role of the Private Sector in the Global Poultry Revolution. IFPRI Discussion Paper 00841. Washington DC: The International Food Policy Research Institute (IFPRI). [Google Scholar]
- Ngure F.M., Humphrey J.H., Mbuya M.N., Majo F., Mutasa K., Govha M., Mazarura E., et al. . 2013. Formative Research on Hygiene Behaviors and Geophagy among Infants and Young Children and Implications of Exposure to Fecal Bacteria. American Journal of Tropical Medicine and Hygiene 89 (4): 709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara F.P. 2011. The Significance of Livestock as a Contributor to Global Greenhouse Gas Emissions Today and in the Near Future. Animal Feed Science and Technology 166–167: 7–15. [Google Scholar]
- Pachón H., Simondon K.B., Fall S.T., Menon P., Ruel M.T., Hotz C., Creed-Kanashiro H., et al. . 2007. Constraints on the Delivery of Animal-Source Foods to Infants and Young Children: Case Studies from Five Countries. Food and Nutrition Bulletin 28 (2): 215–29. [DOI] [PubMed] [Google Scholar]
- Pinstrup-Andersen P., and Caicedo E.. 1978. The Potential Impact of Changes in Income Distribution on Food Demand and Human Nutrition. American Journal of Agricultural Economics 60 (3): 402–15. [Google Scholar]
- Rawlins R., Pimkina S., Barrett C.B., Pedersen S., and Wydick B.. 2014. Got Milk? The Impact of Heifer International’s Livestock Donation Programs in Rwanda on Nutritional Outcomes. Food Policy 44: 202–13. [Google Scholar]
- Semba R.D. 2016. The Rise and Fall of Protein Malnutrition in Global Health. Annals of Nutrition Metabolism 69 (2): 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld H., Gerber P., Wassenaar T., Castel V., and de Haan C.. 2006. Livestock’s Long Shadow: Environmental Issues and Options. Rome: Food and Agriculture Organisation. [Google Scholar]
- Subramanian S., and Deaton A.. 1996. The Demand for Food and Calories. Journal of Political Economy 104 (1): 133–62. [Google Scholar]
- Takahashi E. 1984. Secular Trend in Milk Consumption and Growth in Japan. Human Biology 56 (3): 427–37. [PubMed] [Google Scholar]
- Tang M., Sheng X.-Y., Krebs N.F., and Hambidge K.M.. 2014. Meat as Complementary Food for Older Breastfed Infants and Toddlers: A Randomized, Controlled Trial in Rural China. Food and Nutrition Bulletin 35 (4_suppl3): S188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne-Lyman A., Spiegelman D., and Fawzi W.W.. 2014. Is the Strength of Association between Indicators of Dietary Quality and the Nutritional Status ofChildrenBeing Underestimated? Maternal & Child Nutrition 10 (1): 159–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., de Onis M., Curi Hallal P., Blössner M., and Shrimpton R.. 2010. Worldwide Timing of Growth Faltering: Revisiting Implications for Interventions. Pediatrics 125: 473–80. [DOI] [PubMed] [Google Scholar]
- Walker S.P., Powell C.A., Grantham-McGregor S.M., Himes J.H., and Chang S.M.. 1991. Nutritional Supplementation, Psychosocial Stimulation, and Growth of Stunted Children: The Jamaican Study. American Journal of Clinical Nutrition 54 (4): 642–8. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2006. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatrica Supplement 450: 76–85. [DOI] [PubMed] [Google Scholar]
- Zerfu T.A., Umeta M., and Baye K.. 2016. Dietary Habits, Food Taboos, and Perceptions towards Weight Gain during Pregnancy in Arsi, Rural Central Ethiopia: A Qualitative Cross-Sectional Study. Journal of Health, Population and Nutrition 35 (1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.