Abstract
High-stress occupations (ie, firefighters, military personnel, police officers, etc.) are often plagued by cardiometabolic diseases induced by exposure to chronic stressors. Interrupted sleep cycles, poor dietary patterns, lack of physical activity, and smoke exposure along with simultaneous psychological stressors promote chronic low-grade inflammation and excessive oxidative stress. Collectively, these data suggest that practical interventions which might mitigate the underlying pathologies of these cardiometabolic diseases are warranted. Ketones, specifically R-βHB, modulates intracellular signaling cascades such as the cellular redox ratios of NAD+/NADH, the activity of NAD dependent deacetylases SIRT1 and SIRT3, and promotes a robust mitochondrial environment which favors reductions in oxidative stress and inflammation. To date, the literature examining R-βHB as a signaling metabolite has mostly been performed from endogenous R-βHB production achieved through nutritional ketosis or cell culture and mouse models using exogenous R-βHB. To the authors knowledge, only 1 study has attempted to report on the effects of exogenous ketones and the mitigation of oxidative stress/inflammation. Therefore, the scope of this review is to detail the mechanisms of R-βHB as a signaling metabolite and the role that exogenous ketones might play in mitigating diseases in individuals serving in high-stress occupations.
Keywords: Ketosis, cardiovascular disease, nutrition, metabolism, inflammation, oxidative stress, firefighters, police officers, soldiers
Introduction
Men and women serving in high-stress occupations (HSO; military soldiers, police personnel, firefighters, paramedics, etc.) are exposed to a variety of acute and chronic physiological and psychological stressors. Indeed, HSO present a unique model for studying metabolic dysregulation provided these individuals often demonstrate components of metabolic syndrome while also conducting occupational tasks (ie, short sprints, victim search and rescues, fire suppression, long endurance rucks, etc.) that mimic the physical and cognitive demands of athletes. However, chronic exposure to factors such as disturbed sleep patterns, nutrient poor diets, exposure to micro particulates, post-traumatic stress disorder, and lack of physical activity all potentially result in the prolonged activation of the hypothalamic pituitary adrenal (HPA) and sympathoadrenal (SA) axes. Activation of the HPA and SA axes results in the “fight or flight” response and while these physiological responses (ie, increased cortisol, epinephrine, and norepinephrine levels) are acutely beneficial for immediate stressors, persistent activation of these axes have been linked to chronic low-grade inflammation, excessive oxidative stress (OS), and an elevated risk for developing several cardiometabolic diseases.1 In support of these data, firefighters suffer more fatalities from events related to cardiometabolic diseases, such as sudden heart attacks, than from firefighting.2 Moreover, metabolic syndrome is largely prevalent among military soldiers and law enforcement personnel as approximately 7% of law enforcement deaths are attributed to heart infarctions.3 Subsequent investigations demonstrated that police are also at an increased risk for cardiovascular morbidity compared to the general population4,5 and observational data revealed that 51% of members in the military service are too overweight (body mass index > 25.0) to be considered occupationally ready to complete their military-specific job requirements.6 Collectively these data suggest that interventions are needed which might alleviate the detrimental effects associated with serving in an HSO. Currently, the American Heart Association and American College of Sports Medicine recommend that individuals working to prevent and mitigate cardiometabolic diseases should aim to burn more calories than consumed, follow a dietary approach to stop hypertension diet, participate in 75 to 150 min a week of moderate-to-vigorous intensity aerobic exercise, and eliminate exposure to smoke.7 While the authors do not refute these recommendations as each would dramatically improve the health and well-being of an individual serving in a HSO, currently the data suggest poor adherence to both dietary8,9 and exercise10-13 interventions. Provided these findings, alternative and practical interventions which could be implemented in a HSO are warranted.
Inducing a state of ketosis (βHB ⩾0.5 mM)14 represents 1 intervention for combatting cardiometabolic diseases and simultaneously complimenting the rigorous physical and mental exertion required by individuals in HSO. Ketone bodies acetoacetate, acetone, and β-hydroxybutyrate (2 enantiomers; S- and R-βHB) have re-emerged in recent years for their purported properties as signaling metabolites, provision as an alternative fuel source during exercise, and characteristics which induce a range of pleiotropic effects. More specifically, it is the ketone body R-βHB which has attracted sport scientists and clinicians alike. Produced in the liver during periods of carbohydrate restriction, R-βHB is accumulating evidence as a metabolite which positively affects components of metabolic syndrome,15 acts as an alternative and efficient metabolic fuel substrate compared to either glucose or fatty acids,16 hampers OS via intracellular signaling events,17 and augments training adaptations18,19 which might potentially benefit performance. While the ketogenic diet in past years has arguably been the most common method for elevating S- and R-βHB levels and attaining nutritional ketosis, the ketogenic diet is also one of the more rigorous diets in terms of compliance requirements (carbohydrate intake <50 g a day or <10% total kcals14). However, over the last decade the development of isolated ketone bodies for human consumption has produced various commercially available forms of ketone salts and ketone esters. These exogenous ketones (EK) induce an acute state of ketosis without the limitations imposed by the ketogenic diet. Provided that some of the health benefits (ie, decreased OS, inflammation, skeletal muscle catabolism, and increased cardiac muscle energetics) observed in subjects following a ketogenic diet are potentially a direct or indirect result of elevated R-βHB acting as a signaling metabolite, it is then reasonable to speculate that EK potentially extend the benefits of nutritional ketosis or even afford some of the purported properties of nutritional ketosis without severely limiting carbohydrate intake.
As such, the scope of this review is to discuss the mechanistic data of ketone bodies and highlight the potential field implications, if any, EK might offer individuals serving in HSO such as mitigating markers of cardiometabolic disease and improving the mitochondrial environment to protect against future stressors.
R-βHB as a Signaling Metabolite
Endogenous versus exogenous ketones
The intricate details of ketogenesis and ketolysis exceed the scope of this article and readers are directed to a comprehensive review on ketone metabolism.16 However, a brief overview is provided for the reader. During periods of low glucose availability and subsequent elevated glucagon-to-insulin ratios, free fatty acids are liberated from adipose tissue and circulated to hepatic mitochondria. Following several sequential reactions within the mitochondrial matrix, the ketone body acetoacetate is synthesized and either transported into circulation, converted to acetone and excreted, or the primary pathway is a reduction to S- and R-βHB. Currently, the specific effects of S-βHB as a signaling metabolite and in various metabolic roles are poorly understood. S-βHB is a minor biological intermediate during β-oxidation, a cellular antioxidant and scavenger of reactive oxygen species (ROS),20 and 1 study found that S-βHB might influence the pro-inflammatory pathway NOD-, LRR- and pyrin domain-containing protein-3 (NLRP3).21 However, Stubbs et al. demonstrated that S-βHB remained elevated in circulation longer than R-βHB likely due to differences in metabolic fates. These data support the work of earlier experimental studies in rats22 and suggest that S-βHB is a poor oxidative fuel compared to R-βHB.23 While only speculative, due to S-βHB low oxidative rates and longer elevations in the circulatory system, there is potential that S-βHB serves a more prominent role in redox signaling than currently thought. However, provided the continued discrepancies regarding the intracellular and systemic mechanisms of S-βHB, the rest of the present review will focus solely on R-βHB.
Unlike nutritional ketosis which is a result of inadequate glucose availability and an upregulation of the ketogenesis pathway, an alternative method for achieving acute ketosis is via EK. Currently, EK are available in either a racemic salt mixture (ie, containing both the S-and R-βHB enantiomer in a 50:50 ratio) or several different ketone ester forms. While ketone salts are a practical option for the general public due to greater availability and lower cost compared to the ester, a recent systematic review showed that ketone salts lack an ergogenic effect regarding physical performance and poorly elevate circulating R-βHB levels (⩽1.0 mM);24 It is worth noting that simply elevating R-βHB levels via EK will not independently improve physical performance.24 Muscles seem to display an R-βHB saturation point between 0.8 and 2.0 mM25 which likely evolved as a protective mechanism for sparing circulating R-βHB for extrahepatic tissues. Overall these data suggest a ceiling effect in skeletal muscle where further increases in R-βHB levels might offer no additional ergogenic benefits and potentially put an individual at risk for metabolic acidosis should ketone levels start to exceed 10.0 mM.26 Still, although ketone salts technically achieve a state of ketosis, the lack of findings have led to speculations by several investigators of a hypothesized threshold (~2.0 mM) for physiological benefits that extend beyond merely performance.24,27-29 Circulating R-βHB of 2.0 mM or greater is commonly observed among ketone ester studies,23,30-34 specifically those using the ketone monoester. Additionally, when ergogenic effects exist, improvements to performance tend to only be observed when R-βHB surpasses this hypothesized threshold.30 It is reasonable to then speculate that other beneficial properties (eg, reduced OS and inflammation) might also occur when R-βHB levels are elevated beyond this threshold, such as is observed during ketogenic diet interventions. For more detailed information on the characteristics of EK and nutritional ketosis, the reader is directed to a more comprehensive review.35
Stress, R-βHB, and the mitochondria
When considering the stress-induced redox implications for HSO, it is important to elucidate what is meant by the term “stress.” Stress is widely associated with negative aspects and outcomes.36 However, over 100 years ago, work from Yerkes and Dodson37 demonstrated a positive effect of moderate amounts of physiological arousal (ie, stress) on physical performance which was represented by the inverted U relationship between physiological arousal and performance.38 Interestingly, the concept of the U shape relationship between arousal and performance can also be applied to various aspects of cardiometabolic health, and not only to physical performance.36 For example, while OS is associated with the progression of numerous chronic diseases, acute exposure to ROS is potentially beneficial, as moderate ROS exposure serves as a trigger to up-regulate antioxidant defense mechanisms and initiate signaling for mitochondrial biogenesis.39-41 Thus, the same U shape relationship between stress and performance can also be applied to redox balance and the hermetic stress response. In other words, excessive exposure to ROS leads to OS and chronic low-grade inflammation, while acute/moderate exposure to ROS is beneficial for facilitating favorable mitochondrial adaptations known as mitohormesis.42
HSO regularly encounter numerous chronic stressors (eg, heat exposure, intense physical exertion, disrupted sleep, etc,). Additionally, a unique aspect of HSO is the exposure to concurrent psychological and physiological stressors (ie, dual stress challenges) which have been shown to elicit greater activation of the HPA and SA axes compared to that noted from a single stressor alone. The subsequent impact is exacerbated concentrations of epinephrine, norepinephrine, cortisol, as well as markers of OS and inflammation.43,44 While acutely this response might serve beneficial physical functions, those working in HSO are at greater risk of being exposed to chronic inflammation and OS and subsequently, increased risk for cardiometabolic diseases.1 Although a direct cause and effect relationship between psychological stress and OS is currently lacking, extensive data demonstrate a strong relationship between stress, OS, mitochondrial dysfunction, and an elevated risk for developing numerous chronic cardiovascular, metabolic and neurological diseases.36,45-47
OS is the result of an imbalance in the redox environment such that the production of ROS overwhelms antioxidant defense mechanisms resulting in oxidative damage to endogenous biomolecules. Exposure to environmental, physiological and psychological stress is known to induce OS through numerous mechanisms.40,48 It is well accepted that the mitochondria are common sources of ROS.49 An increased strain on the electron transport complex during mitochondrial respiration is likely to lead to oxygen/electron leakage from complex I and III leading to ROS, specifically formation of superoxide and hydroxyl radicals, and potentially OS.49 However, it is important to clarify that numerous enzyme systems are likely contributors to ROS as well, including (but not limited to) NADPH and xanthine oxidases as well as the endoplasm.50 Regardless where ROS originated, continual metabolic stress challenges the redox environment resulting in depleted antioxidative defense mechanisms and elevated OS, ultimately increasing risk for cardiometabolic dysfunction.
Numerous dietary interventions have been examined as potential methods to reduce OS including ketogenic diets, time restricted feeding, and caloric restriction.51-55 Indeed, the literature on viable methods of restricting calories is somewhat extensive involving both human and animal trials, demonstrating caloric restriction results in a favorable hermetic redox balance and greater resistance to stress as noted by increased antioxidant status, reduced OS, and increased lifespan.55-57 It has been widely speculated that some of the benefits associated with caloric restriction are attributed to endogenous production of ketone bodies.58-61 While more data are needed to clarify this relationship, metabolism of R-βHB has been shown to reduce OS59 and susceptibility to developing several chronic diseases.
Metabolically speaking, R-βHB is often cited as an efficient substrate for cellular work by providing a thermodynamic advantage due to the greater free energy released during ATP hydrolysis compared to either glucose or fat.62 This is supported by earlier work demonstrating ketone body infusion in perfused rat heart models suppressed glycolytic activity and improved the mitochondrial oxygen consumption efficiency.63 These findings are interesting as nutritional ketosis is often cited as a mechanism for increasing mitohormesis.64 When consuming a ketogenic diet, energy demands shift from glycolytic sources to longer chain fatty acids. The shift in substrate metabolism alters the FADH2 to NADH ratio, effectively placing a greater demand on complex II and increasing the chance of mitochondrial ROS production.65,66 The modest increase in ROS production then acts as a signaling stimulus to induce hormetic adaptations.64 However, recent survey data suggests that individuals in HSO would depict low adherence (~10%)67 to a ketogenic diet. An argument could also be made that an intervention which further increased ROS generation in a population potentially suffering from mitochondrial dysfunction, (5-15 days)68 would likely not be an optimal intervention. It is then reasonable to speculate that in this model, chronic EK supplementation paired with a modest caloric restricted diet (~10%-20% total kcal reduction) might be advantageous provided ketones require less oxygen per mole of carbon to oxidize and therefore, reduce the strain on the electron transport complex and related antioxidant defense systems.
The oxidation of endogenous R-βHB has been shown to beneficially impact mitochondrial ROS production and reduce OS susceptibility through a variety of proposed mechanisms.63 Endogenous R-βHB can function as a direct or indirect antioxidant by facilitating reduction reactions, donating electrons to ROS20 and by acting as a trigger to up-regulate mitochondrial signaling proteins.51 The oxidation of R-βHB changes cellular ratios of NAD+/NADH which favors redox changes and results in the activation of signaling proteins such as NAD dependent deacetylases (SIRT1, SIRT3) that are known to increase the activity of antioxidants such as heme oxygenase 1, superoxide dismutases, and catalases through the activation of forkhead box O1 and 3 (FOXO1 & FOXO3).59 It has also been speculated that the mitochondrial consumption of R-βHB activates nuclear factor-erythroid 2-related factor-2 (NRF2) which serves as an additional mechanism for the up-regulation of endogenous antioxidants.59 Collectively, the potential for R-βHB to act as a direct and indirect antioxidant provides evidence that some of the metabolic benefits with caloric restriction may be attributed directly to R-βHB itself. These findings have important implications for EK and HSO who often suffer from OS induced from numerous occupational stressors. To date however, the body of literature surrounding R-βHB as a signaling metabolite in mitigating OS has largely been demonstrated using endogenous ketone bodies, as opposed to EK.
To the authors knowledge, only 1 study has attempted to examine the role of an EK on OS markers. In a randomized, crossover design, McAllister et al. supplemented firefighters for 7 days with either a placebo or racemic ketone salt mixture.69 Markers of OS (ie, reduced and oxidized glutathione, superoxide dismutase, catalase, total antioxidant capacity, and malondialdehyde) were collected at multiple time points pre- and post-exercise task with firefighters exercising in personal protective equipment. Their findings showed that exercising in personal protective equipment did result in OS but EK had no effect on this response. However, a glaring limitation to the investigation was the lack of blood R-βHB analysis throughout the study duration (ie, 7-day treatment periods). While the investigative team reported the supplementation duration as a limitation to the absent changes in OS markers and suggested longer supplementation trials, additional speculation would suggest that the null findings can also be attributed to the team’s incorporation of a racemic ketone salt supplement which arguably only slightly elevated R-βHB levels (~0.5 mM) and put the study’s subjects in the minimum threshold for achieving nutritional ketosis. The present team would then argue it was the non-meaningful rise in R-βHB levels that resulted in a lack of OS changes and perhaps not the study duration. More specifically, data reporting improvements to a subjects cardiometabolic profile are often observed following a prolonged ketogenic diet (⩾3 weeks)26,70, when R-βHB levels consistently approach the hypothesized threshold of ~2.0 mM. Provided these data and our speculation, if therapeutic properties do exist from EK supplementation, then they will likely be revealed through future studies incorporating ketone esters as opposed to racemic ketone salt blends. Indeed, it is the ketone monoester which routinely elevates R-βHB levels above 1.0 mM23,30,34,71 and is generally well tolerated compared to the gastrointestinal issues that accompany the acetoacetate diester.72
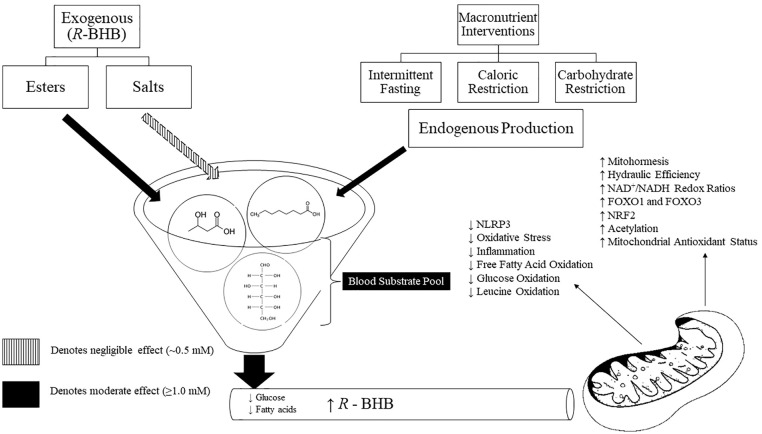
It is difficult to elucidate whether the improvements in cardiometabolic markers are a result of the robust cellular environment induced by nutritional ketosis (eg, mitohormesis, modulation in cellular signaling, downregulation of the NLR3P pathway, etc.) and R-βHB is simply a byproduct of this environment, or if R-βHB is “truly” a signaling metabolite in the absence of these mitochondrial adaptations and adherence to a ketogenic diet. Alternatively, due to the nature of ketogenic diets, ingestion of lipids is substantially increased. A single high-fat feeding is known to induce OS acutely73 and it is possible that the mitochondrial antioxidant defense mechanisms upregulate in response to the dietary pattern to protect against lipid induced OS, not necessarily as a result of rising R-βHB levels. While it does not seem that R-βHB directly induces mitohormesis, mitochondrial health is paramount to R-βHB oxidation considering ketolytic enzymes are highest in type I fibers and located within the mitochondria. In vitro and mouse model studies have shown R-βHB to directly act as a modulator of signaling events by promoting the anti-atherogenic and anti-inflammatory throughout cells of the epithelial wall and immune system.74 Additionally, R-βHB has been shown to directly inhibit histone deacetylase enzymes in a dose-dependent manner and thus promote gene expression and enhanced modulation of the immune system and global reduction in OS.17,75 These data alone should warrant additional research into the potential therapeutic impact that EK might offer clinicians as current mechanistic data is scarce regarding the signaling potential of ketone bodies in human trials. An overview of R-βHB as a signaling metabolite within the mitochondria can be viewed in Figure 1.
Figure 1.
Pleiotropic effects of R-BHB as a signaling molecule within the mitochondria. The mitochondria are often only known as the “powerhouse” of a cell due to the reliance on the mitochondria for adenosine triphosphate production. However, the mitochondria serve an important focal point in improving cellular health and metabolic flexibility. During periods of caloric or carbohydrate restriction or when ingested as a supplement, an elevation in R-BHB can contribute to the overall health of the mitochondria. R-BHB has been shown to mitigate oxidative stress, upregulate antioxidant enzymes and anti-inflammatory transcription factors, and decrease the oxidation of glucose and fatty acids. These effects make R-BHB a favorable metabolite for future projects with an aim to mitigate cardiometabolic diseases in high-stress occupations. R-BHB, R-β-hydroxybutyrate; NLRP3, NOD-, LRR- and pyrin domain-containing protein-3; NAD, nicotinamide adenine dinucleotide; FOXO1 and 3, forkhead box O1 and 3; NRF2, nuclear factor-erythroid 2-related factor-2.
Practical implications
Individuals serving in HSO are plagued by cardiometabolic diseases (ie, insulin resistance, atherosclerosis, obesity) which are exacerbated by the countless stressors encountered during a typical work cycle. While these diseases are multi-factorial in consideration of lifestyle factors, at the cellular level, cardiometabolic diseases tend to stem from chronic low-grade inflammation and excessive OS.76 The present review is not to suggest that EK might act in place of nutrition, exercise, or lifestyle interventions, but rather to direct future research efforts among clinicians and sport scientists alike into the therapeutic properties, if any, that EK supplementation might offer individuals serving in HSO or suffering with metabolic dysregulation.
Current in vitro and mouse data have demonstrated R-βHB to act as a powerful signaling molecule capable of hampering pro-inflammatory pathways. In addition, R-βHB seems to enhance the mitochondrial environment and thus improving the anti-oxidant capabilities and OS reduction properties of cells. R-βHB has also been shown to alter whole-body metabolism, consistently displaying a reduction in plasma glucose concentrations (10%-20%) as well as reductions in circulating free fatty acids. It has been proposed that the reduction observed in lipolysis can be partially explained due to an acute increase in insulin sensitivity71 which would highly favor metabolic health in a diseased population.
It is difficult to suggest EK as a practical intervention to individuals serving in HSO due to the current cost of the ketone esters (~ US$33.00 per serving). However, our team has worked extensively with HSO (military, firefighters, police, and correctional emergency response teams) and the incorporation of EK supplements (ie, ketone salts) as a potential ergogenic aid has become common to observe. Consequently, it is apparent that officers, lieutenants, and some nutritionists working with these individuals have applied data from ketogenic diets interchangeably with EK. A thorough review of the convergence and divergence of these 2 distinct metabolic states was recently published and outlines our similar concerns regarding merging these studies without disentangling the methods utilized to induce nutritional ketosis.35 However, until further data are presented regarding EK and human trials, these practices will likely continue. Therefore, interested individuals are directed towards supplementing with the ketone esters, as opposed to the ketone salts. The present evidence on ketone salts suggests a negligible to weak effect on physical and cognitive performance,24,27,77 as well as lack of potential to mitigate OS.69 In contrast, ketone esters have demonstrated some benefits as an ergogenic aid for both physical and cognitive performance,30 although OS and inflammatory data are missing. In earlier studies, Veech et al. described a protocol of administering divided, oral doses (100-150 g/day) of R-βHB in ester form throughout the day.63,78 It was speculated that maintaining elevated plasma R-βHB levels through the day would result in dramatic reductions to NADP+ and subsequently mitigate OS and cellular metabolic stress. Finally, it should be noted this is not an exhaustive list of the purported properties that ketone esters might offer. Present hypotheses and investigations are ongoing, attempting to provide insight into the role of ketone bodies on appetite regulation,79 muscle recovery,18,19 protection against respiratory viral infections,80 sparing of muscle protein during prolonged exercise81 or during states of muscle wasting,82 and alleviation from type II diabetes mellitus symptoms.83 To date, the most recent collection of EK studies have overwhelmingly focused on the potential substrate (glucose, glycogen, and fatty acids) sparing effects and improvements to physical or cognitive performance when taken acutely and prior to exercise. While the data is not conclusive on EK in this role, it would appear that EK are a more promising application when incorporated as a training and recovery aid and likely supplemented over a longer duration.
Conclusion
Interest into the therapeutic potential of ketone bodies increased when ketogenic diets reemerged as a potential intervention for treating patients suffering from epilepsy in the 1990s and again in the early 2000s from the pioneering work of Dr. Veech whom demonstrated the capabilities of ketone bodies to alleviate underlying symptoms of metabolic diseased states.63 Current speculation suggests that it would be incorrect to assume that EK mimic the robust mitochondrial environment induced from adhering to a ketogenic diet. However, data are limited in human trials, specifically as it relates to the effects of ketone bodies on inflammation and OS markers. If any mitoprotective properties are elicited from EK supplementation, they will likely be observed when plasma R-βHB levels near the hypothesized ~2.0 mM. Currently it is unknown if EK offer HSO an advantage, but until further data are provided, the effects of nutritional ketosis achieved from a ketogenic diet and acute ketosis induced from EK will continue to be conflated.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: H.S.W and M.J.M wrote the manuscript. All authors contributed equally to the manuscript. All authors read and approved the final manuscript.
ORCID iDs: Hunter S Waldman  https://orcid.org/0000-0001-5707-5642
https://orcid.org/0000-0001-5707-5642
Matthew J McAllister  https://orcid.org/0000-0002-4072-317X
https://orcid.org/0000-0002-4072-317X
References
- 1. Huang C, Webb H, Zourdos M, Acevedo E. Cardiovascular reactivity, stress, and physical activity. Front Physiol. 2013;4:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kales SN, Soteriades ES, Christophi CA, Christiani DC. Emergency duties and deaths from heart disease among firefighters in the United States. New Engl J Med. 2007;356(12):1207-1215. [DOI] [PubMed] [Google Scholar]
- 3. Zimmerman FH. Cardiovascular disease and risk factors in law enforcement personnel: a comprehensive review. Cardiol Rev. 2012;20(4):159-166. [DOI] [PubMed] [Google Scholar]
- 4. Calvert GM, Merling JW, Burnett CA. Ischemic heart disease mortality and occupation among 16- to 60-year-old males. J Occup Environ Med. 1999;41(11):960-966. [DOI] [PubMed] [Google Scholar]
- 5. Dubrow R, Burnett CA, Gute DM, Brockert JE. Ischemic heart disease and acute myocardial infarction mortality among police officers. J Occup Med Off Publ Ind Med Assoc. 1988;30(8):650-654. [DOI] [PubMed] [Google Scholar]
- 6. Barlas FM, Higgins WB, Pflieger JC, Diecker K. 2011 Health related behaviors survey of active duty military personnel. ICF International Inc; 2013. [Google Scholar]
- 7. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Part B):2960-2984. [DOI] [PubMed] [Google Scholar]
- 8. Thomas DM, Martin CK, Redman LM, et al. Effect of dietary adherence on the body weight plateau: a mathematical model incorporating intermittent compliance with energy intake prescription. Am J Clin Nutr. 2014;100(3):787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gibson AA, Sainsbury A. Strategies to improve adherence to dietary weight loss interventions in research and real-world settings. Behav Sci. 2017;7(3):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alberga AS, Medd ER, Adamo KB, et al. Top 10 practical lessons learned from physical activity interventions in overweight and obese children and adolescents. Appl Physiol Nutr Metabol. 2013;38(3):249-258. [DOI] [PubMed] [Google Scholar]
- 11. Durbeck DC, Heinzelmann F, Schacter J, et al. The national aeronautics and space administration-us public health service health evaluation and enhancement program: summary of results. Am J Cardiol. 1972;30(7):784-790. [DOI] [PubMed] [Google Scholar]
- 12. Morgan WP. Involvement in vigorous physical activity with special reference to adherence. Paper presented at: Proceedings of the National College Physical Education Association for Men & National Association for Physical Education of College Women National Conference Chicago, IL, 1977. [Google Scholar]
- 13. Taylor H, Buskirk E, Remington R. Exercise in controlled trials of the prevention of coronary heart disease. Paper presented at: Federation proceedings, 1973. [PubMed] [Google Scholar]
- 14. Volek JS, Noakes T, Phinney SD. Rethinking fat as a fuel for endurance exercise. Eur J Sport Sci. 2015;15(1):13-20. [DOI] [PubMed] [Google Scholar]
- 15. Volek JS, Fernandez ML, Feinman RD, Phinney SD. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Progress Lipid Res. 2008;47(5):307-318. [DOI] [PubMed] [Google Scholar]
- 16. Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. Journal Physiol. 2017;595(9):2857-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Newman JC, Verdin E. β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. 2017;37:51-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holdsworth DA, Cox PJ, Kirk T, Stradling H, Impey SG, Clarke K. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sports Exercise. 2017;49(9):1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vandoorne T, De Smet S, Ramaekers M, et al. Intake of a ketone ester drink during recovery from exercise promotes mTORC1 signaling but not glycogen resynthesis in human muscle. Front Physiol. 2017;8:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haces ML, Hernández-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol. 2008;211(1):85-96. [DOI] [PubMed] [Google Scholar]
- 21. Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med. 2015;21(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Webber RJ, Edmond J. Utilization of L (+)-3-hydroxybutyrate, D (-)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid synthesis in the 18-day-old rat. J Biol Chem. 1977;252(15):5222-5226. [PubMed] [Google Scholar]
- 23. Stubbs BJ, Cox PJ, Evans RD, et al. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Margolis LM, O’Fallon KS. Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr. 2020;11(2):412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mikkelsen KH, Seifert T, Secher NH, Grøndal T, van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metabol. 2015;100(2):636-643. [DOI] [PubMed] [Google Scholar]
- 26. Volek J, Phinney SD. The art and science of low carbohydrate performance: A revolutionary program to extend your physical and mental performance envelope. Beyond Obesity; 2012. [Google Scholar]
- 27. Waldman HS, Shepherd BD, Egan B, McAllister MJ. Exogenous ketone salts do not improve cognitive performance during a dual-stress challenge. Int J Sport Nutr Exercise Metabol. 2020;30(2):120-127. [DOI] [PubMed] [Google Scholar]
- 28. Hashim SA, Van Itallie TB. Ketone body therapy: from the ketogenic diet to the oral administration of ketone ester. J Lipid Res. 2014;55(9):1818-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stubbs BJ, Koutnik AP, Poff AM, Ford KM, D’Agostino DP. Commentary: ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2018;9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metabol. 2016;24(2):256-268. [DOI] [PubMed] [Google Scholar]
- 31. Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exercise. 2018;50(11):2330-2338. [DOI] [PubMed] [Google Scholar]
- 32. Poffé C, Ramaekers M, Van Thienen R, Hespel P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol. 2019;597(12):3009-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sports Exercise. 2019;51(12):2506-2515. [DOI] [PubMed] [Google Scholar]
- 34. Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K, de Wet H. A ketone ester drink lowers human ghrelin and appetite. Obesity. 2018;26(2):269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Poff AM, Koutnik AP, Egan B. Nutritional ketosis with ketogenic diets or exogenous ketones: features, convergence, and divergence. Curr Sports Med Rep. 2020;19(7):251-259. [DOI] [PubMed] [Google Scholar]
- 36. Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38(9):1698-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Punishment Issues Exp. 1908:27-41. [Google Scholar]
- 38. Matthews G, Amelang M. Extraversion, arousal theory and performance: a study of individual differences in the EEG. Pers Individ Differ. 1993;14(2):347-363. [Google Scholar]
- 39. Kawamura T, Muraoka I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants. 2018;7(9):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samet JM, Wages PA. Oxidative stress from environmental exposures. Curr Opin Toxicol. 2018;7:60-66. [PMC free article] [PubMed] [Google Scholar]
- 41. Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp Gerontol. 2010;45(6):410-418. [DOI] [PubMed] [Google Scholar]
- 43. Webb HE, Weldy ML, Fabianke-Kadue EC, Orndorff G, Kamimori GH, Acevedo EO. Psychological stress during exercise: cardiorespiratory and hormonal responses. Eur J Appl Physiol. 2008;104(6):973-981. [DOI] [PubMed] [Google Scholar]
- 44. Huang C-J, Webb HE, Evans RK, et al. Psychological stress during exercise: immunoendocrine and oxidative responses. Exp Biol Med. 2010;235(12):1498-1504. [DOI] [PubMed] [Google Scholar]
- 45. Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxidat Med Cell Longevity. 2009;2:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liguori I, Russo G, Curcio F, et al. Oxidative stress, aging, and diseases. Clin Intervent Aging. 2018;13:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35(4):411-429. [DOI] [PubMed] [Google Scholar]
- 48. Ghezzi P, Floridi L, Boraschi D, et al. Oxidative stress and inflammation induced by environmental and psychological stressors: a biomarker perspective. Antioxid Redox Signal. 2018;28(9):852-872. [DOI] [PubMed] [Google Scholar]
- 49. Halliwell B, Gutteridge JM. Free radicals in biology and medicine. Oxford University Press; 2015. [Google Scholar]
- 50. Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer’s disease. Antioxidants. 2018;7(5):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metabol. 2018;27(6):1212-1221.e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Waldman HS, Renteria LI, McAllister MJ. Time-restricted feeding for the prevention of cardiometabolic diseases in high-stress occupations: a mechanistic review. Nutr Rev. 2020;78(6):459-464. [DOI] [PubMed] [Google Scholar]
- 54. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metabol. 2018;27(4):805-815.e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pifferi F, Aujard F. Caloric restriction, longevity and aging: recent contributions from human and non-human primate studies. Progr Neuro-Psychopharmacol Biol Psychiatry. 2019;95:109702. [DOI] [PubMed] [Google Scholar]
- 57. Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78(3):361-369. [DOI] [PubMed] [Google Scholar]
- 58. Seyfried TN. Ketone strong: emerging evidence for a therapeutic role of ketone bodies in neurological and neurodegenerative diseases. J Lipid Res. 2014; 55(9):1815-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rojas-Morales P, Pedraza-Chaverri J, Tapia E. Ketone bodies, stress response, and redox homeostasis. Redox Biol. 2020;29:101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metabol. 2014;25(1):42-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Veech RL, Bradshaw PC, Clarke K, Curtis W, Pawlosky R, King MT. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. 2017;69(5):305-314. [DOI] [PubMed] [Google Scholar]
- 62. Sato K, Kashiwaya Y, Keon C, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9(8):651-658. [DOI] [PubMed] [Google Scholar]
- 63. Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70(3):309-319. [DOI] [PubMed] [Google Scholar]
- 64. Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metabol. 2018;2018:5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem. 2007;282(43):31257-31266. [DOI] [PubMed] [Google Scholar]
- 66. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277(47):44784-44790. [DOI] [PubMed] [Google Scholar]
- 67. Yang J, Farioli A, Korre M, Kales SN. Dietary preferences and nutritional information needs among career firefighters in the United States. Glob Adv Health Med. 2015;4(4):16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Howard EE, Margolis LM. Intramuscular mechanisms mediating adaptation to low-carbohydrate, high-fat diets during exercise training. Nutrients. 2020;12(9):2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McAllister M, Holland A, Chander H, Waldman H, Smith JEW, Basham S. Impact of ketone salt containing supplement on cardiorespiratory and oxidative stress response in firefighters exercising in personal protective equipment. Asian J Sports Med. 2019;10(1):e82404 [Google Scholar]
- 70. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/Metabol Res Rev. 1999;15(6):412-426. [DOI] [PubMed] [Google Scholar]
- 71. Myette-Côté É, Neudorf H, Rafiei H, Clarke K, Little JP. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. The Journal of physiology. 2018;596(8):1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McCarthy CG, Farney TM, Canale RE, Dessoulavy ME, Bloomer RJ. High-fat feeding, but not strenuous exercise, increases blood oxidative stress in trained men. Appl Physiol Nutr Metabol. 2013;38:33-41. [DOI] [PubMed] [Google Scholar]
- 74. Offermanns S. Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol Metabol. 2017;28(3):227-236. [DOI] [PubMed] [Google Scholar]
- 75. Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860-867. [DOI] [PubMed] [Google Scholar]
- 77. Prins PJ, D’Agostino DP, Rogers CQ, et al. Dose response of a novel exogenous ketone supplement on physiological, perceptual and performance parameters. Nutr Metabol. 2020;17(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF., Jr Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51(4):241-247. [DOI] [PubMed] [Google Scholar]
- 79. Deemer SE, Plaisance EP, Martins C. Impact of ketosis on appetite regulation–a review. Nutr Res. 2020;77:1-11. [DOI] [PubMed] [Google Scholar]
- 80. Stubbs BJ, Koutnik AP, Goldberg EL, et al. Investigating ketone bodies as immunometabolic countermeasures against respiratory viral infections. Med. Published online July 15, 2020. doi: 10.1016/j.medj.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koutnik AP, D’Agostino DP, Egan B. Anticatabolic effects of ketone bodies in skeletal muscle. Trends Endocrinol Metabol. 2019;30(4):227-229. [DOI] [PubMed] [Google Scholar]
- 82. Koutnik AP, Poff AM, Ward NP, et al. Ketone bodies attenuate wasting in models of atrophy. J Cachexia Sarcopenia Muscle. 2020;11(4):973-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Egan B. The glucose-lowering effects of exogenous ketones: is there therapeutic potential? J Physiol. 2018;596(8):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]