Abstract
Background –
Stroke patients with unknown time of onset have been previously excluded from thrombolysis. We aimed to determine whether intravenous alteplase is safe and effective in these patients when salvageable tissue is identified using imaging biomarkers.
Methods –
We performed a systematic review and meta-analysis of individual patient data of trials published before 21 September 2020. Randomized trials of intravenous alteplase versus standard of care or placebo in adults with unknown onset stroke using perfusion-diffusion MRI, perfusion CT, or MRI with DWI-FLAIR mismatch were eligible. The primary outcome was favourable functional outcome (score of 0–1 on the modified Rankin Scale [mRS]) at 90 days indicating no disability, secondary outcomes were mRS shift towards a better functional outcome and independent outcome (score 0–2 on the mRS) at 90 days. Safety outcomes included death, severe disability or death (mRS 4–6), and symptomatic intracerebral haemorrhage (sICH). The study is registered with PROSPERO, number CRD42020166903.
Findings –
Four trials met the eligibility criteria: WAKE-UP, EXTEND, THAWS, and ECASS-4. Of the 843 patients, 429 (51%) were assigned to alteplase and 414 (49%) to placebo or standard care. A favourable outcome occurred in 199 of 420 patients (47%) with alteplase and in 160 of 409 patients (39%) among controls (adjusted odds ratio [aOR] 1.50, 95% confidence interval [CI] 1.10–2.04, p=0.010). Alteplase was associated with a significant shift towards better functional outcome (adjusted common odds ratio 1.38, 95% CI 1.05–1.80, p=0.019), and with a higher odds of independent outcome (aOR 1.50, 95% CI 1.06–2.12). In the alteplase group, 90 patients (21%) reached the safety endpoint of being severely disabled or dead (mRS 4–6), compared to 102 patients (25%) in the control group (aOR 0.76, 95% CI 0.52–1.11, p=0.15). Death occurred in 27 patients (6%) with alteplase and in 14 patients (3%) among controls (aOR 2.06, 95% CI 1.03–4.09, p=0.040). sICH was higher with alteplase than among controls (11 [3%] vs. 2 [1%], aOR 5.58, 95% CI1.22–25.50, p=0.024).
Interpretation –
In stroke patients with unknown time of onset with a DWI-FLAIR or perfusion mismatch, intravenous alteplase resulted in better functional outcome at 90 days than placebo or standard care. A net benefit was observed for all functional outcomes despite an increased risk of sICH. While there were numerically higher rates of death with alteplase, rates of severe disability or death were numerically lower in the alteplase group.
Keywords: Acute Ischemic Stroke, unknown time of onset, alteplase, DWI-FLAIR mismatch, penumbral imaging
Intravenous thrombolysis with alteplase is standard of care for acute ischemic stroke. It improves functional outcome and is more effective the earlier treatment is initiated 1. Since its first approval for stroke treatment, intravenous alteplase was restricted to patients with known time of symptom onset within a narrow time window. This was initially less than 3 hours of symptom onset based on the results of the NINDS trial 2 and was extended to a time window of less than 4.5 hours following the positive results of ECASS-3 3 and subsequent meta-analysis 1,4. Patients with unknown time of symptom onset were excluded from randomized controlled trials of intravenous thrombolysis for stroke and from thrombolytic treatment in clinical practice.
The time of symptom onset is unknown in up to 20–25% of stroke patients, mostly due to symptoms being recognized after waking-up from overnight sleep, but also for other reasons such as unwitnessed stroke with aphasia or disturbed level of consciousness. In recent years, several clinical studies have been designed to solve this clinical problem by testing treatment with intravenous thrombolysis based on selection using imaging biomarkers in patients with unknown time of stroke onset by using either penumbral imaging (i.e., perfusion-diffusion MRI or perfusion CT) or MRI-based tissue-clocking, i.e. the mismatch between a visible ischemic lesion on diffusion-weighted imaging (DWI) while there is no marked parenchymal hyperintensity on fluid-attenuated inversion recovery (FLAIR) on MRI (DWI-FLAIR mismatch) 5. The WAKE-UP trial provided evidence of benefit of treatment with intravenous alteplase in patients with unknown onset stroke if the treatment decision was based on DWI-FLAIR mismatch 6. Current US and European guidelines and consensus statements recommend intravenous thrombolysis with alteplase in patients with unknown time of symptom onset if patients meet the WAKE-UP criteria 7,8. Based on selection with MR or CT perfusion imaging, the EXTEND trial also showed better functional outcome in alteplase-treated patients awakening with stroke or treated from 4.5 to 9.0 hours after symptom onset 9.
Both WAKE-UP and EXTEND were started before compelling evidence for stroke thrombectomy was available and both excluded patients in whom thrombectomy was planned. In the meantime, two trials of endovascular stroke treatment, DAWN and DEFUSE-3, provided evidence of a benefit of mechanical thrombectomy guided by penumbral imaging in a late or unknown time-window 10,11.
Nevertheless, the individual trials were still modest in size and there is limited knowledge on efficacy and safety of thrombolysis in subgroups of patients with an unknown time of stroke onset. A recent individual patient-data meta-analysis of the trials using penumbral imaging to guide intravenous thrombolysis in patients in an extended or unknown time window (EXTEND, ECASS4, and EPITHET) indicated that intravenous alteplase improves functional outcome in these patients (n=414), with an overall net clinical benefit despite an increase of the rate of symptomatic intracranial haemorrhage (sICH) and a numerical but non-significant increase in deaths in the thrombolysis group 12.We aimed to determine whether intravenous alteplase is safe and effective in patients with unknown time of onset stroke when salvageable tissue is identified using based on imaging biomarkers. To this end, we performed a meta-analysis of individual patient data (n=843) to test the hypothesis that intravenous alteplase improves functional outcomes compared with placebo or standard of care in patients with acute ischemic stroke with an unknown time of onset if selected using imaging biomarkers including either DWI-FLAIR mismatch or CT or MRI based penumbral imaging.
Methods
Search strategy and selection criteria
For the systematic review we searched PubMed, Web of Science, SciELO, and LILACS for clinical trials published before 21 September 2020 with the following search terms: “stroke” AND (“alteplase” OR “rtPA” OR “tPA” OR “thrombolysis”) AND (“randomised” OR “randomized”) AND (“unknown” OR “unwitnessed” OR “wake-up” OR “extended”) (filters activated: “Clinical Trial”, “Humans”). Randomized trials of intravenous alteplase versus standard of care or placebo in adults (≥ 18 years of age) with acute ischemic stroke and unknown time of symptom onset using advanced brain imaging with either penumbral imaging (i.e., perfusion-diffusion MRI or perfusion CT) or MRI-based tissue-clocking (i.e., DWI-FLAIR mismatch) with >20 patients enrolled were eligible for inclusion. We further searched ClinicalTrials.gov, the European Union Clinical Trials Register, the World Health Organization International Clinical Trials Registry Platform, the ISRCTN Registry, and the Cochrane Central Register of Controlled Trials for clinical trials of intravenous alteplase in unknown onset stroke. All patients included in the primary analyses of individual trials were considered eligible for inclusion in the meta-analysis. Two reviewers (BC and GT) independently reviewed articles and reached a unanimous decision for inclusion.
The steering committees of all included trials agreed to join the Evaluation of unknown Onset Stroke thrombolysis trials (EOS) collaboration and share individual patient-level data for meta-analysis. Ethical approval was obtained for all participating sites for all included trials, and patients or their legal representatives provided written informed consent according to national and local regulations including an exception from explicit informed consent in emergency circumstances in some countries. The protocol for this study was prespecified and followed PRISMA guidelines for meta-analysis of individual patient data (see appendix). The study is registered with PROSPERO, number CRD42020166903).
Outcomes
The prespecified primary outcome was a favourable outcome defined by a score of 0–1 on the modified Rankin Scale (mRS, which ranges from 0 [no symptoms] to 6 [death]) at 90 days after stroke. This identifies patients with no symptoms at all (mRS 0) or only minimal symptoms with no significant disability, being able to carry out all usual activities (mRS 1). Secondary outcomes were functional improvement across the entire mRS (i.e., mRS shift analysis) at 90 days and independent outcome defined by a score of 0–2 on the mRS at 90 days. Safety outcomes were death, severe disability or death (i.e., mRS 4–6), sICH according to the Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS–MOST) 13 and radiologically defined parenchymal haemorrhage type 2 (PH-2) 14. Additional outcomes were death within 7 days of randomization and death or dependence (i.e., MRS 3–6) at 90 days.
Imaging data
We reanalysed all available imaging data as follows. Judgement of vessel occlusion was based on image readings provided by the individual trials. Any vessel occlusion was defined as any visible occlusion of an intracranial brain supplying artery on baseline MR- or CT-angiography. Large vessel occlusion was defined as occlusion of the intracranial internal carotid artery or main stem of the middle cerebral artery. Penumbral mismatch was defined according to the criteria used in recent studies of intravenous alteplase based on perfusion mismatch 12. Penumbral mismatch was considered present with a mismatch ratio between critically hypoperfused tissue and infarct core >1.2, a mismatch volume greater than 10 ml, and an ischemic core volume less than 70 ml. Critically hypoperfused tissue was defined as tissue with a time to maximum (Tmax) >6 s in CT perfusion or MR perfusion. Infarct core was defined as a relative cerebral blood flow <30% of contralateral cerebral blood flow for CT perfusion or an apparent diffusion coefficient of less than 620 μm2/s for diffusion MRI. CT perfusion and MR perfusion and DWI data, if available, were reprocessed using automated processing software RAPID (version 4.6, 4.9 and 5.0; iSchemaView, Menlo Park, CA, USA) as described previously 10,11. DWI-FLAIR mismatch was defined according to the criteria used in the WAKE-UP trial, i.e., a mismatch between an acute ischemic lesion visible on DWI and no marked parenchymal hyperintensity on FLAIR in the corresponding region as assessed by visual inspection 15.
Data Analysis
The full statistical analysis plan is provided in the appendix. The responsible statisticians from each trial extracted the patient-level data from the trial databases on the basis of data fields pre-specified in the study protocol (see supplementary methods in the appendix). The responsible biostatistician for the meta-analysis (FB) collated all data from the individual trials and cross-checked them against the original publications of the individual trials.
The modified Cochrane Collaboration tool to assess risk of bias for randomized controlled trials was applied for qualitative assessment of between trial differences including patient eligibility and assessment of bias (see appendix).
This meta-analysis followed a one-stage approach with the use of relevant mixed-effect logistic regression models with “trial” as a random intercept effect and “treatment assignment” as a random slope effect, allowing the treatment effect to vary across trials. As randomization in the WAKE-UP trial was stratified on age (≤60/>60 years) and severity of symptoms (National Institute of Health Stroke Scale [NIHSS] ≤10/>10) at baseline, all models were adjusted on these two categorical covariates. All outcomes were assessed in the intention-to-treat population, i.e., all randomized patients assigned to their randomized treatment group.
The primary efficacy outcome was analysed using an unconditional mixed-effect logistic-regression model fitted to estimate the treatment effect, reported as an odds ratio (OR) and its 95% confidence interval (CI). Missing primary outcome values were replaced using multiple imputation including the following covariates: allocated treatment, baseline age (continuous), baseline NIHSS score, and NIHSS score at day 7. We also performed a sensitivity analysis without replacement of missing outcomes. The heterogeneity of treatment effect across trials was tested using the Cochran’s Q-statistic. We also report the I2 statistic describing the percentage of variation across studies that is due to heterogeneity rather than chance 16.
The categorical shift in the distribution of mRS scores between the two treatment groups was analysed fitting a proportional-odds logistic regression model, assuming a common OR across all cut points of the mRS with values 5 and 6 collapsed into one category to prevent interpreting mRS 5 as a better outcome than mRS 6. Prior to performing ordinal regression analysis, we tested the proportional odds assumption. Analysis of independent outcome, death, and severe disability or death was performed using the same unconditional mixed-effect logistic-regression model as for the primary outcome. SICH and PH-2 were analysed with two-by-two tables stratified by trials and the common Cochran- Mantel-Haenszel OR and its 95% CI were calculated. The Breslow-Day test was used to test homogeneity of OR across trials. For secondary and safety outcomes missing values were not replaced.
We performed pre-planned analyses in the following subgroups: use of standard dose (0.9 mg/kg bodyweight) vs. low dose (0.6 mg/kg bodyweight) alteplase; age (≤60 vs >60 years); sex; baseline stroke severity (NIHSS ≤10 vs >10); any visualized vessel occlusion; large vessel occlusion; imaging modality (CT vs MRI); history of AF; history of stroke or TIA; prior antiplatelet use; delay from symptom recognition to treatment (≤3 vs >3 hours); penumbral mismatch present; DWI-FLAIR mismatch present. For all subgroup analyses, the same model as for the analysis of the primary outcome was used, with an additional interaction parameter between the treatment and the subgroup covariate entered as a fixed effect.
In an additional analysis, we included available data on outcome and safety of intravenous alteplase in unknown onset stroke from single-arm studies.
All analyses were performed with a two-sided alpha level of 0.05. There was no correction of the alpha-level for multiple comparisons. Statistical analysis was done using SAS software (version 9.4, Windows) and R-software (version 3.3.2).
Role of the funding source
There was no funding source for this meta-analysis. The funders of the trials included in this meta-analysis (WAKE-UP, EXTEND, THAWS, ECASS4) had no role in study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Our search strategy identified four randomized trials that met the eligibility criteria: WAKE-UP6, EXTEND9, THAWS17, and ECASS-418 (for the PRISMA IPD flow diagram see supplemental figure 1 in the appendix). All four trials applied brain imaging beyond non-contrast CT to randomize either exclusively patients with unknown onset stroke, or patients within an extended time window beyond 4.5 hours of symptom onset including unknown onset stroke, to intravenous alteplase or placebo/standard of care.
WAKE-UP was a European randomized placebo-controlled trial that used MRI with DWI-FLAIR mismatch to guide standard dose intravenous alteplase in patients with unknown onset stroke 6. While not mandatory, MR perfusion data were acquired in a subgroup of patients randomized and available for assessment of penumbral mismatch. EXTEND was a trial in Australia, New Zealand, Asia, and Finland that used penumbral imaging with either CT perfusion or perfusion-diffusion MRI to randomize patients in an extended time window 4.5–9 hours of stroke or wake-up stroke (if the midpoint between the time last known well and time of waking up with symptoms was within 9 hours) to standard dose alteplase or placebo 9. THAWS was a Japanese trial using MRI with DWI-FLAIR mismatch according to WAKE-UP to randomize patients to a reduced dose of 0.6 mg/kg alteplase (i.e., the approved dose in Japan) 19 or standard of care 17. ECASS-4 was a European trial that applied the same eligibility criteria as EXTEND but used only perfusion-diffusion MRI (not CT) to randomize patients to standard dose alteplase or placebo 18. ECASS-4 data were also available for assessment of DWI-FLAIR mismatch. WAKE-UP was terminated early due to cessation of funding. EXTEND and THAWS were stopped early after publication of the positive results of the WAKE-UP trial due to lack of equipoise, and ECASS-4 was terminated early due to reduced recruitment following the publication of the positive trials of thrombectomy in an extended time-window 10,11. These four trials were included in the primary analysis. Importantly, from EXTEND and ECASS-4, we only included patients with unknown-onset stroke for this meta-analysis. Overall, the risk of bias in the studies included in the meta-analysis was considered low (see table S2 in the supplementary appendix). For baseline characteristics of individual trials see table S3 in the supplementary appendix. Our search strategy identified another study, which met eligibility criteria except for not being a randomized clinical trial, MR WITNESS20 which we included in a supplementary safety analysis.
We obtained data from all 843 participants with unknown onset stroke randomized in the four trials included in the primary analysis. Of these, 429 (51%) were assigned to receive alteplase and 414 (49%) to receive placebo or standard of care. Baseline characteristics were balanced between the groups (table 1).
Table 1:
Demographic and Baseline Characteristics of the Patients
| Variable | Alteplase Group (n=429) | Control Group (n=414) |
|---|---|---|
| Age – years | ||
| Mean (SD) | 68.5 (12.2) | 68.5 (12.7) |
| Male sex – number (%) | 268 (63%) | 253 (61%) |
| Reason for unknown time of symptom onset – number (%) | ||
| Overnight-sleep | 385 (90%) | 366 (88%) |
| Other | 44 (10%) | 48 (12%) |
| Time between last seen well and symptom recognition – hours | ||
| Median, interquartile range | 7.0 (4.7–9.0) | 7.0 (5.0–9.0) |
| Medical history / risk factors – no. (%) | ||
| Arterial hypertension | 259/428 (61%) | 254/412 (60%) |
| Diabetes mellitus | 83/424 (20%) | 69/413 (17%) |
| Hypercholesterolemia * | 116/311 (63%) | 108/301 (64%) |
| Atrial fibrillation | 86/427 (20%) | 72/408 (18%) |
| History of ischemic stroke or TIA * | 45/323 (14%) | 45/310 (15%) |
| Pretreatment with antiplatelets – no. (%) | 125/397 (32%) | 132/383 (35%) |
| NIHSS score | ||
| Median, interquartile range | 7 (4–12) | 7 (4–12) |
| Imaging modality: CT | 65 (15%) | 64 (16%) |
| Imaging modality: MRI | 364 (85%) | 350 (85%) |
| Vessel occlusion on baseline CT- or MR-angiography † | ||
| Any vessel occlusion – no. (%) | 174/391 (45%) | 168/380 (44%) |
| Large vessel occlusion – no. (%) | 99/391 (25%) | 90/380 (24%) |
| Penumbral mismatch present | 112/208 (45%) | 109/197 (55%) |
| DWI-FLAIR mismatch present | 327/345 (95%) | 315/334 (94%) |
| Alteplase dose 0.9 mg/kg bodyweight | 359 (84%) | 353 (85%) |
| Alteplase dose 0.6 mg/kg bodyweight | 70 (16%) | 61 (15%) |
| Time from symptom recognition to treatment initiation – hours | ||
| Median, interquartile range | 3.3 (2.6–4.1) | 3.4 (2.7–4.1) |
| Time between last seen well and treatment initiation – hours | ||
| Median, interquartile range | 10.6 (8.6–12.4) | 10.5 (8.4–12.3) |
SD denotes standard deviation; TIA = transient ischemic attack; NIHSS = National Institutes of Health Stroke Scale;
not recorded in ECASS-4 and EXTEND;
not available for ECASS-4.
Mean age was 68.5 years (standard deviation [SD] 12.5), 322 (38%) were female. Waking from overnight sleep was the reason for unknown symptom onset in 751 patients (89%). Median time from last seen well to symptom recognition was 7.0 hours (interquartile range [IQR] 5.0–9.0). Median NIHSS on admission was 7 (IQR 4–12). MRI was used for randomization in 714 patients (85%). A DWI-FLAIR mismatch was present in 642 of 679 patients (95%) in whom assessment of DWI-FLAIR mismatch was performed. Penumbral mismatch was present in 211 of 405 patients (52%) in whom perfusion imaging was carried out. Any vessel occlusion was present in 342 of 771 patients (44%) in whom information on vessel status was available, and 189 of them had large vessel occlusion (24%).
Primary outcome data were available for 829 of 843 patients included in the analysis (420 of 429 patients in the alteplase group and 409 of 414 patients in the control group). We used three covariates to impute the missing primary outcome in 14 patients, two were stratification variables (age and NIHSS score at baseline) with no missing data, and one was NIHSS score at 72 hours, for which 36 missing values were imputed.
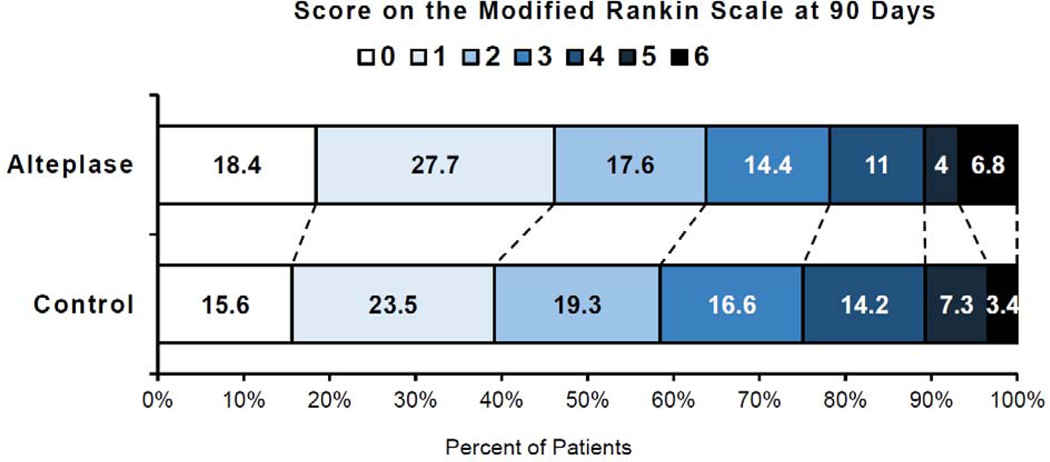
A favourable outcome (mRS score 0–1) at 90 days was observed in 199 of 420 patients (47%) in the alteplase group and in 160 of 409 patients (39%) in the control group (adjusted OR [aOR] 1.49, 95% CI 1.10–2.03, p=0.011; figure 1, table 2). Treatment with alteplase was associated with a significant shift towards better functional outcome, i.e., lower scores on the mRS at 90 days in ordinal analysis (adjusted common OR [acOR] 1.38, 95% CI 1.05–1.80, p=0.019). The proportion of patients achieving functional independence (mRS score 0–2) at 90 days was also significantly higher in the alteplase group than in the control group (aOR 1.50, 95% CI 1.06–2.12, p=0.022). Sensitivity analysis without replacement of primary outcome confirmed a significant benefit of alteplase on the primary endpoint (see supplementary results in the supplementary appendix).
Figure 1. Distribution of Scores on the modified Rankin Scale at 90 Days.
Scores on the modified Rankin Scale range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability, 3 moderate disability, 4 moderately severe disability, 5 severe disability, and 6 death. Numbers indicate the proportion of patients (%) per category.
Table 2:
Efficacy and Safety Outcomes
| Outcome | Alteplase (n=429) | Control (n=414) | Odds ratios (95% CI) * | p-value |
|---|---|---|---|---|
| Primary Efficacy Outcome | ||||
| Favourable Outcome (mRS 0–1) at 90 days – no. (%) † | 199 (47%) | 160 (39%) | 1.49 (1.10–2.03) | 0.011 |
| Secondary Efficacy Outcomes | ||||
| mRS score at 90 days ‡ | 1.38 (1.05–1.80) | 0.019 | ||
| Independent Outcome (mRS 0–2) at 90 days – no. (%) † | 273 (65%) | 239 (58) | 1.50 (1.06–2.12) | 0.022 |
| Safety Outcomes | ||||
| Death at 90 days – no. (%) † | 27 (6%) | 14 (3%) | 2.06 (1.03–4.09) | 0.040 |
| Death at 7 days – no. (%) | 10 (2%) | 4 (1%) | 2.54 (0.78–8.32) | 0.19 |
| Severe disability or death (MRS 4–6) at 90 days – no. (%) † | 90 (21%) | 102 (25%) | 0.76 (0.52–1.11) | 0.15 |
| Dependence or death (MRS 3–6) at 90 days – no. (%) † | 147 (35%) | 170 (42%) | 0.67 (0.47–0.94) | 0.022 |
| Symptomatic intracerebral haemorrhage – no. (%) | 11 (3%) | 2 (<1%) | 5.58 (1.22–25.50) | 0.024 |
| Parenchymal haemorrhage type 2 (PH-2) – no. (%) ‡ | 11 (3%) | 3 (1%) | 3.51 (0.98–12.60) | 0.068 |
mRS denotes modified Rankin scale
Odds ratios were adjusted for age and symptom severity at baseline.
Numbers are given for patients with available data on the primary efficacy endpoint; mRS at day 90 was missing for 9 patients in the alteplase and 5 in the control group; missing primary outcome values were replaced using multiple imputation.
Radiological assessment of parenchymal haemorrhage type 2 (PH-2) was available for 320 patients in the alteplase and 307 in the control group
At 90 days, death was reported in 27 patients (6%) in the alteplase as compared to 14 patients (3%) in the control group (aOR 2.06, 95% CI 1.03–4.09, p=0.04). Of the 27 deaths in the alteplase group, 7 were attributable to sICH, 4 to recurrent or progressive stroke, 2 were of unknown cause, and the remaining 14 deaths were of non-neurological cause and unrelated to treatment or index stroke. In the control group, all 14 deaths were of non-neurological cause and unrelated to treatment or index stroke. Death within seven days occurred in 10 patients (2%) in the alteplase group and in 4 patients (1%) in the control group (aOR 2.54, 95% CI 0.78–8.32, p=0.19). In the alteplase group, 90 patients (21%) reached the safety endpoint of being severely disabled or dead (mRS 4–6), compared to 102 patients (25%) in the control group (aOR 0.76, 95% CI 0.52–1.11, p=0.15). The proportion of patients being dependent or dead (MRS 3–6) at 90 days was lower with alteplase than in controls (aOR 0.67, 95% CI 0.47–0.94, p=0.022). The number of patients with sICH was higher in the alteplase group than in the control group (11 [3%] vs. 2 [<1%], aOR 5.58, 95% CI1.22–25.50, p=0.024). Rates of radiologically defined PH-2 were numerically higher in the alteplase group than control 11 [3%] vs. 3 [1%], aOR 3.51, 95% CI 0.98–12.60, p=0.068).
A sensitivity analysis excluding THAWS, being the only trial that used a lower dose of alteplase, revealed similar findings with a significant benefit of alteplase for the primary endpoint and secondary efficacy endpoints, despite a numerically higher number of deaths with alteplase (see supplementary results in the supplementary appendix).
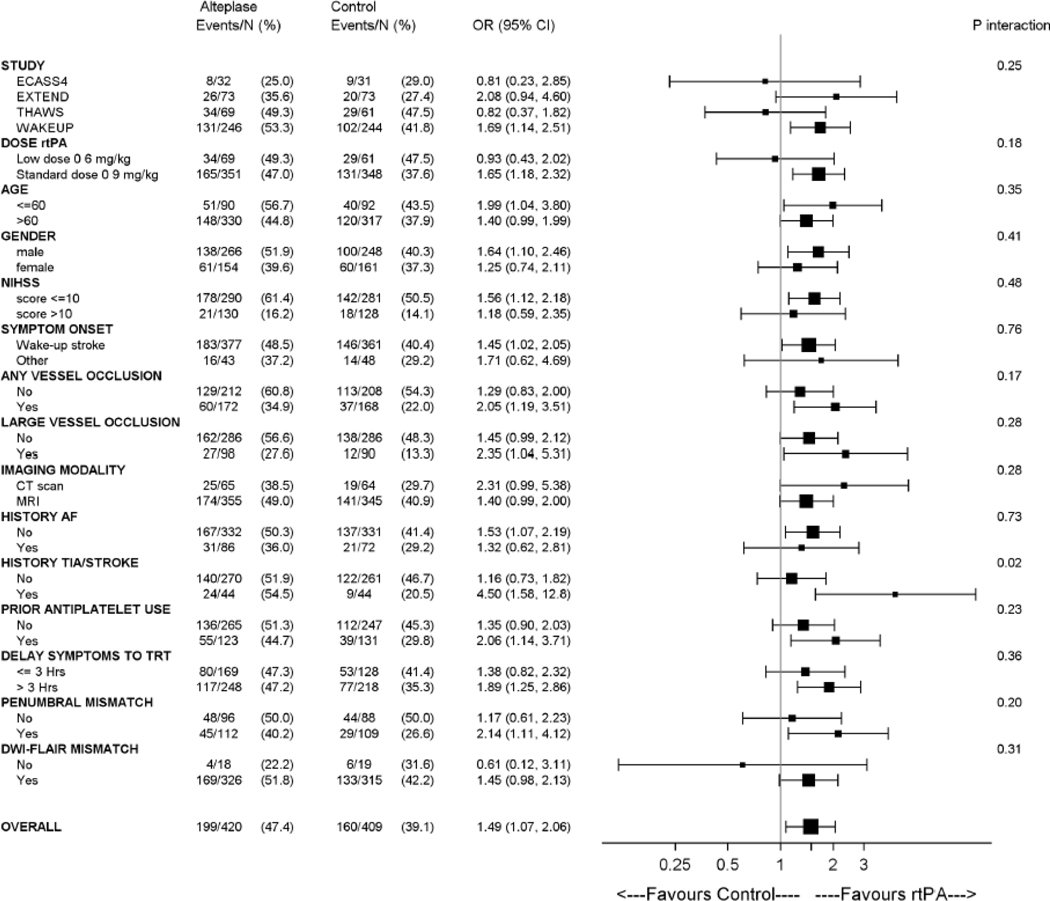
Prespecified subgroup analyses for the primary outcome are shown in figure 2. There was no evidence of significant heterogeneity of the treatment effect across any of the following variables: dose of alteplase (0.9 vs. 0.6 mg/kg bodyweight), age (≤60 vs >60 years), sex, baseline stroke severity (NIHSS ≤10 vs >10), any vessel occlusion, large vessel occlusion, imaging modality (CT vs MRI), history of AF, prior antiplatelet use, delay from symptom recognition to treatment (≤3 vs >3 hours), penumbral mismatch present, or DWI-FLAIR mismatch present. Significant heterogeneity of treatment effect was observed for history of TIA or stroke with larger benefit in the subgroup of patients with history of stroke and TIA (p for interaction = 0.02).
Figure 2. Subgroup analyses.
Forest plots for the primary outcome of favourable outcome (modified Rankin Scale 0–1 at 90 days) in all patients for predefined subgroups.
Subgroup analyses for secondary outcomes and safety outcome are provided in the appendix. We observed no evidence of heterogeneity of treatment effect across any of the subgroups for mortality. Due to the overall small numbers of sICH and PH-2, we did not perform a subgroup analysis on these two safety parameters.
Adding data from the single-arm MR WITNESS trial to the analysis did not alter the main findings (see supplementary appendix).
Discussion
This meta-analysis of individual patient data from four randomized controlled trials showed that intravenous alteplase is beneficial in patients with unknown onset stroke selected by imaging biomarkers using MRI or CT perfusion. Patients with stroke of unknown onset time, who had a DWI-FLAIR mismatch on MRI or a penumbral mismatch on perfusion-diffusion MRI or CT perfusion and who received intravenous alteplase had higher likelihood of a favourable functional outcome at 90 days after stroke compared with controls. Rates of severe disability or death (MRS 4–6) were numerically lower with alteplase, but treatment with alteplase was associated with a small but significant increase in the rate of sICH and a numerical but non-significant increase in mortality. The lower number of deaths in the control group (mRS 6) was offset by an increased proportion of bedridden or nursing home outcomes (mRS 5), (mRS 5 and 6) in the alteplase and control groups. Importantly, there was a significant net and clinically important benefit of intravenous alteplase across the entire range of functional outcome in ordinal analysis of the mRS.
Our analysis strengthens the results of individual trials and extends the information on treatment effect in the subgroup of patients with unknown onset time of stroke. The WAKE-UP trial exclusively randomized patients with unknown onset stroke and demonstrated improved functional outcome with intravenous alteplase guided by MRI with DWI-FLAIR mismatch 6. The EXTEND trial included both patients in a late time window up to 9 hours of stroke and those with unknown onset stroke guided by penumbral imaging, and also showed a benefit of intravenous alteplase on functional outcome in these patients 9. Pooling individual data from only the patients with unknown onset stroke from these trials and two further randomized trials applying imaging biomarker selection to enroll patients resulted in a population of 843 patients with unknown onset stroke randomized based on advanced brain imaging. In this population, the adjusted OR for a favourable outcome with alteplase was 1.48 (95% CI 1.07–2.06), with an absolute increase of 8% patients with favourable outcome translating into a number needed to treat of 12. This treatment effect is comparable to the treatment effect of intravenous alteplase within 4.5 hours of known stroke onset, with an aOR of 1.75 (95% CI 1.35–2.27) within 3 hours and of 1.26 (95% CI 1.05–1.51) after 3–4.5 hours 4. This confirms the validity and clinical utility of the concept of imaging-based selection of acute stroke patients for reperfusion treatment in cases where information on the time of symptom onset is not available.
The trials included in this meta-analysis differed in design and imaging inclusion criteria but they all relied on imaging biomarkers beyond non-contrast CT and vessel imaging. WAKE-UP and THAWS applied the DWI-FLAIR mismatch concept and randomized patients if MRI showed a mismatch between an acute ischemic lesion that was visible on DWI while there was no marked parenchymal hyperintensity on FLAIR based on visual judgement, indicating an ischemic lesion age of less than 4.5 hours and the absence of severe and irreversible tissue damage 5. EXTEND and ECASS-4 used the concept of penumbral imaging for patient selection. They randomized patients who showed a relevant amount of salvageable brain tissue defined by a limited infarct core surrounded by a larger area of critically hypoperfused tissue as shown by perfusion-diffusion MRI or CT perfusion mismatch 12, similar to the approach used to guide endovascular stroke treatment in late or unknown time-window in two recent trials 10,11.
This meta-analysis indicates that both concepts of imaging biomarker selection allow for effective identification of patients for reperfusion treatment after ischemic stroke. Nevertheless, there are inherent advantages and disadvantages of these concepts. DWI-FLAIR mismatch requires MRI, but does not need perfusion imaging. It does not require any postprocessing but is effective with simple visual judgement of routine MRI sequences with a high interrater agreement as compared to quantitative evaluation by measurement of FLAIR signal intensity 21. Moreover, the DWI-FLAIR mismatch allows for treatment of patients with lacunar strokes, a subgroup of the WAKE-UP trial in whom the treatment effect of alteplase was similar compared to patients with other subtypes of stroke 22. These patients would not have met criteria of a relevant amount of salvageable tissue in perfusion-based penumbral mismatch imaging. On the other hand, penumbral mismatch may identify patients with salvageable tissue despite already marked hyperintensity on FLAIR and thus increase of the rate of patients treated with thrombolysis 23. For clinical practice, we conclude that any of the mismatch concepts is effective and can be recommended for guiding intravenous thrombolysis with alteplase in unknown onset stroke.
Our meta-analysis showed increased rates of sICH with alteplase treatment, which was expected based on the results of previous pooled analysis of stroke thrombolysis trials 4. The increased risk of sICH corresponds to the biological effects of alteplase but also relates to higher rates of reperfusion 24. The overall small rate of 3% of intracranial haemorrhages with alteplase in this population of unknown onset stroke is comparable to the 3.5% rate of sICH according to SITS-MOST definition in the pooled analysis of stroke thrombolysis trials in patients with known onset 4. It is reassuring that no excess of intracerebral haemorrhages is observed in unknown onset strokes compared to those with known onset. We also observed an increase in the mortality with alteplase treatment (6% vs. 3% in the control group), while rates of severe disability or death were numerically lower with alteplase, and rates of death and bedridden or nursing home outcomes were comparable for both treatment groups. Slightly increased mortality is not unexpected, as a significant increase of early deaths has been reported in the previous pooled analysis of stroke thrombolysis trials 4, which at least partially can be related to an increased rate of fatal intracerebral haemorrhage. In our analysis, 7/27 (26%) deaths in the alteplase group were attributable to SICH and thus possibly related to treatment with alteplase, while the majority of deaths were considered unrelated and of non-neurological cause. The increased mortality did not negate the net benefit of intravenous alteplase, as the analysis of functional outcome across the entire range of the mRS including death showed a significant benefit with overall better functional outcome with alteplase treatment.
Our analysis did not identify a significant treatment heterogeneity in any relevant subgroups but confirmed a consistent treatment effect across a wide range of subgroups. Subgroup analyses also revealed no interaction of treatment effect with vessel occlusion. We also observed a significant treatment benefit in the subgroup of patients with a large vessel occlusion with an aOR of 2.35 (95% CI 1.04–5.32). This finding is of clinical importance and reinforces the finding in the previous perfusion mismatch meta-analysis of clear benefit of alteplase in large vessel occlusion 12. Doubts concerning the efficacy of intravenous alteplase in large vessel occlusion together with an assumed increased risk for intracranial haemorrhage have led to questioning the rationale for intravenous thrombolysis in these patients prior to thrombectomy 25. The results of our pooled analysis support treatment with alteplase in patients with LVO and unknown time of onset stroke, especially if patients present to centres where thrombectomy is not immediately available.
Our meta-analysis has limitations. We cannot draw any inference on possible effects of the different dose of alteplase, as the THAWS trial was the only trial that used the lower dose of 0.6 mg/kg alteplase. Thus, we cannot separate a possible interaction of treatment effect with alteplase dose from overall trials’ effects. This is even more important, as the THAWS trial differed from the others in that it was the only trial that was not placebo-controlled but open-label and, resulting from this design, allowed for early use of antithrombotic medication in the control group. Beyond early termination and the trial being underpowered, these factors might have been reasons why this individual trial was neutral 17. Moreover, all four randomized trials were terminated early, either due to cessation of funding (WAKE-UP), new evidence (EXTEND, THAWS), or change of clinical practice (ECASS-4). All of these are external reasons, thus, the potential bias is low, but early termination resulted in an overall smaller number of patients available for this individual patient data meta-analysis. With a sample size of 843 patients, statistical power to provide adjusted treatment-effect estimates for smaller subgroups was still limited, as reflected by wide 95% confidence intervals for some of the subgroup analyses. As the majority of patients included in this meta-analysis had rather mild to moderate strokes with a median NIHSS score of 7, results may not be generalizable to patients with severe stroke and large core. Finally, although we observed no heterogeneity of treatment effect between the trials, we have to consider that the results of the meta-analysis are to some extent driven by the WAKE-UP trial, representing almost 60% of the patients included in the analysis.
The requirement for advanced imaging beyond non-contrast CT and vessel imaging, i.e., either perfusion CT or MRI, may currently still represent a potential limitation for implementation of the studied treatment approach in some regions or hospitals. However, given the recent evidence from WAKE-UP and EXTEND, together with the evidence for effective imaging-guided endovascular stroke treatment in an extended or unknown time-window, advanced brain imaging has to be considered a requirement for providing state of the art evidence based stroke treatment 10,11. The results this meta-analysis should further support efforts to make these necessary imaging techniques more widely available to provide access to this effective treatment to as many stroke patients as possible.
In conclusion, intravenous alteplase improved functional outcome in unknown onset stroke patients selected by imaging biomarkers from MRI or CT perfusion. A net benefit was observed across the entire range of functional outcome despite an increased risk of sICH and higher mortality with alteplase. Treatment benefit was consistent across a wide range of subgroups including patients with large vessel occlusion. This individual patient data meta-analysis extends the evidence from individual trials and supports the use of imaging biomarkers to guide treatment with intravenous alteplase in patients with an unknown time of stroke onset.
Panel: Research in context
Evidence before this study
We did a systematic review before 21 September 2020 for randomised trials of intravenous alteplase versus standard of care or placebo in adults with acute ischemic stroke and unknown time of symptom onset using advanced brain imaging with either penumbral imaging (i.e., perfusion-diffusion MRI or perfusion CT) or MRI-based tissue-clocking (i.e., a mismatch on MRI between a visible lesion on diffusion weighted imaging [DWI] and no marked parenchymal hyperintensity on fluid attenuated inversion recovery [FLAIR], DWI-FLAIR mismatch) with >20 patients. This identified four trials. The WAKE-UP trial randomized 503 patients with unknown time of onset stroke to intravenous alteplase or placebo if they had DWI-FLAIR mismatch in visual assessment and found a significant better functional outcome in patients treated with alteplase compared with placebo. The EXTEND trial randomized 225 patients with late time window or unknown time of onset stroke to alteplase after automated CT or MRI perfusion imaging and demonstrated higher rates of excellent functional outcome compared with placebo. The THAWS trial randomized 131 patients with unknown time of onset stroke to treatment with alteplase or standard of care based on visual assessment of the DWI-FLAIR mismatch and was neutral, but was underpowered. The ECASS4-EXTEND trial randomly assigned 119 patients with late time window or unknown time of onset stroke and penumbral mismatch on perfusion-diffusion MRI and was neutral, but also was underpowered. However, all four studies were stopped early for different reasons, and had only modest sample sizes limiting strength and precision of the findings.
Added value of this study
This systematic review and individual patient data meta-analysis of four randomised trials quantifies the benefits and risks of intravenous alteplase for patients with unknown time of onset stroke. Intravenous alteplase resulted in higher rates of excellent functional outcome defined as a score of 0–1 on the modified Rankin Scale (mRS) at 90 days than placebo or standard care. A net benefit was observed for all functional outcomes across the entire range of the mRS despite an increased risk of sICH. While there were numerically higher rates of death with alteplase, rates of severe disability or death (mRS 4–6) were numerically lower with alteplase. Subgroup analysis did not identify a significant treatment heterogeneity in relevant subgroups but confirmed a consistent treatment effect across a wide range of subgroups. We also observed a significant treatment benefit in the subgroup of patients with a large vessel occlusion.
Implications of all the available evidence
Stroke patients with unknown time of symptom onset DWI-FLAIR mismatch or perfusion mismatch, who receive treatment with intravenous alteplase have a better functional outcome at 90 days than patients receiving placebo or standard of care. There was a net benefit for all functional outcomes and also comparable rates of severe disability or death, despite an increased risk of sICH and numerically higher mortality with alteplase. These results extend the findings from individual trials and provide level 1a evidence for the use of brain imaging beyond non-contrast CT to guide treatment with intravenous alteplase in patients with an unknown time of stroke onset.
Supplementary Material
Funding and Acknowledgements
WAKE-UP was supported by a grant (No. 278276) from the European Union Seventh Framework Program. EXTEND was funded by the National Health and Medical Research Council, an Australian Government organization and Commonwealth Scientific and Industrial Research Organisation (CSIRO). Boehringer Ingelheim (BI) provided the study investigational products free of charge. THAWS was supported by the Japan Agency for Medical Research and Development (AMED) (19ek0210091h0003 and 19lk0201094h0001), and the Ministry of Health, Labour, and Welfare, and the Mihara Cerebrovascular Disorder Research Promotion Fund. ECASS IV was an investigator-initiated trial supported by an unrestricted grant from Boehringer Ingelheim (Germany). MR WITNESS was supported by the NIH National Institute of Neurological Disorders and Stroke (NINDS) Specialized Program of Transitional Research in Acute Stroke (SPOTRIAS; P50-NS051343) and NINDS Division of Intramural Research, was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital (MGH), using resources provided by the Center for Functional Neuroimaging Technologies (P41EB015896), a P41 Biotechnology Resource Grant supported by the NIH National Institute of Biomedical Imaging and Bioengineering. Genentech provided alteplase free of charge to the study for distribution to all sites except to the NINDS intramural branch and starting in year 2 provided modest supplemental site payments to permit expansion to 14 sites.
Footnotes
Declaration of interests
MB reports research support from Stryker, European Union, DFG, Hopp foundation, Novartis, Siemens, consultancy for Vascular Dynamics, Boehringer, BBraun and personal fees from Novartis, Grifols, Merck, TEVA and Bayer, outside the submitted work.
SMD reports personal fees from Bayer, Boehringer Ingelheim, Medtronic, and Tide Pharmaceuticals, outside the submitted work.
SMD reports research support from NHMRC (the National Health and Medical Research Council of Australia, modest honoraria for lectures and advisory boards for Medtronic and Boehringer Ingelheim outside of the submitted work.
GAD reports grants from the Australian National Health and Medical Research Council; and personal fees from Allergan, Amgen, Bayer, Boehringer Ingelheim, Pfizer and Servier, outside the submitted work.
MEn reports grants from Bayer and fees paid to the Charité from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, Sanofi, Novartis, Pfizer, all outside the submitted work.
JBF reports consulting and advisory board fees from BioClinica, Cerevast, Artemida, Brainomix, Biogen, BMS, and EISAI, outside the submitted work.
CG reports personal fees from Amgen, Bayer Vital, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi Aventis, Abbott, and Prediction Biosciences outside the submitted work. WH reports grants from Boehringer Ingelheim.
MK reports honoraria from Bayer Yakuhin, BMS/Pfizer, Otsuka, Daiichi-Sankyo, Nippon Boehringer Ingelheim and Takeda, scientific advisory board from Ono, and research supports from Takeda, Daiichi-Sankyo, Nippon Boehringer Ingelheim, Astellas, Pfizer and Shionogi, outside the submitted work.
RL report institutional fees for consultancy and speaker fees from Bayer, Boehringer Ingelheim, Genentech, Ischemaview, Medtronic and Occlutec; outside of the submitted work.
DL reports grants from Bayer, Boehringer Ingelheim, and Pfizer, and fees from the European Stroke Organisation and John Wiley & Sons for being editor of the Eur S J and the Eur J Neurol, outside the submitted work.
KM reports trial support from Boehringer Ingelheim for the ATTEST-2 trial, advisory board & consultancy fees from Boehringer Ingelheim, Bayer, Daiichi-Sankyo and ReNeuron Ltd outside the scope of the submitted work.
MI reports lecturer’s fees from Daiichi Sankyo, Bayer, Bristol-Myers Squibb, Medtronic outside of the submitted work.
MWP reports research support from NHMRC (the National Health and Medical Research Council of Australia, modest honoraria for lectures and advisory boards for Medtronic and Boehringer Ingelheim outside of the submitted work.
PR reports grant support from Boehringer Ingelheim for the ECASS-4 trial, and lecture fees from Boehringer Ingelheim, Bayer, BMS and Daiichi Sankyo.
PDS reports lecture fees and travel stipends from Boehringer Ingelheim as well as Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, Portola, Astra Zeneca and AbbVie outside the submitted work.
SSS reports research support from NIH (P50NS051343) relevant to the submitted work.
LHS reports research support from NIH (P50NS051343), and Genentech (provided alteplase free of charge to Massachusetts General Hospital as well as supplemental per-patient payments to participating sites) relevant to the submitted work. He serves on data safety monitoring boards for Penumbra, Diffusion Pharma and the Charite Hospital B_PROUD trial of mobile stroke units, and serves as a paid consultant to Genentech, Medtronic, and LifeImage; all outside the submitted work.
CZS is supported by a research grant from Novo Nordisk Foundation.
VT reports lecture fees and personal fees from Boehringer Ingelheim, Pfizer, Bayer, Bristol Myers Squibb, Amgen and Allergan outside of the submitted work.
GT reports grant support and lecture fees from Bayer, personal fees from Acandis, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, Portola, and Stryker outside the submitted work.
DT reports advisory boards and speaker’s honoraria from Abbott, Bayer, Boehringer Ingelheim, Daiici Sankyo, Medtronic, Pfizer.
KT reports lecture fees from Bayer, Nippon Boehringer Ingelheim, Bristol-Myers Squibb, and Daiichi Sankyo outside the submitted work.
NW reports an unrestricted grant from Boehringer Ingelheim to SITS International where he was previously chairman.
OW reports research support from NIH (P50NS051343, R01NS059775) relevant to the submitted work. She served as a paid consultant to Penumbra and Genentech; all outside the submitted work. She is a co-inventor of “Delay-compensated calculation of tissue blood flow,” US Patent 7,512,435, which has been licensed to General Electric, Siemens, Imaging Biometrics and Olea Medical.
All author authors report no conflicts of interest.
Data sharing
Individual participant data that underlie the results reported in this article, after de-identification will be made available on request beginning 6 months ending 24 months following article publication to investigators whose proposed use of the data has been approved by the EOS steering committee.
References
- 1.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010; 375(9727): 1695–703. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Neurological D, Stroke rt PASSG. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333(24): 1581–7. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359(13): 1317–29. [DOI] [PubMed] [Google Scholar]
- 4.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384(9958): 1929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol 2011; 10(11): 978–86. [DOI] [PubMed] [Google Scholar]
- 6.Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med 2018; 379(7): 611–22. [DOI] [PubMed] [Google Scholar]
- 7.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019; 50(12): e344–e418. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed N, Audebert H, Turc G, et al. Consensus statements and recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 11–13 November 2018. Eur Stroke J 2019; 4(4): 307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N Engl J Med 2019; 380(19): 1795–803. [DOI] [PubMed] [Google Scholar]
- 10.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 2018; 378(1): 11–21. [DOI] [PubMed] [Google Scholar]
- 12.Campbell BCV, Ma H, Ringleb PA, et al. Extending thrombolysis to 4.5–9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet 2019; 394(10193): 139–47. [DOI] [PubMed] [Google Scholar]
- 13.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369(9558): 275–82. [DOI] [PubMed] [Google Scholar]
- 14.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke 2015; 46(10): 2981–6. [DOI] [PubMed] [Google Scholar]
- 15.Thomalla G, Fiebach JB, Ostergaard L, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP). Int J Stroke 2014; 9(6): 829–36. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–58. [DOI] [PubMed] [Google Scholar]
- 17.Koga M, Yamamoto H, Inoue M, et al. Thrombolysis With Alteplase at 0.6 mg/kg for Stroke With Unknown Time of Onset: A Randomized Controlled Trial. Stroke 2020; 51(5): 1530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ringleb P, Bendszus M, Bluhmki E, et al. Extending the time window for intravenous thrombolysis in acute ischemic stroke using magnetic resonance imaging-based patient selection. Int J Stroke 2019; 14(5): 483–90. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda K, Koga M, Iguchi Y, et al. Guidelines for Intravenous Thrombolysis (Recombinant Tissue-type Plasminogen Activator), the Third Edition, March 2019: A Guideline from the Japan Stroke Society. Neurol Med Chir (Tokyo) 2019; 59(12): 449–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwamm LH, Wu O, Song SS, et al. Intravenous thrombolysis in unwitnessed stroke onset: MR WITNESS trial results. Ann Neurol 2018; 83(5): 980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galinovic I, Puig J, Neeb L, et al. Visual and region of interest-based inter-rater agreement in the assessment of the diffusion-weighted imaging- fluid-attenuated inversion recovery mismatch. Stroke 2014; 45(4): 1170–2. [DOI] [PubMed] [Google Scholar]
- 22.Barow E, Boutitie F, Cheng B, et al. Functional Outcome of Intravenous Thrombolysis in Patients With Lacunar Infarcts in the WAKE-UP Trial. JAMA Neurol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheldeman L, Wouters A, Boutitie F, et al. Different Mismatch Concepts for Magnetic Resonance Imaging-Guided Thrombolysis in Unknown Onset Stroke. Ann Neurol 2020. [DOI] [PubMed] [Google Scholar]
- 24.Thomalla G, Sobesky J, Kohrmann M, et al. Two Tales: Hemorrhagic Transformation but Not Parenchymal Hemorrhage After Thrombolysis Is Related to Severity and Duration of Ischemia. MRI Study of Acute Stroke Patients Treated With Intravenous Tissue Plasminogen Activator Within 6 Hours. Stroke 2007; 38(2): 313–8. [DOI] [PubMed] [Google Scholar]
- 25.Fischer U, Kaesmacher J, Mendes Pereira V, et al. Direct Mechanical Thrombectomy Versus Combined Intravenous and Mechanical Thrombectomy in Large-Artery Anterior Circulation Stroke: A Topical Review. Stroke 2017; 48(10): 2912–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.