Abstract
Background
Coordinated efforts between the National Institutes of Health, the Department of Defense, and the Department of Veterans Affairs have built the capacity for large-scale clinical research investigating the effectiveness of nonpharmacologic pain treatments. This is an encouraging development; however, what constitutes best practice for nonpharmacologic management of low back pain (LBP) is largely unknown.
Design
The Improving Veteran Access to Integrated Management of Back Pain (AIM-Back) trial is an embedded pragmatic cluster-randomized trial that will examine the effectiveness of two different care pathways for LBP. Sixteen primary care clinics will be randomized 1:1 to receive training in delivery of 1) an integrated sequenced-care pathway or 2) a coordinated pain navigator pathway. Primary outcomes are pain interference and physical function (Patient-Reported Outcomes Measurement Information System Short Form [PROMIS-SF]) collected in the electronic health record at 3 months (n=1,680). A subset of veteran participants (n=848) have consented to complete additional surveys at baseline and at 3, 6, and 12 months for supplementary pain and other measures.
Summary
AIM-Back care pathways will be tested for effectiveness, and treatment heterogeneity will be investigated to identify which veterans may respond best to a given pathway. Health care utilization patterns (including opioid use) will also be compared between care pathways. Therefore, the AIM-Back trial will provide important information that can inform the future delivery of nonpharmacologic treatment of LBP.
Keywords: Nonpharmacologic, Pain Management, Physical Function, Pain Interference, Care Pathways
Background and Rationale
A coordinated effort between the National Institutes of Health, the Department of Defense, and the Department of Veterans Affairs (VA) has improved capacity for large-scale clinical research focusing on nonpharmacologic treatment for pain by supporting a coordinating center and demonstration projects [1]. Nonpharmacologic pain treatments have been endorsed as effective, low-risk (i.e., compared with pharmacologic or surgical) approaches by multiple entities, including the Centers for Disease Control and Prevention [2], the National Academy of Medicine [3], and the American College of Physicians [4]. However, this effort to improve capacity for clinical research is still timely because what constitutes best practice for organizing and delivering nonpharmacologic pain management is largely unknown [5]. Observational studies indicate that exposure to nonpharmacologic treatments for low back pain (LBP) can reduce the risk of opioid use [6–8]. These findings are encouraging, but there is still a need for more rigorous designs to address research questions about the structuring of nonpharmacologic care to optimize clinical outcomes (e.g., pain interference, physical function), diminish unwarranted diagnostic testing (e.g., advanced imaging), and limit exposure to higher-risk treatments (e.g., opioids, injections, and/or surgery) [5].
LBP is a high-priority pain condition to include in high-rigor designs [9, 10]. Veterans are more likely to have LBP or joint pain and are more likely to report LBP or joint pain as severe when experienced [11]. The culture of VA pain care has changed over the past decade, with a greater focus on nonpharmacologic approaches; however, many facilities lack clear pain treatment pathways, and providers report confusion about what treatment to recommend when [12]. Additionally, there is significant variability in the frequency and type of nonpharmacologic treatments used for LBP at the individual and facility level across the VA system [13]. The disproportionate impact of LBP on veterans’ quality of life indicates a need for accessible and effective nonpharmacologic care pathways in the VA setting. Indeed, if accessible and effective care pathways were created for veterans, this would represent a critical step forward in the management of LBP [5]. Accordingly, we will conduct the Improving Veteran Access to Integrated Management of Back Pain (AIM-Back) trial. AIM-Back is an embedded pragmatic cluster-randomized clinical trial to 1) develop LBP care pathways designed to improve access to nonpharmacologic treatments and 2) investigate the comparative effectiveness of these care pathways for reducing pain interference and improving physical function.
Methods
Trial Design
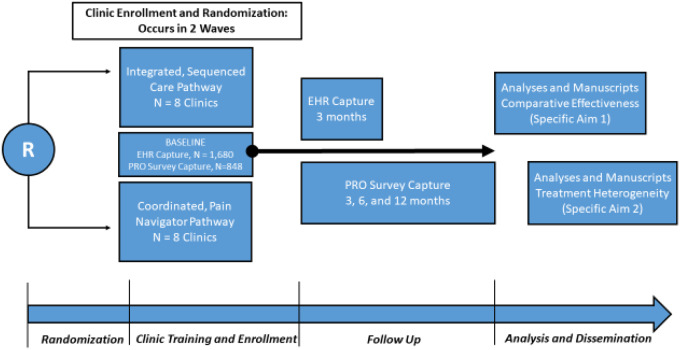
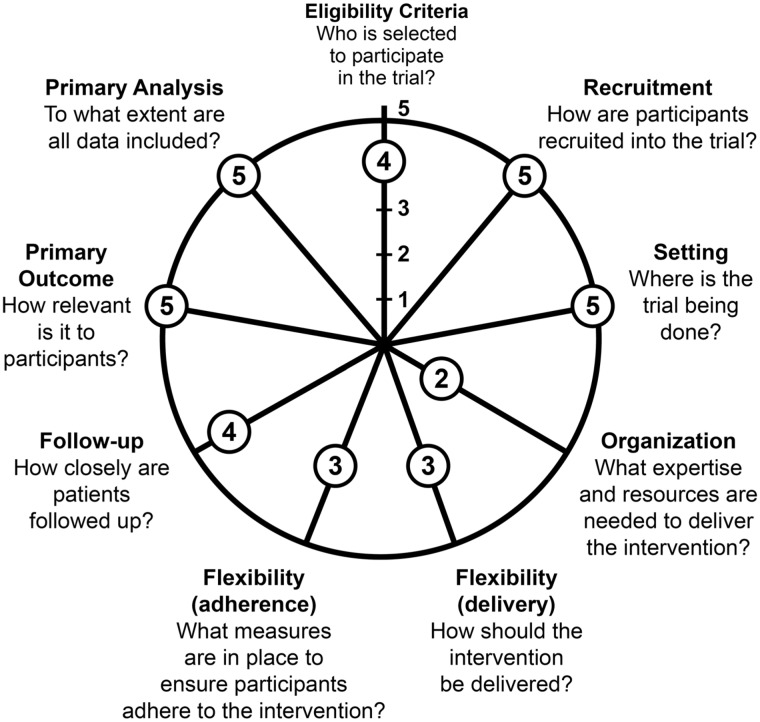
AIM-Back is an embedded cluster-randomized pragmatic clinical trial (PCT) comparing the effectiveness of two care pathways designed to increase access to nonpharmacologic treatment options for LBP. An overview of the AIM-Back trial design is presented in Figure 1, and Figure 2 shows how AIM-Back fits within the Pragmatic-Explanatory Continuum Indicator Summary (PRECIS-2) [14]. It is beyond the scope of a protocol article to describe our rating for each domain, but to provide an example we rated the Eligibility Criteria domain 4 of 5 toward the pragmatic continuum because we have very few exclusion criteria to limit patient enrollment.
Figure 1.
Overview of Improving Veteran Access to Integrated Management of Back Pain (AIM-Back) trial design.
Figure 2.
Pragmatic-Explanatory Continuum Indicator Summary (PRECIS-2) for Improving Veteran Access to Integrated Management of Back Pain (AIM-Back). Adapted by permission from BMJ Publishing Group Limited. [The PRECIS-2 tool: designing trials that are fit for purpose, Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. BMJ 2015;350:h2147]
Trial Overview
The care pathways evaluated in AIM-Back are 1) an integrated sequenced-care pathway (SCP) and 2) a coordinated pain navigator pathway (PNP). VA primary care clinics that agree to have their clinical staff trained in these care pathways as standard care will be randomly assigned to implement one of the care pathways. We are testing these two care pathways in a comparative effectiveness approach because both have the potential to deliver guideline-adherent care that includes nonpharmacologic treatments. However, care delivery is structured to differ for each pathway in terms of staff contact, provider approach, and care progression. AIM-Back will test the central hypothesis that 3 months after initiating care, veterans completing the SCP will have reduced pain interference and improved physical function compared with veterans in the PNP.
Study Sites and Participants
AIM-Back will be conducted in 16 VA primary care clinics, with eight clinics randomized to each care pathway. Site recruitment will occur separately in one block of 10 clinics and one block of up to eight clinics, as discussed in the Randomization Procedures section. The first 10 clinics will be from VA health care systems in Martinsburg, WV (one main medical center, one community); Las Vegas, NV (one community); St. Louis, MO (two main medical centers, two community); and Columbia, SC (one main medical center, two community). The clinics for the second randomization block will be recruited during AIM-Back implementation procedures for the first 10 clinics. See Supplementary Data 1 for the details of study activities by time point. We define “clinic” as a group of providers (i.e., physicians, physical therapists, chiropractors, nurses) practicing in the same geographic location who together provided LBP care for 800 to 5,000 unique patients in the preceding year. Individual clinics that do not meet the threshold of 800 unique patients will be considered for site participation if they are part of a larger health system that already had a qualifying clinic with a range of 800 to 5,000 unique patients. To enroll as a participating site, clinics must agree to provide personnel to deliver either care pathway and to submit participation agreements signed by health system leadership. Within health systems, more than one clinic can participate if contamination can be avoided because the clinics do not share clinical staff in addition to being separated physically. Individual informed consent is not required for patients to receive treatment in the AIM-Back pathways, as these have been deemed nonresearch activities. Site training of the primary care physicians (i.e., provider initiating pathway referral) includes guidelines for referral criteria so there is consistency in the selection of participants for the care pathways.
Electronic Health Record (Primary Outcome Collection)
We anticipate a sample size of 1,680 veterans for the primary analysis. For these participants, all data will be obtained from VA electronic health records (Supplementary Data 1). To be included in the primary analyses, individuals must be ≥18 years old and referred to AIM-Back by a participating provider. Referred patients will be tracked by a specific AIM-Back referral in the electronic health record. We will exclude from analyses individuals receiving or referred for hospice or palliative care (defined by encounter codes and electronic health record consults) or lacking a phone number in the electronic health record.
Telephone Surveys (Secondary Outcome Collection)
From the total sample, we will recruit a subset of veterans (n=848) to participate in a series of telephone interviews conducted at baseline and at 3, 6, and 12 months (Supplementary Data 1). Participants will provide verbal informed consent for participation in this portion of the study and will provide additional information on their LBP experience. Data collected via telephone interviews will complement the data obtained via the electronic health record with patient-reported data across multiple domains of pain and associated comorbidities. These data will also provide rich information about response trajectories and will suggest areas to target for future electronic health record data capture methods.
As additional inclusion criteria for the telephone subset, participants must be able and willing to provide informed consent and a valid telephone number in the electronic health record. Exclusion criteria for the telephone surveys include any of the following: 1) currently in institutional care (nursing home or hospital); 2) cognitive impairment or dementia (identified via International Classification of Diseases diagnosis codes or primary care physician note in the previous 2 years) or lack of decision-making capacity, as documented in the medical record; 3) serious mental illness defined as diagnosis of schizophrenia, bipolar disorder, psychiatric hospitalization in the previous year, or current high-risk suicide flag in their electronic health record medical record; or 4) unable to communicate on the telephone or no telephone access for duration of study.
Care Pathways
The nonpharmacologic services that constitute the care pathways being examined in this study represent restructuring existing clinical practices, not experimental treatments. This position has been officially supported by the VA in a memorandum indicating that implementing the LBP care pathways is a nonresearch activity. Employees at participating clinical sites will receive educational training for delivering the treatment as part of standard care and will perform clinical program–related duties only. All research activities involving human participants (e.g., data collection and analysis) will be carried out by employees of the Durham VA Health Care System and Duke University in accordance with approved institutional review board protocols.
Integrated Sequenced-Care Pathway
The integrated SCP provides both on-site physical therapy services and centrally delivered services via telephone or video. The SCP is initiated with a primary care referral to the pathway to receive on-site physical therapy services for one to two visits. These visits will include examination, pain modulation treatment, and education. After the on-site visit(s), participants will receive weekly calls for 6 weeks of home physical activity counseling. These calls will be conducted by providers at the Durham VA Health Care System. After the home physical activity instruction, veterans will be instructed to return to the on-site physical therapy services to complete risk stratification with the STarT Back Screening Tool (SBST). Veterans at medium or high risk on the SBST will receive an additional 6 weeks of centrally delivered intervention, including psychological and behavioral activation components, from the providers at the Durham VA Health Care System. Veterans at low risk on the SBST will be discharged from the care pathway. The SCP is described in Figure 3, with each step described in more detail in the Figure caption.
Figure 3.
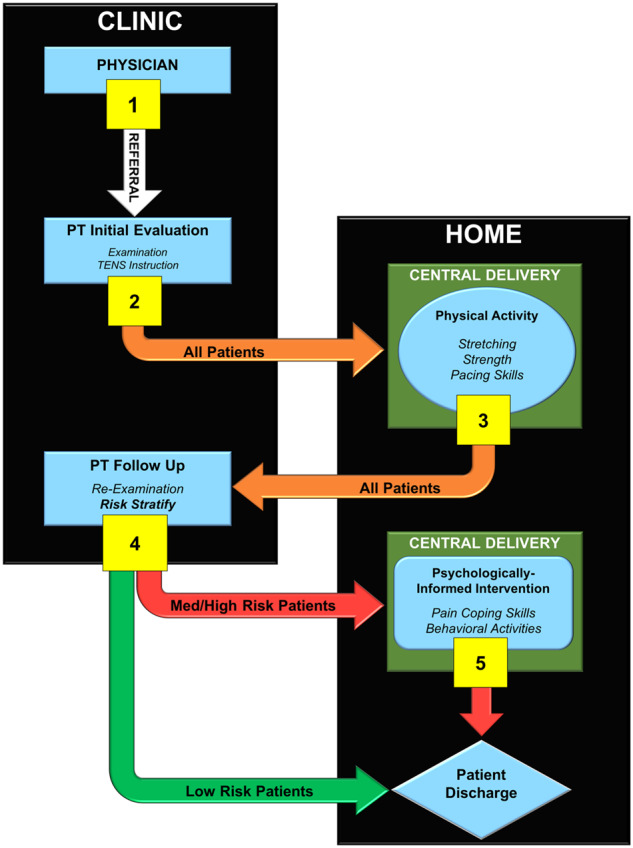
Overview of the integrated sequenced-care pathway for low back pain.
1. Participants are referred from a physician to central delivery to explain the Integrated Sequenced Care Pathway; initiate on-site physical therapy services; and receive an examination, on-site treatment, and a transcutaneous electrical nerve stimulation (TENS) unit.
2. Participants are referred for approximately 6 weeks of centrally delivered physical activity instruction.
3. Participants follow up with Department of Veterans Affairs (VA) physical therapy services for reexamination.
4. Participants complete the STarT Back Screening Tool (SBST). Patients who are considered “low risk” are discharged to home with instructions to continue their physical activity program. Patients who are considered “medium to high risk” are referred for approximately 6 weeks of a centrally delivered, psychologically informed intervention.
5. Participants who receive psychologically informed interventions are discharged to home upon completion.
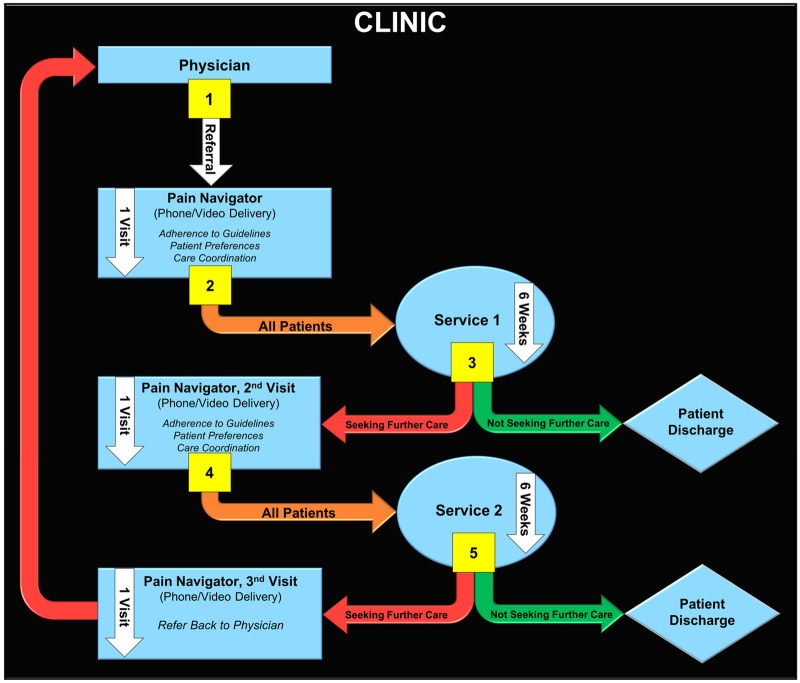
Coordinated Pain Navigator Pathway
The coordinated PNP involves a pain navigator who has received training in the current recommended treatment guidelines for LBP. There is flexibility in personnel who can fulfill the PNP role at participating sites due to the variability in providers and staff involved with patient communication. All personnel will be familiar with services offered at the VA and by VA partners and will complete training delivered by the study team to be competent in a core skill set for the PNP role. The PNP is initiated by a primary care referral to the pathway that activates the pain navigator to engage in a shared decision-making process with veterans to determine appropriate guideline-supported services for LBP. As appropriate, the pain navigator will coordinate and facilitate referrals to services with the referring physician. The VA has adopted the Stepped Care Model for Pain Management (SCM-PM) [15], and this is the model of care that the PNP is designed to follow. The SCM-PM is an evidence-based model that calls for the initial assessment and management of health problems via low-intensity interventions, delivered in the context of primary care teams. Expanded care management is recommended as an initial approach to pain care; however, there has been little guidance as to how teams should accomplish this. Thus, the PNP will implement and test a direct recommendation of the VA’s SCM-PM by training a pain navigator to facilitate access to VA and non-VA pain services. The PNP is described in Figure 4, with each step described in more detail in the Figure caption.
Figure 4.
Coordinated pain navigator pathway for low back pain.
1. Participants are referred from their physician to a clinic pain navigator. The pain navigator contacts patients via telephone or video conference. The pain navigator and patient engage in a shared decision-making process to identify the appropriate Department of Veterans Affairs (VA) service for the patient’s low back pain.
• Pain navigator provides information on current VA-recommended guidelines for nonpharmacologic and nonsurgical pain management as well as availability of services.
• Patient provides their service preferences.
2. Pain navigator coordinates consultation input with physician. Participant attends service.
3. At the completion of service, participants either do not seek further care and are discharged to home or seek further care through the pain navigator.
4. Participants who seek further care through the pain navigator reengage in the shared decision-making process to identify the next appropriate VA service. The pain navigator coordinates consultation input with the physician, and the participant attends a second service.
5. At the completion of service, participants either do not seek further care and are discharged to home or seek further care. Those seeking further care follow up with the pain navigator, who coordinates referral back to the physician.
Training
Training for both pathways takes place in the 2 to 3 months leading up to program launch at each site. The first block of randomization training will be determined remotely due to COVID-19 travel restrictions, and training methods for the second block will be revisited later in the trial. Abridged training programs are available to train new providers in the event of staff turnover during enrollment. To assist each site with implementing the care pathways, we will collect data and provide reports to be used for process improvement. These will include both quantitative (e.g., number of eligible patients, number of referrals, and so on) and qualitative data collected from interviews with participating providers (e.g., exploring referral process and patient response to care pathways).
Training for the central providers in the SCP will include 1) a 2-day virtual workshop that combines didactic and experiential learning to deliver each session in a treatment manual; 2) access to an online video of an experienced provider delivering key elements of each session; and 3) 1-hour supervision sessions to enhance fidelity to the treatment that initially will be weekly and then adjusted to monthly. On-site providers will engage in two conference calls, a virtual training conference, and an online training video, totaling 5 hours of training. The goal of the on-site training is to provide knowledge and competency in 1) the core components of the SCP; 2) SCP documentation, including administration of the SBST; 3) delivery of pain modulatory interventions and patient education; and 4) knowledge of central delivery treatment content to plan for care continuity. On-site providers will be supported by AIM-Back team members via quarterly follow-up calls and on an as-needed basis. Training for the pain navigator includes five remote training sessions composed of didactic information, one-on-one consulting, videos, handouts, and ongoing monthly support meetings. Training focuses on building patient engagement skills, facilitating patient preferences, and matching these preferences to pillars of conservative treatment.
Randomization Procedures
Randomization for AIM-Back will occur at the clinic level via covariate constrained randomization to increase the chances of acceptable study arm balance in cluster trials [16–18]. Due to logistical constraints related to site training, randomization will be conducted in two blocks following a covariate constrained randomization extension from Carter and Hood [29]. Using this procedure, all possible randomizations will be generated for the first block of clinics (n=10) where a balance criterion score is computed using standardized values for the baseline covariates for each randomization scheme. For the second block of clinics (n=6 planned; up to n=8 if there are dropouts in the first block), the balance criterion score is conditional on the balance score for the randomization selected for the first block. For each block, the distribution of balance scores will be examined and a cutoff of the 25th percentile will be used as a criterion for identifying the set of randomizations from which a single randomization will be randomly selected to be used for the block of clinics. As care pathway implementation training cannot occur at the same time for all clinics within a block, the SCP and PNP clinics will be paired and then the pairs of clinics will be ordered for staggered training.
The covariates assessed over the 6-month period prior to enrollment for each clinic are 1) average LBP pain intensity scale scores; 2) average level of opioid exposure for LBP patients; 3) number of participating primary care providers; 4) main medical center or community clinic; and 5) average age of veterans with LBP. These characteristics were selected a priori, as they represent factors likely to be associated with baseline differences that may affect primary outcomes. For pain intensity scores, opioid exposure, and age of veterans, administrative data will be used and averaged over the 6-month period prior to clinic enrollment.
Blinding
AIM-Back is an embedded cluster-randomized pragmatic trial and, as such, participants, intervention delivery personnel, and recruitment personnel will not be blinded to care pathway assignment. Research personnel collecting study surveys by telephone will be blinded to clinic assignment.
Primary Outcomes
AIM-Back has co-primary outcomes of pain interference and functional status assessed by the Patient-Reported Outcomes Measurement Information System Short Form (PROMIS-SF). Baseline data collection is the responsibility of the corresponding care pathway providers (i.e., on-site physical therapy or central delivery provider for the sequenced pathway and pain navigator for the coordinated pathway). The primary outcome time point is 3 months and, consistent with an embedded, pragmatic design, we will rely on the aforementioned clinical providers from the care pathways to collect these data via progress note templates from in-person or telehealth visits. Reports will be generated periodically to evaluate which veterans are due for the 3-month follow-up for clinical providers to administer these measures. The window around the 3-month outcome assessment will be 1 month.
Schedule of Assessments
The timing of primary and secondary outcome measures collection is summarized in Table 1 and Supplementary Data 1. For all veterans (n=1,680), primary outcomes will be collected in the electronic health record at baseline and at the 3-month follow-up. For a subset of veterans (n=848), additional surveys for secondary measures will be administered at baseline with follow-up points at 3, 6, and 12 months.
Table 1.
Summary of AIM-Back trial outcome measures
| Variable | Measure | Data Source | Baseline | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|---|---|
| Primary and Secondary Outcomes | ||||||
| Pain interference | PROMIS-SF | CDW health factor data element | X | X | ||
| PROMIS-SF | Patient report on CATI survey | X | X | X | X | |
| Physical function | PROMIS-SF | CDW health factor data element | X | X | ||
| PROMIS-SF | Patient report on CATI survey | X | X | X | X | |
| Sleep disturbance | PROMIS-SF | CDW health factor data element | X | X | ||
| PROMIS-SF | Patient report on CATI survey | X | X | X | X | |
| Opioid use | Characterized by number supplied, days supplied, and morphine equivalent dosage | Pharmacy benefits management and/or CDW administrative data | X | X | ||
| Exploratory Outcomes | ||||||
| Depressed mood | PHQ-2 | Patient report on CATI survey | X | X | X | X |
| Alcohol use | AUDIT-C | Patient report on CATI survey | X | X | X | X |
| Pain intensity | Numeric scale (0–10) | CDW health factor data element | X | X | ||
| Numeric scale (0–10) | Patient report on CATI survey | X | X | X | X | |
| Pain intensity | PEG Tool | Patient report on CATI survey | X | X | X | X |
| Catastrophizing | NIH Task Force | Patient report on CATI survey | X | X | X | X |
| Self-efficacy | Pain Self-Efficacy Questionnaire | Patient report on CATI survey | X | X | X | X |
| Quality of life | EuroQol | Patient report on CATI survey | X | X | X | X |
AIM-Back = Improving Veteran Access to Integrated Management of Back Pain; PROMIS-SF = Patient-Reported Outcomes Measurement Information System Short Form; CDW = Veterans Health Administration Corporate Data Warehouse; CATI = computer-assisted telephone interviewing; PHQ-2 = Patient Health Questionnaire-2; AUDIT-C = Alcohol Use Disorders Identification Test; PEG = Pain, Enjoyment of Life, and General Activity; NIH = National Institutes of Health.
Adherence
For both the SCP and the PNP, adherence outcomes for each participant will be captured in the electronic health record via referral consults to pathways and health factor data in electronic health record templates used by clinic personnel in each of the pathways (Supplementary Data 2). We define “adherence” according to patient participation in planned sessions with intervention team personnel (telehealth or in person depending on the participating clinic). The number of planned sessions will vary between and within arms (e.g., depending on response to first service in the PNP and depending on risk stratification in the SCP).
Study Objectives
AIM-Back will compare the effectiveness at 3 months of the SCP and the PNP and tests the central hypothesis that the SCP will provide larger reductions in pain interference and improve physical function when compared with the PNP. Although both pathways promote access to guideline-concordant care, we hypothesize that the SCP will be a superior pathway due to the sequenced nature of the pathway (potentially reducing exposure to non–guideline-supported care), inclusion of home-based physical activity, and risk stratification for receiving behavioral treatment. In other planned secondary analyses, AIM-Back will 1) compare the effectiveness of these care pathways on sleep quality; 2) conduct subgroup analysis to examine treatment moderators of previous opioid exposure and high-impact chronic LBP; and 3) identify factors associated with better adherence to each care pathway. We will also perform exploratory analyses to determine baseline characteristics exhibiting heterogeneity of treatment effects for pain interference and physical function.
Sample Size Determination
Supplementary Data 3 presents the range of minimum detectable effect size differences for the primary outcomes for eight clinics randomized to each care pathway. Sample size calculations for the cluster-randomized design were based on the net difference between the two conditions across baseline and at the 3-month follow-up [20]. The standardized effect sizes [21] that we are powered to detect range from 0.30 to 0.50 for the primary outcomes. This range maps to differences of approximately 2.4 to 5.0 points in the PROMIS Pain Interference score, which is in the range from a prior study piloting LBP treatments for veterans [22]. These magnitudes of differences have been reported to be clinically relevant [23]. We assumed correlations of 0.50 for participants and clinics over time based on data from the aforementioned study [22]. Intraclass correlation coefficients of 0.01, 0.02, and 0.05 accounted for clinic clustering and adjusted for the potential group-level randomization of up to five stratification variables as baseline covariate adjustments. For all calculations, the type I error is 2.5% to account for the multiple primary outcomes, and power is conservatively assumed to be 90% to guard against deviations from assumptions. The number needed at baseline was calculated based on an attrition rate of 20%, resulting in target sample sizes for the electronic health record (n=1,680) and survey outcomes (n=848).
Analytic Methods
Primary analyses will be conducted on an intention-to-treat basis; participants will be analyzed in the group to which they were randomized, regardless of intervention adherence, using all available data. The main conclusions drawn from this trial will be based on the prespecified hypotheses outlined in the following sections and will be tested with two-sided P values at the standard 0.05 level, except where previously noted at the 0.025 level due to multiple primary outcomes. Statistical analyses will be performed using the latest release of SAS for Windows (Cary, NC) and R software.
Primary Analyses
The primary outcomes are continuous and will be ascertained at the planned baseline and at the follow-up assessment (3 months) from the electronic health record. Changes in pain interference and physical function scores will be estimated and the primary hypotheses tested via hierarchical linear mixed-effects models with participants nested within clinics and baseline and 3-month values in the response vector [24]. The fixed-effect portion of the model will have the form Yijk = β0 + β1 × (follow-up) + β2 × (follow-up × intervention) for clinic i and participant j at time k. Random effects (clinics and time × clinics) will be included in the model to account for clustering of participants within clinics; random effects will also be included to account for the within-participant correlation between repeated measures over time. The model will be fit in the SAS PROC MIXED procedure using full likelihood approximation, and the hypotheses will be tested by whether the estimated coefficient β2 is positive and significantly different than 0 at the 0.025 level due to two primary outcome variables. We will include covariates used in the covariate constrained randomization [16] in our primary model as well as a limited number of participant-level covariates that are readily available in the electronic health record (i.e., age, gender, race, and comorbidity). We have procedures in place to monitor the timing of the 3-month follow-up assessment for primary outcomes. If we have a significant number of assessments outside the 1-month window, we will conduct a sensitivity analysis to estimate a 3-month treatment effect by including the assessments outside the window.
Subgroup Analyses
We will investigate high-impact chronic LBP and previous opioid use vs opioid naive as a priori subgroups for treatment moderation [25]. “High-impact chronic LBP” will be defined as pain that has persisted for at least 3 months and has resulted in pain on at least half of the days in the past 6 months to be consistent with recent definitions [26, 27]. All participants will have chronicity of back pain assessed via the electronic template at the baseline assessment. Opioid exposure prior to pathway entry will be defined as being prescribed (yes or no) within 6 months prior to entering the pathway [28]. For the planned subgroup, analyses for key moderators on the primary outcomes of pain interference and function will use the same modeling framework as described for the primary analysis with the addition of the indicator variable(s) for the moderator and associated interactions [29]. In exploratory analyses, we will use data-driven statistical methods to identify multidimensional subgroups from combinations of baseline characteristics (e.g., age, sex, opioid use) exhibiting heterogeneous treatment effects for the primary outcomes of pain and function [30].
Secondary Analyses
Secondary outcomes of sleep and opioid use will be obtained from the electronic health record. The sleep PROMIS measure is a continuous outcome that will be assessed at baseline and after 3 months in the electronic health record, and similar modeling procedures as described for the primary outcomes will be used to compare pathway effectiveness. Opioid use will be examined in two ways: 1) as a binary variable based on whether a long-term opioid therapy has been prescribed during the last 12 months and 2) using a continuous measure of morphine equivalents for opioid dose at baseline and after 12 months [31]. For the dichotomous outcomes, we will use a generalized linear mixed model with a logit link function where the main predictor of interest will be the treatment arm and will include baseline opioid use status (long-term therapy prescribed or not), adjusting for clustering of VA clinic either with random effects or by conditioning [24, 32, 33]. For opioid morphine equivalent dose, we will fit a similar model as was described for the primary outcomes, except the follow-up time point in the model will be 12 months.
The survey outcomes for the enrolled subset of participants will be collected at baseline and at 3, 6, and 12 months (Supplementary Data 1). These are all longitudinal continuous outcomes, and a hierarchical linear model similar to that described for the primary aim will be fit. Random coefficient models (e.g., random intercept only, random intercept, and linear slope) will be fit and assessed using Akaike information criterion (AIC) model selection criteria to determine the best model for the covariance structure. Similarly, the best model for the mean structure (e.g., linear, quadratic, dummy coding) will be determined, guided by descriptive plots and model fit assessed using AIC model selection criteria, as there are multiple follow-up measurement occasions. We will describe adherence in both care pathways to identify patient characteristics potentially associated with program participation. We will not test for differences in adherence rates between pathways, as our goal is to gain insight into where to target clinical programs and focus efforts to improve access to and patient engagement with nonpharmacologic pain services. In exploratory analysis within each pathway, we will use data-driven methods to identify multidimensional subgroups from combinations of baseline characteristics (e.g., age, sex, opioid use) that identify subgroups with increased adherence [30].
Procedures for Missing Data
We do not anticipate much missing data in the main predictors of interest: intervention arm and participant characteristics available in the electronic health record or assessed at baseline in survey outcomes. Hierarchical linear mixed models via maximum likelihood estimation—our main analysis technique for the primary outcomes—implicitly accommodate missingness at random (MAR) [24]. Therefore, inferences will be valid even with differential dropout by intervention arm. We will thoroughly explore reasons for dropout, and depending on the type and scope of missing data, we may explore the sensitivity of intervention effects to different missing data mechanisms (MAR vs missingness not at random [MNAR]) [34]. To explore the MAR assumption, outcomes will be multiply imputed using principled methods in SAS (via PROC MI or IVEware) [35]. If we cannot justify the assumption of MAR, we will explore the sensitivity of intervention effects to the MNAR assumption; we will follow the guidelines in the studies conducted by Mallinckrodt [18] and Ratitch et al. [33] for model selection and pattern mixture modeling.
Implementation and Dissemination
A hallmark of AIM-Back is that we have been designing and planning for dissemination and implementation throughout the trial rather than at the end stage only. One advantage of this embedded pragmatic trial is that the care strategies were meant to be sustainable, which will improve translation into routine clinical practice outside the sites that participated in the trial, if the results warrant such translation. The results of this study will be relevant to broad audiences; thus, we plan to disseminate the study results in both academic and nonacademic forums.
At the conclusion of the study, we will provide participating clinics with the training, tools, and resources needed to implement the AIM-Back pathway if one is shown to be superior. If there is not a superior pathway, training materials for the care pathway not implemented during the trial will be shared with the appropriate sites. In addition, we will leverage our VA health system partnerships at the local, regional, and national levels to disseminate training materials to decision-makers beyond participating sites. At the conclusion of the study, the Duke University, Durham VA, and ClinicalTrials.gov websites will host summary data, downloadable versions of key papers, and the manual of procedures and operations for each care pathway.
Discussion
In recognition of the high burden of pain on veterans and associated costs to the health care system, the Veterans Health Administration implemented a National Pain Strategy [15]. A key component of this strategy was to approach pain care within a biopsychosocial framework and to increase access to nonpharmacologic treatments. This led to the VA offering a broad array of services for pain care. However, a major barrier to the organized and timely delivery of nonpharmacologic services has been limited provider and veteran awareness about these treatments and how best to access them. Other barriers include the lack of a standardized process for connecting participants to guideline-recommended nonpharmacologic services (i.e., who is responsible, which services are appropriate). This has led to increased confusion about how and when nonpharmacologic services for LBP should be offered to veterans. Therefore, AIM-Back was designed to address a high-priority question for the VA and veterans—how best to organize and deliver nonpharmacologic services for LBP. An embedded cluster-randomized trial was selected for AIM-Back because it offered the most pragmatic and efficient design to address our aims. Embedded PCTs examine intervention(s) that will be implemented using existing clinical resources under real-world conditions [37]. The VA is a large integrated health care system with a fully implemented electronic health record and mature data warehouse infrastructure. Therefore, it is a prime environment for conducting embedded PCTs such as AIM-Back that compare two different approaches (i.e., A vs B comparison as opposed to A vs A+ comparison) [38].
AIM-Back leveraged an extended planning period to improve the original study design and benefited from Pain Management Collaboratory Working Groups, Office of Clinical Research Administration, Protocol Review Committee, and stakeholder input on key design elements. The planning process led to several changes in our approach. First, the number of clusters was increased (from n=10 to n=16) to adjust for a more conservative interclass correlation coefficient and to be able to detect a smaller effect size difference. Second, in examining our proposed workflow for each pathway, we realized that we would not be able to collect our primary outcome during a baseline period at each site to use in our randomization schema. Thus, we transitioned to the use of a blocked covariate constrained randomization [19]. These changes allowed for some flexibility in site training and also provide some safeguards if any clinics drop out.
Another area of modification that occurred during the planning period was the evolution of the care pathways based on our planned stakeholder engagement process involving VA providers, VA administrators, and veterans. Based on stakeholder feedback, the PNP was updated to include consideration of participant preference in decision-making, monitoring of the number of services received before referral back to the referring provider, and recommended provider types to be the pain navigator (e.g., nursing or social work). The SCP was updated to include fewer on-site physical therapy visits, at-home delivery of psychologically informed care, and return to on-site physical therapy for additional examination. Low intervention uptake at participating sites has been reported as a key challenge in previous embedded PCTs [37], and engaging clinical personnel and participants to modify the pathways was an essential strategy to enhance their acceptability in being fully embedded within existing clinical care.
In summary, AIM-Back was designed to compare the effectiveness of two different care pathways that meet the standards set by the National Pain Strategy and could be used to inform future care delivery models in and out of the VA [39]. As an embedded PCT, AIM-Back will be completed in a real-world environment and has the potential to inform future best practices [40] and lead to learning health systems [38] that routinely test the effectiveness of nonpharmacologic approaches for common musculoskeletal pain conditions such as LBP.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
Supplementary Material
Funding sources: This study was supported by the National Institutes of Health (NIH) through cooperative agreements U24AT009769 (Coordinating Center) and UG3/UH3 AT009790 (Demonstration Project) from the National Center for Complementary and Integrative Health (NCCIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study was also supported in part by the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13–410) within the Durham Veterans Affairs Health Care System. Dr. Hastings received support from the Duke Claude D. Pepper Older Americans Independence Center (NIA P30AG028716). Dr. Simon received support from Physical Resilience to Pain with Activity Influences among Seniors (L30 AG064712). The contents do not represent the views of the US Department of Veterans Affairs or the US government. This article is a product of the NIH–Department of Defense–Veterans Affairs Pain Management Collaboratory. For more information about the Collaboratory, visit http://painmanagementcollaboratory.org.
Disclosure and conflicts of interest: The authors have no conflicts of interest or disclosures to report.
Supplement sponsorship: This article appears as part of the supplement entitled “NIH-DOD-VA Pain Management Collaboratory (PMC)”. This supplement was made possible by Grant Number U24 AT009769 from the National Center for Complementary and Integrative Health (NCCIH), and the Office of Behavioral and Social Sciences Research (OBSSR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH, OBSSR, and the National Institutes of Health.
References
- 1. Kerns RD, Brandt CA, Peduzzi P. NIH-DoD-VA Pain Management Collaboratory. Pain Med 2019;20(12):2336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Academies of Sciences, Engineering, and Medicine. The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop. Washington, DC: National Academies Press; 2019. [PubMed] [Google Scholar]
- 4. Chou R, Deyo R, Friedly J, et al. AHRQ Comparative Effectiveness Reviews. Noninvasive Treatments for Low Back Pain. Rockville, MD: Agency for Healthcare Research and Quality (US; ); 2016. [PubMed] [Google Scholar]
- 5. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: A systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med 2017;166(7):493–505. [DOI] [PubMed] [Google Scholar]
- 6. Childs JD, Fritz JM, Wu SS, et al. Implications of early and guideline adherent physical therapy for low back pain on utilization and costs. BMC Health Serv Res 2015;15(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frogner BK, Harwood K, Andrilla CHA, Schwartz M, Pines JM. Physical therapy as the first point of care to treat low back pain: An instrumental variables approach to estimate impact on opioid prescription, health care utilization, and costs. Health Serv Res 2018;53(6):4629–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kazis LE, Ameli O, Rothendler J, et al. Observational retrospective study of the association of initial healthcare provider for new-onset low back pain with early and long-term opioid use. BMJ Open 2019;9(9):e028633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morbid Mortality Wkly Rep 2018;67(36):1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Io M. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 11. Amtmann D, Kim J, Chung H, Askew RL, Park R, Cook KF. Minimally important differences for Patient Reported Outcomes Measurement Information System pain interference for individuals with back pain. J Pain Res 2016;9:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on research standards for chronic low back pain. J Pain 2014;15(6):569–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods 2001;6(4):330–51. [PubMed] [Google Scholar]
- 14. Gurung T, Ellard DR, Mistry D, Patel S, Underwood M. Identifying potential moderators for response to treatment in low back pain: A systematic review. Physiotherapy 2015;101(3):243–51. [DOI] [PubMed] [Google Scholar]
- 15. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 16. Goode AP, Taylor SS, Hastings SN, Stanwyck C, Coffman CJ, Allen KD. Effects of a home-based telephone-supported physical activity program for older adult veterans with chronic low back pain. Phys Ther 2018;98(5):369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome - When, why, and how? BMC Med Res Methodol 2014;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mallinckrodt CH. Preventing and Treating Missing Data in Longitudinal Clinical Trials: A Practical Guide. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- 19. Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: A proposal. Trials 2010;11(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray DD. Design and Analysis of Group Randomized Trials. New York: Oxford University Press; 1998. [Google Scholar]
- 21. Kerns RD, Philip EJ, Lee AW, Rosenberger PH. Implementation of the Veterans Health Administration national pain management strategy. Transl Behav Med 2011;1(4):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li F, Turner EL, Heagerty PJ, Murray DM, Vollmer WM, DeLong ER. An evaluation of constrained randomization for the design and analysis of group-randomized trials with binary outcomes. Stat Med 2017;36(24):3791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lipkovich I, Dmitrienko A, D’Agostino RB Sr. Tutorial in biostatistics: Data-driven subgroup identification and analysis in clinical trials. Stat Med 2017;36(1):136–96. [DOI] [PubMed] [Google Scholar]
- 24. Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: An overview. Ann Intern Med 2001;135(2):112–23. [DOI] [PubMed] [Google Scholar]
- 25. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: Designing trials that are fit for purpose. BMJ 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 26.Interagency Pain Research Coordinating Committee. National pain strategy: A comprehensive population health level strategy for pain In: HaH Services ed. Washington, DC: National Institutes of Health; 2015. [Google Scholar]
- 27. Mattocks K, Rosen MI, Sellinger J, et al. Pain care in the Department of Veterans Affairs: Understanding how a cultural shift in pain care impacts provider decisions and collaboration. Pain Med 2020;21(5):970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ha G. Longitudinal Data Analysis. Hoboken, NJ: Wiley & Sons; 2006. [Google Scholar]
- 29. Carter BR, Hood K. Balance algorithm for cluster randomized trials. BMC Med Res Methodol 2008;8(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nahin RL. Severe pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain 2017;18(3):247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008;24(6):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raab GM, Butcher I. Balance in cluster randomized trials. Stat Med 2001;20(3):351–65. [DOI] [PubMed] [Google Scholar]
- 33. Ratitch B, O’Kelly M, Tosiello R. Missing data in clinical trials: From clinical assumptions to statistical analysis using pattern mixture models. Pharm Stat 2013;12(6):337–47. [DOI] [PubMed] [Google Scholar]
- 34. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA 2012;307(9):940–7. [DOI] [PubMed] [Google Scholar]
- 35. Simon GE, Platt R, Hernandez AF. Evidence from pragmatic trials during routine care—Slouching toward a learning health system. N Engl J Med 2020;382(16):1488–91. [DOI] [PubMed] [Google Scholar]
- 36. Turner EL, Li F, Gallis JA, Prague M, Murray DM. Review of recent methodological developments in group-randomized trials: Part 1—Design. Am J Public Health 2017;107(6):907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tuzzio L, Larson EB, Chambers DA, et al. Pragmatic clinical trials offer unique opportunities for disseminating, implementing, and sustaining evidence-based practices into clinical care: Proceedings of a workshop. Healthc (Amst) 2019;7(1):51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vanneman ME, Larson MJ, Chen C, et al. Treatment of low back pain with opioids and nonpharmacologic treatment modalities for Army veterans. Med Care 2018;56(10):855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. George SZ, Goertz C, Hastings SN, Fritz JM. Transforming low back pain care delivery in the United States. Pain 2020; Publish Ahead of Print:doi: 10.1097/j.pain.0000000000001989. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weinfurt KP, Hernandez AF, Coronado GD, et al. Pragmatic clinical trials embedded in healthcare systems: Generalizable lessons from the NIH Collaboratory. BMC Med Res Methodol 2017;17(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.