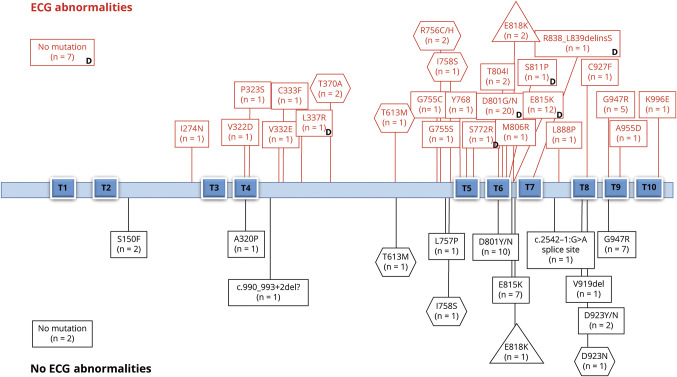
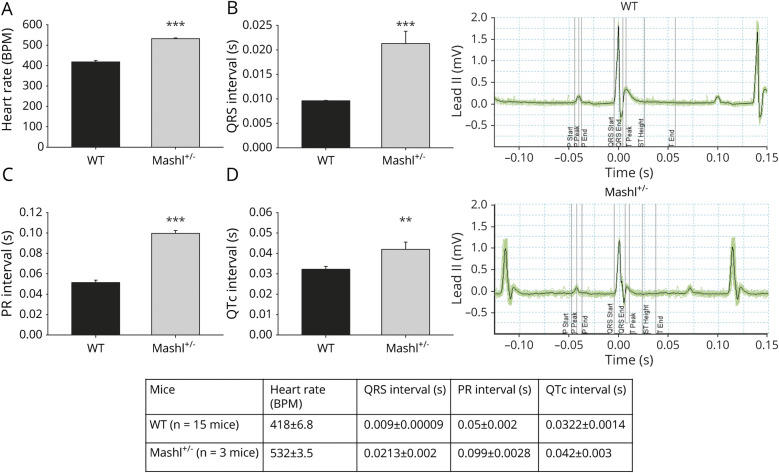
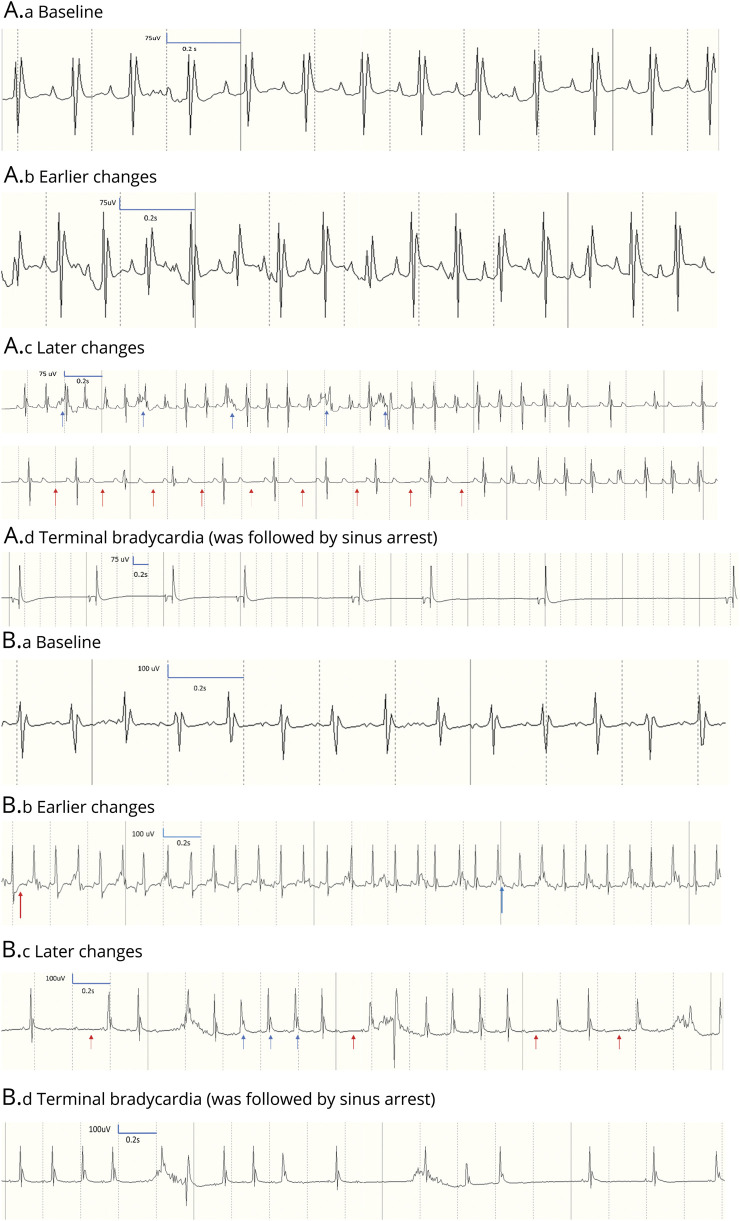
Simona Balestrini
Simona Balestrini, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,*,
Mohamad A Mikati
Mohamad A Mikati, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,†,✉,
Reyes Álvarez-García-Rovés
Reyes Álvarez-García-Rovés, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,*,
Michael Carboni
Michael Carboni, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Arsen S Hunanyan
Arsen S Hunanyan, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Bassil Kherallah
Bassil Kherallah, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Melissa McLean
Melissa McLean, BS
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Lyndsey Prange
Lyndsey Prange, RN
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Elisa De Grandis
Elisa De Grandis, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Alessandra Gagliardi
Alessandra Gagliardi, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Livia Pisciotta
Livia Pisciotta, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Michela Stagnaro
Michela Stagnaro, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Edvige Veneselli
Edvige Veneselli, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Jaume Campistol
Jaume Campistol, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Carmen Fons
Carmen Fons, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Leticia Pias-Peleteiro
Leticia Pias-Peleteiro, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Allison Brashear
Allison Brashear, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Charlotte Miller
Charlotte Miller, AND
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Raquel Samões
Raquel Samões, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Vesna Brankovic
Vesna Brankovic, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Quasar S Padiath
Quasar S Padiath, MBBS, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Ana Potic
Ana Potic, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Jacek Pilch
Jacek Pilch, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Aikaterini Vezyroglou
Aikaterini Vezyroglou, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Ann ME Bye
Ann ME Bye, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Andrew M Davis
Andrew M Davis, MD, FHRS
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Monique M Ryan
Monique M Ryan, MD, FRACP
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Christopher Semsarian
Christopher Semsarian, MBBS, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Georgina Hollingsworth
Georgina Hollingsworth, MGC
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Ingrid E Scheffer
Ingrid E Scheffer, MBBS, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Tiziana Granata
Tiziana Granata, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Nardo Nardocci
Nardo Nardocci, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Francesca Ragona
Francesca Ragona, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Alexis Arzimanoglou
Alexis Arzimanoglou, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Eleni Panagiotakaki
Eleni Panagiotakaki, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Inês Carrilho
Inês Carrilho, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Claudio Zucca
Claudio Zucca, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Jan Novy
Jan Novy, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Karolina Dzieżyc
Karolina Dzieżyc, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Marek Parowicz
Marek Parowicz, Dipl.-Ing.agr.
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Maria Mazurkiewicz-Bełdzińska
Maria Mazurkiewicz-Bełdzińska, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Sarah Weckhuysen
Sarah Weckhuysen, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Roser Pons
Roser Pons, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Sergiu Groppa
Sergiu Groppa, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Daniel S Sinden
Daniel S Sinden, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Geoffrey S Pitt
Geoffrey S Pitt, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Andrew Tinker
Andrew Tinker, FRCP
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Michael Ashworth
Michael Ashworth, MD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Zuzanna Michalak
Zuzanna Michalak, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Maria Thom
Maria Thom, FRCPath
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
J Helen Cross
J Helen Cross, MD, PhD
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Rosaria Vavassori
Rosaria Vavassori, Dr. Eng
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,
Juan P Kaski
Juan P Kaski, FRCP
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,†,✉,
Sanjay M Sisodiya
Sanjay M Sisodiya, PhD, FRCP
1From the Department of Clinical and Experimental Epilepsy (S.B., S.M.S.), UCL Queen Square Institute of Neurology, London; Chalfont Centre for Epilepsy (S.B., S.M.S.), Bucks, UK; Division of Pediatric Neurology (M.A.M., A.S.H., B.K., M.M., L.P.), Department of Neurobiology, and Division of Cardiology (M.C.), Department of Pediatrics, Duke University, School of Medicine, Durham, NC; Centre for Inherited Cardiovascular Diseases (R.A.G.-R., J.P.K.), Great Ormond Street Hospital for Children NHS Foundation Trust; Institute of Cardiovascular Science(R.A.G.-R., J.P.K.), University College London, London, UK; Child Neuropsychiatry Unit (E.D.G., A.G., L.P., M.S., E.V.), IRCCs Istituto Giannina Gaslini, Department of Neurosciences, Rehabilitation, Ophthalmology, Genetics and Maternal and Child Health, DINOG-MI, University of Genoa; Department of Pediatric Neuroscience (A.G., T.G., N.N., F.R.), Fondazione IRCCS Istituto Neurologico Carlo Besta; Unit of Child Neuropsychiatry (L.P.), ASST Fatebenefratelli Sacco, Milan, Italy; Paediatric Neurology Department (J.C., C.F., L.P.-P., A.A.), Hospital Sant Joan de Déu, Esplugues de Llobregat, Barcelona University, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Barcelona, Spain; Department of Neurology (A.B., C.M.), Wake Forest School of Medicine, Winston-Salem, NC; Neurology Department (R.S.), Centro Hospitalar e Universitario do Porto–Hospital de Santo António, Porto, Portugal; Clinic for Child Neurology and Psychiatry (V.B., A.P.), Department of Child Neurology, Medical Faculty University of Belgrade, Serbia; Department of Human Genetics (Q.S.P.), Graduate School of Public Health, University of Pittsburgh, PA; Department of Pediatric Neurology (J.P.), Medical University of Silesia, Katowice, Poland; Clinical Neurosciences (K.V., J.H.C.), Developmental Neuroscience Programme, UCL Great Ormond Street Institute of Child Health, and Great Ormond Street Hospital for Children NHS Foundation Trust, Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, London, UK; Sydney Children's Hospital (A.M.E.B.), Randwick; Department of Cardiology (A.M.D.), The Royal Children's Hospital, Melbourne, University of Melbourne; Department of Neurology (M.M.R.), Royal Children's Hospital, Melbourne; Agnes Ginges Centre for Molecular Cardiology (C.S.), Centenary Institute, University of Sydney; Epilepsy Research Centre (G.H., I.E.S.), Department of Medicine, University of Melbourne, Austin Health, Heidelberg, VIC; Department of Paediatrics (I.E.S.), University of Melbourne, Royal Children's Hospital, Florey and Murdoch Children's Research Institutes, Melbourne, Australia; Department of Clinical Epileptology, Sleep Disorders and Functional Neurology in Children (A.A., E.P.), University Hospitals of Lyon (HCL), Member of the International Alternating Hemiplegia in Childhood Research Consortium IAHCRC and of the European Reference Network ERN EpiCARE, Lyon, France; Paediatric Neurology Unit (I.C.), CMIN, Centro Hospitalar e Universitario Porto, Porto, Portugal; Clinical Neurophysiology Unit (C.Z.), IRCCS “E. Medea,” Bosisio Parini (LC), Italy; Department of Neurology (J.N.), CHUV and Université de Lausanne, Switzerland; Second Department of Neurology (K.D.), Institute Psychiatry and Neurology, Warsaw, Poland; Association AHC18+ e. V. (Germany) and Polish Association for People Affected by AHC, ahc-pl (M.P.); Department of Developmental Neurology (M.M.B.), Medical University of Gdańsk, Poland; Neurology Department (S.W.), University Hospital Antwerp; Neurogenetics Group (S.W.), University Antwerp, Belgium; First Department of Pediatrics (R.P.), “Agia Sofia” Children Hospital, National & Kapodistrian University of Athens, Greece; Department of Neurology (S.G.), University Medical Center of the Johannes Gutenberg University Mainz, Germany; Ion Channel Research Unit (D.S.S.), Department of Medicine/Cardiology and Pharmacology, Duke University Medical Center, Durham, NC; Cardiovascular Research Institute (G.S.P.), Weill Cornell Medical College, New York, NY; The Heart Centre (A.T.), Queen Mary University of London; Department of Pathology (M.A.), Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuropathology (Z.M., M.T.), Institute of Neurology, University College London, UK; and ICT and Data Analysis Section (R.V.), Euro-Mediterranean Institute of Science and Technology (I.E.ME.S.T.), Palermo, Italy.
1,†,✉