Abstract
Nicotinamide phosphoribosyltransferase (NAMPT) is a rate-limiting enzyme in the salvage pathway required for nicotinamide adenine dinucleotide synthesis. The secreted NAMPT protein serves as a master regulatory cytokine involved in activation of evolutionarily-conserved inflammatory networks. Appreciation of the role of NAMPT as a damage-associated molecular pattern protein (DAMP) has linked its activities to several disorders via toll-like receptor 4 (TLR4) binding and inflammatory cascade activation. Information is currently lacking concerning the precise mode of the NAMPT protein functionality due to limited availability of purified protein for use in in vitro and in vivo studies. Here we report successful NAMPT expression using the pET-SUMO expression vector in E. coli strain SHuffle containing a hexa-His tag for purification. The Ulp1 protease was used to cleave the SUMO and hexa-His tags, and the protein was purified by immobilized-metal affinity chromatography. The protein yield was ~4 mg/L and initial biophysical characterization of the protein using circular dichroism revealed the secondary structural elements, while dynamic light scattering demonstrated the presence of oligomeric units. The NAMPT-SUMO showed a predominantly dimeric protein with functional enzymatic activity. Finally, we report NAMPT solubilization in n-dodecyl-β-D-maltopyranoside (DDM) detergent in monomeric form, thus enhancing the opportunity for further structural and functional investigations.
Keywords: NAMPT purification, Biophysical characterization, Ligation-independent cloning, Dynamic light scattering, Circular dichroism, SUMO fusion tag
Graphical Abstract
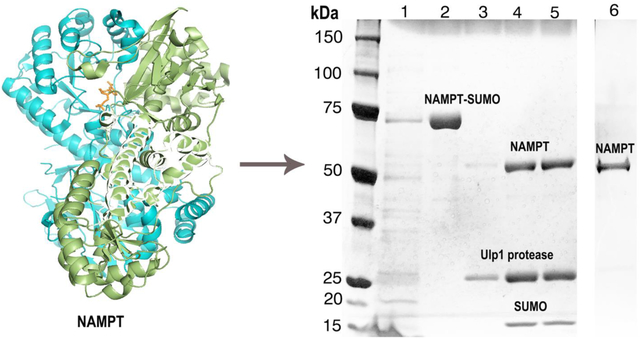
1. Introduction
In recent years a number of methods have been developed for production of recombinant proteins [1, 2], including the commonly used fusion tags glutathione S-transferase (GST), maltose-binding protein (MBP), and small ubiquitin-related modifier (SUMO). Furthermore, postranslational modification of cellular proteins in vivo through covalent attachment and detachment of SUMO modifies key cellular functions, including apoptosis, protein stability, response to stress, and transcriptional regulation. These fusion tags serve to enhance protein expression, solubility, and immunogenicity in E. coli systems [3]. Although the different expression systems have their specific utility, SUMO is recognized as one of the most effective commonly used solubility tags [4]. The expressed SUMO-tagged recombinant proteins exhibit high solubility, and the high fidelity of the SUMO protease allows for efficient removal of the SUMO tag from the recombinant protein, while maintaining the wild-type full-length amino-acid sequence [5–19]. These new advances, including the ligation-independent cloning procedure [20], a revolutionary development that effectively bypasses highly time-consuming ligation steps, pave the way for expression of clinically-relevant and pathobiologically-important proteins as targets for novel drug development.
Nicotinamide phosphoribosyltransferase (NAMPT) ― also known as Visfatin or Pre-B-cell colony-enhancing factor (PBEF) ― is an important evolutionarily-conserved protein involved in biosynthesis of nicotinamide adenine dinucleotide (NAD) [21–23] (Fig. 1). The enzyme catalyzes the rate-limiting step in the salvage pathway, which involves condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate (PRPP) to yield nicotinamide mononucleotide (NMN) as a key metabolite (Fig. 1). The enzyme, consisting of 491 amino acid residues with a relative molar mass (Mr) ≈55 kDa, exhibits pleiotropic cellular functions including growth factor activity, cytokine activity, and enzymatic activity. Additionally, NAMPT is involved in resistance during stress that coordinately regulates metabolism, cell defenses, and diseases of aging [24–29]. Impairment of protein activity alters the pathogenesis of cancer [30, 31], ischemic stress, heart failure [32], cardiovascular diseases, obesity, type-II diabetes [25, 33–36], and acute inflammatory lung injury [25, 34, 35, 37, 38]. Clearly, NAMPT is of immense medical importance, yet there is a lack of understanding of the exact protein function, which can be attributed to the absence of sensitive in vivo assays and appropriate expression systems. Although the protein has been purified from inclusion bodies [39], the exact fold and functionality of the protein extracted from inclusion bodies has proven to be problematic.
Fig. 1.

Schematic representation of nicotinamide phosphoribosyltransferase (NAMPT) biological function. The enzyme is involved in synthesis of nicotinamide adenine dinucleotide (NAD), which is an important metabolite involved in regulation of cellular processes. In mammals, the synthesis of NAD occurs in two steps: first, formation of nicotinamide mononucleotide (NMN) from nicotinamide (NAM) takes place, and then NAD is formed from NMN in the second step. The X-ray structure of NMN-bound NAMPT is shown (PDB ID: 2H3B) representing a functional dimeric form. For completion of NAD cycling, the deacetylation of NAD+ to NAM takes place in presence of Sirtuins (SIRT1,2). The X-ray structure of SIRT2 is depicted (PDB ID: −4Y6L). The NAMPT protein is a potential drug target and the drug-bound NAMPT crystal structure is shown at the top (PDB ID: 4O13)[23].
We hypothesized that for proteins such as NAMPT, information on the secondary structural folds and the multimeric state of the protein can be obtained using experimental methods such as circular dichroism (CD) and dynamic light scattering (DLS), thereby providing further insight into structure and biological function. Furthermore, the extracellular binding of NAMPT to toll-like receptor 4 (TLR4) results in NF-κB phosphorylation and activation, providing information on protein functionality [40]. In our study, we successfully constructed a recombinant plasmid using the SUMO fusion tag involving the ligation-independent cloning technique, and expressed and purified the protein by conventional nickel-nitrilotriacetic acid (Ni-NTA) column chromatography. We found that compared to the protein without the SUMO tag, the SUMO-tagged protein is less self-associated, yet maintains its native secondarty structure (≈40% alpha-helix). Moreover, we demonstrated that NAMPT exists as a monomer in the presence of n-dodecyl-β-D-maltopyranoside (DDM) detergent. These data suggest that hydrophobic effects are responsible for the aggregation of NAMPT, and that oligomerization may be important for amplification of the signal required for its functional pleiotropism. Our studies provide the basis for further mechanistic and biophysical investigations of functional NAMPT signaling pathways using the SUMO-tagged recombinant protein.
2. Materials and methods
2.1. Chemicals and reagents
All experiments were performed in 50 mM Tris HCl, pH 8, and 100 mM sodium chloride. Buffer solutions were filtered and carefully degassed. All buffers and solutions were prepared with ultra-high-quality sterile water from Sigma Aldrich (St. Louis, Mo, U.S.A.). The pETHSUL vector, IPTG, ampicillin, and Ni-NTA resin were obtained from Thermo Scientific (Waltham, MA, U.S.A.). Shuffle T7 competent cells were obtained from New England Biolabs (NEB) (Ipswich, MA, U.S.A.). Lysogeny broth (LB) and super optimal broth (SOB) were from the BIO5 media facility (BIO5 Institute at the University of Arizona, Tucson, Arizona, U.S.A.). Antibodies for western blot immunoreactivity were obtained from Thermo Fisher Scientific (Waltham, MA, U.S.A.), and Coomassie blue (EZ-blue G-250) stain was obtained from Sigma Aldrich (St. Louis, Mo, U.S.A). The SUMO protease was obtained from MCLAB (San Francisco, CA, U.S.A.). The NAMPT protein generated by Northwestern University (Chicago, IL, U.S.A.) was utilized as a positive control for testing our expression system. All other reagents were analytical grade. Protein expression was monitored using both anti-Histidine and rat anti-NAMPT antibodies. The anti-hexa-His antibody, mouse monoclonal MA1–21315, is from ThermoFisher. The goat polyclonal anti-NAMPT antibody was custom generated as described [41].
2.2. Construction of recombinant plasmid
To obtain a high-yield expression system that provides high solubility of the protein, the protein was cloned using the SUMO fusion tag. Furthermore a hexa-His tag was attached at the N-terminus to facilitate protein purification. The vector map showing the important regions is provided in Fig. 2A. Using the ligation-independent cloning (LIC) method, the gene coding for the NAMPT protein was successfully cloned, and tested with agarose gel electrophoresis as shown in Fig. 2B.
Fig. 2.
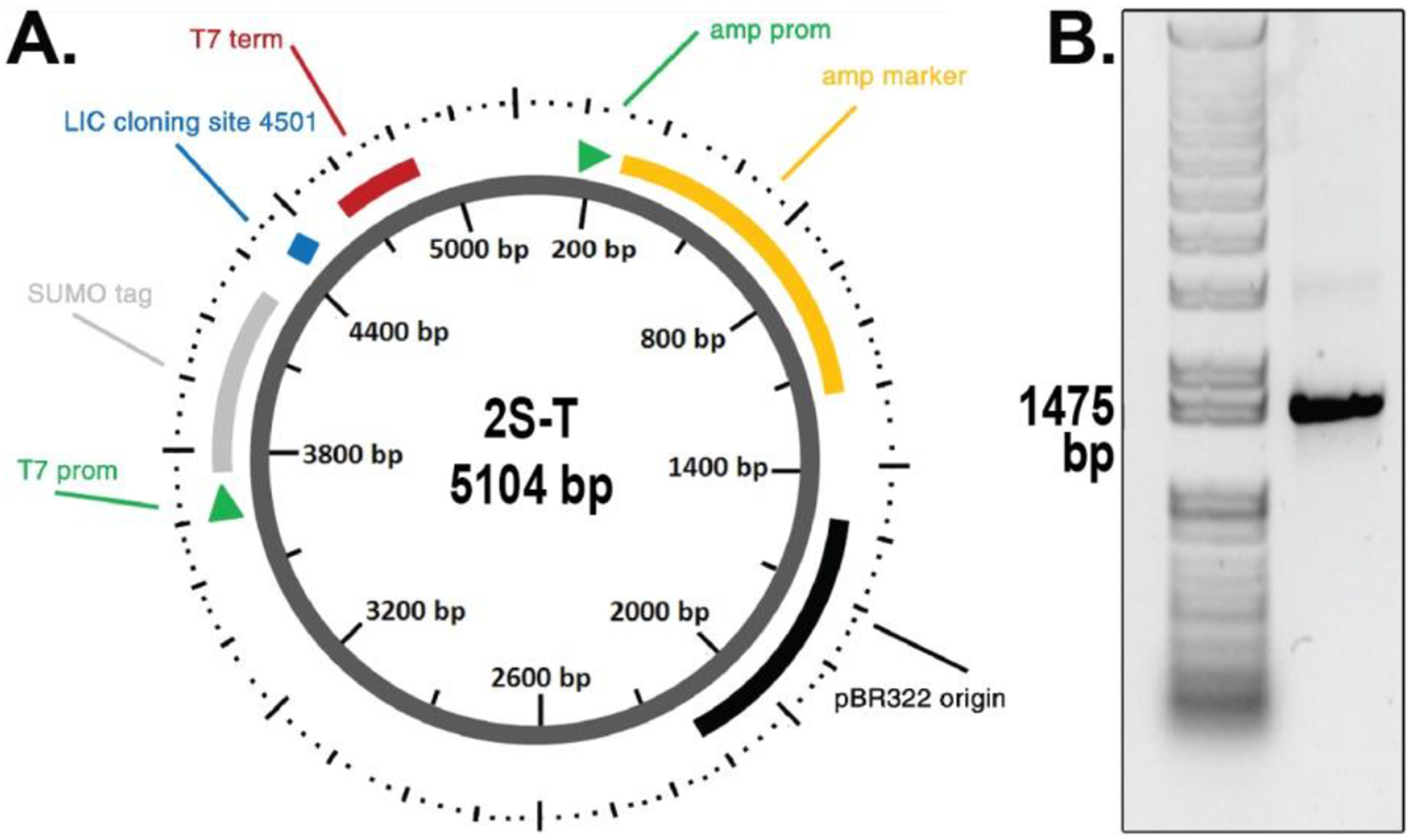
(a) Map of the plasmid used for constructing recombinant NAMPT protein is shown. The sequence of forward and reverse primers are indicated at the bottom. The plasmid contains the SUMO (small ubiquitin-like modifier) tag at the N-terminus for increase solubility and expression of the protein. The six-His tag is present at the N-terminus. Cleavage of the expressed protein using SUMO protease yields the recombinant protein without any extra tag. (b) Agarose gel electrophoresis showing the construct with the correct size of 1473 base pairs. The plasmid was generated using the ligation-independent cloning strategy. Sequencing of the plasmid hPBEF-pETHSUL (shown here) was further carried out to ensure the correct plasmid.
The full length cDNA sequence of human NAMPT was amplified by the polymerase chain reaction (PCR), with two synthetic nucleotide primers: the forward primer AGATTGGTGGCATGAATCCTGCGGCAGAAGC and the reverse primer GAGGAGAGTTTAGACCTAATGATGTGCTGCTTC. The products were cloned in a ligation-independent manner into a pETHSUL vector with both hexa-His and SUMO tags at the N-terminus. Briefly, the pETHSUL plasmid was digested with BseRI, followed by treatment with calf intestinal alkaline phosphate (CIAP) and T4 DNA polymerase in the presence of deoxycytidine triphosphate (dCTP), while the PCR product was treated with T4 DNA polymerase and deoxyguanosine triphosphate (dGTP). After overnight incubation of both the plasmid and PCR product at 37°C, the ligated clones were selected by transformation of DH5α competent cells, and confirmed by PCR and digestion. An agarose gel electrophoresis image confirming the PBEF-pETHSUL construct is shown in Fig. 2.
2.3. Expression of recombinant NAMPT
A confirmed PBEF-pETHSUL clone was further used for transformation of SHuffle T7 express-competent E. coli cells. Cells were grown at 37 °C to an absorbance of 0.6 OD600 in LB media containing 100 μg/ml ampicillin. The expression of the fusion protein was induced by using 1 mM IPTG for 16 h at 30 °C in 1L of LB broth. The absorbance at the induction was 0.8 OD600. Bacterial cells were harvested by centrifuging the induced culture at 5000 rpm (4750 × g) for 20 min at 4 °C. The cell pellet was resuspended in lysis buffer containing (50 mM Tris HCl, pH 7.8, 150 mM NaCl, 100 mM KCl, 10% glycerol, 10 mM imidazole, ≈ 0.2 mg/mL lysozyme, protease inhibitor EDTA-free cocktail complete) incubated on ice for 1 h. The pellet to lysis buffer ratio was 1:20. The suspension was sonicated for 3 min using 15-s on and 15-s off burst cycles with 60% amplitude. Thereafter, 5 μg/mL DNase was added to the cell lysate, kept on ice for 20 min, and then centrifuged at 5000 rpm (4750 × g) for 20 min. The supernatant was collected and stored at −80 °C for purification. Bacterial expression was confirmed by western blot analysis.
2.4. Purification of recombinant NAMPT by affinity chromatography
The lysate was next purified by immobilized-metal affinity chromatography (IMAC) using a Ni-NTA resin column. First, the lysate was allowed to bind to the resin for 12 h with gentle agitation. The resin was then subjected to several wash cycles using 20 mM imidazole to remove nonspecific-binding proteins. The NAMPT protein with hexa-His-tagged SUMO (NAMPT-SUMO) was eluted using an elution buffer (50 mM Tris HCl buffer, pH 8, 250 mM imidazole, 150 mM NaCl) and the purity of the protein tested by SDS-PAGE (Fig. 3). Centrifugal filters were used to eliminate the residual impurities, and the excess imidazole in the solution with buffer exchange (50 mM Tris HCl buffer, pH 8, 150 mM NaCl). At this stage, the yiled of NAMPT-SUMO is estimated to be ~10 mg/L of bacterial culture. Next, SUMO protease (Ulp1) was added (2μL of Ulp1 for 200 μg of protein) to the concentrated protein solution, and incubated at 4 °C overnight with gentle agitation. SDS-PAGE confirmed the complete cleavage of the hexa-His-tagged SUMO (Fig. 4). Typically the solution is eluted from a Ni-NTA resin column to remove the six-His-tagged SUMO and uncleaved NAMPT-SUMO. However, as the NAMPT protein itself showed a tendency towards avid binding to the Ni affinity column, the six-His-tagged SUMO and the protease were removed using centrigugal filters. We used 50-kDa filters to remove the hexa-His-tagged SUMO (Mr ≈16 kDa) and the SUMO protease (Mr ≈ 27 kDa). The final protein concentration after cleaving the SUMO tag was estimated using ELISA (see Supplementary Material for protocol), with the yield of 90% pure NAMPT estimated to be 4 mg/L. The purified NAMPT samples were stored at −80 °C. Final protein samples were analyzed using silver-stained SDS-PAGE images (Fig. 4). The full-length NAMPT-SUMO subsequently showed a dominantly dimeric protein and was fully active (see Figs. S1, S2). The protein samples were tested for aggregation using dynamic light scattering (DLS) techniques (Malvern Instruments, Malvern, UK).
Fig. 3.

(a) Image of the SDS-PAGE of the purified recombinant NAMPT containing SUMO tag stained using Coomassie blue dye. A single protein band around 70 kDa shows the highly purified recombinant protein with SUMO tag (lanes 7 – 10). (b) Dynamic light scattering (DLS) shows the presence of dimers for the purified NAMPT-SUMO protein. (c) Circular dichroism spectroscopy shows the secondary structure of the NAMPT-SUMO fusion protein.
Fig. 4.

Purification of recombinant NAMPT expressed using SUMO tag from E. coli culture. After initial IMAC purification and SUMO tag cleavage, removal of the cleaved tags and SUMO protease was achieved using centrigugal filters. Image shows silver-stained SDS-PAGE of the purified NAMPT. Lane 1: the lysate before purification; lane 2: the NAMPT-SUMO after Ni-NTA column purification at ≈70 kDa; lanes 3, 4 and 5: SUMO-cleaved NAMPT at ≈55 kDa, Ulp1 protease at ≈27 kDa and hexa-His SUMO at ≈16 kDa; lane 6: the NAMPT at ≈55 kDa further purified using centrifugal filters. After electrophoresis the gel was silver stained to check the high purity of the NAMPT protein.
2.5. Mass spectrometry
Mass spectrometry (MS) was next performed to further identify and characterize the recombinant NAMPT, as a complement to gel electrophoresis, utilizing an Ultraflex III MALDI TOF-TOF mass spectrometer (Bruker Daltonic, Germany) in the linear-mode registration for positively charged ions. A senapinic acid matrix was used for the sample preparation, and sample spotting was performed using the thin-layer method. Initially, 1 μl of the senapinic acid matrix solution was spotted onto the MALDI-MS sample plate and allowed to dry. Then 1 μl of the protein sample (500 μg/mL) was dispensed, and allowed to form the matrix-sample crystals through solvent evaporation.
2.6. Circular Dichroism spectroscopy
Circular dichroism spectropolarimetry was used to identify secondary structure elements of the folded NAMPT protein. Spectra were recorded using an OLIS DSM 20 CD spectrophotometer (OLIS, Inc., Bogart, GA, USA) equipped with a Quantum Northwest-CD temperature controller (Northwest Instruments Inc, Liberty lane, WA, USA) with 0.1 mm path length cuvettes at T = 10 °C. The cuvette chamber was continuously flushed with nitrogen gas. For each sample, 10 scans between 190 and 260 nm were averaged, with a resolution of 1 nm and an acquisition time of 10 s/nm. Control experiments were performed using buffer alone and DDM detergent in buffer. The protein concentration was adjusted to 1 mg/mL in 50 mM Tris HCl buffer, pH 8, containing 100 mM NaCl. Secondary structure estimation from CD data used the deconvolution algorithm CDSSTR with SMP56 as a basis reference set (provided by the software CDPro and Dichroweb) [42].
2.7. Dynamic light scattering
Dynamic light scattering (DLS) was utilized to assess the molecular size distribution (molar mass, Mr) and oligomeric state by measuring the time-dependent fluctuations of the scattered light intensity by the NAMPT solution. The DLS method calculates the diffusion coefficient, hydrodynamic radius, relative molar mass, and polydispersity of the samples investigated, and was carried out using a Zetasizer nano-ZS instrument (Malvern Instruments, Malvern, UK) using 100 μL of a 1mg/mL concentration protein samples in a low-volume (100 μL) cuvette. As the raw data (intensity) obtained from DLS is biased towards large particles (which scatter much more light than smaller particles), the Dispersion Technology Software (DTS) (Malvern Instruments, Malvern, UK) was utilized to provide realistic estimates of the volume and number distributions.
3. Results
The goal of this study was to generate large amounts of functionally intact protein at high concentrations suitable for biophysical characterization, and to conduct initial structural studies using spectroscopic methods. Here we describe the expression and purification of recombinant NAMPT protein together with preliminary functional and structural studies.
3.1. Expression and purification of recombinant protein
For protein expression, the recombinant plasmid transformation was performed in E. coli strain SHuffle. This E. coli strain was chosen as it contains mutated genes that further enhance the solubility of the expressed protein. The optimized conditions for protein expression were T = 30°C, using LB media with 1 mM IPTG, 0.1 optical density (OD), and 16 h of induction time. Western blot analysis revealed the presence of a single intense band corresponding to the NAMPT protein with attached SUMO and hexa-His tags, although several impurities were also observed by SDS-PAGE analysis.
The SDS-PAGE image of the Ni-NTA column-purified fusion protein with the SUMO fusion tag is shown in Fig. 3A. Additionally, we incubated the recombinant protein with SUMO-protease to cleave the SUMO and hexa-His tag. Silver stain SDS-PAGE analysis of the purified protein showed a single band (Fig. 4) indicating the presence of purified NAMPT in the final extractions.
3.2. Biophysical characterization of the purified protein
The recombinant protein was analyzed using MS (Fig. 5A), together with CD spectropolarimetry (Fig. 5B) and DLS (Fig. 5C) techniques to characterize the samples. Notably, the recombinant protein does not contain any extra amino acid residues, and it is expected to have unaltered folding and biological function versus the native protein. The secondary structure estimates using CDpro and DichroWeb indicated an approximately 40% α-helical structure, findings consistent with X-ray crystallography studies [43]. This finding ensures the protein has a well-defined secondary structure. The DLS results indicated an aggregated protein (Fig. 5C) that depended reversibly on the concentration of salt and protein (Figs. S3, S4); however, neither lowering of the protein nor increasing the salt concentration increased the likelihood of the protein assuming a functional dimeric form.
Fig. 5.

Biophysical characterization of purified NAMPT protein. (a) Mass spectrometry after purification of NAMPT protein. The purified protein has a molar mass cf 55.98 kDa representing the monomeric protein, and is equal within experimental error to the theoretically predicted molar mass of 55.5 kDa. (b) Circular dichroism spectrum of the purified protein after cleavage of the hexa-His-SUMO tag shows a characteristic secondary structure fold for purified NAMPT protein. (c) Dynamic light scattering (DLS) results of purified recombinant protein with and without DDM detergent. The NAMPT protein exists as higher order oligomers without DDM detergent and as monomer in presence of the detergent.
3.3. Study of the purified protein in detergent micelles
In contrast to the results obtained by denatured SDS-PAGE analysis, dynamic light scattering indicated that the native NAMPT was substantially aggregated. To further address the oligomerization properties of NAMPT, we performed DLS experiments in the presence of detergents, as well as under denaturing conditions. Detergent screening used DLS experiments to search for monomeric or functional dimeric NAMPT by solubilizing the protein in various ionic and nonionic detergents. From the detergent screens, we discovered that in the presence of DDM detergent, NAMPT exists as a monomeric protein (Fig 5C). Solubilizing NAMPT in the detergents LDS likewise resulted in lower-order oligomers or monomeric protein. However, our CD experiments showed that the protein is unfolded in LDS and SDS detergents (Figs. S5, S6), whereas in DDM detergent both purified NAMPT and detergent-solubilized NAMPT retained a similar secondary structure (Fig. 5B). Additional complementary mass spectrometry studies of NAMPT in detergents were performed. The DDM detergent-solubilized NAMPT efficiently showed a peak at the theoretically predicted mass value, indicating the presence of the monomeric form of the protein (Fig. 5A).
4. Discussion
Successful protein expression and purification is key for successful completion of in vitro structural studies such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy [44–46]. Such studies enable structural information to be obtained that increase the understanding of protein function in signaling pathways, and identification of novel drug targets [47, 48]. Despite advancements in techniques for enhancing protein expression, however, there are a number of challenges that limit the protein yield, and as a consequence, the information that can potentially be obtained for drug development studies. Although the yield of the recombinant protein can be lower than compared to the protein obtained from natural sources [49, 50], the latter case presents difficulties for isotopic labeling of proteins. On the other hand, expressed proteins generally contain extra amino acid residues that may hinder the ability of the protein to fold, thereby impairing function. Solubility of the protein also can become an issue when it is expressed under in vitro conditions. Poteins purified from inclusion bodies can introduce additional challenges with regard to their function versus the natural physiological environment [51, 52].
The expression and purification of NAMPT in E. coli. cells in native conditions enables the initial structural and biophysical characterization of this medically important protein in solution. The NAMPT protein obtained with conventional purification methods was analyzed using biophysical methods including circular dichroism (CD) spectroscopy, mass spectrometry (MS), and dynamic light scattering (DLS). Gel electrophoresis methods using SDS PAGE and western blot analysis showed distinct bands, indicating a highly purified protein. The CD spectrum analysis (Fig. 5B) indicated a folded protein, with secondary structural elements consistent with X-ray crystallography [43]. Under these conditions, mass spectroscopy could not identify the protein, raising the question of NAMPT oligomerization, which led us to investigate the use of other possible biophysical methods. Using DLS the coexistence of dimeric and aggregated SUMO-NAMPT protein was confirmed (Fig. 3B), whereas, the NAMPT was present as aggregated protein in a detergent-free aqueous solution (Fig. 5B).
Given the possibility of the strong intermolecular hydrophobic interactions, we further evaluated suitable detergents to potentially increase the solubility of NAMPT, while allowing the native folding to remain unaffected. When solubilized in anionic LDS detergent, the DLS experiment results indicated coexistence of monomeric and dimeric NAMPT, while the CD spectra showed a completely unfolded protein. On the other hand, solubilization of NAMPT with the nonionic DDM detergent successfully showed monomeric NAMPT protein, as assessed using DLS with the CD spectrum identical to the detergent-free NAMPT. Correspondingly, NAMPT solubilized with DDM detergent enabled successful mass spectrometry analysis, which indicated a peak at exactly the predicted theoretical molar mass of NAMPT (Mr = 55979 Da) (Fig. 5A). This finding suggests that NAMPT may be amphiphilic, raising the possibility that protein-protein and/or lipid-protein interactions may play a role in NAMPT function. Our discovery of conditions for the monomeric protein may potentially enhance understanding of the basis for formation of large oligomers in future work.
To summarize, we successfully expressed and purified recombinant NAMPT protein in E. coli with SUMO and hexa-His tags attached at the N-terminus of the protein. Circular dichroism spectroscopy and DLS techniques characterized the folding and oligomeric state of the protein. Expression of NAMPT with a SUMO tag provides a possible way to obtain high yields of the protein by improving the solubility. The purified NAMPT showed a clear tendency to form large oligomers. However, NAMPT with SUMO and hexa-His tags showed dominantly functional dimers and was fully active (Fig. S1). Formation of large multimers could be the reason for the loss of activity in SUMO-cleaved NAMPT; however, further investigations are needed to more fully characterize the aggregation upon cleaving the tags. Understanding the basis of its oligomerization in turn may give important clues for NAMPT biological function. Solubilizing monomeric NAMPT in the presence of DDM detergent, for the first time, can enable future high-resolution NMR studies to explore the structural dynamics and function of the protein. With these insights, future research, involving the amphiphilic nature of NAMPT, can address NAMPT protein function as a dimer and the role of lipid-protein interactions in NAMPT biological activity.
Supplementary Material
Highlights:
Nicotinamide phosphoribosyltransferase was expressed using the pET-SUMO vector containing a hexa-Histidine tag.
The recombinant NAMPT protein was characterized with MALDI-TOF mass spectrometry and does not contain extra amino acids.
Circular dichroism and light scattering showed the NAMPT-SUMO was dimeric and had a structure consistent with X-ray studies.
The NAMPT-SUMO protein is functional with full enzymatic activity.
Monomeric NAMPT solubilized in n-dodecyl-β-D-maltopyranoside has a secondary structure similar to the detergent-free protein.
Acknowledgements
This work was funded and supported by the NIH (grants EY012049, EY02604 to M.F.B. and R01HL094394, P01HL126609 to J.G.N.G.) and by the NSF (grant CHE 1904125 to M.F.B). We thank J. Mascarenhas, X. Wu, and W. Montfort for helpful discussions, L. Breci for expert assistance in analyzing mass spectrometry results, and C. Aspinwall for generous access to the light scattering instrumentation.
Abbreviations:
- CD
circular dichroism
- DDM
n-dodecyl-β-D-maltopyranoside
- DLS
dynamic light scattering
- IPTG
Isopropyl-β-D-thiogalactoside
- LDS
lithium dodecyl sulfate
- LIC
ligation-independent cloning
- MALDI
matrix-assisted laser desorption ionization
- MBP
maltose-binding protein
- NAD
nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- Ni-NTA
nickel-nitrilotriacetic acid
- PAGE
polyacrylamide gel electrophoresis
- PBEF
Pre-B cell colony enhancing factor
- SDS
sodium dodecyl sulfate
- SUMO
small ubiquitin-like modifier
- Visfatin
visceral fat protein
References
- [1].Esposito D, Chatterjee DK, Enhancement of soluble protein expression through the use of fusion tags, Curr. Opin. Biotechno, 17 (2006) 353–358. [DOI] [PubMed] [Google Scholar]
- [2].Kosobokova EN, Skrypnik KA, Kosorukov VS, Overview of fusion tags for recombinant proteins, Biochemistry (Moscow), 81 (2016) 187–200. [DOI] [PubMed] [Google Scholar]
- [3].Costa S, Almeida A, Castro A, Domingues L, Fusion tags for protein solubility, purification, and immunogenicity in Escherichia coli: the novel Fh8 system, Front. Microbiol, 5 (2014) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marblestone JG, Edavettal SC, Lim Y, Lim P, Zuo X, Butt TR, Comparison of SUMO fusion technology with traditional gene fusion systems: Enhanced expression and solubility with SUMO, Prot. Sci, 15 (2006) 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang H, Xiao Y, Fu L, Zhao H, Zhang Y, Wan X, Qin Y, Huang Y, Gao H, Li X, High-level expression and purification of soluble recombinant FGF21 protein by SUMO fusion in Escherichia coli, BMC Biotechnology, 10 (2010) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang J, Movahedi A, Wei Z, Sang M, Wu X, Wang M, Wei H, Pan H, Yin T, Zhuge Q, High-level SUMO-mediated fusion expression of ABP-dHC-cecropin A from multiple joined genes in Escherichia coli, Anal. Biochem, 509 (2016) 15–23. [DOI] [PubMed] [Google Scholar]
- [7].Tileva M, Krachmarova E, Ivanov I, Maskos K, Nacheva G, Production of aggregation prone human interferon gamma and its mutant in highly soluble and biologically active form by SUMO fusion technology, Protein Expres. Purif, 117 (2016) 26–34. [DOI] [PubMed] [Google Scholar]
- [8].Rao S, Zang X, Yang Z, Gao L, Yin Y, Fang W, Soluble expression and purification of the recombinant bioactive peptide precursor BPP-1 in Escherichia coli using a cELP-SUMO dual fusion system, Protein Expres. Purif, 118 (2016) 113–119. [DOI] [PubMed] [Google Scholar]
- [9].Zhang L, Li X, Wei D, Wang J, Shan A, Li Z, Expression of plectasin in Bacillus subtilis using SUMO technology by a maltose-inducible vector, Journal of Industrial Microbiology & Biotechnology, 42 (2015) 1369–1376. [DOI] [PubMed] [Google Scholar]
- [10].Liu Z, Zhang J, Fan H, Yin R, Zheng Z, Xu Q, Liu Q, He H, Peng X, Wang X, Li X, Xiao Y, Expression and purification of soluble single-chain Fv against human fibroblast growth factor receptor 3 fusedwith Sumo tag in Escherichia coli, Electron. J. Biotechnol, 18 (2015) 302–306. [Google Scholar]
- [11].Guerrero F, Ciragan A, Iwai H, Tandem SUMO fusion vectors for improving soluble protein expression and purification, Protein Expres. Purif, 116 (2015) 42–49. [DOI] [PubMed] [Google Scholar]
- [12].Ge W-S, Fan J-G, Chen Y-W, Xu L-M, Expression and purification of functional HMGB1 A box by fusion with SUMO, Mol. Med. Rep, 12 (2015) 6527–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dai XY, Sun ZQ, Liang R, Li Y, Luo HM, Huang YD, Chen MW, Su ZJ, Xiao F, Recombinant Nogo-66 via soluble expression with SUMO fusion in Escherichia coli inhibits neurite outgrowth in vitro, Appl. Microbiol. Biotechnol, 99 (2015) 5997–6007. [DOI] [PubMed] [Google Scholar]
- [14].Lee JE, Kim JH, SUMO modification regulates the protein stability of NDRG1, Biochem. Biophys. Res. Commun, 459 (2015) 161–165. [DOI] [PubMed] [Google Scholar]
- [15].Hartwig S, Frister T, Alemdar S, Li Z, Scheper T, Beuterl S, SUMO-fusion, purification, and characterization of a (+)-zizaene synthase from Chrysopogon zizanioides, Biochem. Biophys. Res. Commun, 458 (2015) 883–889. [DOI] [PubMed] [Google Scholar]
- [16].Zhang J, Ma L, Zhang SQ, Expression and purification of soluble human APRIL in Escherichia coli using ELP-SUMO tag, Protein Expres. Purif, 95 (2014) 177–181. [DOI] [PubMed] [Google Scholar]
- [17].Zhang C, He X, Gu Y, Zhou H, Cao J, Gao Q, Recombinant scorpine produced using SUMO fusion partner in Escherichia coli has the activities against clinically isolated bacteria and inhibits the plasmodium falciparum parasitemia in vitro, Plos One, 9 (2014) e103456–1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peciak K, Tommasi R, Choi J-W, Brocchini S, Laurine E, Expression of soluble and active interferon consensus in SUMO fusion expression system in E. coli, Protein Expres. Purif, 99 (2014) 18–26. [DOI] [PubMed] [Google Scholar]
- [19].Liew OW, Ang CX, Peh YP, Chong PCJ, Ng YX, Hwang L-A, Koh XY, Yip YM, Liu W, Richards AM, A His6-SUMO-eXact tag for producing human prepro-Urocortin 2 in Escherichia coli for raising monoclonal antibodies, J. Immunol. Methods, 403 (2014) 37–51. [DOI] [PubMed] [Google Scholar]
- [20].Weeks SD, Drinker M, Loll PJ, Ligation independent cloning vectors for expression of SUMO fusions, Protein Expres. Purif, 53 (2007) 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Revollo JR, Grimm AA, Imai S.-i., The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals, Curr. Opin. Gastroenterol, 23 (2007) 164–170. [DOI] [PubMed] [Google Scholar]
- [22].Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W, Physiological and pathophysiological roles of NAMPT and NAD metabolism, Nat. Rev. Endocrinol, 11 (2015) 535–546. [DOI] [PubMed] [Google Scholar]
- [23].Wang W, Elkins K, Oh A, Ho Y-C, Wu J, Li H, Xiao Y, Kwong M, Coons M, Brillantes B, Cheng E, Crocker L, Dragovich PS, Sampath D, Zheng X, Bair KW, O’Brien T, Belmont LD, Structural basis for resistance to diverse classes of NAMPT inhibitors, PloS One, 9 (2014) e109366–1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang H, Lavu S, Sinclair DA, Nampt/PBEF/Visfatin: A regulator of mammalian health and longevity?, Exp. Gerontol, 41 (2006) 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou T, Wang T, Garcia JGN, Expression of Nicotinamide phosphoribosyltransferase-influenced genes predicts recurrence-free survival in lung and breast cancers, Sci. Rep, 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ouchi N, Parker JL, Lugus JJ, Walsh K, Adipokines in inflammation and metabolic disease, Nat. Rev. Immunol, 11 (2011) 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Imai S.-i., Guarente L, NAD+ and sirtuins in aging and disease, Trends. Cell. Biol, 24 (2014) 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Partridge L, Finkel T, Amita S, Focus on aging, Cell Metab, 23 (2016) 951–956. [DOI] [PubMed] [Google Scholar]
- [29].Patwardhan B, Mutalik G, Tillu G, Longevity, rejuvenation, and rasayana In Integrative approaches for health, Academic Press, Boston, 2015, pp. 259–291. [Google Scholar]
- [30].Chen X-Y, Zhang H-S, Wu T-C, Sang W-W, Ruan Z, Down-regulation of NAMPT expression by miR-182 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation, Int. J. Biochem. Cell Biol, 45 (2013) 292–298. [DOI] [PubMed] [Google Scholar]
- [31].Garten A, Petzold S, Schuster S, Körner A, Kratzsch J, Kiess W, NAMPT and its potential role in inflammation and type 2 diabetes, Handb. Exp. Pharmacol, (2011) 147–164. [DOI] [PubMed] [Google Scholar]
- [32].Gupta A, Gupta S, Young D, Das B, McMahon J, Sen S, Impairment of ultrastructure and cytoskeleton during progression of cardiac hypertrophy to heart failure, Lab. Investig, 90 (2010) 520–530. [DOI] [PubMed] [Google Scholar]
- [33].Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, Coscia M, Maffei R, Rossi D, Wang T, Inghirami G, Rizzi M, Gaidano G, Garcia JGN, Wolberger C, Raffaelli N, Deaglio S, Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia, Blood, 125 (2015) 111–123. [DOI] [PubMed] [Google Scholar]
- [34].Zhang LQ, Adyshev DM, Singleton P, Li H, Cepeda J, Huang S-Y, Zou X, Verin AD, Tu J, Garcia JGN, Ye SQ, Interactions between PBEF and oxidative stress proteins – A potential new mechanism underlying PBEF in the pathogenesis of acute lung injury, FEBS Lett, 582 (2008) 1802–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhou T, Garcia JGN, Zhang W. Integrating microRNAs into a system biology approach to acute lung injury, Transl. Res, 157 (2011) 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu P, Li H, Cepeda J, Zhang LQ, Cui X, Garcia JGN, Ye SQ, Critical role of PBEF expression in pulmonary cell inflammation and permeability, Cell Biol. Int, 33 (2009) 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garcia JGN, Pulmonary circulation and regulation of fluid balance, in: Mason RJ, Ernst JD, King TE, Lazarus SC, Murray JF, Nadel JA, Slutsky AS, Gotway MB (Eds.) Murray and Nadel’s Textbook of Respiratory Medicine (Sixth Edition), W.B. Saunders, Philadelphia, 2016, pp. 92–110.e118. [Google Scholar]
- [38].Ye SQ, Zhang LQ, Adyshev D, Usatyuk PV, Garcia AN, Lavoie TL, Verin AD, Natarajan V, Garcia JGN, Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation, Microvasc. Res, 70 (2005) 142–151. [DOI] [PubMed] [Google Scholar]
- [39].Liu XY, Ge L, Yu DM, Cloning and expression of visfatin and screening of oligopeptides binding with visfatin, Int. J. Clin. Exp. Med, 7 (2014) 4828–4834. [PMC free article] [PubMed] [Google Scholar]
- [40].Camp SM, Ceco E, Evenoski CL, Danilov SM, Zhou T, Chiang ET, Moreno-Vinasco L, Mapes B, Zhao J, Gursoy G, Brown ME, Adyshev DM, Siddiqui SS, Quijada H, Sammani S, Letsiou E, Saadat L, Yousef M, Wang T, Liang J, Garcia JGN, Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NF kappa B Signaling and Inflammatory Lung Injury, Sci. Rep, 5 (2015) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hong S-B, Huang Y, Moreno-Vinasco L, Sammani S, Moitra J, Barnard JW, Ma S-F, Mirzapoiazova T, Evenoski C, Reeves RR, Chiang ET, Lang GD, Husain AN, Dudek SM, Jacobson JR, Ye SQ, Lussier YA, Garcia JGN, Essential Role of Pre–B-Cell Colony Enhancing Factor in Ventilator-induced Lung Injury, Am. J. Respir. Crit. Care Med, 178 (2008) 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Whitmore L, Wallace BA, Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases, Biopolymers, 89 (2008) 392–400. [DOI] [PubMed] [Google Scholar]
- [43].Wang T, Zhang X, Bheda P, Revollo JR, Imai S.-i., Wolberger C, Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme, Nat. Struct. Mol. Biol, 13 (2006) 661–662. [DOI] [PubMed] [Google Scholar]
- [44].Zook JD, Molugu TR, Jacobsen NE, Lin G, Soll J, Cherry BR, Brown MF, Fromme P, High-resolution NMR reveals secondary structure and folding of amino acid transporter from outer chloroplast membrane, PLoS ONE, 8 (2013) e78116–1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Molugu TR, Lee S, Brown MF, Concepts and methods of solid-state NMR spectroscopy applied to biomembranes, Chem. Rev, 117 (2017) 12087–12132. [DOI] [PubMed] [Google Scholar]
- [46].Kamal MZ, Ahmad S, Molugu TR, Vijayalakshmi A, Deshmukh MV, Sankaranarayanan R, Rao NM, In vitro evolved non-aggregating and thermostable lipase: structural and thermodynamic investigation, J. Mol. Biol, 413 (2011) 726–741. [DOI] [PubMed] [Google Scholar]
- [47].Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu C-C, LeBleu VS, Kalluri R, Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer, Nature, 527 (2015) 525–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hasty J, McMillen D, Collins JJ, Engineered gene circuits, Nature, 420 (2002) 224–230. [DOI] [PubMed] [Google Scholar]
- [49].Struts AV, Barmasov AV, Brown MF, Spectral methods for study of the G-protein-coupled receptor rhodopsin: I. Vibrational and electronic spectroscopy, Opt. Spectrosc, 118 (2015) 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chawla U, Jiang YJ, Zheng W, Kuang LJ, Perera SMDC, Pitman MC, Brown MF, Liang HJ, A usual G-protein-coupled receptor in unusual membranes, Angew. Chem. Int. Ed, 55 (2016) 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hwang PM, Pan JS, Sykes BD, Targeted expression, purification, and cleavage of fusion proteins from inclusion bodies in Escherichia coli, FEBS Lett, 588 (2014) 247–252. [DOI] [PubMed] [Google Scholar]
- [52].Cabrita LD, Bottomley SP, Protein expression and refolding--a practical guide to getting the most out of inclusion bodies, Biotechnol. Annu. Rev, 10 (2004) 31–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


