Abstract
Background
The etiology of postpartum psychopathologies are not well understood, but folate metabolism pathways are of potential interest. Demands for folate increase dramatically during pregnancy, low folate level has been associated with psychiatric disorders, and supplementation may improve symptomatology. The MTHFR C677T variant influences folate metabolism and has been implicated in depression during pregnancy.
Objective
To conduct a prospective longitudinal study to explore the relationship between MTHFR C677T genotype, folate levels, and postpartum psychopathology in at-risk women.
Hypothesis
In the first three months postpartum, folate will moderate a relationship between MTHFR genotype and depression, with TT homozygous women having more symptoms than CC homozygous women.
Methods
We recruited 365 pregnant women with a history of mood or psychotic disorder, and at 3 postpartum timepoints, administered the Edinburgh Postnatal Depression Scale (EPDS); Clinician-Administered Rating Scale for Mania (CARS-M) and the Positive and Negative Symptom Scale (PANSS) and drew blood for genotype/folate level analysis. We used robust linear regression to investigate interactions between genotype and folate level on the highest EPDS and CARS-M scores, and logistic regression to explore interactions with PANSS psychosis scores above/below cut-off.
Results
There was no significant interaction effect between MTHFR genotype and folate level on highest EPDS (p = 0.36), but there was a significant interaction between genotype, folate level and log(CARS-M) (p = 0.02); post-hoc analyses revealed differences in the effect of folate level between CC/CT, and TT genotypes, with folate level in CC and CT having an inverse relationship with symptoms of mania, while there was no relationship in participants with TT genotype. There was no significant interaction between MTHFR genotype and folate level on the likelihood of meeting positive symptom criteria for psychosis on the PANSS (p = 0.86).
Discussion
These data suggest that perhaps there is a relationship between MTHFR C677T, folate level and some symptoms of postpartum psychopathology.
Introduction
Postpartum psychiatric disorders are urgent health concerns that have important implications for mothers, infants, and their families. Although all women are at risk for an episode of mental illness in the postpartum [1, 2], those who have a history of a mood or psychotic disorder are at greater risk compared to the general population [2–10].
Similar to non-perinatal psychiatric disorders, postpartum psychiatric disorders are thought to arise due to the combined effects of genetic and environmental factors. There is an abundance of literature investigating the role of genetic variations in psychiatric disorders such as schizophrenia and bipolar disorder [11], with accumulating research on gene-environment interactions [12]. However, in the context of postpartum psychiatric disorders, the majority of investigations focus on either environmental contributors or the role of genetics [13–18] with few studies investigating gene and environment interactions (e.g. 5HTTLPR/monoaminergic variations and stress [19, 20]).The need for studies of postpartum depression that integrate these elements has been recognized [21], and there are potential gene-environment interactions worthy of investigation in relation to postpartum psychopathology. One such potential example involves variations in the gene encoding the enzyme methylenetetrahydrofolate reductase, and folate.
Methyltetrahydrofolate reductase (MTHFR) is a folate dependent enzyme that has a common, functional, thermolabile variant—MTHFR C677T. It has been studied in the context of non pregnancy related psychiatric disorders, depression, bipolar disorder, and schizophrenia [20–22]–including a study of the relationship between psychopathology, folate, and the MTHFR C677T variant [22].
MTHFR C677T variants and low folate levels have also been separately studied, and associated with depressed mood during pregnancy [23, 24]. Folate deficiency has also been postulated as a contributor to postpartum psychosis and depression [24, 25], but no studies, to our knowledge, have explored whether the MTHFR C677T variant increases risk for postpartum psychiatric disorders and/or explored how postpartum physiological folate levels interact with these genotypes in relation to risk. Further, no studies to our knowledge have investigated the role of MTHFR C677T or folate levels in postpartum mania. Given the increased demands for folate during pregnancy, and the impact of folate deficiency on TT homozygous women [26], there is need to further understand the role of MTHFR C677T and folate levels on postpartum psychopathology, especially since there has been some suggestion of using folic acid or L-methylfolate supplements to treat low mood in the postpartum and the general population in those with MTHFR C677T variants [27, 28]. A more thorough understanding of genetic risk factors that may suggest remediatory biological interventions (e.g. perinatal folic acid supplementation tailored to MTHFR genotype) is critical to improving outcomes for women at risk for postpartum psychopathology.
The purpose of this study was to conduct a prospective, longitudinal, observational study to better understand the relationships between the MTHFR C677T variant, physiological folate levels and postpartum depression in at-risk women. We aimed to test the hypothesis that in the first three months postpartum, compared to MTHFR CC homozygous women, TT homozygous women would have increased symptoms of postpartum depression (PPD) and that this relationship would be moderated by physiological levels of red blood cell (RBC) folate. We also conducted exploratory analyses regarding the impact of MTHFR C677T genotypes and RBC folate levels on: a) postpartum mania (PPM) and b) postpartum psychosis (PPP).
Materials and methods
The study was approved by the University of British Columbia Research Ethics Board (H06-70145). All participants provided written informed consent.
Participants were recruited from the metropolitan Vancouver, Canada area between 2007 and 2016 (N = 365). Women were eligible to participate if they: a) had a history of a mood or psychotic disorder (depression, bipolar disorder, schizophrenia) as confirmed by the Structured Clinical Interview for Diagnosis (SCID) [29]; b) were pregnant; and c) were fluent in English. Details regarding recruitment methods are described elsewhere [30].
The study was observational; no experimental interventions were provided to participants. Data collection occurred at 4 timepoints: T1 (during pregnancy (>15 weeks gestation)); T2 (1–2 week(s) postpartum); T3 (1–2 month(s) postpartum); and T4 (3–4 months postpartum).
At T1, we collected demographic information, and at each timepoint, blood was drawn (for measuring RBC folate, and MTHFR genotyping). Also at each timepoint participants completed the Edinburgh Postnatal Depression Scale (EPDS) to assess depression symptomatology (see below), and a trained researcher administered the Clinician Administered Rating Scale (CARS-M) to assess mania symptomatology (see below), and the Positive and Negative Symptom Scale (PANSS) to assess psychosis symptomatology (see below). Participants also provided information regarding use of folic acid supplements and psychotropic medication at each timepoint.
Data were managed using REDCap (Research Electronic Data Capture) tools hosted at BC Children’s Hospital Research Institute. REDCap is a secure, web-based application designed to support data capture for research studies [31].
Instruments
Edinburgh Postnatal Depression Scale (EPDS)
The EPDS is a 10-item, self-administered, Likert scale-based questionnaire (each item is rated by selecting from 4 options, scored from 0 to 3) that has been validated for measuring both prenatal and postpartum depression [32]. Higher scores indicate greater depression symptomatology.
Clinician Administered Rating Scale for Mania (CARS-M)
The 10-item CARS-M is a clinician rated, reliable and valid measure of the severity of mania symptomatology with or without psychotic features. On the basis of an interview and observation, severity of symptomatology is assessed by rating each item from 0 (absent) to 5 (extreme) [33].
Positive and Negative Syndrome Scale (PANSS)
The PANSS is a well-validated instrument (completed by a clinically trained rater, on the basis of a 30–45 minute semi-structured interview) that measures the presence and severity of 30 psychiatric symptoms [34]. Each symptom is rated on a 7-point scale; a score of 1 means the symptom is not present, and a score of 7 means that it is present to an extreme degree. Five of the PANSS items can be used to assess the presence and severity of psychosis, using specific cut off scores [35, 36]. Specifically we categorized psychosis as present if a participant met at least one of the following threshold scores (delusions ≥3, conceptual disorganization ≥5 [37], hallucinations ≥3, suspiciousness ≥5, unusual thought content ≥4).
To ensure agreement between multiple raters (inter-rater concordance) for the PANSS, we conducted periodic PANSS inter-rater concordance sessions and calculated coefficient of agreement (determined by % agreement within 1 rating point amongst all raters) for each of the five PANSS items for psychosis [38].
Biological measures
Folate
RBC folate was measured using an Abbott Architect i1000 immunoanalyzer (Abbott Diagnostics,Canada, Mississauga Ont), folate reagent kit (#B1P740) and Abbott folate calibrators and quality controls. RBC folate levels were corrected for hematocrit and plasma folate.
MTHFR C677T genotyping
DNA was extracted from buffy coats from whole blood (or if blood samples were unavailable, buccal swabs) using Qiagen QIAmp DNA cultured cells protocol. Real-time PCR using Taqman primers/probes determined genotypes of MTHFR C677T (ie CC, CT, or TT).
Analysis
To allow for differences in timing for the emergence of symptoms of depression and mania we selected the highest postpartum EPDS and CARS-M scores (from all available postpartum timepoints) for each participant and the corresponding RBC folate levels (i.e. RBC folate measured at the time of the highest EPDS or CARS-M) for analysis. When the highest score persisted for more than one timepoint, we used the earliest timepoint with available corresponding RBC folate level. Similarly, to allow for differences in timing for the emergence of key positive symptoms of psychosis, we used the earliest postpartum timepoint for which the participant met criteria for psychosis (determined by the PANSS scores defined above) with available corresponding RBC folate data. If the participant never met criteria for psychosis we used the earliest timepoint with available RBC folate data.
To investigate relationships between: depression (EPDS) or mania (CARS-M) symptoms in the postpartum, MTHFR genotype, and RBC folate levels, we used robust linear regression with MM estimation to mitigate the effects of outliers and highly influential points [39, 40] as implemented in the ‘robustbase’ package [41]. The presence of outliers and influential data was assessed using diagnostic regression plots of non-robust models. Additionally, the results of the robust regressions were different enough from the non-robust regressions to justify their use. Moderation of the relationship between MTHFR and EPDS or CARS-M by RBC folate levels, was tested by including an interaction term in the models [42]. If a significant interaction term was detected, we estimated the difference in slope among the CC, CT, and TT genotypes using asymptotic Chi-square tests [43] with Benjamini-Hochberg false-discovery rate p-value correction [44]. Otherwise, the interaction term was removed and the main effects of MTHFR genotype and RBC folate were estimated. CARS-M scores were log transformed to better meet the assumptions of normality and homogeneity of variance. We calculated Cohen’s d values to estimate the effect size of the difference in mean EPDS and CARS-M scores between CC, CT, and TT genotypes, controlled for RBC folate.
We investigated relationships between: likelihood of meeting criteria for psychosis on the PANSS, MTHFR genotype, and RBC folate levels with logistic regression.
Statistical significance was assumed at p < 0.05.
We used descriptive statistics to report demographic data. To test differences in demographic and clinical variables between genotypes we used Kruskal-Wallis tests for continuous variables and Fisher Exact tests for categorical variables.
Results
The study enrolled 365 women. Sufficient postpartum data was collected from 327 participants to be included in analyses (i.e. completed the PANSS, EPDS, or CARS-M, had corresponding RBC folate data for at least one postpartum timepoint, and were genotyped for MTHFR C677T). There were no significant differences in demographic characteristics between MTHFR genotype groups, and distribution of genotype groups did not deviate from Hardy-Weinberg equilibrium (χ2 = 3.67, df = 1, p = 0.06) (see Table 1).
Table 1. Demographics for each MTHFR C677T genotype.
| MTHFR genotype | |||||
|---|---|---|---|---|---|
| Total | TT | CT | CC | P-value | |
| N = 327 | n = 37 | n = 125 | n = 165 | ||
| Age (years) (n = 326) | |||||
| Mean (SD) | 31.1 (±5.7) | 31.0 (±6.4) | 30.8 (±5.9) | 31.3 (±5.4) | 0.83 |
| Annual Household Income ($ CAD) (n = 316) | |||||
| <$20,000 | 32 (10.1%) | 4 (11.1%) | 12 (9.8%) | 16 (10.1%) | 0.16 |
| $20,000 - $40,000 | 54 (17.1%) | 11 (30.6%) | 24 (19.7%) | 19 (12.0%) | |
| $41,000 - $60,000 | 55 (17.4%) | 6 (16.7%) | 21 (17.2%) | 28 (17.7%) | |
| $61,000 - $80,000 | 62 (19.6%) | 6 (16.7%) | 24 (19.7%) | 32 (20.3%) | |
| $81,000 - $100,000 | 44 (13.9%) | 6 (16.7%) | 19 (15.6%) | 19 (12.0%) | |
| >$100,000 | 69 (21.8%) | 3 (8.3%) | 22 (18.0%) | 44 (27.8%) | |
| Ethnicity (n = 321) | |||||
| European | 243 (75.7%) | 24 (66.7%) | 91 (72.8%) | 128 (80.0%) | 0.38 |
| Asian | 24 (7.5%) | 3 (8.3%) | 8 (6.4%) | 13 (8.1%) | |
| Mixed | 42 (13.1) | 7 (19.4%) | 20 (16.0%) | 15 (9.4%) | |
| Other* (includes African and Aboriginal) | 10 (3.1%) | 2 (5.6%) | 5 (4.0%) | 3 (1.9%) | |
| Highest level of education (n = 315) | |||||
| High school | 24 (7.6%) | 4 (11.4%) | 10 (8.3%) | 10 (6.3%) | 0.79 |
| Less than 4 years of college/university | 121 (38.4%) | 10 (28.6%) | 47 (38.8%) | 64 (40.3%) | |
| 4 or more years of college/university | 157 (49.8%) | 20 (57.1%) | 58 (47.9%) | 79 (49.7%) | |
| Did not complete high school | 13 (4.1%) | 1 (2.9%) | 6 (5.0%) | 6 (3.8%) | |
| Employment (n = 326) | |||||
| Not employed | 48 (14.7%) | 4 (10.8%) | 23 (18.4%) | 21 (12.8%) | 0.34 |
| Employed | 278 (85.3%) | 33 (89.2%) | 102 (81.6%) | 143 (87.2%) | |
| Body Mass Index (BMI) (n = 296) | |||||
| Mean (SD) | 29.1 (±5.6) | 28.7 (±5.4) | 28.7 (±5.5) | 29.4 (±5.7) | 0.74 |
| Gravida (n = 324) | |||||
| Median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.79 |
| Number of children at enrollment (n = 324) | |||||
| Median (IQR) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.83 |
| IVF pregnancy (n = 320) | |||||
| no | 307 (95.9%) | 33 (94.3%) | 115 (94.3%) | 159 (97.5%) | 0.26 |
| yes | 13 (4.1%) | 2 (5.7%) | 7 (5.7%) | 4 (2.5%) | |
| Marital status (n = 324) | |||||
| Married/Common-Law/Partnered | 302 (93.2%) | 33 (97.3%) | 109 (88.6%) | 157 (95.7%) | 0.04 |
| Single | 22 (6.8%) | 1 (2.7%) | 14 (11.4%) | 7 (4.3%) | |
| Psychiatric Diagnosis (n = 326) | |||||
| Bipolar Disorder | 60 (18.4%) | 7 (18.9%) | 21 (16.8%) | 32 (19.5%) | 0.95 |
| Depression | 264 (81.3%) | 30 (81.1%) | 103 (82.4%) | 131 (79.9%) | |
| Schizophrenia | 2 (0.6%) | 0 (0.0%) | 1 (0.8%) | 1 (0.6%) | |
| Previous History of psychotic symptoms (n = 326) | |||||
| no | 251 (77.0%) | 28 (75.7%) | 102 (81.6%) | 121 (73.8%) | 0.28 |
| yes | 75 (23.0%) | 9 (24.3%) | 23 (18.4%) | 43 (26.2%) | |
| Psychotropic medication in the postpartum (n = 325) | |||||
| no | 216 (66.5%) | 24 (64.9%) | 84 (67.7%) | 108 (65.9%) | 0.93 |
| yes | 109 (33.5%) | 13 (35.1%) | 40 (32.3%) | 56 (34.1%) | |
| RBC folate (ng/ml) | |||||
| Mean (SD) | 663.5 (261.7) | 777.0 (332.3) | 650.0 (198.4) | 648.2 (189.8) | 0.097 |
| Taking folic acid supplement throughout postpartum (n = 326) | |||||
| no | 127 (39.0%) | 12 (33.3%) | 48 (38.4%) | 67 (40.6%) | 0.71 |
| yes | 199 (61.0%) | 24 (66.7%) | 77 (61.6%) | 98 (59.4%) | |
Depression
There were 305 participants whose highest postpartum EPDS had a corresponding RBC folate available (see Table 2).
Table 2. Highest EPDS (depression) scores and the corresponding RBC folate levels and medication/supplement data for each MTHFR C677T genotype.
| MTHFR genotype | |||||
|---|---|---|---|---|---|
| Total | TT | CT | CC | p-value | |
| N = 305 | n = 33 | n = 116 | n = 156 | ||
| Highest postpartum EPDS score | |||||
| Mean (SD) | 9.4 (5.2) | 10.4 (5.9) | 9.1 (5.1) | 9.4 (5.2) | 0.48 |
| RBC folate (ng/ml) | |||||
| Mean (SD) | 645.8 (218.5) | 722.0 (257.2) | 648.2 (217.1) | 627.8 (208.4) | 0.08 |
| Taking a Folic Acid Supplement (n = 303) | |||||
| no | 72 (23.8%) | 5 (15.6%) | 23 (19.8%) | 44 (28.3%) | 0.14 |
| yes* | 231 (76.2%) | 27 (84.4%) | 93 (80.2%) | 111 (71.6%) | |
| Taking a daily Psychotropic Medication (n = 304) | |||||
| no | 230 (75.7%) | 23 (69.7%) | 90 (78.3%) | 117 (75.0%) | 0.57 |
| yes | 74 (24.3%) | 10 (30.3%) | 25 (21.7%) | 39 (25.0%) | |
*n = 1 participant took 5-MTHF supplements (not folic acid).
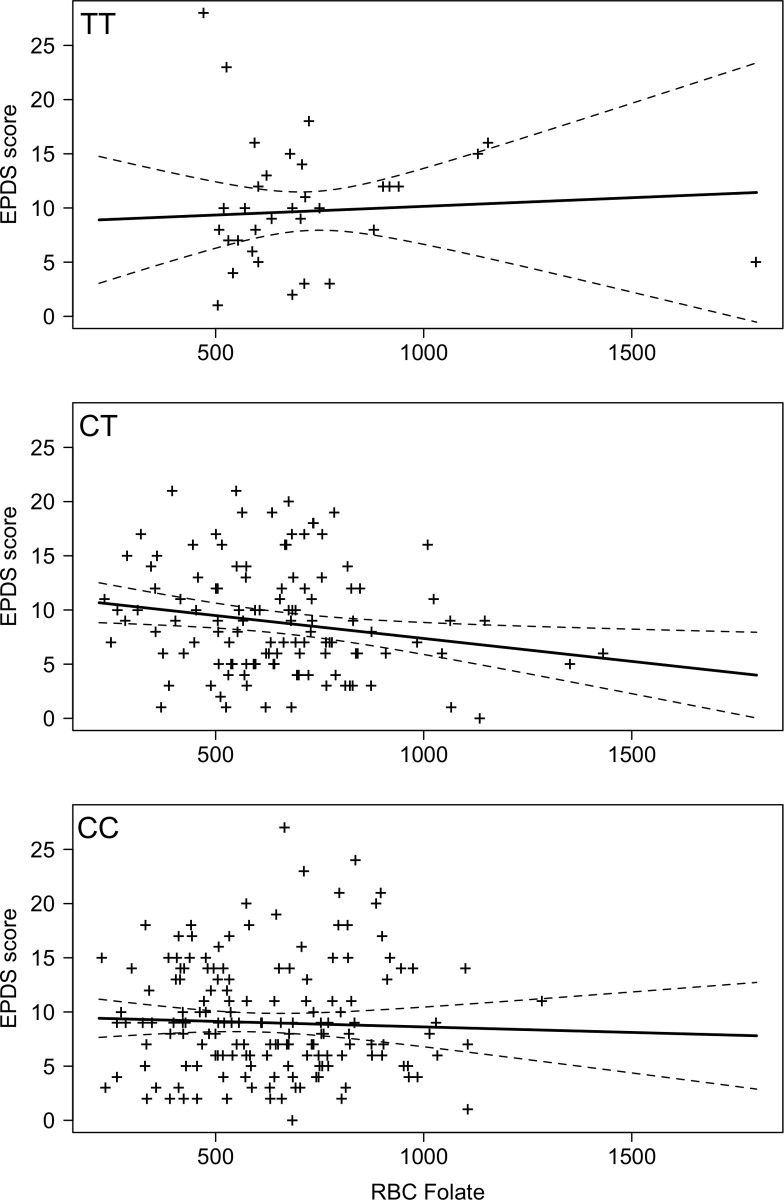
RBC folate and highest postpartum EPDS scores (EPDS vs RBC folate) are shown for each MTHFR genotype in Fig 1. There was no significant interaction between MTHFR genotype and RBC folate level on highest EPDS (p = 0.36). There was also no relationship between RBC folate and EPDS on its own (coefficient = -0.002 (95%CI = -0.004 to 0.0008), p = 0.19, adjusted R-squared = 0.002), and no difference between genotypes for mean EPDS controlling for RBC folate (p = 0.59, Cohen’s d values: CC compared to CT: d = 0.02, CC compared to TT: d = 0.16, and CT compared to TT: d = 0.18).
Fig 1. (n = 305) EPDS (depression) score vs. RBC folate (ng/ml) score for each MTHFR C677T (TT, CT, CC) genotype (dashed line indicates confidence intervals).
Mania
There were 233 women whose highest CARS-M had a corresponding RBC folate level (Table 3).
Table 3. Highest CARS-M (mania) score and the corresponding RBC folate levels and medication/supplement data for each MTHFR C677T genotype.
| MTHFR genotype | |||||
|---|---|---|---|---|---|
| Total | TT | CT | CC | p-value | |
| N = 233 | n = 24 | n = 90 | n = 119 | ||
| Highest postpartum CARS-M score | |||||
| Mean (SD) | 6.8 (3.8) | 7.46 (3.3) | 7.1 (3.7) | 6.5 (3.9) | 0.31 |
| RBC folate (ng/ml) | |||||
| Mean (SD) | 672.4 (227.9) | 818.9 (342.4) | 659.4 (199.1) | 652.7 (210.9) | 0.004 |
| Taking a Folic Acid Supplement (n = 232) | |||||
| no | 52(22.4%) | 4 (17.4%) | 13 (14.4%) | 35 (29.4%) | 0.03 |
| yes* | 180 (77.6%) | 19 (82.6%) | 77 (85.6%) | 84 (70.6%) | |
| Taking a daily psychotropic medication | |||||
| no | 176 (75.5%) | 16 (43.2%) | 73 (81.1%) | 87 (73.1%) | 0.22 |
| yes | 57 (24.5%) | 8 (33.3%) | 17 (18.9%) | 32 (26.9%) | |
* n = 1 participant took 5-MTHF supplements (not folic acid).
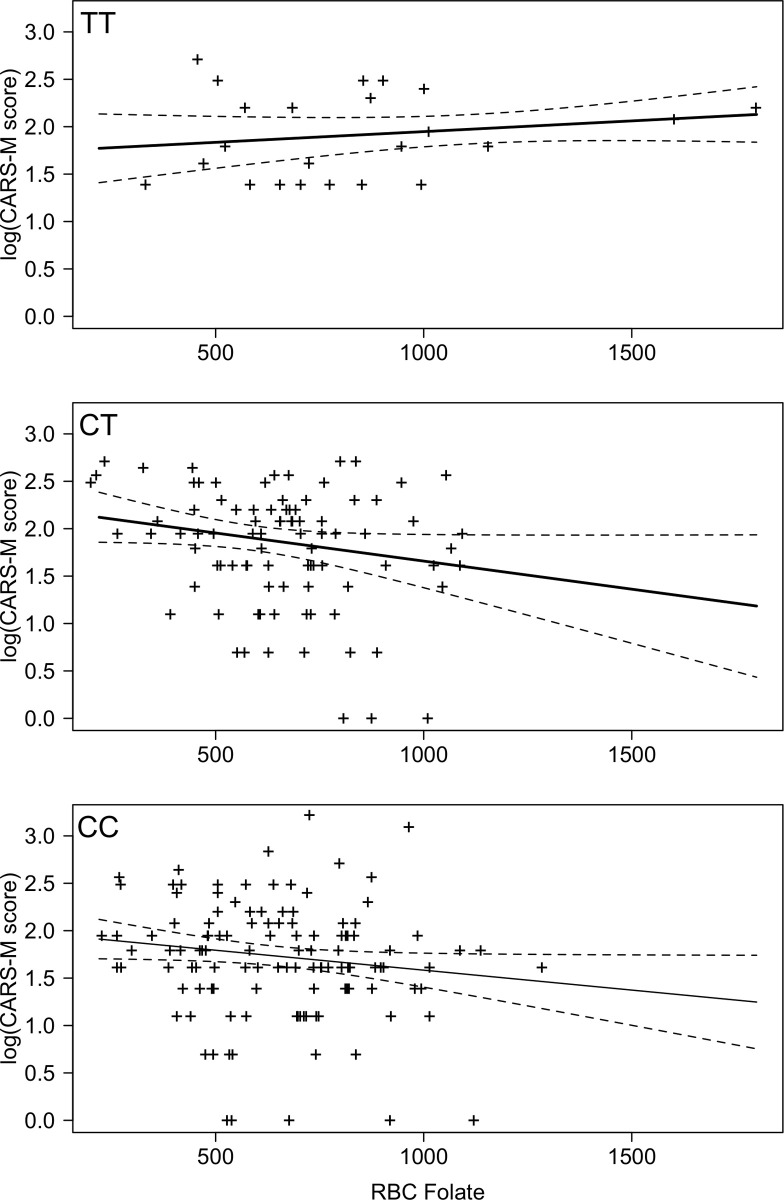
RBC folate level and log transformed CARS-M (CARS-M vs RBC folate) scores are shown for each MTHFR genotype in Fig 2. There was a significant interaction between MTHFR genotype and RBC folate level on highest (log)CARS-M scores (p = 0.02). Post-hoc analyses suggested no significant differences between the slopes (CARS-M vs RBC Folate) for MTHFR CC and CT genotypes (p = 0.65), but suggested that there was a significant difference between the slopes of CARS-M vs RBC folate for MTHFR CC and TT (p = 0.03) genotypes, and between the slopes of CARS-M vs RBC folate for MTHFR CT and TT (p = 0.03) genotypes (Fig 2). The slopes for the CC and CT genotype groups showed an inverse relationship between RBC folate and CARS-M, with higher folate levels associated with lower CARS-M scores. However, the slope for the TT genotype suggests that there was not a strong relationship between CARS-M and RBC folate within the TT genotype group.
Fig 2. (n = 233) CARS-M score vs. RBC folate (ng/ml) score for each MTHFR C677T (TT, CT, CC) genotype (dashed line indicates confidence intervals).
Psychosis
Coefficient of agreement for each of the five PANSS items (delusions, conceptual disorganization, hallucinations, suspiciousness, and unusual thought content) from PANSS interrater concordance sessions held over the course of the study were 0.85, 0.70, 0.90, 0.77, and 0.85 with an overall mean coefficient of agreement of 0.82.
There were 326 women with sufficient postpartum PANSS data and corresponding RBC folate data, for at least one postpartum timepoint (Table 4).
Table 4. Earliest presence of psychotic symptoms and the corresponding RBC folate levels and medication/supplement data for each MTHFR C677T genotype.
| MTHFR genotype | |||||
|---|---|---|---|---|---|
| Total | TT | CT | CC | p-value | |
| N = 326 | n = 37 | n = 124 | n = 165 | ||
| PANSS Psychosis Criteria Met | |||||
| no | 261 (80.0%) | 33 (89.2%) | 96 (77.4%) | 132 (80.0%) | 0.31 |
| yes | 65 (20.1%) | 4 (10.8%) | 28 (22.6%) | 33 (20.0%) | |
| RBC folate (ng/ml) | |||||
| Mean (SD) | 705.0 (238.7) | 830.4 (369.8) | 698.9 (206.9) | 681.4(216.1) | 0.002 |
| Taking a Folic Acid Supplement (n = 324) | |||||
| no | 71 (21.9%) | 6 (16.7%) | 24 (19.4%) | 42 (25.6%) | 0.42 |
| yes* | 253 (78.1%) | 30 (83.3%) | 100 (80.6%) | 122 (73.9%) | |
| Taking a daily psychotropic medication (n = 324) | |||||
| no | 244 (75.3%) | 28 (75.7%) | 95 (77.2%) | 121 (73.8%) | 0.82 |
| yes | 80 (24.7%) | 9 (24.3%) | 28 (22.6%) | 43 (26.2%) | |
* n = 1 participant took 5-MTHF supplements (not folic acid).
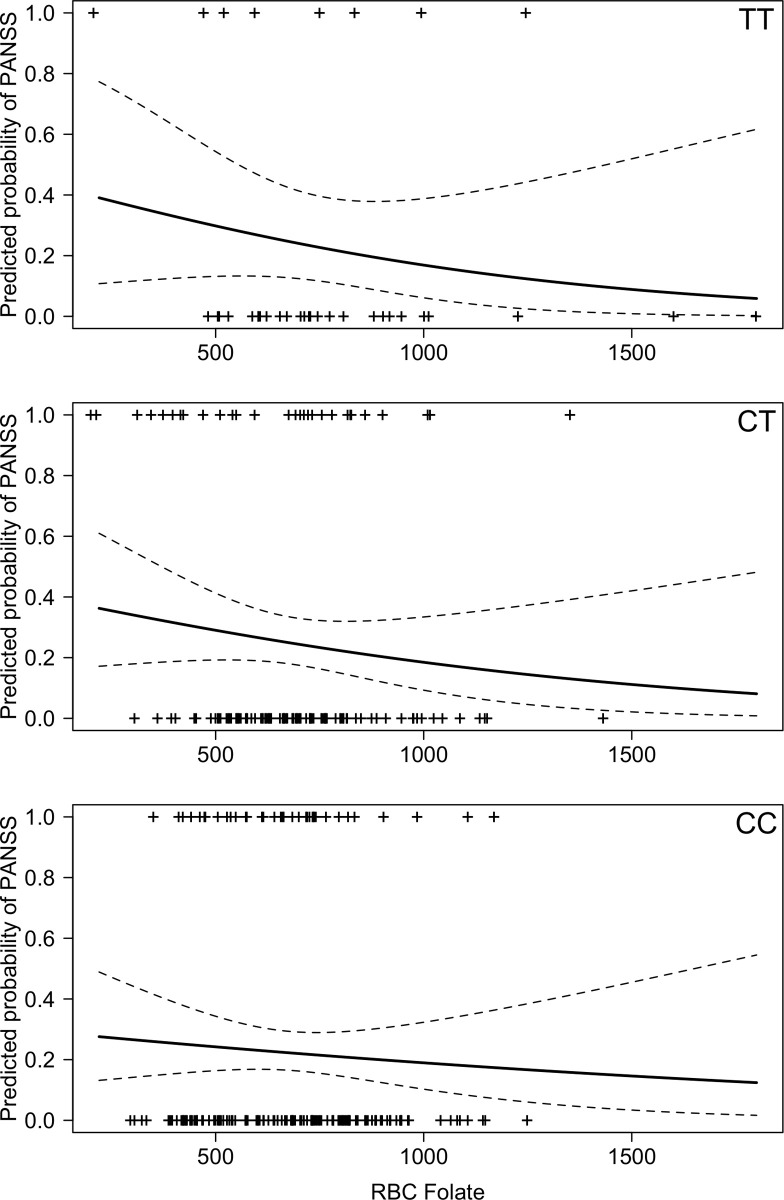
There was no significant interaction between MTHFR genotype and RBC folate level on the probability of meeting psychotic symptom criteria on the PANSS (p = 0.86). RBC folate levels and whether participants met criteria for psychosis for each MTHFR genotype is shown in Fig 3. There was also no relationship between RBC folate and psychotic symptoms on its own (OR = 1.00, 95%CI = 0.99 to 1.01, p = 0.09), and there was also no difference between genotypes for psychotic symptoms controlling for RBC folate (p = 0.86; Odds Ratio (OR) for CT compared to CC = 1.16 (95%CI = 0.66 to 1.03); OR for TT compared to CC = 1.12 (95%CI = 0.44 to 1.60)).
Fig 3. (n = 326) Predicted probably of meeting threshold criteria for psychosis vs. RBC folate (ng/ml) for each MTHFR C677T (TT, CT, CC) genotype (dashed line indicates confidence intervals).
Regression coefficients and data from both linear (depression and mania) and logistic (psychosis) regression analyses are displayed in S1 Table.
Discussion
This is the first study to our knowledge to explore relationships between MTHFR C677T variants, physiological levels of folate, and postpartum psychiatric symptoms, and specifically it is the first study to explore postpartum mania and any associations with MTHFR or folate levels. Our data suggest that, for women with a MTHFR C677T C allele (i.e. CC or CT genotypes), folate levels and mania symptoms may be inversely related, but that there is no relationship between folate levels and mania symptoms in women with a TT genotype. Interestingly, our data also showed that mean mania scores for women with a TT genotype did not significantly differ from those of women with CC or CT genotypes. While visually (see Fig 1) there is an appearance of an inverse relationship between depression scores and folate levels for women with CC and CT genotypes, that reflects the relationships found with mania, in this case, the relationship was not statistically significant. Given the small effect size of the difference in EPDS scores (when controlling for RBC folate levels) between TT genotypes and those with a C allele (CC and CT), it is possible that a relationship between MTHFR genotype and RBC folate levels (like we observed with mania symptoms) also exists for depressive symptoms, but the sample size was underpowered to detect it. Our data do not suggest a relationship between psychosis and folate levels and MTHFR C677T genotype.
MTHFR C677T was historically considered a strong candidate gene for a variety of psychiatric disorders (outside the perinatal setting) and was initially implicated in schizophrenia [45–50], bipolar and unipolar depression [50–53], though this association was not supported by genome wide association study (GWAS) findings [54, 55]. Our data suggest that MTHFR C677T genotype alone does not increase risk for postpartum psychopathology. Our findings are also broadly in line with previous studies that have shown that low folate (and thus subsequent high levels of homocysteine) is associated with various forms of psychopathology [56–59]. However, our findings suggest that this inverse relationship may only exist in the postpartum for women with a MTHFR C677T C allele, at least in terms of mania, and, perhaps, depressive symptoms.
One study that investigated the impact of folate levels on perinatal depression, without accounting for MTHFR genotype, did not find any relationship between plasma folate levels and depression in the postpartum period [24]. While our study also did not find a significant relationship of RBC folate with postpartum depression symptomatology, our results suggest that there may be an inverse relationship with other mood symptoms (mania), and possibly depression, when the effect of MTHFR genotype is considered.
While retrospective observational studies have suggested that self-reported perinatal folic acid supplementation can improve maternal depression symptomology several months postpartum, especially in women with a MTHFR TT genotype [28] our findings suggest that women with a TT genotype do not demonstrate an inverse relationship between physiological folate and mood symptoms (i.e. mania and perhaps, depression). Our results also suggest that overall there is no difference in physiological folate levels based on MTHFR C677T genotype (Table 1), but at times there may be higher physiological folate levels in those with TT genotypes (Tables 3 and 4), despite previous research suggesting that individuals who are TT homozygous would be more prone to folate deficiency [60]. These differences in our study may be due to the fact that a perinatal population is highly supplemented (e.g. in our study population the majority of women were taking 1 mg of folic acid daily, in addition to folate and folic acid consumed through diet) compared to studies in the general population. It is also possible that further studies of MTHFR C677T variants in pregnancy and postpartum cohorts are needed to fully understand the impact of pregnancy on enzyme function.
Limitations
Since most of the women in our study were taking folic acid supplements, and all were living in Canada (a country with folic acid fortified foods), it is possible that an inverse relationship between physiological folate levels and psychiatric symptoms does exist for women who are MTHFR TT homozygotes; however, this relationship is not observable in populations with adequate folic acid supplementation (i.e. at a certain RBC folate level there may be a ceiling effect (for TT homozygotes) for the impact of folate on psychiatric symptoms). While there was no statistical difference in RBC folate levels (overall–see Table 1) between CC, CT, and TT genotypes, those with a TT genotype consistently had the highest RBC folate levels, which reached statistical significance when there was the highest levels of manic symptoms or presence/absence of psychotic symptoms, suggesting that the TT group was more than adequately supplemented, perhaps mitigating any negative impact of the TT genotype on psychopathology that would be observed when folic acid supplementation (and RBC folate) is less abundant. It is also possible that, while overall there was no difference in the proportions of women taking a folic acid supplements across the genotype groups (Table 1), participants with a TT genotype may, by chance, have been taking a greater amount of folic acid.
Our data may be enriched for missing data at timepoints when women were the most depressed or experiencing psychotic symptoms (resulting in data only being available for a single timepoint, where symptoms may be less severe), as women may have cancelled study visits during periods of poorer mental health. This possibility increases the chance of a Type II error, whereby results indicate the absence of relationship that truly exists. Because our study population consisted of women who were already at a high risk for postpartum psychiatric disorders, due to their past history of a mood or psychotic disorder [2–10], our results may not be generalizable to other populations of postpartum women. As our study did not investigate the role of elevated homocysteine, future studies could explore relationships with MTHFR C677T genotype, psychopathology, and homocysteine levels.
Conclusions
There may be a relationship between MTHFR C677T genotype, RBC folate levels, and risk for some forms of postpartum psychopathology, at least in the context of high-risk Canadian women with adequate folic acid supplementation. Further studies with larger samples of women may be needed to characterize the nuances in relationships between postpartum depression and mania symptoms in relation to MTHFR C677T genotype and folate levels.
Supporting information
(DOCX)
Acknowledgments
The authors thank all members of the Translational Psychiatric Genetics Group for their support, and the numerous volunteers over the years who helped with data entry. The authors would like to thank the participants who generously participated in the study.
Data Availability
Our data contains potentially sensitive information. Further, in order to share de-identified data, our Research Ethics Board requires that participants explicitly consent. Unfortunately this was not included in our consent form for this study and we are unable to provide a de-identified data set to be made accessible. Data access requests can be sent to the University of British Columbia Clinical Research Ethics board (ethics.research.ubc.ca).
Funding Statement
This work was made possible through funding from the Canadian Institutes of Health Research (CIHR), JA was supported by BC Mental Health and Substance Use Services, the Canada Research Chairs Program, the Michael Smith Foundation for Health Research, and CIHR. JA received funding from the Canadian Institutes of Health Research (https://cihrirsc.gc.ca/e/193.html) to conduct this work (CIHR MOP 82750). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kendell RE, Chalmers JC, Platz C: Epidemiology of puerperal psychoses. The British journal of psychiatry: the journal of mental science 150:662–673, 1987. 10.1192/bjp.150.5.662 [DOI] [PubMed] [Google Scholar]
- 2.Bosanac P, Buist A, Burrows G: Motherhood and Schizophrenic Illnesses: A Review of the Literature. Aust N Z J Psychiatry 37:24–30, 2003. 10.1046/j.1440-1614.2003.01104.x [DOI] [PubMed] [Google Scholar]
- 3.Howard L: Postnatal depression. American Family Physician 72, 2005 [Google Scholar]
- 4.Viguera AC, Cohen LS, Bouffard S, et al. : Reproductive Decisions by Women With Bipolar Disorder After Prepregnancy Psychiatric Consultation. AJP 159:2102–2104, 2002. 10.1176/appi.ajp.159.12.2102 [DOI] [PubMed] [Google Scholar]
- 5.Jones I, Craddock N: Familiality of the Puerperal Trigger in Bipolar Disorder: Results of a Family Study. AJP 158:913–917, 2001. 10.1176/appi.ajp.158.6.913 [DOI] [PubMed] [Google Scholar]
- 6.Yonkers KA, Ramin SM, Rush AJ, et al. : Onset and Persistence of Postpartum Depression in an Inner-City Maternal Health Clinic System. American Journal of Psychiatry 158:1856–1863, 2001. 10.1176/appi.ajp.158.11.1856 [DOI] [PubMed] [Google Scholar]
- 7.Marks MN, Wieck A, Checkley SA, et al. : Contribution of psychological and social factors to psychotic and non-psychotic relapse after childbirth in women with previous histories of affective disorder. Journal of affective disorders 24:253–263, 1992. 10.1016/0165-0327(92)90110-r [DOI] [PubMed] [Google Scholar]
- 8.Davies A, McIvor RJ, Channi Kumar R: Impact of childbirth on a series of schizophrenic mothers: a comment on the possible influence of oestrogen on schizophrenia. Schizophrenia research 16:25–31, 1995. 10.1016/0920-9964(94)00062-d [DOI] [PubMed] [Google Scholar]
- 9.Freeman MP, Gracious BL, Wisner KL: Pregnancy Outcomes in Schizophrenia. American Journal of Psychiatry 159:1609, 2002. 10.1176/appi.ajp.159.9.1609 [DOI] [PubMed] [Google Scholar]
- 10.Wisner KL, Hanusa BH, Peindl KS, et al. : Prevention of postpartum episodes in women with bipolar disorder. Biological psychiatry 56:592–596, 2004. 10.1016/j.biopsych.2004.07.022 [DOI] [PubMed] [Google Scholar]
- 11.Bray NJ, O'Donovan M,C.: The genetics of neuropsychiatric disorders. Brain and neuroscience advances 2:10.1177/2398212818799271, 2019. 10.1177/2398212818799271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assary E, Vincent JP, Keers R, et al. : Gene-environment interaction and psychiatric disorders: Review and future directions. Seminars in Cell & Developmental Biology 77:133–143, 2018. 10.1016/j.semcdb.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 13.Couto TCe, Brancaglion MYM, Alvim-Soares A, et al. : Postpartum depression: A systematic review of the genetics involved. World Journal of Psychiatry 5:103–111, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coyle N, Jones I, Robertson E, et al. : Variation at the serotonin transporter gene influences susceptibility to bipolar affective puerperal psychosis. Lancet (London, England) 356:1490–1491, 2000. 10.1016/S0140-6736(00)02877-4 [DOI] [PubMed] [Google Scholar]
- 15.Middle F, Jones I, Robertson E, et al. : Tumour necrosis factor alpha and bipolar affective puerperal psychosis. Psychiatric genetics 10:195–198, 2000. 10.1097/00041444-200010040-00008 [DOI] [PubMed] [Google Scholar]
- 16.Jones I, Middle F, McCandless F, et al. : Molecular genetic studies of bipolar disorder and puerperal psychosis at two polymorphisms in the estrogen receptor alpha gene (ESR 1). American Journal of Medical Genetics 96:850–853, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Robertson E, Jones I, Middle F, et al. : No association between two polymorphisms at the 5HT2A gene and bipolar affective puerperal psychosis. Acta Psychiatrica Scandinavica 108:387–391, 2003. 10.1034/j.1600-0447.2003.00167.x [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Gardner CO, Bigdeli T, et al. : Clinical features of and risk factors for major depression with history of postpartum episodes in Han Chinese women: A retrospective study. Journal of affective disorders 183:339–346, 2015. 10.1016/j.jad.2015.05.033 [DOI] [PubMed] [Google Scholar]
- 19.Comasco E, Sylven SM, Papadopoulos FC, et al. : Postpartum depression symptoms: a case-control study on monoaminergic functional polymorphisms and environmental stressors. Psychiatric genetics 21:19–28, 2011. 10.1097/YPG.0b013e328341a3c1 [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Wang L, Huang F, et al. : Gene-environment interaction in postpartum depression: a Chinese clinical study. Journal of affective disorders 165:208–212, 2014. 10.1016/j.jad.2014.04.049 [DOI] [PubMed] [Google Scholar]
- 21.Yim IS, Tanner Stapleton LR, Guardino CM, et al. : Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annual Review of Clinical Psychology 11:99–137, 2015. 10.1146/annurev-clinpsy-101414-020426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjelland I, Tell GS, Vollset SE, et al. : Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Archives of General Psychiatry 60:618–626, 2003. 10.1001/archpsyc.60.6.618 [DOI] [PubMed] [Google Scholar]
- 23.Devlin AM, Brain U, Austin J, et al. : Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS ONE [Electronic Resource] 5:e12201, 2010. 10.1371/journal.pone.0012201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong MF, Wong JX, Colega M, et al. : Relationships of maternal folate and vitamin B12 status during pregnancy with perinatal depression: The GUSTO study. Journal of psychiatric research 55:110–116, 2014. 10.1016/j.jpsychires.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 25.Thornton WE: Folate deficiency in puerperal psychosis. American Journal of Obstetrics and Gynecology 129:222–223, 1977. 10.1016/0002-9378(77)90752-9 [DOI] [PubMed] [Google Scholar]
- 26.Shelnutt KP, Kauwell GP, Gregory JF, et al. : Methylenetetrahydrofolate reductase 677C—>T polymorphism affects DNA methylation in response to controlled folate intake in young women. The Journal of nutritional biochemistry 15:554–560, 2004. 10.1016/j.jnutbio.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 27.Wan L, Li Y, Zhang Z, et al. : Methylenetetrahydrofolate reductase and psychiatric diseases. Translational psychiatry 8:242–6, 2018. 10.1038/s41398-018-0276-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis SJ, Araya R, Leary S, et al. : Folic acid supplementation during pregnancy may protect against depression 21 months after pregnancy, an effect modified by MTHFR C677T genotype. European journal of clinical nutrition 66:97–103, 2012. 10.1038/ejcn.2011.136 [DOI] [PubMed] [Google Scholar]
- 29.First, MB, Spitzer, RL, Gibbon, M, Williams, JBW: Structured clinical interview for DSM-IV Axis I Disorders- Clinician Version (SCID-CV). American Psychiatric Press, 1997
- 30.Yaremco E, Inglis A, Innis SM, et al. : Red blood cell folate levels in pregnant women with a history of mood disorders: a case series. Birth defects research.Part A, Clinical and molecular teratology 97:416–420, 2013. 10.1002/bdra.23144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, et al. : Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 42:377–381, 2008. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox JL, Holden JM, Sagovsky R: Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry 150:782–786, 1987. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 33.Altman EG, Hedeker DR, Janicak PG, et al. : The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biological psychiatry 36:124–134, 1994. 10.1016/0006-3223(94)91193-2 [DOI] [PubMed] [Google Scholar]
- 34.Kay SR, Fiszbein A, Opler LA: The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin 13:261–276, 1987. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 35.Hui CL, Wong GH, Tang JY, et al. : Predicting 1-year risk for relapse in patients who have discontinued or continued quetiapine after remission from first-episode psychosis. Schizophrenia research 150:297–302, 2013. 10.1016/j.schres.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 36.Chen EY, Hui CL, Lam MM, et al. : Maintenance treatment with quetiapine versus discontinuation after one year of treatment in patients with remitted first episode psychosis: randomised controlled trial. BMJ (Clinical research ed.) 341:c4024, 2010. 10.1136/bmj.c4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mighton CE, Inglis AJ, Carrion PB, et al. : Perinatal psychosis in mothers with a history of major depressive disorder. Archives of Women's Mental Health 19:253–258, 2016. 10.1007/s00737-015-0561-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kay SR, Opler LA, Lindenmayer JP: Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry research 23:99–110, 1988. 10.1016/0165-1781(88)90038-8 [DOI] [PubMed] [Google Scholar]
- 39.Yohai VJ: High Breakdown-Point and High Efficiency Robust Estimates for Regression. The Annals of Statistics 15:642–656, 1987 [Google Scholar]
- 40.Koller M, Stahel WA: Sharpening Wald-type inference in robust regression for small samples. Computational Statistics & Data Analysis 55:2504–2515, 2011 [Google Scholar]
- 41.Maechler M, Rousseeuw P, Croux C, et al: robustbase: Basic Robust Statistics. R package version 0.93–6, 2020
- 42.Dawson JF: Moderation in Management Research: What, Why, When, and How. Journal of Business and Psychology 29:1–19, 2014 [Google Scholar]
- 43.Fox J: Applied Regression Analysis and Generalized Linear Models., Sage, 2016
- 44.Benjamini Y, Hochberg Y: Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society.Series B (Methodological) 57:289–300, 1995 [Google Scholar]
- 45.Arinami T, Yamada N, Yamakawa-Kobayashi K, et al. : Methylenetetrahydrofolate reductase variant and schizophrenia/depression. American Journal of Medical Genetics 74:526–528, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Almeida OP, Flicker L, Lautenschlager NT, et al. : Contribution of the MTHFR gene to the causal pathway for depression, anxiety and cognitive impairment in later life. Neurobiology of aging 26:251–257, 2005. 10.1016/j.neurobiolaging.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 47.Peerbooms OLJ, van Os J, Drukker M, et al. : Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: Evidence for a common genetic vulnerability? Brain Behavior and Immunity 25:1530–1543, 2011. 10.1016/j.bbi.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 48.Lewis SJ, Zammit S, Gunnell D, et al. : A meta‐analysis of the MTHFR C677T polymorphism and schizophrenia risk. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 135B:2–4, 2005. 10.1002/ajmg.b.30170 [DOI] [PubMed] [Google Scholar]
- 49.Muntjewerff JW, Hoogendoorn ML, Kahn RS, et al. : Hyperhomocysteinemia, methylenetetrahydrofolate reductase 677TT genotype, and the risk for schizophrenia: a Dutch population based case-control study. American Journal of Medical Genetics.Part b: Neuropsychiatric Genetics 135:69–72, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Kempisty B, Mostowska A, Górska I, et al. : Association of 677C>T polymorphism of methylenetetrahydrofolate reductase (MTHFR) gene with bipolar disorder and schizophrenia. Neuroscience letters 400:267–271, 2006. 10.1016/j.neulet.2006.02.055 [DOI] [PubMed] [Google Scholar]
- 51.McGuffin P, Knight J, Breen G, et al. : Whole genome linkage scan of recurrent depressive disorder from the depression network study. Human molecular genetics 14:3337–3345, 2005. 10.1093/hmg/ddi363 [DOI] [PubMed] [Google Scholar]
- 52.Bjelland I, Tell GS, Vollset SE, et al. : Folate, vitamin B-12, homocysteine, and the MTHFR 677C -> T polymorphism in anxiety and depression—The Hordaland Homocysteine Study. Archives of General Psychiatry 60:618–626, 2003. 10.1001/archpsyc.60.6.618 [DOI] [PubMed] [Google Scholar]
- 53.Lewis SJ, Lawlor DA, Smith GD, et al. : The thermolabile variant of MTHFR is associated with depression in the British Women's Heart and Health Study and a meta-analysis. Molecular psychiatry 11:352–360, 2006. 10.1038/sj.mp.4001790 [DOI] [PubMed] [Google Scholar]
- 54.Wray NR, Ripke S, Mattheisen M, et al. : Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature genetics 50:668–681, 2018. 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schizophrenia Working Group of the Psychiatric Genomics Consortium: Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427, 2014. 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakker RC, Brandjes DP: Hyperhomocysteinaemia and associated disease. Pharmacy World & Science 19:126–132, 1997. 10.1023/a:1008634632501 [DOI] [PubMed] [Google Scholar]
- 57.Monji A, Yanagimoto K, Maekawa T, et al. : Plasma folate and homocysteine levels may be related to interictal "schizophrenia-like" psychosis in patients with epilepsy. Journal of clinical psychopharmacology 25:3–5, 2005. 10.1097/01.jcp.0000150225.76748.73 [DOI] [PubMed] [Google Scholar]
- 58.Muntjewerff JW, Kahn RS, Blom HJ, et al. : Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: a meta-analysis. Molecular psychiatry 11:143–149, 2006. 10.1038/sj.mp.4001746 [DOI] [PubMed] [Google Scholar]
- 59.Bodnar LM, Wisner KL: Nutrition and Depression: Implications for Improving Mental Health Among Childbearing-Aged Women. Biological psychiatry 58:679–685, 2005. 10.1016/j.biopsych.2005.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishio K, Goto Y, Kondo T, et al. : Serum folate and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism adjusted for folate intake. Journal of epidemiology 18:125–131, 2008. 10.2188/jea.je2007417 [DOI] [PMC free article] [PubMed] [Google Scholar]