Abstract
Primary cilia are immotile appendages that have evolved to receive and interpret a variety of different extracellular cues. Cilia play crucial roles in intercellular communication during development and defects in cilia affect multiple tissues accounting for a heterogeneous group of human diseases called ciliopathies. The Hedgehog (Hh) signaling pathway is one of these cues and displays a unique and symbiotic relationship with cilia. Not only does Hh signaling require cilia for its function but the majority of the Hh signaling machinery is physically located within the cilium-centrosome complex. More specifically, cilia are required for both repressing and activating Hh signaling by modifying bifunctional Gli transcription factors into repressors or activators. Defects in balancing, interpreting or establishing these repressor/activator gradients in Hh signaling either require cilia or phenocopy disruption of cilia. Here, we will summarize the current knowledge on how spatiotemporal control of the molecular machinery of the cilium allows for a tight control of basal repression and activation states of the Hh pathway. We will then discuss several paradigms on how cilia influence Hh pathway activity in tissue morphogenesis during development. Last, we will touch on how cilia and Hh signaling are being reactivated and repurposed during adult tissue regeneration. More specifically, we will focus on mesenchymal stem cells within the connective tissue and discuss the similarities and differences of how cilia and ciliary Hh signaling control the formation of fibrotic scar and adipose tissue during fatty fibrosis of several tissues.
Keywords: Cilia, hedgehog, morphogen, repressor, regeneration, fatty fibrosis
1. Introduction.
The primary cilium is a microtubule-based dynamic cellular appendage that extends from the mother centriole of the centrosome [1]. Primary cilia function as sensory organelles and are the prototype for compartmentalized subcellular signaling. Signaling mediated by cilia is an ancient phenomenon; for example, interactions between agglutinins on plus and minus gamete flagella during fertilization in the green algae Chlamydomonas stimulate a signaling pathway leading to cell-cell fusion [2]. This review focuses on hedgehog (Hh) signaling, the main pathway transduced by vertebrate cilia [3]. Other signaling pathways affected by cilia have been recently reviewed elsewhere [4].
Hh signaling regulates cell fate and proliferation in multiple developmental and regeneration paradigms. Ciliary defects, which account for a heterogeneous group of human diseases known as “ciliopathies”, present with a wide variety of clinical manifestations such as neural tube defects, brain malformations, polydactyly and bone deformities [5]. Many of these defects are due to disrupted Hh signaling, emphasizing the strict co-dependence of Hh signaling and cilia.
Here, we will first focus on our current understanding of the molecular mechanisms underlying Hh signaling by cilia. We next describe diseases resulting from dysregulation of Hh signaling, primarily in the context of ciliary involvement. We highlight broad principles underlying generation and interpretation of morphogenetic gradients that regulate phenotypic outcomes in various tissues. We then discuss the diversity and complexity of downstream regulation during development and regeneration of different tissues by Hh family morphogens (Sonic, Desert and Indian hedgehog, abbreviated as Shh/Dhh/Ihh). Understanding the role of ciliary Hh signaling in multiple developmental and adult tissue contexts is highly relevant for uncovering the mechanisms underlying the diverse clinical phenotypes in ciliopathies.
2. Primary cilium in Hh signaling.
Primary cilia are assembled by an active and conserved process called intraflagellar transport (IFT), consisting of trains of multipolypeptide particles moving along the axonemal microtubules by anterograde and retrograde motors [6]. Anterograde transport is mediated by kinesin-II [7], whereas retrograde transport is powered by the dynein 2 motor [8]. The IFT gene Ift88(Tg737) knockout was first shown to lack expression of FoxA2 (Hnf3β) in the floor plate of the neural tube [9], similar to lack of Shh [10]. Mutants in kinesin-II and multiple IFT genes, many of which were isolated in a forward genetic screen, were subsequently shown to lack ventral neural tube cell types and conversely rescue ventral expansion of neuroprogenitors from high Hh signaling [11]. These results demonstrated that Hh signaling in the mouse neural tube require cilia. Work from multiple laboratories have now provided a wealth of knowledge into how cilia organize Hh signaling.
In canonical Hh signaling, the transcriptional output of the Hh pathway is determined by the glioma-associated oncogene transcription factors (Gli) that function as transcriptional activators (GliA) and repressors (GliR) [12, 13] (Figure 1A). Both activator and repressor forms are generated from full-length Gli2/3 proteins and require cilia for their formation [3]. Locally activated protein kinase A (PKA) phosphorylates Gli2 and Gli3, which are partially proteolyzed to generate Gli repressors [14–17]. The cilia-localized class A orphan GPCR, Gpr161 functions as a critical regulator of PKA in Gli3R formation [18]. Hh binding to its receptor Patched (Ptch1) triggers removal of Ptch1 from cilia and promotes enrichment and activation of Smoothened (Smo), the pathway transducer, in cilia [19, 20] (Figure 1B). Ptch1 is a 12-pass transmembrane (TM) receptor, whereas Smo is a class F (frizzled) family GPCR. Smo activation promotes release of Gli proteins from their carrier protein, Suppressor of Fused (Sufu), generating Gli activators [21, 22]. The transcriptional factor Gli1, a pure activator [23], and Ptch1 are among the direct transcriptional targets of Hh signaling. However, these targets might not always reflect lack of repression (derepression) of Hh pathway in different tissue contexts, such as during craniofacial development (section 4.3.3) [24], limb patterning (section 4.4) [25], and endochondral bone development (section 4.2.1) [26].
Figure 1. Organization of Hh signaling by cilia.
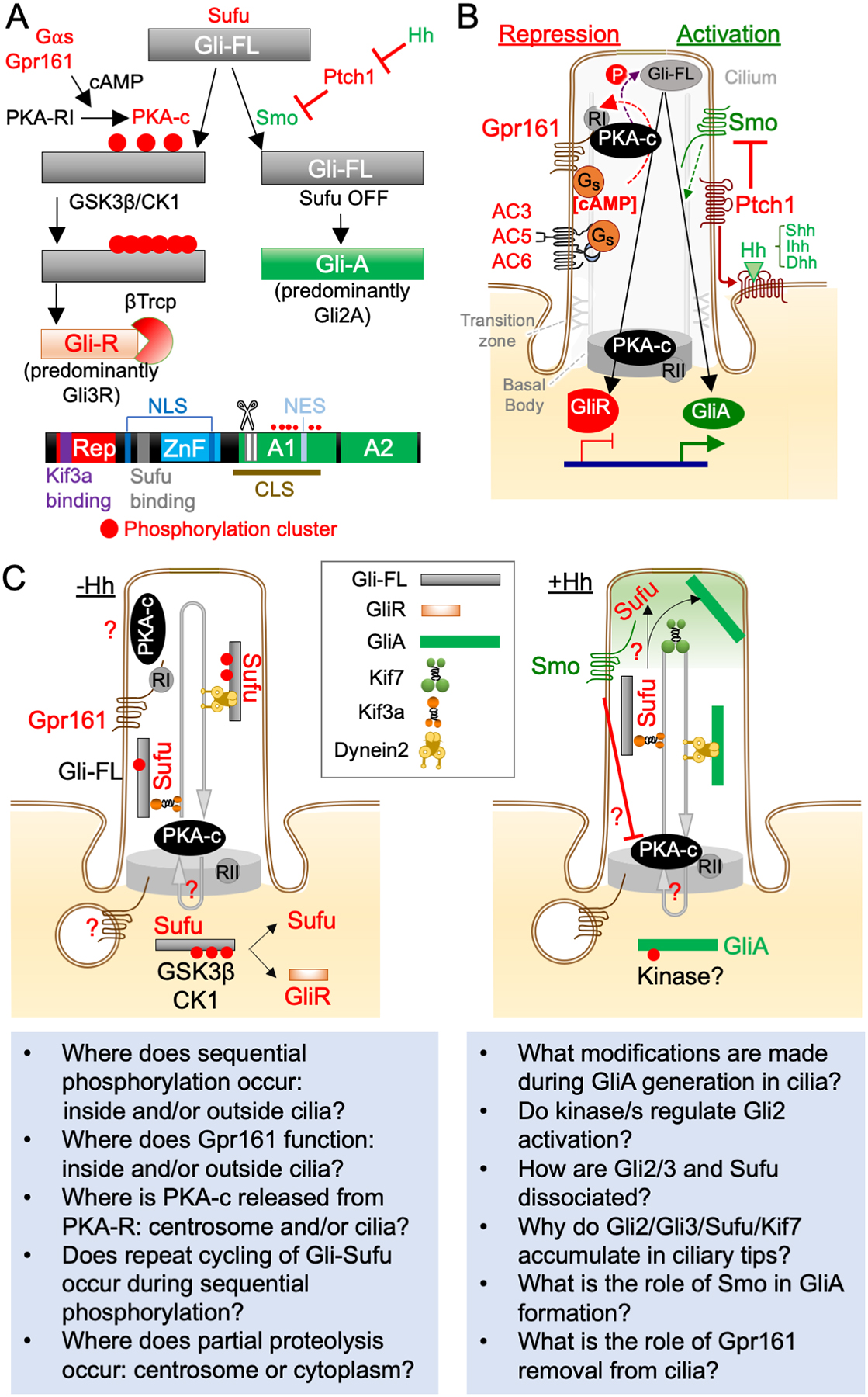
(A) Steps in generation of GliR and GliA. Phosphorylation of Gli-full length (Gli-FL) by PKA, followed by GSK3β/Casein kinase 1 (CK1) causes limited proteolysis after ubiquitination by E3 ligase βTRCP to form GliR. Smo activation by Shh binding to Ptch1 causes dissociation of Gli-FL from Sufu to form Gli-A. Both processes require cilia. Schematic showing Gli2/3 domains below. Abbreviations: Rep, repressor domain; ZnF Zinc Finger domains; NLS, nuclear localization. Signal; NES, nuclear export signal; CLS, cilia localization signal; A1/A2, transactivation domains.
(B) Repression and activation of Hh signaling at cilia. Regulation of GPCR-adenylyl cyclase-PKA and Smo signaling at cilia. RI, PKA-RI; RII, PKA-RII.
(C) Spatiotemporal control of steps in GliR and GliA formation. Unsolved questions are mentioned in text boxes below and discussed in section 2.3 (GliR formation) and 2.4 (GliA formation).
Although there are examples for cilium-independent and non-canonical modes of Hh signaling [27–29], here we only discuss organization of canonical Hh signaling by cilia. Role of cilia, Smo [30], and repression of Hh signaling [31] in the context of left-right asymmetry has been reviewed elsewhere [32].
2.1. Basal repression of Hh signaling.
In the absence of Hh, PKA-mediated phosphorylation of Gli2/3 [14–16] primes sequential phosphorylation events by CK1 and GSK3β [15] (Figure 1A). Phosphorylation results in Gli2/3 binding to the SCF-βTrcp ubiquitin ligase and subsequently partial proteolysis by the proteasome into GliR [33, 34]. Gpr161 regulates PKA activation in Gli3R formation via Gαs coupling and cAMP signaling [18]. Of the downstream factors that regulate cAMP signaling, at least three of the nine adenylyl cyclases Adcy3, 5, and 6 are localized to cilia [35–37] (Figure 1B). Overexpression of ADCY5 and 6 partially represses the Hh pathway in the developing chicken neural tube [38].
GliR formation requires cilia [25, 39] and involves multiple steps. Sufu restrains Gli3 in the cytoplasm and GliR formation requires Gli-Sufu complex [21, 40] (Figure 1A). However, cilia are not required for Hh pathway hyperactivation in Sufu knockouts [41, 42]. It is not clear how multiple steps in GliR formation are coordinated by Sufu and the cilium-centrosome complex. Gli2/3 proteins are likely trafficked by IFT in complex with Sufu (Figure 1C). The following evidence points to cycling of Gli2/3 through cilia. First, Gli2/3 can be enriched in cilia upon dynein-2 inhibition [43]. Second, Gli2/3 proteins also physically interact with kinesin-II subunit Kap3 and Kif3a through a N-terminal motif [44]. As cilia are required for GliR formation, it can be assumed that almost all of the Gli2/3 full length protein that forms repressor has to traverse the ciliary compartment.
Phosphorylation by PKA-c is one of the earliest steps in GliR formation (Figure 1A), but presently it is not clear where Gli phosphorylation occurs. During PKA activation, the catalytic subunit of PKA (PKA-c) is released from its regulatory subunits (PKA-Rs) upon cAMP binding to the PKA-Rs. Upon release from PKA-R, PKA-c is associated with membranes via myristoylation to preferentially phosphorylate membrane substrates [45]. A-kinase anchoring proteins (AKAPs) recruit the PKA-R subunits to distinct subcellular locations [46]. Based on immunofluorescence, PKA-c localizes to the centrosome [17, 47, 48], but cannot be detected in cilia [17] (Figure 1C). Pericentrin is an AKAP that anchors PKA-RII subunits to the centrosome [49]. Gpr161, despite being a GPCR, is a recently described AKAP for targeting PKA-RIα/β to the cilia [50]. Thus, cAMP could be binding to pericentrosomal PKA-RII to release PKA-c or PKA-RI bound to the Gpr161 C-tail [50] and releasing PKA-c in close vicinity. A ciliary-targeted PKA inhibitory peptide (PKI) reduces Gli3R levels [35], suggesting a role for inhibiting ciliary PKA release in GliR formation. It is, therefore, most likely that at least some of the Gli phosphorylation by PKA occurs during Gli-Sufu transit through the cilium-centrosome complex (Figure 1C).
PKA-mediated phosphorylation of Gli2/3 primes sequential phosphorylation events by CK1 and GSK3β [15] at six phosphorylation clusters in the C-terminal halves [16]. As CK1 and GSK3β are soluble proteins and are not enriched in cilia, the phosphorylation by these kinases possibly occurs both inside cilia and in periciliary cytoplasm (Figure 1C). It is not clear if partially phosphorylated Gli-Sufu complexes go back to the cilia for further phosphorylation cycles (Figure 1C). Once phosphorylated, and still in complex with Sufu [21], the Gli2/3 proteins undergo limited proteolysis by the SCF ubiquitin ligase complex (Skp1-Cullin-F-box) containing the E3 ligase and F box protein βTrcp [15]. βTrcp directly binds to the phosphorylated degrons overlapping the first four phosphorylation clusters [15]. Such proteolysis can possibly happen in both centrosome and/or cytoplasm for the following reasons (Figure 1C) for the following reasons. First, components of SCF complex, such as Skp1 and Cul1 localize to centrosomes [51], although βTrcp has not been reported to localize to centrosomes. Second, active proteasomal components are enriched in centrosomes [52, 53].
Gli2/3R are found dissociated from Sufu [21]. Multiple lines of evidence suggest that partial proteolysis and dissociation from Sufu likely occur in the cytoplasm (Figure 1C). First, Sufu promotes the synthesis but does not affect the degradation of Gli3R [21, 54]. Second, Gli2/3 are not accumulated in ciliary tips without Hh pathway activation. Third, Gli3R tagged with GFP does not localize to ciliary tips [55].
2.2. Activation of the canonical Hh pathway in the primary cilium.
The exact role of Ptch1 in inactivating Smo from being activated in cilia is not fully understood. Ptch1 inhibits Smo sub-stoichiometrically, thus it indirectly blocks Smo activity [56]. Recently solved structures of Ptch1 bound or unbound to Shh [57–60] and Smo bound or unbound to cholesterol derivatives [61–63] reveal important insights into functions of Ptch1 and activation of Smo in cilia. The most parsimonious model from these results is that Shh-mediated activation and removal of Ptch1 from cilia increases endogenous ligands for Smo in the ciliary membrane, thereby activating Smo in cilia [64]. The endogenous ligands for Smo are most likely to be cholesterol or cholesterol derivatives [63, 65]. A defined fraction of membrane cholesterol, termed accessible cholesterol, which is increased upon lack of cholesterol sequestration from sphingomyelin depletion potentiates Hh signaling [66]. Smo activation can also be potentiated by PKA catalytic subunit (PKA-c) in centrosome that promotes ciliary translocation of Smo via phosphorylation of Inversin [67], a protein localized in the proximal intraciliary compartment distal to the transition zone [68, 69]. Smo can also be phosphorylated by Casein kinase γ, which localizes to cilia and regulate high pathway activity [70].
The intermediate steps between Smo activation, GliA formation, and Gli2 translocation to the nucleus are not well understood. Downstream effectors for mediating Smo-dependent Gli2 activation are varied, including Gαi proteins [71–73] and other proteins such as the integrin-linked kinase (ILK) [74], and the Evc-Evc2 complex [75, 76]. Efcab7-Iqce module anchors the Evc-Evc2 complex in a signaling microdomain at the base of cilia, and is important in mediating downstream signals for Smo-dependent Gli2 activation [75]. Loss of Ift25 and Ift27, IFT-B subunit that are not required for ciliogenesis, but are linked with BBSome, results in the accumulation of both Smo and Ptch1 in cilia [77–79], suggesting their role is in trafficking these subunits out of cilia. Gli2/3 full-length proteins that are free from Sufu translocate to the nucleus, where they are also targeted for proteasomal degradation via the cullin3-based ubiquitin E3 ligase, in coordination with the substrate-binding adaptor Spop [42, 54, 80].
In the absence of cilia, Gli2/3 full length proteins accumulate as GliR processing is inhibited [21, 24]; however, GliA is not formed, suggesting differences between full length proteins and GliA. Due to the labile nature of GliA, the nature of post-translational modifications and processes that generate GliA has been enigmatic. Two lines of evidence suggest that GliA is a full-length form of Gli2/3 that is not phosphorylated at the C-terminus [16], but probably has another modification in this region, which occurs in a cilia-dependent manner [81]. First, overexpressing a Gli2 variant with non-phosphorylatable alanine mutants of all six PKA sites highly activates Shh pathway in cultured cells and chicken spinal cords [16]. Second, a non-ciliary Gli2 variant that has a deletion from 570–967 aa in Gli2 overlapping all six PKA sites [81] (Figure 1A) fails to localize to the cilium but retains intrinsic transcriptional activity and responds to Sufu inhibition [81]. Knock-in of this variant phenocopies Gli2 knockout embryos. Therefore, it is likely that even a PKA-insensitive Gli2 variant requires ciliary localization to be activated. It is unclear if phosphorylation or any other modification either in this region or allosterically regulated in some other region constitutes the cilia-mediated activation [82]. Gli2 can also be phosphorylated in N-terminus residues by Fused family kinases such as Stk36 and Ulk3, which could regulate Gli2 activity [83], but the subcellular location for these modifications is unknown (Figure 1C).
Gli2/3 proteins accumulate in ciliary tips upon pathway activation [55] suggesting that dissociation from Sufu [21, 22] and activator formation are regulated in the vicinity of cilia. What are the mechanisms causing Sufu-Gli dissociation? Gli-Sufu binding involves a conserved sequence upstream of the C2H2 Zinc finger [84, 85] and also other regions [86] in Gli2/3 (Figure 1A). Release of binding is regulated by a central intrinsically disordered (IDR) sequence in Sufu [84]. Sufu is phosphorylated in the IDR by PKA and GSK3β [87]. IDR phosphorylation stabilizes Sufu and prevents against Hh-induced Sufu degradation [87]. Lack of PKA also cause Gli2 activation [17]. Adenylyl cyclase activation by forskolin treatment can cause persistent Sufu-Gli interactions even upon Smo activation [21]. Whether lack of Gli2/3 or Sufu phosphorylation from inactivation of PKA regulate Gli-Sufu interactions is not known.
Why are Gli/Sufu accumulated in ciliary tips? Gli2, Gli3 and Sufu associate with ciliary tips [55] during activation of the pathway [42, 43]. As Gli-Sufu complexes are on IFT-trains, we speculate that during turnaround and in the absence of Gli phosphorylation, release of Gli2/3 from Sufu might prevent efficient access to the retrograde IFT trains, resulting in accumulation in the tip (Figure 1C). Another microtubule-associated atypical kinesin Kif7 is enriched in ciliary tips [88, 89]. Kif7 functions in both positive and negative regulation of Hh signaling by regulating ciliary architecture [88, 89]. Multiple ciliary tip-like compartments form in the absence of Kif7 [88], and Kif7 not only recognizes but also stabilizes a GTP-form of tubulin to promote its own microtubule-end localization [90] (Figure 1C). Liprin-α1 (PPFIA1) and the protein phosphatase PP2A interact with Kif7 and are important for trafficking of Kif7 and Gli proteins to ciliary tips and transcriptional output of Hh signaling. PPFIA1 also functions with PP2A to promote the dephosphorylation of Kif7, triggering Kif7 localization to the tips of primary cilia and promoting Gli transcriptional activity [91].
What is the role of Smo activation in Sufu-Gli dissociation? Smo activation in cilia is linked to Sufu-Gli dissociation [21, 22]. Lack of repression in PKA null cells also cause pathway activation and Gli2 accumulation in ciliary tips irrespective of Smo activation [17]. Thus the role of Smo in GliA formation could actually involve lack of PKA activity and lack of Gli2/3 phosphorylation that could cause Gli-Sufu dissociation. Gpr161 is also removed from the primary cilia in a Smo- and β-arrestin-dependent manner following pathway activation [92, 93]. Gpr161 removal from cilia would reduce ciliary cAMP signaling [92], as would Smo activation of Gαi [71–73] (Figure 1C). Depletion of the 5’phosphatase, Inpp5e causes accumulation of phosphatidylinositol 4,5-bisphosphate (PIP2) in cilia resulting in increased steady-state levels of Gpr161 [94, 95], irrespective of accumulation of the pathway activator Smo [96]. Such accumulation of Gpr161 prevents full pathway activity in cultured cells [94, 95]. However, the role of Inpp5e in neural tube patterning is complex, with both positive and negative regulatory roles through regulation of the relative timing of GliA and GliR production [97].
3. Dysregulation of cilia regulated Hh pathway in disease.
Recently, bi-allelic loss-of-function variations in SMO in humans have been shown to cause wide phenotypic spectrum of developmental anomalies affecting the brain (hypothalamic hamartoma and microcephaly), heart (atrioventricular septal defect) and skeleton (shortening of long bones) [98]. Activating somatic missense mutations in SMO are found in sporadic basal cell carcinoma and Shh-subtype medulloblastoma [99]. Ptch1 knockout results in high Hh signaling from activation of the Smo-dependent arm of the pathway during neural tube development and cerebellar granule cell proliferation that causes Shh-subtype medulloblastoma [30, 100, 101]. Lack of Ptch1 also causes defects in skeletal morphogenesis [102], limb formation and patterning [103]. Other coreceptors for Hh ligands include Gas1, Cdo and Boc [104, 105], but whether they localize to cilia is not known. These co-receptors are required for cerebellar granule cell progenitor proliferation [104] and ventral neural tube patterning [105], unlike Ptch1 that represses these processes by preventing Smo activation [30, 100, 101].
Diseases from derepression of the pathway are still being unraveled as our knowledge on regulators of repression have been limited. In most tissues, lack of cilia prevents derepression (except in tissues that require GliR formation by cilia for morphogenesis, such as nasopharyngeal processes [24] and limb buds before Shh expression [106, 107]); thus, intact cilia are required for manifestation of phenotypes from derepression. Importantly, lack of basal suppression in Gpr161, PKA, and Sufu mutants causes high Hh signaling during mouse neural tube development [17, 18, 108], similar to Ptch1 knockout that results in activation of Smo [100], but with varying degrees of severity [109]. Formation of dorsal hinge point, a zone of curvature in the dorsolateral neural tube is dependent on low Shh signaling [110]. Thus, multiple mouse models that show high Shh signaling, including mutants of Ptch1 [100], PKA [17], Sufu [108], Gpr161 [111] and Tulp3 [112, 113], present with neural tube closure defects and/or spina bifida [114].
Apart from affecting neural tube development, premature and hyperactive Shh signaling from deletion of Gpr161 in limb mesenchymal cells causes defects in limb and skeletal morphogenesis [26], whereas neural stem cell-specific deletion causes cerebellar granule cell hyperproliferation and Shh-subtype medulloblastoma [115]. Manifestation of these phenotypes require cilia [26, 115]. Forebrain abnormalities from deletion of Gpr161 in neural stem cells include ventriculomegaly-induced hydrocephalus, polymicrogyria and periventricular nodular heterotopia [116]. While polymicrogyria has been reported also with a constitutively active Smo mutant [117], periventricular heterotopia (heterotopic cell clusters adjacent to the lateral ventricle) in human patients has not been associated with dysregulation of the Hh pathway before [118]. Active repression of the Hh pathway is highly relevant to human disease. GPR161 mutations are prevalent in human spina bifida patients [119]. Germ line mutations in SUFU [120] and GPR161 [121] has been linked with predisposition to Shh-subtype medulloblastoma in human patients at rates similar to PTCH1 loss in Gorlin syndrome [121]. Thus, active suppression of Hh pathway is as important as the activation arm of the pathway.
In contrast to Gpr161 knockout tissues in mice [18, 26, 115, 116, 119] and zebrafish [122], genetic manipulation of Gpr161 in Hh-responsive NIH 3T3 cells did not cause increased basal transcription of pathway targets or lack of Gli3R formation [123], pointing to a difference between in vitro and in vivo models for studying basal repression of Shh signaling. A small amount of Gli3R is retained in embryos that lack both PKA catalytic subunits α/β (PKA null) suggesting there are additional PKA-independent mechanisms for Gli3R formation [17], a process that is probably more important in NIH 3T3 cells than in vivo models. Other parameters such as Gli2 accumulation at ciliary tips could be used to correlate with the in vivo results. Nonetheless, such differences emphasize the requirement for studying derepression of Hh signaling in relevant tissue contexts in vivo.
4. Developmental paradigms of ciliary and Hh signaling.
4.1. Hh signaling in differentiation and patterning.
A gradient of Shh secreted from the notochord and floor plate patterns the ventral neural tube in vertebrates during early embryogenesis. The dorso-ventral patterning of the ciliated neuroprogenitors in the neural tube provides a sensitive readout of Hh pathway activity. The Shh gradient promotes floor plate progenitors expressing forkhead box A2 (FoxA2), p3 progenitors expressing NK2 homeobox 2 (Nkx2.2), pMN progenitors expressing oligodendrocyte transcription factor 2 (Olig2), and p3/pMN/p2 progenitors expressing NK6 homeobox 1 (Nkx6.1), while inhibiting specification of lateral and dorsal neural cell types expressing Pax6 and Pax7 [124] (Figure 2A). Absence of Shh results in loss of ventral neural cell fates [10]. Mutants that affect the IFT machinery, including the IFT-B complex and IFT motors, exhibit loss of the ventral cell types that are specified by high levels of Shh [11, 25]. Conversely, increased Shh signaling from loss of Ptch1 [100] or both PKA catalytic subunits α/β (PKA null) [17] causes ectopic specification of ventral cell types at the expense of dorsolateral cell types. Lack of PKA activation from loss of Gpr161 [18], Gαs [125], and factors that traffic Gpr161 to cilia including the tubby family protein Tulp3 [112] and IFT-A complex proteins [126–128] also cause ventral expansion, but with differing levels of severity [109]. Sufu knockout embryos show less severe ventralization than Ptch1 knockout or PKA null embryos [81, 108]. Lack of the cilia localized atypical GTPase, Arl13b, results in both ventral and dorsal expansion of intermediate Shh-dependent cell fates [129]. However, such regulation does not require ciliary pools of Arl13b [130], suggesting Arl13b functions outside cilia in regulation of intermediate cell fates. Regulation of neutral tube patterning by Gli2A and Gli3R is described in Section 4.3.
Figure 2. Hh signaling paradigms during development.
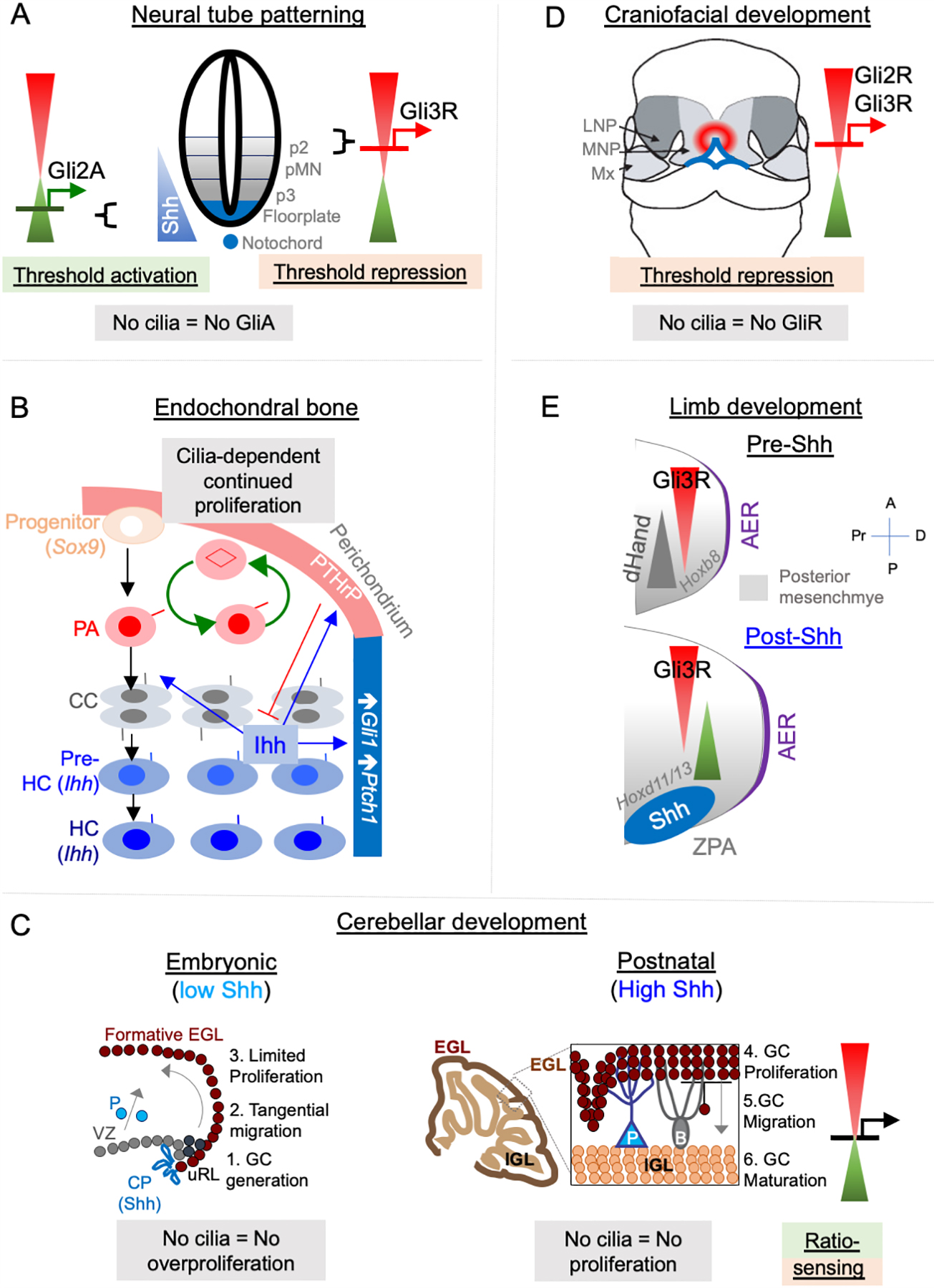
(A) Neural tube patterning. Shh is expressed from the notochord (blue). Gli2A-mediated threshold activation mediates floorplate and ventral most progenitor patterning. Lack of cilia prevents patterning of all ventral progenitors. Gli3R regulates intermediate-level patterning (not shown).
(B) Endochondral bone development. Chondrogenic progenitors differentiate into periarticular chondrocytes (PA), which further differentiate into columnar chondrocytes (CC), followed by forming prehypertrophic chondrocytes (Pre-HC) and hypertrophic chondrocytes (HC), both of which secrete Ihh. All these chondrocytes are ciliated. Ihh increases Gli1 and Ptch1 levels in adjacent perichondrium. Ihh also results in production of parathyroid hormone-like peptide (PTHrP) in periarticular cartilage, which prevents differentiation of CC to pre-HC in a negative-feedback loop. Lack of Gpr161 causes persistent slow proliferation of PA and prevents differentiation of PA into CC. Cilia disruption prevents continued proliferation from lack of Gpr161.
(C) Cerebellar development.
Left, Embryonic development (E15-E18). At this stage, Shh is expressed by choroid plexus (CP), which causes activation in adjacent ventricular zone (VZ). Purkinje neurons (P) are still translocating. Granule cell (GC) progenitors are generated in upper Rhombic lip (uRL), and tangentially migrate to the formative external granule layer (EGL). Proliferation in formative EGL is not affected by loss of cilia (unless overproliferation occurs from derepression arising from Gpr161 loss), and proceeds in the absence of Shh secretion by Purkinje neurons.
Right, Postnatal development (P0-P14). At this stage, Shh secreted by Purkinje neurons (P) causes proliferation of GC progenitors in EGL. Proliferation at this stage requires cilia. After GCs stop proliferating, they migrate radially on Bergmann glia (B) to form the internal granule layer (IGL). Proliferation at this stage can be affected by both lack of GliA and expression of GliR, suggesting sensing of GliA to GliR ratio by the GC progenitors.
(D) Craniofacial development. Threshold repression by both Gli2R and Gli3R prevents midfacial widening. Lack of cilia, or lack of both Gli2/3 causes mid facial widening, which is prevented by forced Gli3R expression. LNP, latera nasal process; MNP, medial nasal process, Mx, maxillary process.
(E) Limb development.
Pre-Shh stage, top. (E9.25-E9.75). Gli3R gradient is set up by posterior dHand gradient that is established in posterior mesenchyme.
Post-Shh stage, bottom. (E9.75 onwards). Shh expression from ZPA establishes posterior gradient of pathway targets such as Ptch1/Gli1. Anterior Gli3R gradient also regulates expression of genes in posterior mesenchyme such as Hoxd11/13. Lack of cilia causes decreased expression of Shh pathway targets but can cause preaxial polydactyly from increased 5’Hoxd gene expression arising from lack of Gli3R. Abbreviations: AER, anterior ectodermal ridge; ZPA, zone of polarizing activity; A, anterior; P, posterior, Pr, proximal; D, distal.
4.2. Hh signaling in proliferation.
4.2.1. Endochondral bone formation.
Endochondral bones are formed from an intermediate cartilaginous template that develop in the limb bud. During endochondral bone formation, periarticular/round chondrocytes mature into columnar chondrocytes. Columnar chondrocytes further differentiate into prehypertrophic and hypertrophic chondrocytes that express and secrete Ihh [131]. Ihh increases Gli1/Ptch1 levels in proliferating chondrocytes and in adjacent perichondrium. Ihh also results in production of Parathyroid hormone-related protein (PTHrP) in periarticular cartilage that prevents differentiation of columnar to prehypertrophic chondrocytes in a negative feedback loop [132, 133] (Figure 2B). Mesenchymal cells of the limb bud, perichondrial cells, chondrocytes, osteoblasts and osteocytes are ciliated. Conditional knockouts of the IFT-B complex protein lft88 that disrupt cilia results in a smaller growth plate during endochondral bone formation, without affecting ossification [134, 135]. Interestingly, sustained proliferation and accumulation of periarticular/round chondrocytes in forelimb long bones is seen from conditional deletion of Gpr161 in forelimb mesenchyme (Prx1-Cre). Persistent periarticular/round chondrocyte proliferation also prevents differentiation and Ihh signaling from later-stage chondrocytes, causing severe lack of bone ossification in the Gpr161 conditional knockout [26]. Hypertrophic chondrocytes are also reduced in Col2a1-cre; Ptch1f/f mutants [102], but bone collar is mineralized unlike Prx1-Cre; Gpr161f/f conditional mutants. Cre-mediated recombination occurs in all chondrocytes in Col2a1Cre but could be inefficient in perichondrium causing these differences. Lack of ossification in Gpr161 conditional knockout is suppressed in the absence of cilia, indicating that the chondrocyte proliferation step is likely to be cilia-dependent [26]. Consistent with a lack of Ihh secreting hypertrophic chondrocytes, Hh signaling targets such as Gli1 and Ptch1 are not upregulated in the periarticular chondrocytes upon conditional deletion of Gpr161. Periarticular chondrocytes possess cilia embedded in the ciliary pocket and are surrounded by the cartilaginous extracellular matrix, raising the possibility that certain unknown, possibly mechanosensory stimuli [136], might regulate chondrocyte proliferation [137, 138] that is actively prevented by Hh pathway repression.
4.2.2. Cerebellum.
Granule cell (GC) progenitors arise from atonal homolog 1 (Atoh1) expressing cells in the upper rhombic lip (uRL) of the embryonic cerebellum (cerebellar anlage) starting from E13 [139, 140]. Shh is secreted by the Purkinje neurons, starting only from E18.5, and serves as a critical mitogen in the postnatal growth spurt of GC progenitors [141–143]. Thus, generation of the formative EGL between E13-E18.5 occurs in the absence of Shh production by Purkinje neurons. Shh signaling upregulates CyclinD1 and N-Myc levels that promote proliferation in the GCs [144, 145]. The GC progenitors proliferate multiple times postnatally in a Shh-dependent manner, before exiting the cell cycle. The post-mitotic GCs extend axons chronologically forming the molecular layer (ML), and migrate radially along the Bergmann glia into their final location for maturation in the inner granule layer (IGL) [146] (Figure 2C). Shh-subtype medulloblastoma result from abnormal expansion of GC progenitors [147, 148]. Shh-subtype medulloblastoma can be initiated in GC progenitors or neural stem cells, but tumorigenesis is associated with commitment to the GC lineage [147, 148]. Thus, pathogenesis of Shh-subtype medulloblastoma can be best understood in the context of normal development of GCs.
Of these stages in GC life cycle, GC progenitors are ciliated and Shh-mediated proliferation of GC progenitors during postnatal development requires primary cilia [149, 150]. Atoh1 also controls the presence of cilia, which maintains responsiveness of GCs to Shh [151]. Atoh1 promotes ciliogenesis by transcriptionally regulating Cep131, a centriolar satellite protein [152]. Shh signaling also prevents Atoh1 from degradation by the E3 ubiquitin ligase Huwe1 [153]. In contrast to postnatal development, baseline proliferation of GC progenitors in the formative EGL during embryogenesis in the low Shh environment does not require cilia [149] (Figure 2C). Nestin-Cre expressing Gpr161 conditional knockout show thickening and increased proliferation in the formative EGL by E15.5, along with increased Gli1 and Ptch1 transcripts and CyclinD1 levels and reduced Gli3R levels. Similar results are seen upon early embryonic deletion of Sufu [154]. Thus, premature high Hh signaling contributes to increased GC proliferation during embryogenesis. Unlike baseline GC proliferation, such overproliferation during embryogenesis is cilia dependent. Premature high Hh signaling from Gpr161 deletion in neural stem cells in Nestin-Cre; Gpr161f/f animals also causes higher preponderance of Shh-subtype medulloblastoma compared to conditional knockout in committed GC lineages [115].
4.3. Balance between activation and repression at the heart of Hh signaling.
Gli2 is the predominant activator, whereas Gli3 is the predominant repressor [155, 156]. Gli3 can also function as an activator to partially rescue Gli2 knockout floor plate and p3 progenitor defects [157] and ventralization in Sufu knockout [158]. Conversely, Gli2 repressor has been proposed to have a role in craniofacial development [24]. However, depending on the tissues, Gli activators or repressors could be the predominant drivers of transcriptional responses during morphogenesis. Gli family members bind to the same consensus DNA sequences. However, regulation of cis-regulatory modules of targets, although having the same Gli binding sites are complex, context-dependent and regulatable by co-activators and repressors [159]. As both activation and repression are regulated by cilia, we discuss how Gli activator and/or repressor are involved in sculpting different tissues. We describe three distinct modules that are regulated by ratio sensing between activator and repressor levels or by detecting thresholds of primarily activator or repressor levels, as originally proposed by the Vokes lab [160].
4.3.1. Ratio sensing model.
In a ratio sensing model, the relative levels of activator vs repressor matter. Thus, if a phenotype is caused by a decrease in repressor and increase of activator, reciprocal changes in either would rescue the respective phenotype. In this case, activator and repressor likely regulate Gli binding sites reciprocally. For example, increased thickness of external granule layer (EGL) in postnatal cerebellum upon conditional knockout of Sufu in mouse granule progenitors (using Math1-Cre) is rescued by Gli2 deletion or introduction of Gli3Δ699 allele [161] that expresses a less potent form of Gli3R [162, 163]. Similarly, lack of cilia prevents SmoM2-induced medulloblastoma formation suggesting a role in Gli2-mediated activation. However, lack of cilia or Gli3 heterozygote background also promotes medulloblastoma formation upon expression of an active non-repressible form of Gli2, suggesting that the cilium-generated Gli3R restricts tumor progression [164] (Figure 2C). Similar results are observed in pathogenesis of basal cell carcinoma in skin [165].
4.3.2. Threshold activator model.
In a threshold activator model, the relative levels of activator matter. Thus, if a phenotype is associated with an increase in activator and decrease in repressor, decrease in activator levels rescue most of the phenotype. For example, Gli3 repressor is reduced in PKA null embryos and Gli2 localizes to ciliary tips without Shh pathway activation in PKA null MEFs suggesting Gli2 activator formation. However, expansion of markers of the floor plate (FoxA2) and V3 interneuron progenitors (Nkx2.2) in the neural tube in PKA deficient mutants is rescued by loss of Gli2 but not Gli3. This scenario suggests a predominant role of Gli2 activator in high level ventralization of these progenitors [17] (Figure 2A). Similarly, expansion of FoxA2 and Nkx2.1 in Ptch1 knockout in thoracic spinal cord is reverted back to wild type levels upon concomitant loss of Gli2 [81], although transcriptional outputs are partially reduced [81, 166]. Another example is provided by the Sufu knockout, where expansion of FoxA2 and Nkx2.1 is Gli2-dependent [158], whereas overexpression of Gli3R using homozygosed Gli3Δ699 allele only partially reverses ventralization [54]. Dosage of Gli2 is also critical in pathogenesis of mouse models of Shh-subtype medulloblastoma from loss of Sufu. Early embryonic deletion of Sufu in mouse cerebellum did not exhibit tumorigenesis [154], while heterozygotes developed Shh-subtype medulloblastomas only in a p53 null background [167]. However, lack of Spop increases Gli2 levels that is required for Shh-subtype medulloblastoma formation from Sufu deletion [168].
4.3.3. Threshold repressor model.
In a threshold repressor model, the relative levels of repressor matter. Thus, if a phenotype is associated with an increase in full length Gli2/3 proteins and decrease in repressor, only increase in repressor levels would rescue the phenotype. Loss of cilia in mid facial tissues causes widening of nasopharangeal processes and duplicated nasal septum [24, 169]. Deletion of both Gli2 and Gli3 phenocopies these phenotypes, which are partially restored from expression of Gli3Δ699 [24], suggesting that ciliary signaling regulate Gli2R and Gli3R formation that is important in morphogenesis of this tissue (Figure 2D). Another example is provided by role for Gli3R in patterning of the intermediate region of the spinal cord that complements the requirement for Gli2 in ventral regions [170] (Figure 2A). Here, the Gli3 knockout causes ventral expansion of progenitors, which is restored by introduction of the homozygosed Gli3Δ699 allele [170].
4.4. Repressor gradient established by Hh morphogen-independent mechanisms.
Gli3 is expressed anteriorly in forelimb bud even prior to Shh expression. Shh expression starts in the posterior forelimb bud (zone of polarizing activity, ZPA) starting from E9.75 [107] and continues until E12 in regulating limb bud patterning [171]. Prior to Shh expression in the posterior forelimb bud, mutual antagonism between Gli3R and the bHLH transcription factor, dHand prepatterns the forelimb mesenchyme in causing posterior expression of bona fide Hh pathway targets and of 5’ Hoxd genes such as Hoxd13 [106, 107] (Figure 2E). Lack of Gli3 also rescues Shh knockout limb patterning defects in the autopod, suggesting that Gli3R-mediated repression is key in phenotypes arising from lack of Shh [172].
There is an increase in premature Hh pathway activity (as apparent from Ptch1/Gli1 RNA in situs before Shh expression) in the conditional knockout of Ptch1 [103] and Gpr161 [26] in limb mesenchyme. In case of Gpr161 knockout, consistent with a lack of Gli3R protein activity, Hoxd13 is also expanded throughout the forelimb buds. Both Gpr161 knockout and Ptch1 conditional knockouts show complete lack or stunting of forelimb buds, respectively, in addition to patterning defects, but the role of Hh signaling in limb formation has remained unexplored.
A hypomorphic Ift88 mutant that causes short cilia causes premature expansion of dHand before Shh expression suggesting compromised Gli3R formation. This mutant also shows reduced Gli1/Ptch1 expression in the post-Shh stage suggesting lack of Gli activator formation. The limbs have preaxial polydactyly but no ectopic Shh expression, suggesting that in this case the polydactyly might be related to lack of Gli3R function [25]. The preaxial polydactyly is also associated with increased anterior expansion of Hoxd11 and Hoxd13. A null knockout of Ift88 also shows low Ptch1/Gli1 levels in forelimb buds [55] despite showing preaxial polydactyly [173], but expression of 5’Hoxd genes were not checked.
5. Ciliary signaling in regeneration
Now that we outlined how important cilia and ciliary Hh signaling are for development, we will focus on how cilia are being used to maintain and repair different tissues in the adult with a focus on skeletal and cardiac muscle as well as white adipose tissue. For each tissue, we will summarize and discuss which cell types carry a cilium and the proposed role cilia may play. We will specifically discuss the role for cilia and ciliary signaling in connective tissue fibroblasts, which display a cilium in virtually every adult tissue [174–176].
Connective tissue fibroblasts are present in the stroma of every adult tissue (Figure 3A–C). They are often called mesenchymal stem cells as they can differentiate into adipocytes, osteoblast or chondroblasts in vitro. Fibroblasts build and maintain the extracellular matrix (ECM), which serves as the scaffold for many adult tissues. In addition, fibroblasts are crucial during the repair of damaged tissues by secreting a multitude of beneficial factors. In chronic diseases, however, fibroblasts get chronically activated leading to their uncontrolled expansion, excessive ECM production and ultimately fibrotic scar formation (Figure 3D) [177]. Fibroblasts are also the cellular origin of pathological fat which replaces skeletal and cardiac muscle tissue ((Figure 3D) [175, 178]. Several pro-fibrotic and anti-adipogenic signaling pathways have been discovered to be pathologically activated during fibrosis such as transforming growth factor-β (TGFβ), platelet-derived growth factor (PDGF), WNT and Hh signaling [177]. Interestingly, all of these pro-fibrotic pathways have been described to require the primary cilium for its function [4]. Given the fact that most fibroblasts possess a cilium, independent of the tissue, highlights the cilium as a pivotal key player in balancing normal tissue homeostasis vs. replacement of healthy tissue with fatty fibrosis.
Figure 3. Ciliary control of fibroblast function during fatty fibrosis.
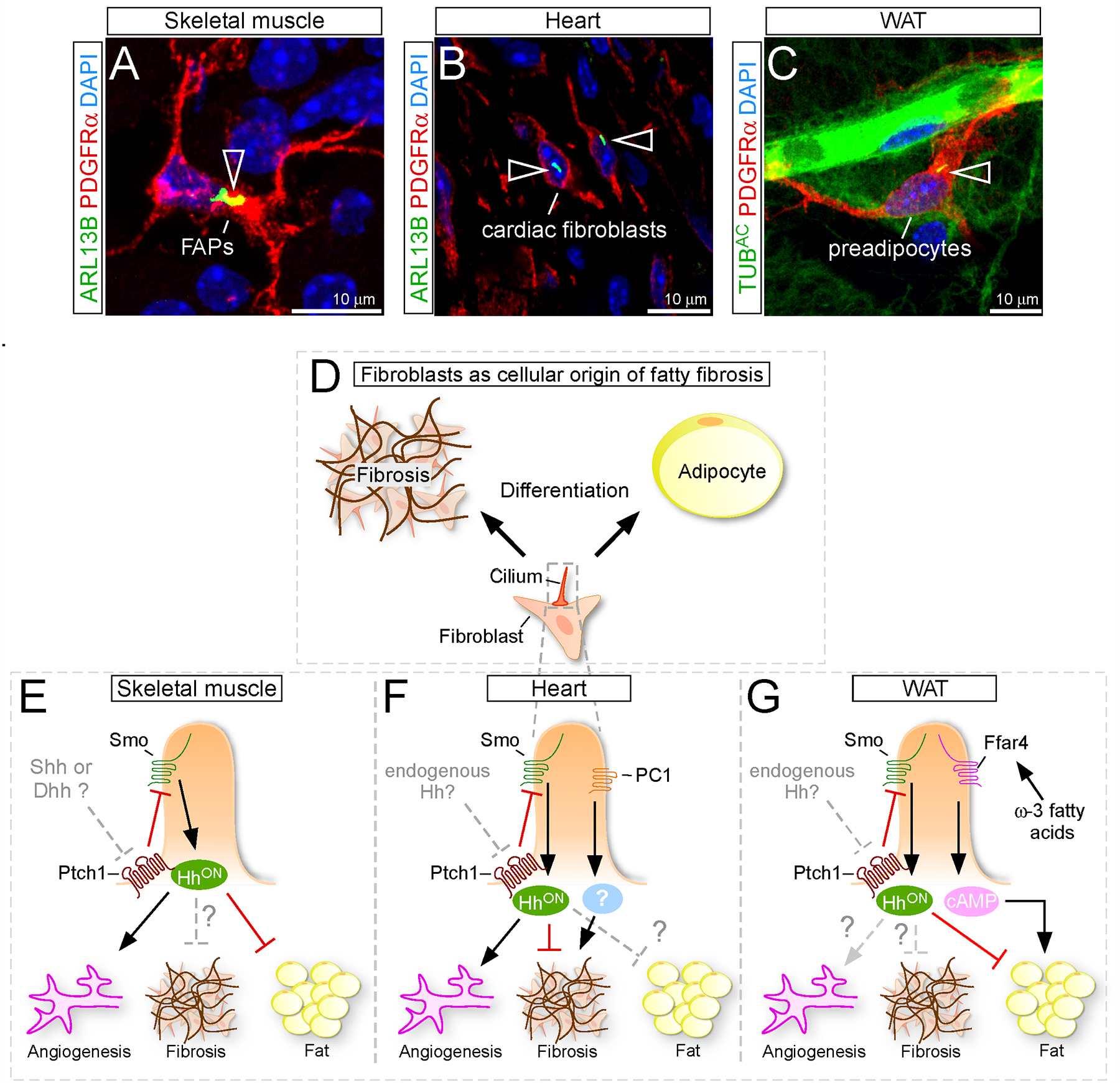
(A-C) Fibroblasts expressing the marker PDGFRα (red) are frequently ciliated (green; arrowhead) in different tissues such as skeletal muscle (A), heart (B) and white adipose tissue (WAT) (C). Nuclei in blue (DAPI).
(D) Fibroblasts are the cellular source of fibrotic scar and fat tissue.
(E) Ciliary Hh signaling has a pro-angiogenic and anti-adipogenic function in skeletal muscle fibroblasts. The role of Hh during fibrosis as well as which endogenous Hh ligand is being used, however, is still unclear.
(F) Hh signaling in cardiac fibroblasts (CFs) blocks fibrosis and promotes angiogenesis during ischemic injuries. It remains to be determined if Hh has an endogenous role and if Hh could also affect fat infiltration. CF cilia also utilize polycystin1 (PC1) to control fibrosis, however the exact mechanism still needs to be determined.
(G) Cilia, present on the preadipocytes in white adipose tissue (WAT), balance adipogenesis by sensing the anti-adipogenic Hh and the pro-adipogenic ω−3 fatty acid signal. If Hh has an endogenous role in WAT, however, to control angiogenesis and/or fibrosis, is unknown.
5.1. Ciliary signaling in skeletal muscle regeneration
Adult skeletal muscle has a remarkable ability to regenerate due to a dedicated muscle stem cell population (MuSCs). Upon injury, MuSCs, also called satellite cells, expand before differentiating into myoblasts and fusing to form new myofibers [179]. Skeletal muscle contains a second type of stem cell, called fibro/adipogenic progenitors (FAPs). FAPs are connective tissue fibroblasts that work with MuSCs to regenerate skeletal muscle [180, 181]. Following acute injury, FAPs transiently expand and promote MuSC differentiation by secreting several pro-myogenic factors [180, 182–184]. In chronic diseases, however, muscle regeneration fails and FAPs produce scar tissue and differentiate into adipocytes [175, 180, 181]. This replacement of healthy muscle tissue with fatty fibrosis is a prominent feature of chronic muscle diseases such as Duchenne muscular dystrophy (DMD), sarcopenia, the age-related loss of skeletal muscle and strength, obesity and diabetes. There are no cures for DMD and no specific therapies for either DMD or sarcopenia.
5.1.1. Cilia in muscle.
Recent work identified FAPs as the main ciliated cell type (Figure 3A). Interestingly, an acute injury insult caused the ciliation frequency to sharply increase within the FAP population before returning to pre-injury levels [175]. This places the cilium at the right time and place to be instrumental in controlling the behavior of FAPs. It is intriguing to speculate what injury-induced factors cause this increase in ciliation and if this is a shared mechanism across tissues. For example, cardiac fibroblasts (CFs) also increase their ciliation frequency post myocardial infarction injury [176]. Genetically removing cilia from FAPs resulted in strong repression of the conversion of FAPs into intramuscular fat after an adipogenic injury (Figure 3E) [175]. Thus, FAP cilia are crucial in balancing fatty fibrosis.
Even though FAPs are the main ciliated cell in skeletal muscle, MuSCs can also be ciliated [185, 186]. While the role for MuSC cilia in vivo remains to be fully determined, knock down of cilia in C2C12 myoblasts increased their proliferation but impaired their differentiation [186]. Similarly, affecting cilia stability via microtubule destabilizing agents in primary myoblasts impaired their self-renewal capacity [185]. As there is also evidence for direct activation of the Hh pathway within the myogenic compartment [187, 188], it is, therefore, conceivable that MuSCs might be able to respond to Hh signaling. Besides these two cell types, endothelial cells have also been proposed to possess a primary cilium [189]. As conditional loss of function of Smo within endothelial cells had no effect on angiogenesis, however, it argues against a direct role for Hh signaling in endothelial cells [190].
5.1.2. Hh in muscle regeneration.
Hh signaling is not only important for embryonic myogenesis but also for the maintenance and repair of mature muscle tissue. During embryogenesis, Hh plays a crucial instructive role in initiating the myogenic program [191–193]. In mature muscle tissue, the Hh pathway only displays low activity under homeostatic conditions. Upon different injuries, however, Hh signaling is robustly induced. For example, Hh signaling is being activated during the early regenerative phase upon an ischemic, cardiotoxin or crush injury, suggesting that Hh signaling might be functionally important during these early regenerative processes [175, 194–197]. Fittingly, administration of recombinant Shh induces pro-angiogenic factors within fibroblasts to increase capillary density and blood flow [195]. In contrast, inhibiting Hh signaling via the Hh-blocking antibody, 5E1, or the SMO antagonist, Cyclopamine, prevents this pro-angiogenic response and reduces blood flow post ischemic injury [194, 196]. Similarly, Dhh null mice displayed severe angiogenesis defects post ischemia [197]. In addition to be required for neovascularization, Hh signaling also has a clear impact on muscle regeneration itself. For example, blocking Hh via cyclopamine treatment increased the fibrotic response, prevented the expansion of MuSCs and resulted in reduced grip strength [196]. Similarly, loss of Dhh reduced the regenerative response after an ischemic injury [197]. Recent evidence suggests that FAPs are the main cell type, which respond to Hh signaling. Genetic loss of cilia resulted in strong loss of the repressor Gli3 leading to Hh derepression and low-level Hh activation. As a consequence, ectopically elevating Hh activity, genetically via loss of Ptch1 in FAPs or pharmacologically via SAG, a Smo agonist, also blocked intramuscular fat formation. Furthermore, Hh activation within FAPs also accelerated muscle regeneration after an acute injury [175]. As these phenotypes are similar to when Hh is activated via Shh gene therapy, Hh signaling in muscle most likely acts through FAP cilia (Figure 3E).
5.1.3. Hh in aged and diseased muscle.
While Hh is being induced upon injury in young and healthy muscle, this activation is severely blunted with age [187, 188]. Interestingly, re-activating Hh signaling in aged animals via a Shh overexpression plasmid increased the number of myogenic progenitors and reduced fibrosis after an acute injury [188]. Interestingly, in mdx mice, a mouse model of Duchenne Muscular Dystrophy, Hh pathway activity is induced during the early stages but is lost as the disease progresses [198]. Given that Hh signaling seems to be induced upon acute injuries and its many beneficial roles during muscle regeneration, it is possible that Hh is also used to repair the initial damage in mdx mice before degeneration takes over [198]. This raises the question if reduced Hh levels could explain why regeneration fails in mdx mice and if, by ectopically keeping Hh elevated, muscle function could be preserved. In support of this hypothesis, activating Hh signaling specifically within FAPs prevented not only fatty fibrosis but also the decline in myofiber size normally seen in mdx mice [175]. Corroborating the beneficial effect of Hh on diseased muscle, Hh activation in isolated MuSCs from mdx mice does increase their proliferation [198].
In summary, Hh signaling is crucial not only for embryonic myogenesis but also for maintaining healthy muscle in the adult. The main cell type responding to Hh are the FAPs, which are also the main ciliated cell type. Once Hh is being sensed by the FAPs, Hh induces a multitude of genes through which it executes its pro-angiogenic, anti-fibrotic, anti-adipogenic and pro-myogenic function (Figure 3E). Thus, restoring Hh signaling within FAPs could be a viable option to combat fatty fibrosis as well as the regeneration defects observed with age and disease.
5.2. Ciliary signaling in cardiac fibrosis and ischemia
Similar to skeletal muscle, the adult heart is also affected by fibrosis. Cardiac fibrosis, characterized by excessive deposition of extracellular matrix, replaces cardiomyocytes following acute insults such as myocardial infarction but also forms in congenital defects, dilated cardiomyopathy and hypertension. This fibrotic response increases stiffness of the heart wall affecting both contraction and relaxation behavior of the heart, ultimately resulting in a decrease in cardiac function. While the fibrotic scar tissue initially may protect the heart from rupturing, it gradually expands to the non-infarcted area leading to a progressive decrease in contractility and finally causes heart failure [reviewed by 199]. While fine-tuning the fibrotic response could preserve cardiac function, there is no current therapy available. A promising cellular target to treat cardiac fibrosis are the cardiac fibroblasts (CFs), which are the cellular origin of cardiac fibrosis. Thus, modifying the fibrotic response of CFs could have enormous health benefits.
5.2.1. Cilia in the heart.
An elegant study recently analyzed murine and human cardiac tissue sections during normal homeostasis as well as after a myocardial infarction focusing on cardiomyocytes, macrophages, endothelial cells and fibroblasts. In addition, this group also studied cultured neonatal and adult myocytes, adult cardiac fibroblasts, a murine macrophage cell line, human umbilical vein endothelial cells and human aortic endothelial cells for the presence of cilia. Interestingly, primary cilia were only found to be present on CFs [176] (Figure 3B). Functionally testing polycystin 1, PC1, which localizes to the cilium and has been previously associated with controlling ECM composition [200], via conditional removal in CFs using a periostin-driven Cre changed the fibrotic response leading to enhanced pathological remodeling after a myocardial infarction [176]. However, it remains to be determined if the function of PC1 in controlling ECM production is relying on the cilium or, as previously suggested, can also be explained in a cilium-independent manner [200].
5.2.2. What is the role of Hh?
Hedgehog signaling appears to be turned off in the adult heart but is being reactivated upon ischemic injury [201, 202]. Ectopically activating the Hh pathway either via a naked Shh overexpression plasmid or via a Smo agonist enhanced neovascularization, reduced fibrosis and improved cardiac dysfunction after an ischemic injury in mice, rabbits and pigs [201, 202]. In addition, erythropoietin treatment, previously shown to improve heart function in patients with congestive heart failure [203, 204], induces Shh expression in cardiomyocytes. Conditional removal of Shh in cardiomyocytes prevented this pro-angiogenic response [205]. In contrast, turning off the endogenous Hh pathway via cyclopamine improved cardiac function after a myocardial infarction similar to turning the Hh pathway on [206]. This would suggest that Hh signaling has a dual role in cardiac ischemia in which high exogenous levels are able to improve tissue repair while endogenous Hh is deleterious. In contrast, Gli3 haploinsufficiency leads to reduced capillary density and worsened myocardial output after an ischemic injury. As Gli3 is the main repressor, Gli3 haploinsufficiency should lead to derepression and low-level activation of Hh signaling. Since elevated Hh signaling seems to have beneficial effects, one would have expected that Gli3 haploinsufficiency improves the outcome post ischemic injury. Gli3 has been shown to also act as a weak activator [157]. Thus, while conceivable that Gli3 haploinsufficiency could lead to reduced Hh signaling, more experiments are clearly needed to fully understand cilia and ciliary Hh signaling in the heart.
5.2.3. Which cells respond to Hh signaling?
Exogenous Shh treatment activates the Hh reporter, Ptch1-LacZ, in fibroblasts but not in endothelial or smooth muscle cells. In addition, Shh treatment in HUVECs, aortic and microvascular endothelial cells did not activate Hh signaling [195, 207]. In contrast, Shh activates Ptch1-LacZ in fibroblasts in vivo and induces several potent pro-angiogenic factors including Vegf, Ang-1 and Ang-2 in isolated fibroblasts in vitro [195]. As CFs are also ciliated, CFs are most likely also responding to Hh signaling in vivo.
As described above, one prerequisite for canonical Hh signaling is the presence of a primary cilium. Cardiomyocytes have been proposed to, at least transiently, carry a cilium [208–210] and to respond to Hh signaling [202, 209, 211]. For example, primary neonatal rat ventricular cardiomyocytes, isolated via the selective adhesion method, known for its impurity [212], activate the Hh pathway upon stimulation [211]. However, a functional role for cilia was recently disproven via conditional mutagenesis to remove cilia specifically in cardiomyocytes. In this study, loss of Kif3a had no effect on cardiomyocyte function [176]. The most severe Hh-related phenotype was observed when Smo was conditionally removed in mature CMs (via the αMHC-MerCreMer allele), which resulted in the death of most mice within 2–5 days after tamoxifen administration. These mice displayed tissue hypoxia and cell death resulting in cardiac failure [213]. However, recent evidence demonstrates that this specific Cre line displays cardiac Cre toxicity [214]. Therefore, it remains to be determined if cardiomyocytes possess a cilium and, if they do, use cilia for their function including to respond to Hh signaling.
5.2.4. What about fat in the heart?
Recent work from the Rossi lab demonstrated that Pdgfra+ CFs are not only responsible for the fibrotic response post ischemic injuries but are also the cellular origin of adipose tissue, which forms in arrhythmogenic cardiomyopathies [178]. It will be interesting to ask if cilia play an anti-adipogenic role during intracardial fat formation as they do in intramuscular fat tissue [175] or a pro-adipogenic role as described in white adipose tissue [174].
Balancing the amount of a scar tissue after an ischemic injury is important: too little and the heart wall can rupture, while too much interferes with contractility and function. Thus, fine tuning the fibrotic response could provide a great medial benefit to patients. Given the ample evidence presented here, it is clear that cilia are a key player in controlling the fibrotic response and strongly argues for further exploration of cilia and ciliary Hh signaling as a novel therapeutic target (Figure 3F).
5. 3. White adipose tissue
White adipose tissue (WAT) is our main energy storage. WAT expands through either hypertrophy, the increase in the individual size of preexisting adipocytes, or by hyperplasia, the de novo generation of new fat cells from adipogenic progenitors called preadipocytes. During obesity, individual adipocytes expand in size causing mechanical and hypoxic stress, which, in turn, leads to adipose tissue inflammation and fibrosis and, ultimately, insulin resistance, diabetes and heart disease. In contrast, WAT consisting of more but smaller adipocytes is considered metabolically more healthy [reviewed by 215]. Thus, by inducing hyperplasia the mechanical and hypoxic stress would be reduced. Here we are discussing the role of cilia and ciliary Hh signaling in the formation and maintenance of white adipose tissue. For an in-depth review on ciliary signaling and obesity see the review by Engle, Bansal, Antonellis and Berbari in this edition.
5.3.1. Cilia during adipogenesis.
Similar to the FAPs in skeletal muscle and the CFs in cardiac tissue, preadipocytes are ciliated both in vitro and in vivo (Figure 3C). Interestingly, this ciliation is only transient as preadipocytes lose their cilium upon differentiation suggesting that the cilium is required for receiving adipogenic cues [174, 175, 216–218]. In fact, removal of cilia from preadipocytes impairs adipogenesis in vitro [174, 217]. Similarly, genetically removing cilia from preadipocytes also prevented the expansion of white adipose tissue in vivo. Mice without preadipocyte cilia remained skinny and displayed smaller fat pads. Screening for pro-adipogenic signals sensed by cilia, the GPCR Ffra4 (free fatty acid receptor 4) was found to localize to preadipocyte cilia. Ffra 4 senses ω−3 fatty acids. Binding of ω−3 fatty acids to ciliary Ffra4 increases ciliary cAMP levels, which induces expansion of the preadipocyte pool followed by efficient adipogenesis [174]. These data suggest a model in which cilia through Ffra4 sense healthy fatty acids leading to WAT hyperplasia. Another pro-adipogenic signaling pathway described to require the cilium is the insulin growth factor pathway. IGF-1R, a crucial pro-adipogenic factor, localizes to the cilium of preadipocytes [217, 219]. This localization to the cilium is required for its pro-adipogenic function [217]. Thus, preadipocyte cilia control adipogenesis by sensing pro-adipogenic factors.
5.3.2. Hh during adipogenesis.
Hh signaling has a known and evolutionary conserved role in preventing the differentiation of preadipocytes into mature fat cells. Activating the Hh pathway in several preadipocyte cell lines completely abolishes adipogenesis [175, 220–222]. Conversely, inactivation Hh resulted in a modest increase in adipogenesis [220, 222]. Similarly, Hh signaling potently blocks the formation of white [221] and brown adipose tissue [223] in vivo. Thus, Hh signaling is a potent guardian of adipogenesis.
5.3.3. What about Hh in obesity?
Interestingly, mice fed a high fat diet for 4 months or genetically obese mice displayed a strong downregulation of the Hh pathway [222]. Fittingly, loss of the Hh co-receptor Boc resulted in excess WAT and overweight mice demonstrating that Hh inactivation is associated with increased adipogenesis [224]. In contrast, re-activating Hh signaling genetically blocked the expansion of WAT induced by high fat diet [225]. These data suggest that Hh signaling serves as endogenous adipogenic break and losing that brake could partially explain why WAT expands upon excess energy.
5.3.5. What about cilia in obesity?
Preadipocytes derived from obese patients displayed reduced cilia length compared to from lean control groups. Obese patient derived preadipocytes also exhibited reduced adipogenic differentiation capacity [226], which could be restored via Aurora A kinase inhibition [227]. These data not only demonstrate that cilia are required for efficient fat formation but also that restoring cilia length could be a potential novel approach to make healthy fat by accelerating hyperplasia. Interestingly, loss of Bbs12, a component of the BBsome, resulted in overweight mice. However, the fat pads were compromised of more but smaller adipocytes due to increased hyperplasia. Fittingly, Bbs12 null mice displayed reduced adipose tissue inflammation and improved oxygenation, which could explain why many metabolic markers including blood glucose levels showed marked improvement over control littermates [228]. As the BBsome is required for the exit of activated signaling receptors from cilia [229], it is possible that one of the pro-angiogenic factors, perhaps even FFAR4 [174], remains longer within Bbs12 null cilia, thereby increasing expansion of the preadipocyte pool. Thus, changing the composition of the fat pads from fewer larger to more smaller adipocytes can indeed protect metabolic health even during obesity.
5.3.6. Does ciliary Hh signaling also affect fibrosis?
Unhealthy adipose tissue displays fibrosis, which is strongly linked to insulin resistance and type II diabetes [230]. Interestingly, Pdgfrα+ fibroblasts have been shown to be the cellular origin of both adipocytes and fibrotic scar tissue [174, 231]. While cilia and ciliary Hh signaling have a strong influence on the differentiation of the Pdgfrα+ preadipocytes into fat cells, it is unclear if they also affect fibrosis similar to skeletal muscle (see above). It will be interesting to determine if Hh signaling can also reduce the fibrotic response in adipose tissue.
It is clear that cilia play an important role in building and maintaining healthy adipose tissue by balancing pro-angiogenic cues such as ω−3 fatty acids and IGF1 to promote adipogenesis and anti-adipogenic cues such as Hh signaling to limit fat (Figure 3G). One possibility to build healthy fat is to stimulate cilia-controlled adipogenesis via ω−3 fatty acids, which have been shown to improve insulin sensitivity and decreased inflammation within adipose tissue [232].
6. Conclusion
Primary cilia have turned out to be the paradigmatic organelle for compartmentalized subcellular signaling in the context of the vertebrate Hh pathway during development, regeneration and disease. By organizing both Gli repressor and activator formation, primary cilia coordinate divergent tissue responses to repressor and/or activator gradients. These pathways converge on differentiation and/or proliferation modules of cellular programs that upon dysregulation can cause a plethora of pathological outcomes. Especially, current studies on negative regulation of Hh pathway are unraveling unknown phenotypes and disease associations. Manipulating these divergent outcomes by targeting ciliary trafficking modules and ciliary architecture promises to provide unique therapeutic opportunities for intervention. One such unique opportunity arises in the context of organ failure due to fibrosis, which contributes to almost half of all deaths in the developed world [233]. To successfully fight fibrosis, we need to fully understand what controls the fate of fibroblasts and ways to manipulate their differentiation. This review highlights that ciliary Hh signaling presents a very exciting target and should be the focus of novel therapeutic approaches.
Highlights.
Primary cilia are required for both repressing and activating Hh signaling.
Active repression is as important as the activation arm of Hh pathway.
Ratio sensing or threshold detection of activator/repressor drive morphogenesis.
Cilia regulate differentiation, patterning or proliferation by Hh pathway.
Hh signaling is reactivated and repurposed during adult tissue regeneration.
7. Acknowledgements
The authors’ work presented in this review was supported by a R01 grant from the National Institutes of Health (1R01GM113023 to S.M.), an A-grant from the Alex’s Lemonade Foundation (to S.M.), and departmental start-up funds (to D.K.). We thank members of the Kopinke and Mukhopadhyay labs for comments on the manuscript.
Abbreviations
- Hh
hedgehog
- GliA
Gli activator
- GliR
Gli repressor
- ECD
extracellular domain
- Ptch1
Patched
- Smo
Smoothened
- GC
granule cell
- uRL
upper rhombic lip
- FAPs
fibro/adipogenic progenitors
- CFs
cardiac fibroblasts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rosenbaum JL, Witman GB, Intraflagellar transport, Nature reviews. Molecular cell biology 3(11) (2002) 813–25. [DOI] [PubMed] [Google Scholar]
- [2].Wang Q, Pan J, Snell WJ, Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas, Cell 125(3) (2006) 549–62. [DOI] [PubMed] [Google Scholar]
- [3].Goetz SC, Anderson KV, The primary cilium: a signalling centre during vertebrate development, Nature reviews. Genetics 11(5) (2010) 331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST, Cellular signalling by primary cilia in development, organ function and disease, Nat Rev Nephrol 15(4) (2019) 199–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hildebrandt F, Benzing T, Katsanis N, Ciliopathies N Engl J Med 364(16) (2011) 1533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL, A motility in the eukaryotic flagellum unrelated to flagellar beating, Proceedings of the National Academy of Sciences of the United States of America 90(12) (1993) 5519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kozminski KG, Beech PL, Rosenbaum JL, The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane, J Cell Biol 131(6 Pt 1) (1995) 1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pazour GJ, Dickert BL, Witman GB, The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly, J Cell Biol 144(3) (1999) 473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP, The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination, Development 127(11) (2000) 2347–55. [DOI] [PubMed] [Google Scholar]
- [10].Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA, Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function, Nature 383(6599) (1996) 407–13. [DOI] [PubMed] [Google Scholar]
- [11].Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV, Hedgehog signalling in the mouse requires intraflagellar transport proteins, Nature 426(6962) (2003) 83–7. [DOI] [PubMed] [Google Scholar]
- [12].Ruiz i Altaba A, Gli proteins encode context-dependent positive and negative functions: implications for development and disease, Development 126(14) (1999) 3205–16. [DOI] [PubMed] [Google Scholar]
- [13].Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H, Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling, Development 126(17) (1999) 3915–24. [DOI] [PubMed] [Google Scholar]
- [14].Wang B, Fallon JF, Beachy PA, Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb, Cell 100(4) (2000) 423–34. [DOI] [PubMed] [Google Scholar]
- [15].Tempe D, Casas M, Karaz S, Blanchet-Tournier MF, Concordet JP, Multisite protein kinase A and glycogen synthase kinase 3beta phosphorylation leads to Gli3 ubiquitination by SCFbetaTrCP, Mol Cell Biol 26(11) (2006) 4316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Niewiadomski P, Kong JH, Ahrends R, Ma Y, Humke EW, Khan S, Teruel MN, Novitch BG, Rohatgi R, Gli protein activity is controlled by multisite phosphorylation in vertebrate Hedgehog signaling, Cell reports 6(1) (2014) 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tuson M, He M, Anderson KV, Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube, Development 138(22) (2011) 4921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK, The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling, Cell 152(1–2) (2013) 210–23. [DOI] [PubMed] [Google Scholar]
- [19].Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF, Vertebrate Smoothened functions at the primary cilium, Nature 437(7061) (2005) 1018–21. [DOI] [PubMed] [Google Scholar]
- [20].Rohatgi R, Milenkovic L, Scott MP, Patched1 regulates hedgehog signaling at the primary cilium, Science 317(5836) (2007) 372–6. [DOI] [PubMed] [Google Scholar]
- [21].Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R, The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins, Genes Dev 24(7) (2010) 670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tukachinsky H, Lopez LV, Salic A, A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes, The Journal of cell biology 191(2) (2010) 415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee J, Platt KA, Censullo P, Ruiz i Altaba A, Gli1 is a target of Sonic hedgehog that induces ventral neural tube development, Development 124(13) (1997) 2537–52. [DOI] [PubMed] [Google Scholar]
- [24].Chang CF, Chang YT, Millington G, Brugmann SA, Craniofacial Ciliopathies Reveal Specific Requirements for GLI Proteins during Development of the Facial Midline, PLoS Genet 12(11) (2016) e1006351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu A, Wang B, Niswander LA, Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors, Development 132(13) (2005) 3103–11. [DOI] [PubMed] [Google Scholar]
- [26].Hwang SH, White KA, Somatilaka BN, Shelton JM, Richardson JA, Mukhopadhyay S, The G protein-coupled receptor Gpr161 regulates forelimb formation, limb patterning and skeletal morphogenesis in a primary cilium-dependent manner, Development 145(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yam PT, Langlois SD, Morin S, Charron F, Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway, Neuron 62(3) (2009) 349–62. [DOI] [PubMed] [Google Scholar]
- [28].Ferent J, Constable S, Gigante ED, Yam PT, Mariani LE, Legue E, Liem KF Jr., Caspary T, Charron F, The Ciliary Protein Arl13b Functions Outside of the Primary Cilium in Shh-Mediated Axon Guidance, Cell Rep 29(11) (2019) 3356–3366 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bijlsma MF, Borensztajn KS, Roelink H, Peppelenbosch MP, Spek CA, Sonic hedgehog induces transcription-independent cytoskeletal rearrangement and migration regulated by arachidonate metabolites, Cell Signal 19(12) (2007) 2596–604. [DOI] [PubMed] [Google Scholar]
- [30].Zhang XM, Ramalho-Santos M, McMahon AP, Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node, Cell 105(6) (2001) 781–92. [PubMed] [Google Scholar]
- [31].Tsiairis CD, McMahon AP, An Hh-dependent pathway in lateral plate mesoderm enables the generation of left/right asymmetry, Curr Biol 19(22) (2009) 1912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Norris DP, Cilia, calcium and the basis of left-right asymmetry, BMC Biol 10 (2012) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pan Y, Wang B, A novel protein-processing domain in Gli2 and Gli3 differentially blocks complete protein degradation by the proteasome, The Journal of biological chemistry 282(15) (2007) 10846–52. [DOI] [PubMed] [Google Scholar]
- [34].Schrader EK, Harstad KG, Holmgren RA, Matouschek A, A three-part signal governs differential processing of Gli1 and Gli3 proteins by the proteasome, J Biol Chem 286(45) (2011) 39051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV, Proteomics of Primary Cilia by Proximity Labeling, Dev Cell 35(4) (2015) 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Choi YH, Suzuki A, Hajarnis S, Ma Z, Chapin HC, Caplan MJ, Pontoglio M, Somlo S, Igarashi P, Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases, Proc Natl Acad Sci U S A 108(26) (2011) 10679–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bishop GA, Berbari NF, Lewis J, Mykytyn K, Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain, J Comp Neurol 505(5) (2007) 562–71. [DOI] [PubMed] [Google Scholar]
- [38].Vuolo L, Herrera A, Torroba B, Menendez A, Pons S, Ciliary adenylyl cyclases control the Hedgehog pathway, J Cell Sci 128(15) (2015) 2928–37. [DOI] [PubMed] [Google Scholar]
- [39].Huangfu D, Anderson KV, Cilia and Hedgehog responsiveness in the mouse, Proc Natl Acad Sci U S A 102(32) (2005) 11325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kise Y, Morinaka A, Teglund S, Miki H, Sufu recruits GSK3beta for efficient processing of Gli3, Biochem Biophys Res Commun 387(3) (2009) 569–74. [DOI] [PubMed] [Google Scholar]
- [41].Jia J, Kolterud A, Zeng H, Hoover A, Teglund S, Toftgard R, Liu A, Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia, Dev Biol 330(2) (2009) 452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT, Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved, Genes Dev 23(16) (2009) 1910–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim J, Kato M, Beachy PA, Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus, Proc Natl Acad Sci U S A 106(51) (2009) 21666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Carpenter BS, Barry RL, Verhey KJ, Allen BL, The heterotrimeric kinesin-2 complex interacts with and regulates GLI protein function, J Cell Sci 128(5) (2015) 1034–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tillo SE, Xiong WH, Takahashi M, Miao S, Andrade AL, Fortin DA, Yang G, Qin M, Smoody BF, Stork PJS, Zhong H, Liberated PKA Catalytic Subunits Associate with the Membrane via Myristoylation to Preferentially Phosphorylate Membrane Substrates, Cell Rep 19(3) (2017) 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Scott JD, Dessauer CW, Tasken K, Creating order from chaos: cellular regulation by kinase anchoring, Annu Rev Pharmacol Toxicol 53 (2013) 187–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Saade M, Gonzalez-Gobartt E, Escalona R, Usieto S, Marti E, Shh-mediated centrosomal recruitment of PKA promotes symmetric proliferative neuroepithelial cell division, Nat Cell Biol 19(5) (2017) 493–503. [DOI] [PubMed] [Google Scholar]
- [48].Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R, Pons S, Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base, J Cell Sci 123(Pt 1) (2010) 62–9. [DOI] [PubMed] [Google Scholar]
- [49].Diviani D, Langeberg LK, Doxsey SJ, Scott JD, Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain, Curr Biol 10(7) (2000) 417–20. [DOI] [PubMed] [Google Scholar]
- [50].Bachmann VA, Mayrhofer JE, Ilouz R, Tschaikner P, Raffeiner P, Rock R, Courcelles M, Apelt F, Lu TW, Baillie GS, Thibault P, Aanstad P, Stelzl U, Taylor SS, Stefan E, Gpr161 anchoring of PKA consolidates GPCR and cAMP signaling, Proc Natl Acad Sci U S A 113(28) (2016) 7786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Freed E, Lacey KR, Huie P, Lyapina SA, Deshaies RJ, Stearns T, Jackson PK, Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle, Genes Dev 13(17) (1999) 2242–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, Thomas PJ, Dynamic association of proteasomal machinery with the centrosome, J Cell Biol 145(3) (1999) 481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fabunmi RP, Wigley WC, Thomas PJ, DeMartino GN, Activity and regulation of the centrosome-associated proteasome, J Biol Chem 275(1) (2000) 409–13. [DOI] [PubMed] [Google Scholar]
- [54].Wang C, Pan Y, Wang B, Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors, Development 137(12) (2010) 2001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK, Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function, PLoS Genet 1(4) (2005) e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Taipale J, Cooper MK, Maiti T, Beachy PA, Patched acts catalytically to suppress the activity of Smoothened, Nature 418(6900) (2002) 892–7. [DOI] [PubMed] [Google Scholar]
- [57].Gong X, Qian H, Cao P, Zhao X, Zhou Q, Lei J, Yan N, Structural basis for the recognition of Sonic Hedgehog by human Patched1, Science 361(6402) (2018). [DOI] [PubMed] [Google Scholar]
- [58].Qi X, Schmiege P, Coutavas E, Wang J, Li X, Structures of human Patched and its complex with native palmitoylated sonic hedgehog, Nature 560(7716) (2018) 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Qi X, Schmiege P, Coutavas E, Li X, Two Patched molecules engage distinct sites on Hedgehog yielding a signaling-competent complex, Science 362(6410) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang Y, Bulkley DP, Xin Y, Roberts KJ, Asarnow DE, Sharma A, Myers BR, Cho W, Cheng Y, Beachy PA, Structural Basis for Cholesterol Transport-like Activity of the Hedgehog Receptor Patched, Cell 175(5) (2018) 1352–1364 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Huang P, Zheng S, Wierbowski BM, Kim Y, Nedelcu D, Aravena L, Liu J, Kruse AC, Salic A, Structural Basis of Smoothened Activation in Hedgehog Signaling, Cell 174(2) (2018) 312–324 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Deshpande I, Liang J, Hedeen D, Roberts KJ, Zhang Y, Ha B, Latorraca NR, Faust B, Dror RO, Beachy PA, Myers BR, Manglik A, Smoothened stimulation by membrane sterols drives Hedgehog pathway activity, Nature 571(7764) (2019) 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Qi X, Liu H, Thompson B, McDonald J, Zhang C, Li X, Cryo-EM structure of oxysterol-bound human Smoothened coupled to a heterotrimeric Gi, Nature 571(7764) (2019) 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kong JH, Siebold C, Rohatgi R, Biochemical mechanisms of vertebrate hedgehog signaling, Development 146(10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Raleigh DR, Sever N, Choksi PK, Sigg MA, Hines KM, Thompson BM, Elnatan D, Jaishankar P, Bisignano P, Garcia-Gonzalo FR, Krup AL, Eberl M, Byrne EFX, Siebold C, Wong SY, Renslo AR, Grabe M, McDonald JG, Xu L, Beachy PA, Reiter JF, Cilia-Associated Oxysterols Activate Smoothened, Mol Cell 72(2) (2018) 316–327 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kinnebrew M, Iverson EJ, Patel BB, Pusapati GV, Kong JH, Johnson KA, Luchetti G, Eckert KM, McDonald JG, Covey DF, Siebold C, Radhakrishnan A, Rohatgi R, Cholesterol accessibility at the ciliary membrane controls hedgehog signaling, eLife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang B, Zhuang T, Lin Q, Yang B, Xu X, Xin G, Zhu S, Wang G, Yu B, Zhang T, Jiang Q, Zhang C, Patched1-ArhGAP36-PKA-Inversin axis determines the ciliary translocation of Smoothened for Sonic Hedgehog pathway activation, Proceedings of the National Academy of Sciences of the United States of America 116(3) (2019) 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shiba D, Yamaoka Y, Hagiwara H, Takamatsu T, Hamada H, Yokoyama T, Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region, J Cell Sci 122(Pt 1) (2009) 44–54. [DOI] [PubMed] [Google Scholar]
- [69].Bennett HW, Gustavsson AK, Bayas CA, Petrov PN, Mooney N, Moerner WE, Jackson PK, Novel fibrillar structure in the inversin compartment of primary cilia revealed by 3D single-molecule super-resolution microscopy, Molecular biology of the cell (2020) mbcE19090499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Li S, Li S, Han Y, Tong C, Wang B, Chen Y, Jiang J, Regulation of Smoothened Phosphorylation and High-Level Hedgehog Signaling Activity by a Plasma Membrane Associated Kinase, PLoS Biol 14(6) (2016) e1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Barzi M, Kostrz D, Menendez A, Pons S, Sonic Hedgehog-induced proliferation requires specific Galpha inhibitory proteins, J Biol Chem 286(10) (2011) 8067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Villanueva H, Visbal AP, Obeid NF, Ta AQ, Faruki AA, Wu MF, Hilsenbeck SG, Shaw CA, Yu P, Plummer NW, Birnbaumer L, Lewis MT, An essential role for Galpha(i2) in Smoothened-stimulated epithelial cell proliferation in the mammary gland, Sci Signal 8(394) (2015) ra92. [DOI] [PubMed] [Google Scholar]
- [73].Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ, G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling, Nature 456(7224) (2008) 967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Barakat B, Yu L, Lo C, Vu D, De Luca E, Cain JE, Martelotto LG, Dedhar S, Sadler AJ, Wang D, Watkins DN, Hannigan GE, Interaction of smoothened with integrin-linked kinase in primary cilia mediates Hedgehog signalling, EMBO reports 14(9) (2013) 837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Pusapati GV, Hughes CE, Dorn KV, Zhang D, Sugianto P, Aravind L, Rohatgi R, EFCAB7 and IQCE Regulate Hedgehog Signaling by Tethering the EVC-EVC2 Complex to the Base of Primary Cilia, Developmental cell (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Dorn KV, Hughes CE, Rohatgi R, A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia, Developmental cell 23(4) (2012) 823–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ, IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport, Developmental cell 22(5) (2012) 940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, Tobita K, Johnson CA, Abdelhamed ZA, Lo CW, Pazour GJ, IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment, Dev Cell 31(3) (2014) 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow DK, Gygi SP, Nachury MV, The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3, Dev Cell 31(3) (2014) 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wen X, Lai CK, Evangelista M, Hongo JA, de Sauvage FJ, Scales SJ, Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation, Mol Cell Biol 30(8) (2010) 1910–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Liu J, Zeng H, Liu A, The loss of Hh responsiveness by a non-ciliary Gli2 variant, Development 142(9) (2015) 1651–60. [DOI] [PubMed] [Google Scholar]
- [82].Niewiadomski P, Niedziolka SM, Markiewicz L, Uspienski T, Baran B, Chojnowska K, Gli Proteins: Regulation in Development and Cancer, Cells 8(2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Han Y, Wang B, Cho YS, Zhu J, Wu J, Chen Y, Jiang J, Phosphorylation of Ci/Gli by Fused Family Kinases Promotes Hedgehog Signaling, Dev Cell 50(5) (2019) 610–626 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cherry AL, Finta C, Karlstrom M, Jin Q, Schwend T, Astorga-Wells J, Zubarev RA, Del Campo M, Criswell AR, de Sanctis D, Jovine L, Toftgard R, Structural basis of SUFU-GLI interaction in human Hedgehog signalling regulation, Acta Crystallogr D Biol Crystallogr 69(Pt 12) (2013) 2563–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dunaeva M, Michelson P, Kogerman P, Toftgard R, Characterization of the physical interaction of Gli proteins with SUFU proteins, J Biol Chem 278(7) (2003) 5116–22. [DOI] [PubMed] [Google Scholar]
- [86].Merchant M, Vajdos FF, Ultsch M, Maun HR, Wendt U, Cannon J, Desmarais W, Lazarus RA, de Vos AM, de Sauvage FJ, Suppressor of fused regulates Gli activity through a dual binding mechanism, Mol Cell Biol 24(19) (2004) 8627–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Chen Y, Yue S, Xie L, Pu XH, Jin T, Cheng SY, Dual Phosphorylation of suppressor of fused (Sufu) by PKA and GSK3beta regulates its stability and localization in the primary cilium, J Biol Chem 286(15) (2011) 13502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].He M, Subramanian R, Bangs F, Omelchenko T, Liem KF Jr., Kapoor TM, Anderson KV, The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment, Nat Cell Biol 16(7) (2014) 663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Liem KF Jr., He M, Ocbina PJ, Anderson KV, Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling, Proc Natl Acad Sci U S A 106(32) (2009) 13377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jiang S, Mani N, Wilson-Kubalek EM, Ku PI, Milligan RA, Subramanian R, Interplay between the Kinesin and Tubulin Mechanochemical Cycles Underlies Microtubule Tip Tracking by the Non-motile Ciliary Kinesin Kif7, Dev Cell 49(5) (2019) 711–730 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Liu YC, Couzens AL, Deshwar AR, LD BM-C, Zhang X, Puviindran V, Scott IC, Gingras AC, Hui CC, Angers S, The PPFIA1-PP2A protein complex promotes trafficking of Kif7 to the ciliary tip and Hedgehog signaling, Sci Signal 7(355) (2014) ra117. [DOI] [PubMed] [Google Scholar]
- [92].Pal K, Hwang SH, Somatilaka B, Badgandi H, Jackson PK, DeFea K, Mukhopadhyay S, Smoothened determines beta-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium, J Cell Biol 212(7) (2016) 861–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nager AR, Goldstein JS, Herranz-Perez V, Portran D, Ye F, Garcia-Verdugo JM, Nachury MV, An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling, Cell 168(1–2) (2017) 252–263 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Garcia-Gonzalo FR, Phua SC, Roberson EC, Garcia G 3rd, Abedin M, Schurmans S, Inoue T, Reiter JF, Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling, Dev Cell 34(4) (2015) 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chavez M, Ena S, Van Sande J, de Kerchove d’Exaerde A, Schurmans S, Schiffmann SN, Modulation of Ciliary Phosphoinositide Content Regulates Trafficking and Sonic Hedgehog Signaling Output, Dev Cell 34(3) (2015) 338–50. [DOI] [PubMed] [Google Scholar]
- [96].Badgandi HB, Hwang SH, Shimada IS, Loriot E, Mukhopadhyay S, Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins, J Cell Biol 216(3) (2017) 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Constable S, Long AB, Floyd KA, Schurmans S, Caspary T, The ciliary phosphatidylinositol phosphatase Inpp5e plays positive and negative regulatory roles in Shh signaling, Development 147(3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Le TL, Sribudiani Y, Dong X, Huber C, Kois C, Baujat G, Gordon CT, Mayne V, Galmiche L, Serre V, Goudin N, Zarhrate M, Bole-Feysot C, Masson C, Nitschke P, Verheijen FW, Pais L, Pelet A, Sadedin S, Pugh JA, Shur N, White SM, El Chehadeh S, Christodoulou J, Cormier-Daire V, Hofstra RMW, Lyonnet S, Tan TY, Attie-Bitach T, Kerstjens-Frederikse WS, Amiel J, Thomas S, Bi-allelic Variations of SMO in Humans Cause a Broad Spectrum of Developmental Anomalies Due to Abnormal Hedgehog Signaling, Am J Hum Genet (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH Jr., de Sauvage FJ, Activating Smoothened mutations in sporadic basal-cell carcinoma, Nature 391(6662) (1998) 90–2. [DOI] [PubMed] [Google Scholar]
- [100].Goodrich LV, Milenkovic L, Higgins KM, Scott MP, Altered neural cell fates and medulloblastoma in mouse patched mutants, Science 277(5329) (1997) 1109–13. [DOI] [PubMed] [Google Scholar]
- [101].Oliver TG, Read TA, Kessler JD, Mehmeti A, Wells JF, Huynh TT, Lin SM, Wechsler-Reya RJ, Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma, Development 132(10) (2005) 2425–39. [DOI] [PubMed] [Google Scholar]
- [102].Mak KK, Chen MH, Day TF, Chuang PT, Yang Y, Wnt/beta-catenin signaling interacts differentially with Ihh signaling in controlling endochondral bone and synovial joint formation, Development 133(18) (2006) 3695–707. [DOI] [PubMed] [Google Scholar]
- [103].Zhulyn O, Li D, Deimling S, Vakili NA, Mo R, Puviindran V, Chen MH, Chuang PT, Hopyan S, Hui CC, A switch from low to high Shh activity regulates establishment of limb progenitors and signaling centers, Dev Cell 29(2) (2014) 241–9. [DOI] [PubMed] [Google Scholar]
- [104].Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F, Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation, Dev Cell 20(6) (2011) 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, Krauss RS, McMahon AP, Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function, Dev Cell 20(6) (2011) 775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].te Welscher P, Fernandez-Teran M, Ros MA, Zeller R, Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling, Genes Dev 16(4) (2002) 421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Charite J, McFadden DG, Olson EN, The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development, Development 127(11) (2000) 2461–70. [DOI] [PubMed] [Google Scholar]
- [108].Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S, Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway, Dev Cell 10(2) (2006) 187–97. [DOI] [PubMed] [Google Scholar]
- [109].Hwang SH, Mukhopadhyay S, G-protein-coupled receptors and localized signaling in the primary cilium during ventral neural tube patterning, Birth Defects Res A Clin Mol Teratol 103(1) (2015) 12–9. [DOI] [PubMed] [Google Scholar]
- [110].Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ, Sonic hedgehog and the molecular regulation of mouse neural tube closure, Development 129(10) (2002) 2507–17. [DOI] [PubMed] [Google Scholar]
- [111].Matteson PG, Desai J, Korstanje R, Lazar G, Borsuk TE, Rollins J, Kadambi S, Joseph J, Rahman T, Wink J, Benayed R, Paigen B, Millonig JH, The orphan G protein-coupled receptor, Gpr161, encodes the vacuolated lens locus and controls neurulation and lens development, Proc Natl Acad Sci U S A 105(6) (2008) 2088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Norman RX, Ko HW, Huang V, Eun CM, Abler LL, Zhang Z, Sun X, Eggenschwiler JT, Tubby-like protein 3 (TULP3) regulates patterning in the mouse embryo through inhibition of Hedgehog signaling, Hum Mol Genet 18(10) (2009) 1740–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Patterson VL, Damrau C, Paudyal A, Reeve B, Grimes DT, Stewart ME, Williams DJ, Siggers P, Greenfield A, Murdoch JN, Mouse hitchhiker mutants have spina bifida, dorso-ventral patterning defects and polydactyly: identification of Tulp3 as a novel negative regulator of the Sonic hedgehog pathway, Hum Mol Genet 18(10) (2009) 1719–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Murdoch JN, Copp AJ, The relationship between sonic Hedgehog signaling, cilia, and neural tube defects, Birth Defects Res A Clin Mol Teratol 88(8) (2010) 633–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Shimada IS, Hwang SH, Somatilaka BN, Wang X, Skowron P, Kim J, Kim M, Shelton JM, Rajaram V, Xuan Z, Taylor MD, Mukhopadhyay S, Basal Suppression of the Sonic Hedgehog Pathway by the G-Protein-Coupled Receptor Gpr161 Restricts Medulloblastoma Pathogenesis, Cell reports 22(5) (2018) 1169–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Shimada IS, Somatilaka BN, Hwang SH, Anderson AG, Shelton JM, Rajaram V, Konopka G, Mukhopadhyay S, Derepression of sonic hedgehog signaling upon Gpr161 deletion unravels forebrain and ventricular abnormalities, Dev Biol 450(1) (2019) 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang L, Hou S, Han YG, Hedgehog signaling promotes basal progenitor expansion and the growth and folding of the neocortex, Nat Neurosci 19(7) (2016) 888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Sheen VL, Basel-Vanagaite L, Goodman JR, Scheffer IE, Bodell A, Ganesh VS, Ravenscroft R, Hill RS, Cherry TJ, Shugart YY, Barkovich J, Straussberg R, Walsh CA, Etiological heterogeneity of familial periventricular heterotopia and hydrocephalus, Brain Dev 26(5) (2004) 326–34. [DOI] [PubMed] [Google Scholar]
- [119].Kim SE, Lei Y, Hwang SH, Wlodarczyk BJ, Mukhopadhyay S, Shaw GM, Ross ME, Finnell RH, Dominant negative GPR161 rare variants are risk factors of human spina bifida, Hum Mol Genet 28(2) (2019) 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, Hao A, Goldstein AM, Stavrou T, Scherer SW, Dura WT, Wainwright B, Squire JA, Rutka JT, Hogg D, Mutations in SUFU predispose to medulloblastoma, Nat Genet 31(3) (2002) 306–10. [DOI] [PubMed] [Google Scholar]
- [121].Begemann M, Waszak SM, Robinson GW, Jager N, Sharma T, Knopp C, Kraft F, Moser O, Mynarek M, Guerrini-Rousseau L, Brugieres L, Varlet P, Pietsch T, Bowers DC, Chintagumpala M, Sahm F, Korbel JO, Rutkowski S, Eggermann T, Gajjar A, Northcott P, Elbracht M, Pfister SM, Kontny U, Kurth I, Germline GPR161 Mutations Predispose to Pediatric Medulloblastoma, J Clin Oncol 38(1) (2020) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Tschaikner P, Regele D, Salvenmoser W, Geley S, Stefan E, Aanstad P, Zebrafish GPR161 Contributes to Basal Hedgehog Repression in a Tissue-specific Manner, bioRxiv (2019) 616482. [DOI] [PubMed] [Google Scholar]
- [123].Pusapati GV, Kong JH, Patel BB, Gouti M, Sagner A, Sircar R, Luchetti G, Ingham PW, Briscoe J, Rohatgi R, G protein-coupled receptors control the sensitivity of cells to the morphogen Sonic Hedgehog, Sci Signal 11(516) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM, Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube, Cold Spring Harb Symp Quant Biol 62 (1997) 451–66. [PubMed] [Google Scholar]
- [125].Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM, Kaplan FS, Yang Y, Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification, Nat Med 19(11) (2013) 1505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT, Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components, Proc Natl Acad Sci U S A 108(4) (2011) 1456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, Yoder BK, Beier DR, THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia, Nat Genet 40(4) (2008) 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Liem KF Jr., Ashe A, He M, Satir P, Moran J, Beier D, Wicking C, Anderson KV, The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking, J Cell Biol 197(6) (2012) 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Caspary T, Larkins CE, Anderson KV, The graded response to Sonic Hedgehog depends on cilia architecture, Dev Cell 12(5) (2007) 767–78. [DOI] [PubMed] [Google Scholar]
- [130].Gigante ED, Taylor MR, Ivanova AA, Kahn RA, Caspary T, ARL13B regulates Sonic hedgehog signaling from outside primary cilia, Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kronenberg HM, Developmental regulation of the growth plate, Nature 423(6937) (2003) 332–6. [DOI] [PubMed] [Google Scholar]
- [132].Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM, PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth, Science 273(5275) (1996) 663–6. [DOI] [PubMed] [Google Scholar]
- [133].Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ, Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein, Science 273(5275) (1996) 613–22. [DOI] [PubMed] [Google Scholar]
- [134].Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK, Intraflagellar transport is essential for endochondral bone formation, Development 134(2) (2007) 307–16. [DOI] [PubMed] [Google Scholar]
- [135].Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R, Development of the post-natal growth plate requires intraflagellar transport proteins, Dev Biol 305(1) (2007) 202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].R Ferreira R, Fukui H, Chow R, Vilfan A, Vermot J, The cilium as a force sensor-myth versus reality, J Cell Sci 132(14) (2019). [DOI] [PubMed] [Google Scholar]
- [137].Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR, Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism, Proceedings of the National Academy of Sciences of the United States of America 104(33) (2007) 13325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD, Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression, J Biol Chem 281(41) (2006) 30884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Machold R, Fishell G, Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors, Neuron 48(1) (2005) 17–24. [DOI] [PubMed] [Google Scholar]
- [140].Wang VY, Rose MF, Zoghbi HY, Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum, Neuron 48(1) (2005) 31–43. [DOI] [PubMed] [Google Scholar]
- [141].Dahmane N, Ruiz i Altaba A, Sonic hedgehog regulates the growth and patterning of the cerebellum, Development 126(14) (1999) 3089–100. [DOI] [PubMed] [Google Scholar]
- [142].Wallace VA, Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum, Curr Biol 9(8) (1999) 445–8. [DOI] [PubMed] [Google Scholar]
- [143].Wechsler-Reya RJ, Scott MP, Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog, Neuron 22(1) (1999) 103–14. [DOI] [PubMed] [Google Scholar]
- [144].Kenney AM, Cole MD, Rowitch DH, Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors, Development 130(1) (2003) 15–28. [DOI] [PubMed] [Google Scholar]
- [145].Oliver TG, Grasfeder LL, Carroll AL, Kaiser C, Gillingham CL, Lin SM, Wickramasinghe R, Scott MP, Wechsler-Reya RJ, Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors, Proc Natl Acad Sci U S A 100(12) (2003) 7331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Rakic P, Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus, J Comp Neurol 141(3) (1971) 283–312. [DOI] [PubMed] [Google Scholar]
- [147].Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL, Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma, Cancer Cell 14(2) (2008) 123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schuller U, Machold R, Fishell G, Rowitch DH, Wainwright BJ, Wechsler-Reya RJ, Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells, Cancer Cell 14(2) (2008) 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A, Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool, Dev Biol 317(1) (2008) 246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ, Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool, J Neurosci 27(36) (2007) 9780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Chang CH, Zanini M, Shirvani H, Cheng JS, Yu H, Feng CH, Mercier AL, Hung SY, Forget A, Wang CH, Cigna SM, Lu IL, Chen WY, Leboucher S, Wang WJ, Ruat M, Spassky N, Tsai JW, Ayrault O, Atoh1 Controls Primary Cilia Formation to Allow for SHH-Triggered Granule Neuron Progenitor Proliferation, Dev Cell 48(2) (2019) 184–199 e5. [DOI] [PubMed] [Google Scholar]
- [152].Staples CJ, Myers KN, Beveridge RD, Patil AA, Lee AJ, Swanton C, Howell M, Boulton SJ, Collis SJ, The centriolar satellite protein Cep131 is important for genome stability, J Cell Sci 125(Pt 20) (2012) 4770–9. [DOI] [PubMed] [Google Scholar]
- [153].Forget A, Bihannic L, Cigna SM, Lefevre C, Remke M, Barnat M, Dodier S, Shirvani H, Mercier A, Mensah A, Garcia M, Humbert S, Taylor MD, Lasorella A, Ayrault O, Shh signaling protects Atoh1 from degradation mediated by the E3 ubiquitin ligase Huwe1 in neural precursors, Dev Cell 29(6) (2014) 649–61. [DOI] [PubMed] [Google Scholar]
- [154].Kim JJ, Gill PS, Rotin L, van Eede M, Henkelman RM, Hui CC, Rosenblum ND, Suppressor of fused controls mid-hindbrain patterning and cerebellar morphogenesis via GLI3 repressor, The Journal of neuroscience : the official journal of the Society for Neuroscience 31(5) (2011) 1825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bai CB, Joyner AL, Gli1 can rescue the in vivo function of Gli2, Development 128(24) (2001) 5161–72. [DOI] [PubMed] [Google Scholar]
- [156].Litingtung Y, Chiang C, Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3, Nat Neurosci 3(10) (2000) 979–85. [DOI] [PubMed] [Google Scholar]
- [157].Bai CB, Stephen D, Joyner AL, All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3, Dev Cell 6(1) (2004) 103–15. [DOI] [PubMed] [Google Scholar]
- [158].Liu J, Heydeck W, Zeng H, Liu A, Dual function of suppressor of fused in Hh pathway activation and mouse spinal cord patterning, Dev Biol 362(2) (2012) 141–53. [DOI] [PubMed] [Google Scholar]
- [159].Oosterveen T, Kurdija S, Alekseenko Z, Uhde CW, Bergsland M, Sandberg M, Andersson E, Dias JM, Muhr J, Ericson J, Mechanistic differences in the transcriptional interpretation of local and long-range Shh morphogen signaling, Dev Cell 23(5) (2012) 1006–19. [DOI] [PubMed] [Google Scholar]
- [160].Falkenstein KN, Vokes SA, Transcriptional regulation of graded Hedgehog signaling, Semin Cell Dev Biol 33 (2014) 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Jiwani T, Kim JJ, Rosenblum ND, Suppressor of fused controls cerebellum granule cell proliferation by suppressing Fgf8 and spatially regulating Gli proteins, Development 147(3) (2020). [DOI] [PubMed] [Google Scholar]
- [162].Bose J, Grotewold L, Ruther U, Pallister-Hall syndrome phenotype in mice mutant for Gli3, Hum Mol Genet 11(9) (2002) 1129–35. [DOI] [PubMed] [Google Scholar]
- [163].Cao T, Wang C, Yang M, Wu C, Wang B, Mouse limbs expressing only the Gli3 repressor resemble those of Sonic hedgehog mutants, Dev Biol 379(2) (2013) 221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A, Dual and opposing roles of primary cilia in medulloblastoma development, Nat Med 15(9) (2009) 1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH Jr., Dlugosz AA, Reiter JF, Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis, Nat Med 15(9) (2009) 1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Motoyama J, Milenkovic L, Iwama M, Shikata Y, Scott MP, Hui CC, Differential requirement for Gli2 and Gli3 in ventral neural cell fate specification, Dev Biol 259(1) (2003) 150–61. [DOI] [PubMed] [Google Scholar]
- [167].Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, McKinnon PJ, Loss of suppressor-of-fused function promotes tumorigenesis, Oncogene 26(44) (2007) 6442–7. [DOI] [PubMed] [Google Scholar]
- [168].Yin WC, Satkunendran T, Mo R, Morrissy S, Zhang X, Huang ES, Uuskula-Reimand L, Hou H, Son JE, Liu W, Liu YC, Zhang J, Parker J, Wang X, Farooq H, Selvadurai H, Chen X, Ngan ES, Cheng SY, Dirks PB, Angers S, Wilson MD, Taylor MD, Hui CC, Dual Regulatory Functions of SUFU and Targetome of GLI2 in SHH Subgroup Medulloblastoma, Dev Cell 48(2) (2019) 167–183 e5. [DOI] [PubMed] [Google Scholar]
- [169].Brugmann SA, Allen NC, James AW, Mekonnen Z, Madan E, Helms JA, A primary cilia-dependent etiology for midline facial disorders, Hum Mol Genet 19(8) (2010) 1577–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, Ericson J, Briscoe J, Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity, Genes Dev 16(22) (2002) 2865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, Mackem S, Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud, Dev Cell 14(4) (2008) 624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C, Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity, Nature 418(6901) (2002) 979–83. [DOI] [PubMed] [Google Scholar]
- [173].Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, Yoder BK, Loss of the Tg737 protein results in skeletal patterning defects, Dev Dyn 227(1) (2003) 78–90. [DOI] [PubMed] [Google Scholar]
- [174].Hilgendorf KI, Johnson CT, Mezger A, Rice SL, Norris AM, Demeter J, Greenleaf WJ, Reiter JF, Kopinke D, Jackson PK, Omega-3 Fatty Acids Activate Ciliary FFAR4 to Control Adipogenesis, Cell 179(6) (2019) 1289–1305 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Kopinke D, Roberson EC, Reiter JF, Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis, Cell 170(2) (2017) 340–351 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Villalobos E, Criollo A, Schiattarella GG, Altamirano F, French KM, May HI, Jiang N, Nguyen NUN, Romero D, Roa JC, Garcia L, Diaz-Araya G, Morselli E, Ferdous A, Conway SJ, Sadek HA, Gillette TG, Lavandero S, Hill JA, Fibroblast Primary Cilia Are Required for Cardiac Fibrosis, Circulation 139(20) (2019) 2342–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Distler JHW, Gyorfi AH, Ramanujam M, Whitfield ML, Konigshoff M, Lafyatis R, Shared and distinct mechanisms of fibrosis, Nat Rev Rheumatol 15(12) (2019) 705–730. [DOI] [PubMed] [Google Scholar]
- [178].Soliman H, Paylor B, Scott RW, Lemos DR, Chang C, Arostegui M, Low M, Lee C, Fiore D, Braghetta P, Pospichalova V, Barkauskas CE, Korinek V, Rampazzo A, MacLeod K, Underhill TM, Rossi FMV, Pathogenic Potential of Hic1-Expressing Cardiac Stromal Progenitors, Cell Stem Cell 26(2) (2020) 205–220 e8. [DOI] [PubMed] [Google Scholar]
- [179].Relaix F, Zammit PS, Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage, Development 139(16) (2012) 2845–56. [DOI] [PubMed] [Google Scholar]
- [180].Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM, Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis, Nat Cell Biol 12(2) (2010) 153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K, Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle, Nat Cell Biol 12(2) (2010) 143–52. [DOI] [PubMed] [Google Scholar]
- [182].Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G, Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration, Development 138(17) (2011) 3625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA, Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle, Cell Rep 27(7) (2019) 2029–2035 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lukjanenko L, Karaz S, Stuelsatz P, Gurriaran-Rodriguez U, Michaud J, Dammone G, Sizzano F, Mashinchian O, Ancel S, Migliavacca E, Liot S, Jacot G, Metairon S, Raymond F, Descombes P, Palini A, Chazaud B, Rudnicki MA, Bentzinger CF, Feige JN, Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors, Cell Stem Cell 24(3) (2019) 433–446 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Jaafar Marican NH, Cruz-Migoni SB, Borycki AG, Asymmetric Distribution of Primary Cilia Allocates Satellite Cells for Self-Renewal, Stem Cell Reports 6(6) (2016) 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Fu W, Asp P, Canter B, Dynlacht BD, Primary cilia control hedgehog signaling during muscle differentiation and are deregulated in rhabdomyosarcoma, Proc Natl Acad Sci U S A 111(25) (2014) 9151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Renault MA, Robbesyn F, Chapouly C, Yao Q, Vandierdonck S, Reynaud A, Belloc I, Traiffort E, Ruat M, Desgranges C, Gadeau AP, Hedgehog-dependent regulation of angiogenesis and myogenesis is impaired in aged mice, Arterioscler Thromb Vasc Biol 33(12) (2013) 2858–66. [DOI] [PubMed] [Google Scholar]
- [188].Piccioni A, Gaetani E, Neri V, Gatto I, Palladino M, Silver M, Smith RC, Giarretta I, Pola E, Hlatky L, Pola R, Sonic hedgehog therapy in a mouse model of age-associated impairment of skeletal muscle regeneration, J Gerontol A Biol Sci Med Sci 69(3) (2014) 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Lim YC, McGlashan SR, Cooling MT, Long DS, Culture and detection of primary cilia in endothelial cell models, Cilia 4 (2015) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Gupta R, Mackie AR, Misener S, Liu L, Losordo DW, Kishore R, Endothelial smoothened-dependent hedgehog signaling is not required for sonic hedgehog induced angiogenesis or ischemic tissue repair, Lab Invest 98(5) (2018) 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Hu JK, McGlinn E, Harfe BD, Kardon G, Tabin CJ, Autonomous and nonautonomous roles of Hedgehog signaling in regulating limb muscle formation, Genes Dev 26(18) (2012) 2088–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Anderson C, Williams VC, Moyon B, Daubas P, Tajbakhsh S, Buckingham ME, Shiroishi T, Hughes SM, Borycki AG, Sonic hedgehog acts cell-autonomously on muscle precursor cells to generate limb muscle diversity, Genes Dev 26(18) (2012) 2103–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Borycki AG, <Borycki, Emerson, 1999, Dev, Sonic hedgehog controls epaxial muscle determination through Myf5 activation.pdf>, Development 126 (1999). [DOI] [PubMed] [Google Scholar]
- [194].Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C, Corbley M, Kearney M, Isner JM, Losordo DW, Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia, Circulation 108(4) (2003) 479–85. [DOI] [PubMed] [Google Scholar]
- [195].Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM, The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors, Nat Med 7(6) (2001) 706–11. [DOI] [PubMed] [Google Scholar]
- [196].Straface G, Aprahamian T, Flex A, Gaetani E, Biscetti F, Smith RC, Pecorini G, Pola E, Angelini F, Stigliano E, Castellot JJ Jr., Losordo DW, Pola R, Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration, J Cell Mol Med 13(8B) (2009) 2424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Renault MA, Chapouly C, Yao Q, Larrieu-Lahargue F, Vandierdonck S, Reynaud A, Petit M, Jaspard-Vinassa B, Belloc I, Traiffort E, Ruat M, Duplaa C, Couffinhal T, Desgranges C, Gadeau AP, Desert hedgehog promotes ischemia-induced angiogenesis by ensuring peripheral nerve survival, Circ Res 112(5) (2013) 762–70. [DOI] [PubMed] [Google Scholar]
- [198].Piccioni A, Gaetani E, Palladino M, Gatto I, Smith RC, Neri V, Marcantoni M, Giarretta I, Silver M, Straino S, Capogrossi M, Landolfi R, Pola R, Sonic hedgehog gene therapy increases the ability of the dystrophic skeletal muscle to regenerate after injury, Gene Ther 21(4) (2014) 413–21. [DOI] [PubMed] [Google Scholar]
- [199].Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC, Cardiac Fibrosis: The Fibroblast Awakens, Circ Res 118(6) (2016) 1021–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Mangos S, Lam PY, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA, The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation, Dis Model Mech 3(5–6) (2010) 354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Xiao Q, Hou N, Wang YP, He LS, He YH, Zhang GP, Yi Q, Liu SM, Chen MS, Luo JD, Impaired sonic hedgehog pathway contributes to cardiac dysfunction in type 1 diabetic mice with myocardial infarction, Cardiovasc Res 95(4) (2012) 507–16. [DOI] [PubMed] [Google Scholar]
- [202].Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, Thorne T, Takenaka H, Aikawa R, Goukassian D, von Samson P, Hamada H, Yoon YS, Silver M, Eaton E, Ma H, Heyd L, Kearney M, Munger W, Porter JA, Kishore R, Losordo DW, Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling, Nat Med 11(11) (2005) 1197–204. [DOI] [PubMed] [Google Scholar]
- [203].Ponikowski P, Anker SD, Szachniewicz J, Okonko D, Ledwidge M, Zymlinski R, Ryan E, Wasserman SM, Baker N, Rosser D, Rosen SD, Poole-Wilson PA, Banasiak W, Coats AJ, McDonald K, Effect of darbepoetin alfa on exercise tolerance in anemic patients with symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled trial, J Am Coll Cardiol 49(7) (2007) 753–62. [DOI] [PubMed] [Google Scholar]
- [204].Silverberg DS, Wexler D, Sheps D, Blum M, Keren G, Baruch R, Schwartz D, Yachnin T, Steinbruch S, Shapira I, Laniado S, Iaina A, The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study, J Am Coll Cardiol 37(7) (2001) 1775–80. [DOI] [PubMed] [Google Scholar]
- [205].Ueda K, Takano H, Niitsuma Y, Hasegawa H, Uchiyama R, Oka T, Miyazaki M, Nakaya H, Komuro I, Sonic hedgehog is a critical mediator of erythropoietin-induced cardiac protection in mice, J Clin Invest 120(6) (2010) 2016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Bijlsma MF, Leenders PJ, Janssen BJ, Peppelenbosch MP, Ten Cate H, Spek CA, Endogenous hedgehog expression contributes to myocardial ischemia-reperfusion-induced injury, Exp Biol Med (Maywood) 233(8) (2008) 989–96. [DOI] [PubMed] [Google Scholar]
- [207].Chinchilla P, Xiao L, Kazanietz MG, Riobo NA, Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways, Cell Cycle 9(3) (2010) 570–79. [DOI] [PubMed] [Google Scholar]
- [208].Rash B, <Rash, Biesele, 1969, Cilia in cardiac differentiation.pdf>, (1969). [DOI] [PubMed] [Google Scholar]
- [209].Clement CA, Kristensen SG, Mollgard K, Pazour GJ, Yoder BK, Larsen LA, Christensen ST, The primary cilium coordinates early cardiogenesis and hedgehog signaling in cardiomyocyte differentiation, J Cell Sci 122(Pt 17) (2009) 3070–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Kaur S, McGlashan SR, Ward ML, Evidence of primary cilia in the developing rat heart, Cilia 7 (2018) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Carbe CJ, Cheng L, Addya S, Gold JI, Gao E, Koch WJ, Riobo NA, Gi proteins mediate activation of the canonical hedgehog pathway in the myocardium, Am J Physiol Heart Circ Physiol 307(1) (2014) H66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Louch WE, Sheehan KA, Wolska BM, Methods in cardiomyocyte isolation, culture, and gene transfer, J Mol Cell Cardiol 51(3) (2011) 288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Lavine KJ, Kovacs A, Ornitz DM, Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice, J Clin Invest 118(7) (2008) 2404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Bersell K, Choudhury S, Mollova M, Polizzotti BD, Ganapathy B, Walsh S, Wadugu B, Arab S, Kuhn B, Moderate and high amounts of tamoxifen in alphaMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death, Dis Model Mech 6(6) (2013) 1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Ghaben AL, Scherer PE, Adipogenesis and metabolic health, Nat Rev Mol Cell Biol 20(4) (2019) 242–258. [DOI] [PubMed] [Google Scholar]
- [216].Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H, Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation, Proc Natl Acad Sci U S A 106(6) (2009) 1820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Zhu D, Shi S, Wang H, Liao K, Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes, J Cell Sci 122(Pt 15) (2009) 2760–8. [DOI] [PubMed] [Google Scholar]
- [218].Forcioli-Conti N, Lacas-Gervais S, Dani C, Peraldi P, The primary cilium undergoes dynamic size modifications during adipocyte differentiation of human adipose stem cells, Biochem Biophys Res Commun 458(1) (2015) 117–22. [DOI] [PubMed] [Google Scholar]
- [219].Dalbay MT, Thorpe SD, Connelly JT, Chapple JP, Knight MM, Adipogenic Differentiation of hMSCs is Mediated by Recruitment of IGF-1r Onto the Primary Cilium Associated With Cilia Elongation, Stem Cells 33(6) (2015) 1952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].James AW, Leucht P, Levi B, Carre AL, Xu Y, Helms JA, Longaker MT, Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells, Tissue Eng Part A 16(8) (2010) 2605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui CC, Esterbauer H, Penninger JM, Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate, Cell 140(1) (2010) 148–60. [DOI] [PubMed] [Google Scholar]
- [222].Suh JM, Gao X, McKay J, McKay R, Salo Z, Graff JM, Hedgehog signaling plays a conserved role in inhibiting fat formation, Cell Metab 3(1) (2006) 25–34. [DOI] [PubMed] [Google Scholar]
- [223].Nosavanh L, Yu DH, Jaehnig EJ, Tong Q, Shen L, Chen MH, Cell-autonomous activation of Hedgehog signaling inhibits brown adipose tissue development, Proc Natl Acad Sci U S A 112(16) (2015) 5069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Lee HJ, Jo SB, Romer AI, Lim HJ, Kim MJ, Koo SH, Krauss RS, Kang JS, Overweight in mice and enhanced adipogenesis in vitro are associated with lack of the hedgehog coreceptor boc, Diabetes 64(6) (2015) 2092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Shi Y, Long F, Hedgehog signaling via Gli2 prevents obesity induced by high-fat diet in adult mice, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Ritter A, Friemel A, Kreis NN, Hoock SC, Roth S, Kielland-Kaisen U, Bruggmann D, Solbach C, Louwen F, Yuan J, Primary Cilia Are Dysfunctional in Obese Adipose-Derived Mesenchymal Stem Cells, Stem Cell Reports 10(2) (2018) 583–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Ritter A, Kreis NN, Roth S, Friemel A, Jennewein L, Eichbaum C, Solbach C, Louwen F, Yuan J, Restoration of primary cilia in obese adipose-derived mesenchymal stem cells by inhibiting Aurora A or extracellular signal-regulated kinase, Stem Cell Res Ther 10(1) (2019) 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Marion V, Mockel A, De Melo C, Obringer C, Claussmann A, Simon A, Messaddeq N, Durand M, Dupuis L, Loeffler JP, King P, Mutter-Schmidt C, Petrovsky N, Stoetzel C, Dollfus H, BBS-induced ciliary defect enhances adipogenesis, causing paradoxical higher-insulin sensitivity, glucose usage, and decreased inflammatory response, Cell Metab 16(3) (2012) 363–77. [DOI] [PubMed] [Google Scholar]
- [229].Ye F, Nager AR, Nachury MV, BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone, J Cell Biol 217(5) (2018) 1847–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Sun K, Tordjman J, Clement K, Scherer PE, Fibrosis and adipose tissue dysfunction, Cell Metab 18(4) (2013) 470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Marcelin G, Ferreira A, Liu Y, Atlan M, Aron-Wisnewsky J, Pelloux V, Botbol Y, Ambrosini M, Fradet M, Rouault C, Henegar C, Hulot JS, Poitou C, Torcivia A, Nail-Barthelemy R, Bichet JC, Gautier EL, Clement K, A PDGFRalpha-Mediated Switch toward CD9(high) Adipocyte Progenitors Controls Obesity-Induced Adipose Tissue Fibrosis, Cell Metab 25(3) (2017) 673–685. [DOI] [PubMed] [Google Scholar]
- [232].Gao H, Geng T, Huang T, Zhao Q, Fish oil supplementation and insulin sensitivity: a systematic review and meta-analysis, Lipids Health Dis 16(1) (2017) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Friedman SL, Sheppard D, Duffield JS, Violette S, Therapy for fibrotic diseases: nearing the starting line, Sci Transl Med 5(167) (2013) 167sr1. [DOI] [PubMed] [Google Scholar]


