Abstract
Background
Multiple myeloma (MM) with the translocation, t(11;14) may have inferior outcomes compared to other standard risk MM and has been suggested to portend a worse prognosis in African Americans compared to Whites. We utilized the Center for International Blood and Marrow Transplant Research® (CIBMTR®) database to study the impact of t(11;14) in clinical outcome of MM patients with African American and White descent.
Methods
We evaluated 3,538 patients who underwent autologous hematopoietic cell transplantation (autoHCT) for MM from 2008–2016 and reported to the CIBMTR®. Patients were analyzed in four groups: African American with t(11;14) (n=117), African American without t(11;14) (n= 968), Whites with t(11;14) (n= 266) and Whites without t(11;14) (n=2,187).
Results
African Americans with t(11;14) were younger, had lower Karnofsky score, more advanced stage, with higher Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI). Fewer African Americans with t(11;14) (21%) had a coexistent high-risk marker compared to Whites with t(11;14) (27%). On multivariate analysis, race and t(11;14) had no association with progression-free survival. However, overall survival was superior among African Americans with t(11;14) compared to Whites with t(11;14), hazard ratio 0.53 (95% confidence interval, 0.30–0.93) (p= 0.03). Survival was also associated with female sex, stage, time from diagnosis to transplant, low HCT-CI, and receipt of maintenance.
Conclusion
We conclude that race may have a differential impact on survival of patients with t(11;14) MM who undergo autoHCT and needs to be further studied.
Keywords: race, t(11;14), myeloma, Transplant, Outcomes
Precis:
Race has a differential impact on survival of patients with t(11;14) multiple myeloma who undergo autologous hematopoietic cell transplantation. African Americans with t(11;14) have superior survival compared to Whites after adjusting for other prognostic factors.
Introduction
Multiple myeloma (MM) is a heterogeneous malignant plasma cell cancer characterized by an abnormal proliferation of plasma cells in the bone marrow.1 Cytogenetic abnormalities are an important determinant of prognosis in patients with MM.2–4 The translocation t(11;14), detected by fluorescence in situ hybridization (FISH) testing in MM, has been considered a standard risk marker with favorable prognosis.2, 5 In the era of novel therapies, several retrospective studies have demonstrated an inferior outcome in MM patients harboring t(11;14) compared to other standard risk MM.6–9
Race also plays an important role in MM particularly in the United States where the disease is more prevalent in African Americans than Whites and may be associated with outcome disparities.10–14 Moreover, African American patients with MM may have a higher prevalence of t(11;14) compared to Whites.15, 16 Recently, an analysis from the Connect Multiple Myeloma Disease Registry (Connect MM), demonstrated that the t(11;14) abnormality was an independent, negative prognostic factor for overall survival (OS) among African American patients compared to other races.17 The difference in outcome was observed in patients receiving similar induction regimens, comparable autologous hematopoietic cell transplant (autoHCT) rates and overall length of treatment.
Historically, the discrepancy in clinical outcome between African Americans and Whites in MM has been linked to inadequate access to health care and underutilization of autoHCT in African American patients.12–14 With equal access, African American patients have been shown to have similar, and in some studies superior, survival compared to Whites.13, 18 In order to study the impact of t(11;14) on race in MM patients uniformly treated with autoHCT in the US, we conducted this observational study using the Center for International Blood and Marrow Transplant Research® (CIBMTR®) database.
Patients and Methods
Data source:
The CIBMTR is a prospectively maintained transplant registry that collects transplant data from over 500 centers worldwide. Data are submitted to the Statistical Center at the Medical College of Wisconsin (MCW) in Milwaukee, where computerized checks for discrepancies, physician’s review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants and are MCW Institutional Review Board-approved. Transplant data are collected at two levels: registration and research. Registration data include disease type, age, sex, date of diagnosis, graft type, conditioning regimen, post-transplantation disease progression, survival, and cause of death, and includes all transplantations reported to the CIBMTR. More detailed clinical data are collected from a subgroup of registered patients selected for research data by using a weighted randomization scheme. Both the registration data and the research data are collected pre-transplantation, at 100 days and 6 months post-transplantation, and annually thereafter until death or last follow-up. We included patients with research level data in this study.
Patient Selection:
The selection criteria for the study are summarized in Supplementary Table 1. Briefly, US adult MM patients who underwent a first peripheral blood autoHCT between 2008 and 2016 with melphalan conditioning, self-identified as White or African American, with available cytogenetic/FISH data, and registered in the research track in the CIBMTR were included (N=3,538). Among them 383 (11%) patients had t(11;14) and 3,155 (89%) patients were without t(11;14). High risk markers included the presence of 17pdel, t(4;14), t(14;16), +1q21, and 1pdel.
Definition of outcomes:
The endpoints of interest included transplant-related mortality (TRM), relapse/progression, progression-free survival (PFS), and overall survival (OS) after transplant. Disease response was assessed using the International Myeloma Working Group (IMWG) consensus criteria.19 PFS was defined as survival without disease progression and/or relapse from the time of autoHCT; progression and/or relapse and death were considered events. OS was defined as time from autoHCT till death from any cause with censoring of surviving patients at last follow-up.
Statistical Analysis:
Patient-, disease- and treatment-related factors were described and compared using the Pearson chi-square test for categorical and the Mann-Whitney test for continuous variables. Estimates of outcomes were reported as probabilities with 95% confidence intervals (CI). Probability of PFS and OS was calculated using the Kaplan-Meier estimator while competing risk endpoints were summarized using cumulative incidence estimates. Comparison of survival and cumulative incidence curves was done using the log-rank test and Gray’s test, respectively. Multivariate Cox proportional hazards regression models were fitted using the stepwise variable selection to identifying prognostic factors using the above clinical outcomes. Race with or without t(11;14) was considered the main effect in the model during the variable selection process with 4 resultant groups (1) African Americans with t(11;14) and Whites with t(11;14) (2) African Americans without t(11;14) and Whites without t(11;14). During the group analyses we also checked to see if any new covariates entered the models. Other covariates tested in the multivariate models included age, sex, Karnofsky performance status (KPS), Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), insurance status, marital status, employment status, education, stage at diagnosis, presence of high risk molecular markers, time from diagnosis to transplant, creatinine at diagnosis, lines of treatment, induction with doublets/triplets, disease status at transplant, melphalan dose, year of transplant, and post-transplant maintenance. Once the final model was determined, we explored interactions between the main effect and other prognostic variables. The assumption of proportionality was tested using a time-dependent interaction term.
Results
Baseline characteristics are summarized in Table 1. These were evenly balanced between African Americans and Whites with or without (w/o) t(11;14) with the following differences. The median age of patients was younger in the African Americans than Whites with 57 (range, 31–75) and 58 (range, 20–78) years with or w/o t(11;14) in African Americans versus 61 (range, 30–79) and 60 (range,23–80) years with or w/o t(11;14) in Whites, respectively. African Americans had lower KPS score than Whites: KPS <90 was seen in 49% and 53% of patients African Americans with or w/o t(11;14) compared to 40% each in Whites with or w/o t(11;14), respectively. A higher proportion of patients with t(11;14) had light chain myeloma in African Americans (27% vs. 17%) and Whites (35% vs. 17%). Similarly, a higher proportion of patients with t(11;14) MM had elevated creatinine at diagnosis (≥ 2 mg/dl), 17% vs 13%, and more stage III (59% vs 55%). A higher percentage of patients in African American (44% and 42%) had HCT-CI score ≥ 3 than Whites (39% and 33%), with or w/o t(11;14), respectively. Among the patients with t(11;14), Whites were more likely to harbor high-risk abnormality compared to African Americans (27% vs 21%). More African American patients were seen in recent years of the study compared to Whites resulting in a shorter median follow-up in the African American cohort compared to the White cohort. Post-transplant maintenance was similar by race and 24–30% of patients received no maintenance (Table 2).
Table 1.
Characteristics of adult patients who underwent PB and melphalan based first AHCT for multiple myeloma with t(11;14) abnormality and reported with CIBMTR in 2008–2016
| Characteristic | AA with t(11;14) N=117 |
AA w/o t(11;14) N=968 |
Whites with t(11;14) N=266 |
Whites w/o t(11;14) N=2,187 |
|---|---|---|---|---|
| No. of centers | 45 | 98 | 71 | 115 |
| Median age (range) | 57 (31–75) | 58 (20–78) | 61 (30–79) | 60 (23–80) |
| Gender, Male (%) | 63 (54) | 463 (48) | 143 (54) | 1323 (60) |
| Karnofsky score <90 | 57 (49) | 512 (53) | 107 (40) | 882 (40) |
| HCT-CI score ≥ 3 | 51 (44) | 410 (42) | 103 (39) | 729 (33) |
| Insurance | ||||
| Medicare/Medicaid | 44 (38) | 403 (42) | 94 (35) | 723 (33) |
| Employer/Private Insurance | 73 (62) | 542 (56) | 169 (64) | 1438 (66) |
| Not reported | 0 | 23 (2) | 3 (1) | 26 (1) |
| Education | ||||
| High school or less | 54 (46) | 465 (48) | 91 (34) | 873 (40) |
| College | 37 (32) | 266 (27) | 84 (32) | 650 (30) |
| Graduate school | 5 (4) | 26 (3) | 20 (8) | 90 (4) |
| Not reported | 21 (18) | 211 (22) | 71 (27) | 574 (26) |
| Marriage status | ||||
| Single/Divorced | 46 (39) | 393 (41) | 67 (25) | 456 (21) |
| Married, live with partner | 66 (56) | 530 (55) | 193 (73) | 1684 (77) |
| Not reported | 5 (4) | 45 (5) | 6 (2) | 47 (2) |
| Employment status | ||||
| Unemployed | 45 (38) | 316 (33) | 64 (24) | 455 (21) |
| Employed | 38 (32) | 295 (30) | 89 (33) | 756 (35) |
| Retired | 29 (25) | 265 (27) | 89 (33) | 758 (35) |
| Not reported | 5 (4) | 92 (10) | 24 (9) | 218 (10) |
| Immunochemical subtype | ||||
| IgG | 62 (53) | 640 (66) | 116 (44) | 1294 (59) |
| IgA | 18 (15) | 154 (16) | 43 (16) | 469 (21) |
| Light chain | 32 (27) | 163 (17) | 92 (35) | 379 (17) |
| Non-secretory | 3 (3) | 8 (1) | 6 (2) | 27 (1) |
| Others | 2 (2) | 3 (0) | 9 (3) | 18 (1) |
| Stage at diagnosis (ISS/DSS), stage III | 72 (62) | 534 (55) | 153 (58) | 1189 (54) |
| Cytogenetics | ||||
| No abnormality | 0 | 397 (41) | 0 | 918 (42) |
| t(11;14) only | 39 (33) | 0 | 75 (28) | 0 |
| t(11;14) + other standard risk | 53 (45) | 0 | 118 (44) | 0 |
| t(11;14) + other high risk | 25 (21) | 0 | 73 (27) | 0 |
| Standard risk | 0 | 339 (35) | 0 | 770 (35) |
| High risk abnormality (not t(11;14)) | 0 | 232 (24) | 0 | 499 (23) |
| Serum creatinine at diagnosis, mg/dl ≥ 2 mg/dl | 19 (16) | 140 (14) | 45 (17) | 266 (12) |
| Lines of induction chemotherapy, 1 | 77 (66) | 683 (71) | 162 (61) | 1487 (68) |
| Induction Chemotherapy | ||||
| Triples (VTD/VCD/VRD) | 88 (75) | 678 (70) | 196 (74) | 1320 (60) |
| Doublets (TD/RD/VD) | 24 (21) | 251 (26) | 63 (24) | 767 (35) |
| Other | 4 (3) | 30 (3) | 5 (2) | 79 (4) |
| Not reported | 1 (1) | 9 (1) | 2 (1) | 21 (1) |
| Disease status prior to transplant | ||||
| ≥VGPR | 45 (38) | 471 (49) | 103 (38) | 1002 (46) |
| ≤PR | 72 (62) | 495 (52) | 163 (62) | 1182 (54) |
| Not reported | 0 | 2 (0) | 0 | 3 (0) |
| Melphalan 200 mg/m2 conditioning | 83 (71) | 661 (68) | 197 (74) | 1594 (73) |
| Time from diagnosis to transplant | ||||
| <6 months | 28 (24) | 221 (23) | 80 (30) | 716 (33) |
| 6 – 12 months | 62 (53) | 468 (48) | 131 (49) | 999 (46) |
| 12 – 24 months | 14 (12) | 175 (18) | 38 (14) | 275 (13) |
| ≥ 24 months | 13 (11) | 104 (11) | 17 (6) | 197 (9) |
| Type of transplant | ||||
| Single HCT | 106 (91) | 928 (96) | 253 (95) | 2034 (93) |
| Tandem AutoHCT | 11 (9) | 40 (4) | 13 (5) | 153 (7) |
| Year of transplant | ||||
| 2008 | 9 (8) | 96 (10) | 36 (14) | 475 (22) |
| 2009 | 1 (1) | 53 (5) | 14 (5) | 168 (8) |
| 2010 | 10 (9) | 68 (7) | 7 (3) | 127 (6) |
| 2011 | 5 (4) | 47 (5) | 24 (9) | 201 (9) |
| 2012 | 2 (2) | 42 (4) | 27 (10) | 192 (9) |
| 2013 | 13 (11) | 92 (10) | 42 (16) | 316 (14) |
| 2014 | 20 (17) | 146 (15) | 31 (12) | 218 (10) |
| 2015 | 26 (22) | 196 (20) | 36 (14) | 261 (12) |
| 2016 | 31 (26) | 228 (24) | 49 (18) | 229 (10) |
| Median follow-up of survivors (range), months | 25 (4–79) | 27 (3–110) | 37 (4–111) | 51 (3–120) |
w/o; without, VTD; bortezomib (V), thalidomide (T), dexamethasone (D), VCD; bortezomib, cyclophosphamide (C), dexamethasone (D), VRD; bortezomib (V), lenalidomide (R), dexamethasone (D)
Table 2.
Post auto-HCT maintenance therapy
| AA w t(11;14) | AA w/o t(11;14) | Whites w t(11;14) | Whites w/o t(11;14) | |
|---|---|---|---|---|
| Number of patients | 117 | 968 | 266 | 2,187 |
| Bortezomib/Lenalidomide ± other | 14 (12) | 97 (10) | 42 (16) | 304 (14) |
| Bortezomib ± other (no Lenalidomide) | 6 (5) | 71 (7) | 17 (6) | 132 (6) |
| Lenalidomide ± other (no Bortezomib) | 46 (39) | 461 (48) | 106 (40) | 861 (39) |
| Other | 15 (13) | 103 (11) | 28 (11) | 304 (14) |
| No maintenance | 35 (30) | 236 (24) | 73 (27) | 580 (27) |
| Missing | 1 (<1) | 0 | 0 | 6 (<1) |
w: with, w/o: without. AA- African American
Univariate outcomes:
Univariate outcomes are shown in Table 3. Treatment-related mortality at multiple time points post autoHCT was not significantly different in African Americans and Whites with or w/o t(11;14). There was no statistically significant difference in overall p-value for PFS (p 0.06) and OS (p 0.41) by race and t(11;14). When restricted to 2013–2016 years, African Americans had superior OS at 2 years compared to Whites (p 0.02), with 91 (83–96)% vs 88 (82–90)% for African Americans with t(11;14) versus Whites with t(11;14) and 93 (91–95)% versus 88 (86–90%) for African Americans without t(11;14) versus Whites without t(11;14) (Supplementary table 2).
Table 3.
Univariate outcome in African American and Caucasians with or without t(11;14)
| AA w t(11;14) (N = 117) |
AA w/o t(11;14) (N = 968) |
Whites w t(11;14) (N = 266) |
Whites w/o t(11;14) (N = 2,187) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | N | Prob (95% CI) |
N | Prob (95% CI) |
N | Prob (95% CI) |
N | Prob (95% CI) |
p-value |
| TRM | 115 | 959 | 259 | 2164 | 0.68 | ||||
| 100-day | 1 (0–3)% | 1 (0–1)% | 1 (0–3)% | 1 (0–1)% | 0.77 | ||||
| 1-year | 2 (0–5)% | 1 (1–2)% | 3 (1–5)% | 2 (1–2)% | 0.66 | ||||
| 2-year | 2 (0–5)% | 2 (1–3)% | 3 (1–6)% | 2 (2–3)% | 0.79 | ||||
| PFS | 115 | 959 | 259 | 2164 | 0.06 | ||||
| 1-year | 84 (76–90)% | 81 (78–83)% | 76 (70–81)% | 79 (78–81)% | 0.20 | ||||
| 2-year | 65 (54–74)% | 65 (61–68)% | 54 (47–60)% | 61 (59–63)% | 0.02 | ||||
| OS | 117 | 968 | 266 | 2187 | 0.41 | ||||
| 1-year | 95 (90–98)% | 95 (94–97)% | 93 (90–96)% | 95 (94–96)% | 0.69 | ||||
| 2-year | 91 (85–96)% | 89 (86–91)% | 86 (81–90)% | 87 (86–89)% | 0.35 | ||||
AA; Africans Americans, N; number, Prob; probability, w; with, w/o without, TRM; transplant related mortality, PFS; progression free survival, OS; overall survival, N; number evaluated
Multivariate analysis of survival outcomes
Progression-free survival:
In multivariate analysis, there was no difference in PFS by the main effect (p 0.08) (Table 4). Factors associated with worse PFS included a KPS score < 90 (HR; 1.19, 95% CI 1.14–1.02, p= 0.02), HCT-CI > 2 (HR; 1.21, 95% CI; 1.05–1.39, p=0.01) and receipt of more than 1 line of induction chemotherapy (HR; 1.46, 95% CI 1.29–1.64, p <0.0001). Female sex (HR 0.88, 95% CI 0.78–0.98, p=0.02), disease status of VGPR or better at transplant (HR; 0.8, 95% CI 0.76–0.9, p= 0.0001), use of melphalan conditioning dose of 200 mg/m2 (HR; 0.86, 95% CI 0.76–0.97, p= 0.02) and use of post-HCT maintenance (HR; 0.55, 95% CI 0.49–0.61, p < 0.0001) were associated with favorable PFS in multivariate analysis.
Table 4.
Multivariate analysis for progression free survival by main groups
| Parameter | Level | N | Hazard Ratio | 95% Hazard Ratio Confidence Limits | p-value | |
|---|---|---|---|---|---|---|
| Race | AA w t(11;14) | 114 | 1.00 | 0.08 | ||
| AA w/o t(11;14) | 956 | 1.05 | 0.76 | 1.46 | 0.96 | |
| Whites w t(11;14) | 258 | 1.31 | 0.91 | 1.89 | 0.14 | |
| Whites w/o t(11;14) | 2157 | 1.01 | 0.73 | 1.39 | 0.77 | |
| Age at transplant | < 60 | 1773 | 1.00 | |||
| 60+ | 1712 | 1.06 | 0.95 | 1.19 | 0.28 | |
| Sex | Male | 1969 | 1.00 | |||
| Female | 1516 | 0.88 | 0.78 | 0.98 | 0.02 | |
| Karnofsky | ≥90 | 1872 | 1.00 | 0.04 | ||
| < 90 | 1531 | 1.19 | 1.14 | 1.02 | 0.02 | |
| Missing | 82 | 1.25 | 0.89 | 1.76 | 0.19 | |
| HCT-CI | 0 | 1074 | 1.00 | 0.03 | ||
| 1 | 548 | 1.23 | 1.04 | 1.46 | 0.02 | |
| 2 | 588 | 1.10 | 0.93 | 1.31 | 0.27 | |
| >2 | 1275 | 1.21 | 1.05 | 1.39 | 0.01 | |
| Lines of chemo | 1 | 2373 | 1.00 | <.0001 | ||
| >1 | 1079 | 1.46 | 1.29 | 1.64 | <.0001 | |
| Missing | 33 | 1.00 | 0.55 | 1.82 | 1.00 | |
| Disease status | PR/SD/PD/Relapse | 1888 | 1.00 | |||
| sCR/CR/VGPR | 1597 | 0.80 | 0.76 | 0.90 | 0.0001 | |
| Melphalan dose | MEL 140 | 990 | 1.00 | |||
| MEL 200 | 2495 | 0.86 | 0.76 | 0.97 | 0.02 | |
| Time from diagnosis to transplant | <6 | 1032 | 1.00 | 0.0014 | ||
| 6–12 | 1636 | 0.79 | 0.69 | 0.90 | 0.0005 | |
| > 12 | 817 | 0.91 | 0.77 | 1.06 | 0.23 | |
| Post-transplant rx | No | 911 | 1.00 | |||
| Yes | 2574 | 0.55 | 0.49 | 0.61 | <.0001 | |
AA; African Americans, w; with, w/o; without, HCT-CI; hematopoietic stem cell transplantation comorbidity index, PR; partial response, SD; stable disease, PD; progressive disease, sCR; stringent complete response, VGPR; very good partial response, MEL; melphalan
Overall survival:
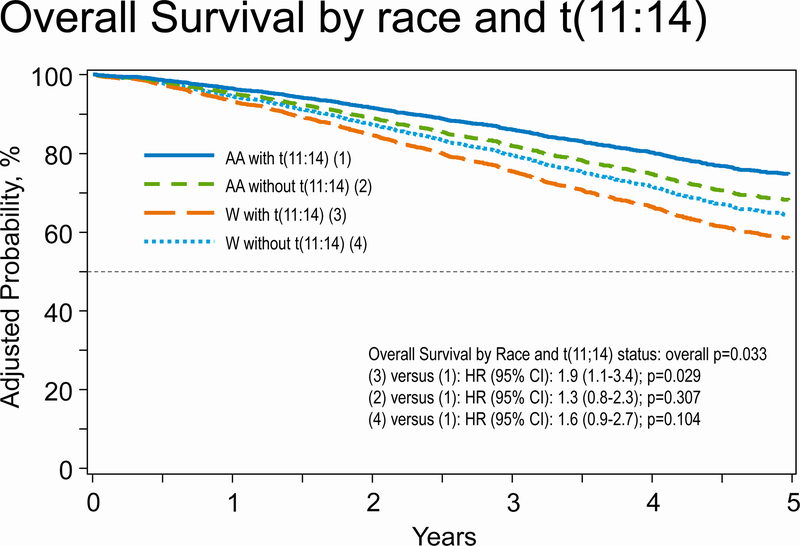
In multivariate analysis for OS (table 5), we observed a significant difference in OS by the 4 groups studied as our main effect (p = 0.04). Compared to African Americans with t(11;14) as the reference group, Whites with t(11;14) had significantly inferior OS (HR; 1.90, 95% CI 1.07–3.38, p= 0.03). Other predictors of worse OS included time from diagnosis to transplant >12 months compared to autoHCT within 6 months from diagnosis (overall p <0.0001), HCT-CI score (overall p <0.0001), higher stage disease at diagnosis (overall p <0.0001), male sex (overall p 0.005), and no post-HCT maintenance therapy (p <0.001). Figure 1 shows survival by race and t(11;14) subgroups.
Table 5.
Multivariate analysis for overall survival by main groups
| Parameter | Level | N | Hazard Ratio | 95% Confidence Limits | P-value | |
|---|---|---|---|---|---|---|
| Race | AA w/ t(11,14) | 114 | 1.00 | 0.04 | ||
| AA w/o t(11,14) | 959 | 1.35 | 0.78 | 2.33 | 0.29 | |
| White w/ t(11,14) | 259 | 1.90 | 1.07 | 3.38 | 0.03 | |
| White w/o t(11,14) | 2162 | 1.55 | 0.91 | 2.64 | 0.11 | |
| Age at transplant (years) | < 60 | 1778 | 1.00 | |||
| 60+ | 1716 | 1.12 | 0.97 | 1.30 | 0.11 | |
| Gender | Female | 1521 | ||||
| Male | 1973 | 1.23 | 1.06 | 1.42 | 0.005 | |
| HCT-CI | 0 | 1076 | 1.00 | <.0001 | ||
| 1 | 549 | 1.41 | 1.14 | 1.75 | 0.002 | |
| 2 | 589 | 1.20 | 0.96 | 1.50 | 0.12 | |
| 3+ | 1276 | 1.52 | 1.28 | 1.81 | <.0001 | |
| Missing | 4 | 1.85 | 0.46 | 7.47 | 0.39 | |
| Stage at diagnosis | Stage I-II | 1482 | 1.00 | <.0001 | ||
| Stage III | 1925 | 1.62 | 1.40 | 1.88 | <.0001 | |
| Missing | 87 | 1.14 | 0.68 | 1.91 | 0.63 | |
| Time from diagnosis to transplant (months) | < 6 | 1035 | 1.00 | <.0001 | ||
| 6–12 | 1640 | 0.97 | 0.81 | 1.15 | 0.70 | |
| 12+ | 819 | 1.52 | 1.25 | 1.84 | <.0001 | |
| Post-transplant Maintenance | Yes | 2581 | 1.00 | |||
| No | 913 | 2.25 | 1.95 | 2.60 | <.0001 | |
Figure 1.
Overall survival by four main groups; African American (AA) and Whites (W) with and without t(11;14).
Subset univariate analysis by race and t(11;14) with other molecular markers
Because the presence of high-risk markers was different by race among patients with t(11;14), and though we tested high risk markers in our multivariate analysis and these were not associated with PFS or OS in our Cox multivariate models, we conducted a subset analysis to further understand the impact of high-risk markers in those with t(11;14) in this study (Table 6). This analysis showed that African Americans with t(11;14) without high risk cytogenetics (N=92) had 2-year PFS of 61% (95% CI 50–72%) compared to Whites with t(11;14) without high risk cytogenetics 59% (95% CI 51–66%) versus Whites with t(11;14) with high-risk cytogenetics 38% (95% CI 25–51%), p-value 0.003. Similarly, for 2-year OS, African Americans with t(11;14) without high risk cytogenetics (N=92) had 2-year 0S of 93% (95% CI 86–97%) compared to Whites with t(11;14) without high risk cytogenetics 89% (95% CI 84–93%) versus Whites with t(11;14) with high-risk cytogenetics 75% (95% CI 62–85%), p-value 0.06. Unfortunately, the group with African Americans with t(11;14) with high risk cytogenetics had too small of a number to assess 2-year outcomes.
Table 6.
Univariate analysis by race and cytogenetic risk
| Whites w t(11;14) w/o high risk cytogenetics (N = 193) |
AA w t(11;14) w/o high risk cytogenetics (N = 92) |
Whites w t(11;14) w high risk cytogenetics (N = 73) |
AA w t(11;14) w high risk cytogenetics (N = 25) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | N | Prob (95% CI) | N | Prob (95% CI) | N | Prob (95% CI) | N | Prob (95% CI) | p-value |
| Progression free survival | 188 | 92 | 71 | 23 | 0.02 | ||||
| 1-year | 78 (72–84)% | 83 (75–90)% | 69 (57–79)% | 86 (70–97)% | 0.14 | ||||
| 2-year | 59 (51–66)% | 61 (50–72)% | 38 (25–51)% | NE | 0.003 | ||||
| Overall survival | 193 | 92 | 73 | 25 | 0.13 | ||||
| 1-year | 93 (89–96)% | 96 (90–99)% | 94 (88–98)% | 92 (78–99)% | 0.83 | ||||
| 2-year | 89 (84–93)% | 93 (86–97)% | 75 (62–85)% | NE | 0.06 | ||||
Patients without t(11;14) were excluded for this analysis
AA; African Americans, w; with, w/o; without, Prob; probability, CI; confidence interval
Discussion
In the present study focusing on the effect of race and t(11;14) in a contemporaneous US population treated with autoHCT, we make the following observations: 1) the presence of t(11;14) is associated with light chain myeloma, advanced stage and higher creatinine at diagnosis; 2) African American MM patients are younger with equal gender distribution with lower KPS, higher HCT-CI with several socioeconomic differences; 3) t(11;14) was associated as the sole abnormality in approximately a third of cases, among the other two thirds, t(11;14) was more commonly associated with high-risk cytogenetics in Whites (27%) compared to African Americans (21%); 4) on multivariate analysis while race and t(11;14) had no impact on PFS, race and t(11;14) were significantly associated with OS, with African Americans with t(11;14) having the best survival and significantly worse survival in Whites with t(11;14).
Primary translocations in MM typically involve immunoglobulin heavy chain gene locus on chromosome 14 and one of frequently occurring chromosomal partners: 4, 6, 11, 14 and 20.1 Among them, the t(11;14) abnormality is one of the most common chromosomal translocation seen in MM, however, the incidence is lower in MM than what is observed in monoclonal gammopathy of undetermined significance (MGUS) or light chain (AL) amyloidosis.20 Our analysis showed that t(11;14) is associated with higher likelihood of light chain myeloma, and as expected these patients are more likely to have renal disease owing to cast nephropathy and consequently a more advanced stage. This observation was seen irrespective of race.
Our analysis which was restricted to MM patients who received autoHCT showed significant differences in the baseline characteristics by race. As shown in prior analyses of the US autoHCT population13, African American patients receiving autoHCT were younger but with lower KPS and higher HCT-CI compared to Whites. However, our analysis did not show that this impacted TRM and overall TRM was low and not impacted by race or t(11;14). Further, gender was more equally distributed among the African American cohort (49% male, 51% female) compared to Whites (60% male, 40% female). We also observed that our study was enriched for more African Americans in more recent years (related to the likelihood of being selected to the CRF track).
Lakshman et al. conducted a retrospective analysis of 1,095 MM patients diagnosed between 2004–14 and compared outcomes of patients with t(11;14) (n= 365), non- (11;14) translocations (n= 132) and no translocation (n= 598) seen at the Mayo Clinic.9 The median PFS in MM patients with t(11;14) was inferior (23 months) compared with no translocation (28 months) but better than non-(11;14) translocation group (19 months). Further, 11% of patients with t(11;14) had del17p or monosomy 17 and 34% had 13q- or monosomy 13. In our study, we found co-occurrence of any high-risk abnormality in approximately 25% of t(11;14) patients although this appeared to be higher among Whites (27%) compared to African Americans (21%). In this study9, in the presence of 17p abnormality, no difference in survival was observed between t(11;14) and the non-(11;14) translocation group. The group of patients with t(11;14), age > 65 years, ISS III and 17p abnormality appeared to have a poor outcome, while induction therapy with novel agents was associated with improved outcome in the t(11;14) group. Only 61% of patients received autoHCT in this study, and the racial breakdown of the study population was not available. Sasaki, et al. compared the outcomes of three group of MM patients who received autoHCT: (1) MM with t(11;14), ((2) MM with normal cytogenetics and (3) MM with high-risk cytogenetics seen at the M.D. Anderson Cancer Center.7 Among 993 MM patients analyzed, only 27 (3%) patients had t(11;14), 97 (10%) had high-risk cytogenetics and the rest (87%) had normal cytogenetics. Progression-free survival and OS in the t(11;14) group was inferior to the normal cytogenetics group but better than the high-risk cytogenetics group. In multivariate analysis for PFS and OS, high risk cytogenetics and t(11;14) retained an adverse prognostic significance. The Connect MM registry evaluated the impact of race on survival of MM patients with t(11;14) and found a significantly inferior OS in African Americans with t(11;14) compared to non-African Americans with t(11;14). The survival analysis was adjusted for age, ISS stage, t(4;14) and transplant intent.17 Of note, the proportion of patients who received HCT was not reported in the abstract and a publication has not been reported yet, to the best of our knowledge. Our analysis is different from the above studies in that it was restricted to patients who underwent autoHCT. Our study is the largest cohort of t(11:14) MM patients treated with autoHCT and demonstrates that when adjusted for baseline-, disease- and transplant-related covariates, the overall effect of the translocation does not confer inferior prognosis in African Americans in the context of autoHCT. Indeed, our results show that African Americans with t(11;14) treated with autoHCT, in fact have better OS compared to Whites with t(11:14). This novel finding needs to be validated by further studies.
Multiple prior reports from the CIBMTR have shown that transplant utilization is lower among African Americans compared to Whites in the US, but among transplanted patients, no survival differences exist by race.12, 13, 23 Gonzalez Velez et al., in a study of 87 MM patients with t(11;14) reported that patients had no benefit from novel agents compared to pre-novel therapy data, but had better outcomes if they received autoHCT despite lack of response to novel agents.24 In AL amyloidosis, a plasma cell neoplasm related to MM, similar findings have been observed; AL patients with t(11;14) have been shown to have reduced response to bortezomib but superior response to autoHCT.25 Our study, which is concordant to these findings, may indicate that among MM patients receiving autoHCT, the negative impact of t(11;14) if any, may be overcome. However, it is not clear why there is a racial difference in this finding. Differential treatment effects based on the t(11:14) mutation has also been demonstrated with other drugs, such as with venetoclax that has shown to preferentially benefit patients with t(11:14).26 Additionally, we found a PFS benefit only in the univariate and not multivariate analysis, though it is possible that given the small numbers in the t(11;14) groups, we did not have enough power to identify a statistically significant difference as the HR does point toward a favorable outcome for African American with t(11;14) subgroup. We note that our study had more African Americans in recent years. While we adjusted for year of transplant, it is possible that the superior survival is related to other treatment-related modifiers that we are unable to adjust for. Therefore, we additionally did a subset analysis of survival by the 4 groups restricting our study population to 2013–2016 to understand better if African Americans had better outcomes owing to time of diagnosis. We still found that African Americans had better overall survival than Whites in this subset though the effect of t(11;14) was less clear. It is to be noted that we likely lost significant statistical power to study the differential effect of t(11;14) as 4 distinct groups in the subset.
This study which involves over 3,500 patients also endorses prior reports regarding the significance of early autoHCT in the treatment course of MM and the survival benefit of maintenance therapy post autoHCT.27–30 In our study, multivariate analysis for PFS and OS suggest that patient who had delayed autoHCT more than 12 months after diagnosis had inferior outcomes compared to those who had autoHCT in less than 12 months from diagnosis, though our analysis is biased in that we do not have data on patients who never received transplant. Similarly, post-transplant maintenance therapy retained its favorable significance for PFS and OS in multivariate analysis. The benefit of early autoHCT and maintenance therapy was observed across all 4 subgroups. The CIBMTR currently captures over 80% of autoHCT activity in the US and thus represents outcome data from the general US practice.31 However, because these data are obtained from a transplant registry, it does not include patients who did not receive transplant due to lack of health care resources, personal or physician choice, socioeconomic disparities, and under referrals for transplant. We also acknowledge other limitations, such as self-reported race, shorter follow up in African American patients compared to Whites, as well as our inability to identify and adjust for other potential treatment effect modifiers. The prevalence of t(11;14) is reported to be 15–18% in the reported literature5 but was only 11% in our series, although in another transplant study, only 3% of autoHCT MM patients had t(11;14).7 These differences may be reflective of the time-period of study and different FISH procedure practices (e.g. plasma cell enrichment) over the time period of study. The sizable numbers of patients and uniform induction treatment with autoHCT followed by maintenance indicate that these results are representative of transplant outcomes in this subset of MM patients. In conclusion, our analyses show that African Americans with t(11;14) who undergo autoHCT may have better overall survival compared to Whites with t(11;14). Additional studies are needed to confirm this finding.
Supplementary Material
Acknowledgment:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; OT3HL147741, R21HL140314 and U01HL128568 from the NHLBI; HHSH250201700006C, SC1MC31881-01-00 and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-18-1-2850, N00014-18-1-2888, and N00014-20-1-2705 from the Office of Naval Research; Additional federal support is provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01HL126589, R01AI128775, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612, and BARDA. Support is also provided by Be the Match Foundation, Boston Children’s Hospital, Dana Farber, Japan Hematopoietic Cell Transplantation Data Center, St. Baldrick’s Foundation, the National Marrow Donor Program, the Medical College of Wisconsin and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; Adienne SA; Allovir, Inc.; Amgen, Inc.; Anthem, Inc.; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; Chimerix, Inc.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Gamida-Cell, Ltd.; Genzyme; GlaxoSmithKline (GSK); HistoGenetics, Inc.; Incyte Corporation; Janssen Biotech, Inc.; Janssen Pharmaceuticals, Inc.; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc.; Kiadis Pharma; Kite Pharma; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt LLC; Medac GmbH; Merck & Company, Inc.; Merck Sharp & Dohme Corp.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Novartis Oncology; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Phamacyclics, LLC; Regeneron Pharmaceuticals, Inc.; REGiMMUNE Corp.; Sanofi Genzyme; Seattle Genetics; Sobi, Inc.; Takeda Oncology; Takeda Pharma; Terumo BCT; Viracor Eurofins and Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR releases only de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality
Conflicts of interest: The following authors have reported conflicts of interest: Dr. D’Souza reports funding from Merck, Prothena, Sanofi, Mundipharma EDO, TeneoBio, Takeda and has received consulting fees from Prothena, Pfizer, Imbrium, and Akcea. Dr. Berdeja reports funding from Abbvie, Amgen, Acetylon, Bluebird, BMS, Celgene, Celularity, Constellation, CURIS, EMD Sorono, Glenmark, Janseen, Kesios, Lilly, Novartis, Poseida, Sanofi, Takeda, Teva, Vivolux and Consultancy from Takeda, BMS, Karyopharm, CRISPR Therapeutics, Celgene, Kite Pharma Inc, Legend, Servier, Janssen, Amgen, BioClinica, Prothena. Dr. Dhakal reports Advisory Board from Amgen, Takeda, GSK, Janssen and Honorarium from Celgene. Dr. Ganguly reports personal fees from Seattle Genetics. Dr. Hildebrandt reports Advisory Role from Pfizer, Kite Pharma, Incyte, Jazz pharmaceuticals; Research funding from Pharmacyclics, Takeda, and Jazz Pharmaceuticals; Travel/Accommodations/Expenses from Kite Pharma, Incyte, Pfizer, Falk Foundation, Jazz Pharmaceuticals, Astellas Pharma. Dr. Kharfan-Dabaja reports Consultancy for Pharmacyclics and Daiichi Sankyo. Dr. Kumar reports Grants/clinical trial support to institution from BMS/Celgene, Takeda, Abbvie, Roche, Medimmune, Tenebio, Carsgen, Janssen and personal fees (consulting) from Oncopeptides. Dr. Landau reports grants from Genzyme Corporation, Amge, Ortho Biotech Clinical Affairs, LLC, Millennium Pharmaceuticals, Inc, Berlex, Philanthropic Funds, Astex, Amyloidosis Foundation Takeda and Personal Fees from Takeda. Dr. Lazarus reports DSMB for CAR-t studies from Celgene. Dr. Nishihori reports Research support to institution from Novartis and Karyopharm. Dr. Vesole reports Speaker’s bureaus at Amgen, Celgene, Takeda.
References
- 1.Kumar SK, Rajkumar SV. The multiple myelomas - current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol. 2018;15: 409–421. [DOI] [PubMed] [Google Scholar]
- 2.Mikhael JR, Dingli D, Roy V, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88: 360–376. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33: 2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127: 2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109: 3489–3495. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman GP, Gertz MA, Dispenzieri A, et al. Impact of cytogenetic classification on outcomes following early high-dose therapy in multiple myeloma. Leukemia. 2016;30: 633–639. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki K, Lu G, Saliba RM, et al. Impact of t(11;14)(q13;q32) on the outcome of autologous hematopoietic cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2013;19: 1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin HJ, Kim K, Lee JJ, et al. The t(11;14)(q13;q32) translocation as a poor prognostic parameter for autologous stem cell transplantation in myeloma patients with extramedullary plasmacytoma. Clin Lymphoma Myeloma Leuk. 2015;15: 227–235. [DOI] [PubMed] [Google Scholar]
- 9.Lakshman A, Alhaj Moustafa M, Rajkumar SV, et al. Natural history of t(11;14) multiple myeloma. Leukemia. 2018;32: 131–138. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin M, Reddy S, Brawley OW. Myeloma and race: a review of the literature. Cancer Metastasis Rev. 2003;22: 87–93. [DOI] [PubMed] [Google Scholar]
- 11.Verma PS, Howard RS, Weiss BM. The impact of race on outcomes of autologous transplantation in patients with multiple myeloma. Am J Hematol. 2008;83: 355–358. [DOI] [PubMed] [Google Scholar]
- 12.Costa LJ, Huang JX, Hari PN. Disparities in utilization of autologous hematopoietic cell transplantation for treatment of multiple myeloma. Biol Blood Marrow Transplant. 2015;21: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hari PN, Majhail NS, Zhang MJ, et al. Race and outcomes of autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2010;16: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshua TV, Rizzo JD, Zhang MJ, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116: 3469–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazandjian D, Hill E, Hultcrantz M, et al. Molecular underpinnings of clinical disparity patterns in African American vs. Caucasian American multiple myeloma patients. Blood Cancer J. 2019;9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baughn LB, Pearce K, Larson D, et al. Differences in genomic abnormalities among African individuals with monoclonal gammopathies using calculated ancestry. Blood Cancer J. 2018;8: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasparetto CJ, Abonour R, Jagannath S, et al. Differential Effect of t(11;14) Abnormality on Survival and Depth of Response in African American (AA) and Non-AA (NAA) Patients (Pts) with Newly Diagnosed Multiple Myeloma (NDMM) in the Connect® MM Registry. Blood. 2017;130: 3101–3101. [Google Scholar]
- 18.Saraf S, Chen YH, Dobogai LC, et al. Prolonged responses after autologous stem cell transplantation in African-American patients with multiple myeloma. Bone Marrow Transplant. 2006;37: 1099–1102. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17: e328–e346. [DOI] [PubMed] [Google Scholar]
- 20.Fonseca R, Blood EA, Oken MM, et al. Myeloma and the t(11;14)(q13;q32); evidence for a biologically defined unique subset of patients. Blood. 2002;99: 3735–3741. [DOI] [PubMed] [Google Scholar]
- 21.Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2: 175–187. [DOI] [PubMed] [Google Scholar]
- 22.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351: 1860–1873. [DOI] [PubMed] [Google Scholar]
- 23.Schriber JR, Hari PN, Ahn KW, et al. Hispanics have the lowest stem cell transplant utilization rate for autologous hematopoietic cell transplantation for multiple myeloma in the United States: A CIBMTR report. Cancer. 2017;123: 3141–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velez MG, Parrondo RD, Andrews T, et al. Prognostic significance of t(11;14) expression by FISH in patients with newly diagnosed multiple myeloma in the era of novel therapies 2017;35: e19525–e19525. [Google Scholar]
- 25.Bochtler T, Hegenbart U, Kunz C, et al. Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study. Blood. 2016;128: 594–602. [DOI] [PubMed] [Google Scholar]
- 26.Gonsalves WI, Buadi FK, Kumar SK. Combination therapy incorporating Bcl-2 inhibition with Venetoclax for the treatment of refractory primary plasma cell leukemia with t (11;14) 2018;100: 215–217. [DOI] [PubMed] [Google Scholar]
- 27.Dhakal B, Szabo A, Chhabra S, et al. Autologous Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Novel Agent Induction: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stadtmauer EA, Pasquini MC, Blackwell B, et al. Comparison of Autologous Hematopoietic Cell Transplant (autoHCT), Bortezomib, Lenalidomide (Len) and Dexamethasone (RVD) Consolidation with Len Maintenance (ACM), Tandem Autohct with Len Maintenance (TAM) and Autohct with Len Maintenance (AM) for up-Front Treatment of Patients with Multiple Myeloma (MM): Primary Results from the Randomized Phase III Trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 - StaMINA Trial). Blood. 2016;128: LBA-1–LBA-1. [Google Scholar]
- 29.Holstein SA, Jung SH, Richardson PG, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol. 2017;4: e431–e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366: 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Souza A, Lee S, Zhu X, Pasquini M. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant. 2017;23: 1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.