Abstract
Cerebellar development has a remarkably protracted morphogenetic timeline that is coordinated by multiple cell types. Here, we discuss the intriguing cellular consequences of interactions between inhibitory Purkinje cells and excitatory granule cells during embryonic and postnatal development. Purkinje cells are central to all cerebellar circuits, they are the first cerebellar cortical neurons to be born, and based on their cellular and molecular signaling, they are considered the master regulators of cerebellar development. Although rudimentary Purkinje cell circuits are already present at birth, their connectivity is morphologically and functionally distinct from their mature counterparts. The establishment of the Purkinje cell circuit with its mature firing properties has a temporal dependence on cues provided by granule cells. Granule cells are the latest born, yet most populous, neuronal type in the cerebellar cortex. They provide a combination of mechanical, molecular and activity-based cues that shape the maturation of Purkinje cell structure, connectivity and function. We propose that the wiring of Purkinje cells for function falls into two developmental phases: an initial phase that is guided by intrinsic mechanisms and a later phase that is guided by dynamically-acting cues, some of which are provided by granule cells. In this review, we highlight the mechanisms that granule cells use to help establish the unique properties of Purkinje cell firing.
Keywords: cerebellum, Purkinje cells, granule cells, development, electrophysiology, behavior
Introduction
The cerebellum was initially described as a motor region, but it is now receiving much attention for its roles in neurocognitive behaviors (Gill and Sillitoe, 2019; Manto et al., 2012; Sathyanesan et al., 2019). Towards this, there is mounting evidence from studies in humans and rodents showing that the cerebellum controls movement as well as language, emotion, and cognition by modulating diverse brain regions (Badura et al., 2018; Carta et al., 2019; King et al., 2019). Therefore, one could hypothesize that there are regional differences in structure that are related to its function, as is seen in the cerebral cortex. Yet, there are very few differences in the anatomical architecture of the cerebellar cortex that could fully explain the functional heterogeneity of the cerebellum, as the cerebellar cortex consists of highly repetitive local circuits (Beckinghausen and Sillitoe, 2019). Although, in accordance with the cerebellum’s functional heterogeneity, the underlying features that promote such diversity likely emerge from specific circuit wiring plans that are initiated during development (Apps and Hawkes, 2009; Dastjerdi et al., 2012; Leto et al., 2016). The extensive embryonic to postnatal timeline of cerebellar development, that encompasses over two years in humans and nearly two months in rodents, corroborates this hypothesis (Sathyanesan et al., 2019; White and Sillitoe, 2013). During this period, anatomically homogeneous cerebellar circuits acquire a striking level of molecular and synaptic specializations that could account for the expansive contribution of the cerebellum to different behaviors (Dastjerdi et al., 2012). The lengthy window allocated for cerebellar development could also explain why a wide range of genetic and physical insults in the cerebellum can cause a large number of different neurological and neuropsychiatric disorders including movement disorders such as ataxia, dystonia and tremor, as well as neuropsychiatric disorders including autism spectrum disorders (ASD), attention deficit hyperactivity disorder (ADHD) and schizophrenia (Stoodley and Limperopoulos, 2016). Thus, a more comprehensive understanding of the temporal features of cerebellar circuit development may provide key insights into the origins, pathogenesis and pathophysiology of brain diseases ranging from movement disorders to complex conditions that cause neurocognitive dysfunction.
Cerebellar development starts before birth (~30 days after conception in human and ~7 days in mice) and Purkinje cells are among the first of the major cell types to be born (Hashimoto and Mikoshiba, 2003; Hoshino et al., 2005; Miale and Sidman, 1961); in mice this occurs after embryonic day 10. Purkinje cells ultimately form the core of all cerebellar circuits and are the sole output of the cerebellar cortex. In adults, they perform critical computations for function whereas during development they guide the wiring of afferent fibers and interneurons. Thus, the centrality of Purkinje cells in the circuit is reflected in their influence over cerebellar development and later, in their control of circuit function (Dastjerdi et al., 2012).
The protracted timeline of Purkinje cell development is accompanied by the later-onset development of a second key population in the cerebellar cortex, the excitatory granule cells. Granule cells are the most numerous cell type in the mammalian brain (Herculano-Houzel et al., 2015) and the peak of granule cell neurogenesis occurs postnatally between 20 and 40 weeks post conception in humans and postnatally between the first to second weeks of life in mice, which is after most other cerebellar cell types are born and have started migrating to their final locations in the cerebellar cortex (Beckinghausen and Sillitoe, 2019; Haldipur et al., 2019; Leto et al., 2016; Sathyanesan et al., 2019; White and Sillitoe, 2013). There are multiple morphogenetic and signaling mechanisms that Purkinje cells use to guide granule cell development (Caddy and Biscoe, 1979; Chen and Hillman, 1989; Corrales et al., 2004, 2006; Dahmane and Ruiz i Altaba, 1999; Lackey et al., 2018; Sidman et al., 1962; Wallace, 1999; Wang and Liu, 2019; Wechsler-Reya and Scott, 1999; Wetts and Herrup, 1982, 1983), although the reverse signaling from granule cells to Purkinje cells is also critical, especially for correct circuit wiring. For example, there is evidence that granule cells and their parallel fiber projections influence the late phase of supernumerary elimination of climbing fibers from Purkinje cells (Hashimoto and Kano, 2003). Eliminating granule cell synaptic signaling, however, results in relatively specific motor phenotypes compared to those observed in mice that lack granule cells throughout development (Galliano et al., 2013). This shows that, in addition to neuronal communication at granule cell to Purkinje cell synapses, other granule cell cues must also be necessary for the final maturation of Purkinje cells, in addition to their roles in mediating other cerebellar processes. These cues include mechanical forces due to clonal cell expansion and heterologous molecular signaling from granule cells to Purkinje cells (D’Arcangelo et al., 1995; Dastjerdi et al., 2012; Jensen et al., 2002).
Thus, Purkinje cells orchestrate the development of rudimentary circuits. Yet, upon their temporal intersection with excitatory granule cells, major rearrangements shape Purkinje cell structure and connectivity. These rearrangements appear to be fundamental to circuit function as they coincide with the refinement of cerebellar-guided behaviors and are dependent on a diverse range of signals. Here, we discuss the bi-phasic development of Purkinje cells. There is an early phase that is guided by cell-intrinsic mechanisms such as cellular differentiation and a late phase that is guided by cell extrinsic mechanisms including mechanical, molecular, and activity cues provided by granule cells. We discuss the developmental events that occur in both phases in terms of Purkinje cell migration, morphology, connectivity, and electrophysiology. We further briefly discuss, how, as Purkinje cell circuit maturation proceeds, the cerebellum acquires its diverse functions for behaviors such as locomotion, learning, and ultrasonic vocalization (Lalonde and Strazielle, 2015). We also discuss how the temporal patterns that shape cerebellar development impact disease. Before considering cerebellar wiring, and specifically how Purkinje cells acquire their functional connectivity, we will review the basic anatomy and circuitry of the cerebellum. We also introduce how cell lineage and molecular expression contribute to circuit identity.
An overview integrating the morphology, cytoarchitecture and patterning of the cerebellum
Gross anatomy:
The cerebellum is anatomically partitioned in both the anterior-posterior and the medial-lateral axes (Sillitoe et al., 2012). In the anterior-posterior axis, the cerebellum is folded into ten lobules that are separated by distinct fissures (Figure 1A) (Larsell, 1952). In the medial-lateral axis, the cerebellum is organized into four major regions: the vermis (and paravermis), hemispheres, floculus, and paraflocculus (Figure 1B). We will not consider the details of basic cerebellar anatomy any further since they have been extensively described in many previous publications (Glickstein and Voogd, 1995; Sillitoe and Joyner, 2007; Voogd and Glickstein, 1998).
Figure 1: The basic architecture of the cerebellum in a mature mouse.
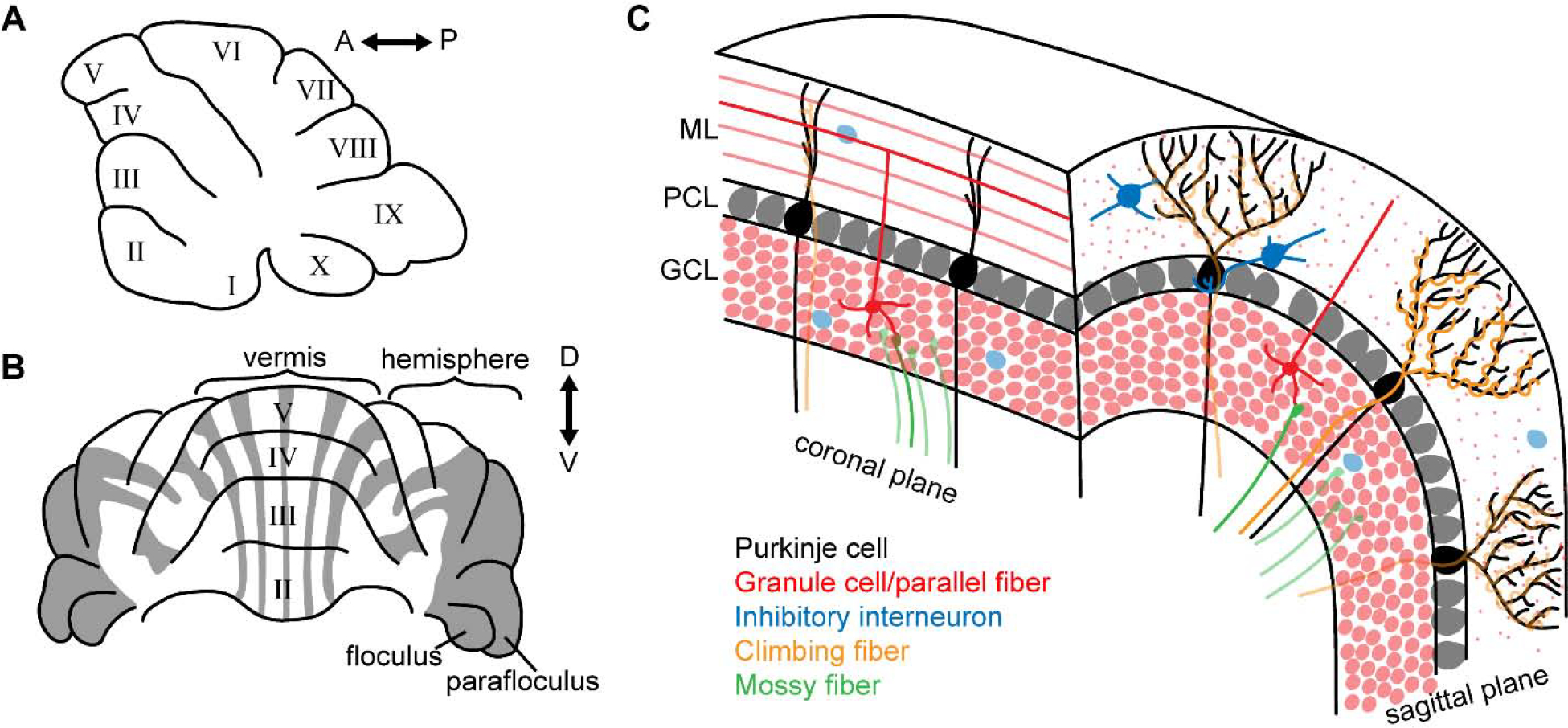
A. Schematic of a sagittal section of the cerebellum. Lobules are labelled with Roman numbers. B. Schematic of a coronal section of the cerebellum. Gray areas depict the ZebrinII-positive domains. ZebrinII expression is the best studied molecular marker of adult zones. Lobules are labelled with Roman numbers. C. Schematic of a cross section of the cerebellum illustrating the major excitatory and inhibitory circuit components of the canonical cerebellar circuit. ML = molecular layer; PCL = Purkinje cell layer; GCL = granule cell layer.
Cerebellar patterns:
In addition to its main anatomical divisions, the medial-lateral axis of the cerebellum is also organized around a finer scale. Neurons and glia are organized into an array of patterns that are best revealed using molecular expression patterns, although afferent terminal field organization, cell lineage, and genetic phenotypes all conform to the same plan (Beckinghausen and Sillitoe, 2019; Miterko et al., 2018; Zhou et al., 2020). Importantly, the Purkinje cell plan is set first, and then acquired by the different cerebellar cell types, together forming parasagittal stripes, or zones (Apps and Hawkes, 2009), that then operate as functional cerebellar modules (Pijpers et al., 2006; Ruigrok, 2011). The patterns have been extensively described using molecular markers (Dastjerdi et al., 2012; Hawkes, 2014; White and Sillitoe, 2013). The most well characterized molecular marker of cerebellar zones is Zebrin II, an antigen on the Aldolase C protein (Ahn et al., 1994; Brochu et al., 1990; Hawkes and Leclerc, 1987). Zebrin II is expressed by a subset of Purkinje cells in highly conspicuous and evolutionarily conserved parasagittal bands (Figure 1B) (Apps and Hawkes, 2009; Sillitoe et al., 2004). Similarly, it should be noted that the anterior-posterior axis also has finer structure-related divisions. Based on the projection patterns of afferent fiber subsets to specific cerebellar regions, it is possible that the type of information that gets processed by the Purkinje cells is distinct depending on afferent terminal localization patterns in the cerebellum (Nishiyama and Linden, 2004; Serapide et al., 2001; Vogel et al., 1996).
Circuit architecture:
Cerebellar circuits are carefully arranged according to a three-layered structure across the cerebellar cortex (Figure 1C). The most superficial layer, called the molecular layer, is occupied by the large dendritic arbors of Purkinje cells, and is the site for innervation by excitatory parallel and climbing fibers. Parallel fibers are the axons of cerebellar granule cells. Each Purkinje cell receives extensive synaptic inputs from parallel fibers; ~50 thousand in mice, ~150 thousand in rats, and ~7 million in humans (Huang et al., 2014). Purkinje cells are also innervated by climbing fibers, which originate in the inferior olive of the brainstem. In the adult, every Purkinje cell receives synaptic contacts from a single climbing fiber. In addition to the Purkinje cell dendrites, parallel fibers, and climbing fibers, two types of molecular layer interneurons (MLI) also reside in the molecular layer, namely the stellate cells and basket cells. The second and middle layer of the cerebellar cortex is the Purkinje cell layer, where the cell bodies of Purkinje cells and some interneurons are found. Purkinje cell projections are restricted to select brain regions including the vestibular, cerebellar, and parabrachial nuclei (Hashimoto et al., 2018; Sillitoe et al., 2009). There is also a recently discovered Purkinje cell projection to the locus coeruleus(Schwarz et al., 2015). Sandwiched between the Purkinje cells are the large Bergmann glia and interneurons called candelabrum cells. The third and deepest layer is called the granule cell layer. This layer is densely packed with the cell bodies of excitatory granule cells. The granule cell layer also contains inhibitory Golgi, Lugaro and globular cells (Hirono et al., 2012), and a unique population of excitatory interneurons called unipolar brush cells. The unipolar brush cells are localized mainly in the vermis of lobules IX and X, with a smaller number localized to lobules VI and VII (Mugnaini et al., 2011). The granule cells, Golgi cells, and unipolar brush cells are innervated by mossy fibers, which relay information to the cerebellum from the cortex (via the pons), the vestibular nuclei and primary vestibular nerve, and spinal cord, among other regions. The cerebellar nuclei are situated in the core of the cerebellum and form the primary connection between the cerebellar cortex and other brain regions and the spinal cord. In addition to Purkinje cell input, the cerebellar nuclei cells receive collateral inputs from mossy and climbing fibers. We will not cover the beaded neuromodulatory fibers, although their existence should be noted.
Purkinje cells and granule cells are derived from spatially distinct germinal zones
Mature inhibitory and excitatory neurons are tightly intermingled in the cerebellar circuit, which contrasts with their progenitors that are initially segregated in two germinal zones (the ventricular zone and rhombic lip) that produce the separate lineages (Ben-Arie et al., 1997; Hoshino et al., 2005; Rose et al., 2009; Sellick et al., 2004; Wang et al., 2005). All inhibitory neurons derive from the ventricular zone and are marked by the expression of pancreas specific transcription factor 1a (Ptf1a) (Hoshino et al., 2005), whereas the excitatory cerebellar neurons develop from the rostral rhombic lip and require the expression of atonal homolog 1 (Atoh1) (Figure 2A) (Ben-Arie et al., 1997; Rose et al., 2009; Wang et al., 2005). Cellular identity is also defined by birthdate (Hoshino et al., 2005; Rose et al., 2009; Wang et al., 2005). Here, all developmental timepoints are defined according to mouse development, but the order in which different cell types are born is conserved across species (Butts et al., 2011, 2014; Sathyanesan et al., 2019). From here on, the postnatal days are indicated by a “P” and the embryonic days are indicated by an “E” (Figure 2B). The earliest born cells (~E10–11) in the ventricular zone and rhombic lip become inhibitory and excitatory cerebellar nuclei cells, respectively (Hoshino et al., 2005; Rose et al., 2009; Wang et al., 2005). Purkinje cells are the earliest born cerebellar cortical neurons (~E10–13) and are derived from the ventricular zone (Miale and Sidman, 1961). Their birth is followed by the genesis of a variety of cerebellar inhibitory (~E14-P7, ventricular zone) and excitatory (~E14–18, rhombic lip) interneurons. Granule cell precursor cells (~E12–16, rhombic lip) form a secondary germinal matrix called the external granular layer (Chizhikov et al., 2006, 2010), which covers the surface of the cerebellar anlage. There, the precursor cells further proliferate for several weeks (~E14-P20) and then produce differentiated granule cells. Granule cells ultimately migrate to the inner granular layer (Butts et al., 2014), although during this period they also extend parallel fiber axons that form synaptic connections with the Purkinje cells (Espinosa and Luo, 2008; Park et al., 2019).
Figure 2. Cerebellar cell-type identities are spatially and temporally determined in the embryo according to birthplace and birthdate.
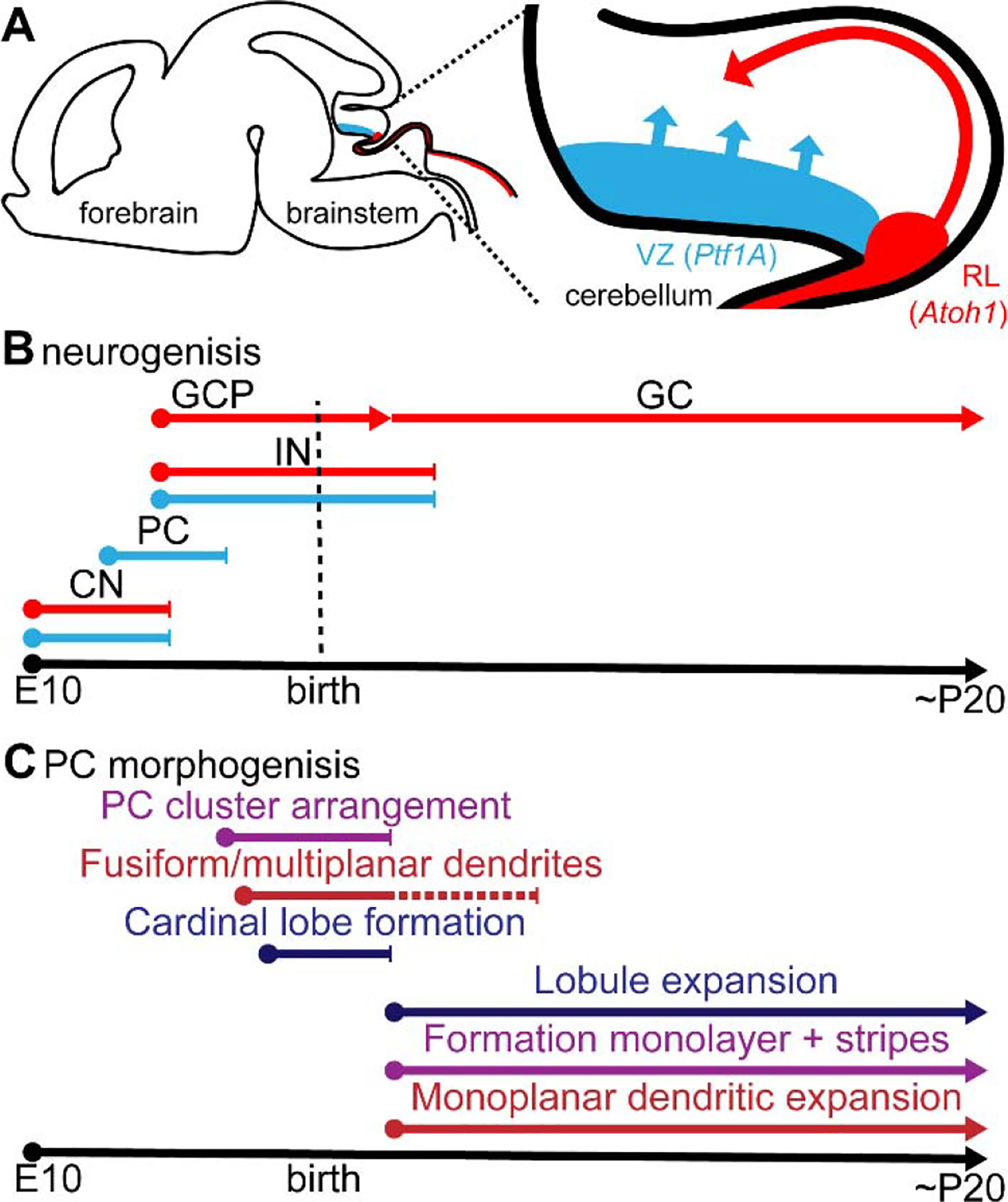
A. Schematic of a sagittal section of an embryonic mouse brain (left). The inset (right) is zoomed in onto the cerebellar anlage. The ventricular zone (VZ) is shown in blue, progenitor cells in this region express the transcription factor Ptf1a and migrate radially into the core of the cerebellar anlage after final mitosis. The rhombic lip (RL) is shown in red, progenitor cells in this region express the transcription factor Atoh1 and migrate over the cerebellar surface in a tangential manner. B. Neurogenesis of diverse cerebellar cell types occurs in a temporally controlled manner. Blue lines indicate neurons developing from the VZ, red lines indicate neurons developing from the RL. CN = cerebellar nuclei; PC = Purkinje cell; IN = interneurons; GCP = granule cell precursor; GC = granule cell. C. Purkinje cell morphogenesis occurs in two distinct phases (the panel is split into processes, at top and bottom of the timeline). The processes shown at the bottom are dependent on granule cells. Purple lines indicate temporally distinct migratory paths. Red lines indicate the morphogenesis of the Purkinje cell dendritic arbor. Blue lines indicate lobule formation.
The assembly of Purkinje cell circuitry starts during embryogenesis
Shortly after neurogenesis in the ventricular zone, Purkinje cells start to migrate towards the inner core of the cerebellar anlage (E13-E17) (Figure 2C) (Morales and Hatten, 2006). Placement of Purkinje cells in the embryonic cerebellum is based on their birthdate; lateral, dorsal, and posterior Purkinje cells are born prior to the medial, ventral, and anterior Purkinje cells (Altman and Bayer, 1985; Yuasa et al., 1991). Purkinje cells migrate radially and are guided by radial glia (Yuasa et al., 1991, 1996). Their migration is, in part, mediated by reelin signaling from granule cell precursors and the developing cerebellar nuclei (Heckroth et al., 1989; Jensen et al., 2002). Accordingly, mice lacking all rhombic-lip derived neurons (Atoh1 null mice) show Purkinje cell ectopia due to improper migration (Jensen et al., 2002). Interestingly, recent work shows that in addition to providing cues for migration, the excitatory cerebellar nuclei neurons also regulate the number of Purkinje cells, thereby influencing their integration into the cerebellar circuit through an inter-cellular matching mechanism that has not been fully resolved (Willett et al., 2019).
The molecular differentiation of Purkinje cells occurs shortly after terminal mitosis (E10-E13), prior to the establishment of synaptic contacts and is thus likely coordinated by genetic programs rather than synaptic and extra-synaptic inputs (Wassef et al., 1990; Wizeman et al., 2019). Analysis of Purkinje cell development in chimeric mice shows that clones of Purkinje cells are situated in close proximity to each other in the mature cerebellum (Oster-Granite and Gearhart, 1981) with lineage studies in chick revealing their routes of dispersion (Lin and Cepko, 1999). More recent studies have confirmed that birthdate and embryonic identity correlate with molecular identity in the mature cerebellum: early- and late-born cells become ZebrinII-negative and ZebrinII-positive Purkinje cells, respectively (Hashimoto and Mikoshiba, 2003; Namba et al., 2011; Sillitoe et al., 2009). There is evidence that the transcription factor Ebf2 plays a role in early Purkinje cell differentiation, with Ebf2 repressing ZebrinII-identity (Chung et al., 2008). After Purkinje cell identity is established, they initiate expression of different markers. Among the early marker molecules are transcription factors (FoxP1, FoxP2, En1/En2 and Etv1), but also signaling and guidance molecules (EphA4 and Pcdh10), as well as molecules that are known to form zonal patterns in the developing postnatal and adult cerebellum (L7/Pcp2, PLCβ4) (Fujita and Sugihara, 2012; Fujita et al., 2012; Wizeman et al., 2019). Thus, Purkinje cells differentiate early during cerebellar development, soon after their final mitosis, and then they form clusters that can be distinguished by birthdate, localization, and molecular identity (Fujita et al., 2012; Sugihara and Fujita, 2013). Clusters are thought to be the developmental equivalent of adult zones, although the process of how clusters segregate and transform into sharp zones is not yet fully understood (Fujita et al., 2012; Larouche and Hawkes, 2006; Marzban et al., 2007; Sillitoe et al., 2009).
In addition to promoting specific molecular identities, Purkinje cell differentiation is also essential for guiding the formation of neuronal connections, which starts shortly after Purkinje cells have invaded the core of the embryonic cerebellum. The efferent connectivity of the Purkinje cells onto the cerebellar and vestibular nuclei neurons starts to form during embryogenesis (before E15 and E17 in rodents, respectively; (Eisenman et al., 1991; Sillitoe et al., 2009)). Purkinje cell clusters project to multiple cerebellar nuclei, but form the connections predominantly with nuclei in their own medial-lateral plane-that is, vermis Purkinje cells project heavily to the fastigial (medial) nucleus, paravermis cells to the interposed (middle) nucleus, and hemisphere cells to the dentate (lateral) nucleus. Thus, the overall impact of Purkinje cells on cerebellar output function may be, at least partially, determined by their distinct birthdates because the general medial-lateral organization of Purkinje cell patterning is established during embryogenesis, by the time the cells leave the ventricular zone (Sillitoe et al., 2009).
Afferent connections onto Purkinje cells start to form shortly after Purkinje cells begin to settle in the cerebellar anlage. In the initial stages of Purkinje cell synaptogenesis, the Purkinje cells receive direct input from both types of the main extra-cerebellar pathways: mossy fibers and climbing fibers (Mason and Gregory, 1984) (Figure 3). Climbing fibers interact with Purkinje cells as early as E15 and follow a strict topographical organization to form an olivocerebellar map, which conforms to the zonal plan (Chédotal et al., 1997; Sotelo and Chédotal, 2005). Inferior olive neurons send their climbing fibers to the contralateral cerebellum to specific Purkinje cell clusters that have similar molecular identities as those of the inferior olive neurons themselves (Paradies and Eisenman, 1993). The connectivity between afferents and Purkinje cell clusters is thought to depend on a molecular matching mechanism that may involve Eph/ephrin signaling (Chédotal et al., 1997; Nishida et al., 2002). A similar Eph/ephrin mechanism may also be required for mossy fiber topography (see below, Sillitoe and Lackey, 2020). Immature climbing fibers differ from mature fibers in their cellular localization. The name climbing fiber derives from the manner in which the mature axons “climb” through the large Purkinje cell dendritic arbor, yet early postnatal climbing fibers congregate around the Purkinje cell somata and at the base of the primary dendrite. Furthermore, during the first two weeks of life (in rodents), multiple climbing fibers innervate each Purkinje cell, although individual climbing fibers may prefer a single Purkinje cell as early as P3 (Wilson et al., 2019), which sets the stage for the “winner” fiber to translocate to the dendrite of a given Purkinje cell. The early pruning of climbing fiber inputs is thought to be independent of granule cells (Hashimoto and Kano, 2003; Hashimoto et al., 2009; Kano and Hashimoto, 2012).
Figure 3. Reorganization of the Purkinje cell connectome during normal development.
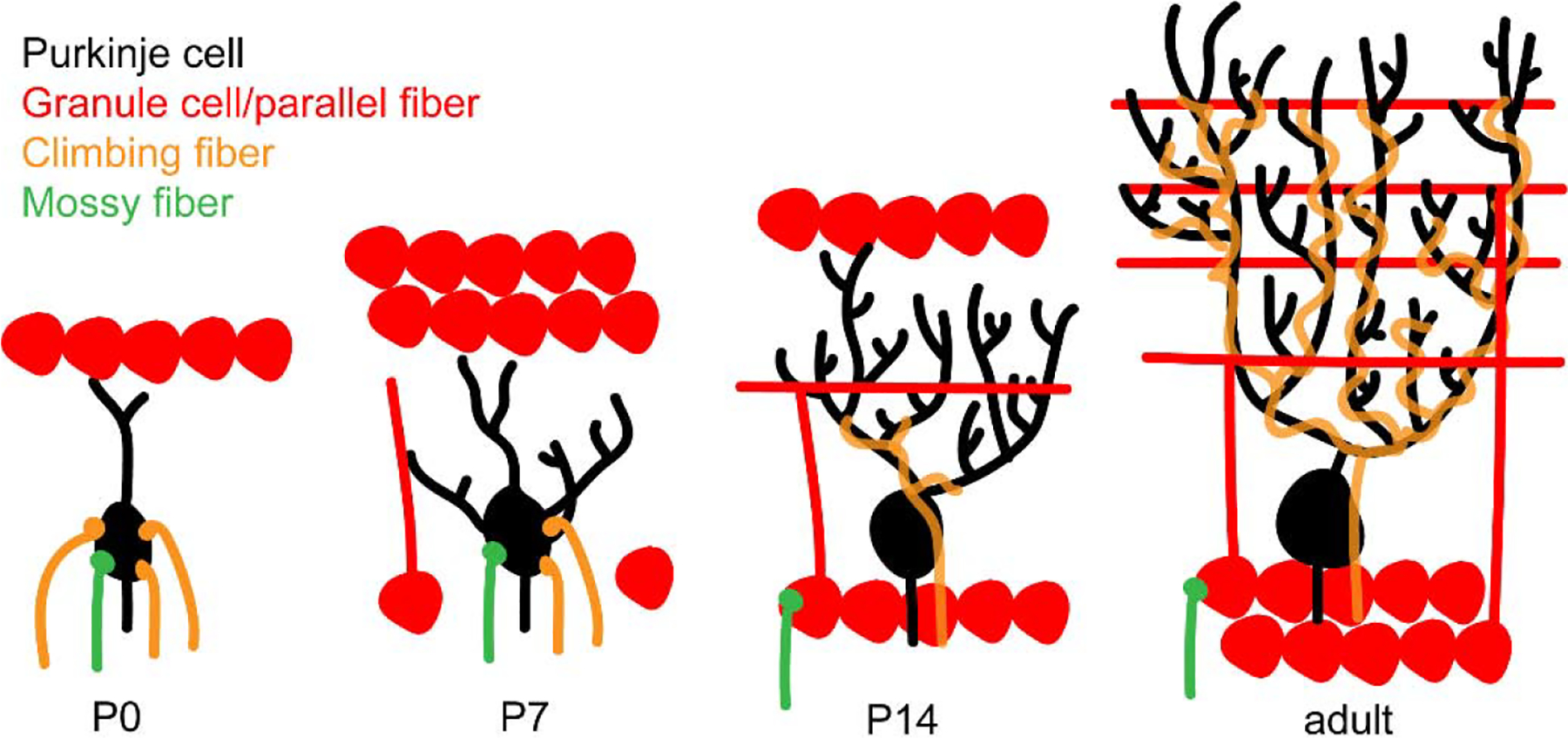
After birth (P0), Purkinje cells (black) are innervated by extra-cerebellar afferents: mossy fibers (green) and climbing fibers (orange). The afferents synapse on the Purkinje cell body. Within the first week after birth (P7), some granule cells (red) start to migrate inwardly into the granule cell layer. Granule-cell independent pruning of climbing fiber synapses initiates during this stage of development. Major synaptic rearrangements occur in the second postnatal week (P14). Granule cells contact Purkinje cells through parallel fibers, Purkinje cells are only innervated by one climbing fiber that now contact the Purkinje cells on their dendrites, and mossy fibers contact granule cells. As Purkinje cell connectivity matures, they receive parallel fiber input in an inside-out manner and climbing fiber inputs on the basal two thirds of the large dendritic tree.
During this early phase of circuit assembly, Purkinje cells are also directly innervated by mossy fibers (Lackey and Sillitoe, 2020; Sillitoe et al., 2010). Like the climbing fibers, mossy fibers are targeted to specific Purkinje cell clusters and anterior-posterior locations (Ji and Hawkes, 1995; Sillitoe, 2016; Sotelo and Wassef, 1991). This process requires Purkinje cell signaling and relies on the expression of the homeobox genes En1 and En2 (Sillitoe et al., 2010). However, recent work demonstrates that similar to their interactions with the developing climbing fibers, Purkinje cells likely also use Eph/ephrin signaling to establish the zonal topography of mossy fibers (Lackey and Sillitoe, 2020; Sillitoe et al., 2010). It is intriguing that the synapses between immature Purkinje cells and mossy fibers are functional (Takeda and Maekawa, 1989), although they do not form a rosette-like shape as mossy fiber synapses on granule cell dendrites do (Kalinovsky et al., 2011). Therefore, based on the identities of afferents that provide direct contacts, the cellular localization of the synapses they form, and the number of afferents per Purkinje cell, one could hypothesize that the neonatal Purkinje cell circuit is unique-both in its structure and function-compared to that of the mature cerebellum (Figure 3).
One of the most remarkable features of the mature Purkinje cell is its large and complex dendritic tree. The development of the dendritic arbor is dependent on cell-autonomous processes, as Purkinje cells devoid of molecular or synaptic signals from surrounding cells still initiate dendritogenesis and form some branches (reviewed in (Sotelo and Dusart, 2009)). Similar to mature Purkinje cells, embryonic Purkinje cells have a polarized morphology, with the dendritic processes protruding from the cell body on one side and the opposite side projecting an axon. At this stage, the cells are referred to as being “fusiform”. During early postnatal development, the number of branches become more numerous and dendrite architecture increases in complexity. Because the perinatal Purkinje cells have not yet formed a monolayer, the immature dendrites are not all oriented in the same direction as “palisades”, as is the case in the mature cerebellum.
Taken together, we discussed evidence that the assembly of the rudimentary cerebellar circuit is mediated by intrinsic Purkinje cell mechanisms. These mechanisms include structural morphogenesis and molecular signaling that are initiated from within the Purkinje cells. Immature Purkinje cell circuits then incorporate the many components of the adult cerebellar circuit, including mossy fiber and climbing fiber afferents. However, compared to adults, there are some key differences in the anatomy and connectivity of immature Purkinje cell circuits. The mechanisms that transform rudimentary Purkinje cell circuits into mature circuits with vast computational power coincide with the proliferation, migration and differentiation of granule cells (Figure 3). We next consider the cellular, genetic and circuit mechanisms of granule cell development, but we keep the discussion focused on their relationship with Purkinje cells.
Experimentally manipulating developing granule cells leads to cerebellar defects
Peak granule cell neurogenesis occurs between 24 to 40 weeks post-conception in humans, which corresponds to the first two postnatal weeks in mice (Sathyanesan et al., 2019; Sillitoe and Joyner, 2007). Thus, most granule cells are born well after the Purkinje cells have formed their initial connections and interactions with the cerebellar afferents and nuclei. The arrival of granule cells into the internal granular layer reorganizes the rudimentary cerebellar circuit both on a micro (single Purkinje cell) and macro (cerebellum at-large) level. Because of the many changes that occur in temporally overlapping time windows, it has been a challenge to tease out which of the changes to Purkinje cells occur due to cell-autonomous maturation versus active guidance by granule cells. Still, we have gained a wealth of knowledge about how granule cells contribute to Purkinje cell development by studying genetic models that have altered granule cell development or using the neonatal X-irradiation model that causes severe loss of granule cells. The mice with granule cell deficits are collectively referred to as “agranular mice”. Before discussing the findings from these different manipulations, we introduce the key lines of agranular mouse models.
There is a long and fascinating history of how different mutant mice became precious experimental models after the recognition that their peculiar behaviors were due to spontaneous mutations in genes critical for cerebellar development (Dusart et al., 2006; Gold et al., 2007; Lalonde and Strazielle, 2019). These mouse lines are named according to their gait abnormalities, because the identities of the causal mutated genes were not known at the time; staggerer (later identified as a mutation in RORα) is one example. Studies over the past six decades have found that abnormal motor control can often be attributed to altered cerebellar development, with the resulting phenotypes reflecting the functional impact of the specific cell-type(s) that expresses the mutated gene. Among the most studied spontaneous agranular mutants are the aforementioned staggerer mice (Hamilton et al., 1996; Herrup, 1983; Sidman et al., 1962), weaver mice that have a mutation in the gene encoding the G-protein inward rectifying potassium channel, Girk2 (Patil et al., 1995; Rezai and Yoon, 1972; Sidman et al., 1965), reeler mice that have a mutation in the gene encoding the extra-cellular matrix protein, reelin (D’Arcangelo et al., 1995; Falconer, 1951; Hamburgh, 1960), and scrambler mice that have a mutation in the reelin-receptor encoding gene, disabled (Goldowitz et al., 1997; Sheldon et al., 1997; Sweet et al., 1996). Of these mutations, only the weaver mutation causes cell loss through a granule cell intrinsic Girk2 mutation. RORα (staggerer) is only expressed in inhibitory cells and likely impairs granule cell survival and proliferation through reduced sonic hedgehog (SHH) signaling. Finally, reeler and disabled mice have low numbers of granule cells that may occur as a secondary consequence to abnormal Purkinje cell migration, or perhaps secondary to abnormal SHH signaling (Cendelin, 2014; Lalonde and Strazielle, 2019). While the mutated genes in these mice are distinct, their anatomical phenotypes overlap in that most granule cells do not survive after final mitosis and, as a result, adequate numbers of granule cells fail to integrate into the developing cerebellar circuit. Interestingly though, in these four agranular mutant mouse models, to some degree an external granular layer still forms during early development.
The only well-known mutant mice to have an almost complete absence of granule cells, as well as the granule cell precursors, are engineered mutant mice that have a deletion of Atoh1, which encodes a basic helix-loop-helix transcription factor (Ben-Arie et al., 1997; van der Heijden and Zoghbi, 2018). Unfortunately, the use of Atoh1 knockout mice to study the role of granule cells on Purkinje cell development has been limited because Atoh1 is a necessary gene for neonatal survival. Interestingly, studies investigating cerebellar development in Atoh1 mosaic animals have confirmed a resemblance between these mice and Reeler mice (Jensen et al., 2002, 2004).
A third model of cerebellar agranularity is X-irradiation of the early postnatal cerebellum. X-irradiation preferentially kills mitotic neurons, and therefore its postnatal application has a major bias towards developing granule cells, which results in anatomical and behavioral phenotypes that are similar to those observed in the different genetic models (Altman and Anderson, 1971).
A common complication of these genetic and experimental manipulations that cause a loss (or lack) of granule cells is that the primary effects are not restricted to the granule cell population. For instance, in regard to the spontaneous mutants staggerer, weaver, reeler, and scrambler, multiple cerebellar cell-types express the proteins for which the mutated genes encode. Likewise, Atoh1 is not only important for the development of granule cells, but it is also critical for establishing the excitatory cerebellar nuclei neurons and unipolar brush cells (Rose et al., 2009). Finally, X-irradiation affects all proliferating cells at the time of radiation. Therefore, this experimental manipulation can also affect the molecular layer interneurons (Altman and Anderson, 1971), or, conversely, cause an incomplete depletion of granule cells due to adaptive mechanisms (Wojcinski et al., 2017). Nevertheless, the striking overlap in the phenotypes between different agranular mice, and specifically in the way Purkinje cell development is affected, supports the hypothesis that the lack of granule cell cues is a primary driving force for the cerebellar defects.
Observations of abnormal Purkinje cell development in agranular mice are further corroborated by results from studies in which the synapses between granule cells and Purkinje cells are specifically impaired. Some studies focused on eliminating presynaptic vesicle release by targeting the expression of tetanus toxin to granule cells (Kim et al., 2009; Park et al., 2019) or eliminating calcium-dependent vesicle fusion by genetically deleting presynaptic calcium channels (Galliano et al., 2013), whereas others studies focused on parallel fiber-Purkinje cell signaling by eliminating glutamate receptors on Purkinje cells (Aiba et al., 1994; Hashimoto et al., 2001; Kashiwabuchi et al., 1995). In all cases, the development of Purkinje cell structure was altered and as a consequence of altering the wiring process, cerebellar circuit function was compromised. We use these studies as a motivation to raise the issue of how intrinsic and extrinsic factors cooperate to sculpt Purkinje cell development and establish function from embryonic through postnatal life.
Granule cells mediate the normal reorganization of Purkinje cell circuits
With the rapid expansion of the granule cell population, the cerebellum starts to acquire its structurally mature morphology, characterized by its ten primary lobules and three-layered cortex (Figure 1). These developmental milestones coincide with a second phase of Purkinje cell development (Figure 2C). Purkinje cells form part of an anchor center required for the formation of primary fissures between lobules (Sudarov and Joyner, 2007), but lobule formation is mostly dependent on the massive expansion of cerebellar granule cells from P0 and onwards, and is completely absent in mice lacking granule cells (Ben-Arie et al., 1997; Jensen et al., 2002, 2004). Likewise, reduction of the number of granule cells by interfering with SHH signaling results in a smaller number of folia (Corrales et al., 2004, 2006; Lewis et al., 2004). Granule cell progenitors start to secrete reelin during neurogenesis, which aids in the dispersion of embryonic Purkinje cell clusters (D’Arcangelo et al., 1995). With the formation of the lobules and increase in surface area, Purkinje cells start to spread out and rearrange into a single monolayer (Figure 4). As the cerebellum primarily expands in the anterior-posterior orientation due to lobule formation (the vermal surface extends ~25-fold in the anterior-posterior direction and only ~1.5-fold in the medial-lateral direction), the embryonic clusters also segregate in an anterior-posterior manner, which eventually results in the classical longitudinal zones (Figure 1B). In agranular mice, lobule architecture, layer formation and zonal patterning are all severely disrupted (reeler, Larouche et al., 2008; Gli2 conditional mutant, Sillitoe et al., 2010). Accordingly, when the absence of granule cells is regionally restricted, Purkinje cell clusters do not disperse to form a monolayer and distinct molecular zones fail to sharpen, with the cells instead remaining in embryonic-like clusters with poorly defined boundaries (Armstrong et al., 2009; Reeber et al., 2013; Vig et al., 2005).
Figure 4. Expansion of the cerebellar cortex in the anterior-posterior axis during development.
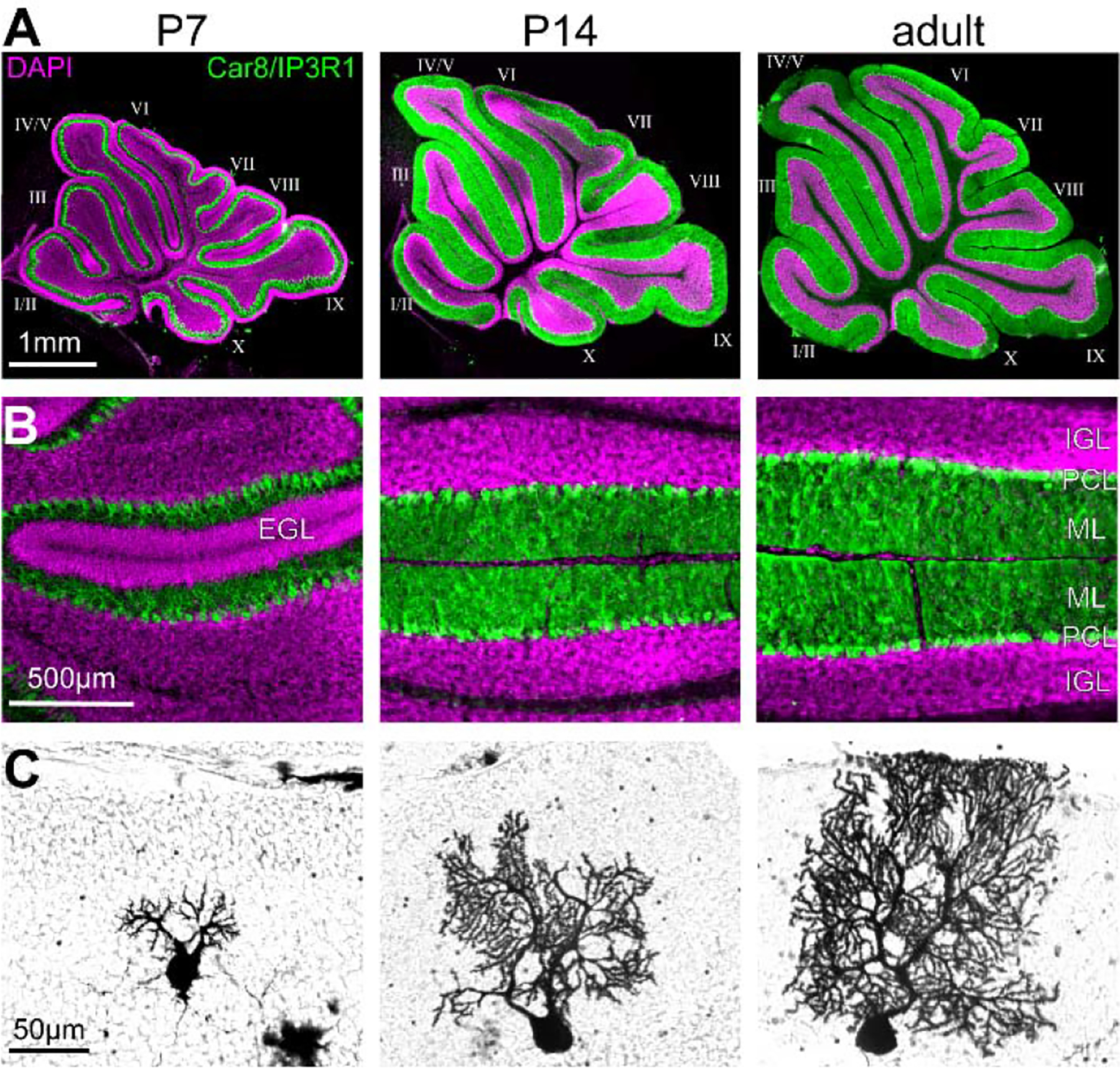
A. Sections cut through the cerebellar vermis of P7, P14, and adult mice stained for the nuclear marker DAPI and the Purkinje cell markers, Car8 and IP3R1. B. High-power images of the cerebellar cortex stained for the nuclear marker DAPI and the Purkinje cell markers, Car8 and IP3R1. Note the large external granular cell layer (EGL) in P7 animals and the increase in the thickness of the molecular layer (ML) between the different time points. C. Example of Golgi-Cox-stained Purkinje cells at P7, P14, and adult.
In addition to localizing Purkinje cells in the cerebellar cortex, expansion of the granule cell population also guides Purkinje cell dendritic morphology, orientation, and planar organization. Starting in the second postnatal week, the Purkinje cell dendritic arbor starts to expand mainly along the transverse plane, following the general growth of the cerebellar cortex in the anterior-posterior direction (Figure 4C) (Fujishima et al., 2018; Sotelo and Dusart, 2009). In the third postnatal week, when the Purkinje cells are situated in a monolayer, the Purkinje cell dendritic arbor expands more in the vertical direction (Sotelo and Dusart, 2009). Purkinje cell dendrites in agranular mice are smaller and maintain their multi-polar dendritic orientation (Bradley and Berry, 1976; Mariani et al., 1977). Slowed migration and decreased granule cell survival in astrotactin null mice results in impaired Purkinje cell monolayer formation and dysmorphic dendritic arborization (Adams et al., 2002). However, the expansion of the dendritic arbor may dependent on the formation and physical presence of synapses, rather than synaptic signaling, as partially blocking parallel fiber input onto Purkinje cells results in only a slightly thinner molecular layer and less dendritic branches (Kim et al., 2009; Park et al., 2019).
Finally, granule cells are essential for the reorganization of the Purkinje cell connectome, a process that mostly occurs between postnatal day 7 and 14 (although it does continue until ~P21 in mice). Granule cells modify the connectivity of the Purkinje cell afferents in three major ways (Figure 3). First, granule cells make contacts with Purkinje cells via their parallel fiber projections. Second, granule cells are necessary for the displacement of mossy fibers from the Purkinje cell bodies to the granule cell dendrites. And third, synaptic signaling at granule cell to Purkinje cell synapses is necessary for the pruning of extra-numerous climbing fibers from the Purkinje cells.
The thousands of parallel fiber synapses that terminate on Purkinje cells (Huang et al., 2014) are arranged in an inside-out manner, with the first born and earlier migrating granule cells synapsing on the portion of the Purkinje cell dendrites that are closest to the Purkinje cell somata (Park et al., 2019). The first parallel fiber-Purkinje cell synapses start to form around the second postnatal week in rats (Shimono et al., 1976), but granule cells continue to migrate until around P20 in rodents and the parallel fiber-Purkinje cell synapses continue to form until the fourth postnatal week in mice (Espinosa and Luo, 2008; Park et al., 2019). In accordance with these anatomical data, in vivo electrophysiology recordings demonstrated that the mature properties of Purkinje cell activity (frequency, pattern, etc.) are also set at around P30 (Arancillo et al., 2015).
The relocation of mossy fibers from Purkinje cells to their mature synaptic partners, the granule cells, encompasses a process involving positive and negative signals. As granule cells invade the internal granular layer, the expression of Purkinje cell derived BMP4 is thought to provide a negative regulator of the embryonic plan. In BMP4 knock-out mice, postnatal mossy fibers erroneously maintain their connectivity to Purkinje cells, and the placement of postnatal mossy fibers was shifted towards the Purkinje cell layer (Kalinovsky et al., 2011). Conversely, granule cells might provide positive signals. They express FGF22, Wnt7a and neuroligins, molecules that are all thought to provide a positive synaptogenic influence over mossy fiber afferents (Hall et al., 2000; Scheiffele et al., 2000; Umemori et al., 2004). In this scenario, the granule cells would attract their own afferent input. It is interesting to speculate that granule cells attract the mossy fibers that reside on Purkinje cells in their closest proximity. If this is the case, then the clustered mossy fiber-Purkinje cell map (Sillitoe, 2016) may provide a developmental plan that dictates the formation of an equivalent map of mossy fiber-granule cell connections. In accordance with the data from normal mice (Sillitoe, 2016), in agranular scrambler mice, mossy fibers intermingle with ectopic zonal clusters of Purkinje cells (Reeber et al., 2013).
The manner in which granule cells mediate the pruning of climbing fiber synaptic contacts onto Purkinje cells should be considered from a functional perspective. Previous work showed that Purkinje cells in the agranular cerebellum receive functional inputs from multiple climbing fibers, even in the adult stage (Crepel et al., 1981; Mariani and Changeux, 1981; Woodward et al., 1974). More recent studies have shown that partially eliminating parallel-fiber inputs onto Purkinje cells, by eliminating postsynaptic glutamate receptors, also results in the multi-innervation of Purkinje cells by climbing fibers (Hashimoto and Kano, 2003; Hashimoto et al., 2001, 2009; Kano and Hashimoto, 2012). In the normal cerebellum, the parallel fiber-Purkinje cell synapses likely contribute to climbing fiber elimination through two distinct processes. First, the parallel-fiber synapses directly compete with climbing fiber synapses for innervation territory, specifically on the most distal sites of the Purkinje cell dendrites. Second, synaptic signaling from the parallel fibers is suggested to initiate downstream signaling cascades that are required for the elimination of climbing fiber synapses (reviewed in (Hashimoto et al., 2009).
Together, this extensive literature lends support to the hypothesis that granule cells provide essential cues for the anatomical maturation of Purkinje cell as they integrate into functional circuits. Granule cells are important for controlling both the gross morphology of the cerebellum, as their numbers and migration drive the growth and foliation of the cerebellum, as well as the processes that promote layering of the different cell types in the cerebellar cortex. They are also indispensable for the reorganization of the rudimentary Purkinje cell circuit into the adult circuit, which occurs during the second postnatal week in rodents. In summary, although cell-autonomous mechanisms initiate the development of Purkinje cells, many aspects of Purkinje cell circuit maturation, including their structure and connectivity, are highly dependent on granule cell cues. But, what about Purkinje cell activity? How might these granule cell-dependent developmental processes influence the firing properties of their target Purkinje cells?
The wiring of functional connections and maturation of Purkinje cell firing
A lot is known about the anatomical maturation of Purkinje cells and the contribution of granule cells herein. Similarly, Purkinje cell firing patterns have been studied extensively. Purkinje cells in adult animals have a very distinct electrophysiological signature, hallmarked by intrinsically generated simple spikes and climbing fiber-initiated complex spikes (indicated with “*” in Figure 5) (Davie et al., 2008; Schmolesky et al., 2002). The complex spikes can be distinguished from the simple spikes because they typically initiate a large amplitude spike, which is followed by 3–5 smaller spikelets, and they induce a pause in simple spike firing. However, surprisingly little is known about how Purkinje cells acquire these unique firing properties or how their spikes change in vivo during the rapid period of circuit reorganization in the first to second postnatal weeks.
Figure 5. The electrophysiological signature of Purkinje cells changes during development.
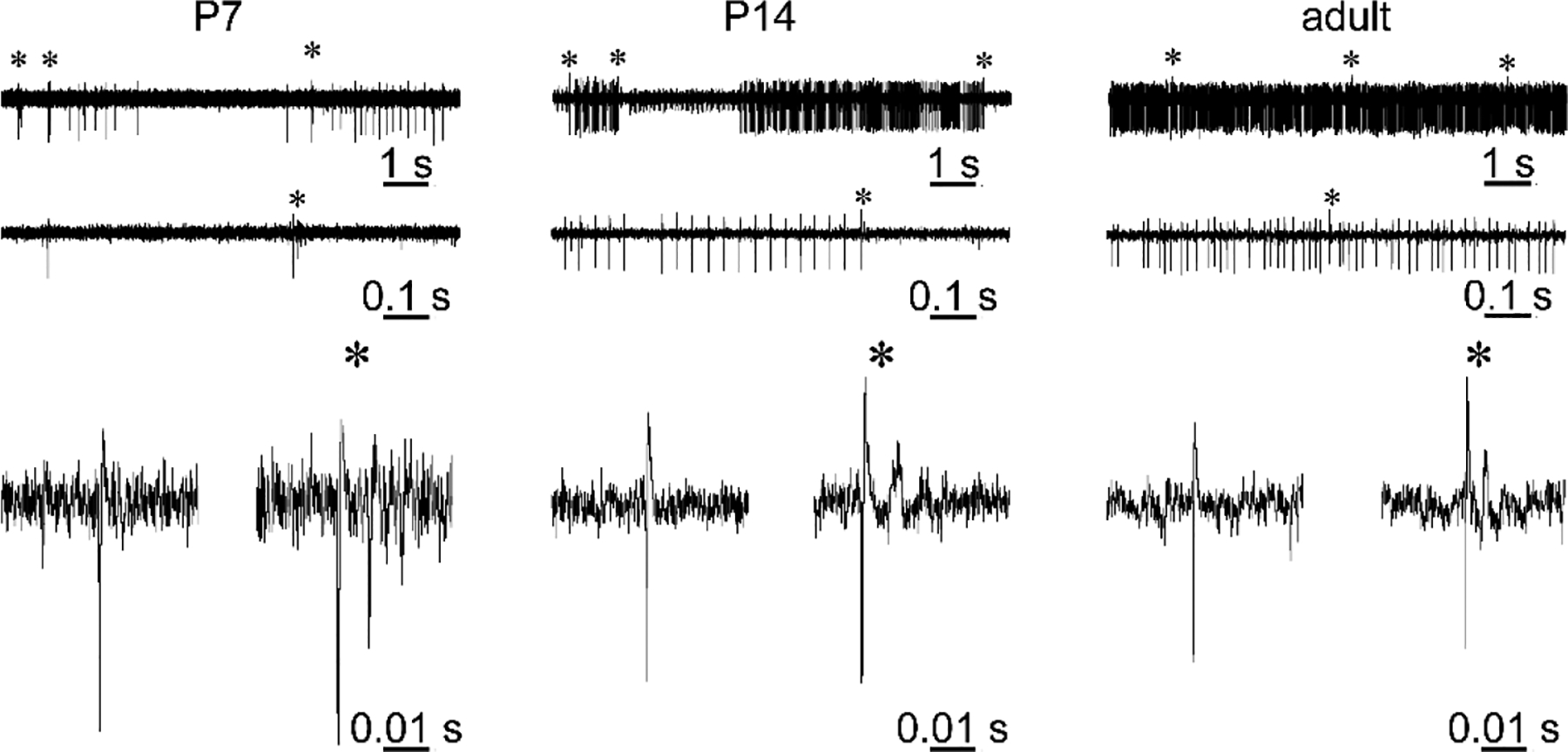
Example traces of in vivo extracellular electrophysiology recordings from anesthetized P7, P14, and adult mice. Top traces are 10 seconds of recordings, middle traces are 1 second of recording, and bottom traces are examples of simple spikes (left) and complex spikes (right) from each different age. Complex spikes are indicated with an asterisk (*). During development, Purkinje cells start to fire more frequently and more regularly with less pauses between simple spike trains.
In rodents, Purkinje cells start to fire spontaneous action potentials early in the first postnatal week, coinciding with the integration of the first granule cells into the cerebellar circuit. But, the rate and shape of these action potentials differ from those in mature Purkinje cells. Slice recordings in rats show that Purkinje cells increase their excitability and firing frequencies as they age, with many parameters of Purkinje cell function continuing to mature until after the second postnatal week (McKay and Turner, 2005). In vivo recordings in rats show that the firing rate of Purkinje cells increases from P4 to P12 (Sokoloff et al., 2015), and similarly in vivo recordings in mice show that Purkinje cells start to fire at higher rates and with more regularity from P15 onwards (Arancillo et al., 2015). Even though climbing fiber synapses are present on Purkinje cells in the first postnatal week, climbing fiber-induced spikes trains can have a different profile in neonates (Figure 5) (Crepel, 1972; Dupont and Crepel, 1979; Good et al., 2017; Puro and Woodward, 1977a, 1977b; Sokoloff et al., 2015). Specifically, climbing fiber-induced spikes in immature Purkinje cells have the form of either “doublets” or typical complex spikes. On electrophysiological traces recorder in vivo, the doublets can be recognized by the presence of an initial spike, which is followed by a smaller spike within ~ 20 ms (see Figure 5 for an example). Complex spikes in neighboring Purkinje cells measured in early neonatal mice (~P3–5) are highly temporally correlated. This correlation declines with age (P8–10) and is impaired in mice that lack functional parallel fiber synapses (abnormal until P14), suggesting that the extra-numerous climbing fiber innervation in neighboring neurons drives temporally correlated complex spikes (Good et al., 2017). It is unclear how this correlated activity impacts behavior in young mice.
None of the aforementioned studies, however, provided a comprehensive developmental analysis of intrinsically generated Purkinje cell simple spike firing rate and patterns during the ages that experience the most striking anatomical rearrangement in vivo (P7–P14). It is therefore unclear to what extent functional Purkinje cell maturation is guided cell-autonomously or driven by synaptic inputs, or which inputs are most important for early Purkinje cell function. Some important insights can be gained from the few studies that have examined Purkinje cells in very young mice. Electrophysiological recordings performed in the first postnatal week show that Purkinje cells acquire immature, yet functional, electrophysiological properties before they receive inputs from parallel fibers, during a time when the Purkinje cell circuit is fundamentally different from the adult (Crepel, 1972). In addition, X-irradiated, weaver and reeler mice have Purkinje cell firing rates that are similar to control mice (Dupont et al., 1983; Siggins et al., 1976; Woodward et al., 1974), and Purkinje cells isolated in culture can be excited in the absence of proper cerebellar architecture (Gruol and Franklin, 1987; Hockberger et al., 1989). Together, these results provide evidence that some of the Purkinje cell electrophysiological properties may develop independent of granule cells (or at least independent of a complete granule cell population), despite the importance of granule cells for the formation of Purkinje cell anatomy. Indeed, while an initial decrease in Purkinje cell simple spike firing rate and regularity was observed in adolescent mice lacking functional glutamatergic inputs from climbing fibers, the firing patterns normalized by adulthood (White and Sillitoe, 2017). Thus, Purkinje cells may be refractory to the loss of specific excitatory inputs as their firing properties can recover over time, potentially as a consequence of homeostatic regulatory processes.
The observation that Purkinje cell activity is only subtly affected in the absence of granule cells raises an interesting question: how do parallel fibers influence Purkinje cell activity, especially given that they provide the most extensive excitatory input on Purkinje cells? Using a genetic approach, the influence of silencing, rather than eliminating, parallel fibers was shown to alter the spontaneous firing of Purkinje cells (Galliano et al., 2013). In that study, the authors showed that the firing frequency of Purkinje cell simple spikes and complex spikes was not changed, but the firing pattern of the simple spikes was more regular than in control Purkinje cells. Additionally, the modulation of Purkinje cell simple spikes was relatively attenuated in response to stimuli. These findings suggest that the basic electrophysiological properties of Purkinje cells develop independent of granule cell inputs, but that granule cell inputs are essential for modulating Purkinje cells during behavior.
Acquisition of cerebellar-dependent behaviors by granule cell-Purkinje cell interactions
Based on their local (Heck et al., 2007) and network level (Giovannucci et al., 2017; Wagner et al., 2017) connectivity, the lack of granule cells (or their signals) may have specific consequences on the function of adult Purkinje cells (Lackey et al., 2018). In addition to their roles during motor learning (Galliano et al., 2013), recent work also implicates granule cells during cognitive behavior (Wagner et al., 2017). This raises a fundamental question: how do granule cells contribute to cerebellar function during ongoing behavior in developing and adult animals?
Of specific relevance to the current discussion, one wonders how these proposed granule cell functions influence behaviors that are of vital importance for neonates to thrive in the early postnatal weeks. These behaviors include ultrasonic vocalizations, righting reflex, and gait control (Al-Afif et al., 2013; Fujita et al., 2008). Studies in agranular mice show that the lack of granule cells impairs cerebellar control in very early postnatal mice, before granule cells make functional contacts onto Purkinje cells. Rats X-irradiated immediately after birth showed behavioral abnormalities as early as P10 (Guelman et al., 1993). Interestingly, early X-irradiation in rats causes more severe motor abnormalities than later insults, suggesting a temporal dependence on granule cells on behavioral outcomes (Ferguson, 1996; Le Marec et al., 1997). Genetic models of agranularity also caused early observable phenotypes, weaver mice have abnormal swimming behavior at P3 (Bolivar et al., 1996), staggerer mice show abnormal righting behavior at P3 (Heuzé et al., 1997) and scrambler mice have abnormal gait and tremor as early as P8 (Jacquelin et al., 2012). As mentioned previously, the mutations in the genetically-induced agranular mice are not cerebellum specific and therefore it is hard to pinpoint whether all observed phenotypes are a direct result of cerebellar dysfunction. However, the onset and specificity of the behavioral abnormalities are reminiscent of similar impairments that have been observed in mice that have genetic mutations restricted to the Purkinje cells (Lalonde and Strazielle, 2015).
These observations have two major implications for cerebellar function. First, the immature cerebellar circuit, where Purkinje cells are innervated by mossy fibers and multiple climbing fibers, contributes to gait control in neonatal mice. And second, granule cells are somehow necessary for the function of this “transient” circuit, even though their axons are not a stable component of the circuit and granule cell development is far from complete. This highlights the problem of how granule cells contribute to cerebellar function in the earliest stages of circuit development and function, because many of the granule cell axon-dependent processes that are required for Purkinje cell morphogenesis are not initiated until after ~P10 in mice. Note, however, that granule cell axonal growth is initiated during the first postnatal week in rodents (Stegmüller et al., 2006). This indicates that immature parallel fibers could, in theory, have a dynamic interaction with nearby Purkinje cells, even though the granule cell somata are still making their descending journey from the external granule layer. We therefore postulate that granule cells form transient contacts with Purkinje cells as they migrate through the molecular and Purkinje cell layers. This hypothesis could be tested by studying the anatomy and function of cerebellar circuits at these specific ages, as well as examining the emergence of cerebellar-dependent behaviors in mouse models in which the dynamic interactions between parallel fibers and Purkinje cells are impaired.
Although cell-type specific mutational analyses provide powerful mechanistic insight, one has to consider that most natural and disease-related alterations involve several different classes of neurons (and glia). Accordingly, while behaviors in mice with impaired parallel fiber connections were not tested during the dynamic period of circuit reorganization, these mouse models all show abnormal motor coordination and motor learning in adulthood (Aiba et al., 1994; Galliano et al., 2013; Park et al., 2019). Of these examples, mice lacking the postsynaptic glutamate receptor delta 2 (GluRδ2) potentially provide the best example of how cerebellar circuit formation influences the function of that circuit. GluRδ2 is an important component of the postsynaptic glutamate receptor in parallel fiber synapses. In addition to decreased parallel fiber connectivity, GluRδ2-deficient mice also have impaired synaptic pruning of climbing fiber connections onto Purkinje cells. The more generalized abnormal motor performance in GluRδ2-deficient mice is accompanied severe essential tremor (Aiba et al., 1994; Pan et al., 2020). The lack of climbing fiber pruning was also found in post-mortem tissue of human patients with essential tremor (Pan et al., 2020) and excessive climbing fiber signaling is causative in a pharmacological (harmaline-induced) model of cerebellar-dependent tremor (Brown et al., 2020; Handforth, 2012). It should be noted, however, that lack of climbing fiber signaling also results in tremor (Sausbier et al., 2004; White and Sillitoe, 2017). Together, these observations show that too much and too little climbing fiber activity are both detrimental to cerebellar function, and that granule cells play a pivotal function in pruning climbing fiber synapses to perfectly balance the amount of excitation that reaches Purkinje cells from the inferior olive. Additional studies with specific circuit manipulations will have to be conducted in order to gain further insights in the link between cerebellar formation and function. Such studies will provide information about the contribution of specific cerebellar cell-types to different neurological diseases and neuropsychiatric disorders with early and late onset symptoms.
Clinical relevance of studying Purkinje cell and granule cell development in mice
Compared to mice, the human cerebellum contains 80% of all neurons in the central nervous system (vs. ~60% in mice), has a longer developmental timeline (2 years vs 2 months), is ~750 times larger (Herculano-Houzel et al., 2015; Sathyanesan et al., 2019), and has a morphologically distinct rhombic lip (Haldipur et al., 2019). Yet, gene expression programs and the temporal sequence of cellular development are generally conserved between rodents and human (Haldipur et al., 2019). The clinical outcomes of impaired cerebellar development, and specifically the vulnerability of the cerebellum during its peak granule cell expansion and growth, have been discussed in detail in several recent review articles (Gill and Sillitoe, 2019; Sathyanesan et al., 2019; Stoodley and Limperopoulos, 2016). Instead of summarizing these resources, we highlight here several examples of the clinical value of studying mouse models of cerebellar development.
One good example of genetic conservation in a disease context is the complete cerebellar agenesis that occurs upon loss of functional copies of a gene encoding a transcription factor, Ptf1a (PTF1A in human), which is expressed in the ventricular zone and is required for the proper formation of inhibitory neurons in the cerebellum (Hoshino et al., 2005). Homozygotic loss-of-function mutations in PTF1A in consanguineous families result in complete cerebellar agenesis (Al-Shammari et al., 2011; Sellick et al., 2004). While abnormal movements were observed in one of the infants, early neonatal lethality due to pancreatic comorbidities prevented further study of PTF1A mutations in human. Coincidentally, a specific mutation in mouse Ptf1a prevents cerebellar development in mice and results in severe motor impairments including ataxic gait and tremor (Hoshino et al., 2005). Despite the lethality of the mutations in humans, we can conclude that in humans and mice, development of the Ptf1a-inhibitory lineage is essential for the formation of a cerebellum.
The predictive value of mouse models is further exemplified by the similarities in cerebellar malformations upon mutations in the reelin signaling pathway. Homozygote loss-of-function mutations in the human homolog of reelin, RELN, causes lissencephaly and cerebellar hypoplasia (Hong et al., 2000) and results in severe neurocognitive delay and hypotonia. Interestingly, mutations in a gene encoding for a RELN receptor, VLDLR, were found in a patient presenting with developmental delay and ataxia. These VLDLR mutations caused cerebellar hypoplasia and only minor malformations of the cerebral cortex (Boycott et al., 2009). Curiously, while homozygosity for a single point mutation in VLDLR was also found in Eurasier dogs with cerebellar hypoplasia and ataxia (Gerber et al., 2015), knockout of mouse Vldlr did not have overt neurological deficits (Frykman et al., 1995). Instead, only a double knockout approach of Vldlr and ApoE2, another reelin receptor, was able to produce migration and foliation deficits in the mouse cerebellum (Trommsdorff et al., 1999). These findings show that the roles of specific signaling pathways are largely conserved between mouse and human, but that the human cerebellum may be more sensitive to specific loss-of-function mutations.
The difference in sensitivity to gene dosage was also found when studying the transcription factor Foxc1. FOXC1 mutations can cause Dandy Walker Malformation (DWM) (Aldinger et al., 2009), a disorder hallmarked by smaller cerebellar size and a concomitant increase in the size of the fourth ventricle (Pinchefsky et al., 2019). Although mice are resistant to loss of a single copy of Foxc1, cerebellar malformations in Foxc1-null mice and human patients are strikingly similar, with abnormalities being largely restricted to posterior foliation (Haldipur et al., 2017). Additional studies using the Foxc1 mutant mouse model have shown that Foxc1 is in fact expressed in the mesenchyme early during cerebellar development, where it regulates the expression of signaling molecules that in turn promote proliferation and the correct migration of ventricular zone and rhombic lip neurons (Haldipur et al., 2014). These studies show how FOXC1 loss-of-function mutations indirectly impair cerebellar development even though the gene itself is not normally expressed within the cerebellar anlage.
These three examples of specific genetic disruptions exemplify how mutations in a single gene can cause severe cerebellar malformations. However, most cases of DWM and cerebellar impairments are sporadic and have no known genetic cause (Aldinger et al., 2019). For example, premature birth and cerebellar hemorrhage (that often occur in preterm infants) lead to smaller cerebellar size and surviving preterm infants have a high risk for deficient cognitive function and autism spectrum disorders (Limperopoulos et al., 2005, 2007; Stoodley and Limperopoulos, 2016; Volpe, 2009). In these infants, the relative cerebellar size correlates with the severity of neurological impairment, with smaller size and earliest born infants having the highest occurrence of severe neurodevelopmental disorders (Limperopoulos et al., 2007; Volpe, 2009). Furthermore, isolated cerebellar hemorrhages in preterm infants result in abnormal cortical development, suggesting direct and widespread neurological dependence on normal cerebellar development (Dijkshoorn et al., 2020). At a cellular level, premature birth may preferentially interrupt the proliferation of granule cells due to a rapid decrease in SHH levels that occurs after birth, a molecular change which may also depend on the extent of prematurity (Haldipur et al., 2011). A baboon model (Barron and Kim, 2020), a pig model (Iskusnykh et al., 2018) and a mouse model of ischemic insult (Yoo et al., 2014) all confirmed specific impairments in granule cell proliferation and a concomitant decrease in cerebellar size. Thus, premature birth may partially halt cerebellar development due to impaired (but not blocked) granule cell neurogenesis and result in relatively milder neurological features compared to those observed in infants with genetic disorders that impair developmental programs directly involving multiple neuron types.
Altogether, the above disease-relevant examples underscore the value of understanding cerebellar developmental programs in mouse models. They show that although mice may be more resistant to changes in gene dosage, genetic programs for cerebellar development are largely conserved. Genetic insults to key developmental programs cause severe cerebellar malformations and devastating neurological dysfunctions. Less severe anatomical changes, including more modest decreases in cerebellum size that are observed in some surviving prematurely born infants, can result in relatively milder neurological symptoms and correlate with specific neurocognitive disorders. The next question is whether, in addition to face and construct validity, mouse models also have predictive validity and can be used to develop treatments to overcome the functional deficits that may be secondary to abnormal development. For this, however, we must first bridge the gap between form and function (Miterko et al., 2018), and we could use mouse models to understand how abnormal cerebellar development leads to distinctly impaired brain functions.
Future directions
The timeline required for establishing Purkinje cell architecture and the role of granule cells therein, are well defined (Haldipur et al., 2019; Leto et al., 2016). Similarly, the correlation between abnormal cerebellar development and impaired neurocognitive functions and motor impairments have been well-established in human (Limperopoulos et al., 2007; Stoodley and Limperopoulos, 2016; Volpe, 2009). However, there remains a major knowledge gap in how Purkinje cell function emerges and then matures during the course of development. There is a limited understanding of how their dynamic structural (e.g. dendrite expansion) and functional (e.g. changes in complex spike firing) properties influence one another. This leaves several major outstanding questions: How do Purkinje cells change their firing properties during the dynamic developmental time window? How does the early loss of each cerebellar afferent sub-type (based on the source) affect Purkinje cell function? How does the integration of granule cells into the cerebellar circuit influence the functional properties of Purkinje cells? How do Purkinje cells develop without granule cell inputs and alternatively how would they develop with less granule cell input? In other words, how does cerebellar form shape cerebellar function? We have started to address some of these questions using a combination of conditional genetic approaches and in vivo electrophysiology in mice (van der Heijden et al., 2020), cementing the hypothesis that granule development indeed shapes Purkinje cell activity. Several temporally-dependent processes and interactions are likely at play, especially given the multiple stages of granule cell development.
During the period when granule cells are proliferating (Corrales et al., 2004, 2006), they produce signals that help organize Purkinje cell settling (Larouche et al., 2008; Miyata et al., 1997), and by bridging these two morphogenetic processes they may also help sculp zones through their migratory routes that occur in specialized “raphes” (Karam et al., 2001; Lin and Cepko, 1998; Redies et al., 2002). In an interesting parallel to the role of granule cells during such patterning events, the Purkinje cell firing pattern is more regular in the absence of granule cell activity, although the rate of Purkinje cell firing is not affected (Dupont et al., 1983; Galliano et al., 2013; Siggins et al., 1976; Woodward et al., 1974). Thus, one critical function of granule cells in the developing and mature cerebellum may be to increase the dimensionality of Purkinje cell function. This hypothesis is supported by developmental anatomy findings, that show that granule cell number, their clonal expansion, and the Cartesian coordinate-like matrix of zones and lobules are all likely to increase the efficiency of processing different sensorimotor modalities (Butts et al., 2011; Gill and Sillitoe, 2019). The outcome of the highly patterned cellular interactions, during development and in the adult, may be to support the cerebellum’s role in behavioral flexibility.
Understanding the functional differences between the rudimentary and mature Purkinje cell circuits will provide key insights into the mechanisms that establish the computational processes that are essential for cerebellar-dependent behaviors (Dean and Porrill, 2008). Moreover, a more comprehensive and mechanistic understanding of how Purkinje cells acquire their unique functions during the period of dynamic synapse reorganization may give insights into the reasons that cerebellar development is so susceptible to genetic and physical insults. In turn, solving these problems will improve our appreciation for how the cerebellum contributes to neurodevelopmental disorders that result in motor and cognitive impairments. Finally, a more complete and integrated knowledge of cerebellar development, structure, function, and behavior may inspire the design of more efficacious treatments for cerebellar and related brain disorders.
Highlights.
Purkinje cells are born early in cerebellar development and they orchestrate the formation of a transient circuit
Granule cells are born later and reorganize cerebellar connectivity and shape cerebellar morphology
Cerebellar function is dependent upon Purkinje cell and granule cell-mediated developmental processes
Impairments during cerebellar development can lead to a wide range of neurological and neuropsychiatric conditions
The relationship between abnormal cerebellar circuit formation and specific functional deficits remains unresolved
Acknowledgements:
This work was supported by Baylor College of Medicine (BCM), Texas Children’s Hospital, The Hamill Foundation, BCM IDDRC U54HD083092 (Neurovisualization Core), and the National Institutes of Neurological Disorders and Stroke (NINDS) R01NS089664 and R01NS100874.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: We have nothing to disclose.
Bibliography
- Adams NC, Tomoda T, Cooper M, Dietz G, and Hatten ME (2002). Mice that lack astrotactin have slowed neuronal migration. Development 129, 965–972. [DOI] [PubMed] [Google Scholar]
- Ahn AH, Dziennis S, Hawkes R, and Herrup K (1994). The cloning of zebrin II reveals its identity with aldolase C. Development 120, 2081–2090. [DOI] [PubMed] [Google Scholar]
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, and Tonegawa S (1994). Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 79, 377–388. [PubMed] [Google Scholar]
- Al-Afif S, Staden M, Krauss JK, Schwabe K, and Hermann EJ (2013). Splitting of the cerebellar vermis in juvenile rats--effects on social behavior, vocalization and motor activity. Behav. Brain Res 250, 293–298. [DOI] [PubMed] [Google Scholar]
- Al-Shammari M, Al-Husain M, Al-Kharfy T, and Alkuraya FS (2011). A novel PTF1A mutation in a patient with severe pancreatic and cerebellar involvement. Clin. Genet 80, 196–198. [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, Krantz ID, Dobyns WB, and Millen KJ (2009). FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat. Genet 41, 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger KA, Timms AE, Thomson Z, Mirzaa GM, Bennett JT, Rosenberg AB, Roco CM, Hirano M, Abidi F, Haldipur P, et al. (2019). Redefining the etiologic landscape of cerebellar malformations. Am. J. Hum. Genet 105, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, and Anderson WJ (1971). Irradiation of the cerebellum in infant rats with low-level x-ray: histological and cytological effects during infancy and adulthood. Exp. Neurol 30, 492–509. [DOI] [PubMed] [Google Scholar]
- Altman J, and Bayer SA (1985). Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J. Comp. Neurol 231, 42–65. [DOI] [PubMed] [Google Scholar]
- Apps R, and Hawkes R (2009). Cerebellar cortical organization: a one-map hypothesis. Nat. Rev. Neurosci 10, 670–681. [DOI] [PubMed] [Google Scholar]
- Arancillo M, White JJ, Lin T, Stay TL, and Sillitoe RV (2015). In vivo analysis of Purkinje cell firing properties during postnatal mouse development. J. Neurophysiol 113, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CL, Chung SH, Armstrong JN, Hochgeschwender U, Jeong YG, and Hawkes R (2009). A novel somatostatin-immunoreactive mossy fiber pathway associated with HSP25-immunoreactive purkinje cell stripes in the mouse cerebellum. J. Comp. Neurol 517, 524–538. [DOI] [PubMed] [Google Scholar]
- Badura A, Verpeut JL, Metzger JW, Pereira TD, Pisano TJ, Deverett B, Bakshinskaya DE, and Wang SS-H (2018). Normal cognitive and social development require posterior cerebellar activity. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron T, and Kim JH (2020). Preterm birth impedes structural and functional development of cerebellar Purkinje cells in the developing baboon cerebellum. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckinghausen J, and Sillitoe RV (2019). Insights into cerebellar development and connectivity. Neurosci. Lett 688, 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, and Zoghbi HY (1997). Math1 is essential for genesis of cerebellar granule neurons. Nature 390, 169–172. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Manley K, and Fentress JC (1996). The development of swimming behavior in the neurological mutant weaver mouse. Dev. Psychobiol 29, 123–137. [DOI] [PubMed] [Google Scholar]
- Boycott KM, Bonnemann C, Herz J, Neuert S, Beaulieu C, Scott JN, Venkatasubramanian A, and Parboosingh JS (2009). Mutations in VLDLR as a cause for autosomal recessive cerebellar ataxia with mental retardation (dysequilibrium syndrome). J. Child Neurol 24, 1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P, and Berry M (1976). The effects of reduced climbing and parallel fibre input on Purkinje cell dendritic growth. Brain Res. 109, 133–151. [DOI] [PubMed] [Google Scholar]
- Brochu G, Maler L, and Hawkes R (1990). Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J. Comp. Neurol 291, 538–552. [DOI] [PubMed] [Google Scholar]
- Brown AM, White JJ, van der Heijden ME, Zhou J, Lin T, and Sillitoe RV (2020). Purkinje cell misfiring generates high-amplitude action tremors that are corrected by cerebellar deep brain stimulation. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts T, Chaplin N, and Wingate RJT (2011). Can clues from evolution unlock the molecular development of the cerebellum? Mol. Neurobiol 43, 67–76. [DOI] [PubMed] [Google Scholar]
- Butts T, Green MJ, and Wingate RJT (2014). Development of the cerebellum: simple steps to make a “little brain”. Development 141, 4031–4041. [DOI] [PubMed] [Google Scholar]
- Caddy KW, and Biscoe TJ (1979). Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos. Trans. R. Soc. Lond. B, Biol. Sci 287, 167–201. [DOI] [PubMed] [Google Scholar]
- Carta I, Chen CH, Schott AL, Dorizan S, and Khodakhah K (2019). Cerebellar modulation of the reward circuitry and social behavior. Science 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendelin J (2014). From mice to men: lessons from mutant ataxic mice. Cerebellum Ataxias 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédotal A, Bloch-Gallego E, and Sotelo C (1997). The embryonic cerebellum contains topographic cues that guide developing inferior olivary axons. Development 124, 861–870. [DOI] [PubMed] [Google Scholar]
- Chen S, and Hillman DE (1989). Regulation of granule cell number by a predetermined number of Purkinje cells in development. Developmental Brain Research 45, 137–147. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Currle DS, Rose MF, Monuki ES, and Millen KJ (2006). The roof plate regulates cerebellar cell-type specification and proliferation. Development 133, 2793–2804. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Mishima Y, Roberts RW, Aldinger KA, Miesegaes GR, Currle DS, Monuki ES, and Millen KJ (2010). Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc. Natl. Acad. Sci. USA 107, 10725–10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Marzban H, Croci L, Consalez GG, and Hawkes R (2008). Purkinje cell subtype specification in the cerebellar cortex: early B-cell factor 2 acts to repress the zebrin II-positive Purkinje cell phenotype. Neuroscience 153, 721–732. [DOI] [PubMed] [Google Scholar]
- Corrales JD, Rocco GL, Blaess S, Guo Q, and Joyner AL (2004). Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development 131, 5581–5590. [DOI] [PubMed] [Google Scholar]
- Corrales JD, Blaess S, Mahoney EM, and Joyner AL (2006). The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development 133, 1811–1821. [DOI] [PubMed] [Google Scholar]
- Crepel F (1972). Maturation of the cerebellar Purkinje cells. Exp. Brain Res 14, 472–479. [DOI] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N, and Dupont JL (1981). Fate of the multiple innervation of cerebellar Purkinje cells by climbing fibers in immature control, X-irradiated and hypothyroid rats. Developmental Brain Research 1, 59–71. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, and Curran T (1995). A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723. [DOI] [PubMed] [Google Scholar]
- Dahmane N, and Ruiz i Altaba A (1999). Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126, 3089–3100. [DOI] [PubMed] [Google Scholar]
- Dastjerdi FV, Consalez GG, and Hawkes R (2012). Pattern formation during development of the embryonic cerebellum. Front. Neuroanat 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JT, Clark BA, and Häusser M (2008). The origin of the complex spike in cerebellar Purkinje cells. J. Neurosci 28, 7599–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, and Porrill J (2008). Adaptive-filter models of the cerebellum: computational analysis. Cerebellum 7, 567–571. [DOI] [PubMed] [Google Scholar]
- Dijkshoorn ABC, Turk E, Hortensius LM, van der Aa NE, Hoebeek FE, Groenendaal F, Benders MJNL, and Dudink J (2020). Preterm infants with isolated cerebellar hemorrhage show bilateral cortical alterations at term equivalent age. Sci. Rep 10, 5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont JL, and Crepel F (1979). Correlations among climbing fiber responses of nearby cerebellar Purkinje cells in the immature rat. Exp. Brain Res 37, 525–535. [DOI] [PubMed] [Google Scholar]
- Dupont JL, Gardette R, and Crepel F (1983). Bioelectrical properties of cerebellar Purkinje cells in reeler mutant mice. Brain Res. 274, 350–353. [DOI] [PubMed] [Google Scholar]
- Dusart I, Guenet JL, and Sotelo C (2006). Purkinje cell death: differences between developmental cell death and neurodegenerative death in mutant mice. Cerebellum 5, 163–173. [DOI] [PubMed] [Google Scholar]
- Eisenman LM, Schalekamp MP, and Voogd J (1991). Development of the cerebellar cortical efferent projection: an in-vitro anterograde tracing study in rat brain slices. Brain Res. Dev. Brain Res 60, 261–266. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, and Luo L (2008). Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells. J. Neurosci 28, 2301–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS (1951). Two new mutants, “trembler” and “reeler”, with neurological actions in the house mouse (Mus musculus L.). J Genet 50, 192–201. [DOI] [PubMed] [Google Scholar]
- Ferguson SA (1996). Neuroanatomical and functional alterations resulting from early postnatal cerebellar insults in rodents. Pharmacology Biochemistry and Behavior 55, 663–671. [DOI] [PubMed] [Google Scholar]
- Frykman PK, Brown MS, Yamamoto T, Goldstein JL, and Herz J (1995). Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 92, 8453–8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima K, Kawabata Galbraith K, and Kengaku M (2018). Dendritic Self-Avoidance and Morphological Development of Cerebellar Purkinje Cells. Cerebellum 17, 701–708. [DOI] [PubMed] [Google Scholar]
- Fujita H, and Sugihara I (2012). FoxP2 expression in the cerebellum and inferior olive: development of the transverse stripe-shaped expression pattern in the mouse cerebellar cortex. J. Comp. Neurol 520, 656–677. [DOI] [PubMed] [Google Scholar]
- Fujita E, Tanabe Y, Shiota A, Ueda M, Suwa K, Momoi MY, and Momoi T (2008). Ultrasonic vocalization impairment of Foxp2 (R552H) knockin mice related to speech-language disorder and abnormality of Purkinje cells. Proc. Natl. Acad. Sci. USA 105, 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Morita N, Furuichi T, and Sugihara I (2012). Clustered fine compartmentalization of the mouse embryonic cerebellar cortex and its rearrangement into the postnatal striped configuration. J. Neurosci 32, 15688–15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano E, Gao Z, Schonewille M, Todorov B, Simons E, Pop AS, D’Angelo E, van den Maagdenberg AMJM, Hoebeek FE, and De Zeeuw CI (2013). Silencing the majority of cerebellar granule cells uncovers their essential role in motor learning and consolidation. Cell Rep. 3, 1239–1251. [DOI] [PubMed] [Google Scholar]
- Gerber M, Fischer A, Jagannathan V, Drögemüller M, Drögemüller C, Schmidt MJ, Bernardino F, Manz E, Matiasek K, Rentmeister K, et al. (2015). A deletion in the VLDLR gene in Eurasier dogs with cerebellar hypoplasia resembling a Dandy-Walker-like malformation (DWLM). PLoS One 10, e0108917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JS, and Sillitoe RV (2019). Functional outcomes of cerebellar malformations. Front. Cell Neurosci 13, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci A, Badura A, Deverett B, Najafi F, Pereira TD, Gao Z, Ozden I, Kloth AD, Pnevmatikakis E, Paninski L, et al. (2017). Cerebellar granule cells acquire a widespread predictive feedback signal during motor learning. Nat. Neurosci 20, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, and Voogd J (1995). Lodewijk Bolk and the comparative anatomy of the cerebellum. Trends Neurosci. 18, 206–210. [DOI] [PubMed] [Google Scholar]
- Gold DA, Gent PM, and Hamilton BA (2007). ROR alpha in genetic control of cerebellum development: 50 staggering years. Brain Res. 1140, 19–25. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Cushing RC, Laywell E, D’Arcangelo G, Sheldon M, Sweet HO, Davisson M, Steindler D, and Curran T (1997). Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of reelin. J. Neurosci 17, 8767–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good J-M, Mahoney M, Miyazaki T, Tanaka KF, Sakimura K, Watanabe M, Kitamura K, and Kano M (2017). Maturation of Cerebellar Purkinje Cell Population Activity during Postnatal Refinement of Climbing Fiber Network. Cell Rep. 21, 2066–2073. [DOI] [PubMed] [Google Scholar]
- Gruol DL, and Franklin CL (1987). Morphological and physiological differentiation of Purkinje neurons in cultures of rat cerebellum. J. Neurosci 7, 1271–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guelman LR, Zieher LM, Ríos H, Mayo J, and Dopico AM (1993). Motor abnormalities and changes in the noradrenaline content and the cytoarchitecture of developing cerebellum following X-irradiation at birth. Mol. Chem. Neuropathol 20, 45–57. [DOI] [PubMed] [Google Scholar]
- Haldipur P, Bharti U, Alberti C, Sarkar C, Gulati G, Iyengar S, Gressens P, and Mani S (2011). Preterm delivery disrupts the developmental program of the cerebellum. PLoS One 6, e23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P, Gillies GS, Janson OK, Chizhikov VV, Mithal DS, Miller RJ, and Millen KJ (2014). Foxc1 dependent mesenchymal signalling drives embryonic cerebellar growth. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P, Dang D, Aldinger KA, Janson OK, Guimiot F, Adle-Biasette H, Dobyns WB, Siebert JR, Russo R, and Millen KJ (2017). Phenotypic outcomes in Mouse and Human Foxc1 dependent Dandy-Walker cerebellar malformation suggest shared mechanisms. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P, Aldinger KA, Bernardo S, Deng M, Timms AE, Overman LM, Winter C, Lisgo SN, Razavi F, Silvestri E, et al. (2019). Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 366, 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, and Salinas PC (2000). Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 100, 525–535. [DOI] [PubMed] [Google Scholar]
- Hamburgh M (1960). Observations on the neuropathology of “reeler”, a neurological mutation in mice. Experientia 16, 460–461. [Google Scholar]
- Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, van Berkel V, Birren BW, et al. (1996). Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature 379, 736–739. [DOI] [PubMed] [Google Scholar]
- Handforth A (2012). Harmaline tremor: underlying mechanisms in a potential animal model of essential tremor. Tremor Other Hyperkinet. Mov. (N. Y.) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, and Kano M (2003). Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron 38, 785–796. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, and Mikoshiba K (2003). Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. J. Neurosci 23, 11342–11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Takechi H, Inoue Y, Aiba A, Sakimura K, Mishina M, Hashikawa T, Konnerth A, Watanabe M, et al. (2001). Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J. Neurosci 21, 9701–9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Yoshida T, Sakimura K, Mishina M, Watanabe M, and Kano M (2009). Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum. Neuroscience 162, 601–611. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Yamanaka A, Kato S, Tanifuji M, Kobayashi K, and Yaginuma H (2018). Anatomical Evidence for a Direct Projection from Purkinje Cells in the Mouse Cerebellar Vermis to Medial Parabrachial Nucleus. Front. Neural Circuits 12, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R (2014). Purkinje cell stripes and long-term depression at the parallel fiber-Purkinje cell synapse. Front. Syst. Neurosci 8, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R, and Leclerc N (1987). Antigenic map of the rat cerebellar cortex: the distribution of parasagittal bands as revealed by monoclonal anti-Purkinje cell antibody mabQ113. J. Comp. Neurol 256, 29–41. [DOI] [PubMed] [Google Scholar]
- Heck DH, Thach WT, and Keating JG (2007). On-beam synchrony in the cerebellum as the mechanism for the timing and coordination of movement. Proc. Natl. Acad. Sci. USA 104, 7658–7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckroth JA, Goldowitz D, and Eisenman LM (1989). Purkinje cell reduction in the reeler mutant mouse: a quantitative immunohistochemical study. J. Comp. Neurol 279, 546–555. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Catania K, Manger PR, and Kaas JH (2015). Mammalian Brains Are Made of These: A Dataset of the Numbers and Densities of Neuronal and Nonneuronal Cells in the Brain of Glires, Primates, Scandentia, Eulipotyphlans, Afrotherians and Artiodactyls, and Their Relationship with Body Mass. Brain Behav. Evol 86, 145–163. [DOI] [PubMed] [Google Scholar]
- Herrup K (1983). Role of staggerer gene in determining cell number in cerebellar cortex. I. Granule cell death is an indirect consequence of staggerer gene action. Developmental Brain Research 11, 267–274. [DOI] [PubMed] [Google Scholar]
- Heuzé P, Féron C, and Baudoin C (1997). Early behavioral development of mice is affected by staggerer mutation as soon as postnatal day three. Brain Res. Dev. Brain Res 101, 81–84. [DOI] [PubMed] [Google Scholar]
- Hirono M, Saitow F, Kudo M, Suzuki H, Yanagawa Y, Yamada M, Nagao S, Konishi S, and Obata K (2012). Cerebellar globular cells receive monoaminergic excitation and monosynaptic inhibition from Purkinje cells. PLoS One 7, e29663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger PE, Tseng HY, and Connor JA (1989). Development of rat cerebellar Purkinje cells: electrophysiological properties following acute isolation and in long-term culture. J. Neurosci 9, 2258–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, and Walsh CA (2000). Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet 26, 93–96. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, et al. (2005). Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron 47, 201–213. [DOI] [PubMed] [Google Scholar]
- Huang C, Gammon SJ, Dieterle M, Huang RH, Likins L, and Ricklefs RE (2014). Dramatic increases in number of cerebellar granule-cell-Purkinje-cell synapses across several mammals. Mammalian Biology 79, 163–169. [Google Scholar]
- Iskusnykh IY, Buddington RK, and Chizhikov VV (2018). Preterm birth disrupts cerebellar development by affecting granule cell proliferation program and Bergmann glia. Exp. Neurol 306, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelin C, Strazielle C, and Lalonde R (2012). Neurologic function during developmental and adult stages in Dab1(scm) (scrambler) mutant mice. Behav. Brain Res 226, 265–273. [DOI] [PubMed] [Google Scholar]
- Jensen P, Zoghbi HY, and Goldowitz D (2002). Dissection of the cellular and molecular events that position cerebellar Purkinje cells: a study of the math1 null-mutant mouse. J. Neurosci 22, 8110–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Smeyne R, and Goldowitz D (2004). Analysis of cerebellar development in math1 null embryos and chimeras. J. Neurosci 24, 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, and Hawkes R (1995). Developing mossy fiber terminal fields in the rat cerebellar cortex may segregate because of Purkinje cell compartmentation and not competition. J. Comp. Neurol 359, 197–212. [DOI] [PubMed] [Google Scholar]
- Kalinovsky A, Boukhtouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, and Scheiffele P (2011). Development of axon-target specificity of ponto-cerebellar afferents. PLoS Biol. 9, e1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, and Hashimoto K (2012). Activity-dependent maturation of climbing fiber to Purkinje cell synapses during postnatal cerebellar development. Cerebellum 11, 449–450. [DOI] [PubMed] [Google Scholar]
- Karam SD, Kim YS, and Bothwell M (2001). Granule cells migrate within raphes in the developing cerebellum: an evolutionarily conserved morphogenic event. J. Comp. Neurol 440, 127–135. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, and Kang Y (1995). Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell 81, 245–252. [DOI] [PubMed] [Google Scholar]
- Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, and Dymecki SM (2009). Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron 63, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, and Diedrichsen J (2019). Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat. Neurosci 22, 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey EP, and Sillitoe RV (2020). Eph/ephrin function contributes to the patterning of spinocerebellar mossy fibers into parasagittal zones. Front. Syst. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey EP, Heck DH, and Sillitoe RV (2018). Recent advances in understanding the mechanisms of cerebellar granule cell development and function and their contribution to behavior. [version 1; peer review: 3 approved]. F1000Res. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, and Strazielle C (2015). Behavioral effects of neonatal lesions on the cerebellar system. Int. J. Dev. Neurosci 43, 58–65. [DOI] [PubMed] [Google Scholar]
- Lalonde R, and Strazielle C (2019). Motor Performances of Spontaneous and Genetically Modified Mutants with Cerebellar Atrophy. Cerebellum 18, 615–634. [DOI] [PubMed] [Google Scholar]
- Larouche M, and Hawkes R (2006). From clusters to stripes: the developmental origins of adult cerebellar compartmentation. Cerebellum 5, 77–88. [DOI] [PubMed] [Google Scholar]
- Larouche M, Beffert U, Herz J, and Hawkes R (2008). The Reelin receptors Apoer2 and Vldlr coordinate the patterning of Purkinje cell topography in the developing mouse cerebellum. PLoS One 3, e1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsell O (1952). The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J. Comp. Neurol 97, 281–356. [DOI] [PubMed] [Google Scholar]
- Leto K, Arancillo M, Becker EBE, Buffo A, Chiang C, Ding B, Dobyns WB, Dusart I, Haldipur P, Hatten ME, et al. (2016). Consensus paper: cerebellar development. Cerebellum 15, 789–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, and McMahon AP (2004). Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev. Biol 270, 393–410. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, and du Plessis AJ (2005). Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics 115, 688–695. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL, Sullivan NR, Benson CB, Avery L, Stewart J, Soul JS, Ringer SA, et al. (2007). Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120, 584–593. [DOI] [PubMed] [Google Scholar]
- Lin JC, and Cepko CL (1998). Granule cell raphes and parasagittal domains of Purkinje cells: complementary patterns in the developing chick cerebellum. J. Neurosci 18, 9342–9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, and Cepko CL (1999). Biphasic dispersion of clones containing Purkinje cells and glia in the developing chick cerebellum. Dev. Biol 211, 177–197. [DOI] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, Habas C, Hagura N, Ivry RB, Mariën P, et al. (2012). Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 11, 457–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marec N, Stelz T, Delhaye-Bouchaud N, Mariani J, and Caston J (1997). Effect of cerebellar granule cell depletion on learning of the equilibrium behaviour: study in postnatally X-irradiated rats. Eur. J. Neurosci 9, 2472–2478. [DOI] [PubMed] [Google Scholar]
- Mariani J, and Changeux JP (1981). Ontogenesis of olivocerebellar relationships. II. Spontaneous activity of inferior olivary neurons and climbing fiber-mediated activity of cerebellar Purkinje cells in developing rats. J. Neurosci 1, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Crepel F, Mikoshiba K, Changeux JP, and Sotelo C (1977). Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Philos. Trans. R. Soc. Lond. B, Biol. Sci 281, 1–28. [DOI] [PubMed] [Google Scholar]
- Marzban H, Chung S, Watanabe M, and Hawkes R (2007). Phospholipase Cbeta4 expression reveals the continuity of cerebellar topography through development. J. Comp. Neurol 502, 857–871. [DOI] [PubMed] [Google Scholar]
- Mason CA, and Gregory E (1984). Postnatal maturation of cerebellar mossy and climbing fibers: transient expression of dual features on single axons. J. Neurosci 4, 1715–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BE, and Turner RW (2005). Physiological and morphological development of the rat cerebellar Purkinje cell. J. Physiol. (Lond.) 567, 829–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miale IL, and Sidman RL (1961). An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp. Neurol 4, 277–296. [DOI] [PubMed] [Google Scholar]
- Miterko LN, Lackey EP, Heck DH, and Sillitoe RV (2018). Shaping diversity into the brain’s form and function. Front. Neural Circuits 12, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Nakajima K, Mikoshiba K, and Ogawa M (1997). Regulation of Purkinje cell alignment by reelin as revealed with CR-50 antibody. J. Neurosci 17, 3599–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales D, and Hatten ME (2006). Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J. Neurosci 26, 12226–12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E, Sekerková G, and Martina M (2011). The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res. Rev 66, 220–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba K, Sugihara I, and Hashimoto M (2011). Close correlation between the birth date of Purkinje cells and the longitudinal compartmentalization of the mouse adult cerebellum. J. Comp. Neurol 519, 2594–2614. [DOI] [PubMed] [Google Scholar]
- Nishida K, Flanagan JG, and Nakamoto M (2002). Domain-specific olivocerebellar projection regulated by the EphA-ephrin-A interaction. Development 129, 5647–5658. [DOI] [PubMed] [Google Scholar]
- Nishiyama H, and Linden DJ (2004). Differential maturation of climbing fiber innervation in cerebellar vermis. J. Neurosci 24, 3926–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster-Granite ML, and Gearhart J (1981). Cell lineage analysis of cerebellar Purkinje cells in mouse chimeras. Dev. Biol 85, 199–208. [DOI] [PubMed] [Google Scholar]
- Pan M-K, Li Y-S, Wong S-B, Ni C-L, Wang Y-M, Liu W-C, Lu L-Y, Lee J-C, Cortes EP, Vonsattel J-PG, et al. (2020). Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci. Transl. Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies MA, and Eisenman LM (1993). Evidence of early topographic organization in the embryonic olivocerebellar projection: a model system for the study of pattern formation processes in the central nervous system. Dev. Dyn 197, 125–145. [DOI] [PubMed] [Google Scholar]
- Park H, Kim T, Kim J, Yamamoto Y, and Tanaka-Yamamoto K (2019). Inputs from Sequentially Developed Parallel Fibers Are Required for Cerebellar Organization. Cell Rep. 28, 2939–2954.e5. [DOI] [PubMed] [Google Scholar]
- Patil N, Cox DR, Bhat D, Faham M, Myers RM, and Peterson AS (1995). A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat. Genet 11, 126–129. [DOI] [PubMed] [Google Scholar]
- Pijpers A, Apps R, Pardoe J, Voogd J, and Ruigrok TJH (2006). Precise spatial relationships between mossy fibers and climbing fibers in rat cerebellar cortical zones. J. Neurosci 26, 12067–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchefsky EF, Accogli A, Shevell MI, Saint-Martin C, and Srour M (2019). Developmental outcomes in children with congenital cerebellar malformations. Dev. Med. Child Neurol 61, 350–358. [DOI] [PubMed] [Google Scholar]
- Puro DG, and Woodward DJ (1977a). Maturation of evoked climbing fiber input to rat cerebellar purkinje cells (I.). Exp. Brain Res 28, 85–100. [DOI] [PubMed] [Google Scholar]
- Puro DG, and Woodward DJ (1977b). The climbing fiber system in the Weaver mutant. Brain Res. 129, 141–146. [DOI] [PubMed] [Google Scholar]
- Redies C, Luckner R, and Arndt K (2002). Granule cell raphes in the cerebellar cortex of chicken and mouse. Brain Res. Bull 57, 341–343. [DOI] [PubMed] [Google Scholar]
- Reeber SL, Loeschel CA, Franklin A, and Sillitoe RV (2013). Establishment of topographic circuit zones in the cerebellum of scrambler mutant mice. Front. Neural Circuits 7, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai Z, and Yoon CH (1972). Abnormal rate of granule cell migration in the cerebellum of “weaver” mutant mice. Dev. Biol 29, 17–26. [DOI] [PubMed] [Google Scholar]
- Rose MF, Ahmad KA, Thaller C, and Zoghbi HY (2009). Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc. Natl. Acad. Sci. USA 106, 22462–22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok TJH (2011). Ins and outs of cerebellar modules. Cerebellum 10, 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanesan A, Zhou J, Scafidi J, Heck DH, Sillitoe RV, and Gallo V (2019). Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat. Rev. Neurosci 20, 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, et al. (2004). Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc. Natl. Acad. Sci. USA 101, 9474–9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, and Serafini T (2000). Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Weber JT, De Zeeuw CI, and Hansel C (2002). The making of a complex spike: ionic composition and plasticity. Ann. N. Y. Acad. Sci 978, 359–390. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, et al. (2015). Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, Gloyn AL, Edghill EL, Hattersley AT, Wellauer PK, et al. (2004). Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat. Genet 36, 1301–1305. [DOI] [PubMed] [Google Scholar]
- Serapide MF, Pantó MR, Parenti R, Zappalá A, and Cicirata F (2001). Multiple zonal projections of the basilar pontine nuclei to the cerebellar cortex of the rat. J. Comp. Neurol 430, 471–484. [DOI] [PubMed] [Google Scholar]
- Sheldon M, Rice DS, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell BW, Cooper JA, Goldowitz D, and Curran T (1997). Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 389, 730–733. [DOI] [PubMed] [Google Scholar]
- Shimono T, Nosaka S, and Sasaki K (1976). Electrophysiological study on the postnatal development of neuronal mechanisms in the rat cerebellar cortex. Brain Res. 108, 279–294. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Lane PW, and Dickie MM (1962). Staggerer, a new mutation in the mouse affecting the cerebellum. Science 137, 610–612. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Green MC, and Appel SH (1965). Catalog of the neurological mutants of the mouse (Cambridge, MA and London, England: Harvard University Press; ). [Google Scholar]
- Siggins GR, Henriksen SJ, and Landis SC (1976). Electrophysiology of Purkinje neurons in the weaver mouse: iontophoresis of neurotransmitters and cyclic nucleotides, and stimulation of the nucleus locus coeruleus. Brain Res. 114, 53–69. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV (2016). Mossy fibers terminate directly within purkinje cell zones during mouse development. Cerebellum 15, 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe RV, and Joyner AL (2007). Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol 23, 549–577. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Malz CR, Rockland K, and Hawkes R (2004). Antigenic compartmentation of the primate and tree shrew cerebellum: a common topography of zebrin II in Macaca mulatta and Tupaia belangeri. J. Anat 204, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe RV, Gopal N, and Joyner AL (2009). Embryonic origins of ZebrinII parasagittal stripes and establishment of topographic Purkinje cell projections. Neuroscience 162, 574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe RV, Vogel MW, and Joyner AL (2010). Engrailed homeobox genes regulate establishment of the cerebellar afferent circuit map. J. Neurosci 30, 10015–10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe RV, Fu Y, and Watson C (2012). Cerebellum. In The mouse nervous system, (Elsevier; ), pp. 360–397. [Google Scholar]
- Sokoloff G, Plumeau AM, Mukherjee D, and Blumberg MS (2015). Twitch-related and rhythmic activation of the developing cerebellar cortex. J. Neurophysiol 114, 1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C, and Chédotal A (2005). Development of the olivocerebellar system: migration and formation of cerebellar maps. Prog. Brain Res 148, 1–20. [DOI] [PubMed] [Google Scholar]
- Sotelo C, and Dusart I (2009). Intrinsic versus extrinsic determinants during the development of Purkinje cell dendrites. Neuroscience 162, 589–600. [DOI] [PubMed] [Google Scholar]
- Sotelo C, and Wassef M (1991). Cerebellar development: afferent organization and Purkinje cell heterogeneity. Philos. Trans. R. Soc. Lond. B, Biol. Sci 331, 307–313. [DOI] [PubMed] [Google Scholar]
- Stegmüller J, Konishi Y, Huynh MA, Yuan Z, Dibacco S, and Bonni A (2006). Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron 50, 389–400. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, and Limperopoulos C (2016). Structure-function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med 21, 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarov A, and Joyner AL (2007). Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev. 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I, and Fujita H (2013). Peri- and postnatal development of cerebellar compartments in the mouse. Cerebellum 12, 325–327. [DOI] [PubMed] [Google Scholar]
- Sweet HO, Bronson RT, Johnson KR, Cook SA, and Davisson MT (1996). Scrambler, a new neurological mutation of the mouse with abnormalities of neuronal migration. Mamm. Genome 7, 798–802. [DOI] [PubMed] [Google Scholar]
- Takeda T, and Maekawa K (1989). Transient direct connection of vestibular mossy fibers to the vestibulocerebellar Purkinje cells in early postnatal development of kittens. Neuroscience 32, 99–111. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, and Herz J (1999). Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701. [DOI] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, and Sanes JR (2004). FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 118, 257–270. [DOI] [PubMed] [Google Scholar]
- van der Heijden ME, and Zoghbi HY (2018). Loss of Atoh1 from neurons regulating hypoxic and hypercapnic chemoresponses causes neonatal respiratory failure in mice. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden ME, Lackey EP, Işleyen FS, Brown AM, Perez R, Lin T, Zoghbi HY, and Sillitoe RV (2020). Maturation of Purkinje cell firing properties relies on granule cell neurogenesis. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig J, Goldowitz D, Steindler DA, and Eisenman LM (2005). Compartmentation of the reeler cerebellum: segregation and overlap of spinocerebellar and secondary vestibulocerebellar fibers and their target cells. Neuroscience 130, 735–744. [DOI] [PubMed] [Google Scholar]
- Vogel MW, Ji Z, Millen K, and Joyner AL (1996). The Engrailed-2 homeobox gene and patterning of spinocerebellar mossy fiber afferents. Brain Res. Dev. Brain Res 96, 210–218. [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2009). Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol 24, 1085–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, and Glickstein M (1998). The anatomy of the cerebellum. Trends Cogn. Sci. (Regul. Ed.) 2, 307–313. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, and Luo L (2017). Cerebellar granule cells encode the expectation of reward. Nature 544, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VA (1999). Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol 9, 445–448. [DOI] [PubMed] [Google Scholar]
- Wang L, and Liu Y (2019). Signaling pathways in cerebellar granule cells development. Am. J. Stem Cells 8, 1–6. [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Rose MF, and Zoghbi HY (2005). Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48, 31–43. [DOI] [PubMed] [Google Scholar]
- Wassef M, Sotelo C, Thomasset M, Granholm AC, Leclerc N, Rafrafi J, and Hawkes R (1990). Expression of compartmentation antigen zebrin I in cerebellar transplants. J. Comp. Neurol 294, 223–234. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, and Scott MP (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103–114. [DOI] [PubMed] [Google Scholar]
- Wetts R, and Herrup K (1982). Cerebellar Purkinje cells are descended from a small number of progenitors committed during early development: quantitative analysis of lurcher chimeric mice. J. Neurosci 2, 1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetts R, and Herrup K (1983). Direct correlation between Purkinje and granule cell number in the cerebella of lurcher chimeras and wild-type mice. Brain Res. 312, 41–47. [DOI] [PubMed] [Google Scholar]
- White JJ, and Sillitoe RV (2013). Development of the cerebellum: from gene expression patterns to circuit maps. Wiley Interdiscip Rev Dev Biol 2, 149–164. [DOI] [PubMed] [Google Scholar]
- White JJ, and Sillitoe RV (2017). Genetic silencing of olivocerebellar synapses causes dystonia-like behaviour in mice. Nat. Commun 8, 14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett RT, Bayin NS, Lee AS, Krishnamurthy A, Wojcinski A, Lao Z, Stephen D, Rosello-Diez A, Dauber-Decker KL, Orvis GD, et al. (2019). Cerebellar nuclei excitatory neurons regulate developmental scaling of presynaptic Purkinje cell number and organ growth. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, Schalek R, Suissa-Peleg A, Jones TR, Knowles-Barley S, Pfister H, and Lichtman JW (2019). Developmental Rewiring between Cerebellar Climbing Fibers and Purkinje Cells Begins with Positive Feedback Synapse Addition. Cell Rep. 29, 2849–2861.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wizeman JW, Guo Q, Wilion EM, and Li JY (2019). Specification of diverse cell types during early neurogenesis of the mouse cerebellum. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcinski A, Lawton AK, Bayin NS, Lao Z, Stephen DN, and Joyner AL (2017). Cerebellar granule cell replenishment postinjury by adaptive reprogramming of Nestin+ progenitors. Nat. Neurosci 20, 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DJ, Hoffer BJ, and Altman J (1974). Physiological and pharmacological properties of Purkinje cells in rat cerebellum degranulated by postnatal x-irradiation. J. Neurobiol 5, 283–304. [DOI] [PubMed] [Google Scholar]
- Yoo JYJ, Mak GK, and Goldowitz D (2014). The effect of hemorrhage on the development of the postnatal mouse cerebellum. Exp. Neurol 252, 85–94. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Kawamura K, Ono K, Yamakuni T, and Takahashi Y (1991). Development and migration of Purkinje cells in the mouse cerebellar primordium. Anat. Embryol. (Berl.) 184, 195–212. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Kawamura K, Kuwano R, and Ono K (1996). Neuron-glia interrelations during migration of Purkinje cells in the mouse embryonic cerebellum. Int. J. Dev. Neurosci 14, 429–438. [PubMed] [Google Scholar]
- Zhou Y. (Joy), Brown AM, Lackey EP, Arancillo M, Lin T, and Sillitoe RV (2020). Purkinje cell neurotransmission patterns cerebellar basket cells into zonal modules that are defined by distinct pinceau sizes. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]


