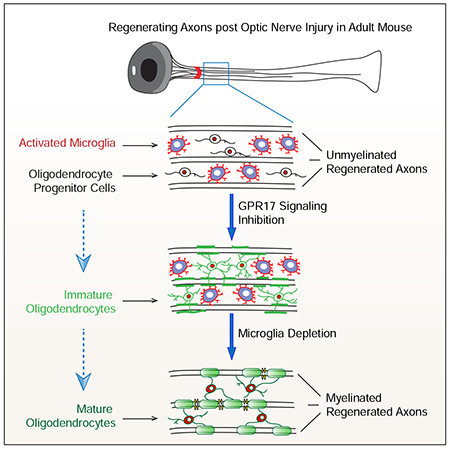
Abstract
Myelination facilitates rapid axonal conduction thereby enabling efficient communication across different parts of the nervous system. Here we examined mechanisms controlling myelination after injury and during axon regeneration in the central nervous system (CNS). Previously we discovered multiple molecular pathways and strategies that could promote robust axon regrowth after optic nerve injury. However, regenerated axons remain unmyelinated and the underlying mechanisms were elusive. In this study, we found that in injured optic nerves, oligodendrocyte precursor cells (OPCs) undergo transient proliferation, but fail to differentiate into mature myelination-competent oligodendrocytes, reminiscent of what is observed in human progressive multiple sclerosis. Mechanistically, we showed that both OPC-intrinsic GPR17 signaling and sustained activation of microglia inhibit different stages of OPC differentiation. Importantly, co-manipulation of GPR17 and microglia led to extensive myelination of regenerated axons. The regulatory mechanisms of stage-dependent OPC differentiation uncovered here suggest a translatable strategy for efficient de novo myelination after CNS injury.
Graphical Abstract
INTRODUCTION
Functional deficits caused by CNS injuries have been largely attributed to severing of long-projection axons. Despite tremendous progress towards developing strategies to promote axon regeneration, behavioral and functional improvements achieved with these methodologies are still limited even in experimental models (He and Jin, 2016; Hilton and Bradke, 2017, Benowitz et al., 2017). For example, our recent studies showed that the methods activating mTOR and STAT3 pathways in retinal ganglion cells (RGCs) promoted robust axon regeneration after optic nerve injury (Park et al., 2008; Duan et al., 2015), and these regenerated axons could make functional synapses with their appropriate targets, such as the superior colliculus. However, regenerated RGC axons remain unmyelinated and ineffective at supporting visual functions (Bei et al., 2016). In light of the role of myelin in facilitating axon conduction, these observations pointed to a need to uncover regulatory mechanisms of myelination after CNS injury.
For myelination in adult CNS, residential oligodendrocyte precursor cells (OPCs) need to proliferate and then undergo a poorly understood multi-step differentiation process before ultimately becoming myelination-competent oligodendrocytes (Simons and Nave, 2015; Chang et al., 2016; Monje, 2018). Demyelination and failure to re-myelinate underlie a number of neurological diseases, such as multiple sclerosis (MS) and Alzheimer’s disease (Fumagalli et al., 2016; Mathys et al., 2019). In the advanced stage of progressive MS, some proliferating OPCs remain in the lesions but fail to differentiate into mature oligodendrocytes (Wolswijk, 2002, Kuhlmann et al., 2008). Therefore, numerous efforts have been made to develop strategies that promote the proliferation and differentiation of OPCs (Franklin and Ffrench-Constant, 2017). However, in most available demyelination models, remyelination occur spontaneously, thereby preventing the precise examination of pro-myelination treatments that initiate de novo myelination. Furthermore, given the nature of the multi-step differentiation process required for transforming OPCs into mature oligodendrocytes, experimental perturbations targeting multiple steps may be required. In this regard, the regenerated axons without spontaneous myelination in our optic nerve injury model serves as a “clean” model to assess the regulatory mechanisms of de novo myelination in the adult CNS. In this study, we asked how OPC proliferation and differentiation occur in injured optic nerves, and how barriers obstructing myelination of regenerated axons can be overcome. Our results revealed a set of translatable manipulations that enable robust myelination of regenerated axons in this model.
RESULTS
Injury-induced OPC proliferation
Our previous studies revealed that multiple different methods could elevate the intrinsic regenerative ability of RGCs and enabled robust axon regeneration after injury. Intriguingly, these regenerated axons were not coupled with myelin associated glycoprotein (MAG), implying that they were not myelinated (Bei et al., 2016). However, other work has reported the myelination of regenerated axons induced by different strategies (de Lima, et al., 2012; Marin et al., 2016). Therefore, we assessed the myelination of regenerated axons induced by PTEN deletion in RGCs after an optic nerve crush injury. Among several thousands of axons analyzed, only two regenerated axons had thin myelin (Figure S1A, B). These results verified that most, if not all, regenerated optic nerve axons failed to undergo spontaneous myelination in our injury models.
Since OPCs are responsible for myelination in adults, we first assessed the proliferation of OPCs in injured (ipsilateral) and control (contralateral) optic nerves of PDGFRα-H2B-GFP transgenic mice, in which all OPCs express nuclear H2B-GFP (Figure 1A). Because these mice also expressed GFP in <5% of vascular and leptomeningeal cells (Marques et al., 2016), we co-stained optic nerve sections with an oligodendrocyte lineage marker, Olig2, and defined the GFP+/Olig2+ double positive cells as OPCs (Figures 1A–C and S1C). As shown in Figures 1B, 1C and S1C, the total numbers of OPCs in the crushed nerves increased significantly at 1 and 2 weeks after injury but returned to the basal levels at 4 weeks. In contrast, low numbers of GFP+/Olig2+ OPCs were seen in intact nerves at all time points. To further assess injury-induced OPC proliferation, we administered BrdU at various time points after injury and evaluated BrdU incorporation just 3 hours post-injection, with an expectation to label dividing OPCs at specific time points (Figure 1D and 1E). The results revealed that injury-induced OPC proliferation was significantly increased around 3-5 days after injury and subsequently reduced to basal levels at later time points (Figure 1E). Together, our results suggest that an optic nerve crush injury triggers rapid and reversible OPC proliferation.
Figure 1. Increased proliferation and failed differentiation of OPCs in injured optic nerves.
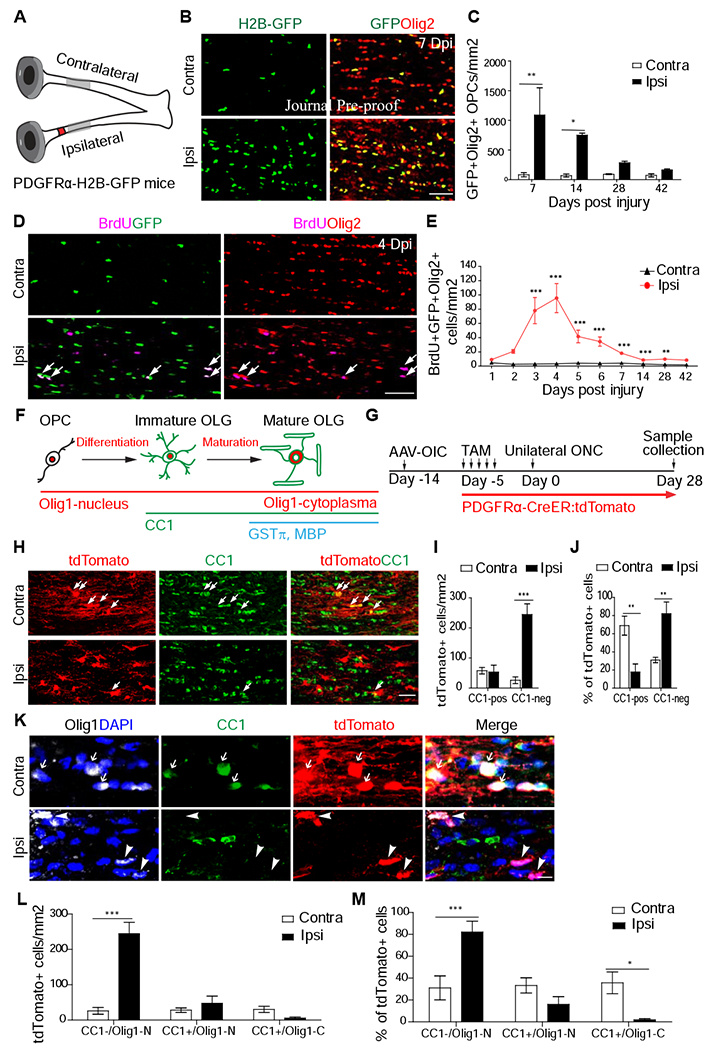
(A) Scheme for experiments in Figure 1A-C. The red spot indicates crush injury site and the gray region indicates the regions that were analyzed for this study.
(B-C) Images and quantification of OPC numbers in injured optic nerves at different time points after injury. N = 3-8 mice per group.
(D-E) Images and quantification of BrdU+/Olig2+ cells in injured optic nerves.
(F) Illustration of the differentiation stages of OPCs and their respective markers.
(G) Scheme for experiments in Figure 1H-M. AAV-OIC: AAVs expressing osteopontin/IGF1/CNTF1. TAM: tamoxifen. ONC: optic nerve crush.
(H-J) Images (H) and quantitation (cell number in I and proportion in J) of CC1+ and tdTomato+ cells. N = 6 mice per group.
(K-M) Images (K) and quantitation of three different populations. In K, arrows in the contralateral side indicate tdTomato+/CC1+/Olig1-C, arrowheads in ipsilateral side indicate tdTomato+/CC1−/Olig1-N (un-differentiated cells). N = 6 mice per group. Scale bar: 100 μm (B, D), 50 μm (H), 10 μm (K). *, **, *** P < 0.05, 0.01, 0.001, respectively. Olig1-N: nuclear Olig1; Olig1-C: cytoplasmic Olig1.
Differentiation failure of proliferated OPCs in injured optic nerves
To trace the differentiation of proliferated OPCs, we utilized a different reporter mouse line, namely PDGFRα-CreER (Young et al., 2013) crossed with Rosa26-STOP-tdTomato mice (Arenkiel et al., 2011), or PDGFRα-CreER:tdTomato mice. Upon tamoxifen administration, Cre expression is induced in PDGFRα+ OPCs, resulting in tdTomato expression in OPCs and their progenies. The differentiation stages of these cells were assessed by immunohistochemistry with different markers, CC1, for all differentiated oligodendrocytes, and Olig1, whose translocation from the nucleus to the cytoplasm as a hallmark for maturation into myelinating oligodendrocytes (Arnett et al., 2004; Gibson et al., 2019). Based on the results, lineage-traced cells could be divided to three stages: 1) un-differentiated OPCs (CC1− with nuclear Olig1), 2) immature oligodendrocytes (CC1+ with nuclear Oligl), and 3) mature oligodendrocytes (CC1+ with cytoplasmic Oligl) (Figure 1F). The identity of mature oligodendrocytes was also verified by additional markers of mature oligodendrocytes, GSTπ (or GST-pi) (Tansey et al., 1991; Duncan et al., 2017) and myelin basic protein (MBP, Duncan et al., 2017) (Figure 1F).
To promote axon regeneration, we injected AAVs expressing osteopontin/IGF1/CNTF (AAVs-OIC) to the vitreous bodies of PDGFRα-CreER:tdTomato mice, 2 weeks prior to optic nerve injury. To label pre-existing OPCs, tamoxifen was injected to these mice right before optic nerve crush, (Figure 1G). At 4 weeks after injury, although the total tdTomato+ number is lower in intact optic nerves, 68% of tdTomato+ cells became CC1+ oligodendrocytes and about half of them exhibited cytoplasmic Olig1+ (Figure 1H–M). Noticeably, tdTomato+ cells had extensive processes in parallel with axons, indicating mature myelinating oligodendrocytes (Figure 1H). However, in the injured optic nerves, only 18% tdTomato+ cells were CC1+ oligodendrocytes and most had nuclear, but not cytoplasmic Olig1 (Figure 1H–M). Consistently, the majority of these tdTomato+ cells had short process, indicative of undifferentiated OPCs (Figure 1K). These results suggest that in injured optic nerves OPC differentiation is suppressed. Consistently, many GSTπ+/tdTomato+ cells were seen in intact, but not injured optic nerves (Figure S1D–F). In addition, although OPCs could differentiate into astrocytes during development (Levison and Goldman, 1993), we did not observe any tdTomato+ cells expressing astrocyte marker GFAP (Figure S1G). Together, these data suggest that proliferated OPCs exhibit differentiation blockades in injured nerves, resembling what observed in the lesions of progressive multiple sclerosis patients.
Injury-induced GPR17 up-regulation contributes to the early differentiation failure of OPCs
Previous studies, with cultured cells and EAE models, identified a variety of compounds that could promote OPC proliferation and/or differentiation. However, it is unknown whether any of these agents could facilitate myelination of regenerated axons. To address this, we screened a set of small molecule compounds, with the goal to identify those that could increase OPC differentiation in injured optic nerves (Figure 2A–C). Individual compounds, capable of penetrating the blood brain barrier, were systematically administrated for 4 weeks after optic nerve injury in wild-type mice. To monitor the differentiation of proliferating OPCs, we applied daily BrdU injection from days 4-10 after injury when OPCs exhibited a high proliferation rate (Figure 1E). The pro-differentiation effect of each compound was evaluated 3 weeks post administration (Figure 2A–B). As shown in Figures 2B and 2C, three compounds, specifically Montelukast, a GPR17 antagonist (Fumagalli et al., 2011), Benztropine mesylate (a M1/M3 muscarinic receptor antagonist (Deshmukh et al., 2013), and Solifenacin, a M3 muscarinic receptor antagonist (Abiraman et al., 2015), significantly increased the numbers of BrdU+/CC1+ double positive cells. Since Montelukast had the strongest effect, our further studies focused on this compound and its putative target GPR17.
Figure 2. GPR17 is an intrinsic blocker of early oligodendrocyte differentiation of OPCs in injured optic nerves.
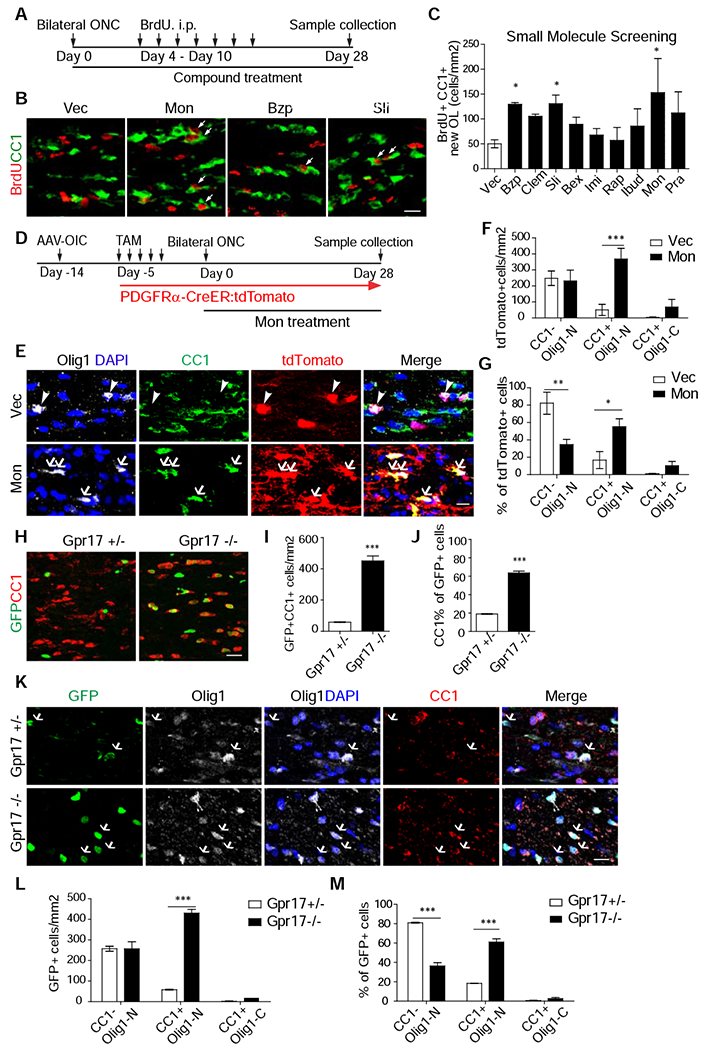
(A) Scheme of compound screening.
(B, C) Images of injured optic nerves stained with anti-CC1 and BrdU (B) and quantification (C). N = 4-13 mice per group. Vec (Vehicle). Mon (Montelukast), Bzp (Benztropine Mesylate), and Sli (Solifenacin succinate).
(D) Design for experiments in Figure 2E-G. N = 6 mice per group. AAV-OIC: AAVs expressing osteopontin/IGF1/CNTF1. TAM: tamoxifen. ONC: optic nerve crush.
(E-G) Images of injured or intact optic nerves stained with antibodies against Olig1, CC1, tdTomato, and DAPI (E) and quantification results of the densities (F) or proportions (G) of different populations. In E, arrowheads indicate CC1−/ Olig1-N cells in Vec, while arrows indicate CC1+/Olig1-N cells after treatment.
(H-J) Images of injured optic nerves at 28 days after injury (H) and quantification results of the densities of GFP+/CC1+ cells (I) or proportion of CC1+ among GFP+ cells (J).
(K-M) Images (K) of injured optic nerves stained with indicated antibodies and BrdU and quantification results of the densities (L) or proportions (M) of different populations. N = 6 mice per group. Scale bar: 20 μm (B, H), 10 μm (E), 20 μm (K). *, **, *** P < 0.05, 0.01, 0.001, respectively. Olig1-N: nuclear Olig1; Olig1-C: cytoplasmic Olig1.
As an initial verification, we applied Montelukast treatment to PDGFRα-CreER/tdTomato mice after injury for 4 weeks (Figure 2D). As shown in Figure 2E–G, 65% of tdTomato+ cells became CC1+, in contrast to 18% in vehicle-treated mice. Surprisingly, the majority of these CC1+/tdTomato+ cells had nuclear but not cytoplasmic Olig1 (Figure 2E–G). In addition, the total tdTomato+ cell number increased after Montelukast treatment (Figure 2F). As cell death is associated with failed OPC differentiation (Hughes et al., 2018), such an increase in tdTomato+ cells may be secondary to the improved differentiation. Together, our results suggest that while Montelukast treatment promoted the early differentiation of OPCs, these cells fail to advance into mature oligodendrocytes.
As an antagonist of leukotriene receptors, including GPR17 (Fumagalli et al., 2016), Montelukast is a clinically approved treatment for asthma and seasonal allergies. GPR17 was previously implicated as an inhibitor of oligodendrocyte differentiation (Chen et al., 2009, Simon et al., 2016; Ou et al., 2016). However, GPR17’s expression is down-regulated in the adult CNS and myelination appears normal in adult GPR17 knockout mice (Chen et al., 2009). By in situ hybridization, we found that GPR17 expression was rarely detectable in intact optic nerves of adult mice. However, optic nerve crush injury triggers significant up-regulation of GPR17 in injured nerves (Fig. S2A and S2B).
In addition to GPR17, Montelukast may inhibit other leukotriene receptors (Leff et al., 1998). Thus we assessed the effects of genetic deletion of GPR17 on OPC differentiation in injured optic nerves by utilizing GPR17 knock-in mice (Chen et al., 2009). In this line, the GPR17 coding region is replaced with the H2B-GFP sequence. Thus, these mice could be used for monitoring GPR17 expression (by GFP signal in both heterozygotes and homozygotes) and for loss-of-function studies (homozygotes). Consistent with injury-induced GPR17 expression, GFP+ (GPR17+) cells were significantly increased in both GPR17+/− and GPR17−/− mice at 7 days after injury (Figure S2C–E). Most of these GFP+ cells were also co-stained with anti-Olig2, consistent with their restricted expression in OPC lineage (Figure S2C). By 30 days after injury, many GFP+ cells were CC1+ in GPR17−/− mice (Figures 2H–J, S2F–H). However, these cells had nuclear, but not cytoplasmic Olig1 signals (Figure 2L–M for dpi 28 and Figure S2I–K for dpi 7), similar to Montelukast treatment. In addition, the number of GFP+ cells was significantly higher in GPR17−/− mice (Figures S2F–H). But these mice did not show increase proliferation by BrdU labeling (Figure S2C, S2E). Thus, increased GFP+ cells are likely secondary to improved differentiation. Thus, similar to Montelukast treatment, GPR17 knockout facilitated the initial differentiation, but not late maturation, of proliferated OPCs in injured optic nerves.
Differential effects of acutely or sustained activated microglia on OPC proliferation and maturation
Because of the partial effects of GPR17 inhibition on OPC differentiation, we attempted to identify additional blocker(s) for the late maturation step of OPC differentiation. An important hint was the differing numbers of CC1+ cells with cytoplasmic Olig1 or GSTπ in injured versus their control uninjured nerves (Figure 1K–M, S1D–F), suggesting a possible contribution of environmental factors. While similar levels of GFAP immunoreactivity were detected in both injured and non-injured optic nerves (Figure S3A–B), microglia became rapidly and sustainably activated only in injured ones (Figure S3A–F). As neuroinflammation has been shown to regulate OPC proliferation and differentiation (Franklin and Ffrench-Constant, 2017; Lassmann et al., 2018; Lloyd and Miron, 2019), we further examined the role of microglia in injured optic nerves on OPC proliferation and differentiation.
Taking advantage of the observation that systematic application of PLX3397, an inhibitor of colony stimulating factor 1 receptor, depleted microglia in vivo (Figure S3J–K, Elmore et al., 2014), we first pre-treated PDGFRα-H2B-GFP mice with PLX3397 or control vehicle for 3 days before injury and continued the treatment for additional 14 days before examining OPC proliferation by BrdU injection 48 hours before euthanasia (Figure 3A). As shown in Figures 3B and 3C, PLX3397 profoundly reduced the total numbers of OPCs (BrdU+/GFP+/Olig2+). Thus microglia activation is required for injury-induced OPC proliferation, consistent with early reports about a positive role of activated microglia in promoting myelination (Franklin and Ffrench-Constant, 2008; Miron et al., 2013; Lloyd and Miron, 2019).
Figure 3. Microglia are required for OPC proliferation but detrimental for their maturation.
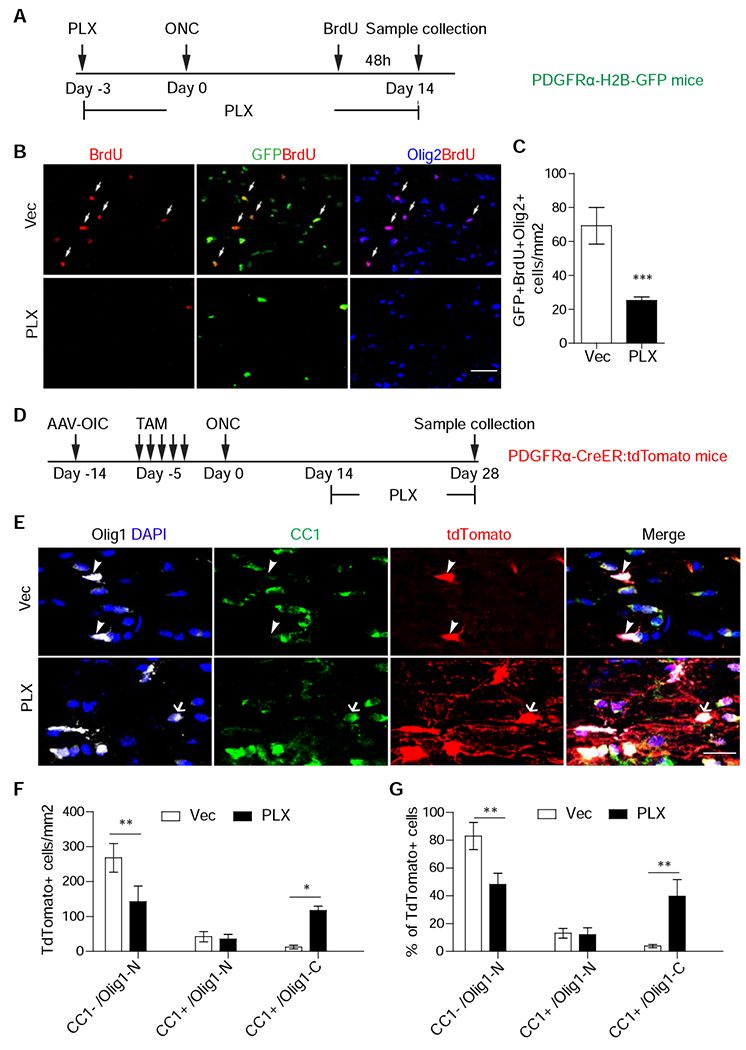
(A) Scheme for experiments in Figure 3B, C.
(B, C). Images of injured optic nerves stained with GFP, Olig2, or BrdU (B) and quantification of the densities of GFP+/Olig2+/BrdU+ cells (C). N = 6 mice per group.
(D) Scheme for experiments in Figure 3E-G.
(E-G) Images of injured optic nerves stained with indicated antibodies or DAPI (E) and quantification results of the densities (F) or proportions (G) of different populations. E, arrowheads indicate CC1−/Olig1-N cells in Vec, while arrows indicate CC1+/Olig1-C cells after PLX treatment. N = 6 mice per group. Scale bar: 100 μm (B), 25 μm (E). *, **, *** P < 0.05, 0.01, 0.001, respectively.
Given that the majority of OPC proliferation occurs in the first week after injury (Figure 1E), we reasoned that a delayed PLX3397 treatment at 2-4 weeks after injury could bypass its inhibition on OPC proliferation, permitting us to assess its effects on OPC differentiation. Thus, PLX3397 was administrated from 2 to 4 weeks after injury in PDGFRα-CreER:tdTomato mice, as used in Figures 1G–M (Figure 3D). This delayed PLX3397 treatment did not alter early differentiated OPCs (tdTomato+/CC1+/nuclear Olig1), but decreased the number of undifferentiated OPCs (tdTomato+/CC1−/nuclear Olig1) and, importantly, increased the number and proportion of mature oligodendrocytes (tdTomato+/CC1+/cytoplasmic Olig1) (Figure 3E–G). Consistently, many of tdTomato+ cells had cytoplasmic Olig1 (Figure 3E–G), were positive for GSTπ (Figure S4B), and exhibited elongated process, likely undergoing myelination (Figure 3E).
To examine the activation states of microglia in injured optic nerves, we performed immunohistochemistry with antibodies against iNOS or arginase 1, markers for M1 and M2 microglia subtypes, respectively (Miron et al., 2013). As shown in Fig. S3G–I, the numbers of Arg1+/Iba1 + cells were much lower than the overall number of iNOS+/Iba1+ cells. However, their expression patterns were similar at 7 dpi and 21 dpi, suggesting that other mechanisms, rather than M1/M2 dichotomy, account for the different activities of microglia in acute and chronic conditions (Marschallinger et al., 2020). Together, unlike from GPR17 inhibition (Figure 2), delayed ablation of microglia preferentially promoted the maturation of early differentiated OPCs into myelinating oligodendrocytes.
Combinatorial treatment of Montelukast and PLX3397 led to robust myelination of regenerated axons
Our observations concerning the differential effects of GPR17 inhibition and delayed microglia ablation on OPC differentiation prompted us to assess the effects of combined treatments on the myelination of regenerated axons. PDGFRα-CreER:tdTomato mice were treated with Montelukast (for 4 weeks from dpi 1 to dpi 28) and/or PLX3397 (for 2 weeks during dpi 15-28) after optic nerve crush. As shown in Figures 4A–C, the combined treatment dramatically increased the numbers of CC1+/tdTomato+ cells and the majority of these CC1+ cells had cytoplasmic Olig1, implying that combinatorial treatment promoted both early and late differentiation of OPCs. Similar results were also obtained from immunohistochemistry with antibodies against GFTπ and MBP (Figure S4A–F). Consistently, most of these tdTomato+ cells exhibited extensive elongating processes, indicative of myelination (Figure 4A).
Figure 4. Combinatorial treatment of Montelukast and PLX3397 lead to robust myelination of regenerated axons in injured optic nerves.
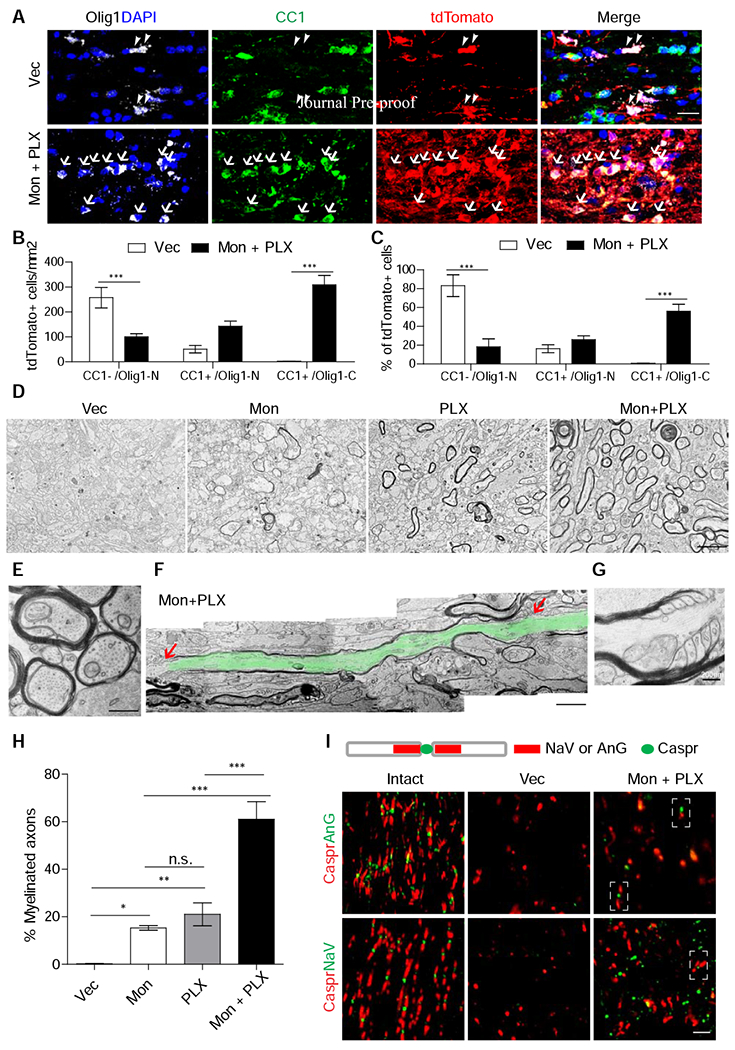
(A-C) Images of injured optic nerves stained with antibodies against Olig1, CC1, tdTomato, and DAPI (A) and quantification results of the densities (B) or proportions (C) of different populations. Arrowheads: CC1−/Olig1-N cells; arrows: CC1+/Olig1-N cells. N = 6 mice per group.
(D-H) Transmission electron microscopic images (D-G) and quantification (H) of myelination of regenerated axons of injured optic nerves from the mice with the treatment of Montelukast and/or PLX3397. (D, H): Low magnification of coronal sections (D) and quantification (H) of different groups. n=4 for each group. An enlarged image (E) showing ongoing myelination, an image montage showing a complete internode highlighted in green color (F), and an enlarged image of half of nodes of Ranvier (G) from the mice with combined treatments.
(I) Images of injured optic nerves with the combined treatments stained with nodes of Ranvier markers. Scale bar: 20 μm (A), 2 μm (D), 500 μm (E), 1400 nm (F), 3.5 μm (I). *, **, *** P < 0.05, 0.01, 0.001, respectively.
Subsets of mice in each treatment group were subjected to electron microscopic (EM) analysis (Figures 4D–H) and additional immunohistochemistry (Figure 4I). As shown in Figures 4D and 4H, approximately 20% of regenerated axons were myelinated in mice treated with either Montelukast (15%) or PLX3397 (21%). However, the myelin structures after Montelukast treatment were noticeably thinner, consistent with our results that this compound promotes the generation of early differentiated OPCs, which are only able to ensheath axons (Nave and Werner, 2014; Bercury and Macklin, 2015; Osso and Chan, 2017). In contrast, in the mice with the combined treatment, the majority (60%) of regenerated axons were myelinated (Figures 4D and 4H). Many of these myelin structures were still thin and had large inner tongues, suggesting ongoing myelination (Figure 4E). Importantly, the nodes of Ranvier and sometimes semi-nodes could be detected by EM (Figures 4F and 4G) or immunohistochemistry (Figure 4I).
It is interesting to note that most of these regenerated axons have not crossed the optic chiasm, suggesting that the induced myelination occurs before these regenerated axons form functional synapses with their functional targets. Additionally, we observed significantly more and longer regenerated axons with myelination-promoting treatments (Figure S4G–I), possibly relevant to protective effects of myelination on nascent axons (Simons and Nave, 2015; Morrison et al., 2013).
DISCUSSION
Together, our studies established a combinatorial treatment enabling robust myelination of regenerated axons in injured optic nerves. Thus, these results provide important insights for removing a major roadblock towards rebuilding functionally meaningful neuronal circuits. Importantly, the OPC dynamics observed in injured optic nerves share remarkable similarities with lesions in patients with progressive multiple sclerosis. Furthermore, persistently activated microglia dominate in injured optic nerves (our results) and in multiple sclerosis lesions (Zrzavy et al., 2017). Thus the results reported here could be informative for designing myelination-promoting interventions for progressive MS patients and other conditions.
Although Montelukast could target GPR17 and other cysteinyl-leukotriene receptors (Yokomizo et al., 2018), similar results observed in GPR17 knockout and Montelukast treated mice point towards GPR17 as a most relevant target. Interestingly, the cell numbers from OPC lineage are significantly increased after GPR17 inhibition. As this was not observed in knockout mice during development (Chen et al., 2009), this might be relevant to injury-associated factors. Indeed, GPR17 is known to be activated by cysteinyl-leukotrienes (Ciana et al., 2006). Thus, inflammation-elicited factors may activate GPR17, preventing GPR17-expressing OPCs from differentiation and even proliferating. These results highlight the importance of the interactions between environmental factors and OPC-intrinsic mechanisms in regulating their differentiation. In addition, other regulators may also play a role in this process, as two M1/M3 muscarinic receptor antagonists also significantly increased OPC differentiation. Further studies will examine the effects of these molecules and their respective targets.
Our results also suggested a binary role of microglia in OPC dynamics. While the majority of previous studies mainly emphasized a positive role of these activated microglia (Miron, 2013, Lloyd et al, 2019), Gibson et. al. showed that chemotherapy-induced persistent activation of microglia contributes to the impairment of OPC differentiation (Gibson et al., 2019). Thus, our results may reconcile these prior findings. However, the molecular mechanisms underlying these different activities of microglia are still unclear. Furthermore, our results showed that co-manipulation of intrinsic (GPR17) and extrinsic (microglia) factors can achieve robust myelination of regenerated axons. As myelination requires a sufficient number of mature oligodendrocytes differentiated from OPCs, our results highlight the need to target multiple steps and respective regulatory mechanisms for achieving robust (re)myelination. Future studies will examine whether such treatments enhance behavioral improvements in injury models and other pathological conditions.
STAR*METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information will be addressed by the Lead Contact, Zhigang He (zhigang.he@childrens.harvard.edu).
Materials Availability
Further requests for reagents, please contact the Lead Contact, Zhigang He (zhigang.he@childrens.harvard.edu).
Data and code Availability
This study did not generate datasets or codes that are available at current stage.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse Strains
All experimental procedures were performed in compliance with animal protocols approved by the Institutional Animal Care and Use Committee at Boston Children’s Hospital. GPR17 transgenic mice were from Dr. Richard Lu (Chen et al., 2009). Other mouse strains were obtained from The Jackson Laboratory (KEY RESOURCE TABLE). Experiments started when mice reached 6-8 weeks old. Both male and female mice were randomized and assigned to different treatment groups, prior to injury, and no other specific randomization was used for the animal studies. Quantifications were examined blindly.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-Olig1 | Dr. Charles D Stiles | |
| Rabbit anti-Olig2 | Novus biologicals | NBP1-28667 |
| Rat anti-PDGFRα (CD140a) | BD Bioscience | 558774 |
| Mouse anti-CC1(APC) | Millipore | OP80 |
| Rat anti-BrdU | Abcam | ab6326 |
| Mouse anti-Nav1.6 | Antibodies incorporated | 75-026 |
| Mouse anti-Ankyrin-G (AnkG) | Antibodies incorporated | 75-146 |
| Rabbit anti-Caspr | Abcam | ab34151 |
| Rat anti-MBP | Abcam | ab7349 |
| Mouse anti-MAG | Millipore | MAB1567 |
| Rat anti-CD68 | Bio-Rad | MCA1957 |
| Rabbit anti-Iba1 | WAKO Pure Chemicals | 019-19741 |
| Rabbit anti-P2Y12 | AnaSpec | AS-55043A |
| Rat anti-GFAP | Thermo Fisher | 13-0300 |
| Mouse anti-GST-π | BD Transduction Laboratories | 610718 |
| Mouse anti-iNOS | BD Transduction Laboratories | 610329 |
| Mouse anti-Arginase 1 | Santa Cruz Biotechnology | Sc-166920 |
| Rabbit anti-RFP | Abcam | ab34771 |
| In situ hybridization reagents | ||
| HCR v3.0 kits | Molecular Instruments | |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Alexa-conjugated cholera toxin subunit B | Thermo Fisher | C34776 |
| Fluoromont-G with DAPI | SouthernBiotech | 0100-20 |
| Tamoxifen | VWR | IC15673883 |
| Bromodeoxyuridine (BrdU) | Sigma | B5002-1G |
| Glutaraldehyde | Electron Microscopy Sciences | 16210 |
| Benztropine mesylate | PharmaBlock | Cat#N/A |
| Bexarotene | PharmaBlock | Cat#N/A |
| Clemastine Fumarate | PharmaBlock | Cat#N/A |
| Ibudilast | Selleckchem | S4837 |
| Imidazole | Sigma | I5513-5G |
| Montelukast | PharmaBlock | Cat#N/A |
| Pranlukast | PharmaBlock | Cat#N/A |
| Rapamycin | PharmaBlock | Cat#N/A |
| Solifenacin succinate | PharmaBlock | Cat#N/A |
| Pexidartinib (PLX-3397) | PharmaBlock | Cat#N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 | The Jackson Laboratory | Stock No: 000664 |
| Mouse: PDGFRα-CreER | The Jackson Laboratory | Stock No: 018280 |
| Mouse: PDGFRα-H2B-GFP | The Jackson Laboratory | Stock No: 007669 |
| Mouse: Rosa26-STOP-tdTomato mice | Fan Wang lab | Arenkiel et al., 2011 |
| Mouse: PTENf/f | The Jackson Laboratory | Stock No: 034621 |
| Recombinant DNA | ||
| pAAV-CAG-Cre | BCH Viral Core | N/A |
| pAAV-CAG-IGF1 | BCH Viral Core | N/A |
| pAAV-CAG-CNTF | BCH Viral Core | N/A |
| pAAV-CAG-OPN | BCH Viral Core | N/A |
| Software and Algorithms | ||
| ImageJ | NIH | RRID: SCR_003070 |
| Prism 7.0 | GraphPad Software | RRID: SCR_002798 |
| Matlab | Mathworks | N/A |
| Others | ||
| LSM 700 scanning confocal microscope | Zeiss | N/A |
| LSM 710 scanning confocal microscope | Zeiss | N/A |
| TEM microscope | JEOL 1200EX - 80kV | N/A |
Antibodies
Primary antibodies used were: Rabbit anti-Olig1 (1:50, a gift from Dr. Charles D Stiles), rabbit anti-Olig2 (1:300, Novus biologicals, NBP1-28667), rat anti PDGFRα (CD140a) (1:100, BD Bioscience, 558774), mouse anti-CC1(APC) (1:100, Millipore, OP80), rat anti-BrdU (1:300, Abcam, ab6326), mouse Anti-Nav1.6 (1:50, Antibodies Incorporated, 75-026), mouse anti-Ankyrin-G (AnkG) (1:50, Antibodies Incorporated, 75-146), rabbit anti-Caspr (1:1000, Abcam, ab34151), rat anti-MBP (1:300, Abcam, ab7349), mouse anti-MAG (1:100,Millipore,MAB1567), rat anti-CD68 (1:300, Bio-Rad, MCA1957), rabbit anti-Iba1(1:500, WAKO Pure Chemicals, 019-19741), rabbit anti-P2Y12 (1:500, AnaSpec, AS-55043A), rat anti-GFAP (1:1000, Thermo Fisher, 13-0300), moues anti-GST pi (1:100, BD Transduction Laboratories, 610718), mouse anti-iNOS (1:200, BD Transduction Laboratories, 610329), mouse anti-Arginase 1 (1:100, Santa Cruz Biotechnology, sc-166920), and rabbit anti-RFP (1:500, Abcam, ab34771). Secondary antibodies with conjugated fluorophores were from Invitrogen.
METHOD DETAILS
Virus Production
All AAV viral vectors were made by Boston Children’s Hospital Viral Core. AAV serotype 2 were used in our study as follows: AAV2-Cre; AAV2-CNTF; AAV2-IGF1; AAV2-OPN. The titers of all viral preparations were at least 1.0 X 1013 GC/ml.
Surgical Procedures
For all surgical procedures, mice were anaesthetized with ketamine and xylazine and received buprenorphine as a postoperative analgesic.
AAV virus injections:
As previously described, intravitreal AAV injection was performed two weeks before optic nerve crush injury to enable axon regeneration. Briefly, a pulled-glass micropipette was inserted near the peripheral retina, behind the ora serrata, and deliberately angled to avoid damage to the lens. 2 μl of AAV2/2-CAG-Cre virus was injected in PTEN f/f mice (Park et al., 2008). 2 μl of combined AAV2/2-CAG-CNTF, AAV2/2-CAG-IGF and AAV2/2-CAG-OPN (1:1:1 mix) was injected for other mouse strains (Bei et al., 2016).
Optic Nerve Injury:
As previously described, the optic nerve was exposed intraorbitally and crushed with fine forceps (Dumont #5 FST) for 2 seconds, approximately 1 mm behind the optic disc. Afterwards, eye ointment was applied postoperatively to protect the cornea. Robust axon regeneration could be observed at 2 weeks post-crush by Alexa-conjugated cholera toxin subunit B labeling.
Compound Administration
For PDGFRα-CreER mice, Tamoxifen (100 mg/kg, oral gavage) was administrated daily for 5 days immediately preceding optic nerve crush. For OPC proliferation assays, BrdU (100 mg/kg, intraperitoneal injection) was injected at either 3 hours or 48 hours before sample collection. For compound screening, BrdU was injected from day 4-10 post optic nerve crush. Each compound or the vehicle was administrated daily for four weeks, starting from day 1 post optic nerve crush. Tested compounds include: Benztropine mesylate (Bzp), a M1/M3 muscarinic receptor antagonist (Deshmukh et al., 2013, 10 mg/kg, i.p.), clemastine (Clem), an antihistamine and anticholinergic agent, M1/M3 muscarinic receptor antagonist (Mei et al., 2014, 10 mg/kg, p.o.), Solifenacin (Sli), a M3 muscarinic receptor antagonist (Abiraman et al., 2015, 20 mg/kg, i.p.), Bexarotene (Bex), a retinoid X receptor agonist (Natrajan et al., 2015, 100 mg/kg, p.o.), imidazole (Imi), an anti-cholesterol synthesis compound (Hubler et al., 2018, 10 mg/kg, i.p.), Ibudilast (Ibud), a clinically-approved phosphodiesterase (PDE) inhibitor (Fox et al., 2018, 10 mg/kg, i.p.), and Montelukast (Mon, 25 mg/kg, p.o.),) and Pranlukast (Pra, 0.5 mg/kg, i.p.), two different GPR17 antagonists (Fumagalli et al., 2011, Marschallinger et al., 2015; Ou et al., 2016). Rapamycin (Rap, 6 mg/kg, i.p.), a mTOR inhibitor, was also included in our screening as it was shown to improve myelination in TSC1 knockout mice (Meikle et al., 2008). Pexidartinib (PLX 3397) was mixed in food chow at 290 mg/kg by LabDiet laboratory animal nutrition.
Perfusions and Tissue Processing
For immunostaining, mice were given an overdose of anesthesia and transcardiacally perfused with ice cold PBS followed by 4% paraformaldehyde (PFA, Sigma-Aldrich). After perfusion, optic nerves were dissected out and post-fixed in 4% PFA overnight at 4°C. Tissues were cryoprotected by sinking in 30% sucrose (in 1X phosphate buffered saline (PBS) for 48 hours. Samples were frozen in Optimal Cutting Temperature compound (Tissue Tek) using dry ice and then sectioned at 12 mm for optic nerves.
Immunostaining and Imaging Analysis
Cryosections (12 μm thick) were permeabilized, incubated in blocking buffer (0.5% Triton X-100 and 5% normal goat serum in PBS) for 1 h at room temperature, and overlaid with primary antibodies overnight at 4°C. For BrdU staining, cells or tissue sections were denatured with HCl (2N) for 30 minutes at 37°C and then neutralized with 0.1 M sodium borate buffer for 10 min before proceeding to the normal blocking procedure. On the next day, the corresponding Alexa Fluor 488-, 594- or 647-conjugated secondary antibodies were applied (all secondary antibodies were purchased from Invitrogen). All stained sections were mounted with DAPI-containing mounting solution and sealed with glass coverslips. All immunofluorescence-labeled images were acquired using a Zeiss 700 or Zeiss 710 confocal microscope. Images were taken within 1mm distal region from the crush site since this area contains the highest number of regenerated axons (shadow area in Figure 1A). For each biological sample, 3-5 sections of each optic nerve were imaged under 10x or 20x objectives for quantification. For whole nerve images, Tiles function was applied to stitch individual scanning image on Zeiss 710 (Figure S3E). Positive cell numbers were then quantified manually using the Plugins/ Analyze /Cell Counter function in ImageJ software. For fluorescent intensity analysis, the images were first converted to 8-bit depth in ImageJ software and then the mean intensity value was calculated by the build-in function: Analyze/Measure.
Tissue Clearing, Imaging, and Quantification of Optic Nerve Regeneration
Mice injected with fluorophore tagged Cholera Toxin B (CTB) were perfused with 4% paraformaldehyde. Dissected optic nerves were then subjected to a modified procedure from previously published iDISCO tissue clearing method (Renier et al., 2014). Briefly, optic nerve samples were incubated in the dark for 0.5 h of 80% tetrahydrofuran (THF, Sigma-Aldrich 360589-500ML)/H2O and then switched to 100% THF for 1 hour for dehydration. Then, samples were incubated in Dichloromethane (DCM, Sigma-Aldrich 270997-1L) for 20 minutes. Samples were finally switched to dibenzyl ether (DBE, Sigma-Aldrich 33630-250ML) until completely transparent (at least 3 hours, but overnight is recommended). For imaging, processed nerves can be mounted in DBE and imaged under Zeiss 710 confocal microscope using Z-stack and Tiles functions. Z-stack scanning and maximum projection of images were used in order to capture all regenerated axons. For image analysis, fluorescent intensity profile along the nerve was generated by the build-in function of ImageJ: Analyze/Plot Profile. To calculate the integral of fluorescent intensity across the entire length of the nerve, a custom Matlab algorithm was developed by our lab to quantify the “area under curve” from the plot profile data generated by ImageJ.
Electron Microscopy and Morphometric Analysis.
Mice were perfused with 4% paraformaldehyde containing 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. Optic nerves were dissected and placed in this fixative overnight. Samples were then processed by EM Core facility at Harvard Medical School, with the following procedure: samples were rinsed in PBS, post-fixed in 1% OsO4 in PBS for 1 h, dehydrated in a graded ethanol series, infiltrated with propylene oxide, and embedded in Epon. Semithin sections were stained with toluidine blue, and ultrathin sections were stained with lead citrate. Ultrathin sections were taken under JEOL 1200EX – 80kV electron microscope. The number of myelinated axons per nerve was analyzed in ultrathin sections at magnifications 3,000x to 20,000x. To better distinguish new myelin from pre-existing myelin, areas with regenerated axons and no myelin debris were selected for quantification. For longitudinal images of Nodes of Ranvier (Figure 4F), individual images were stitched in photoshop software by automatic stitching.
In situ hybridization (FISH)
To assess the expression pattern of GPR17, we performed in situ hybridization by hybridization chain reaction (HCR) (Choi et al., 2018) with a commercial kit containing a DNA probe set, a DNA HCR amplifier, and different buffers (Molecular Instruments). To prepare sections, anesthetized mice were perfused with DEPC-PBS followed by 4% paraformaldehyde (PFA). Dissected optic nerves were fixed in 4% PFA overnight, dehydrated in 30% sucrose/DEPC-PBS at 4°C, embedded in OCT and cryosectioned at 14 μm. Tissues were permeabilized in 5% SDS for 20 min at room temperature (RT) and pre-hybridized in hybridization buffer for 3 hours at 37°C. Then slides were incubated in pre-warmed hybridization buffer including probes (2.5 nM for each) at 37°C overnight. After hybridization, slices were washed for 1 hour at 37°C with wash buffer followed by 2xSSC or 15 minutes at room temperature. The amplification step was performed with B3 HCR amplifiers for overnight.
QUANTIFICATION AND STATISTICAL ANALYSIS
Normality and variance similarity were measured by STATA before we applied any parametric tests. Two-tailed student’s t-test was used for single comparisons between two groups. Other data were analyzed using one-way or two-way ANOVA depending on the appropriate design. Post hoc comparisons were carried out only when the primary measure showed statistical significance. P-value of multiple comparisons was adjusted using Bonferroni’s correction. Error bars in all figures represent mean ± S.E.M. Mice with different litters, body weights, and sexes were randomized and assigned to different treatment groups, and no other specific randomization was used for the animal studies.
Supplementary Material
Proliferated OPCs exhibit differentiation blockade in injured optic nerves
GPR17 inhibition promotes OPC differentiation
Chronically activated microglia prevents oligodendrocyte maturation
Co-manipulation of GPR17 and microglia promotes robust myelination
Acknowledgments
We thank J. Alberta and C. Stiles for providing anti-Olig1 antibodies, A. Effenberger for assistance, Tracey Suter for critical reading the manuscript. This study was supported by NIH grants R01EY021526 and R01EY026939 (Z.H.), and grants from Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (Z.H.). J.C.P. is supported by F32 fellowship from NCCIH (F32AT011155). We thank IDDRC and viral cores supported by the NIH grants HD018655 and P30EY012196.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
A patent based on the results in this manuscript was filed by Boston Children’s Hospital (Z.H., J.W. and X.H. are co-inventors).
SUPPLEMENTAL INFORMATION
Supplemental information includes four supplementary figures and legend.
REFERENCES
- Abiraman K, Pol SU, O’Bara MA, Chen G. Di Khaku ZM, Wang J, Thorn D, Vedia BH, Ekwegbalu EC, Li JX, et al. (2015). Anti-muscarinic adjunct therapy accelerates functional human oligodendrocyte repair. J. Neurosci 35, 3676–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Hasegawa H, Yi JJ, Larsen RS, Wallace ML, Philpot BD, Wang F, and Ehlers MD (2011). Activity-induced remodeling of olfactory bulb microcircuits revealed by monosynaptic tracing. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett HA, Fancy SPJ, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJM, and Stiles CD (2004). bHLH transcription factor Oligl is required to repair demyelinated lesions in the CNS. Science 306, 2111–2115. [DOI] [PubMed] [Google Scholar]
- Bei F, Lee HHC, Liu X, Gunner G, Jin H, Ma L, Wang C, Hou L, Hensch TK, Frank E, et al. (2016). Restoration of Visual Function by Enhancing Conduction in Regenerated Axons. Cell 164, 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, He Z, Goldberg JL. (2017). Reaching the brain: Advances in optic nerve regeneration. 287, 365–373. [DOI] [PubMed] [Google Scholar]
- Bercury KK, and Macklin WB (2015). Dynamics and mechanisms of CNS myelination. Dev. Cell 32, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KJ, Redmond SA, Chan JR (2016). Remodeling myelination: implications for mechanisms of neural plasticity. Nat Neurosci 19, 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP (2006). The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 25, 4615–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, et al. (2009). The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci 12, 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L (2012). Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 109, 9149–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, et al. (2013). A regenerative approach to the treatment of multiple sclerosis. Nature 502, 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Qiao M, Bei F, Kim IJ, He Z, and Sanes JR (2015). Subtype-Specific regeneration of retinal ganglion cells following axotomy: Effects of osteopontin and mtor signaling. Neuron 85, 1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Plemel JR, Assinck P, Manesh SB, Muir FGW, Hirata R, Berson M, Liu J, Wegner M, Emery B, Moore GRW, Tetzlaff W (2017). Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol. 134, 403–422. [DOI] [PubMed] [Google Scholar]
- Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, et al. (2014). Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RJ, Coffey CS, Conwit R, Cudkowicz ME, Gleason T, Goodman A, Klawiter EC, Matsuda K, McGovern M, Naismith RT, et al. (2018). Phase 2 trial of ibudilast in progressive multiple sclerosis. N. Engl. J. Med 379, 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM, and Ffrench-Constant C (2017). Regenerating CNS myelin - From mechanisms to experimental medicines. Nat. Rev. Neurosci 18, 753–769. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Daniele S, Lecca D, Lee PR, Parravicini C, Douglas Fields R, Rosa P, Antonucci F, Verderio C, Letizia Trincavelli M, et al. (2011). Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J. Biol. Chem 286, 10593–10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Lecca D, and Abbracchio MP (2016). CNS remyelination as a novel reparative approach to neurodegenerative diseases: The roles of purinergic signaling and the P2Y-like receptor GPR17. Neuropharmacology 104, 82–93. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, et al. (2019). Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 176, 43–55.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, and Jin Y (2016). Intrinsic Control of Axon Regeneration. Neuron 90, 437–451. [DOI] [PubMed] [Google Scholar]
- Hilton BJ, and Bradke F (2017). Can injured adult CNS axons regenerate by recapitulating development? Dev. 144, 3417–3429. [DOI] [PubMed] [Google Scholar]
- Hennen S, Wang H, Peters L, Merten N, Simon K, Spinrath A, Blättermann S, Akkari R, Schrage R, Schröder R, Schulz D, Vermeiren C, Zimmermann K, Kehraus S, Drewke C, Pfeifer A, König GM, Mohr K, Gillard M, Müller CE, Lu QR, Gomeza J, Kostenis E. (2013). Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci Signal 6, ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubler Z, Allimuthu D, Bederman I, Elitt MS, Madhavan M, Allan KC, Shick HE, Garrison E, T. Karl M, Factor DC, et al. (2018). Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature 560, 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Orthmann-Murphy JL, Langseth AJ, and Bergles DE (2018). Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci 21, 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, and Brück W (2008). Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 132, 1118. [DOI] [PubMed] [Google Scholar]
- Laha B, Stafford BK, and Huberman AD (2017). Regenerating optic pathways from the eye to the brain. Science 356, 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H (2018). Multiple sclerosis pathology. Cold Spring Harbor Perspect Med 8, a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff JA, Busse WW, Pearlman D, Bronsky EA, Kemp J, Hendeles L, Dockhorn R, Kundu S, Zhang J, Seidenberg BC, et al. (1998). Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N. Engl. J. Med 339, 147–152. [DOI] [PubMed] [Google Scholar]
- Lloyd AF, and Miron VE (2019). The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol 15, 447–458. [DOI] [PubMed] [Google Scholar]
- Marin MA, de Lima S, Gilbert HY, Giger RJ, Benowitz L, Rasband MN (2016). Reassembly of Excitable Domains after CNS Axon Regeneration. 36, 9148–9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, Van Bruggen D, Falcão AM, Xiao L, Li H, Häring M, Hochgerner H, Romanov RA, et al. (2016). Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science (80-.). 352, 1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, Kim J, Tevini J, Felder TK, Wolinski H, Bertozzi CR, Bassik MC, Aigner L, Wyss-Coray T (2020). Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nature Neurosci. 23, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, et al. (2019). Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Fancy SPJ, Shen YAA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, et al. (2014). Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med 20, 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, and Kwiatkowski DJ (2008). Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: Effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci 28, 5422–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, Van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, et al. (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci 16, 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BM, Lee Y, Rothstein JD. (2013). Oligodendroglia: metabolic supporters of axons. Trends Cell Biol. 23, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M (2018). Myelin Plasticity and Nervous System Function. Annu Rev Neurosci 41, 61–76. [DOI] [PubMed] [Google Scholar]
- Natrajan MS, De La Fuente AG, Crawford AH, Linehan E, Nuñez V, Johnson KR, Wu T, Fitzgerald DC, Ricote M, Bielekova B, et al. (2015). Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain 138, 3581–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K-A, and Werner HB (2014). Myelination of the Nervous System: Mechanisms and Functions. Annu. Rev. Cell Dev. Biol 30, 503–533. [DOI] [PubMed] [Google Scholar]
- Osso LA, and Chan JR (2017). Architecting the myelin landscape. Curr. Opin. Neurobiol 47, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z, Sun Y, Lin L, You N, Liu X, Li H, Ma Y, Cao L, Han Y, Liu M, et al. (2016). Olig2-targeted G-protein-coupled receptor Gpr17 regulates oligodendrocyte survival in response to lysolecithin-induced demyelination. J. Neurosci 36, 10560–10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, et al. (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science (80-.). 322, 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K, Hennen S, Merten N, Blättermann S, Gillard M, Kostenis E, Gomeza J (2016). The Orphan G Protein-coupled Receptor GPR17 Negatively Regulates Oligodendrocyte Differentiation via Gαi/o and Its Downstream Effector Molecules. J. Biol. Chem 291, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M and Nave KA (2015). Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect Biol. 8, a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey FA, Cammer W (1991). A Pi Form of Glutathione-S-Transferase Is a Myelin- and Oligodendrocyte- Associated Enzyme in Mouse Brain. J Neurochem. 57, 95–102. [DOI] [PubMed] [Google Scholar]
- Wolswijk G (2002). Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 125, 338–349. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Nakamura M, and Shimizu T (2018). Leukotriene receptors as potential therapeutic targets. J. Clin. Invest 128, 2691–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, and Richardson WD (2013). Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron 77, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, and Lassmann H (2017). Loss of “homeostatic” microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets or codes that are available at current stage.


