Abstract
An estimated 50-90% of individuals with cocaine use disorder (CUD) also report using alcohol. Cocaine users report co-abusing alcohol to ‘self-medicate’ against the negative emotional side effects of the cocaine ‘crash’, including the onset of anxiety. Thus, pharmaceutical strategies to treat CUD would ideally reduce the motivational properties of cocaine, alcohol, and their combination, as well as reduce the onset of anxiety during drug withdrawal. The hypothalamic orexin (hypocretin) neuropeptide system offers a promising target, as orexin neurons are critically involved in activating behavioral and physiological states to respond to both positive and negative motivators. Here, we seek to describe studies demonstrating efficacy of orexin receptor antagonists in reducing cocaine, alcohol- and stress-related behaviors, but note that these studies have largely focused on each of these phenomena in isolation. For orexin-based compounds to be viable in the clinical setting, we argue that it is imperative that their efficacy be tested in animal models that account for polysubstance use patterns. To begin to examine this, we present new data showing that rats’ preferred level of cocaine intake is significantly increased following chronic homecage access to alcohol. We also report that cocaine intake and motivation are reduced by a selective orexin-1 receptor antagonist when rats have a history of cocaine + alcohol, but not a limited history of cocaine alone. In light of these proof-of-principle data, we outline what we believe to be the key priorities going forward with respect to further examining the orexin system in models of polysubstance use.
Keywords: addiction, anxiety, behavioral economics, cocaethylene, ethanol, polysubstance, stress
1.0. Introduction: Coabuse of alcohol in cocaine use disorder
The overwhelming majority of individuals with substance use disorder report using more than one drug of abuse either simultaneously or at different times. Among individuals with cocaine use disorder (CUD), alcohol represents the most commonly used alternative drug, with estimates of coabuse ranging from 50-90% (Anthony et al., 1994; Brookoff et al., 1996; Grant and Harford, 1990; Kedia et al., 2007; Liu et al., 2018; Rounsaville et al., 1991). Indeed, cocaine + alcohol represents the most prevalent 2-drug combination among patients seeking treatment for substance use disorder (12.4% of treatment seekers), with cocaine + cannabis a distant second (3.4% of treatment seekers; (Kedia et al., 2007)). When taken in combination, cocaine and alcohol results in the production of cocaethylene (CE), a cocaine metabolite that has a plasma half-life of 3-5 times that of cocaine and is associated with seizure, liver damage and compromised immune system function (Andrews, 1997; Dasgupta, 2017). Notably, the LD50 of CE is significantly lower than cocaine, and thus is associated with an 18-25-fold risk of immediate death compared to cocaine alone (Andrews, 1997; Dasgupta, 2017). Moreover, those who use cocaine and alcohol simultaneously report that the amount of each substance consumed increases significantly compared to when either drug are used alone (Gossop et al., 2006).
Despite the prevalence of alcohol coabuse in CUD, preclinical research overwhelmingly tends to focus on these substances in isolation (Crummy et al., 2020; Liu et al., 2018). This phenomenon may in part explain why several pharmacotherapies designed to treat CUD have failed in clinical trials, despite promising data pointing to their efficacy in the laboratory setting. One interesting example is the dopamine transporter inhibitor modafanil, which is highly effective at reducing a range of cocaine-related behaviors in rat. When tested in a double blind, placebo-controlled study in cocaine-dependent patients, modafinil was effective at reducing the percentage of non-use days only in patients who did not have a history of alcohol dependence (Anderson et al., 2009). Examples such as this highlight the need for greater emphasis on animal models that incorporate drug use patterns that mimic those in clinical populations – indeed, they are critical to identify candidate brain systems for novel pharmacotherapies to treat CUD with associated alcohol coabuse.
When considering the neurobiological mechanisms contributing to cocaine + alcohol coabuse, one first step might be to focus on those brain systems identified as governing cocaine and alcohol behaviors when studied in isolation. One strong candidate system is the hypothalamic orexin (hypocretin) system, given its common role in mediating reward seeking, including both cocaine and alcohol seeking (Campbell et al., 2020a; James et al., 2017b; Mahler et al., 2014). Indeed, an extensive literature of preclinical studies now indicate that orexin receptor antagonists are highly effective at protecting against cocaine and alcohol use and a series of ongoing clinical studies are seeking to extend these observations to human patients (Campbell et al., 2020a; James and Aston-Jones, 2020; Suchting et al., 2019). Critically with respect to cocaine + alcohol coabuse, orexin receptor antagonists also protect against the negative aspects of drug reward, including stress and anxiety behaviors during withdrawal (Georgescu et al., 2003; Li et al., 2010; Li et al., 2011; Yeoh et al., 2014), which appear to be a major driving factor for alcohol use in CUD populations (Higgins et al., 1993; McCance et al., 1995; McCance-Katz et al., 1993). Despite this promise, to date there currently exists no study, in laboratory animals or humans, that has explicitly examined the orexin system in the context of cocaine + alcohol coabuse. To this end, here we seek to review the evidence pointing to the potential utility of orexin-based therapies designed to treat cocaine + alcohol coabuse, as well as identify the key steps forward for future studies designed to test this hypothesis. First, we briefly explore the factors contributing to alcohol use in CUD, highlighting the perceived heightened reinforcing efficacy and anxiogenic properties of CE. Next, we review the extant literature relating to the orexin system in reward as it relates to cocaine and alcohol seeking, with a focus given the neural circuits through which the orexin system mediates the seeking of both drugs, as well as the receptor systems involved in these behaviors. Similarly, we discuss the orexin system as a key mediator of stress and anxiety behaviors, including those related to drug withdrawal, highlighting the anxiolytic properties of compounds that block orexin signaling. Finally, we outline what we believe to be the key priorities going forward with respect to examining orexin system function in cocaine + alcohol co-abusing populations. As a practical step forward, we offer preliminary empirical data providing proof-of-principle support for the efficacy of a selective orexin-1 receptor antagonist in reducing cocaine demand in rats with a history of cocaine and alcohol self-administration. Our overarching intention is to give impetus to future efforts designed to explore the orexin system as a candidate for novel pharmacotherapies to address alcohol coabuse in CUD populations.
2.0. Factors contributing to cocaine and alcohol coabuse
The reasons underlying polysubstance abuse are varied, and include opportunistic access, experimentation and social conformity (Connor et al., 2014). In the case of cocaine + alcohol coabuse, there is a perception that the euphoric effects of cocaine are enhanced by simultaneous alcohol consumption. Two unique metabolic phenomena appear to contribute to this enhanced subjective experience (Liu et al., 2018). First, simultaneous consumption of cocaine + alcohol is associated with a reduction in the amount of cocaine metabolized to benzoylecgonine, resulting in enhanced plasma levels of cocaine (Pan and Hedaya, 1999). Second, as noted above, simultaneous consumption of cocaine + alcohol results in the production of CE, which unlike other cocaine metabolites, is psychoactive and rewarding in and of itself. Indeed, CE causes euphoria in humans as measured by self-rating scales of the intensity of the “high” (Hart et al., 2000; McCance et al., 1995). CE is self-administered by primates (Jatlow et al., 1991) and rats (Raven et al., 2000) and produces CPP in rat to a similar magnitude as cocaine (Knackstedt et al., 2002). There is some evidence to indicate that the combination of cocaine and alcohol is more reinforcing than either drug alone. Rats consumed higher amounts of alcohol in their home cage following a non-contingent cocaine injection (Knackstedt et al., 2006), and mice showed enhanced cocaine-induced psychomotor stimulation when co-administered with alcohol (Masur et al., 1989). In rats tested on an ICSS task, the combination of low intraperiotoneal doses of cocaine and alcohol reduced reward thresholds and increased response rate despite neither dose having any effect on its own (Lewis and June, 1994). In monkeys, daily access to sweetened alcohol 4h after cocaine access was associated with self-administration of cocaine at doses that were previously non-reinforcing (Czoty, 2015). These effects appear to be dependent on the temporal patterns of cocaine + alcohol co-consumption, as another study in monkey reported that cocaine self-administration behavior was unaffected by i.v. alcohol administration 10min prior to cocaine availability (Aspen and Winger, 1997). Additionally, in rats cocaine can attenuate the anxiolytic effects of alcohol, but simultaneously enhance motor impairments (Aston-Jones et al., 1984).
Cocaine users also report ingesting alcohol to avoid the undesirable side effects of cocaine. In particular, users report using alcohol to reduce the anxiety and other negative emotional states that characterize the cocaine “crash” (Higgins et al., 1993; Knackstedt et al., 2002; McCance et al., 1995; McCance-Katz et al., 1993). In addition to the psychoactive and anxiolytic properties of alcohol, it has been hypothesized that the co-administration of alcohol reduces cocaine-related anxiety via CE-mediated processes (Knackstedt et al., 2002). First, whereas cocaine binds the serotonin transporter and subsequently blocks serotonergic reuptake (Ritz et al., 1987), CE has weaker effects on the serotonergic transporter (Bradberry et al., 1993; Nobiletti et al., 1994), thereby resulting in comparatively higher serotonin availability. Second, studies in rat indicate that the anxiogenic side effects of CE are less than those observed for cocaine (Knackstedt et al., 2002; Raven et al., 2000). Third, rats develop a conditioned place aversion to an environment when pairings are made 15mins after cocaine injection, compared to 30mins for CE, indicating that the onset of CE’s aversive properties are delayed relative to cocaine (Knackstedt et al., 2002). Combined with the significantly longer half-life of CE compared to cocaine, CE may serve to blunt or mask the onset of cocaine’s anxiogenic actions (Knackstedt et al., 2002). In addition, given the high comborbidity between SUDs (including CUD) and chronic anxiety-disorders (Brady et al., 2013), alcohol is also likely sought to ‘self-medicate’ against chronic underlying anxiety states. Future studies should also seek to examine other potential reasons for polydrug use, including the possibility that persons use cocaine and alcohol to ‘balance out’ the stimulant and depressive effects of these drugs, respectively.
Together, these data indicate that coabuse of alcohol in CUD populations is driven, at least in part, by both positive and negative reinforcing factors. Thus, a pharmacotherapy targeting a general motivational system, regardless of valence, might offer a solution to ameliorating both contributing factors. To this end, we speculate that the hypothalamic orexin system holds significant promise, given its common role in broadly mediating motivational states (Mahler et al., 2014). Below, we review evidence linking the recruitment of orexin neurons to drug seeking, both for cocaine and alcohol, and by various stress states including withdrawal. Moreover, we highlight evidence that compounds that block orexin signaling are highly effective at reducing drug seeking as well as reducing behavioral and physiological responses to stress (James et al., 2017a; James et al., 2017b). Collectively, these studies demand that future studies examine the potential utility of orexin-based compounds as a novel therapeutic approach to managing cocaine + alcohol coabuse – an effort that we seek to catalyze by describing preliminary data indicating efficacy of such an approach.
3.0. The orexin/hypocretin system – role in addiction and stress
3.1. Introduction to the orexin system
Orexins (orexin-A and orexin-B) are neuropeptides that are exclusively synthesized in neurons in the caudal hypothalamus (de Lecea et al., 1998; Sakurai et al., 1998). Orexin-producing neurons project widely throughout the brain (Peyron et al., 1998), where orexin peptides act on two G protein coupled receptors - orexin 1 receptor (Ox1R) and orexin 2 receptor (Ox2R) (de Lecea et al., 1998; Marcus et al., 2001; Sakurai et al., 1998). Ox1R and Ox2R have differing binding affinities for the peptide, such that orexin A has greater binding affinity for Ox1R, while orexin B has an equal binding affinity for both receptors (de Lecea et al., 1998; Sakurai et al., 1998). The orexin system is implicated in a range of physiological and behavioral functions, including sleep, arousal, cardiorespiratory responses, stress and motivation (Abreu et al., 2020; Barson, 2020; Burdakov, 2020; Carrive and Kuwaki, 2017; Freeman et al., 2020; James et al., 2017a; Krystal et al., 2013; Li et al., 2017; Mahler et al., 2014; Simmons and Gentile, 2019; Soya and Sakurai, 2020; Summers et al., 2020). We have argued that these broad functions reflect a fundamentally unified role for the orexin system in translating motivational states into organized and adaptive behaviors (Mahler et al., 2014). That is, orexin neurons are recruited by homeostatic signals of physiological disequilibrium and/or exposure to important environmental stimuli, especially when they are relevant to a current physiological need. Orexin neurons, in turn, facilitate suites of psychological (e.g. appetitive/aversive motivation) and physiological (e.g. cardiovascular activation, suppression of sleep) processes via mechanisms described below. Periods of rapid firing during phasically adaptive behavior are overlaid on circadian oscillations in tonic orexin neuron activity, which contribute to orexin’s broad involvement in regulating sleep and arousal (Mahler et al., 2014).
Diversity of orexin system functionality is achieved in three ways. First is heterogeneity of processing in efferent targets. Orexin neurons provide input to a broad range of neural sites, including those important for arousal/stress (e.g. locus coeruleus, paraventricular nucleus of the hypothalamus, amygdala) and reward (ventral tegmental area, lateral septum, nucleus accumbens) (Peyron et al., 1998). Interestingly, although orexin axons are collateralized and often target several adjacent regions, there is evidence that orexin neurons participate largely in multiple parallel but anatomically segregated neural circuits (Berridge et al., 2010; Gompf and Aston-Jones, 2008). In addition, the expression of Ox1R and Ox2R are partially non-overlapping, allowing for target-specific effects of orexin release (Marcus et al., 2001). Studies generally indicate that actions at Ox1Rs are more important in mediating reward and feeding as well as the regulation of dopamine signaling (Harris et al., 2005; Haynes et al., 2000; James et al., 2017b; Prince et al., 2015b) whereas actions at Ox2Rs have been implicated more strongly with arousal and stress related functions (Sakurai and Mieda, 2011; Tsujino and Sakurai, 2013; Yamanaka et al., 2002; Yamanaka and Tsunematsu, 2010). Second is heterogeneity of inputs. Retrograde mapping studies indicate that orexin afferents arise from a broad number of regions, and may selectively target subsets of orexin neurons (Giardino et al., 2018; Sakurai et al., 2005; Yoshida et al., 2006) allowing for differential recruitment of functional subpopulations of orexin neurons (Mahler et al., 2014). Moreover, orexin neurons exhibit heterogeneity in receptor expression and diversity in their responsivity to inputs (Burdakov et al., 2013). Third, there is some evidence of heterogeneity of orexin neuron function based on anatomical location. In rat, DMH/PF but not LH orexin neurons respond to stress and arousal (Estabrooke et al., 2001; Harris and Aston-Jones, 2006; Sakamoto et al., 2004; Winsky-Sommerer et al., 2004), whereas LH neurons are preferentially recruited by stimuli associated with reward (Harris et al., 2005; James et al., 2019b). Moreover, there is a close association between the number of LH, but not DMH/PF, orexin neurons and the expression of addiction-like behavior (discussed below; (Fragale et al., 2020; James et al., 2019b; Pantazis et al., 2020). Together, these three factors contribute to the orexin system’s facilitation of a broad range of behaviors and physiological functions. Indeed, it is through this heterogeneity that orexin neurons simultaneously underlie the expression of both appetitive and aversive behavioral states, including drug seeking and anxiety/stress behaviors. Below, we elaborate on these functions as they apply to cocaine and alcohol coabuse.
3.2. Orexin as a mediator of cocaine and alcohol behaviors
In 2005, our lab provided the first evidence that the orexin system mediates drug seeking behavior, reporting that the expression of conditioned place preference for cocaine (and morphine) is associated with Fos recruitment in LH orexin neurons (Harris et al., 2005). In the ~15 years since, a role for the orexin system has been extended beyond cocaine and morphine to all drugs of abuse tested, including alcohol (Brodnik et al., 2018; Hopf, 2020; James et al., 2017b; Matzeu and Martin-Fardon, 2020; Moorman, 2018; Walker and Lawrence, 2017). Fos activation of orexin neurons has been reported following alcohol sensitization in mice (Macedo et al., 2013) and in response to discriminative stimuli predictive of ethanol and cocaine availability (Dayas et al., 2008; Martin-Fardon et al., 2018). Consistent with these findings, Fos expression in orexin neurons is positively correlated with context-induced renewal of extinguished alcohol seeking (Hamlin et al., 2007), and we found that activation of orexin neurons predicts both homecage ethanol preference and the degree of context-induced ethanol seeking (Moorman et al., 2016). As noted above, we reported that orexin neurons are activated in response to context associated with cocaine reward (Harris et al., 2005). We also found that rats with a history of intermittent access to cocaine, a self-administration paradigm known to induce a robust addiction-like state (Fragale, 2020; James et al., 2018b; Kawa et al., 2016), exhibit greater activation of orexin neurons in response to a drug-associated environment compared to subjects with a milder addiction-like profile (James et al., 2018b).
Significant evidence now indicates that cocaine- and alcohol-induced changes to orexin system function extend beyond plasticity of activity to persistent changes in the expression of orexin peptide levels (Barson et al., 2015; Collier et al., 2019; Collier et al., 2020; James et al., 2018b; Lawrence et al., 2006; Sterling et al., 2015). Chronic voluntary ethanol consumption was reported to increase the area of prepro-orexin mRNA in LH of inbred alcohol preferring (iP) rats (Lawrence et al., 2006), while acute ethanol exposure increased orexin peptide expression in LH of Sprague Dawley rats (Morganstern et al., 2010). Moreover, increased numbers of orexin neurons, particularly in the left hemisphere, are observed in zebrafish following direct embryonic exposure or maternal ethanol consumption prior to paternal fertilization (Collier et al., 2019; Collier et al., 2020). Similar to these findings, we found that rats that exhibit enhanced cocaine motivation following intermittent access to cocaine exhibit greater numbers of orexin neurons, and that this increase persists for at least 150d after the cessation of intermittent cocaine (James et al., 2019b). We also found that the number of orexin neurons predicts baseline or ‘trait’ motivation for cocaine on a behavioral economics task, such that a higher number of LH orexin neurons is associated with higher motivated responding, but not baseline intake, for cocaine (Pantazis et al., 2019).
Much of the work examining the receptor systems through which orexin mediates the reinforcing and motivational properties of alcohol and cocaine has focused on the Ox1R. Pharmacological agents that block signaling at Ox1R reduce compulsive ethanol drinking (Lei et al., 2016a) as well as ethanol self-administration on both fixed (FR) and progressive ratio (PR) schedules of reinforcement (Jupp et al., 2011; Lawrence et al., 2006; Moorman et al., 2017; Richards et al., 2008). A significant body of evidence now indicates that Ox1Rs play a particularly important role in regulating alcohol and cocaine seeking under conditions of increased motivation (James et al., 2017b). We have shown that systemic pretreatment with the Ox1R antagonist SB selectively reduces homecage ethanol drinking, self-administration and cue-induced reinstatement in high alcohol preferring rats (Moorman et al., 2017). This is consistent with reports showing that Ox1R antagonism preferentially reduces cocaine seeking under conditions of increased motivation. For example, Ox1R blockade reduces cocaine consumption under PR but not FR1 schedules of reinforcement (Borgland et al., 2009; Boutrel et al., 2005; Espana et al., 2010; Smith et al., 2009b). Moreover, using a behavioral economics task, we found that systemic SB decreases motivation for cocaine, but that this effect is strongest in rats with high baseline demand (James et al., 2018a). Our lab and others have also shown that rats highly motivated for cocaine are more sensitive to lower doses of SB to decrease drug motivation (James et al., 2018b; Schmeichel et al., 2017a).
Both cocaine and alcohol use disorders are chronically relapsing conditions, whereby re-initiation of drug seeking can be triggered by several factors, including exposure to drug-associated cues and contexts. The orexin system seems to play a consistent role in the reinstatement of extinguished alcohol and cocaine seeking. Inhibition of the orexin cells themselves is sufficient to block reinstatement of cocaine seeking elicited by discriminative stimuli (Yeoh et al., 2018). Ox1R blockade attenuates ethanol and cocaine seeking in response to discrete and contextual cues (James et al., 2019b; Jupp et al., 2011; Lawrence et al., 2006; Martin-Fardon and Weiss, 2014; Moorman et al., 2017; Smith et al., 2009a; Smith et al., 2010). Similar to their effects on drug demand, Ox1R antagonists are most effective at blocking reinstatement in animals that exhibit a stronger addiction-like phenotype, such that lower doses are required to achieve behavioral effects in these animals (James et al., 2019a; James et al., 2019b; Moorman et al., 2017). In addition to drug cues, relapse to drug seeking can also be triggered by stress. Ox1R antagonists are also highly effective at preventing reinstatement of both alcohol and cocaine seeking elicited by stressful/aversive stimuli, consistent with a role for the orexin system in both reward and stress/arousal (discussed below) (Boutrel et al., 2005; Richards et al., 2008; Wang et al., 2009).
Ox1Rs regulate the reinforcing properties of alcohol and cocaine through classic reward-associated brain areas including VTA. Local microinjection of SB into VTA reduced ethanol consumption in mice and attenuated cue-induced reinstatement in iP rats (Olney et al., 2017) (Brown et al., 2013). Similarly, intra-VTA SB reduced motivation for cocaine on a BE task and attenuates discriminative cue-induced reinstatement of extinguished cocaine seeking (Espana et al., 2010; James et al., 2011). Ox1Rs may also regulate consumption of both drugs through stress-associated brain regions. Microinfusion of SB into CeA reduced binge-like alcohol drinking in mice (Olney et al., 2017) and reduced cocaine self-administration in highly motivated rats given extended access to cocaine (Schmeichel et al., 2017a). Parventricular thalamus also appears to be an important locus of orexin signaling in drug seeking, as infusions of orexin peptides reinstate extinguished drug seeking and stimulates alcohol drinking (Barson et al., 2015; Barson et al., 2017; Matzeu et al., 2016). Similarly, local injections of an Ox1R antagonist into nucleus accumbens shell disrupts both cocaine and alcohol behaviors (Lei et al., 2019; Lei et al., 2016b; Yang et al., 2020).
Although there is a high degree of convergence with respect to Ox1R signaling in cocaine and alcohol behaviors, evidence to date largely points to a unique role for Ox2R signaling in alcohol reward. For example, systemic administration of the Ox2R antagonist JNJ-10397049 attenuated ethanol self-administration and CPP (Shoblock et al., 2011). In addition, i.c.v. administration of the Ox2R antagonist TCS-OX2-29 (TCS) reduced ethanol self-administration (Brown et al., 2013) and intra-VTA microinjections of TCS or a dual orexin receptor antagonist decreased ethanol drinking (Olney et al., 2017; Srinivasan et al., 2012). Moreover, intermittent-access to 20% ethanol increased Ox2R mRNA in the anterior portion of PVT, and infusions of TCS into this region decreased this type of drinking behavior (Barson et al., 2015). In contrast, there is little evidence to support a role of Ox2R signaling in cocaine reward. Systemic pretreatment with the selective Ox2R antagonist 4PT had no effect on cued reinstatement of extinguished cocaine seeking (Smith et al., 2009b), and the dual Ox1R/Ox2R antagonist almorexant has similarly limited effects as a selective Ox1R antagonist on cocaine self-administration (Prince et al., 2015a). Notably however, one study reported that intra-PVT infusions of orexin-A reinstated extinguished cocaine seeking and that this was blocked by co-administration of TCS (Matzeu et al., 2016). Importantly, the role of Ox2R signaling in cocaine behaviors remains to be fully explored, in part due to a paucity of selective Ox2R antagonists that can be administered systemically; thus, this should be the focus of efforts going forward.
To date, a role for the orexin system in combined cocaine + alcohol consumption remains entirely untested. One interesting question relates to whether polydrug use might have additive effects on orexin cell upregulation, such that a greater increase in orexin cell numbers is observed following cocaine + alcohol self-administration as compared to either drug on its own. It is likely that there is a ceiling on how many cells in the hypothalamus are capable of producing orexin peptides, however this remains to be tested directly. Similar outstanding questions relate to whether the rewarding properties of CE, as a result of concomitant cocaine + alcohol consumption, enhances the salience of drug-paired cues/contexts during self-administration, thus enhancing the role for the orexin system in this type of drug seeking. Such an effect might lead to increased efficacy of orexin receptor antagonists in reducing drug seeking/taking (as is seen in rats with excessive motivation for either cocaine or alcohol, as described above), however this also remains to be tested. One important first step to address this might be to test the effects of orexin receptor antagonism on responding for CE. Although we acknowledge that as a field, we are far from understanding the role of the orexin system in the context of individual drugs of abuse, we suggest that if orexin-based therapeutics are to be effective in the clinic, as we and others have suggested (Campbell et al., 2020a; James and Aston-Jones, 2020; James et al., 2020; Yeoh et al., 2014), it is imperative that investigations going forward begin to examine their efficacy in preclinical models that take polydrug use into account.
3.3. The orexin system in stress and anxiety
As outlined above, CUD populations report co-abusing alcohol to avoid the anxiogenic side effects associated with cocaine withdrawal. To this end, it is interesting that extensive evidence now indicates that orexin neurons are critically involved in coordinating behavioral and physiological responses to stress. This is achieved, in part, via direct innervation of key arousal, autonomic and emotion regions (for recent reviews, see (Abreu et al., 2020; Grafe and Bhatnagar, 2018; James et al., 2017a; Summers et al., 2020)). Moreover, orexin neurons directly modulate the hypothalamic pituitary adrenal (HPA) axis at various levels via direct innervation of the CRF-containing medial parvocellular division of the paraventricular nucleus, and orexin peptides act at the pituitary and adrenal glands themselves (Date et al., 2000; Lo Pez et al., 1999; López et al., 2001; Malendowicz et al., 2001; Voisin et al., 2003). Consistent with this, chemogenetic inhibition of orexin neurons abolishes heightened HPA axis responsivity to repeated restraint stress in female rats (Grafe et al., 2017a).
Exposure to a range of stressors results in recruitment of orexin neurons. Psychological stressors, including exposure to fear-associated contexts, novel environments, restraint stress, foot shock and the forced swim test all increase orexin mRNA or Fos protein expression in orexin cells (Chen et al., 2014; Chen et al., 2013; Flores et al., 2015; Furlong et al., 2009; Harris and Aston-Jones, 2006; James et al., 2014; Martins et al., 2004). Similarly, Fos induction is observed in orexin neurons in response to physiological stressors, including hypercapnia (C02 stress), intraperioteneal injections and food restriction, as well as pharmacological agents that induce stress behaviors including caffeine and the alpha2 receptor antagonist yohimbine (Abreu et al., 2020; Johnson et al., 2012; Kastman et al., 2016; Kurose et al., 2002; Panhelainen and Korpi, 2012). Moreover, prepro-orexin mRNA levels are directly related to behavioral responsivity to repeated social defeat stress, such that lower orexin levels correspond with greater resilience to this form of stress (Grafe et al., 2018). As noted above, there is some evidence of selective recruitment of DMH/PF orexin neurons by some types of stressors, however this has not been observed in all studies (James et al., 2017a).
Acute orexin receptor antagonism is highly effective at ameliorating behavioral responses to anxiogenic stimuli. For example, systemic treatment with selective Ox1R antagonists reduces the expression of anxiety-like behaviors in response to cat odor exposure, nicotine, novelty, yohimbine and fear associated contexts (Beig et al., 2015; Gozzi et al., 2013; Plaza-Zabala et al., 2010; Staples and Cornish, 2014; Vanderhaven et al., 2015), although see (Blume et al., 2018). These effects appear to be mediated, at least in part, by reduced recruitment of the HPA axis (Heydendael et al., 2013; Vanderhaven et al., 2015). There is also a role for Ox2R signaling in the expression of stress behaviors. Mice lacking Ox2R exhibit a blunted stress response (Yun et al., 2017) and administration of selective Ox2R antagonists blocks behavioral, cardiovascular and HPA axis responsivity to restraint stress (Grafe et al., 2017b), quinporole (Abounoori et al., 2020), cage exchange stress (Yun et al., 2017) and novelty (Beig et al., 2015) (although note that there is some evidence that signaling at Ox2R can be protective against the expression of anxiety; see (Arendt et al., 2014; Arendt et al., 2013; Staton et al., 2018; Summers et al., 2020)). Similarly, there is emerging evidence that dual orexin receptor antagonists (DORAs) may be effective anxiolytics. For example, a clinical study recently reported that suvorexant attenuates heart rate and cortisol release in response to the cold pressor test (Suchting et al., 2019) and there are several clinical trials currently underway investigating the utility of suvorexant for panic disorder (NCT02593682) and PTSD (NCT03642028). In animals, DORAs prevent the behavioral and autonomic responses to stress (Chen et al., 2013), and there is some evidence that these compounds have additive protective effects beyond Ox1R and Ox2R antagonists alone (Beig et al., 2015).
Of particular relevance here is the question of whether orexin receptor antagonists might be effective at reducing stress and anxiety states associated with cocaine withdrawal. The majority of work on the orexin system in drug withdrawal has been carried out using Ox1R antagonists in models of opioid withdrawal, which is characterized by a range of physiological and emotional symptoms, including anxiety. In morphine-dependent rats, naloxone-precipitated withdrawal is associated with Fos induction in orexin neurons and an increase in orexin mRNA. Moreover, Ox1R antagonists reduce morphine withdrawal (Laorden et al., 2012) and withdrawal symptoms are less severe in orexin knockout mice (Georgescu et al., 2003). There is some evidence that orexins might be involved in withdrawal from other drugs of abuse, including a report that orexin levels in the CSF are elevated during acute abstinence from methamphetamine (Chen et al., 2016). To date, however, orexin signaling during cocaine withdrawal remains largely uncharacterized. One study reported that acute (24h) withdrawal from chronic escalating-dose cocaine administration was associated with increased LH orexin mRNA levels (Zhou et al., 2008). Similarly, we reported that orexin cell numbers and reactivity was higher in intermittent vs. short access rats after drug context exposure following 24h withdrawal (James et al., 2019b). In that study we also reported that intermittent access rats exhibited greater negative emotional behaviors, including anxiety-like behavior, after 5 months of homecage abstinence; these rats also exhibited greater reactivity and number of orexin neurons after protracted abstinence, however the relevance of this increase to negative emotional behaviors was not directly tested. Given the role for orexin in both acute and chronic anxiety-like states, we suggest that targeting this system could have particularly beneficial therapeutic effects in cocaine and alcohol coabuse, both in terms of reducing the onset of anxiety during the cocaine crash (and thus reducing ‘self-medication’ with alcohol), as well as ameliorating more chronic underlying anxiety states that contribute to ongoing drug use. Of course, significant work is required to test this hypothesis, especially considering that no studies to date have investigated the orexin system, nor the efficacy of orexin receptor antagonists, in the context of cocaine and alcohol coabuse.
4.0. Orexin-1 receptor antagonists are more effective at reducing cocaine demand following homecage alcohol exposure.
As outlined above, studies examining the neurobiological basis of cocaine + alcohol coabuse are limited. Significant evidence indicates that the orexin system might underlie cocaine + alcohol coabuse, as activity of these neurons is linked to seeking and taking both drugs, as well as the expression of withdrawal behaviors, including anxiety. Although there is extensive literature demonstrating the efficacy of Ox1R antagonists at reducing drug-related behaviors in animals with a history of either cocaine or alcohol self-administration, the efficacy of these compounds in animals with a history of cocaine and alcohol coabuse has not been tested. To begin to examine this, we sought to test the efficacy of the selective Ox1R antagonist SB334867 (SB) on motivated cocaine seeking in rats, both before and after cocaine self-administration was made coincident with intermittent home cage alcohol access. To do this, we leveraged the behavioral economics procedure, which allows repeated assessment of SB treatment on cocaine motivation in the same animal. We predicted that with prolonged cocaine self-administration, home cage alcohol intake would increase and that this might, in turn, increase demand for cocaine. Because Ox1R antagonists are most effective in rats with stronger addiction profiles (James et al., 2019; Fragale et al. 2020), we also predicted that SB would be more effective at reducing cocaine demand when cocaine self-administration was coincident with home cage alcohol access.
All new experiments were carried out in male Sprague Dawley rats (n=10; 250-300g, Charles River) that were given ad libitum access to regular rat chow and water, and maintained on a reverse 12:12 light/dark cycle (lights on at 06:00h). All protocols and animal care procedures were approved by the Rutgers University Institutional Animal Care and Use Committee, and in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Rats were implanted with an intravenous jugular catheter and trained to self-administer cocaine on a FR1 schedule (0.2mg/50μl infusion; minimum of 6 sessions) before being tested for ‘baseline’ economic demand on a within-session behavioral economics procedure over a period of ~1w (Bentzley et al., 2013). Rats were then given access to alcohol (20% ethanol) in their homecage 3d/week (Monday, Wednesday, Friday) over a period of 5w, during which time demand for cocaine was assessed daily (Monday-Friday). Over the 5w alcohol access period, homecage intake of alcohol increased significantly from an average of 1.94 ± 0.50 g/kg to 3.40 ± 0.81 g/kg per session (W10 = 41.00, p = 0.037; Wilcoxon test; Fig. 1a and b). This increase in alcohol intake was paralleled by a significant increase in the animals’ preferred level of cocaine intake (Q0; W10 = 53.00, p = 0.004; Wilcoxon test; Fig. 1c), but no change in their motivation for cocaine (α; p > 0.05; Fig. 1d). To test the role of orexin signaling in these rats, we assessed the effect of SB on both Q0 and α prior to any alcohol experience and at d36-40 (after 5w of home cage alcohol exposure). Rats were administered a single intraperitoneal (i.p.) dose of SB (30mg/kg) at both time points and tested for cocaine demand 30 min later. Interestingly, SB had no effect on cocaine Q0 prior to home cage alcohol exposure (consistent with prior studies; (Bentzley and Aston-Jones, 2015; James et al., 2019a; James et al., 2019b)), but significantly decreased preferred cocaine levels following home cage alcohol (Time x Treatment interaction, F1,18=6.986, p=0.0165; Sidak’s post-hoc test, p=0.012; Figure 1e). Similarly, SB increased α (decreased motivation) only following alcohol experience (Treatment main effect, F1,18=6.691, p=0.019; Sidak’s post-hoc test, p=0.029).
Figure 1:
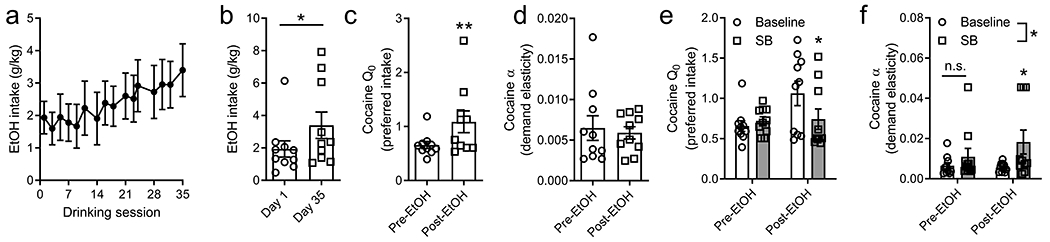
Rats were tested for economic demand for cocaine prior to alcohol exposure. Rats were then given home cage access to alcohol three times a week (Monday, Wednesday, Friday; 12h/d), during which time they were tested daily for cocaine demand. (a, b) Alcohol consumption escalated significantly across the 5w of home cage alcohol access, such that intake was significantly higher on the final alcohol session compared to the first. (c) Rats had a higher preferred level of cocaine intake (Q0) after 5w of home cage alcohol. (d) There was no change in cocaine motivation as a result of home cage alcohol. (e) SB had no effect on preferred cocaine levels (Q0) prior to alcohol access (Pre-EtOH), but was associated with a significant reduction in Q0 values when tested following 5w alcohol (post-EtOH). (f) We observed a main effect of SB treatment on cocaine motivation (α) across both test days, however post-hoc analyses revealed that SB significantly increased α (decreased cocaine motivation) only following alcohol (post-EtOH). Data reflects mean ± SEM. *p<0.05. **p<0.01. Baseline in Panels e and f reflects values from the day prior to SB testing (i.e. no treatment).
Two key findings can be derived from these preliminary data. First, rats that self-administered cocaine and had concurrent homecage access to alcohol had preferred levels of cocaine intake almost twice that of when they were given access to cocaine alone. It is unlikely that this reflects the development of cocaine ‘tolerance’ with prolonged cocaine intake, as baseline demand parameters (including Q0) remain stable for several weeks in rats given limited (so called ‘short access’) daily access to cocaine, or across multiple BE testing days (James et al., 2019a), as was the case here (although we acknowledge that the inclusion of a cocaine-only group in future studies would be informative in this regard). Rather, increased cocaine intake might reflect reduced anxiogenic effects of cocaine resulting from concomitant alcohol consumption, either due to the production of CE on days where both cocaine and alcohol were available, or because of the general anxiolytic properties of alcohol. Anxiety-like behavior was not assessed here, and thus it will be important for future studies to examine whether the aversive properties of cocaine are attenuated in animals that have concurrent access to cocaine + alcohol compared to cocaine alone. Second, we observed an increase in SB efficacy following alcohol, such that SB was effective at reducing preferred cocaine levels and cocaine motivation only after 5w home cage alcohol exposure. This finding is generally consistent with previous studies showing that SB is more effective in animals that exhibit a stronger addiction phenotype, including after self-administration procedures that induce an addiction phenotype, such as extended or intermittent access (Fragale et al., 2020; James and Aston-Jones, 2017; James et al., 2019a; James et al., 2019b; Schmeichel et al., 2017b). Thus, the present data might indicate that concurrent cocaine + alcohol use precipitated a stronger addiction phenotype that was orexin-dependent. Although SB is reported to be most effective at reducing motivation for drug rather than its intake per se, our findings are also consistent with studies that have reported that SB or orexin knockdown reduces cocaine intake in animals that exhibited escalation of intake (Schmeichel et al., 2017b; Schmeichel et al., 2018). An interesting question going forward relates to the effect of cocaine + alcohol co-administration on orexin cell expression. Cocaine and alcohol each increase orexin cell and mRNA levels (James et al., 2019b; Lawrence et al., 2005) and greater SB efficacy has previously been reported in rats with higher orexin cell numbers (Fragale et al., 2020; James et al., 2019b), and thus we might expect that the introduction of alcohol in this experiment resulted in a concurrent increase in the number of orexin cells. Moreover, here, alcohol was available in the home cage before and after cocaine sessions, however intake patterns were not studied in a way that allows estimations of possible cocaine x alcohol interactions (or CE production). Future studies should seek to better understand how the temporal patterns of cocaine and alcohol intake contribute to the effects observed here. Finally, it will also be important for future studies to understand the precise circuits through which orexin neurons mediate cocaine intake versus motivation after a history of cocaine + alcohol use, and whether these differ to those involved in each drug on their own.
5.0. Conclusions and considerations for future studies
Here we propose that the hypothalamic orexin neuropeptide system represents a potential target for novel therapeutics designed to reduce drug seeking in cocaine + alcohol co-abusing populations. Evidence reviewed here indicates that the orexin peptides act primarily at Ox1R to mediate cocaine seeking, whereas alcohol behaviors are mediated by actions at both Ox1R and Ox2R, raising the possibility that compounds that target both receptor subtypes (DORAs) might be most effective in cases of dual-dependence. Moreover, the role for orexin signaling in stress and anxiety appears to involve signaling at both receptors, and thus the anxiolytic properties of a DORA might be most effective to reduce the tendency to ‘self-medicate’ against the negative emotional aspects of cocaine withdrawal with alcohol. To this end, the FDA-approved DORA suvorexant (Belsomra™) represents an attractive therapeutic option with the possibility of being readily repurposed. Indeed, we note that several clinical trials are currently underway examining the efficacy of suvorexant in CUD and AUD populations (ClinicalTrials.gov; (Campbell et al., 2020b; James et al., 2020); it is not clear to what extent these studies will include polydrug use as a key outcome measure. Notably, suvorexant and another recently approved DORA (lemborexant) are hypnotics designed to promote sleep in an insomnia indication (Kishi et al., 2020), thus any studies examining their repurposing for addiction must consider whether the soporific effects of these compounds might be enhanced by concomitant drug use. Indeed, prescribing guidelines for suvorexant lists alcohol use as a contraindication, based on evidence that their combined use has negative effects on tests of reaction time, vigilance, working/episodic memory, postural stability and alertness (Sun et al., 2015). Nonetheless, it is possible that the sleep-promoting properties of DORAs might have additional therapeutic benefits for addiction outcomes, given that both cocaine and alcohol use are associated with severe sleep disruptions that contribute to relapse risk (Angarita et al., 2014a; Angarita et al., 2014b; Roehrs et al., 2020). Focus should also be given to examining any potential effects of DORAs on mood, given that low orexin signaling can be associated with depression symptomology, including suicidality (Arendt et al., 2013; Bowrey et al., 2017; Brundin et al., 2007a; Brundin et al., 2007b; James et al., 2014; Lutter et al., 2008). Moreover, based on preclinical studies finding higher efficacy of orexin receptor antagonists in rats with stronger addiction phenotypes (Fragale et al., 2020; Fragale et al., 2019; James et al., 2019a; Mohammadkhani et al., 2019a), it is possible that low, non-sedating doses of suvorexant will be effective at reducing craving. Additionally, evidence indicating that the anti-drug seeking properties of orexin receptor antagonists, including suvorexant, extend beyond their bioavailability (up to 48h) suggests that daily dosing might not be necessary (Brodnik et al., 2020; Mohammadkhani et al., 2019b; O’Connor et al., 2020). Clearly, significant work is required to address these questions around potential clinical use of orexin receptor antagonists for polysubstance use, as well as the more mechanistic questions raised throughout this article. Regardless, there exists a significant foundation of work pointing to the potential utility of orexin-based compounds; we suggest that extending these investigations to the context of polysubstance abuse should be the focus of studies moving forward.
Acknowledgements:
We would like to thank Elisabeth Kilroy for her assistance with the behavioral studies described in this manuscript.
Funding sources: This work was supported by the National Institute on Drug Abuse (K99DA045765 to MHJ; RO1 DA006214-25S1 to GAJ), National Institute of Health (K12 GM093854 to JEF) and the National Health and Medical Research Council of Australia (CJ Martin Fellowship 1072706 to MHJ). The funding sources had no involvement in the study design, collection, analysis or interpretation of the data; in the writing of the report; or the decision to submit the manuscript for publication.
Footnotes
Declarations of interest: None.
References
- Abounoori M, Maddah MM, Akbari E, Houshmand G, Ardeshiri MR, 2020. The Effect of Orexin Receptor Antagonism on Quinpirole-Induced Compulsive-Like Checking Behavior in Rats. Neurotox Res 38, 18–26. [DOI] [PubMed] [Google Scholar]
- Abreu AR, Molosh AI, Johnson PL, Shekhar A, 2020. Role of medial hypothalamic orexin system in panic, phobia and hypertension. Brain Res 1731, 145942. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC 3rd, Elkashef AM, 2009. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend 104, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P, 1997. Cocaethylene toxicity. J Addict Dis 16, 75–84. [DOI] [PubMed] [Google Scholar]
- Angarita GA, Canavan SV, Forselius E, Bessette A, Morgan PT, 2014a. Correlates of polysomnographic sleep changes in cocaine dependence: self-administration and clinical outcomes. Drug Alcohol Depend 143, 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angarita GA, Canavan SV, Forselius E, Bessette A, Pittman B, Morgan PT, 2014b. Abstinence-related changes in sleep during treatment for cocaine dependence. Drug Alcohol Depend 134, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC, 1994. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Experimental and Clinical Psychopharmacology 2, 244–268. [Google Scholar]
- Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, Dileone RJ, Ronan PJ, Summers CH, 2014. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology 40, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH, 2013. Depressive behavior and activation of the orexin/hypocretin system. Behav Neurosci 127, 86–94. [DOI] [PubMed] [Google Scholar]
- Aspen JM, Winger G, 1997. Ethanol effects on self-administration of alfentanil, cocaine, and nomifensine in rhesus monkeys. Psychopharmacology (Berl) 130, 222–227. [DOI] [PubMed] [Google Scholar]
- Aston-Jones S, Aston-Jones G, Koob GF, 1984. Cocaine antagonizes anxiolytic effects of ethanol. Psychopharmacology (Berl) 84, 28–31. [DOI] [PubMed] [Google Scholar]
- Barson JR, 2020. Orexin/hypocretin and dysregulated eating: Promotion of foraging behavior. Brain Res 1731, 145915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF, 2015. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol 20, 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Poon K, Ho HT, Alam MI, Sanzalone L, Leibowitz SF, 2017. Substance P in the anterior thalamic paraventricular nucleus: promotion of ethanol drinking in response to orexin from the hypothalamus. Addict Biol 22, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beig MI, Dampney BW, Carrive P, 2015. Both Ox1r and Ox2r orexin receptors contribute to the cardiovascular and locomotor components of the novelty stress response in the rat. Neuropharmacology 89, 146–156. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G, 2015. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41, 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G, 2013. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 226, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Espana RA, Vittoz NM, 2010. Hypocretin/orexin in arousal and stress. Brain Res 1314, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume SR, Nam H, Luz S, Bangasser DA, Bhatnagar S, 2018. Sex- and Age-dependent Effects of Orexin 1 Receptor Blockade on Open-Field Behavior and Neuronal Activity. Neuroscience 381, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A, 2009. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29, 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L, 2005. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A 102, 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowrey HE, James MH, Aston-Jones G, 2017. New directions for the treatment of depression: Targeting the photic regulation of arousal and mood (PRAM) pathway. Depress Anxiety 34, 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH, 1993. Cocaine and cocaethylene: microdialysis comparison of brain drug levels and effects on dopamine and serotonin. J Neurochem 60, 1429–1435. [DOI] [PubMed] [Google Scholar]
- Brady KT, Haynes LF, Hartwell KJ, Killeen TK, 2013. Substance use disorders and anxiety: a treatment challenge for social workers. Social work in public health 28, 407–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Alonso IP, Xu W, Zhang Y, Kortagere S, Espana RA, 2018. Hypocretin receptor 1 involvement in cocaine-associated behavior: Therapeutic potential and novel mechanistic insights. Brain Res. [DOI] [PubMed] [Google Scholar]
- Brodnik ZD, Alonso IP, Xu W, Zhang Y, Kortagere S, España RA, 2020. Hypocretin receptor 1 involvement in cocaine-associated behavior: Therapeutic potential and novel mechanistic insights. Brain Res 1731, 145894. [DOI] [PubMed] [Google Scholar]
- Brookoff D, Rotondo MF, Shaw LM, Campbell EA, Fields L, 1996. Coacaethylene levels in patients who test positive for cocaine. Ann Emerg Med 27, 316–320. [DOI] [PubMed] [Google Scholar]
- Brown RM, Khoo SY, Lawrence AJ, 2013. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol 16, 2067–2079. [DOI] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Petersen A, Traskman-Bendz L, 2007a. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol 17, 573–579. [DOI] [PubMed] [Google Scholar]
- Brundin L, Petersén A, Björkqvist M, Träskman-Bendz L, 2007b. Orexin and psychiatric symptoms in suicide attempters. J Affect Disord 100, 259–263. [DOI] [PubMed] [Google Scholar]
- Burdakov D, 2020. How orexin signals bias action: Hypothalamic and accumbal circuits. Brain Res 1731, 145943. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Karnani MM, Gonzalez A, 2013. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol Behav 121, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Marchant NJ, Lawrence AJ, 2020a. A sleeping giant: Suvorexant for the treatment of alcohol use disorder? Brain Res 1731, 145902. [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Norman A, Bonomo Y, Lawrence AJ, 2020b. Suvorexant to treat alcohol use disorder and comorbid insomnia: Plan for a phase II trial. Brain Res 1728, 146597. [DOI] [PubMed] [Google Scholar]
- Carrive P, Kuwaki T, 2017. Orexin and Central Modulation of Cardiovascular and Respiratory Function. Curr Top Behav Neurosci 33, 157–196. [DOI] [PubMed] [Google Scholar]
- Chen WY, Kao CF, Chen PY, Lin SK, Huang MC, 2016. Orexin-A level elevation in recently abstinent male methamphetamine abusers. Psychiatry Res 239, 9–11. [DOI] [PubMed] [Google Scholar]
- Chen X, Li S, Kirouac GJ, 2014. Blocking of corticotrophin releasing factor receptor-1 during footshock attenuates context fear but not the upregulation of prepro-orexin mRNA in rats. Pharmacol Biochem Behav 120, 1–6. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, Vrontakis ME, Kirouac GJ, 2013. Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct Funct. [DOI] [PubMed] [Google Scholar]
- Collier AD, Halkina V, Min SS, Roberts MY, Campbell SD, Camidge K, Leibowitz SF, 2019. Embryonic Ethanol Exposure Affects the Early Development, Migration, and Location of Hypocretin/Orexin Neurons in Zebrafish. Alcohol Clin Exp Res 43, 1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AD, Min SS, Campbell SD, Roberts MY, Camidge K, Leibowitz SF, 2020. Maternal ethanol consumption before paternal fertilization: Stimulation of hypocretin neurogenesis and ethanol intake in zebrafish offspring. Prog Neuropsychopharmacol Biol Psychiatry 96, 109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JP, Gullo MJ, White A, Kelly AB, 2014. Polysubstance use: diagnostic challenges, patterns of use and health. Curr Opin Psychiatry 27, 269–275. [DOI] [PubMed] [Google Scholar]
- Crummy EA, O’Neal TJ, Baskin BM, Ferguson SM, 2020. One Is Not Enough: Understanding and Modeling Polysubstance Use. Front Neurosci 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, 2015. Effects of chronic binge-like ethanol consumption on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend 153, 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, 2017. 4 - Combined alcohol and drug abuse: A potentially deadly mix In: Dasgupta A, (Ed), Alcohol, Drugs, Genes and the Clinical Laboratory. Academic Press, pp. 75–88. [Google Scholar]
- Date Y, Mondal MS, Matsukura S, Ueta Y, Yamashita H, Kaiya H, Kangawa K, Nakazato M, 2000. Distribution of orexin/hypocretin in the rat median eminence and pituitary. Brain Res Mol Brain Res 76, 1–6. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F, 2008. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry 63, 152–157. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, 1998. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR, 2010. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci 31, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE, 2001. Fos expression in orexin neurons varies with behavioral state. J Neurosci 21, 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Saravia R, Maldonado R, Berrendero F, 2015. Orexins and fear: implications for the treatment of anxiety disorders. Trends Neurosci 38, 550–559. [DOI] [PubMed] [Google Scholar]
- Fragale JE, James MH, Aston-Jones G, 2020. Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. bioRxiv, 2020.2004.2023.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, James MH, and Aston-Jones G, 2020. Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, Pantazis CB, James MH, Aston-Jones G, 2019. The role of orexin-1 receptor signaling in demand for the opioid fentanyl. Neuropsychopharmacology 44, 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LR, Bentzley BS, James MH, Aston-Jones G, 2020. Sex Differences in Demand for Highly Palatable Foods: Role of the Orexin System. Int J Neuropsychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L, Carrive P, 2009. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 30, 1603–1614. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ, 2003. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23, 3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li SB, Malenka RC, de Lecea L, 2018. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat Neurosci 21, 1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompf HS, Aston-Jones G, 2008. Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity. Brain Res 1224, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Manning V, Ridge G, 2006. Concurrent use and order of use of cocaine and alcohol: behavioural differences between users of crack cocaine and cocaine powder. Addiction 101, 1292–1298. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Lepore S, Vicentini E, Merlo-Pich E, Bifone A, 2013. Differential effect of orexin-1 and CRF-1 antagonism on stress circuits: a fMRI study in the rat with the pharmacological stressor Yohimbine. Neuropsychopharmacology 38, 2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Bhatnagar S, 2018. Orexins and stress. Front Neuroendocrinol 51, 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S, 2017a. Orexins Mediate Sex Differences in the Stress Response and in Cognitive Flexibility. Biol Psychiatry 81, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Eacret D, Dobkin J, Bhatnagar S, 2018. Reduced Orexin System Function Contributes to Resilience to Repeated Social Stress. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Eacret D, Luz S, Gotter AL, Renger JJ, Winrow CJ, Bhatnagar S, 2017b. Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience 348, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Harford TC, 1990. Concurrent and simultaneous use of alcohol with cocaine: results of national survey. Drug Alcohol Depend 25, 97–104. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP, 2007. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience 146, 525–536. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G, 2006. Arousal and reward: a dichotomy in orexin function. Trends Neurosci 29, 571–577. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G, 2005. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559. [DOI] [PubMed] [Google Scholar]
- Hart CL, Jatlow P, Sevarino KA, McCance-Katz EF, 2000. Comparison of intravenous cocaethylene and cocaine in humans. Psychopharmacology (Berl) 149, 153–162. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR, 2000. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept 96, 45–51. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Sengupta A, Beck S, Bhatnagar S, 2013. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Rush CR, Bickel WK, Hughes JR, Lynn M, Capeless MA, 1993. Acute behavioral and cardiac effects of cocaine and alcohol combinations in humans. Psychopharmacology (Berl) 111, 285–294. [DOI] [PubMed] [Google Scholar]
- Hopf FW, 2020. Recent perspectives on orexin/hypocretin promotion of addiction-related behaviors. Neuropharmacology 168, 108013. [DOI] [PubMed] [Google Scholar]
- James MH, Aston-Jones G, 2017. Orexin/Hypocretin, Central Amygdala, and Escalation of Cocaine Intake. Biol Psychiatry 81, 552–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Aston-Jones G, 2020. Introduction to the Special Issue: “Making orexin-based therapies for addiction a reality: What are the steps from here?”. Brain Res 1731, 146665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Bowrey HE, Stopper CM, Aston-Jones G, 2018a. Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Bowrey HE, Stopper CM, Aston-Jones G, 2019a. Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci 50, 2602–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Dayas CV, 2017a. Role of the Orexin/Hypocretin System in Stress-Related Psychiatric Disorders. Curr Top Behav Neurosci 33, 197–219. [DOI] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Walker FR, Smith DW, Richardson HN, Hodgson DM, Dayas CV, 2014. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front Behav Neurosci 8, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV, 2011. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol 14, 684–690. [DOI] [PubMed] [Google Scholar]
- James MH, Fragale JE, Aurora RN, Cooperman NA, Langleben DD, Aston-Jones G, 2020. Repurposing the dual orexin receptor antagonist suvorexant for the treatment of opioid use disorder: why sleep on this any longer? Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G, 2017b. A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci 33, 247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G, 2018b. Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G, 2019b. Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry 85, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatlow P, Elsworth JD, Bradberry CW, Winger G, Taylor JR, Russell R, Roth RH, 1991. Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci 48, 1787–1794. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, Shekhar A, 2012. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav 107, 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ, 2011. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res 1391, 54–59. [DOI] [PubMed] [Google Scholar]
- Kastman HE, Blasiak A, Walker L, Siwiec M, Krstew EV, Gundlach AL, Lawrence AJ, 2016. Nucleus incertus Orexin2 receptors mediate alcohol seeking in rats. Neuropharmacology 110, Part A, 82–91. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE, 2016. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl) 233, 3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia S, Sell MA, Relyea G, 2007. Mono- versus polydrug abuse patterns among publicly funded clients. Subst Abuse Treat Prev Policy 2, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Nomura I, Matsuda Y, Sakuma K, Okuya M, Ikuta T, Iwata N, 2020. Lemborexant vs suvorexant for insomnia: A systematic review and network meta-analysis. J Psychiatr Res 128, 68–74. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Ben-Shahar O, Ettenberg A, 2006. Alcohol consumption is preferred to water in rats pretreated with intravenous cocaine. Pharmacol Biochem Behav 85, 281–286. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Samimi MM, Ettenberg A, 2002. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol Biochem Behav 72, 931–936. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Benca RM, Kilduff TS, 2013. Understanding the sleep-wake cycle: sleep, insomnia, and the orexin system. J Clin Psychiatry 74 Suppl 1, 3–20. [DOI] [PubMed] [Google Scholar]
- Kurose T, Ueta Y, Yamamoto Y, Serino R, Ozaki Y, Saito J, Nagata S, Yamashita H, 2002. Effects of restricted feeding on the activity of hypothalamic Orexin (OX)-A containing neurons and OX2 receptor mRNA level in the paraventricular nucleus of rats. Regul Pept 104, 145–151. [DOI] [PubMed] [Google Scholar]
- Laorden ML, Ferenczi S, Pintér-Kübler B, González-Martín LL, Lasheras MC, Kovács KJ, Milanés MV, Núñez C, 2012. Hypothalamic orexin--a neurons are involved in the response of the brain stress system to morphine withdrawal. PLoS One 7, e36871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B, 2006. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Kwok C, Darevsky D, Wegner SA, Yu J, Nakayama L, Pedrozo V, Anderson L, Ghotra S, Fouad M, Hopf FW, 2019. Nucleus Accumbens Shell Orexin-1 Receptors Are Critical Mediators of Binge Intake in Excessive-Drinking Individuals. Front Neurosci 13, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Hopf FW, 2016a. Orexin-1 receptor blockade suppresses compulsive-like alcohol drinking in mice. Neuropharmacology 110, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Mototake A, Hu B, Hopf FW, 2016b. Nucleus Accumbens Shell and mPFC but Not Insula Orexin-1 Receptors Promote Excessive Alcohol Drinking. Front Neurosci 10, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, June HL, 1994. Synergistic effects of ethanol and cocaine on brain stimulation reward. J Exp Anal Behav 61, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SB, Giardino WJ, de Lecea L, 2017. Hypocretins and Arousal. Curr Top Behav Neurosci 33, 93–104. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ, 2010. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 212, 251–265. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang H, Qi K, Chen X, Li S, Sui N, Kirouac GJ, 2011. Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol Behav 102, 42–50. [DOI] [PubMed] [Google Scholar]
- Liu Y, Williamson V, Setlow B, Cottler LB, Knackstedt LA, 2018. The importance of considering polysubstance use: lessons from cocaine research. Drug Alcohol Depend 192, 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Pez M, Sen Aris R, Gallego R, Garci ACT, Lago F, Seoane L, Casanueva F, Die Guez C, 1999. Orexin Receptors Are Expressed in the Adrenal Medulla of the Rat. Endocrinology 140, 5991–5994. [DOI] [PubMed] [Google Scholar]
- López M, Seoane L, Señarís RM, Diéguez C, 2001. Prepro-orexin mRNA levels in the rat hypothalamus, and orexin receptors mRNA levels in the rat hypothalamus and adrenal gland are not influenced by the thyroid status. Neurosci Lett 300, 171–175. [DOI] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ, 2008. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci 28, 3071–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo GC, Kawakami SE, Vignoli T, Sinigaglia-Coimbra R, Suchecki D, 2013. The influence of orexins on ethanol-induced behavioral sensitization in male mice. Neurosci Lett 551, 84–88. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G, 2014. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17, 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malendowicz LK, Jedrzejczak N, Belloni AS, Trejter M, Hochól A, Nussdorfer GG, 2001. Effects of orexins A and B on the secretory and proliferative activity of immature and regenerating rat adrenal glands. Histol Histopathol 16, 713–717. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Cauvi G, Kerr TM, Weiss F, 2018. Differential role of hypothalamic orexin/hypocretin neurons in reward seeking motivated by cocaine versus palatable food. Addict Biol 23, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F, 2014. N-(2-methyl-6-benzoxazolyl)-N′-1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol 19, 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins PJ, D’Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S, 2004. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept 117, 155–158. [DOI] [PubMed] [Google Scholar]
- Masur J, Souza-Formigoni ML, Pires ML, 1989. Increased stimulatory effect by the combined administration of cocaine and alcohol in mice. Alcohol 6, 181–182. [DOI] [PubMed] [Google Scholar]
- Matzeu A, Kerr TM, Weiss F, Martin-Fardon R, 2016. Orexin-A/Hypocretin-1 Mediates Cocaine-Seeking Behavior in the Posterior Paraventricular Nucleus of the Thalamus via Orexin/Hypocretin Receptor-2. J Pharmacol Exp Ther 359, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Martin-Fardon R, 2020. Targeting the Orexin System for Prescription Opioid Use Disorder. Brain Sci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance EF, Price LH, Kosten TR, Jatlow PI, 1995. Cocaethylene: pharmacology, physiology and behavioral effects in humans. J Pharmacol Exp Ther 274, 215–223. [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI, 1993. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology (Berl) 111, 39–46. [DOI] [PubMed] [Google Scholar]
- Mohammadkhani A, Fragale JE, Pantazis CB, Bowrey HE, James MH, Aston-Jones G, 2019a. Orexin-1 receptor signaling in ventral pallidum regulates motivation for the opioid remifentanil. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadkhani A, James MH, Pantazis CB, Aston-Jones G, 2019b. Persistent effects of the orexin-1 receptor antagonist SB-334867 on motivation for the fast acting opioid remifentanil. Brain Res, 146461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, 2018. The hypocretin/orexin system as a target for excessive motivation in alcohol use disorders. Psychopharmacology (Berl) 235, 1663–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G, 2016. Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci 43, 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G, 2017. Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res 1654, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF, 2010. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res 34, 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobiletti JB, Jatlow PI, Bradberry CW, 1994. Differences in bioavailability between cocaine and cocaethylene and their implications for drug-reward studies. Psychopharmacology (Berl) 116, 273–278. [DOI] [PubMed] [Google Scholar]
- O’Connor SL, Fragale JE, James MH, Aston-Jones G, 2020. The dual orexin/hypocretin receptor antagonist suvorexant reduces addiction-like behaviors for the opioid fentanyl. bioRxiv, 2020.2004.2025.061887. [Google Scholar]
- Olney JJ, Navarro M, Thiele TE, 2017. The Role of Orexin Signaling in the Ventral Tegmental Area and Central Amygdala in Modulating Binge-Like Ethanol Drinking Behavior. Alcohol Clin Exp Res 41, 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Hedaya MA, 1999. Cocaine and alcohol interactions in the rat: effect of cocaine and alcohol pretreatments on cocaine pharmacokinetics and pharmacodynamics. J Pharm Sci 88, 1266–1274. [DOI] [PubMed] [Google Scholar]
- Panhelainen AE, Korpi ER, 2012. Evidence for a role of inhibition of orexinergic neurons in the anxiolytic and sedative effects of diazepam: A c-Fos study. Pharmacol Biochem Behav 101, 115–124. [DOI] [PubMed] [Google Scholar]
- Pantazis C, James MH, Bentzley BS, Aston-Jones G, 2019. The number of lateral hypothalamus orexin/hypocretin neurons contributes to individual differences in cocaine demand. bioRxiv, 547836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis CB, James MH, Bentzley BS, Aston-Jones G, 2020. The number of lateral hypothalamus orexin/hypocretin neurons contributes to individual differences in cocaine demand. Addict Biol 25, e12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F, 2010. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci 30, 2300–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, Espana RA, 2015a. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci 6, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, España RA, 2015b. Hypocretin/Orexin Regulation of Dopamine Signaling and Cocaine Self-Administration Is Mediated Predominantly by Hypocretin Receptor 1. ACS Chemical Neuroscience 6, 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A, 2000. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol 8, 117–124. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE, 2008. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 199, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ, 1987. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237, 1219–1223. [DOI] [PubMed] [Google Scholar]
- Roehrs TA, Auciello J, Tseng J, Whiteside G, 2020. Current and potential pharmacological treatment options for insomnia in patients with alcohol use disorder in recovery. Neuropsychopharmacol Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F, 1991. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry 48, 43–51. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y, 2004. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept 118, 183–191. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, 2011. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci 32, 451–462. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M, 2005. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 46, 297–308. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Herman MA, Roberto M, Koob GF, 2017a. Hypocretin Neurotransmission Within the Central Amygdala Mediates Escalated Cocaine Self-administration and Stress-Induced Reinstatement in Rats. Biol Psychiatry 81, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BE, Herman MA, Roberto M, Koob GF, 2017b. Hypocretin Neurotransmission within the Central Amygdala Mediates Escalated Cocaine Self-Administration and Stress-induced Reinstatement in Rats. Biol Psychiatry 81, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BE, Matzeu A, Koebel P, Vendruscolo LF, Sidhu H, Shahryari R, Kieffer BL, Koob GF, Martin-Fardon R, Contet C, 2018. Knockdown of hypocretin attenuates extended access of cocaine self-administration in rats. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW, Boggs J, Galici R, 2011. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl) 215, 191–203. [DOI] [PubMed] [Google Scholar]
- Simmons SJ, Gentile TA, 2019. Cocaine abuse and midbrain circuits: Functional anatomy of hypocretin/orexin transmission and therapeutic prospect. Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G, 2009a. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. European Journal of Neuroscience 30, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G, 2009b. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci 30, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G, 2010. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology 58, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Sakurai T, 2020. Orexin as a modulator of fear-related behavior: Hypothalamic control of noradrenaline circuit. Brain Res 1731, 146037. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, Hopf FW, Bonci A, Bartlett SE, 2012. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One 7, e44726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples LG, Cornish JL, 2014. The orexin-1 receptor antagonist SB-334867 attenuates anxiety in rats exposed to cat odor but not the elevated plus maze: An investigation of Trial 1 and Trial 2 effects. Horm Behav 65, 294–300. [DOI] [PubMed] [Google Scholar]
- Staton CD, Yaeger JDW, Khalid D, Haroun F, Fernandez BS, Fernandez JS, Summers BK, Summers TR, Sathyanesan M, Newton SS, Summers CH, 2018. Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology 143, 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling ME, Karatayev O, Chang GQ, Algava DB, Leibowitz SF, 2015. Model of voluntary ethanol intake in zebrafish: effect on behavior and hypothalamic orexigenic peptides. Behav Brain Res 278, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting R, Yoon JH, Miguel GGS, Green CE, Weaver MF, Vincent JN, Fries GR, Schmitz JM, Lane SD, 2019. Preliminary examination of the orexin system on relapse-related factors in cocaine use disorder. Brain Res, 146359. [DOI] [PubMed] [Google Scholar]
- Summers CH, Yaeger JDW, Staton CD, Arendt DH, Summers TR, 2020. Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: Potential for therapy. Brain Res 1731, 146085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Yee KL, Gill S, Liu W, Li X, Panebianco D, Mangin E, Morrison D, McCrea J, Wagner JA, Troyer MD, 2015. Psychomotor effects, pharmacokinetics and safety of the orexin receptor antagonist suvorexant administered in combination with alcohol in healthy subjects. J Psychopharmacol 29, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T, 2013. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaven MW, Cornish JL, Staples LG, 2015. The orexin-1 receptor antagonist SB-334867 decreases anxiety-like behavior and c-Fos expression in the hypothalamus of rats exposed to cat odor. Behav Brain Res 278, 563–568. [DOI] [PubMed] [Google Scholar]
- Voisin T, Rouet-Benzineb P, Reuter N, Laburthe M, 2003. Orexins and their receptors: structural aspects and role in peripheral tissues. Cell Mol Life Sci 60, 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Lawrence AJ, 2017. The Role of Orexins/Hypocretins in Alcohol Use and Abuse. Curr Top Behav Neurosci 33, 221–246. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA, 2009. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry 65, 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L, 2004. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci 24, 11439–11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JL, Wang QP, Tominaga M, Goto K, Shioda S, Sakurai T, 2002. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun 290, 1237–1245. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Tsunematsu T, 2010. New approaches for the study of orexin function. J Neuroendocrinol 22, 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ma H, Jia M, Li Y, Miao D, Cui C, Wu L, 2020. The role of the nucleus accumbens OXR1 in cocaine-induced locomotor sensitization. Behav Brain Res 379, 112365. [DOI] [PubMed] [Google Scholar]
- Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV, 2014. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh JW, James MH, Adams CD, Bains JS, Sakurai T, Aston-Jones G, Graham BA, Dayas CV, 2018. Activation of lateral hypothalamic group III metabotropic glutamate receptors suppresses cocaine-seeking following abstinence and normalizes drug-associated increases in excitatory drive to orexin/hypocretin cells. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE, 2006. Afferents to the orexin neurons of the rat brain. J Comp Neurol 494, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S, Wennerholm M, Shelton JE, Bonaventure P, Letavic MA, Shireman BT, Lovenberg TW, Dugovic C, 2017. Selective Inhibition of Orexin-2 Receptors Prevents Stress-Induced ACTH Release in Mice. Front Behav Neurosci 11, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ, 2008. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience 153, 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]


