Abstract
The adrenal cortex functions to produce steroid hormones necessary for life. To maintain its functional capacity throughout life, the adrenal cortex must be continually replenished and rapidly repaired following injury. Moreover, the adrenal responds to endocrine-mediated organismal needs, which are highly dynamic and necessitate a precise steroidogenic response. To meet these diverse needs, the adrenal employs multiple cell populations with stem cell function. Here, we discuss the literature on adrenocortical stem cells using hematopoietic stem cells as a benchmark to examine the functional capacity of particular cell populations, including those located in the capsule and peripheral cortex. These populations are coordinately regulated by paracrine and endocrine signaling mechanisms, and display remarkable plasticity to adapt to different physiological and pathological conditions. Some populations also exhibit sex-specific activity, which contributes to highly divergent proliferation rates between sexes. Understanding mechanisms that govern adrenocortical renewal has broad implications for both regenerative medicine and cancer.
Keywords: Stem cell function, adrenal homeostasis, adrenocortical zonation, Wnt/β-catenin, Sonic hedgehog, sex-specific renewal
1. Introduction
The adult adrenal cortex is an essential endocrine organ that produces distinct steroid hormones in response to different physiological demands. In order to achieve a rapid and precise response, the adrenal cortex is organized into three histologically and functionally distinct zones, each controlled by unique hormone signals. The outer zona glomerulosa (zG) produces aldosterone, a mineralocorticoid that regulates sodium retention and intravascular volume, thereby controlling blood pressure. The intermediate zona fasciculata (zF) produces glucocorticoids, which play a key role in glucose metabolism and the immune response, and the innermost zona reticularis (zR) is responsible for the production and secretion of androgens. Since adrenal-derived steroid hormones are necessary for life, the adrenal cortex must be continually maintained throughout life and rapidly repaired following injury. Thus, like many tissues, the adrenal cortex relies upon adult stem cells for both homeostatic maintenance and repair.
The identification and characterization of adult stem cells in the adrenal cortex continues to be a major area of research with important clinical implications for both regenerative medicine and cancer. Among the human diseases that could be treated with a combination of genetic and stem cell-based therapies, there are several conditions of adrenal hypofunction that arise from varying etiologies. These include congenital adrenal hypoplasia1 (caused by mutations in genes including NR0B1, NR5A1, and WNT4), congenital adrenal hyperplasia2 (caused by mutations in genes including CYP21A2, HSD3B2, CYP11B1, POR, CYP17A1, and STAR), autoimmune-mediated destruction of the adrenal glands3, and atrophy from chronic exogenous glucocorticoid therapy4. Additionally, the presence of normal adult stem cells capable of long-term tissue renewal has significant implications for cancer, where deregulation of normal stem cell function is thought to contribute to both tumor initiation and progression5. Some cancers might originate in a normal stem cell with a high intrinsic capacity for self-renewal or in a non-stem cell that acquires an increased capacity for self-renewal following oncogene activation. Thus, understanding mechanisms of normal self-renewal in the adrenal cortex has broad implications for regenerative medicine, where tissue engineering strategies could be used to restore tissue function6,7, as well as cancer, where blocking self-renewal could be used to help eradicate tumor cells8,9.
Here, we aim to provide a comprehensive overview of the literature on adult stem cells in the adrenal cortex. As an overall framework, we first briefly discuss hematopoietic stem cells (HSCs) and other mammalian tissues where the defining characteristics of adult stem cells are well established. We then examine the evidence supporting different cell populations with stem cell function in the adrenal cortex. Many of the fundamental concepts about stem cells in the adrenal cortex originated from studies performed in the early-to-mid 1900s. With more recent advances in biological techniques, these early principles have been further refined at both the cellular and molecular level. We discuss adrenocortical stem cell populations, including the historical context for their discovery, as well as the paracrine and endocrine signaling mechanisms that differentially regulate these cell populations under different physiological conditions.
1.1. Defining adult stem cells
The definition and identification of adult stem cells in the adrenal cortex and other mammalian tissues is largely based on decades of pioneering research on HSCs. These classic studies led to the general paradigm that adult stem cells are a rare and quiescent cell population10–12. HSC division, although infrequent, is asymmetric13 and yields one new stem cell and one actively dividing daughter cell that progressively differentiates in a unidirectional manner to give rise to all blood cell types. Thus, HSCs are by definition long-term retained and multipotent, the latter of which has been functionally demonstrated using classic transplantation assays14,15.
This stem cell paradigm established in HSCs serves as the model by which stem cells in solid tissues have been studied. However, different solid tissues are confronted with varying demands to replace lost cells16. Tissues that provide a barrier function from the environment, which include the epidermis and intestinal tract epithelia, are subject to frequent damage and maintain relatively high rates of turnover (days to months). In contrast, many other tissues like the liver and prostate have a much lower physiological turnover rate (>6 months), but can acutely increase cell renewal in response to damage. Along the spectrum from high- to low-turnover tissues, the adrenal cortex more closely aligns with those of low rates that do not maintain barrier function or have the ability to directly discard cells externally. Consistent with these general characteristics of low-turnover tissues, the mouse adrenal cortex renews in an average of 6 months, albeit with a known sexual dimorphism17 that we will discuss in greater detail.
The application of the original HSC paradigm to solid tissues of varying renewal capacities has led to the development of divergent theories and terminologies, much of which remains debated18. Some tissues contain a dedicated population of so-called ‘professional’ stem cells that follow an HSC-like hierarchy (e.x. satellite cells of skeletal muscle) while others rely more heavily on facultative stem cells that can dedifferentiate following injury in order to support regeneration (e.x. cholangiocytes of the liver). The adrenal cortex employs divergent cell populations, including both professional and facultative stem cells, to establish and maintain homeostasis and to promote regeneration and repair following injury. This diverse strategy enables the adrenal cortex to rapidly respond to unique endocrine demands.
In this review, we define an adrenocortical ‘stem cell’ based on the classic HSC paradigm as cells that are long-term retained (i.e. rare and quiescent) and multipotent. However, we also adopt a recently proposed operational definition of ‘stem cell function’, which more broadly describes the ability of unique cell populations to replace lost cells through cell division16. This newly developed nomenclature encompasses ‘progenitors’, which are actively dividing cells with the capacity to differentiate, as well as more differentiated populations that retain high proliferative potential. Homeostatic maintenance and repair of the adrenal cortex requires coordination of both stem cells and cells with stem cell function. These varying cell populations display remarkable plasticity and are differentially activated in response to a range of factors, including developmental window, endocrine demand, and injury.
1.2. Adrenal anatomy, development, and zonation
The anatomy and development of the adrenal gland as well as functional zonation of the adrenal cortex has been extensively reviewed elsewhere19–23. Here, we simply introduce major concepts and their historical framework that will be informative as we further examine literature specific to adrenocortical stem cells. In addition, we highlight several molecular markers that are used to determine cell lineage and function in the adrenal, which are essential for evaluating the stem cell potential of any given cell population.
1.2.1. The adrenal gland is composed of two separate organs, the cortex and the medulla, that are supported by an outer mesenchymal capsule
The adrenal gland is comprised of two functionally distinct organs that derive from different embryonic origins. The medulla, which is centrally located, is derived from neural crest cells of the neuroectoderm lineage and primarily functions to synthesize catecholamines for the “fight-or-flight” response. The adrenal cortex, which is derived from intermediate mesoderm, surrounds the medulla and functions to produce steroid hormones. Finally, an outer mesenchymal capsule encases the entire gland. Consistent with a distinct origin and function, the adrenal cortex maintains its renewal capacity independent from the medulla. This was first observed experimentally as early as 1926 in transplantation assays performed in rats. Jaffee and Plavska cut the adrenal gland in half and transplanted it between the fascia and abdominal wall24. They observed that the medulla degenerated, but the adrenal cortex remained and increased in size. Further, the transplanted adrenal cortex was functional, as evidenced by a 6% mortality rate compared to the 40% observed with complete adrenalectomy. These findings were corroborated by other transplantation approaches25,26 and further substantiated by physiological testing for epinephrine activity, which confirmed the lack of medullary function following transplantation27. Together, these early studies made the fundamental observation that regeneration of the adrenal cortex is uncoupled from the medulla, which has significantly impacted the way in which we study tissue renewal in the adrenal gland.
1.2.2. The adrenal gland and gonad share a common origin marked by expression of Steroidogenic factor 1 (SF1)
The basic anatomy of the adrenal gland, with inner medullary cells surrounded by the cortex and encased by the outer mesenchymal capsule, is established early in embryonic development. The adrenal anlage is derived from the adreno-gonadal primordium (AGP), which is a common precursor to both the adrenal and the gonad28. The AGP forms from cells of the coelomic epithelium and is marked by expression of Steroidogenic factor 1 (SF1, encoded by Nr5a1). The adrenal primordium then separates from the AGP and tissue-specific enhancer activity independently maintains SF1 expression in each tissue29,30. Following AGP separation, the adrenal primordium is invaded by neural crest cells31 that further differentiate into chromaffin cells of the medulla in the presence of glucocorticoids32. Mesenchymal cells then coalescence and encapsulate the gland, and the cortex proceeds with functional differentiation and zonation.
The developing adrenal cortex is initially comprised of fetal cells, which give rise to the cells that will persist into adulthood. These adult cortical cells were originally termed the ‘true’ cortex33, later renamed the ‘permanent’ cortex34, and finally called the ‘definitive’ cortex, which is the terminology now widely adopted. Although the origin and potential relationship between the fetal and definitive cortex was debated for decades, lineage tracing studies in mice have demonstrated that the definitive cortex arises from the fetal cortex35. However, this ability of fetal cells to generate adult definitive cortex is lost by E14.5. Thus, the fetal adrenal cortex acts as a precursor for the definitive cortex during a defined developmental window.
During the conversion from fetal to definitive cortex, the Sf1-fetal adrenal enhancer (FAdE) that drives Sf1 expression in early adrenal development is shut off. Sf1 expression is however maintained in the adult cortex by a proposed, but yet to be identified, definitive adrenal enhancer (DAdE)36. Combined with the fact that SF1 is necessary for adrenal development37, the continued expression of SF1 throughout the fetal and definitive cortex has made SF1 one of the most widely used molecular markers of the cortical lineage. Steroidogenic cells of the cortex also notably express cholesterol side-chain cleavage enzyme (P450SCC or CYP11A1) and 3β-hydroxysteroid dehydrogenase (3βHSD), which are also used as lineage markers to evaluate the differentiation potential of proposed stem cell populations.
1.2.3. The adrenal cortex is organized into three histologically and functionally distinct zones
Within the adrenal cortex, cells are organized into three concentric zones that were famously first described by Julius Arnold in 186638. Largely derived from his observations of human and bovine adrenals, Arnold described three subdivisions or zones within the cortex that he called the glomerulosa, fasciculata, and reticularis. These terms reflect the unique organization of cortical cells in each region, but as Arnold observed, this is truly shaped by the vasculature and connective tissue. Cells of the outer glomerulosa are organized into round clusters (Latin glomus, ball), cells of the intermediate fasciculata form radial pillars or cords (Latin fascicle, bundle), and cells of the innermost reticularis are spread across a mesh or net-like structure (Latin rete, net).
The terminology established by Arnold was initially morphological and topological. However, it eventually became clear that function largely follows morphology. Starting with the outermost zone, or zG, this region functions in response to angiotensin II (Ang II) as part of the renin-angiotensin-aldosterone system (RAAS) to produce mineralcorticoids39. Aldosterone, the main mineralocorticoid, regulates sodium balance, thereby controlling intravascular volume and blood pressure. Based on this function, one molecular marker widely used to identify cells of the glomerulosa is CYP11B2 (aldosterone synthase)40. While CYP11B2 is highly specific for cells of the zG that are actively producing aldosterone41, there are also non-aldosterone producing, CYP11B2− cells in the glomerulosa, particularly when dietary sodium levels are high and intravascular volume is replete42. This discrepancy highlights the challenge and ongoing debate over whether adrenocortical zonation is best defined histologically or functionally. Given these considerations, we regard CYP11B2 as a functional marker of the zG that identifies differentiated glomerulosa cells with the capacity to produce aldosterone. More broadly, disabled homolog 2 (DAB2) serves as a histological marker that identifies all SF1+ cortical cells of the zG43,44.
Directly adjacent to the zG, the zF functions in response to adrenocorticotropin hormone (ACTH) as part of the hypothalamic-pituitary-adrenal (HPA) axis to produce glucocorticoids. Cortisol is the main glucocorticoid produced in humans, but many rodent species instead synthesize corticosterone due to a lack of Cyp17a1 expression in the adrenal after fetal development45. Despite these species differences in steroid production, the zF is molecularly identified by the expression of CYP11B140 (11β-hydroxylase) or aldose reductase (AKR1B) proteins46,47.
The innermost zone, or zR, functions to produce androgens, particularly dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S)48. DHEA acts as a weak androgen and estrogen receptor agonist, but can be converted peripherally into more potent steroids, including testosterone and estradiol. DHEA is produced from pregnenolone through a multistep conversion process that requires both the 17α-hydroxylase and 17,20-lyase enzyme activity of CYP17A149. Importantly, the 17,20-lyase activity of CYP17A1 is significantly enhanced by the cofactor cytochrome b5 (CYB5A). Thus, while CYP17A1 is expressed and functions throughout both the zF and zR, CYB5A is expressed at much higher levels in zR and therefore helps to zonally restrict androgen production.
The zG and zF are highly conserved between rodents and humans, but rodents are widely thought to lack a functional zR due to the absence of adrenal Cyp17a1. The notable exception to this rule has been the spiny mouse, which expresses CYP17A1 and produces both cortisol and DHEA50. However, Cyp17a1 is transiently expressed in mice during fetal adrenal development between E12.5 and E16.545. This has been observed by both in situ hybridization and qPCR, and functionally validated in fetal adrenal lysates based on pregnenolone 17α-hydroxylase activity. Further, these results are consistent with the observed peak in fetal cortisol production in late gestation in rats51. Cyp17a1 however becomes undetectable in the adrenal after fetal development, despite its continue gonadal expression52,53. This is mediated by tissue-specific methylation-induced silencing, which can be reversed with either methyltransferase or histone deacetylase inhibitors54. However, newly emerging data suggests that the epigenetic regulation of Cyp17a1 in the mouse adrenal cortex may be strain specific. Much of the previously published work was performed in C57BL/6 mice, which do not have detectable adrenal androgens by gas chromatography-tandem mass spectrometry (GC-MS/MS)55. Recently published studies in C.B-17 SCID mice (derived from the BALB/C strain) reported adrenal Cyp17a1 expression in adulthood, which correlated with unmethylated promoter DNA and detectable CYP17A1-derived steroids in the serum56. Moreover, adrenalectomy significantly reduced tumor growth beyond the effect of castration alone in two patient-derived xenograft (PDX) models of castration resistant prostate cancer (CRPC), suggesting that adrenal-derived androgens in this model were functional in a pathological setting. These results raise the possibility that in addition to the spiny mouse, other mouse strains may contain a functional adrenal reticularis. Additional follow-up studies are required to quantify the expression and function of CYP17A1 across a wider panel of mouse strains. However, as genetically tractable models of zR biology have been limited in the field57, this could have significant implications for the study of many human diseases where elevated adrenal-derived androgens are thought to play an important role, including premature adrenarche, CRPC, congenital adrenal hyperplasia (CAH), and polycystic ovary syndrome (PCOS)48.
While many of the widely studied mouse models of adrenal biology do not contain a functional zR, they do contain a transient inner zone called the ‘X zone’. Evelyn Howard first coined this term in 1927 to describe the inner region of the mouse adrenal cortex58. Prior studies59 had presumed this to be the reticularis described by Arnold, but Howard proposed that it might instead represent something special to the mouse since it was notably absent in sexually mature adult males58. Lineage tracing studies have since revealed that the X zone is derived from the fetal35 rather than the definitive60 adrenal cortex. The X zone is initially quite narrow and indistinct just after birth, but becomes considerably more evident by 3 weeks of age and can be identified by two molecular markers, 20αHSD61 (encoded by Akr1c18) and Pik3c2g62. The X zone regresses in males during puberty and in females either during pregnancy or spontaneously in aged nulliparous animals58. Despite this marked loss of the X zone, these cells are known to re-emerge in males following castration58,63,64, suggesting their renewal capacity can be activated by the hypothalamic-pituitary-gonadal (HPG) axis.
1.3. The ‘centripetal’ versus ‘zonal’ model of cortical renewal
Given the subdivision of the adrenal cortex into three histologically and functionally distinct zones, one of the central questions in the field has been how each of these zones is maintained and replenished throughout life. The first theory proposed was the ‘centripetal model’ or ‘escalator theory’, whereby adrenocortical cells originate in the outer capsule and migrate inward over time, ultimately dying at the medullary boundary. Gottschau proposed this model in 188365 and it gained further support from histological observations reported by Zwemer in 193666. Zwemer studied a range of species and employed a wide variety of experimental procedures to induce adrenal injury. This approach enabled Zwemer to directly observe both normal adrenal maintenance as well as how the gland responds to varying types of injury, and to identify shared mechanisms of cellular renewal. From these experiments, Zwemer described, “that the glomerular cells arise from indifferent connective tissue-like cells in the capsule. These capsular cells lose their long processes, become short ovals and take up lipoid droplets.”
This model championed by Zwemer in which the adrenal capsule is multipotent (i.e. able to self renew and give rise to the cortical lineage) was functionally supported by prior transplantation studies. Experiments first performed in rats clearly demonstrated that removal of the capsule was unfavorable for successful transplantation. In one approach, Wyman and Walker cut the adrenal in half and transplanted it to the abdomen67. If the capsule was left intact, 97.4% of tissues regenerated, compared to just 12.8% when the capsule was removed. Similar results were later obtained using a different autotransplantation approach where adrenal glands from adult female rats were removed and grafted to the ovaries68. Following an initial period of atrophy, transplanted whole adrenals regenerated to their original cortical size within ~6 weeks. Strikingly, if the adrenal was first ‘enucleated’ to remove all tissue except the capsule, grafts still regenerated to the same extent. However, if the inner portion of the gland was engrafted without the capsule, all animals died from adrenal insufficiency within 6 weeks. These transplantation studies strongly suggested that the mesenchymal capsule contains functional stem cell capacity.
In addition to providing functional evidence for a multipotent stem cell population in the capsule, these early transplantation studies also indirectly revealed that growth of the adrenal gland begins at the periphery and proceeds towards the center. This directional growth pattern was subsequently visualized more formally using trypan blue labeling69. Adrenal glands from rats injected with trypan blue showed initial uptake of the dye in the capsule. A band of blue-stained cells was then observed in the glomerulosa and progressively further inward towards the medulla at later stages. Together with the initial histological and transplantation studies, results from these primitive labeling experiments strongly backed the centripetal model, whereby cortical cells arise from the capsule and gradually migrate towards the medulla over time.
Despite mounting evidence for the centripetal model, a competing ‘zonal model’ began to take shape in the 1940s70. This new model emerged as it was first becoming evident that the distinct histological zones of the adrenal cortex had unique functions71. The zonal model was based in large part on work showing that while removal of the pituitary caused atrophy of the adrenal cortex, it was only the inner cortex that was affected. The glomerulosa remained unchanged or even increased in size72. Furthermore, carbohydrate metabolism was altered by hypophysectomy, but electrolyte balance remained normal. Conversely, alterations in sodium or potassium intake induced morphological and cytochemical changes in the glomerulosa, but left the fasciculata unaltered73. These apparent functional differences led to the new theory that each zone of the adrenal cortex was self-maintaining70,72. Skepticism over the centripetal model was further fueled by technical considerations regarding the initial transplantation approaches. Specifically, while enucleation highly enriched for capsular cells, it was noted that some glomerulosa cells certainly persisted and likely contributed to regeneration of the cortical tissue74. Attempts to reproduce results from the early enucleation studies further demonstrated that a few rows of cortical cells remained attached to the transplanted capsule, and that the majority of mitoses during regeneration occurred in the cortex just below the capsule. Moreover, while inner cortical cells were found arising from the glomerulosa, these follow-up studies did not observe glomerulosa cells stemming from the capsule, like what Zwemer had described. These new studies concluded that the glomerulosa rather than the capsule largely drove renewal, and that the cortical cells directly below the capsule acted as the “seed” for cortical growth.
The centripetal model and the zonal model were the predominate theories first proposed to explain cellular renewal in the adrenal cortex. Although originally presented as such, these two paradigms are not necessarily mutually exclusive and components of both models have proven to be true with further studies and more advanced experimental approaches. It is now clear that the centripetal model explains the primary growth and renewal pattern of the adrenal cortex under normal conditions. This was in part established by later tritiated thymidine labeling approaches, which determined that proliferation rates are highest at the periphery of the gland75–78 and that the label is displaced inward over time79. Further, lineage tracing studies have now conclusively demonstrated that adrenocortical cells arise in the outer portion of the gland and progressively move towards the medulla, adopting different functional states as they advance60,80. However, during this process of centripetal migration and differentiation, cells from both the capsule and the glomerulosa participate in cellular renewal. Moreover, the glomerulosa contributes to cortical turnover at significantly higher rates than the capsule, which is consistent with some aspects proposed by the zonal model. Finally, while the centripetal paradigm largely explains normal development and homeostasis, there are a number of perturbations that induce zone-specific changes in cellular renewal. Thus, both models as originally proposed are seemingly too simplistic to fully explain adrenocortical homeostasis and repair. The adrenal gland instead employs multiple cell populations with differing renewal capacity (Fig. 1). This enables the adrenal cortex to rapidly respond to endocrine-mediated organismal needs, which require steroidogenic output from individual zones, while maintaining long-term continual replenishment of cortical cells through paracrine-mediated centripetal migration. Such an integrated approach allows the gland to maintain high plasticity and adaptability to a wide range of both physiological and pathological conditions.
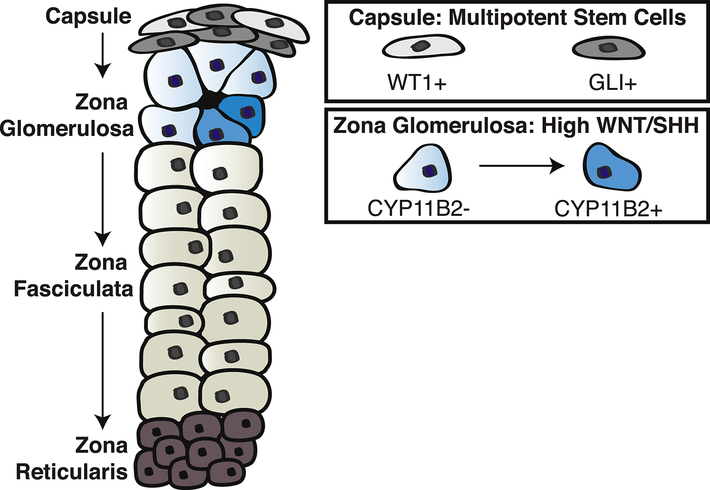
Figure 1. The adrenal cortex employs multiple cell populations with stem cell function to support cellular renewal.
The outer mesenchymal capsule contains distinct populations of multipotent stem cells, including GLI+ and WT1+ cells, which are capable of giving rise to steroidogenic cells. These populations contribute at relatively low rates to homeostatic adrenocortical renewal, but can be acutely activated in response to severe stress. The main driver of homeostatic centripetal renewal is the zona glomerulosa (zG), which is characterized by high Wnt pathway activation and the production of SHH ligands. The zG contains CYP11B2− cells as well as more differentiated CYP11B2+ cells, which can repopulate the inner cortex through transdifferentiation. The ability of the adrenal cortex to maintain cellular renewal through a diverse set of mechanisms enables the tissue to adapt to a wide range of physiological and pathological conditions.
2. Multipotent stem cells in the mesenchymal capsule
Newly developed genetic lineage tracing tools have led to the identification and characterization of at least two bona fide stem cell populations in the mesenchymal capsule that are capable of giving rise to the underlying adrenal cortex: GLI1+ cells80 and WT1+ cells81. Although the contribution of each of these lineages to adult homeostatic maintenance of the cortex is low, these populations meet the original HSC-based criteria of an adult stem cell, including multipotency that requires the ability to both self-renew and give rise to steroidogenic cells. Thus, as originally implicated by Gottschau and Zwemer, the mesenchymal capsule serves as an adrenal stem cell niche.
2.1. GLI1+ cells
2.1.1. GLI1+ stem cells contribute to homeostatic maintenance of the cortex
The first direct evidence that a non-steroidogenic cell of the mesenchymal capsule could give rise to steroidogenic cells of the cortex came from King et al in 200980. These studies used a LacZ-based reporter allele to identify a population of Gli1+ cells within the inner capsule. Notably, some Gli1+ non-steroidogenic cells were also found sparsely distributed throughout the cortex. To test whether this novel population of Gli1+/SF1− cells could give rise to steroidogenic cells, the authors performed lineage tracing using a tamoxifen inducible Gli1:CreERT2 allele combined with the R26-X reporter. This approach permanently marks Gli1+ cells and their descendants with EGFP following the administration of tamoxifen. By administering tamoxifen at E14.5 and analyzing adrenal tissue thereafter, the authors first measured the contribution of the prenatal Gli1+ lineage to adrenal development. After 1 day, EGFP+ cells were present at the periphery of the gland and were largely SF1−. However, SF1+ clusters began to appear after 5 days and progressively extend into the cortex, as far as 20-cell diameters by 21 days. Most notably, at 4 months, EGFP+ cells were retained in the capsule and steroidogenic EGFP+ cell clusters extended the entire length of the cortex to the medullary boundary. These results provided strong evidence that embryonic Gli1+ cells are long-term retained and multipotent. To further extend these observations to the postnatal period, tamoxifen was administered at P23 and analysis was performed 3 weeks later. Consistent with prenatal lineage tracing, EGFP+ cells were detected in the capsule and steroidogenic EGFP+ cells were observed extending from the capsule into the outer layers of cortex. While 3 weeks was not sufficient to determine whether these cells were long-term retained, these results suggested that the Gli1 lineage is present and can contribute to cortical renewal postnatally.
Building on these initial observations, more recent studies have strengthened the evidence that the Gli1 lineage retains its stem cell capacity after birth. Using a similar lineage tracing approach, Grabek and colleagues17 administered tamoxifen at 3-, 6-, or 12 weeks of age and observed Gli1-derived steroidogenic cells 4 weeks later in all cases. Further, the GFP label was maintained in the capsule throughout an entire 6-month experimental period, indicating that Gli1+ capsular stem cells are long-term retained, even after birth. The most striking observation in these studies however was that the stem cell capacity of the Gli1+ lineage became sexually dimorphic after puberty. Gli1+ stem cells contributed to homeostatic maintenance of the adrenal cortex to a similar extent in both males and females until 3 weeks of age. Following puberty, Gli1-driven cortical renewal was maintained throughout adulthood in females, but rarely observed in males. This sex-specific stem cell activity will be discussed in greater detail later in this review (section 4.1).
2.1.2. GLI1+ stem cells are acutely stimulated during regenerative repair of the cortex
These prior pre- and postnatal lineage tracing studies clearly demonstrated that Gli1+ cells are long-term retained, multipotent stem cells. However, the contribution of the Gli1 lineage to adrenocortical renewal is relatively low, particularly during adult homeostasis. Quantification methods vary across studies and are limited by the efficiency of tamoxifen-induced recombination, but we estimate that roughly 1–2% of the adult male cortex is derived from Gli1+ cells17,80,82, which is ~16-fold higher in adult females17. Despite this low level of homeostatic activity, Gli1+ cells are acutely activated to regenerate the adrenal cortex following glucocorticoid-induced atrophy82. This capacity of Gli1+ stem cells to increase their renewal capacity has important clinical implications given the prevalence of high-dose glucocorticoid therapy used to treat inflammatory, allergic, and immunological disorders in humans83. Chronic glucocorticoid therapy suppresses the HPA axis through a classic endocrine negative feedback loop that inhibits ACTH. This can lead to secondary adrenal insufficiency, but the HPA axis typically recovers following the cessation of pharmacological glucocorticoids84. However, the timing of recovery is variable4 and requires regeneration of the zF, which is responsible for endogenous glucocorticoid production and is known to undergo significant atrophy in the absence of ACTH.
To model this clinical challenge in the laboratory, dexamethasone can be administered to mice60,82. This potent synthetic glucocorticoid inhibits the HPA axis and leads to a significant loss of fasciculata cells60,82 through apoptotic cell death82. Following the withdrawal of dexamethasone, the adrenal cortex progressively regenerates over the course of several weeks. This regeneration is in part driven by an acute activation of capsular Gli1+ stem cells. Lineage tracing performed in our laboratory in adult male mice showed an ~7-fold increase in SF1+ cortical cells derived from the Gli1 lineage at the peak of regeneration82. These Gli1-descendents helped repopulate steroidogenic cells of the cortex, but were not retained after 3 months. Thus, activation of Gli1+ stem cells during glucocorticoid-induced regeneration is a transient and acute response, likely driven at least in part by rising ACTH levels. However, since the primary site of action for ACTH is the zF, stimulation of capsular Gli+ stem cells during this process necessitates cortical-to-capsular signaling, which we will discuss further in a later segment of this review (section 3.1). This regeneration paradigm is one example of how adrenocortical stem cells are hormonally regulated.
2.2. WT1+ cells
2.2.1. WT1+ stem cells contribute at low levels to homeostatic maintenance of the cortex
In addition to Gli1+ cells, the adrenal capsule also contains a population of cells that express Wilms tumor suppressor gene 1 (WT1). WT1 is a transcriptional regulator that is first expressed very early in the developing AGP81,85,86 and is required for its formation87,88. Consistent with WT1 expression marking the earliest step of adrenal formation, lineage tracing using a constitutive Wt1:Cre driver and the R26RmTmG reporter labeled the entire adrenal cortex81. The R26RmTmG allele is designed such that Cre-induced recombination activates expression of membrane-targeted GFP (mG)89. When combined with Wt1:Cre, this approach irreversibly marks all WT1+ cells and their descendants81. Thus, the fully GFP+ adrenal cortex observed in Wt1:Cre;R26RmTmG mice demonstrates that all adrenocortical cells derive from a cell that once expressed WT1.
Despite being expressed very early in development, WT1 is repressed in the adrenal primordium soon after AGP separation81,86 and is not expressed in adrenocortical cells thereafter. WT1 silencing occurs upon activation of SF1 and is critical for subsequent steroidogenic differentiation81. However, following AGP separation, WT1 is expressed in a high proportion of mesenchymal cells that ultimately encapsulate the gland. This was experimentally demonstrated using a tamoxifen inducible Wt1:CreERT2 driver combined with the R26RmTmG reporter, which labeled a portion of the capsule following the administration of tamoxifen at E11.5 and E12.581. To evaluate the potential overlap between WT1-expressing cells and the previously described Gli1+ cell population, Wt1:CreERT2;R26RmTmG;Gli1:LacZ embryos were analyzed at E18.5 following the administration of tamoxifen at E12.5. This experiment showed only partial overlap, with WT1+ cells primarily at the periphery and Gli1+ cells in the innermost capsular layers.
To trace the fate of the WT1 lineage, Wt1:CreERT2;R26RmTmG mice were analyzed following the administration of tamoxifen at different embryonic, postnatal, and adult time points81. Tamoxifen injection at E12.5 or E14.5 resulted in a large number of GFP+ cells within the capsule as well as a small number of GFP+/SF1+/WT− cells in the cortex at E18.5. These labeled WT1-derived cortical cells extended centripetally over time and by 7 months, GFP+ cords reached from the capsule to the medullary boundary. Notably, the GFP label was retained in the capsule at 7 months and cortical GFP+ cells expressed multiple steroidogenic markers, including SF1, AKR1B7, and 3βHSD2. Thus, these results demonstrate that embryonic WT1+ capsular cells are long-term retained and multipotent. The same labeling approach was used at 3- or 10–12 weeks of age to test whether this stem cell activity is retained postnatally. Analysis 10 days, 1 month, and 7 months later demonstrated that WT1+ capsular cells do maintain stem cell capacity into adulthood, but WT1-descendants comprise only a small proportion of the overall cortex.
2.2.2. WT1+ stem cells maintain gonadal potential that can be stimulated by gonadectomy
Although WT1+ cells are not a major source of steroidogenic cells in the adrenal, they can be acutely activated by gonadectomy (GDX). Previously, GDX has been shown to increase gonadotropins90, which results in an accumulation of spindle-shaped “A cells” beneath the capsule91–94. A proportion of these cells then differentiate into gonadal-like cells, or “B cells”, that produce sex hormones95. Together with the fact that WT1 is initially expressed in the AGP prior to separation of the adrenal and gonadal primordia, these observations led to the hypothesis that capsular WT1+ cells may possess gonadal potential that can be stimulated upon GDX. To test this hypothesis, GDX was performed in adult Wt1:CreERT2;R26mTmG mice just after tamoxifen injection81. Analysis 10 weeks later showed a significant expansion of GFP+ cells. Further, the majority of GFP+ cells had lost expression of WT1 and instead expressed SF1, GATA4, CYP17, and Lhr, which collectively mark gonadal steroidogenic fate. These results suggest that capsular WT1+ cells can adopt a gonadal differentiation fate in response to elevated gonadotropins. Thus, WT1+ cells seemingly represent a reserve stem cell pool with high cellular plasticity that can respond to extreme hormonal stress.
2.3. Summary and perspectives on capsular stem cells
The mesenchymal capsule was first proposed as a niche for multipotent stem cells in the adrenal gland more than a century ago. With advances in molecular biology and new genetic tools, two capsular stem cell populations have been identified and characterized – GLI1+ cells and WT1+ cells. These cell populations, which are largely distinct from one another, both contribute to pre- and postnatal adrenal development as well as adult homeostasis. The proportion however of adrenal cortical cells derived from each of these populations is typically low, especially for the WT1 lineage. Despite these low levels of normal renewal, both of these cell populations are uniquely activated in response to severe endocrine stress. GLI1+ cells respond to adrenal atrophy induced by excess glucocorticoid therapy and rising ACTH levels, while WT1+ cells respond to gonadectomy and increased gonadotropin levels. The observed histological and functional differences between these two cell populations suggests that the mesenchymal capsule is comprised of heterogeneous cell populations. With the recent emergence of single-cell RNA sequencing (scRNAseq), we expect that this observed heterogeneity will become even more granular at the transcriptional level and may reveal additional capsular cell populations with stem cell capacity.
3. Stem cell function within the zona glomerulosa
Given that the capsule typically contributes at relatively low levels to cortical renewal, other cell populations are necessary to augment continual replenishment of the cortex. In particular, the glomerulosa contains a high capacity for cellular renewal that is critical for both homeostatic maintenance and repair. Lineage tracing studies have provided clear evidence that the zG gives rise to the zF, but these cells are all within the steroidogenic lineage. Therefore, based on the original HSC criteria, the zG is not multipotent and does not meet the classical criteria of a true bona fide stem cell population. However, much like early proponents of the zonal model postulated, the zG has very high potential to replace lost cells through cell division and thus can be ascribed to have stem cell function. The zG if often referred to as a singular region owing to its sole primary function to produce aldosterone, yet within the zG, there are multiple cell populations capable of sustaining cortical renewal.
3.1. SHH+ cells
One of the first molecular pathways associated with zG stem cell function was Sonic Hedgehog (SHH) signaling. SHH is a secreted molecule that is required for the growth and patterning of a wide variety of tissues96. SHH and its other mammalian orthologs, Indian Hedgehog (IHH) and Desert Hedgehog (DHH), act by binding to the transmembrane receptor Patched1 (PTCH1). In the absence of ligand, PTCH1 inhibits the activity of a transmembrane protein called Smoothened (SMO). Ligand binding inactivates PTCH1 and subsequently derepresses SMO, which leads to intracellular signal transduction and the ultimate activation of GLI transcription factors (Fig. 2). Mutations in SHH and other pathway components were identified in human patients with holoprosencephaly and Pallister-Hall syndrome, who often have endocrine disorders, including adrenal hypoplasia97–100. This association in human patients first suggested that SHH signaling might play an important role in adrenal development and homeostasis.
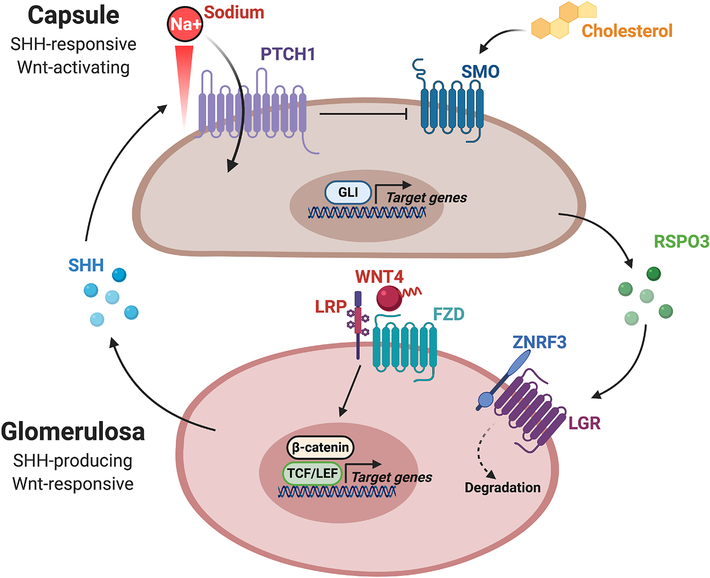
Figure 2. Reciprocal signaling between the capsule and cortex coordinately regulates SHH and Wnt pathway activation.
Schematic representation of the crosstalk between the mesenchymal capsule and underlying adrenal cortex that is required for SHH and Wnt activity. (Top) Adrenocortical cells in the glomerulosa produce SHH ligands, while capsular cells express the central components of the SHH signaling response, PTCH1 and SMO. In the absence of ligand, PTCH1 inhibits the activity of SMO. SHH ligand binding inactivates PTCH1 and subsequently derepresses SMO, which leads to intracellular signal transduction and the activation of GLI transcription factors. Additionally, PTCH1 and SMO are respectively dependent upon the transmembrane flux of sodium ions and cholesterol availability. (Bottom) Capsular-to-cortical signaling regulates Wnt pathway activation. RSPO3 produced by the capsule helps facilitate degradation of ZNRF3 in underlying adrenal cortex. ZNRF3 is a transmembrane E3 ubiquitin ligase that acts to promote the turnover of Wnt receptor complexes. Thus, RSPO3-mediated degradation of ZNRF3 potentiates Wnt pathway activation by increasing the availability of Wnt receptors on the cell surface. Schematic created with BioRender.com.
3.1.1. SHH+ cells in the outer cortex are important for adrenal development and centripetal renewal
Following the initial observations in humans implicating the SHH pathway in adrenal growth, in situ hybridization studies demonstrated strong expression of Shh mRNA in the peripheral cortex at E14.5101. These results were later confirmed and refined based on a wider analysis of developmental time points, which revealed that Shh is expressed as early as E12.5 and remains confined to the outer cortex after birth80,102. To test the functional importance of SHH signaling in the adrenal, three independent groups generated conditional knockout (cKO) mouse models80,102,103 using the cortical-specific driver, SF1-Cre104. While there are differences in the exact time- and endpoints analyzed, a common phenotype emerged from these studies. The loss of Shh resulted in significantly reduced adrenal mass, marked by thinning of both the capsule and cortex. Notably, all three models displayed an asymmetry, with the defect being more severe in the right as compared to the left adrenal. Histologically, normal zonation was maintained, yet there were fewer cells in both the zG and zF. Despite this reduction in cell number, some differentiated zG cells expressing CYP11B2 were maintained80,103 and normal serum aldosterone levels were observed when measured102. However, the zF appeared to be more severely affected with the vast majority of cells displaying cellular hypertrophy characteristic of elevated ACTH. Consistent with this observation, plasma ACTH levels were increased, but no changes in corticosterone were detected. This phenotype could be secondary to defects in the HPA axis since SF1-Cre is active in the pituitary (albeit not in ACTH-producing corticotropes104,105). However, a similar phenotype was observed in late embryonic development when pituitary function is not required for adrenocortical development106–108. Taken together, these results suggest that loss of Shh in the glomerulosa reduces the number of cortical cells in both the zG and zF. This reduction has a particularly high impact on glucocorticoid-producing cells within the fasciculata, leading to a compensatory rise in ACTH and subsequent cellular hypertrophy.
The baseline phenotype of Shh cKO mice, with loss of Shh in the zG manifesting in large part as a functional loss of the underlying zF, is consistent with the centripetal model of homeostatic maintenance. These results support a model in which Shh-expressing cells in the glomerulosa significantly contribute to the generation of inner cortex. This was directly demonstrated by lineage tracing studies80. A tamoxifen-inducible Shh:CreERT2 allele was combined with the R26-X or the R26-YFP reporter. Following tamoxifen injection at E14.5, EGFP+/SCC+ cells were observed in the periphery of the gland after 5 days. These small cell clusters extended into longer columns that reached towards the medulla by 28 days. These results demonstrated that during embryonic development, Shh-expressing cells give rise to adrenocortical cells of both the glomerulosa and fasciculata. To extend these observations to the postnatal period, tamoxifen was administered to adult male mice and analysis was performed 1- and 2-weeks later. After 1 week, ~45% of YFP+ cells expressed CYP11B2, ~5% expressed CYP11B1, and ~50% were negative for both markers. These proportions significantly changed after 2 weeks, with nearly 25% of YFP+ cells expressing CYP11B1. Thus, these results revealed that Shh-expressing cells give rise to differentiated CYP11B2+ cells in the glomerulosa and CYP11B1+ cells in the fasciculata. This progenitor capacity of Shh-expressing cells is observed in both embryonic and adult stages, but additional studies are needed to determine whether these cells are long-term retained.
3.1.2. Cortical-to-capsular SHH signaling supports homeostatic cortical renewal
The phenotype of Shh cKO mice combined with lineage tracing analysis presents a model in which Shh-expressing cells in the glomerulosa normally contribute at significant levels to the generation of inner cortex. Upon loss of Shh, this renewal capacity is reduced and leads to cortical thinning characterized by loss of differentiated zG cells, but an even more profound effect on the zF that manifests functionally. This model is consistent at the cellular level, but creates a significant gap at the molecular level that necessitates an intermediate role for the capsule. As a ligand, SHH acts by binding to the receptor PTCH1 and inhibiting its activity. In the adrenal gland, Ptch1 expression is restricted to the mesenchymal capsule, which has been visualized by in situ hybridization80 and Ptch1-LacZ103,109 reporter analysis. Thus, it would follow that SHH secreted by the zG must bind to PTCH1 in the capsule in order to activate downstream signaling (Fig. 2). Consistent with this paradigm, expression of Gli1, a transcriptional target and mediator of SHH signaling, is also restricted to the capsule80,103,109. Further, inactivation of SMO within the steroidogenic lineage had no effect on adrenocortical development80. These genetic experiments further demonstrate that the mesenchymal capsule rather than steroidogenic cells of the cortex transduces the SHH signal.
Collectively, studies on SHH have established a key signaling link between the cortex and capsule that functions to sustain centripetal renewal of the inner cortex. However, this raises the next big question of how SHH pathway activation in the capsule stimulates renewal of the underlying cortex. One likely mechanism is through direct maintenance of the Gli1+ lineage itself. As we previously discussed, lineage tracing studies have demonstrated that Gli1+ cells in the capsule give rise to steroidogenic cells of the cortex during embryonic and postnatal development as well as adult homeostasis. This may partially explain the phenotype of Shh cKO mice – that SHH signaling from the cortex is important for the maintenance of Gli1+ capsular stem cells. Consistent with this idea, the capsule of Shh cKO mice had significantly lower levels of proliferation103 and was thinner than control mice80,103. However, while maintenance of the Gli1+ stem cell pool is likely an important factor, there is an apparent discrepancy between the severity of the Shh cKO phenotype and the relatively low levels of Gli1-driven cortical renewal observed under normal conditions. These observations suggest that SHH-responsive cells in the capsule act through additional mechanisms to facilitate cortical renewal.
3.1.3. SHH-responsive cells initiate reciprocal capsule-to-cortical signaling through the Wnt pathway
In addition to a demonstrated contribution to cellular lineage, GLI1 also participates in cellular signaling. Gli1 is a transcriptional target of active hedgehog (HH) signaling that functions in a feed-forward loop to amplify the pathway. There are also two additional GLI family members, GLI2 and GLI3, which act to regulate HH-dependent transcription and are expressed in the mouse, rat, and human adrenal80,103,110. In contrast to Gli1, these additional family members are primarily regulated post-transcriptionally and are processed to a truncated form that favors repression when the pathway is off96. Upon HH activation, full-length GLI2 and GLI3 are stabilized and act alongside GLI1 to drive target gene expression. GLI family members are known to stimulate a wide range of target genes in many tissues that promote cell survival, proliferation, and tissue patterning.
In the adrenal capsule, the direct targets of each GLI family member have yet to be elucidated. ChIP-sequencing (ChIP-seq) would be a valuable approach to define the transcriptional program regulated by each family member and particularly to identify secreted factors that could potentially signal between the capsule and the cortex to promote cortical cell renewal. While ChIP-seq has not been performed, a complementary genetic approach has been used to constitutively activate SMO in capsular cells and analyze downstream HH-dependent transcriptional changes82. SmoM2 mice, which harbor an activating W539L point mutation111, were combined with the Gli1:CreERT2 driver in order to induce and restrict constitutive HH pathway activation to the capsule. Following 3 weeks of tamoxifen chow, male mice were analyzed at 6 weeks of age. The full transcriptome was not analyzed, but a qPCR array was used to assess differential gene expression in particular signaling pathways. These studies revealed a significant increase in Wnt pathway activation marked by more than a 2-fold increase in Ctnnb1 and Wnt4, and a significant decrease in the Wnt inhibitors, Sfrp4 and Wif1.
The Wnt pathway is a well established mechanism that facilitates paracrine signaling between cells in close proximity112. Further, previous studies have demonstrated that Wnt activity in the adrenal gland is highest in the peripheral cortex113–116 and that cortical-specific loss of the central signaling molecule, β-catenin, results in progressive cortical decline over time113. Taken together, these studies suggest that HH activation in the capsule likely promotes Wnt signaling in the underlying adrenal cortex to facilitate renewal. Consistent with this paracrine-signaling mechanism, SmoM2 mice displayed enhanced β-catenin staining in the outer cortex and increased proliferation82. Overall, these studies present a dynamic and reciprocal signaling paradigm between the cortex and capsule that functions to promote cortical renewal through the coordination of SHH and Wnt signaling (Fig. 2). This SHH-Wnt relay is also critical during adrenocortical regeneration to acutely increase the rate of cellular renewal, which we will further discuss (section 3.1.6).
3.1.4. SHH-responsive cells may initiate reciprocal capsule-to-cortical signaling through additional pathways
In addition to the Wnt pathway, other reciprocal signaling mechanisms may facilitate HH-dependent crosstalk between the capsule and cortex. Other candidates include the fibroblast growth factor (FGF) and transforming growth factor-β (TGF-β) pathways, which both signal in a paracrine fashion and are known to interact with HH signaling in other tissues. The FGF signaling pathway is comprised of 22 secreted ligands that can activate four FGF receptors (FGFRs)117. Additionally, FGFR1–3 can be alternatively spliced into multiple isoforms. This level of complexity allows for an extremely high number of potential ligand-receptor interactions.
Previous studies surveyed expression of FGF signaling components using laser capture microdissection coupled with RT-PCR and identified just three ligands expressed in the adrenal gland. Fgf2 and Fgf9 were detected in the capsule and Fgf1 was identified in the cortex118. If FGF signaling functions in part to transduce HH activation from the capsule to the cortex, Fgf2 and Fgf9 would be predicted to be downstream of SMO activation, which has not been assessed. However, FGFRs in the underlying cortex would also be expected to play an essential role in binding secreted FGF ligands to initiate downstream signaling, and the function of FGFRs in cortical renewal has been partially investigated. All four FGFRs have been detected in the cortex118,119, but functional studies have focused on FGFR1 and FGFR2. Inactivation of FGFR1 had no effect on adrenocortical growth120. However, multiple FGFR2 loss-of-function (LOF) models, including germline loss of Fgfr2-IIIb118,121 and conditional deletion of Fgfr2 using different Cre drivers with adrenocortical activity120,122,123 have consistently demonstrated a severe phenotype marked by reduced adrenal size. Particular aspects of the Fgfr2 LOF phenotype are similar to that observed with loss of Shh, including a thinner cortex and reduced cortical cell proliferation. However, loss of Fgfr2 also increased apoptosis within the cortex120, which was not observed with Shh LOF102,103. These results indicate that FGF signaling is critical for maintaining cortical cell growth and proliferation, but it is unclear whether this pathway is directly downstream of capsular HH activation or perhaps acting in parallel.
The TGF-β superfamily is comprised of a large number of structurally related proteins including TGF-β proteins, bone morphogenic proteins (BMPs), activins, inhibins, and growth differentiation factors (GDFs), among others124. These signaling molecules act through SMAD proteins, at least in part, and collectively regulate a diverse range of developmental and physiological processes. While studies on the TGF-β subfamily are sparse in the adrenal cortex, work in our laboratory defined a unique role of TGF-β2 in the gonadal-specification of adrenal cells under high luteinizing hormone (LH)125. Adrenal inhibin serves to specifically antagonize TGF-β2 and hence prevent LH-dependent, GATA4-driven gonadal differentiation (theca and granulosa lineage) of GATA6-specified adrenocortical cells. In addition to highlighting the shared AGP origin of the gonad and adrenal cortex, these studies were some of the first to demonstrate the high cellular plasticity of adrenocortical cells that is coordinated by paracrine and endocrine signaling mechanisms. Using an elegant in vivo system to allow for graded Tgf-β1 expression in mice, a separate study demonstrated that increasing levels of Tgf-β1 repressed zG differentation (Cyp11b2 and aldosterone production)126. Together, these studies suggest that TGF-β family members may serve to regulate an undifferentiated, progenitor-like state in the adrenal cortex. However, any direct signaling link between TGF-β1 or TGF-β2 and HH production in the cortex or signaling activation in the capsule remains to be examined.
3.1.5. Endocrine factors that additionally regulate HH pathway activation
In addition to potential crosstalk with other paracrine signaling pathways, HH activity has recently been shown to be highly sensitive to sodium ion flux and cholesterol metabolism – two major players in the endocrine function of the adrenal gland. This work is primary based on efforts to understand the basic molecular mechanism that mediates communication between PTCH1 and SMO, which has been an outstanding question in the HH field for decades. While early models postulated that PTCH1 and SMO form a heteromeric complex that either stabilizes or dissociates upon ligand binding, subsequent work demonstrated that PTCH1 and SMO do not directly interact127. Further, sub-stoichiometric levels of PTCH1 were sufficient to suppress SMO, which strongly suggested that a secondary messenger regulates PTCH1 catalytic activity.
More recent work has provided new insights on the mechanism of signal transmission between these two spatially separated receptors. Surprisingly, this has revealed that SMO activity is cholesterol-dependent and the ability of SMO to bind cholesterol relies on PTCH1 activity, which is an ion transporter driven by sodium ion flux (Fig. 2). This model progressively emerged from pharmacological, genetic, and structural studies that made several key observations, including; 1) depletion of cellular cholesterol blocks SMO activation independent of HH biogenesis or PTCH1128,129, 2) increasing cholesterol is sufficient for SMO activation130,131, 3) SMO contains two sterol-binding sites, one located in the extracellular cysteine-rich domain (CRD)132–134 and the other deep within a seven-transmembrane (7TM) pocket135, 4) CRD mutations do not alter sensitivity to PTCH1 activity or changes in cholesterol130,132,136, and 5) PTCH1 is an ion transporter powered by the extracellular sodium ion gradient130 that inhibits SMO by altering accessibility to its ligand (i.e. cholesterol)137. Notably, when extracellular sodium is depleted, PTCH1 loses its ability to repress SMO130. Taken together, these observations support a current working model that cholesterol binding within the 7TM site is critical for SMO activation. PTCH1 regulates SMO by controlling access to this key ligand-binding site and PTCH1 activity itself is dependent on HH ligands as well as the flux of sodium ions across the membrane (Fig. 2). Most studies in the adrenal cortex to date have primarily focused on paracrine regulation of HH signaling. However, the ability of sodium ions and cholesterol biosynthesis to respectively control the activity of PTCH1 and SMO highlights the need for further investigation in the adrenal to understand how these endocrine factors interplay with paracrine signaling to mediate cortical renewal.
3.1.6. SHH signaling supports adrenocortical repair following injury
In addition to SHH signaling playing an important role in adrenocortical homeostasis, it also functions to accelerate regeneration following glucocorticoid-induced atrophy. In the previously described regeneration model, whereby dexamethasone is administered to mice to suppress the HPA axis and ablate the zF, our lab found that Shh was among the significantly up regulated genes following dexamethasone withdrawal82. Further, the size of the Shh expression domain within the peripheral cortex was expanded based on a Shh-LacZ reporter, suggesting that SHH signaling might participate in recovery of the cortex. To measure the contribution of the Shh lineage to cortical regeneration, we combined Shh:CreERT2 mice with the R26REGFP reporter and administered tamoxifen during the final two days of dexamethasone. This approach permanently marked Shh-expressing cells and their descendants with EGFP and showed that a significantly higher number of Shh-derived EGFP+ cortical cells were present throughout the recovery period compared to baseline (i.e. no dexamethasone). Pharmacologic inhibition of SHH signaling impaired zF regeneration and conversely, constitutive activation of HH signaling in the Gli1:CreERT2-SmoM2 model accelerated recovery. Taken together, these experiments demonstrated that SHH signaling functionally contributes to adrenocortical regeneration.
Given that Gli1:CreERT2-SmoM2 mice activate HH signaling specifically in the capsule, the enhanced recovery from dexamethasone observed in the SmoM2 model suggested that capsular-cortical signaling mechanisms likely facilitate regeneration, similar to the homeostatic paradigm. Consistent with this hypothesis, Wnt signaling was activated in the cortex early in regeneration82. This was evident based on gene expression analysis, a Wnt-GFP reporter, and lineage tracing studies. Further, genetic ablation of β-catenin significantly impaired regrowth of the zF. These studies further support a critical signaling paradigm between the cortex and capsule that is coordinated by HH and Wnt signaling.
3.1.7. Summary on the role of SHH signaling in adrenocortical homeostasis and repair
Taken together, studies on SHH signaling in the adrenal gland have clearly established an important role for this pathway in supporting renewal of the adrenal cortex during both homeostatic maintenance and regeneration. Collectively, these studies suggest a model (Fig. 2) in which SHH-producing cells in the zG signal to the overlying capsule where PTCH1 is expressed. SHH binding represses PTCH1 activity, which importantly is also modulated by the transmembrane flux of sodium ions. Inhibition of PTCH1 subsequently de-represses SMO and leads to activation of the GLI family of transcription factors. Notably, SMO activity is dependent on cholesterol availability. Once activated, GLI transcription factors stimulate a wide range of target genes that promote cell survival and proliferation, although the exact identity of this gene expression program is largely unexplored. At least some of these target genes are thought to support the Gli1-capsular stem cell lineage, which helps generate new cortical cells, albeit at a relatively low rate during homeostatic conditions. However, in addition to this cell autonomous effect, the activation of GLI transcription factors also up regulates a number of secreted factors that can signal to the underlying cortex to stimulate proliferation. To date, Wnt activation in the peripheral cortex is the major mechanism known to be stimulated by capsular HH activity. This reciprocal signaling paradigm between the cortex and the capsule supports both homeostatic maintenance and regeneration of the adrenal cortex.
3.2. Wnt-responsive cells
The Wnt pathway is critical for proper development and homeostasis in many tissues112, including the adrenal cortex. Signaling is initiated by a family of 19 secreted glycoproteins, which bind to receptor complexes on the surface of cells in close proximity138. Activation of these receptor complexes triggers an intracellular response that acts through a β-catenin-dependent (i.e. canonical or Wnt/β-catenin) or β-catenin-independent (i.e. non-canonical) mechanism. Here, we will focus our discussion on Wnt/β-catenin signaling since functional studies testing the potential role of non-canonical Wnt signaling in the adrenal cortex have yet to be published.
β-catenin is the central signaling molecule in the canonical Wnt pathway112. Briefly, in the absence of Wnt ligands, β-catenin is targeted for degradation by a complex of proteins. Upon ligand activation, β-catenin is stabilized and translocates to the nucleus, where it acts in association with TCF/LEF family members to promote expression of a number of target genes, including Axin2 and Lef1. In addition to this central signaling role, β-catenin also participates in the formation of adherens junctions at the cell membrane. As a result, immunohistochemistry (IHC) for β-catenin is often used to assess canonical Wnt activation, but careful assessment of its subcellular localization is necessary to infer pathway activity. Other readouts of Wnt activation include the use of transgenic reporter constructs, such as TCF/LEF-LacZ139 and TCF/LEF-H2B:GFP140. These reporters have high specificity, but lower sensitivity than endogenous readouts, which manifests in a patchy expression pattern113,140. Even though these reporters do not completely mark all Wnt active cells, they are highly useful to isolate Wnt-responsive cells for further characterization and manipulation. Finally, Wnt/β-catenin activity can also be measured based on target gene expression using qPCR or in situ hybridization (ISH).
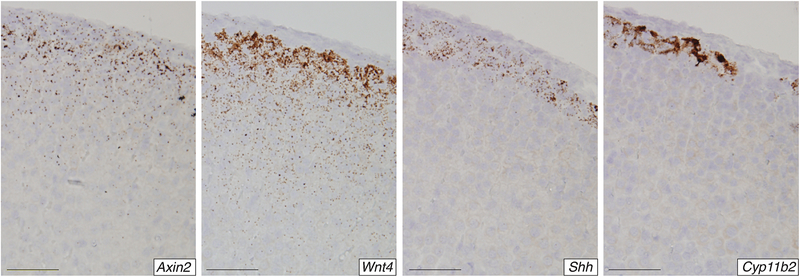
IHC for β-catenin along with TCF/LEF-LacZ reporter activity were some of the first methods used to assess the temporal and spatial pattern of Wnt activity in the adrenal gland. These experiments demonstrated that canonical Wnt activation begins early in adrenal development between E12.5 and E14.5113. Notably, encapsulation is one of the major developmental events that occur during this period81, suggesting that the capsule may act as an important source of paracrine factors to initiate Wnt/β-catenin signaling in the cortex. Once activated, the highest levels of Wnt/β-catenin activation are observed in the peripheral cortex beneath the capsule113, which corresponds to the histological glomerulosa. This expression pattern is maintained after birth and throughout adulthood113–115.
3.2.1. High Wnt pathway activation promotes glomerulosa differentiation
High activation of Wnt/β-catenin signaling in the outer adrenal suggested that this pathway might play an important role in centripetal renewal of the cortex. To test this hypothesis, several LOF and gain-of-function (GOF) mouse models have been generated. Complete inactivation of Ctnnb1 (the gene encoding β-catenin) early in development using the SF1-Crehigh driver resulted in adrenal aplasia, marked by severe loss of adrenocortical cells as early as E14.5 and a complete loss of the entire adrenal gland after E16.5113. These results suggest that β-catenin is required for early adrenal development. However, the converse experiment to constitutively activate β-catenin in all SF1-expressing cortical cells also produced a similar phenotype. SF1-Crehigh-driven β-catenin stabilization, either directly141,142 or indirectly through the loss of APC116 (a member of the destruction complex that degrades β-catenin), resulted in severely hypoplastic adrenals. Taken together, these results suggest that the level of Wnt activity during early adrenal development must be tightly regulated since both too little and too much activation blocks normal growth and development.
The severity of the phenotype observed with both complete loss or constitutive activation of β-catenin early in adrenal development precluded the ability to perform further analysis in these models on the potential role of Wnt signaling in adrenocortical renewal. To bypass these β-catenin-dependent developmental defects, other Cre drivers were employed that exhibit mosaic activity. These include the SF1-Crelow driver, which has only one copy of the SF1-Cre transgene and mediates recombination in a subset of SF1+ adrenocortical cells113. Using SF1-Crelow, inactivation of Ctnnb1 in a proportion of cortical cells circumvented the early lethality and permitted analysis of the adrenal gland after birth. This showed that partial loss of β-catenin caused a progressive thinning of the adrenal cortex over time, with the most profound defects observed after 6 months of age. These results suggest that Wnt/β-catenin signaling plays an important role in maintaining the adrenal cortex.
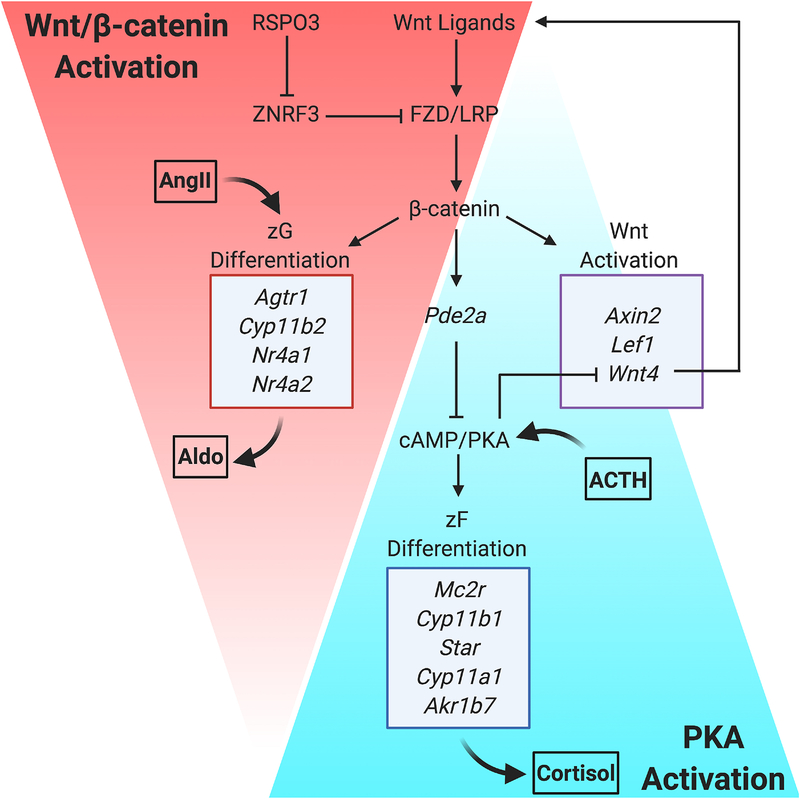
To further examine the role of β-catenin signaling in adrenocortical renewal, GOF models using stochastic Cre drivers were generated. The first approach used Akr1b7-Cre143, which is active beginning at E14.5 in all steroidogenic zones of the adrenal cortex, but has incomplete penetrance of transgene expression. This driver was combined with Catnbflox(ex3)/+ mice144 to directly stabilize β-catenin in the adrenal cortex, which successfully bypassed the early developmental defects observed with SF1-Crehigh. The resulting mice, termed Δcat, showed progressive dysplasia with accumulation of both steroidogenic and non-steroidogenic cells within the cortex at 5 and 10 months of age115. There was an increase in proliferation, although variable by region, and a corresponding ~1.5-fold increase in adrenal weight by 10 months of age. One of the more striking features of the Δcat model was ectopic glomerulosa differentiation marked by CYP11B2 expression in the central adrenal cortex and a significant increase in aldosterone production. These results suggested that high Wnt/β-catenin signaling was sufficient to promote glomerulosa identity and function. Follow-up studies further supported this notion at the molecular level by demonstrating that β-catenin directly activated genes important for aldosterone secretion, including Agtr1 (Angiotensin II Receptor Type 1), Nr4a2 (Nuclear Receptor Subfamily 4 Group A Member 2 or Nurr1), and Nr4a1 (Nuclear Receptor Subfamily 4 Group A Member 1 or Nur77)145. In addition to the Δcat model, a complementary approach used SF1-Crelow combined with APCfx/fx mice to stochastically increase Wnt/β-catenin signaling in the adrenal cortex116. APC KO mice similarly showed a mild and variable increase in proliferation along with progressive dysplasia, but no increase in adrenal weight. Taken together, these models of mosaic β-catenin stabilization in the adrenal cortex support a central role for Wnt/β-catenin signaling in glomerulosa differentiation.
More recently, an additional β-catenin GOF model was generated using Catnbflox(ex3)/+ mice combined with the aldosterone synthase (AS)-Cre driver, which is a knock-in allele that expresses Cre from the Cyp11b2 genetic locus60. Consistent with Cyp11b2 expression just before birth, lineage tracing based on the R26RmTmG reporter previously demonstrated that ASCre/+ is first active in a few cells of the peripheral cortex by P1. During postnatal adrenal development, the zG is progressively marked by GFP, as expected by the zonal expression pattern of CYP11B2. Ultimately, as centripetal migration drives renewal of the inner cortex, the zF gradually becomes GFP+ and is fully recombined by ~12 weeks of age in female mice. Using this Cre driver combined with lineage tracing, Pignatti and Ling et al recently showed that stabilization of β-catenin within in the AS-expressing lineage expands the zG146. This was demonstrated based on immunofluorescence (IF) for glomerulosa markers, including DAB2 and Gαq, concurrent with the lack of fasciculata marker expression (e.x. AKR1B7). Interestingly, there was no increase in proliferation with β-catenin stabilization, but rather a block in lineage conversion from zG to zF that caused a progressive accumulation of zG cells, which was evidenced by R26RmTmG reporter analysis. In agreement with a non-proliferative increase in cell number, adrenal weight wasn’t significantly increased until after 38 weeks of age (~2-fold). Stabilization of β-catenin and subsequent zG expansion also enhanced aldosterone production similar to the Δcat model, with transcriptional up regulation of genes including Agtr1 and Nr4a2. These results further suggest that high Wnt/β-catenin signaling drives functional zG differentiation while having a limited impact on proliferation.
Although the direct stabilization of β-catenin alone did not significantly accelerate proliferation, endocrine disruption of RAAS activity was found to cooperate with aberrant Wnt activation to promote adrenal hyperplasia. This was demonstrated as an extension of the AS-Cre-driven β-catenin GOF model using homozygous AScre/cre mice, which are deficient for Cyp11b2 (AS-KO). The complete lack of aldosterone synthase in AScre/cre animals leads to compensatory RAAS activity (~5-fold)60 and proliferative expansion of the zG146. Notably, increased zG proliferation in AS-KO mice can be fully rescued by treatment with candesartan (AngII antagonist) demonstrating that this effect is in part RAAS-driven146. Similar to AS-KO mice, AScre/cre:Catnbflox(ex3)/+ mice exhibited elevated proliferation in the zG (relative to AScre/+:Catnbflox(ex3)/+) that could be rescued by candesartan. This suggests that RAAS dysregulation rather than increased Wnt/β-catenin signaling is the main proliferative driver. However, zG area was also significantly expanded in AScre/cre:Catnbflox(ex3)/+ mice. This effect on zG size was more significant than either AS-KO or βcat GOF alone, and it was unaffected by treatment with candesartan. Moreover, AScre/cre:Catnbflox(ex3)/+ mice exhibited significantly increased adrenal weight (>2-fold) as compared to all other groups. These results indicate that there is a synergistic effect between paracrine Wnt/β-catenin activation and enhanced endocrine RAAS activity that results in hyperplastic growth of the glomerulosa. These findings have important clinical implications for patients who may have underlying germline or somatic Wnt pathway mutations in addition to chronic conditions that increase RAAS activation.
3.2.2. Upstream Wnt pathway components regulate β-catenin activation
The models described thus far interrogate Wnt/β-catenin signaling in the adrenal gland by directly targeting the expression and stabilization of β-catenin. These studies are particularly informative for understanding the potential consequences of activating mutations in CTNNB1, which are recurrent in both human adrenocortical adenoma (ACA) and adrenocortical carcinoma (ACC) tumors147–149. However, in addition to β-catenin stability, canonical Wnt activation can be modulated by alternative upstream mechanisms that affect ligand or receptor availability.
Of the 19 Wnt ligands, Wnt4 is the dominant family member expressed in the adult adrenal cortex and consistent with a critical role in canonical Wnt activation, Wnt4 is most highly expressed in the peripheral cortex105,142,150. LOF mouse models, including global Wnt4 KO150 and SF1-Crehigh-mediated cKO142,151, have collectively demonstrated that loss of Wnt4 results in significantly reduced β-catenin activation, as measured by IHC and target gene expression142, and a significant reduction in glomerulosa differentiation, as measured by CYP11B2 and aldosterone production150. Global loss of Wnt4 was not associated with a significant change in adrenal size at birth150 and conditional Wnt4 deletion resulted in only a modest reduction in adrenal weight by 12 weeks of age151. These studies are highly complementary to results from β-catenin GOF models and suggest that Wnt4 primarily functions to promote glomerulosa differentiation through Wnt/β-catenin activation. In addition to Wnt4, our lab has surveyed expression of all 19 Wnt ligands in the adult adrenal cortex using single-molecule ISH105 (i.e. RNAscope). Along with strong Wnt4 expression, we additionally detected Wnt5a as well as lower levels of Wnt5b in the adult cortex, which we have confirmed by scRNAseq (unpublished). Wnt5a and Wnt5b are more classically associated with non-canonical Wnt signaling, but functional studies have not yet been performed to determine how these ligands signal in the adrenal cortex.
In addition to ligand expression, Wnt signaling is also regulated upstream by the R-spondin (RSPO)-ZNRF3/RNF43 signaling module, which controls the availability of Wnt receptors on the cell surface. ZNRF3 and RNF43 are homologs that were first described as β-catenin target genes152 that are also enriched in LGR5+ intestinal stem cells153. These transmembrane E3 ubiquitin ligases specifically promote the ubiquitination and degradation of Wnt receptors, thus acting in a negative feedback mechanism to reduce pathway activation152,153. However, R-spondin (RSPO) proteins control the activity of ZNRF3/RNF43154. RSPOs are secreted agonists that induce membrane clearance of ZNRF3/RNF43 through both LGR-dependent152 and LGR-independent mechanisms155,156. Accordingly, the balance between RSPOs and ZNRF3/RNF43 determines cell surface receptor expression, which establishes the upper threshold for ligand-mediated Wnt pathway activation.
There are four RSPO family members, but only Rspo1 and Rspo3 are expressed in the adrenal and their expression is confined to the outer mesenchymal capsule105,151. Global Rspo1 KO mice have no discernable adrenal phenotype, but a series of Rspo3 LOF models showed profound defects in both glomerulosa differentiation and adrenocortical renewal151. Using a tamoxifen-inducible approach, either with ubiquitously expressed cCAG:CreERT or capsular restricted Gli1:CreERT2, Rspo3 loss during embryonic development resulted in a significant reduction in Wnt pathway activation (Axin2 and Wnt4), loss of zG differentiation (CYP11B2 and DAB2), and decreased cellular renewal marked by reduced cortical cell number and loss of Shh and Gli1 expression151. A similar phenotype was observed following tamoxifen administration in adult mice, suggesting that Rspo3 has a critical role in both embryonic development and adult homeostasis. Strikingly, just 1 day after tamoxifen administration in adult mice, there was near complete loss of Shh, Wnt4, DAB2, and CYP11B2 in the zG. Since there was no associated increase in cell death following Rspo3 ablation, these results indicate that Rspo3-dependent signaling is required for zG identity. Has well as IHC for the active, non-phosphorylated form h Wnt/β-catenin, it does not directly activate Wnt receptors as a ligand. RSPO3 rather potentiates signaling by remodeling receptor complexes on the cell surface through ZNRF3/RNF43. As a result, the effects of Rspo3 loss would be predicted to phenocopy the loss Wnt ligands, yet the phenotype of Wnt4 cKO mice142,151 is notably less severe than Rspo3 LOF. Cortical-specific loss of porcupine (Porcn), an enzyme required for secretion of all Wnt ligands, using SF1-Crehigh also showed only a mild adrenal phenotype105. These results suggest that an additional Wnt ligand, likely expressed in the capsule, acts alongside RSPO3 to initiate Wnt/β-catenin signaling and promote zG identity.
3.2.3. Low levels of Wnt pathway activation promote adrenocortical proliferation
Studies on Wnt4 and Rspo3 LOF in the adrenal gland helped elucidate some of the first upstream Wnt pathway components that play a significant role in Wnt/β-catenin activation and subsequent zG differentiation. Interest in the RSPO-ZNRF3/RNF43 signaling module in the adrenal further intensified when ZNRF3 was identified as one of the most frequently altered genes in human ACC patients148,149. Specifically, biallelic inactivation of ZNRF3 primarily due to homozygous deletion was seen in ~20% of cases. These observations suggested that ZNRF3 might function as a tumor suppressor in ACC, potentially by inhibiting Wnt/β-catenin signaling. Consistent with this hypothesis, loss of function ZNRF3 alterations were mutually exclusive with activating mutations in CTNNB1, suggesting a shared mechanism of action.
Based on this analysis in human ACC patients, loss of Znrf3 in the mouse adrenal cortex was predicted to phenocopy Ctnnb1 GOF. However, we tested this hypothesis and found that loss of Znrf3, either with SF1-Crehigh or ASCre/+, resulted in a profoundly different phenotype as compared to β-catenin GOF models. Contrary to the severe hypoplasia induced by Ctnnb1 GOF116,141,142, SF1-Crehigh-mediated loss of Znrf3 resulted in a nearly 8.5-fold increase in adrenal weight by 6 weeks of age105. AScre/+ similarly caused progressive adrenal hyperplasia with an ~5.5-fold increase in adrenal weight at 52 weeks105 (compared to the ~2-fold increase in aged AScre/+:Catnbflox(ex3)/+ mice146). Consistent with a significant increase in adrenal weight, Znrf3 LOF mice showed significantly increased proliferation based on both Ki67 and EdU incorporation. Surprisingly, and in contrast to Δcat and AScre/+:Catnbflox(ex3)/+ mice, loss of Znrf3 had no effect on glomerulosa differentiation or zG size. DAB2 and CYP11B2 were normal and lineage tracing using AScre/+ showed unimpaired zG-to-zF lineage conversion. Instead, loss of Znrf3 caused hyperplasia due to proliferative expansion of the zF. Increased Ki67 and EdU were specific to the enlarged CYP11B1+ region, and ACTH was suppressed concurrent with normal plasma corticosterone, which is consistent with negative feedback induced by an increased number of steroidogenic zF cells.
A primary zF defect coupled with no effect on the Wnt-high glomerulosa in Znrf3 cKO mice was unexpected based on the presumed function of ZNRF3 as a Wnt pathway inhibitor. However, Porcn inactivation significantly rescued the Znrf3-deficient phenotype, suggesting that the mechanism was in fact Wnt-dependent. To further examine the potential role of Wnt signaling in mediating the effects of Znrf3 loss, we performed high-resolution single-molecule ISH for β-catenin target genes as well as IHC for the active, non-phosphorylated form of β-catenin. These approaches confirmed that Wnt/β-catenin signaling in the normal adrenal gland is highest in the zG. Moreover, consistent with normal glomerulosa differentiation, Znrf3 cKOs showed high-intensity activated β-catenin, Axin2, and Wnt4 in the zG similar to control mice. However, in addition to the expected Wnt-high zG population, we found that Wnt/β-catenin activity extended along a gradient into the zF of the normal adrenal. A Wnt-responsive population characterized by lower β-catenin activity was identified in the upper zF, which expressed moderate levels of activated β-catenin, Axin2, and Wnt4. In Znrf3 cKO mice, the gradient of Wnt/β-catenin activation was disrupted and low levels of pathway activity were observed throughout the cortex. These results demonstrated that the loss of Znrf3 resulted in a precise, low-level increase in Wnt signaling that was not sufficient to promote glomerulosa differentiation, but rather sustained proliferation. To functionally test this hypothesis, we genetically reduced the dosage of β-catenin by inactivating a single copy of Ctnnb1, which significantly reversed the Znrf3-deficient phenotype. These results suggest that adrenocortical cells are highly sensitive to varying levels of Wnt/β-catenin activation and that low rather than high β-catenin activity drives proliferative renewal.
3.2.4. The level of Wnt pathway activation is in part established by reciprocal PKA signaling
The discovery of a proliferative, Wnt-active cell population in the upper zF characterized by low pathway activation was contrary to the previously held notion that Wnt/β-catenin signaling abruptly shuts off at the zG/zF boundary. However, this model whereby Wnt/β-catenin signaling gradually decreases as cells adopt a zF identity is consistent with the reciprocal signaling paradigm between the Wnt and cAMP/PKA pathway that has been previously established (Fig. 3). The cAMP/PKA pathway is an essential mediator of zF identity, which promotes glucocorticoid and androgen production in response to ACTH157. Briefly, ACTH binds to hetero-hexameric receptor complexes on the cell surface composed of MRAP and MC2R subunits. ACTH binding triggers an increase in intracellular cAMP that subsequently leads to PKA activation. Once activated, this signaling cascade initiates phosphorylation of several key transcription factors, including CREB and SF1, which collectively induce expression of cAMP-responsive genes. This transcriptional program includes many of the major steroidogenic enzymes such as Star, Cyp11a1, and Cyp11b1.
Figure 3. Antagonism between Wnt/β-catenin and cAMP/PKA signaling helps determine adrenocortical cell fate.
Activation of Wnt/β-catenin signaling induces a transcriptional program that promotes zG differentiation, which includes several genes that support the production of aldosterone (e.x. Agtr1, Cyp11b2, Nr4a1, and Nr4a2). Simultaneously, Wnt/β-catenin activation represses zF identity through mechanisms that include direct up regulation of Pde2a. Conversely, cAMP/PKA signaling promotes zF differentiation by increasing expression of several genes that support the production of glucocorticoids (e.x. Mc2r, Cyp11b1, Star, Cyp11a1, and Akr1b7). PKA signaling additionally represses Wnt/β-catenin activation through mechanisms that include inhibition of Wnt4. The balance between Wnt and PKA signaling in part determines adrenocortical cell fate. Schematic created with BioRender.com.
Crosstalk between PKA and Wnt signaling was first suggested by in vitro studies using the ATC7-L cell line. This mouse adrenocortical tumor cell line maintains a zF-like identity and produces basal levels of corticosterone that can be further enhanced by ACTH treatment158,159. Interestingly, pharmacological activation of Wnt/β-catenin signaling using the GSK3β inhibitor, BIO, suppressed both basal and ACTH-induced corticosterone114. At the molecular level, Wnt/β-catenin stimulation reversed ACTH-induced CREB phosphorylation and decreased SF1 expression, consistent with reduced cAMP/PKA signaling. These changes corresponded to diminished SF1 occupancy on steroidogenic promoters and subsequent repression of basal and ACTH-induced gene expression, including Star, Mc2r, Cyp11a1, and Cyp11b1. These results suggest that enhanced Wnt/β-catenin signaling functionally inhibits cAMP/PKA activation and suppresses zF identity.
In addition to these in vitro-based studies, the recently published ASCre/+;Catnbflox(ex3)/+ mouse model revealed a new potential mechanism through which canonical Wnt activation represses zF identity146. In this β-catenin GOF model, the phosphodiesterase Pde2a was identified as a direct β-catenin target gene. Phosphodiesterses are known to hydrolyze and inactivate cAMP, thus blocking PKA signaling. PDE2A was significantly up regulated in β-catenin GOF adrenals compared to controls. Moreover, ChIP analysis in extracts from whole adrenals showed that β-catenin binds at two consensus TCF/LEF sites within the promoter and first intron of the Pde2a locus. The identification and characterization of Pde2a as a novel β-catenin target gene provided new molecular insights on the mechanism by which Wnt activation inhibits zF identity.
These in vitro and in vivo studies demonstrate that Wnt/β-catenin activation inhibits cAMP/PKA signaling. To examine the converse relationship, in vivo mouse models of PKA activation were employed. Using either ACTH administration in normal control mice or SF1-Crehigh-mediated deletion of Prkar1a (i.e. constitutive PKA activation), Drelon et al demonstrated that both pharmacological and genetic activation of PKA antagonizes canonical Wnt signaling142. This was observed based on IHC for β-catenin as well as Axin2 and Lef1 target gene expression. Moreover, PKA stimulation caused a near complete loss of CYP11B2 and significantly reduced Agtr1 expression, consistent with decreased glomerulosa differentiation. Concurrent with repression of the zG, PKA activation increased Star and expanded the AKR1B7 expression domain. To examine the molecular mechanism by which PKA suppressed Wnt/β-catenin signaling, gene expression analysis was performed on adrenals from SF1-Crehigh;Prkar1afx/fx mice. Targeted analysis of Wnt-associated genes identified Wnt4 as one of the most significantly down regulated genes following PKA activation. Further, Wnt4 was rapidly decreased in normal mice following a single injection of ACTH, suggesting that the PKA pathway can rapidly repress Wnt4 expression. This hypothesis was further substantiated by in vitro studies in H295R cells as well as analysis of Wnt4 cKO mice (SF1-Crehigh;Wnt4fx/fx), which phenocopy constitutive PKA activation. Taken together, these studies suggest that PKA activation inhibits Wnt/β-catenin signaling, at least in part through controlling the expression of Wnt4.
Collectively, these studies on Wnt and PKA reveal a reciprocal signaling paradigm in the adrenal cortex that helps guide cell fate (Fig. 3). High Wnt activation directly activates a transcriptional program that promotes zG differentiation and represses zF identity. Conversely, cAMP/PKA signaling promotes zF differentiation and represses zG identity. Rather than an abrupt zG/zF switch, cells gradually transition from the Wnt-driven glomerulosa to the PKA-driven fasciculate, and experimental manipulations that shift the balance between Wnt and PKA accordingly influence the differentiation state along the glomerulosa-to-fasiculata spectrum.
3.2.5. Summary on the effects of differential Wnt pathway activation on adrenocortical proliferation and differentiation
Taken together, Wnt LOF and GOF models in the adrenal suggest that spatiotemporal control of Wnt/β-catenin signaling is essential for proper development and homeostatic renewal (Table 1). Early in embryogenesis, either too much or too little β-catenin blocks adrenal growth and development (SF1high-βcat GOF, SF1high-APC LOF, SF1high-βcat LOF). After this critical developmental window, high Wnt/β-catenin signaling is necessary (Wnt4 LOF, Rspo3 LOF) and sufficient (Δcat, AS-βcat GOF) in the outer cortex to drive zG differentiation, while low Wnt/β-catenin signaling in the upper zF promotes proliferation (Znrf3 LOF). When sustained, high-level β-catenin signaling blocks zG-to-zF conversion, enhances zG differentiation, and increases aldosterone production, whereas low-level β-catenin signaling increases zF proliferation and results in severe adrenal hyperplasia.
Table 1. Summary of Wnt/β-catenin LOF and GOF mouse models.
Spatiotemporal control of Wnt/β-catenin signaling is essential for proper development and homeostatic renewal in the adrenal cortex.
| Model | Cre driver | Cre activation | Cell population targeted | Allele | Result of recombination | Change in Wnt/β-catenin activation | Phenotype | Age | References |
|---|---|---|---|---|---|---|---|---|---|
| β-catenin LOF | SF1-Crehigh | E10.5 | Entire adrenal cortex | Ctnnb1tm2kem | Loss of β-catenin | Loss of β-catenin (IHC) | Adrenal hypoplasia progressing to aplasia | E12.5, E14.5, E16.5, and E18.5 | Kim et al 2008, Development |
| SF1-Crehigh | E10.5 | Entire adrenal cortex | Ctnnb1tm2kem | Loss of β-catenin | Data not shown | Adrenal hypoplasia progressing to aplasia | E16.5, E18.5, and P0 | Huang et al 2012, Molecular and Cellular Endocrinology | |
| SF1-Crelow | E10.5 | Stochastic within cortex | Ctnnb1tm2kem | Loss of β-catenin | Reduced TCF/LEF-LacZ and Axin2 (qPCR) | Progressive thinning of adrenal cortex | 15, 30, and 45 weeks of age | Kim et al 2008, Development | |
| RSPO3 LOF | cCAG:CreERT | +tamoxifen | Ubiquitous | Rspo3Fx/Fx | Loss of Rspo3 | Reduced Axin2 (qPCR) and Wnt4 (ISH) | Reduced cortical cell number and adrenal weight Loss of zG differentiation (DAB2 and CYP11B2) and loss of Shh and Gli1 expression | E16.5 (tamoxifen @ E11.5). 1 day or 2-, 4-, and 6-weeks post tamoxifen in adult mice. | Vidal et al, 2016, Genes and Development |
| Gli1:CreERT2 | +tamoxifen | Gli1+ capsular cells | Rspo3Fx/Fx | Loss of Rspo3 | Reduced Axin2 (qPCR) | Reduced adrenal weight Loss of zG differentiation (DAB2) | E18.5 (tamoxifen @ E13.5). 4 weeks post tamoxifen in adult mice. | Vidal et al, 2016, Genes and Development | |
| WNT4 LOF | SF1-Crehigh | E10.5 | Entire adrenal cortex | Wnt4Fx/Fx | Loss of Wnt4 | Reduced β-catenin (IHC), LEF1 (IHC and qPCR) and Axin2 (qPCR) | Mildly reduced adrenal weight Reduced expression of CYP11B2 and Agtr1 | 12 weeks of age | Drelon et al 2016, Nature Communications; Vidal et al, 2016, Genes and Development |
| N/A | N/A | Global knockout | Wnt4 global knockout | Global loss of Wnt4 | Data not shown | No change in adrenal size Reduced CYP11B2 expression and aldosterone production | E14.5, E18.5, and newborn | Heikkila et al 2002, Endocrinology | |
| PORCN LOF | SF1-Crehigh | E10.5 | Entire adrenal cortex | PorcnFx/Fx | Loss of Porcn | Data not shown | No change in adrenal size or proliferation Reduced DAB2 and increased AKR1B7 | 6 weeks of age | Basham et al, 2019, Genes and Development |
| RSPO1 LOF | N/A | N/A | Global knockout | Rspo1 global knockout | Global loss of Rspo1 | No change | No defect in adrenal weight or zonation | 4, 15, and 30 weeks of age | Vidal et al, 2016, Genes and Development |
| RNF43 LOF | SF1-Crehigh | E10.5 | Entire adrenal cortex | Rnf43Fx/Fx | Loss of Rnf43 | No change | No defect | 6 weeks of age | Basham et al, 2019, Genes and Development |
| ZNRF3 LOF | SF1-Crehigh | E10.5 | Entire adrenal cortex | Znrf3Fx/Fx | Loss of Znrf3 | Increased low-level β-catenin (IHC), Axin2 and Wnt4 (RNAscope) | Progressive hyperplasia from P0 to 6 weeks. ~8.5-fold increased adrenal weight by 6 weeks | P0, P14, and 6 weeks of age | Basham et al, 2019, Genes and Development |
| ASCre/+ | E18.5 | Cyp11b2+ glomerulosa | Znrf3Fx/Fx | Loss of Znrf3 | Increased low-level Axin2 and Wnt4 (RNAscope) | Progressive hyperplasia after 15 weeks. ~5.5-fold increased adrenal weight by 52 weeks | 15, 30, and 52 weeks of age | Basham et al, 2019, Genes and Development | |
| β-catenin GOF | SF1-Crelow | E10.5 | Stochastic within cortex | ApcFx/Fx | Loss of Apc | Increased β-catenin (W. blot and IHC), Axin2 and Lef1 (qPCR) | Increased proliferation with no change in adrenal weight Progressive dysplasia | 15, 30, 45, and >45 weeeks of age | Heaton et al 2012, American Journal of Pathology |
| 0.5 akr1b7:Cre | E14.5 | Stochastic within cortex | Catnblox(ex3) | β-catenin stabilization | Increased β-catenin (W. blot and IHC) and Axin2 (qPCR) | Variable increase in proliferation with ~1.5-fold increased adrenal weight (10 months) Enhanced zG differentiation (increased CYP11B2 and aldosterone) | 5 months (~20 weeks) and 10 months (~40 weeks) | Berthon et al 2010, Human Molecular Genetics | |
| ASCre/+ | E18.5 | Cyp11b2+ glomerulosa | Catnblox(ex3) | β-catenin stabilization | Increased β-catenin (IF), Axin2 and Lef1 (qPCR) | No increase in proliferation. No increase in adrenal weight until after 38 weeks of age (~2-fold) Block in lineage conversion from zG to zF with increased aldosterone production | 5, 10, 15–21, and 38–53 weeks of age | Ling and Pignatti et al 2020, Nature Communications; Pignatti and Ling et al 2020, Cell Reports | |
| ASCre/Cre | E18.5 | Cyp11b2+ glomerulosa | Catnblox(ex3) | β-catenin stabilization | Increased β-catenin (IF) | Increased zG proliferation and increased adrenal weight (~2-fold) zG expansion not rescued by candesartan | Adult (age not specified) | Pignatti and Ling et al 2020, Cell Reports | |
| SF1-Crehigh | E10.5 | Entire adrenal cortex | Catnblox(ex3) | β-catenin stabilization | Data not shown | Significant adrenal hypoplasia | Timecourse from E16.5 to P21 | Huang et al 2012, Molecular and Cellular Endocrinology | |
| SF1-Crehigh | E10.5 | Entire adrenal cortex | Catnblox(ex3) | β-catenin stabilization | Data not shown | Significant left adrenal hypoplasia and right adrenal agenesis | 1 month of age (~4 weeks) | Drelon et al 2016, Nature Communications | |
| SF1-Crehigh | E10.5 | Entire adrenal cortex | ApcFx/Fx | Loss of Apc | Data not shown | Significant adrenal hypoplasia | E16.5 | Heaton et al 2012, American Journal of Pathology | |
3.3. CYP11B2+ cells
In addition to SHH and Wnt activation, a subset of glomerulosa cells are marked by expression of CYP11B2 (Aldosterone synthase, AS) (Fig. 4). This enzyme mediates the final step of aldosterone production is therefore classically associated with terminal zG differentiation. Notably, the population of CYP11B2+ cells is highly dynamic depending on the levels of dietary sodium42. When sodium intake is restricted, CYP11B2 is elevated, and vice versa. Since CYP11B2 is thought to mark the most highly differentiated cells of the glomerulosa, it was initially uncertain whether this cell population contributed to centripetal renewal of the inner cortex.
Figure 4. The zona glomerulosa displays heterogeneity and contains several populations that support adrenocortical renewal.
Wnt/β-catenin pathway activation, as measured by Axin2 and Wnt4 expression, follows a gradient in the normal adrenal cortex with highest pathway activation in the outermost cortex. Shh is expressed in the zona glomerulosa (zG) and a subset of more differentiated zG cells express Cyp11b2. Single-molecule in situ hybridizations were performed on serial sections from 6-week-old female mice as previously described105. Representative images are shown. Scale bars, 100μm.
3.3.1. The zF arises from CYP11B2+ cells through transdifferentiation during both homeostasis and regeneration
To directly measure the contribution of CYP11B2+ cells to adrenocortical renewal, the previously described AScre/+ mouse model was employed. This knock-in allele expresses Cre from the endogenous Cyp11b2 gene locus60. When combined with the R26RmTmG reporter, Cyp11b2+ cells and their descendants are permanently marked by GFP. Using this lineage tracing approach, Freedman et al found that GFP+ cells were initially located in the peripheral cortex, as expected. During postnatal adrenal development, the number of GFP+ cells increased in the zG and extended into the zF in a radial and age-dependent manner. The entire cortex was GFP+ in female mice by 12 weeks of age, which was significantly accelerated in AScre/cre mice (~5 weeks), consistent with increased RAAS-driven proliferation. Moreover, in the dexamethasone-induced regeneration paradigm, the regenerated zF was fully recombined (i.e. GFP+). These studies demonstrate that differentiated cells within the zG significantly contribute to centripetal renewal of the inner cortex during both homeostasis and repair.
3.3.2. The inner cortex can be maintained independent of Cyp11b2+ cells
Although lineage tracing studies with the AS-Cre driver demonstrated a clear path of transdifferentiation between the zG and zF, additional experiments revealed that this is not the only mechanism able to maintain the inner cortex. Using AScre/+, adrenocortical renewal was evaluated following the deletion of Nr5a1 (i.e. SF1) or Nr0b1 (i.e. DAX1). SF1 is a master transcriptional regulator of the steroidogenic lineage in adrenal development and homeostasis37, and DAX1 is a transcription factor proposed to be important for adrenocortical maintenance160. Mutations in DAX1 cause X-linked adrenal hypoplasia congenita in humans, which often presents with adrenocortical failure161,162. Interestingly, the entire adrenal cortex was GFP+ in Dax1-deficient animals, suggesting that AS-Cre-driven loss of Dax1 does not impact the ability of differentiated zG cells to give rise to the zF. In contrast, targeted loss of SF1 in the AS-expressing lineage significantly impaired zG function. This was evident based on histological analysis as well as decreased P450SCC expression specifically in the zG. Moreover, while these mice had normal aldosterone levels, plasma renin was significantly elevated, consistent with a compensatory response to hypoaldosteronism. Surprisingly, despite this marked change in the glomerulosa, the zF was completely normal based on both histological markers and corticosterone levels. Further analysis using the R26RmTmG reporter revealed that the zF was GFP− and therefore not derived from the CYP11B2−expressing cell population. These results demonstrate that although the zF is typically derived from cells that once express Cyp11b2, alternative mechanisms can maintain the zF.
3.3.3. Summary on transdifferentiation of CYP11B2+ cells
Lineage tracing studies based on AScre/+ clearly demonstrate that differentiated CYP11B2−expressing cells in the zG significantly contribute to renewal of the inner cortex postnatally. Further, complete recombination of the R26RmTmG reporter throughout the entire adrenal cortex suggests that all zF cells typically arise through this transdifferentiation process during both homeostasis and dexamethasone-induced regeneration. However, under certain conditions (including SF1 ablation in the CYP11B2 lineage), additional CYP11B2−independent mechanisms can be engaged to maintain the zF. These alternative pathways have not been fully elucidated, but could include the activation of other cell populations in the cortex with stem cell function (e.x. SHH+/CYP11B2− cells in the zG or a novel facultative zF population) or simply enhanced self-renewal of the pre-existing zF cells that are derived prior to zG impairment. In addition to these unresolved questions, it also remains unclear the extent to which differentiated CYP11B2+ cells in the zG are long-term retained. Generation and characterization of a tamoxifen-inducible AS-Cre driver will be a valuable tool to answer this question.
3.4. Summary and perspectives on populations with stem cell function in the zG
Despite not fulfilling the original HSC-based criteria of a multipotent stem cell, adrenocortical cells in the zG maintain high stem cell function. During both normal homeostatic maintenance and regeneration, the zG is the primary driver of cellular renewal in the adrenal cortex. SHH and Wnt/β-catenin signaling are two of the most well characterized pathways that help maintain the renewal capacity of the zG. Notably, these pathways are interdependent and require reciprocal signaling between the cortex and capsule. Additionally, a subset of more highly differentiated cells marked by expression of CYP11B2 also reside in the zG. These cells typically support renewal of the inner cortex through direct zG-to-zF transdifferentiation. However, the inner cortex can be maintained through alternative pathways when the zG is impaired. These pathways have yet to be fully defined, but highlight the dynamic nature of the adrenal cortex. Such plasticity enables adrenocortical cells to rapidly respond to subtle changes in endocrine demand as well as significant injury.
In addition to SHH and Wnt/β-catenin signaling, other pathways are also likely to help maintain zG stem cell function. In particular, delta-like homologue 1 (DLK1) is co-expressed with Shh in the rat adrenal gland163 and regulates Gli1 expression in vitro. DLK1 is a member of the Notch/Delta/Serrate family that is thought to function as an atypical ligand, although its exact mechanism remains debated164. Interestingly, DLK1 and SHH are coordinately regulated by the RAAS and inversely correlated with CYP11B2 expression163. Moreover, DLK1 is expressed in the peripheral cortex in humans and becomes restricted to relatively undifferentiated clusters of cells with increased age165. Taken together, these observations suggest that DLK1 may act to help maintain a progenitor-like cell state in the adrenal cortex. Lineage tracing, mechanistic, and functional studies will help further elucidate the role of DLK1 in adrenocortical renewal.
4. Sexual dimorphisms in adrenal stem cell function
In some of the first autotransplantation studies performed in the 1930s, there was a striking difference in the regeneration capacity of male and female rats. Based upon cortical diameter and tissue weight after the regrowth period, females consistently regenerated more tissue than males166. These early observations suggested that there was a fundamental difference in adrenal stem cell capacity between males and females. Consistent with this notion, the female adrenal gland was later found to be significantly larger than males70. This difference first becomes evident in mice at puberty167, which could potentially be explained by differences in X zone regression. However, the female adrenal remains larger even after accounting for the X zone, either mathematically with zone-specific quantification methods167 or simply by comparing adult parous females to age-matched males70. Instead, the sex-specific difference in adrenal size has been primarily attributed to increased zF growth in females beginning at puberty, which also corresponds to higher glucocorticoid production167. Taken together, these observations suggest that there is an underlying sexual dimorphism in adrenal stem cell function that is linked to the onset of puberty.
4.1. Sex-dependent recruitment of GLI1+ capsular stem cells
While a fundamental difference in adrenocortical renewal between males and females was noted as early as the 1930s, the first study to provide a cellular and molecular basis for this sexual dimorphism was only recently published. Grabek and colleagues first performed a detailed quantification of BrdU incorporation in adult male and female mice to confirm that the larger gland size in females was in fact a refection of enhanced cortical renewal17. In mature adult mice (12 weeks old), proliferation of steroidogenic cells was ~6-fold higher in females compared to males. Interestingly, the most profound difference in proliferation rates between males and females was specifically in the outer zF, which corresponds to the previously described Wnt-low population (section 3.2.3). In this region, proliferation was ~15-fold higher in females compared to males. To further visualize and quantify cortical renewal in both sexes, lineage tracing was performed using the R26RmTmG reporter in combination with either Axin2-CreERT2 or Wnt4-CreERT2. Following tamoxifen injection, these Wnt/β-catenin-dependent Cre drivers mark the majority of cells within the Wnt-high zG. All cells that descend from this marked population also express GFP, which provides a quantitative readout of the dynamics of cellular renewal. Importantly, the proportion of cells initially labeled by tamoxifen was equivalent between the sexes. However, the entire adrenal cortex was GFP+ in females by 3 months of age, while the male cortex was only ~40% labeled. Even after 6 months, only ~75% of the male cortex was GFP+. Based on these results, complete renewal of the adult male cortex was estimated to occur in ~9 months, compared to just 3 months in females. These results are consistent with the observed lower proliferation rates in males as well as previous estimates of cortical turnover from studies using AS-Cre57,60.
In addition to steroidogenic cells, Grabek and colleagues also noted a significantly higher proliferation rate in the capsular compartment of female mice compared to males (~4-fold)17. Given the known role of Gli1+ stem cells in adrenocortical maintenance, Gli1-CreERT2 mice were used to perform additional lineage tracing experiments in order to specifically compare the recruitment of Gli1+ capsular stem cells between sexes. These studies revealed that the number of capsule-derived steroidogenic cells was nearly 16-fold higher in adult female mice compared to males. However, this dimorphism in stem cell recruitment only emerged after puberty. Both sexes displayed a high and comparable rate of Gli1-derived cortical cells at 3 weeks of age. The recruitment of Gli1+ capsular stem cells was sustained in females throughout maturity, but dramatically suppressed in males by 6 weeks of age. This observed dimorphism in adrenal stem cell recruitment could potentially be explained by a difference in sex chromosomes or sex hormones. However, an elegant series of genetic sex reversal models ruled out a sex-chromosome-dependent mechanism. Instead, recruitment of capsule-derived adrenocortical stem cells was found to be androgen-dependent. Gonadectomy (GDX) in adult male mice significantly increased the proliferative index and enhanced recruitment of Gli1+ stem cells to the steroidogenic lineage. Furthermore, ovariectomy had no effect on females, but treatment with exogenous androgens (i.e. dihydrotestosterone, DHT) was sufficient to repress capsular proliferation and block stem cell recruitment. Together, these experiments demonstrate that androgens negatively regulate Gli1+ adrenal stem cells.
4.2. Androgen-dependent regulation of DAX1
In addition to capsular stem cell recruitment, androgens have also been shown to repress DAX1 expression in the adrenal cortex. As we briefly described previously, DAX1 is a transcription factor that is mutated in human patients with X-linked adrenal hypoplasia congenita (AHC)161,162. Interestingly, DAX1 is an atypical nuclear receptor and co-repressor of SF1-mediated transcription. Dax1-deficient mice display normal adrenal development until puberty, when the X zone fails to regress in male mice168, which may be an indirect effect due to hypogonadism169,170. However, adult Dax1-deficient animals become hyper-responsive to ACTH before ultimately showing signs of significant adrenocortical dysplasia at late stages160. This biphasic phenotype, with early adrenal hyperfunction followed by late-stage failure, is similar to what has been observed in some AHC patients171, and suggests that DAX1 may function in part to maintain adrenocortical progenitor cells.
To investigate molecular mechanisms that regulate DAX1, a detailed expression analysis was performed in postnatal mice. These studies revealed that DAX1 is initially expressed throughout the definitive cortex in both males and females. However, beginning at P14 with the onset of puberty, DAX1 expression becomes progressively restricted to the zG in males. The dynamics and sex-specific nature of this expression pattern suggested that DAX1 is actively repressed in males. To test a potential role for androgens in down regulating DAX1, a series of GDX and hormone replacement studies were performed. These experiments showed that GDX in male mice restored DAX1 expression throughout the cortex, which could be reversed by testosterone treatment. Similarly, testosterone treatment in females restricted DAX1 expression to the zG, thus mimicking the male pattern. These results strongly implicated a role for androgens in repressing DAX1 in the inner cortex. Consistent with this hypothesis, the androgen receptor (AR) was more highly expressed in the zF as compared to the zG. Subsequent in vitro studies showed that upon ligand activation, AR directly interacts with SF1 and suppresses SF1 transcriptional targets, including Dax1. Taken together, these experiments suggest a model in which DAX1 is initially expressed throughout the definitive adrenal cortex. With the onset of puberty, androgens activate AR primarily in the inner cortex, which promotes a direct interaction between AR and SF1 that alters the transcriptional activity of SF1. Based on the proposed role of DAX1 in regulating adrenocortical progenitor activity, androgen-dependent DAX1 expression may contribute to the observed differences in proliferation between males and females in the adrenal cortex.
4.3. Sex-dependent proliferation of the X zone
In addition to the definitive adrenal cortex, the X zone also displays differences in cellular renewal based on sex. The X zone is a fetal-derived region of the mouse adrenal cortex that normally regresses in males during puberty and in females either during pregnancy or spontaneously in aged nulliparous animals58. Aside from these sex-dependent differences in regression, proliferation of the X zone is also differentially regulated in males and females. Specifically, castration has been shown to stimulate proliferation of the X zone in male mice. When performed before puberty, GDX induces proliferation of the existing X zone. This results in an enlarged ‘primary X zone’, which can grow to become more than 1/3 of the entire cortex64. Interestingly, even after puberty-induced regression, castration in male mice causes re-emergence of a so-called ‘secondary X zone.’ This is thought to arise from the expansion of cell remnants remaining after regression, which is supported by lineage tracing studies57. These observations suggest that cellular renewal of the X zone is independent from the definitive cortex. Moreover, these studies suggest that X zone proliferation is at least in part regulated by the HPG axis. Further studies are needed to examine the molecular mechanism that control cellular renewal of the X zone.
4.4. Summary and implications of sexually dimorphic stem cell function
Historic transplantation studies first suggested that the functional stem cell capacity of the adrenal gland is significantly higher in females compared to males. More recent studies have discovered a molecular basis for these early observations and demonstrated that androgens actively restrict the recruitment of Gli1+ capsular stem cells to the steroidogenic lineage. Moreover, AR signaling represses DAX1 expression in the inner cortex. These observations have important implications for genetic models of adrenal disease since the rate of adrenocortical renewal significantly impacts Cre-mediated recombination. This may at least in part explain some of the sex-dependent phenotypes reported in prior mouse models. However, the observed sexual dimorphism in adrenocortical renewal also has potential implications for human patients, where adrenal pathologies are often more frequent in women than in men. This includes the incidence of both benign and malignant adrenocortical tumors172–174. As a higher number of cell divisions are predicted to lead to an accumulation of more mutations, it is tempting to speculate that sex hormone-dependent differences in cellular renewal may contribute to the higher risk in women for developing adrenal tumors. However, additional studies in human tissues are needed to assess how well these mechanisms are conserved across species.
5. Concluding Remarks
The adrenal cortex is a highly dynamic tissue. During homeostasis, adrenocortical cells must be continually replenished while also remaining poised for steroidogenic output in response to varying physiological demands. Moreover, the adrenal cortex maintains a high regenerative capacity to repair itself following injury. In order to successfully meet these diverse needs, the adrenal cortex employs multiple cell populations with stem cell function (Fig. 1). The outer mesenchymal capsule contains multipotent stem cells that give rise to steroidogenic cells of the underlying cortex. The relative proportion of adrenocortical cells derived from the capsule is low during development and homeostasis, but these cell populations can be acutely activated in response to severe stress. The bulk of homeostatic turnover in the cortex is instead governed by the glomerulosa, which contains multiple populations that are capable of sustaining cortical renewal. These include progenitor-like cells as well as more differentiated CYP11B2+ cells that repopulate the inner cortex through transdifferentiation. The ability of the adrenal cortex to maintain cellular renewal through a diverse set of mechanisms enables the tissue to adapt to a wide range of physiological and pathological conditions. Understanding the molecular mechanisms that control cellular plasticity and renewal in the adrenal cortex will help inform new approaches for regenerative medicine to restore tissue function as well as new therapeutic strategies for the treatment of cancer.
Acknowledgements
The National Institutes of Health (R01-DK062027 to G.D.H.), the Heather Rose Kornick Research Fund (K.J.B), and the American Cancer Society (Michigan Cancer Research Fund Postdoctoral Fellowship PF-17-227-01-DDC to K.J.B.) supported this work. We are also grateful to Typhanie Dumontet (Ann Arbor, MI) for critically reading this manuscript.
Footnotes
Conflicts of Interest
Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Achermann JC & Vilain EJ in GeneReviews((R)) (eds Adam MP et al. ) (1993). [Google Scholar]
- 2.Speiser PW & White PC Congenital adrenal hyperplasia. N Engl J Med 349, 776–788, doi: 10.1056/NEJMra021561 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Saverino S & Falorni A Autoimmune Addison’s disease. Best Pract Res Clin Endocrinol Metab, 101379, doi: 10.1016/j.beem.2020.101379 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Joseph RM, Hunter AL, Ray DW & Dixon WG Systemic glucocorticoid therapy and adrenal insufficiency in adults: A systematic review. Semin Arthritis Rheum 46, 133–141, doi: 10.1016/j.semarthrit.2016.03.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shackleton M Normal stem cells and cancer stem cells: similar and different. Semin Cancer Biol 20, 85–92, doi: 10.1016/j.semcancer.2010.04.002 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Cable J et al. Adult stem cells and regenerative medicine-a symposium report. Ann N Y Acad Sci 1462, 27–36, doi: 10.1111/nyas.14243 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanpain C & Fuchs E Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344, 1242281, doi: 10.1126/science.1242281 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil J, Stembalska A, Pesz KA & Sasiadek MM Cancer stem cells: the theory and perspectives in cancer therapy. J Appl Genet 49, 193–199, doi: 10.1007/BF03195612 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Batlle E & Clevers H Cancer stem cells revisited. Nat Med 23, 1124–1134, doi: 10.1038/nm.4409 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Morrison SJ, Uchida N & Weissman IL The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol 11, 35–71, doi: 10.1146/annurev.cb.11.110195.000343 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Zon LI Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature 453, 306–313, doi: 10.1038/nature07038 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Laurenti E & Gottgens B From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418–426, doi: 10.1038/nature25022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suda T, Suda J & Ogawa M Disparate differentiation in mouse hemopoietic colonies derived from paired progenitors. Proc Natl Acad Sci U S A 81, 2520–2524, doi: 10.1073/pnas.81.8.2520 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spangrude GJ, Heimfeld S & Weissman IL Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62, doi: 10.1126/science.2898810 (1988). [DOI] [PubMed] [Google Scholar]
- 15.Smith LG, Weissman IL & Heimfeld S Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A 88, 2788–2792, doi: 10.1073/pnas.88.7.2788 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post Y & Clevers H Defining Adult Stem Cell Function at Its Simplest: The Ability to Replace Lost Cells through Mitosis. Cell Stem Cell 25, 174–183, doi: 10.1016/j.stem.2019.07.002 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Grabek A et al. The Adult Adrenal Cortex Undergoes Rapid Tissue Renewal in a Sex-Specific Manner. Cell Stem Cell, doi: 10.1016/j.stem.2019.04.012 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Munoz-Canoves P & Huch M Definitions for adult stem cells debated. Nature 563, 328–329, doi: 10.1038/d41586-018-07175-6 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Xing Y, Lerario AM, Rainey W & Hammer GD Development of Adrenal Cortex Zonation. Endocrinol Metab Clin North Am 44, 243–274, doi: 10.1016/j.ecl.2015.02.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drelon C, Berthon A, Mathieu M, Martinez A & Val P Adrenal cortex tissue homeostasis and zonation: A WNT perspective. Mol Cell Endocrinol 408, 156–164, doi: 10.1016/j.mce.2014.12.014 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Pignatti E, Leng S, Carlone DL & Breault DT Regulation of zonation and homeostasis in the adrenal cortex. Mol Cell Endocrinol 441, 146–155, doi: 10.1016/j.mce.2016.09.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinson GP Functional Zonation of the Adult Mammalian Adrenal Cortex. Front Neurosci 10, 238, doi: 10.3389/fnins.2016.00238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pihlajoki M, Dorner J, Cochran RS, Heikinheimo M & Wilson DB Adrenocortical zonation, renewal, and remodeling. Front Endocrinol (Lausanne) 6, 27, doi: 10.3389/fendo.2015.00027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plavska H. L. J. a. A. Functioning autoplastic suprarenal transplants. Proceedings of the Society for Experimental Biology and Medicine 23, 528–530 (1926). [Google Scholar]
- 25.Ingle DJ Adrenal Insufficiency and Capacity for Sustained Work Output. Proceedings of the Society for Experimental Biology and Medicine 31, 163–165 (1933). [Google Scholar]
- 26.Nilson HW & Ingle DJ Recovery of Viable Adrenal Cortical Tissue. Science 84, 424, doi: 10.1126/science.84.2184.424 (1936). [DOI] [PubMed] [Google Scholar]
- 27.Harris D. J. I. a. R. E. Voluntary activity of the rat after destruction of the adrenal medulla. American Journal of Physiology 114, 657–660 (1936). [Google Scholar]
- 28.Hatano O, Takakusu A, Nomura M & Morohashi K Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1, 663–671, doi: 10.1046/j.1365-2443.1996.00254.x (1996). [DOI] [PubMed] [Google Scholar]
- 29.Zubair M, Ishihara S, Oka S, Okumura K & Morohashi K Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol 26, 4111–4121, doi: 10.1128/MCB.00222-06 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shima Y et al. Identification of an enhancer in the Ad4BP/SF-1 gene specific for fetal Leydig cells. Endocrinology 153, 417–425, doi: 10.1210/en.2011-1407 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Pankratz DS The development of the suprarenal gland in the albino rat, with a consideration of its possible relation to the origin of foetal movements. The Anatomical Record 49, 31–49 (1931). [Google Scholar]
- 32.Anderson DJ & Axel R A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell 47, 1079–1090, doi: 10.1016/0092-8674(86)90823-8 (1986). [DOI] [PubMed] [Google Scholar]
- 33.Keene MF, & Hewer EE Observations on the Development of the Human Suprarenal Gland. J Anat 61, 302–324 (1927). [PMC free article] [PubMed] [Google Scholar]
- 34.Uotila U The early embryological development of the fetal and permanent adrenal cortex in man. American Journal of Anatomy 6, 259–390 (1940). [Google Scholar]
- 35.Zubair M, Parker KL & Morohashi K Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol 28, 7030–7040, doi: 10.1128/MCB.00900-08 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morohashi K & Zubair M The fetal and adult adrenal cortex. Mol Cell Endocrinol 336, 193–197, doi: 10.1016/j.mce.2010.11.026 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Luo X, Ikeda Y & Parker KL A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77, 481–490, doi: 10.1016/0092-8674(94)90211-9 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Arnold J Ein Beitrag zu der feineren Structur und dem Chemismus der Nebennieren. Virchows Archiv Pathologie, Anatomie, Physiologie 39, 64–117 (1866). [Google Scholar]
- 39.Seccia TM, Caroccia B, Gomez-Sanchez EP, Gomez-Sanchez CE & Rossi GP The Biology of Normal Zona Glomerulosa and Aldosterone-Producing Adenoma: Pathological Implications. Endocr Rev 39, 1029–1056, doi: 10.1210/er.2018-00060 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wotus C, Levay-Young BK, Rogers LM, Gomez-Sanchez CE & Engeland WC Development of adrenal zonation in fetal rats defined by expression of aldosterone synthase and 11beta-hydroxylase. Endocrinology 139, 4397–4403, doi: 10.1210/endo.139.10.6230 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Bassett MH, White PC & Rainey WE The regulation of aldosterone synthase expression. Mol Cell Endocrinol 217, 67–74, doi: 10.1016/j.mce.2003.10.011 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Shelton JH & Jones AL The fine structure of the mouse adrenal cortex and the ultrastructural changes in the zona glomerulosa with low and high sodium diets. Anat Rec 170, 147–181, doi: 10.1002/ar.1091700204 (1971). [DOI] [PubMed] [Google Scholar]
- 43.Romero DG et al. Disabled-2 is expressed in adrenal zona glomerulosa and is involved in aldosterone secretion. Endocrinology 148, 2644–2652, doi: 10.1210/en.2006-1509 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Boulkroun S et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology 152, 4753–4763, doi: 10.1210/en.2011-1205 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Keeney DS, Jenkins CM & Waterman MR Developmentally regulated expression of adrenal 17 alpha-hydroxylase cytochrome P450 in the mouse embryo. Endocrinology 136, 4872–4879, doi: 10.1210/endo.136.11.7588219 (1995). [DOI] [PubMed] [Google Scholar]
- 46.Aigueperse C, Martinez A, Lefrancois-Martinez AM, Veyssiere G & Jean CI Cyclic AMP regulates expression of the gene coding for a mouse vas deferens protein related to the aldo-keto reductase superfamily in human and murine adrenocortical cells. J Endocrinol 160, 147–154, doi: 10.1677/joe.0.1600147 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Pastel E, Pointud JC, Martinez A & Lefrancois-Martinez AM Aldo-Keto Reductases 1B in Adrenal Cortex Physiology. Front Endocrinol (Lausanne) 7, 97, doi: 10.3389/fendo.2016.00097 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turcu A, Smith JM, Auchus R & Rainey WE Adrenal androgens and androgen precursors-definition, synthesis, regulation and physiologic actions. Compr Physiol 4, 1369–1381, doi: 10.1002/cphy.c140006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuber MX, Simpson ER & Waterman MR Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science 234, 1258–1261, doi: 10.1126/science.3535074 (1986). [DOI] [PubMed] [Google Scholar]
- 50.Quinn TA et al. Ontogeny of the adrenal gland in the spiny mouse, with particular reference to production of the steroids cortisol and dehydroepiandrosterone. Endocrinology 154, 1190–1201, doi: 10.1210/en.2012-1953 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Kalavsky SM Fetal rat adrenal steroidogenesis. Biol Neonate 17, 427–435, doi: 10.1159/000240335 (1971). [DOI] [PubMed] [Google Scholar]
- 52.Le Goascogne C et al. Immunoreactive cytochrome P-450(17 alpha) in rat and guinea-pig gonads, adrenal glands and brain. J Reprod Fertil 93, 609–622, doi: 10.1530/jrf.0.0930609 (1991). [DOI] [PubMed] [Google Scholar]
- 53.Perkins LM & Payne AH Quantification of P450scc, P450(17) alpha, and iron sulfur protein reductase in Leydig cells and adrenals of inbred strains of mice. Endocrinology 123, 2675–2682, doi: 10.1210/endo-123-6-2675 (1988). [DOI] [PubMed] [Google Scholar]
- 54.Missaghian E et al. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J Endocrinol 202, 99–109, doi: 10.1677/JOE-08-0353 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Nilsson ME et al. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology 156, 2492–2502, doi: 10.1210/en.2014-1890 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Mostaghel EA et al. Contribution of Adrenal Glands to Intratumor Androgens and Growth of Castration-Resistant Prostate Cancer. Clin Cancer Res 25, 426–439, doi: 10.1158/1078-0432.CCR-18-1431 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dumontet T et al. PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal. JCI Insight 3, doi: 10.1172/jci.insight.98394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howard-Miller E A transitory zone in the adrenal cortex which shows age and sex relationships. The American Journal of Anatomy 40, 251–293 (1927). [Google Scholar]
- 59.Masui K, and Tamura Y The effect of gonadectomy on the structure of the suprrenal glands of mice, with special reference to the functional relation between this gland and the sex gland of the female. Japanese Journal of Zootechnicl Science 1, 55–70 (1924). [Google Scholar]
- 60.Freedman BD et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell 26, 666–673, doi: 10.1016/j.devcel.2013.07.016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hershkovitz L, Beuschlein F, Klammer S, Krup M & Weinstein Y Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology 148, 976–988, doi: 10.1210/en.2006-1100 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Pihlajoki M et al. Conditional mutagenesis of Gata6 in SF1-positive cells causes gonadal-like differentiation in the adrenal cortex of mice. Endocrinology 154, 1754–1767, doi: 10.1210/en.2012-1892 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callow RK & Deanesly R Effect of androsterone and of male hormone concentrates on the accessory reproductive organs of castrated rats, mice and guinea-pigs. Biochem J 29, 1424–1445, doi: 10.1042/bj0291424 (1935). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howard E Effects of castration on the seminal vesicles as influenced by age, considered in relation to the degree of development of the adrenal X zone. American Journal of Anatomy 65, 105–149 (1939). [Google Scholar]
- 65.Gottschau M Struktur und embryonale Entwickelung der Nebennieren bei Saugethieren. Archiv. Anat. Physiol. 9, 412–458 (1883). [Google Scholar]
- 66.Zwemer RL A Study of Adrenal Cortex Morphology. Am J Pathol 12, 107–114 101, doi: 10.1097/00005053-193607000-00010 (1936). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker L. C. W. a. B. S. Studies on suprarenal insufficiency. American Journal of Physiology 89, 215–222 (1929). [Google Scholar]
- 68.Ingle Dwight J., a. G. M. H. Autotransplantation and regeneration of the adrenal gland. Endocrinology 22, 458–464 (1938). [Google Scholar]
- 69.Salmon TN & Zwemer RL A study of the life history of cortico-adrenal gland cells of the rat by means of trypan blue injections. The Anatomical Record 80, 421–429 (1941). [Google Scholar]
- 70.Jones IC Variation in the mouse adrenal cortex with special reference to the zona reticularis and to brown degeneration, together with a discussion of the cell migration theory. Q J Microsc Sci 89, 53–74 (1948). [PubMed] [Google Scholar]
- 71.Swann HG The pituitary-adrenocortical relationship. Physiol. Rev. 20, 493–521 (1940). [Google Scholar]
- 72.Deane HW & Greep RO A morphological and histochemical study of the rat’s adrenal cortex after hypoph ysectomy, with comments on the liver. Am J Anat 79, 117–145, doi: 10.1002/aja.1000790104 (1946). [DOI] [PubMed] [Google Scholar]
- 73.Deane HW, Shaw JH & Greep RO The effect of altered sodium or potassium intake on the width and cytochemistry of the zona glomerulosa of the rat’s adrenal cortex. Endocrinology 43, 133–153, doi: 10.1210/endo-43-3-133 (1948). [DOI] [PubMed] [Google Scholar]
- 74.Greep RO & Deane HW Histological, cytochemical and physiological observations on the regeneration of the rat’s adrenal gland following enucleation. Endocrinology 45, 42–56, doi: 10.1210/endo-45-1-42 (1949). [DOI] [PubMed] [Google Scholar]
- 75.Wright NA Cell proliferation in the prepubertal male rat adrenal cortex: an autoradiographic study. J Endocrinol 49, 599–609, doi: 10.1677/joe.0.0490599 (1971). [DOI] [PubMed] [Google Scholar]
- 76.Wright NA Variation in tritiated thymidine uptake during DNA synthesis in the adrenal cortex. Histochemie 28, 99–102, doi: 10.1007/bf00279854 (1971). [DOI] [PubMed] [Google Scholar]
- 77.Wright NA, Voncina D & Morley AR An attempt to demonstrate cell migration from the zona glomerulosa in the prepubertal male rat adrenal cortex. J Endocrinol 59, 451–459, doi: 10.1677/joe.0.0590451 (1973). [DOI] [PubMed] [Google Scholar]
- 78.Bertholet JY Proliferative activity and cell migration in the adrenal cortex of fetal and neonatal rats: an autoradiographic study. J Endocrinol 87, 1–9, doi: 10.1677/joe.0.0870001 (1980). [DOI] [PubMed] [Google Scholar]
- 79.Zajicek G, Ariel I & Arber N The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol 111, 477–482, doi: 10.1677/joe.0.1110477 (1986). [DOI] [PubMed] [Google Scholar]
- 80.King P, Paul A & Laufer E Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A 106, 21185–21190, doi: 10.1073/pnas.0909471106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bandiera R et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev Cell 27, 5–18, doi: 10.1016/j.devcel.2013.09.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finco I, Lerario AM & Hammer GD Sonic Hedgehog and WNT Signaling Promote Adrenal Gland Regeneration in Male Mice. Endocrinology 159, 579–596, doi: 10.1210/en.2017-03061 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nicolaides NC, Pavlaki AN, Maria Alexandra MA & Chrousos GP in Endotext (eds Feingold KR et al. ) (2000). [Google Scholar]
- 84.Younes AK & Younes NK Recovery of steroid induced adrenal insufficiency. Transl Pediatr 6, 269–273, doi: 10.21037/tp.2017.10.01 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilhelm D & Englert C The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev 16, 1839–1851, doi: 10.1101/gad.220102 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore AW et al. YAC transgenic analysis reveals Wilms’ tumour 1 gene activity in the proliferating coelomic epithelium, developing diaphragm and limb. Mech Dev 79, 169–184, doi: 10.1016/s0925-4773(98)00188-9 (1998). [DOI] [PubMed] [Google Scholar]
- 87.Kreidberg JA et al. WT-1 is required for early kidney development. Cell 74, 679–691, doi: 10.1016/0092-8674(93)90515-r (1993). [DOI] [PubMed] [Google Scholar]
- 88.Moore AW, McInnes L, Kreidberg J, Hastie ND & Schedl A YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126, 1845–1857 (1999). [DOI] [PubMed] [Google Scholar]
- 89.Muzumdar MD, Tasic B, Miyamichi K, Li L & Luo L A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605, doi: 10.1002/dvg.20335 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Kero J et al. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J Clin Invest 105, 633–641, doi: 10.1172/JCI7716 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woolley GW, Dickie MM & Little CC Adrenal tumors and other pathological changes in reciprocal crosses in mice. I. Strain DBA x strain CE and the reciprocal. Cancer Res 12, 142–152 (1952). [PubMed] [Google Scholar]
- 92.Woolley GW, Dickie MM & Little CC Adrenal tumors and other pathological changes in reciprocal crosses in mice. II. An introduction to results of four reciprocal crosses. Cancer Res 13, 231–245 (1953). [PubMed] [Google Scholar]
- 93.Bielinska M et al. Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology 144, 4123–4133, doi: 10.1210/en.2003-0126 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Fekete E, Woolley G & Little CC HISTOLOGICAL CHANGES FOLLOWING OVARIECTOMY IN MICE : I. dba HIGH TUMOR STRAIN. J Exp Med 74, 1–8, doi: 10.1084/jem.74.1.1 (1941). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosner JM, Charreau E, Houssay AB & Epper C Biosynthesis of sexual steroids by hyperplastic adrenal glands of castrated female C3H/Ep mice. Endocrinology 79, 681–686, doi: 10.1210/endo-79-4-681 (1966). [DOI] [PubMed] [Google Scholar]
- 96.Wu F, Zhang Y, Sun B, McMahon AP & Wang Y Hedgehog Signaling: From Basic Biology to Cancer Therapy. Cell Chem Biol 24, 252–280, doi: 10.1016/j.chembiol.2017.02.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.King PJ, Guasti L & Laufer E Hedgehog signalling in endocrine development and disease. J Endocrinol 198, 439–450, doi: 10.1677/JOE-08-0161 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Begleiter ML & Harris DJ Holoprosencephaly and endocrine dysgenesis in brothers. Am J Med Genet 7, 315–318, doi: 10.1002/ajmg.1320070312 (1980). [DOI] [PubMed] [Google Scholar]
- 99.Dubourg C et al. Holoprosencephaly. Orphanet J Rare Dis 2, 8, doi: 10.1186/1750-1172-2-8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang S, Graham JM Jr., Olney AH & Biesecker LG GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet 15, 266–268, doi: 10.1038/ng0397-266 (1997). [DOI] [PubMed] [Google Scholar]
- 101.Bitgood MJ & McMahon AP Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172, 126–138, doi: 10.1006/dbio.1995.0010 (1995). [DOI] [PubMed] [Google Scholar]
- 102.Ching S & Vilain E Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis 47, 628–637, doi: 10.1002/dvg.20532 (2009). [DOI] [PubMed] [Google Scholar]
- 103.Huang CC, Miyagawa S, Matsumaru D, Parker KL & Yao HH Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology 151, 1119–1128, doi: 10.1210/en.2009-0814 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bingham NC, Verma-Kurvari S, Parada LF & Parker KL Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis 44, 419–424, doi: 10.1002/dvg.20231 (2006). [DOI] [PubMed] [Google Scholar]
- 105.Basham KJ et al. A ZNRF3-dependent Wnt/beta-catenin signaling gradient is required for adrenal homeostasis. Genes Dev 33, 209–220, doi: 10.1101/gad.317412.118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karpac J et al. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology 146, 2555–2562, doi: 10.1210/en.2004-1290 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Yaswen L, Diehl N, Brennan MB & Hochgeschwender U Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5, 1066–1070, doi: 10.1038/12506 (1999). [DOI] [PubMed] [Google Scholar]
- 108.Chida D et al. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci U S A 104, 18205–18210, doi: 10.1073/pnas.0706953104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Finco I, LaPensee CR, Krill KT & Hammer GD Hedgehog signaling and steroidogenesis. Annu Rev Physiol 77, 105–129, doi: 10.1146/annurev-physiol-061214-111754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nishimoto K et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A 112, E4591–4599, doi: 10.1073/pnas.1505529112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mao J et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res 66, 10171–10178, doi: 10.1158/0008-5472.CAN-06-0657 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nusse R & Clevers H Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 169, 985–999, doi: 10.1016/j.cell.2017.05.016 (2017). [DOI] [PubMed] [Google Scholar]
- 113.Kim AC et al. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135, 2593–2602, doi: 10.1242/dev.021493 (2008). [DOI] [PubMed] [Google Scholar]
- 114.Walczak EM et al. Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol Endocrinol 28, 1471–1486, doi: 10.1210/me.2014-1060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berthon A et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet 19, 1561–1576, doi: 10.1093/hmg/ddq029 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Heaton JH et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and beta-catenin. Am J Pathol 181, 1017–1033, doi: 10.1016/j.ajpath.2012.05.026 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brewer JR, Mazot P & Soriano P Genetic insights into the mechanisms of Fgf signaling. Genes Dev 30, 751–771, doi: 10.1101/gad.277137.115 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guasti L, Candy Sze WC, McKay T, Grose R & King PJ FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development. Mol Cell Endocrinol 371, 182–188, doi: 10.1016/j.mce.2013.01.014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stark KL, McMahon JA & McMahon AP FGFR-4, a new member of the fibroblast growth factor receptor family, expressed in the definitive endoderm and skeletal muscle lineages of the mouse. Development 113, 641–651 (1991). [DOI] [PubMed] [Google Scholar]
- 120.Hafner R, Bohnenpoll T, Rudat C, Schultheiss TM & Kispert A Fgfr2 is required for the expansion of the early adrenocortical primordium. Mol Cell Endocrinol 413, 168–177, doi: 10.1016/j.mce.2015.06.022 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Revest JM et al. Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev Biol 231, 47–62, doi: 10.1006/dbio.2000.0144 (2001). [DOI] [PubMed] [Google Scholar]
- 122.Kim Y et al. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A 104, 16558–16563, doi: 10.1073/pnas.0702581104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leng S et al. beta-Catenin and FGFR2 regulate postnatal rosette-based adrenocortical morphogenesis. Nat Commun 11, 1680, doi: 10.1038/s41467-020-15332-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Massague J TGFbeta signalling in context. Nat Rev Mol Cell Biol 13, 616–630, doi: 10.1038/nrm3434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Looyenga BD, Wiater E, Vale W & Hammer GD Inhibin-A antagonizes TGFbeta2 signaling by down-regulating cell surface expression of the TGFbeta coreceptor betaglycan. Mol Endocrinol 24, 608–620, doi: 10.1210/me.2008-0374 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kakoki M et al. Primary aldosteronism and impaired natriuresis in mice underexpressing TGFbeta1. Proc Natl Acad Sci U S A 110, 5600–5605, doi: 10.1073/pnas.1302641110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taipale J, Cooper MK, Maiti T & Beachy PA Patched acts catalytically to suppress the activity of Smoothened. Nature 418, 892–897, doi: 10.1038/nature00989 (2002). [DOI] [PubMed] [Google Scholar]
- 128.Cooper MK et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet 33, 508–513, doi: 10.1038/ng1134 (2003). [DOI] [PubMed] [Google Scholar]
- 129.Blassberg R, Macrae JI, Briscoe J & Jacob J Reduced cholesterol levels impair Smoothened activation in Smith-Lemli-Opitz syndrome. Hum Mol Genet 25, 693–705, doi: 10.1093/hmg/ddv507 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Myers BR, Neahring L, Zhang Y, Roberts KJ & Beachy PA Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc Natl Acad Sci U S A 114, E11141–E11150, doi: 10.1073/pnas.1717891115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luchetti G et al. Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. Elife 5, doi: 10.7554/eLife.20304 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Myers BR et al. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev Cell 26, 346–357, doi: 10.1016/j.devcel.2013.07.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang P et al. Cellular Cholesterol Directly Activates Smoothened in Hedgehog Signaling. Cell 166, 1176–1187 e1114, doi: 10.1016/j.cell.2016.08.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang P et al. Structural Basis of Smoothened Activation in Hedgehog Signaling. Cell 174, 312–324 e316, doi: 10.1016/j.cell.2018.04.029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deshpande I et al. Smoothened stimulation by membrane sterols drives Hedgehog pathway activity. Nature 571, 284–288, doi: 10.1038/s41586-019-1355-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Byrne EFX et al. Structural basis of Smoothened regulation by its extracellular domains. Nature 535, 517–522, doi: 10.1038/nature18934 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Briscoe J & Therond PP The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol 14, 416–429, doi: 10.1038/nrm3598 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Clevers H & Nusse R Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205, doi: 10.1016/j.cell.2012.05.012 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Mohamed OA, Clarke HJ & Dufort D Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn 231, 416–424, doi: 10.1002/dvdy.20135 (2004). [DOI] [PubMed] [Google Scholar]
- 140.Ferrer-Vaquer A et al. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol 10, 121, doi: 10.1186/1471-213X-10-121 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang CC, Liu C & Yao HH Investigating the role of adrenal cortex in organization and differentiation of the adrenal medulla in mice. Mol Cell Endocrinol 361, 165–171, doi: 10.1016/j.mce.2012.04.004 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Drelon C et al. PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development. Nat Commun 7, 12751, doi: 10.1038/ncomms12751 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lambert-Langlais S et al. A transgenic mouse line with specific Cre recombinase expression in the adrenal cortex. Mol Cell Endocrinol 300, 197–204, doi: 10.1016/j.mce.2008.10.045 (2009). [DOI] [PubMed] [Google Scholar]
- 144.Harada N et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 18, 5931–5942, doi: 10.1093/emboj/18.21.5931 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Berthon A et al. WNT/beta-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet 23, 889–905, doi: 10.1093/hmg/ddt484 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Pignatti E et al. Beta-Catenin Causes Adrenal Hyperplasia by Blocking Zonal Transdifferentiation. Cell Rep 31, 107524, doi: 10.1016/j.celrep.2020.107524 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tissier F et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 65, 7622–7627, doi: 10.1158/0008-5472.CAN-05-0593 (2005). [DOI] [PubMed] [Google Scholar]
- 148.Zheng S et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 29, 723–736, doi: 10.1016/j.ccell.2016.04.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Assie G et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 46, 607–612, doi: 10.1038/ng.2953 (2014). [DOI] [PubMed] [Google Scholar]
- 150.Heikkila M et al. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology 143, 4358–4365, doi: 10.1210/en.2002-220275 (2002). [DOI] [PubMed] [Google Scholar]
- 151.Vidal V et al. The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3. Genes Dev 30, 1389–1394, doi: 10.1101/gad.277756.116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hao HX et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200, doi: 10.1038/nature11019 (2012). [DOI] [PubMed] [Google Scholar]
- 153.Koo BK et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669, doi: 10.1038/nature11308 (2012). [DOI] [PubMed] [Google Scholar]
- 154.de Lau WB, Snel B & Clevers HC The R-spondin protein family. Genome Biol 13, 242, doi: 10.1186/gb-2012-13-3-242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Szenker-Ravi E et al. RSPO2 inhibition of RNF43 and ZNRF3 governs limb development independently of LGR4/5/6. Nature 557, 564–569, doi: 10.1038/s41586-018-0118-y (2018). [DOI] [PubMed] [Google Scholar]
- 156.Lebensohn AM & Rohatgi R R-spondins can potentiate WNT signaling without LGRs. Elife 7, doi: 10.7554/eLife.33126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ruggiero C & Lalli E Impact of ACTH Signaling on Transcriptional Regulation of Steroidogenic Genes. Front Endocrinol (Lausanne) 7, 24, doi: 10.3389/fendo.2016.00024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Soltani Y et al. Hormonal regulation of the mouse adrenal melanocortinergic system. J Endocrinol Invest 32, 46–51, doi: 10.1007/BF03345678 (2009). [DOI] [PubMed] [Google Scholar]
- 159.Ragazzon B et al. Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis. Endocrinology 147, 1805–1818, doi: 10.1210/en.2005-1279 (2006). [DOI] [PubMed] [Google Scholar]
- 160.Scheys JO, Heaton JH & Hammer GD Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology 152, 3430–3439, doi: 10.1210/en.2010-0986 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zanaria E et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372, 635–641, doi: 10.1038/372635a0 (1994). [DOI] [PubMed] [Google Scholar]
- 162.Muscatelli F et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372, 672–676, doi: 10.1038/372672a0 (1994). [DOI] [PubMed] [Google Scholar]
- 163.Guasti L et al. Dlk1 up-regulates Gli1 expression in male rat adrenal capsule cells through the activation of beta1 integrin and ERK1/2. Endocrinology 154, 4675–4684, doi: 10.1210/en.2013-1211 (2013). [DOI] [PubMed] [Google Scholar]
- 164.Traustadottir GA et al. The imprinted gene Delta like non-canonical Notch ligand 1 (Dlk1) is conserved in mammals, and serves a growth modulatory role during tissue development and regeneration through Notch dependent and independent mechanisms. Cytokine Growth Factor Rev 46, 17–27, doi: 10.1016/j.cytogfr.2019.03.006 (2019). [DOI] [PubMed] [Google Scholar]
- 165.Hadjidemetriou I et al. DLK1/PREF1 marks a novel cell population in the human adrenal cortex. J Steroid Biochem Mol Biol 193, 105422, doi: 10.1016/j.jsbmb.2019.105422 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wyman LC a. C. t. S. Studies on suprarenal insufficiency; the growth of transplanted cortical tissue in the rat. American Journal of Physiology 101, 662 (1932). [Google Scholar]
- 167.Bielohuby M et al. Growth analysis of the mouse adrenal gland from weaning to adulthood: time- and gender-dependent alterations of cell size and number in the cortical compartment. Am J Physiol Endocrinol Metab 293, E139–146, doi: 10.1152/ajpendo.00705.2006 (2007). [DOI] [PubMed] [Google Scholar]
- 168.Yu RN, Ito M, Saunders TL, Camper SA & Jameson JL Role of Ahch in gonadal development and gametogenesis. Nat Genet 20, 353–357, doi: 10.1038/3822 (1998). [DOI] [PubMed] [Google Scholar]
- 169.Wang ZJ et al. Aromatase (Cyp19) expression is up-regulated by targeted disruption of Dax1. Proc Natl Acad Sci U S A 98, 7988–7993, doi: 10.1073/pnas.141543298 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tomooka Y & Yasui T Electron microscopic study of the response of the adrenocortical X-zone in mice treated with sex steroids. Cell Tissue Res 194, 269–277, doi: 10.1007/BF00220393 (1978). [DOI] [PubMed] [Google Scholar]
- 171.Achermann JC, Silverman BL, Habiby RL & Jameson JL Presymptomatic diagnosis of X-linked adrenal hypoplasia congenita by analysis of DAX1. J Pediatr 137, 878–881, doi: 10.1067/mpd.2000.108567 (2000). [DOI] [PubMed] [Google Scholar]
- 172.Steffensen C, Bak AM, Rubeck KZ & Jorgensen JO Epidemiology of Cushing’s syndrome. Neuroendocrinology 92 Suppl 1, 1–5, doi: 10.1159/000314297 (2010). [DOI] [PubMed] [Google Scholar]
- 173.Audenet F, Mejean A, Chartier-Kastler E & Roupret M Adrenal tumours are more predominant in females regardless of their histological subtype: a review. World J Urol 31, 1037–1043, doi: 10.1007/s00345-012-1011-1 (2013). [DOI] [PubMed] [Google Scholar]
- 174.Else T et al. Adrenocortical carcinoma. Endocr Rev 35, 282–326, doi: 10.1210/er.2013-1029 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]