Abstract
Background
Little is known on the association between local signs and intravascular catheter infections. This study aimed to evaluate the association between local signs at removal and catheter-related bloodstream infections (CRBSI), and which clinical conditions may predict CRBSIs if inflammation at insertion site is present.
Methods
We used individual data from four multicenter randomized controlled trials in intensive care units (ICUs) that evaluated various prevention strategies for arterial and central venous catheters. We used multivariate logistic regressions in order to evaluate the association between ≥ 1 local sign, redness, pain, non-purulent discharge and purulent discharge, and CRBSI. Moreover, we assessed the probability for each local sign to observe CRBSI in subgroups of clinically relevant conditions.
Results
A total of 6976 patients and 14,590 catheters (101,182 catheter-days) and 114 CRBSI from 25 ICUs with described local signs were included. More than one local sign, redness, pain, non-purulent discharge, and purulent discharge at removal were observed in 1938 (13.3%), 1633 (11.2%), 59 (0.4%), 251 (1.7%), and 102 (0.7%) episodes, respectively. After adjusting on confounders, ≥ 1 local sign, redness, non-purulent discharge, and purulent discharge were associated with CRBSI. The presence of ≥ 1 local sign increased the probability to observe CRBSI in the first 7 days of catheter maintenance (OR 6.30 vs. 2.61 [> 7 catheter-days], pheterogeneity = 0.02).
Conclusions
Local signs were significantly associated with CRBSI in the ICU. In the first 7 days of catheter maintenance, local signs increased the probability to observe CRBSI.
Keywords: Insertion site, Exit-site, Intravascular catheter, Intravascular catheter infection, Catheter-related bloodstream infection, Local sign
Background
Infections due to central venous catheters and arterial catheters significantly increase hospitalization duration, hospital costs, patient morbidity and mortality in critically ill adult patients [1–3]. Moreover, intravascular catheter-related bloodstream infections are frequent events in the intensive care unit (ICU) setting [4]. Numerous risk factors associated with catheter-related bloodstream infections (CRBSIs) have been identified in several studies [5–8]. More specifically, in the last 20 years, only one old study assessed the exit-site signs as a predictor for intravascular catheter infections [9]. These data reflect practices from an arguably bygone era [10]. Indeed, the use of intravascular catheter bundles has recently changed the landscape of the risk factors [11] and, probably, of the clinical predictors for intravascular catheter infections. Moreover, recent studies suggested that the epidemiology of intravascular catheter-related infections is changing [12, 13]. To the best of our knowledge, there are no recent data on the role of exit-site signs with regards to intravascular catheter infections. In short-term intravascular catheters, the extraluminal route of infection (i.e., originating from the dermal surface) predominates [14]: it is conceivable that local signs at catheter insertion may be linked to intravascular short-term catheter infections. Our primary objective was to determine whether local symptoms and signs at insertion site were associated with CRBSI using data from four randomized controlled trials (RCTs). The secondary objective was to determine which clinical conditions increase the probability to observe CRBSI if inflammation at insertion site is present.
Material and methods
Data sources
This study included four longitudinal databases from four RCTs: DRESSING1 [15], DRESSING2 [16], ELVIS [17] and CLEAN [18]. Merging the data was facilitated by the fact that all these studies rely on the same definitions and similar inclusion criteria. These four studies had similar objectives: to assess the impact of specific prevention strategies on the rate of colonization and infection of intravascular catheters in ICU. Details on the studies are in the Additional file 1. The studies were approved by the national ethics committee. This post hoc analysis was not pre-planned. All RCTs complied with CONSORT guidelines and the current analysis complied with the STROBE guidelines for observational studies [19, 20].
Study patients
The patients were recruited from 2006 to 2014 in various ICUs in France. The patients were included if they required a catheterization with a short-term central venous catheter, a short-term dialysis catheter, or peripheral arterial catheter (AC), with an expected duration of use of more than 48 h (see Additional file 1). The patients underwent follow-up until 48 h after ICU discharge.
Study catheters
For the current analysis, short-term dialysis catheters were considered as central venous catheters (CVCs). All catheters in a given patient were managed in the same way, and all study centers complied with the French recommendations for catheter insertion and care, which are similar to CDC recommendations [21] (see Additional file 1). Catheters were removed if no longer needed, in the case of dysfunction or thrombosis or if an infection was suspected. Catheter tips were cultured using quantitative culture techniques.
Definitions and evaluation criteria
Each study patient was evaluated daily by a team of research nurses. The patient was asked about discomfort at the insertion site, and the site was visually inspected for inflammation. These data were routinely collected at catheter removal. Local symptom (i.e., “pain”) or signs as redness (i.e., redness ≥ 0.5 cm), pain, non-purulent discharge and purulent discharge were noted as either absent or present. We mostly focused on a composite variable including “≥ 1 local signs or symptom.” For sake of simplicity, we used the term “local signs” instead of “local signs or symptom” throughout the text. Of note, the center investigators reported whether a catheter infection was subjectively suspected (“suspicion of infection”). The information on pathological body temperature (body temperature ≥ 38·5 °C or hypothermia with body temperature ≤ 36·5 °C) 24 h before catheter removal was collected and analyzed.
According to French [22] and American guidelines [23], the following definitions were used. A CRBSI (or “infected catheter”) was a combination of (outcome): (1) one or more positive peripheral blood cultures sampled 48 h before or after catheter removal; (2) isolation of the same organism (same species and same susceptibility pattern) from the colonized catheter or from the catheter insertion site, or a blood culture differential time to positivity of 2 h or more [24]; and (3) no apparent source of bacteremia other than the catheter. If a patient had a positive blood culture for coagulase-negative staphylococci (CoNS), a CoNS CRBSI was diagnosed solely if the pulsotype was the same among the strains recovered from the catheter and blood culture. Alternatively, at least two positive cultures with CoNS from separate blood samples were required. Catheter colonization was defined as a quantitative catheter tip culture yielding ≥ 1000 colony-forming units/mL [25]. For patients without catheter cultures, a masked adjudication committee determined whether bloodstream infections were classified as catheter related. The skin colonization was evaluated using semi-quantitative insertion-site cultures: the insertion site was sampled immediately before catheter removal. Because the size of the counting surface was different across studies, we created a semi-quantitative variable with sterile, low-grade colonization, and high-grade colonization according to the median of quantitative cultures obtained in each study.
Statistical analysis
Characteristics of patients and catheters were described as count (percent) or median (interquartile range) for qualitative and quantitative variables, respectively.
The statistical plan had two steps: (1) The primary objective was to evaluate the association between local sign and CRBSI after adjusting for the other CRBSI confounders; (2) the secondary objective was to evaluate the role of the different local signs for observing CRBSI in subgroup of clinically relevant populations. Moreover, we added an explanatory analysis including skin colonization at removal.
We used univariate logistic regressions in order to identify variable associated with CRBSI, and we calculated odds ratios (ORs) for ≥ 1 local sign, redness, pain, non-purulent discharge, and purulent discharge. We then performed multivariate logistic regression forcing the “local sign” (i.e., ≥ 1 local sign, redness, pain, non-purulent discharge, and purulent discharge) variables and adjusting for the other variables associated CRBSI (i.e., outcome) in order to determine the association between local signs and CRBSI. Of note, the final choice of adjustment variables was based on the following clinical well-known risk factors for CRBSI [26]: sex, SOFA score, duration of catheter maintenance, experience of the operator, catheter type, insertion site, skin antisepsis, and antibiotic at insertion [5–7, 18, 27]. Logistic models were stratified for the different centers included in the analysis. Moreover, to evaluate the possible clustering effect of multiple catheters per patient, we performed a sensitivity analysis for the first catheter inserted in an individual patient.
The risk to observe CRBSI for the different local signs in subgroup of clinically relevant populations (i.e., suspicion of catheter infection vs. any suspicion of infection, pathological temperature within the 24 h before removal vs. physiological temperature, duration of catheter maintenance ≤ 7 days vs. > 7 days, catheter type CVC vs. AC, presence of immunosuppression or not, or SOFA score ≤ 11 points vs. > 11 points) was calculated with univariate logistic regression. The heterogeneity between each subgroup of clinically relevant populations was assessed using the Cochran's Q test.
For the explanatory analyses including skin colonization, we compared groups using Chi-square or Fisher tests, as appropriate.
All statistical analyses were performed with SAS (version 9.4) and R (Version 3.5.3) [28, 29].
Results
Description of patients and catheters
A total of 6976 patients and 14,590 catheters (101,182 catheter-days) from 25 ICUs with described local signs were included in this study (see Additional file 1: Figure E1): 2033 (29.1%) from the CLEAN study, 1460 (20.9%) from ELVIS, 1614 (23.1%) from DRESSING1 and 1869 (26.8%) from DRESSING2. The characteristics of the patients and catheters are illustrated in Tables 1, 2, and 3.
Table 1.
Patients’ (n = 6976) characteristics
| Variable | n (%) |
|---|---|
| Sex | |
| Male | 4471 (64.1) |
| Age/median [IQR] | 64 [53; 74] |
| Main reason for ICU admission | |
| Septic shock | 1508 (21.6) |
| Scheduled surgery | 237 (3.4) |
| Trauma | 422 (6) |
| Multi-organ failure | 215 (3.1) |
| Cardiogenic shock | 568 (8.1) |
| Hemorrhagic shock | 294 (4.2) |
| Other shock | 202 (2.9) |
| De novo respiratory failure | 1585 (22.7) |
| COPD exacerbation | 127 (1.8) |
| Renal failure | 533 (7.6) |
| Coma | 662 (9.5) |
| Continuous surveillance | 623 (8.9) |
| No comorbidity | 4098 (58.7) |
| Cancer | 377 (5.4) |
| Chronic renal failure | 348 (5) |
| Chronic heart failure | 513 (7.4) |
| AIDS | 158 (2.3) |
| Immunosuppression | 394 (5.6) |
| Solid organ transplantation | 269 (3.9) |
| Hematologic neoplasia or metastatic cancer | 353 (5.1) |
| Diabetes mellitus | 557 (8) |
| Chronic respiratory failure | 359 (5.1) |
| Mechanical ventilation at admission | 5167 (74.1) |
| Vasopressor at admission | 2548 (36.5) |
| SOFA score, median (IQR) | 10.5 [7; 14] |
| Length of stay in hospital, median (IQR) | 24 [12; 46] |
| ICU mortality | 2343 (33.6) |
IQR, interquantile range; ICU, intensive care unit; AIDS, acquired immunodeficiency syndrome; SOFA, Sequential Organ Failure Assessment
Table 2.
Catheters’ (n = 14,590) characteristics
| Variable | n (%) |
|---|---|
| Catheter days, mean (SD)/median [IQR] | 6.9 (6.5)/5 [2; 9] |
| CVC | 8500 (58.3) |
| Experience of the operator | |
| Junior (< 50 procedures) | 8828 (60.5) |
| Senior (≥ 50 procedures) | 5762 (39.5) |
| Insertion site | |
| Jugular | 2706 (18.5) |
| Subclavian | 2160 (14.8) |
| Femoral | 5795 (39.7) |
| Radial | 3929 (26.9) |
| Mechanical ventilation at insertion | 11,104 (76.1) |
| Vasopressor at insertion | 7804 (53.5) |
| Antibiotic at insertion | 9149 (62.7) |
SD, standard deviation; IQR, interquantile range; CVC, central venous catheter
Table 3.
Symptoms, local signs and outcomes at catheter removal (n = 14,590)
| Variable | |
|---|---|
| Catheter removal for suspected infection | 2034 (13.9) |
| Temperature ≤ 36.5 or ≥ 38.5 at removal | 7979 (54.7) |
| Local signs or symptom | |
| ≥ 1 local sign | 1938 (13.3) |
| Redness | 1633 (11.2) |
| Pain | 59 (0.4) |
| Non-purulent discharge | 251 (1.7) |
| Purulent discharge | 102 (0.7) |
| Outcomes | |
| Catheter colonization | 1186 (8.1) |
| CRBSI | 114 (0.8) |
| Reason for removal | |
| Death | 3183 (21.8) |
| Catheter no longer needed | 4577 (31.4) |
| Suspicion of infection | 1981 (13.6) |
| Exit ICU | 3098 (21.2) |
| Symptoms or signs at removal | |
| Catheter removal for suspected infection | 2034 (13.9) |
| Temperature ≤ 36.5 or ≥ 38.5 at removal | 7979 (54.7) |
CRBSI, catheter-related bloodstream infection
There were 8500 (58.3%) CVCs and 6090 (41.7%) ACs.
Overall, 13.9% (2034) catheters were removed for suspected infection, whereas pathological body temperature was present in 54.7% (7979) of catheters at removal. At least one local sign, redness, pain, non-purulent discharge, and purulent discharge were observed in 13.3% (1938), 11.2% (1633), 0.4% (59), 1.7% (251), 0.7% (102) of cases, respectively.
The incidence-density per 1000 catheter-days was 1.1 for CRBSI (0.8% of the total number of catheters, 114 events) and 11.7 for catheter colonization (8.1%, 1186).
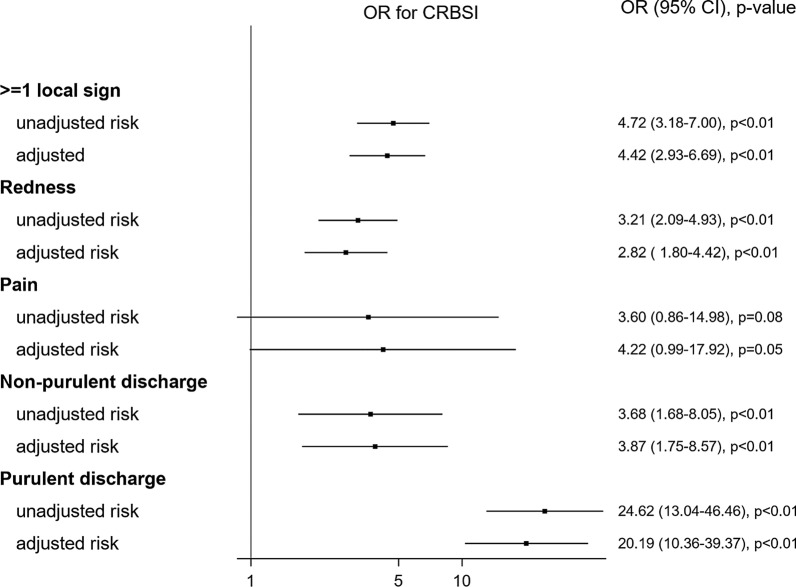
Odds ratios (ORs) for unadjusted and adjusted local signs for CRBSI are illustrated in Fig. 1.
Fig. 1.
Unadjusted and adjusted local sign risk for catheter-related bloodstream infection. We adjusted for the following confounding factors for CRBSI: Sex, SOFA, catheter days, catheter type, experience of the operator, insertion site, skin antisepsis, CHG-dressing and antibiotics at insertion. OR, odds ratio; CI, confidence interval; CRBSI, catheter-related bloodstream infection
At least one local sign
At least one local sign was associated with CRBSI in the univariate (OR 4.72, 95% confidence interval [CI] 3.18–7.00, p < 0.01, Fig. 1 and Additional file 1: Table E1) and after adjusting on confounders (OR 4.42, 95% CI 2.93–6.69, p < 0.01, Fig. 1 and Additional file 1: Table E2). For ≥ 1 local sign, similar results were observed only when the first catheter in an individual patient was considered (OR 4.63, 95% CI 2.35–9.12, p < 0.01). This association remained statistically significant from 2007 to 2014 (data not shown).
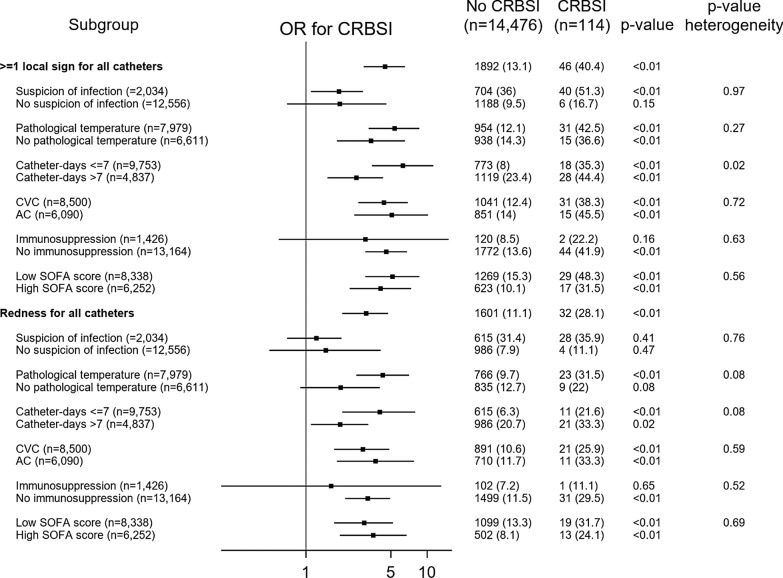
At least one sign was present in 40.4% of the infected catheters (vs. 13.1% of the non-infected catheters, p < 0.01, Fig. 2), and its probability to observe CRBSI was higher in the first 7 days of catheter maintenance (OR 6.30 vs. OR 2.61 for > 7 days, p for heterogeneity = 0.02).
Fig. 2.
Probability to observe catheter-related bloodstream infection for the variable ≥ 1 local sign or redness in different subgroups. The group immunosuppression included AIDS patients, solid organ transplantation and other immunosuppression (see Table 1). CRBSI, catheter-related bloodstream infection (or infected catheter); CVC, central venous catheter; AC, arterial catheter; SOFA, sequential organ failure assessment. Low SOFA: ≤ 11 points. High SOFA: > 11 points
However, suspicion of infection (pfor heterogeneity = 0.97), pathological temperature at removal (pfor heterogeneity = 0.27), the catheter type (pfor heterogeneity = 0.72), and SOFA score (pfor heterogeneity = 0.56) did not increase the probability to observe CRBSI. We observed a non-significantly increased OR for ≥ 1 local sign (3.1, 95% CI 0.64–15.03, p = 0.16) in the immunosuppressed population (n = 1426), whereas in non-immunosuppressed patients (n = 13,164) a significantly increased CRBSI risk for ≥ 1 local sign was observed (OR 4.59, 95% CI 3.11–6.79, p < 0.01). We did not identify a significant heterogeneity between these two groups (pfor heterogeneity = 0.63).
Redness
In the univariate logistic regression, redness was associated with CRBSI (OR 3.21, 95% CI 2.09–4.93, p < 0.01, Fig. 1 and Additional file 1: Table E1). After adjusting for CRBSI confounding factors, the OR for redness was 2.82 (95% CI 1.80–4.42, p < 0.01, Fig. 1 and Additional file 1: Table E3). Similar results for CRBSI were observed only when the first catheter in an individual patient was considered (OR 3.48, 95% CI 1.69–7.15, p < 0.01).
Among CRBSI, 28.1% showed redness at the insertion site (vs. non CRBSI 11.1%, p < 0.01, Fig. 2). Redness at insertion site was less prevalent for a catheter maintenance ≤ 7 days, but increased the probability to observe CRBSI (OR 4.06 vs. OR 1.92 [> 7 days], pfor heterogeneity = 0.08, Fig. 2). Similarly, temperature at removal showed marginal significance (pfor heterogeneity = 0.08). However, the catheter type (pfor heterogeneity = 0.59), immunosuppression (pfor heterogeneity = 0.52) and SOFA score (pfor heterogeneity = 0.66) did not increase the probability to observe CRBSI.
Pain
In the univariate analysis, pain was not significantly associated with CRBSI (OR 3.60, 95% CI 0.86–14.98, p = 0.08, Fig. 1 and Additional file 1: Table E1). After adjusting for confounding factors, pain was marginally associated with CRBSI (OR 4.22, 95% CI 0.99–17.92, p = 0.05, Fig. 1 and Additional file 1: Table E4). No heterogeneity was observed between specific subgroups in predicting CRBSI (data not shown).
Non-purulent discharge
In the univariate analysis, non-purulent discharge was associated with CRBSI (OR 3.68, 95% CI 1.68–8.05, p < 0.01, Fig. 1 and Additional file 1: Table E1). After adjusting for confounding factors, the OR for non-purulent discharge was 3.87 (95% CI 1.75–8.57, p < 0.01, Fig. 1, and Additional file 1: Table E5). Non-purulent discharge at insertion for a catheter maintenance ≤ 7 days increased the probability to observe CRBSI (OR 7.37 vs. OR 1.49 for > 7 days, pfor heterogeneity = 0.07, Additional file 1: Figure E2).
Purulent discharge
In the univariate analysis, purulent discharge was associated with CRBSI (OR 24.62, 95% CI 13.04–46.46, p < 0.01, Fig. 1, and Additional file 1: Table E1). After adjusting for confounding factors, purulent discharge was associated with CRBSI (OR 20.19, 95% CI 10.36–39.37, p < 0.01, Fig. 1, and Additional file 1: Table E6). Purulence was more frequently observed in infected catheters (11.4% vs. 0.6% of non-infected catheters, p < 0.01, Additional file 1: Figure E2).
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive and negative likelihood ratio
The sensitivity, specificity, and PPV, NPV of each local sign for predicting CRBSI are illustrated in Table 4.
Table 4.
Sensitivity, Specificity, positive predictive value, negative predictive value, positive and negative likelihood ratio for CRBSI (n = 114)
| Prevalence of local sign | Sensitivity | Specificity | PPV | NPV | Positive LR | Negative LR | |
|---|---|---|---|---|---|---|---|
| ≥ 1 local sign (n = 1938) | 13.3 | 40.4 | 86.9 | 2.4 | 99.5 | 3.09 | 0.69 |
| Redness (n = 1633) | 11.2 | 28.1 | 88.9 | 2.0 | 99.4 | 2.54 | 0.81 |
| Pain (n = 59) | 0.4 | 1.7 | 99.6 | 3.3 | 99.2 | 4.46 | 0.99 |
| Non-purulent discharge (n = 251) | 1.7 | 6.1 | 98.3 | 2.8 | 99.3 | 3.64 | 0.95 |
| Purulent discharge (n = 102) | 0.7 | 11.4 | 99.4 | 12.7 | 99.3 | 18.55 | 0.99 |
PPV, positive predictive value; NPV, negative predictive value; LR likelihood ratio
The sensitivity for ≥ 1 local sign was 40.4%, whereas the highest specificities were observed for pain (99.6%) and purulent discharge (98.4%). PPV was low for redness (2%), pain (3%), non-purulent discharge (3%) and ≥ 1 local sign (2%), whereas PPV increased for purulent-discharge (12.7%). NPVs were high for all local signs.
Purulent discharge showed the highest positive likelihood ratio (18.55, 95% CI 10.68–32.21), whereas ≥ 1 local sign reduced the evidence for CRBSI (negative likelihood ratio 0.69, 95% CI 0.59–0.80). Among catheter removed for suspected infection (n = 2034), the NPV for ≥ 1 local sign was 97% (95% CI 95.97–97.91, data not shown). Interestingly, within the first 7 days of catheter maintenance, the positive likelihood ratio for redness and ≥ 1 local sign increased to 8.84 (95% CI 7.26–10.77) and 4.43 (95% CI 3.04–6.46, data not shown), respectively.
Microorganism identified among CRBSI
CRBSIs were more frequently caused by Enterobacteriaceae (n = 25), polymicrobial (n = 24), S. aureus (n = 23), and non-fermenting Gram-negative bacilli (n = 16, Additional file 1: Table E7). Coagulase negative Staphylococci (CoNS) were observed in 15 CRBSI episodes.
Skin colonization at catheter removal
The skin colonization at catheter removal was significantly more often colonized in case of ≥ 1 local sign (p < 0.01), redness (p < 0.01), non-purulent (p = 0.01), and purulent discharge (p < 0.01, Additional file 1: Table E8). The skin colonization did not significantly differ according to pain at removal (p = 0.74).
Discussion
Through high quality data from four multi-centric RCTs and after correction for other confounders, this post hoc analysis showed that local signs at the exit site were clearly associated with intravascular catheter infections in both CVCs and ACs. This finding was more pronounced for redness, non-purulent discharge, and purulent discharge.
Three old studies examined the condition of the insertion site as a clinical predictor of CRBSI in adults. The first study included 169 patients, and the authors illustrated that infection was also associated with redness at the insertion site greater than 4 mm in diameter [30]. The second was a prospective, observational study that enrolled 1353 CVCs and showed that inflammation at the catheter site was absent in approximately 70% of CRBSIs [31]. To the best of our knowledge, only one observational study exhaustively explored this research question in the last 20 years [9]. In 2002, Safdar et al. showed that the insertion site appearance was not associated with catheter colonization or CVC-related bloodstream infections [9]. However, this study (1) reported old data collected from 1998 to 2000, probably reflecting the era prior to the routine implementation of infection prevention bundles; (2) included less than one-tenth of all catheters included in our post hoc analysis; (3) yielded a large proportion of CoNS among CRBSI; and (4) analyzed only CVC, without including AC. Similarly to the DRESSING1 and CLEAN trials, Safdar et al. also included patients from two RCTs [9], one evaluating chlorhexidine-gluconate sponge dressings [32] and the other evaluating chlorhexidine skin disinfection for prevention of CRBSI [33]. In contrast to Safdar et al., local signs in our analysis were significantly associated with an increased risk for CRBSI. To support our findings, we found that skin colonization at catheter removal occurred more frequently if local signs were present. Moreover, we observed that an important proportion of CRBSI were due to S. aureus and Gram-negative bacilli, organisms that elicit more inflammation compared to CoNS which are less virulent [34] and in our study represented only 13% of CRBSI. In contrast, in the Safdar’s study, 87% of microorganisms identified were CoNS. In this context, and consistently with our findings, a change in the epidemiology of intravascular catheter infection toward lower prevalence of CoNS and increasing proportion of Gram-negative microorganisms has been documented in several recent studies [12, 13, 35]. In light of these considerations, the description of this cohort may probably better represent this issue.
Our findings have some important clinical Implications which, to date, have never been assessed. First, compared to the most recent literature available, local signs are associated with CRBSI, and their presence should elicit investigations for diagnosing potential intravascular catheter infections. Second, local signs may help clinicians in a specific clinical condition: if at least one local sign is present within the first 7 days of catheter maintenance, it further increases the probability to observe a catheter infection. Therefore, clinicians should deserve particular attention to the catheter insertion site in the first week after the insertion and a promptly catheter removal should be considered. Moreover, and not surprisingly, purulence at insertion site is a strong reason to remove a catheter. Interestingly, immunosuppression, pathological temperature at removal, catheter type, and severity of illness with the presence of local signs did not significantly help clinicians in predicting CRBSI. Moreover, the solely intuition of the physician who subjectively suspected an intravascular catheter infection in the presence of local signs did not increase the probability to observe a CRBSI. On the other hand, in catheters removed for suspected infection without any local signs, the probability to get infected remained low. An old RCT including a relatively low number of hemodynamically stable critically ill patients without proven bacteremia and local sign at insertion site illustrated that a “watchful waiting strategy” (versus immediate catheter removal) permitted a substantial decrease in the number of unnecessarily removed CVCs without increased morbidity [36]. In the case of suspicion of infection without any local signs, a catheter retention may be considered and would reduce unessentially removals. A post hoc analysis for this subpopulation in our cohort showed that CRBSI were not associated with increased mortality (OR 1.37, 95% CI 0.68–2.77, p = 0.38), thus suggesting, at least when catheters were routinely removed, a low mortality risk for this population.
Our study has important limitations. First, it was an observational study, and unmeasured factors may persist and cause residual confounding. However, we presented high quality exhaustive data that were prospectively collected by trained investigators and study monitors during all RCTs. Second, all RCTs were conducted in University-affiliated ICUs in France from 2006 to 2014 and included only selected short-term intravascular catheters, thus limiting the generalizability of our results. Third, we did not have any data reported for the variable “pain” in intubated or comatose patients at removal, thus probably leading to an underestimation of the proportion of this specific symptom. Fourth, redness was defined as ≥ 5 mm diameter: a smaller threshold compared to the IDSA guidelines which declared 2 cm as relevant for CRBSI [23]. We selected our cutoff in reason of the results of an old study which illustrated that infection was associated with redness > 4 mm in diameter [30]. Fifth, we described a large database designed to investigate the impact of certain prevention measures, and interactions may have occurred among the various study groups. Finally, PPVs of local signs were low, thus limiting the utilization of local signs to create algorithms for better decision-making in all patients with short-term intravascular catheters.
Conclusions
Using the largest dataset ever collected from four multi-centric RCTs conducted with consistent catheter care, we showed that local signs were clearly associated with infections in short-term catheters in the ICU. In the first 7 days of catheter maintenance, local signs increased the probability to observe CRBSI.
Supplementary Information
Additional file 1: Supplementary Material of “Local signs at insertion site and catheter-related bloodstream infections. A post hoc analysis using individual data of four RCTs”. Description: supplementary methods (data sources, patients and study catheters), supplementary Figures (Figure E1 flow-chart; Figure E2 Risk for developing CRBSI for non-purulent discharge and purulent discharge by different subgroups), supplementary Tables (Table E1: Univariate logistic model for catheter-related bloodstream infection, stratification by ICU; Table E2: Multivariate logistic models for CRBSI forcing the variable ≥ 1 local sign, stratification by ICU; Table E3: Multivariate logistic models for CRBSI forcing the variable redness, stratication by ICU; Table E4: Multivariate logistic models for CRBSI forcing the variable pain, stratification by ICU; Table E5: Multivariate logistic models for CRBSI forcing the variable non-purulent discharge, stratification by ICU; Table E6: Multivariate logistic model for CRBSI stratified by ICU forcing the variable purulent discharge; Table E7: Microorganism identified in CRBSI, E8 Skin colonization at catheter removal), supplementary references.
Acknowledgement
The authors thank Céline Féger, M.D., (EMIBiotech) for her editorial support.
Abbreviations
- AC
Peripheral arterial catheter
- CoNS
Coagulase-negative staphylococci
- CRBSI
Catheter-related bloodstream infections
- CVC
Central venous catheter
- ICU
Intensive care unit
- NPV
Negative predictive value (NPV)
- OR
Odds ratio
- PPV
Positive predictive value
- RCT
Randomized controlled-trial
- SOFA score
Sequential Organ Failure Assessment Score
Authors’ contributions
NB, SR and JFT analyzed and interpreted the data. OM, BS, MGO, CS, JCL, OM, LB were responsible for the data collection. NB and JFT were the major contributors in writing the manuscript. All authors read and approved the final manuscript.
Funding
NB is currently receiving a Mobility grant from the Swiss National Science Foundation (Grant Number: P400PM_183865) and a grant from the Bangerter-Rhyner Foundation. These grants support his fellowship in France.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All studies were approved by the national ethics committees.
Consent for publication
Not applicable.
Competing interests
The authors have disclosed that they do not have conflict of interest. JFT received fees for lectures to 3M, MSD, Pfizer, and Biomerieux. JFT received research grants from Astellas, 3M, MSD, and Pfizer. JFT participated to advisory boards of 3M, MSD, Bayer Pharma, Nabriva, and Pfizer. OM received fees for lectures for 3M and BD. OM received research grants from BD.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-020-03425-0.
References
- 1.Leistner R, Hirsemann E, Bloch A, Gastmeier P, Geffers C. Costs and prolonged length of stay of central venous catheter-associated bloodstream infections (CVC BSI): a matched prospective cohort study. Infection. 2014;42(1):31–36. doi: 10.1007/s15010-013-0494-z. [DOI] [PubMed] [Google Scholar]
- 2.Stevens V, Geiger K, Concannon C, Nelson RE, Brown J, Dumyati G. Inpatient costs, mortality and 30-day re-admission in patients with central-line-associated bloodstream infections. Clin Microbiol Infect. 2014;20(5):O318–O324. doi: 10.1111/1469-0691.12407. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29–36. doi: 10.1007/s15010-014-0689-y. [DOI] [PubMed] [Google Scholar]
- 4.ECDC. Healthcare associated infections acquired in intensive care units - annual epidemiological report for 2016. Stockholm (Sweden): European Centre for Disease Prevention and Control; 2018. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2016-HAI_0.pdf. Accessed 3 Oct 2020.
- 5.Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O'Grady NP, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753–771. doi: 10.1086/676533. [DOI] [PubMed] [Google Scholar]
- 6.Buetti N, Ruckly S, Lucet JC, Mimoz O, Souweine B, Timsit JF. Short-term dialysis catheter versus central venous catheter infections in ICU patients: a post hoc analysis of individual data of 4 multi-centric randomized trials. Intensive Care Med. 2019;45(12):1774–1782. doi: 10.1007/s00134-019-05812-w. [DOI] [PubMed] [Google Scholar]
- 7.Lucet JC, Bouadma L, Zahar JR, Schwebel C, Geffroy A, Pease S, et al. Infectious risk associated with arterial catheters compared with central venous catheters. Crit Care Med. 2010;38(4):1030–1035. doi: 10.1097/CCM.0b013e3181d4502e. [DOI] [PubMed] [Google Scholar]
- 8.Timsit JF, Bouadma L, Mimoz O, Parienti JJ, Garrouste-Orgeas M, Alfandari S, et al. Jugular versus femoral short-term catheterization and risk of infection in intensive care unit patients. Causal analysis of two randomized trials. Am J Respir Crit Care Med. 2013;188(10):1232–1239. doi: 10.1164/rccm.201303-0460OC. [DOI] [PubMed] [Google Scholar]
- 9.Safdar N, Maki DG. Inflammation at the insertion site is not predictive of catheter-related bloodstream infection with short-term, noncuffed central venous catheters. Crit Care Med. 2002;30(12):2632–2635. doi: 10.1097/00003246-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Brun-Buisson C. New technologies and infection control practices to prevent intravascular catheter-related infections. Am J Respir Crit Care Med. 2001;164(9):1557–1558. doi: 10.1164/ajrccm.164.9.2109051. [DOI] [PubMed] [Google Scholar]
- 11.Callister D, Limchaiyawat P, Eells SJ, Miller LG. Risk factors for central line-associated bloodstream infections in the era of prevention bundles. Infect Control Hosp Epidemiol. 2015;36(2):214–216. doi: 10.1017/ice.2014.32. [DOI] [PubMed] [Google Scholar]
- 12.Braun E, Hussein K, Geffen Y, Rabino G, Bar-Lavie Y, Paul M. Predominance of Gram-negative bacilli among patients with catheter-related bloodstream infections. Clin Microbiol Infect. 2014;20(10):O627–O629. doi: 10.1111/1469-0691.12565. [DOI] [PubMed] [Google Scholar]
- 13.Buetti N, Lo Priore E, Atkinson A, Widmer AF, Kronenberg A, Marschall J, et al. Catheter-related infections: does the spectrum of microbial causes change over time? A nationwide surveillance study. BMJ open. 2018;8(12):e023824. doi: 10.1136/bmjopen-2018-023824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mermel LA. What is the predominant source of intravascular catheter infections? Clin Infect Dis. 2011;52(2):211–212. doi: 10.1093/cid/ciq108. [DOI] [PubMed] [Google Scholar]
- 15.Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste-Orgeas M, Pease S, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231–1241. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 16.Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste-Orgeas M, Alfandari S, et al. Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J Respir Crit Care Med. 2012;186(12):1272–1278. doi: 10.1164/rccm.201206-1038OC. [DOI] [PubMed] [Google Scholar]
- 17.Souweine B, Lautrette A, Gruson D, Canet E, Klouche K, Argaud L, et al. Ethanol lock and risk of hemodialysis catheter infection in critically ill patients. A randomized controlled trial. Am J Respir Crit Care Med. 2015;191(9):1024–1032. doi: 10.1164/rccm.201408-1431OC. [DOI] [PubMed] [Google Scholar]
- 18.Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V, et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet. 2015;386(10008):2069–2077. doi: 10.1016/S0140-6736(15)00244-5. [DOI] [PubMed] [Google Scholar]
- 19.Eaton LA. CONSORT guidelines. In: Gellman MD, Turner JR, editors. Encyclopedia of behavioral medicine. New York: Springer; 2013. pp. 486–487. [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timsit JF. Updating of the 12th consensus conference of the Societe de Reanimation de langue francaise (SRLF): catheter related infections in the intensive care unit. Ann Fr Anesth Reanim. 2005;24(3):315–322. doi: 10.1016/j.annfar.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blot F, Nitenberg G, Chachaty E, Raynard B, Germann N, Antoun S, et al. Diagnosis of catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet. 1999;354(9184):1071–1077. doi: 10.1016/S0140-6736(98)11134-0. [DOI] [PubMed] [Google Scholar]
- 25.Brun-Buisson C, Abrouk F, Legrand P, Huet Y, Larabi S, Rapin M. Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch Intern Med. 1987;147(5):873–877. doi: 10.1001/archinte.1987.00370050069012. [DOI] [PubMed] [Google Scholar]
- 26.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann t Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 27.Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700–707. doi: 10.1001/jama.286.6.700. [DOI] [PubMed] [Google Scholar]
- 28.The SAS system for Windows. Version 9.4. SAS Inst.
- 29.R Development Core Team .R. 2005.
- 30.Armstrong CW, Mayhall CG, Miller KB, Newsome HH, Jr, Sugerman HJ, Dalton HP, et al. Clinical predictors of infection of central venous catheters used for total parenteral nutrition. Infect Control Hosp Epidemiol. 1990;11(2):71–78. doi: 10.2307/30144265. [DOI] [PubMed] [Google Scholar]
- 31.Pittet D, Rae AC, Auckenthaler R. Clinical diagnosis of central venous catheter line infections: a difficult job. Abstract 453. Programs and abstracts of the 31st interscience conference on antimicrobial agents and chemotherapy. Washington, DC: American Society for Microbiology; 1991. [Google Scholar]
- 32.Maki DG NL, Knasinski V et al. Prospective randomized investigator masked trial of a novel chlorhexidine-impregnated sponge (Biopatch) on central venous and arterial catheters. In: Programs and proceedings of the 4th decennial conference on nosocomial and healthcare associated infection. Atlanta, GA, March 5–9, 2000. Infection Control & Hospital Epidemiology 2000; 21:96.
- 33.Maki DG KV, Narans LL, et al. A randomized trial of a novel 1% chlorhexidine-75% alcohol tincture versus 10% povidone-iodine for cutaneous disinfection with vascular catheters. Abstr. In: 31st annual society for hospital epidemiology of America meeting. Society for Hospital Epidemiology of America, 2001, Toronto, Canada.
- 34.von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002;2(11):677–685. doi: 10.1016/S1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- 35.Novosad SA, Fike L, Dudeck MA, Allen-Bridson K, Edwards JR, Edens C, et al. Pathogens causing central-line-associated bloodstream infections in acute-care hospitals-United States, 2011–2017. Infect Control Hosp Epidemiol. 2020;41(3):313–319. doi: 10.1017/ice.2019.303. [DOI] [PubMed] [Google Scholar]
- 36.Rijnders BJ, Peetermans WE, Verwaest C, Wilmer A, Van Wijngaerden E. Watchful waiting versus immediate catheter removal in ICU patients with suspected catheter-related infection: a randomized trial. Intensive Care Med. 2004;30(6):1073–1080. doi: 10.1007/s00134-004-2212-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Material of “Local signs at insertion site and catheter-related bloodstream infections. A post hoc analysis using individual data of four RCTs”. Description: supplementary methods (data sources, patients and study catheters), supplementary Figures (Figure E1 flow-chart; Figure E2 Risk for developing CRBSI for non-purulent discharge and purulent discharge by different subgroups), supplementary Tables (Table E1: Univariate logistic model for catheter-related bloodstream infection, stratification by ICU; Table E2: Multivariate logistic models for CRBSI forcing the variable ≥ 1 local sign, stratification by ICU; Table E3: Multivariate logistic models for CRBSI forcing the variable redness, stratication by ICU; Table E4: Multivariate logistic models for CRBSI forcing the variable pain, stratification by ICU; Table E5: Multivariate logistic models for CRBSI forcing the variable non-purulent discharge, stratification by ICU; Table E6: Multivariate logistic model for CRBSI stratified by ICU forcing the variable purulent discharge; Table E7: Microorganism identified in CRBSI, E8 Skin colonization at catheter removal), supplementary references.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.