Abstract
Like axon guidance, the tuning of vascular tip cells during angiogenesis is an intriguing but puzzling developmental process. A new study in zebrafish (Liu et al., 2020) now demonstrates a critical role of the Piezo1 mechanosensitive ion channel in guiding vascular tip cells in pathfinding.
The vascular system is the first to be functional during embryonic development. Vascular development starts as angioblasts form a primitive vascular plexus through a process known as vasculogenesis. Next, new blood vessels sprout from the existing vessel network and vascularize nearby tissue. This process, known as angiogenesis, requires a fate switch of endothelial progenitor cells to tip cells at the leading edge. A tip cell is analogous to the growth cone of a developing axon. It sends out dynamic filopodia to sense the microenvironment and finds its target vessel by balancing pro- and anti-angiogenic cues, including vascular growth factors, extracellular matrix, cell adhesion, and guidance molecules. As the tip cell advances, it induces adjacent endothelial progenitors to become the stalk cells and proliferate, resulting in a growing bud (Figure 1) (Chung and Ferrara, 2011). The tip cell will further navigate the new sprouting vessel though the tissue and find a vessel target. Although vascular endothelial growth factor (VEGF) and lateral inhibition from Delta-Notch signaling coordinately determine cell fate and initiate budding (Blanco and Gerhardt, 2013), how tip cells make appropriate pathfinding decisions to achieve a highly organized 3D vessel network in tissue remains an open question.
Figure 1. Biochemical and Mechanical Signaling in Angiogenesis.
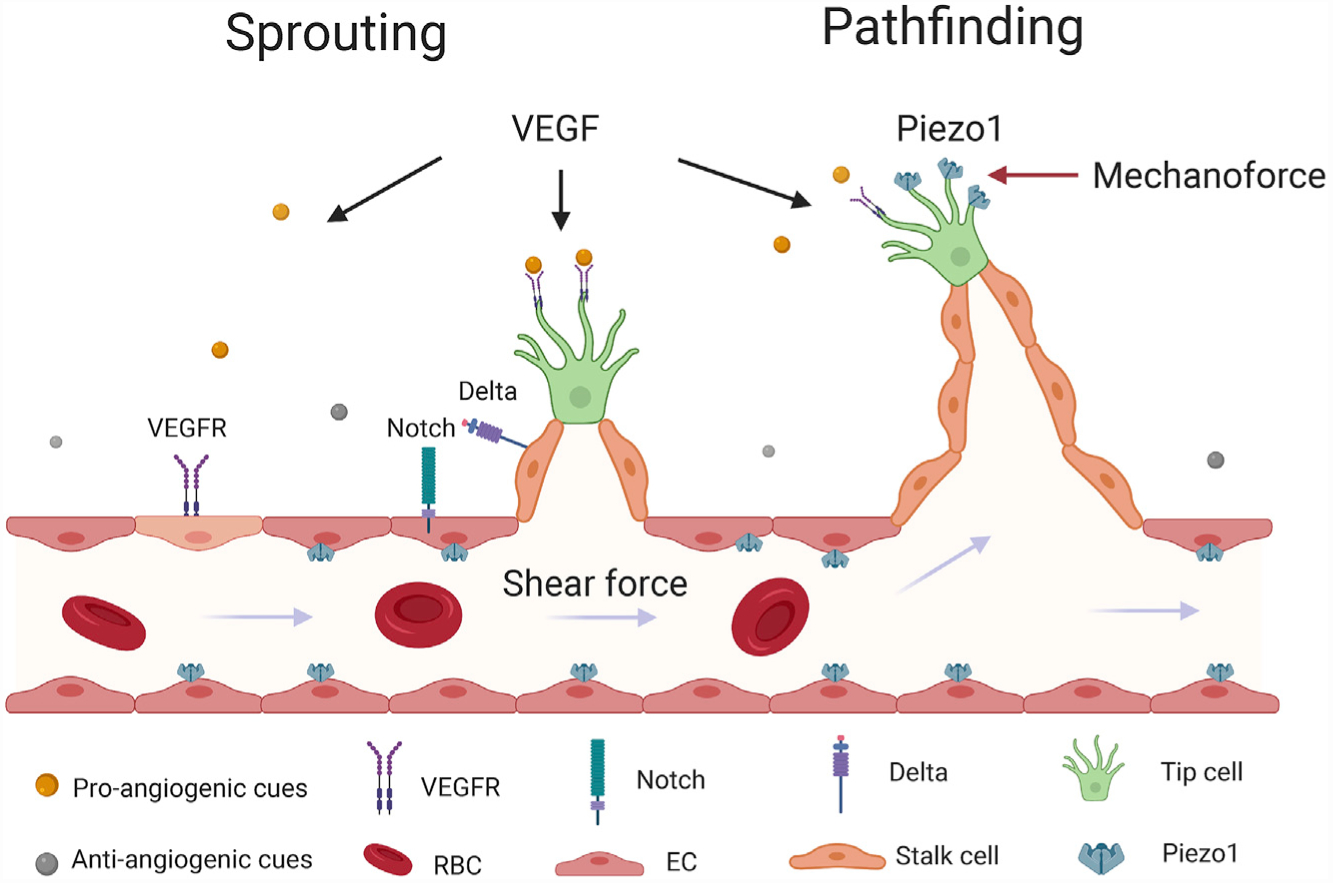
The spatial patterning of the pro- and anti-angiogenic cues determine the site of angiogenesis and the fate conversion of tip cells. During the extension of sprouting vessels, tip cells sense not only VEGF signals, but also local mechanical properties and membrane tension with Piezo1 mechanosensitive channels on their branches, which collaboratively determine the extension/retraction of the branches and tip cell pathfinding. In the stabilized vessels, Piezo1 also senses the shear force from the blood flow and maintains vascular tone and function. VEGF, vascular growth factors; VEGFR, vascular growth factor receptors; RBC, red blood cell; EC, endothelial cell.
Zebrafish larvae are an excellent model for monitoring early vascular development, including the dynamic behavior of tip cells and their intracellular responses to pro- and anti-angiogenic cues occurring around 3 days post-fertilization (Betz et al., 2016). Using this model, previous studies have demonstrated that calcium dynamics are critical for intersegmental vessel budding from the dorsal aorta. For example, VEGF signaling activates phospholipase C and subsequently opens the inositol triphosphate receptor (IP3R) calcium channel on the endoplasmic reticulum, resulting in robust cytosolic calcium oscillations. In this issue of Neuron, a new study from Liu et al. closely followed the sprouting vessels that grow into the zebrafish midbrain with high-resolution in vivo confocal imaging and found calcium dynamics exist in the branches of cerebral tip cells (Liu et al., 2020). More interestingly, branches with strong and frequent calcium transients (> 1 event per 10 min) almost all retracted within minutes, while branches with infrequent calcium transients were able to continue their journey. The authors also found that blocking the frequent calcium transients using a photoactivatable chelator, Diazo-2, prevented the branches from retraction, whereas completely abolishing calcium transients with a cell-permeant chelator, BAPTA-AM, stabilized the branches, suggesting that the frequency of calcium dynamics determines the fate of tip cell branches. The authors confirmed this hypothesis with controlled calcium release from a photolabile chelator, Nitrophenyl-EGTA, with laser pulses, as 5 pulses per 10 min triggered branch retraction, whereas 0.5 pulse per 10 min induced branch extension. They further found that the differential effects on branch selection are caused by two distinct mechanisms. First, frequent calcium transients activate calpain, a calcium-dependent cysteine protease that dismantles the focal adhesion and causes branch retraction, which could be blocked by a selective inhibitor: calpeptin. On the other hand, infrequent calcium transients are sufficient to maintain endothelial nitric oxide synthase (NOS) activity via a calmodulin-dependent mechanism (Förstermann and Sessa, 2012), and nitric oxide production is required for branch extension (Liu et al., 2020).
The Piezo is a family of pore-forming ion channels involved in converting mechano-stimulation to intracellular calcium signal (Ranade et al., 2015). As the mechanosensory ion channel Piezo1 conducts stretch-evoked calcium influx and flow-induced calcium transients and activates both calpain and NOS in endothelial cells (Li et al., 2014), the authors next tested if Piezo1 was the master regulator of pathfinding. For this the authors created piezo1 knockout zebrafish (piezo1−/−) with CRISPR-Cas9-mediated genome editing on exon 19. Surprisingly, the calcium transients in branches of midbrain tip cells were nearly abolished in the piezo1−/− larvae. Piezo1−/− zebrafish also exhibited abnormal tip cell development with excessive branches and a loss of balance between extension and retraction, resulting in an over-vascularized phenotype in midbrain (Liu et al., 2020). The authors further showed that Piezo1 on the branches is sensitive to mechanical stimulation induced by either negative pressure or substrate stiffness, which resulted in a robust calcium influx and retraction of the targeted branch. Pharmacological manipulation of Piezo1 function in vivo with the spider venom peptide GsMTx4 blocked the mechanically induced calcium transients and branch retraction, whereas Piezo1-specific agonist Yoda1 (Li et al., 2014) triggered high-frequency calcium oscillations in the quiescent branches, sufficient to induce retraction. From these observations, the authors conclude that Piezo1 converts the tissue mechanoproperties to local calcium dynamics in tip cells and controls branch retraction through calpain and branch extension through NOS, respectively, which is key to the pathfinding of sprouting vessels and brain vascular patterning (Liu et al., 2020).
Endothelial cells’ capability in integrating both biochemical and mechanical signals is key to successful angiogenesis (Santos-Oliveira et al., 2015). They are known to express a repertoire of mechanosensitive machineries, including adhesion complexes formed by platelet-endothelial cell adhesion molecule (PECAM-1); integrins and cytoskeletal proteins; the surface proteoglycan/glycoprotein layer known as the glycocalyx; and ion channels including members of the potassium channels, transient receptor potential family, and the Piezo channels. This is evolutionarily conserved, at least in vertebrates, and suggests that endothelial cells are constantly experiencing various mechanical forces from blood flow and surrounding tissue. As mentioned before, Piezo channels are involved in converting mechanostimulation to intracellular calcium signal (Ranade et al., 2015). Between the two mammalian homologs, Piezo1 is a cell stretch sensor for tension and shear force, whereas Piezo2 plays a key role in light-touch sensation (Ranade et al., 2015). Piezo1 is highly expressed in the vascular system. Besides its function in sensing shear stress by endothelial cells, it is also critical for vascular development and cell alignment in major arteries in mice (Li et al., 2014). Although Piezo1 may have different functions in different species, the current study now shows for the first time that the Piezo1 mechanosensitive channel also plays a role in tip cells and their pathfinding.
As the data suggested that Piezo1 is responsible for ~80% of frequent and half of infrequent calcium transients (Liu et al., 2020), it is still important to determine the contributions of biochemical signals and other mechanosensitive components in tip cells, as well as the crosstalk between biochemical and mechanical pathways. For example, whether VEGF signaling contributes to the rest of infrequent calcium influx and how tip cells integrate Piezo1 mechanotransduction and VEGF signals remain elusive. The current findings might also suggest that Piezo1 is perhaps another indicator that nervous and vascular systems are developed through similar mechanisms, since recent findings suggested that Piezo1-dependent mechanotransduction is also critical for axonal development and regeneration (Song et al., 2019).
More importantly, mutations in the PIEZO1 gene are linked to dehydrated hereditary stomatocytosis and lymphatic malformation (Ranade et al., 2015), and its upregulations have been reported in cells near the amyloid plaques. Therefore, it would be interesting and relevant to investigate its function in diseased vascular and lymphatic systems, and perhaps its contributions to vascular dysfunction in Alzheimer’s disease (Sweeney et al., 2019) as well.
ACKNOWLEDGMENTS
The work of B.V.Z. is supported by NIH grant nos. R01AG023084, R01NS090904, R01NS034467, R01AG039452, 1R01NS100459, 5P01AG052350, and 5P50AG005142 in addition to the Alzheimer’s Association strategic 509279 grant, Cure Alzheimer’s Fund, and the Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease reference no. 16 CVD 05. The work of Z.Z. is supported by NIH grant nos. R01AG061288, R01NS110687, R03AG063287, and R21AG066090 and by Bright Focus Foundation.
REFERENCES
- Betz C, Lenard A, Belting H-G, and Affolter M (2016). Cell behaviors and dynamics during angiogenesis. Development 143, 2249–2260. [DOI] [PubMed] [Google Scholar]
- Blanco R, and Gerhardt H (2013). VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med 3, a006569, a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AS, and Ferrara N (2011). Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol 27, 563–584. [DOI] [PubMed] [Google Scholar]
- Förstermann U, and Sessa WC (2012). Nitric oxide synthases: regulation and function. Eur. Heart J 33, 829–837, 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, et al. (2014). Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Du X, Zhang B, Zi H, Yan Y, Yin J, Hou H, Gu S, Chen Q, and Du J (2020). Piezo1-Mediated Ca2+ Activities Regulate Brain Vascular Pathfinding during Development. Neuron 108, this issue, 180–192. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Syeda R, and Patapoutian A (2015). Mechanically Activated Ion Channels. Neuron 87, 1162–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Oliveira P, Correia A, Rodrigues T, Ribeiro-Rodrigues TM, Matafome P, Rodríguez-Manzaneque JC, Seiça R, Girão H, and Travasso RDM (2015). The Force at the Tip–Modelling Tension and Proliferation in Sprouting Angiogenesis. PLoS Comput. Biol 11, e1004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Li D, Farrelly O, Miles L, Li F, Kim SE, Lo TY, Wang F, Li T, Thompson-Peer KL, et al. (2019). The Mechanosensitive Ion Channel Piezo Inhibits Axon Regeneration. Neuron 102, 373–389.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, and Zlokovic BV (2019). Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev 99, 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


