Abstract
Solid cancers progress from primordial lesions through complex interactions between tumor-promoting and anti-tumor immune cell types, ultimately leading to the orchestration of humoral and T cell adaptive immune responses, albeit in an immunosuppressive environment. B cells infiltrating most established tumors have been associated with a dual role: Some studies have associated antibodies produced by tumor-associated B cells with the promotion of regulatory activities on myeloid cells, and also with direct immunosuppression through the production of IL-10, IL-35 or TGF-β. In contrast, recent studies in multiple human malignancies identify B cell responses with delayed malignant progression and coordinated T cell protective responses. This includes the elusive role of Tertiary Lymphoid Structures identified in many human tumors, where the function of B cells remains unknown. Here, we discuss emerging data on the dual role of B cell responses in the pathophysiology of human cancer, providing a perspective on future directions and possible novel interventions to restore the coordinated action of both branches of the adaptive immune response, with the goal of maximizing immunotherapeutic effectiveness.
Keywords: B cell, Cancer antibodies, tertiary lymphoid structure, tumor immunology
1. Introduction
Today, tumor immunology is primarily focused on T cells. However, T cells do not work in isolation. Within tumor beds, for instance, T and B cells often interact to orchestrate humoral and cellular responses that are incompletely understood.
B cell activation is initiated when the IgD and IgM on the surface of naïve B cells bind to specific antigens, initiating a differentiation process that culminates with the formation of antibody-producing plasma cells. Plasma cells can then migrate to the bone marrow, where they can produce antibodies for years after the antigen has been cleared. Recognition of the same antigen by CD4+ T cells initiates a crosstalk between T and B cells that reinforces both cellular and humoral responses. On the one hand, this can occur through the up-regulation of co-stimulatory molecules and antigen presentation on the B cell surface, enhancing T cell priming. On the other hand, CD40L:CD40 interactions result in B cell activation. CD40 signaling is additionally required for isotype-switching by antigen-specific B cells, which is the phenotype consistently found in most human cancers, as discussed below. Optimal adaptive immune responses therefore require coordinated activation of B and T lymphocytes. In cancer, however, the role of B cell responses has remained controversial for years. This is the result, first, of possible differences between humoral responses in human cancer versus rapidly progressing murine tumors. Second, functional heterogeneity between B cell subsets includes populations of regulatory B cells (Breg) that are known to counteract immune responses in inflammation and autoimmunity. Those cell types could be co-opted by some tumors. Third, antibodies in the tumor microenvironment have been found to enhance the immunosuppressive activity of myeloid cells in some mouse models. While the role of these mechanisms in attenuating anti-tumor immunity is supported by multiple independent studies (although primarily in mouse models), recent reports support that the overall role of B cells in human cancer is primarily associated with delayed malignant progression, suggesting protective activity.
The anti-tumor activity of B cell-derived antibodies makes sense because antibodies can theoretically induce the killing of tumor cells through antibody-dependent cellular cytotoxicity (ADCC), complement activation, or by inducing NK-dependent cytotoxicity. In addition, specific B cell subtypes can act as antigen presenting cells [1], thus fostering T cell-mediated responses. B cells also have the capacity to directly kill tumor cells through FASL:FAS-mediated interactions [2], or through the TRAIL/Apo-2L pathway [3]. Nevertheless, it still remains unclear whether antibody production at tumor beds is simply associated with a productive cellular response; or whether humoral responses are meaningful contributors to immune pressure against the progression of human cancer.
In this review, we will discuss recent advances on our understanding of the dual role of humoral responses in cancer, including the role of poorly understood Tertiary Lymphoid Structures where T and B cells interact very closely at tumor beds.
2. Regulatory B Cells are associated with tumor-promoting activities in specific murine models
B cells have been associated with accelerated tumor growth by independent studies in multiple mouse models of cancer. FcR-dependent myeloid cell activation, for instance, was found to drive tumor-promoting chronic inflammation in autochthonous models of epithelial carcinogenesis by Lisa Coussens and colleagues [4, 5]. Interestingly, accelerated carcinoma development was elicited by isotype-switched IgGs, rather than the more abundant IgM isotypes typically found in quickly progressing mouse models, as accelerated transplantable tumor growth was abrogated in the absence of activating (but not inhibitory) FcγR [5]. Using the same model, the team also found that release of C5a by macrophages during premalignant progression also enhances the immunosuppressive activity of myeloid cells against CD8+ T cells [6], which supports previous reports on the tumor-promoting activity of C5a in different preclinical systems [7]. Furthermore, recent reports suggest that inflammation-induced IgA+ cells counteract anti-liver cancer immunity in different mouse models [8].
Besides antibody-dependent effects, other authors have reported that B2 cells at tumor beds produce prostate cancer-promoting lymphotoxin, which activates NF-κB and STAT3 signaling in tumor cells, acting as a survival factor [9]. Lymphotoxin, however, plays a dual role in anti-tumor immunity, as it drives the assembly of Tertiary Lymphoid Structures (TLS). As discussed below, TLS are consistently associated with immune protection in most human cancers [10–12].
In addition to the aforementioned non-specific tumorigenic activities, tumors co-opt specific populations of B cells with regulatory/suppressor activity, generically termed regulatory B cells (Breg). Breg cells represent less than 10% of total B cells in circulation in the steady state [13, 14], and promote peripheral tolerance. Breg have been associated with an IgM+CD5+CD24hiCD27+CD38hi phenotype and the secretion of IL-10 [15, 16]. Recent studies support that Breg cell development depends on the inflammatory milieu in which B cells at different stages of differentiation find themselves [17]. Thus, recent studies defined multiple signals, that, in combination, promote the formation of immunosuppressive IL-10-producing Breg cells. These signals include the activation of different TLRs, CD40, the BCR, CD80, CD86, and cytokines [17, 18], with multiple subsets of Breg cells emerging from exposure to different milieus, at least in mice. The acquisition of immunosuppressive activity by B cell subsets also appears to be driven by cancer-derived metabolites, including leukotrienes produced by 5-lipoxygenase activity [19]. In addition, other hematopoietic cells can also drive regulatory activities on B cells, including Treg [20]. Finally, IL-35 has been associated with conversion of resting B into IL-10/IL-35-producing Breg cells [21]. The role of expression specific molecular drivers governing the suppressive activity of Breg cells remains controversial, although Interleukin 4 (IL4)-induced gene 1 (IL4I1), an oxidase that degrades l-phenylalanine, has been found specifically expressed in melanoma-infiltrating Breg cells, in association with immunosuppressive activity [22]. Because inflammation, including in the context of productive anti-tumor immunity, is a potent driver of Breg development, it is not surprising to find Breg cells in the tumor microenvironment. However, the dominant role of tumor-associated B cells in human cancer appears to be antagonizing malignant progression, rather than accelerating tumor growth. Thus, similar to Treg, Breg also exist in human cancer, but immune pressure against malignant progression appears to depend on coordinated humoral and cellular adaptive responses, similar to infections and autoimmunity. This is important because at the moment there is no intervention to specifically deplete Breg while preserving non-immunosuppressive B cells. Thus, while overall B cell depletion in some mouse models is associated with enhanced T cell-mediated protected immunity [23], unselected anti-CD20 depletion of B cells impairs T cell-mediated antitumor immunity in other systems [24]. Furthermore, in different preclinical models of diseases previously associated with tumor-promoting B cells (i.e., pancreatic cancer), B cells are identified as immunostimulatory cell types that support anti-tumor immunity [25], suggesting that the negative effects of B cells are tumor model-dependent. Accordingly, a recent study by Hollern and colleagues demonstrates how responses to immune checkpoint inhibitors using immunogenic mouse models of triple-negative breast cancer are actually dependent on B cell activation of T cells and the generation of antibody [26]. Most importantly, studies in humans have seen no additional effect of rituximab on IL-2 therapy for renal cell carcinoma and melanoma patients [27].
Although differences between mice and humans, and a growing range of phenotypic markers, have complicated the characterization of the relevant Breg population/s, what they have in common is the secretion of immunosuppressive cytokines such as IL-10 [28], TGF-β and IL-35 [16, 29–36]. The immunosuppressive activity of B cells has been primarily associated with IL-10 production because IL-10-producing Breg are clearly protective against autoimmunity, but IL-35-producing B cells have been also proposed as targets for autoimmune disease and infections [37]. Production of these immunosuppressive mediators by tumor-associated Breg has been proposed to enhance the suppressive activity of Myeloid-Derived Suppressor Cells (MDSCs) [36]. Collectively, heterogeneous Breg cells inhibit Th1 and Th17 responses, and drive the conversion of CD4+ T cells into FoxP3+ Treg cells [14, 38]. In addition, Breg cells can inhibit IFN-γ production by CD8+ T cells in the context of viral infection [39]; TNF-α production by monocytes [40]; and IFN-α secretion by plasmacytoid dendritic cells, thus contributing to immunosuppression through multiple mechanisms. Furthermore, the immunosuppressive activity associated with murine plasma cells has been shown to thwart the immunostimulatory effects of oxaliplatin in models of prostate cancer [41]. T cell inhibition by Breg producing these immunosuppressive mediators has been identified in a variety of tumors, including breast, gastric, pancreatic, colorectal and prostate cancer [35, 42–45].
3. In contrast, B cell infiltration is associated with superior outcome in many human malignancies
In contrast to the regulatory role associated with B cells in mouse models, converging independent evidence indicates that humoral responses are associated with immune protection in multiple human cancers. For instance, dense B-cell infiltration has been correlated with better clinical outcome in human hepatocarcinoma, which in fact orchestrates mechanisms to decrease B cell infiltration [46]. This positive association needs to be reconciled with the fact that isotype-switched humoral responses have been correlated with disease progression in mouse models of the same disease [8]. The most logical explanation, again, is that tumor-promoting effects are dependent on specific tumor models.
Other malignancies where denser B cell infiltrates have been associated with better outcome include cutaneous melanoma [47], colorectal cancer [48, 49], and breast carcinoma [50]. Critically, recent independent studies demonstrate that infiltration of B cells in melanoma [51–53], sarcoma [54] and renal cancer [51] identifies patients with better outcome in response to immunotherapy. In fact, B cell activity remained prognostic factor in sarcoma even in the context of high or low CD8+ T cells [54]. In both melanoma and sarcoma, a protective B cell signature was predominantly associated with the formation of tertiary lymphoid structures [51, 52, 54]. Not counting TCGA datasets, these studies combine the analysis of 480 patients, strongly supporting what immunologists have been teaching for years; namely, that T and B cell responses work in coordination to maximize protective immunity. Besides these seminal studies, at the mRNA level, positive associations between B cell markers and better outcome have been identified in non-small cell lung [55] and gastric [56] cancers.
In head and neck squamous carcinomas (HNSCC), on the other hand, some studies have shown that B cell subpopulations can drive both tumor-promoting and antitumor activities [57]. In support of the latter, exhaustive genomic and histological studies have recently identified an expression signature associated with CD4+ T follicular helper cells (and therefore germinal center activity), in association with longer progression-free survival [58]. However, germinal center reactions are more prominent in HPV+, which can explain differences between patients.
In ovarian cancer, both plasma cell and B-cell infiltration, including the presence of Tertiary Lymphoid Structures (TLS [59]), have been recently associated with T-cell cytolytic activity at ovarian cancer beds, and improved outcome [60–62]. Although these studies strongly suggest that humoral responses potentiate T-cell immune protection, specific isotypes expressed by these cells, how B cells interact with other immune and non-immune players, and to what extent antibodies impede malignant progression, all remained unknown. This is relevant because a recent study showed that only ovarian tumors containing both CD8+ and CD20+ lymphocytes (as opposed to CD8+ T cells alone) are associated with better outcomes [63]. Because ovarian cancer is indisputably an immunogenic disease [64–66], but ovarian cancer patients remain resistant to monotherapeutic immune checkpoint inhibitors [67, 68], boosting coordinated T and B cell responses could elicit superior immune protection against malignant progression. In support of this proposition, our analysis of 575 high-grade serous ovarian cancers demonstrated that intraepithelial T cells are associated with better outcome in treatment-naïve patients only when intraepithelial B cells are also present (manuscript under review). The predictive value of B cell infiltration in ovarian cancer extends therefore beyond TLS assembly, which is only found in ~20% of tumors.
The bottom line suggested by these converging studies is that coordinated T and B cell responses determine immune pressure against human cancer, with B cell responses clearly identifying a greater protective response in patients, rather than global inhibitory activity. The important next question is whether humoral responses are truly protective on their own (i.e., through the production of relevant antibodies), and not merely a reflection of enhanced adaptive immunity. Because B cells also express PD-1, humoral responses can be boosted by checkpoint inhibitors or vaccines [69]. In fact, PD-1 is expressed by B cells, where it inhibits lymphocyte activation as it does in T cells [70]. However, the contribution of B cell responses to the effectiveness of checkpoint inhibitors remains uninvestigated. Equally important, all truly efficacious vaccines work through the production of neutralizing antibodies, as it is much easier to boost B cell memory than prolonged antigen-specific T cell activity. A better definition of the nature of B cell responses in different human tumors would help clarifying whether personalized anti-cancer vaccines should be primarily designed to foster B cell memory.
4. What is the isotype and reactivity of antibodies produced in the tumor microenvironment?
Despite increasing evidence that B cell responses play a crucial role in spontaneous and immunotherapeutically-driven immune pressure against established malignancies in humans, the full range of functions that B cells perform in cancer remains to be clarified. B cells could boost T cell responses, for instance, through antigen presentation; or could secrete antibodies that neutralize oncogenic drivers, or target tumor cells for elimination by other immune cells. An array of more than two decade-old studies has supported the view that anti-cancer antibodies primarily recognize intracellular proteins, including classical “cancer testis antigens” [71–73]. Intracellular antigens can still promote tumor cell killing through complement activation [74], and externalization of intracellular targets can occur in the tumor microenvironment (i.e., in exosomes). In addition, antibodies can be engineered to traffic inside tumor cells [75, 76]. However, antibody-dependent cellular cytotoxicity (ADCC) by NK cells (in the case of IgG) or myeloid cells (in the case of both IgG and IgA) depends on the Fc portion of the antibody, as well as extracellular accessibility. Interestingly, antibodies that recognize proteins on the cell membrane [77] or tumor-promoting cytokines [69] have been also recently identified in cancer patients. In human gastric cancer, for instance, sulfated glycosaminoglycans have been found to elicit major B cell responses [78]. Furthermore, we have identified antibodies spontaneously produced in the ovarian cancer microenvironment that recognize secreted molecules or oncodrivers with transmembrane motifs (manuscript under review). The feasibility of cloning human tumor-derived immunoglobulins to generate CAR T cells or novel therapeutic antibodies is currently being investigated.
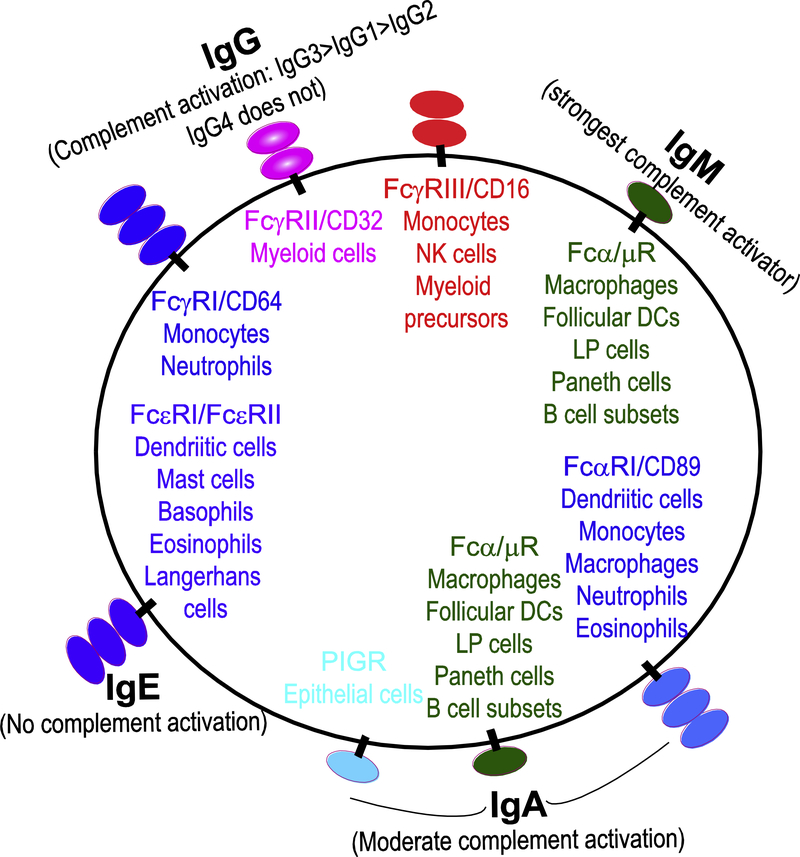
Another important issue is whether humoral responses at tumor beds are primarily dependent on robustly activated B cells secreting isotype-switched antibodies, or whether IgM is the predominant Ig in the tumor microenvironment (Figure 1). Converging evidence suggests that both responses co-exist in solid tumors. In breast cancer, isotype-switched IgG and IgA antibodies were found to be equally abundant at tumor beds. Interestingly, breast cancer antigen-reactive IgA production was associated with the presence of tertiary lymphoid structures [79]. In older studies using clinical samples, IgG and IgA were found in melanoma extracts, and melanoma antigen-specific IgG were identified [80]. In more recent studies in metastatic lesions of the same disease, Tertiary Lymphoid Structures were also found to contain B cells producing IgG and IgA with attributes of affinity maturation [81]. In esophageal adenocarcinoma, plasma cells producing IgG4 were found to accumulate after chemoradiotherapy [82].
Figure 1. Summary of Fc Receptors binding to different antibodies on different cell types.
Strong activators of complement are also illustrated.
Overall, it is becoming increasingly clear that isotype-switched humoral responses occur in many human tumors, indicative of strong antigenic responses. Because IgA and IgG bind to different Fc receptors and elicit different biological activities, future studies should dissect the relative contribution of these antibodies to immune protection against malignant growth.
If at least some humoral responses in cancer are associated with isotype-switched antibodies, the implication is that they occur in coordination with CD4+ T cell priming, which is required to activate CD40 signaling. What is the nature of antigens concurrently recognized by T and B cells? Affinity maturation of B cells occurs in germinal centers, which are found in many human tumors, suggesting the generation of high affinity antibodies. While the field of tumor immunology is now focused on mutated neoantigens, all current evidence suggests that tumor-associated humoral responses are consistently associated with self-antigen overexpression, or expression of developmental antigens, rather than single-nucleotide mutations associated with T cell activity in recent studies. The implication is that many tumor-specific B cells produce autoantibodies that can only arise from previously anergic autoreactive B cells, which could overcome tolerance in the presence of high antigen levels and the right inflammatory conditions. Thus, while a combination of elimination of highly autoreactive B cells, receptor editing and peripheral clonal deletion are the major B cell tolerance mechanisms, many autoreactive B cells persist in the periphery in a state of unresponsiveness or anergy [83]. In humans, anergic B cells have been associated with CD21−/lo subsets [84] as well as B cells that express intrinsically autoreactive BCRs encoded by the VH4–34 gene [85], or B cells that down-regulated IgM expression [86]. There is evidence that anergic, autoreactive B cells can become activated with sufficient stimulation [87]. Future studies should confirm the relative contribution of autoreactive B cells versus B cells recognizing mutated neoantigens to anti-tumor immune pressure. It is possible that conformational changes in extracellular domains drive reactive antibody responses, but at this moment evidence suggests that overexpressed shared antigens are the primary target of humoral immunity in cancer. It will be also important to determine how many of these antibodies can access tumor-expressed molecules with extracellular domains (i.e., transmembrane receptors or secreted mediators), to use them as potential therapeutic agents (i.e., in CAR T cells or antibody-drug conjugates), depending on the specificity of target expression.
5. Composition and prognostic value of Tertiary Lymphoid Structures (TLS)
In the tumor microenvironment, T and B cells often co-localize in aggregates of different mass and different degrees of organization. Some of these interactions result in highly organized structures similar to lymph nodes, termed tertiary lymphoid structures (TLS). TLS contain a discrete T-cell zone occupied by CD4 and CD8 T cells, and high endothelial venules, adjacent to B-cell follicles, including germinal centers with interdigitating networks of follicular dendritic cells (DCs; Figure 2) [88]. However, less organized adjacent conglomerates of T and B cells with at an earlier degree of organization are found in many tumors, and they are often also identified as “lymphoid structures”. These characteristic structures are reminiscent of TLS developing in noncancerous conditions such as autoimmunity and transplantation, where TLS are associated with tissue damage [88]. Depending on the degree of maturation of these structures, some elements commonly found include infiltrates of CD19+CD20+ B cells forming a CD95+GL7+BCL6+ germinal center, interdigitating CD21+CD35+ follicular and DC-LAMP+ Dendritic Cells, CD138+ plasma cells, CD3+CD4+ (including CXCR5+PD-1highICOS+BCL6+ T follicular helper (TFH) cells), and CD3+CD8+ T cells, some CD15+ neutrophils and CD68+ macrophages [59]. Besides the presence of germinal centers, where affinity maturation and formation of memory B cells and long lived plasma cells should take place, the most distinctive feature of TLS is the orchestration of high endothelial venules (HEVs), identified by the PNAd marker.
Figure 2. Typical elements of Tertiary Lymphoid Structures and proposed mechanism of anti-tumor protection fueled by them.
TLS are orchestrated by adjacently distributed B and T cells in differentiated structures irrigated by HEVs. TFH cells support germinal centers, while cells are supported by follicular DCs, CD40:CD40L interactions, and the production of IL-21 by TFH cells. T cells in turn are primed by B cells under the protection of HEVs, while B cells produce isotype-switched antibodies that recognize tumor antigens. Enhanced T cell responses act in coordination with antibody responses to target tumor cells outside TLS.
TLS are more frequently located in tumor margins or the stroma, compared to central tumor areas [59]. TLS are rarely found in mouse models, underscoring another biological difference between mice and humans in terms of B cell responses. Interestingly, the mouse models in which TLS are found are frequently associated with intraperitoneal masses, rather than conventional flank tumors [89].
Notably, the presence of TLS is associated with better outcomes in more than 10 different types of human cancer [88, 90–96], further supporting that optimal anti-tumor immunity is, in general, associated with coordinated T cell and antibody-mediated responses. For instance, recent studies in ovarian cancer patients indicate that favorable CD8+ TIL responses are associated with the presence of TLS, which are present in ~23% of ovarian carcinoma specimens [61]. TLS are also important sites of immune activation in breast cancer, particularly in tumors with dense immune infiltrates [97–99]. Intratumoral tertiary lymphoid organs are also favorable prognosticators in patients with pancreatic cancer [96], colo-rectal carcinoma [92], bladder cancer [100] and non-small cell lung cancer [101], among other malignancies. As aforementioned, a protective B cell signature was also predominantly associated with TLS formation in both melanoma and sarcoma [51, 52, 54].
Active research in multiple laboratories should offer deeper insight into how TLS are orchestrated and their actual function in upcoming years, but it seems logical that the crosstalk between multiple immune cell types in these peculiar structures enhances both arms of the adaptive immune system in a reciprocal manner: First, B cells can present tumor antigens to tumor-reactive T cells present in TLS, strengthening their effector activity and promoting T cell memory differentiation. Second, tumor-reactive T cells in TLS can be better protected from immunosuppressive networks in the tumor microenvironment through high endothelial venules (i.e., PD-L1 or CD277 [102]), providing new waves of anti-tumor effector cells. Third, plasma cells in TLS could produce antibodies with strong anti-tumor activity. Thus, our unpublished studies have identified highly oligoclonal IgA responses in different TLS in human ovarian cancer. Although their targets remain unknown, it is tempting to speculate that these antibodies could target oncodrivers overexpressed in tumors, causing complement activation, exosome neutralization, increased uptake of tumor antigen by follicular dendritic cells, and even direct targeting of transmembrane receptors expressed in tumor cells. In turn, activated CD4 T cells could maintain B cell activity in TLS through cytokine and CD40L signals. A fourth possibility is that the metabolic restrictions caused by the Warburg effect, or production of Amino Acid-depleting enzymes such as IDO, L-Arginase or SLC43A2 [103] by tumor cells are alleviated in the microenvironment of intra-tumoral TLS, thus preventing the mechanisms of metabolically-driven ER stress that paralyze T cells [104, 105]. Alternatively, TLS formation could simply illustrate a strong protective response at tumor beds. Characterizing the crosstalk between different immune cell types in TLS is the subject of intensive investigations in multiple laboratories, which should offer new light into the best way of exploiting these protective responses.
6. How are TLS assembled?
As aforementioned, understanding the precise role/s, composition and development of TLS still requires extensive investigation. Recapitulating the orchestration of TLS is particularly important because this insight could pave the way for novel therapeutic interventions to boost coordinated lymphocytes responses against unresectable tumors, including metastases and masses in vital organs. TLS assembly, however, remain poorly understood, due in part to the paucity of mouse models that recapitulate bona fide TLS.
During development, the formation of secondary lymphoid organs is initiated by lymphoid tissue inducer cells (LTi), which drive endothelial cells to express PNAd and become HEV, and stromal cells to differentiate into FDC and fibroblastic reticular cells [81, 106]. In cancer, delivery of the cytokine LIGHT, a member of the TNF-α family, to tumor vessels was recently found to be sufficient to induce TLS formation [107]. The role of lymphotoxin has been underscored by seminal studies from Engelhard and colleagues [12], which unveiled how the generation of “lymph node-like” vasculature and the recruitment of T cells populations during TLS development is driven by effector lymphocytes (both CD8+ T cells and NK cells) that produce lymphotoxin and IFNγ. Differentiation of this peculiar vasculature is associated with organized conglomerates of B lymphocytes and fibroblasts, similar to the structure of conventional secondary lymph nodes.
The importance of chemokine- and cytokine-signaling pathways in the assembly of TLS is also supported by the studies of Storkus and colleagues [108]. The team identified CCR7, CXCR5, lymphotoxin and IL-36 produced at tumor beds as orchestrators of the recruitment of T cells, B cells, dendritic cells and other specialized subsets to form these complex structures. Cooperation between Tbet and IL-36γ expression in dendritic cells was demonstrated in elegant experiments where the injection of tumors with dendritic cells engineered to secrete an active form of IL-36γ upregulated Tbet expression, driving TLS formation, and ultimately resulting in delayed tumor growth [109].
In independent studies, Mule and colleagues defined a 12 chemokine signature that reliably identifies the presence of TLS across multiple human tumors [94]. This signature contains interesting elements that offer additional light into the mechanisms of TLS assembly at tumor beds. Thus, common elements of this signature found in other studies include CCL21, primarily produced by fibroblasts and endothelial cells (i.e, in response to LIGHT signaling [107]); IFN-γ-responsive CXCR3 chemokines; and, interestingly, CXCL13, a chemokine primarily produced by T Follicular Helper (TFH) cells that mediates the recruitment of CXCR5+ B cells. The lab is currently working in collaboration with the Artzi lab at the MIT on bioengineering approaches to induce the formation of TLS through scaffolds [110]. In complementary approaches, the same group has demonstrated that TLS with antitumor activity can be driven by the infusion of a lymph node-derived stromal cell line [111], opening new opportunities for in vivo interventions in the clinic. Overall, much more mechanistic insight into the sequence of events leading to the formation of TLS in cancer is needed to be able to promote these protective structures in unresectable tumors in patients, but a strong rationale for inducing TLS is emerging. TLS assembly could be effectively driven through intra-tumoral administration of TLS-associated cytokines, dendritic cell vaccination, or perhaps adoptive transfer of TFH cells. This could provide immune pressure against accelerated progression of metastatic, unresectable tumors, with substantial therapeutic benefits. It will be finally interesting to monitor the capacity of multiple oncolytic viruses currently undergoing early clinical testing to alter the immuno-environment of established tumors and promote TLS assembly.
7. A role for TFH cells and TGF-β signaling in TLS formation?
Although the process leading to the orchestration of TLS remains incompletely understood, together, the aforementioned studies convey the importance of dendritic cell-mediated T cell priming in the generation of a chemokine milieu that promotes the recruitment of all the cellular types that, properly assembled and maintained through the right cytokine setting, eventually establish an organized TLS. However, there is another crucial element that, as established in responses to microorganisms, is required for the generation and maintenance of germinal centers, as well as sustained isotype-switched antibody responses; namely, TFH cells [112]. In support of the importance of TFH, CXCL13, one of the chemokines included in the signature defined by Mule and colleagues, is heavily produced by TFH cells [113] and has been identified as a biomarker of germinal center activity that drives the recruitment of CXCR5+ B cells [114, 115]. TFH cells also produce IL-21, which drives the proliferation and maturation of B cells producing plasma cells that secrete high-affinity antibody [116]. TFH cells could therefore represent the cornerstone of TLS formation.
The rationale for a role of TFH (and CD4+ T cells in general) in TLS assembly is also supported by recent understanding of TLS generation in autoimmune conditions, whereby activated CD4+ T cells substitute LTi cells [117, 118]. In addition, CD4+ T cell transfer into transgenic mice overexpressing CCL21 in the thyroid has been found to be sufficient for causing inflammatory lymphangiogenesis [119].
If TFH cells are necessary contributors to the orchestration and maintenance of TLS, the role of TGF-β also needs to be addressed. Thus, although TGF-β has been conclusively identified as an immunosuppressive cytokine in the tumor microenvironment [120, 121], this cytokine has multiple activities on different immune cell types. We have previously demonstrated that TGF-β signaling downregulates the genomic organizer SATB1 through direct binding of Smad3 to the SATB1 promoter region, driving PD-1 de-repression [120]. The role of TGF-β in PD-1 overexpression was simultaneously unveiled by Park and colleagues [122]. Interestingly, PD-1 upregulation is one of the hallmarks of TFH cells, and independent reports support that TGF-β in fact promotes the TFH differentiation of CD4+ T cells, and isotype-switched antibody responses in humans and mice [112, 123–125]. In fact, only a single report associates TGF-β signaling with defective TFH formation, but this is due to developmental defects [126]. Successful conditions for the differentiation of human CD4+ T cells into functional TFH in vitro actually include the addition of TGF-β [113, 124]. It is important to note that TGF-β is not effective at inducing mouse CD4+ T cells TFH differentiation, underscoring another difference between humoral responses in both the species.
8. Concluding remarks
Immunologists have taught for years that optimal adaptive immune responses progress through the coordinated activity of both T and B cells, which potentiate each other. Although B cells have multiple immunostimulatory activities on T cells and have the capacity to produce antibodies that kill tumor cells, today Immuno-Oncology remains almost exclusively focused on T cell responses. The role of B cell responses in cancer has been traditionally disregarded as irrelevant, or primarily associated with immunosuppressive responses. However, an array of novel clinical studies primarily associate B cell responses with superior immune protection and delayed malignant progression. In addition, several laboratories have started characterizing the nature of B cells in TLS commonly found in many human tumors, which in most cases are also associated with better outcome. Compared to our understanding of anti-tumor T cell responses, however, our current knowledge of humoral responses in human cancer is still in its infancy. This obviously includes how to manipulate B cell responses for eliciting immune protection through immunotherapies or protective vaccines. However, emerging independent reports indicate that isotype-switched antibodies, in some cases with attributes of affinity maturation, spontaneously target tumor antigens at tumor beds. Because virtually all vaccines that are or have been successful in the history of Immunology work through the production of neutralizing antibodies, modulating humoral responses could synergize with existing immunotherapies to elicit coordinated immune control of human cancer.
ACKNOWLEDGEMENTS
This study was supported by R01CA157664, R01CA124515, R01CA178687, R01CA211913 and U01CA232758 to JRCG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Chen X, Jensen PE, The role of B lymphocytes as antigen-presenting cells, Arch Immunol Ther Exp (Warsz) 56(2) (2008) 77–83. [DOI] [PubMed] [Google Scholar]
- [2].Tao H, Lu L, Xia Y, Dai F, Wang Y, Bao Y, Lundy SK, Ito F, Pan Q, Zhang X, Zheng F, Shu G, Fang B, Jiang J, Xia J, Huang S, Li Q, Chang AE, Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10, Eur J Immunol 45(4) (2015) 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kemp TJ, Moore JM, Griffith TS, Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide stimulation, J Immunol 173(2) (2004) 892–9. [DOI] [PubMed] [Google Scholar]
- [4].de Visser KE, Korets LV, Coussens LM, De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent, Cancer Cell 7(5) (2005) 411–23. [DOI] [PubMed] [Google Scholar]
- [5].Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM, FcRgamma activation regulates inflammation-associated squamous carcinogenesis, Cancer Cell 17(2) (2010) 121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Medler TR, Murugan D, Horton W, Kumar S, Cotechini T, Forsyth AM, Leyshock P, Leitenberger JJ, Kulesz-Martin M, Margolin AA, Werb Z, Coussens LM, Complement C5a Fosters Squamous Carcinogenesis and Limits T Cell Response to Chemotherapy, Cancer Cell 34(4) (2018) 561–578 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD, Modulation of the antitumor immune response by complement, Nat Immunol 9(11) (2008) 1225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M, Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity, Nature 551(7680) (2017) 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M, B-cell-derived lymphotoxin promotes castration-resistant prostate cancer, Nature 464(7286) (2010) 302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodriguez AB, Peske JD, Engelhard VH, Identification and Characterization of Tertiary Lymphoid Structures in Murine Melanoma, Methods Mol Biol 1845 (2018) 241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kratz A, Campos-Neto A, Hanson MS, Ruddle NH, Chronic inflammation caused by lymphotoxin is lymphoid neogenesis, J Exp Med 183(4) (1996) 1461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peske JD, Thompson ED, Gemta L, Baylis RA, Fu YX, Engelhard VH, Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity, Nat Commun 6 (2015) 7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Esteve-Sole A, Luo Y, Vlagea A, Deya-Martinez A, Yague J, Plaza-Martin AM, Juan M, Alsina L, B Regulatory Cells: Players in Pregnancy and Early Life, Int J Mol Sci 19(7) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C, CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients, Immunity 32(1) (2010) 129–40. [DOI] [PubMed] [Google Scholar]
- [15].Mauri C, Menon M, The expanding family of regulatory B cells, Int Immunol 27(10) (2015) 479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR, B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis, Proc Natl Acad Sci U S A 108(26) (2011) 10662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rosser EC, Mauri C, Regulatory B cells: origin, phenotype, and function, Immunity 42(4) (2015) 607–12. [DOI] [PubMed] [Google Scholar]
- [18].Mauri C, Bosma A, Immune regulatory function of B cells, Annu Rev Immunol 30 (2012) 221–41. [DOI] [PubMed] [Google Scholar]
- [19].Wejksza K, Lee-Chang C, Bodogai M, Bonzo J, Gonzalez FJ, Lehrmann E, Becker K, Biragyn A, Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor alpha, J Immunol 190(6) (2013) 2575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, Beyer T, Reister F, Fabricius D, Lotfi R, Lunov O, Nienhaus GU, Simmet T, Kreienberg R, Moller P, Schrezenmeier H, Jahrsdorfer B, Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells, Cancer Res 73(8) (2013) 2468–79. [DOI] [PubMed] [Google Scholar]
- [21].Dambuza IM, He C, Choi JK, Yu CR, Wang R, Mattapallil MJ, Wingfield PT, Caspi RR, Egwuagu CE, IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease, Nat Commun 8(1) (2017) 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Prevost-Blondel A, Richard Y, Interleukin 4-Induced Gene 1 as an Emerging Regulator of B-Cell Biology and its Role in Cutaneous Melanoma, Crit Rev Immunol 39(1) (2019) 39–57. [DOI] [PubMed] [Google Scholar]
- [23].Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, Ma Y, Wiesen JF, Wong MH, Kulesz-Martin M, Irving B, Coussens LM, B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas, Cancer Cell 25(6) (2014) 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Candolfi M, Curtin JF, Yagiz K, Assi H, Wibowo MK, Alzadeh GE, Foulad D, Muhammad AK, Salehi S, Keech N, Puntel M, Liu C, Sanderson NR, Kroeger KM, Dunn R, Martins G, Lowenstein PR, Castro MG, B cells are critical to T-cell-mediated antitumor immunity induced by a combined immune-stimulatory/conditionally cytotoxic therapy for glioblastoma, Neoplasia 13(10) (2011) 947–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Spear S, Candido JB, McDermott JR, Ghirelli C, Maniati E, Beers SA, Balkwill FR, Kocher HM, Capasso M, Discrepancies in the Tumor Microenvironment of Spontaneous and Orthotopic Murine Models of Pancreatic Cancer Uncover a New Immunostimulatory Phenotype for B Cells, Front Immunol 10 (2019) 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, He X, Garay JP, Carey-Ewend K, Marron D, Ford J, Liu S, Vick SC, Martin M, Parker JS, Vincent BG, Serody JS, Perou CM, B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer, Cell 179(5) (2019) 1191–1206 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, Gajewski TF, Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma, Ann Oncol 15(7) (2004) 1109–14. [DOI] [PubMed] [Google Scholar]
- [28].Inoue S, Leitner WW, Golding B, Scott D, Inhibitory effects of B cells on antitumor immunity, Cancer Res 66(15) (2006) 7741–7. [DOI] [PubMed] [Google Scholar]
- [29].Ganti SN, Albershardt TC, Iritani BM, Ruddell A, Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth, Sci Rep 5 (2015) 12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lykken JM, Candando KM, Tedder TF, Regulatory B10 cell development and function, Int Immunol 27(10) (2015) 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sarvaria A, Basar R, Mehta RS, Shaim H, Muftuoglu M, Khoder A, Sekine T, Gokdemir E, Kondo K, Marin D, Daher M, Alousi AM, Alsuliman A, Liu E, Oran B, Olson A, Jones RB, Popat U, Hosing C, Champlin R, Shpall EJ, Rezvani K, IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation, Blood 128(10) (2016) 1346–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Das S, Bar-Sagi D, BTK signaling drives CD1d(hi)CD5(+) regulatory B-cell differentiation to promote pancreatic carcinogenesis, Oncogene 38(17) (2019) 3316–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang Y, Morgan R, Chen C, Cai Y, Clark E, Khan WN, Shin SU, Cho HM, Al Bayati A, Pimentel A, Rosenblatt JD, Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses, Int Immunol 28(9) (2016) 423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC, Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia, Cancer Discov 6(3) (2016) 256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yuen GJ, Demissie E, Pillai S, B lymphocytes and cancer: a love-hate relationship, Trends Cancer 2(12) (2016) 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, Araki Y, Miyoshi I, Yang L, Trinchieri G, Biragyn A, Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells, Cancer Res 75(17) (2015) 3456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SHE, Anderton SM, Fillatreau S, IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases, Nature 507(7492) (2014) 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mauri C, Menon M, Human regulatory B cells in health and disease: therapeutic potential, J Clin Invest 127(3) (2017) 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, Kennedy PT, Brunetto M, Lampertico P, Mauri C, Maini MK, IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection, J Immunol 189(8) (2012) 3925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF, Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells, Blood 117(2) (2011) 530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE, Jamieson C, Kane CJ, Klatte T, Birner P, Kenner L, Karin M, Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy, Nature 521(7550) (2015) 94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sarvaria A, Madrigal JA, Saudemont A, B cell regulation in cancer and anti-tumor immunity, Cell Mol Immunol 14(8) (2017) 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang K, Liu J, Li J, IL-35-producing B cells in gastric cancer patients, Medicine (Baltimore) 97(19) (2018) e0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang K, Gong H, Chai R, Yuan H, Chen Y, Liu J, Aberrant frequency of IL-35 producing B cells in colorectal cancer patients, Cytokine 102 (2018) 206–210. [DOI] [PubMed] [Google Scholar]
- [45].Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A, Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells, Cancer Res 71(10) (2011) 3505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang Z, Ma L, Goswami S, Ma J, Zheng B, Duan M, Liu L, Zhang L, Shi J, Dong L, Sun Y, Tian L, Gao Q, Zhang X, Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma, Oncoimmunology 8(4) (2019) e1571388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ladanyi A, Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma, Pigment Cell Melanoma Res 28(5) (2015) 490–500. [DOI] [PubMed] [Google Scholar]
- [48].Meshcheryakova A, Tamandl D, Bajna E, Stift J, Mittlboeck M, Svoboda M, Heiden D, Stremitzer S, Jensen-Jarolim E, Grunberger T, Bergmann M, Mechtcheriakova D, B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer, PLoS One 9(6) (2014) e99008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Richards CH, Flegg KM, Roxburgh CS, Going JJ, Mohammed Z, Horgan PG, McMillan DC, The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer, Br J Cancer 106(12) (2012) 2010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang Z, Zhu Y, Wang Z, Zhang T, Wu P, Huang J, Yin-yang effect of tumor infiltrating B cells in breast cancer: From mechanism to immunotherapy, Cancer Lett 393 (2017) 1–7. [DOI] [PubMed] [Google Scholar]
- [51].Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautes-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA, B cells and tertiary lymphoid structures promote immunotherapy response, Nature 577(7791) (2020) 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lovgren K, Warren S, Jirstrom K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jonsson G, Tertiary lymphoid structures improve immunotherapy and survival in melanoma, Nature 577(7791) (2020) 561–565. [DOI] [PubMed] [Google Scholar]
- [53].Griss J, Bauer W, Wagner C, Simon M, Chen M, Grabmeier-Pfistershammer K, Maurer-Granofszky M, Roka F, Penz T, Bock C, Zhang G, Herlyn M, Glatz K, Laubli H, Mertz KD, Petzelbauer P, Wiesner T, Hartl M, Pickl WF, Somasundaram R, Steinberger P, Wagner SN, B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma, Nat Commun 10(1) (2019) 4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautes-Fridman C, Tawbi HA, Fridman WH, B cells are associated with survival and immunotherapy response in sarcoma, Nature 577(7791) (2020) 556–560. [DOI] [PubMed] [Google Scholar]
- [55].Ho KH, Chang CJ, Huang TW, Shih CM, Liu AJ, Chen PH, Cheng KT, Chen KC, Gene landscape and correlation between B-cell infiltration and programmed death ligand 1 expression in lung adenocarcinoma patients from The Cancer Genome Atlas data set, PLoS One 13(12) (2018) e0208459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hennequin A, Derangere V, Boidot R, Apetoh L, Vincent J, Orry D, Fraisse J, Causeret S, Martin F, Arnould L, Beltjens F, Ghiringhelli F, Ladoire S, Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients, Oncoimmunology 5(2) (2016) e1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lechner A, Schlosser HA, Thelen M, Wennhold K, Rothschild SI, Gilles R, Quaas A, Siefer OG, Huebbers CU, Cukuroglu E, Goke J, Hillmer A, Gathof B, Meyer MF, Klussmann JP, Shimabukuro-Vornhagen A, Theurich S, Beutner D, von Bergwelt-Baildon M, Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma, Oncoimmunology 8(3) (2019) 1535293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cillo AR, Kurten CHL, Tabib T, Qi Z, Onkar S, Wang T, Liu A, Duvvuri U, Kim S, Soose RJ, Oesterreich S, Chen W, Lafyatis R, Bruno TC, Ferris RL, Vignali DAA, Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer, Immunity 52(1) (2020) 183–199 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH, Tertiary lymphoid structures in the era of cancer immunotherapy, Nat Rev Cancer (2019). [DOI] [PubMed] [Google Scholar]
- [60].Montfort A, Pearce O, Maniati E, Vincent BG, Bixby L, Bohm S, Dowe T, Wilkes EH, Chakravarty P, Thompson R, Topping J, Cutillas PR, Lockley M, Serody JS, Capasso M, Balkwill FR, A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases, Clin Cancer Res 23(1) (2017) 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kroeger DR, Milne K, Nelson BH, Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer, Clin Cancer Res 22(12) (2016) 3005–15. [DOI] [PubMed] [Google Scholar]
- [62].Iglesia MD, Vincent BG, Parker JS, Hoadley KA, Carey LA, Perou CM, Serody JS, Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer, Clin Cancer Res 20(14) (2014) 3818–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH, CD20+ tumor-infiltrating lymphocytes have an atypical CD27− memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer, Clin Cancer Res 18(12) (2012) 3281–92. [DOI] [PubMed] [Google Scholar]
- [64].Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W, Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival, Nat Med 10(9) (2004) 942–9. [DOI] [PubMed] [Google Scholar]
- [65].Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G, Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer, N Engl J Med 348(3) (2003) 203–13. [DOI] [PubMed] [Google Scholar]
- [66].Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K, Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer, Proc Natl Acad Sci U S A 102(51) (2005) 18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Boland JL, Zhou Q, Martin M, Callahan MK, Konner J, O’Cearbhaill RE, Friedman CF, Tew W, Makker V, Grisham RN, Hensley ML, Zecca N, Iasonos AE, Snyder A, Hyman DM, Sabbatini P, Aghajanian C, Cadoo KA, Zamarin D, Early disease progression and treatment discontinuation in patients with advanced ovarian cancer receiving immune checkpoint blockade, Gynecol Oncol 152(2) (2019) 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hamanishi J, Mandai M, Konishi I, Immune checkpoint inhibition in ovarian cancer, Int Immunol 28(7) (2016) 339–48. [DOI] [PubMed] [Google Scholar]
- [69].Wu X, Li J, Connolly EM, Liao X, Ouyang J, Giobbie-Hurder A, Lawrence D, McDermott D, Murphy G, Zhou J, Piesche M, Dranoff G, Rodig S, Shipp M, Hodi FS, Combined Anti-VEGF and Anti-CTLA-4 Therapy Elicits Humoral Immunity to Galectin-1 Which Is Associated with Favorable Clinical Outcomes, Cancer Immunol Res 5(6) (2017) 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Keir ME, Butte MJ, Freeman GJ, Sharpe AH, PD-1 and its ligands in tolerance and immunity, Annu Rev Immunol 26 (2008) 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kawakami Y, Rosenberg SA, Human tumor antigens recognized by T-cells, Immunol Res 16(4) (1997) 313–39. [DOI] [PubMed] [Google Scholar]
- [72].Old LJ, Chen YT, New paths in human cancer serology, J Exp Med 187(8) (1998) 1163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ, Cancer/testis antigens, gametogenesis and cancer, Nat Rev Cancer 5(8) (2005) 615–25. [DOI] [PubMed] [Google Scholar]
- [74].Teillaud JL, Dieu-Nosjean MC, Tertiary Lymphoid Structures: An Anti-tumor School for Adaptive Immune Cells and an Antibody Factory to Fight Cancer?, Front Immunol 8 (2017) 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rhodes DA, Isenberg DA, TRIM21 and the Function of Antibodies inside Cells, Trends Immunol 38(12) (2017) 916–926. [DOI] [PubMed] [Google Scholar]
- [76].Veomett N, Dao T, Scheinberg DA, Therapeutic antibodies to intracellular targets in cancer therapy, Expert Opin Biol Ther 13(11) (2013) 1485–8. [DOI] [PubMed] [Google Scholar]
- [77].Jinushi M, Hodi FS, Dranoff G, Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity, Proc Natl Acad Sci U S A 103(24) (2006) 9190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Katoh H, Komura D, Konishi H, Suzuki R, Yamamoto A, Kakiuchi M, Sato R, Ushiku T, Yamamoto S, Tatsuno K, Oshima T, Nomura S, Seto Y, Fukayama M, Aburatani H, Ishikawa S, Immunogenetic Profiling for Gastric Cancers Identifies Sulfated Glycosaminoglycans as Major and Functional B Cell Antigens in Human Malignancies, Cell Rep 20(5) (2017) 1073–1087. [DOI] [PubMed] [Google Scholar]
- [79].Garaud S, Zayakin P, Buisseret L, Rulle U, Silina K, de Wind A, Van den Eyden G, Larsimont D, Willard-Gallo K, Line A, Antigen Specificity and Clinical Significance of IgG and IgA Autoantibodies Produced in situ by Tumor-Infiltrating B Cells in Breast Cancer, Front Immunol 9 (2018) 2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kristensen E, Langvad E, Reimann R, Humoral immunity in malignant skin melanoma. Isolation of melanoma specific IgG from melanoma metastases, Eur J Cancer 12(12) (1976) 945–50. [DOI] [PubMed] [Google Scholar]
- [81].Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N, Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases, Cancer Res 72(16) (2012) 3997–4007. [DOI] [PubMed] [Google Scholar]
- [82].Yakirevich E, Lu S, Allen D, Mangray S, Fanion JR, Lombardo KA, Safran H, Resnick MB, Prognostic significance of IgG4+ plasma cell infiltrates following neoadjuvant chemoradiation therapy for esophageal adenocarcinoma, Hum Pathol 66 (2017) 126–135. [DOI] [PubMed] [Google Scholar]
- [83].Getahun A, Beavers NA, Larson SR, Shlomchik MJ, Cambier JC, Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells, J Exp Med 213(5) (2016) 751–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, Meffre E, Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones, Blood 115(24) (2010) 5026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Cappione A 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I, Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus, J Clin Invest 115(11) (2005) 3205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, Garman L, Helms C, Nakken B, Smith K, Farris AD, Wilson PC, Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors, J Exp Med 206(1) (2009) 139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Andrews SF, Wilson PC, The anergic B cell, Blood 115(24) (2010) 4976–8. [DOI] [PubMed] [Google Scholar]
- [88].Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautes-Fridman C, Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers, Immunol Rev 271(1) (2016) 260–75. [DOI] [PubMed] [Google Scholar]
- [89].Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL Jr., Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity, J Immunol 200(2) (2018) 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sautes-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC, Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention, Front Immunol 7 (2016) 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].McMullen TP, Lai R, Dabbagh L, Wallace TM, de Gara CJ, Survival in rectal cancer is predicted by T cell infiltration of tumour-associated lymphoid nodules, Clin Exp Immunol 161(1) (2010) 81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, Laghi L, Allavena P, Mantovani A, Marchesi F, Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers, Clin Cancer Res 20(8) (2014) 2147–58. [DOI] [PubMed] [Google Scholar]
- [93].Ladanyi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, Gaudi I, Timar J, Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor, Cancer Immunol Immunother 56(9) (2007) 1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, Schell MJ, Sondak VK, Weber JS, Mule JJ, 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy?, Sci Rep 2 (2012) 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E, Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma, BMC Clin Pathol 14 (2014) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K, Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer, Br J Cancer 112(11) (2015) 1782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lee HJ, Park IA, Song IH, Shin SJ, Kim JY, Yu JH, Gong G, Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer, J Clin Pathol 69(5) (2016) 422–30. [DOI] [PubMed] [Google Scholar]
- [98].Lee M, Heo SH, Song IH, Rajayi H, Park HS, Park IA, Kim YA, Lee H, Gong G, Lee HJ, Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis, Mod Pathol 32(1) (2019) 70–80. [DOI] [PubMed] [Google Scholar]
- [99].Solinas C, Garaud S, De Silva P, Boisson A, Van den Eynden G, de Wind A, Risso P, Rodrigues Vitoria J, Richard F, Migliori E, Noel G, Duvillier H, Craciun L, Veys I, Awada A, Detours V, Larsimont D, Piccart-Gebhart M, Willard-Gallo K, Immune Checkpoint Molecules on Tumor-Infiltrating Lymphocytes and Their Association with Tertiary Lymphoid Structures in Human Breast Cancer, Front Immunol 8 (2017) 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Pfannstiel C, Strissel PL, Chiappinelli KB, Sikic D, Wach S, Wirtz RM, Wullweber A, Taubert H, Breyer J, Otto W, Worst T, Burger M, Wullich B, Bolenz C, Fuhrich N, Geppert CI, Weyerer V, Stoehr R, Bertz S, Keck B, Erlmeier F, Erben P, Hartmann A, Strick R, Eckstein M, G. Bridge Consortium, G. Bridge Consortium, G. Bridge Consortium, G. Bridge Consortium, The Tumor Immune Microenvironment Drives a Prognostic Relevance That Correlates with Bladder Cancer Subtypes, Cancer Immunol Res 7(6) (2019) 923–938. [DOI] [PubMed] [Google Scholar]
- [101].Silina K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P, Curioni-Fontecedro A, Foukas P, Levesque MP, Moch H, Line A, van den Broek M, Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma, Cancer Res 78(5) (2018) 1308–1320. [DOI] [PubMed] [Google Scholar]
- [102].Payne KK, Mine JA, Biswas S, Chaurio RA, Perales-Puchalt A, Anadon CM, Costich TL, Harro CM, Walrath J, Ming Q, Tcyganov E, Buras AL, Rigolizzo KE, Mandal G, Lajoie J, Ophir M, Tchou J, Marchion D, Luca VC, Bobrowicz P, McLaughlin B, Eskiocak U, Schmidt M, Cubillos-Ruiz JR, Rodriguez PC, Gabrilovich DI, Conejo-Garcia JR, BTN3A1 governs antitumor responses by coordinating alphabeta and gammadelta T cells, Science 369(6506) (2020) 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Bian Y, Li W, Kremer DM, Sajjakulnukit P, Li S, Crespo J, Nwosu ZC, Zhang L, Czerwonka A, Pawlowska A, Xia H, Li J, Liao P, Yu J, Vatan L, Szeliga W, Wei S, Grove S, Liu JR, McLean K, Cieslik M, Chinnaiyan AM, Zgodzinski W, Wallner G, Wertel I, Okla K, Kryczek I, Lyssiotis CA, Zou W, Cancer SLC43A2 alters T cell methionine metabolism and histone methylation, Nature 585(7824) (2020) 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Cao Y, Trillo-Tinoco J, Sierra RA, Anadon C, Dai W, Mohamed E, Cen L, Costich TL, Magliocco A, Marchion D, Klar R, Michel S, Jaschinski F, Reich RR, Mehrotra S, Cubillos-Ruiz JR, Munn DH, Conejo-Garcia JR, Rodriguez PC, ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression, Nat Commun 10(1) (2019) 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Song M, Sandoval TA, Chae CS, Chopra S, Tan C, Rutkowski MR, Raundhal M, Chaurio RA, Payne KK, Konrad C, Bettigole SE, Shin HR, Crowley MJP, Cerliani JP, Kossenkov AV, Motorykin I, Zhang S, Manfredi G, Zamarin D, Holcomb K, Rodriguez PC, Rabinovich GA, Conejo-Garcia JR, Glimcher LH, Cubillos-Ruiz JR, IRE1alpha-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity, Nature 562(7727) (2018) 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Germain C, Gnjatic S, Dieu-Nosjean MC, Tertiary Lymphoid Structure-Associated B Cells are Key Players in Anti-Tumor Immunity, Front Immunol 6 (2015) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Johansson-Percival A, He B, Li ZJ, Kjellen A, Russell K, Li J, Larma I, Ganss R, De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors, Nat Immunol 18(11) (2017) 1207–1217. [DOI] [PubMed] [Google Scholar]
- [108].Weinstein AM, Storkus WJ, Therapeutic Lymphoid Organogenesis in the Tumor Microenvironment, Adv Cancer Res 128 (2015) 197–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Weinstein AM, Chen L, Brzana EA, Patil PR, Taylor JL, Fabian KL, Wallace CT, Jones SD, Watkins SC, Lu B, Stroncek DF, Denning TL, Fu YX, Cohen PA, Storkus WJ, Tbet and IL-36gamma cooperate in therapeutic DC-mediated promotion of ectopic lymphoid organogenesis in the tumor microenvironment, Oncoimmunology 6(6) (2017) e1322238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhu G, Falahat R, Wang K, Mailloux A, Artzi N, Mule JJ, Tumor-Associated Tertiary Lymphoid Structures: Gene-Expression Profiling and Their Bioengineering, Front Immunol 8 (2017) 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhu G, Nemoto S, Mailloux AW, Perez-Villarroel P, Nakagawa R, Falahat R, Berglund AE, Mule JJ, Induction of Tertiary Lymphoid Structures With Antitumor Function by a Lymph Node-Derived Stromal Cell Line, Front Immunol 9 (2018) 1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Marshall HD, Ray JP, Laidlaw BJ, Zhang N, Gawande D, Staron MM, Craft J, Kaech SM, The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa, Elife 4 (2015) e04851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Crotty S, T follicular helper cell differentiation, function, and roles in disease, Immunity 41(4) (2014) 529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Belanger S, Kasturi SP, Landais E, Akondy RS, McGuire HM, Bothwell M, Vagefi PA, Scully E, Investigators IPCP, Tomaras GD, Davis MM, Poignard P, Ahmed R, Walker BD, Pulendran B, McElrath MJ, Kaufmann DE, Crotty S, CXCL13 is a plasma biomarker of germinal center activity, Proc Natl Acad Sci U S A 113(10) (2016) 2702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothe F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K, CD4(+) follicular helper T cell infiltration predicts breast cancer survival, J Clin Invest 123(7) (2013) 2873–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Walters GD, Vinuesa CG, T Follicular Helper Cells in Transplantation, Transplantation 100(8) (2016) 1650–5. [DOI] [PubMed] [Google Scholar]
- [117].Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA, Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid, J Clin Invest 116(10) (2006) 2622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Aloisi F, Pujol-Borrell R, Lymphoid neogenesis in chronic inflammatory diseases, Nat Rev Immunol 6(3) (2006) 205–17. [DOI] [PubMed] [Google Scholar]
- [119].Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, Chen BK, Genden E, Skobe M, Lira SA, Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid, Proc Natl Acad Sci U S A 104(12) (2007) 5026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Stephen TL, Payne KK, Chaurio RA, Allegrezza MJ, Zhu H, Perez-Sanz J, Perales-Puchalt A, Nguyen JM, Vara-Ailor AE, Eruslanov EB, Borowsky ME, Zhang R, Laufer TM, Conejo-Garcia JR, SATB1 Expression Governs Epigenetic Repression of PD-1 in Tumor-Reactive T Cells, Immunity 46(1) (2017) 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Stephen TL, Rutkowski MR, Allegrezza MJ, Perales-Puchalt A, Tesone AJ, Svoronos N, Nguyen JM, Sarmin F, Borowsky ME, Tchou J, Conejo-Garcia JR, Transforming Growth Factor beta-Mediated Suppression of Antitumor T Cells Requires FoxP1 Transcription Factor Expression, Immunity 41(3) (2014) 427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Park BV, Freeman ZT, Ghasemzadeh A, Chattergoon MA, Rutebemberwa A, Steigner J, Winter ME, Huynh TV, Sebald SM, Lee SJ, Pan F, Pardoll DM, Cox AL, TGFbeta1-Mediated SMAD3 Enhances PD-1 Expression on Antigen-Specific T Cells in Cancer, Cancer Discov 6(12) (2016) 1366–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Sanjabi S, Oh SA, Li MO, Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection, Cold Spring Harb Perspect Biol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H, The cytokine TGF-beta co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells, Nat Immunol 15(9) (2014) 856–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kobayashi S, Watanabe T, Suzuki R, Furu M, Ito H, Ito J, Matsuda S, Yoshitomi H, TGF-beta induces the differentiation of human CXCL13-producing CD4(+) T cells, Eur J Immunol 46(2) (2016) 360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].McCarron MJ, Marie JC, TGF-beta prevents T follicular helper cell accumulation and B cell autoreactivity, J Clin Invest 124(10) (2014) 4375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]