Abstract
Cholinergic neuromodulation plays an important role in numerous cognitive functions including regulating arousal and attention, as well as associative learning and extinction processes. Further, studies demonstrate that cholinergic inputs from the basal forebrain cholinergic system influence physiological responses in the basolateral amygdala (BLA) as well as fear extinction processes. Since rodent models display individual differences in conditioned fear and extinction responses, this study investigated if cholinergic transmission in the BLA during fear extinction could contribute to differences between extinction resistant and extinction competent phenotypes in outbred Long-Evans male rats. Experiment 1 used in vivo microdialysis to test the hypothesis that acetylcholine (ACH) efflux in the BLA would increase with presentation of an auditory conditioned stimulus (CS+) during extinction learning. Acetylcholine efflux was compared in rats exposed to the CS+, a CS- (the tone never paired with a footshock), or to a context shift alone (without CS+ tone presentation). Consistent with acetylcholine's role in attention and arousal, ACH efflux in the BLA was increased in all three groups (CS+, CS-, Shift Alone) by the initial context shift into the extinction learning chamber, but returned more rapidly to baseline levels in the Shift Alone group (no CS+). In contrast, in the group exposed to the CS+, ACH efflux in the BLA remained elevated during continued presentation of conditioned cues and returned to baseline more slowly, leading to an overall increase in ACH efflux compared with the Shift Alone group. Based on the very dense staining in the BLA for acetylcholinesterase (ACHE), Experiment 2 examined if individual differences in fear extinction were associated with differences in cholinesterase enzyme activity (CHE) in the BLA and/or plasma with a separate cohort of animals. Cholinesterase activity (post-testing) in both the BLA and plasma was higher in extinction competent rats versus rats resistant to extinction learning. There was also a significant negative correlation between BLA CHE activity and freezing during extinction learning. Taken together, our results support a role for ACH efflux in the BLA during cued fear extinction that may be modulated by individual differences in ACHE activity, and are associated with behavioral responses during fear extinction. These findings implicate individual differences in cholinergic regulation in the susceptibility to disorders with dysregulation of extinction learning, such post-traumatic stress disorder (PTSD) in humans.
Keywords: Amygdala, Acetylcholine, Acetylcholinesterase, Fear extinction, Individual differences, Microdialysis
Highlights
-
•
Basolateral amygdala acetylcholine efflux is increased during extinction learning.
-
•
Acetylcholine efflux also increased transiently in amygdala during a context shift.
-
•
Acetylcholinesterase activity in amygdala differed between extinction phenotypes.
-
•
Amygdala cholinergic regulation contributes to variations in extinction learning.
1. Introduction
As demonstrated across a variety of studies, cholinergic signaling is heavily implicated in cognitive function, arousal, and attention, including the consolidation of long-term memories (see (Gold 2003; Power et al., 2003; Tinsley et al., 2004; Hasselmo and Sarter 2011; Robinson et al., 2011; Konig et al., 2018)). This cholinergic regulation is most evident in various studies using systemic and region-specific pharmacological manipulations during associative fear learning and extinction paradigms (Gold 2003; Power et al., 2003; Tinsley et al., 2004; Robinson et al., 2011; Gould and Leach 2014; Knox 2016; Wilson and Fadel 2017). During Pavlovian fear learning a neutral conditioned stimulus (CS+) such as a tone is paired with an aversive unconditioned stimulus such as a footshock (US). The temporal pairing of the CS+ and US enables both the context and the CS+ (the tone), to elicit freezing and other defensive responses even when the CS+ is presented in a novel context (Fendt and Fanselow 1999). Extinction of cue-conditioned fear is elicited by repeated re-exposure to the CS+ in the absence of the US (Baldi and Bucherelli 2015). Plasticity within and among neural regions in the fear circuit that includes the amygdala, hippocampus and prefrontal cortex (PFC) is thought to contribute to these conditioned fear and extinction behaviors (Fendt and Fanselow 1999; Milad and Quirk 2012; Baldi and Bucherelli 2015). In contrast to the mechanisms underlying the original learning of the contextual or cue-conditioned response, extinction learning is thought to involve disparate neuronal populations and signaling processes specifically within prefrontal-amygdalar circuits (Herry et al., 2008; Tronson et al., 2009; Orsini and Maren 2012; Baldi and Bucherelli 2015; Rozeske et al., 2015).
The basal forebrain cholinergic system (BFCS) provides dense neuromodulatory inputs to the basolateral amygdala (BLA) complex, targeting both calcium/calmodulin protein kinase II (CaMK)-positive (presumably glutamatergic) pyramidal neurons and interneurons in the BLA (Muller et al., 2011; Lee and Kim 2019). Recent studies have implicated a critical role for these inputs in cued fear responses, with a particularly important influence on cued fear extinction. Optogenetic stimulation of cholinergic inputs from the BFCS to the BLA can influence physiological responses of both interneurons and pyramidal cells in the BLA (Unal et al., 2015; Jiang et al., 2016; Aitta-Aho et al., 2018; Lee and Kim 2019), and also suggest that cholinergic inputs enhance the “signal to noise ratio” (Unal et al., 2015). Both muscarinic and nicotinic cholinergic receptors have been implicated in these electrophysiological responses (Unal et al., 2015; Jiang et al., 2016; Aitta-Aho et al., 2018; Lee and Kim 2019). Further, optogenetic stimulation of amygdala cholinergic inputs during acquisition disrupts the extinction, but not the acquisition, of cued fear memories (Jiang et al., 2016). In addition, increasing acetylcholine (ACH) release in the BLA using histaminergic agonists improves the expression of fear memories (Cangioli et al., 2002), while lesions of the BFCS system disrupt fear learning and extinction (Knox 2016; Knox and Keller 2016). Pharmacological studies support the role of both muscarinic and nicotinic receptor activation in extinction memory formation and recall [see (Maruki et al., 2003; Elias et al., 2010; Santini et al., 2012; Zeitlin et al., 2012; Zelikowsky et al., 2013; Barreto et al., 2015; Wilson and Fadel 2017; Sharp 2019). These studies are all consistent with the notion that the BFCS is activated and enhances ACH efflux in projection sites including PFC and BLA, resulting in increased endogenous tone on cholinergic receptors during both exposure to conditioned cues and extinction training. The role of ACH in cue detection and attentional performance (Hasselmo and Sarter 2011), combined with the indication that BLA pyramidal neuron activity is critical for determining the salience of a conditioned stimulus during an aversive learning task (Sengupta et al., 2018), also suggest that cholinergic regulation of BLA activity may be important during cued fear learning and/or extinction. Although some studies have examined acetylcholine release during associative learning responses, none of these studies have focused on measuring ACH efflux in the BLA during Pavlovian fear extinction (Acquas et al., 1996; Nail-Boucherie et al., 2000; Izaki et al., 2001; Calandreau et al., 2006), which is one of the goals of the present study. We also examined glutamate efflux since anatomical and electrophysiological evidence suggests that cholinergic inputs into the BLA modulate glutamate neurotransmission via both muscarinic and nicotinic receptors (Yajeya et al., 2000; Jiang and Role 2008; McDonald et al., 2019), and inputs from BFCS may co-release acetylcholine and glutamate (Nickerson Poulin et al., 2006).
The BLA has very dense staining for acetylcholinesterase (ACHE), the enzyme that breaks down acetylcholine in the synaptic cleft to terminate neurotransmission. Various stressors can alter ACHE enzyme activity and alter the expression of ACHE splice variants in multiple brain regions including amygdala (Kaufer et al., 1998; Birikh et al., 2003; Nijholt et al., 2004; Sklan et al., 2004; Das et al., 2005; Meshorer et al., 2005; Perrier et al., 2005; Salmon et al., 2005; Dori et al., 2011; Valuskova et al., 2017; Ketenci et al., 2020). Human studies also support an association between plasma ACHE activity, ACHE genotypes, and trait or state anxiety (Sklan et al., 2004). Further, a neuroimaging study in humans identified individual variation in genetic markers for ACH synthesis and signaling that were correlated with differences in BFCS modulation of amygdalar functional connectivity during processing of salient stimuli, suggesting underlying neurobiological differences in cholinergic regulation of this fear circuit (Gorka et al., 2015). In humans, dysregulation of fear learning or extinction processes may predict susceptibility to traumatic stress disorders, and patients with post-traumatic stress disorder (PTSD) show impairments in fear learning and extinction (Guthrie and Bryant 2006; Shin and Liberzon 2010; Milad and Quirk 2012; Pitman et al., 2012; Holmes and Singewald 2013; Lommen et al., 2013; Kutlu and Gould 2015). Rodent models allow for examining the mechanisms underlying individual differences in risk and resilience following traumatic stress (Holmes and Singewald 2013; Deslauriers et al., 2018), and such models have demonstrated individual differences in fear extinction (Bush et al., 2007; Galatzer-Levy et al., 2013; Holmes and Singewald 2013; Shumake et al., 2014; Gruene et al., 2015; Sharko et al., 2017; Monfils et al., 2019) as well as various indices of cholinergic function including ACH release in the PFC (van der Zee et al., 1997; Izaki et al., 2001; McIntyre et al., 2002; Gold 2003). Some of these cholinergic markers are correlated with behavioral responses during learning tasks, including conditioned fear or extinction responses. Consistent with similar rodent models, we have seen individual differences in cued fear extinction in outbred Long-Evans rats (Sharko et al., 2017), and given the role of ACHE in cholinergic signaling, we hypothesized that cholinesterase activity (CHE) in BLA might differ between extinction resistant and extinction competent phenotypes.
Therefore, in the present study we investigated if cholinergic transmission in the BLA induced by repeated cue presentation during fear extinction could contribute to the differences between extinction resistant and extinction competent rats. Experiment 1 used in vivo microdialysis to test the hypothesis that ACH efflux would increase with presentation of an aversive CS during extinction learning in the BLA. In a separate cohort of animals, Experiment 2 examined if individual differences in fear extinction were associated with differences in BLA or plasma CHE activity. Our results demonstrate that acetylcholine efflux in BLA is induced during cued fear extinction, and that individual differences in CHE activity in this region are associated with freezing responses during fear extinction.
2. Materials and methods
2.1. Experiment 1: subjects and surgery for implanting microdialysis cannulas
2.1.1. Subjects
The timeline for Experiment 1 is shown in Fig. 1A. Adult male Long Evans rats (150–175 g on arrival; Envigo Indianapolis, IN) were singly housed in an AAALAC accredited, temperature controlled vivarium under a 12:12 light/dark cycle with ad libitum food and water. Rats were habituated to brief handling prior to surgery. The University of South Carolina Institutional Animal Care and Use Committee (IACUC) approved all animal procedures. For experiment 1, rats were briefly restrained to collect a tail blood sample (≤0.5 mL) before behavioral testing.
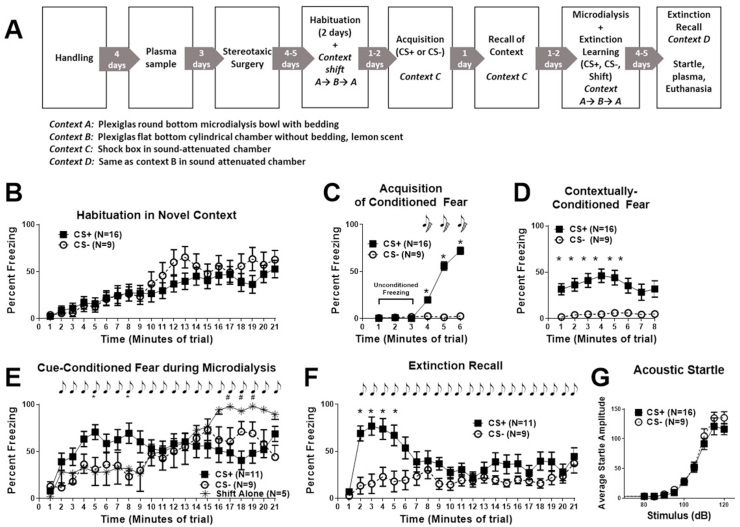
Fig. 1.
Timeline and behavior during the microdialysis experiment (Experiment 1). Panel A shows the timeline for Experiment 1. Panels B–F show percent freezing (immobility) during each minute of habituation on day one (B) and all conditioning trials (C, D, E, F) for all rats, including animals without probe placement in the BLA (Panels B–G). Acoustic startle responses are also shown (Panel G). After taking pre-test tail blood sample, male Long-Evans rats were implanted with a microdialysis guide cannula above the basolateral amygdala. Following 4–5 days of post-operative care, rats were habituated to the microdialysis procedure including transfer into Contexts A and B on two separate days (Habituation, Panel B). Rats then underwent auditory fear conditioning with three tone-shock pairings (CS+, N = 16) or were exposed to tones without shock (CS-, N = 9) followed by contextual recall 24 h later (both in Context C; Panel C). Microdialysis was performed during extinction learning following a context shift and 20 tone presentations (CS+, N = 11, CS- N = 5) or a context shift without tone presentation (Shift Alone, N = 5, Panel E). Extinction recall in Context D (Panel F) and auditory startle responses (to confirm intact hearing, Panel G) were done prior to euthanasia and brain harvesting for microdialysis probe placement. Panel B shows that immobility behavior during day one of habituation increased during the 21 min exposure in the new context, without differences between CS+ and CS- groups. Note the similarity between habituation and the CS- and Shift Alone group in Panel C. Rats exposed to 3 tone-shock pairings (CS+, N = 16) froze more during the tone-shock pairings during acquisition (Panel C) and contextual fear recall (Panel D) than rats exposed to tones without shock (CS-, N = 9). Panel E shows immobility during the microdialysis session that was initiated with a context shift followed by 20 tone presentations at a 1 min ISI. CS+ animals (N = 11) exposed to the tones during the session showed greater freezing behavior during the first 10 min compared to the CS- (N = 9) and Shift Alone (N = 5, no tone presentation) groups. During the last 10 min of the session, the Shift Alone and CS- groups showed increasing immobility that was visually assessed as resting rather than freezing and was similar to habituation (time × group interaction, # = P < 0.05 between CS+ and Shift Alone groups). Panel F shows CS+ rats exhibited more freezing during extinction recall then CS- groups; note that Shift Alone rats are not included in this data set since they did not undergo extinction trial at the same time as other groups during microdialysis. Acoustic startle responses (Panel G) assessed after conditioning trials were intact in both CS+ and CS- groups, indicating that both groups responded similarly to auditory cues. * = P < 0.05 CS+ versus CS- groups; # = P < 0.05 CS+ versus Shift Alone groups. Data are represented as mean ± SEM. dB = decibels.
2.1.2. Stereotaxic surgery
On days 7–8 post-arrival, rats (N = 30) to be used for microdialysis were surgically implanted with a unilateral cannula [MD-2251, Bioanalytical Systems, Inc. (BAS), West Lafayette, IN, USA] aimed just above the BLA via stereotaxic surgery with blunt ear bars. Rats were anesthetized with isoflurane (1–5%) in oxygen, and local anesthetic (2% carbocaine, s. c.) was injected at the scalp incision site and behind the ears. Body temperature was maintained using a homeothermic blanket system (Harvard Apparatus, Holliston, MA). A unilateral cannula with a removable stylet for maintaining patency was inserted above either the right or left BLA using the following coordinates measured from Bregma with skull flat: A/P −2.0/2.7, M/L ± 4.8, and D/V 6.8 (Paxinos and Watson 1997). Cannulas were stabilized using 2–3 skull screws surrounded by dental cement (DuraLay Inlay Resin, Reliance Dental Manufacturing, Alsip, IL). Rats were outfitted with a rat collar (MF-5371, BASi) for tethering during microdialysis testing. Nalbuphine (1 mg/kg, s. c.) was administered postoperatively for pain management, the diet was supplemented with bacon softies (Bio-serve, Frenchtown, NJ) to maintain postoperative weight, and topical nitrofurazone powder (NFZ puffer, Neogen Corporation) was used for prevention of infection at the incision site. Animals were allowed 4–6 days of recovery from surgery, during which all rats were habituated to stylet removal.
2.2. Experiment 1: behavioral analysis of microdialysis subjects
2.2.1. Habituation
Following surgical recovery, rats were habituated on 2 consecutive days (4 h/day) to the microdialysis procedure in order to minimize responses to handling and a context shift during the microdialysis session. Two rats were habituated and tested at a time, although microdialysis chambers were separated by sound attenuating barriers. Habituation included transportation to the microdialysis room, connection to a tethering system (without probe insertion), 3 h in a Plexiglas microdialysis bowl (Context A, BASi Rat-turn bowl, 42 cm diameter x 35 cm height with Aspen bedding), a 21 min context shift into a distinct flat-bottom cylindrical enclosure (Context B, BASi, 31 cm diameter x 33 cm height without bedding; lemon scent), and then a return to Context A for 39 min. The 21 min context shift was recorded overhead via video camera and freezing behavior (immobility) was analyzed using Freezescan software (CleverSys, Inc., Reston, VA) as described below.
2.2.2. Fear conditioning and extinction
After the two days of habituation, a fear conditioning and extinction protocol described previously was used (Sharko et al., 2017), although cue-conditioned recall and extinction learning occurred during microdialysis. For all fear conditioning, microdialysis, and extinction trials, Freezescan software (CleverSys, Inc., Reston, VA) was used to acquire and analyze freezing behavior in 1-min bins that included tone presentation and the inter-tone interval; Freezescan parameters were set such that freezing behavior was detected as immobility (absence of movement other than breathing). For fear acquisition, animals were put in a sound-attenuating shock box (Context C; Med Associates, Inc., Fairfax, VT). Following 3 min of unconditioned freezing, a conditioned CS+ group of rats (N = 16) were exposed to three 10 s (sec) tones (80 dB, 2 kHz) co-terminating with foot shock (1 s, 1 mA) presented at 60 s interstimulus intervals (ISI). The unconditioned CS- group (N = 9) was exposed to the same cue presentation protocol without footshock. Ammonium hydroxide (7%) was used to clean the shock box between rats. The following day, context-conditioned freezing was assessed via an 8 min re-exposure to the shock box (Context C). One-two days later (4 rats at 4–5 days later), microdialysis sampling for analysis of ACH and glutamate (GLU) efflux was done during re-exposure to 20 tones (10 s, 80 dB, 2 kHZ, 1 min ISI) in Context B (see section 2.3.1 below); twenty tones allowed assessment of cue-conditioned recall and extinction learning in a single trial (Likhtik et al., 2008; Sharko et al., 2017). Following the 20 tone presentations, rats were switched back to Context A for 90 min sampling during recovery. For extinction recall, 4–5 days later rats were exposed to an additional 20 tones (10 s, 80 dB, 2 kHz, 1 min ISI) in a chamber identical to that used for Context B, except it was located in a sound attenuated chamber (Context D). Context B and D chambers for extinction learning and extinction recall were cleaned with 70% ethanol between subjects and were scented with lemon. After extinction recall, animals were tested for acoustic startle responses (to ensure adequate hearing), anesthetized for a tail blood sample for CHE analysis, and perfused to collect brains for verification of cannula placement.
2.2.3. Acoustic startle response
After the extinction recall trial, rats were tested for auditory startle responses using SR-LAB™ Startle Response System hardware and automated software (San Diego Instruments, San Diego, CA) using a protocol modified from Kelly et al. (2007) in mice (Kelly et al., 2007). Rats were placed in a cylindrical enclosure with an underlying piezoelectric accelerometer within a sound attenuating isolation cabinet for automated recording of startle responses. Chambers were cleaned with 70% ethanol between animals. After a 5-min acclimation period with 65 dB background noise, rats were presented with 40 msec white noise auditory stimuli with a variable ISI of 10–17 s. After five 120 dB stimuli to determine baseline responding, five stimuli at 0, 80, 85, 90, 95, 100, 105, 110, 115 and 120 dB were presented in a random order and average startle amplitude from the five trials at each intensity (80–120 dB) are reported. This was followed by trials to assess habituation (five 120 dB exposures), pre-pulse inhibition (PPI), and a second habituation period (five 120 dB exposures) (data not reported).
2.3. Experiment 1: In vivo microdialysis for analysis of acetylcholine and glutamate efflux in BLA during extinction learning
2.3.1. Microdialysis during Extinction Learning
Microdialysis was conducted as described previously (Reznikov et al., 2009; Carrero et al., 2019) during the extinction learning trial. On the day of the microdialysis session, guide cannula stylets were switched to semi-permeable (30 kDa cutoff) microdialysis probes (MD-2200, BASi) that projected 2 mm further than the guide cannula. The probe was perfused at a rate of 2 μL/min with artificial cerebrospinal fluid (aCSF) containing 150 mM NaCl, 3.0 mM KCl, 1.7 mM CaCl2 dihydrate, 0.9 mM MgCl2 hexahydrate, and 4.9 mM D-glucose, plus 100 nM neostigmine bromide to promote reliable recovery of detectable levels of ACH during collection (Konig et al., 2018). The outlet line was connected to an FC-90 fraction collector (Amuza, San Diego, CA) with chilled storage tubes (4 °C) for dialysate collection in 10 min intervals. Following a 3-h discard period in context A, six collections were used to sample 1 h of baseline neurotransmitter efflux. Rats were then transferred into context B for the extinction learning trial. After 1 min for unconditioned freezing, two 10 min samples were collected during the presentation of 20 tones (10 s, ~80 dB, 2 kHZ, 1 min ISI) while freezing was determined using a camera mounted above the chamber using FreezeScan (CleverSys, Inc., Reston, VA). After the extinction learning trial, rats were returned to context A for dialysate collections during a 90 min recovery period. Some conditioned rats in the CS+ group (N = 5 total; N = 3 with placement in BLA) were exposed to this procedure without tone presentation to determine the effects of the context shift alone on ACH efflux (shift alone group). ACH and GLU efflux was sampled during this initial microdialysis testing condition (CS+, CS-, or Shift Alone) and the following 90 min recovery period. After recovery, rats were shifted back into context B for 20 min without tones (except for shift alone group, which now received tones) followed by an additional 90 min recovery period (data from second test session not included). A 10 μL aliquot of each dialysate was stored at −80 °C for ACH analysis with the remaining dialysate being stored for GLU analysis.
2.3.2. Analysis of acetylcholine levels
High performance liquid chromatography (HPLC) for ACH was done as described previously (Calva et al., 2018; Macht et al., 2019) using 10 μl of dialysates and external standards. ACH was analyzed with an HPLC-ECD, HTEC-510 (Amuza, San Diego, CA) and separated from other analytes via an AC-GEL analytical column (2.0 mm internal diameter x 150 mm length, Amuza, San Diego CA) in mobile phase (pH ~8.5, 49.4 mM potassium bicarbonate, 134.3 μM disodium ethylenediamine tetraacetate (EDTA-2Na), and 1.23 mM 1-decanesulfonic acid, sodium salt). Subsequently, an AC-Enzympak II enzyme reactor (1 mm internal diameter x 4 mm length, Amuza, San Diego, CA) provided post-column derivatization of ACH to hydrogen peroxide for detection at a platinum electrode (applied potential = +450 mV). A standard curve based on external standards (0.1, 1.0, and 10 pmol) was utilized to quantify ACH in each dialysate (limit of detection ~5 fmol).
2.3.3. Analysis of glutamate levels
For GLU, 5 μl of dialysate was diluted with 5 μl aCSF for HPLC analysis (see (Calva et al., 2018)). GLU was analyzed on a separate HTEC-510 HPLC coupled to an AS-700 insight autosampler (Amuza, San Diego, CA) used for automated derivatization and injection. Precolumn derivatization was achieved by adding 2 μl of o-phthaldialdehyde reagent to each 10 μl dialysate, then injecting 10 μl of the derivatized dialysate onto a FA-3ODS 3 × 75 mm analytical column (Amuza, San Diego, CA) for separation of GLU from other amino acids and metabolites using a mobile phase consisting of 80% 0.1 mM phosphate buffer, 7% methanol, and 13% acetonitrile by volume with 148.6 nM EDTA-2Na. Derivatized GLU was oxidized and detected on a glassy carbon electrode with an applied potential of +750 mV. A standard curve (100, 1000, and 10,000 nM) was utilized to quantify GLU in each diluted dialysate (limit of detection ~100 nM).
2.4. Experiment 1: microdialysis probe placement
After behavioral testing, rats were deeply anesthetized (5% isoflurane) followed by intracardiac perfusion with clearing solution (0.1 M phosphate buffer, 0.5 mM EDTA, 0.05% NaNO2) followed by 10% formalin in 0.05 M phosphate-buffered saline to collect brains for cannula placement (see (Carrero et al., 2019)). After removal and post-fixation in buffered 10% formalin, coronal sections (100 μm) were stored in 0.1 M phosphate buffer at 4° C. Sections were stained for ACHE (0.2 M Tris maleate buffer (pH 5.7), 0.1 M sodium citrate, 0.03 M cupric sulfate, 5 mM potassium ferricyanide, and 1.7 mM acetylthiocholine iodide) for ~60 min at room temperature followed by a 70% ethanol rinse and coverslipping (Hedreen et al., 1985; Carrero et al., 2019). Rats lacking probe placement in BLA (mainly central amygdala or lateral ventricle) were analyzed as a separate group to examine the specificity of effects to the BLA (N = 5 CS+, N = 4 CS- rats).
2.5. Experiment 1: statistical analyses
For microdialysis experiments ACH and GLU efflux is presented as percent of baseline, averaged over the six 10 min collections. Five rats were removed from all analyses since a full set of dialysate samples at all time points was not collected; additional animals were removed from GLU analysis due to insufficient sample to complete analysis. Analysis of variance (ANOVA) with time as a repeated measure was used to compare efflux during baseline, tone presentation, and 90 min recovery in CS+ (N = 7), CS- (N = 5), and Shift Alone (no tone; N = 3) groups with probe placement in the BLA; post-hoc Bonferroni analysis was used to determine differences between the three treatment groups following a significant main effect. In addition, both the average percent increase and the area under the curve (AUC; based on pmol ACH changes) during the 20 min tone presentation and 90 min recovery period were calculated for ACH and GLU. These were analyzed using one-way ANOVA in the CS+ (N = 7), CS- (N = 5), Shift Alone (N = 3), and CS+ rats with placement outside the BLA (N = 4) groups. Freezing behavior and startle responses in the CS+ and CS- groups were compared using ANOVA with repeated measures (time) for each behavioral trial. Pearson correlation coefficients were used to explore relationships between behavioral endpoints (freezing, startle) and changes in ACH and GLU efflux in BLA. All data were analyzed using GraphPad Prism 8 (La Jolla, CA, USA). Significance was set as alpha = 0.05. Data are expressed as means ± standard error of the mean (SEM).
2.6. Experiment 2: cholinesterase activity in plasma and BLA
CHE activity was assessed in both BLA and plasma immediately after the extinction recall trial in a separate cohort of rats exposed to the CS+ protocol (N = 30). The timeline for Experiment 2 is shown in Fig. 4A. Prior to fear conditioning, rats were outfitted with indwelling cardiotransmitters [Data Sciences International (DSI), St. Paul MN) HD-S11] implanted in the intraperitoneal cavity for monitoring cardiovascular responses during testing ~12–16 days prior to behavioral testing (cardiovascular data not included; see (Finnell et al., 2018) for surgical methods).
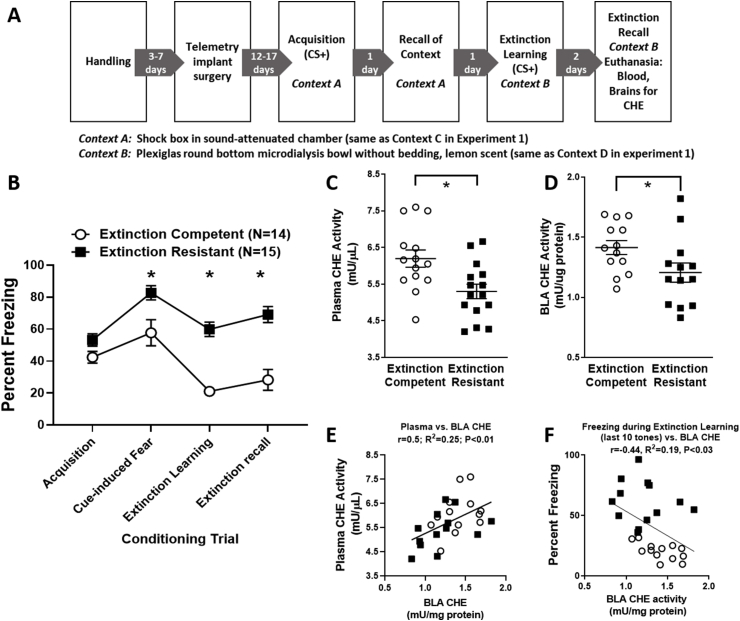
Fig. 4.
Timeline (Panel A), fear extinction behavior (Panel B), and cholinesterase activity (CHE) in plasma and BLA (Panels C–F) for Experiment 2. Rats were implanted with a DSI telemetry device for cardiovascular recordings (data not reported). Rats then underwent auditory fear conditioning acquisition via three tone-shock pairings (CS+, N = 30) followed by contextual fear recall 24 h later (both in Context A, identical to Context C in Experiment 1). Extinction learning was then assessed 24 h later with 3 min in Context B (identical to Context D in Experiment 1) to assess unconditioned freezing followed by exposure to 20 tones. Extinction recall was assessed 48 h later followed immediately by euthanasia and collection of blood and brains for CHE analysis. Panel B shows group differences between extinction resistant (ER) and extinction competent (EC) rats emerged during cue-induced fear (first 5 tone presentations), extinction learning (last 10 tone presentations of extinction learning), and extinction recall (first 5 tones of extinction recall), but not acquisition (during three tone-shock pairings). Rats were divided into ER and EC groups based a median split of the average freezing during extinction learning (see Sharko et al., 2017). Panels C and D show that ER rats had lower levels of post-test plasma and BLA CHE activity compared EC rats. Panels E and F demonstrate that not only were BLA and plasma CHE positively correlated (r = 0.49, P < 0.01), but BLA CHE activity was negatively correlated with the average percent freezing during the last ten tones of extinction learning (r = −0.44, P = 0.02). * = P < 0.05 for ER versus EC. Data are represented as mean ± SEM. Closed squares = ER rats, open circles = EC rats.
2.7. Experiment 2: fear conditioning and extinction
Except for the extinction learning trial, which had to be modified for conducting microdialysis, fear conditioning and extinction protocols were as described in Experiment 1 and in (Sharko et al., 2017). All animals received three tone-shock pairings (like CS+ group in Experiment 1) during fear acquisition in a shock box (Context A) and were tested for contextual fear responses in the same context 24 h later (context recall data not included). The following day, for cue-conditioned recall and within-trial extinction learning, rats were placed in a novel context (Context B) similar to Context D in Experiment 1. After a 3 min period to measure unconditioned freezing, 20 tones (10 s, 80 dB, 2 kHz, 1 min ISI) were presented. Forty-eight hours later, rats were returned to Context B and extinction recall was assessed via the presentation of 20 tones (10 s, 80 dB, 2 kHZ, 1 min ISI) following 1 min of unconditioned freezing. Freezing behavior in each trial was calculated using Freezescan as described in Experiment 1 (CleverSys, Inc., Reston, VA). Freezing behavior was averaged over the following periods: acquisition during the three tone-shock pairings, 8 min of re-exposure to context A, the first 5 presentations of the conditioned tone in Context B (Cue-induced Fear), the last 10 tones in Context B (Extinction Learning), and the first 5 tones of Extinction Recall. Rats were divided into Extinction Resistant (ER) and Extinction Competent (EC) groups using a median split of average freezing during Extinction Learning (last 10 tones) (see (Sharko et al., 2017)).
2.8. Experiment 2: analysis of BLA and plasma cholinesterase activity
Immediately after extinction recall, rats were euthanized by rapid decapitation for brain and blood collection and CHE analysis. Trunk blood was collected into chilled tubes containing 100 μl of 30 mg/ml EDTA and 10 μl Aprotinin (11,117KIU/ml), then centrifuged for 15 min at 1500×g (4 °C) and serum was separated and stored at −20 °C until analysis. Brains were removed, rapidly frozen using powdered dry ice, and stored at −80 °C until analysis. For analysis of CHE in BLA, a 1 mm brain slice was cut on a freezing microtome and placed on a chilled Peltier plate while a 1 mm diameter BLA punch was taken. Punches were placed in 400 μl of 0.1 M phosphate buffer on ice and homogenized using a Next Advance Bullet Blender tissue homogenizer (Next Advance, Troy, NY; 100 μl of beads, 3 min, speed 8). The supernatant was removed and aliquots for protein analysis and CHE assay were stored at −80 °C until assay.
A CHE activity assay (measuring both acetylcholinesterase and butyrylcholinesterase activity) was performed on BLA and plasma using the Abcam kit (#ab138871, Abcam, Cambridge, MA; see (Macht et al., 2018)). Plasma was diluted 1:50 and BLA samples were diluted 1:2 using the assay buffer. Fifty μl of standards ranging from 0 to 300 mU/mL and diluted samples were added to a 96-well plate in duplicates, followed by 50 μl of acetylthiocholine reaction mixture. Following 30 min incubation in the dark, the plate was read at 410 nm absorbance using a microplate reader (BioTek Synergy 2 Microplate Reader, Winooski, VT). Sample CHE activity was interpolated from the linear standard curve. Plasma values are expressed as mU CHE/μl; BLA activity is expressed as mU CHE/mg protein. Protein was determined by analyzing 10 μl of BLA homogenate using the Pierce™ BCA Protein Assay-Low Protein Standard Curve (Thermo Fisher Scientific, Waltham, MA). One BLA sample from the ER group was removed as an outlier.
2.9. Experiment 2: statistical analyses
Based on our prior results (Sharko et al., 2017) and other reports (Bush et al., 2007; Galatzer-Levy et al., 2013; Shumake et al., 2014; Monfils et al., 2019) demonstrating a non-normal distribution of freezing during extinction learning, animals were divided into EC and ER groups using a median split of the average percent freezing during the last 10 tones of the extinction learning trial (Sharko et al., 2017). Freezing in ER and EC groups was compared using two-way ANOVA during cue-induced fear (first five tone presentations during extinction learning), extinction learning (last 10 tones during extinction learning), and extinction recall (first five tones of extinction recall trial). Bonferroni post-hoc analysis was used to determine specific group differences once a main effect was identified. CHE activity between the two groups were compared in plasma and the BLA using t-tests. Pearson correlation coefficients were used to determine significant relationships between behavioral endpoints (freezing) and CHE activity. All data were analyzed using GraphPad Prism 8 (La Jolla, CA, USA). Outliers were identified using the ROUT method (GraphPad Prism 8, Q = 2%). Significance was set as alpha = 0.05. Data are expressed as means ± SEM.
3. Results
3.1. Behavioral analysis and microdialysis during fear conditioning and fear extinction
As seen in Fig. 1 (panels C. D, E, F), rats exposed to the conditioned cue paired with shock (CS+ group; N = 16) showed more freezing behavior in all conditioning trials than rats that were exposed to the tones without shock during acquisition (CS- group; N = 9). The behavioral analysis includes all rats regardless of probe placement (both BLA and outside BLA). The CS+/CS- effect was significant for acquisition [F (1,23) = 129, P < 0.0001], contextually-conditioned freezing [F (1,23) = 15.9, P < 0.001], and extinction recall [F (1,18) = 13.1, P = 0.002], and the interaction between CS+ vs. CS- over time was also significant during both acquisition [F (5,115) = 90.2, P < 0.0001] and extinction recall [F (20,360) = 2.72, P = 0.0001]. Note that the CS+ group during extinction recall (Fig. 1F) does not include the rats that were exposed to Shift Alone (no tones) during microdialysis, since there was no extinction learning trial during the first context shift.
Freezing during the microdialysis session with the presentation of the conditioned cue in the CS+ group was higher during the first 10 tone presentations (Fig. 1E) compared to freezing in the CS- and Shift Alone (no tones) groups. Immobility gradually increased during the 21 min trial in the CS- and Shift Alone groups (with or without tone presentations, respectively). This pattern during microdialysis resulted in a significant effect of time [F (20,400) = 7.85, P < 0.0001] and a time by group interaction [F (40,440) = 2.65, P < 0.0001], but not a main effect of group (CS+, CS- versus Shift Alone; [F (2,22) = 0.86, P = 0.4]). This interaction resulted from greater freezing in the CS+ group compared to the CS- group during the first 10 tones, and higher immobility in the Shift Alone group during the later times in the trial (Fig. 1E). This pattern in the CS- and Shift Alone groups was very similar to the behavioral changes seen during the prior habituation to the same context (Fig. 1B), which was associated with the animals transitioning into sleeping or resting during the later parts of the trial after the context shift. Using the automated Freezescan system, the absence of movement (except breathing) used to detect freezing behavior also scores sleeping/resting as freezing. Visual examination of the behavioral files confirmed behavior in the CS+ group was indeed freezing, while later in the trial CS- and Shift Alone groups were resting or sleeping (but not freezing). The first habituation trial (Fig. 1B) also showed an effect of time [F (20,460) = 9.5, P < 0.0001] but no difference between CS+ and CS- groups [F (1,23) = 0.9, P = 0.35]. A comparison of freezing between CS+ and CS- rats during the tone presentation (10 s) versus the inter-tone interval (50 s) showed that immobility during the first 10 tone presentations in the CS+ group (48.2 ± 8.0%) was twice that seen in CS- rats (24.1 ± 5.2%). A similar difference was seen during the inter-tone intervals (58.7 ± 6.5% in CS+; 32.6 ± 5.2% in CS-; P < 0.05). Further, CS+ rats showed similar levels of freezing during the tones and inter-tone intervals through the trial. There was also no difference between CS+ versus CS- groups in the pre-tone interval (first minute of session; 8.1 ± 3.4 in CS+; 12.9 ± 4.9% in CS-, P > 0.05). We also used a median split of freezing during extinction learning (during microdialysis) to divide the N = 11 CS+ rats (including rats with placement outside the BLA) into ER and EC phenotypes (like in Experiment 2), however only two of seven rats in the CS+ group with probe placement in the BLA showed the ER phenotype (N = 5 EC). This ER/EC comparison may have been confounded by using an ACHE inhibitor in the perfusate to allow reliable detection of ACH release during microdialysis, which may have also modified freezing during the extinction learning trial and therefore the extinction phenotype of the animal.
After the extinction recall session, animals used for the microdialysis study were tested for acoustic startle responses to ensure similar responses to auditory cues. As seen in Fig. 1G, startle responses to increasing auditory cues were robust [F (12,276) = 140.5 P < 0.0001] and did not differ between the CS+ and CS- groups [F (1,23) = 0.71, P = 0.4].
3.2. Acetylcholine efflux during presentation of conditioned cue and extinction learning
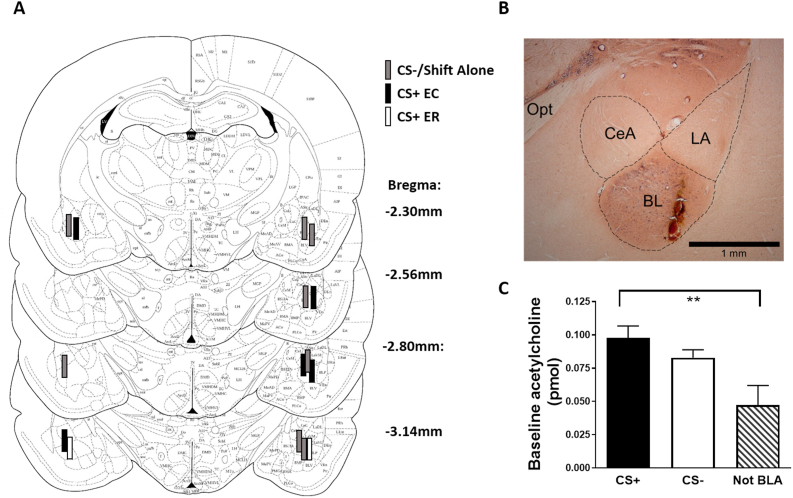
Fig. 2 shows the location of the probes in the BLA used for analysis of ACH efflux in the CS+ (N = 7), CS- (N = 5) and Shift Alone (N = 3) groups. Not unexpectedly, baseline levels of acetylcholine were higher in the BLA than in rats with placement outside the BLA (central amygdala, lateral ventricle; Fig. 2C). This resulted in a main effect (F (2,21) = 5.56, P = 0.01) between the three groups, but no difference between CS+ (N = 10) and CS- (N = 5) groups with placement in the BLA. The extinction phenotype of the animals in the CS+ group with probe placement in the BLA (N = 7 rats) is indicated in Fig. 2A (ER = white bars; EC = black bars), although we did not compare ACH efflux between phenotypes due to the low N (N = 2) in the ER group.
Fig. 2.
Microdialysis probe placement and baseline acetylcholine levels (Experiment 1). Panel A shows probe placement in the basolateral amygdala (BLA) (Paxinos and Watson, 1997). Panel B shows an example of a section stained with acetylcholinesterase demonstrating probe placement in the BLA [(taken at 4x magnification; the optic tract (Opt) is located medially and dotted lines indicate central amygdala (CeA), lateral amygdala (LA), and basal amygdala (BL)] Panel C shows average 1 h baseline acetylcholine levels. The CS+ and CS- groups with probe placement in the BLA showed no differences, but higher baseline levels were seen in the BLA CS+ group compared to levels if probe placement was outside the BLA (central amygdala or lateral ventricle). Since placement within the BLA may have influenced the ability to detect differences in ACH efflux between extinction resistant (ER) and extinction competent (EC) groups, we have indicated probes from ER (white bars) or EC (black bars) rats from the CS+ rats exposed to tones during extinction learning having probe placement in the BLA (N = 7). Other probes are from animals in the CS- or Shift Alone groups (grey bars). ** = P < 0.05 for CS+ versus not-BLA groups. Data are represented as mean ± SEM.
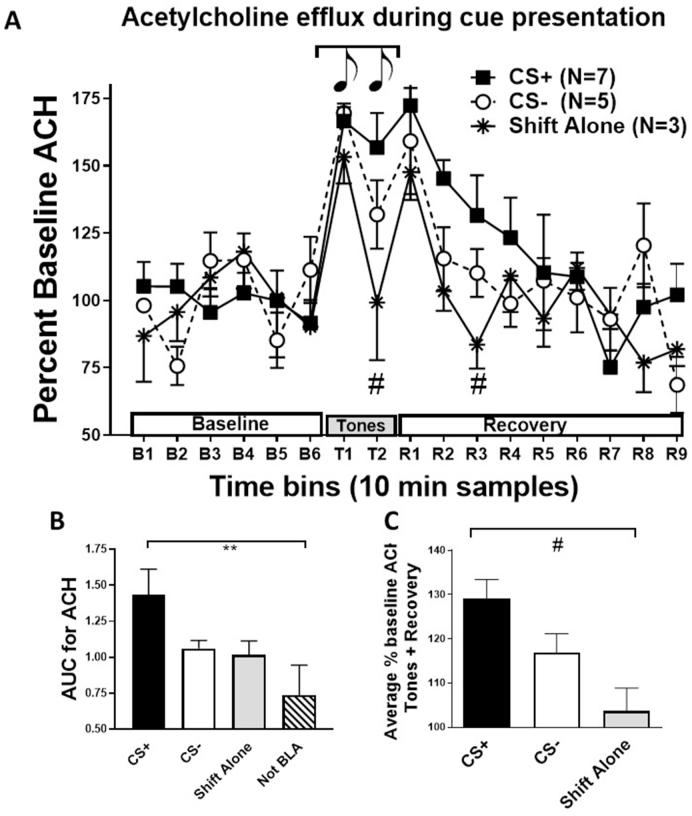
As seen in Fig. 3, presentation of 20 conditioned cues after shifting to a new context in the CS+ group increased ACH efflux in the BLA, followed by a slow recovery back to baseline. This increase during the first 10 min collection (T1, context shift plus first 10 tones) and the shift back to Context A (R1) was similar between all groups (CS+, CS-, Shift Alone), resulting in a significant effect of time [F (16,192) = 10.3, P < 0.0001]. This suggests that the context shift alone increases ACH efflux and is further supported by the ACH efflux pattern in the Shift Alone group showing a peak at each context shift. In contrast, the percent increase in ACH efflux remained elevated during the second set of ten conditioned tones and the recovery period in the CS+ group, especially compared to the Shift Alone group (Fig. 3A, T2; # indicates P < 0.05 between CS+ and Shift Alone groups). This resulted in a significant difference between the three groups with placement in the BLA [F (2,12) = 4.06, P = 0.045], but not a significant interaction [F (33,192) = 1.4, P = 0.075]. This enhanced ACH efflux in the CS+ group is supported by a significant average percent increase in ACH efflux during the tones and recovery period in animals with placement in the BLA (Fig. 3C; [F (2,12) = 6.4, P = 0.013]. Further, AUC analysis showed that the CS+ group had a greater total response than the other groups (Fig. 3B), including animals exposed to the CS+ with placement outside the BLA, demonstrating the regional specificity of the effect [F (3,15) = 3.4, P < 0.05]. Overall these results suggest there is a significant efflux of ACH in the BLA just from a context shift alone, although the presentation of a conditioned cue (CS+) prolongs this response leading to enhanced overall ACH efflux in BLA compared to groups during the Shift Alone or rats with placement outside of the BLA.
Fig. 3.
Acetylcholine (ACH) efflux in basolateral amygdala (BLA) during cue-conditioned recall and extinction learning (Experiment 1). Panel A shows ACH as percent baseline release in all three groups with placement in the BLA (CS+, N = 7; CS-, N = 5; Shift Alone, N = 3) over the six baseline collections (B1-6), two collections during 20 tone presentations (T1, T2) and 90 min recovery (R1-9). Acetylcholine efflux increased relative to baseline following the context shift (T1) in all groups, but remained elevated in the CS+ group during the second tone collection (T2) and during the recovery period compared with the Shift Alone (no tone presentation) group. The elevation of ACH release in the CS+ group is highlighted by significant group differences for the average percent baseline when collapsing across the tones and recovery bins (Panel CC; F (2,12) = 6.4, P < 0.02) and higher area under the curve (AUC; Panel BB; F (3,15) = 3.4, P < 0.05). The CS+ group had a higher average percent baseline ACH release than the Shift Alone group (Panel CC) and the animals with placement outside the BLA (Panel B). The low AUC for the animals with placement outside the BLA suggest this effect is restricted to the BLA. # = P < 0.05 between CS+ and Shift Alone groups. ** = P < 0.05 for CS+ versus not-BLA groups. Data are represented as mean ± SEM.
3.3. Glutamate efflux during presentation of conditioned cue and extinction learning
The same dialysate samples were also analyzed for GLU efflux during cue-conditioned freezing and extinction learning (one sample was identified as an outlier based on baseline levels). Overall there was no change in BLA GLU efflux during the tone presentation or recovery period [F (16,166) = 1.2, P = 0.24] (data not shown). Further neither the percent change in GLU [F (2,10) = 0.63, P = 0.55] nor the AUC for GLU [F (2,10) = 1.3, P = 0.32] differed between the CS+, CS- and Shift Alone groups. The percent change from baseline for GLU in the groups were 109 ± 15, 177 ± 66, and 157 ± 78 for the BLA CS+ (N = 6), BLA CS- (N = 4), and BLA Shift Alone (N = 3) groups, respectively.
3.4. Extinction competent (EC) and extinction resistant (ER) rats show different levels of cholinesterase (CHE) activity in BLA and plasma
As seen previously (Sharko et al., 2017), a median split of the animals based on extinction learning (last 10 tones) produced two groups of rats with different freezing profiles in Experiment 2 (Fig. 4B). Extinction Competent rats showed lower levels of freezing during exposure to the conditioned cue (first five tone presentations), as well as the last 10 tones of extinction learning and extinction recall (first 5 tones) when compared to Extinction Resistant rats [F (1,27) = 35, P < 0.0001 for EC vs. ER difference across all trials]. Since extinction trials lowered freezing, there was also a main effect of trial [F (3,81)] = 18.4, P < 0.0001]. Since as seen previously there was no significant difference between ER and EC groups during acquisition or context (not shown), this also resulted in a significant interaction [F (3.81) = 5.4, P < 0.002].
As seen in Fig. 4C and D, EC rats had higher levels of CHE activity in both the BLA [t (24) = 2.1, P < 0.05] and plasma [t (27) = 2.9, P = 0.007] than ER rats. There was also a significant correlation between CHE activity in the plasma and BLA [r = 0.5, R2 = 0.25, P < 0.01; Fig. 4E], suggesting plasma CHE might be a proxy for brain (at least BLA) CHE activity. Furthermore, there was a significant negative correlation between BLA CHE activity (Fig. 4F, r = −0.44, R2 = 0.19, P < 0.03) and freezing during the last 10 tones of extinction learning. A similar trend was seen for plasma (data not shown; [r = −0.36, R2 = 0.13, P = 0.056]). No significant correlations were seen between BLA CHE activity and freezing during acquisition (r = −0.19, R2 = 0.04, P = 0.36), cue-induced fear (first 5 tones of extinction learning, r = −0.26, R2 = 0.07, P = 0.2), or extinction recall (first 5 tones; r = −0.26, R2 = 0.07, P = 0.2). Interestingly, a comparable negative correlation was seen between the plasma CHE activity in the blood sample taken after extinction recall in Experiment 1, and freezing during the last 5 tones of extinction learning during microdialysis in the CS+ group [N = 7, r = −0.78, R2 = 0.61, P < 0.04].
4. Discussion
Overall, these findings suggest that BLA ACH levels are increased during presentation of a conditioned cue during extinction learning, although ACH is also more transiently increased by exposure to a new context. This finding contributes to evidence that ACH plays an important role in attentional or arousal processing during learning tasks, and is one of the first demonstrations that ACH efflux in the BLA is indeed increased during an extinction learning trial, which is critical for activation of muscarinic or nicotinic receptors in this region as suggested by the literature. Although a few studies have examined ACH release during associative learning responses, none of these studies have focused on the BLA during Pavlovian fear extinction (Acquas et al., 1996; Nail-Boucherie et al., 2000; Izaki et al., 2001; Calandreau et al., 2006). In addition, we also found that cholinesterase activity in both the BLA and plasma was higher in animals showing effective extinction learning (EC rats) versus rats resistant to extinction learning (ER rats), suggesting a significant relationship between CHE activity in the BLA and extinction learning. Taken together, our results support a role for ACH neurotransmission in the BLA during cued fear extinction processes, and suggest a potential cholinergic basis in this region for individual differences in extinction learning.
Using in vivo microdialysis, which samples the extracellular space, we demonstrated that ACH was released during the extinction learning trial and that the group exposed to the conditioned cue showed a more prolonged elevation in ACH in the BLA compared to the Shift Alone group given a context shift without any cue presentation (e.g., no tones). Despite extensive handling and habituation to the microdialysis procedure including the context shift, all animals showed an initial increase in ACH release in the BLA during the first 10 min (tones) of the extinction learning trial, although this rapidly returned to baseline in the Shift Alone group. In contrast, ACH levels remained elevated throughout the rest of the tone presentations and recovery period in the CS+ group when exposed to a conditioned cue. Further, comparison of AUC based on pmol ACH release showed that the CS+ group had the greatest ACH efflux in the BLA compared to other groups (CS-, Shift Alone), which was significantly greater than CS+ animals with probes outside the BLA suggesting the regional specificity of the increase in ACH. These results are similar to other studies demonstrating that the handling associated with moving rats into a testing chamber for microdialysis produces a rapid and robust increase in ACH in both hippocampus and PFC, including animals that had been extensively habituated to conditioned auditory and visual cues (Acquas et al., 1996). The same study showed unconditioned sensory stimuli also increased ACH efflux in these two brain regions, similar to our results in the CS- group. Similarly, hippocampal ACH efflux increased following placement of the animals into the conditioning chamber during acquisition and upon return to the chamber in the unconditioned group, although there was a greater ACH efflux in conditioned animals returned to the context (Nail-Boucherie et al., 2000). The lack of striking differences in ACH efflux in the BLA between CS+ and CS- groups may have been related not only to the context shift enhancing ACH efflux on its own, but also the role of cholinergic processes in locomotion and immobility (see (Konig et al., 2018)). In both the CS- and Shift Alone groups, animals became immobile over the course of microdialysis testing and visual inspection demonstrated that this was due to a transition to resting or sleeping similar to that observed during habituation. This is perhaps not surprising since this was a familiar environment without the presentation of any conditioned stimuli. Further, microdialysis and testing was performed during the light (inactive) phase of the circadian cycle, which also influences cholinergic tone and fear responses (see (Albrecht and Stork 2017)). Increases in ACH release in the cortex and hippocampus during the dark (active) phase and in response to a novel environment have been associated with motor activity (see (Konig et al., 2018)) and a putative relationship between locomotor behavior and ACH release was also seen in the BLA (Sturgill et al., 2020). In the present study, exploratory post-hoc analysis revealed a negative correlation (r = −0.8, R2 = 0.56, P < 0.002) between immobility (freezing or resting) and ACH increases during the second 10 min bin (T2) of fear extinction when all groups were collapsed (N = 15). Further, since freezing and active behaviors such as locomotion are mutually exclusive, differences in freezing during extinction between ER and EC groups could be linked to differences in locomotor behaviors in these phenotypes. In a separate (unpublished) study, however, our analysis of activity in a runway task during the control condition (neutral object) suggested the distance traveled did not differ between ER and EC groups; we have not directly examined these phenotypes for locomotion in an open field test so this remains a possibility. Nevertheless, increases in ACH efflux, which are prolonged in the CS+ group, might still be important in fear conditioning or extinction learning, since it has been postulated that cholinergic activation of BLA muscarinic receptors that regulate neural plasticity may play a critical role in the influences of stress and arousal levels on fear extinction (Knox 2016).
Like our previous study, in a separate cohort of Long Evans rats we demonstrate individual differences in freezing that emerged during the extinction learning trial, and divided animals into extinction resistant and extinction competent groups based on average freezing during the last 10 tone presentations (as in (Sharko et al., 2017)). Such individual variations in fear extinction learning or recall have been seen in various outbred and inbred strains of rats or mice (Herry and Mons 2004; Burgos-Robles et al., 2007; Bush et al., 2007; Burgos-Robles et al., 2009; Galatzer-Levy et al., 2013; Holmes and Singewald 2013; Shumake et al., 2014; Gruene et al., 2015; Monfils et al., 2019), although these studies used distinct methods to distinguish different extinction phenotypes. Our behavioral differences between ER and EC groups appear quite similar to previous studies in Long Evans or Sprague Dawley rats, since differences were seen during extinction learning and recall, but generally not in acquisition of learned fear or contextually-conditioned freezing (Bush et al., 2007; Galatzer-Levy et al., 2013; Shumake et al., 2014; Sharko et al., 2017; Monfils et al., 2019). The ER-EC difference in freezing during cue-induced fear (the first few tones before extinction learning) could suggest there are pre-existing factors and/or disparities in the consolidation of the original fear memory that impact subsequent extinction learning. This possibility is supported by evidence that activation of histaminergic H3 receptors, which increases ACH release in the BLA, improves expression of fear memories (Cangioli et al., 2002). An intriguing possibility that awaits further study is that these differences during fear acquisition contribute to the emergence of phenotypic variations in ACHE activity that we observed after extinction recall.
In this study we also demonstrate that after behavioral testing, BLA CHE activity was negatively correlated with freezing behavior during extinction learning, with lower levels of CHE in ER compared to EC rats. Since ACHE terminates cholinergic neurotransmission by hydrolyzing acetylcholine, this suggests that reduced CHE activity in the BLA during the extinction learning trial might drive sustained cholinergic signaling in ER animals compared with EC subjects. Cholinesterase activity was sampled after the extinction recall trial, so it is unclear if the differences in CHE activity between ER and EC rats were preexisting, perhaps genetic differences, or if the differences emerged as a result of fear learning and/or extinction learning. A few studies have examined changes in ACHE enzyme activity after various stressors, but these studies suggest the changes are dependent on the brain region and the type of stress (Kaufer et al., 1998; Birikh et al., 2003; Das et al., 2005; Valuskova et al., 2017; Ketenci et al., 2020). Interestingly, several studies demonstrate stress-induced increases in the monomeric read-through variant of ACHE (ACHE-R) in various brain areas, including the amygdala (Kaufer et al., 1998; Nijholt et al., 2004; Meshorer et al., 2005; Perrier et al., 2005; Salmon et al., 2005; Dori et al., 2011; Valuskova et al., 2017), although the influence of the ACHE-R variant on ACHE activity is unclear since even after stress the variant is only ~1.5% of total ACHE expression (Perrier et al., 2005). Nevertheless, human serum ACHE levels have been demonstrated to correlate with trait and state anxiety levels, and high expression of serum ACHE-R was associated with low trait-anxiety scores in human (Sklan et al., 2004). Prior studies have also demonstrated individual differences in other markers of cholinergic signaling or correlations between indices of cholinergic function and behavioral endpoints in aversive learning tasks (van der Zee et al., 1997; McIntyre et al., 2002; Gold 2003), although none of these studies examined ACHE activity directly. For example, the expression of choline acetyltransferase (ChAT) mRNA in BFCS is enhanced by cue conditioning (Oh et al., 1992) and muscarinic receptor immunoreactivity in the central amygdala was positively correlated with immobility in a conditioned task (van der Zee et al., 1997; van der Zee and Luiten 1999). In an operant task, ACH release in the PFC was negatively correlated with lever presses during extinction, further suggesting that ACH efflux can be dissociated from activity (Izaki et al., 2001). This latter finding suggesting heightened ACH release was associated with enhanced extinction learning differs from our data in the BLA showing elevated CHE activity, and potentially reduced cholinergic signaling, in EC animals. Taken together, these findings may indicate there are region-specific influences of cholinergic processes during extinction learning. Alternatively, since we assessed CHE activity after extinction recall, there might be differences between extinction learning and extinction recall time points in CHE activity. This will require further analysis at different time points, as well as comparison with additional brain regions such as PFC. Although there was an association between CHE activity and fear extinction behaviors, further analysis will be needed to demonstrate whether or not phenotypic CHE differences contribute to altered ACH efflux. A recent paper showed injections of an organophosphate ACHE inhibitor resulted in impaired contextual fear extinction that were accompanied by relatively small decreases in hippocampal CHE activity (Rodrigues et al., 2020). These results led the authors to suggest that low ACHE activity and enhanced cholinergic stimulation was associated with contextual extinction resistance, however, ACHE changes with different doses examined at different time points suggested that behavioral changes do not necessary always accompany ACHE changes in all brain areas. As decreases in ACHE activity induced by ACHE inhibition after fear conditioning were capable of altering subsequent contextual fear extinction (Rodrigues et al., 2020), knowing the timing of the alterations in BLA CHE activity will be critical in understanding how these differences contribute to cued fear extinction. Additional reports demonstrate decreased ACh efflux alongside increased ACHE in the thalamus of tau-transgenic JNPL3 mice compared to wild-types, but not for the hippocampus which showed decreased ACh efflux in tau-transgenic JNPL3 mice compared to wild types independent of a significant change in ACHE (Stein et al., 2019). Collectively, these studies suggest that phenotypic differences in CHE may indeed influence ACH release in BLA and fear extinction, but that such a relationship is likely to be time and even brain region specific. Further, our studies used only male subjects, but other groups have indicated distinct extinction phenotypes are also seen in females (Shumake et al., 2014; Gruene et al., 2015). Future analysis will be needed to determine if the differences in CHE seen in males are also seen in females. Our finding of individual differences in CHE activity in a rodent model also provides insight into a potential neurobiological basis for dysregulation of fear extinction in patients with PTSD (Guthrie and Bryant 2006; Shin and Liberzon 2010; Milad and Quirk 2012; Pitman et al., 2012; Holmes and Singewald 2013; Lommen et al., 2013; Kutlu and Gould 2015). Although additional studies are needed, our findings of an association between BLA ACHE activity and freezing during extinction learning suggest that differential changes in ACHE expression and/or alternative splicing could modulate cholinergic signaling in the BLA and contributes to an individual's extinction phenotype.
Our findings contribute to an emerging literature that the dense BFCS inputs to BLA (Muller et al., 2011; Lee and Kim 2019), shown to modulate electrophysiological properties of BLA pyramidal neurons and interneurons (Unal et al., 2015; Jiang et al., 2016; Aitta-Aho et al., 2018; Lee and Kim 2019), are critical to associative processes involving a discrete conditioned stimulus. Emerging evidence also indicates that activation of the BFCS increases oscillations in the BLA (Aitta-Aho et al., 2018), suggesting that ACH efflux in BLA could play a role in the oscillatory coupling between the amygdala, PFC and hippocampus during extinction learning and recall (Lesting et al., 2011; Lesting et al., 2013; Gorka et al., 2015). This might even be coded by distinct neuron types, firing patterns, or both in the BFCS (Laszlovszky et al., 2020). Our demonstration of increased ACH efflux during the extinction learning trial support findings that optogenetically stimulating BFCS inputs to the BLA during acquisition disrupts the extinction, but not the acquisition, of cued fear memories (Jiang et al., 2016). Further, since ER showed lower ACHE activity in the BLA than EC rats, this also suggests that prolonged or enhanced ACH efflux in the BLA might influence cued extinction learning processes. It has been suggested that cholinergic inputs enhance the “signal to noise ratio” in the BLA (Unal et al., 2015), so prolonged ACH signaling might accentuate the “signal” in extinction resistant animals, and attenuate extinction learning. This would be congruent with the overall notion that acetylcholine plays a critical role in cue detection and attentional performance (Hasselmo and Sarter 2011) and the recent suggestion that BLA pyramidal neuron activity is critical for determining the salience of a conditioned stimulus during an aversive learning task (Sengupta et al., 2018). Studies using acetylcholine biosensors in the BLA also demonstrated that ACH release increased during learning of a cue-reward contingency, further supporting a role for BLA ACH in cue salience and the formation of associations between cues and either rewarding or aversive outcomes (Crouse et al., 2020). Further, studies showing activation of histaminergic H3 receptors which increase ACH release in the BLA enhances the expression of fear memories (Cangioli et al., 2002), which could also make extinction learning more difficult. Mineur and Picciotto, 2019 suggest that heightened levels of ACH from either genetic or environmental factors can lead to attentional bias toward negative stimuli, and thus maladaptive behavior by biasing the encoding of negative memories (Mineur and Picciotto, 2019). Our data suggesting that cholinergic transmission might be enhanced in the ER phenotype due to reduced ACHE activity in the BLA would support this notion. Unfortunately, due to both the low number of subjects and the need to have the animals tethered during extinction learning, it was not reasonable to assign individual subjects into clear extinction phenotypes based on the last 10 min of extinction learning in Experiment 1 (microdialysis) as was done in Experiment 2. When the N = 11 CS+ rats (including rats with placement outside the BLA) were divided into ER and EC phenotypes based on freezing during extinction learning with microdialysis, only two of seven rats in the CS+ group with probe placement in the BLA showed the ER phenotype (N = 5 EC); two subjects in one of these groups was not sufficiently powered to conduct reliable statistical analysis on any endpoints. This ER/EC comparison was also confounded by using an ACHE inhibitor in the perfusate to allow reliable detection of ACH release during microdialysis, which may have also modified freezing during the extinction learning trial and therefore the extinction phenotype of the animal (Fadel, 2011; Konig et al., 2018).
Given that many forms of stress increase glutamate release in the amygdala (see (Reznikov et al., 2007; Wilson et al., 2015)), and the anatomical evidence suggesting that acetylcholine and glutamate might be co-released (Nickerson Poulin et al., 2006), it was somewhat surprising that we found no differences in glutamate efflux even in the CS + group. Prior studies have shown rapid, transient glutamate release in amygdala during various auditory and contextual fear trials using a rapid-sampling procedure (Venton et al., 2006), so it is possible that we were unable to see brief transients averaged over our much longer 10 min sampling periods during microdialysis. Alternatively, it is possible that the habituation to the microdialysis procedure blunted glutamate responses due to the context shift alone, as we saw previously in the central amygdala (Carrero et al., 2019), suggesting CS+ presentation was not sufficient to induce additional glutamate efflux. Further, the prolonged ACH release, or even modified choline levels, in the BLA during microdialysis, may have attenuated glutamate release either directly or via activation of inhibitory GABAergic neurons (Yajeya et al., 2000; Pidoplichko et al., 2013; Unal et al., 2015; Jiang et al., 2016; Aitta-Aho et al., 2018; Lee and Kim 2019).
5. Conclusion
We have demonstrated acetylcholine efflux is induced in the basolateral amygdala during cued extinction learning. Further, we demonstrate individual differences in cholinesterase activity in this region that could modify the intensity or duration of cholinergic signaling during fear extinction. These studies support the emerging evidence for an important role of cholinergic processes, particularly in the BLA, in fear extinction that could contribute to phenotypic differences in extinction learning. Further, the results suggest that the cholinergic system might be a viable target for identifying biomarkers or individualized therapeutic approaches for some of the symptoms associated with PTSD.
Funding
This work was supported by the Veterans Administration [VA Merit Awards BX001374 (Wilson PI), BX001804 (Reagan PI) and BX002664 (Reagan PI)], the National Institutes of Health [NIH R01AG050518 to JRF; GM076277 for PREP support of EW; R01 MH113892 (Wood PI)], and the University of South Carolina VP for Research [ASPIRE II award to MAW].
CRediT authorship contribution statement
Devin M. Kellis: Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Kris Ford Kaigler: Investigation, Formal analysis. Eric Witherspoon: Investigation. Jim R. Fadel: Conceptualization, Funding acquisition, Resources, Supervision, Methodology, Writing - review & editing. Marlene A. Wilson: Conceptualization, Project administration, Funding acquisition, Methodology, Supervision, Visualization, Formal analysis, Writing - review & editing, Writing - review & editing.
Declaration of competing interest
Authors (DMK, KFK, EW, JRF, MAW) have no conflicts of interest and no competing financial interests in relation to the work described.
Acknowledgements
The authors thank Dr. Sarah C. Tryon, Ph.D. for insightful comments on the manuscript.
References
- Acquas E., Wilson C., Fibiger H.C. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J. Neurosci. 1996;16(9):3089–3096. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitta-Aho T., Hay Y.A., Phillips B.U., Saksida L.M., Bussey T.J., Paulsen O., Apergis-Schoute J. Basal forebrain and brainstem cholinergic neurons differentially impact amygdala circuits and learning-related behavior. Curr. Biol. 2018;28(16):2557–2569. doi: 10.1016/j.cub.2018.06.064. e2554. [DOI] [PubMed] [Google Scholar]
- Albrecht A., Stork O. Circadian rhythms in fear conditioning: an overview of behavioral, brain system, and molecular interactions. Neural Plast. 2017:3750307. doi: 10.1155/2017/3750307. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E., Bucherelli C. Brain sites involved in fear memory reconsolidation and extinction of rodents. Neurosci. Biobehav. Rev. 2015;53:160–190. doi: 10.1016/j.neubiorev.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Barreto G.E., Yarkov A., Avila-Rodriguez M., Aliev G., Echeverria V. Nicotine-Derived compounds as therapeutic tools against post-traumatic stress disorder. Curr. Pharmaceut. Des. 2015;21(25):3589–3595. doi: 10.2174/1381612821666150710145250. [DOI] [PubMed] [Google Scholar]
- Birikh K.R., Sklan E.H., Shoham S., Soreq H. Interaction of "readthrough" acetylcholinesterase with RACK1 and PKCbeta II correlates with intensified fear-induced conflict behavior. Proc. Natl. Acad. Sci. U. S. A. 2003;100(1):283–288. doi: 10.1073/pnas.0135647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A., Vidal-Gonzalez I., Quirk G.J. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J. Neurosci. 2009;29(26):8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A., Vidal-Gonzalez I., Santini E., Quirk G.J. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53(6):871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Bush D.E., Sotres-Bayon F., LeDoux J.E. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20(4):413–422. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- Calandreau L., Trifilieff P., Mons N., Costes L., Marien M., Marighetto A., Micheau J., Jaffard R., Desmedt A. Extracellular hippocampal acetylcholine level controls amygdala function and promotes adaptive conditioned emotional response. J. Neurosci. 2006;26(52):13556–13566. doi: 10.1523/JNEUROSCI.3713-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calva C.B., Fayyaz H., Fadel J.R. Increased acetylcholine and glutamate efflux in the prefrontal cortex following intranasal orexin-A (hypocretin-1) J. Neurochem. 2018;145(3):232–244. doi: 10.1111/jnc.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangioli I., Baldi E., Mannaioni P.F., Bucherelli C., Blandina P., Passani M.B. Activation of histaminergic H3 receptors in the rat basolateral amygdala improves expression of fear memory and enhances acetylcholine release. Eur. J. Neurosci. 2002;16(3):521–528. doi: 10.1046/j.1460-9568.2002.02092.x. [DOI] [PubMed] [Google Scholar]
- Carrero J.P., Kaigler K.F., Hartshorn G.H., Fadel J.R., Wilson M.A. Mu opioid receptor regulation of glutamate efflux in the central amygdala in response to predator odor. Neurobiol Stress. 2019;11:100197. doi: 10.1016/j.ynstr.2019.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse R.B., Kim K., Batchelor H.M., Girardi E.M., Kamaletdinova R., Chan J., Rajebhosale P., Pittenger S.T., Role L.W., Talmage D.A., Jing M., Li Y., Gao X.B., Mineur Y.S., Picciotto M.R. Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances the learning of cue-reward contingency. Elife. 2020;9 doi: 10.7554/eLife.57335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Rai D., Dikshit M., Palit G., Nath C. Nature of stress: differential effects on brain acetylcholinesterase activity and memory in rats. Life Sci. 2005;77(18):2299–2311. doi: 10.1016/j.lfs.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Deslauriers J., Toth M., Der-Avakian A., Risbrough V.B. Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol. Psychiatr. 2018;83(10):895–907. doi: 10.1016/j.biopsych.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dori A., Oriel S., Livneh U., Duek O., Lin T., Kofman O. Acetylcholinesterase inhibitor pretreatment alters stress-induced expression of acetylcholinesterase transcripts in the mouse brain. Neuroscience. 2011;183:90–98. doi: 10.1016/j.neuroscience.2011.03.044. [DOI] [PubMed] [Google Scholar]
- Elias G.A., Gulick D., Wilkinson D.S., Gould T.J. Nicotine and extinction of fear conditioning. Neuroscience. 2010;165(4):1063–1073. doi: 10.1016/j.neuroscience.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J.R. Regulation of cortical acetylcholine release: insights from in vivo microdialysis studies. Behav. Brain Res. 2011;221(2):527–536. doi: 10.1016/j.bbr.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M., Fanselow M.S. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 1999;23(5):743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Finnell J.E., Muniz B.L., Padi A.R., Lombard C.M., Moffitt C.M., Wood C.S., Wilson L.B., Reagan L.P., Wilson M.A., Wood S.K. Essential role of ovarian hormones in susceptibility to the consequences of witnessing social defeat in female rats. Biol. Psychiatr. 2018;84(5):372–382. doi: 10.1016/j.biopsych.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy I.R., Bonanno G.A., Bush D.E., Ledoux J.E. Heterogeneity in threat extinction learning: substantive and methodological considerations for identifying individual difference in response to stress. Front. Behav. Neurosci. 2013;7:55. doi: 10.3389/fnbeh.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P.E. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol. Learn. Mem. 2003;80(3):194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Gorka A.X., Knodt A.R., Hariri A.R. Basal forebrain moderates the magnitude of task-dependent amygdala functional connectivity. Soc. Cognit. Affect Neurosci. 2015;10(4):501–507. doi: 10.1093/scan/nsu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould T.J., Leach P.T. Cellular, molecular, and genetic substrates underlying the impact of nicotine on learning. Neurobiol. Learn. Mem. 2014;107:108–132. doi: 10.1016/j.nlm.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruene T.M., Roberts E., Thomas V., Ronzio A., Shansky R.M. Sex-specific neuroanatomical correlates of fear expression in prefrontal-amygdala circuits. Biol. Psychiatr. 2015;78(3):186–193. doi: 10.1016/j.biopsych.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie R.M., Bryant R.A. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom. Med. 2006;68(2):307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- Hasselmo M.E., Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedreen J.C., Bacon S.J., Price D.L. A modified histochemical technique to visualize acetylcholinesterase-containing axons. J. Histochem. Cytochem. 1985;33(2):134–140. doi: 10.1177/33.2.2578498. [DOI] [PubMed] [Google Scholar]
- Herry C., Ciocchi S., Senn V., Demmou L., Muller C., Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C., Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur. J. Neurosci. 2004;20(3):781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Holmes A., Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36(1):23–31. doi: 10.1016/j.tins.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki Y., Hori K., Nomura M. Elevation of prefrontal acetylcholine is related to the extinction of learned behavior in rats. Neurosci. Lett. 2001;306(1–2):33–36. doi: 10.1016/s0304-3940(01)01863-8. [DOI] [PubMed] [Google Scholar]
- Jiang L., Kundu S., Lederman J.D., Lopez-Hernandez G.Y., Ballinger E.C., Wang S., Talmage D.A., Role L.W. Cholinergic signaling controls conditioned fear behaviors and enhances plasticity of cortical-amygdala circuits. Neuron. 2016;90(5):1057–1070. doi: 10.1016/j.neuron.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Role L.W. Facilitation of cortico-amygdala synapses by nicotine: activity-dependent modulation of glutamatergic transmission. J. Neurophysiol. 2008;99(4):1988–1999. doi: 10.1152/jn.00933.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer D., Friedman A., Seidman S., Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393(6683):373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- Kelly M.P., Isiegas C., Cheung Y.F., Tokarczyk J., Yang X., Esposito M.F., Rapoport D.A., Fabian S.A., Siegel S.J., Wand G., Houslay M.D., Kanes S.J., Abel T. Constitutive activation of Galphas within forebrain neurons causes deficits in sensorimotor gating because of PKA-dependent decreases in cAMP. Neuropsychopharmacology. 2007;32(3):577–588. doi: 10.1038/sj.npp.1301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketenci S., Acet N.G., Saridogan G.E., Aydin B., Cabadak H., Goren M.Z. The neurochemical effects of prazosin treatment on fear circuitry in a rat traumatic stress model. Clin Psychopharmacol Neurosci. 2020;18(2):219–230. doi: 10.9758/cpn.2020.18.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. The role of basal forebrain cholinergic neurons in fear and extinction memory. Neurobiol. Learn. Mem. 2016;133:39–52. doi: 10.1016/j.nlm.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D., Keller S.M. Cholinergic neuronal lesions in the medial septum and vertical limb of the diagonal bands of Broca induce contextual fear memory generalization and impair acquisition of fear extinction. Hippocampus. 2016;26(6):718–726. doi: 10.1002/hipo.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M., Thinnes A., Klein J. Microdialysis and its use in behavioural studies: focus on acetylcholine. J. Neurosci. Methods. 2018;300:206–215. doi: 10.1016/j.jneumeth.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Kutlu M.G., Gould T.J. Nicotine modulation of fear memories and anxiety: implications for learning and anxiety disorders. Biochem. Pharmacol. 2015;97(4):498–511. doi: 10.1016/j.bcp.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlovszky T., Schlingloff D., Hegedus P., Freund T.F., Gulyas A., Kepecs A., Hangya B. Distinct synchronization, cortical coupling and behavioral function of two basal forebrain cholinergic neuron types. Nat. Neurosci. 2020;23(8):992–1003. doi: 10.1038/s41593-020-0648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kim J.H. Basal forebrain cholinergic-induced activation of cholecystokinin inhibitory neurons in the basolateral amygdala. Exp Neurobiol. 2019;28(3):320–328. doi: 10.5607/en.2019.28.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J., Daldrup T., Narayanan V., Himpe C., Seidenbecher T., Pape H.C. Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear extinction. PloS One. 2013;8(10) doi: 10.1371/journal.pone.0077707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesting J., Narayanan R.T., Kluge C., Sangha S., Seidenbecher T., Pape H.C. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PloS One. 2011;6(6) doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E., Popa D., Apergis-Schoute J., Fidacaro G.A., Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommen M.J., Engelhard I.M., Sijbrandij M., van den Hout M.A., Hermans D. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behav. Res. Ther. 2013;51(2):63–67. doi: 10.1016/j.brat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Macht V.A., Woodruff J.L., Grillo C.A., Wood C.S., Wilson M.A., Reagan L.P. Pathophysiology in a model of gulf war illness: contributions of pyridostigmine bromide and stress. Psychoneuroendocrinology. 2018;96:195–202. doi: 10.1016/j.psyneuen.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Macht V.A., Woodruff J.L., Maissy E.S., Grillo C.A., Wilson M.A., Fadel J.R., Reagan L.P. Pyridostigmine bromide and stress interact to impact immune function, cholinergic neurochemistry and behavior in a rat model of Gulf War Illness. Brain Behav. Immun. 2019;80:384–393. doi: 10.1016/j.bbi.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruki K., Izaki Y., Akema T., Nomura M. Effects of acetylcholine antagonist injection into the prefrontal cortex on the progress of lever-press extinction in rats. Neurosci. Lett. 2003;351(2):95–98. doi: 10.1016/j.neulet.2003.07.012. [DOI] [PubMed] [Google Scholar]
- McDonald A.J., Jones G.C., Mott D.D. Diverse glutamatergic inputs target spines expressing M1 muscarinic receptors in the basolateral amygdala: an ultrastructural analysis. Brain Res. 2019;1722:146349. doi: 10.1016/j.brainres.2019.146349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C.K., Pal S.N., Marriott L.K., Gold P.E. Competition between memory systems: acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. J. Neurosci. 2002;22(3):1171–1176. doi: 10.1523/JNEUROSCI.22-03-01171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshorer E., Bryk B., Toiber D., Cohen J., Podoly E., Dori A., Soreq H. SC35 promotes sustainable stress-induced alternative splicing of neuronal acetylcholinesterase mRNA. Mol. Psychiatr. 2005;10(11):985–997. doi: 10.1038/sj.mp.4001735. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Quirk G.J. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur Y.S., Picciotto M.R. The role of acetylcholine in negative encoding bias: too much of a good thing? Eur. J. Neurosci. 2019:1–12. doi: 10.1111/ejn.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils M.H., Lee H.J., Keller N.E., Roquet R.F., Quevedo S., Agee L., Cofresi R., Shumake J. Predicting extinction phenotype to optimize fear reduction. Psychopharmacology (Berlin) 2019;236(1):99–110. doi: 10.1007/s00213-018-5005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J.F., Mascagni F., McDonald A.J. Cholinergic innervation of pyramidal cells and parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. J. Comp. Neurol. 2011;519(4):790–805. doi: 10.1002/cne.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nail-Boucherie K., Dourmap N., Jaffard R., Costentin J. Contextual fear conditioning is associated with an increase of acetylcholine release in the hippocampus of rat. Brain Res Cogn Brain Res. 2000;9(2):193–197. doi: 10.1016/s0926-6410(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Nickerson Poulin A., Guerci A., El Mestikawy S., Semba K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J. Comp. Neurol. 2006;498(5):690–711. doi: 10.1002/cne.21081. [DOI] [PubMed] [Google Scholar]
- Nijholt I., Farchi N., Kye M., Sklan E.H., Shoham S., Verbeure B., Owen D., Hochner B., Spiess J., Soreq H., Blank T. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol. Psychiatr. 2004;9(2):174–183. doi: 10.1038/sj.mp.4001446. [DOI] [PubMed] [Google Scholar]
- Oh J.D., Woolf N.J., Roghani A., Edwards R.H., Butcher L.L. Cholinergic neurons in the rat central nervous system demonstrated by in situ hybridization of choline acetyltransferase mRNA. Neuroscience. 1992;47(4):807–822. doi: 10.1016/0306-4522(92)90031-v. [DOI] [PubMed] [Google Scholar]
- Orsini C.A., Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. Rev. 2012;36(7):1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Academic Press; San Diego: 1997. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Perrier N.A., Salani M., Falasca C., Bon S., Augusti-Tocco G., Massoulie J. The readthrough variant of acetylcholinesterase remains very minor after heat shock, organophosphate inhibition and stress, in cell culture and in vivo. J. Neurochem. 2005;94(3):629–638. doi: 10.1111/j.1471-4159.2005.03140.x. [DOI] [PubMed] [Google Scholar]
- Pidoplichko V.I., Prager E.M., Aroniadou-Anderjaska V., Braga M.F. alpha7-Containing nicotinic acetylcholine receptors on interneurons of the basolateral amygdala and their role in the regulation of the network excitability. J. Neurophysiol. 2013;110(10):2358–2369. doi: 10.1152/jn.01030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power A.E., Vazdarjanova A., McGaugh J.L. Muscarinic cholinergic influences in memory consolidation. Neurobiol. Learn. Mem. 2003;80(3):178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Reznikov L.R., Grillo C.A., Piroli G.G., Pasumarthi R.K., Reagan L.P., Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur. J. Neurosci. 2007;25(10):3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- Reznikov L.R., Reagan L.P., Fadel J.R. Effects of acute and repeated restraint stress on GABA efflux in the rat basolateral and central amygdala. Brain Res. 2009;1256:61–68. doi: 10.1016/j.brainres.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Robinson L., Platt B., Riedel G. Involvement of the cholinergic system in conditioning and perceptual memory. Behav. Brain Res. 2011;221(2):443–465. doi: 10.1016/j.bbr.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Rodrigues J.V.F., Vidigal A.P.P., Minassa V.S., Batista T.J., de Lima R.M.S., Funck V.R., Antero L.S., Resstel L.B.M., Coitinho J.B., Bertoglio L.J., Sampaio K.N., Beijamini V. A single dose of the organophosphate triazophos induces fear extinction deficits accompanied by hippocampal acetylcholinesterase inhibition. Neurotoxicol. Teratol. 2020;82:106929. doi: 10.1016/j.ntt.2020.106929. [DOI] [PubMed] [Google Scholar]
- Rozeske R.R., Valerio S., Chaudun F., Herry C. Prefrontal neuronal circuits of contextual fear conditioning. Gene Brain Behav. 2015;14(1):22–36. doi: 10.1111/gbb.12181. [DOI] [PubMed] [Google Scholar]
- Salmon A., Erb C., Meshorer E., Ginzberg D., Adani Y., Rabinovitz I., Amitai G., Soreq H. Muscarinic modulations of neuronal anticholinesterase responses. Chem. Biol. Interact. 2005;157–158:105–113. doi: 10.1016/j.cbi.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Santini E., Sepulveda-Orengo M., Porter J.T. Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology. 2012;37(9):2047–2056. doi: 10.1038/npp.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A., Yau J.O.Y., Jean-Richard-Dit-Bressel P., Liu Y., Millan E.Z., Power J.M., McNally G.P. Basolateral amygdala neurons maintain aversive emotional salience. J. Neurosci. 2018;38(12):3001–3012. doi: 10.1523/JNEUROSCI.2460-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko A.C., Fadel J.R., Kaigler K.F., Wilson M.A. Activation of orexin/hypocretin neurons is associated with individual differences in cued fear extinction. Physiol. Behav. 2017;178:93–102. doi: 10.1016/j.physbeh.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp B.M. Basolateral amygdala, nicotinic cholinergic receptors, and nicotine: pharmacological effects and addiction in animal models and humans. Eur. J. Neurosci. 2019;50(3):2247–2254. doi: 10.1111/ejn.13970. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J., Furgeson-Moreira S., Monfils M.H. Predictability and heritability of individual differences in fear learning. Anim. Cognit. 2014;17(5):1207–1221. doi: 10.1007/s10071-014-0752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklan E.H., Lowenthal A., Korner M., Ritov Y., Landers D.M., Rankinen T., Bouchard C., Leon A.S., Rice T., Rao D.C., Wilmore J.H., Skinner J.S., Soreq H. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in Health, Risk Factors, Exercise Training, and Genetics study. Proc. Natl. Acad. Sci. U. S. A. 2004;101(15):5512–5517. doi: 10.1073/pnas.0307659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C., Koch K., Hopfeld J., Lobentanzer S., Lau H., Klein J. Impaired hippocampal and thalamic acetylcholine release in P301L tau-transgenic mice. Brain Res. Bull. 2019;152:134–142. doi: 10.1016/j.brainresbull.2019.07.014. [DOI] [PubMed] [Google Scholar]
- Sturgill J.F., Hegedus P., Li S.J., Chevy Q., Siebels A., Jing M., Li Y., Hangya B., Kepecs A. Basal foebrain-derived acetylcholine encodes valence free reinforcement prediction error. bioRxiv. 2020 doi: 10.1101/2020.02.17.953141. [DOI] [Google Scholar]
- Tinsley M.R., Quinn J.J., Fanselow M.S. The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing pavlovian fear conditioning and inhibitory avoidance. Learn. Mem. 2004;11(1):35–42. doi: 10.1101/lm.70204. [DOI] [PubMed] [Google Scholar]
- Tronson N.C., Schrick C., Guzman Y.F., Huh K.H., Srivastava D.P., Penzes P., Guedea A.L., Gao C., Radulovic J. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J. Neurosci. 2009;29(11):3387–3394. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal C.T., Pare D., Zaborszky L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J. Neurosci. 2015;35(2):853–863. doi: 10.1523/JNEUROSCI.2706-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valuskova P., Farar V., Janisova K., Ondicova K., Mravec B., Kvetnansky R., Myslivecek J. Brain region-specific effects of immobilization stress on cholinesterases in mice. Stress. 2017;20(1):36–43. doi: 10.1080/10253890.2016.1263836. [DOI] [PubMed] [Google Scholar]
- van der Zee E.A., Luiten P.G. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: a review of immunocytochemical localization in relation to learning and memory. Prog. Neurobiol. 1999;58(5):409–471. doi: 10.1016/s0301-0082(98)00092-6. [DOI] [PubMed] [Google Scholar]
- van der Zee E.A., Roozendaal B., Bohus B., Koolhaas J.M., Luiten P.G. Muscarinic acetylcholine receptor immunoreactivity in the amygdala--I. Cellular distribution correlated with fear-induced behavior. Neuroscience. 1997;76(1):63–73. doi: 10.1016/s0306-4522(96)00359-4. [DOI] [PubMed] [Google Scholar]
- Venton B.J., Robinson T.E., Kennedy R.T., Maren S. Dynamic amino acid increases in the basolateral amygdala during acquisition and expression of conditioned fear. Eur. J. Neurosci. 2006;23(12):3391–3398. doi: 10.1111/j.1460-9568.2006.04841.x. [DOI] [PubMed] [Google Scholar]
- Wilson M.A., Fadel J.R. Cholinergic regulation of fear learning and extinction. J. Neurosci. Res. 2017;95(3):836–852. doi: 10.1002/jnr.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.A., Grillo C.A., Fadel J.R., Reagan L.P. Stress as a one-armed bandit: differential effects of stress paradigms on the morphology, neurochemistry and behavior in the rodent amygdala. Neurobiol Stress. 2015;1:195–208. doi: 10.1016/j.ynstr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajeya J., De La Fuente A., Criado J.M., Bajo V., Sanchez-Riolobos A., Heredia M. Muscarinic agonist carbachol depresses excitatory synaptic transmission in the rat basolateral amygdala in vitro. Synapse. 2000;38(2):151–160. doi: 10.1002/1098-2396(200011)38:2<151::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Zeitlin R., Patel S., Solomon R., Tran J., Weeber E.J., Echeverria V. Cotinine enhances the extinction of contextual fear memory and reduces anxiety after fear conditioning. Behav. Brain Res. 2012;228(2):284–293. doi: 10.1016/j.bbr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M., Hast T.A., Bennett R.Z., Merjanian M., Nocera N.A., Ponnusamy R., Fanselow M.S. Cholinergic blockade frees fear extinction from its contextual dependency. Biol. Psychiatr. 2013;73(4):345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]