Abstract
Most cancer patients with brain metastases are treated with radiation therapy, yet this modality has not yet been meaningfully incorporated into preclinical experimental brain metastasis models. We applied two forms of whole brain radiation therapy (WBRT) to the brain-tropic 231-BR experimental brain metastasis model of triple-negative breast cancer. When compared to sham controls, WBRT as 3 Gy × 10 fractions (3 × 10) reduced the number of micrometastases and large metastases by 87.7 and 54.5 %, respectively (both p < 0.01); whereas a single radiation dose of 15 Gy × 1 (15 × 1) was less effective, reducing metastases by 58.4 % (p < 0.01) and 47.1 % (p = 0.41), respectively. Neuroinflammation in the adjacent brain parenchyma was due solely to a reaction from metastases, and not radiotherapy, while adult neurogenesis in brains was adversely affected following both radiation regimens. The nature of radiation resistance was investigated by ex vivo culture of tumor cells that survived initial WBRT (“Surviving” cultures). The Surviving cultures surprisingly demonstrated increased radiosensitivity ex vivo. In contrast, re-injection of Surviving cultures and re-treatment with a 3 × 10 WBRT regimen significantly reduced the number of large and micrometastases that developed in vivo, suggesting a role for the microenvironment. Micrometastases derived from tumor cells surviving initial 3 × 10 WBRT demonstrated a trend toward radioresistance upon repeat treatment (p = 0.09). The data confirm the potency of a fractionated 3 × 10 WBRT regimen and identify the brain microenvironment as a potential determinant of radiation efficacy. The data also nominate the Surviving cultures as a potential new translational model for radiotherapy.
Keywords: Metastasis, Brain, Radiation, Breast, Cancer, Radiosensitivity
Introduction
Brain metastases are one of the most challenging conditions to treat, often with unsatisfactory results for both patients and clinicians [1]. Over 200,000 new cases and 100,000 deaths from brain metastases occur per year in the United States, with 20 % of cases attributable to a breast cancer primary tumor [2]. Within breast cancer, the incidence of brain metastases is 37 % of metastatic patients with HER2+ tumors, often in the setting of stable or responding disease, or a first relapse after metastatic treatment; for metastatic patients with “triple-negative” (TN) tumors, a comparable incidence is reported but in the setting of uncontrolled systemic disease [3, 4]. As improvements in systemic therapies occur, the number of patients who will develop brain metastases is expected to increase.
Current standard treatments are only palliative and include surgery, stereotactic radiosurgery (SRS) for discrete lesions, and whole brain radiotherapy (WBRT) for diffuse disease. WBRT is delivered to the entire brain in equally divided, daily treatments or “fractions”, designed to treat multiple lesions and occult micrometastases. By contrast, SRS employs a single, large dose of radiation focused on the metastatic lesion(s) as the target. The larger fraction size of radiation in SRS is used to overcome the effect of hypoxia in larger lesions not amenable to resection and to induce endothelial apoptosis and vascular collapse [5]. While dependent on total dose, fractionation schedule, and volume treated, both types of radiation treatments can have side effects to include fatigue, cognitive alterations in short-term memory and concentration and, in rare cases, dementia [6-8]. Thus the goals of radiation therapy research are to enhance efficacy and reduce side effects.
Recently, experimental models of brain metastasis from breast cancer have been developed based on intracardiac or carotid artery injection of tumor cells [9, 10]. These models have been used to explore the mechanistic and molecular basis of brain metastasis development and to preclinically validate imaging, metastasis prevention and treatment strategies [10-24]. Most prior brain radiation therapy research has used a different model system, in which a bolus of tumor cells was injected intracranially, a single radiation treatment was given, and survival was the primary endpoint. We hypothesize that the use of a hematogenous model of brain metastasis incorporating fractionated radiation therapy, comparable to clinically used regimens, will lead to promising preclinical leads for future clinical testing. Herein, we evaluate WBRT in a well-established experimental metastasis model of TN breast cancer to the brain, 231-BR, derived from MDA-MB-231 tumor cells [24, 25]. Two schedules of WBRT are compared, 3 Gy × 10 fractions (3 × 10), comparable to the most commonly delivered clinical regimen, versus a single 15 Gy fraction (15 × 1). In addition to quantifying the effect of WBRT in a hematogenous model, we preclinically evaluated several important questions in the field: whether fractionation is better for eliminating metastases and reducing tumor cell viability, whether WBRT is responsible for producing neuroinflammation or damage to proliferating adult stem cell populations, and whether post-radiotherapy surviving tumor cells maintain continued radiosensitivity.
Materials and Methods
Cell culture
A brain metastatic derivative of the breast cancer cell line, MDA-MB-231, was isolated and maintained as described previously [24]. Although the cells had been previously characterized, we performed further cell authentication testing through ATCC to ensure a match to MDA-MB-231 (May, 2012). These cells, designated 231-BR, were maintained in DMEM supplemented with 10 % fetal bovine serum, 1 % Penicillin-Streptomycin and 1 % Essential Amino Acid supplement (Invitrogen) at 37 °C in 5 % CO2. Cells were passaged within 4 months of resuscitation according to ATCC protocol and routinely tested for Mycoplasma infection using MycoAlert™ Mycoplasma Detection Kit (Lonza).
Animal experiments
231-BR experimental brain metastasis model
Experiments were conducted under protocol ROB-144, approved by the Animal Care and Use Committee of the National Cancer Institute, National Institutes of Health. Female athymic nu/nu NRC mice (5–7 weeks old, Charles River Laboratories) were inoculated with 175,000 231-BR cells into the cardiac left ventricle. Mice injected with vehicle (DMEM) alone were used as “no metastasis” controls. Brain metastases were allowed to develop until day 14, at which time the mice were randomized to receive WBRT on a schedule of 30 Gy in 10 daily fractions, 15 Gy given as a single dose, or sham irradiation with equivalent daily anesthesia administrations of ketamine (90 mg/kg)/xylazine (10 mg/kg) intraperitoneally for immobilization. One group of controls received sham irradiation without ketamine/xylazine. Whole brain radiation was delivered on day 14 for 15 × 1 and days 14-23 for 3 × 10. Mice were euthanized if they showed signs of distress, 20 % weight loss, or paralysis. Otherwise, all mice were sacrificed on day 28 following intracardiac injection, with brains excised and bisected along the sagittal plane. Ten serial sections (10 microns thick) every 300 microns through the right hemisphere were H&E stained and analyzed at ×50 magnification using an ocular grid with 0.8 mm2 squares. Every micro or large metastasis (cutpoint of 300 microns along the longest axis) in each section was tabulated. All brains were examined to confirm the presence of metastases. These experiments were performed in triplicate, with each treatment and control group having at least 10 animals per group, for a minimum total of 30 animals per group overall.
231-BR intracranial implantation
To generate intracerebral xenografts, athymic nude mice were anesthetized and each brain sterilely injected with 1 × 106 231-BR cells at 1.0 mm anterior and 2.0 mm lateral to the bregma to a depth of 3.5 mm using a small animal stereotactic apparatus (Stoelting). A single resulting tumor was allowed to develop until day 5, followed by randomization to receive 3 Gy × 10 fractions WBRT, 15 Gy × 1 fraction WBRT, or sham irradiation. All mice meeting euthanasia criteria did so as a result of observed direct progression of intracranial tumor.
Irradiation
For in vivo irradiation, mice were immobilized with ketamine (90 mg/kg)/xylazine (10 mg/kg) intraperitoneally and placed in well-ventilated Plexiglass jigs designed with an aperture to allow exposure for whole brain radiation, and to provide shielding for the mouse along the torso and critical normal structures of the head. Irradiation was conducted with a Pentak X-irradiator (Inspection Systems) at 300 kV, 10 mA, dose rate 253 cGy/min.
Isolation of 231-BR cells surviving (S) radiotherapy
Metastases were extracted from brains that received sham, 3 × 10 or 15 × 1 treatment. The left hemisphere from five mice per experimental arm was minced under sterile conditions in MEM, treated with 0.25 % trypsin for 10 min, and cultured in DMEM as above with 1 % amphotericin B (Invitrogen). Brain tissue was removed after 48 h, and attached cells allowed to proliferate. Viable 231-BR cells were designated “S” as surviving radiation therapy (S-Sham, S-15 × 1, S-3 × 10), individual cultures pooled, and GFP labeling was confirmed by direct visualization.
Adult hippocampal neurogenesis
From the animal experiments previously described, brains were extracted on day 28 post-injection, fixed in 4 % paraformaldehyde, and cryoprotected. Coronal 40 μm sections were collected in a 1:12 series throughout the dentate gyrus, and processed for doublecortin (DCX) peroxidase immunohistochemistry, using goat anti-DCX (Santa Cruz Biotechnology, 1/200), biotinylated secondary antibodies (Vector Labs, 1/250), avidin–biotin-horseradish peroxidase (Vector Labs), and diaminobenzidine (Sigma) as previously described [26, 27]. Stereological counts of DCX-positive neurons in the subgranular zone (SGZ) and granule cell layer (GCL) of the dentate gyrus of each animal were collected and multiplied by 12 to estimate total numbers of DCX-positive neurons.
Immunofluorescence
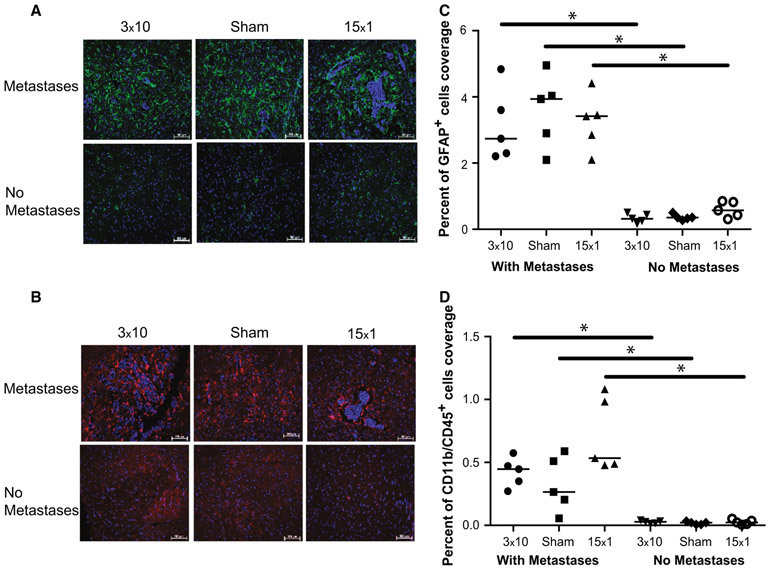
Brains from metastases/radiation treatment and control groups were immunostained as described previously [28]. The following antibodies were used: rat anti-mouse CD11b (Millipore, 1/50), rat anti-mouse CD45 (Millipore, 1/200), rabbit anti-human GFAP ready to use (Dako), and rabbit anti-Ki67 (Vector Labs, 1/200). AxioVision4 software was used for image acquisition and quantification. To quantify GFAP-expressing astrocytes and CD11b/CD45-expressing microglia, five clusters of metastatic lesions within one matched sagittal brain section per mouse, from five mice per group, were randomly selected and analyzed. The percentage of area covered by the immunostaining was reported.
In vitro analyses
Analyses of cell cycle distribution, proliferation, DNA double strand formation and repair and protein/mRNAs associated with this process are described in Supplemental Methods.
Statistical analysis
Calculations were performed using GraphPad Prism 6.0 (GraphPad Software, Inc.). Mixed model analysis of variance (ANOVA) was performed on continuous data (cell viability and in vivo brain metastasis data). Data were transformed as appropriate to meet ANOVA assumptions. Immunoreactivity differences in tissue were compared using the Mann- Whitney test. Neurogenesis data were log transformed to achieve homogeneity of variance and compared using 2-way ANOVA excluding the sham radiation group (which lacked a “no metastases” subgroup). Actuarial analysis was performed on survival data using the Kaplan– Meier method, and log-rank values were calculated for the intracranial implant model. Statistical analyses for in vivo analysis of surviving cells were performed using the unpaired Student’s t test, with statistical significance defined as p < 0.05.
Results
WBRT of mice bearing 231-BR experimental brain metastases
The standard one month 231- BR experimental brain metastasis model system was adapted to treat mice with WBRT using: (a) a single 15 Gy fraction dosing schedule (15 × 1); (b) a standard clinical fractionated radiation schedule of 3 Gy × 10 fractions (3 × 10); or (c) sham irradiation, including anesthesia for immobilization and placing the animals in jigs within the irradiator, as the control. Treatments began on day 14 post-injection, a time at which multiple micrometastases and an occasional large metastasis can be present. These schedules were devised to achieve a biologically equivalent dose of tumor cell kill corresponding to 37 ± 1 Gy, assuming a α/β ratio of 10 (BED10) [29]. After necropsy on day 28 post-injection, one hemisphere of each brain was step- sectioned for a manual count of micrometastases and large metastases, using 300 microns in a single dimension as a cutoff; H&E stained sections of the metastases are shown on Supplemental Figure 1. Table 1 lists the brain metastatic burden resulting from each treatment. Brains treated with either WBRT regimen demonstrated fewer micrometastases and large metastases when compared to sham-irradiation. Neither radiation regimen eliminated large metastases, with a 54.5 % reduction in the fractionated (3 × 10) group versus a 47.1 % reduction in the 15 × 1 group compared to sham-irradiated controls (both p < 0.01). For large metastases the two radiation treatment regimens were not statistically distinct from each other (p = 0.54). However, for micometastases, the fractionated regimen reduced the number of lesions by 87.7 % compared to sham, while the 15 × 1 regimen was less effective, reducing micrometastases by only 58.4 % (both p < 0.01 vs. sham). The WBRT regimens were statistically distinct from each other (p < 0.01), suggesting that in this model, fractionated radiation showed a more pronounced reduction in the number of micrometastases.
Table 1.
Effect of fractionated and single dose WBRT regimens in the 231-BR model
| Regimen | Large metastases |
Micrometastases |
Ki67 immunoreactivity |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean (95 % CI) |
% change | P value | Mean (95 % CI) |
% change | P value | Mean (95 % CI) |
% change (P value) |
|
| Sham irradiation | 0.68 (0.2–1.2) | – | – | 121.02 (44.6–197.5) | – | – | 238.28 (124.5–397.0) | – |
| 3 × 10 WBRT | 0.31 (0.01–0.6) | −54.5 | <0.01 (vs. Sham) | 27.14 (14.5–39.8) | −87.7 | <0.01 (vs. Sham) | 68.68 (19.1–118.2) | −71.2 (<0.01) |
| 15 × 1 WBRT | 0.36 (0.0–0.8) | −47.1 | <0.01 (vs. Sham) | 50.35 (21.9–78.8) | −58.4 | <0.01 (vs. Sham) | 213.12 (72.4–353.8) | −24.8 (0.41) |
| 0.54 (vs. 3 × 10) | <0.01 (vs. 3 × 10) | |||||||
Mice were injected with 231-BR cells on day 0. On day 14 post-injection, mice were randomized to the indicated regimens. Mice were necropsied on day 28 post-injection. Results of manual counts of step sections through one brain hemisphere of each mouse are enumerated, using a 300 micron in a single dimension cutoff between large- and micrometastases. Data are representative of 3 experiments conducted, with at least 10 animals per group for a minimum total of 30 animals per group
The superiority of fractionated WBRT could result from greater tumor kill and/or reduced proliferation. Ki67 immunofluorescence was performed on fixed, frozen sections of tumor cells in brain tissue across 5 randomly selected animals per group (Table 1). The compiled data reveal a significant reduction (71.2 %, p < 0.01) in the number of Ki67 + tumor cells following fractionated WBRT as compared to sham. No significant reduction was observed between single-dose WBRT and sham (24.8 %, p = 0.41). Cleaved caspase 3, a marker of apoptosis, was not appreciably present on day 28 post-injection (data not shown). Thus, fractionated radiation therapy decreased the proliferative potential of tumor cells that survived treatment.
Experimental brain metastases and human craniotomy specimens are known to be infiltrated and surrounded by a neuroinflammatory response [21]. To determine the neuroinflammatory response within the tumor microenvironment following WBRT, brain tissue from mice that received 3 × 10, 15 × 1 WBRT treatments or sham-irradiation were evaluated by immunofluorescence using markers against activated astrocytes (GFAP) and activated microglia (CD11b/CD45) (Fig. 1a, b, Metastases). Virtually all metastatic lesions were surrounded by a zone of activated astrocytes and microglia, as previously reported [21]. For activated astrocytes, the density of GFAP+ infiltrates did not vary by radiation treatment. In order to determine whether neuroinflammation resulted from the metastasis, the radiation therapy, or a combination, comparable tissue sections were also stained from animals that had been injected with vehicle alone and therefore did not develop metastases (Fig. 1b. No Metastases). Activated astrocytes were infrequently observed in brains that did not develop metastases (vehicle injected mice) (p < 0.01) and did not vary with radiation treatment (Fig. 1c). Similar trends were observed for microglia with CD11b/CD45 staining (Fig. 1d). Using these histological markers, these data suggest that only the presence of metastases dictates the neuroinflammatory response in the 231-BR model, and that this response is unaffected by either radiation or the fractionation schedule of radiation that is given.
Fig. 1.
Effect of radiation therapy on the neuroinflammatory microenvironment in the 231-BR experimental brain metastasis model of breast cancer. a Anti-GFAP (green) and b Anti- CD11b/CD45 (red) antibodies were used to stain for activated astrocytes and reactive microglia, respectively, across 10 μM brain sections from 5 animals per treatment group (Sham, 3 × 10 WBRT, and 15 × 1 WBRT from Table 1). Nuclei are stained blue with DAPI. The percentage of area covered by immunostaining was reported (c and d). In all cases, immunoreactivity was statistically greater in brains with metastases compared to those without, despite treatment received (*p < 0.01), suggesting that the neuroinflammatory response in the 231-BR model is independent of radiation therapy
Neurogenesis, a process by which new neurons are produced in the brain, persists throughout life in discrete regions of the adult brain, including the hippocampus. It has been long established that radiation exposure has a negative impact on neurogenesis [30-33]. The effects of tumors and radiation therapy on naturally-occurring neurogenesis were assessed by counting the number of immature, doublecortin (DCX)-expressing neurons in the hippocampus. Neurogenesis was unaffected by metastases in the control brains (Fig. 2). Radiation therapy alone strongly inhibited adult neurogenesis in metastasis-containing brains with a reduction of 99 % by fractionated WBRT (p < 0.01) and 79 % by the single-dose WBRT regimen (p = 0.01). Surprisingly, following single-dose treatment, more new neurons were observed in brains containing metastases than in brains without metastases (p = 0.02), suggesting that metastases may confer some resilience against radiation to neuronal endogenous precursor cells.
Fig. 2.
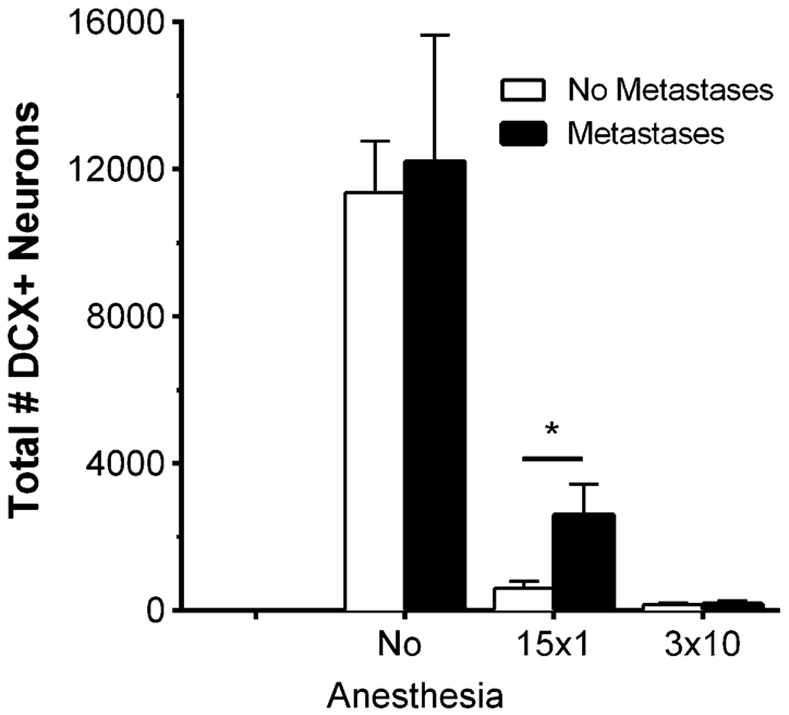
Effect of radiation therapy on adult neurogenesis in the subgranular zone (SGZ)/granule cell layer (GCL) of the dentate gyrus in the 231-BR model. Coronal step sections (40 μm) were cut through the hippocampus of mice detailed in Table 1, and doublecortin (DCX)-positive neurons in the subgranular zone (SGZ) and granule cell layer (GCL) of the dentate gyrus were identified and counted. The data are represented as average number of DCX-positive neurons as a function of treatment group. Adult neurogenesis was detectable in the sham-irradiated metastatic brains, and was reduced 99 % by fractionated WBRT (p < 0.01) and 79 % by the single dose regimen (p = 0.01). There was a significant difference (*p = 0.02) toward increased adult neurogenesis in those brains containing metastases after 15 × 1 compared to no metastasis controls, suggesting an interplay between metastases and stem cells in the SGZ following irradiation
WBRT using intracranial injection
Estimating survival in the 231-BR model via intracardiac injection is problematic in that extracranial bone metastases can also occasionally grow and produce paralysis symptoms similar to brain lesions. Traditional intracranial inoculation was therefore used to estimate mouse brain-specific survival [34]. Direct implantation of 1 × 106 231-BR cells into the right hemisphere of athymic nudes established a large, single tumor in each animal, each of whom were randomized to receive sham irradiation, 15 × 1 WBRT, or 3 × 10 WBRT beginning day 5 post-injection [35]. All mice died from tumor progression or were censored at day 280 (Fig. 3). The median survival of 24 days in the sham-irradiated arm was extended to 46 days with 3 × 10. 15 × 1 treatment increased median survival to 81 days post-injection, and 40 % of the animals survived 280 days, at which time they were sacrificed. Necropsy evaluation of these long-term survivors revealed no evidence of intracranial tumor. Thus, the overall efficacy of fractionated versus unfractionated WBRT differed depending on the route of tumor cell injection.
Fig. 3.
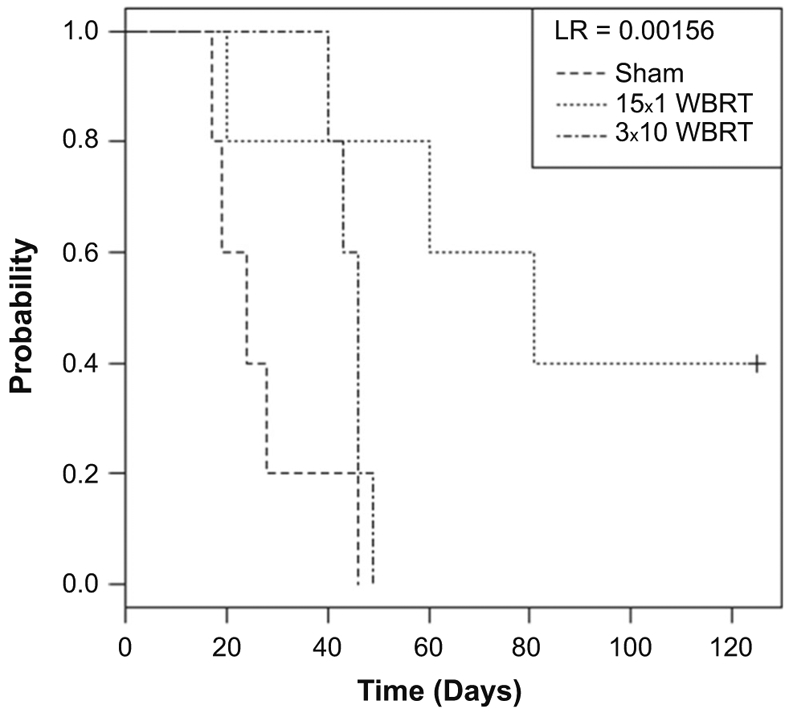
Overall survival was increased with WBRT in an intracranial preclinical model system. 231-BR cells were injected into the right hemisphere. On day 5 post-injection, mice were randomized to receive sham irradiation (no radiation control), 3 × 10 WBRT, or 15 × 1 WBRT. Mice were observed daily until death from direct tumor progression, and are represented by Kaplan–Meier curves of each group that are statistically distinct (log-rank = 0.00156). Median survival was 24 days in the sham-irradiated arm, and was extended to 46 days with 3 × 10 WBRT. 15 × 1 WBRT produced a greater increase in median survival to 81 days post-injection, and 40 % of the animals survived 280 days (denoted by +), at which time they were sacrificed. Necropsy revealed no evidence of viable tumor in the long-term survivors
Isolation and analysis of “Surviving” brain metastatic tumor cells
An understanding of the mechanisms of radiosensitivity will be key to improving radiation therapy for patients with brain metastases. Currently it is assumed that cells surviving radiotherapy are resistant to further treatment with radiation [36]. We asked whether brain metastatic tumor cells that survived radiation therapy remained radiosensi-tive, or exhibited stable aspects of radioresistance. “Surviving” 231-BR cells (S-cells) were cultured and expanded ex vivo from mice that had received Sham, 3 × 10 WBRT, or 15 × 1 WBRT; multiple cultures from one set of treatments were combined to reflect potential heterogeneity. These cells were designated as S-Sham, S-3 × 10, and S-15 × 1, respectively.
Clonogenic assays, a standard in vitro test of radiation sensitivity, were performed on the surviving (S-) cultures as well as the parental 231-BR cells used for intracardiac injection (Fig. 4). S-Sham cultures were incrementally more sensitive to 2–8 Gy radiation doses than parental 231-BR cells. The dose enhancement factor calculation at 10 % survival (DEF10), where ratios >1 suggest radiosensitization and values <1 suggest radioprotection, was 1.07 for S-Sham cells compared to parental 231-BR cells (p < 0.01). Within the S-cultures, there was no significant enhancement seen for S-Sham compared to S-15 × 1 cells (DEF10 = 1.0). However, the DEF10 was greatest for cells surviving fractionated WBRT compared to S-Sham (DEF10 = 1.34, p < 0.01), a finding repeatable over 12 experiments conducted, at virtually all radiation doses tested. These data suggest the unexpected conclusion that tumor cells surviving radiation therapy in the 231-BR model, theoretically expected to be resistant, were actually more radiosensitive in this ex vivo assay. Correlates of the increased in vitro radiosensitivity of the S-3 × 10 tumor cells included altered Ki67 proliferative status, reduced repair of DNA double strand breaks and altered mRNA and protein levels of DNA repair components (Supplemental Figures 2-3, Supplemental Tables 1-2). Increased γH2AX and phosphorylated CHK2 following radiation re-exposure in the S-cultures suggests continued capacity for DNA damage response. Likewise, decreased Bcl2 and phosphorylated AKT following repeated radiation exposure in the S-cultures correlates with the potential for decreased survivability in vitro (Supplemental Figure 3A, Supplemental Table 2).
Fig. 4.
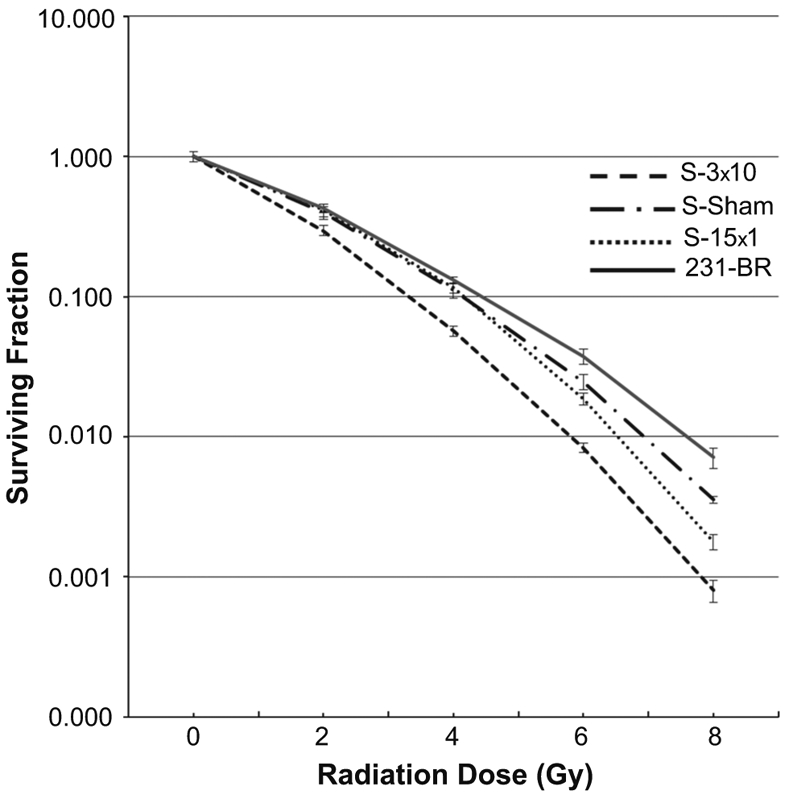
Clonogenic assay of surviving (S) tumor cells. Surviving brain metastases were harvested from sham-irradiated mice (S-Sham), mice that received 15 × 1 WBRT (S-15 × 1), and 3 × 10 WBRT (S-3 × 10) using 5 brains per treatment group and compared to 231-BR cells used for intracardiac injection. 250-40,000 cells/well were treated with sham or single doses of radiation. Colonies containing at least 50 cells were counted, and survival curves were generated after normalizing for plating efficiency (25–30 %). Data presented are the mean ± SE from 12 independent experiments. The S-3 × 10 cells were repeatedly the most radiosensitive at virtually all radiation doses tested with a Dose enhancing factor (DEF10) = 1.34 (p < 0.01)
To determine the radiosensitivity of the S cultures in vivo, passage 8 S-cells were re- injected into the left cardiac ventricle of nude mice. Again, metastases were allowed to form for 14 days, after which the mice were randomized to receive Sham-irradiation or 3 × 10 WBRT. The mice were sacrificed on day 27, and metastases were quantified (Table 2). S-Sham tumor cells, when re-injected and treated with 3 × 10, demonstrated sensitivity to radiation therapy with 87.5 and 79.3 % decreases in large and micrometastases, respectively. S-15 × 1 tumor cells produced similar results, with an 86.9 and 82.8 % reduction in large and micrometastases, respectively (p = 0.01). Tumor cells surviving 3 × 10 evidenced somewhat distinct growth patterns following re- irradiation. The S-3 × 10 culture retained comparable radiosensitivity of large metastases, with a 79.6 % reduction following a second exposure to 3 × 10 (p = 0.03). However, the potency of a repeat 3 × 10 radiation treatment on micrometastases demonstrated a trend toward radioresistance compared to the other surviving cultures, a 58.6 % reduction (p = 0.09), warranting further investigation. Thus, the in vitro radiosensitivity of the S-fractionated therapy cultures was not replicated in vivo, suggesting the host, and the brain microenvironment in particular, as a contributing factor.
Table 2.
Effect of 3 × 10 WBRT on Cells Surviving previous WBRT in the 231-BR Model
| S-tumor cells |
Large metastases |
Micrometastases |
|||||
|---|---|---|---|---|---|---|---|
| Initial WBRT treatment |
Second WBRT treatment |
No (95 % CI) | % difference from Sham |
P value | No (95 % CI) | % difference from Sham |
P value |
| Sham | Sham | 14.8 (3.4–26.2) | – | – | 439.8 (130.4–308.5) | – | – |
| 3 × 10 | 1.85 (1.0–2.7) | −87.5 | 0.05 | 90.8 (58.8–122.8) | −79.2 | 0.05 | |
| 3 × 10 | Sham | 20.6 (8.4–32.8) | – | – | 378.4 (160.9–596.0) | – | – |
| 3 × 10 | 4.2 (2.4–6.0) | −79.6 | 0.05 | 156.7 (100.0–213.3) | −58.6 | 0.09 | |
| 15 × 1 | Sham | 34.25 (10.6–57.9) | – | – | 1165.5 (467.4–1863.7) | – | – |
| 3 × 10 | 4.5 (1.9–7.1) | −86.9 | 0.01 | 200.5 (106.9–294.1) | −82.8 | 0.01 | |
Mice were injected with “Surviving” S-cells on day 0. On day 14 post-injection, mice were randomized to Sham irradiation versus 3 Gy × 10 fractions of whole brain radiation therapy (WBRT). Mice were necropsied on day 27 post-injection, with a minimum of 10 animals per group evaluated. Results of manual counts of step sections through one brain hemisphere of each mouse are enumerated, using a 300 micron in a single dimension cutoff between large and micrometastases. P values were calculated using the unpaired t test
Discussion
Our long-term goal is to develop preclinical models for radiation therapy of brain metastases of breast cancer that best reflect patient experience, as a vehicle for understanding the biology, identifying synergistic combination therapies, and reducing side effects. Most previous preclinical modeling employed intracranial implantation of a bolus of tumor cells followed by a single dose of radiation which mimicked neither SRS nor WBRT, with survival as the only endpoint. Our experiments significantly modified this regimen, but could not incorporate all of the possible attributes of the human clinical situation. Brain metastases formed from a hematogenous route, and varied in size from micrometastases to the equivalent of MRI detectable lesions in a human brain. The mice were randomized to receive WBRT as a fractionated regimen, similar to WBRT administered clinically, versus a single dose. Radiation therapy commenced on day 14 post-injection in order to provide adequate time for mitotic catastrophe of lethally damaged cells to occur, and to ensure the ability to appreciate a visible difference in the volume and number of metastases. Because mice receiving fractionated therapy had less time post-treatment for recovery, finishing radiation treatment on day 23 post-injection as opposed to day 14 post-injection with single-dose WBRT, both fractionation and recovery time were potential causative variables in the differences between regimens. This report is based on a single brain tropic cell line and further research is needed to expand this approach to other brain metastatic model systems. We were not able to model stereotactic radiosurgery (SRS), the other common form of radiation therapy for brain metastases, in that the entire brain was irradiated.
Using a histologic count of micrometastases and large metastases, fractionated and single-dose WBRT were distinct across three replicate experiments. Fractionation resulted in the greatest suppression of micrometastasis development (87.7 vs. 58.4 % after single dose), but failed to completely eliminate progression, in keeping with the clinical scenario. Fractionated radiotherapy was also accompanied by the greatest decrease in tumor cell Ki67 staining, indicating the importance of the anti-proliferative effect of radiation for brain metastasis control. All animals were sacrificed at Day 28 after injection, with whole brain radiation being delivered on day 14 for 15 × 1, and days 14–23 for 3 × 10. Therefore, there were 5 days between the completion of WBRT and sacrifice for the 3 × 10 mice versus 14 days between the completion of WBRT and sacrifice for the 15 × 1 mice. Given the time differences, one might hypothesize that the mice who received 15 × 1 might have the advantage in reducing Ki67 and cleaved Caspase 3 staining, due to the advantage of having more time for cells to succumb to the effects of radiation exposure. However, we observed the opposite. Brains that received fractionated radiation, with the shortest time interval between completion of radiation and sacrifice, demonstrated the least Ki67 and cleaved Caspase 3 staining. Alternatively, the 15 × 1 regimen could permit those tumor cells surviving radiation therapy to repair any damage and then restart proliferation.
A different trend was observed if the same tumor cells were implanted intracranially as a bolus. Using this more traditional model system, the 15 × 1 treatment provided superior median survival, and long term survival to 40 % of the mice. Single, large necrotic tumors generated by direct implantation model may explain why a larger fraction size was required to overcome the effect of hypoxia on the tumors as compared to hematogenous delivery, and why fractionated radiation is less effective on single, large necrotic lesions [34, 37]. Use of the 231-BR model as treated herein, or similar models to preclinically validate tumor radiosensitization or normal brain neuroprotection strategies, can be compared to eventual clinical trial data to determine whether the current findings represent a preclinical modeling improvement.
We analyzed several aspects of brain metastasis and radiation therapy in the 231-BR hematogenous model. Neuroinflammation is a prominent feature of many CNS diseases including stroke, Alzheimer’s disease, Parkinson’s disease, and mild cognitive impairment [38-40]. The neuroinflammatory response has been hypothesized to contribute to radiation-induced cognitive losses [41-43]. When activated astrocytes and microglia adjacent to metastases (or throughout the brain, in animals that were injected with DMEM only) were quantified, it was clear that the neuroinflammatory response was metastasis-driven and not radiation treatment- dependent. Essentially no neuroinflammation was seen in brains without metastases, and the degree of neuroinflammation was similar in metastatic brains with and without radiation treatment. The current findings are consistent with a recent report in which the brains of old and young mice received WBRT and few changes in microglial or astrocytic activation were noted [44]. Our data do not rule out a role for neuroinflammation in cognitive impairment, as this has been described to be a late effect, months to a year after radiation treatment [6]. Herein, we have described an acute neuroinflammatory process.
The effect of metastasis development and radiation therapy on adult hippocampal neurogenesis was also quantified. The dentate gyrus of the hippocampus is capable of generating new neurons throughout life, and this process is thought to influence aspects of learning and memory, spatial recognition/pattern separation, and emotional control/affective behavior [45-47]. The impact of radiation therapy on neural stem cells and progenitors and the neurovascular niche remains incompletely defined [31, 42, 48-55], and little is known about the effect of brain metastases on adult hippocampal neurogenesis. Here, we report that WBRT was toxic for the number of DCX + neurons of the dentate gyrus. Fractionation was more potent than single- dosing, likely because fractionated radiation has repeated opportunities to affect the non-synchronous dividing cell population during the most sensitive period of the cell cycle. However, even WBRT did not completely abrogate adult hippocampal neurogenesis, suggesting a potential for recovery of the newborn neuron population given sufficient time and/or growth-promoting treatments [56-60]. Surprisingly, 15 × 1 radiation treatment was less destructive to neurogenesis in the metastatic brain than in the non-metastatic brain (76.0 vs. 94.5 % inhibition, p = 0.02). The causes of this interaction are unknown but may involve tumor-secreted survival signals.
Perhaps the most interesting set of experiments reported herein concern the isolation and characterization of radiation therapy “resistant” tumor cells, the Surviving (S) cultures. In the clinic, it is impossible to distinguish between the generation of radioresistant tumor cells following WBRT, from the continual intravasation of radiation therapy-naïve tumor cells. Herein, tumor cells were delivered in one injection, the brains irradiated after micrometastasis formation starting on day 14 post-injection, and surviving tumor cells were harvested; there was little extracranial disease to generate circulating tumor cells and new brain metastases. A prominent disconnect was observed in the response of Surviving cultures in vitro and in vivo. In clonogenic assays, the S-3 × 10 tumor cells were radiosensitive upon rechallenge, accompanied by decreased Ki67 and inefficient DNA repair. A simple hypothesis to explain the unexpected in vitro radiosensitivity of micrometastases observed in cells that had received prior fractionated WBRT posits that stable damage by radiation therapy, resulting in poor viability, can perpetuate through multiple generations of cellular progeny. When the S-cultures were reintroduced in vivo and randomized to sham or fractionated WBRT, no preferential radiosensitivity of the S-3 × 10 culture was observed in micrometastases. Both the S-3 × 10 and S-1 × 15 cultures responded to fractionated WBRT; micrometastases produced by the 3 × 10 treated S-3 × 10 cells showed a trend of augmented radioresistance. These data argue a functional contribution of the in vivo condition, possibly the brain microenvironment, to radioresistance, which will be the subject of continued investigation. Isolation and characterization of additional rounds of surviving tumor cells is planned, as well as application of the experimental strategy to other brain metastasis hematogenous models. Many radiation therapy clinical trials enroll patients after relapse from WBRT. We propose that the intracardiac model in combination with clinically-relevant radiation schedules may provide a better model than direct intracranial implantation to evaluate the effect of investigational therapies prior to their use in clinical trials.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research program of the National Cancer Institute and by the Department of Defense Center of Excellence Breast Cancer Research Program Award W81XWH-06-0033. The authors thank Dr. Yong Qian for excellent animal procedures.
Abbreviations
- SRS
Stereotactic radiosurgery
- TN
Triple-negative
- WBRT
Whole brain radiation therapy
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10585-015-9739-9) contains supplementary material, which is available to authorized users.
References
- 1.Davis FG, Dolecek TA, McCarthy BJ, Villano JL (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14:1171–1177. doi: 10.1093/neuonc/nos152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75:5–14 [DOI] [PubMed] [Google Scholar]
- 3.Heitz F, Harter P, Lueck HJ, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A, du Bois A (2009) Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer 45:2792–2798. doi: 10.1016/j.ejca.2009.06.027 [DOI] [PubMed] [Google Scholar]
- 4.Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, Tudor IC, Wang LI, Brammer MG, Shing M, Yood MU, Yardley DA (2011) Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res 17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnik R (2003) Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 300:1155–1159 [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis LM, Delattre JY, Posner JB (1989) Radiation-induced dementia in patients cured of brain metastases. Neurology 39:789–796 [DOI] [PubMed] [Google Scholar]
- 7.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3 [DOI] [PubMed] [Google Scholar]
- 8.Baschnagel A, Wolters PL, Camphausen K (2008) Neuropsychological testing and biomarkers in the management of brain metastases. Radiat Oncol 3:26. doi: 10.1186/1748-717X-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steeg PS, Camphausen KA, Smith QR (2011) Brain metastases as preventive and therapeutic targets. Nat Rev Cancer 11:352–363. doi: 10.1038/nrc3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Huang WC, Zhang L, Zhang C, Lowery FJ, Ding Z, Guo H, Wang H, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D (2013) Src family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res 73:5764–5774. doi: 10.1158/0008-5472.CAN-12-1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR (2010) Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16:5664–5678. doi: 10.1158/1078-0432.CCR-10-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmieri D, Duchnowska R, Woditschka S, Hua E, Qian Y, Biernat W, Sosinkska-Mielcarek K, Gril B, Stark AM, Hewitt SM, Liewehr DJ, Steinberg SM, Jassem J, Steeg PS (2014) Profound prevention of experimental brain metastases of breast cancer by temozolomide in an MGMT-dependent manner. Clin Cancer Res 20:2727–2739. doi: 10.1158/1078-0432.CCR-13-2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gril B, Palmieri D, Qian Y, Smart D, Ileva L, Liewehr DJ, Steinberg SM, Steeg PS (2011) Pazopanib reveals a role for tumor cell B-raf in the prevention of HER2(+) breast cancer brain metastasis. Clin Cancer Res 17:142–153. doi: 10.1158/1078-0432.CCR-10-1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Percy DB, Ribot EJ, Chen Y, McFadden C, Simedrea C, Steeg PS, Chambers AF, Foster PJ (2011) In vivo characterization of changing blood-tumor barrier permeability in a mouse model of breast cancer metastasis a complementary magnetic resonance imaging approach. Invest Radiol 46:718–725. doi: 10.1097/RLI.0b013e318226c427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Ridgway LD, Wetzel MD, Ngo J, Yin W, Kumar D, Goodman JC, Groves MD, Marchetti D (2013) The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci Trans Med 5:180ra48. doi: 10.1126/scitranslmed.3005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGowan PM, Simedrea C, Ribot EJ, Foster PJ, Palmieri D, Steeg PS, Allan AL, Chambers AF (2011) Notch1 inhibition alters the CD44(hi)/CD24(lo) population and reduces the formation of brain metastases from breast cancer. Mol Cancer Res 9:834–844. doi: 10.1158/1541-7786.MCR-10-0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha D, Dunn H, Zhou H, Harada H, Hiraoka M, Mason RP, Zhao D (2011) In vivo bioluminescence imaging of tumor hypoxia dynamics of breast cancer brain metastasis in a mouse model. J Vis Exp 56. doi: 10.3791/3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorger M, Felding-Habermann B (2010) Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol 176:2958–2971. doi: 10.2353/ajpath.2010.090838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16:116–122. doi: 10.1038/nm.2072 [DOI] [PubMed] [Google Scholar]
- 20.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459:1005–1009. doi: 10.1038/nature08021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, Vega-Valle E, Weil RJ, Stark AM, Vortmeyer AO, Steeg PS (2008) Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis 25:799–810. doi: 10.1007/s10585-008-9193-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, Becker K, Yates JR 3rd, Felding-Habermann B (2007) Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res 67:1472–1486 [DOI] [PubMed] [Google Scholar]
- 23.Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT, Mikulis DJ, Palimieri D, Bronder JL, Steeg PS, Yoneda T, MacDonald IC, Chambers AF, Rutt BK, Foster PJ (2006) In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med 56:1001–1010 [DOI] [PubMed] [Google Scholar]
- 24.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L, Halverson D, Vortmeyer AO, Steinberg SM, Aldape K, Steeg PS (2007) Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res 67:4190–4198 [DOI] [PubMed] [Google Scholar]
- 25.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R (2001) A bone seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 16:1486–1495 [DOI] [PubMed] [Google Scholar]
- 26.Olariu A, Cleaver KM, Cameron HA (2007) Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol 501:659–667 [DOI] [PubMed] [Google Scholar]
- 27.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA (2009) Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci 29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gril B, Palmieri D, Qian Y, Anwar T, Liewehr DJ, Steinberg SM, Andreu Z, Masana D, Fernandez P, Steeg PS, Vidal-Vanaclocha F (2013) Pazopanib inhibits the activation of PDGFRbeta-expressing astrocytes in the brain metastatic microenvironment of breast cancer cells. Am J Pathol 182:2368–2379. doi: 10.1016/j.ajpath.2013.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall EJ, Giaccia AJ (2006) Radiobiology for the radiologist, 6th edn. Lippencott Williams and Wilkins, Philadelphia [Google Scholar]
- 30.Yamazaki JN, Schull WJ (1990) Perinatal loss and neurological abnormalities among children of the atomic bomb. Nagasaki and Hiroshima revisited, 1949 to 1989. JAMA 264:605–609 [PubMed] [Google Scholar]
- 31.Andres-Mach M, Rola R, Fike JR (2008) Radiation effects on neural precursor cells in the dentate gyrus. Cell Tissue Res 331:251–262 [DOI] [PubMed] [Google Scholar]
- 32.Monje M (2008) Cranial radiation therapy and damage to hippocampal neurogenesis. Dev Disabil Res Rev 14:238–242. doi: 10.1002/ddrr.26 [DOI] [PubMed] [Google Scholar]
- 33.Wojtowicz JM (2006) Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus 16:261–266 [DOI] [PubMed] [Google Scholar]
- 34.Baschnagel A, Russo A, Burgan WE, Carter D, Beam K, Palmieri D, Steeg PS, Tofilon P, Camphausen K (2009) Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts. Mol Cancer Ther 8:1589–1595. doi: 10.1158/1535-7163.MCT-09-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camphausen K, Scott T, Sproull M, Tofilon PJ (2004) Enhancement of xenograft tumor radiosensitivity by the histone deacetylase inhibitor MS-275 and correlation with histone hyperacetylation. Clin Cancer Res 10:6066–6071 [DOI] [PubMed] [Google Scholar]
- 36.Jagannathan J, Bourne TD, Schlesinger D, Yen CP, Shaffrey ME, Laws ER Jr, Sheehan JP (2010) Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery 66:208–217. doi: 10.1227/01.NEU.0000359318.90478.69 [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald DP, Subramanian P, Deshpande M, Graves C, Gordon I, Qian Y, Snitkovsky Y, Liewehr DJ, Steinberg SM, Paltan-Ortiz JD, Herman MM, Camphausen K, Palmieri D, Becerra SP, Steeg PS (2012) Opposing effects of pigment epithelium-derived factor on breast cancer cell versus neuronal survival: implication for brain metastasis and metastasis-induced brain damage. Cancer Res 72:144–153. doi: 10.1158/0008-5472.CAN-11-1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan Z, Aman Y, Ahmed I, Chetelat G, Landeau B, Ray Chaudhuri K, Brooks DJ, Edison P (2014) Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimers Dement. doi: 10.1016/j.jalz.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 39.Davis DH, Skelly DT, Murray C, Hennessy E, Bowen J, Norton S, Brayne C, Rahkonen T, Sulkava R, Sanderson DJ, Rawlins JN, Bannerman DM, MacLullich AM, Cunningham C (2015) Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry 23:403–415. doi: 10.1016/j.jagp.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurata T, Lukic V, Kozuki M, Wada D, Miyazaki K, Morimoto N, Ohta Y, Deguchi K, Ideda Y, Kamiya T, Abe K (2014) Telmisartan reduces progressive accumulation of cellular amyloid beta and phosphorylated tau with inflammatory responses in aged spontaneously hypertensive stroke resistant rat. J Stroke Cerebrovasc Dis 23:2580–2590. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 41.Schnegg CI, Greene-Schloesser D, Kooshki M, Payne VS, Hsu FC, Robbins ME (2013) The PPARdelta agonist GW0742 inhibits neuroinflammation, but does not restore neurogenesis or prevent early delayed hippocampal-dependent cognitive impairment after whole-brain irradiation. Free Radic Biol Med 61:1–9. doi: 10.1016/j.freeradbiomed.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen AR, Eilertson K, Sharma S, Schneider D, Baure J, Allen B, Rossi S, Raber J, Fike JR (2013) Effects of radiation combined injury on hippocampal function are modulated in mice deficient in chemokine receptor 2 (CCR2). Radiat Res 180:78–88. doi: 10.1667/RR3344.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenrow KA, Brown SL, Lapanowski K, Naei H, Kolozsvary A, Kim JH (2013) Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat Res 179:549–556. doi: 10.1667/RR3026.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen AR, Eilertson K, Chakraborti A, Sharma S, Baure J, Habdank-Kolczkowski J, Allen B, Rosi S, Raber J, Fike JR (2014) Radiation exposure prior to traumatic brain injury induces responses that differ as a function of animal age. Int J Radiat Biol 90:214–223. doi: 10.3109/09553002.2014.859761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hannesson DK, Howland J, Pollock M, Mohapel P, Wallace AE, Corcoran ME (2001) Dorsal hippocampal kindling produces a selective and enduring disruption of hippocampally mediated behavior. J Neurosci 21:4443–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aimone JB, Li Y, Lee SW, Clemenson DG, Deng W, Gage FH (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94:991–1026. doi: 10.1152/physrev.00004.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron HA, Glover LR (2014) Adult neurogenesis: beyond learning and memory. Annu Rev Psychol 66:53–81. doi: 10.1146/annurev-psych-010814-015006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fike JR, Rola R, Limoli CL (2007) Radiation response of neural precursor cells. Neurosurg Clin N Am 18:115–127 [DOI] [PubMed] [Google Scholar]
- 49.Greene-Schloesser DM, Kooshki M, Payne V, D’Agostino RV Jr, Wheeler KT, Metheny-Barlow LJ, Robbins ME (2014) Cellular response of the rat brain to single doses of (137)Cs gamma rays does not predict its response to prolonged ‘biologically equivalent’ fractionated doses. Int J Radiat Biol 90:790–798. doi: 10.3109/09553002.2014.933915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee TC, Greene-Schloesser D, Payne V, Diz DI, Hsu FC, Kooshki M, Mustafa R, Riddle DR, Zhao W, Chan MD, Robbins ME (2012) Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res 178:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parihar VK, Archaya MM, Roa DE, Bosch O, Christie LA, Limoli CL (2014) Defining functional changes in the brain caused by targeted stereotaxic radiosurgery. Transl Cancer Res 3:124–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR (2004) Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat Res 161:17–27 [DOI] [PubMed] [Google Scholar]
- 53.Walker AK, Rivera PD, Wang Q, Chuang JC, Tran S, Osborne-Lawrence S, Estill SJ, Starwalt R, Huntington P, Morlock L, Naidoo J, Williams NS, Ready JM, Eisch AJ, Pieper AA, Zigman JM (2015) The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatry 20:500–508. doi: 10.1038/mp.2014.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bostrom M, Kalm M, Karlsson N, Hellstrom Erkenstam N, Blomgren K (2013) Irradiation to the young mouse brain caused long-term, progressive depletion of neurogenesis but did not disrupt the neurovascular niche. J Cereb Blood Flow Metab 33:935–943. doi: 10.1038/jcbfm.2013.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou Y, Leu D, Chui J, Fike JR, Huang TT (2013) Effects of altered levels of extracellular superoxide dismutase and irradiation on hippocampal neurogenesis in female mice. Int J Radiat Oncol Biol Phys 87:777–784. doi: 10.1016/j.ijrobp.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monje ML, Palmer T (2003) Radiation injury and neurogenesis. Curr Opin Neurol 16:129–134 [DOI] [PubMed] [Google Scholar]
- 57.Gondi V, Tome WA, Mehta MP (2010) Why avoid the hippocampus? A comprehensive review. Radiother Oncol 97:370–376. doi: 10.1016/j.radonc.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gondi V, Hermann BP, Mehta MP, Tome WA (2013) Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys 85:348–354. doi: 10.1016/j.ijrobp.2012.11.031 [DOI] [PubMed] [Google Scholar]
- 59.Wan JF, Zhang SJ, Wang L, Zhao KL (2013) Implications for preserving neural stem cells in whole brain radiotherapy and prophylactic cranial irradiation: a review of 2270 metastases in 488 patients. J Radiat Res 54:285–291. doi: 10.1093/jrr/rrs085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghia A, Tome WA, Thomas S, Cannon G, Khuntia D, Kuo JS, Mehta MP (2007) Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys 68:971–977 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.