Abstract
Intraflagellar transport (IFT) is essential for assembling primary cilia required for bone formation. Disruption of IFT frequently leads to bone defects in humans. While it has been well studied about the function of IFT in osteogenic cell proliferation and differentiation, little is known about its role in collagen biosynthesis during bone formation. Here we show that IFT20, the smallest IFT protein in the IFT-B complex, is important for collagen biosynthesis in mice. Deletion of Ift20 in craniofacial osteoblasts displayed bone defects in the face. While collagen protein levels are unaffected by loss of Ift20, collagen cross-linking was significantly altered. In both Ift20:Wnt1-Cre and Ift20:Ocn-Cre mice the bones exhibit increased hydroxylysine-aldehyde deived cross-linking, and decreased lysine-aldehyde derived cross-linking. To obtain insight into the molecular mechanisms, we examined the expression levels of telopeptidyl lysyl hydroxylase 2 (LH2), and associated chaperone complexes. The results demonstrated that, while LH2 levels were unaffected by loss of Ift20, its chaperone, FKBP65, was significantly increased in Ift20:Wnt1-Cre and Ift20:Ocn-Cre mouse calvaria as well as femurs. These results suggest that IFT20 plays a pivotal role in collagen biosynthesis by regulating, in part, telopeptidyl lysine hydroxylation and cross-linking in bone. To the best of our knowledge, this is the first to demonstrate that the IFT components control collagen post-translational modifications. This provides a novel insight into the craniofacial bone defects associated with craniofacial skeletal ciliopathies.
Keywords: Craniofacial bone, Collagen, Intraflagellar transport, Mice, Post-translational modifications
Introduction
A fundamental role of intraflagellar transport (IFT) is to assemble primary cilia(1, 2). Once overlooked as an evolutionary vestige, primary cilia are now considered to be a critical organelles indispensable for regulating tissue development and homeostasis(3–5). In humans, mutations in ciliary genes can affect development of the skeletal system(6–9). We and others demonstrated that the IFTs are critical for regulating skeletogenic cell proliferation, survival and differentiation(10–15). However, at present, little is known about their roles in the biosynthesis of the organic matrix critical for regulating bone mineralization.
Bone is mainly composed of two phases, an organic matrix, principally fibrillar type I collagen, and inorganic mineral crystals. The minerals are encased within and around collagen fibrils in a highly organized manner, indicating that collagen controls spatial aspects of mineralization(16, 17). To perform this structural function, not only the quantity of collagen but also its quality, as determined in part by its post-translational modifications (PTMs), is vitally important. In the past, we proposed that the final collagen PTM, covalent intermolecular cross-linking, plays a key role in spatially organizing the mineral deposition and growth in bone(18–22). Recent studies have also demonstrated that mutations in genes encoding the collagen PTM enzymes and associated ER chaperones results in various types of recessive osteogenesis imperfecta (OI)(23–25). Lys hydroxylation of collagen, catalyzed by lysyl hydroxylases 1-3 (LH1-3), is a critical collagen PTM to determine the fate of cross-linking chemistry(26–28). Among the LH isoforms, LH2 (mostly a longer isoform of LH2, i.e. LH2b), which is encoded by the PLOD2 gene, is the only LH that is capable of hydroxylating Lys in the telopeptides, thus, critical for the formation of stable Hylald-derived cross-links(29, 30). This LH2-catalyzed PTM is associated with a number of diseases including Bruck syndrome/OI(31, 32), fibrosis(33, 34) and cancer metastasis(35–38). Several groups including ours have demonstrated that LH2 expression directs the cross-linking pathway and regulates matrix mineralization in vitro(29, 39, 40). Interestingly, both hypo- and hyper-LH2 activities resulted in defective mineralization, indicating that a specific level of LH2-catalyzed telopeptidyl modification and resulting cross-linking are necessary for proper bone mineralization(35, 39). This is also well exemplified in that OI cases can be caused by loss-, and gain-of-function of LH2(31, 41, 42). A series of recent studies have revealed that LH1 and 2 activities are regulated by a number of endoplasmic reticulum (ER) chaperone-complexes. LH1 is regulated by cyclophilin B, Synaptonemal Complex 65 (SC65), and prolyl 3-hydroxylase 3 (P3H3) (22, 43, 44), while LH2 is regulated by FKBP65(45), HSP47 and Bip(41). These components may control LH activities positively or negatively, ultimately leading to a specific cross-linking pattern that is critical for proper mineralization. However, at present, to the best of our knowledge, there is no study on the association of collagen PTMs with skeletal ciliopathies.
The aim of this study is to investigate the role of IFT20 in collagen biosynthesis in bone development. Our study may shed light on the pathogenesis of not only for skeletal ciliopathies, but also for other skeletal disorders related to abnormal collagen biosynthesis, including OI.
Materials and methods
Animals
The Animal Welfare Committee and the Institutional Animal Care and Use Committee of The University of Texas Medical School at Houston approved the experimental protocol. Ift20-floxed mice (#012565), Ocn-Cre mice (#019509), Wntl-Cre mice (#009107) and Rosa26 reporter mice (#007906) were obtained from the Jackson Laboratory.
Histology and immunohistochemistry
Picrosirius red staining was performed using 1% picrosirius red solution (Sigma-Aldrich; 365548 and P6744). FKBP65 (Proteintech; 12172-1-AP, 1:200) and EGFP (abcam; ab13970, 1:1,000) antibodies were used for immunostaining. Images were captured with an Olympus FluoView 1000 confocal microscope.
Micro-computed tomography (pCT) analysis
The distal femoral metaphyses were scanned using a μCT system at 90kV of energy and 88μA of intensity (CosmoScanGX: Rigaku corporation, Tokyo Japan). One hundred slices of metaphyses under the growth plate, constituting 1.0 mm in length, were selected and reconstructed to produce 2D and 3D images (Analyze12.0: AnalyzeDirect Inc., Overland Park, KS).
Cross-link analysis
Skull were harvested at E18.5 and p90, pulverized, demineralized with EDTA, reduced with standardized NaB3H4, acid hydrolyzed and subjected to amino acid and cross-link analyses as reported(46). The reducible cross-links, dehydro (deH)-dihydroxylysinonorleucine/its ketoamine, deH-hydroxylysinonorleucine/its ketoamine, and deH-histidinohydroxymerodesmosine were analyzed as their reduced forms, i.e., DHLNL, HLNL, and HHMD, respectively, and the mature trivalent cross-link, pyridinoline (Pyr), was simultaneously quantified by their fluorescence. All cross-links were quantified as mol mol−1 of collagen based on the value of 300 residues of hydroxyproline (Hyp) per collagen molecule. The Hyl content in collagen was calculated as Hyl Hyp−1 x 300. Results represent the mean values from triplicate biological samples in a single experiment.
Quantitative real-time RT-PCR
Total RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific; 15596-026). Quantitative RT-PCR was carried out using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad; 1725274). The conditions for qRT-PCR were 95°C for 2 min, 95°C for 5 sec, and 60°C for 30 sec, for 40 cycles. Primers for Fkbp10 were purchased (Bio-Rad, qMmuCID0009528). Primer sequences for Ift20 were 5’-TGTGGAGCTCAAGGAGGAGT-3’ and 5’-TGGCCTTCATCTTCTCGTTC-3’. Primer sequences for Plod2 were 5’-CATCCGAGAGTTCATTGCTCCAG-3’ and 5’-GCGCTGTCTTTCAGGTGAGTAC-3’. Primer sequences for Gapdh were 5’-CGTCCCGTAGACAAAATGGT-3’ and 5’-TCAATGAAGGGGTCGTTGAT-3’. Data were normalized to Gapdh levels and quantified using the 2−ΔΔCT method.
Western blotting analysis
Cell lysates from skull tissues were subjected to SDS-PAGE (Bio-Rad; 4561036). Anti-IFT20 (Proteintech; 13615-AP, 1:1,000), FKBP65 (Proteintech; 12172-1-AP, 1:1,000) anti-GAPDH (Cell Signaling technology; 14C10, 1:5,000), and Goat anti-rabbit IgG HRP-conjugate (Millipore sigma; 12-348, 1:5,000) antibodies were used for western blotting. The Clarity Max ECL Substrate (Bio-Rad; 1705061) was used for chemiluminescent detection, and the signals were quantified with the image-J software.
Statistical analysis
A two-tailed Student’s t test was used for the two groups. A p value of less than 0.05 was considered statistically significant.
Results and Discussion
Disruption of Ift20 results in craniofacial bone defects in the adults
To characterize the function of IFT-B complex in intramembranous bone formation in the face, we previously disrupted Ift20 in a neural crest-specific manner in mice (hereafter Ift20:Wnt1-Cre mice) and found that Ift20:Wnt1-Cre mice displayed craniofacial bone defects(11). Since Ift20:Wnt1-Cre mice died soon after birth due to the severe craniofacial abnormalities including cleft palate(11), this does not allow us to investigate the function of IFT20 in bones during postnatal and adult stages. In addition, while there is strong evidence that the primary cilia control embryonic bone development(47, 48), the focus on mice with IFT mutations has been on osteogenic proliferation and differentiation(6, 7). However, the role of IFT in biosynthesis of collagen, a key organizer of bone mineralization, is unknown. To address these questions, we utilized the osteocalcin-Cre driver to disrupt Ift20 in osteoblasts postnatally in mice (hereafter Ift20:Ocn-Cre mice). Consistent with craniofacial bone abnormalities observed in Ift20:Wnt1-Cre mice during embryogenesis(11), Ift20:Ocn-Cre mice displayed osteopenia-like phenotypes in skulls (Fig. 1A). Micro-CT analysis revealed that mineralization of trunk bones (e.g., femurs) was also attenuated in Ift20:Ocn-Cre mice (Supplemental Fig. 1). Picrosirius red staining in skull tissues further confirmed that the area of collagen matrices in Ift20:Ocn-Cre mice was smaller than that of controls (Fig. 1B), suggesting poor bone formation. These data suggest that, in addition to embryonic stages, IFT20 also plays a critical role in controlling bone formation in the adults.
Fig. 1. Disruption of Ift20 in mature osteoblasts causes bone defects in Ift20:Ocn-Cre mice.
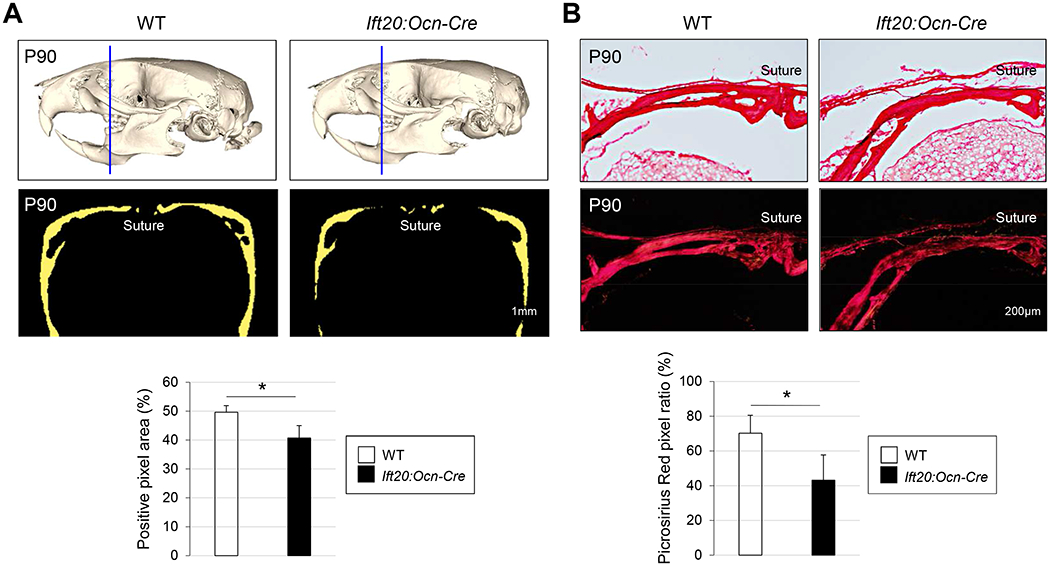
(A) Gross morphology of craniofacial bones were examined by Micro-CT (μCT) at post natal stage (P) P90 (upper panel). Thickness of skulls were quantified (lower panel). Blue lines represent the position of facial view for quantification. (B) Skull tissues were stained by Picrosirius red (upper panel). Signal strength of Picrosirius red was quantified (lower panel). Data in (A) and (B) are represented as mean ± SD; n=5 in each group. *P<0.05.
IFT20 plays a role in lysine (Lys) PTMs of collagen in bone maturations
To explore the role of IFT20 in bone formation, we biochemically characterized the major organic matrix, collagen, focusing on its post-translational modifications (PTMs) using skulls of Ift20:Ocn-Cre mice. The results shown in Fig 2 demonstrated that: (i) Collagen content (collagen/total proteins) (A) and Hyl content (Hyl/collagen) (B) were identical between WT and Ift20:Ocn-Cre mice; (ii) 26% and 64% more Hylald- derived cross-links, i.e. dihydroxylysinonorleucine (DHLNL) (C) and pyridinoline (Pyr) (D), respectively; (iii) In contrast, the Lysald-dederived cross-link, histidinohydroxymerodesmosine (HHMD) (E), was significantly lower in Ift20:Ocn-Cre mice by ~27%; and (iv) These changes in cross-links resulted in a significant increase by ~76% in the ratio of the Hylald- to Lysald-derived collagen cross-links in Ift20:Ocn-Cre mice (F). The HLNL cross-link (G) that can be derived from either Hylald or Lysald showed no difference between the WT and mutant groups (27). Interestingly, the total number of aldehydes involved in cross-linking showed no difference between the mutant and wild-type mice (H), indicating the major difference in cross-linking is the “type” not “quantity”. The cross-linking pattern of Ift20:Ocn-Cre bone collagen also indicated that telopeptidyl Lys in this mutant was overhydroxylated leading to increases in the stable Hylald-derived cross-links and a decrease in the Lysald-derived cross-link.
Fig. 2. Disruption of Ift20 results in abnormal PTMs in Ift20:Ocn-Cre mice.
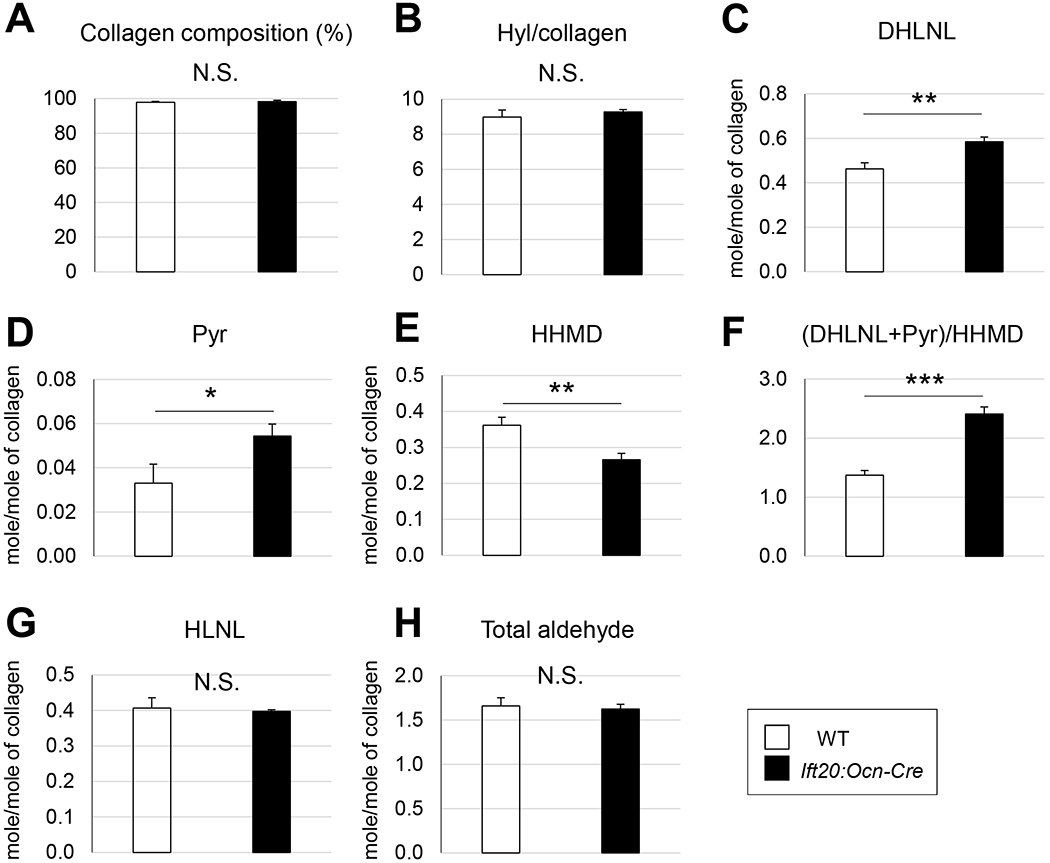
(A) Collagen composition (% of total proteins). (B) Hyl content in collagen calculated as Hyl/Hyp X300. (C) Dihydroxylysinonorleucine (DHLNL). (D) Pyridinoline (Pyr). (E) Histidinohydroxymerodesmosine (HHMD). (F) Ratio of the hydroxylysine-(DHLNL+Pyr) to lysine-aldehyde derived (HHMD) cross-links. (G) Hydroxylysinonorleucine (HLNL). (H) Total aldehydes involved in cross-linking. Data are represented as mean ± SD; n=4 in WT and n=3 in Ift20:Ocn-Cre mice. *P<0.05. **P<0.01. ***P<0.001.
To investigate whether these abnormal molecular phenotypes are also seen during embryonic stages, we analyzed skull tissues obtained from Ift20:Wnt1-Cre mice. The results were identical to those seen in the Ift20:Ocn-Cre mice, i.e., a significant increase in DHLNL and a decrease in HHMD when compared to controls (Supplemental Fig. 2). At this embryonic stage, a mature cross-link, Pyr, was below detection level. Together, these results suggest that IFT20 plays a critical role in collagen PTMs in craniofacial bone formation.
IFT20 modulates collagen biosynthesis via FKBP65 regulation
Since the cross-link analysis indicates that Ift20 disruption causes over-hydroxylation of telopeptidyl Lys, which may have caused the osteopenia-like phenotype (Fig. 1, Supplemental Fig. 1), we hypothesized that IFT20 may regulate the expression of the enzyme and/or its chaperone complex critical for telopeptidyl Lys hydroxylation. We first confirmed that ciliogenesis was completely disrupted in Ift20:Wnt1-Cre osteoblasts (Supplemental Fig. 3). Next, we analyzed the telopeptidyl LH, i.e. LH2, and associated chaperone complex(41, 45). The results showed, while the expression of LH2 encoded by Plod2 were comparable between WT and Ift20:Wnt1-Cre skulls, the expression of FKBP65 encoded by Fkbp10 was significantly increased in the latter (Fig. 3A). Protein levels of LH2 and FKBP65 were consistent with the respective gene expression (Fig. 3B). Other ER chaperone complex members such as HSP47 and Bip were comparable between WT and Ift20:Wnt1-Cre mice (Supplemental Fig. 4). To examine further whether or not the disruption of IFT20 is associated with increased FKBP65 expression in vivo, we superimposed ROSA26 EGFP reporter alleles in Ift20:Ocn-Cre mice. Consistent with our observation in Ift20:Wnt1-Cre mice (Fig. 3A, B), the levels of FKBP65 was significantly increased in a cell autonomous manner in Ift20:Ocn-Cre skulls (Fig. 3C). These results suggest that IFT20 plays a critical role in the regulation of FKBP65 expression presumably via a ciliary dependent function of IFT20 essential for regulating quality of craniofacial bone (Fig. 3D).
Fig. 3. IFT20 plays a role in the regulation of FKBP65 expression.
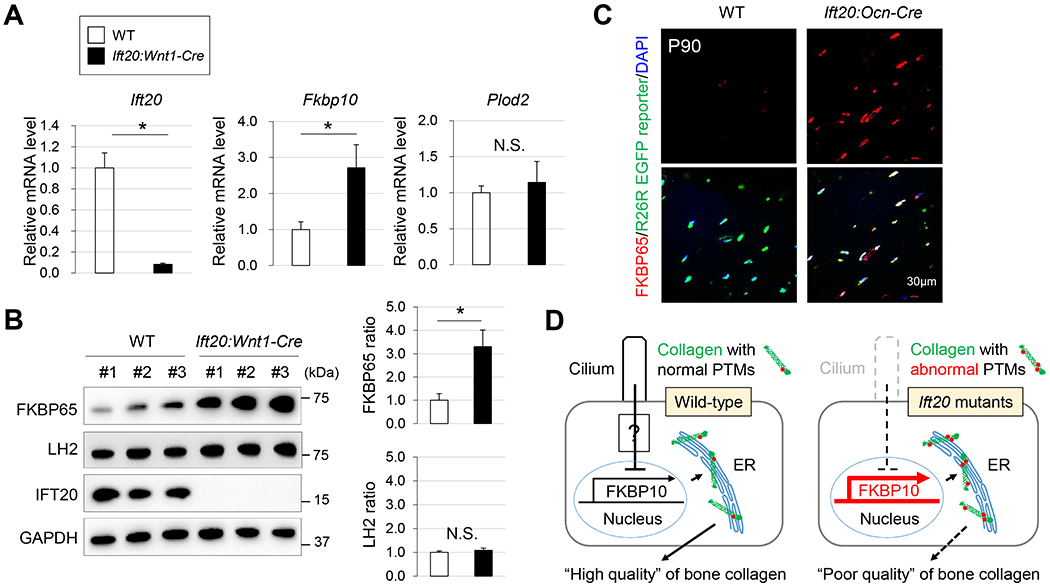
(A) qRT-PCR analysis for Ift20, and Fkbp10, and Plod2 in WT and Ift20 mutant osteoblasts from Ift20:Wnt1-Cre skull tissues. (B) Western blot analysis for FKBP65, LH2 and IFT20 from WT and Ift20:Wnt1-Cre skull tissues. (C) FKBP65 expression in Ift20:Ocn-Cre skull tissues was examined by immunohistochemistry. (D) A mechanism by which IFT20 controls the expression of PTM regulator (FKBP65) in bone formation. Data in (A) and (B) represented as mean ± SD; n=3 in each group. *P<0.05.
In summary, utilizing both Wnt1-Cre and Ocn-Cre drivers, we found that IFT20 regulates collagen biosynthesis in craniofacial and trunk bone formation. It would be of great interest to examine the cellular mechanisms by which ciliary-dependent signaling controls the expression of collagen PTM regulators in the future. Our study strongly indicates the importance of IFT to establish the bone matrix network, and may aid in uncovering the etiology of skeletal ciliopathies and, possibly, other craniofacial and bone diseases.
Supplementary Material
Highlights:
IFT20 regulates collagen biosynthesis in craniofacial and trunk bone formation.
IFT20 plays a pivotal role in collagen biosynthesis by regulating, in part, telopeptidyl lysine hydroxylation and cross-linking.
IFT20 plays a critical role in the regulation of FKBP65 expression presumably via a ciliary dependent function of IFT20.
Acknowledgments
We gratefully acknowledge Dr. Mark Corkins and Dr. Masaru Kaku for fruitful discussion. This study was supported in part by NIH/NIAMS R21AR060978 (M.Y.), NIDCR/NIH R01DE025897 (Y.K.) and by a fellowship from the Uehara Memorial Foundation (H.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors state that they have no conflicts of interest.
References
- 1.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL, A motility in the eukaryotic flagellum unrelated to flagellar beating. Proceedings of the National Academy of Sciences of the United States of America 90, 5519–5523 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum JL, Witman GB, Intraflagellar transport. Nature reviews. Molecular cell biology 3, 813–825 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Pazour GJ, Witman GB, The vertebrate primary cilium is a sensory organelle. Current opinion in cell biology 15, 105–110 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Corbit KC et al. , Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Huangfu D, Anderson KV, Cilia and Hedgehog responsiveness in the mouse. Proceedings of the National Academy of Sciences of the United States of America 102, 11325–11330 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serra R, Role of intraflagellar transport and primary cilia in skeletal development. Anatomical record 291, 1049–1061 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Yuan X, Serra RA, Yang S, Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Annals of the New York Academy of Sciences 1335, 78–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrill AE et al. , Ciliary abnormalities due to defects in the retrograde transport protein DYNC2H1 in short-rib polydactyly syndrome. American journal of human genetics 84, 542–549 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor SP et al. , Mutations in DYNC2LI1 disrupt cilia function and cause short rib polydactyly syndrome. Nature communications 6, 7092 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitami M et al. , IFT20 is required for the maintenance of cartilaginous matrix in condylar cartilage. Biochemical and biophysical research communications 509, 222–226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noda K, Kitami M, Kitami K, Kaku M, Komatsu Y, Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proceedings of the National Academy of Sciences of the United States of America 113, E2589–2597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M, Alharbi M, Graves D, Yang S, IFT80 Is Required for Fracture Healing Through Controlling the Regulation of TGF-beta Signaling in Chondrocyte Differentiation and Function. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 10.1002/jbmr.3902 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R, Development of the post-natal growth plate requires intraflagellar transport proteins. Developmental biology 305, 202–216 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haycraft CJ et al. , Intraflagellar transport is essential for endochondral bone formation. Development 134, 307–316 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Yuan X et al. , Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nature communications 7, 11024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver FH, Landis WJ, Deposition of apatite in mineralizing vertebrate extracellular matrices: A model of possible nucleation sites on type I collagen. Connective tissue research 52, 242–254 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Landis WJ, Jacquet R, Association of calcium and phosphate ions with collagen in the mineralization of vertebrate tissues. Calcified tissue international 93, 329–337 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Otsubo K, Katz EP, Mechanic GL, Yamauchi M, Cross-linking connectivity in bone collagen fibrils: the COOH-terminal locus of free aldehyde. Biochemistry 31, 396–402 (1992). [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi M, Katz EP, Otsubo K, Teraoka K, Mechanic GL, Cross-linking and stereospecific structure of collagen in mineralized and nonmineralized skeletal tissues. Connective tissue research 21, 159–167; discussion 168-159 (1989). [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi M, Katz EP, The post-translational chemistry and molecular packing of mineralizing tendon collagens. Connective tissue research 29, 81–98 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Shiiba M, Arnaud SB, Tanzawa H, Kitamura E, Yamauchi M, Regional alterations of type I collagen in rat tibia induced by skeletal unloading. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 17, 1639–1645 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Terajima M et al. , Cyclophilin B Deficiency Causes Abnormal Dentin Collagen Matrix. Journal of proteome research 16, 2914–2923 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Lim J, Grafe I, Alexander S, Lee B, Genetic causes and mechanisms of Osteogenesis Imperfecta. Bone 102, 40–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marom R, Lee YC, Grafe I, Lee B, Pharmacological and biological therapeutic strategies for osteogenesis imperfecta. Am J Med Genet C Semin Med Genet 172, 367–383 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Marini JC et al. , Osteogenesis imperfecta. Nat Rev Dis Primers 3, 17052 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi M, Barker TH, Gibbons DL, Kurie JM, The fibrotic tumor stroma. The Journal of clinical investigation 128, 16–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamauchi M, Sricholpech M, Lysine post-translational modifications of collagen. Essays in biochemistry 52, 113–133 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyre DR, Weis MA, Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcified tissue international 93, 338–347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer DK, Nicol PF, Kimbembe C, Robins SP, Identification, expression, and tissue distribution of the three rat lysyl hydroxylase isoforms. Biochemical and biophysical research communications 307, 803–809 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Piersma B, Bank RA, Collagen cross-linking mediated by lysyl hydroxylase 2: an enzymatic battlefield to combat fibrosis. Essays in biochemistry 63, 377–387 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Bank RA et al. , Defective collagen crosslinking in bone, but not in ligament or cartilage, in Bruck syndrome: indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. Proceedings of the National Academy of Sciences of the United States of America 96, 1054–1058 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gistelinck C et al. , Loss of Type I Collagen Telopeptide Lysyl Hydroxylation Causes Musculoskeletal Abnormalities in a Zebrafish Model of Bruck Syndrome. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 31, 1930–1942 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Slot AJ et al. , Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix biology : journal of the International Society for Matrix Biology 23, 251–257 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Uzawa K et al. , Altered posttranslational modifications of collagen in keloid. Biochemical and biophysical research communications 249, 652–655 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Chen Y et al. , Lysyl hydroxylase 2 induces a collagen cross-link switch in tumor stroma. The Journal of clinical investigation 125, 1147–1162 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisinger-Mathason TS et al. , Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer discovery 3, 1190–1205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilkes DM et al. , Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Molecular cancer research : MCR 11, 456–466 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Saito T et al. , Aberrant Collagen Cross-linking in Human Oral Squamous Cell Carcinoma. Journal of dental research 98, 517–525 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M, Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 19, 1349–1355 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Uzawa K et al. , Differential expression of human lysyl hydroxylase genes, lysine hydroxylation, and cross-linking of type I collagen during osteoblastic differentiation in vitro. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 14, 1272–1280 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Duran I et al. , A Chaperone Complex Formed by HSP47, FKBP65, and BiP Modulates Telopeptide Lysyl Hydroxylation of Type I Procollagen. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 32, 1309–1319 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindert U et al. , Molecular Consequences of the SERPINH1/HSP47 Mutation in the Dachshund Natural Model of Osteogenesis Imperfecta. The Journal of biological chemistry 290, 17679–17689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heard ME et al. , Sc65-Null Mice Provide Evidence for a Novel Endoplasmic Reticulum Complex Regulating Collagen Lysyl Hydroxylation. PLoS genetics 12, e1006002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cabral WA et al. , Abnormal type I collagen post-translational modification and crosslinking in a cyclophilin B KO mouse model of recessive osteogenesis imperfecta. PLoS genetics 10, e1004465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gjaltema RA, van der Stoel MM, Boersema M, Bank RA, Disentangling mechanisms involved in collagen pyridinoline cross-linking: The immunophilin FKBP65 is critical for dimerization of lysyl hydroxylase 2. Proceedings of the National Academy of Sciences of the United States of America 113, 7142–7147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terajima M et al. , Cyclophilin B control of lysine post-translational modifications of skin type I collagen. PLoS genetics 15, e1008196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singla V, Reiter JF, The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313, 629–633 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Eggenschwiler JT, Anderson KV, Cilia and developmental signaling. Annual review of cell and developmental biology 23, 345–373 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


