Abstract
The signaling nucleotide (p)ppGpp has been the subject of intense research in the past two decades. Initially discovered as the effector molecule of the stringent response, a bacterial stress response that reprograms cell physiology during amino acid starvation, follow-up studies indicated that many effects of (p)ppGpp on cell physiology occur at levels that are lower than those needed to fully activate the stringent response, and that the repertoire of enzymes involved in (p)ppGpp metabolism is more diverse than initially thought. Of particular interest, (p)ppGpp regulation has been consistently linked to bacterial persistence and virulence, such that the scientific pursuit to discover molecules that interfere with (p)ppGpp signaling as a way to develop new antimicrobials has grown substantially in recent years. Here, we highlight contemporary studies that have further supported the intimate relationship of (p)ppGpp with bacterial virulence and studies that provided new insights into the different mechanisms by which (p)ppGpp modulates bacterial virulence.
Keywords: (p)ppGpp, stringent response (SR), bacterial virulence, bacterial stress response, regulatory nucleotides
Introduction
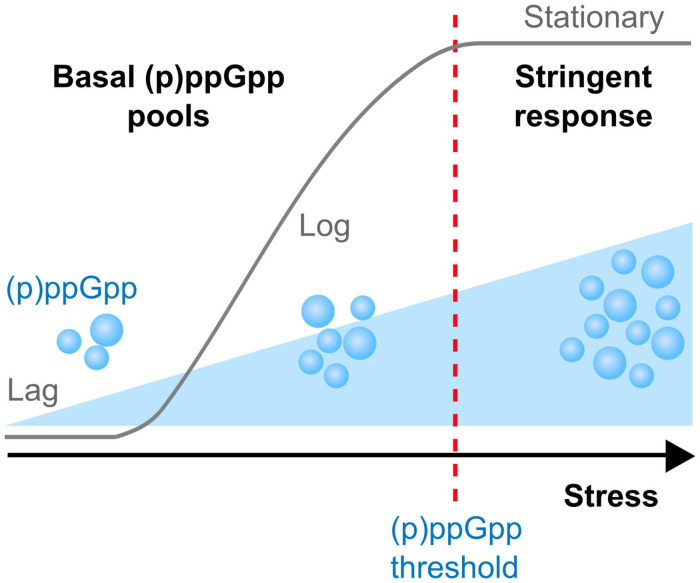
In response to changes in the surrounding environment, bacteria utilize a variety of sophisticated sensory mechanisms that reprogram cell physiology to facilitate adaptation to the new environment. Among those mechanisms are the production of signaling nucleotides such as (i) cAMP, the first regulatory nucleotide ever described, (ii) a growing family of cyclic nucleotides such as c-di-GMP, c-di-AMP, and cAMP-GMP, and (iii) hyperphosphorylated nucleotides, including (p)ppGpp and newly described analogs pGpp and (p)ppApp (Potrykus and Cashel, 2008; Kalia et al., 2013; Gaca et al., 2015b; Yang et al., 2019, 2020; Fung et al., 2020; Jimmy et al., 2020). The commonly used (p)ppGpp abbreviation indicates two guanosine derivatives – ppGpp (GDP, 3′-diphosphate) and pppGpp (GTP, 3′-diphosphate) – initially known as the magic spot or nutritional alarmone (Cashel and Kalbacher, 1970; Potrykus and Cashel, 2008). Seminal studies discovered that in response to stress conditions, (p)ppGpp rapidly accumulates to high levels within the cell and reprograms cell physiology through transcriptional and allosteric mechanisms that ultimately reallocate cellular resources from an active growth state toward a semi-dormant state (Potrykus and Cashel, 2008). When fully active, this process termed the stringent response (SR), has been shown to lower the activity of metabolic pathways associated with rapid cell growth, while activating pathways associated with nutrient uptake, amino acid biosynthesis and stress survival thereby facilitating cell survival under severe adverse conditions (Potrykus and Cashel, 2008; Kanjee et al., 2012; Gaca et al., 2015a). While initially discovered as a response to amino acid starvation, the SR was also shown to be induced by non-nutritional stresses such as heat stress and antibiotics (Gallant et al., 1977; Glass et al., 1979; Wells and Gaynor, 2006; Hobbs and Boraston, 2019; Schafer et al., 2020). In addition to acting as the effector molecule of the SR, contemporary studies revealed that (p)ppGpp plays a fundamental role in the control of core cellular processes at concentrations that are well below those needed to activate the SR (Figure 1). Indeed, during balanced (non-stressed) conditions, relatively small fluctuations in basal (p)ppGpp pools were shown to influence transcription of hundreds of genes, with a complete loss of (p)ppGpp regulation impairing cell fitness even in the absence of stress (Gaca et al., 2013; Colomer-Winter et al., 2019; Sanchez-Vazquez et al., 2019; Fernandez-Coll et al., 2020; Pletzer et al., 2020). Given the critical role of (p)ppGpp in cell physiology, it is not surprising that several studies have implicated (p)ppGpp with bacterial virulence and antibiotic tolerance (Table 1 and Figure 2). Specifically, the production of (p)ppGpp has been associated with expression of virulence traits, which includes but is not limited to adhesion, biofilm formation, toxin production, motility, sporulation, and antibiotic tolerance (Dalebroux et al., 2010). Importantly, several of those studies indicate that the intimate relationship of (p)ppGpp with bacterial persistence and virulence might be more closely linked to basal levels of (p)ppGpp than to those needed to activate the SR. In 2010, Dalebroux and colleagues published a comprehensive review linking (p)ppGpp to bacterial virulence in plants, animals and humans (Dalebroux et al., 2010). Here, we will highlight recent studies that have further supported the intimate relationship of (p)ppGpp with bacterial virulence, with an emphasis on studies that directly tested (p)ppGpp-deficient strains in animal infection models that are relevant to human infectious diseases. For historical and contemporary perspectives of other aspects of the field, we direct the reader to other reviews (Dalebroux and Swanson, 2012; Gaca et al., 2015a; Hauryliuk et al., 2015; Liu et al., 2015; Steinchen and Bange, 2016; Ronneau and Hallez, 2019; Zhu et al., 2019b).
FIGURE 1.
(p)ppGpp modulates bacterial physiology at all growth stages. During adaptation (lag) and exponential (log) growth phases in the absence of stresses, basal levels of (p)ppGpp are important to maintain a balanced metabolism avoiding uncontrolled consumption of energy stores and toxic accumulation of metabolic byproducts. Entry into stationary growth phase or adverse condtions such as nutrient starvation, heat shock or selected antibiotic stresses triggers the accumulation of (p)ppGpp that rapidly reaches the threshold necessary to mount the SR responsible for remodeling cell physiology from a growth mode to a survival mode.
TABLE 1.
Virulence of (p)ppGpp-defective mutants.
| Bacterial Pathogen | Strain | Basal (p)ppGpp | Stringent response | Animal Model/Virulence | References |
| Acinetobacter baumanii | ΔrelA | Yes | No | G. mellonella: attenuated | Perez-Varela et al. (2020) |
| Bacillus anthracis | Δrel | Yes | No | Mouse subcutaneous: ND1 | van Schaik et al. (2007) |
| Borrelia burgdorferi | Δrel | No | No | Mouse intradermal: avirulent | Bugrysheva et al. (2005) |
| Brucella melitensis | Δrel | No | No | Mouse systemic: attenuated | Dozot et al. (2006) |
| Burkholderia pseudomallei | ΔrelAΔspoT | No | No | G. mellonella and mouse: attenuated | Muller et al. (2012) |
| Enterococcus faecalis | Δrel | Yes | No | G. mellonella or C. elegans: ND; Mouse CAUTI: ND; Rabbit abscess: ND; Rabbit endocarditis: attenuated | Abranches et al. (2009); Yan et al. (2009), Gaca et al. (2012); Frank et al. (2014), Colomer-Winter et al. (2018, 2019) |
| ΔrelQ | Yes | Yes | G. mellonella or C. elegans: ND; Mouse CAUTI: ND; Rabbit abscess: ND; Rabbit endocarditis: ND | Abranches et al. (2009); Gaca et al. (2012), Frank et al. (2014); Colomer-Winter et al. (2018),Colomer-Winter et al. (2019) | |
| ΔrelΔrelQ | No | No | G. mellonella or C. elegans: attenuated; Mouse CAUTI: attenuated; Rabbit abscess: attenuated; Rabbit endocarditis: ND | Abranches et al. (2009); Gaca et al. (2012), Frank et al. (2014); Colomer-Winter et al. (2018),Colomer-Winter et al. (2019) | |
| Francisella novocida | ΔrelA | Yes | No | Mouse (intranasal): attenuated | Dean et al. (2009) |
| Francisella tularensis | ΔrelAΔspoT | No | No | Mouse (intranasal): avirulent | Charity et al. (2009); Ma et al. (2019) |
| Haemophilus ducreyi | ΔrelAΔspoT | No | No | Human pustule: attenuated | Holley et al. (2014) |
| Listeria monocytogenes | Δrel | Yes | No | Mouse systemic: avirulent | Taylor et al. (2002), Bennett et al. (2007) |
| rel:Tn | ? | ? | Mouse systemic: attenuated | Taylor et al. (2002) | |
| ΔrelΔrelPΔrelQ | No | No | Mouse systemic: attenuated | Whiteley et al. (2015) | |
| Mycobacterium tuberculosis | relH344Y | Yes | No | Mouse lung: attenuated | Weiss and Stallings (2013) |
| relH80A | Yes | Yes | Mouse lung: attenuated | Weiss and Stallings (2013) | |
| ΔrelΔSAS | No | No | Mouse lung: attenuated | Weiss and Stallings (2013) | |
| Δrel | Yes | No | Mouse lung and guinea pig lung: attenuated | Dahl et al. (2003); Klinkenberg et al. (2010) | |
| Pseudomonas aeruginosa | ΔrelA | Yes | No | D. melanogaster: attenuated; Mouse abscess: ND | Vogt et al. (2011); Pletzer et al. (2017) |
| ΔrelAΔspoT | No | No | D. melanogaster, Mouse pneumonia: avirulent/attenuated; Mouse abscess/skin: attenuated | Vogt et al. (2011); Xu et al. (2016), Pletzer et al. (2017) | |
| Salmonella Gallinarum | ΔrelAΔspoT | No | No | Chicken (oral): attenuated | Park et al. (2010) |
| Salmonella Typhi | ΔrelAΔspoT | No | No | Mouse (oral): avirulent | Dasgupta et al. (2019) |
| Salmonella Typhimurium | ΔrelA | Yes | No | Mouse (intragastric): attenuated | Pizarro-Cerda and Tedin (2004) |
| spoT-Δctd | Yes | Yes | Mouse (oral): attenuated | Fitzsimmons et al. (2020) | |
| ΔrelAΔspoT | No | No | Mouse (intragastric): avirulent | Pizarro-Cerda and Tedin (2004) | |
| Staphylococcus aureus | relSaF128Y | High | Yes | G. mellonella: attenuated | Gao et al. (2010) |
| relSasyn | Yes | No | Mouse kidney: attenuated; Mouse skin: attenuated | Geiger et al. (2010); Mansour et al. (2016) | |
| ΔrelP | Yes | Yes | Rabbit endocarditis: ND | Li et al. (2020) | |
| Streptococcus pneumoniae | ΔrelSpn | Yes | No | Mouse pneumonia: attenuated | Kazmierczak et al. (2009) |
| Streptococcus suis | ΔrelΔrelQ | No | No | Mouse systemic: avirulent | Zhu et al. (2016) |
| Vibrio cholerae | ΔrelA | Yes | No | Suckling mouse: attenuated/ND 2; Rabbit ileal loop: attenuated | Haralalka et al. (2003) |
| ΔrelAΔspoT | Yes | No | Infant mouse: ND | Oh et al. (2014) | |
| ΔrelAΔspoTΔrelV | No | No | Infant mouse: attenuated | Oh et al. (2014) | |
| Yersinia pestis | ΔrelA | Yes | No | Mouse: ND | Sun et al. (2009) |
| ΔrelAΔspoT | No | Yes | Mouse: attenuated | Sun et al. (2009) |
ND, no differences when compared to parent strain. Conflicting data from different laboratories.
FIGURE 2.
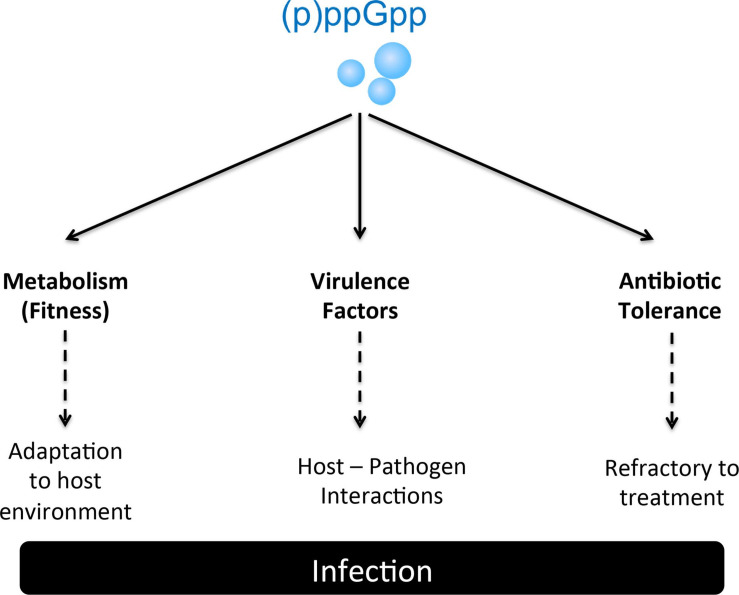
(p)ppGpp participates in the regulation of several processes critical to bacterial pathogenesis. The roles of (p)ppGpp in adaptation to the host environment and antibioric tolerance are likely universal whereas the association with the expression of virulence factors appears to be species-specific.
Gram-Positive Pathogens
Most of the Gram-positive bacteria that cause disease in humans belong to the Firmicutes phylum, which is primarily comprised of bacteria with a low-GC content. In Firmicutes, (p)ppGpp metabolism is primarily controlled by the bifunctional synthetase/hydrolase Rel enzyme, also known as RelA or Rsh (for RelA SpoT homolog) (Atkinson et al., 2011). The use of different designations for the (p)ppGpp synthetase/hydrolase of Gram-positive bacteria has created confusion in the field, since the Gram-negative RelA protein (originally described in Escherichia coli) is a monofunctional synthetase without hydrolase activity. Though some studies might have originally referred to the bifunctional (p)ppGpp synthetase/hydrolase of Gram-positive bacteria as RelA or Rsh, herein, we will adopt the nomenclature proposed by Atkinson and colleagues (Atkinson et al., 2011) and refer to the bifunctional Gram-positive enzyme as Rel.
While Rel-dependent (p)ppGpp synthesis is primarily responsible for activation of the SR in all Gram-positive bacteria investigated to date, the (p)ppGpp hydrolase activity of this enzyme is essential to some species by avoiding the accumulation of (p)ppGpp to toxic levels (Geiger et al., 2010; Weiss and Stallings, 2013; Gratani et al., 2018; Ronneau and Hallez, 2019). In addition to Rel, Firmicutes express one or two short/small (p)ppGpp synthetases (SASs), termed RelP and RelQ (known as YjbM and YwaC, respectively, in Bacillus subtilis) (Atkinson et al., 2011). All SASs appear to be primarily involved in maintainence of basal (p)ppGpp pools during growth, though have been also linked to a timely activation of the SR (Gaca et al., 2012) and to cell envelope stress tolerance (Geiger et al., 2014; Bhawini et al., 2019). While the majority of Firmicutes encode both RelP and RelQ, few species including Streptococcus pneumoniae, Streptococcus suis and all members of the Enterococcus genus only encode RelQ (Atkinson et al., 2011). Below, we review studies that, either through the use of animal models, clinical evidence, or both, provided conclusive evidence of the association of (p)ppGpp with Gram-positive bacterial virulence. When discussing these studies it is important to mention that different than Gram-negative bacteria, (p)ppGpp does not control global gene transcription by physically interacting with the RNAP and by partnering with the transcriptional regulator DksA (Wolz et al., 2010). Instead, (p)ppGpp indirectly affects Gram-positive gene transcription through modulation of intracellular purine concentrations thereby changing the availability of initiating nucleotides of transcription (Krasny and Gourse, 2004; Krasny et al., 2008; Kriel et al., 2012). In addition, the sharp drop in GTP and concomitant increase in ATP directly affects activity of CodY, a nutrient-sensing transcriptional regulator that is widespread in Firmicutes and that controls activation of nutrient-acquisition and nutrient biosynthesis pathways and virulence determinants (Sonenshein, 2005). Because CodY is a GTP-responsive regulator, there is an inverse relationship between (p)ppGpp and CodY where the accumulation of (p)ppGpp typically leads to alleviation of CodY regulation (Geiger and Wolz, 2014). As a result, the (p)ppGpp and CodY networks are intertwined, with several reports linking both regulatory networks to bacterial virulence and, in most cases, having an antagonistic relationship (Geiger and Wolz, 2014). In addition to CodY, (p)ppGpp control of bacterial physiology is also exherted through direct interaction with enzymes and riboswitches (Kanjee et al., 2012; Sherlock et al., 2018).
Staphylococcus aureus
A common inhabitant of the human upper respiratory tract, S. aureus is also a major threat to public health as they can cause a wide range of opportunistic infections that range from mild skin infections to life-threatening pneumonia, osteomyelitis, endocarditis and sepsis. In S. aureus, (p)ppGpp is metabolized by the bifunctional RelSa (RshSa) and the monofucntional RelP and RelQ. While the RelSa synthetase was dispensable, its hydrolase activity was found to be essential in the presence of functional RelP and RelQ by preventing toxic accumulation of (p)ppGpp due to the activities of SASs (Geiger et al., 2010). The contribution of (p)ppGpp and the SR to staphylococcal virulence was first demonstrated in a synthetase-dead/hydrolase-active Rel mutant strain (relsyn), where loss of RelSa synthetase activity significantly attenuated S. aureus virulence in a mouse model of kidney infection (Geiger et al., 2010). Follow-up studies testing this mutant confirmed that Rel-dependent (p)ppGpp accumulation was also required for lesion formation in a cutaneous mouse model (Mansour et al., 2016). Notably, virulence of the relsyn mutant was restored by deletion of codY, providing clear evidence of the (p)ppGpp-CodY relationship during infection (Geiger et al., 2010). Subsequent studies revealed that most genes activated by (p)ppGpp in S. aureus were under CodY control (Geiger et al., 2012), indicating that activation of the SR (i.e., high levels of (p)ppGpp) promotes virulence by, at least in part, alleviation of CodY regulation (Geiger et al., 2010, 2012). In addition to virulence, the SR has been associated to antibiotic tolerance (often referred as antibiotic persistence). For instance, a drug-resistant clinical isolate obtained from a persistent bloodstream infection treated with the protein synthesis inhibitor linezolid was shown to harbor point mutations in the relSa gene that led to constitutive activation of the SR (Gao et al., 2010).
In addition to activation of the SR, more recent studies indicated that basal levels of (p)ppGpp (below those needed to activate the SR) contribute to antibiotic tolerance. Specifically, point mutations on the relSa gene lowered the (p)ppGpp hydrolase activity of RelSa causing a slight increase in basal (p)ppGpp pools that was linked to broad antibiotic tolerance (Mwangi et al., 2013; Bryson et al., 2020). In another study, the relPSa and relQSa genes were shown to be strongly induced upon treatment with cell wall-active antibiotics (ampicillin and vancomycin) and simultaneous inactivation of both genes significantly decreased tolerance of S. aureus toward these antibiotics (Geiger et al., 2014). In a more recent study, the association of SASs with antibiotic tolerance was traced to RelQ as inactivation of relQSa decreased tolerance to β-lactam antibiotics in methicillin-resistant S. aureus (MRSA) by interfering with mecA gene expression, which codes for an alternative penicillin-binding protein (Bhawini et al., 2019). Interestingly, the importance of RelQSa in β-lactam tolerance could be bypassed by activation of the SR with mupirocin (an isoleucine analog/iletRNA inhibitor that triggers the SR), suggesting that mecA transcription requires a certain (p)ppGpp threshold (Bhawini et al., 2019). Unexpectedly, single inactivation of relPSa increased β-lactam resistance, which the authors attributed to a sizeable increase in the expression of the relQSa promoter in the ΔrelP strain (Bhawini et al., 2019). MRSA isolates from a clinical case with high tolerance to vancomycin displayed high levels of (p)ppGpp, high expression of cytotoxic PSMs (phenol-soluble modulins), increased PMN lysis and intracellular survival, and enhanced adherence to fibronectin/endothelial cells in in vitro assays (Li et al., 2020). Interestingly, transcription activation of PSMs was found to be both (p)ppGpp- and CodY-independent (Geiger et al., 2012). Finally, virulence of the relP mutant was not affected in a rabbit infective endocarditis (IE) model but treatment of the heart vegetation colonized by the relP mutant with vancomycin reduced spread in the cardiac vegetation and dissemination to other tissues (Li et al., 2020). Collectively, these studies reveal that the SR and CodY mediate S. aureus virulence and antibiotic tolerance, but also indicate that basal levels of (p)ppGpp, primarily mediated by RelP and RelQ, play a role in supporting staphylococcal cell attachment, survival to immune cells and antibiotic tolerance. In the future, it will be important to determine the relevance of (p)ppGpp and the SR in other types of infection and evaluate the virulence potential of a triple ΔrelΔrelPΔrelQ mutant [(p)ppGpp0 strain].
Enterococci
A natural inhabitant of the gastrointestinal tract of animals, enterococci are among the leading causes of life-threatening nosocomial infections (Arias and Murray, 2012). While E. faecalis accounts for the majority of enterococcal infections in humans (∼75%), E. faecium responds for the majority of vancomycin resistant enterococci (VRE) infections. The genome of E. faecalis encodes a bifunctional Rel (RelEf) and a single SAS termed RelQEf. Similar to S. aureus, RelEf is the major enzyme responsible for (p)ppGpp accumulation and activation of the SR (Abranches et al., 2009; Gaca et al., 2012), an observation that has been confirmed with all other Gram-positive bacteria that have been studied to date. In the past decade, the importance of (p)ppGpp and the SR to E. faecalis pathophysiology has been probed to some detail by our group. Collectively, our studies indicate that the association of (p)ppGpp with virulence does not necessarily relate to activation of the SR but to basal levels of (p)ppGpp (Abranches et al., 2009; Gaca et al., 2012, 2013). Specifically, we and others showed that the consequences of single inactivation of relEf or relQEf to E. faecalis pathophysiology are for the most part negligible or fairly modest (Yan et al., 2009; Frank et al., 2014; Colomer-Winter et al., 2018, 2019) whereas a ΔrelΔrelQ double mutant [(p)ppGpp0] strain showed multiple phenotypes including impaired survival within the macrophage cell line J774.A1, growth/survival defects in human fluids (blood and urine) ex vivo, and attenuated virulence in invertebrate (Caenorhabditis elegans and Galleria mellonella) and vertebrate [rabbit subdermal abscess and mouse (CAUTI) catheter-associated urinary tract infection] models (Abranches et al., 2009; Gaca et al., 2012; Frank et al., 2014; Colomer-Winter et al., 2019). Through transcriptome and biochemical analyses, the defective phenotypes of the (p)ppGpp0 strain were traced to a metabolic dysregulation that resulted in toxic accumulation of endogenously produced reactive oxygen species (ROS) and an impaired metal homeostasis (Gaca et al., 2013; Colomer-Winter et al., 2017). In several cases, growth/survival defects of the (p)ppGpp0 strain could be rescued by addition of glutathione or manganese supplementation, which either directly or indirectly mitigate ROS damage (Colomer-Winter et al., 2017). In agreement with previous findings obtained with other Firmicutes (Bennett et al., 2007; Geiger et al., 2012; Whiteley et al., 2015), inactivation of codY restored the virulence of the (p)ppGpp0 strain in the G. mellonella and mouse CAUTI models (Colomer-Winter et al., 2017, 2019). Still, the molecular mechanisms by which (p)ppGpp supports virulence in this species cannot be solely attributed to association of (p)ppGpp with the CodY regulon as (i) basal levels of (p)ppGpp that are unlikely to interfere with CodY activity are more relevant to enterococcal pathogenesis than the SR, and (ii) (p)ppGpp controls several genes in a CodY-independent manner during active growth and during the SR (Gaca et al., 2012; Colomer-Winter et al., 2019).
Similar to clinical observations made with S. aureus persistent infections (see text above), whole genome sequencing identified a single missense mutation in the RelEfm gene of an antibiotic resistant E. faecium that was isolated from a bacteremia case (Honsa et al., 2017). This SNP resulted in constitutively high levels of ppGpp directly linking (p)ppGpp to antibiotic tolerance in E. faecium. Interesting, this mutation did not change the minimum inhibitory concentration (MIC) for several antibiotics under in vitro conditions, including antibiotics that had been administered to the infected patient (Honsa et al., 2017). Collectively, the picture that emerges is that, similarly to staphylococci, increases in (p)ppGpp basal pools can be directly linked to antibiotic tolerance without necessarily conferring antibiotic resistance.
Streptococci
The genus Streptococcus harbors some of the most commonhuman and animal pathogens. Even though seminal studies that helpedshape our understanding of how (p)ppGpp is metabolized in Gram-positive bacteria were performed with streptococci, from the first insights into the intramolecular regulation of the two catalytic domains of the Rel enzyme (Mechold et al., 2002; Sajish et al., 2007) to the discovery of SASs (Lemos et al., 2007), very few studies directly probed the significance of (p)ppGpp (and the SR) to streptococcal pathogenesis. In S. pneumoniae, which only harbors a single SAS (RelQSp), inactivation of the bifunctional relSp supported that RelSp is the primary source of (p)ppGpp and fully responsible for SR activation (Kazmierczak et al., 2009). Moreover, RelSp was shown to play a major role in disease progression in a pneumonia mouse model, and was linked to induction of the ply operon, which encodes the pneumolysin toxin involved in early infection and tissue invasion (Kazmierczak et al., 2009). However, the exact mechanism by which RelSpn/(p)ppGpp regulates this virulence factor remains to be elucidated. In S. suis, an important pathogen of pigs, a (p)ppGpp0 strain lacking both relSs and relQSs displayed a number of phenotypic defects that can be linked to S. suis pathogenesis including decreased capacity to adhere and invade Hep-2 cells, lower survival in whole blood and decreased anti-phagocytic capacity (Zhu et al., 2016). Not surprisingly, virulence of the S. suis (p)ppGpp0 strain was attenuated in a systemic infection mouse model (Zhu et al., 2016). However, different than S. aureus, E. faecalis, and Listeria monocytogenes (see below), the S. suis ΔrelΔrelQΔcodY triple mutant phenocopied the ΔrelΔrelQ double mutant in the mouse infection model (Zhu et al., 2019a). Moreover, CodY was shown to interact with the RelSs promoter in a GTP-independent manner, suggesting a new mechanism of (p)ppGpp and CodY crosstalk.
Listeria monocytogenes
Listeria monocytogenes is an intracellular foodborne pathogen and the causative agent of human listeriosis (Radoshevich and Cossart, 2018). Similar to S. aureus and Streptococcus mutans, the genome of L. monocytogenes encodes a bifunctional RelLm and the SASs RelPLm and RelQLm. In one of the first studies to provide direct evidence of the association of (p)ppGpp with bacterial virulence, a transposon insertion library identified relLm as an essential gene in a murine model of listeriosis (Taylor et al., 2002). In a study that explored the relationship between (p)ppGpp and CodY, the attenuated virulence of a ΔrelLm strain could be partially linked to continued CodY-dependent repression as inactivation of codY in the ΔrelLm background strain (ΔrelLmΔcodY) partially restored L. monocytogenes virulence (Bennett et al., 2007). However, the reduced survival of the ΔrelLm strain in culture cell lines was not restored in the ΔrelLmΔcodY strain (Bennett et al., 2007). Follow up studies demonstrated that virulence of a triple mutant strain lacking all three (p)ppGpp synthetases (ΔrelLmΔrelPLmΔrelQLm) was severely attenuated in an in vitro plaque assay (used as a surrogate for Listeria virulence), and in a mouse infection model (Whiteley et al., 2015). Similar to what was observed with the single ΔrelLm strain (Bennett et al., 2007), inactivation of codY partially restored virulence of the (p)ppGpp0 strain (Whiteley et al., 2015) collectively suggesting that (p)ppGpp synthesis promotes virulence in L. monocytogenes in a CodY-dependent manner. Of note, L. monocytogenes was the first bacterial pathogen to identify a regulatory crosstalk between the (p)ppGpp, CodY, and cyclic diadenosine monophosphate (c-di-AMP) signaling pathways (Whiteley et al., 2015).
Bacillus anthracis
The spore-forming Bacillus anthracis is the etiological agent of anthrax (Pilo and Frey, 2018). In B. anthracis, activation of the SR, mediated by RelBa, was linked to sporulation but did not affect expression of virulence factors or virulence in a subcutaneous infection mouse model (van Schaik et al., 2007). It should be noted that B. anthracis as well as other Bacillus species also encode RelP and RelQ (YjbM and YwaC, respectively) such that inactivation of relBa alone can be anticipated to abolish activation of the SR but not basal production of (p)ppGpp. Based on evidence from other Firmicutes, there is a great likelihood that loss of RelBa hydrolase activity in the presence of active RelP and/or RelQ results in a SR-defective strain with high basal levels of (p)ppGpp. Given the mounting evidence that the association of (p)ppGpp with virulence goes beyond activation of the SR, it will be important to assess the virulence potential of a B. anthracis triple ΔrelΔrelPΔrelQ mutant [(p)ppGpp0] strain in future investigations.
Gram-Negative Pathogens
The textbook description of how (p)ppGpp controls bacterial physiology largely derives from studies conducted with the Gram-negative paradigm E. coli. In this group of bacteria, (p)ppGpp regulates transcription through direct interaction with the interface of the β′ and ϖ subunits of the RNAP (Ross et al., 2013). Of note, most transcriptional effects triggered by artificially induced early (p)ppGpp accumulation in the absence of stress were recently shown to be caused by (p)ppGpp-RNAP interaction (Sanchez-Vazquez et al., 2019). Moreover, many of the targets subjected to allosteric regulation by (p)ppGpp including proteins involved in translation, DNA replication and purine biosynthesis were fisrt identified in E. coli (Dalebroux and Swanson, 2012). In the absence of clinical reports or in vivo studies clearly linking (p)ppGpp regulation to E. coli virulence, the bulk of our understanding of the importance of (p)ppGpp to the virulence of Gram-negative pathogens derives from studies conducted with other Gammaproteobacteria such as Pseudomonas aeruginosa, Salmonella enterica, and Vibrio cholerae (Table 1). Different than Firmicutes, (p)ppGpp is metabolized by a monofunctional synthetase (RelA) and a bifunctional synthetase/hydrolase (SpoT) in Gammaproteobacteria (Atkinson et al., 2011). In all organisms with the RelA and SpoT enzyme arrangement, RelA has strong enzymatic activity and is the primary driver of the SR while SpoT has a strong hydrolase activity and weak synthetase activity that is triggered by specific conditions including iron and fatty acid starvation (Vinella et al., 2005; Battesti and Bouveret, 2006). The exception among Gammaproteobacteria, V. cholerae encodes a third (p)ppGpp synthetase, which is a SAS-like monofuntional enzyme named RelV (Das et al., 2009). Different than Gammaproteobacteria, Alphaproteobacteria such as Brucella sp. and Epsilonproteobacteria such as Helicobacter pylori encode a single bifunctional (p)ppGpp synthetase/hydrolase known as Rsh, Rel or SpoT, that seems to be functionally analog to the Gram-positive Rel enzyme as it possess equally strong synthetase and hydrolase activities (Atkinson et al., 2011). While there are numerous studies linking (p)ppGpp to virulence expression in Gram-negative pathogens, many covered in a previous review (Dalebroux et al., 2010), the sections below will focus on recent studies that have directly linked (p)ppGpp to Gram-negative bacterial virulence using animal infection models.
Salmonella
Salmonella enterica is responsible for a variety of human infections ranging from gastroenteritis to typhoid fever. Early studies with (p)ppGpp0 strains (ΔrelAΔspoT) of S. enterica serovar Typhimurium, Typhi, and Gallinarum clearly demonstrated the essentiality of (p)ppGpp to Salmonella pathogenesis (Pizarro-Cerda and Tedin, 2004; Jeong et al., 2008; Park et al., 2010; Dasgupta et al., 2019). Interestingly, (p)ppGpp was found to regulate the expression of Salmonella pathogenicity islands 1 (SPI-1) and 2 (SPI-2), which are required for Salmonella virulence (Pizarro-Cerda and Tedin, 2004; Song et al., 2004). Follow up RNA sequencing (RNAseq) analysis revealed that transcription of more than 30% of the Salmonella coding regions and approximately 20% of non-coding regions was affected by fluctuations in (p)ppGpp levels (Ramachandran et al., 2012), further confirming the far-reaching scope of (p)ppGpp regulation. To better understand the contributions of RelA and SpoT, a recent study characterized a spoT mutant (spoTΔctd strain), unable to synthesize (p)ppGpp via SpoT due to the deletion of the C-terminal domain (ctd) regulatory element of the enzyme without affectting its hydrolase activity, and revealed that RelA was the primary enzyme responsible for nutrient, nitrosative and oxidative stresses, while (p)ppGpp synthesized by SpoT was important for adaptation and survival within phagocytes (Fitzsimmons et al., 2020). More specifically, the spoTΔctd strain failed to induce SPI-2 genes in response to the acidic pH of the phagosome, had a major defect in cation metal uptake, and was highly attenuated in a murine model of acute salmonellosis (Fitzsimmons et al., 2020). Thus, in addition to its well-recognized role in environmental stress adaptation, (p)ppGpp appears to also function as an intracellular signal for Salmonella virulence. Finally, single immunization of mice with a live (p)ppGpp0 strain elicited both systemic and mucosal antibody responses and protected vaccinated animals from a subsequent challenge with a lethal dose of wild-type S. typhimurium (Na et al., 2006). Given the high potential of targeting (p)ppGpp metabolism for the development of anti-infective therapies, additional studies are warranted to determine the distinct roles of RelA, SpoT, and of basal (p)ppGpp pools in Salmonella.
Pseudomonas aeruginosa
This environmental organism is well known for its remarkable ability to form biofilms on different types of surfaces, intrinsic and acquired tolerance to multiple antibiotics, and association with chronic lung infections in cystic fibrosis patients, burn wound infections and serious nosocomial infections (Gellatly and Hancock, 2013). In the absence of RelA, cells treated with the serine analog serine hydroxamate failed to accumulate (p)ppGpp, confirming previous observations that RelA is responsible for activation of the SR during amino acid starvation (Erickson et al., 2004). While virulence of ΔrelA and ΔrelAΔspoT [(p)ppGpp0] mutant strains was significantly attenuated in a Drosophila melanogaster feeding model of infection (Erickson et al., 2004), only the double mutant showed loss of virulence in a rat lung agar bead and in a murine skin infection model (Vogt et al., 2011; Pletzer et al., 2017). Furthermore, basal levels of (p)ppGpp was linked to expression of important virulence determinants such as alginate, type-three secretion system (T3SS), pyocyanin, proteases, siderophores, swarming, twitching and other forms of motility, at least in part due to a crosstalk with the quinolone quorum-sensing system (Vogt et al., 2011). In addition, a P. aeruginosa (p)ppGpp0 strain showed decreased cytotoxicity toward human alveolar adenocarcinoma cell lines and human epithelial cells, reduced hemolytic activity, impaired virulence in a cutaneous abscess model, and reduced mortality, lung edema and inflammatory cell infiltration in a mouse model of acute pneumonia (Xu et al., 2016). In terms of antibiotic tolerance, (p)ppGpp has been associated to P. aeruginosa multidrug tolerance upon stationary phase entry through activation of antioxidant defenses. Specifically, lack of (p)ppGpp led to loss of superoxide dismutase (SOD) activity, while genetic or chemical complementation of SOD activity in the ΔrelAΔspoT strain restored antibiotic tolerance (Martins et al., 2018).
Burkholderia pseudomallei
Burkholderia pseudomallei (formally Pseudomonas pseudomallei) is the causative agent of melioidosis, a disease of both humans and animals, classified by the CDC as a category B select agent (Wiersinga et al., 2018). Burkholderia spp. are known to be metabolically versatile, to thrive under adverse conditions and to tolerate antibiotic treatment. A B. pseudomallei strain lacking the relA and spoT genes [(p)ppGpp0], displayed defects in stationary-phase survival, replication within macrophages, and attenuated virulence in the G. mellonella invertebrate model as well as acute and chronic mouse models of melioidosis (Muller et al., 2012). Similar to Salmonella, vaccination of mice with the (p)ppGpp0 strain conferred partial protection against subsequent infection with wild-type B. pseudomallei (Muller et al., 2012). The distinct roles of RelA and SpoT in the infection process and in antibiotic tolerance, and the extent of the (p)ppGpp regulatory network remain to be explored in B. pseudomallei and other pathogenic Burkholderia spp.
Vibrio cholerae
A water-borne pathogen and the causative agent of cholera, V. cholerae is a versatile pathogen with important virulence factors such as the cholera toxin (CT) and the toxin co-regulated pilus (TCP) (Childers and Klose, 2007). As indicated above, in addition to the canonical RelA and SpoT, the genome of V. cholerae encodes a SAS named RelV that is unique to Vibrio species (Das et al., 2009). Early studies linked RelA-dependent (p)ppGpp production with optimal expression of CT, TCP, and two major virulence regulators (ToxR and ToxT), and with virulence in rabbit ileal loop and suckling mouse infection models (Haralalka et al., 2003). However, contradictory findings were subsequently made with a new relA mutant that produced normal levels of CT and TCP and displayed no colonization defects in the suckling mouse model (Silva and Benitez, 2006). Later reports using a double ΔrelAΔspoT mutant strain – an overproducer of basal (p)ppGpp levels due to the activity of RelV – produced higher levels of CT, whereas anaerobic growth via trimethylamine oxide respiration was severely inhibited (Oh et al., 2014). In contrast, a ppGpp0 strain (ΔrelAΔspoTΔrelV) grew substantially better, but produced no CT, collectively suggesting that CT production and bacterial growth are inversely regulated in response to (p)ppGpp accumulation (Oh et al., 2014). Moreover, virulence of the ΔrelAΔspoTΔrelV strain was significantly attenuated in the infant suckling mouse model (Oh et al., 2014). More recently, (p)ppGpp was also shown to contribute to antibiotic tolerance of V. cholerae, possibly by suppressing TCA cycle activity that lowered ROS production (Kim et al., 2018).
Acinetobacter baumannii
An opportunistic pathogen, Acinetobacter baumannii has emerged as a leading cause of hospital-acquired infections, in large part due to its stress resilience and multidrug tolerance (Harding et al., 2018). A ΔrelA strain failed to produce detectable levels of ppGpp during amino acid starvation and was hypermotile while showing reduced tolerance to antibiotics and attenuated virulence in the G. mellonella model (Perez-Varela et al., 2020). In a separate study, lack of (p)ppGpp resulted in lower expression of several efflux pump genes, providing a possible explanation for the association of (p)ppGpp with antibiotic tolerance in this organism (Jung et al., 2020). Studies to determine the consequences of a complete loss of (p)ppGpp that should only be achieved in a ΔrelAΔspoT double mutant strain to the pathophysiology of A. baumannii are still warranted.
Haemophilus ducreyi
Haemophilus ducreyi causes the sexually transmitted disease chancroid, a major cause of genital ulceration in developing countries (Lewis and Mitjà, 2016). The conditions encountered in human lesions are thought to resemble those found during stationary phase in vitro growth and; in line with this, a (p)ppGpp0 ΔrelAΔspoT mutant was attenuated for pustule formation in human volunteers (Holley et al., 2014). However, the (p)ppGpp0 strain displayed conflicting phenotypes in vitro as it was more sensitive to oxidative stress, but showed increased resistance to phagocytosis and prolonged survival in the stationary phase (Holley et al., 2014). RNAseq analysis of H. ducreyi grown to stationary phase indicated that loss of (p)ppGpp resulted in the dysregulation of several of its virulence determinants, including reduced production of Flp adhesin proteins (Holley et al., 2015). More recently, the H. ducreyi transcriptome in biopsy specimens of human lesions was compared to bacteria grown to mid-log, transition or stationary phases. While many of the genes previously shown to be regulated by (p)ppGpp were not differentially expressed in this study, genes coding for proteins involved in nutrient transport and alternative carbon utilization pathways, which are typically controlled by (p)ppGpp, were upregulated during infection (Gangaiah et al., 2016). Further characterization of the impact of the SR to H. ducreyi virulence and antibiotic tolerance by using relA and spoT single mutants coupled with cellular (p)ppGpp quantifications might shed new light on these findings.
Francisella tularensis
Francisella tularensis is the causative agent of tularemia, which is transmitted to humans upon contact with infected animals (Jones et al., 2014). Because of its high lethality in its pneumonic form and extremely low infectious dose, F. tularensis is classified by the United States Center for Disease Control (CDC) as a Category A select agent. Several studies have implicated (p)ppGpp with virulence gene expression of F. tularensis, with the most current model indicating that (p)ppGpp promotes virulence in this pathogen by activating transcription of the Francisella pathogenicity island (FPI) through interactions with the DNA-binding protein PigR and the MglA-SspA-RNAP complex (Cuthbert et al., 2017; Rohlfing et al., 2018). Indeed, virulence of a double ΔrelAΔspoT strain was shown to be attenuated in intranasally infected mice (Ma et al., 2019). Similar to observations made with P. aeruginosa and V. cholerae, (p)ppGpp was shown to govern global transcriptional changes in response to oxidative stress and required for tolerance to oxidants, most likely supporting intraphagocytic survival (Ma et al., 2019). In the closely related F. novocida – a laboratory surrogate of F. tularensis – survival of a relA mutant was impaired in the J774. A macrophage cell line, and its virulence was attenuated in the mouse model of tularemia (Dean et al., 2009). To date, relA and spoT single mutants of F. tularensis have not been characterized and it is unknown how loss of RelA-mediated SR (rather than complete absence of (p)ppGpp of a relA spoT double mutant strain) will impact F. tularensis virulence. Similar to Salmonella and Burkholderia (p)ppGpp-deficient strains, infection with the ΔrelA mutant elicited a protective immune response in mice, further supporting the potential of (p)ppGpp-deficient strains as attenuated live vaccines (Dean et al., 2009).
Brucella
The intracellular pathogen Brucella melitensis, the causative agent of brucellosis, possesses a single (p)ppGpp synthetase/hydrolase enzyme known as rsh. Inactivation of rsh resulted in altered morphology, reduced intracellular growth/survival in HeLa and ovine macrophages, and attenuated virulence in a mouse infection model (Dozot et al., 2006). The attenuated virulence of the (p)ppGpp0 strain was attributed, at least in part, to (p)ppGpp controlling the expression of the type four secretion system (T4SS) VirB, a major virulence factor of Brucellae (Dozot et al., 2006).
Other Bacteria
Mycobacterium
A distinguishing characteristic of Mycobacterium species is the presence of a hydrophobic mycolate layer attached to the peptidoglycan by an intermediate arabinogalactan layer. The most relevant member of this genus is M. tuberculosis, which causes tuberculosis in humans, while some other species are opportunistic animal and human pathogens. The genome of M. tuberculosis encodes a bifunctional Rel enzyme and a SAS enzyme which is phylogenetically distinct from the RelP and RelQ enzymes of Firmicutes and the V. cholerae RelV (Atkinson et al., 2011).
In M. tuberculosis, (p)ppGpp regulates multiple phenotypes, including biofilm formation, latency and antibiotic tolerance (Primm et al., 2000; Dahl et al., 2003; Weiss and Stallings, 2013). Similar to Firmicutes, activation of the SR in M. tuberculosis is primarily mediated by RelMtb. Inactivation of relMtb significantly attenuated M. tuberculosis virulence in a mouse model of chronic lung infection (Dahl et al., 2003) and in a guinea pig lung infection model (Klinkenberg et al., 2010). Notably, the pathology of guinea pig lungs infected with the relMtb mutant was markedly different showing a delayed hypersensitive response. Collectively, these studies indicate that the SR is not required for initial colonization and growth in the lungs, but essential for chronic infection. In a separate study, a strain harboring a point mutation in relMtb that silenced its synthetase activity without disrupting the hydrolase activity phenocopied the relMtb deletion strain, as it failed to persist in the lungs of infected mice (Weiss and Stallings, 2013). A double mutant strain lacking both relMtb and the SAS-encoding gene, presumably a (p)ppGpp0 strain, also phenocopied the relMtb single mutant in this mouse model (Weiss and Stallings, 2013), suggesting that the SR might be more relevant to M. tuberculosis pathophysiology than basal levels of (p)ppGpp. In addition to playing an essential role in the latency stage, likely through regulation of central metabolism, transcriptional studies indicate that (p)ppGpp also controls the expression of virulence genes. Specifically, the expression of several polyketide synthases that function as immune modulators and surface proteins important for granuloma formation are regulated in a RelMtb-dependent manner (Dahl et al., 2003).
Borrelia burgdorferi
The etiological agent of Lyme disease is a tick-borne obligate intracellular pathogen and a member of the phylum Spirochetes (Steere et al., 2016). The zoonotic life cycle of Borrelia burgdorferi involves adapting to a variety of stresses, including nutrient starvation in arthropod hosts. In addition, B. burgdorferi must tolerate adverse conditions during infection of the human host. In Spirochetes, a single bifunctional RelBbu enzyme (also known as SpoT) is responsible for (p)ppGpp metabolism. Despite conflicting results on whether (p)ppGpp levels increase during conditions that mimic the nutrient-limiting conditions encountered by B. burgdorferi in vivo (Bugrysheva et al., 2003; Drecktrah et al., 2015), relBbu was required for full virulence in an intradermal infection mouse model (Bugrysheva et al., 2005). Subsequent studies revealed that (p)ppGpp modulates glycerol uptake and utilization, the morphological conversion that occurs during nutrient starvation, and persistence in the tick (Bugrysheva et al., 2015; Drecktrah et al., 2015).
ppGpp Signaling as a Therapeutic Target
As the current literature strongly supports that (p)ppGpp is critical for bacterial fitness, virulence and antibiotic tolerance, the identification of molecules that interfere with (p)ppGpp signaling has been actively pursued by investigators around the globe. The first antibiotic to be associated with decreases in alarmone levels was chloramphenicol (Rodionov and Ishiguro, 1995), which was later confirmed and expanded to include other protein synthesis inhibitors (Kudrin et al., 2017). However, the effects of these antibiotics to (p)ppGpp metabolism and activation of the SR were not specific. To date, the lead compounds identified as ppGpp and/or SR inhibitors are classified into two groups: (i) molecules that directly inhibit (p)ppGpp synthesis by interfering with the activity of (p)ppGpp synthetases, and (ii) molecules that promote (p)ppGpp degradation. In the group of (p)ppGpp synthesis inhibitors, the first identified compound was relacin, a synthetic (p)ppGpp analog based on the crystal structure of the S. equisimilis Rel (RelSeq) enzyme (Wexselblatt et al., 2012). While shown to inhibit (p)ppGpp synthetic activity of both the Gram-negative RelA and Gram-positive Rel enzymes in vitro (Wexselblatt et al., 2012), relacin does not penetrate the intracellular compartment of Gram-negative bacteria. Thus, the antimicrobial properties of relacin was restricted to Gram-positive pathogens as it was shown to impair cell survival, biofilm formation of S. pyogenes and B. anthracis and sporulation of B. anthracis (Wexselblatt et al., 2012). Based on these promising results, follow up studies modified the relacin structure to develop more potent inhibitors, with two new relacin analogs shown to effectively lower intracellular (p)ppGpp and impair biofilm formation and survival of Myocobacterium smegmatis and biofilm formation of M. tuberculosis (Wexselblatt et al., 2013; Syal et al., 2017b). Importantly, these compounds were shown to be permeable and non-toxic to human cells (Syal et al., 2017b). However, even the most effective relacin analog showed inhibitory effects in the millimolar range such that further improvements to achieve inhibition in the nanomolar range are deemed necessary prior to testing in human subjects. It should be noted that relacin does not inhibit the activity of the purified E. faecalis RelQ enzyme (Gaca et al., 2015b), such that production of (p)ppGpp will most likely not be completely abolished in organisms that encode SASs when treated with relacin or its current analogs.
In addition to relacin, other compounds have been shown to inhibit alarmone synthesis, albeit with low specificity and at even higher concentrations. For example, vitamin C inhibited Rel-dependent (p)ppGpp production in M. smegmatis decreasing its long term survival and biofilm formation capacities (Syal et al., 2017a). In another study, a high-throughput screening assay of a library containing ∼2 million compounds against a recombinant RelMtb identified one compound with RelMtb-specific inhibitory activity that showed synergy with isoniazide in the treatment of M. tuberculosis in a mouse lung infection model (Dutta et al., 2019).
As mentioned above, another useful strategy to interfere with (p)ppGpp signaling is to promote (p)ppGpp hydrolysis rather than interfere with its synthesis. Along this line of thought, the anti-biofilm activity of peptide 1018, a synthetic peptide based on the host defense protein bactenecin, was linked to increased (p)ppGpp degradation (de la Fuente-Nunez et al., 2014) albeit the specific association of peptide 1018 with (p)ppGpp has been challenged with its activity suggested to derive from its general physicochemical properties (Andresen et al., 2016a). Apart from the controversial interpretation of the antimicrobial properties of peptide 1018, the synthetically modified DJK-5 and DJK-6 analogs were found to be more potent than peptide 1018 and to confer protection against P. aeruginosa infection in two invertebrate models (de la Fuente-Nunez et al., 2015). Taking a step further, DJK-5 was shown to reduce tissue damage and lesion size caused by either S. aureus or P. aeruginosa in a murine cutaneous abscess model (Mansour et al., 2016). Finally, this same group showed that P. aeruginosa spoT promoter activity was suppressed by treatment with peptides DJK-5 and 1018, and that a peptide-treated relA complemented SR double mutant strain exhibited reduced peptide susceptibility in the murine subcutaneous abscess model (Pletzer et al., 2017).
To date, several high-throughput screening assays are in place (Andresen et al., 2016b; Beljantseva et al., 2017) and compounds such as relacin can provide proof-of-principle evidence of the potential of (p)ppGpp signaling as the target for antimicrobial drug development. However, more studies are needed before (p)ppGpp signaling inhibitors can be tested in the clinical setting. For instance, even if more potent compounds are identified, additional studies will be necessary to assess their toxicity to humans, biodistribution and pharmacokinetics. Initially thought to be absent in eukaryotes, studies conducted in the past decade have identified SpoT orthologs in plants, insects, and humans (Sun et al., 2010; Tozawa and Nomura, 2011). While the insect and human genes appear to code for the (p)ppGpp hydrolytic domain with no evidence of functioning as a syhthetase, inactivation of the D. melanogaster spoT ortholog led to phenotypes that resemble those found in (p)ppGpp-deficient bacteria (Sun et al., 2010). In addition, one must also take into consideration that the significance of (p)ppGpp signaling and the enzymes responsible for (p)ppGpp metabolism may vary among bacterial groups. Specifically, while in some cases persistence and virulence can be associated with activation of the SR (mediated by RelA/Rel enzymes), in other cases basal (p)ppGpp pools during active growth and below the levels needed to activate the SR, appear to mediate those phenotypes. Thus, the ideal (p)ppGpp inhibitor must be capable of inhibiting the enzymatic activity of the so-called long RSHs (RelA, SpoT, Rel) and of SASs (RelP, RelQ, RelV). Alternatively, an effective antimicrobial might function by tipping the balance of bifunctional enzymes toward (p)ppGpp degradation. Apart from these challenges, the development of antibacterial strategies that target (p)ppGpp signaling have several advantages when compared to the antibiotics that are currently available. First, as non-essential enzymes, (p)ppGpp inhibitors will interfere with bacterial fitness and virulence expression but not cell viability such that drug resistance mechanisms may not arise rapidly or may not be acquired at all. Second, perhaps the most promising strategy, considering accumulating evidence that (p)ppGpp mediates bacterial persistence, (p)ppGpp inhibitors could be used in combination with currently available antibiotics that depend on actively growing cells to be effective.
Concluding Remarks
In addition to the expression of classic virulence factors such as toxins, capsule and fimbriae, contemporary investigations into the mechanisms of bacterial pathogenesis revealed that core cellular processes associated with metabolism and stress tolerance can be equally or even more critical to bacterial pathogenesis. As a global stress regulator, (p)ppGpp signaling appears to provide bacteria with an “extra edge,” increasing cell fitness by controlling central metabolism adjustments in response to environmental fluctuations, activating stress responses, and coordinating expression of classic virulence factors (Figure 2). Importantly, the regulatory effects of (p)ppGpp that initially were thought to be linked to the SR activation is now known to occur in an incremental manner as opposed to the on/off switch that is characteristic of the SR (Gaca et al., 2015a). The picture that emerges from recent studies is that the role of either the SR or basal levels of (p)ppGpp to bacterial virulence depends on the lifestyle and metabolic versatility of each organism.
Despite much progress made in recent years, there are still several aspects of (p)ppGpp regulation that are not well understood. In this regard, the relatively recent discovery of a crosstalk between the (p)ppGpp and c-di-AMP signaling networks may provide new clues. c-di-AMP is an essential signaling nucleotide reported to regulate a variety of cellular functions, in particular osmoregulation (Stulke and Kruger, 2020). Parallel studies conducted with B. subtilis, L. monocytogenes, and S. aureus identified a link between the c-di-AMP and (p)ppGpp signaling pathways (Rao et al., 2010; Corrigan et al., 2015; Huynh et al., 2015). It follows that the phosphodiesterases that are responsible for c-di-AMP degradation are subject to allosteric inhibition by ppGpp, such that high levels of (p)ppGpp correlate with high levels of c-di-AMP; studies with S. aureus have also shown an overlap between the c-di-AMP and SR transcriptional signatures (Corrigan et al., 2015; Huynh et al., 2015). In L. monocytogenes, a diadenylate cyclase (dacA) mutant harbored suppressor mutations in the synthetase domain of the bifunctional Rel enzyme, which led to reduced (p)ppGpp levels (Whiteley et al., 2015). Mutational analysis confirmed that dacA was essential in wild-type but not in a (p)ppGpp0 strain (Whiteley et al., 2015). Further studies revealed that c-di-AMP was essential because accumulated (p)ppGpp altered GTP concentrations, thereby affecting CodY activity (Whiteley et al., 2015). While the details of the relationship between c-di-AMP and the (p)ppGpp-CodY networks are not well understood, one can hypothesize that (p)ppGpp acts in concert with c-di-AMP to regulate bacterial activities important for adaptation to new environments. Therefore, a better understanding of the c-di-AMP regulatory mechanisms and identification of its targets may fill some of the gaps in our current understanding of how (p)ppGpp promote cell fitness and virulence.
Author Contributions
SK, CC-W, and JL wrote and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work in this field was supported by NIH-NIAID award R21 AI135158. We thank Dr. Marta Monguió-Tortajada for helping with the design of Figure 1.
Footnotes
Funding. This study was partially funded by National Institute of Allergy and Infections Diseases.
References
- Abranches J., Martinez A. R., Kajfasz J. K., Chavez V., Garsin D. A., Lemos J. A. (2009). The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J. Bacteriol. 191 2248–2256. 10.1128/jb.01726-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen L., Tenson T., Hauryliuk V. (2016a). Cationic bactericidal peptide 1018 does not specifically target the stringent response alarmone (p)ppGpp. Sci. Rep. 6:36549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen L., Varik V., Tozawa Y., Jimmy S., Lindberg S., Tenson T., et al. (2016b). Auxotrophy-based high throughput screening assay for the identification of Bacillus subtilis stringent response inhibitors. Sci. Rep. 6:35824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C. A., Murray B. E. (2012). The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 10 266–278. 10.1038/nrmicro2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson G. C., Tenson T., Hauryliuk V. (2011). The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One 6:e23479. 10.1371/journal.pone.0023479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battesti A., Bouveret E. (2006). Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 62 1048–1063. 10.1111/j.1365-2958.2006.05442.x [DOI] [PubMed] [Google Scholar]
- Beljantseva J., Kudrin P., Jimmy S., Ehn M., Pohl R., Varik V., et al. (2017). Molecular mutagenesis of ppGpp: turning a RelA activator into an inhibitor. Sci. Rep. 7:41839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett H. J., Pearce D. M., Glenn S., Taylor C. M., Kuhn M., Sonenshein A. L., et al. (2007). Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol. Microbiol. 63 1453–1467. 10.1111/j.1365-2958.2007.05597.x [DOI] [PubMed] [Google Scholar]
- Bhawini A., Pandey P., Dubey A. P., Zehra A., Nath G., Mishra M. N. (2019). RelQ mediates the expression of beta-Lactam resistance in methicillin-resistant Staphylococcus aureus. Front. Microbiol. 10:339. 10.3389/fmicb.2019.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson D., Hettle A. G., Boraston A. B., Hobbs J. K. (2020). Clinical mutations that partially activate the stringent response confer multidrug tolerance in Staphylococcus aureus. Antimicrob. Agents Chemother. 64 e2103–e2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva J., Dobrikova E. Y., Sartakova M. L., Caimano M. J., Daniels T. J., Radolf J. D., et al. (2003). Characterization of the stringent response and rel(Bbu) expression in Borrelia burgdorferi. J. Bacteriol. 185 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva J. V., Bryksin A. V., Godfrey H. P., Cabello F. C. (2005). Borrelia burgdorferi rel is responsible for generation of guanosine-3’-diphosphate-5’-triphosphate and growth control. Infect. Immun. 73 4972–4981. 10.1128/iai.73.8.4972-4981.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugrysheva J. V., Pappas C. J., Terekhova D. A., Iyer R., Godfrey H. P., Schwartz I., et al. (2015). Characterization of the RelBbu Regulon in Borrelia burgdorferi reveals modulation of glycerol metabolism by (p)ppGpp. PLoS One 10:e0118063. 10.1371/journal.pone.0118063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. (1970). The control of ribonucleic acid synthesis in Escherichia coli, V. Characterization of a nucleotide associated with the stringent response. J. Biol. Chem. 245 2309–2318. [PubMed] [Google Scholar]
- Charity J. C., Blalock L. T., Costante-Hamm M. M., Kasper D. L., Dove S. L. (2009). Small molecule control of virulence gene expression in Francisella tularensis. PLoS Pathog. 5:e1000641. 10.1371/journal.ppat.1000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers B. M., Klose K. E. (2007). Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol. 2 335–344. 10.2217/17460913.2.3.335 [DOI] [PubMed] [Google Scholar]
- Colomer-Winter C., Flores-Mireles A. L., Kundra S., Hultgren S. J., Lemos J. A. (2019). (p)ppGpp and CodY promote Enterococcus faecalis virulence in a Murine model of catheter-associated urinary tract infection. mSphere 4:e392-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer-Winter C., Gaca A. O., Chuang-Smith O. N., Lemos J. A., Frank K. L. (2018). Basal levels of (p)ppGpp differentially affect the pathogenesis of infective endocarditis in Enterococcus faecalis. Microbiology 164 1254–1265. 10.1099/mic.0.000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer-Winter C., Gaca A. O., Lemos J. A. (2017). Association of metal homeostasis and (p)ppGpp regulation in the pathophysiology of Enterococcus faecalis. Infect. Immun. 85:e00260-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan R. M., Bowman L., Willis A. R., Kaever V., Grundling A. (2015). Cross-talk between two nucleotide-signaling pathways in Staphylococcus aureus. J. Biol. Chem. 290 5826–5839. 10.1074/jbc.m114.598300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B. J., Ross W., Rohlfing A. E., Dove S. L., Gourse R. L., Brennan R. G., et al. (2017). Dissection of the molecular circuitry controlling virulence in Francisella tularensis. Genes Dev. 31 1549–1560. 10.1101/gad.303701.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. L., Kraus C. N., Boshoff H. I., Doan B., Foley K., Avarbock D., et al. (2003). The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. U.S.A. 100 10026–10031. 10.1073/pnas.1631248100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalebroux Z. D., Svensson S. L., Gaynor E. C., Swanson M. S. (2010). ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 74 171–199. 10.1128/mmbr.00046-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalebroux Z. D., Swanson M. S. (2012). ppGpp: magic beyond RNA polymerase. Nat. Rev. Microbiol. 10 203–212. 10.1038/nrmicro2720 [DOI] [PubMed] [Google Scholar]
- Das B., Pal R. R., Bag S., Bhadra R. K. (2009). Stringent response in Vibrio cholerae: genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene. Mol. Microbiol. 72 380–398. 10.1111/j.1365-2958.2009.06653.x [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Das S., Biswas A., Bhadra R. K., Das S. (2019). Small alarmones (p)ppGpp regulate virulence associated traits and pathogenesis of Salmonella enterica serovar Typhi. Cell. Microbiol. 21:e13034. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Nunez C., Reffuveille F., Haney E. F., Straus S. K., Hancock R. E. (2014). Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 10:e1004152. 10.1371/journal.ppat.1004152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Nunez C., Reffuveille F., Mansour S. C., Reckseidler-Zenteno S. L., Hernandez D., Brackman G., et al. (2015). D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 22 196–205. 10.1016/j.chembiol.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. E., Ireland P. M., Jordan J. E., Titball R. W., Oyston P. C. F. (2009). RelA regulates virulence and intracellular survival of Francisella novicida. Microbiology 155 4104–4113. 10.1099/mic.0.031021-0 [DOI] [PubMed] [Google Scholar]
- Dozot M., Boigegrain R. A., Delrue R. M., Hallez R., Ouahrani-Bettache S., Danese I., et al. (2006). The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB. Cell. Microbiol. 8 1791–1802. 10.1111/j.1462-5822.2006.00749.x [DOI] [PubMed] [Google Scholar]
- Drecktrah D., Lybecker M., Popitsch N., Rescheneder P., Hall L. S., Samuels D. S. (2015). The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathog. 11:e1005160. 10.1371/journal.ppat.1005160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta N. K., Klinkenberg L. G., Vazquez M. J., Segura-Carro D., Colmenarejo G., Ramon F., et al. (2019). Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Sci. Adv. 5:eaav2104. 10.1126/sciadv.aav2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson D. L., Lines J. L., Pesci E. C., Venturi V., Storey D. G. (2004). Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect. Immun. 72 5638–5645. 10.1128/iai.72.10.5638-5645.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Coll L., Maciag-Dorszynska M., Tailor K., Vadia S., Levin P. A., Szalewska-Palasz A., et al. (2020). The absence of (p)ppGpp renders initiation of Escherichia coli chromosomal DNA synthesis independent of growth rates. mBio 11:e03223-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons L. F., Liu L., Kant S., Kim J. S., Till J. K., Jones-Carson J., et al. (2020). SpoT induces intracellular Salmonella virulence programs in the phagosome. mBio 11:e3397-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. L., Colomer-Winter C., Grindle S. M., Lemos J. A., Schlievert P. M., Dunny G. M. (2014). Transcriptome analysis of Enterococcus faecalis during mammalian infection shows cells undergo adaptation and exist in a stringent response state. PLoS One 9:e115839. 10.1371/journal.pone.0115839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung D. K., Yang J., Stevenson D. M., Amador-Noguez D., Wang J. D. (2020). Small alarmone synthetase SasA expression leads to concomitant accumulation of pGpp, ppApp, and AppppA in Bacillus subtilis. Front. Microbiol. 11:2083. 10.3389/fmicb.2020.02083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaca A. O., Abranches J., Kajfasz J. K., Lemos J. A. (2012). Global transcriptional analysis of the stringent response in Enterococcus faecalis. Microbiology 158 1994–2004. 10.1099/mic.0.060236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaca A. O., Colomer-Winter C., Lemos J. A. (2015a). Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J. Bacteriol. 197 1146–1156. 10.1128/jb.02577-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaca A. O., Kudrin P., Colomer-Winter C., Beljantseva J., Liu K., Anderson B., et al. (2015b). From (p)ppGpp to (pp)pGpp: characterization of regulatory effects of pGpp synthesized by the small alarmone synthetase of Enterococcus faecalis. J. Bacteriol. 197 2908–2919. 10.1128/jb.00324-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaca A. O., Kajfasz J. K., Miller J. H., Liu K., Wang J. D., Abranches J., et al. (2013). Basal levels of (p)ppGpp in Enterococcus faecalis: the magic beyond the stringent response. mBio 4:e00646-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Palmer L., Pao C. C. (1977). Anomalous synthesis of ppGpp in growing cells. Cell 11 181–185. 10.1016/0092-8674(77)90329-4 [DOI] [PubMed] [Google Scholar]
- Gangaiah D., Zhang X., Baker B., Fortney K. R., Gao H., Holley C. L., et al. (2016). Haemophilus ducreyi seeks alternative carbon sources and adapts to nutrient stress and anaerobiosis during experimental infection of human volunteers. Infect. Immun. 84 1514–1525. 10.1128/iai.00048-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Chua K., Davies J. K., Newton H. J., Seemann T., Harrison P. F., et al. (2010). Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog. 6:e1000944. 10.1371/journal.ppat.1000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Francois P., Liebeke M., Fraunholz M., Goerke C., Krismer B., et al. (2012). The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog. 8:e1003016. 10.1371/journal.ppat.1003016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Goerke C., Fritz M., Schafer T., Ohlsen K., Liebeke M., et al. (2010). Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect. Immun. 78 1873–1883. 10.1128/iai.01439-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Kastle B., Gratani F. L., Goerke C., Wolz C. (2014). Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J. Bacteriol. 196 894–902. 10.1128/jb.01201-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T., Wolz C. (2014). Intersection of the stringent response and the CodY regulon in low GC Gram-positive bacteria. Int. J. Med. Microbiol. 304 150–155. 10.1016/j.ijmm.2013.11.013 [DOI] [PubMed] [Google Scholar]
- Gellatly S. L., Hancock R. E. (2013). Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog. Dis. 67 159–173. 10.1111/2049-632x.12033 [DOI] [PubMed] [Google Scholar]
- Glass T. L., Holmes W. M., Hylemon P. B., Stellwag E. J. (1979). Synthesis of guanosine tetra- and pentaphosphates by the obligately anaerobic bacterium Bacteroides thetaiotaomicron in response to molecular oxygen. J. Bacteriol. 137 956–962. 10.1128/jb.137.2.956-962.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratani F. L., Horvatek P., Geiger T., Borisova M., Mayer C., Grin I., et al. (2018). Regulation of the opposing (p)ppGpp synthetase and hydrolase activities in a bifunctional RelA/SpoT homologue from Staphylococcus aureus. PLoS Genet. 14:e1007514. 10.1371/journal.pgen.1007514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralalka S., Nandi S., Bhadra R. K. (2003). Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors. J. Bacteriol. 185 4672–4682. 10.1128/jb.185.16.4672-4682.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. M., Hennon S. W., Feldman M. F. (2018). Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 16 91–102. 10.1038/nrmicro.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauryliuk V., Atkinson G. C., Murakami K. S., Tenson T., Gerdes K. (2015). Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 13 298–309. 10.1038/nrmicro3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J. K., Boraston A. B. (2019). (p)ppGpp and the stringent response: an emerging threat to antibiotic therapy. ACS Infect. Dis. 5 1505–1517. 10.1021/acsinfecdis.9b00204 [DOI] [PubMed] [Google Scholar]
- Holley C., Gangaiah D., Li W., Fortney K. R., Janowicz D. M., Ellinger S., et al. (2014). A (p)ppGpp-null mutant of Haemophilus ducreyi is partially attenuated in humans due to multiple conflicting phenotypes. Infect. Immun. 82 3492–3502. 10.1128/iai.01994-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley C. L., Zhang X., Fortney K. R., Ellinger S., Johnson P., Baker B., et al. (2015). DksA and (p)ppGpp have unique and overlapping contributions to Haemophilus ducreyi pathogenesis in humans. Infect. Immun. 83 3281–3292. 10.1128/iai.00692-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honsa E. S., Cooper V. S., Mhaissen M. N., Frank M., Shaker J., Iverson A., et al. (2017). RelA mutant Enterococcus faecium with multiantibiotic tolerance arising in an immunocompromised host. mBio 8:e02124-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T. N., Luo S., Pensinger D., Sauer J. D., Tong L., Woodward J. J. (2015). An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence. Proc. Natl. Acad. Sci. U.S.A. 112 E747–E756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J. H., Song M., Park S. I., Cho K. O., Rhee J. H., Choy H. E. (2008). Salmonella enterica serovar gallinarum requires ppGpp for internalization and survival in animal cells. J. Bacteriol. 190 6340–6350. 10.1128/jb.00385-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimmy S., Saha C. K., Kurata T., Stavropoulos C., Oliveira S. R. A., Koh A., et al. (2020). A widespread toxin-antitoxin system exploiting growth control via alarmone signaling. Proc. Natl. Acad. Sci. U.S.A. 117 10500–10510. 10.1073/pnas.1916617117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Faron M., Rasmussen J. A., Fletcher J. R. (2014). Uncovering the components of the Francisella tularensis virulence stealth strategy. Front. Cell. Infect. Microbiol. 7:32. 10.3389/fcimb.2014.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. W., Kim K., Islam M. M., Lee J. C., Shin M. (2020). Role of ppGpp-regulated efflux genes in Acinetobacter baumannii. J. Antimicrob. Chemother. 75 1130–1134. 10.1093/jac/dkaa014 [DOI] [PubMed] [Google Scholar]
- Kalia D., Merey G., Nakayama S., Zheng Y., Zhou J., Luo Y., et al. (2013). Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p)ppGpp signaling in bacteria and implications in pathogenesis. Chem. Soc. Rev. 42 305–341. 10.1039/c2cs35206k [DOI] [PubMed] [Google Scholar]
- Kanjee U., Ogata K., Houry W. A. (2012). Direct binding targets of the stringent response alarmone (p)ppGpp. Mol. Microbiol. 85 1029–1043. 10.1111/j.1365-2958.2012.08177.x [DOI] [PubMed] [Google Scholar]
- Kazmierczak K. M., Wayne K. J., Rechtsteiner A., Winkler M. E. (2009). Roles of rel(Spn) in stringent response, global regulation and virulence of serotype 2 Streptococcus pneumoniae D39. Mol. Microbiol. 72 590–611. 10.1111/j.1365-2958.2009.06669.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Y., Go J., Lee K. M., Oh Y. T., Yoon S. S. (2018). Guanosine tetra- and pentaphosphate increase antibiotic tolerance by reducing reactive oxygen species production in Vibrio cholerae. J. Biol. Chem. 293 5679–5694. 10.1074/jbc.ra117.000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg L. G., Lee J. H., Bishai W. R., Karakousis P. C. (2010). The stringent response is required for full virulence of Mycobacterium tuberculosis in guinea pigs. J. Infect. Dis. 202 1397–1404. 10.1086/656524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasny L., Gourse R. L. (2004). An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 23 4473–4483. 10.1038/sj.emboj.7600423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasny L., Tiserova H., Jonak J., Rejman D., Sanderova H. (2008). The identity of the transcription +1 position is crucial for changes in gene expression in response to amino acid starvation in Bacillus subtilis. Mol. Microbiol. 69 42–54. 10.1111/j.1365-2958.2008.06256.x [DOI] [PubMed] [Google Scholar]
- Kriel A., Bittner A. N., Kim S. H., Liu K., Tehranchi A. K., Zou W. Y., et al. (2012). Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Mol. Cell 48 231–241. 10.1016/j.molcel.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrin P., Varik V., Oliveira S. R., Beljantseva J., Del Peso Santos T., Dzhygyr I., et al. (2017). Subinhibitory concentrations of bacteriostatic antibiotics induce relA-dependent and relA-independent tolerance to beta-Lactams. Antimicrob. Agents Chemother. 61:e2173-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. A., Lin V. K., Nascimento M. M., Abranches J., Burne R. A. (2007). Three gene products govern (p)ppGpp production by Streptococcus mutans. Mol. Microbiol. 65 1568–1581. 10.1111/j.1365-2958.2007.05897.x [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Mitjà O. (2016). Haemophilusducreyi: from sexually transmitted infection to skin ulcer pathogen. Curr. Opin. Infect. Dis. 29 52–57. 10.1097/QCO.0000000000000226 [DOI] [PubMed] [Google Scholar]
- Li L., Bayer A. S., Cheung A., Lu L., Abdelhady W., Donegan N. P., et al. (2020). The stringent response contributes to persistent methicillin-resistant Staphylococcus aureus endovascular infection through the purine biosynthetic pathway. J. Infect. Dis. 222 1188–1198. 10.1093/infdis/jiaa202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Bittner A. N., Wang J. D. (2015). Diversity in (p)ppGpp metabolism and effectors. Curr. Opin. Microbiol. 24 72–79. 10.1016/j.mib.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., King K., Alqahtani M., Worden M., Muthuraman P., Cioffi C. L., et al. (2019). Stringent response governs the oxidative stress resistance and virulence of Francisella tularensis. PLoS One 14:e0224094. 10.1371/journal.pone.0224094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. C., Pletzer D., de la Fuente-Nunez C., Kim P., Cheung G. Y. C., Joo H. S., et al. (2016). Bacterial abscess formation is controlled by the stringent stress response and can be targeted therapeutically. EBioMedicine 12 219–226. 10.1016/j.ebiom.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D., McKay G., Sampathkumar G., Khakimova M., English A. M., Nguyen D. (2018). Superoxide dismutase activity confers (p)ppGpp-mediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 115 9797–9802. 10.1073/pnas.1804525115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechold U., Murphy H., Brown L., Cashel M. (2002). Intramolecular regulation of the opposing (p)ppGpp catalytic activities of Rel(Seq), the Rel/Spo enzyme from Streptococcus equisimilis. J. Bacteriol. 184 2878–2888. 10.1128/jb.184.11.2878-2888.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C. M., Conejero L., Spink N., Wand M. E., Bancroft G. J., Titball R. W. (2012). Role of RelA and SpoT in Burkholderia pseudomallei virulence and immunity. Infect. Immun. 80 3247–3255. 10.1128/iai.00178-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangi M. M., Kim C., Chung M., Tsai J., Vijayadamodar G., Benitez M., et al. (2013). Whole-genome sequencing reveals a link between beta-lactam resistance and synthetases of the alarmone (p)ppGpp in Staphylococcus aureus. Microb. Drug Resist. 19 153–159. 10.1089/mdr.2013.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na H. S., Kim H. J., Lee H. C., Hong Y., Rhee J. H., Choy H. E. (2006). Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine 24 2027–2034. 10.1016/j.vaccine.2005.11.031 [DOI] [PubMed] [Google Scholar]
- Oh Y. T., Park Y., Yoon M. Y., Bari W., Go J., Min K. B., et al. (2014). Cholera toxin production during anaerobic trimethylamine N-oxide respiration is mediated by stringent response in Vibrio cholerae. J. Biol. Chem. 289 13232–13242. 10.1074/jbc.m113.540088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. I., Jeong J. H., Choy H. E., Rhee J. H., Na H. S., Lee T. H., et al. (2010). Immune response induced by ppGpp-defective Salmonella enterica serovar Gallinarum in chickens. J. Microbiol. 48 674–681. 10.1007/s12275-010-0179-6 [DOI] [PubMed] [Google Scholar]
- Perez-Varela M., Tierney A. R. P., Kim J. S., Vazquez-Torres A., Rather P. (2020). Characterization of RelA in Acinetobacter baumannii. J. Bacteriol. 202:e00045-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilo P., Frey J. (2018). Pathogenicity, population genetics and dissemination of Bacillus anthracis. Infect. Genet. Evol. 64 115–125. 10.1016/j.meegid.2018.06.024 [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda J., Tedin K. (2004). The bacterial signal molecule, ppGpp, regulates Salmonella virulence gene expression. Mol. Microbiol. 52 1827–1844. 10.1111/j.1365-2958.2004.04122.x [DOI] [PubMed] [Google Scholar]
- Pletzer D., Blimkie T. M., Wolfmeier H., Li Y., Baghela A., Lee A. H. Y., et al. (2020). The stringent stress response controls proteases and global regulators under optimal growth conditions in Pseudomonas aeruginosa. mSystems 5:e00495-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer D., Wolfmeier H., Bains M., Hancock R. E. W. (2017). Synthetic peptides to target stringent response-controlled virulence in a Pseudomonas aeruginosa murine cutaneous infection model. Front. Microbiol. 8:1867. 10.3389/fmicb.2017.01867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrykus K., Cashel M. (2008). (p)ppGpp: still magical? Annu. Rev. Microbiol. 62 35–51. 10.1146/annurev.micro.62.081307.162903 [DOI] [PubMed] [Google Scholar]
- Primm T. P., Andersen S. J., Mizrahi V., Avarbock D., Rubin H., Barry C. E., III. (2000). The stringent response of Mycobacterium tuberculosis is required for long-term survival. J. Bacteriol. 182 4889–4898. 10.1128/jb.182.17.4889-4898.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoshevich L., Cossart P. (2018). Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol. 16 32–46. 10.1038/nrmicro.2017.126 [DOI] [PubMed] [Google Scholar]
- Ramachandran V. K., Shearer N., Jacob J. J., Sharma C. M., Thompson A. (2012). The architecture and ppGpp-dependent expression of the primary transcriptome of Salmonella Typhimurium during invasion gene expression. BMC Genomics 13:25. 10.1186/1471-2164-13-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao F., See R. Y., Zhang D., Toh D. C., Ji Q., Liang Z. X. (2010). YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J. Biol. Chem. 285 473–482. 10.1074/jbc.m109.040238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov D. G., Ishiguro E. E. (1995). Direct correlation between overproduction of guanosine 3’,5’-bispyrophosphate (ppGpp) and penicillin tolerance in Escherichia coli. J. Bacteriol. 177 4224–4229. 10.1128/jb.177.15.4224-4229.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing A. E., Ramsey K. M., Dove S. L. (2018). Polyphosphate kinase antagonizes virulence gene expression in Francisella tularensis. J. Bacteriol. 200:e00460-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronneau S., Hallez R. (2019). Make and break the alarmone: regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol. Rev. 43 389–400. 10.1093/femsre/fuz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Vrentas C. E., Sanchez-Vazquez P., Gaal T., Gourse R. L. (2013). The magic spot: a ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation. Mol. Cell 50 420–429. 10.1016/j.molcel.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajish M., Tiwari D., Rananaware D., Nandicoori V. K., Prakash B. (2007). A charge reversal differentiates (p)ppGpp synthesis by monofunctional and bifunctional Rel proteins. J. Biol. Chem. 282 34977–34983. 10.1074/jbc.m704828200 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vazquez P., Dewey C. N., Kitten N., Ross W., Gourse R. L. (2019). Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 116 8310–8319. 10.1073/pnas.1819682116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer H., Beckert B., Frese C. K., Steinchen W., Nuss A. M., Beckstette M., et al. (2020). The alarmones (p)ppGpp are part of the heat shock response of Bacillus subtilis. PLoS Genet. 16:e1008275. 10.1371/journal.pgen.1008275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock M. E., Sudarsan N., Breaker R. R. (2018). Riboswitches for the alarmone ppGpp expand the collection of RNA-based signaling systems. Proc. Natl. Acad. Sci. U.S.A. 115 6052–6057. 10.1073/pnas.1720406115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. J., Benitez J. A. (2006). A Vibrio cholerae relaxed (relA) mutant expresses major virulence factors, exhibits biofilm formation and motility, and colonizes the suckling mouse intestine. J. Bacteriol. 188 794–800. 10.1128/jb.188.2.794-800.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L. (2005). CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8 203–207. 10.1016/j.mib.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Song M., Kim H. J., Kim E. Y., Shin M., Lee H. C., Hong Y., et al. (2004). ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J. Biol. Chem. 279 34183–34190. 10.1074/jbc.m313491200 [DOI] [PubMed] [Google Scholar]
- Steere A. C., Strle F., Wormser G. P., Hu L. T., Branda J. A., Hovius J. W., et al. (2016). Lyme borreliosis. Nat. Rev. Dis. Primers 2:16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinchen W., Bange G. (2016). The magic dance of the alarmones (p)ppGpp. Mol. Microbiol. 101 531–544. 10.1111/mmi.13412 [DOI] [PubMed] [Google Scholar]
- Stulke J., Kruger L. (2020). Cyclic di-AMP signaling in bacteria. Annu. Rev. Microbiol. 74 159–179. 10.1146/annurev-micro-020518-115943 [DOI] [PubMed] [Google Scholar]
- Sun D., Lee G., Lee J. H., Kim H. Y., Rhee H. W., Park S. Y., et al. (2010). A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat. Struct. Mol. Biol. 17 1188–1194. 10.1038/nsmb.1906 [DOI] [PubMed] [Google Scholar]
- Sun W., Roland K. L., Branger C. G., Kuang X., Curtiss R., III. (2009). The role of relA and spoT in Yersinia pestis KIM5 pathogenicity. PLoS One 4:e6720. 10.1371/journal.pone.0006720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal K., Bhardwaj N., Chatterji D. (2017a). Vitamin C targets (p)ppGpp synthesis leading to stalling of long-term survival and biofilm formation in Mycobacterium smegmatis. FEMS Microbiol. Lett. 364:fnw282. 10.1093/femsle/fnw282 [DOI] [PubMed] [Google Scholar]
- Syal K., Flentie K., Bhardwaj N., Maiti K., Jayaraman N., Stallings C. L., et al. (2017b). Synthetic (p)ppGpp analogue is an inhibitor of stringent response in Mycobacteria. Antimicrob. Agents Chemother. 61:e443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. M., Beresford M., Epton H. A., Sigee D. C., Shama G., Andrew P. W., et al. (2002). Listeria monocytogenes relA and hpt mutants are impaired in surface- attached growth and virulence. J. Bacteriol. 184 621–628. 10.1128/jb.184.3.621-628.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozawa Y., Nomura Y. (2011). Signalling by the global regulatory molecule ppGpp in bacteria and chloroplasts of land plants. Plant Biol. 13 699–709. 10.1111/j.1438-8677.2011.00484.x [DOI] [PubMed] [Google Scholar]
- van Schaik W., Prigent J., Fouet A. (2007). The stringent response of Bacillus anthracis contributes to sporulation but not to virulence. Microbiology 153 4234–4239. 10.1099/mic.0.2007/010355-0 [DOI] [PubMed] [Google Scholar]
- Vinella D., Albrecht C., Cashel M., D’Ari R. (2005). Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol. Microbiol. 56 958–970. 10.1111/j.1365-2958.2005.04601.x [DOI] [PubMed] [Google Scholar]
- Vogt S. L., Green C., Stevens K. M., Day B., Erickson D. L., Woods D. E., et al. (2011). The stringent response is essential for Pseudomonas aeruginosa virulence in the rat lung agar bead and Drosophila melanogaster feeding models of infection. Infect. Immun. 79 4094–4104. 10.1128/iai.00193-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. A., Stallings C. L. (2013). Essential roles for Mycobacterium tuberculosis Rel beyond the production of (p)ppGpp. J. Bacteriol. 195 5629–5638. 10.1128/jb.00759-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. H., Gaynor E. C. (2006). Helicobacter pylori initiates the stringent response upon nutrient and pH downshift. J. Bacteriol. 188 3726–3729. 10.1128/jb.188.10.3726-3729.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexselblatt E., Kaspy I., Glaser G., Katzhendler J., Yavin E. (2013). Design, synthesis and structure-activity relationship of novel Relacin analogs as inhibitors of Rel proteins. Eur. J. Med. Chem. 70 497–504. 10.1016/j.ejmech.2013.10.036 [DOI] [PubMed] [Google Scholar]
- Wexselblatt E., Oppenheimer-Shaanan Y., Kaspy I., London N., Schueler-Furman O., Yavin E., et al. (2012). Relacin, a novel antibacterial agent targeting the Stringent Response. PLoS Pathog. 8:e1002925. 10.1371/journal.ppat.1002925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley A. T., Pollock A. J., Portnoy D. A. (2015). The PAMP c-di-AMP is essential for Listeria monocytogenes growth in rich but not minimal media due to a toxic increase in (p)ppGpp. Cell Host Microbe 17 788–798. 10.1016/j.chom.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W. J., Virk H. S., Torres A. G., Currie B. J., Peacock S. J., Dance D. A. B., et al. (2018). Melioidosis. Nat. Rev. Dis. Primers 4:17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolz C., Geiger T., Goerke C. (2010). The synthesis and function of the alarmone (p)ppGpp in firmicutes. Int. J. Med. Microbiol. 300 142–147. 10.1016/j.ijmm.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Xu X., Yu H., Zhang D., Xiong J., Qiu J., Xin R., et al. (2016). Role of ppGpp in Pseudomonas aeruginosa acute pulmonary infection and virulence regulation. Microbiol. Res. 192 84–95. 10.1016/j.micres.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Yan X., Zhao C., Budin-Verneuil A., Hartke A., Rince A., Gilmore M. S., et al. (2009). The (p)ppGpp synthetase RelA contributes to stress adaptation and virulence in Enterococcus faecalis V583. Microbiology 155 3226–3237. 10.1099/mic.0.026146-0 [DOI] [PubMed] [Google Scholar]
- Yang J., Anderson B. W., Turdiev A., Turdiev H., Stevenson D. M., Amador-Noguez D., et al. (2020). The nucleotide pGpp acts as a third alarmone in Bacillus, with functions distinct from those of (p) ppGpp. Nat. Commun. 11:5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Xie S., Tang N. Y., Choi M. Y., Wang Y., Watt R. M. (2019). The Ps and Qs of alarmone synthesis in Staphylococcus aureus. PLoS One 14:e0213630. 10.1371/journal.pone.0213630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zhang T., Su Z., Feng L., Liu H., Xu Z., et al. (2019a). Co-regulation of CodY and (p)ppGpp synthetases on morphology and pathogenesis of Streptococcus suis. Microbiol. Res. 223 88–98. 10.1016/j.micres.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Zhu M., Pan Y., Dai X. (2019b). (p)ppGpp: the magic governor of bacterial growth economy. Curr. Genet. 65 1121–1125. 10.1007/s00294-019-00973-z [DOI] [PubMed] [Google Scholar]
- Zhu J., Zhang T., Su Z., Li L., Wang D., Xiao R. (2016). (p)ppGpp synthetases regulate the pathogenesis of zoonotic Streptococcus suis. Microbiol. Res. 191 1–11. 10.1016/j.micres.2016.05.007 [DOI] [PubMed] [Google Scholar]