Abstract
Current diagnostic tests for tuberculosis (TB) are not able to predict reactivation disease progression from latent TB infection (LTBI). The main barrier to predicting reactivation disease is the lack of our understanding of host biomarkers associated with progression from latent infection to active disease. Here, we applied an immune-based gene expression profile by NanoString platform to identify whole blood markers that can distinguish active TB from other lung diseases (OPD), and that could be further evaluated as a reactivation TB predictor. Among 23 candidate genes that differentiated patients with active TB from those with OPD, nine genes (CD274, CEACAM1, CR1, FCGR1A/B, IFITM1, IRAK3, LILRA6, MAPK14, PDCD1LG2) demonstrated sensitivity and specificity of 100%. Seven genes (C1QB, C2, CCR2, CCRL2, LILRB4, MAPK14, MSR1) distinguished TB from LTBI with sensitivity and specificity between 82 and 100%. This study identified single gene candidates that distinguished TB from OPD and LTBI with high sensitivity and specificity (both > 82%), which may be further evaluated as diagnostic for disease and as predictive markers for reactivation TB.
Subject terms: Computational biology and bioinformatics, Microbiology, Immunology, Infectious diseases, Diagnostic markers, Predictive markers
Introduction
Tuberculosis (TB), an aerosol-borne disease caused by Mycobacterium tuberculosis (Mtb), is one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent1. About a quarter of the world’s population is estimated to have latent TB infection (LTBI), and about 10% of these individuals will progress to have active TB disease during their lifetime (reactivation TB)1,2. Despite longstanding intense efforts to control this disease, TB remains a global health problem that mandates better diagnostic tests and preventive strategies.
Worldwide, the diagnosis of active TB is mostly dependent on sputum smear microscopy by Mtb acid-fast staining or culture3. Microscopy suffers from low sensitivity, and culture can take several weeks to yield results, and neither can be applied to extrapulmonary TB4. Although the GeneXpert MTB/RIF test offers a fast result for active TB, the test can be a challenge for TB diagnosis of children and the elderly due to difficulties in obtaining sputum samples from these groups5,6. Also, the GeneXpert MTB/RIF requires sophisticated technology and a well-trained staff, and thus not affordable or sustainable in most healthcare systems7. Finally, none of these sputum-based tests can predict reactivation TB.
Multiple populations of immune cells have distinct functions that cooperate for Mtb infection control when the bacillus enters the lungs8. During infection, alterations of immune processes in the host lead to changes in the transcriptional profiles of circulating immune cells9. The immune response-based biomarker identification for TB diagnosis has extensively been researched10 and many of them have focused on distinguishing latent infection from active TB11–13. Unfortunately, none of these gene signatures has so far been translated into a point of care (POC) diagnostic test. The translation into the clinical practice of gene signature-based assays is challenged by the difficulty in determining which of the multiple gene signatures can be implemented as a diagnostic platform that is simple and cost-effective.
Here, we report the results of an immune-based gene expression profile study based on the NanoString technology in patients with active TB and other pulmonary diseases (OPD), healthy donors with latent TB infection (LTBI), and uninfected health controls (HC). The aim of this study was to identify whole blood markers that can distinguish active TB from OPD, HC, and LTBI. We identified 23 and seven genes associated with inflammatory mechanisms that distinguished with high sensitivity and specificity, patients with TB from OPD and LTBI, respectively.
Results
Demographic and clinical characteristics of the study population
The demographic, clinical, and laboratory features of the 35 study participants are shown in Table 1. Of the 17 TB patients, 13 (76.5%) had sputum smear test positive, three were positive by Mtb culture and one patient had the TB confirmed by Mtb molecular test (XPERT TB/RIF). Of all TB patients, eight (47.1%) were screened by Mtb culture. The median age was 41.9 (± 14.04) years in the TB group, 42.7 (± 17.06) in the LTBI group, 43.8 (± 9.70) in the OPD group, and 32.5 (± 3.53) in the HC group.
Table 1.
Demographic and clinical data of study population.
| Active TB 17 |
LTBI 7 |
OPD 5 |
HC 6 |
|
|---|---|---|---|---|
| Gender, n (%)a | ||||
| Men | 11 (68.75) | 4 (57.14) | 2 (40) | 1 (20) |
| Women | 5 (31.25) | 3 (42.86) | 3 (60) | 4 (80) |
| Age ± SDc | 41.93 ± 14.04 | 42.67 ± 17.06 | 43.8 ± 9.70 | 32.5 ± 3.53 |
| TB diagnosis, n (%) | ||||
| Sputum Smear | ||||
| Pos | 13 (76.5) | – | 0 | |
| Neg | 3 (23.5) | – | 5 (100) | – |
| Culture | ||||
| Pos | 8 (47.1) | – | 0 | – |
| Neg | 0 | – | 5 (100) | – |
| N/S | 9 (52.9) | – | – | – |
| Xpert MTB/RIF | 1 (5.9) | – | – | – |
| IGRA: n (%) | ||||
| Pos | – | 7 (100) | – | 0 |
| Neg | – | 0 | – | 6 (100) |
TB tuberculosis, LTBI latent tuberculosis infection, OPD other pulmonary disease, HC health communicants, SD standard deviation, N/S not screened.
aMissing data: gender (TB = 1 and HC = 1) and age (TB = 1, TBL = 1 and HC = 1).
Sample clustering
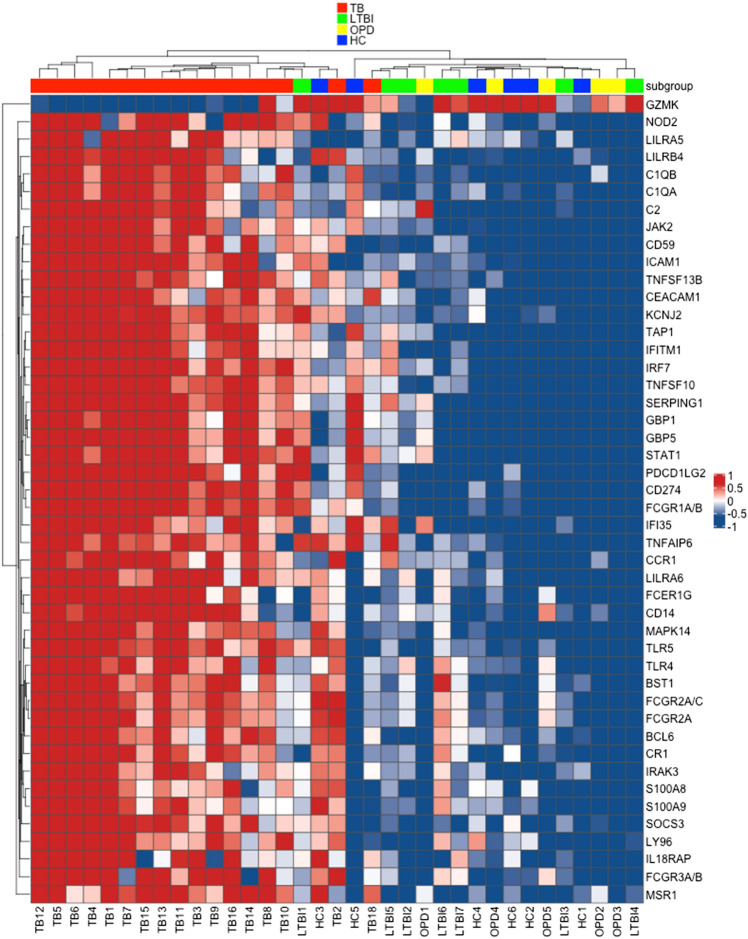
We evaluated 594 inflammatory genes in whole blood from 17 TB patients and 18 controls (seven with LTBI, six HC and five with OPD). We further organized these groups in order to identify whole blood biomarkers to diagnose active TB (TB vs. OPD) and candidate to predict TB reactivation (LTBI vs. TB). First, we evaluated all four study groups together to verify whether the gene panel would be able to distinguish them. Figure 1 shows a heatmap of the normalized data generated via unsupervised hierarchical clustering. The mRNA expression levels of 46 of 594 genes segregated the study groups into two large groups. Transcripts that showed increased expression (red) clustered among TB patients while those that showed decreased expression clustered among non-active TB groups. Two individuals belonged to the groups LTBI (LTBI1) and HC (HC3) clustered with patients with active TB.
Figure 1.
Heatmap showing different expression pattern of 46 proinflammatory genes out of 549 genes. Heatmap of gene expression levels in patients diagnosed with active TB (red), LTBI subjects (green), OPD patients (yellow) and uninfected donors HC (blue). Expression levels are scaled from dark blue (low expression) to dark red (high expression). Heatmap was generated in R (version 3.6.3) with the ComplexHeatmap package (version 2.0.0, https://bioconductor.org/packages/release/bioc/html/ComplexHeatmap.html).
Gene expression data of TB and OPD donors
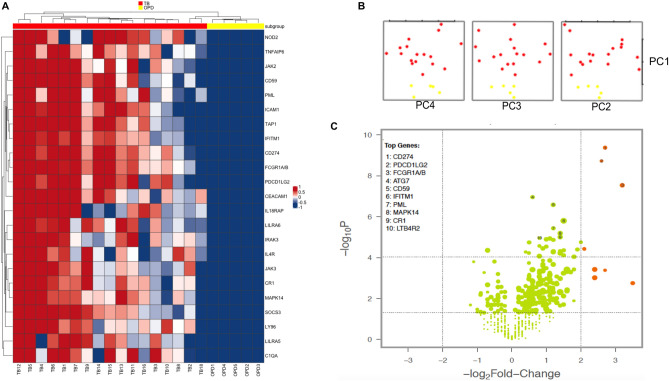
Asthma represents a chronic non-infectious inflammatory airways disease and needs to be promptly distinguished from TB by healthcare providers. We identified 23 candidates genes that differentiated most of the TB patients from asthma (OPD group) (p < 0.001 and fold change [FC] > 2) (Fig. 2A). Principal component analyses (PCA) of the gene expression data showed significant separation between TB and OPD patients (Fig. 2B). The findings are also presented by the volcano plots of all data displayed in orange at a significance level of p < 0.05 and at a log2-fold change higher than 2 for both groups (Fig. 2C). These analyses identify genes that can be used to distinguish TB and OPD patients, which included CD274, PDCD1LG2 and FCGR1A/B (p-value < 0.0001 and log2-fold change ratio > 2.6) (Fig. 2C).
Figure 2.
Identification of markers for TB diagnosis. (A) Heatmap of 23 gene expression levels of TB (red) and OPD (yellow) patients. (B) PCA score plot of TB and OPD patients. (C) Volcano plots showing the distribution of the gene expression fold changes in TB patients relative to OPD patients. Genes with absolute fold change ≥ 4 and p-value ≤ 0.05 are indicated in orange. Expression levels are scaled from dark blue (low expression) to dark red (high expression). Figures were generated with R (version 3.6.3) using ComplexHeatmap package (version 2.0.0, https://bioconductor.org/packages/release/bioc/html/ComplexHeatmap.html), prcomp function from stats package (version 3.6.3, https://www.r-project.org/), and NanoStringNorm package (version 1.2.1.1, https://cran.r-project.org/web/packages/NanoStringNorm/index.html) for heatmap, pca and Volcano plot, respectively.
Gene expression data of TB and LTBI donors
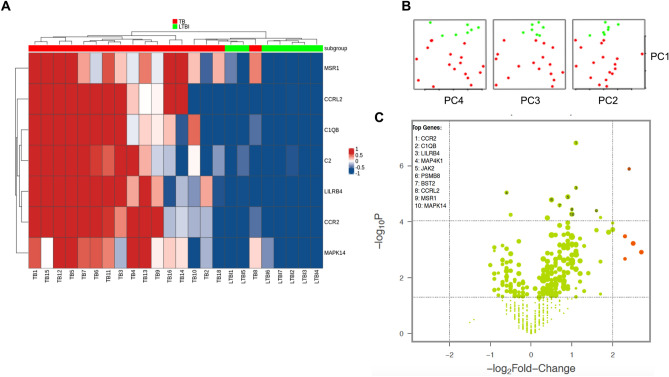
We also compared the gene expression levels between TB and LTBI groups aiming to identify candidate markers able to differentiate these groups. Both heatmap (Fig. 3A) and PCA analysis (Fig. 3B) show 7 of 594 inflammatory genes that significantly differentiate those groups (p < 0.001 and FC > 2). Volcano plots analyses revealed two promising genes (CCR2 and CIQB, p-value < 0.0001 and log2-fold change ratio > 1.1 and 2.4, respectively) that can be further tested as a possible marker of TB reactivation (Fig. 3C).
Figure 3.
Identification of markers for TB progression. (A) Heatmap of seven gene expression levels of TB (red) and LTBI (green) subjects. (B) PCA score plot of TB and LTBI subjects. (C) Volcano plot showing the distribution of the gene expression fold changes in TB patients relative to LTBI. Genes with absolute fold change ≥ 4 and p-value ≤ 0.05 are indicated in orange. Expression levels are scaled from dark blue (low expression) to dark red (high expression). Figures were generated with R (version 3.6.3) using ComplexHeatmap package (version 2.0.0, https://bioconductor.org/packages/release/bioc/html/ComplexHeatmap.html), prcomp function from stats package (version 3.6.3, https://www.r-project.org/), and NanoStringNorm package (version 1.2.1.1, https://cran.r-project.org/web/packages/NanoStringNorm/index.html) for heatmap, pca and Volcano plot, respectively.
Receiver operating characteristic (ROC) curve analysis
ROC analysis was used to evaluate the individual discriminatory performance of the genes that showed a p-value < 0.001 on the heatmap for the study group’s comparison. The values of area under the curve (AUC), sensitivity, specificity, and the optimal cut-off points for TB diagnostic tests (Table 2) and to differentiate TB and LTBI subjects (Table 3) are shown. CD274, CEACAM1, CR1, FCGR1A/B, IFITM1, IRAK3, LILRA6, MAPK14, PDCD1LG2 genes (all of them presented AUC = 1.0, 100% of sensitivity and specificity) seems promising targets to distinguish TB and OPD patients (Table 2) (see Supplementary Fig. S1). Table 3 presents seven possible candidates to be further evaluated as a predictor of TB progression, including the CCR2, which showed an AUC = 1.0 and both sensitivity and specificity of 100% (see Supplementary Fig. S2).
Table 2.
ROC analysis, sensibility and specificity of candidate genes to TB diagnosis.
| Gene | ROC AUC (95% CI) | ROC p-value | Sensitivity % (95% CI) | Specificity % (95% CI) | Cut-off |
|---|---|---|---|---|---|
| C1QA | 0.98 (0.95–1.02) | < 0.0001 | 88.24 (63.56–98.54) | 100 (66.37–100) | > 3.74 |
| CD274 | 1.00 | < 0.0001 | 100 (80.49–100) | 100 (66.37–100) | > 5.71 |
| CD59 | 0.98 (0.95–1.02) | < 0.0001 | 94.12 (71.31–99.85) | 100 (66.37–100) | > 8.63 |
| CEACAM1 | 1.00 | < 0.0001 | 100 (80.49–100) | 100 (66.37–100) | > 6.02 |
| CR1 | 1.00 | < 0.0001 | 100 (80.49–100) | 100 (66.37–100) | > 10.02 |
| FCGR1A/B | 1.00 | < 0.0001 | 100 (80.49–100) | 100 (66.37–100) | > 7.83 |
| ICAM1 | 0.98 (0.95–1.02) | < 0.0001 | 88.24 (63.56–98.54) | 100 (66.37–100) | > 7.54 |
| IFITM1 | 1.00 | < 0.0001 | 100 (80.49–100) | 100 (66.37–100) | > 12.86 |
| IL18RAP | 0.98 (0.95–1.02) | < 0.0001 | 88.24 (63.56–98.54) | 100 (66.37–100) | > 8.97 |
| IL4R | 0.98 (0.95–1.02) | < 0.0001 | 94.12 (71.31–99.85) | 100 (66.37–100) | > 9.08 |
| IRAK3 | 1.00 | < 0.0001 | 100 (80.49–100) | 100 (66.37–100) | > 6.86 |
| JAK2 | 0.99 (0.97–1.01) | < 0.0001 | 94.12 (71.31–99.85) | 100 (66.37–100) | > 9.86 |
| JAK3 | 0.99 (0.97–1.01) | < 0.0001 | 94.12 (71.31–99.85) | 100 (66.37–100) | > 8.59 |
| LILRA5 | 0.97 (0.92–1.02) | < 0.0001 | 88.24 (63.56–98.54) | 100 (66.37–100) | > 9.87 |
| LILRA6 | 1.00 | < 0.0001 | 100 (80.49–100) | 100 (66.37–100) | > 7.79 |
| LY96 | 0.99 (0.97–1.01) | < 0.0001 | 94.12 (71.31–99.85) | 100 (66.37–100) | > 7.69 |
| MAPK14 | 1.00 | 0.0008757 | 100 (80.49–100) | 100 (47.82–100) | > 10.03 |
| NOD2 | 0.92 (0.81–1.04) | 0.004259 | 82.35 (56.57–96.20) | 100 (47.82–100) | > 8.79 |
| PDCD1LG2 | 1.00 | 0.0008757 | 100 (80.49–100) | 100 (47.82–100) | > 4.13 |
| PML | 0.98 (0.95–1.02) | 0.001156 | 94.12 (71.31–99.85) | 100 (47.82–100) | > 7.72 |
| SOCS3 | 0.96 (0.88–1.04) | 0.001981 | 94.12 (71.31–99.85) | 100 (47.82–100) | > 7.40 |
| TAP1 | 0.97 (0.92–1.03) | 0.001518 | 88.24 (63.56–98.54) | 100 (47.82–100) | > 8.63 |
| TNFAIP6 | 0.98 (0.95–1.02) | 0.001156 | 94.12 (71.31–99.85) | 100 (47.82–100) | > 6.02 |
Table 3.
ROC analysis, sensibility and specificity of candidate genes to predict TB reactivation.
| Gene | ROC AUC (95% CI) | ROC p-value | Sensitivity % (95% CI) | Specificity % (95% CI) | Cut-off |
|---|---|---|---|---|---|
| C1QB | 0.97 (0.92–1.02) | 0.0003360 | 88.24 (63.56–98.54) | 100 (59.04–100) | > 4.15 |
| C2 | 0.92 (0.82–1.02) | 0.001348 | 82.35(56.57–100) | 100 (59.04–100) | > 4.16 |
| CCR2 | 1.00 | 0.0001594 | 100 (80.49–100) | 100 (59.04–100) | > 8.61 |
| CCRL2 | 0.86 (0.71–1.01) | 0.005753 | 82.35 (56.57–96.20) | 85.71 (42.13–99.64) | > 5.31 |
| LILRB4 | 0.94 (0.82–1.05) | 0.0008613 | 94.12 (71.31–99.85) | 100 (59.04–100) | > 6.56 |
| MAPK14 | 0.95 (0.88–1.03) | 0.0005420 | 88.24 (63.56–98.54) | 100 (59.04–100) | > 10.54 |
| MSR1 | 0.99 (0.96–1.01) | 0.0002051 | 94.12 (71.31–99.85) | 100 (59.04–100) | > 4.72 |
Discussion
The World Health Organization (WHO) identified the need for a non-sputum-based test as a high-priority for TB diagnosis and suggested that a rapid biomarker-based test should be easy to perform and implement at health posts; should increase the number of patients diagnosed with TB; should have sensitivity > 98% among patients with smear-positive, culture-positive, and ≥ 68% for smear-negative and culture-positive pulmonary TB in adults; and the test would ideally be able to diagnose adults and children, and pulmonary TB and extrapulmonary TB alike14. Here, we performed a multiplex gene expression analysis in a single assay for more than 500 inflammatory genes in whole blood samples. By this approach, of all 30 genes herein identified, 23 were candidate targets to diagnose active TB and seven can be validated as biomarkers to distinguish LTBI and TB. All those 30 genes showed sensitivity and specificity > 82%, and ROC AUC > 0.8.
A major challenge to interrupt the TB transmission cycle is to predict when an individual with LTBI will develop active TB. Here, we identified seven genes that were able to discriminate TB patients from LTBI individuals, all presenting high sensitivity and specificity in ROC curve analysis (Table 3). The expression of five (CCRL2, C1QB, C2, LILRB4, and CCR2) of seven genes placed the donor TB8 (TB patient) in the cluster enriched by the LTBI group (Fig. 3). It is possible that the other two genes (MSR1 and MAPK14), which shared a pattern of expression similar to the TB patients, maybe the first set of genes to undergo a change in the level of expression during progression to active TB. To confirm these findings, it is necessary to carry out an evaluation of the expression of these genes in a cohort with LTBI subjects.
We identified 30 candidate genes to be further tested for TB diagnosis and as biomarkers for TB progression. From 23 genes suggested to be suitable for TB diagnosis, ten were related to adaptive immune response, ten were involved in innate immune response, and the other three genes (JAK2, JAK3, and LY96) were not specifically related to either. Conversely, for TB progression, five of seven genes were components of the innate immune system and were increased in TB patients relative to LTBI volunteers (Table 4). These data suggest the involvement of activation of the innate immune response during progression to active TB in latently infected subjects.
Table 4.
Annotation of selected genes based on ROC curve analysis.
| Symbol | Name | Annotation | Target for |
|---|---|---|---|
| C1QA | Complement C1q A chain | Complement system, host pathogen interaction, innate immune system | TB diagnosis |
| C1QB | Complement C1q B chain | Complement system, host pathogen interaction, innate immune system | TB progression |
| C2 | Complement component 2 | Complement system, host pathogen interaction, innate immune system | TB progression |
| CCR2 | C–C chemokine receptor type 2 | Chemokine signaling, cytokine signaling, innate immune system, lymphocyte activation | TB progression |
| CCRL2 | C–C chemokine ligand type 2 | Chemokine signaling | TB progression |
| CD59 | CD59 molecule | Complement system, innate immune system, lymphocyte activation | TB diagnosis |
| CD274 | CD274 molecule | Adaptive immune system, cell adhesion, lymphocyte activation | TB diagnosis |
| CEACAM1 | CEA cell adhesion molecule 1 | Hemostasis, innate immune system and lymphocyte activation | TB diagnosis |
| CR1 | Complement receptor type 1 | Complement system, host pathogen interaction, innate immune system | TB diagnosis |
| FCGR1A/B | Fc fragment of IgG receptor Ia | Adaptive immune system, cytokine signaling, host pathogen interaction, innate immune system, MHC class I antigen presentation, phagocytosis and degradation and Type II interferon signaling | TB diagnosis |
| ICAM1 | Intercellular adhesion molecule 1 | Adaptive immune system, cell adhesion, innate immune system, lymphocyte activation | TB diagnosis |
| IFITM1 | Interferon induced transmembrane protein 1 | Adaptive immune system, B cell receptor signaling, cytokine signaling and Type I interferon signaling | TB diagnosis |
| IL18RAP | Interleukin 18 receptor accessory protein | Cytokine signaling, oxidative stress | TB diagnosis |
| IL4R | Interleukin 4 receptor | Cytokine signaling, lymphocyte activation and Th2 differentiation | TB diagnosis |
| IRAK3 | Interleukin 1 receptor associated kinase 3 | Cytokine signaling, innate immune system and TLR signaling | TB diagnosis |
| JAK2 | Janus kinase 2 | Chemokine signaling, cytokine signaling, host pathogen interaction, hemostasis, oxidative stress, Th1 and Th17 differentiation, Type II interferon signaling | TB diagnosis |
| JAK3 | Janus kinase 3 | Chemokine signaling, cytokine signaling, host pathogen interaction, hemostasis, lymphocyte activation, Th2 and Th17 differentiation | TB diagnosis |
| LILRA5 | Leukocyte immunoglobulin like receptor A5 | Adaptive immune system | TB diagnosis |
| LILRA6 | Leukocyte immunoglobulin like receptor A6 | Adaptive immune system and MHC class I antigen presentation | TB diagnosis |
| LILRB4 | Leukocyte immunoglobulin like receptor B4 | Adaptive immune system | TB progression |
| LY96 | Lymphocyte antigen 96 | Adaptive immune system, apoptosis, host pathogen interaction, innate immune system, MHC class I antigen presentation, NF-κB signaling, TLR signaling | TB diagnosis |
| MAPK14 | Mitogen-activated protein kinase 14 | Cytokine signaling, hemostasis, host pathogen interaction, innate immune system, lymphocyte trafficking, NLR signaling, T cell receptor signaling, Th17 differentiation, TNF family signaling and TLR signaling | TB diagnosis and TB progression |
| NOD 2 | Nucleotide binding oligomerization domain containing 2 | Cytokine signaling, host pathogen interaction, innate immune system, lymphocyte activation, NLR signaling, TNF family signaling, TLR signaling | TB diagnosis |
| MSR1 | Macrophage scavenger receptor 1 | Phagocytosis and degradation | TB progression |
| PDCD1LG2 | Programmed cell death 1 ligand 2 | Adaptive immune system, cell adhesion and lymphocyte activation | TB diagnosis |
| PML | Promyelocytic leukemia | Cytokine signaling, host pathogen interaction, oxidative stress, Type II interferon signaling | TB diagnosis |
| SOCS3 | Suppressor of cytokine signaling 3 | Adaptive immune system, cytokine signaling, host pathogen interaction, MHC class I antigen presentation, TNF family signaling, Type I interferon signaling and Type II interferon signaling | TB diagnosis |
| TAP1 | Transporter 1, ATP binding cassette subfamily B member | Adaptive immune system, host pathogen interaction, MHC class I antigen presentation, phagocytosis and degradation | TB diagnosis |
| TNFAIP6 | Tumor necrosis factor alpha induced protein 6 | Innate immune system | TB diagnosis |
TB Tuberculosis, LTBI latent tuberculosis infection.
Previously identified genes that can discriminate TB patient from non-TB patients and TB risk11,13,15–25 either do not fill the minimum sensitivity requirements in adults regardless of HIV status for a POC test (95% in smear-positive culture-confirmed cases and 60–80% in smear-negative culture-confirmed cases), or they proposed gene signatures-based tests which are very difficult to implement. Here, although the number of participants was a limiting issue, we identified single candidate genes for TB diagnosis and progression, all of them presenting high levels of AUC, sensitivity, and specificity.
This study provided valuable information on the development of new diagnostic tests for TB. When validated in a larger population-based study, the expression of the genes herein identified can compose new tools that will overcome the limitations of the currently available diagnostic tests, including low sensibility, long time consuming to perform, and requirement of sputum samples collection. Besides, some of the genes can distinguish seek people with TB from those latently infected. These targets need to be further validated as a possible biomarker to predict TB reactivation in a prospective cohort study.
Methods
Study participants
Subjects were recruited between November 2015 to December 2016. Written informed consent was obtained from all participants. Our study included 35 participants, 17 active TB, and 18 controls from which seven were healthy donors with latent M. tuberculosis infection (LTBI), six were uninfected health controls (HC), and five were patients with asthma (OPD). All participants were recruited at the Instituto Brasileiro para Investigação de Tuberculose (IBIT), Bahia, Brazil and 2° Centro de Saúde Rodrigo Argolo, Bahia, Brazil. TB patients were confirmed to have active pulmonary TB by chest X-ray and at least sputum smear microscopy and/or culture positive. Symptomatic patients with sputum smear microscopy negative had TB confirmed by TB culture. TB patients with no sputum smear microscopy and/or culture screened had TB diagnosis by the Xpert MTB-Rif system. The blood sample was collected prior to TB treatment. Household contacts of TB patients were defined as belonging to either LTBI or HC groups, according to QuantiFERON-TB (QFT) Gold In-Tube test. Those with QTF Gold In-tube test negative (cut-off ≤ 0.35 IU/mL) were considered healthy controls while the household contacts with positive results (cut-off > 0.35 IU/mL) were considered LTBI patients. OPD group was composed of patients who sought care with suspected pulmonary TB but were negative to both sputum smear microscopy and culture. Individuals who tested positive for human immunodeficiency virus and patients taking immunosuppressive drugs were excluded. All subjects were between 18 and 65 years old.
RNA isolation
For each donor, we collected 2.5 mL peripheral blood in a PAXgene blood RNA tube (PreAnalytiX). RNA was isolated and purified with the PAXgene Blood RNA kit (Qiagen), according to the manufacturer’s protocol for gene expression analysis by NanoString technology. RNA quantification and quality were assessed by Nanodrop.
NanoString
We performed gene expression assays at the Molecular Oncology Research Center, Barretos Cancer Hospital, Barretos, Brazil using the NanoString technology with nCounter Immunology Panel that contains 594 targets and 15 internal reference genes. Up to 100 ng of total RNA per sample was used and protocol was performed according to manufacturer’s recommendations. Briefly, RNA was hybridized with reporter and capture probes (NanoString Technologies) and incubated at 67 °C for 21 h. Samples were then loaded onto automated nCounter Prep Station (NanoString, Technologies) for sample purification and immobilization in cartridges. Finally, cartridges were transferred to nCounter Digital Analyzer (NanoString Technologies) to capture image in 280 fields of view (FOVs) providing all gene counts.
Data analysis
The files corresponding to each cartridge were initially analyzed in nSolver Software (NanoString Technologies) for quality control assessment. Then, we analyzed the data in R statistical environment (version 3.6.3)26. Distributions of raw counts were evaluated in quantro package27. Normalization and differential expression were carried out with NanoStringNorm package28. Raw data were normalized with the geometric mean of positive control and housekeeping genes. Hierarchical clustering with Pearson correlation coefficient distance of differentially expressed genes was performed on ComplexHeatmap package29. The ability of genes to discriminate the study groups was evaluated with receiver operating characteristic (ROC) curves and the graphic representation was created by the statistical analysis system GraphPad Prism.
Ethical statement
The study was approved by the Research Ethics Council (CEP) of Maternidade Climério de Oliveira from Universidade Federal da Bahia, CAAE: 48844315.8.0000.5543. Following the basic norms of CEP, Resolution 466/12, all study participants were verbally and in writing informed about the objectives of the study, their participation and IRB contacts and the study coordinator. All participants signed the Consent Form, assuring confidentiality and liberty to leave the study and all methods were performed in accordance with the relevant guidelines and regulations.
Supplementary Information
Acknowledgements
The authors would like to thank the clinical staff from the Instituto Brasileiro para Investigação de Tuberculose for help in examining patients and volunteer recruitment. This work was supported by the Global Health Equity Scholars (GHES) program, Fogarty International Center [TW009338] and in part by the Research Program for SUS-PPSUS/BA [SUS 0027/2018]. JP was a recipient of a Ph.D. fellowship from Oswaldo Cruz Foundation/FIOCRUZ and AQ was a recipient of the GHES fellowship. LSS is supported by the Public Ministry of Labor Campinas (Research, Prevention and Education of Occupational Cancer) in Campinas, Brazil.
Author contributions
J.D.P., L.E.A. and L.S. wrote the main manuscript text and make substantial contributions to conception and design, acquisition of data and analysis and interpretation of data; A.C.L. and I.M. make substantial contributions to acquisition of data; R.M.R., E.M.N., L.W.R. and S.A. participate in drafting the article or revising it critically for important intellectual content; and A.Q. give final approval of the version to be submitted and any revised version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jéssica D. Petrilli and Luana E. Araújo.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-78793-2.
References
- 1.World Health Organization . Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 2.Pai M, et al. Tuberculosis. Nat. Rev. Dis. Primer. 2016;2:16076. doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 3.Vilchèze, C. & Kremer, L. Acid-fast positive and acid-fast negative Mycobacterium tuberculosis: The Koch Paradox. Microbiol. Spectr.5, TBTB2-0003-2015 (2017). [DOI] [PubMed]
- 4.Squire SB, et al. ‘Lost’ smear-positive pulmonary tuberculosis cases: Where are they and why did we lose them? Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2005;9:25–31. [PubMed] [Google Scholar]
- 5.Giang DC, et al. Prospective evaluation of GeneXpert for the diagnosis of HIV-negative pediatric TB cases. BMC Infect. Dis. 2015;15:70. doi: 10.1186/s12879-015-0814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartung TK, Maulu A, Nash J, Fredlund VG. Suspected pulmonary tuberculosis in rural South Africa–sputum induction as a simple diagnostic tool? South Afr. Med. J. Suid-Afr. Tydskr. Vir Geneeskd. 2002;92:455–458. [PubMed] [Google Scholar]
- 7.Boehme CC, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer-Barber KD, Barber DL. Innate and adaptive cellular immune responses to Mycobacterium tuberculosis infection. Cold Spring Harb. Perspect. Med. 2015;5:a018424. doi: 10.1101/cshperspect.a018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maertzdorf J, et al. Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PLoS ONE. 2011;6:e26938. doi: 10.1371/journal.pone.0026938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty M, Wallis RS, Zumla A. WHO-Tropical Disease Research/European Commission joint expert consultation group. Biomarkers for tuberculosis disease status and diagnosis. Curr. Opin. Pulm. Med. 2009;15:181–187. doi: 10.1097/MCP.0b013e328326f42c. [DOI] [PubMed] [Google Scholar]
- 11.Berry MPR, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S-W, et al. Gene expression profiling identifies candidate biomarkers for active and latent tuberculosis. BMC Bioinform. 2016;17:S3. doi: 10.1186/s12859-015-0848-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen M, et al. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J. Mol. Med. Berl. Ger. 2007;85:613–621. doi: 10.1007/s00109-007-0157-6. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. High-PriorityTargetProductProfilesforNewTuberculosisDiagnostics:ReportofaConsensusMeeting:WorldHealthOrganization. (2014).
- 15.Zak DE, et al. A blood RNA signature for tuberculosis disease risk: A prospective cohort study. Lancet Lond. Engl. 2016;387:2312–2322. doi: 10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darboe F, et al. Detection of tuberculosis recurrence, diagnosis and treatment response by a blood transcriptomic risk signature in HIV-infected persons on antiretroviral therapy. Front. Microbiol. 2019;10:1441. doi: 10.3389/fmicb.2019.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Costa LL, et al. A real-time PCR signature to discriminate between tuberculosis and other pulmonary diseases. Tuberc. Edinb. Scotl. 2015;95:421–425. doi: 10.1016/j.tube.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney TE, Braviak L, Tato CM, Khatri P. Genome-wide expression for diagnosis of pulmonary tuberculosis: A multicohort analysis. Lancet Respir. Med. 2016;4:213–224. doi: 10.1016/S2213-2600(16)00048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson ST, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N. Engl. J. Med. 2014;370:1712–1723. doi: 10.1056/NEJMoa1303657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaforou M, et al. Detection of tuberculosis in HIV-infected and -uninfected African adults using whole blood RNA expression signatures: A case-control study. PLoS Med. 2013;10:e1001538. doi: 10.1371/journal.pmed.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom CI, et al. Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PLoS ONE. 2013;8:e70630. doi: 10.1371/journal.pone.0070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leong S, et al. Existing blood transcriptional classifiers accurately discriminate active tuberculosis from latent infection in individuals from south India. Tuberculosis. 2018;109:41–51. doi: 10.1016/j.tube.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Sambarey A, et al. Unbiased identification of blood-based biomarkers for pulmonary tuberculosis by modeling and mining molecular interaction networks. EBioMedicine. 2017;15:112–126. doi: 10.1016/j.ebiom.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhagen LM, et al. A predictive signature gene set for discriminating active from latent tuberculosis in Warao Amerindian children. BMC Genom. 2013;14:74. doi: 10.1186/1471-2164-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suliman S, et al. Four-gene Pan-African blood signature predicts progression to tuberculosis. Am. J. Respir. Crit. Care Med. 2018 doi: 10.1164/rccm.201711-2340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. R: A Language and Environment for Statistical Computing, 2019. (2019).
- 27.Hicks SC, Irizarry RA. quantro: A data-driven approach to guide the choice of an appropriate normalization method. Genome Biol. 2015;16:117. doi: 10.1186/s13059-015-0679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waggott D, et al. NanoStringNorm: An extensible R package for the pre-processing of NanoString mRNA and miRNA data. Bioinformatics. 2012;28:1546–1548. doi: 10.1093/bioinformatics/bts188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinform. Oxf. Engl. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.