Abstract
Objective
Association of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) use with coronavirus disease 2019 (COVID-19) remains controversial. We aimed to investigate the impact of ACEI/ARB use on all-cause mortality in severe COVID-19 patients with hypertension.
Methods
We enrolled 650 COVID-19 patients from Changsha and Wuhan city between 17 January 2020 and 8 March 2020. Demographic, clinical characteristics, and outcomes were collected. Multivariable analysis and propensity-score matching were performed to assess the impact of ACEI/ARB therapy on mortality.
Results
Among the 650 patients, 126 who had severe COVID-19 concomitant with hypertension were analyzed. The average age was 66 years and 56 (44.4%) were men. There were 37 ACEI/ARB users and 21 in-hospital deaths (mortality rate, 16.7%). Male sex (odds ratio [OR], 5.13; 95% confidence interval [CI], 1.75 to 17.8), but not ACEI/ARB use (OR, 1.09; 95%CI, 0.31 to 3.43), was an independent risk factor for mortality in severe COVID-19 patients with hypertension. After propensity-score matching, 60 severe COVID-19 patients were included and no significant correlation between use of ACEI/ARB and mortality was observed.
Conclusions
There was no significant association of ACEI/ARB use with mortality in severe COVID-19 patients with hypertension. These findings support the continuation of ACEI/ARB therapy for such patients.
Keywords: Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, coronavirus disease 2019, hypertension, renin–angiotensin system inhibitors, severe, mortality
Introduction
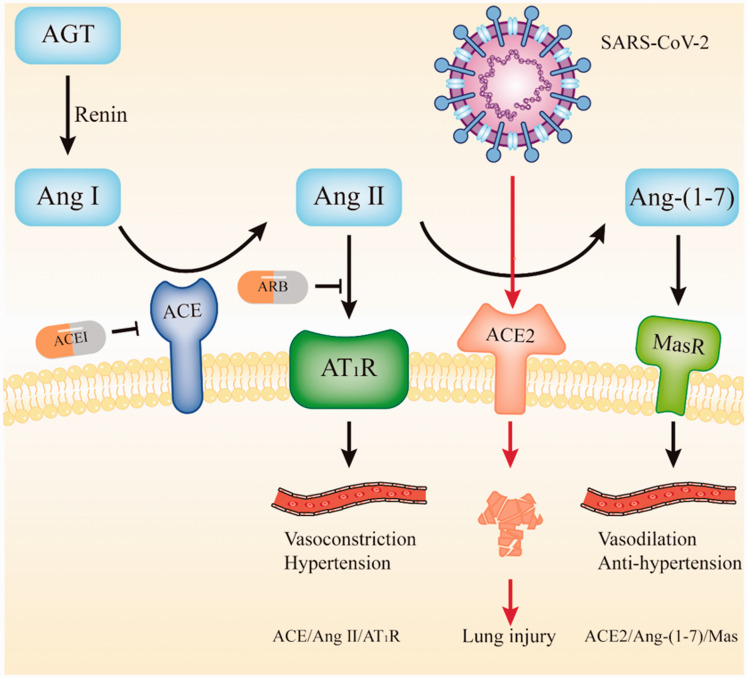
In late 2019, a novel coronavirus disease 2019 (COVID-19) was identified in Wuhan, China, and this disease is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2 COVID-19 has since developed into a global pandemic, and it has brought significant challenges to human society. Angiotensin-converting enzyme 2 (ACE2), which is both the cell entry receptor of SARS-CoV-23 and a member of the renin–angiotensin system (RAS), plays a crucial role in SARS-CoV-2 infection and blood pressure regulation (Figure 1). ACE inhibitors (ACEI) and angiotensin receptor blockers (ARB), which are two RAS inhibitors, are commonly prescribed drugs for hypertension.4 On the one hand, some studies show that ACEI/ARB increases ACE2 expression, which plays a role in promoting viral cell entry and disease progression in hypertensive patients.5,6 On the other hand, evidence confirms that the binding of SARS-Cov Spike protein to ACE2 downregulates ACE2 expression and causes acute lung injury, but it can be attenuated by ARB.7 Similarly, RAS inhibitors may promote both viral cell entry and lung injury intervention by enhancing ACE2 expression (Figure 1). Therefore, the impact of RAS inhibitors use on the clinical condition of COVID-19 patients is conflicting and remains to be further explored.
Figure 1.
The renin–angiotensin system and SARS-COV-2 infection.
AGT, angiotensinogen; Ang I, angiotensin I; Ang II, angiotensin II; Ang-(1–7), angiotensin-(1–7); AT1R, angiotensin II type 1 receptor; ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Multiple studies have investigated the effects of ACEI/ARB use on COVID-19 patients.8–16 Some studies consistently proposed that ACEI/ARB therapy does not affect the risk of SARS-CoV-2 infection8,9,12,15 or the risk of developing severe disease.10,12,14 However, no unanimous conclusion has been reached regarding the impacts of ACEI/ARB therapy on the risk of death. Death often occurs in severe COVID-19 patients with comorbidities such as hypertension.17,18 There is a lack of clinical data on the association of ACEI/ARB use with mortality in severe COVID-19 patients. Therefore, this study was performed to investigate whether ACEI/ARB administration influences all-cause mortality in severe COVID-19 patients with hypertension.
Methods
Patients and study design
This retrospective observational study enrolled 650 COVID-19 patients who were admitted to the Public Health Treatment Center of Changsha and Tongji Hospital, Tongji Medical College of Huazhong University of Science & Technology between 17 January 2020 and 8 March 2020. Severe COVID-19 patients with concomitant hypertension were selected and further analyzed. Demographic, clinical characteristics, drug use for comorbidities, and outcomes were collected. Clinical characteristics included comorbidities, symptoms, and time from illness onset to admission. The study was approved by the institutional ethics board at the Second Xiangya Hospital, Central South University (No. 2020001 and No. 2020026). Written informed consent was obtained from all patients during hospitalization.
COVID-19 was confirmed using next-generation sequencing or real-time reverse transcription-polymerase chain reaction (RT-PCR) in specimens from the respiratory tract.1 COVID-19 patients with severe events included both severe and critically ill patients who met one of the following: respiratory rate ≥30/minute; finger oxygen saturation at rest ≤93%; oxygenation index (arterial blood oxygen partial pressure/oxygen concentration) ≤300 mmHg; mechanical ventilation; shock; or intensive care unit admission because of other organ failure.19 All hypertensive patients were diagnosed before admission, and they self-reported this condition.
Definition of antihypertensive drug administration
Antihypertensive drugs were categorized as follows: ARB, ACEI, calcium channel blockers, beta-blockers, and diuretics. Antihypertensive drug administration was defined as taking medication regularly as recommended by doctors until admission. Compound antihypertensive agents were determined by the active ingredients.
Propensity-score matching analysis
Propensity-score matching was used to minimize the effect of potential confounders. ACEI/ARB users were matched 1:1 with non-ACEI/ARB users on the basis of age, sex, coronary heart disease, and statin use using exact matching with a caliper size of 0.02. Propensity-score matching was performed using EmpowerStats (Solutions, Inc., Boston, MA, USA, R 3.4.3).
Statistical analysis
Continuous variables with a normal distribution were expressed as the mean and standard deviation (SD) and compared using an independent group t-test. Non-normally distributed continual variables were described using the median and interquartile range and compared using the Mann–Whitney U-test. Categorical variables were described as numbers and percentages and compared using the Pearson chi-square test or Fisher’s exact test. The adjusted odds ratios (OR) and the corresponding 95% confidence intervals (CI) were calculated in the multivariable analysis.
To analyze the impact of treatment with ACEI/ARB on mortality in severe COVID-19 patients with hypertension, binary multivariate logistic regression analysis was performed. Statistically significant variables in the univariate analysis and some known confounders were included in the multivariate model. Statistical analyses were conducted using IBM SPSS Statistics 22.0 (IBM Corp., Armonk, NY, USA). A two-sided α of less than .05 was considered to be statistically significant.
Results
As of 8 March 2020, 126 severe COVID-19 patients with hypertension in Changsha and Wuhan city were included in the analysis. The average age was 66 years, and 46 (36.5%) patients were older than 70 years old. There were 56 (44.4%) men, 37 (29.4%) ACEI/ARB users, and 21 in-hospital deaths (mortality rate, 16.7%). Clinical characteristics and outcomes were included and compared between ACEI/ARB users and non-ACEI/ARB users, as shown in Table 1. Patients with and without ACEI/ARB therapy had similar comorbidities except for coronary artery disease, clinical symptoms, and time from illness onset to admission. ACEI/ARB users had a higher prevalence of coronary artery disease and a higher proportion of patients who were administered the corresponding drugs such as statins and antiplatelet drugs than those patients who did not use ACEI/ARB.
Table 1.
Characteristics of severe COVID-19 patients with hypertension in the ACEI/ARB and non-ACEI/ARB groups.
| All patients (n = 126) | ACEI/ARB(n = 37) | Non-ACEI/ARB(n = 89) | p value | |
|---|---|---|---|---|
| Age, mean (SD), years | 66.3 (10.6) | 65.6 (11.6) | 66.6 (10.1) | 0.628 |
| ≤70 | 80 (63.5) | 22 (59.5) | 58 (65.2) | 0.544 |
| >70 | 46 (36.5) | 15 (40.5) | 31 (34.8) | |
| Sex, male, n (%) | 56 (44.4) | 16 (43.2) | 40 (44.9) | 0.861 |
| Other comorbidities, n (%) | ||||
| Diabetes | 41 (32.5) | 11 (29.7) | 30 (33.7) | 0.664 |
| Coronary artery disease | 21 (16.7) | 11 (29.7) | 10 (11.2) | 0.011 |
| Chronic pulmonary disease | 8 (6.3) | 3 (8.1) | 5 (5.6) | 0.602 |
| Cerebrovascular disease | 10 (7.9) | 3 (8.1) | 7 (7.9) | 1.000 |
| Malignant tumors | 8 (6.3) | 3 (8.1) | 5 (5.6) | 0.692 |
| Chronic hepatorenal disease | 3 (2.4) | 0 (0) | 3 (3.4) | 0.555 |
| Symptoms, n (%) | ||||
| Fever | 96 (76.2) | 29 (78.4) | 67 (75.3) | 0.710 |
| Cough | 86 (68.3) | 22 (59.5) | 64 (71.9) | 0.172 |
| Expectoration | 39 (31.0) | 6 (11.5) | 33 (37.1) | 0.021 |
| Fatigue | 56 (44.4) | 19 (51.4) | 37 (41.6) | 0.314 |
| Chill | 26 (20.6) | 6 (16.2) | 20 (22.5) | 0.429 |
| Anorexia | 30 (23.8) | 9 (24.3) | 21 (23.6) | 0.930 |
| Myalgia | 28 (22.2) | 9 (24.3) | 19 (21.3) | 0.714 |
| Dyspnea | 69 (54.8) | 17 (45.9) | 52 (58.4) | 0.200 |
| Pharyngalgia | 7 (5.6) | 1 (2.7) | 6 (6.7) | 0.673 |
| Diarrhea | 45 (35.7) | 10 (27.0) | 35 (39.3) | 0.189 |
| Time from onset to admission, median (IQR), days | 19 (15.1) | 10 (6.0–13.0) | 9 (5.0–14.0) | 0.818 |
| ≥10 | 61 (48.4) | 19 (51.4) | 42 (47.2) | 0.670 |
| <10 | 65 (51.6) | 18 (48.6) | 47 (52.8) | |
| Treatment, n (%) | ||||
| Beta-blocker | 15 (11.9) | 6 (16.2) | 9 (10.1) | 0.371 |
| CCB | 96 (76.2) | 21 (56.8) | 75 (84.3) | 0.001 |
| Diuretic | 5 (4.0) | 4 (10.8) | 1 (1.1) | 0.026 |
| Statins | 11 (8.7) | 9 (24.3) | 2 (2.2) | <0.001 |
| Antidiabetic | 30 (23.8) | 7 (18.9) | 23 (25.8) | 0.406 |
| Antiplatelet drugs | 18 (14.3) | 11 (29.7) | 7 (7.9) | 0.001 |
| Deaths | 21 (16.7) | 6 (16.2) | 15 (16.9) | 0.930 |
COVID-19, coronavirus disease 2019; SD, standard deviation; IQR, interquartile range; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; CCB, calcium channel blockers.
To analyze the risk factors for death, 126 severe COVID-19 patients were divided into the following two groups: survivors (n = 21) and non-survivors (n = 105). The characteristics of these two groups were compared (Table 2). Death was more common in men compared with women (p = 0.001), and non-survivors were older than survivors (p=0.035). ACEI/ARB administration was not significantly different between survivors and non-survivors in the univariable analysis (29.5% vs. 28.6%). Other comorbidities, clinical symptoms, and the median time from illness onset to admission were also not significantly different between these groups.
Table 2.
Characteristics of severe COVID-19 patients with hypertension between survivors and non-survivors.
| Survivors (n = 105) | Non-survivors (n = 21) | p value | |
|---|---|---|---|
| Age, mean (SD), years | 65.4 (10.6) | 70.8 (9.4) | 0.035 |
| ≤70 | 70 (66.7) | 10 (47.6) | 0.098 |
| >70 | 35 (33.3) | 11 (52.4) | |
| Sex, male, n (%) | 40 (38.1) | 16 (76.2) | 0.001 |
| Other comorbidities, n (%) | |||
| Diabetes | 36 (34.3) | 5 (23.8) | 0.350 |
| Coronary artery disease | 16 (15.2) | 5 (23.8) | 0.344 |
| Chronic pulmonary disease | 5 (4.8) | 3 (14.3) | 0.128 |
| Cerebrovascular disease | 10 (9.5) | 0 (0) | 0.211 |
| Malignant tumors | 7 (6.7) | 1 (4.8) | 1.000 |
| Chronic hepatorenal disease | 1 (1.0) | 2 (9.5) | 0.072 |
| Symptoms, n (%) | |||
| Fever | 78 (74.3) | 18 (85.7) | 0.262 |
| Cough | 70 (66.7) | 16 (76.2) | 0.392 |
| Expectoration | 31 (29.5) | 8 (38.1) | 0.438 |
| Fatigue | 46 (43.8) | 10 (47.6) | 0.748 |
| Chill | 20 (19.0) | 6 (28.6) | 0.377 |
| Anorexia | 22 (21.0) | 8 (38.1) | 0.092 |
| Myalgia | 21 (20.0) | 7 (33.3) | 0.248 |
| Dyspnea | 54 (51.4) | 15 (71.4) | 0.093 |
| Pharyngalgia | 6 (5.7) | 1 (4.8) | 1.000 |
| Diarrhea | 37 (35.2) | 8 (38.1) | 0.803 |
| Time from onset to admission, median (IQR), days | 10 (5.5–14.0) | 7 (5.0–10.5) | 0.425 |
| ≥10 | 53 (50.5) | 6 (38.1) | 0.300 |
| <10 | 52 (49.5) | 13 (61.9) | |
| Treatment, n (%) | |||
| ACEI/ARB administration | 31 (29.5) | 6 (28.6) | 0.930 |
| Beta-blocker | 14 (13.3) | 1 (4.8) | 0.463 |
| CCB | 81 (77.1) | 15 (71.4) | 0.575 |
| Diuretic | 5 (4.8) | 0 (0) | 0.589 |
| Statins | 9 (8.6) | 2 (9.5) | 1.000 |
| Antidiabetic | 26 (24.8) | 4 (19.0) | 0.575 |
| Antiplatelet drugs | 14 (13.3) | 4 (19.0) | 0.500 |
COVID-19, coronavirus disease 2019; SD, standard deviation; IQR, interquartile range; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; CCB, calcium channel blockers.
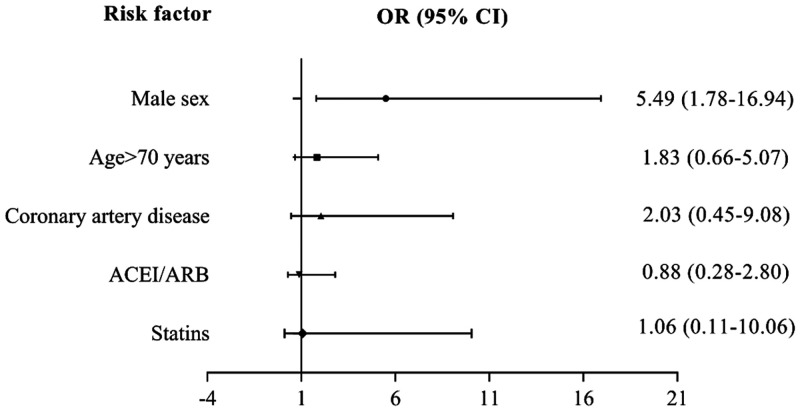
To further investigate the impact of ACRI/ARB use on mortality in severe COVID-19 patients with hypertension, we included ACEI/ARB, age >70 years, sex, coronary artery disease, and statin use in the multivariable logistic model. Only male sex (adjusted OR, 5.49; 95% CI, 1.78 to 16.94) was an independent risk factor for death (Figure 2). We observed no significant association between ACEI/ARB and mortality in severe COVID-19 patients with hypertension (adjusted OR, 0.88; 95% CI, 0.28 to 2.80).
Figure 2.
Multivariable analysis of risk factors for death in severe COVID-19 patients with hypertension.
COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers.
To increase the credibility of the conclusion, we also performed propensity-score matching. Thirty ACEI/ARB users and 30 non-ACEI/ARB users (1:1) were matched, and their characteristics were summarized in Table 3. Age, sex, comorbidities, and drugs that were used for comorbidities were similar between ACEI/ARB users and non-ACEI/ARB users after propensity-score matching. Additionally, ACEI/ARB use did not correlate with mortality in severe COVID-19 patients with hypertension (16.7% vs. 20.0%).
Table 3.
Characteristics and clinical outcomes of severe COVID-19 patients with hypertension after propensity-score matching.
| ACEI/ARB(n = 30) | Non-ACEI/ARB(n = 30) | p value | |
|---|---|---|---|
| Age, mean (SD), years | 64.4 (11.8) | 67.8 (11.5) | 0.257 |
| ≤70 | 18 (60.0) | 15 (50.0) | 0.436 |
| >70 | 12 (40.0) | 15 (50.0) | |
| Sex, male, n (%) | 15 (50.0) | 17 (56.7) | 0.605 |
| Other comorbidities, n (%) | |||
| Diabetes | 8 (26.7) | 8 (26.7) | 1.000 |
| Coronary artery disease | 5 (16.7) | 5 (16.7) | 1.000 |
| Chronic pulmonary disease | 3 (10.0) | 3 (10.0) | 1.000 |
| Cerebrovascular disease | 2 (6.7) | 2 (6.7) | 1.000 |
| Malignant tumors | 2 (6.7) | 0 (0.0) | 0.492 |
| Chronic hepatorenal disease | 0 (0.0) | 1 (3.3) | 1.000 |
| Time from onset to admission, median (IQR), days | |||
| ≥10 | 14 (46.7) | 14 (46.7) | 1.000 |
| <10 | 16 (53.3) | 16 (53.3) | |
| Treatment, n (%) | |||
| Beta-blocker | 4 (13.3) | 4 (13.3) | 1.000 |
| CCB | 18 (60.0) | 25 (83.3) | 0.045 |
| Diuretic | 3 (10.0) | 1 (3.3) | 0.612 |
| Statins | 2 (6.7) | 2 (6.7) | 1.000 |
| Antidiabetic | 5 (16.7) | 5 (16.7) | 1.000 |
| Antiplatelet drugs | 7 (23.3) | 3 (10.0) | 0.299 |
| Deaths | 5 (16.7) | 6 (20.0) | 0.739 |
COVID-19, coronavirus disease 2019; SD, standard deviation; IQR, interquartile range; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; CCB, calcium channel blockers.
Discussion
In this retrospective study, we found that there was no significant association between ACEI/ARB use and mortality in severe COVID-19 patients with hypertension after adjusting for potential confounders in the multivariable analysis and the propensity-score matching analysis.
The COVID-19 pandemic has raised some serious challenges, and hypertension correlated with both severity and mortality.17,20,21 Therefore, better management of hypertensive patients is of great importance to reduce mortality and improve the overall prognosis of COVID-19 patients. In the multivariable analysis, male sex was an independent risk factor for in-hospital death in severe COVID-19 patients, which was reported in multiple studies.22,23 Another study compared clinical characteristics between 113 deceased and 161 recovered patients with COVID-19, and that study also showed that male sex was associated with death.21 A longitudinal study enrolling 98 COVID-19 patients found that there were higher levels of innate inflammatory cytokines (interleukin [IL]-8 and IL-18) and the chemokine CCL5 in male patients.24 The uncontrolled production of pro-inflammatory factors such as IL-8 and chemokines such as CCL-5, which is also called cytokine storm, could contribute to pulmonary fibrosis and death.25 This may, to some extent, explain the higher mortality rate in male compared with female COVID-19 patients. Severe COVID-19 patients were often in an immunodeficient state that was characterized by fewer multifunctional CD4+ T cells and non-exhausted CD8+ T cells.26 Female COVID-19 patients had higher levels of activated CD38 and HLA-DR-positive T cells and terminally differentiated T cells compared with male COVID-19 patients,24 which may account for better viral resistance and lower mortality in female COVID-19 patients. Moreover, genetic and hormonal mediators, environmental factors (nutrition and microbiota), age, and reproductive status could also differentially modulate the immune system between men and women.27 Future studies on these factors are required to clarify the mechanism of sex differences in COVID-19 patient mortality.
In the clinical setting, whether hypertensive patients with COVID-19 should discontinue ACEI/ARB therapy has been the subject of recent debate. The Heart Failure Society of America, the American College of Cardiology, and the American Heart Association published a joint declaration calling for more studies, and they suggested continuing ACEI/ARB therapy until the harmful effects of ACEI/ARB on COVID-19 patients with hypertension could be verified.28 Recent studies analyzed the influence of ACEI/ARB use on all-cause mortality in COVID-19 patients with various degrees of severity.10–14,16 Some studies showed no significant correlation between ACEI/ARB use and mortality in COVID-19 patients.10,12,13 One of these studies enrolled 362 hypertensive patients,10 while others included both hypertensive and non-hypertensive patients.12,13 However, other studies proposed that ACEI/ARB use lowered the risk of death in hypertensive patients with COVID-19.11,14,16 Whether ACEI/ARB users have a potential survival advantage remains to be elucidated in larger cohorts or randomized controlled trials. Generally, there was no evidence that ACEI/ARB was detrimental to COVID-19 patient survival. The hypothesis that ACEI/ARB administration promotes viral cell entry and disease progression was mainly based on the fact that RAS inhibitors increase ACE2 expression in animal experiments.5,29 Studies in humans did not consistently show upregulation of ACE2 expression by RAS inhibitors.30–32 Therefore, not enough evidence supports that RAS inhibitors aggravate the clinical condition and increase all-cause mortality in COVID-19 patients. Additionally, we revealed that there was no significant association between ACEI/ARB use and mortality in severe COVID-19 patients with hypertension. The findings support the recommendations that severe COVID-19 patients with hypertension should continue to take ACEI/ARB to maintain their blood pressure stability.
This study has some limitations. First, we have a relatively small sample size, so we could not bring more potential confounders into the multivariate model when analyzing the effect of ACEI/ARB use on mortality in severe COVID-19 patients with hypertension. However, potential confounders such as comorbidities, statin use, and antiplatelet drugs were balanced after propensity-score matching. Therefore, this may not affect the validity of the finding that ACEI/ARB use has no significant association with mortality in severe COVID-19 patients. Second, the analysis is retrospective, and thus, selection bias may be present. Additionally, some data such as smoking history are not available. Future prospective studies or randomized controlled trials may help to address this issue. Third, we considered ACEI and ARB to be a single variable, and we could not detect if their effects are different. This requires additional studies with a larger sample size to analyze ACEI and ARB separately. Fourth, only severe COVID-19 patients with hypertension were included in the analysis for risk factors of death. The findings have great implications for severe COVID-19 patients, but these results are not generalizable to all COVID-19 patients.
Conclusion
We demonstrated that ACEI/ARB use had no significant association with mortality in severe COVID-19 patients with concomitant hypertension. These clinical data support the previous guidelines, and continuation of ACEI/ARB therapy is recommended for severe COVID-19 patients with hypertension during the pandemic.
Acknowledgements
The authors thank Trevor Bihl, PhD and Olouwa Shegoun M. Babalao for English language editing.
Footnotes
Authors’ contributions: ZYJ, LXL, and WF provided the data and designed the research. ZLS wrote this paper and analyzed the data. WGB, HCH, WCF, XM, DHY, ZQ, WGY, YB, LJY, WC, ZSY, and LXL collected the data. SL and PY analyzed the data. CCH contributed to the figure. All authors read and approved the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Fang Wu https://orcid.org/0000-0002-6627-3437
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. DOI: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das S, Das S, Ghangrekar MM. The COVID-19 pandemic: biological evolution, treatment options and consequences. Innov Infrastruct Solut 2020; 5: 76. DOI: 10.1007/s41062-020-00325-8. [Google Scholar]
- 3.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 590: 270–273. DOI: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev 2018; 98: 505–553. DOI: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111: 2605–2610. DOI: 10.1161/circulationaha.104.510461. [DOI] [PubMed] [Google Scholar]
- 6.Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 2020; 27: taaa041. DOI: 10.1093/jtm/taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11: 875–879. DOI: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med 2020; 382: 2431–2440. DOI: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020; 5: 1020–1026. DOI: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Wang X, Chen J, et al. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for Coronavirus Disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol 2020; 5: 825–830. DOI: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020; 126: 1671–1681. DOI: 10.1161/circresaha.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020; 324: 168–177. DOI: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung SY, Choi JC, You SH, et al. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis Epub ahead of print 22 May 2020. DOI: 10.1093/cid/ciaa624. [DOI] [PMC free article] [PubMed]
- 14.Guo X, Zhu Y, Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension 2020; 76: e13–e14. DOI: 10.1161/hypertensionaha.120.15572. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med 2020; 382: 2441–2448. DOI: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Wang F, Chen P, et al. Mortality and pre-hospitalization use of renin-angiotensin system inhibitors in hypertensive COVID-19 patients. J Am Heart Assoc Epub ahead of print 18 August 2020. DOI: 10.1161/jaha.120.017736. [DOI] [PMC free article] [PubMed]
- 17.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. DOI: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. DOI: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.New coronavirus pneumonia treatment protocol (5th ed.) (in Chinese), http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440/files/7260301a393845fc87fcf6dd52965ecb.pdf (2020, accessed 27 March 2020).
- 20.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. DOI: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. DOI: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020; 108: 154262. DOI: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020; 146: 110–118. DOI: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature Epub ahead of print 28 August 2020. DOI: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed]
- 25.Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev 2020; 53: 38–42. DOI: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 2020; 19: 102567. DOI: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16: 626–638. DOI: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 28.American College of Cardiology. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19, https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 (2020, accessed 19 May 2020).
- 29.Wang J, He W, Guo L, et al. The ACE2-Ang (1-7)-Mas receptor axis attenuates cardiac remodeling and fibrosis in post-myocardial infarction. Mol Med Rep 2017; 16: 1973–1981. DOI: 10.3892/mmr.2017.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriram K, Insel PA. Risks of ACE inhibitor and ARB usage in COVID-19: evaluating the evidence. Clin Pharmacol Ther 2020; 108: 236–241. DOI: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreutz R, Algharably EAE, Azizi M, et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res 2020; 116: 1688–1699. DOI: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med 2020; 382: 1653–1659. DOI: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]