Abstract
Benign prostatic hyperplasia (BPH) is an age-related debilitating prostatic disease that is frequently associated with prostatic inflammation and bothersome lower urinary tract symptoms (LUTS). Animal models have shown that formalin- and bacterial-induced prostatic inflammation can induce bladder dysfunction; however, the underlying mechanisms contributing to prostatic inflammation in BPH and bladder dysfunction are not clear. We previously reported that E-cadherin expression in BPH is downregulated in hyperplastic nodules compared with expression in adjacent normal tissues. Here, we explored the potential consequences of prostatic E-cadherin downregulation on the prostate and bladder in vivo using an inducible murine model of prostate luminal epithelial-specific deletion of Cdh1. The prostate-specific antigen (PSA)-CreERT2 transgenic mouse strain expressing tamoxifen-inducible CreERT2 recombinase driven by a 6-kb human PSA promoter/enhancer was crossed with the B6.129-Cdh1tm2Kem/J mouse to generate bigenic PSA-CreERT2/Cdh1-/- mice. Deletion of E-cadherin was induced by transient administration of tamoxifen when mice reached sexual maturity (7 weeks of age). At 21 to 23 weeks of age, the prostate, bladder, and prostatic urethra were examined histologically, and bladder function was assessed using void spot assays and cystometry. Mice with Cdh1 deletion had increased prostatic inflammation, prostatic epithelial hyperplasia, and stromal changes at 21 to 23 weeks of age, as well as changes in bladder voiding function compared with age-matched controls. Thus, loss of E-cadherin in the murine prostate could result in prostatic defects that are characteristic of BPH and LUTS, suggesting that E-cadherin downregulation could be a driving force in human BPH development and progression.
Keywords: CDH1, E-cadherin, prostatic inflammation, BPH, bladder overactivity, LUTS
The adherens junction protein E-cadherin is expressed in all mammalian epithelia and plays an important role in epithelial barrier maintenance. In the prostate, repression of E-cadherin gene transcription during development stimulates the maturation and differentiation of the prostatic bud epithelium (1), suggesting that E-cadherin expression in adult prostate is important for maintaining homeostasis. Loss of E-cadherin in adult prostate is thought to play an important role in the development and progression of prostate cancer (reviewed in (2)). Downregulation of E-cadherin expression is also frequently observed in the hyperplastic nodules of benign prostatic hyperplasia (BPH), as compared with expression in adjacent normal prostate (3, 4), implicating a potential role for E-cadherin loss in BPH pathogenesis. Our recent studies revealed that luminal epithelial permeability was increased in BPH tissues (4, 5), which could permit chronic leakage of prostatic secretions into the stromal compartment and subsequently cause prostatic inflammation, bladder overactivity, and voiding abnormality.
We previously identified the presence of secretory proteins PSA and kallikrein-related peptidase 2 (KLK2) in the stromal compartment of BPH specimens accompanied by decreased E-cadherin expression (5), suggesting that E-cadherin downregulation may contribute to “leakiness” of the epithelial barrier of BPH glands. Moreover, PSA and KLK2 were not observed in the stroma of normal prostate adjacent to BPH or prostate cancer tissues (5). More recently, we reported a decrease in tight junction “kiss points” in BPH luminal epithelial cells compared with adjacent normal prostate glands (4). Knockdown of E-cadherin in benign prostatic cell lines BHPrE1 and BPH-1 induced an increase in epithelial barrier permeability and a decrease in the formation of tight junction “kiss points” (4), suggesting that E-cadherin downregulation in BPH might result in an altered epithelial barrier potentially contributing to the leakage of secretory proteins such as PSA into the surrounding stroma observed in mixed BPH nodules (5). The presence of secretory proteins in the prostate stroma might induce stromal inflammation, proliferation, or fibrosis, which are also frequently observed in BPH (6, 7). Chronic prostatic inflammation and increased transforming growth factor β1 (TGF-β1) have been associated with age-related prostatic disease and the onset of lower urinary tract symptoms (LUTS) (reviewed in (8)). TGF-β1 suppressed levels of E-cadherin protein, but not mRNA level, in BPH-1 and BHPrE1 cells (4, 9), suggesting that prostatic inflammation could downregulate E-cadherin. BPH is also the most common cause of LUTS in men, and BPH patients with inflammation were shown to be at increased risk of clinical progression to acute urinary retention or urinary incontinence (10). Inflammation in the rat prostatitis model also results in the downregulation of E-cadherin in prostate epithelium (5). Prostatic E-cadherin downregulation may be associated with a decline in prostate epithelial barrier function and may thus contribute to prostatic inflammation and BPH pathogenesis.
Rodent models of BPH and LUTS have demonstrated that bacterial- or chemical-induced prostatic inflammation can induce bladder overactivity (11-14), suggesting that prostatic inflammation may alter bladder function. Recently, we showed that prostatic inflammation can lower the electrical threshold for the activation of afferent axons in the pelvic nerve, resulting in bladder overactivity and upregulation of nerve growth factor in the bladder (12). These results suggest that prostatic inflammation could cross-sensitize the bladder through primary afferent pathways in the pelvic nerve and contribute to the development of LUTS and symptomatic BPH.
E-cadherin deletion studies in murine prostate epithelial cells have demonstrated context-dependent and cell-type specific effects. In human prostate epithelium, E-cadherin expression has been reported in both basal and luminal cells (15), and murine lineage tracing studies have shown that while E-cadherin is expressed predominantly in luminal epithelial cells of the prostate, it is also expressed in basal epithelial cells (16). In an inducible model of E-cadherin deletion in Nkx3.1 expressing prostate epithelial cells in adult mice, luminal epithelial cells undergo anoikis and are replaced by luminal cells derived from E-cadherin positive basal epithelial cells (17). In this model, the prostate epithelium is replenished without impacting the surrounding stroma or inducing an inflammatory response (17). In another model, probasin-driven deletion of E-cadherin is constitutive and early onset, which induced hyperplasia during development and adulthood and the development of prostate adenocarcinoma in aged animals in one study (16); in a second study, mice developed prostatic intraepithelial neoplasia (PIN)-like lesions that failed to progress to tumors (18). The different phenotypes observed in these 2 knockout models support a role for E-cadherin in both prostate development and adult homeostasis and also suggest that the method and timing of E-cadherin deletion could profoundly affect the prostatic phenotype. However, whether E-cadherin loss in the adult prostate could impact bladder function has not been investigated.
Here we explored the potential functional consequences of prostatic luminal epithelial E-cadherin loss on the adult prostate and bladder in a murine model. Mice were generated with tamoxifen-inducible prostate luminal epithelial-specific deletion of E-cadherin. Cre-mediated recombination was induced at sexual maturity (7 weeks of age) and mice were followed to 21 to 23 weeks of age. Prostate tissues were examined histologically for defects and bladder function was determined using void spot assays and awake cystometry.
Materials and Methods
Generation of prostate-specific Cdh1-deletion mice
Our approach to conditionally delete Cdh1 in the mouse prostate differed from related transgenic models (16, 17) by limiting Cdh1 deletion to adult prostatic luminal epithelial cells. Specifically, mice with prostate luminal epithelial cell–specific deletion of the Cdh1 gene were generated by cross-breeding the B6.129-Cdh1tm2Kem/J mouse (19) (JAX stock #005319, The Jackson Laboratory, Bar Harbor, ME, USA) with the PSA-CreERT2 mouse (generously provided by Dr. Pierre Chambon and Dr. Daniel Metzger, IGBMC, Illkirch, France) on a C57BL/6J background (20). Experimental cohorts were Cre-positive and Cre-negative control animals with homozygous expression of Cdh1 floxed alleles (Supplemental Figure S1A, breeding scheme (21)). Male mice used for breeding purposes were not treated with tamoxifen and were not included in experimental cohorts. The average litter size was 4.46 mice per litter, the average number of males per litter was 2.31 and the average number of females was 2.16. Genotyping was performed using polymerase chain reaction analysis of mouse tail genomic DNA at age 21 days and muscle genomic DNA after euthanization (Supplemental Figure S1B, genotyping gel (21)). Primers specific for Cre recombinase mice were upstream primer 3′-TTGCCTGCATTACCGGTCGATG-5′ and downstream primer 3′-TCCAGCCACCAGCTTGCATG-5′. Primers specific for Cdh1 were upstream primer 3′- GGGTCTCACCGTAGTCCTCA-5′, and downstream primer 3′- GATCTTTGGGAGAGCAGTCG-5′.
Tamoxifen (TAM) induction of Cre activity in mice containing the PSA-CreERT2 allele was performed as previously (22). TAM (Sigma Chemical Co., St. Louis, MO) suspended in Kolliphor EL (Sigma) 3 mg/40 g of body weight was injected intraperitoneally daily for 5 consecutive days in adult mice at 7 weeks of age and animals were followed to 21 to 23 weeks of age (Supplemental Figure S1C (21)). A second cohort of Cre-positive and Cre-negative animals was treated with an additional regimen of tamoxifen injections (3 mg/40 g of body weight) at 19 weeks of age (Supplemental Figure S1C (21)). For each cohort of animals, both Cre-positive and Cre-negative animals were treated with the same regimens of tamoxifen injections. Tamoxifen-treated Cre-negative Cdh1fl/fl mice were utilized as controls, similar to other prostate studies using Cre-inducible murine models (23-25). A total of 20 control and 18 Cdh1-/- mice were generated in cohort 1, and 13 control and 11 Cdh1-/- mice in cohort 2. The number of animals analyzed in each experiment is designated in figure legends and each datapoint represents 1 animal. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh and were conducted in strict accordance with the standards for humane animal care and use as set by the Animal Welfare Act and the National Institutes of Health guidelines for the use of laboratory animals under Animal Welfare Assurance number A3187-01. Animals were weighed every 20 days on a triple beam balance starting at 40 days of age. Lower urinary tract necropsy was performed at euthanization and organs were cleaned of excess fat and membrane with phosphate-buffered saline. The prostate lobes were microdissected away from the urethra. The ventral and the lateral part of the prostate (designated as ventral-lateral prostate, vlp), dorsal (dp) and anterior (ap) prostate lobes were identified in accordance with Sugimara et al. description of the murine prostate lobes, (26) for subsequent microdissection. Lobe mass was determined for the isolated ventral-lateral prostate, dorsal prostate, and anterior prostate lobes after blotting with filtration paper to remove excess liquid. Bladder mass was determined after gently squeezing all urine out and blotting with filtration paper.
Histopathologic analysis
Samples were fixed in 10% formalin for at least 24 hours, then embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. All tissues were examined by a board-certified animal pathologist and a board-certified genitourinary pathologist in a blinded fashion (L.H.R., DMV and R.D., MD). Prostatic defects were identified as epithelial hyperplasia, stromal hyperplasia, inflammation and mouse prostatic intraepithelial neoplasia (mPIN) per the criteria published by Shappell et al, which is commonly used to score prostate lesions in transgenic mouse models (27).
Immunohistochemistry, Masson’s trichrome staining and terminal transferase-mediated DNA end labeling assay
Immunohistochemical stains were performed on 5-μm sections of paraffin-embedded murine tissue specimens as described previously (28). Briefly, sections were de-paraffinized and rehydrated through a graded series of ethanol. Heat-induced epitope retrieval was performed using a BioCare Decloaking Chamber (Biocare Medical, Pacheco, CA), followed by 5 minutes of rinsing in TBS buffer. Primary antibodies for immunostaining of murine tissue sections were mouse monoclonal anti-E-cadherin (29), rabbit monoclonal anti-Ki-67 (30), rabbit monoclonal anti-CD3 (31), rabbit monoclonal anti-CD19 (32), and mouse monoclonal anti-CD68 (33). Slides were then counterstained in hematoxylin and coverslipped. Masson’s trichrome histochemical staining was performed using HT15 (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. Terminal transferase-mediated DNA end labeling (TUNEL) histochemical staining was performed using Apoptag TUNEL Stain (EMD Millipore, Burlington, MA, USA) according to the manufacturer’s protocol. Stained sections were imaged with a Leica DM LB microscope (Leica Microsystems Inc., Bannockburn, IL, USA) equipped with an Imaging Source NII 770 camera (The Imaging Source Europe GmbH, Bremen, Germany) and NIS-Elements Documentation v 4.6 software (Nikon Instruments, Inc., Mellville, NY). All tissues were examined by a board-certified veterinary pathologist (L.H.R.) and a board-certified genitourinary pathologist (R.D.) using light microscopy. For all immunohistochemical analyses, lobes that had been washed away during staining process were not included in quantification analyses. Animal numbers are identified in each graph and reflect the final number of animals with tissues available for quantification after the staining process.
For murine tissues, Ki-67-positive cell density and TUNEL-positive cell density were determined by analysis of sections from at least 5 independent mice from each genotype and from all lobes of the prostate. The average number of Ki-67-positive luminal epithelial cells or stromal cells in each prostate lobe for each mouse was determined from at least 4 nonoverlapping fields imaged at 20× magnification. The average number of TUNEL-positive luminal epithelial cells or stromal cells in each prostate lobe for each mouse was determined from at least 4 fields imaged at 40× magnification with no overlap. The average number of proliferating and apoptotic cells in the ventral, lateral, dorsal, and anterior prostate lobes was determined for each mouse. Inflammatory cell density was determined similarly from at least 4 fields per prostate lobe imaged at 40× magnification with no overlap. The average number of CD19-positive B cells, CD68-positive macrophages, and CD3-positive T cells was also determined for each prostate lobe in each mouse.
Extracellular matrix (ECM) was quantified by calculating the intensity of blue staining in Masson’s trichrome stain using the color deconvolution plugin to separate the blue staining and then ImageJ (34). Five fields from each section were analyzed and an average score was determined for each mouse.
Void spot assay
Mice were placed in metabolic cages and voiding behavior was evaluated for 4 hours. Filter paper was placed under the wire mesh bottom of each metabolic cage and urine stains on the paper were analyzed to determine voiding behavior and voided volumes using the voided stain on paper (VSOP) method (35). Before each analysis, mice were treated with subcutaneous injections of sterile water (20 µL/g body weight) to enhance urine production. The voided volume for each urine spot was calculated using ImageJ (NIH, Bethesda, MD).
Cystometry
Mice were examined by cystometric investigation under a conscious condition as previously (11). Under isoflurane anesthesia, following a lower midline abdominal incision, the bladder was exposed and catheterized with PE-50 tubing (Clay Adams Division of Becton Dickinson, Parsippany, NJ) inserted through the bladder dome. After recovery from anesthesia, conscious mice were placed in a restraining cage (Economy holder 15 to 30 g, Kent Scientific, Torrington, CT, USA). After 1 to 2 hours of acclimation, saline was infused into the bladder at 0.01 mL/min to induce micturition. At least 3 reproducible micturition cycles were recorded after an initial stabilization period (60 minutes). After several micturition cycles, the saline infusion was stopped to evaluate intercontraction intervals, and nonvoiding contractions (NVCs) using Chart software (AD Instruments, Colorado Springs, CO). NVCs were defined as rises in intravesical pressure that exceeded 20% of voiding pressure over the baseline without fluid elimination from the urethral orifice during bladder filling.
Statistical analysis
Comparisons between groups were calculated using the Student t test or 2-tailed Fisher exact test as appropriate. A P value of P < 0.05 was considered significant. GraphPad Prism version 6 was used for graphics (GraphPad Software, San Diego, CA, USA). Values are expressed as means ± standard deviation.
Results
Conditional deletion of E-cadherin in the luminal prostate epithelial cell resulted in epithelial and stromal hyperplasia
Transgenic PSA-CreERT2+/-:Cdh1fl/fl mice (referred to herein as Cdh1-/-) and Cre-negative Cdh1fl/fl control (referred to herein as control) mice were generated and Cre-mediated recombination was induced during the seventh week of age for male mice via a single TAM pulse of 5 daily injections (Supplemental Figure S1C, Cohort 1 (21)) examined for histologic defects at 21 to 23 weeks of age. A second cohort of mice was treated with a second series of tamoxifen injections (double TAM pulse) at 19 weeks of age (Supplemental Figure S1C, Cohort 2 (21)). Control mice were treated similarly, to control for potential changes induced by tamoxifen. Perinatal exposure to tamoxifen was previously reported to induce a decrease in the weight of the lateral prostate lobe and an increase in inflammation in both the lateral and ventral prostate lobes (36). Animals were weighed every 20 days starting at 40 days of age (1 week before tamoxifen induction). Animals in both groups gained weight at similar rates (Fig. 1A). All of the Cdh1-/- and control mice generated and followed in the study survived to the experimental endpoints and exhibited healthy behavior and showed no signs of morbidity or distress up to 23 weeks of age (data not shown).
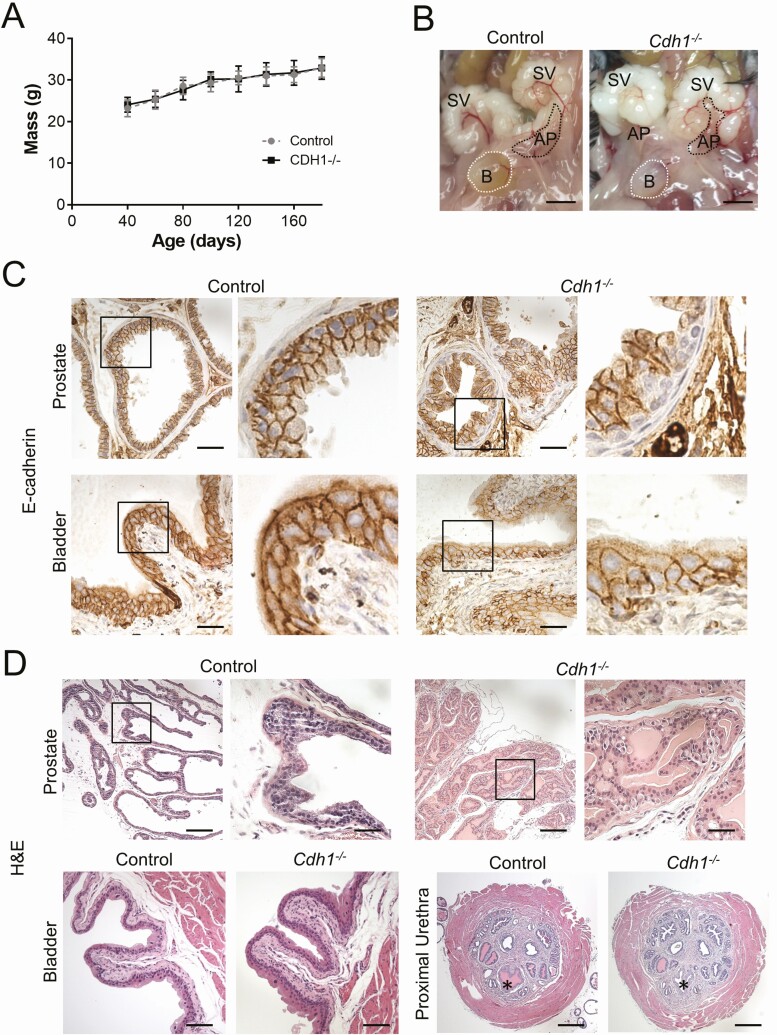
Figure 1.
Impact of prostate epithelial-specific E-cadherin loss on the lower urogenital tract of male mice. A, Body mass in grams (g) for PSA-CreERT2+/-:Cdh1fl/fl (Cdh1-/-) and Cdh1fl/fl Control mice during the study period. Mass was measured every 20 days starting at 40 days of age until 160 days of age. Mass data was collected for 70 mice total, 35 from each group. B, Gross appearance of lower urogenital tract in Control and Cdh1-/- mice. Bladder (B, outlined by white dotted line), both seminal vesicles (SV) and the left anterior prostate lobes are visible (AP, outlined by black dotted line). Scale bars indicate 5 mm. C, Expression of E-cadherin in the ventral lobes of the murine prostate (top panels) and bladder urothelium (bottom panels) in Control or Cdh1-/- knockout mice at 21 to 23 weeks of age. Original magnification, 40×, inset 40×, scale bars indicate 50 µm. D, Histology of murine prostate, bladder, and prostatic urethra. H&E staining of transverse sections of prostate lateral lobes (top panels), bladder (bottom left panels) and prostatic urethra (bottom right panels, *) from Control and Cdh1-/- mice at 21 to 23 weeks of age. Cdh1-/- mice displayed prostatic epithelial hyperplasia (red arrow). Original magnification for prostate, 10×, inset 40×, bladder 20×, and prostatic urethra 5×. Scale bars indicate 200 µm in 10×, 50 µm 40×, 100 µm 20×, and 400 µm in 5×.
Gross examination of the lower urinary tract of Control and Cdh1-/- mice revealed no morphologic differences (Fig. 1B). The bladder, seminal vesicle, and prostate lobes did not appear to be enlarged in Cdh1-/- mice upon microdissection, suggesting the absence of an enlarged bladder which can be induced by outlet obstruction and has been observed in other murine BPH models (37-39). The prostates of control animals displayed a contiguous layer of E-cadherin positive luminal epithelial cells lining the lumen of all glands in each prostate lobe, while Cdh1-/- mice displayed a mosaic pattern of E-cadherin negative epithelial cells which were frequently in a basal position surrounded by E-cadherin positive luminal epithelial cells within multiple glands in each prostate lobe (Fig. 1C, upper panels). E-cadherin expression in the bladder urothelium was similar in both groups, confirming that E-cadherin deletion was specific to the prostate in Cdh1-/- mice (Fig. 1C, lower panels). Prostate epithelial defects, stromal fibrosis, and inflammation were obvious changes in Cdh1-/- mice when sections were compared with those from control animals at 21 to 23 weeks of age (Fig. 1D, upper panels, Supplemental Figure S2A (21)). Subepithelial atypical cells were present in many sections, often under a normal overlying cuboidal epithelium. These cells were similarly sized to the overlying cuboidal epithelium or slightly larger and haphazardly arranged. Cells were round and sometimes individualized with round nuclei and foamy lightly eosinophilic cytoplasm (Fig. 1D, right panels). The prostates from age-matched, tamoxifen-treated Cre-positive animals with heterozygous expression of Cdh1, which were also examined as additional controls to determine the potential impact of PSA-CreERT2 on the prostate, displayed no apparent phenotype (Supplemental Figure S2B (21)). Histologic examination of the bladder and prostatic urethra showed no apparent infiltration of inflammatory cells such as neutrophils in either group (Fig. 1D, lower panels).
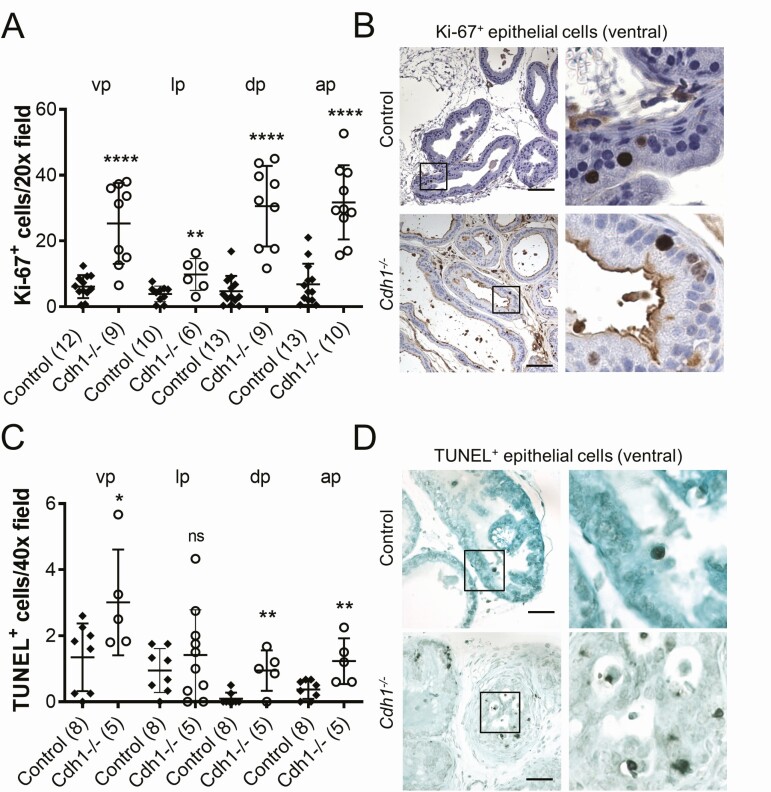
Analysis of prostate histology was performed by a board-certified veterinary pathologist (L.H.R., DVM) and animals were scored for prostatic defects in the epithelium, stroma, and the presence of inflammation (Table 1). Three (3/13, 23%) Cdh1-/- animals displayed moderate-to-severe PIN lesions in the lateral prostate lobes. No epithelial defects were observed in control animals (0/9). Stromal defects were observed in both Cdh1-/- and control mice and included mild-to-moderate increased edema and more prominent fibroblast nuclei. Prostate mass was also measured for comparison between groups. The dorsal lobes of the prostates of Cdh1-/- mice had a statistically significant increased mass compared with age-matched controls but no significant difference was observed in the mass of the ventral-lateral or anterior prostate lobes (Supplemental Figure S3 (21)). There was no significant difference in the bladder mass between the 2 groups (data not shown). The proliferative marker Ki-67 was used to detect dividing cells in the prostates of Cdh1-/- and control mice. The number of Ki-67-positive luminal epithelial cells was significantly increased in all lobes of the Cdh1-/- mice compared with controls (Fig. 2A). Ki-67-positive proliferating cells were frequently observed in clusters in Cdh1-/- mice, while control animals frequently displayed a more dispersed proliferation with 1 or 2 Ki-67-positive cells in close proximity (Fig. 2B). Immunostaining for apoptosis with TUNEL indicated an increase in the number of TUNEL-positive apoptotic cells in all lobes of the Cdh1-/- mice compared with controls (Fig. 2C, 2D).
Table 1.
Average and Lobe-Specific Score of Male Mice Studied for Prostatic Defects
| Epithelial defects | Cdh1 fl/fl (n = 9) | PSA-CreERT2+/-: Cdh1fl/fl (n = 13) | Fisher exact test P value |
|---|---|---|---|
| Atypical cells | 0/9 | 13/13 | <0.0001 |
| PIN Lesions | 0/9 | 3/13 | 0.24 |
Abbreviation: PIN, prostatic intraepithelial neoplasia.
Figure 2.
Effect of Cdh1-deficiency on epithelial proliferation in the murine prostate. A, Quantification of Ki-67+ luminal epithelial cells in the lobes of the prostate from Control and Cdh1-/- mice at 21 to 23 weeks of age. B, Ki-67 immunostaining in prostate ventral lobes. C, Quantification of TUNEL staining in prostate luminal epithelial cells. D, TUNEL staining in prostate ventral lobes, TUNEL-positive apoptotic cells (red arrows). Original magnification, 20×, inset 40×. Scale bars indicate 100 µm 20×, 50 µm in 40×. Data represent mean ± SD, number of mice in each group in parentheses. Lobes that had been washed away during staining process were not quantified. Abbreviations: ap, anterior prostate; dp, dorsal prostate; lp, lateral prostate; ns, nonsignificant; vp, ventral prostate. * P < 0.05; ** P < 0.01; **** P < 0.0001.
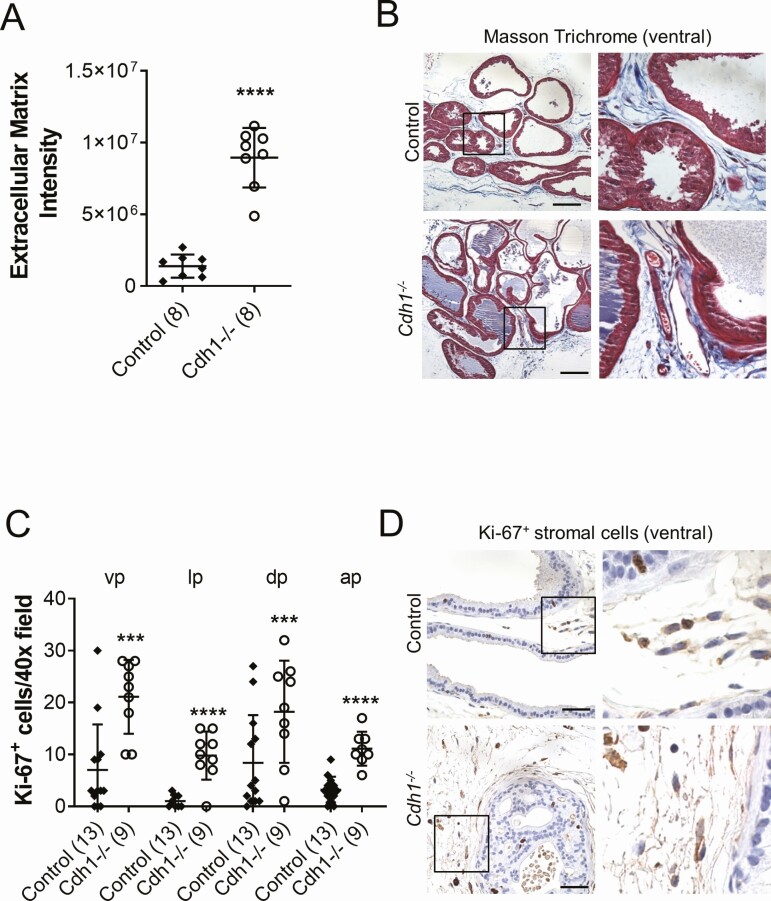
Cdh1 -/- mice also displayed considerable changes in the stromal compartment of the prostate compared with control animals. Masson’s trichrome staining showed an increase in extracellular matrix deposition in Cdh1-/- mice compared with control prostates (Fig. 3A, 3B). Increased Ki-67-positive proliferating cells were also observed in the stromal compartment of Cdh1-/- mice compared with controls, suggesting stromal hyperplasia (Fig. 3C, 3D). TUNEL analysis of stromal cells did not reveal a statistically significant increase in stromal apoptosis (data not shown).
Figure 3.
Effects of Cdh1-deficiency on stromal compartment in the murine prostate. A, Quantification of Masson’s trichrome staining of extracellular matrix (blue) in the stroma surrounding prostate glands of Cdh1-/- mice at 21 to 23 weeks of age. Extracellular matrix (ECM) was quantified by calculating the intensity of blue staining as previously (54), using the color deconvolution plugin to separate the blue staining and then ImageJ (34). Five fields from each section were analyzed and an average score was determined for each mouse. B, Masson’s trichrome staining in transverse sections of prostate ventral lobes. C, Quantification of Ki-67+ stromal cells in prostates from Control and Cdh1-/- mice. D, Ki-67 immunostaining in transverse sections of prostate ventral lobes. Data represent mean ± SD, number of mice in each group in parentheses. Lobes that had been washed away during staining process were not quantified. Original magnification, 40×, scale bars indicate 50 µm. *** P < 0.001; **** P < 0.0001.
Cdh1 gene deletion is associated with increased infiltration of inflammatory cells in the prostate
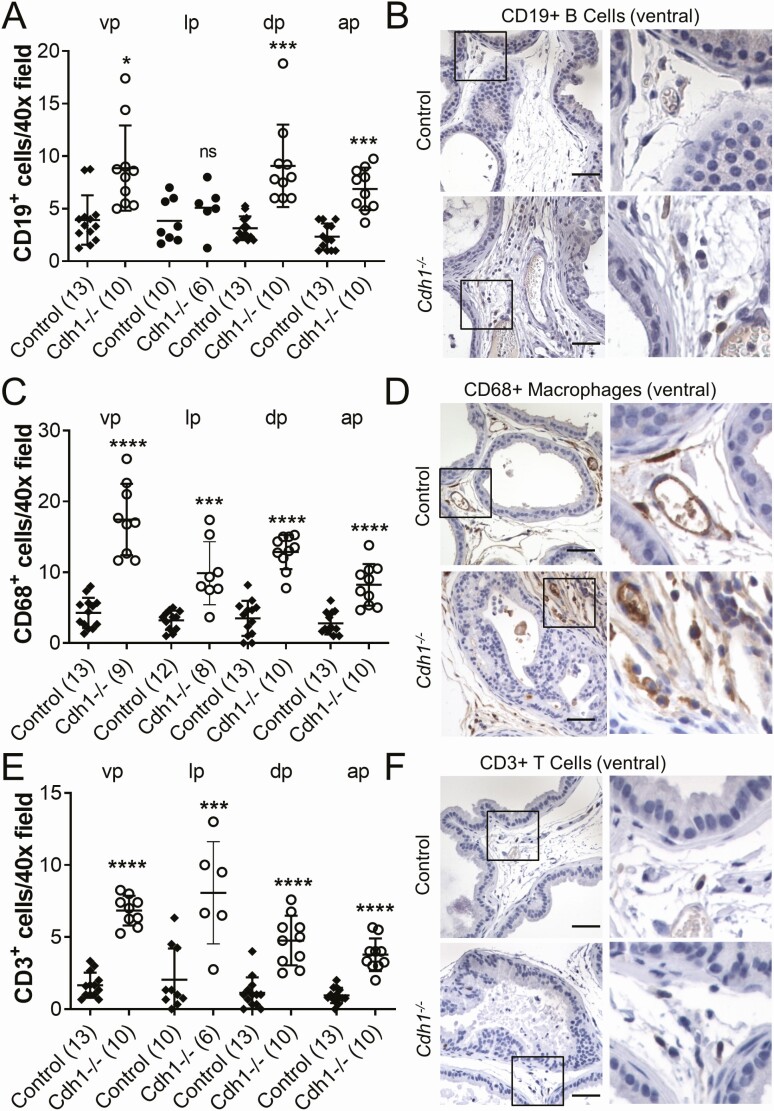
Histological analysis of prostate tissue in Cdh1 knockout mice revealed increased inflammation compared with controls. Mild inflammation, including inflammatory cells around vessels and in the stroma, was observed in control prostates, possibly due to tamoxifen treatment (36). Cdh1 knockout mice displayed increased mild-to-moderate inflammatory cells in the stroma compared with control prostates; therefore, we analyzed the number of CD19-positive B cells, CD68-positive macrophages, and CD3-positive T cells in the prostates of mice with Cdh1 deficiency. Immunostaining analysis revealed a statistically significant increase in the number of CD19-positive B cells in all prostate lobes of Cdh1-/- mice compared with controls (Fig. 4A, 4B). Similar increases were also observed in the number of CD68-positive macrophages and CD3-positive T cells in the prostates of mice with Cdh1 deficiency (Fig. 4C-4E). CD19-positive B cells in all lobes of control and Cdh1-/- mouse prostates were most frequently localized in the interglandular stroma and were rarely observed in contact with the smooth-muscle stroma or within the glandular epithelium. CD68-positive macrophages were also most frequently localized in the interglandular stroma of control mouse prostates with rare or no intraglandular or periglandular cells, however in Cdh1-/- mouse prostates there was an increased number of interglandular, intraglandular, and periglandular macrophages observed in all lobes. CD3-positive T cells were observed most frequently localized in the interglandular stroma of control mice prostates with rare or no intraglandular or periglandular cells with an increased number of interglandular stroma observed in all lobes of Cdh1-/- mouse prostates.
Figure 4.
Effects of Cdh1-deficiency on inflammatory cells in the murine prostate. A, Quantification of CD19+ B cells in the prostate from Control and Cdh1-/- mice at 21 to 23 weeks of age. B, CD19 immunostaining in transverse sections of prostate ventral lobes. C, Quantification of CD68+ macrophages in prostate. D, CD68 immunostaining in transverse sections of prostate ventral lobes. E, Quantification of CD3+ T cells in prostate. F, CD3 immunostaining in transverse sections of prostate ventral lobes. Data represent mean ± SD, number of mice in each group in parentheses. Lobes that had been washed away during staining process were not quantified. Original magnification, 40×, scale bars indicate 50 µm. * P < 0.05; *** P < 0.001; **** P < 0.0001. Abbreviation: ns, nonsignificant.
Voiding behavioral studies in Cdh1-/- mice
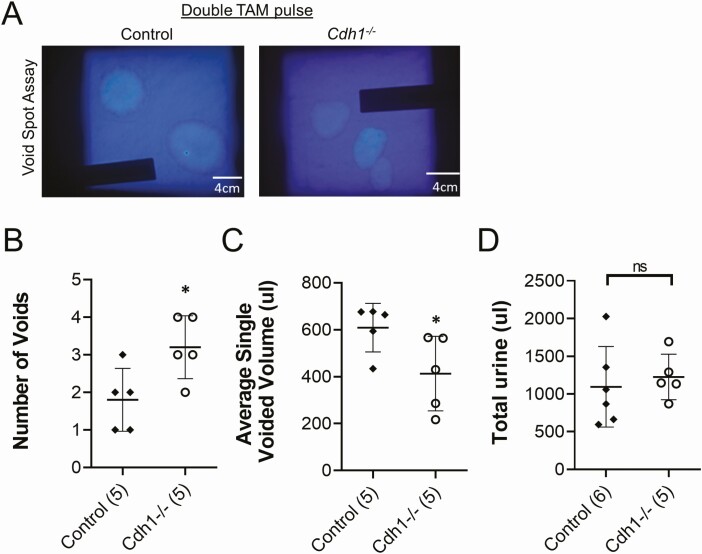
Previously, we and others have reported an increased urinary frequency (40) and bladder overactivity in mice and rat models of bacterial- or chemical-induced prostatic inflammation (11, 41). In an initial group of mice treated with a single TAM pulse (see Supplemental Figure 1C, Cohort 1 (21)), although there was an increased trend in urinary frequency and altered bladder function, the results were not statistically significant (Supplemental Figure S4 (21)). Thus, we performed a second series of TAM injections in a group of Cdh1-/- and control mice at 19 weeks of age and mice were analyzed 2 weeks later (Supplemental Figure S1C, Cohort 2 (21)). The second series of TAM injections induced a similar loss of E-cadherin positive epithelial cells in the prostate but not in the bladder (Supplemental Figure S5A (21)) and an increase in stromal inflammation and ECM (Supplemental Figure S5B, C (21)) compared with the phenotype displayed by mice treated with a single TAM pulse. In Cdh1-/- mice with double TAM pulses, voiding spot assays demonstrated a significant increase in the number of voids compared with control mice (Fig. 5A, B). The average single-voided volume was decreased in Cdh1-/- mice compared with control mice, while the total volume of urine was not different between the 2 groups (Fig. 5C, D).
Figure 5.
Effects of Cdh1-deficiency on bladder voiding in mice treated with a double series of tamoxifen and analyzed at 21 to 23 weeks of age (Double TAM pulse). A, Void spot on paper assay results from Control and Cdh1-/- mice over 4 hours. B, Quantification of the number of voids, and the C, Average single voiding volume, and D, Total urine volume over the 4-hour testing period. Number of mice in each group in parentheses. * P < 0.05. Abbreviation: ns, nonsignificant.
Conscious cystometry in Cdh1-/- mice
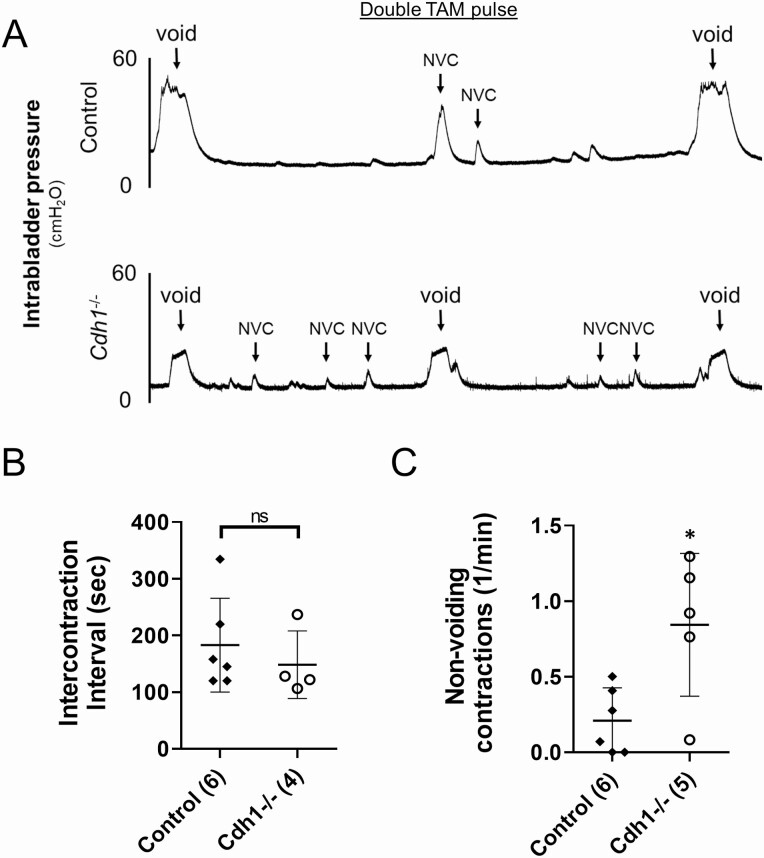
The initial group of mice treated with a single TAM pulse (Supplemental Figure 1C, Cohort 1 (21)) did not exhibit a significant difference in the mean number of nonvoiding contractions (NVC) during bladder filling (0.36 in control vs 0.69 in Cdh1-/-; P = 0.053, Supplemental Figure S6 (21)). Thus, cystometry analysis of bladder function was performed in an additional group of Cdh1-/- and control mice treated with a double pulse of TAM at 19 weeks of age (Supplemental Figure S1C, Cohort 2 (21)). Cystometric tracing of awake Cdh1-/- mice treated with a double pulse of TAM demonstrated a significantly increased mean number of NVC per minute without altering intercontraction intervals compared with controls (Fig. 6).
Figure 6.
Effects of Cdh1-deficiency on bladder cystometry in mice treated with a double series of tamoxifen and analyzed at 21 to 23 weeks of age (Double TAM pulse). A, Representative cystometric tracings for Control and double TAM Cdh1-/- mice at 21 to 23 weeks of age. B, Intercontraction interval for Control and double TAM Cdh1-/- mice. C, Number of nonvoiding contractions for Control and Cdh1-/- mice. Number of mice in each group in parentheses. * P < 0.05. Abbreviation: ns, nonsignificant.
Discussion
E-cadherin downregulation is a frequent histological observation in BPH (5, 42), but its potential role in BPH pathogenesis and progression to LUTS has not been fully elucidated. Advanced age and inflammation have also been linked to prostatitis and BPH development and progression to LUTS (43, 44). In BPH, which is the most common cause of LUTS in men (43), tissues frequently exhibit inflammation, increased epithelial proliferation and remodeling of the stroma. Inflammation in BPH is characterized predominantly by infiltration of T-lymphocytes and macrophages (43). BPH prostatic glands are frequently associated with increased CD8+ T-lymphocytes and the stroma contains clusters of B-lymphocytes and CD4+ T-lymphocytes (45). Ki-67-positive proliferating cells are increased (46, 47) and the stromal compartment is characterized by increased fibrosis and ECM remodeling (48).
E-cadherin expression in clinical BPH specimens was less intense and displayed a discontinuous pattern relative to that in normal adjacent prostatic tissue but was not completely absent (4). This Cdh1 knockout model will likely be more relevant to BPH patients exhibiting more dramatic E-cadherin downregulation. More studies will be needed to determine if patients with more dramatic E-cadherin downregulation have increased BPH symptom severity. The Cdh1 knockout model also did not display a significant increase in prostate size compared with controls, although epithelial and stromal proliferation was observed. Clinical studies have demonstrated an association of BPH with increased prostate size, and men with enlarged prostates are much more likely to develop LUTS presumed due to BPH in the future (49, 50). However, men without enlarged prostates can also develop severe LUTS, suggesting that factors other than prostatic enlargement can contribute to BPH/LUTS (51, 52).
Here, we show that deletion of E-cadherin in the murine prostate induced prostatic inflammation accompanied by prostate epithelial and stromal hyperplasia and subsequent alterations in bladder function consistent with lower urinary tract symptoms displayed by BPH and BPH/LUTS patients. Cdh1-deficient mice had increased T cells, B cells, and macrophages, and increased epithelial and stromal proliferation and stromal fibrosis in the prostate. The finding of prostatic inflammation and voiding abnormality in the Cdh1 KO mice suggests that loss of E-cadherin in prostatic luminal epithelial cells is one of the mechanisms leading to prostatic inflammation and BPH symptoms. In cell line studies, the inflammatory cytokine TGF-β1 suppressed levels of E-cadherin protein level, but not mRNA, in BPH-1 and BHPrE1 benign prostatic epithelial cells (4, 9). Taken together, these studies suggest that loss of E-cadherin can induce prostatic inflammation, which could further reduce E-cadherin, thereby setting up a vicious cycle of epithelial barrier alteration triggering a chronic inflammatory response, which in turn could further reduce epithelial barrier integrity. Considering the complexity of BPH, BPH development and progression will likely involve multiple factors. Other factors could also lead to prostatic inflammation, which could further exacerbate epithelial barrier alterations.
The role of stromal changes in BPH development and progression is not fully understood. Increased prostatic ECM and fibrosis have been associated with LUTS (53); however, a more recent study detected no difference in ECM content between normal prostate and glandular BPH using Masson’s trichrome staining (54). Increased collagen deposition and ECM were recently reported in aged mice compared with young mice (55). Future studies will be required to determine whether targeting the stromal changes induced by loss of E-cadherin in the murine prostate could improve bladder function.
Cdh1-deficient mice also displayed an increased number of NVC as well as an increased number of voids and a smaller average voiding volume without changes in total urine volume. The bladder and prostatic urethra displayed no histologic sign of inflammatory cell infiltration or narrowing urethral lumen, suggesting that the knockout of Cdh1 in the murine prostate luminal epithelial cell does not induce bladder obstruction.
A major roadblock to address the role of inflammation in BPH/LUTS is the lack of suitable animal models. Previous reports of prostatic inflammation models include Escherichia coli-induced prostatic inflammation (56). However, only about 20% of the BPH specimens have bacterial infections (57). Also, E. coli inoculation could affect the prostate in other ways such as colonization inside prostatic cells (58). Our formalin-induced prostatic inflammation model demonstrated that chemically induced prostatic inflammation in rats induces pelvic nerve-dependent bladder overactivity and bladder/prostate afferent hyperexcitability up to 8 weeks after intraprostatic formalin injection and mimicked the upregulation of androgen- and TGFβ-signaling pathways seen in human BPH specimens (12, 41). However, the severity of nonbacterial prostatic inflammation in rodents (59-61) is not fully representative of the grade and extent of prostatic inflammation seen in the majority of BPH patients (62), and these models are not considered physiologically sufficient to fully understand human BPH prostatic inflammation pathophysiology. More recently, a model of genetically induced prostate-specific inflammation by conditional expression of interleukin-1β in mouse prostatic epithelium was reported (63), but the histological and voiding function changes in this model have yet to be characterized. Thus, our newly developed Cdh1-deficient mice, which can recapitulate the major molecular changes and pathophysiology in human BPH, seem to be a better animal model to further characterize the chronic effects of prostatic inflammation on both prostatic re-landscaping and bladder dysfunction.
The phenotype of our Cdh1-deficient mice is different from 3 previously published Cdh1-deficient mouse lines. These 4 Cdh1 knockout lines, including ours, used different knockout approaches, which resulted in 4 very different phenotypes, ranging from: (i) no abnormality in tamoxifen-inducible Nkx3.1-CreERT2 driver (17), (ii) prostate cancer in constitutive probasin (Pb)-Cre driver (16), (iii) PIN-like lesions with no progression to tumor in a separate study using constitutive probasin (Pb)-Cre driver (18), and (iv) prostatic inflammation, proliferation, and some mPIN lesions in our model using the tamoxifen-inducible PSA-CreERT2 driver. The variations of Cdh1 knockout phenotypes in the 4 different lines are most likely due to the different ways Cdh1 was deleted in prostatic luminal epithelial cells. Factors involved in carcinogenesis (eg, androgens, TGF-β1 and E-cadherin) could also play important roles in normal development, tissue homeostasis, and/or benign diseases and vice versa. The dosage and timing of their addition, overexpression, and/or deletion could profoundly affect the phenotype development of treated animals. In the Nkx3.1-CreERT2 driven model, Cdh1 knockout was induced in adult prostatic luminal epithelial cells only in the presence of tamoxifen. Cdh1 loss caused anoikis of luminal cells and transdifferentiation of basal cells to generate new normal luminal cells in adult prostate. Although no abnormality was observed in this model at 30 days postinduction, rare E-cadherin negative cells in basal positions of the epithelium were observed in the Nkx3.1-CreERT2 driven model (17), similar to those we observed here.
In the Pb-Cre driven models, Cdh1 knockout was constitutive in prostatic luminal epithelial cells. Loss of Cdh1 should also cause anoikis of luminal cells and basal-to-luminal transdifferentiation to generate new normal luminal cells and was reported in one of these studies (18). The Pb-Cre should be activated in the newly generated normal luminal cells, which should induce Cdh1 knockout in these cells. Thus, the luminal epithelial layer would not be repaired via basal-to-luminal transdifferentiation in this model. It is not clear if new basal-derived luminal cells would behave differently from preexisting luminal cells. Secondly, Cdh1 deletion in the Pb-Cre driven model would occur during prostatic development, as soon as the Pb promoter is activated. The early-onset continuous deletion of Cdh1 in luminal prostatic cells could drive prostatic carcinogenesis in the Pb-Cre driven model. In our model using the tamoxifen-inducible PSA-CreERT2 driver, Cdh1 knockout was induced in adult prostatic luminal epithelial cells by tamoxifen injection, similar to the Nkx3.1-CreERT2 driven model. In our model, loss of Cdh1 should also cause anoikis of luminal cells and basal-to-luminal transdifferentiation to generate new normal luminal cells. In addition, we observed persistent inflammation and a mosaic pattern of E-cadherin negative and E-cadherin positive cells in the prostate epithelium 4 months after tamoxifen induction similar to that observed in the Pb-Cre model described by Olson et al (18), suggesting that basal-to-luminal transdifferentiation did not fully repair the damage caused by Cdh1 knockout via the PSA-CreERT2 driver and that E-cadherin loss could induce prostatic inflammation. Tamoxifen induction of the PSA-CreERT2 mouse resulted in ~70% recombination in our previous studies (64, 65). Loss of E-cadherin in previous murine models induced proliferation in E-cadherin negative luminal epithelial cells and differentiation of basal epithelial cells to luminal cells (16, 17). Here we likely see similar consequences in the prostate epithelial cell populations, however in this model, persistent prostatic inflammation in the stromal compartment was also evident up to 4 months after tamoxifen induction. In our previous studies, we have shown that prostatic inflammation induced by bacteria or formalin can result in bladder dysfunction (11-13, 41). Here we show that prostatic inflammation triggered by loss of E-cadherin can also induce bladder dysfunction in the murine model.
The inducible prostate luminal epithelial cell–specific Cdh1 knockout mouse model provides an opportunity to determine the long-term consequences of losing the integrity of the luminal epithelial barrier, which is also evident in human BPH specimens (4). Our chronic model displayed prostatic inflammation and subsequent bladder overactivity up to 4 months after induction, similar to previous models of more acute chemically or bacterially induced prostatic inflammation (11-14, 40, 41), suggesting that both acute and chronic prostatic inflammation can trigger BPH/LUTS-like symptoms in rodents. Our chronic model has the following similarities to human BPH: (i) loss of prostatic luminal epithelial junctional integrity, (ii) subsequent prostatic inflammation, (iii) elevated luminal epithelial proliferation, (iv) prostatic fibrosis, and (v) lower urinary tract dysfunction, such as bladder overactivity. Prostatic inflammation in the Cdh1 knockout model appears to be mild and thus may be more relevant to prostatic inflammation in human BPH. Therefore, our animal model has the potential to mimic several key aspects of human BPH and will be a valuable tool in future studies to further elucidate the roles of elevated luminal epithelial barrier permeability and prostatic inflammation in BPH pathogenesis, potentially leading to the development of targeted therapies and preventive treatments for BPH. Our studies also suggest that E-cadherin downregulation could lead to prostatic inflammation and drive BPH and LUTS pathogenesis in patients.
Acknowledgments
We thank Dr. Pierre Chambon and Dr. Daniel Metzger (IGBMC, Illkirch, France) for generously providing the PSA-CreERT2 mouse for this study. We are grateful to Anthony Green, Megan Lambert, Robin Frederick, Elaine Isherwood, Paul Knizner, Jianhua Zhou, and Aiyuan Zhang for technical support.
Financial support : This work was funded in part by National Institutes of Health grants U54 from National Institute of Diabetes and Digestive and Kidney Diseases, DK112079 (Z.W.), R56 DK107492 (Z.W.), and National Cancer Institute 1R50 CA211242 (L.E.P.), and by an American Urology Association Award (W.C.). This project used the Tissue Resources and Morphology Core (TRMC) of the O’Brien Urology Research Center at University of Pittsburgh (DK112079). This project used the UPMC Hillman Cancer Center Animal Facility and the Pitt Biospecimen Core and was supported in part by award P30CA047904.
Author Contributions: L.E.P., S.M., R.D., N.Y., and Z.W. designed experiments. L.E.P., S.M., W.C., and T.I. performed research experiments and analyzed data. L.H.R. and R.D. analyzed murine prostate pathology. Z.W. and Z.Y. assisted with tamoxifen injections and animal care. L.E.P. wrote the manuscript with help from S.M., R.D., P.T., W.C.D., D.B.F., N.Y.,and Z.W.; all authors reviewed and edited the manuscript.
Glossary
Abbreviations
- BPH
benign prostatic hyperplasia
- ECM
extracellular matrix
- KLK2
kallikrein-related peptidase 2
- LUTS
lower urinary tract symptoms
- NVC
nonvoiding contraction
- PSA
prostate-specific antigen
- TAM
tamoxifen
- TGF-β1
transforming growth factor β1
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References. Data generated or analyzed during this study are included in this published article or in the data repositories listed in References for Supplemental Figures S1-S6, https://doi.org/10.5061/dryad.p2ngf1vp4.
References
- 1. Keil KP, Abler LL, Mehta V, et al. DNA methylation of E-cadherin is a priming mechanism for prostate development. Dev Biol. 2014;387(2):142-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morton RA Jr, Ewing CM, Watkins JJ, Isaacs WB. The E-cadherin cell-cell adhesion pathway in urologic malignancies. World J Urol. 1995;13(6):364-368. [DOI] [PubMed] [Google Scholar]
- 3. Wang W, Bian K, Vallabhaneni S, et al. ERK3 promotes endothelial cell functions by upregulating SRC-3/SP1-mediated VEGFR2 expression. J Cell Physiol. 2014;229(10): 1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li F, Pascal LE, Stolz DB, et al. E-cadherin is downregulated in benign prostatic hyperplasia and required for tight junction formation and permeability barrier in the prostatic epithelial cell monolayer. Prostate. 2019;79(11):1226-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O’Malley KJ, Eisermann K, Pascal LE, et al. Proteomic analysis of patient tissue reveals PSA protein in the stroma of benign prostatic hyperplasia. Prostate. 2014;74(8):892-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol. 2013;10(9): 546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fibbi B, Penna G, Morelli A, Adorini L, Maggi M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33(3):475-488. [DOI] [PubMed] [Google Scholar]
- 8. De Nunzio C, Presicce F, Tubaro A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol. 2016;13(10):613-626. [DOI] [PubMed] [Google Scholar]
- 9. Wang K, Pascal LE, Li F, et al. Tight junction protein claudin-1 is downregulated by TGF-beta1 via MEK signaling in benign prostatic epithelial cells. Prostate. 2020;80(14):1203-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torkko KC, Wilson RS, Smith EE, Kusek JW, van Bokhoven A, Lucia MS. Prostate biopsy markers of inflammation are associated with risk of clinical progression of benign prostatic hyperplasia: findings from the MTOPS study. J Urol. 2015;194(2):454-461. [DOI] [PubMed] [Google Scholar]
- 11. Mizoguchi S, Mori K, Wang Z, et al. Effects of estrogen receptor β stimulation in a rat model of non-bacterial prostatic inflammation. Prostate. 2017;77(7):803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Funahashi Y, Takahashi R, Mizoguchi S, et al. Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. J Physiol. 2019;597(7):2063-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni J, Mizoguchi S, Bernardi K, et al. Long-lasting bladder overactivity and bladder afferent hyperexcitability in rats with chemically-induced prostatic inflammation. Prostate. 2019;79(8):872-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y, Wu X, Liu J, Tang W, Zhao T, Zhang J. Distribution of convergent afferents innervating bladder and prostate at dorsal root Ganglia in rats. Urology. 2010;76(3):764.e1-764.e6. [DOI] [PubMed] [Google Scholar]
- 15. Harper ME, Glynne-Jones E, Goddard L, Mathews P, Nicholson RI. Expression of androgen receptor and growth factors in premalignant lesions of the prostate. J Pathol. 1998;186(2):169-177. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Dong B, Zhang K, et al. E-cadherin bridges cell polarity and spindle orientation to ensure prostate epithelial integrity and prevent carcinogenesis in vivo. Plos Genet. 2018;14(8):e1007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toivanen R, Mohan A, Shen MM. Basal progenitors contribute to repair of the prostate epithelium following induced luminal anoikis. Stem Cell Reports. 2016;6(5):660-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olson A, Le V, Aldahl J, et al. The comprehensive role of E-cadherin in maintaining prostatic epithelial integrity during oncogenic transformation and tumor progression. Plos Genet. 2019;15(10):e1008451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115(1-2):53-62. [DOI] [PubMed] [Google Scholar]
- 20. Ratnacaram CK, Teletin M, Jiang M, Meng X, Chambon P, Metzger D. Temporally controlled ablation of PTEN in adult mouse prostate epithelium generates a model of invasive prostatic adenocarcinoma. Proc Natl Acad Sci U S A. 2008;105(7):2521-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pascal L, et al. Prostate-specific deletion of Cdh1 induces murine prostatic inflammation and bladder overactivity, Dryad, Dataset, 2020. 10.5061/dryad.p2ngf1vp4 [DOI] [PMC free article] [PubMed]
- 22. Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305-318. [DOI] [PubMed] [Google Scholar]
- 23. Ma X, Ziel-van der Made AC, Autar B, et al. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res. 2005;65(13):5730-5739. [DOI] [PubMed] [Google Scholar]
- 24. Zou M, Toivanen R, Mitrofanova A, et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 2017;7(7):736-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwon OJ, Zhang L, Ittmann MM, Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci U S A. 2014;111(5):E592-E600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34(5):961-971. [DOI] [PubMed] [Google Scholar]
- 27. Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64(6):2270-2305. [DOI] [PubMed] [Google Scholar]
- 28. Pascal LE, Ai J, Rigatti LH, et al. EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate. Angiogenesis. 2011;14(3):331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. RRID:AB_397580, https://scicrunch.org/resolver/AB_397580. [Google Scholar]
- 30. RRID:AB_2687824, https://scicrunch.org/resolver/AB_2687824. [Google Scholar]
- 31. RRID:AB_2755035, https://scicrunch.org/resolver/AB_2755035. [Google Scholar]
- 32. RRID:AB_2800152, https://scicrunch.org/resolver/AB_2800152. [Google Scholar]
- 33. RRID:AB_563621, https://scicrunch.org/resolver/AB_563621. [Google Scholar]
- 34. Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23(4):291-299. [PubMed] [Google Scholar]
- 35. Sugino Y, Kanematsu A, Hayashi Y, et al. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn. 2008;27(6):548-552. [DOI] [PubMed] [Google Scholar]
- 36. Stoker TE, Robinette CL, Cooper RL. Perinatal exposure to estrogenic compounds and the subsequent effects on the prostate of the adult rat: evaluation of inflammation in the ventral and lateral lobes. Reprod Toxicol. 1999;13(6): 463-472. [DOI] [PubMed] [Google Scholar]
- 37. Taylor JA, Jones MB, Besch-Williford CL, Berendzen AF, Ricke WA, vom Saal FS. Interactive effects of perinatal BPA or DES and adult testosterone and estradiol exposure on adult urethral obstruction and bladder, kidney, and prostate pathology in male mice. Int J Mol Sci. 2020;21(11):3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicholson TM, Ricke EA, Marker PC, et al. Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology. 2012;153(11):5556-5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomas S, Hao L, DeLaney K, et al. Spatiotemporal proteomics reveals the molecular consequences of hormone treatment in a mouse model of lower urinary tract dysfunction. J Proteome Res. 2020;19(4):1375-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee S, Yang G, Bushman W. Prostatic inflammation induces urinary frequency in adult mice. Plos One. 2015;10(2): e0116827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Funahashi Y, O’Malley KJ, Kawamorita N, et al. Upregulation of androgen-responsive genes and transforming growth factor-β1 cascade genes in a rat model of non-bacterial prostatic inflammation. Prostate. 2014;74(4): 337-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arenas MI, Romo E, Royuela M, Fraile B, Paniagua R. E-, N- and P-cadherin, and alpha-, beta- and gamma-catenin protein expression in normal, hyperplastic and carcinomatous human prostate. Histochem J. 2000;32(11):659-667. [DOI] [PubMed] [Google Scholar]
- 43. Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51(5):1202-1216. [DOI] [PubMed] [Google Scholar]
- 44. Krušlin B, Tomas D, Džombeta T, Milković-Periša M, Ulamec M. Inflammation in prostatic hyperplasia and carcinoma-basic scientific approach. Front Oncol. 2017;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60(1):106-117. [DOI] [PubMed] [Google Scholar]
- 46. Gangkak G, Bhattar R, Mittal A, et al. Immunohistochemical analysis of estrogen receptors in prostate and clinical correlation in men with benign prostatic hyperplasia. Investig Clin Urol. 2017;58(2):117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shariat SF, Ashfaq R, Roehrborn CG, Slawin KM, Lotan Y. Expression of survivin and apoptotic biomarkers in benign prostatic hyperplasia. J Urol. 2005;174(5):2046-2050. [DOI] [PubMed] [Google Scholar]
- 48. Schauer IG, Rowley DR. The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation. 2011;82(4-5):200-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simon RM, Howard LE, Moreira DM, et al. Does prostate size predict the development of incident lower urinary tract symptoms in men with mild to no current symptoms? Results from the REDUCE Trial. Eur Urol. 2016;69(5):885-891. [DOI] [PubMed] [Google Scholar]
- 50. Roehrborn CG. Definition of at-risk patients: baseline variables. BJU Int. 2006;97 Suppl 2:7–11; discussion 21-12. [DOI] [PubMed] [Google Scholar]
- 51. Turkbey B, Huang R, Vourganti S, et al. Age-related changes in prostate zonal volumes as measured by high-resolution magnetic resonance imaging (MRI): a cross-sectional study in over 500 patients. BJU Int. 2012;110(11):1642-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abler LL, Vezina CM. Links between lower urinary tract symptoms, intermittent hypoxia and diabetes: Causes or cures? Respir Physiol Neurobiol. 2018;256:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ma J, Gharaee-Kermani M, Kunju L, et al. Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol. 2012;188(4):1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bauman TM, Nicholson TM, Abler LL, et al. Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. Plos One. 2014;9(10): e109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu TT, Thomas S, Mclean DT, et al. Prostate enlargement and altered urinary function are part of the aging process. Aging (Albany NY). 2019;11(9):2653-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wong L, Hutson PR, Bushman W. Prostatic inflammation induces fibrosis in a mouse model of chronic bacterial infection. Plos One. 2014;9(6):e100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 1999;84(9):976-981. [DOI] [PubMed] [Google Scholar]
- 58. Ho CH, Fan CK, Yu HJ, et al. Testosterone suppresses uropathogenic Escherichia coli invasion and colonization within prostate cells and inhibits inflammatory responses through JAK/STAT-1 signaling pathway. Plos One. 2017;12(6):e0180244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen CS, Chang PJ, Lin WY, Huang YC, Ho DR. Evidences of the inflammasome pathway in chronic prostatitis and chronic pelvic pain syndrome in an animal model. Prostate. 2013;73(4):391-397. [DOI] [PubMed] [Google Scholar]
- 60. Popovics P, Cai R, Sha W, Rick FG, Schally AV. Growth hormone-releasing hormone antagonists reduce prostatic enlargement and inflammation in carrageenan-induced chronic prostatitis. Prostate. 2018;78(13):970-980. [DOI] [PubMed] [Google Scholar]
- 61. Kashyap M, Pore S, Wang Z, Gingrich J, Yoshimura N, Tyagi P. Inflammasomes are important mediators of prostatic inflammation associated with BPH. J Inflamm (Lond). 2015;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tyagi P, Wang Z, Yoshimura N. Urinary biomarkers and benign prostatic hyperplasia. Curr Bladder Dysfunct Rep. 2019;14:31-40. [Google Scholar]
- 63. Hao L, Thomas S, Greer T, et al. Quantitative proteomic analysis of a genetically induced prostate inflammation mouse model via custom 4-plex DiLeu isobaric labeling. Am J Physiol Renal Physiol. 2019;316(6):F1236-F1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pascal LE, Rigatti LH, Ai J, et al. EAF2 loss induces prostatic intraepithelial neoplasia from luminal epithelial cells in mice. Am J Clin Exp Urol. 2020;8(1):18-27. [PMC free article] [PubMed] [Google Scholar]
- 65. Liu J, Pascal LE, Isharwal S, et al. Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol Endocrinol. 2011;25(11):1849-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References. Data generated or analyzed during this study are included in this published article or in the data repositories listed in References for Supplemental Figures S1-S6, https://doi.org/10.5061/dryad.p2ngf1vp4.