Abstract
The Third International Conference on Controversies in Vitamin D was held in Gubbio, Italy, September 10–13, 2019. The conference was held as a follow‐up to previous meetings held in 2017 and 2018 to address topics of controversy in vitamin D research. The specific topics were selected by the steering committee of the conference and based upon areas that remain controversial from the preceding conferences. Other topics were selected anew that reflect specific topics that have surfaced since the last international conference. Consensus was achieved after formal presentations and open discussions among experts. As will be detailed in this article, consensus was achieved with regard to the following: the importance and prevalence of nutritional rickets, amounts of vitamin D that are typically generated by sun exposure, worldwide prevalence of vitamin D deficiency, the importance of circulating concentrations of 25OHD as the best index of vitamin D stores, definitions and thresholds of vitamin D deficiency, and efficacy of vitamin D analogues in the treatment of psoriasis. Areas of uncertainly and controversy include the following: daily doses of vitamin D needed to maintain a normal level of 25OHD in the general population, recommendations for supplementation in patients with metabolic bone diseases, cutaneous production of vitamin D by UVB exposure, hepatic regulation of 25OHD metabolites, definition of vitamin D excess, vitamin D deficiency in acute illness, vitamin D requirements during reproduction, potential for a broad spectrum of cellular and organ activities under the influence of the vitamin D receptor, and potential links between vitamin D and major human diseases. With specific regard to the latter area, the proceedings of the conference led to recommendations for areas in need of further investigation through appropriately designed intervention trials. © 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC. on behalf of American Society for Bone and Mineral Research.
Keywords: VITAMIN D, VITAMIN D DEFICIENCY, NUTRITION, ENDOCRINE PATHWAYS, OSTEOMALACIA, RICKETS, METABOLIC BONE DISEASES
Introduction
Following meetings held in 2017( 1 ) and 2018,( 2 ) the Third International Conference on Controversies in Vitamin D was held in Gubbio, Italy, September 10–13, 2019. The aim of the conference was to convene leading worldwide experts in vitamin D research to address ongoing controversies and current topics of debate in vitamin D research. Following formal presentations on specific topics, discussions among experts were used to help resolve lingering issues and to clarify areas of uncertainty. Several core issues from the previous conference in 2018 were revisited, such as assays to determine serum 25OHD concentration, which remains a critical and controversial issue for defining vitamin D status. Definitions of vitamin D nutritional status were also revisited. New areas were discussed, including the epidemiology of vitamin D in developing countries and 25OHD threshold values and how they should be defined in the context of health and disease in different stages of human development. Therapeutic roles of vitamin D and findings from recent randomized clinical trials were also discussed for cancer, cardiovascular disease, and diabetes mellitus (DM). It was evident that results from recent trials are inconclusive because of questionable design, the treatment regimen adopted, or the baseline vitamin D status of the study subjects. Here we also identify issues concerning vitamin D in both skeletal and nonskeletal diseases where consensus is becoming established or is still lacking.
Topics Considered for Consensus
Nutritional rickets
Nutritional rickets, caused by a simple vitamin D or calcium deficiency or both, still affects a significant number of infants and children worldwide.( 3 ) Vitamin D‐deficiency rickets is cured by vitamin D administration.( 3 ) There is consensus that infants and most children require approximately 400 IU (or 600 IU for older children) of vitamin D per day to prevent rickets because direct exposure to sunlight is often avoided and not recommended for the very young.( 4 ) However, such a supplementation policy is either not or not fully implemented in many countries.
Although countries in Asia and the Middle East are most often affected by nutritional vitamin D deficiency, African and some Asian countries also encounter rickets caused by calcium deficiency.( 3 ) For newborns 0 to 6 and infants 6 to 12 months of age, adequate calcium intake is 200 and 260 mg/day, respectively, whereas for children over 12 months of age, a dietary calcium intake of <300 mg/day increases the risk of rickets independent of serum 25OHD levels.( 5 ) For children over 12 months of age, classification of dietary calcium intake can be defined as: sufficiency = >500 mg/day; insufficiency = 300 to 500 mg/day, and deficiency = <300 mg/day.( 6 )
The pathogenesis of calcium deficiency rickets is probably more complex than previously thought. However, we do know that reduced calcium intake increases PTH secretion, which in turn increases FGF‐23. Increases in both PTH and in FGF‐23 lead to an increase in urinary phosphate excretion. This pathophysiological sequence leads to reduced serum phosphate, which, along with PTH, increases the 1,25‐dihydroxyvitamin D [1,25(OH)D] level. Elevated 1,25(OH)D upregulates a number of genes causing an increase in pyrophosphate, a known inhibitor of bone mineralization, along with osteopontin and small integrin‐binding ligand N‐linked glycoproteins (SIBLINGS; Fig. 1).( 7 , 8 , 9 , 10 ) These abnormalities, along with low calcium and low phosphate levels, are primarily responsible for the osteomalacia characteristic of calcium deficiency. Although this pathophysiological sequence has been demonstrated in animals, it is likely that humans are affected in the same way.
Fig 1.
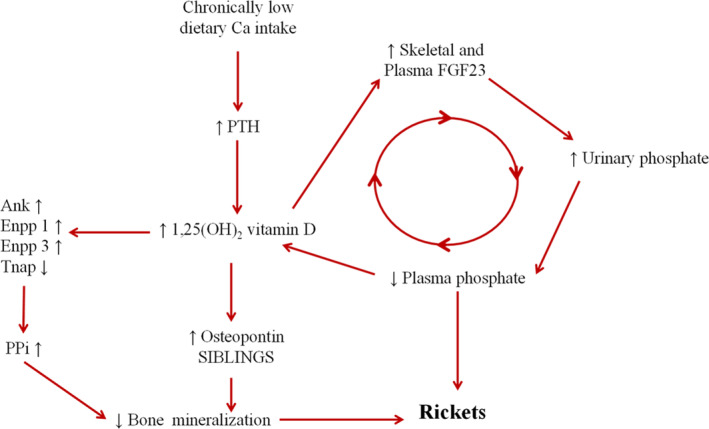
Mechanisms involved in the pathogenesis of rickets caused by a chronic low calcium intake. ANK = ankylosis protein; Ca = calcium; ENPP = ectonucleotide pyrophosphatase/phosphodiesterase; FGF‐23 = fibroblast growth factor 23; PPi = inorganic pyrophosphate; PTH = parathyroid hormone; SIBLINGS = small integrin‐binding ligand, N‐linked glycoproteins; Tnap = tissue nonspecific alkaline phosphatase.
Currently, there is still a high incidence of rickets, mainly based on clinical signs, in different countries around the world (Table 1).( 11 ) Based on the widespread global prevalence of rickets, a task force should be established to deal with this problem. Such a task force comprised of representatives from societies such as the International Society of Endocrinology, the International Federation of Musculoskeletal Research Societies, the Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology, as well as representatives from the vitamin D conference should prepare and present a plan to the WHO to eradicate rickets before 2030.( 3 )
Table 1.
Prevalence of Rickets Worldwide
| Country | Year | Rate (%) | Method |
|---|---|---|---|
| Mongolia | 1998 | 70 | Rickets signs |
| Tibet | 1994 | 66 | Rickets signs |
| Ethiopia | 1997 | 42 | X‐rays |
| Yemen | 1987 | 27 | – |
| Turkey | 1994 | 10 | – |
| Nigeria | 1998 | 9 | Rickets signs |
| Iran | 1975 | 15 | X‐rays |
| China | 1977–83 | 47 | Rickets signs |
| 3.7 | X‐rays/biochem | ||
| The Gambia (West Kiang) | 2007 | 3.3 | Rickets signs |
| 0.6 | Physician exam | ||
| Bangladesh (Chittagong) | 2008 | 2.2 | Rickets signs |
| 1.0 | X‐rays |
Vitamin D is produced by UVB light from the sun
UVB light (wavelength of approximately 280 to 310 nm) opens the B ring of 7‐dehydrocholesterol, the last step in the de novo synthesis of cholesterol, and generates previtamin D, which undergoes thermally induced isomerization into vitamin D3 before being transferred into the circulation by binding to the serum vitamin D binding protein (DBP).
Short periods of exposure to sunlight are beneficial for vitamin D production, whereas prolonged UVB exposure leads to sunburn and DNA damage.( 12 ) Larger doses result in more intense peak reactions in a roughly linear fashion, with the actual slope of the lines defined by individual variability, which in turn is probably accounted for, at least in part, by genetic determinants. As UV doses increase, simple tanning is replaced by more advanced degrees of sunburn. In contrast, vitamin D formation is instantaneous and increases linearly in a time‐dependent fashion from very small to very large UV exposures. The dose response for dermal photosynthesis of vitamin D increases linearly at small UV doses, but differs strikingly from the other dose–response curves in reaching a plateau well below the threshold dose for erythema; Fig. 2). ( 13 , 14 ) Thus, short UVB exposure times increase vitamin D photosynthesis. However, many other variables can influence vitamin D dermal photosynthesis such as age, skin color, sunscreen use, latitude, time of day, and season. As a result, there is no consensus on what constitutes safe and effective exposure to sunlight for the general population.( 6 ) Moreover, given the above‐noted individual differences, attempting blanket guidance seems ill‐advised.
Fig 2.
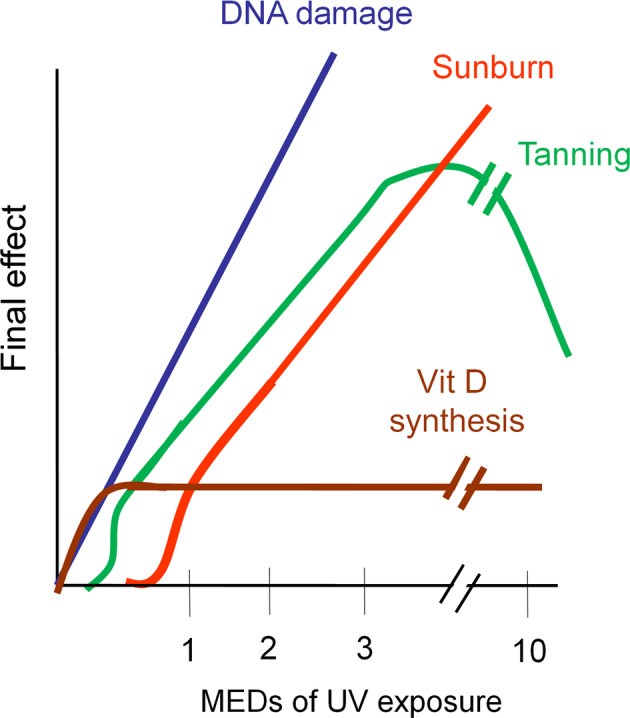
Relationship between minimal erythema dose (MED) of UV exposure and level of DNA damage, suntan/tanning, and vitamin D synthesis.
Vitamin D deficiency is prevalent
Although cutaneous vitamin D3 synthesis occurs rapidly in the presence of adequate solar UVB because of human behavior—indoor work, sun avoidance, etc.—vitamin D deficiency is widespread.( 15 ) Using a definition of <20 ng/mL (<50 nmol/L),( 16 ) as many as one third of the world's population is deficient, with a percentage as high as 40% in Europe (Table 2). Severe vitamin D deficiency, defined as <30 nmol/L (or <12 ng/mL), is seen in approximately 7% of the population worldwide, with considerable variation observed between different countries and populations. Nevertheless, severe vitamin D deficiency occurs in high‐risk populations worldwide.( 17 ) High‐risk groups for vitamin D deficiency include those who lack effective exposure to sunlight. This could be because of a variety of climatologic, cultural, or religious reasons, as well to skin pigmentation. Vitamin D deficiency was long considered rare in Africa, but a systematic analysis of African countries revealed that severe vitamin D deficiency is present in 18% of all African subjects, with clusters having a high prevalence of deficiency widely dispersed based upon cultural/behavioral practices.( 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 )
Table 2.
Vitamin D Deficiency Around the World
25OHD is the “best” marker of vitamin D status
The circulating 25OHD concentration is widely accepted as the best marker of an individual's vitamin D status, and has been used by numerous agencies in the establishment of vitamin D dietary requirements and for population surveillance of vitamin D deficiency or inadequacy.( 27 ) However, circulating 25OHD has, at least historically, been felt to have little physiologic regulation, thus other measures could potentially be better indicators of vitamin D status. Notably, there is ongoing debate with regard to whether free 25OHD (unbound to carrier proteins) or the ratio of 24,25‐dihydroxyvitamin D [24,25(OH)2D]:25OHD is a superior marker than total 25OHD.( 28 )
The ratio of 25(OH)D3:24,25(OH)2D3 has been developed as a diagnostic tool for idiopathic infantile hypercalcemia caused by mutations of CYP24A1. However, the ratio is also elevated in patients with vitamin D deficiency, who undergo dialysis for chronic kidney disease caused by downregulation of the CYP24A1 enzyme.( 29 , 30 ) It is also possible in certain circumstances that the ratio of 1,25(OH)2D:25OHD could be a useful marker for CYP27B1 activity.( 31 )
Importantly, vitamin D research data are plagued by variation in the quality of serum total 25OHD assay methods—which has compromised, and continues to compromise—the ability to distinguish among the different guidelines currently in use.( 32 ) Similarly, uncertainty about the quality of free 25OHD measurement hinders its evaluation compared with serum total 25OHD. For 25OHD and 24,25(OH)D2, reference methods are available that are used to improve the standardization of these analytes. Standardization is encouraged by the Vitamin D Standardization Program (VDSP) and by the Vitamin D External Quality Assurance Scheme (DEQAS). DEQAS, backed‐up by CDC‐standardized target values, has monitored the performance of 700 to 1000 laboratories assaying 25OHD quarterly for 30 years. Over the decades, it has documented problematic assays and kit manufacturers.( 33 , 34 ) DEQAS also promotes an accurate assay of 24,25(OH)2D3 and 1,25(OH)2D by circulating serum samples.
Currently, the VDSP is coordinating an effort to harmonize direct free 25OHD measurement by the development of “trueness” controls (Personal Communication, Professor Chris T Sempos). Finally, the NIH Office of Dietary Supplements, as part of the VDSP, is sponsoring the development of a reference method for 1,25(OH)2D, which will help to standardize its measurement in vitamin D research and bring clarity to its role.( 26 ) Such standardization efforts are essential to advance clarification of what truly constitutes vitamin D deficiency.
However, standardization is not the only analytical challenge in the measurement of vitamin D metabolites. Patient‐ or matrix‐dependent deviations are a well‐known confounder in many 25OHD immunoassays leading to inaccurate results, for example, in pregnant women or hemodialysis patients. In addition, differences in the affinity for, or release from DBP for 25(OH)D3 and 25(OH)D2 within immunoassays lead to important problems in the determination of the serum 25OHD concentration in subjects taking D2 supplements.( 35 ) These problems cannot be solved by standardization initiatives, but are inherent in the specific immunoassays; it is therefore essential that these immunoassays should be improved as well. This is particularly important in regions where ergocalciferol is commonly used and for vegans who may choose to avoid cholecalciferol.
Definition/thresholds of vitamin D deficiency
There is an ongoing debate regarding the definition of vitamin D deficiency as noted by different recommendations from various expert groups.( 4 ) However, there is consensus on two points: 25OHD levels below 12 ng/mL (30 nmol/L) are clearly deficient at all ages and levels above 30 ng/mL (75 nmol/L) are clearly sufficient. In contrast, there is disagreement on how to regard levels between 12 and 30 ng/mL (30 and 75 nmol/L). Some guidelines recommend a threshold value of 20 ng/mL (50 nmol/L),( 36 ) whereas others aim for ≥30 ng/mL (≥ 75 nmol/L).( 37 ) This discussion is based in large part on the lack of 25OHD assay standardization.( 32 )
These cut points have key implications for randomized clinical trials (RCTs). There are few clinical trials that enrolled clearly vitamin D‐deficient subjects; one example is the work of Chapuy and colleagues.( 38 , 39 ) The importance of studying the effect(s) of vitamin D supplementation only in deficient subjects cannot be overemphasized because vitamin D is a threshold nutrient,( 40 ) which means that a physiological endpoint, such as calcium absorption, is enhanced in dose–response fashion up to the threshold value above which higher levels do not lead to a greater effect. If a clinical trial enrolls subjects whose 25OHD levels are above the threshold, randomizing subjects to receive additional vitamin D greatly reduces the likelihood of showing a benefit of supplementation. Recent well‐publicized RCTs published in major peer‐reviewed journals illustrate this confounding point well.( 41 , 42 , 43 , 44 ) One would not expect to see an effect of a threshold nutrient if both the control and the supplemented groups started at baseline with sufficient levels of 25OHD.( 45 )
Vitamin D analogues are the preferred local therapy for psoriasis
The benefits of vitamin D analogues for the treatment of psoriasis are well‐established.( 46 , 47 , 48 ) A topical vitamin D analogue is a first‐line choice in the management of psoriasis, either alone or in combination with topical corticosteroids.( 49 , 50 , 51 , 52 , 53 , 54 ) Unlike corticosteroids, which can be associated with tachyphylaxis, topically administered vitamin D analogue treatment is effective long‐term without side‐effects in patients of all ages.( 55 , 56 , 57 , 58 , 59 )
Topics for Which Consensus Is Not Established
What daily doses of vitamin D are recommended to maintain a normal level of 25OHD in the general population?
The Institute of Medicine recommends 400–600–800 IU/day vitamin D supplementation if there is no exposure to sunlight for infants, children/adults, and elderly, respectively.( 36 ) These recommendations are endorsed by guidelines formulated by Nordic and DACH (German‐speaking) countries, Australia and New Zealand, the European Food Safety Agency, the European Calcified Tissue Society, and the International Osteoporosis Foundation.( 60 , 61 , 62 , 63 , 64 , 65 ) The Endocrine Society recommends 600 IU/day up to 2000 IU/day for so‐called risk groups.( 37 ) UK guidelines (the Scientific Advisory Committee on Nutrition) recommend 400 IU/day for any age.( 66 ) A few other organizations suggest much higher doses (4000 to 10,000 IU/day).( 67 ) These recommendations are for individuals who do not have osteoporosis or other metabolic bone disease. Unfortunately, this point has not been appreciated by many organizations or practitioners. Errors can occur in two ways. First, subjects who are overly concerned about their skeletal health could conceivably take too much if recommendations by some bodies of up to 10,000 IU per day are followed. It is estimated, for example, that 3% of adults in the United States take a vitamin D supplement of >4000 IU/day.( 68 ) Such amounts could potentially be deleterious as such doses may decrease rather than increase BMD or bone strength.( 69 ) On the other hand, use of relatively low doses could be deleterious for those in whom requirements are higher (eg, malabsorption or obesity). It is clearly essential to define and reach consensus regarding what constitutes deficiency to allow resolution of existing differences in daily supplementation dose recommendations.
What are the recommendations for supplementation in patients with metabolic bone diseases?
In patients who have osteoporosis or other metabolic bone diseases, the discussion about vitamin D is different from that for the general population.( 70 ) Clearly, greater emphasis is placed upon first ensuring that the 25OHD level is sufficiently above the threshold, whichever one is being followed, either 20 or 30 ng/mL (50 or 75 nmol/L). Furthermore, there is evidence that the response to antiosteoporosis drugs may be enhanced when vitamin D and calcium sufficiency are ensured.( 71 , 72 )
This consensus has recently been questioned by a meta‐analysis conducted by Bolland and colleagues.( 73 ) In their review, they stated: “Our findings suggest that vitamin D supplementation does not prevent fractures or falls or have clinically meaningful effects on bone mineral density.” They concluded their discussion with the following statement: “There is little justification to use vitamin D supplements to maintain or improve musculoskeletal health” and “This conclusion should be reflected in clinical guidelines.”( 73 ) First, other experts, who have taken issue with their statements, have questioned such conclusions. Lips, Bilezikian, and Bouillon( 74 ) note that this meta‐analysis excluded all studies that compared calcium plus vitamin D versus double placebo. Boonen and colleagues showed many years ago that it is necessary to administer both calcium and vitamin D in sufficient amounts to observe a reduction in fractures.( 75 ) Weaver and colleagues, representing the National Osteoporosis Foundation, came to the same conclusion,( 76 ) as did Yao and colleagues in a recent meta‐analysis.( 77 ) Second, over 60% of the studies were short‐term, <1 year. It is unreasonable to expect a beneficial effect of antiosteoporotic nutrients on fracture risk over such a short period. Third, vitamin D‐deficient individuals (25OHD <12 ng/mL or 30 nmol/L) represented a miniscule percentage of the entire population studied: <2.1%. Fourth, the trial that constituted individuals at highest fracture risk (18%) was hampered by poor compliance (~50%).( 78 ) Another flaw in this meta‐analysis was inclusion of studies that utilized high intermittent boluses of vitamin D, which might increase fracture risk.( 79 ) Moreover, the two main authors of this meta‐analysis have independently published separate meta‐analyses in which they conclude that combined vitamin D and calcium supplements can reduce the risks of hip and nonvertebral fractures in the elderly.( 73 , 80 ) This earlier review was not mentioned or discussed in their latest meta‐analysis. Other experts have reached similar conclusions.( 74 , 81 ) Nevertheless, the debate is alive with contrary views still being expressed as recently as the 2019 meeting of the ASBMR.( 82 )
Cutaneous production of vitamin D by UVB exposure
Studies led by Holick and colleagues have repeatedly stated that a full day of sun exposure can produce 10,000 to 25,000 IU of vitamin D.( 83 , 84 , 85 ) To this point, in other studies it has been shown that the indigenous, very dark‐skinned Masai people are said to make 10,000 or 20,000 IU per day. More recent studies have raised questions about the magnitude of the sun's effect on dermal vitamin D production. Young Danish women exposed to intensive sun in the Canary Islands showed an increase in 25OHD that was equivalent to only 600 to 1000 IU/per day. A similar increase in serum 25OHD was induced by comparing total‐body UVB exposure three times per week with an oral daily intake of only 800 IU of vitamin D.( 86 , 87 ) Another study from the Canary Islands of young Danish women, exposed to 1 week of daily sunlight, showed that serum levels of 25OHD increased by only 20 nmol/L (8 ng/mL), equivalent to about 800 IU of oral vitamin D per day.( 88 ) In yet another study from the Canary Islands, young Polish volunteers with near total body sun exposure achieved a change in 25OHD of 28 nmol/L or approximately 12 ng/mL equivalent to approximately 600 to 1200 IU (~15 to 30 μg) of oral vitamin D per day.( 89 ) Finally, exposure of 1000 cm 2 on the back three times per week at half the minimal erythematous dose in nursing home residents increased median serum 25OHD in 3 months from 7.2 to 24 ng/mL (18 nmol/L to 60 nmol/L), equivalent to a supplement of 400 IU/day.( 90 ) It is at this time unclear what full daily exposure to sun produces. Is it about 1000 IU or closer to 10,000 IU? An answer to that question may be helpful in the interpretation of the daily requirements of vitamin D in subjects with little exposure to sunlight.
Hepatic regulation of 25OHD metabolism
Biochemical dogma states that vitamin D is hydroxylated in the liver to 25OHD by the constitutively active hydroxylase, CYP2R1. Although this enzyme is clearly the major converting enzyme, it is not the only way in which hydroxylation occurs.( 91 ) Recent data have also called into question the constitutive nature of this reaction by evidence suggesting this enzyme is subject to several different control mechanisms. For example, in the fasting state, a significant reduction in the expression of CYP2R1 can be observed.( 92 ) In a murine model of DM, a 50% reduction in mRNA and protein expression of CYP2R1 is demonstrable.( 92 ) This study identified novel molecular mechanisms (involving PPAR γ coactivator a1 and estrogen‐related receptor) for vitamin D deficiency in DM and showed a novel negative feedback mechanism that controls cross‐talk between energy homeostasis and the vitamin D pathway. Activation of the glucocorticoid receptor (by dexamethasone or other corticosteroids) also supresses the activity of CYP2R1. Thus, rather than viewing the liver as a constitutive factory for the quantitative conversion of vitamin D to 25OHD via an unregulated CYP2R1 enzyme, metabolic and hormonal mechanisms are operative. More research is clearly needed to understand better how the production of 25OHD is regulated in the liver.
Definition of vitamin D excess
The classical concept of vitamin D toxicity was thought to be that level above which hypercalcemia was likely to occur. Serum 25OHD values in excess of 100 or 150 ng/mL (250 or 375 nmol/L) may lead to hypercalcemia and, thus, these cut points became frames of reference for a number of authoritative bodies, such as the Institute of Medicine, the Endocrine Society, and reference laboratories.( 36 , 37 , 93 )
Although it would seem reasonable to identify hypercalcemia as a threshold of toxicity, other indices of toxicity, such as hypercalciuria could occur at much lower levels.( 94 ) In the study by Gallagher and colleagues, hypercalciuria occurred in 30% of vitamin D‐deficient individuals administered only 800 to 2000 IU per day for 1 year, whereas hypercalcemia occurred in 9%.
Further human studies conducted by Gallagher and colleagues and reanalyzed by Kaufmann and colleagues showed that doses up to 4000 IU/day for a year resulted in serum 25OHD <90 ng/mL.( 29 )
There was no relationship between the administered amount of vitamin D and the level of urinary calcium excretion or hypercalcemia. Moreover, in half of these subjects, the hypercalciuria was transient. Adding further uncertainty to these data, however, was the observation that those receiving placebo experienced the same incidence of hypercalciuria. These data do not provide compelling support for the idea that such low‐dose regimens may be harmful. In fact, most experts agree that doses up to 4000 IU are probably safe.( 41 , 95 ) A more compelling discussion focuses upon fall risk associated with high doses of vitamin D.( 69 , 96 , 97 ) Intermittent high boluses or administration of vitamin D to older individuals on a regular basis, associated with levels of 25OHD >45 ng/mL (>113 nmol/L), may lead to an increase risk of falls.( 79 ) Further research is needed to further clarify whether such 25OHD levels do indeed increase falls risk.
Skeletal health has also been a focus of recent studies related to adverse effects of high vitamin D dosing. In the Calgary study performed on healthy volunteers without osteoporosis whose mean baseline 25OHD was approximately 31 to 32 ng/mL, treatment with vitamin D for 3 years at a dose of 4000 IU/day or 10,000 IU/day, compared with 400 IU/day, resulted in statistically significant reduction in radial volumetric BMD.( 69 ) However, no significant differences in bone strength at either the radius or tibia were observed. Burt and colleagues concluded from this study that there was no benefit from doses of vitamin D at 4000 IU or higher as an adjunct to bone health.
Vitamin D deficiency in acute illness
In the setting of acute illness, levels of 25OHD may be low because of the acute reduction in circulating DBP.( 98 ) Dilutional effects of acute fluid shifts in the intravascular space may also be a factor. Additionally, pre‐existing vitamin D nutritional status is also a factor. This latter point leads to the suggestion that correction of poor vitamin D status may decrease morbidity and mortality. Christopher and colleagues suggest that very high doses of vitamin D may be needed to see a benefit in patients in the intensive care unit.( 99 ) The higher doses may be needed because acutely ill patients may have secreted stress amounts of cortisol, which in turn could impair hepatic and renal hydroxylation of vitamin D.( 100 , 101 )
Vitamin D requirements during reproduction
There is a lack of consensus on the use of vitamin D during reproduction. On the one hand, maternal vitamin D requirements are not increased during pregnancy or lactation. The achieved 25OHD level is not affected by either reproductive state, and there is no evidence that women should maintain higher 25OHD levels when pregnant or breastfeeding as compared with the healthy nonpregnant ideal. On the other hand, poor maternal vitamin D status during pregnancy can affect fetal and neonatal health; so it certainly makes sense to ensure that maternal vitamin D status is optimized during pregnancy. This does not mean that women require “more” vitamin D when pregnant than when nonpregnant. During lactation maternal vitamin D status does not matter directly because little vitamin D gets into milk, and especially because RCTS have shown that across a range of low to high 25OHD levels, the calcium content of milk is independent of maternal vitamin D status. Breastfed babies need supplemental vitamin D, whereas formula‐fed babies get their vitamin D in the supplemented formula.
Although variability exists among different studies, evidence from RCTs and systematic reviews suggests a benefit of vitamin D repletion with up to 2000 IU/day for preeclampsia and gestational DM,( 102 ) as well as for neonatal outcomes.( 103 , 104 , 105 , 106 , 107 ) It seems reasonable to recommend that normal vitamin D status should be ascertained in pregnancy.
The potential for a broad spectrum of cellular and organ activities under the influence of the vitamin D receptor
The vitamin D receptor (VDR) is present in virtually all cells and tissues. The 1a‐hydroxylase, CYP27B1, is also found throughout the body and in many cell types.( 108 ) It has been estimated that 3% to 10% of all genes in vertebrates, from zebrafish to mice to humans, are under the direct or indirect control of 1,25(OH)2D3.( 109 ) This evolutionary omnipresence suggests a fundamental role for vitamin D in the functioning of all organs. Experiments to delete this gene in a tissue‐specific manner in mice have confirmed this expectation. A sampling of tissue‐specific KO experiments shows that mammary glands are more prone to breast cancer,( 110 ) cardiac muscle develops cardiac hypertrophy,( 111 ) the liver becomes fatty (nonalcoholic fatty liver syndrome),( 112 , 113 ) the prostate develops hyperplasia,( 114 ) atherosclerosis is accelerated,( 115 ) and mice become resistant to diet‐induced obesity.( 116 ) Conversely, overexpression of the VDR leads to obesity( 117 ) in the mouse, but not in humans.( 118 ) More work is needed to understand how these KO and overexpression models in mice relate to human pathophysiology.
Recent mortality data show an association between low 25OHD and increased risk of all‐cause mortality.( 119 , 120 ) These findings were also observed in a European consortium.( 121 ) Several association analyses of overall mortality and cardiovascular mortality have shown a U‐shaped curve with increases at both ends.( 122 ) A meta‐analysis based on 75,000 patients from 38 supplementation trials also showed a small, but significant reduction in mortality (relative risk [RR], 0.94; 95% CI, 0.91–0.98).( 123 )
From Mendelian randomization studies examining the effects of vitamin D on autoimmune diseases, three independent findings show that decreased vitamin D levels (5% to 7% lower than normal levels) significantly increased the susceptibility to developing multiple sclerosis.( 124 , 125 , 126 ) Finally, one Mendelian randomization study showed an association with type 1 diabetes mellitus (T1DM) risk.( 127 )
The data on cancer in mice are also of interest. 1,25(OH)2D3‐deficient mice have a greater chance of developing cancer with increasing age,( 128 ) and an increased rate of proliferation in intestinal and breast cells. Although VDR‐null mice usually do not spontaneously develop more cancers, they are more likely to develop a range of malignancies, such as breast,( 129 ) colon,( 130 ) and skin( 131 , 132 ) cancer, when exposed to oncogenes, loss of antioncogenes, or exposure to carcinogens or UVB light.( 133 , 134 ) This is in line with the “cancer hypothesis,” where the risk of cancer development is associated with multiple events. Although these mice data appear to be compelling, Mendelian randomized studies in humans have not been supportive.( 135 )
Potential links between vitamin D and major human diseases
Many cross‐sectional, observational, and retrospective studies have associated low vitamin D status with many human diseases.( 136 , 137 , 138 , 139 ) In the aggregate, these reports suggest a pervasive influence of vitamin D on the health of most human organ systems. Preclinical evidence for a role of vitamin D in immune system regulation is perhaps strongest as the VDR and CYP27B1 are expressed in cells of both the innate and adaptive arms of the immune system. Moreover, CYP27B1 expression in immune cells is regulated by a complex innate immune and cytokine network.( 136 , 137 , 138 , 139 , 140 , 141 ) There is widespread clinical evidence in both pediatric and adult populations that maintenance of vitamin D sufficiency should lower the incidence of infections of viral or bacterial origin.( 142 ) Accumulated evidence suggests that any role for vitamin D in autoimmune conditions would be preventive rather than therapeutic. One condition for which vitamin D supplementation may be of benefit is in the treatment of the inflammatory bowel condition Crohn disease, where meta‐analyses of a series of small‐scale trials suggest that supplementation reduces disease severity.( 143 , 144 ) It would be important to conduct a large‐scale RCT in patients with Crohn disease to solidify these findings. Large‐scale RCTs are essential to determine whether the relationship between vitamin D deficiency and disease is causal or simply an association.
Another disorder to which vitamin D deficiency has been linked is DM. It has been shown that vitamin D prevents insulitis and the development of experimental DM by acting on the defective suppressor cellular function or by cytokine‐expression modulation. These observations have been confirmed, in part, by clinical findings showing that supplementation with vitamin D during early childhood may decrease the risk of developing T1DM.( 145 , 146 ) However, further studies have not shown any significant effect of calcitriol supplementation on insulin secretion, insulin sensitivity, or insulin requirement or improvement in bone turnover in patients with newly diagnosed T1DM.( 147 , 148 )
It is uncertain whether 25OHD levels in pregnancy or at birth reduce the risk of childhood T1DM. However, when the interaction with genetic variants is taken in consideration, higher 25OHD levels at birth predict a decreased risk of developing T1D or islet autoimmunity.( 149 , 150 ) Both child or maternal VDR SNPs may lower VDR expression, and by consequence, inhibit T‐cell proliferation, thus increasing the risk of autoimmunity.
The recent Vitamin D Assessment (VIDA), Vitamin D and Omega‐3 (VITAL), and Vitamin D and Type 2 Diabetes (D2d) trials represent examples of attempts to translate these observations into clinical relevance.( 43 , 151 , 152 )
Cardiovascular disease
The VIDA trial tested the effect of a monthly dose of 100,000 IU of vitamin D3 compared with a placebo over a mean period of 3.4 years on cardiovascular disease among 5110 subjects.( 153 ) There was no statistical difference between the two groups. In the VITAL trial, there were no significant differences between the vitamin D and placebo groups in any individual cardiovascular event, such as myocardial ischemia, or in the composite cardiovascular end point.( 145 )
Cancer risk and survival
The much larger VITAL trial( 151 ) of 25,871 men and women aged over 50 years tested the effects of 2000 IU/day of vitamin D3 over 5 years on cardiovascular events and cancer. There was no significant difference between the vitamin D and placebo groups on the risk of developing any invasive cancer or individually in breast, prostate, or colorectal cancer. However, among those with a BMI <25, there was a significant reduction in any invasive cancer. Excluding the first 2 years of the study, there was also a reduction in the incidence of death from cancer. The study by Lappe and colleagues is noteworthy in this context; they show that calcium and vitamin D appeared to have an effect to reduce new cancer risk, but statistical significance was not achieved.( 154 )
A subsequent meta‐analysis by some of the invesitigators from the VITAL trial is noteworthy.( 155 ) Whereas VITAL appreciated a “signal” of improved survival in the vitamin‐D–supplemented group, the meta‐analysis of VITAL and several additional studies found a highly significant benefit on survival in the vitamin‐D–supplemented subjects, but again no benefit on risk of developing cancer. For total cancer incidence, 10 trials were included (6537 cases; 3 to 10 years of follow‐up; 54–135 nmol/L of attained levels of circulating 25OHD in the intervention group). The summary for cancer risk remained null across the subgroups tested, including when attained 25OHD levels exceeded 100 nmol/L. For total cancer mortality, five trials were included (1591 deaths; 3 to 10 years of follow‐up; 54 to 135 nmol/L of attained levels of circulating 25OHD in the intervention group). The summary RR was 0.87 (95% CI, 0.79–0.96; p = 0.005), which was largely attributable to interventions with daily dosing (as opposed to infrequent bolus dosing). Thus, this updated meta‐analysis of RCTs showed that vitamin D supplementation significantly reduced total cancer mortality, but did not reduce total cancer incidence. In the Torfadottir study, the goal was to explore whether prediagnostic circulating levels of 25OHD among older individuals were associated with overall and cancer‐specific survival after diagnosis.( 156 ) They used data from the AGES‐ (Gene/Environment Susceptibility‐) Reykjavik study on participants (n = 4619) without cancer at entry, when blood samples were taken for 25OHD standardized measurements. The association with cancer risk and all‐cause‐ and cancer‐specific mortality was assessed among those later diagnosed with cancer, comparing four 25OHD categories, using 50 to 69.9 nmol/L (20–28 ng/mL) as the reference category. Cancer was diagnosed in 919 participants on average 8.3 years after initial sampling. No association was observed between the reference group and other 25OHD groups and total cancer incidence. Mean age at diagnosis was 80.9 (± 5.7) years. Of those diagnosed, 552 died during follow‐up: 67% from cancer. Importantly, low prediagnostic levels of 25OHD <30 nmol/L (<12 ng/mL) were significantly associated with increased total mortality (hazard ratio [HR], 1.39; 95% CI, 1.03–1.88) and not significantly with cancer‐specific mortality (HR, 1.33; 95% CI, 0.93–1.90). Among patients surviving more than 2 years after diagnosis, higher prediagnostic 25OHD levels (≥70 nmol/L) were associated with lower risk of overall (HR, 0.68; 95% CI, 0.46–0.99) and cancer‐specific mortality (HR, 0.47; 95% CI, 0.26–0.99). It appeared that among elderly cancer patients, low prediagnostic serum 25OHD levels (<30 nmol/L [<12 ng/mL]) were associated with increased overall mortality.
Diabetes mellitus
The D2d trial examined the effect of vitamin D3 at 4000 IU/day on the development of overt DM among 2423 men and women aged >30 years, who had risk factors for DM. There was no difference in the probability of developing DM over this period between the vitamin D and placebo groups. However, a post hoc analysis in participants with a baseline 25OHD <12 ng/mL (or < 30 nmol/L) showed a 62% reduction in DM in the vitamin D group.
Pulmonary, blood pressure, and other effects
Additionally, from the VIDA trial, central blood pressure was significantly reduced in patients taking vitamin D supplementation (−7.5 mmHg, p = 0.03),( 157 ) and the number of patients taking NSAIDs was significantly reduced (RR, 0.87, p = 0.01).( 158 ) Furthermore, giving vitamin D to the normal population and to the vitamin D‐deficient population improves lung function,( 159 ) in line with a meta‐analysis,( 160 ) as well as reducing age‐related bone loss.( 161 , 162 ) In an individual participant data meta‐analysis of 15 RCTs, daily or weekly supplementation in individuals with vitamin D deficiency, defined as a serum 25OHD level <10 ng/mL, reduced risk of acute respiratory infection by 30% (odds ratio, 0.30; 95% CI, 0.17–0.53).( 142 )
Methodological Issues
Unfortunately, what VIDA, VITAL, and D2d studies share is that baseline 25OHD levels were not deficient in the majority of participants. Mean baseline levels from VIDA (24.2 ng/mL or 60.5 nmol/L), VITAL (30.8 ng/mL or 77.0 nmol/L), and D2d (28.2 ng/mL or 70.5 nmol/L) were all within the normal range as defined by the Institute of Medicine. Levels below 20 ng/mL (50 nmol/L) were seen in only 33% of the VIDA, 12.7% of the VITAL, and 20.7% of the D2d populations. One important conclusion from these studies is that they did not show that a vitamin D‐deficient population would benefit by vitamin D repletion because the populations were already replete. As noted earlier, if subjects are already above the level for a threshold nutrient, giving more will not necessarily lead to beneficial effects. Therefore, it is not evidence‐based to claim, based on these studies, that vitamin D has no effects on cancer, the cardiovascular system, or the development of DM. A clue to the importance of this statement is the post hoc analysis of the D2d study in which subjects who were frankly vitamin D deficient, namely with levels of 25OHD <12 ng/mL (30 nmol/L) at baseline, were at reduced risk of developing DM (HR, 0.38; 95% CI, 0.18–0.80) if they were in the vitamin‐D–supplemented group (Fig. 3). Other clues are noted above with regard to blood pressure and pulmonary infections in which vitamin D did appear to have beneficial effects.
Fig 3.
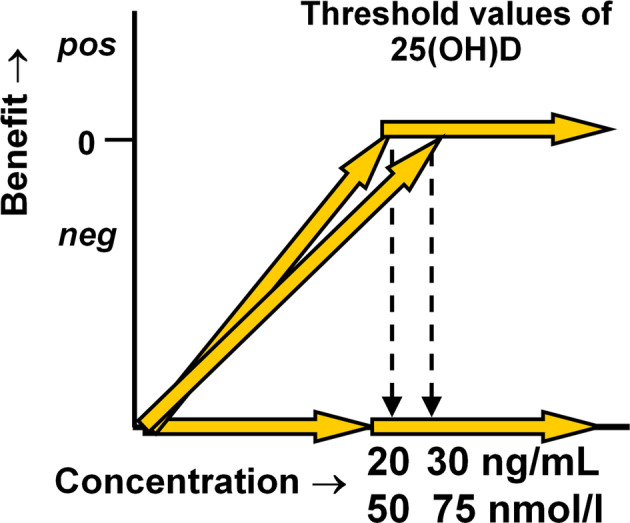
Threshold value of 25OHD where positive effects can be observed.
Another methodological issue is the duration of the studies. One has to consider how long prior to the development of cancer or cardiovascular disease or DM must an intervention have to influence the development of overt disease. For example, the Torfadottir study had a mean duration of 8.3 years from no sign of cancer to the cancer diagnosis.( 156 ) Is it likely that giving a vitamin D supplement for 5 years or less will alter the time course of cancer becoming apparent? Carcinogenesis is usually a slow process that proceeds undiagnosed in a stepwise fashion, perhaps for many years prior to the diagnosis. Thus, the finding that vitamin D supplementation can improve survival once the cancer is apparent, even if it does not reduce the risk of developing cancer over a 5‐year period of intervention, is nevertheless a major factor demonstrating the benefit of maintaining adequate levels of vitamin D.
Rigorous studies of vitamin D supplementation in subject cohorts deficient in vitamin D compared with adequate levels are needed to resolve the controversy surrounding potential/purported nonskeletal effects of vitamin D. For endpoints like cancer and cardiovascular disease, studies need to be carried out for longer duration than 5 years to clearly demonstrate the presence or absence of a benefit on risk. The benefit on cancer survival seems to be solidly demonstrated.
Conclusions
In this review, we have highlighted areas of consensus and uncertainty with regard to vitamin D as a nutrient and regulator of cellular action. Although nutritional rickets is well‐defined and highly prevalent worldwide, a concerted global effort is required to eradicate this eminently curable condition. A better understanding of the endogenous production of vitamin D and the regulation of its metabolism along with the development of universally useful assays with proper quality control remain worthy goals. Although animal data provide a useful backdrop to hypotheses arguing for nonskeletal effects of vitamin D, human studies both in terms of several meta‐analyses, as well as recent RCTs, have not been designed to permit any definitive conclusions. We look forward to future well‐designed studies that can clearly establish the extent to which vitamin D's actions are pervasive and extend beyond the skeleton.
Disclosure
The coauthors of this article have no conflicts to declare with regard to its content.
Author contributions
Andrea Giustina and John P. Bilezikian: Conceptualization; data curation; methodology; supervision; writing‐original draft; writing‐review and editing. Roger Bouillon: Writing‐review and editing. Neil Binkley: Writing‐review and editing. Christopher Sempos: Writing‐review and editing. Robert Adler: Writing‐review and editing. Jens Bollerslev: Writing‐review and editing. Bess Dawson‐Hughes: Writing‐review and editing. Peter Ebeling: Writing‐review and editing. David Feldman: Writing‐review and editing. Annemieke Heijboer: Writing‐review and editing. Glenville Jones: Writing‐review and editing. Christopher Kovacs: Writing‐review and editing. Marise Lazaretti‐Castro: Writing‐review and editing. Paul Lips: Writing‐review and editing. Claudio Marcocci: Writing‐review and editing. Salvatore Minisola: Writing‐review and editing. Nicola Napoli: Writing‐review and editing. René Rizzoli: Writing‐review and editing. Robert Scragg: Writing‐review and editing. John White: Writing‐review and editing. Anna Maria Formenti: Writing‐review and editing.
Peer review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbm4.10417.
Acknowledgments
This report summarizes the proceedings of the Third International Conference on Controversies in Vitamin D. It was held on September 10–13, 2019, Gubbio, Italy. The meeting was funded by an unrestricted grant provided by Abiogen Pharma, Pisa, Italy. Abiogen Pharma had no role in the selection of discussion topics, speakers/authors, preparation or review of this paper. The authors would like to acknowledge the support of Dr Colin Egan in the writing of the manuscript. We wish to acknowledge those who also participated in this meeting, but are not listed as co‐authors: D Bikle, L Degli Esposti, G El‐Haj Fuleihan, S Giannini, AR Martineau, M Hewison, UA Liberman, A Mithal, F Nannipieri, L Plum, A Pittas, S Sciacchitano, and J Virtanen.
Authors’ roles: AG and JPB served as Program Coordinators of the Third International Conference on Controversies in Vitamin D. All authors researched data for the conference. AG and JPB wrote the manuscript, and all authors reviewed and/or edited the manuscript before submission.
References
- 1. Giustina A, Adler RA, Binkley N, et al. Controversies in vitamin D: summary statement from an international conference. J Clin Endocrinol Metab. 2019;104(2):234–40. [DOI] [PubMed] [Google Scholar]
- 2. Giustina A, Adler RA, Binkley N, et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev Endocr Metab Disord. 2020;21(1):89–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouillon R, Antonio L. Nutritional rickets: historic overview and plan for worldwide eradication. J Steroid Biochem Mol Biol. 2019;198:105563. [DOI] [PubMed] [Google Scholar]
- 4. Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13(8):466–79. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed S, Goldberg GR, Raqib R, et al. Aetiology of nutritional rickets in rural Bangladeshi children. Bone. 2020;136:115357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101(2):394–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michigami T. Skeletal mineralization: mechanisms and diseases. Ann Pediatr Endocrinol Metab. 2019;24(4):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jan De Beur SM, Levine MA. Molecular pathogenesis of hypophosphatemic rickets. J Clin Endocrinol Metab. 2002;87(6):2467–73. [DOI] [PubMed] [Google Scholar]
- 9. Huang X, Jiang Y, Xia W. FGF23 and phosphate wasting disorders. Bone Res. 2013;1(1):120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imel EA, Biggin A, Schindeler A, Munns CF. FGF23, hypophosphatemia, and emerging treatments. JBMR Plus. 2019;3(8):e10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prentice A. Nutritional rickets around the world. J Steroid Biochem Mol Biol. 2013;136:201–6. [DOI] [PubMed] [Google Scholar]
- 12. Walker S, Hawk JLM, Young AR. Acute and chronic effects of ultraviolet radiation on the skin In Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds. Fitzpatrick's dermatology in general medicine. New York: McGraw‐Hill New York; 2003. pp 1275–82. [Google Scholar]
- 13. Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–5. [DOI] [PubMed] [Google Scholar]
- 14. McKenzie R, Scragg R, Liley B, et al. Serum 25‐hydroxyvitamin‐D responses to multiple UV exposures from solaria: inferences for exposure to sunlight. Photochem Photobiol Sci. 2012;11(7):1174–85. [DOI] [PubMed] [Google Scholar]
- 15. Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010;121(1–2):297–300. [DOI] [PubMed] [Google Scholar]
- 16. Mogire RM, Mutua A, Kimita W, et al. Prevalence of vitamin D deficiency in Africa: a systematic review and meta‐analysis. Lancet Glob Health. 2020;8(1):e134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hilger J, Friedel A, Herr R, et al. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111(1):23–45. [DOI] [PubMed] [Google Scholar]
- 18. Ayadi ID, Nouaili EBH, Talbi E, et al. Prevalence of vitamin D deficiency in mothers and their newborns in a Tunisian population. Int J Gynaecol Obstet. 2016;133(2):192–5. [DOI] [PubMed] [Google Scholar]
- 19. Feleke Y, Abdulkadir J, Mshana R, et al. Low levels of serum calcidiol in an African population compared to a north European population. Eur J Endocrinol. 1999;141(4):358–60. [DOI] [PubMed] [Google Scholar]
- 20. El Maghraoui A, Ouzzif Z, Mounach A, et al. Hypovitaminosis D and prevalent asymptomatic vertebral fractures in Moroccan postmenopausal women. BMC Womens Health. 2012;12(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Botros RM, Sabry IM, Abdelbaky RS, Eid YM, Nasr MS, Hendawy LM. Vitamin D deficiency among healthy Egyptian females. Endocrinol Nutr. 2015;62(7):314–21. [DOI] [PubMed] [Google Scholar]
- 22.Schleicher RL, Sternberg MR, Lacher DA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25‐hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cashman KD, van den Heuvel EG, Schoemaker RJ, Prévéraud DP, Macdonald HM, Arcot J. 25‐hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv Nutr. 2017;8(6):947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arabi A, El Rassi R, El‐Hajj Fuleihan G. Hypovitaminosis D in developing countries‐prevalence, risk factors and outcomes. Nat Rev Endocrinol. 2010. 6(10):550–61. [DOI] [PubMed] [Google Scholar]
- 25.Durazo‐Arvizu RA, Camacho P, Bovet P et al. 25‐Hydroxyvitamin D in African‐origin populations at varying latitudes challenges the construct of a physiologic norm. Am J Clin Nutr. 2014;100(3):908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition.. 2013;29(7‐8):953–7. [DOI] [PubMed] [Google Scholar]
- 27. Sempos CT, Heijboer AC, Bikle DD, et al. Vitamin D assays and the definition of hypovitaminosis D: results from the first international conference on controversies in vitamin D. Br J Clin Pharmacol. 2018;84(10):2194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bouillon R. Free or total 25OHD as marker for vitamin D status? J Bone Miner Res. 2016;31(6):1124–7. [DOI] [PubMed] [Google Scholar]
- 29. Kaufmann M, Gallagher JC, Peacock M, et al. Clinical utility of simultaneous quantitation of 25‐hydroxyvitamin D and 24,25‐dihydroxyvitamin D by LC‐MS/MS involving derivatization with DMEQ‐TAD. J Clin Endocrinol Metab. 2014;99(7):2567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graeff‐Armas LA, Kaufmann M, Lyden E, Jones G. Serum 24,25‐dihydroxyvitamin D3 response to native vitamin D2 and D3 supplementation in patients with chronic kidney disease on hemodialysis. Clin Nutr. 2018;37(3):1041–5. [DOI] [PubMed] [Google Scholar]
- 31. Bikle DD, Vitamin D. metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sempos CT, Binkley N. 25‐hydroxyvitamin D assay standardisation and vitamin D guidelines paralysis. Public Health Nutr. 2020;23(7):1153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter GD, Berry J, Durazo‐Arvizu R, et al. Hydroxyvitamin D assays: an historical perspective from DEQAS. J Steroid Biochem Mol Biol. 2018;177:30–5. [DOI] [PubMed] [Google Scholar]
- 34. DEQAS: Participant portal . Available at: http://www.deqas.org/. Accessed May 17, 2020.
- 35. Binkley NC, Wiebe DA. It's time to stop prescribing ergocalciferol. Endocr Pract. 2018;24(12):1099–102. [DOI] [PubMed] [Google Scholar]
- 36. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 38. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327(23):1637–42. [DOI] [PubMed] [Google Scholar]
- 39. Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257–64. [DOI] [PubMed] [Google Scholar]
- 40. Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. 2014;72(1):48–54. [DOI] [PubMed] [Google Scholar]
- 41. Khaw K‐T, Stewart AW, Waayer D, et al. Effect of monthly high‐dose vitamin D supplementation on falls and non‐vertebral fractures: secondary and post‐hoc outcomes from the randomised, double‐blind, placebo‐controlled ViDA trial. Lancet Diabetes Endocrinol. 2017;5(6):438–47. [DOI] [PubMed] [Google Scholar]
- 42. Manson JE, Bassuk SS, Lee I‐M, et al. The VITamin D and OmegA‐3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega‐3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scragg R, Waayer D, Stewart AW, et al. The Vitamin D Assessment (ViDA) study: design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non‐vertebral fractures. J Steroid Biochem Mol Biol. 2016;164:318–25. [DOI] [PubMed] [Google Scholar]
- 44. Pittas AG, Dawson‐Hughes B, Sheehan PR, et al. Rationale and design of the vitamin D and type 2 diabetes (D2d) study: a diabetes prevention trial. Diabetes Care. 2014;37(12):3227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rejnmark L, Bislev LS, Cashman KD, et al. Non‐skeletal health effects of vitamin D supplementation: a systematic review on findings from meta‐analyses summarizing trial data. PLoS One. 2017;12(7):e0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holick MF, Smith E, Pincus S. Skin as the site of vitamin D synthesis and target tissue for 1,25‐dihydroxyvitamin D3. Use of calcitriol (1,25‐dihydroxyvitamin D3) for treatment of psoriasis. Arch Dermatol. 1987;123(12):1677–83a. [PubMed] [Google Scholar]
- 47. Zuchi MF, Azevedo PO, Tanaka AA, Schmitt JV, LEAM M. Serum levels of 25‐hydroxy vitamin D in psoriatic patients. An Bras Dermatol. 2015;90(3):430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miyachi Y, Ohkawara A, Ohkido M, et al. Long‐term safety and efficacy of high‐concentration (20 microg/g) tacalcitol ointment in psoriasis vulgaris. Eur J Dermatol. 2002;12(5):463–8. [PubMed] [Google Scholar]
- 49. Kircik L. Efficacy and safety of topical calcitriol 3 microg/g ointment, a new topical therapy for chronic plaque psoriasis. J Drugs Dermatol. 2009;8(8 Suppl):s9–16. [PubMed] [Google Scholar]
- 50. Oquendo M, Abramovits W, Morrell P. Topical vitamin D analogs available to treat psoriasis. Skinmed. 2012;10(6):356–60. [PubMed] [Google Scholar]
- 51. Mason AR, Mason J, Cork M, Dooley G, Edwards G. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2009;2:CD005028. [DOI] [PubMed] [Google Scholar]
- 52. Prieto‐Pérez R, Cabaleiro T, Daudén E, Ochoa D, Román M, Abad‐Santos F. Pharmacogenetics of topical and systemic treatment of psoriasis. Pharmacogenomics. 2013;14(13):1623–34. [DOI] [PubMed] [Google Scholar]
- 53. Ahn CS, Awadalla F, Huang KE, Yentzer B, Dabade TS, Feldman SR. Patterns of vitamin D analog use for the treatment of psoriasis. J Drugs Dermatol. 2013;12(8):906–10. [PubMed] [Google Scholar]
- 54. Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6(6):793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Balato N, Patruno C, Napolitano M, Patrì A, Ayala F, Scarpa R. Managing moderate‐to‐severe psoriasis in the elderly. Drugs Aging. 2014;31(4):233–8. [DOI] [PubMed] [Google Scholar]
- 56. Napolitano M, Megna M, Balato A, et al. Systemic treatment of pediatric psoriasis: a review. Dermatol Ther (Heidelb). 2016;6(2):125–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Megna M, Napolitano M, Balato N, et al. Efficacy and safety of ustekinumab in a group of 22 elderly patients with psoriasis over a 2‐year period. Clin Exp Dermatol. 2016;41(5):564–6. [DOI] [PubMed] [Google Scholar]
- 58. Mattozzi C, Paolino G, Richetta AG, Calvieri S. Psoriasis, vitamin D and the importance of the cutaneous barrier's integrity: an update. J Dermatol. 2016;43(5):507–14. [DOI] [PubMed] [Google Scholar]
- 59. Trémezaygues L, Reichrath J. Vitamin D analogs in the treatment of psoriasis: where are we standing and where will we be going? Dermatoendocrinol. 2011;3(3):180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fogelholm M. New Nordic nutrition recommendations are here. Food Nutr Res. 2013. Oct;3:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. German Nutrition Society . New reference values for vitamin D. Ann Nutr Metab. 2012;60(4):241–6. [DOI] [PubMed] [Google Scholar]
- 62. Paxton GA, Teale GR, Nowson CA, et al. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: a position statement. Med J Aust. 2013;198(3):142–3. [DOI] [PubMed] [Google Scholar]
- 63. European Food Safety Authority . Vitamin D: EFSA sets dietary reference values. 2016. [cited 2020 Mar 12]. Available from: https://www.efsa.europa.eu/en/press/news/161028.
- 64. Lips P, Cashman KD, Lamberg‐Allardt C, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23–54. [DOI] [PubMed] [Google Scholar]
- 65. Dawson‐Hughes B, Mithal A, Bonjour J‐P, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151–4. [DOI] [PubMed] [Google Scholar]
- 66. GOV.UK . SACN vitamin D and health report. [cited 2018 Oct 17] Available from: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report.
- 67. Baggerly CA, Cuomo RE, French CB, et al. Sunlight and vitamin D: necessary for public health. J Am Coll Nutr. 2015;34(4):359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rooney MR, Harnack L, Michos ED, Ogilvie RP, Sempos CT, Lutsey PL. Trends in use of high‐dose vitamin D supplements exceeding 1000 or 4000 international units daily, 1999‐2014. JAMA. 2017;317(23):2448–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of high‐dose vitamin D supplementation on volumetric bone density and bone strength: a randomized clinical trial. JAMA. 2019;322(8):736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ebeling PR, Adler RA, Jones G, et al. Management of endocrine disease: therapeutics of vitamin D. Eur J Endocrinol. 2018;179(5):R239–59. [DOI] [PubMed] [Google Scholar]
- 71. Carmel AS, Shieh A, Bang H, Bockman RS. The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/ml. Osteoporos Int. 2012;23(10):2479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Peris P, Martínez‐Ferrer A, Monegal A, Martínez de Osaba MJ, Muxi A, Guañabens N. 25 hydroxyvitamin D serum levels influence adequate response to bisphosphonate treatment in postmenopausal osteoporosis. Bone. 2012;51(1):54–8. [DOI] [PubMed] [Google Scholar]
- 73. Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta‐analysis. Lancet Diabetes Endocrinol. 2014;2(4):307–20. [DOI] [PubMed] [Google Scholar]
- 74. Bouillon R, Lips P, Bilezikian JP. Vitamin D supplementation and musculoskeletal health. Lancet Diabetes Endocrinol. 2019;7(2):85–6. [DOI] [PubMed] [Google Scholar]
- 75. Boonen S, Lips P, Bouillon R, Bischoff‐Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92(4):1415–23. [DOI] [PubMed] [Google Scholar]
- 76. Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta‐analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yao P, Bennett D, Mafham M, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta‐analysis. JAMA Netw Open. 2019;2(12):e1917789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low‐trauma fractures in elderly people (randomised evaluation of calcium or vitamin D, RECORD): a randomised placebo‐controlled trial. Lancet. 2005;365(9471):1621–8. [DOI] [PubMed] [Google Scholar]
- 79. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high‐dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–22. [DOI] [PubMed] [Google Scholar]
- 80. Avenell A, Mak JCS, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post‐menopausal women and older men. Cochrane Database Syst Rev. 2014;4:CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martineau AR. Vitamin D supplementation and musculoskeletal health. Lancet Diabetes Endocrinol. 2019;7(2):86–7. [DOI] [PubMed] [Google Scholar]
- 82. American Society for Bone and Mineral Research. Congratulations to Juliet Compston, M.D., for Winning the Golden Femur on Behalf of ASBMR in the ASBMR‐ECTS Debate! 2019. [cited 2020 Mar 13]. Available from: https://www.asbmr.org/Publications/News/NewsDetail.aspx?cid=b61732b2-561b-4076-81c7-e7d586d63e13.
- 83. Holick MF. Vitamin D: a D‐lightful solution for health. J Invest Med. 2011;59(6):872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Holick MF. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11):2739S–48S. [DOI] [PubMed] [Google Scholar]
- 85. Dowdy JC, Sayre RM, Holick MF. Holick's rule and vitamin D from sunlight. J Steroid Biochem Mol Biol. 2010;121(1–2):328–30. [DOI] [PubMed] [Google Scholar]
- 86. Ala‐Houhala MJ, Vähävihu K, Hasan T, et al. Comparison of narrowband ultraviolet B exposure and oral vitamin D substitution on serum 25‐hydroxyvitamin D concentration. Br J Dermatol. 2012;167(1):160–4. [DOI] [PubMed] [Google Scholar]
- 87. Passeron T, Bouillon R, Callender V, et al. Sunscreen photoprotection and vitamin D status. Br J Dermatol. 2019;181(5):916–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Petersen B, Wulf HC, Triguero‐Mas M, et al. Sun and ski holidays improve vitamin D status, but are associated with high levels of DNA damage. J Invest Dermatol. 2014;134(11):2806–13. [DOI] [PubMed] [Google Scholar]
- 89. Young AR, Narbutt J, Harrison GI, et al. Optimal sunscreen use, during a sun holiday with a very high ultraviolet index, allows vitamin D synthesis without sunburn. Br J Dermatol. 2019;181(5):1052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chel VGM, Ooms ME, Popp‐Snijders C, et al. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J Bone Miner Res. 1998;13(8):1238–42. [DOI] [PubMed] [Google Scholar]
- 91. Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF. CYP2R1 is a major, but not exclusive, contributor to 25‐hydroxyvitamin D production in vivo. Proc Natl Acad Sci U S A. 2013;110(39):15650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Aatsinki S‐M, Elkhwanky M‐S, Kummu O, et al. Fasting‐induced transcription factors repress vitamin D bioactivation, a mechanism for vitamin D deficiency in diabetes. Diabetes. 2019;68(5):918–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hossein‐nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(7):720–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gallagher JC, Smith LM, Yalamanchili V. Incidence of hypercalciuria and hypercalcemia during vitamin D and calcium supplementation in older women. Menopause. 2014;21(11):1173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rosen CJ, Gallagher JC. The 2011 IOM report on vitamin D and calcium requirements for north america: clinical implications for providers treating patients with low bone mineral density. J Clin Densitom. 2011;14(2):79–84. [DOI] [PubMed] [Google Scholar]
- 96. Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. J Steroid Biochem Mol Biol. 2017;173:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bischoff‐Ferrari HA, Dawson‐Hughes B, Orav EJ, et al. Monthly high‐dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175–83. [DOI] [PubMed] [Google Scholar]
- 98. Amrein K, Venkatesh B. Vitamin D and the critically ill patient. Curr Opin Clin Nutr Metab Care. 2012;15(2):188–93. [DOI] [PubMed] [Google Scholar]
- 99. Christopher KB. Vitamin D and critical illness outcomes. Curr Opin Crit Care. 2016;22(4):332–8. [DOI] [PubMed] [Google Scholar]
- 100. Roizen JD, Levine MA. The role of genetic variation in CYP2R1, the principal vitamin D 25‐hydroxylase, in vitamin D homeostasis In Feldman D, ed. Vitamin D. 4th ed Boca Raton, FL: Academic Press; 2018. pp 303–15. [Google Scholar]
- 101. Al‐Dujaili EAS, Munir N, Iniesta RR. Effect of vitamin D supplementation on cardiovascular disease risk factors and exercise performance in healthy participants: a randomized placebo‐controlled preliminary study. Ther Adv Endocrinol Metab. 2016;7(4):153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rostami M, Tehrani FR, Simbar M, et al. Effectiveness of prenatal vitamin D deficiency screening and treatment program: a stratified randomized field trial. J Clin Endocrinol Metab. 2018;103(8):2936–48. [DOI] [PubMed] [Google Scholar]
- 103. Harvey NC, Holroyd C, Ntani G, et al. Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess. 2014;18(45):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Karras SN, Fakhoury H, Muscogiuri G, et al. Maternal vitamin D levels during pregnancy and neonatal health: evidence to date and clinical implications. Ther Adv Musculoskelet Dis. 2016;8(4):124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wei SQ. Vitamin D and pregnancy outcomes. Curr Opin Obstet Gynecol. 2014;26(6):438–47. [DOI] [PubMed] [Google Scholar]
- 106. Hornsby E, Pfeffer PE, Laranjo N, et al. Vitamin D supplementation during pregnancy: effect on the neonatal immune system in a randomized controlled trial. J Allergy Clin Immunol. 2018;141(1):269–78.e1. [DOI] [PubMed] [Google Scholar]
- 107. Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E. Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials. BMJ. 2017;359:j5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523(1):123–33. [DOI] [PubMed] [Google Scholar]
- 109. Wang T‐T, Tavera‐Mendoza LE, Laperriere D, et al. Large‐scale in silico and microarray‐based identification of direct 1,25‐dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19(11):2685–95. [DOI] [PubMed] [Google Scholar]
- 110. Lopes N, Paredes J, Costa JL, Ylstra B, Schmitt F. Vitamin D and the mammary gland: a review on its role in normal development and breast cancer. Breast Cancer Res. 2012;14(3):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chen S, Law CS, Grigsby CL, et al. Cardiomyocyte‐specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation. 2011;124(17):1838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bozic M, Guzmán C, Benet M, et al. Hepatocyte vitamin D receptor regulates lipid metabolism and mediates experimental diet‐induced steatosis. J Hepatol. 2016;65(4):748–57. [DOI] [PubMed] [Google Scholar]
- 113. Barchetta I, Carotti S, Labbadia G, et al. Liver vitamin D receptor, CYP2R1, and CYP27A1 expression: relationship with liver histology and vitamin D3 levels in patients with nonalcoholic steatohepatitis or hepatitis C virus. Hepatology. 2012;56(6):2180–7. [DOI] [PubMed] [Google Scholar]
- 114. Mordan‐McCombs S, Brown T, Wang W‐LW, Gaupel A‐C, Welsh J, Tenniswood M. Tumor progression in the LPB‐tag transgenic model of prostate cancer is altered by vitamin D receptor and serum testosterone status. J Steroid Biochem Mol Biol. 2010;121(1–2):368–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Oh J, Riek AE, Darwech I, et al. Deletion of macrophage vitamin D receptor promotes insulin resistance and monocyte cholesterol transport to accelerate atherosclerosis in mice. Cell Rep. 2015;10(11):1872–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet‐induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein‐1 in white adipose tissue. Endocrinology. 2009;150(2):651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wong KE, Kong J, Zhang W, et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J Biol Chem. 2011;286(39):33804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bouillon R, Carmeliet G, Lieben L, et al. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol. 2014;10(2):79–87. [DOI] [PubMed] [Google Scholar]
- 119. Sempos CT, Durazo‐Arvizu RA, Dawson‐Hughes B, et al. Is there a reverse J‐shaped association between 25‐hydroxyvitamin D and all‐cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab. 2013;98(7):3001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Durazo‐Arvizu RA, Dawson‐Hughes B, Kramer H, et al. The reverse J‐shaped association between serum total 25‐hydroxyvitamin D concentration and all‐cause mortality: the impact of assay standardization. Am J Epidemiol. 2017;185(8):720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gaksch M, Jorde R, Grimnes G, et al. Vitamin D and mortality: individual participant data meta‐analysis of standardized 25‐hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12(2):e0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Crowe FL, Thayakaran R, Gittoes N, et al. Non‐linear associations of 25‐hydroxyvitamin D concentrations with risk of cardiovascular disease and all‐cause mortality: results from the health improvement network (THIN) database. J Steroid Biochem Mol Biol. 2019;195:105480. [DOI] [PubMed] [Google Scholar]
- 123. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;1:CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gianfrancesco MA, Stridh P, Rhead BL, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric‐onset MS. Neurology. 2017. Apr 25;88(17):1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mokry LE, Ross S, Ahmad OS, et al. Vitamin D and risk of multiple sclerosis: a Mendelian randomization study. PLoS Med. 2015;12(8):e1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rhead BL, Bäärnhielm M, Gianfrancesco MA, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genetics. 2016. Sep 13;2(5):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cooper JD, Smyth DJ, Walker NM, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60(5):1624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chen L, Yang R, Qiao W, et al. 1,25‐dihydroxy vitamin D prevents tumorigenesis by inhibiting oxidative stress and inducing tumor cellular senescence in mice. Int J Cancer. 2018;143(2):368–82. [DOI] [PubMed] [Google Scholar]
- 129. Welsh J. Function of the vitamin D endocrine system in mammary gland and breast cancer. Mol Cell Endocrinol. 2017;453:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tong WM, Kállay E, Hofer H, et al. Growth regulation of human colon cancer cells by epidermal growth factor and 1,25‐dihydroxyvitamin D3 is mediated by mutual modulation of receptor expression. Eur J Cancer. 1998;34(13):2119–25. [DOI] [PubMed] [Google Scholar]
- 131.Bikle DD, Jiang Y, Nguyen T, Oda Y, Tu CL. Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front Physiol. 2016;12:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jiang YJ, Teichert AE, Fong F, Oda Y, Bikle DD. 1α,25(OH)2‐dihydroxyvitamin D3/VDR protects the skin from UVB‐induced tumor formation by interacting with the β‐catenin pathway. J Steroid Biochem Mol Biol. 2013;136:229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vitamin D: from photosynthesis, metabolism, and action to clinical applications . Clinical Gate; 2015. [cited 2020 Mar 16]. Available from: https://clinicalgate.com/vitamin-d-from-photosynthesis-metabolism-and-action-to-clinical-applications/.
- 134. Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–57. [DOI] [PubMed] [Google Scholar]
- 135. Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev. 2019;40(4):1109–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: an exposure‐wide umbrella review of meta‐analyses. PLoS One. 2018;13(3):e0194127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst. 2019;111(2):158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Estébanez N, Gómez‐Acebo I, Palazuelos C, Llorca J, Dierssen‐Sotos T. Vitamin D exposure and risk of breast cancer: a meta‐analysis. Sci Rep. 2018;8(1):9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Hewison M, Burke F, Evans KN, et al. Extra‐renal 25‐hydroxyvitamin D3‐1alpha‐hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103(3–5):316–21. [DOI] [PubMed] [Google Scholar]
- 141. Liu PT, Stenger S, Li H, et al. Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science. 2006;311(5768):1770–3. [DOI] [PubMed] [Google Scholar]
- 142. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li J, Chen N, Wang D, Zhang J, Gong X. Efficacy of vitamin D in treatment of inflammatory bowel disease: A meta‐analysis. Medicine (Baltimore). 2018;97(46):e12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gubatan J, Chou ND, Nielsen OH, Moss AC. Systematic review with meta‐analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50(11–12):1146–58. [DOI] [PubMed] [Google Scholar]
- 145. Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth‐cohort study. Lancet. 2001;358(9292):1500–3. [DOI] [PubMed] [Google Scholar]
- 146. Napoli N, Chandran M, Pierroz DD, et al. Mechanisms of diabetes mellitus‐induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–19. [DOI] [PubMed] [Google Scholar]
- 147. Bizzarri C, Pitocco D, Napoli N, et al. No protective effect of calcitriol on beta‐cell function in recent‐onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care. 2010;33(9):1962–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Napoli N, Strollo R, Pitocco D, et al. Effect of calcitriol on bone turnover and osteocalcin in recent‐onset type 1 diabetes. PLoS One. 2013;8(2):e56488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tapia G, Mårild K, Dahl SR, et al. Maternal and newborn vitamin D‐binding protein, vitamin D levels, vitamin D receptor genotype, and childhood type 1 diabetes. Diabetes Care. 2019;42(4):553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Norris JM, Lee HS, Frederiksen B, et al. Plasma 25‐hydroxyvitamin D concentration and risk of islet autoimmunity. Diabetes. 2018;67(1):146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Manson JE, Cook NR, Lee I‐M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pittas AG, Dawson‐Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381(6):520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high‐dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol. 2017;2(6):608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lappe J, Watson P, Travers‐Gustafson D, et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317(12):1234–43. [DOI] [PubMed] [Google Scholar]
- 155. Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta‐analysis of randomized controlled trials. Ann Oncol. 2019;30(5):733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Torfadottir JE, Aspelund T, Valdimarsdottir UA, et al. Pre‐diagnostic 25‐hydroxyvitamin D levels and survival in cancer patients. Cancer Causes Control. 2019;30(4):333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sluyter JD, Camargo CA Jr, Stewart AW et al. Effect of Monthly, High‐Dose, Long‐Term Vitamin D Supplementation on Central Blood Pressure Parameters: A Randomized Controlled Trial Substudy. J Am Heart Assoc. 2017;24;6(10):e006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wu Z, Camargo CA, Malihi Z, et al. Monthly vitamin D supplementation, pain, and pattern of analgesic prescription: secondary analysis from the randomized, double‐blind, placebo‐controlled vitamin D assessment study. Pain. 2018;159(6):1074–82. [DOI] [PubMed] [Google Scholar]
- 159.Sluyter JD, Camargo CA, Waayer D et al. Effect of Monthly, High‐Dose, Long‐Term Vitamin D on Lung Function: A Randomized Controlled Trial. Nutrients. 2017;9(12):1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Liu J, Dong Y‐Q, Yin J, et al. Meta‐analysis of vitamin D and lung function in patients with asthma. Respir Res. 2019;20(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Reid IR, Horne AM, Mihov B, et al. Effect of monthly high‐dose vitamin D on bone density in community‐dwelling older adults substudy of a randomized controlled trial. J Intern Med. 2017;282(5):452–60. [DOI] [PubMed] [Google Scholar]
- 162. Macdonald HM, Reid IR, Gamble GD, Fraser WD, Tang JC, Wood AD. 25‐hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled trial. J Bone Miner Res. 2018;33(8):1464–9. [DOI] [PubMed] [Google Scholar]


