Abstract
The connexin 37 (Cx37) channel is clustered at gap junctions between cells in the renal vasculature or the renal tubule where it is abundant in basolateral cell interdigitations and infoldings of epithelial cells in the proximal tubule, thick ascending limb, distal convoluted tubule and collecting duct; however, physiological data regarding its role are limited. In this study, we investigated the role of Cx37 in fluid homeostasis using mice with a global deletion of Cx37 (Cx37-/- mice). Under baseline conditions, Cx37-/- had ~40% higher fluid intake associated with ~40% lower urine osmolality compared to wild-type (WT) mice. No differences were observed between genotypes in urinary adenosine triphosphate or prostaglandin E2, paracrine factors that alter renal water handling. After 18-hours of water deprivation, plasma aldosterone and urine osmolality increased significantly in Cx37-/- and WT mice; however, the latter remained ~375 mmol/kg lower in Cx37-/- mice, an effect associated with a more pronounced body weight loss despite higher urinary AVP/creatinine ratios compared to WT mice. Consistent with this, fluid intake in the first 3 hours after water deprivation was 37% greater in Cx37-/- vs WT mice. Cx37-/- mice showed significantly lower renal AQP2 abundance and AQP2 phosphorylation at serine 256 than WT mice in response to vehicle or dDAVP, suggesting a partial contribution of the kidney to the lower urine osmolality. The abundance and responses of the vasopressin V2 receptor, AQP3, NHE3, NKCC2, NCC, H+-ATPase, αENaC, γENaC or Na+/K+-ATPase were not significantly different between genotypes. In summary, these results demonstrate that Cx37 is important for body water handling.
Introduction
Connexins (Cx) are a family of four-pass transmembrane proteins, which are major gap junction proteins in vertebrates [1]. Six Cx assemble to form a connexon, or hemichannel, and two connexons of adjacent cells interact to form a gap junction channel, which assemble into gap junction plaques. These play important roles in intercellular communication and exchange of small molecules, including ions, metabolites, and second messengers [2,3]. At least 20 connexin isoforms have been identified in humans and rodents [4]. The pathophysiological relevance of Cx are evident from disease causing mutations, with mutations in 10 Cx isoforms underlying 28 genetic diseases [5].
Multiple Cx isoforms, including Cx26, Cx30, Cx30.3, Cx31, Cx32, Cx37, Cx40, Cx43, Cx45, and Cx46 are detected in the kidney [6–15]. Cx37, Cx40, Cx43 and Cx45 are expressed in the renal vasculature, with a species-dependent distribution pattern (for review see [6]). Cx37 is expressed in afferent arterioles; however, functional studies failed to identify a role of Cx37 for renin secretion in response to salt depletion or angiotensin II perfusion [16]. Cx40 is expressed in endothelial cells of arcuate and interlobular arteries, and renin-secreting cells of the afferent arterioles. Functionally, Cx40 is involved in renal autoregulation [17,18], tubuloglomerular feedback (TGF) [19] and renin release [16]. Cx43 is found in various vascular endothelial cells in the kidney [10], where it is hypothesized to modulate renin secretion and blood pressure [20]. Cx45 is expressed in the vascular smooth muscle cells of interlobular arteries, afferent and efferent arterioles and the juxtaglomerular apparatus [18,21,22].
Along the renal tubule epithelium, Cx26, Cx30, Cx30.3, Cx32, Cx37 and Cx43 have been identified. Cx26 and Cx32 are co-expressed in the proximal tubule [23–25], but currently no functional studies have determined their renal role. In rats, Cx30 is expressed from the thick ascending limb of the loop of Henle through the collecting duct, including intercalated cells; however, principal cells were devoid of Cx30 [26]. Cx30 levels are altered by dietary salt [26], and Cx30 plays an integral role in pressure natriuresis by releasing ATP into the tubular fluid, which inhibits salt and water reabsorption [27]. Cx30.3 is expressed in the thin ascending limb of the loop of Henle and in the intercalated cells of the collecting duct [28,29], but its renal role is unknown [29]. Cx32 is observed in mouse proximal tubules [25], and whilst its normal physiological role is not described, knockout of Cx32 attenuated ischemia reperfusion-induced acute kidney injury [30].
Cx37 is one of the most abundantly expressed Cx isoforms throughout the renal vasculature and renal tubule. In the renal tubule, Cx37 is abundant in basolateral cell interdigitations and infoldings of cells in the proximal tubule, thick ascending limb, distal convoluted tubule and collecting duct [31]. A comparison between low and high salt diet showed inverse regulation of Cx37 mRNA and protein expression levels with salt intake [31]. Cx43 is localized to inner medullary collecting ducts of the rat [8] and studies indicate a possible role in the progression of chronic kidney disease [32–34].
Despite its high abundance, the physiological role of Cx37 in the kidney is unclear. In the current study, we describe a novel role of Cx37 for body fluid homeostasis, with Cx37 knockout mice (Cx37-/- mice) having greater fluid intake and lower urine osmolality relative to WT. This phenotype is associated with reduced abundance and phosphorylation of the water channel aquaporin 2 (AQP2).
Materials and methods
Animals and ethics
All animal experimentation was conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD) and was approved by the local Institutional Animal Care and Use Committee at the University of South Florida (3338R). The generation of the Cx37-/- mice model has been described previously [35], all animals were bred in-house at the University of South Florida. WT and Cx37-/- mice were housed under a 12:12-hour light-dark cycle in isolated ventilated cages with free access to standard rodent chow (TD.2018; Envigo, Madison, WI) and tap water. A total of 57 age-matched, 3- to 6-month old male and female mice (WT, Cx37-/-) were used for experiments.
Urine and plasma analysis
Fluid and food intake were measured over 9 days in isolated ventilated cages and averaged. Spontaneously voided urine was collected for determination of Na+, K+, creatinine, urea, and osmolality. Blood was drawn from the retroorbital plexus under brief isoflurane anesthesia (5%) for determination of plasma osmolality and urea. Osmolality was measured by an Osmomat 3000 (Gonotec GmbH, Berlin, Germany), Na+ and K+ by flame photometry (BWB Technologies Ltd, Berkshire, UK), and creatinine and urea using commercial enzymatic assays (Thermo Fisher Scientific, Waltham, MA). Plasma aldosterone was determined using a solid phase enzyme-linked immunosorbent assay (IBL America, Minneapolis, MN). Urinary arginine-vasopressin and prostaglandin E2 (PGE2) were determined by enzyme-linked immunosorbent assays (Enzo Life Sciences, Farmingdale, NY). Urinary ATP was determined using an ATP colorimetric assay (BioVision, Milpitas, CA).
Water deprivation
Water deprivation was conducted overnight (18 hours), followed by determination of changes in body weight and collection of spontaneous voided urine to measure osmolality and concentrations of Na+, K+, urea and creatinine. Blood was taken from the retroorbital plexus for determination of osmolality after brief isoflurane anesthesia. After re-introducing the water bottle, fluid intake was determined in 3-hour intervals for the subsequent 6 hours.
Response to exogenous activation of vasopressin type 2 receptors (V2R)
Mice were treated with 5% dextrose/1% ethanol solution overnight to suppress endogenous arginine-vasopressin (AVP) levels [36,37]. The next day, the mice were intraperitoneally injected with vehicle (sterile water, 2 μl/g bw) or the V2R agonist D-amino D-arginine vasopressin (dDAVP) (0.1 μg/kg in sterile water, 2 μl/g bw; Sigma-Aldrich, St. Louis, MO). Mice were observed in their home cages and euthanized by isoflurane overdose (5% until cessation of breathing followed by cervical dislocation as secondary method) 60 minutes after injection, and kidneys were harvested for Western blotting [38].
Immunoblot analysis
Renal tissue was homogenized in buffer containing protease inhibitor cocktail (Roche Applied Science, Penzberg, Germany) and Halt phosphatase inhibitor cocktail (Thermo Fisher Scientific) as described previously [39,40]. Plasma membrane-enriched samples (by centrifugation at 17,000 x g) were prepared for Western blotting. Proteins were transferred to polyvinylidene difluoride membranes and immunoblotted with AQP2 [36], pS256 AQP2 [41], V2R [42], AQP3 [43], NHE3 (AB3085, Millipore, Billerica MA; characterized in [44]), NKCC2 [37], NCC (SPC-402, StressMarq Biosciences, Cadboro Bay, Victoria, BC, Canada; characterized in [45]), H+-ATPase [46], αENaC (provided by Johannes Loffing, Institute of Anatomy, Zürich, Switzerland [47]), γENaC [48,49] and Na+/K+-ATPase (06–520, Merck Millipore, Darmstadt, Germany [48]). Chemiluminescent detection was performed with ECL Plus (Amersham, Piscataway, NJ). Signal intensity in specific bands was quantified using Image Studio Lite (Qiagen, Hilden, Germany) densitometry analysis. Secondary antibodies were from DAKO (Jena, Germany), and sites of antibody/antigen interaction were visualized using the Enhanced Chemiluminescence System (GE Healthcare, Buckinghamshire, Great Britain) and an ImageQuant LAS 4000 imager (GE Healthcare). Coomassie-stained gels were used to adjust for equal protein loading for immunoblotting. Densitometric analyses were performed using Image Studio Lite (Qiagen).
Statistical analyses
The data are expressed as mean±SEM. Unpaired and paired Student’s t-tests were performed, as appropriate, to analyze for statistical differences between groups. All data were analyzed via GraphPad Prism (version 8, San Diego, CA, USA) or SigmaPlot (version 12.5, San Jose, CA, USA). Significance was considered at P<0.05.
Results
Basal analysis of Cx37-/- mice with free access to fluid and food
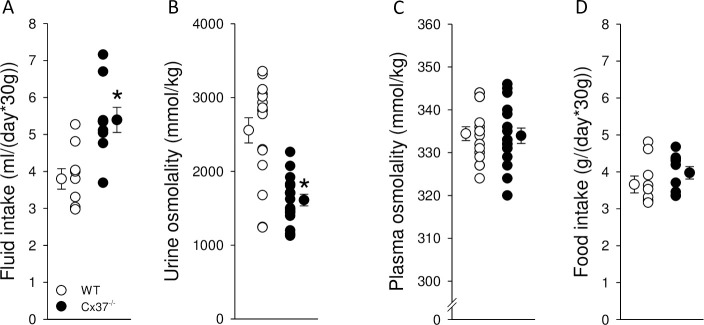
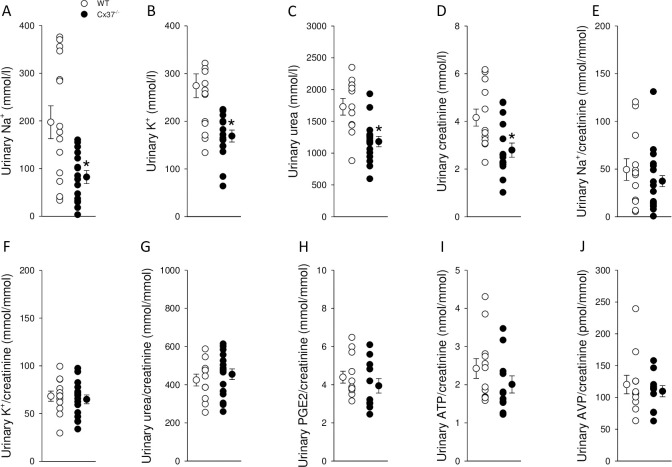
Under baseline conditions with free access to drinking water, Cx37-/- mice had a ~40% higher fluid intake (Fig 1A) associated with a ~40% lower urine osmolality (Fig 1B) compared to WT mice. Plasma osmolality was not significantly different between genotypes (Fig 1C), indicating that Cx37-/- mice were in fluid balance. No differences in food intake were observed (Fig 1D). Consistent with the lower urine osmolality, Cx37-/- mice had lower urinary concentrations of Na+ (Fig 2A), K+ (Fig 2B), urea (Fig 2C) and creatinine (Fig 2D). When Na+, K+ and urea were corrected by urinary creatinine, no significant differences were observed between genotypes (Fig 2E–2G). Urinary PGE2/creatinine (Fig 2H), ATP/creatinine (Fig 2I) and AVP/creatinine ratios (Fig 2J) were not significantly different between genotypes.
Fig 1. Increased fluid intake and lower urine osmolality in Cx37-/- mice with free access to food and water.
(A) Cx37-/- mice have greater fluid intake associated with (B) lower urinary osmolality than WT mice (~142% and ~63% of WT, respectively). Intact water balance is indicated by similar (C) plasma osmolality and (D) food intake between genotypes. Data are expressed as mean ± SEM; n = 9 for fluid and food intake, n = 12–17 for urine and plasma osmolalities. Data were analyzed by Student’s t-test, *P<0.05 versus WT.
Fig 2. Urinary concentrations of Na+, K+, urea and creatinine were reduced in Cx37-/- compared to WT mice.
Urinary concentrations of (A) Na+, (B) K+, (C) urea and (D) creatinine were reduced to ~42%, ~62%, ~68% and ~67% in Cx37-/- compared to WT, respectively. Corrected by urinary creatinine no significant differences were observed for (E) Na+, (F) K+, (G) urea, PGE2 (H), ATP (I) or AVP (J). Data are expressed as mean ± SEM; n = 12–17. Data were analyzed by Student’s t-test, *P<0.05 versus WT.
The urine-to-plasma ratio for osmolality was reduced by approximately 60% in Cx37-/- compared to WT mice (4.6±0.2 versus 7.4±0.5, n = 14–15; P<0.05), highlighting polyuria but maintained body water balance. Plasma levels of urea were not different between Cx37-/- and WT mice (10.1±0.3 versus 9.1±0.5 mmol/l, n = 14–15; not significant [NS]), but urinary urea concentration (Fig 2C) was reduced in the same proportion as urinary osmolality. As observed for the total osmoles, the urine-to-plasma ratio for urea was reduced by approximately 60% in Cx37-/- versus WT mice (119±10 versus 206±24, n = 14–15; P<0.05).
Response to water deprivation
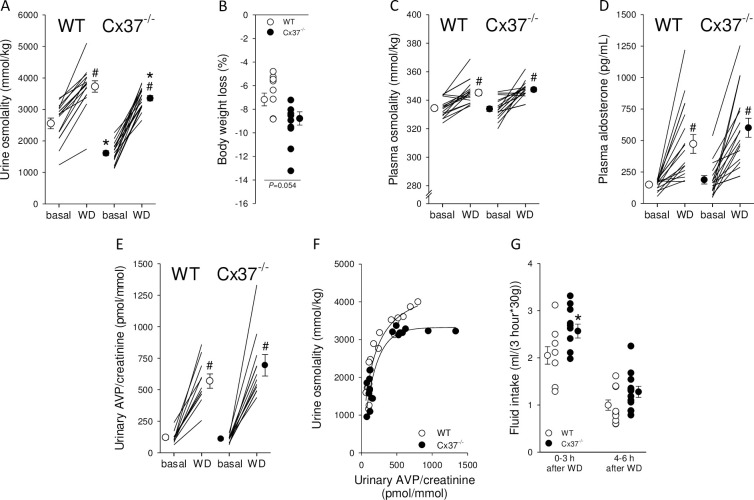
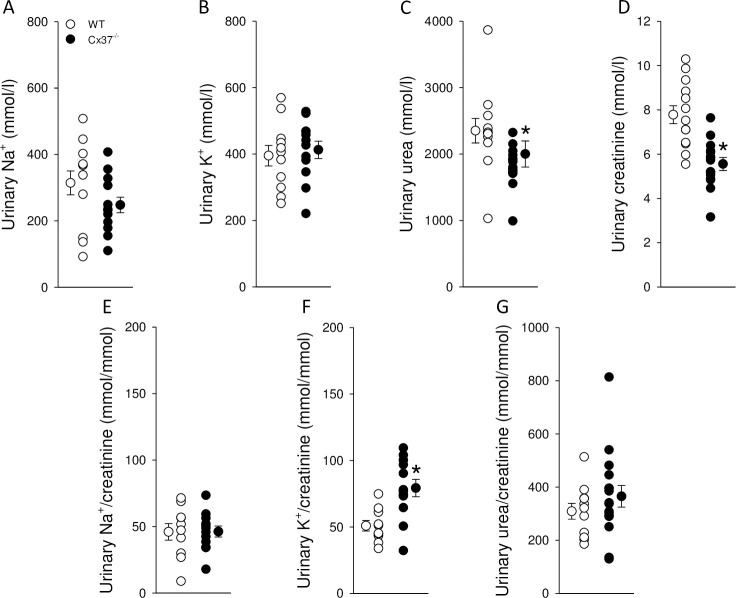
To test for a role of Cx37 in urinary concentrating ability, mice were challenged by an 18-hour water deprivation. Urine osmolality increased significantly in both groups (Fig 3A) but remained ~375 mmol/kg lower in Cx37-/- compared to WT mice. This was associated with a tendency for greater body weight loss (Fig 3B). In response to water deprivation, both genotypes showed a significant increase in plasma osmolality (Fig 3C) but the increase was not significantly different between Cx37-/- and WT mice (Δ change: 14±3 versus 11±2 mmol/kg, NS). Baseline plasma aldosterone levels and alterations in response to water deprivation (Fig 3D) were comparable between genotypes (Δ change: 413±69 versus 326±71 pg/mL, NS). The increase in urinary AVP/creatinine in response to water deprivation compared to baseline (Fig 3E) was not significantly different between WT and Cx37-/- mice (5±1 and 6±1 fold, NS). Despite Cx37-/- mice reaching a maximum urine osmolality of ~3250 mmol/kg they required higher urinary AVP/creatinine levels (Fig 3F). Fluid intake, up to 3 hours after water deprivation, was ~25% greater in Cx37-/- compared to WT mice (Fig 3G). This difference decreased in the next 3 hours. Urinary concentrations of Na+ and K+ were not significantly different between genotypes after water deprivation (Fig 4A and 4B); however, urinary urea (Fig 4C) and creatinine (Fig 4D) concentrations remained significantly lower in Cx37-/- compared to WT mice. When corrected by urinary creatinine, the ratios for Na+ (Fig 4E) and urea (Fig 4G) were not significantly different between genotypes, whereas urinary K+/creatinine was significantly higher in Cx37-/- compared to WT mice (Fig 4F).
Fig 3. Water homeostasis in WT and Cx37-/- mice in response to 18-hour water deprivation (WD).
(A) WD significantly increased urine osmolality in Cx37-/- mice but not to the levels achieved in WT mice, remaining ~10% lower. (B) WD was associated with a tendency for greater loss in body weight. Increases in plasma osmolality (C) and plasma aldosterone (D) were not different between genotypes. (E) There was no difference between genotypes in urinary AVP/creatinine in response to WD compared to baseline. (F) Relationship between urine osmolality and urinary AVP/creatinine ratio between genotypes. Data under baseline conditions and after WD have been plotted. (G) Cx37-/- mice showed ~25% higher fluid intake in the 1st 3 hours after water was re-introduced and a tendency (~28%) for higher fluid intake from 4–6 hours. Data are expressed as mean ± SEM; n = 15–16 for urine osmolality, body weight and plasma osmolality, n = 10–12 for fluid intake and AVP. Data were analyzed by unpaired and paired Student’s t-test, *P<0.05 versus WT.
Fig 4. Effects of 18-hour WD on urinary electrolytes and urea in Cx37-/- and WT mice.
Urinary concentrations of (A) Na+ and (B) K+ were not significantly different between genotypes after WD. In contrast, concentrations of (C) urea and (D) creatinine were ~15% and ~29% lower in Cx37-/- compared to WT mice, respectively. Urinary Na+/creatinine and urea/creatinine were not different between genotypes (E and G, respectively); however, urinary K+/creatinine was significantly higher in in Cx37-/- compared to WT mice (F). Data are expressed as mean ± SEM; n = 11–16. Data were analyzed by Student’s t-test, *P<0.05 versus WT.
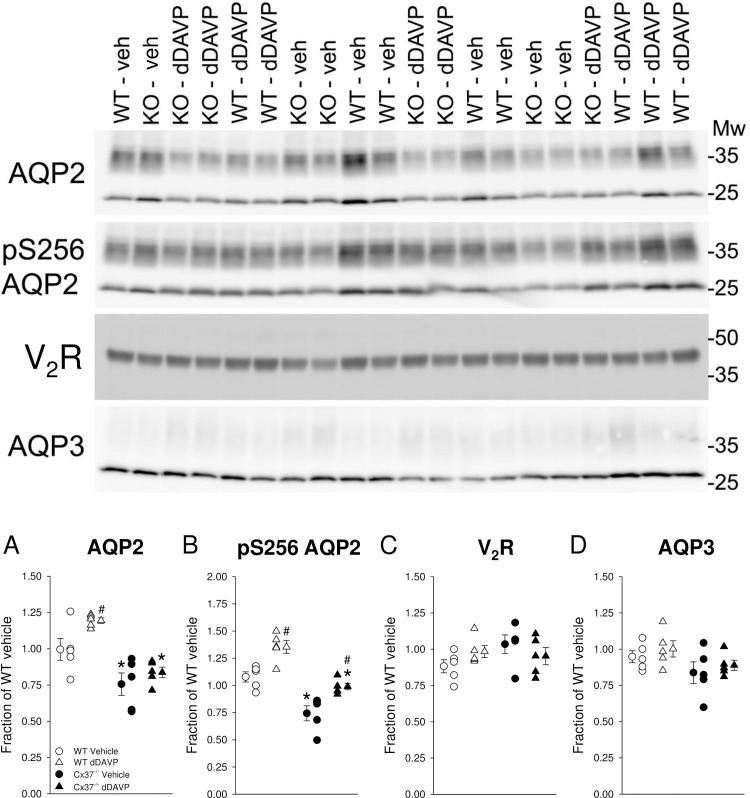
Effect of DAVP on AQP2 abundance and phosphorylation in Cx37-/- mice
To study the in vivo response to V2R activation, we measured the abundance and phosphorylation of AQP2 by Western blotting in response to dDAVP or vehicle treatment after suppression of endogenous AVP by ethanol/water loading overnight. This maneuver caused a significant reduction of urine osmolality that was not different between genotypes (WT: 232±43 versus Cx37-/-: 189±24 mmol/kg, NS). Due to the short duration of the dDAVP stimulation, 60 minutes, we were not able to collect enough urine for analysis. With vehicle, in kidney lysates enriched for plasma membranes, total AQP2 expression was significantly lower in Cx37-/- versus WT mice (Fig 5A). WT mice responded to dDAVP with an increase in total AQP2 abundance. This response was limited in Cx37-/- mice (Fig 5A). Consistent with total AQP2, pS256-AQP2 abundance was significantly lower in Cx37-/- compared to WT mice in response to vehicle application (Fig 5B). Both genotypes showed a significant increase in pS256-AQP2 abundance (an important site for AQP2 plasma membrane targeting in response to dDAVP [50]; however, the effect in Cx37-/- mice remained significantly lower compared to WT mice (Fig 5B). These differences were not caused by differences or changes in V2R abundances (Fig 5C). AQP3 abundance (expressed on the basolateral membrane of principal cells) was not significantly different between genotypes in response to vehicle application and no changes in AQP3 abundance in response to dDAVP were observed (Fig 5D).
Fig 5. Renal abundance and phosphorylation of AQP2 is reduced under vehicle and impaired in Cx37-/- compared to WT mice in response to V2R activation.
Sixty minutes after vehicle application Cx37-/- mice had lower abundance of (A) total and (B) phosphorylated serine 256 AQP2 compared with WT mice. Systemic administration of dDAVP following suppression of endogenous arginine-vasopressin overnight (see Materials and methods for details) increased the abundance of (A) total and (B) phosphorylated serine 256 AQP2 in both genotypes; however, the effect was attenuated in Cx37-/- mice. No differences in the abundance of V2R or AQP3 were observed between genotypes or treatments. Data are expressed as mean ± SEM; n = 5 each genotype and condition. Data were analyzed by paired and unpaired Student’s t-test, *P<0.05 versus WT same condition, #P<0.05 versus vehicle same genotype.
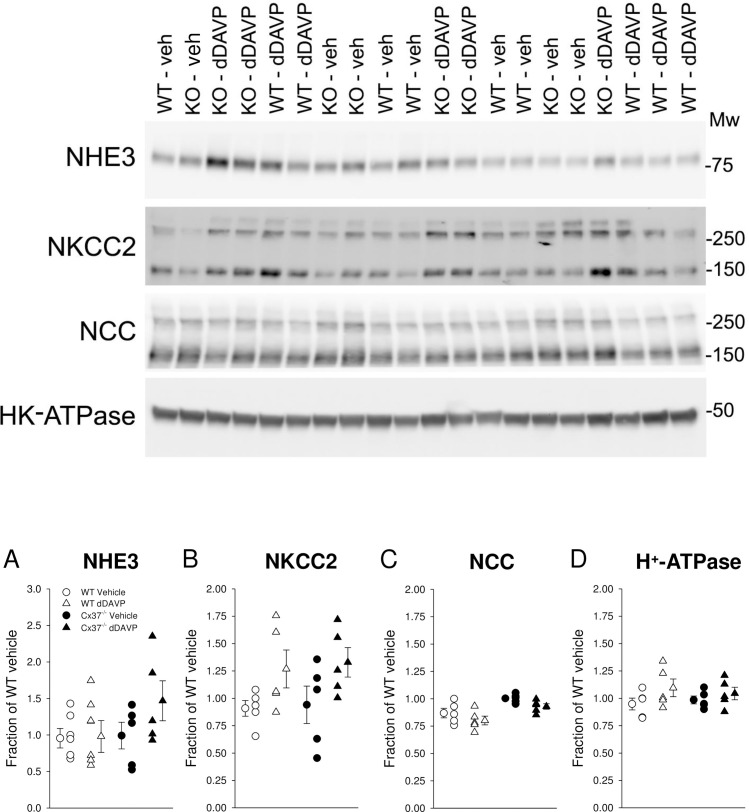
Responses of renal salt transporters and channels in WT and Cx37-/- mice to dDAVP
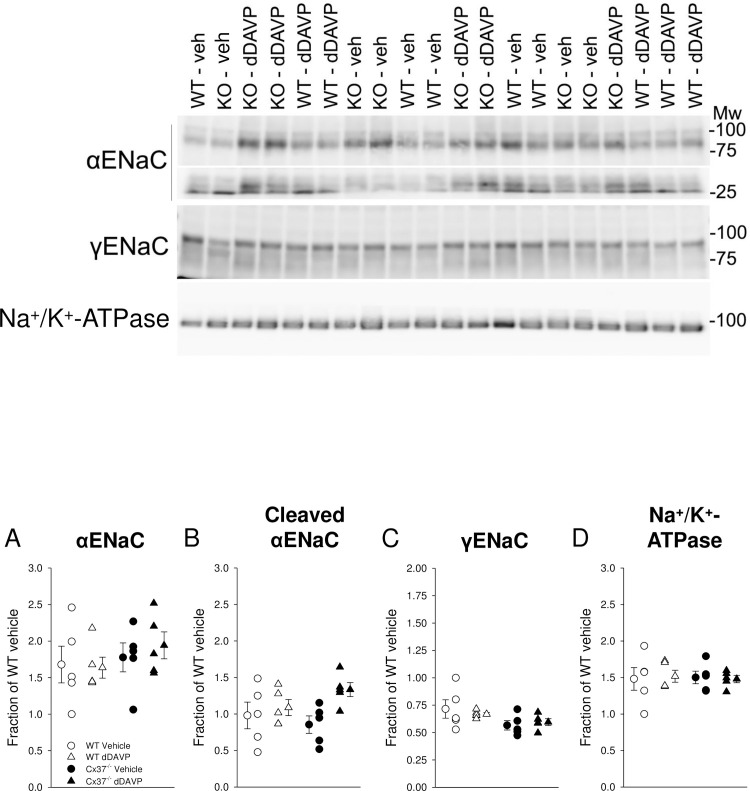
In order to determine if reduced abundance of other transporters and channels that may be involved in renal salt and water handling could explain the water handling defect, the major salt transporters and channels along the nephron were profiled in kidney lysates enriched for plasma membranes. NHE3 abundance, mainly expressed in the proximal tubule and thick ascending limb (TAL) [51], was not significantly different between genotypes after vehicle application and no dDAVP effects were observed (Fig 6A). NKCC2 abundance, mainly expressed in the TAL, was not significantly different after vehicle application (Fig 6B). dDAVP was previously shown to regulate NKCC2 in the renal medulla [37] and both genotypes showed tendencies for increased NKCC2 abundance that did not reach statistical significance (Fig 6B). NCC, expressed in the distal convoluted tubule, was not significantly different between genotypes after vehicle application and no dDAVP effects were observed (Fig 6C). Similar findings were observed for H+-ATPase (Fig 6D; expressed in the proximal tubule and collecting duct), α-subunit of ENaC (Fig 7A; principal cells), cleaved α-subunit of ENaC (Fig 7B; indicative of proteolytic activation), γ-subunit of ENaC (Fig 7C; principal cells), and Na+/K+-ATPase (Fig 7D; expressed in the basolateral membrane).
Fig 6. Renal abundance of major salt and proton transporting proteins.
Sixty minutes after vehicle application no differences were observed in (A) NHE3, (B) NKCC2, (C) NCC or (D) H+-ATPase abundance. Systemic administration of dDAVP following suppression of endogenous vasopressin overnight (see Materials and methods for details) did not affect abundances of these proteins. Data are expressed as mean ± SEM and analyzed by paired and unpaired Student’s t-test, n = 5 each genotype and condition.
Fig 7. Renal abundance of the epithelial sodium channel and Na+/K+-ATPase.
Sixty minutes after vehicle application no differences were observed in (A) αENaC, (B) cleaved αENaC, (C) γENaC or (D) Na+/K+-ATPase abundance. Systemic administration of dDAVP following suppression of endogenous arginine-vasopressin overnight (see Materials and methods for details) did not affect abundances of these proteins. Data are expressed as mean ± SEM and analyzed by paired and unpaired Student’s t-test, n = 5 each genotype and condition.
Discussion
Connexins play critical roles in intercellular communication, e.g. electrical coupling, autocrine/paracrine signaling, and development. Cx37 is expressed in the kidney; however, little is known about its physiological function(s). Classical gap junctions have only been localized (using freeze fracture) in the proximal tubule of rat [52] and human kidneys [53], implying that Cx37 along the further distal segments and collecting ducts rather function as hemichannels [6]. The major finding in this study is that we show for the first time that Cx37 contributes to water homeostasis in vivo. Cx37-/- mice have higher fluid intake and lower urine osmolality, but Cx37-/- mice are in fluid balance, with no signs of plasma electrolyte defects or growth retardation. So what is the basis for the alteration in fluid intake and urine volume–is it of renal or of central origin [54]? One way to differentiate between the two possibilities is based on plasma osmolality, which in patients with nephrogenic diabetes insipidus (NDI) is either normal [36,41,55] or slightly increased [56–58], and in primary polydipsia is often lower [59]. However, mice with primary polydipsia do not show differences in plasma osmolality [60,61]. Based on our studies, we hypothesize that the phenotype in Cx37-/- mice is more consistent with primary polydipsia.
To categorically determine if Cx37-/- mice had NDI, we assessed the response of these mice to water deprivation. After water deprivation, although the urine osmolality of Cx37-/- mice was still significantly lower than WT mice, accompanied by a tendency for greater body weight loss, the Cx37-/- mice still concentrated their urine to > 3000 mmol/kg suggesting an intact urinary concentrating mechanism. However, the Cx37-/- mice do not concentrate their urine above ~3250 mmol/kg despite higher AVP levels. The increased fluid intake for 3 hours proceeding water deprivation fits with these mice having either to initially compensate more than WT mice to the water deprivation (as their maximal urine concentration is less), or that the reintroduction of water immediately stimulates their compulsive water drinking–or it could be a combination of both. Of note, although the phenotype of Cx37-/- mice fits better with primary polydipsia, Cx37 has not been identified in cells with neuronal morphology in the brain [62]. The Cx37-/- phenotype is also not likely related to increased salt appetite [63] because urinary Na+/creatinine ratios and expression of αENaC and proteolytically cleaved αENaC were not significantly different between genotypes. Along those lines, we did not observe differences in plasma aldosterone levels before and in response to water deprivation between genotypes, excluding that aldosterone accounts for differences in urinary K+/creatinine in response to water deprivation. If changes in Cx37-mediated Ca2+-signaling affects K+ secretion via Ca2+-dependent big-conductance potassium channels [64], a channel activated by increases in flow, remains to be determined.
Changes in collecting duct water permeability, mediated by AVP-induced AQP2 trafficking, play a critical role in urinary concentration and water balance [65–67]. This process involves adenylyl cyclase 6 (AC6)-mediated cAMP formation in principal cells [36,41], alongside other mechanisms [68] such as enhanced AQP2 phosphorylation [50]. The importance of serine 256 AQP2 phosphorylation in membrane targeting is highlighted in mice with a substitution of serine with a leucine at residue 256, which lack any form of AQP2 phosphorylation and have NDI [69]. The reduced total AQP2 and phosphorylated pS256-AQP2 abundance in Cx37-/- mice fits with the lower baseline urine osmolality, but whether this is the cause–or an effect–is complicated by AQP2 being stimulated by interstitial osmolality [70] that is predicted to be lower in Cx37-/- mice. Of note, the phenotype is consistent with other studies reporting that AQP2 expression is reduced in primary polydipsia [71,72] without affecting V2R expression [72]. PGE2 is an important regulator of urinary concentrating ability [73,74] and Cx43 has been shown to regulate PGE2 release from osteocytes [75]. Our data do not show differences in urinary PGE2/creatinine ratios between genotypes, suggesting that PGE2 in Cx37-/- mice does not contribute to the differences in fluid homeostasis. In line with this, differences in V2R expression can be excluded to contribute to these differences because no differences in the expression levels were observed between genotypes. Eighteen-hour water restriction resulted in urine osmolarities that were slightly but significantly lower in Cx37-/- versus WT mice, which could be explained by lower total AQP2 and lower phosphorylation of S256-AQP2 in response to dDAVP. A clear response to dDAVP indicates again that the mice do not have NDI. Of note, treating mice with 5% dextrose/1% ethanol solution should completely suppress endogenous vasopressin levels; this maneuver was not intended to dissect between NDI versus primary polydipsia but rather to eliminate differences in endogenous AVP levels between genotypes. The data from our experiments show similarities to other models with defects in water handling but with partially preserved urinary concentrating ability, e.g. AC6 knockout mice [36,41], glycogen synthase kinase 3β knockout mice [76] or calcineurin Aα knockout mice [57].
If not solely compulsive water drinking, what else may contribute to the higher fluid intake and lower urine osmolality in Cx37-/- mice? Connexins and pannexins are also involved in the release of adenosine triphosphate (ATP) and uridine triphosphate (UTP) [6,77], e.g. ATP release through Cx37 occurs in monocytes [78]; however, our data do not show differences in urinary ATP/creatinine ratios. We have previously shown that ATP can inhibit vasopressin-induced cAMP formation and urinary concentrating ability; a process mediated by P2Y2 receptors [79]. However, our current data argue against a primary role of Cx37-mediated ATP/UTP release because Cx37-/- mice show lower and not higher urine osmolality. Likewise, the relatively normal abundance of major transporters and channels involved in renal salt and water handling excludes a major role of these in the observed phenotype. If differences in crosstalk between principal cell and intercalated cells exist in Cx37-/- mice remains to be determined. Our data show that urinary ATP/creatinine ratios were comparable between genotypes; however, Cx30-mediated ATP release from intercalated cells has been shown to affect ENaC open probability in neighboring principal cells [27].
Another major contributor to body water balance is the renal handling of urea [80]. However, as urinary urea concentrations increased in response to water deprivation in both genotypes and there are no differences in urinary urea to total osmolality ratios, altered urea handling is unlikely to be involved. Cx37 polymorphisms can affect endothelial cell function [81,82]. Based on the expression of Cx37 in the vasculature [82], in particular the descending vasa recta [31], we cannot exclude that changes in local renal blood flow also alter body water handling and further studies are needed to better understand the contribution of Cx37 in the vasculature. Of note, no differences in renal blood flow were found between Cx37-/- and WT mice [83].
In summary, this study shows for the first time that Cx37 plays a role in body water handling, with deletion of Cx37 resulting in polyuria and polydipsia. Some of these effects may be mediated by lower AQP2 abundance and phosphorylation at serine 256.
Supporting information
(PPTX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant 1R01DK110621 (to Dr. Rieg) and American Heart Association Transformational Research Award 19TPA34850116 (to Dr. Rieg). Funding (to Dr. Fenton) was provided by the LeDucq Foundation, the Danish Medical Research Council, and the Novo Nordisk Foundation. Mr. Xue was supported by an American Heart Association predoctoral fellowship (18PRE33990236) and Dr. Thomas by a postdoctoral fellowship (19POST34400026). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. 10.1146/annurev.bi.65.070196.002355 [DOI] [PubMed] [Google Scholar]
- 2.Sáez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiological reviews. 2003;83(4):1359–400. 10.1152/physrev.00007.2003 [DOI] [PubMed] [Google Scholar]
- 3.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383(5):725–37. 10.1515/BC.2002.076 [DOI] [PubMed] [Google Scholar]
- 4.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62(2):228–32. 10.1016/j.cardiores.2003.11.013 [DOI] [PubMed] [Google Scholar]
- 5.Srinivas M, Verselis VK, White TW. Human diseases associated with connexin mutations. Biochim Biophys Acta Biomembr. 2018;1860(1):192–201. 10.1016/j.bbamem.2017.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol. 2010;298(5):R1143–55. 10.1152/ajpregu.00808.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner C. Function of connexins in the renal circulation. Kidney Int. 2008;73(5):547–55. 10.1038/sj.ki.5002720 [DOI] [PubMed] [Google Scholar]
- 8.Barajas L, Liu L, Tucker M. Localization of connexin43 in rat kidney. Kidney Int. 1994;46(3):621–6. 10.1038/ki.1994.314 [DOI] [PubMed] [Google Scholar]
- 9.Haefliger JA, Bruzzone R, Jenkins NA, Gilbert DJ, Copeland NG, Paul DL. Four novel members of the connexin family of gap junction proteins. Molecular cloning, expression, and chromosome mapping. J Biol Chem. 1992;267(3):2057–64. [PubMed] [Google Scholar]
- 10.Hwan Seul K, Beyer EC. Heterogeneous localization of connexin40 in the renal vasculature. Microvasc Res. 2000;59(1):140–8. 10.1006/mvre.1999.2216 [DOI] [PubMed] [Google Scholar]
- 11.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115(4):1077–89. 10.1083/jcb.115.4.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker MA, Barajas L. Rat connexins 30.3 and 31 are expressed in the kidney. Exp Cell Res. 1994;213(1):224–30. 10.1006/excr.1994.1194 [DOI] [PubMed] [Google Scholar]
- 13.Zhang JT, Nicholson BJ. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989;109(6 Pt 2):3391–401. 10.1083/jcb.109.6.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Hill CE. Differential connexin expression in preglomerular and postglomerular vasculature: accentuation during diabetes. Kidney Int. 2005;68(3):1171–85. 10.1111/j.1523-1755.2005.00509.x [DOI] [PubMed] [Google Scholar]
- 15.Hanner F, Schnichels M, Zheng-Fischhofer Q, Yang LE, Toma I, Willecke K, et al. Connexin 30.3 is expressed in the kidney but not regulated by dietary salt or high blood pressure. Cell Commun Adhes. 2008;15(1):219–30. 10.1080/15419060802013836 [DOI] [PubMed] [Google Scholar]
- 16.Wagner C, Kurtz L, Schweda F, Simon AM, Kurtz A. Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflugers Arch. 2009;459(1):151–8. 10.1007/s00424-009-0707-6 [DOI] [PubMed] [Google Scholar]
- 17.Sorensen CM, Giese I, Braunstein TH, Brasen JC, Salomonsson M, Holstein-Rathlou NH. Role of connexin40 in the autoregulatory response of the afferent arteriole. Am J Physiol Renal Physiol. 2012;303(6):F855–63. 10.1152/ajprenal.00026.2012 [DOI] [PubMed] [Google Scholar]
- 18.Just A, Kurtz L, de Wit C, Wagner C, Kurtz A, Arendshorst WJ. Connexin 40 mediates the tubuloglomerular feedback contribution to renal blood flow autoregulation. J Am Soc Nephrol. 2009;20(7):1577–85. 10.1681/ASN.2008090943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oppermann M, Carota I, Schiessl I, Eisner C, Castrop H, Schnermann J. Direct assessment of tubuloglomerular feedback responsiveness in connexin 40-deficient mice. Am J Physiol Renal Physiol. 2013;304(9):F1181–6. 10.1152/ajprenal.00721.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haefliger JA, Krattinger N, Martin D, Pedrazzini T, Capponi A, Doring B, et al. Connexin43-dependent mechanism modulates renin secretion and hypertension. J Clin Invest. 2006;116(2):405–13. 10.1172/JCI23327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanner F, von Maltzahn J, Maxeiner S, Toma I, Sipos A, Kruger O, et al. Connexin45 is expressed in the juxtaglomerular apparatus and is involved in the regulation of renin secretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R371–80. 10.1152/ajpregu.00468.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz L, Janssen-Bienhold U, Kurtz A, Wagner C. Connexin expression in renin-producing cells. J Am Soc Nephrol. 2009;20(3):506–12. 10.1681/ASN.2008030252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J-T, Nicholson BJ. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. The Journal of cell biology. 1989;109(6):3391–401. 10.1083/jcb.109.6.3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sainio K, Gilbert SF, Lehtonen E, Nishi M, Kumar NM, Gilula NB, et al. Differential expression of gap junction mRNAs and proteins in the developing murine kidney and in experimentally induced nephric mesenchymes. Development. 1992;115(3):827–37. [DOI] [PubMed] [Google Scholar]
- 25.Butterweck A, Gergs U, Elfgang C, Willecke K, Traub O. Immunochemical characterization of the gap junction protein connexin45 in mouse kidney and transfected human HeLa cells. J Membr Biol. 1994;141(3):247–56. 10.1007/BF00235134 [DOI] [PubMed] [Google Scholar]
- 26.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol. 2005;289(6):F1304–12. 10.1152/ajprenal.00203.2005 [DOI] [PubMed] [Google Scholar]
- 27.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol. 2009;20(8):1724–32. 10.1681/ASN.2008101099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng-Fischhofer Q, Schnichels M, Dere E, Strotmann J, Loscher N, McCulloch F, et al. Characterization of connexin30.3-deficient mice suggests a possible role of connexin30.3 in olfaction. Eur J Cell Biol. 2007;86(11–12):683–700. 10.1016/j.ejcb.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 29.Hanner F, Schnichels M, Zheng-Fischhöfer Q, Yang LE, Toma I, Willecke K, et al. Connexin 30.3 is expressed in the kidney but not regulated by dietary salt or high blood pressure. Cell communication & adhesion. 2008;15(1–2):219–30. 10.1080/15419060802013836 [DOI] [PubMed] [Google Scholar]
- 30.Gu Y, Huang F, Wang Y, Chen C, Wu S, Zhou S, et al. Connexin32 plays a crucial role in ROS-mediated endoplasmic reticulum stress apoptosis signaling pathway in ischemia reperfusion-induced acute kidney injury. J Transl Med. 2018;16(1):117 10.1186/s12967-018-1493-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoessel A, Himmerkus N, Bleich M, Bachmann S, Theilig F. Connexin 37 is localized in renal epithelia and responds to changes in dietary salt intake. Am J Physiol Renal Physiol. 2010;298(1):F216–23. 10.1152/ajprenal.00295.2009 [DOI] [PubMed] [Google Scholar]
- 32.Hills CE, Bland R, Wheelans DC, Bennett J, Ronco PM, Squires PE. Glucose-evoked alterations in connexin43-mediated cell-to-cell communication in human collecting duct: a possible role in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;291(5):F1045–51. 10.1152/ajprenal.00344.2005 [DOI] [PubMed] [Google Scholar]
- 33.Abed A, Toubas J, Kavvadas P, Authier F, Cathelin D, Alfieri C, et al. Targeting connexin 43 protects against the progression of experimental chronic kidney disease in mice. Kidney Int. 2014;86(4):768–79. 10.1038/ki.2014.108 [DOI] [PubMed] [Google Scholar]
- 34.Kavvadas P, Abed A, Poulain C, Authier F, Labejof LP, Calmont A, et al. Decreased Expression of Connexin 43 Blunts the Progression of Experimental GN. J Am Soc Nephrol. 2017;28(10):2915–30. 10.1681/ASN.2016111211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang JS, Angelov SN, Simon AM, Burt JM. Cx37 deletion enhances vascular growth and facilitates ischemic limb recovery. Am J Physiol Heart Circ Physiol. 2011;301(5):H1872–81. 10.1152/ajpheart.00683.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieg T, Tang T, Murray F, Schroth J, Insel PA, Fenton RA, et al. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol. 2010;21(12):2059–68. 10.1681/ASN.2010040409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieg T, Tang T, Uchida S, Hammond HK, Fenton RA, Vallon V. Adenylyl cyclase 6 enhances NKCC2 expression and mediates vasopressin-induced phosphorylation of NKCC2 and NCC. Am J Pathol. 2013;182(1):96–106. 10.1016/j.ajpath.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas L, Xue J, Murali SK, Fenton RA, Dominguez Rieg JA, Rieg T. Pharmacological Npt2a Inhibition Causes Phosphaturia and Reduces Plasma Phosphate in Mice with Normal and Reduced Kidney Function. J Am Soc Nephrol. 2019. 10.1681/ASN.2018121250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominguez Rieg JA, Chirasani VR, Koepsell H, Senapati S, Mahata SK, Rieg T. Regulation of intestinal SGLT1 by catestatin in hyperleptinemic type 2 diabetic mice. Lab Invest. 2016;96(1):98–111. 10.1038/labinvest.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenton RA, Murray F, Dominguez Rieg JA, Tang T, Levi M, Rieg T. Renal phosphate wasting in the absence of adenylyl cyclase 6. J Am Soc Nephrol. 2014;25(12):2822–34. 10.1681/ASN.2013101102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulsen SB, Kristensen TB, Brooks HL, Kohan DE, Rieg T, Fenton RA. Role of adenylyl cyclase 6 in the development of lithium-induced nephrogenic diabetes insipidus. JCI Insight. 2017;2(7):e91042 10.1172/jci.insight.91042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenton RA, Brond L, Nielsen S, Praetorius J. Cellular and subcellular distribution of the type-2 vasopressin receptor in the kidney. Am J Physiol Renal Physiol. 2007;293(3):F748–60. 10.1152/ajprenal.00316.2006 [DOI] [PubMed] [Google Scholar]
- 43.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, et al. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol. 1995;269(5 Pt 2):F663–72. 10.1152/ajprenal.1995.269.5.F663 [DOI] [PubMed] [Google Scholar]
- 44.Xue J, Thomas L, Tahmasbi M, Valdez A, Dominguez Rieg JA, Fenton RA, et al. An inducible intestinal epithelial cell-specific NHE3 knockout mouse model mimicking congenital sodium diarrhea. Clin Sci (Lond). 2020;134(8):941–53. 10.1042/CS20200065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int. 2010;78(2):160–9. 10.1038/ki.2010.130 [DOI] [PubMed] [Google Scholar]
- 46.Poulsen SB, Marin De Evsikova C, Murali SK, Praetorius J, Chern Y, Fenton RA, et al. Adenylyl cyclase 6 is required for maintaining acid-base homeostasis. Clin Sci (Lond). 2018;132(16):1779–96. 10.1042/CS20180060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83(5):811–24. 10.1038/ki.2013.14 [DOI] [PubMed] [Google Scholar]
- 48.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Dominguez Rieg JA, Rieg T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int. 2017;92(2):397–414. 10.1016/j.kint.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104(7):R19–23. 10.1172/JCI7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenton RA, Murali SK, Moeller HB. Advances in Aquaporin-2 trafficking mechanisms and their implications for treatment of water balance disorders. Am J Physiol Cell Physiol. 2020. 10.1152/ajpcell.00150.2020 [DOI] [PubMed] [Google Scholar]
- 51.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Busslinger M, Dominguez Rieg JA, et al. Caffeine-induced diuresis and natriuresis is independent of renal tubular NHE3. Am J Physiol Renal Physiol. 2015;308(12):F1409–20. 10.1152/ajprenal.00129.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pricam C, Humbert F, Perrelet A, Orci L. A freeze-etch study of the tight junctions of the rat kidney tubules. Lab Invest. 1974;30(3):286–91. [PubMed] [Google Scholar]
- 53.Kuhn K, Reale E. Junctional complexes of the tubular cells in the human kidney as revealed with freeze-fracture. Cell Tissue Res. 1975;160(2):193–205. 10.1007/BF00220577 [DOI] [PubMed] [Google Scholar]
- 54.Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev. 2013;34(2):278–301. 10.1210/er.2012-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy CR, Xiong H, Rahal S, Vanderluit J, Slack RS, Zhang Y, et al. Urine concentrating defect in prostaglandin EP1-deficient mice. Am J Physiol Renal Physiol. 2007;292(2):F868–75. 10.1152/ajprenal.00183.2005 [DOI] [PubMed] [Google Scholar]
- 56.Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Neonatal mortality in an aquaporin-2 knock-in mouse model of recessive nephrogenic diabetes insipidus. J Biol Chem. 2001;276(4):2775–9. 10.1074/jbc.M008216200 [DOI] [PubMed] [Google Scholar]
- 57.Gooch JL, Guler RL, Barnes JL, Toro JJ. Loss of calcineurin Aalpha results in altered trafficking of AQP2 and in nephrogenic diabetes insipidus. J Cell Sci. 2006;119(Pt 12):2468–76. 10.1242/jcs.02971 [DOI] [PubMed] [Google Scholar]
- 58.Shi PP, Cao XR, Qu J, Volk KA, Kirby P, Williamson RA, et al. Nephrogenic diabetes insipidus in mice caused by deleting COOH-terminal tail of aquaporin-2. Am J Physiol Renal Physiol. 2007;292(5):F1334–44. 10.1152/ajprenal.00308.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barlow ED, De Wardener HE. Compulsive water drinking. Q J Med. 1959;28(110):235–58. [PubMed] [Google Scholar]
- 60.Hisaki H, Matsuda J, Tadano-Aritomi K, Uchida S, Okinaga H, Miyagawa M, et al. Primary polydipsia, but not accumulated ceramide, causes lethal renal damage in saposin D-deficient mice. Am J Physiol Renal Physiol. 2012;303(7):F1049–59. 10.1152/ajprenal.00047.2012 [DOI] [PubMed] [Google Scholar]
- 61.Kong J, Zhang Z, Li D, Wong KE, Zhang Y, Szeto FL, et al. Loss of vitamin D receptor produces polyuria by increasing thirst. J Am Soc Nephrol. 2008;19(12):2396–405. 10.1681/ASN.2008010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Condorelli DF, Trovato-Salinaro A, Mudo G, Mirone MB, Belluardo N. Cellular expression of connexins in the rat brain: neuronal localization, effects of kainate-induced seizures and expression in apoptotic neuronal cells. Eur J Neurosci. 2003;18(7):1807–27. 10.1046/j.1460-9568.2003.02910.x [DOI] [PubMed] [Google Scholar]
- 63.Costales M, Fitzsimons JT, Vijande M. Increased sodium appetite and polydipsia induced by partial aortic occlusion in the rat. J Physiol. 1984;352:467–81. 10.1113/jphysiol.1984.sp015303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, et al. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72(5):566–73. 10.1038/sj.ki.5002369 [DOI] [PubMed] [Google Scholar]
- 65.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995;92(4):1013–7. 10.1073/pnas.92.4.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabolic I, Katsura T, Verbavatz JM, Brown D. The AQP2 water channel: effect of vasopressin treatment, microtubule disruption, and distribution in neonatal rats. J Membr Biol. 1995;143(3):165–75. 10.1007/BF00233445 [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto T, Sasaki S, Fushimi K, Ishibashi K, Yaoita E, Kawasaki K, et al. Vasopressin increases AQP-CD water channel in apical membrane of collecting duct cells in Brattleboro rats. Am J Physiol. 1995;268(6 Pt 1):C1546–51. 10.1152/ajpcell.1995.268.6.C1546 [DOI] [PubMed] [Google Scholar]
- 68.Olesen ET, Fenton RA. Aquaporin-2 membrane targeting: still a conundrum. Am J Physiol Renal Physiol. 2017;312(4):F744–F7. 10.1152/ajprenal.00010.2017 [DOI] [PubMed] [Google Scholar]
- 69.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, et al. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem. 2008;283(36):24617–27. 10.1074/jbc.M803074200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev. 2007;87(4):1083–112. 10.1152/physrev.00053.2006 [DOI] [PubMed] [Google Scholar]
- 71.Tsumura K, Li X, Murdiastuti K, Parvin MN, Akamatsu T, Yao C, et al. Downregulation of AQP2 expression in the kidney of polydipsic STR/N mice. Am J Physiol Renal Physiol. 2006;290(2):F478–85. 10.1152/ajprenal.00029.2005 [DOI] [PubMed] [Google Scholar]
- 72.Cadnapaphornchai MA, Summer SN, Falk S, Thurman JM, Knepper MA, Schrier RW. Effect of primary polydipsia on aquaporin and sodium transporter abundance. Am J Physiol Renal Physiol. 2003;285(5):F965–71. 10.1152/ajprenal.00085.2003 [DOI] [PubMed] [Google Scholar]
- 73.Olesen ET, Fenton RA. Is there a role for PGE2 in urinary concentration? J Am Soc Nephrol. 2013;24(2):169–78. 10.1681/ASN.2012020217 [DOI] [PubMed] [Google Scholar]
- 74.Olesen ET, Rutzler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci U S A. 2011;108(31):12949–54. 10.1073/pnas.1104691108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16(7):3100–6. 10.1091/mbc.e04-10-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao R, Patel S, Hao C, Woodgett J, Harris R. GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol. 2010;21(3):428–37. 10.1681/ASN.2009060672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. Am J Physiol Renal Physiol. 2011;301(3):F463–75. 10.1152/ajprenal.00236.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, et al. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12(8):950–4. 10.1038/nm1441 [DOI] [PubMed] [Google Scholar]
- 79.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, et al. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 2007;21(13):3717–26. 10.1096/fj.07-8807com [DOI] [PubMed] [Google Scholar]
- 80.Fenton RA. Urea transporters and renal function: lessons from knockout mice. Curr Opin Nephrol Hypertens. 2008;17(5):513–8. 10.1097/MNH.0b013e3283050969 [DOI] [PubMed] [Google Scholar]
- 81.Collings A, Raitakari OT, Juonala M, Mansikkaniemi K, Kahonen M, Hutri-Kahonen N, et al. The influence of smoking and homocysteine on subclinical atherosclerosis is modified by the connexin37 C1019T polymorphism—The Cardiovascular Risk in Young Finns Study. Clin Chem Lab Med. 2008;46(8):1102–8. 10.1515/CCLM.2008.216 [DOI] [PubMed] [Google Scholar]
- 82.Dominguez Rieg JA, Burt JM, Ruth P, Rieg T. P2Y(2) receptor activation decreases blood pressure via intermediate conductance potassium channels and connexin 37. Acta Physiol (Oxf). 2015;213(3):628–41. 10.1111/apha.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Just AW C; Simon A; Dautzenberg M; Kurtz A. Connexin 37 contributes to resting arterial pressure but is not essential for renal and systemic agonist-induced vasomotor responses. Acta Physiol (Oxf). 2010;198:P-SUN-52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.