Abstract
Bacterial secondary metabolites play important roles in promoting survival, though few have been carefully studied in their natural context. Numerous gene clusters code for secondary metabolites in the genomes of members of the Bptm group, made up of three closely related species with distinctly different lifestyles: the opportunistic pathogen Burkholderia pseudomallei, the non-pathogenic saprophyte Burkholderia thailandensis, and the host-adapted pathogen Burkholderia mallei. Several biosynthetic gene clusters are conserved across two or all three species, and this provides an opportunity to understand how the corresponding secondary metabolites contribute to survival in different contexts in nature. In this review, we discuss three secondary metabolites from the Bptm group: bactobolin, malleilactone (and malleicyprol), and the 4-hydroxy-3-methyl-2-alkylquinolines, providing an overview of each of their biosynthetic pathways and insight into their potential ecological roles. Results of studies on these secondary metabolites provide a window into how secondary metabolites contribute to bacterial survival in different environments, from host infections to polymicrobial soil communities.
Keywords: Burkholderia, secondary metabolite, antibiotic
INTRODUCTION
The Bptm group is comprised of three closely related species in the Burkholderia genus: Burkholderia pseudomallei, an opportunistic pathogen that causes the disease melioidosis; Burkholderia thailandensis, a non-pathogenic saprophyte; and Burkholderia mallei, a host-restricted animal pathogen. These three species have distinctly different lifestyles despite their close sequence relatedness, providing a unique opportunity to address questions regarding adaptations that have evolved to benefit bacteria in different environments. Of particular interest, there are many gene clusters coding for the biosynthesis of secondary metabolites in these three species. In all, at least 24 unique biosynthetic gene clusters have been identified (summarized in [7,43]). The products of 12 of those gene clusters have been characterized [71,51,31]. While 18 are unique to just one or two members of the Bptm group, five are conserved across all three species (Table 1).
Table 1.
Secondary metabolite biosynthetic gene clusters conserved in B. thailandensis (Bt) and B. pseudomallei (Bp) or in all three strains within the Bptm group.a,b
| Cluster | Bt Locusa | Other | Natural Product | Reference |
|---|---|---|---|---|
| 1 | BTH_11952 | Bp, Bm | Unknown | |
| 2 | BTH_I2418 | Bp, Bm | Malleobactin | [3, 28] |
| 3 | BTH_II0204 | Bp | Terphenyl | [8] |
| 4 | BTH_II0229 | Bp, Bm | Isonitrile | [10] |
| 5 | BTH_II0562 | Bp | Unknown | |
| 6 | BTH_II1209 | Bp | Unknown | |
| 7 | BTH_II1233 | Bp | Bactobolin | [69] |
| 8 | BTH_II1828 | Bp | Pyochelin | [28] |
| 9 | BTH_II1930 | Bp | HMAQ | [77] |
| 10 | BTH_II2088 | Bp, Bm | Malleilactone | [9,27] |
| 11 | BTH_II2349 | Bp, Bm | Thailandene | [65] |
The gene ID for the first gene within the gene cluster is given. Biosynthetic gene clusters for which the product has been identified are shown in bold type.
Note that Bt and Bm do not share any secondary metabolite biosynthetic gene clusters that are absent in Bp. Three biosynthetic gene clusters shared by Bp and Bm are not listed.
The Bptm group is a useful set of model bacterial species with which to further deepen our understanding of the biology and ecology of secondary metabolites. This is because of the genetic tractability of all three species, their ease of growth in standard laboratory conditions, and the abundance and diversity of encoded secondary metabolite biosynthetic gene clusters. Understanding more about the functions of the small molecule products could give insight into the conditions under which they are most helpful to the producing bacteria (or conditions when they are harmful) and uncover important new biology about the Bptm group. Additionally, these studies could expand our existing knowledge of small-molecule biochemistry. Metabolites produced by the Bptm group could serve as lead compounds or scaffolds to be modified for alternative medicinal or therapeutic purposes [44].
In this review, we will focus on three of the secondary metabolites produced by members of the Bptm group: the bactobolin antibiotics, malleilactone, and the hydroxyalkylquinolines. We focus on these three because results of recent studies have provided insight into their importance in natural environments. For each, we will briefly describe what is known about their structure, function, and biosynthesis. Then we will discuss the ecological importance of these metabolites and how they might benefit populations during infections or surviving within complex soil communities.
BACTOBOLIN
Structure, function, and biosynthesis
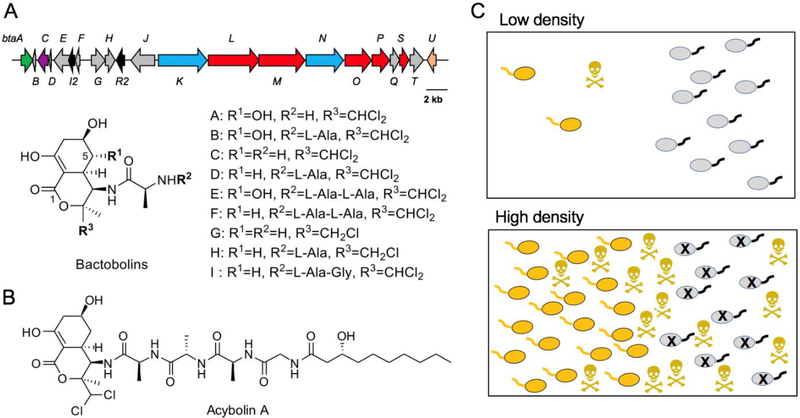
The bactobolins are a family of polar, hybrid polyketide-non-ribosomal peptide antibiotics first discovered in the 1970s in spent cultures of a “Pseudomonas” species [42]. Recent studies initiated by the Greenberg and Clardy labs provided more insight into the structural diversity of this family, the biosynthetic genes and biosynthesis, and its mechanism of action (for a review of their discovery, see ref. [29]). Bactobolins are produced by both B. thailandensis and B. pseudomallei. They consist of a bicyclic enol lactone core that is formed in part with an unusual amino acid building block, 3-hydroxy-4,4-dichloro-L-valine (3-OH-4,4-Cl2-Val, Fig. 1A). Nine bactobolin derivatives have been characterized to date, as well as a family of related molecules, acybolins, which are produced by the same gene cluster (Fig. 1B) [69,11,64]. Bactobolin A, the most abundant variant, inhibits the growth of a variety of bacterial species with minimal inhibitory concentrations (MICs) in the range of 0.1–10 μg/mL. It targets both Gram-positive bacteria, e.g. Staphyococcus aureus with an MIC of ~0.3 μg/mL, and Gram-negative bacteria, e.g. Escherichia coli with an MIC of ~1.5 μg/mL [14,69]. Bactobolin A binds to the 50S ribosome, where it interacts directly with the tRNA in the P-site. Both the C-5 OH and the dichloride groups are important for ribosome interaction and activity [69,11,4]. Binding to the ribosome is thought to block the peptidyl transfer step during protein synthesis. This activity is similar to that of another antibiotic, blasticidin S, although the precise binding site of blasticidin S differs from that of bactobolin [4]. The bactobolin target site is also unique from other antibiotics known to interact with or interfere with the peptidyl transferase center [14]. Thus, bactobolin blocks translation through a unique mode of action.
Fig. 1. Bactobolins.
(A) The bta gene cluster and structure of bactobolin variants A-H. The bta genes are indicated here by color: btaKLMNOPS encode nonribosomal peptide synthetases (blue) and polyketide synthases (red), btaC encodes the enzyme that adds chloride substituents (purple), btaA encodes the enzyme that adds the hydroxy group onto the valine (green), btaU encodes the hydroxylase that adds the OH onto C5 (salmon) and the quorum-sensing genes are encoded by btaI2 and btaR2 (black). All other genes encode predicted transporters, bactobolin biosynthetic genes, or are of unknown function. (B) Structure of acybolin A with the bactobolin warhead. (C) Density-dependent effects of antibiotic production. Top panel: at a low density, antibiotic-producing bacteria (yellow) produce little antibiotic and are unlikely to kill the competitor. This production might slow the growth of the producing bacteria or enable the competitor (grey) to mount a defense response. Bottom panel: at high cell density, enough antibiotics are produced to kill the competitor (black X). Quorum sensing delays antibiotic production until a sufficient killing dose can be produced.
The B. thailandensis bactobolin biosynthetic gene cluster has 21 genes that are highly conserved in B. pseudomallei. The entire gene cluster is notably absent from B. mallei, suggesting that it is important specifically in non-host environments. Within the gene cluster, btaLMO code for large modular type I polyketide synthases, while btaKN code for non-ribosomal peptide synthetases. A small operon, btaABCDE, is responsible for synthesizing and incorporating the non-canonical valine analog (3-OH-4,4-Cl2-Val), with btaE coding for the adenylation domain, and btaA and btaC for an iron-dependent hydroxylase and an iron-dependent chlorinase, respectively. A third iron-dependent enzyme, BtaU, is responsible for adding the OH group at the C-5 position. Two other genes embedded in the bactobolin gene cluster are involved in quorum sensing, a population density-dependent type of cell-cell signaling that relies on acyl-homoserine lactone (AHL) signals. The genes are btaI2 and btaR2, which code for an AHL synthase and an AHL receptor, respectively. AHLs can vary in structure, in which differences in the length or substitutions of the acyl group determine the specificity of the particular system. In this case, BtaI2 synthesizes the AHLs N-(3-hydroxyoctanoyl)-L-homoserine lactone (3OHC8-HSL) and N-(3-hydroxydecanoyl)-L-homoserine lactone (3OHC10-HSL) [24]. BtaR2 specifically recognizes the AHLs synthesized by BtaI2 and upon binding these AHLs, activates expression of the bactobolin biosynthetic genes [24]. Thus, bactobolin biosynthesis is induced at high cell density by quorum sensing.
Interestingly, the bactobolin gene cluster is also activated by antibiotics, notably trimethoprim. Trimethoprim-mediated induction leads to divergent production of molecules related to bactobolins called the acybolins [64]. Acybolins share the bicyclic core and dichloride substituents of bactobolin, but they contain a much longer peptide linker, acylated at the N-terminus with a 3-hydroxydecanoyl group, the same acyl group found in the 3OHC10-HSL signal. Acybolins may be the prodrug form of the bactobolins. However, they are by themselves active against bactobolin-resistant Bacillus subtilis [29], suggesting that they may also serve as antibiotics with a different target than the bactobolins. Much remains to be learned about the biology of acybolins and their relationship to quorum sensing.
Ecological role
Production of antibiotics might be important for competition with other microbes in soil. Interactions within natural microbial communities are quite complex, which can present many barriers to their study. However, reductionist binary co-culture approaches provide an alternative for studying microbial interactions without the added complexity of natural communities. Bactobolin production by B. thailandensis provided an opportunity to develop such a model that could be used for studying the link between quorum sensing and interspecies competition. A laboratory co-culture model was developed with B. thailandensis and another saprophytic soil bacterium, Chromobacterium subtsugae (formerly Chromobacterium violaceum) [13]. The model was also used to test the importance of the BtaR2-BtaI2 quorum-sensing circuit that controls bactobolin production. Specifically, these results showed that genetic disruptions of btaR2, btaI2 or the bactobolin biosynthesis gene btaK reduce the ability of B. thailandensis to compete with C. substugae [13]. These results support the view that bactobolin and the BtaR2-BtaI2 quorum-sensing system are important for interspecies competition.
Quorum sensing commonly controls the production of antibiotics, and there is growing evidence that quorum sensing-controlled antibiotics are important for interspecies competition in other bacteria [59,60,5,70,58]. Several hypotheses have been proposed to explain how quorum control of antibiotic production might benefit microbes during competition (Fig. 1C). Quorum sensing might provide a cost-savings strategy to ensure that metabolically costly antibiotics are produced only when the population density is sufficient to produce a killing dose [37]. An alternative hypothesis is that delaying antibiotic production deprives a competitor of the ability to mount defenses to sublethal antibiotic and survive accumulated concentrations that are lethal [37]. These two strategies do not need to be mutually exclusive.
To explore the potential benefits of using quorum sensing to control antibiotic production, results of the B. thailandensis-C. substugae laboratory co-culture model were used to develop an in silico model where antibiotic production costs and timing could be manipulated through mathematical equations. The in silico models showed that early antibiotic production delayed growth during a time when too little antibiotic was produced to be effective, thereby reducing competitiveness [13]. The in silico results are consistent with the hypothesis that quorum sensing provides a metabolic cost-savings to antibiotic-producing bacteria that is important for competition with other species. The idea that quorum sensing mitigates costs of producing metabolically expensive goods is supported by other studies outside of the context of competition [80,63,36]. These studies provide new insight into how quorum sensing might promote the survival of bacterial populations in natural communities.
The B. thailandensis-C. subtsugae co-culture model was also used to explore the role of antibiotic defense mechanisms during competition [6]. C. subtsugae has two antibiotic efflux pumps that can recognize bactobolin as a substrate. Cells treated with sublethal concentrations of bactobolin activate production of one of these efflux pumps, CseAB-OprN. This activation increases resistance to subsequent higher bactobolin doses. In co-cultures, treatment with sublethal bactobolin concentrations increases C. subtsugae survival during higher-dose treatment by 1,000-fold compared with cultures that did not receive the sublethal treatment. These results show that the activation of antibiotic resistance mechanisms following exposure to sublethal antibiotic concentrations is an important survival strategy during competition. Quorum sensing could be useful to coordinate the delivery of a sudden killing dose of antibiotic during competition to avert the induction of antibiotic resistance.
MALLEILACTONE AND MALLEICYPROL
Structure, function, and biosynthesis
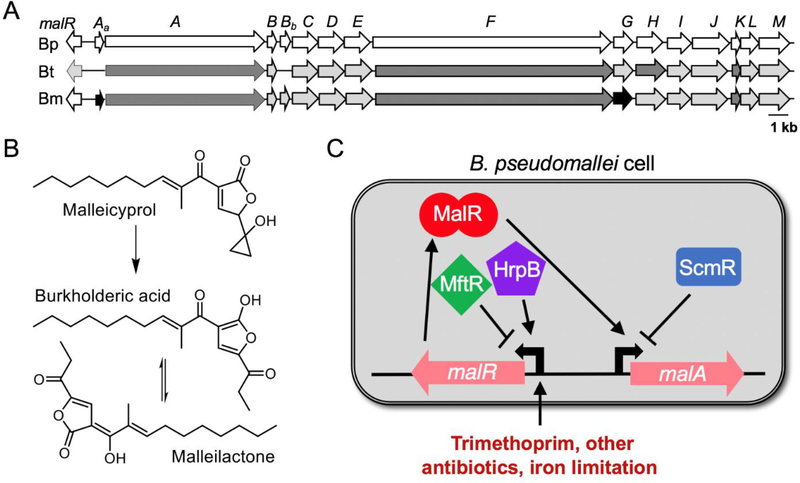
The malleilactone and malleicyprol (mal or bur) biosynthetic genes are encoded in a ~35 kb region that is conserved in all three species of the Bptm group. This region includes the genes malA-M encoding polyketide synthases and non-ribosomal peptide synthetases as well as a divergently-encoded transcriptional regular gene malR (Fig. 2A). Two other genes are found only in B. pseudomallei and B. mallei; malAa and malBb, encoding hypothetical proteins. Otherwise, the gene organization is highly conserved with the encoded proteins sharing ~80–90% amino acid identity in all three species. Early studies identified malleilactone and its tautomer burkholderic acid as the products of the mal genes [9,27]. However, recent studies by Hertweck and colleagues have shown that malleicyprol is the immediate and toxic bioactive product of this pathway in B. thailandensis [72]. Malleicyprol contains an unusual and unstable cyclopropanol warhead, which converts to malleilactone/burkholderic acid under typical work-up conditions. These products have been detected in both B. thailandensis and B. pseudomallei. It is safe to assume, therefore, that the pathway in B. mallei also gives rise to malleicyprol, though the mal genes and gene product from this strain remains to be studied [40,9,27] (Fig. 2B).
Fig. 2. Malleilactone and malleicyprol.
(A) The mal gene cluster from B. pseudomallei (Bp), B. thailandensis (Bt) and B. mallei (Bm). Shading indicates % identity to the B. pseudomallei protein sequences. White, 100%; light gray, 90–99%; dark gray, 80–89%; black <80%. Lowercase letters indicate genes that are present in B. pseudomallei and B. mallei but missing in B. thailandensis. (B) Structures of malleicyprol, malleilactone and burkholderic acid. It is thought that malleicyprol degrades to form malleilactone and its tautomer burkholderic acid. (C) Model of mal gene regulation. Regulation occurs at the promoter of the first mal gene (malA) by the transcriptional activator MalR, encoded upstream from malA. Expression of malR is influenced by the urate-responsive repressor MftR, the LysR-type repressor ScmR, the host-responsive activator HrpB, trimethoprim, and conditions of iron limitation.
To date, the steps of malleilactone and malleicyprol biosynthesis have only been studied in B. thailandensis [27,9,73]. A key piece to the puzzle has recently been reported regarding the initial reactions in the biosynthetic pathway and the installation of the unusual cyclopropanol group. The three-carbon unit of the warhead is derived from methionine. In the current proposed pathway, a set of four enzymes (BurBCDE or MalBCDE) convert methionine to dimethylsulfoniopropionate (DMSP) [73]. The adenylation domain of MalA then charges an adjacent thiolation domain with DMSP. This intermediate condenses with malonyl-CoA to form a thioesterified gonyol intermediate. Lending credence to this model is the detection of gonyol in B. thailandensis overexpressing the mal biosynthetic genes and when malA is heterologously expressed in E. coli [73]. Additional steps in the biosynthetic pathway have not yet been examined experimentally, but the long linear arm of malleicyprol is likely constructed independently on MalF, and the two polyketides are then joined to deliver the final product [9]. Specifically, bioinformatic analyses suggest that MalF elongates a caprylic acid starter unit via two condensation reactions, the first with methylmalonyl-CoA, followed by reduction and dehydration to form a branched olefin, and the second with malonyl-CoA. MalF could subsequently join the two polyketides, one derived from elongated caprylic acid and the second from DMSP [9]. Formation of the substituted furan lactone may also occur on MalF, though this proposal awaits experimental verification. Studies with the malleilactone gene cluster have brought new and interesting biosynthetic reactions to the fore and identified the cytotoxic product that is important for host infection.
Little is known about the biological activities of malleilactone and malleicyprol. The mal biosynthetic genes contribute to B. thailandensis virulence in the social amoeba Dictyostelium discoidium [9] and to virulence of B. thailandensis and B. pseudomallei in Caenorhabditis elegans nematodes [40,9]. Results of two independent studies in a mouse model of melioidosis also suggest these genes are important for infection in mammals. The mouse studies used pools of transposon mutants to infect mice, and the effects of a particular gene on virulence was determined by tracking the frequency of mutants with particular gene disruptions in the pooled infection population (input population) vs. that recovered from the infection (recovered population). For the studies, mice were infected with pools of ~100 [62] or ~20,000 [33] B. pseudomallei transposon mutants, and mal-disrupted mutants were found to be 3–7-fold reduced in the lung (both studies) and 40-fold reduced in the spleen [62] in the recovered population relative to the input population. These results suggest that the mal genes might play a role in B. pseudomallei dissemination to different organs in a mouse. The mechanism of virulence may be through the toxic effects of malleicyprol, which have been demonstrated in vitro to be in the 0.5 μg/mL range [72]. Malleicyprol toxicity has been proposed to be through its biologically active warhead [72], although its specific mode of action has not been tested. Malleilactone has also been shown to coordinate iron [9]; thus it is also possible malleilactone contributes to virulence by sequestering essentially needed iron in the host where its abundance is limited.
Ecological role
The conservation of the mal genes in all three species of the Bptm group suggests that the mal gene products are important for survival in both host- and non-host environments. However, few studies have directly tested the importance of this gene cluster in or outside of the host. The toxic effects of malleicyprol might contribute to infections in vivo or be important for evading eukaryotic predators in soil or killing microbial competitors in polymicrobial communities. The molecules might also have other, as-yet unknown functions in promoting survival in each of these environments. While many questions remain about the biological importance of malleilactone and/or malleicyprol, studies into the regulation of the mal genes do provide some insight into where and when this gene cluster might be important.
Like other secondary metabolites, expression of the mal genes is very low or absent in standard laboratory conditions [9,27,74,68]. For early characterization of malleilactone, the promoter of the native mal cluster was replaced with a synthetic inducible promoter to enable production and isolation from lab-grown cultures [9,27]. In a later study [68], a small-molecule screen was used to search for elicitors of this gene cluster. The results revealed several antibiotics that can promote mal gene transcription [68], which has since been corroborated by other studies [74,64]. Both trimethoprim and another folate biosynthesis pathway inhibitor, sulfamethoxazole, induce the mal genes. In addition, piperacillin and cephalosporins are strong inducers while the fluoroquinolone antibiotics and mitomycin C (a DNA crosslinker) cause moderate mal induction [68]. Of note, trimethoprim and sulfamethoxazole are widely used to treat melioidosis caused by B. pseudomallei infections in humans, though it remains unclear what, if any, biological significance this finding has in vivo.
The discovery that antibiotics induce mal gene expression led to the hypothesis that this regulation might be through the MalR regulator encoded upstream of the mal genes. MalR belongs to the LuxR family of quorum sensing signal-responsive transcriptional regulators. LuxR family receptors typically activate gene expression upon binding to AHL quorum-sensing signals, which are produced by LuxI family signal synthases (for reviews, see [1,78]). Although many cognate LuxR-LuxI pairs are genetically linked, MalR is not encoded near any LuxI genes, and for this reason, MalR is termed an orphan LuxR. MalR is also not responsive to any of the three native AHL signals produced by B. thailandensis and B. pseudomallei or a range of other AHLs [74,40]. However, MalR is important for trimethoprim induction of the mal genes in both B. thailandensis and B. pseudomallei [74,40]. Trimethoprim activates MalR by driving expression of malR, which appears to occur in response to an accumulation of folate and methionine biosynthesis intermediates [48]. The finding that mal gene expression is triggered in response to certain antibiotics supports the idea that the products of the mal genes might enhance Burkholderia survival in polymicrobial soil communities.
The importance of the mal genes during host infections suggests that there are likely specific regulatory systems driving their expression in the host. While direct links have yet to be experimentally demonstrated in vivo, at least four possibilities have emerged. First, the MarR-family transcriptional repressor MftR de-represses malR to drive up malleilactone production in response to its inducer urate [30–32]. Urate is commonly produced by host-supplied xanthine oxidoreductase as part of the host’s antimicrobial response (for a review, see [57]). Thus, MftR could be driving expression of the mal genes by responding to host-produced urate. Second, expression of the AraC-family transcriptional regulator HrpB increases malR transcription [50]. HrpB activates other genes, specifically those encoding a type 3 secretion system (T3SS) in response to plant cell contact in the plant pathogen Ralstonia solanacearum [56]. However, it is inconclusive whether HrpB functions similarly in Burkholderia in response to host cues [50]. Third, microarray [75] analyses revealed that malR is activated during growth in iron-limited conditions relative to iron-replete conditions. These latter results support the idea that malleilactone might be important for sequestering iron during conditions of iron starvation, a notion that is consistent with the ability of malleilactone to bind iron [9]. Finally, the mal genes are also controlled by ScmR, a LysR-type transcriptional regulator that largely suppresses secondary metabolism and virulence in a quorum sensing-regulated fashion in B. thailandensis [55,46,40]. Therefore, interference with the function of ScmR provides yet another route for activating the mal genes.
Together, the regulatory pathways controlling the expression of the mal genes are illustrated in Fig. 2C. Results from studies of these pathways suggest that products of the mal genes are important for promoting survival under stress, which may be growth-inhibiting antibiotics, host immune responses, and/or nutrient (iron) limitation.
4-HYDROXY-3-METHYL-2-ALKYLQUINOLINES
Structure, function and synthesis
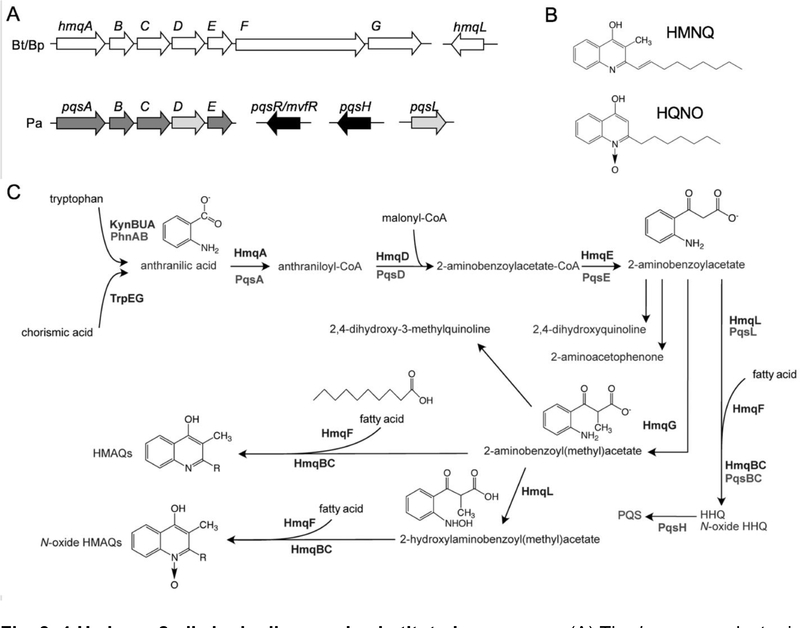
A few bacteria produce 2-alkyl-4(1H)-quinolones, also known as 4-hydroxy-2-alkylquinolines (HAQs), as secondary metabolites. These molecules are involved in interbacterial competition (acting as antibiotics), virulence, iron acquisition, and cell-cell signaling, among other processes [67,38,39,52,61,49,19,21]. The HAQs mostly produced by B. thailandensis and B. pseudomallei are 4-hydroxy-3-methyl-2-alkenylquinolines (HMAQs), which are biosynthesized from products of the hmqABCDEFG genes (Fig. 3A & B). Based on bioinformatics analyses, these genes are present in 98% of B. pseudomallei and 79% of B. thailandensis sequenced strains [17]. Interestingly, the cluster is only present in 36% of sequenced Burkholderia cepacia complex (Bcc) strains [17], and thus is more highly prevalent in B. pseudomallei and B. thailandensis than the Bcc group. It is noteworthy that the hmq genes are entirely absent in host-adapted B. mallei, supporting the idea that they may have a role in the saprophytic lifestyle of the other species.
Fig. 3. 4-Hydroxy-2-alkylquinolines and substituted congeners.
(A) The hmq gene cluster in B. thailandensis (Bt) and B. pseudomallei (Bp) and the homologous pqs gene cluster in P. aeruginosa (Pa). Shading of Pa proteins indicates % sequence identity to Bt sequences. Light grey, 50–60%; dark grey, 30–40%; black, no homology. Bt and Bp proteins are >85% identical. (B) Structures of HMNQ (4-hydroxy-3-methyl-2-nonenylquinoline) and HQNO (2-heptyl-4(1H)-quinoline N-oxide). (C) Biosynthesis of hydroxyl-alkylquinolines by hmq gene products in B. thailandensis/B. pseudomallei and by pqs gene products in P. aeruginosa. The specific steps are described in the text.
The hmqABCDE genes share high similarity to the pqsABCDE genes involved in the biosynthesis of the Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) [77,17]. Among the P. aeruginosa HAQs are the Pseudomonas quinolone signal (PQS) 3,4-dihydroxy-2-heptylquinoline and its precursor 4-hydroxy-2-heptylquinoline (HHQ) [77,17]. Both PQS and HHQ are quorum-sensing signals that bind to the transcriptional regulator MvfR (also called PqsR) [81], which activates transcription of >100 genes in a population density-dependent manner [18,22]. The P. aeruginosa pqs genes differ from the Burkholderia hmq genes in several important ways. First, Burkholderia species uniquely code for HmqF, which adds a double bond to the alkyl chain [2], and HmqG, which adds the methyl group on the quinoline cycle [77]. Second, the Burkholderia genomes are missing a pqsH homolog, which is responsible for the conversion of HHQ into PQS. There is also no homolog of the MvfR/PqsR receptor in the vicinity. Although there seems to be no Pseudomonas-type PQS-dependent quorum-sensing system in the Burkholderias, HMAQs do modulate transcription of the hmq genes through an unknown mechanism [66,16,45]. There are also complex regulatory interactions between the hmq system and the AHL quorum-sensing systems [53,54,45,15].
Despite these key differences, other aspects of HMAQ and HAQ biosynthesis are largely similar (Fig. 3C, [77,17]). Anthranilic acid is the precursor of both HMAQ and HAQ biosynthesis [19,77]. In both Burkholderia and Pseudomonas, anthranilic acid is synthesized via tryptophan degradation by the kynBUA-encoded kynurenine pathway, and/or from chorismic acid via the TrpEG anthranilate synthase [26]. A second anthranilate synthase encoded by phnAB is unique to P. aeruginosa. From anthranilic acid, HmqA/PqsA synthesizes anthraniloyl-CoA. HmqD/PqsD then transfers malonyl-CoA to anthraniloyl-CoA to form 2-aminobenzoylacetate-CoA [23]. Next, the putative 2-aminobenzoylacetyl-CoA thioesterase HmqE/PqsE can remove the CoA from 2-aminobenzoylacetyl-CoA to yield 2-aminobenzoylacetate (2-ABA) [23]. In Burkholderia, the HmqG methylase is thought to add a methyl group onto 2-ABA to make methyl-2-ABA [77]. In both Burkholderia and P. aeruginosa, (methyl)-2-ABA is modified by PqsBC/HmqBC by incorporating fatty acid-derived alkyl groups to make the hydroxy (methyl) alkylquinolone molecules. In P. aeruginosa, PqsL also converts 2-ABA to 2-hydroxyl-ABA (2-HABA) [47,23]. 2-HABA is then alkylated by PqsBC to make N-oxide derivatives of HAQs. In Burkholderia HmqL is thought to play a similar role as PqsL in N-oxide HMAQ biosynthesis [41].
Ecological role
The ecological role and biological function of HMAQs remain poorly understood despite their prevalence across many different Burkholderia species. Several studies support the idea that HMAQs could be important for competing with other microbes in mixed soil communities. These molecules have antibacterial [49,66] and antifungal activities [38,39,52]. In addition, in a recent study [41], HMAQs were shown to be important for B. thailandensis to kill B. subtilis in co-cultures. Genetic disruption of the B. thailandensis hmqL abolished production of the N-oxide derivatives of HMAQs, confirming the role of HmqL in the biosynthesis of the N-oxides. Further, the most abundant N-oxide congener, HMNQ-NO (4-hydroxy-3-methyl-2-nonenyl-quinoline N-oxide, see Fig. 3B), has potent killing effects against B. subtilis [41]. These results support the role of HmqL and HMAQs, in particular HMAQ-NO such as HMNQ-NO, in competition at least against Gram-positive bacteria [66]. Interestingly, the hmqL gene needed for biosynthesis of N-oxide derivatives is missing from the genome of the Bcc [77]. It is possible HMAQs are sufficient to kill competitors in these species [66]. The finding that some of the Burkholderia species lack an hmqL gene suggests another interesting possibility: that HMAQs have other important functions in soil bacteria that remain as-yet undiscovered.
B. thailandensis and B. pseudomallei predominantly produce C9 congeners, while the Bcc species predominantly produce C7 congeners [77,20]. The C9 congeners are synthesized from decanoic acid while C7 congeners are synthesized from octanoic acid, as they are in P. aeruginosa and probably also in other Burkholderia [2,25]. It is unclear what evolutionary factors drive alkyl chain length and substitution in different species of Burkholderia, although it is tempting to speculate that the particular hydroxylalkylquinoline(s) produced by each species promotes survival in that bacterium’s particular environment. For example, differences in alkyl chain length or quinolone substitution could alter the killing activity against different target species. A recently developed procedure for HMAQ chemical synthesis [66] provided an avenue to explore this idea. Studies with these synthetic molecules showed that differences in the alkyl chain length and substitution do alter antimicrobial activities against different species [66,41]. Although the basis for these differences are currently unknown, it is possible that changes in the alkyl group length or substitution change the specific target site or ability to penetrate different types of bacterial cells. These results suggest the possibility that the antimicrobial activity against different types of competitors might drive the evolution of the structure of the HMAQ.
There is also a diversity of hmq biosynthesis products within each organism. It has been proposed that synthesizing diverse secondary metabolites from the same pathway could provide certain advantages, such as synergistic activities [12]. In support of this idea, two different products of the B. thailandensis HMAQ biosynthesis pathway were shown to have the synergistic ability to kill other bacteria [79]. One of these was the major B. thailandensis product HMNQ. The other was HQNO (2-heptyl-4(1H)-quinoline N-oxide (see Fig. 3B), which is a minor product of B. thailandensis HMAQ biosynthesis and which is readily produced by P. aeruginosa. It was shown that HMNQ and HQNO have distinctly different targets; HMNQ disrupts the proton motive force [79], while HQNO blocks the cytochrome bc1 complex [34,35,76]. Thus, by blocking energy production through two different mechanisms, these two molecules act synergistically to inhibit bacterial growth [79]. Divergent molecule synthesis could be important for competing with other bacteria because it could enhance the effects of the antimicrobials produced from the pathway. Divergent molecule synthesis could also avert the development of antibiotic resistance in competitors, as it would require mutations in several pathways occurring simultaneously to overcome the dual-killing mode of action.
CONCLUSIONS AND FUTURE DIRECTIONS
Secondary metabolites can have important and often underappreciated roles in promoting survival in different host and non-host environments. Secondary metabolites might also have other useful purposes, such as expanding the existing knowledge of small-molecule biochemistry or in the development of new antimicrobial compounds to treat bacterial infections. Secondary metabolite studies are slowed down by technical challenges such as their low production under laboratory conditions or issues with purification and stability. It is also challenging to demonstrate their role in promoting survival of the producing microorganisms in their natural ecological niches. However, genetic and chemical tools to elicit secondary metabolite production as well as the development of laboratory co-culture models have helped to overcome some of these challenges. With these tools, we are now beginning to understand how some of these secondary metabolites are synthesized and regulated, and what their functions might be. Results of secondary metabolite studies provide useful knowledge both toward advancing research applications and toward appreciating how and when these molecules might become activated (or suppressed) in biologically-relevant conditions.
We believe that the Bptm group provides an excellent opportunity to further understand the biology of secondary metabolites. This is because of the abundance and diversity of encoded secondary metabolite gene clusters as well as the advancement of genetic, biochemical, and ecological systems for studying these species. There is a growing body of work describing the Bptm metabolites and in particular bactobolin, malleilactone and HMAQs. The results generated provide a strong foundation with which to develop a better understanding of how secondary metabolites affect the ecology of these bacteria. Many questions remain to be answered, such as discovering targets and other activities of the metabolites, the regulatory interplay between the metabolite biosynthesis gene clusters, and uncovering the role of these metabolites in more complex natural communities. Addressing some of these questions will require already-existing genetic and chemical tools or altogether new approaches, such as synthetic ecology systems that expand on existing ones by introducing more species or other variables. Application of the tools and approaches developed in the Bptm group could also provide insights into roles of secondary metabolites in the ecology and physiology of other bacteria.
ACKNOWLEDGEMENTS
This work was supported by the NIH through grant R35GM133572 and a pilot award from the COBRE Chemical Biology of Infectious Disease Program (P20 GM113117) to J.R.C. and grant DP2-AI-124786 to M.R.S. Support for E.D. was from the Canadian Institutes of Health Research (CIHR) under award number MOP-142466.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR (2018) Bacterial quorum sensing and microbial community interactions. mBio 9:e02331–02317. doi: 10.1128/mBio.02331-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Kahyaoglu C, Hansen DB (2012) Characterization of HmqF, a protein involved in the biosynthesis of unsaturated quinolones produced by Burkholderia thailandensis. Biochemistry 51:1648–1657. doi: 10.1021/bi201625w [DOI] [PubMed] [Google Scholar]
- 3.Alice AF, Lopez CS, Lowe CA, Ledesma MA, Crosa JH (2006) Genetic and transcriptional analysis of the siderophore malleobactin biosynthesis and transport genes in the human pathogen Burkholderia pseudomallei K96243. Journal of bacteriology 188:1551–1566. doi: 10.1128/JB.188.4.1551-1566.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amunts A, Fiedorczuk K, Truong TT, Chandler J, Peter Greenberg E, Ramakrishnan V (2015) Bactobolin A binds to a site on the 70S ribosome distinct from previously seen antibiotics. Journal of molecular biology 427:753–755. doi: 10.1016/j.jmb.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An D, Danhorn T, Fuqua C, Parsek MR (2006) Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures. Proceedings of the National Academy of Sciences of the United States of America 103:3828–3833. doi: 10.1073/pnas.0511323103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benomar S, Evans KC, Unckless RL, Chandler JR (2019) Efflux pumps in Chromobacterium species increase antibiotic resistance and promote survival in a co-culture competition model. Applied and environmental microbiology 85:e00908–00919. doi: 10.1128/AEM.00908-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggins JB, Kang HS, Ternei MA, DeShazer D, Brady SF (2014) The chemical arsenal of Burkholderia pseudomallei is essential for pathogenicity. Journal of the American Chemical Society 136:9484–9490. doi: 10.1021/ja504617n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biggins JB, Liu X, Feng Z, Brady SF (2011) Metabolites from the induced expression of cryptic single operons found in the genome of Burkholderia pseudomallei. Journal of the American Chemical Society 133:1638–1641. doi: 10.1021/ja1087369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biggins JB, Ternei MA, Brady SF (2012) Malleilactone, a polyketide synthase-derived virulence factor encoded by the cryptic secondary metabolome of Burkholderia pseudomallei group pathogens. Journal of the American Chemical Society 134:13192–13195. doi: 10.1021/ja3052156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady SF, Bauer JD, Clarke-Pearson MF, Daniels R (2007) Natural products from isnA-containing biosynthetic gene clusters recovered from the genomes of cultured and uncultured bacteria. Journal of the American Chemical Society 129:12102–12103. doi: 10.1021/ja075492v [DOI] [PubMed] [Google Scholar]
- 11.Carr G, Seyedsayamdost MR, Chandler JR, Greenberg EP, Clardy J (2011) Sources of diversity in bactobolin biosynthesis by Burkholderia thailandensis E264. Organic letters 13:3048–3051. doi: 10.1021/ol200922s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Challis GL, Hopwood DA (2003) Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proceedings of the National Academy of Sciences of the United States of America 100 Suppl 2:14555–14561. doi: 10.1073/pnas.1934677100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandler JR, Heilmann S, Mittler JE, Greenberg EP (2012) Acyl-homoserine lactone-dependent eavesdropping promotes competition in a laboratory co-culture model. The ISME journal 6:2219–2228. doi: 10.1038/ismej.2012.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandler JR, Truong TT, Silva PM, Seyedsayamdost MR, Carr G, Radey M, Jacobs MA, Sims EH, Clardy J, Greenberg EP (2012) Bactobolin resistance is conferred by mutations in the L2 ribosomal protein. mBio 3:e00499–00412. doi: 10.1128/mBio.00499-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapalain A, Groleau M-C, Le Guillouzer S, Miomandre A, Vial L, Milot S, Déziel E (2017) Interplay between 4-Hydroxy-3-Methyl-2-Alkylquinoline and N-Acyl-Homoserine Lactone Signaling in a Burkholderia cepacia Complex Clinical Strain. Frontiers in Microbiology 8. doi: 10.3389/fmicb.2017.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapalain A, Groleau MC, Le Guillouzer S, Miomandre A, Vial L, Milot S, Déziel E (2017) Interplay between 4-Hydroxy-3-Methyl-2-Alkylquinoline and N-Acyl-Homoserine lactone signaling in a Burkholderia cepacia complex clinical strain. Front Microbiol 8:1021. doi: 10.3389/fmicb.2017.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coulon PML, Groleau MC, Déziel E (2019) Potential of the Burkholderia cepacia complex to produce 4-hydroxy-3-methyl-2-alkyquinolines. Front Cell Infect Microbiol 9:33. doi: 10.3389/fcimb.2019.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Déziel E, Gopalan S, Tampakaki AP, Lépine F, Padfield KE, Saucier M, Xiao G, Rahme LG (2005) The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Molecular microbiology 55:998–1014. doi: 10.1111/j.1365-2958.2004.04448.x [DOI] [PubMed] [Google Scholar]
- 19.Déziel E, Lépine F, Milot S, He J, Mindrinos MN, Tompkins RG, Rahme LG (2004) Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proceedings of the National Academy of Sciences of the United States of America 101:1339–1344. doi: 10.1073/pnas.0307694100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diggle SP, Lumjiaktase P, Dipilato F, Winzer K, Kunakorn M, Barrett DA, Chhabra SR, Camara M, Williams P (2006) Functional genetic analysis reveals a 2-alkyl-4-quinolone signaling system in the human pathogen Burkholderia pseudomallei and related bacteria. Chem Biol 13:701–710. doi: 10.1016/j.chembiol.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 21.Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, Kong X, Hider RC, Cornelis P, Camara M, Williams P (2007) The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol 14:87–96. doi: 10.1016/j.chembiol.2006.11.014 [DOI] [PubMed] [Google Scholar]
- 22.Diggle SP, Winzer K, Chhabra SR, Worrall KE, Camara M, Williams P (2003) The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Molecular microbiology 50:29–43 [DOI] [PubMed] [Google Scholar]
- 23.Drees SL, Ernst S, Belviso BD, Jagmann N, Hennecke U, Fetzner S (2018) PqsL uses reduced flavin to produce 2-hydroxylaminobenzoylacetate, a preferred PqsBC substrate in alkyl quinolone biosynthesis in Pseudomonas aeruginosa. J Biol Chem 293:9345–9357. doi: 10.1074/jbc.RA117.000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duerkop BA, Varga J, Chandler JR, Peterson SB, Herman JP, Churchill ME, Parsek MR, Nierman WC, Greenberg EP (2009) Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. Journal of bacteriology 191:3909–3918. doi: 10.1128/JB.00200-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dulcey CE, Dekimpe V, Fauvelle DA, Milot S, Groleau MC, Doucet N, Rahme LG, Lépine F, Déziel E (2013) The end of an old hypothesis: the Pseudomonas signaling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem Biol 20:1481–1491. doi: 10.1016/j.chembiol.2013.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrow JM 3rd, Pesci EC (2007) Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. Journal of bacteriology 189:3425–3433. doi: 10.1128/JB.00209-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franke J, Ishida K, Hertweck C (2012) Genomics-driven discovery of burkholderic acid, a noncanonical, cryptic polyketide from human pathogenic Burkholderia species. Angewandte Chemie 51:11611–11615. doi: 10.1002/anie.201205566 [DOI] [PubMed] [Google Scholar]
- 28.Franke J, Ishida K, Ishida-Ito M, Hertweck C (2013) Nitro versus hydroxamate in siderophores of pathogenic bacteria: effect of missing hydroxylamine protection in malleobactin biosynthesis. Angewandte Chemie 52:8271–8275. doi: 10.1002/anie.201303196 [DOI] [PubMed] [Google Scholar]
- 29.Greenberg EP, Chandler JR, Seyedsayamdost MR (2020) The Chemistry and Biology of Bactobolin: A 10-Year Collaboration with Natural Product Chemist Extraordinaire Jon Clardy. J Nat Prod 83:738–743. doi: 10.1021/acs.jnatprod.9b01237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grove A (2010) Urate-responsive MarR homologs from Burkholderia. Mol Biosyst 6:2133–2142. doi: 10.1039/c0mb00086h [DOI] [PubMed] [Google Scholar]
- 31.Gupta A, Bedre R, Thapa SS, Sabrin A, Wang G, Dassanayake M, Grove A (2017) Global Awakening of Cryptic Biosynthetic Gene Clusters in Burkholderia thailandensis. ACS Chem Biol 12:3012–3021. doi: 10.1021/acschembio.7b00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A, Grove A (2014) Ligand-binding pocket bridges DNA-binding and dimerization domains of the urate-responsive MarR homologue MftR from Burkholderia thailandensis. Biochemistry 53:4368–4380. doi: 10.1021/bi500219t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez MG, Yoder-Himes DR, Warawa JM (2015) Comprehensive identification of virulence factors required for respiratory melioidosis using Tn-seq mutagenesis. Front Cell Infect Microbiol 5:78. doi: 10.3389/fcimb.2015.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hacker B, Barquera B, Crofts AR, Gennis RB (1993) Characterization of mutations in the cytochrome b subunit of the bc1 complex of Rhodobacter sphaeroides that affect the quinone reductase site (Qc). Biochemistry 32:4403–4410. doi: 10.1021/bi00067a033 [DOI] [PubMed] [Google Scholar]
- 35.Hazan R, Que YA, Maura D, Strobel B, Majcherczyk PA, Hopper LR, Wilbur DJ, Hreha TN, Barquera B, Rahme LG (2016) Auto Poisoning of the Respiratory Chain by a Quorum-Sensing-Regulated Molecule Favors Biofilm Formation and Antibiotic Tolerance. Current biology : CB 26:195–206. doi: 10.1016/j.cub.2015.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heilmann S, Krishna S, Kerr B (2015) Why do bacteria regulate public goods by quorum sensing?-How the shapes of cost and benefit functions determine the form of optimal regulation. Front Microbiol 6:767. doi: 10.3389/fmicb.2015.00767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hibbing ME, Fuqua C, Parsek MR, Peterson SB (2010) Bacterial competition: surviving and thriving in the microbial jungle. Nature reviews Microbiology 8:15–25. doi: 10.1038/nrmicro2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilani-Feki O, Culioli G, Ortalo-Magne A, Zouari N, Blache Y, Jaoua S (2011) Environmental Burkholderia cepacia strain Cs5 acting by two analogous alkyl-quinolones and a didecylphthalate against a broad spectrum of phytopathogens fungi. Curr Microbiol 62:1490–1495. doi: 10.1007/s00284-011-9892-6 [DOI] [PubMed] [Google Scholar]
- 39.Kilani-Feki O, Zouari I, Culioli G, Ortalo-Magne A, Zouari N, Blache Y, Jaoua S (2012) Correlation between synthesis variation of 2-alkylquinolones and the antifungal activity of a Burkholderia cepacia strain collection. World J Microbiol Biotechnol 28:275–281. doi: 10.1007/s11274-011-0817-0 [DOI] [PubMed] [Google Scholar]
- 40.Klaus JR, Deay J, Neuenswander B, Hursh W, Gao Z, Bouddhara T, Williams TD, Douglas J, Monize K, Martins P, Majerczyk C, Seyedsayamdost MR, Peterson BR, Rivera M, Chandler JR (2018) Malleilactone Is a Burkholderia pseudomallei virulence factor regulated by antibiotics and quorum sensing. Journal of bacteriology 200:e00008–00018. doi: 10.1128/JB.00008-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klaus JR, Majerczyk C, Eppler NA, Ball P, Groleau M-C, Asfahl KL, Smalley NE, Hayden H, Piochon M, Dandekar AA, Gauthier C, Déziel E, Chandler JR (2020) Burkholderia thailandensis methylated hydroxy-alkylquinolines: biosynthesis and antimicrobial activity in co-culture experiments. App Environ Microbiol. 2020.05.27.120295 DOI: 10.1101/2020.05.27.120295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo S, Horiuchi Y, Hamada M, Takeuchi T, Umezawa H (1979) A new antitumor antibiotic, bactobolin produced by Pseudomonas. J Antibiot (Tokyo) 32:1069–1071. doi: 10.7164/antibiotics.32.1069 [DOI] [PubMed] [Google Scholar]
- 43.Kunakom S, Eustaquio AS (2019) Burkholderia as a source of natural products. J Nat Prod 82:2018–2037. doi: 10.1021/acs.jnatprod.8b01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunakom S, Eustáquio AS (2019) Burkholderia as a Source of Natural Products. Journal of Natural Products 82:2018–2037. doi: 10.1021/acs.jnatprod.8b01068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Guillouzer S, Groleau MC, Déziel E (2017) The complex quorum sensing circuitry of Burkholderia thailandensis is both hierarchically and homeostatically organized. mBio 8:e01861–01817. doi: 10.1128/mBio.01861-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Guillouzer S, Groleau MC, Mauffrey F, Déziel E (2020) ScmR, a global regulator of gene expression, quorum sensing, pH homeostasis, and virulence in Burkholderia thailandensis. Journal of bacteriology 202:e00776–00719. doi: 10.1128/JB.00776-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lépine F, Milot S, Déziel E, He J, Rahme LG (2004) Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa. J Am Soc Mass Spectrom 15:862–869. doi: 10.1016/j.jasms.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 48.Li A, Mao D, Yoshimura A, Rosen PC, Martin WL, Gallant E, Wuhr M, Seyedsayamdost MR (2020) Multi-Omic Analyses Provide Links between Low-Dose Antibiotic Treatment and Induction of Secondary Metabolism in Burkholderia thailandensis. mBio 11:e03210–03219. doi: 10.1128/mBio.03210-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li D, Oku N, Hasada A, Shimizu M, Igarashi Y (2018) Two new 2-alkylquinolones, inhibitory to the fish skin ulcer pathogen Tenacibaculum maritimum, produced by a rhizobacterium of the genus Burkholderia sp. Beilstein J Org Chem 14:1446–1451. doi: 10.3762/bjoc.14.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipscomb L, Schell MA (2011) Elucidation of the regulon and cis-acting regulatory element of HrpB, the AraC-type regulator of a plant pathogen-like type III secretion system in Burkholderia pseudomallei. Journal of bacteriology 193:1991–2001. doi: 10.1128/JB.01379-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Cheng YQ (2014) Genome-guided discovery of diverse natural products from Burkholderia sp. J Ind Microbiol Biotechnol 41:275–284. doi: 10.1007/s10295-013-1376-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahenthiralingam E, Song L, Sass A, White J, Wilmot C, Marchbank A, Boaisha O, Paine J, Knight D, Challis GL (2011) Enacyloxins are products of an unusual hybrid modular polyketide synthase encoded by a cryptic Burkholderia ambifaria Genomic Island. Chem Biol 18:665–677. doi: 10.1016/j.chembiol.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 53.Majerczyk C, Brittnacher M, Jacobs M, Armour CD, Radey M, Schneider E, Phattarasokul S, Bunt R, Greenberg EP (2014) Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulon. Journal of bacteriology 196:1412–1424. doi: 10.1128/JB.01405-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majerczyk CD, Brittnacher MJ, Jacobs MA, Armour CD, Radey MC, Bunt R, Hayden HS, Bydalek R, Greenberg EP (2014) Cross-species comparison of the Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei quorum-sensing regulons. Journal of bacteriology 196:3862–3871. doi: 10.1128/JB.01974-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mao D, Bushin LB, Moon K, Wu Y, Seyedsayamdost MR (2017) Discovery of scmR as a global regulator of secondary metabolism and virulence in Burkholderia thailandensis E264. Proceedings of the National Academy of Sciences of the United States of America 114:E2920–E2928. doi: 10.1073/pnas.1619529114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marenda M, Brito B, Callard D, Genin S, Barberis P, Boucher C, Arlat M (1998) PrhA controls a novel regulatory pathway required for the specific induction of Ralstonia solanacearum hrp genes in the presence of plant cells. Molecular microbiology 27:437–453 [DOI] [PubMed] [Google Scholar]
- 57.Martin HM, Hancock JT, Salisbury V, Harrison R (2004) Role of xanthine oxidoreductase as an antimicrobial agent. Infect Immun 72:4933–4939. doi: 10.1128/IAI.72.9.4933-4939.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazzola M, Cook RJ, Thomashow LS, Weller DM, Pierson LS 3rd (1992) Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Applied and environmental microbiology 58:2616–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moons P, Van Houdt R, Aertsen A, Vanoirbeek K, Engelborghs Y, Michiels CW (2006) Role of quorum sensing and antimicrobial component production by Serratia plymuthica in formation of biofilms, including mixed biofilms with Escherichia coli. Applied and environmental microbiology 72:7294–7300. doi: 10.1128/AEM.01708-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moons P, Van Houdt R, Aertsen A, Vanoirbeek K, Michiels CW (2005) Quorum sensing dependent production of antimicrobial component influences establishment of E. coli in dual species biofilms with Serratia plymuthica. Commun Agric Appl Biol Sci 70:195–198 [PubMed] [Google Scholar]
- 61.Mori T, Yamashita T, Furihata K, Nagai K, Suzuki K, Hayakawa Y, Shin-Ya K (2007) Burkholone, a new cytotoxic antibiotic against IGF-I dependent cells from Burkholderia sp. J Antibiot (Tokyo) 60:713–716. doi: 10.1038/ja.2007.92 [DOI] [PubMed] [Google Scholar]
- 62.Moule MG, Spink N, Willcocks S, Lim J, Guerra-Assuncao JA, Cia F, Champion OL, Senior NJ, Atkins HS, Clark T, Bancroft GJ, Cuccui J, Wren BW (2015) Characterization of new virulence factors involved in the intracellular growth and survival of Burkholderia pseudomallei. Infection and immunity 84:701–710. doi: 10.1128/IAI.01102-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nickzad A, Déziel E (2016) Adaptive significance of quorum sensing-dependent regulation of rhamnolipids by integration of growth rate in Burkholderia glumae: A trade-off between survival and efficiency. Front Microbiol 7:1215. doi: 10.3389/fmicb.2016.01215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okada BK, Wu Y, Mao D, Bushin LB, Seyedsayamdost MR (2016) Mapping the Trimethoprim-Induced Secondary Metabolome of Burkholderia thailandensis. ACS Chem Biol 11:2124–2130. doi: 10.1021/acschembio.6b00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park JD, Moon K, Miller C, Rose J, Xu F, Ebmeier CC, Jacobsen JR, Mao D, Old WM, DeShazer D, Seyedsayamdost MR (2020) Thailandenes, Cryptic Polyene Natural Products Isolated from Burkholderia thailandensis Using Phenotype-Guided Transposon Mutagenesis. ACS Chem Biol. doi: 10.1021/acschembio.9b00883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piochon M, Coulon PML, Caulet A, Groleau M-C, Déziel E, Gauthier C (2020) Synthesis and antimicrobial activity of Burkholderia-related 4-hydroxy-3-methyl-2-alkenylquinolones (HMAQs) and their N-oxide counterparts. J Nat Prod 83:2145–2154. doi: 10.1021/acs.jnatprod.0c00171 [DOI] [PubMed] [Google Scholar]
- 67.Reen FJ, McGlacken GP, O’Gara F (2018) The expanding horizon of alkyl quinolone signalling and communication in polycellular interactomes. FEMS Microbiol Lett 365. doi: 10.1093/femsle/fny076 [DOI] [PubMed] [Google Scholar]
- 68.Seyedsayamdost MR (2014) High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proceedings of the National Academy of Sciences of the United States of America 111:7266–7271. doi: 10.1073/pnas.1400019111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seyedsayamdost MR, Chandler JR, Blodgett JA, Lima PS, Duerkop BA, Oinuma K, Greenberg EP, Clardy J (2010) Quorum-sensing-regulated bactobolin production by Burkholderia thailandensis E264. Organic letters 12:716–719. doi: 10.1021/ol902751x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smalley NE, An D, Parsek MR, Chandler JR, Dandekar AA (2015) Quorum sensing protects Pseudomonas aeruginosa against cheating by other species in a laboratory coculture model. Journal of bacteriology 197:3154–3159. doi: 10.1128/JB.00482-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thapa SS, Grove A (2019) Do global regulators hold the key to production of bacterial secondary metabolites? Antibiotics (Basel) 8. doi: 10.3390/antibiotics8040160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trottmann F, Franke J, Richter I, Ishida K, Cyrulies M, Dahse HM, Regestein L, Hertweck C (2019) Cyclopropanol warhead in malleicyprol confers virulence of human- and animal-pathogenic Burkholderia species. Angewandte Chemie 58:14129–14133. doi: 10.1002/anie.201907324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trottmann F, Ishida K, Franke J, Stanisic A, Ishida-Ito M, Kries H, Pohnert G, Hertweck C (2020) Sulfonium acids loaded onto an unusual thiotemplate assembly line construct the cyclopropanol warhead of a Burkholderia virulence factor. Angewandte Chemie. doi: 10.1002/anie.202003958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Truong TT, Seyedsayamdost M, Greenberg EP, Chandler JR (2015) A Burkholderia thailandensis acyl-homoserine lactone-independent orphan LuxR homolog that activates production of the cytotoxin malleilactone. Journal of bacteriology 197:3456–3462. doi: 10.1128/JB.00425-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tuanyok A, Kim HS, Nierman WC, Yu Y, Dunbar J, Moore RA, Baker P, Tom M, Ling JM, Woods DE (2005) Genome-wide expression analysis of iron regulation in Burkholderia pseudomallei and Burkholderia mallei using DNA microarrays. FEMS microbiology letters 252:327–335. doi: 10.1016/j.femsle.2005.09.043 [DOI] [PubMed] [Google Scholar]
- 76.Van Ark G, Berden JA (1977) Binding of HQNO to beef-heart sub-mitochondrial particles. Biochimica et biophysica acta 459:119–127. doi: 10.1016/0005-2728(77)90014-7 [DOI] [PubMed] [Google Scholar]
- 77.Vial L, Lépine F, Milot S, Groleau MC, Dekimpe V, Woods DE, Déziel E (2008) Burkholderia pseudomallei, B. thailandensis, and B. ambifaria produce 4-hydroxy-2-alkylquinoline analogues with a methyl group at the 3 position that is required for quorum-sensing regulation. Journal of bacteriology 190:5339–5352. doi: 10.1128/JB.00400-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waters CM, Bassler BL (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001 [DOI] [PubMed] [Google Scholar]
- 79.Wu Y, Seyedsayamdost MR (2017) Synergy and target promiscuity drive structural divergence in bacterial alkylquinolone biosynthesis. Cell Chem Biol 24:1437–1444 e1433. doi: 10.1016/j.chembiol.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xavier JB, Kim W, Foster KR (2011) A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Molecular microbiology 79:166–179. doi: 10.1111/j.1365-2958.2010.07436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao G, Déziel E, He J, Lépine F, Lesic B, Castonguay MH, Milot S, Tampakaki AP, Stachel SE, Rahme LG (2006) MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Molecular microbiology 62:1689–1699. doi: 10.1111/j.1365-2958.2006.05462.x [DOI] [PubMed] [Google Scholar]