Abstract
Double unit cord blood (dCB) transplantation (dCBT) is associated with high engraftment rates but delayed myeloid recovery. We investigated adding haplo-identical CD34+ cells to dCB grafts to facilitate early haplo-identical donor-derived neutrophil recovery (optimal bridging) prior to CB engraftment. Seventy-eight adults underwent myeloablation with cyclosporine-A/mycophenolate mofetil immunoprophylaxis (no anti-thymocyte globulin, ATG). CB units (median CD34+ dose 1.1 × 105/kg/unit) had a median 5/8 unit-recipient HLA-match. Haplo-identical grafts had a median CD34+ dose of 5.2 × 106/kg. Of 77 evaluable patients, 75 had sustained CB engraftment that was mediated by a dominant unit and heralded by dominant unit-derived T-cells. Optimal haplo-identical donor-derived myeloid bridging was observed in 34/77 (44%) patients (median recovery 12 days). Other engrafting patients had transient bridging with second nadir preceding CB engraftment [20/77 (26%), median first recovery 12 and second 26.5 days] or no bridge [21/77 (27%), median recovery 25 days]. The 2 (3%) remaining patients had graft failure. Higher haplo-CD34+ dose and better dominant unit-haplo-CD34+ HLA-match significantly improved the likelihood of optimal bridging. Optimally bridged patients were discharged earlier [median 28 versus 36 days]. ATG-free haplo-dCBT can speed neutrophil recovery but successful bridging is not guaranteed due to rapid haplo-identical graft rejection.
Keywords: Cord blood transplantation, haplo-identical transplantation, engraftment, chimerism, anti-thymocyte globulin
INTRODUCTION
Double unit cord blood (CB) transplantation (dCBT) is efficacious for adults with high-risk hematologic malignancies and has been associated with comparable progression-free survival to that of unrelated donor transplantation in multiple series(1–3). While we and others have demonstrated high rates of sustained donor engraftment after dCBT(4–7), delayed count recovery is common. For example, myeloablated dCBT recipients at our center engraft at a median of 24 days(5). Slow engraftment can increase morbidity, prolong hospitalization, and increase costs. A novel approach to abrogate prolonged cytopenia pioneered by Fernandez et al(8–11) and others(12–14), and an alternative to ex vivo expansion, is the combination of a CB graft with peripheral blood-derived haplo-identical or third-party donor CD34+ cells. This strategy aims to facilitate early haplo-identical (or third-party) donor-derived neutrophil recovery (myeloid bridging) until CB engraftment is achieved. This platform, however, is not standardized and the efficacy of myeloid bridging in the absence of anti-thymocyte globulin (ATG) is unknown.
To address this question, we have investigated adding haplo-identical CD34+ cells (haplo-CD34+) to dCB grafts (haplo-dCB) in patients transplanted with myeloablative conditioning and no ATG. Herein, we report the kinetics of engraftment after these serotherapy-free haplo-dCB transplants (haplo-dCBT). Adult patients received standard immunoprophylaxis with cyclosporine-A (CSA) and mycophenolate mofetil (MMF). ATG was not used due to its adverse impact on immune reconstitution(15–21) and the substantial evidence of increased mortality in ATG-based CBT(19, 20, 22–27). Double unit CB grafts were used to enhance safety given transplantation of CB combined with haplo-CD34+ cells has not previously been investigated in an ATG-free setting. Additionally, use of dCB grafts permits comparison of engraftment with historical dCBT controls transplanted with identical conditioning and immunosuppression but without haplo-CD34+ cells. Our primary aim was to determine the speed and success of sustained neutrophil recovery after haplo-dCBT. Our hypothesis was that the addition of a haplo-CD34+ graft would provide a haplo-identical donor-derived myeloid bridge prior to sustained CB-derived engraftment.
METHODS
Patients
Patients were treated on a phase II trial (clinicaltrials.gov NCT01682226) between September 2012 and December 2017. The trial was conducted in accordance with the Declaration of Helsinki and was approved by the Memorial Sloan Kettering Cancer Center Institutional Review/ Privacy Board. This trial enrolled pediatric and adult patients with high-risk hematologic malignancies without a suitable HLA-matched related or unrelated donor, who had a suitable CB graft and a suitable haplo-identical donor. For the purposes of this analysis, only adult haplo-dCBT recipients were included to permit comparison with the engraftment kinetics of historic adult dCBT controls. Additionally, two patients who underwent identical haplo-dCBT under Single-Patient Use were included (1 severe aplastic anemia, 1 whose insurance denied clinical trial participation). All patients were assayed for HLA-antibodies as previously described(28). Antibody titers with mean fluorescence intensity > 1000 were considered positive.
CB Graft Selection
Unit selection was based on unit quality/ bank of origin, total nucleated cell (TNC) dose and donor-recipient human leukocyte antigen (HLA)-match. Units contained a minimum cryopreserved TNC dose of 1.5 × 107/kg and were ≥ 4/6 HLA-A, -B antigen, -DRB1 allele matched to the recipient. Cryopreserved CD34+ cell dose and 8-allele HLA-match were also considered in CB graft selection(5, 29, 30). The presence of donor-specific HLA antibodies (DSA) against one or both CB units was not a contraindication to unit selection(28). The HLA-match of the units to each other or the haplo-identical donor was not considered.
Haplo-identical Donor Selection and Collection
Haplo-identical grafts were derived from mobilized peripheral blood; bone marrow harvests were not permitted even in the setting of poor mobilization. Younger adult donors were given priority with emphasis upon availability, compliance, avoidance of a large donor-recipient weight discrepancy, and adequacy of peripheral access. Donors against whom the recipient had DSA were avoided in the latter phase of the trial.
Donors were mobilized with 10 mcg/kg of granulocyte colony stimulating factor (G-CSF) rounded to vial size subcutaneously daily for 5 days. Initially only one collection was performed. The study was later amended to allow a second leukapheresis if the first yielded < 3 × 106/kg CD34+ cells (before CD34+ selection). Grafts were CD34+ cell-selected using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany) under an Investigational New Device from the U.S. Food and Drug Administration. To guard against permanent haplo-identical donor engraftment, the goal for the maximum haplo-graft CD3+ cell dose was 8 × 103/kg. Initially the haplo-CD34+ cell dose was capped at 3 × 106/kg. Subsequently, the target CD34+ cell dose was increased to approximately 5 × 106/kg without an upper limit.
Conditioning Regimens, Immunoprophylaxis and Growth Factor Support
Patients received myeloablative conditioning(4, 31). The intensity was based on diagnosis, disease status, age and hematopoietic cell transplant co-morbidity index (HCT-CI)(32) score. High dose conditioning [cyclophosphamide (Cy) 120 mg/kg, fludarabine (Flu) 75 mg/m2 and total body irradiation (TBI) 1375 cGy (Cy 120/ Flu 75/ TBI 1375)] was considered for fit patients < 30 years with hematologic malignancies. Remaining patients received intermediate intensity conditioning [Cy 50 mg/kg, Flu 150 mg/m2, thiotepa (Thio) 10 mg/kg, TBI 400 cGy (Cy 50/ Flu 150/ Thio 10/ TBI 400)] with a reduced thiotepa dose (5mg/kg) in patients 60–70 years or those with HCT-CI score ≥ 5.
CSA and MMF (15 mg/kg every 8 hours) were started intravenously on day −3 for graft-versus-host disease (GVHD) prophylaxis. No patient received ATG. All patients received G-CSF 5 mcg/kg/day from day 7 post-transplant until neutrophil recovery. In patients with a second neutrophil nadir, G-CSF was resumed until sustained engraftment was achieved.
Engraftment Monitoring and Definitions
A white cell count (WCC) differential was obtained once the WCC was > 0.5 × 109/L. Neutrophil recovery was defined as the first of three consecutive days of neutrophils ≥ 0.5 × 109/L. Platelet recovery was the first day of ≥ 20 × 109/L platelets without transfusion for 7 consecutive days. Graft failure was defined as requirement for a second stem cell infusion or death without neutrophil recovery on day 28 or later.
Haplo-CD34+ and CB donor chimerism was monitored using PCR-amplification of informative recipient and donor short tandem repeats. Whole blood assays were done on days 14, 28, 60, 100, 180 and 365 post-transplant. White cell subset chimerism analyses were performed in sorted myeloid, T-, B- and NK-cell subsets (purity ≥ 95%) on days 28, 100 and 365 post-transplant. Analysis of lineage-specific chimerism was foregone if the purity threshold was not achieved or if the specific cell subset count was too low. Of the two CB units infused, the dominant (or engrafting) CB unit was the only one detected or the one with sustained > 50% contribution to CB-derived chimerism.
Statistical Methods
Our objective was to determine the speed and success of sustained myeloid recovery after haplo-dCBT. Success was arbitrarily defined as neutrophil recovery by 2 weeks post-transplant (prior to or on day 14). Cumulative incidences of neutrophil and platelet recovery were estimated considering early death as a competing risk. Chimerism was analyzed using summary statistics and box-and-whisker diagrams. Correlation of haplo-CD34+ and CB cell doses with days to neutrophil recovery was evaluated using Spearman’s rank correlation coefficient. Cell doses of dominant and non-dominant CB units were compared using the Wilcoxon signed-rank test. Univariate and multivariate logistic regression analyses were performed in patients who achieved sustained CB engraftment to evaluate factors associated with higher odds of successful haplo-CD34+ myeloid bridging. All variables with p < 0.10 in univariate analysis were included in the multivariate model. Transplant-related mortality (TRM) was compared across WCC recovery groups using Gray’s test in a day 28 landmark analysis considering relapse as a competing risk. Results with two-tailed p-values < 0.05 were considered significant. All analyses were conducted using R statistical software, version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient and Graft Characteristics
Seventy-eight patients [median age 48 years (range 21–68), median weight 82 kg (range 48–138)] underwent haplo-dCBT. Thirty-seven patients (47%) were male and 44 (56%) were CMV seropositive. Diagnoses included 54 (69%) acute leukemias, 10 (13%) myelodysplasia/ myeloproliferative diseases, 13 (17%) lymphomas and 1 aplastic anemia. Three patients were second allograft recipients. Conditioning was high-dose (Cy 120/ Flu 75/ TBI 1375, n = 1) or intermediate intensity [Cy 50/ Flu 150/ Thio 10/ TBI 400 (n = 64), Cy 50/ Flu 150/ Thio 5/ TBI 400 (n = 13)].
Infused CB unit (n = 156) and haplo-CD34+ (n = 78) graft characteristics are shown in Table 1. CB units had a median infused TNC dose of 2.3 (range 1.0–5.7) × 107/kg/unit and median infused viable CD34+ cell dose of 1.1 (range 0.1–3.1) × 105/kg/unit. The median infused viable CD3+ cell dose was 2.9 (range 0.3–8.0) × 106/unit. The majority of units were 4/6 HLA-A, -B antigen, -DRB1 allele matched to the patient, and the median CB unit-recipient HLA-allele match was 5/8 (range 2–7/8). Seven patients (9%) had DSA against their CB graft.
Table 1.
Infused CB and haplo-identical graft characteristics.
| Baseline Graft Characteristics | Value |
|---|---|
| Cord Blood Units (n = 156 infused units) | |
| Infused TNC dose × 107/kg/unit, median (range) | 2.3 (1.0–5.7) |
| Infused viable CD34+ dose × 105/kg/unit, median (range) | 1.1 (0.1–3.1) |
| Infused viable CD3+ dose × 106/kg/unit, median (range) | 2.9 (0.3–8.0) |
| 6/6 | 3 (2%) |
| 6–7/8 | 29 (19%) |
| Haplo-CD34+ Grafts (n = 78) | |
| Extended family | 8 (10%) |
| Cryopreserved, N (%) | 12 (15%) |
| Infused CD34+ dose × 106/kg, median (range) | 5.2 (1.1–16.8) |
| Infused CD3+ dose × 103/kg, median (range) | 1.6 (0.3–13.7)* |
| 7/8 | 1 (1%) |
A single haplo-identical graft had a CD3+ cell dose above the 8 × 103/kg cap due to inadequate purity of CD34+ selection. To avoid compromising the CD34+ dose, further T-cell depletion was not performed.
Haplo-CD34+ grafts were most commonly procured from children (46%) or siblings (31%). Haplo-identical donors had a median age of 33 years (range 15–71). The median infused CD34+ dose was 5.2 (range 1.1–16.8) × 106/kg. The median infused CD3+ cell dose was 1.6 (range 0.3–13.7) × 103/kg and approximately 3 logs lower than that of the CB units. The majority of haplo-CD34+ grafts (n = 61, 78%) were 4/8 HLA-allele matched to the patient. Eleven patients (14%) had DSA against their haplo-identical graft.
Overall Hematopoietic Engraftment
Of the 78 analyzed patients, 75 engrafted, 2 had graft failure, and one heavily pre-treated patient died on day 14 from veno-occlusive disease and multi-organ failure. The cumulative incidence of sustained neutrophil recovery for the entire cohort was 96% (95%CI: 87–99). The day 100 cumulative incidence of platelet recovery was 87% (95%CI: 77–93).
In the 75 engrafting patients, sustained engraftment was mediated by a dominant CB unit. The dominant units had a median infused viable CD34+ cell dose of 1.23 × 105/kg (range 0.24–2.95) and a median infused viable CD3+ cell dose of 1.02 × 106/kg (range 0.14–3.4). The median HLA-match of the dominant CB unit to the recipient and to the haplo-identical graft were 5/8 (range 3–7/8) and 3/8 (range 1–7/8), respectively. The pattern of whole blood chimerism is shown in Figure 1. Overall, haplo-identical grafts predominated early post-transplant [median day 14 chimerism 88% (range 0–100)]. However, no haplo-identical graft was detected in 51% of evaluable patients at day 28, in 61% at day 60, and in 78% at day 100 post-transplant. Additionally, the haplo-identical graft comprised only a minor component of donor chimerism in nearly all remaining patients. Concurrently, the dominant CB unit whole blood chimerism increased from a median of 10% (range 0–100) at day 14 to 91% (range 0–100) at day 28, 100% (range 0–100) at day 60, and 100% (range 12–100) at day 100 and beyond. White cell subset chimerism analysis revealed that loss of the haplo-identical graft was associated with early dominant CB unit chimerism in the T-cell fraction.
Figure 1: Pattern of whole blood chimerism in engrafting patients in the first year after dCBT combined with haplo-identical CD34+ cells (n = 75).
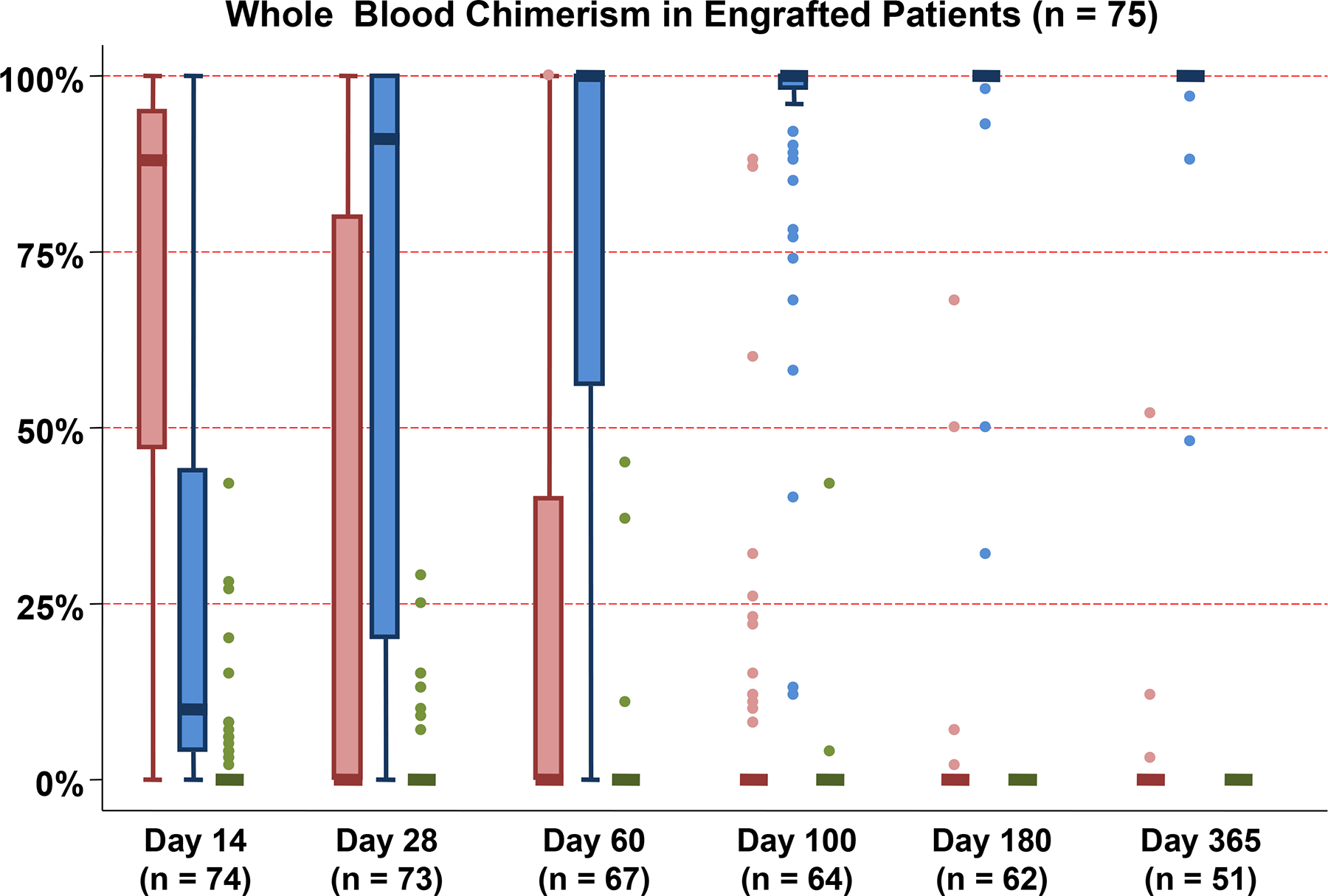
The contribution of the haplo-identical donor (Red), the dominant (engrafting) CB unit (Blue) and the non-dominant (non-engrafting) unit (Green) to whole blood chimerism at each time point post-transplant is shown. While the haplo-identical donors contributed to initial hematopoiesis, one CB unit predominated by day 28 in the majority of patients with the median donor chimerism being 100% the dominant CB unit by day 100 and beyond.
At a median survivor follow-up of 3 years, 9 months (range 1–6 years) all evaluable patients maintain engraftment with a dominant CB unit.
Patterns of Hematopoietic Recovery
While 75 of 77 evaluable patients had sustained CB engraftment that was mediated by a dominant CB unit, the success of obtaining an early haplo-derived myeloid bridge was variable between patients. Three distinct engraftment patterns were observed (Table 2, Figures 2A–D) and were characterized by distinct chimerism patterns associated with the speed of rejection of the haplo-CD34+ graft by the dominant CB unit (Figures 3–5).
Table 2:
Engraftment patterns in haplo-dCBT recipients (n = 77 evaluable patients).
| Engraftment Group | Engraftment Median (range) |
|---|---|
| Group 1: CB engraftment with optimal (early and sustained) haplo-derived myeloid bridge (n = 34, 44%) | Neutrophils: 12 days (10–14) Platelets: 19 days (14–41) in 32 patients |
| Group 2: CB engraftment with transient haplo-derived myeloid bridge and second nadir (n = 20, 26%) | Neutrophils: 12 (11–18) and 26.5 days (20–46) [duration of 2nd nadir: 8.5 days (4–19)] Platelets: 43.5 days (range 14–67) in 20 patients |
| Group 3: CB engraftment with no haplo-derived myeloid bridge (n = 21, 27%) | Neutrophils: 25 days (15–33) Platelets: 45.5 days (range 37–62) in 16 patients |
| Group 4: CB graft failure (n = 2, 3%) | Patient 1: Failure of both haplo-identical donor and CB engraftment in the presence of DSA**. Patient 2: Transient haplo-CD34+ derived myeloid bridge (day 12), followed by CB graft failure in the setting of low infused viable CD34+ cell dose of both CB units. |
1 patient not evaluable for engraftment due to early TRM on day 14.
Of the 7 patients with DSA against the CB graft, one patient had graft failure. The remaining 6 patients had sustained CB engraftment (4 Group 1, 2 Group 3).
Figure 2: Probability of neutrophil recovery after haplo-dCBT by engraftment group (Groups 1–3, n = 75).
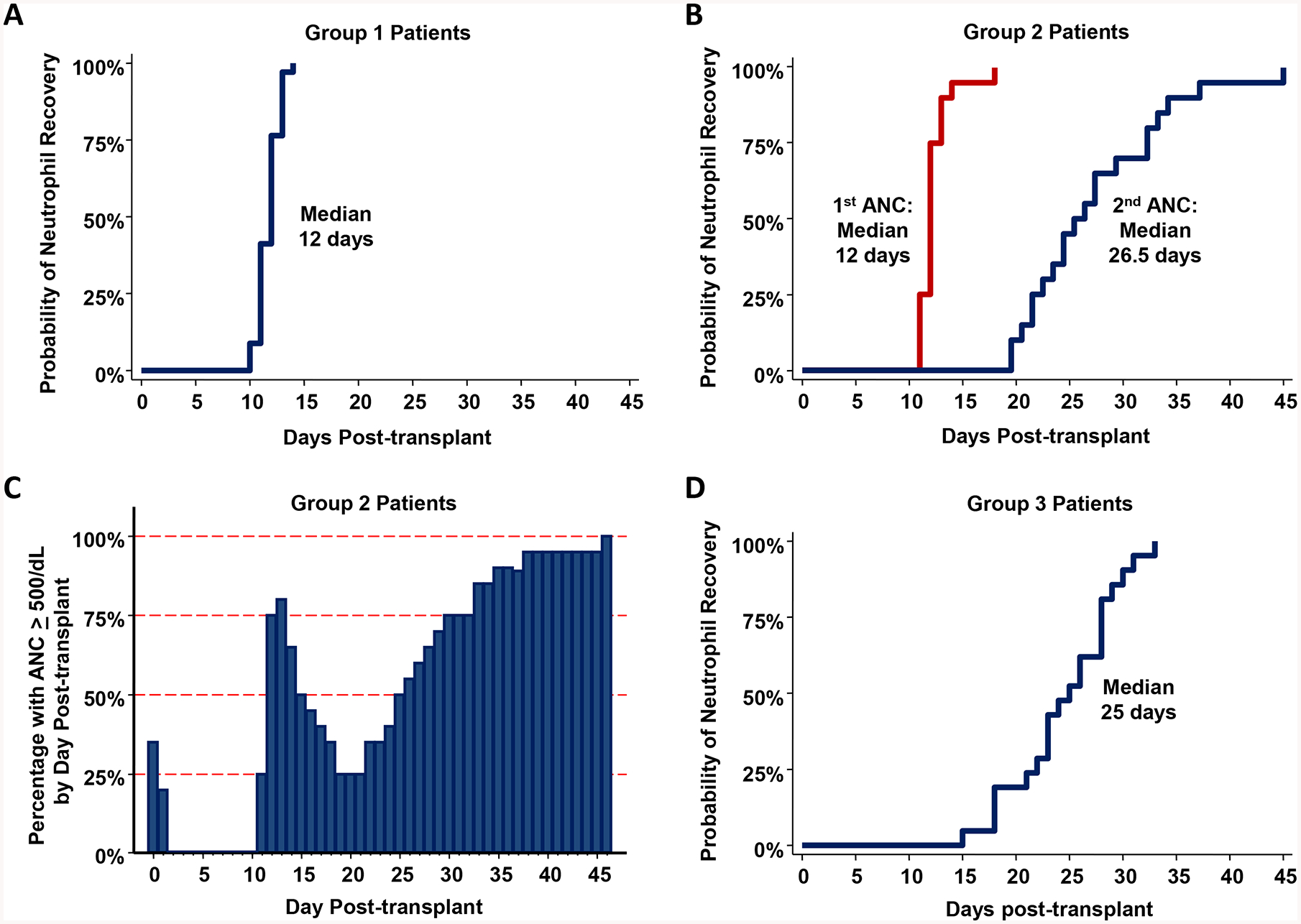
Figure 2A: Group 1 patients (n = 34) had early sustained myeloid recovery by day 14 post-transplant (median 12 days).
Figure 2B: Group 2 patients (n = 20) had transient myeloid recovery (median neutrophil recovery 12 days) followed by a second nadir preceding sustained engraftment (median second neutrophil recovery 26.5 days).
Figure 2C: Percentage of Group 2 patients with a neutrophil count ≥ 500/dL per post-transplant day.
Figure 2D: Group 3 patients (n = 21) had delayed myeloid recovery (median 25 days).
Figure 3. Chimerism patterns after haplo-dCBT in patients with an early myeloid bridge (Group 1, n = 34).
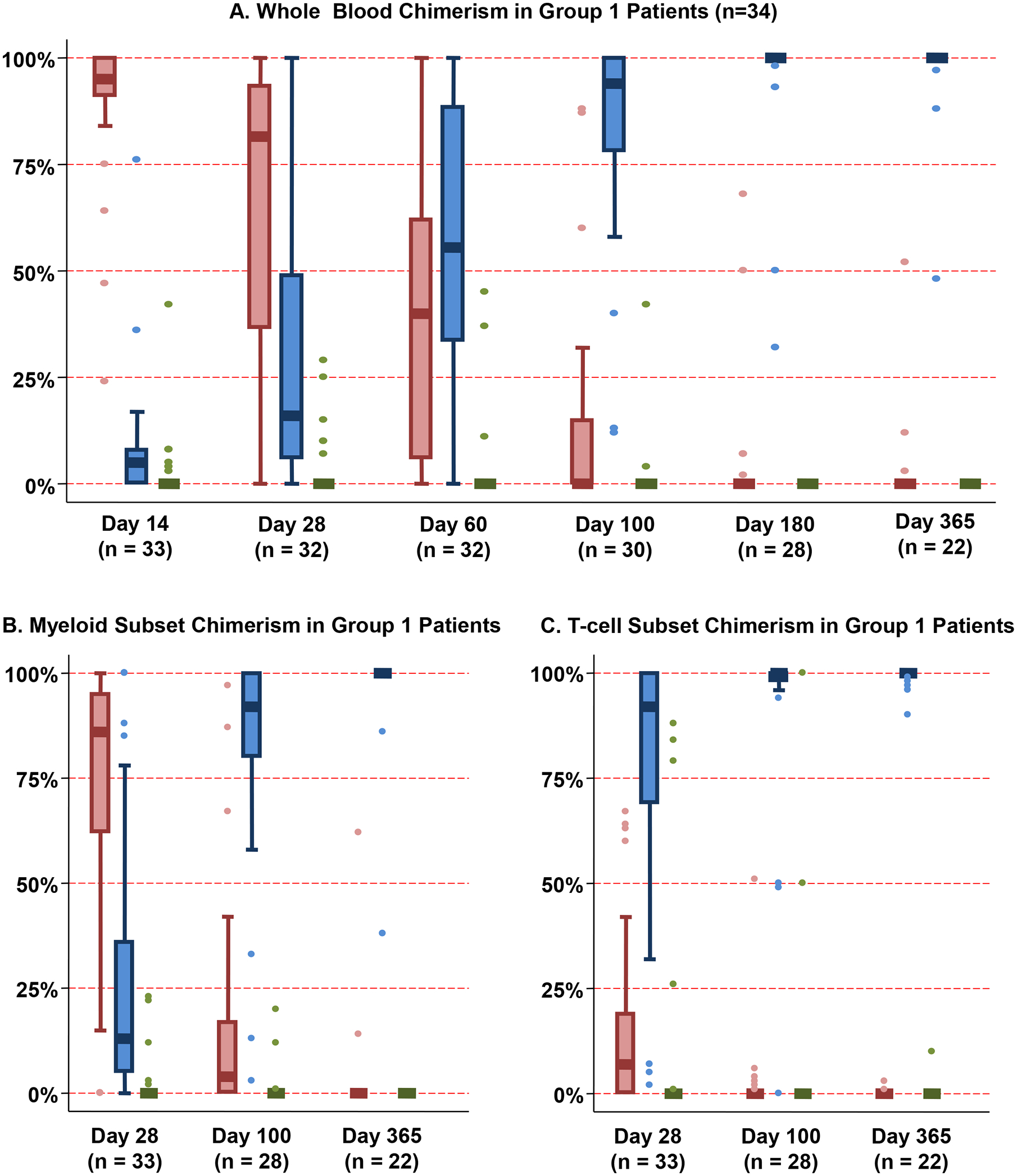
Whole blood chimerism analysis (Figure 3A) revealed predominant engraftment of the haplo-identical donor early post-transplant (median day 14 chimerism 95%, range 24–100) with subsequent increasing dominant CB unit chimerism. As of day 28 post-transplant, the majority of myeloid cells (Figure 3B) were derived from the haplo-identical donor, whereas T-cells (Figure 3C) were primarily derived from the dominant CB unit, with progressive increase in dominant CB unit-derived chimerism in all lineages thereafter.
*Footnote: Red: Haplo-identical donor; Blue: Dominant (engrafting) CB unit; Green: Non-dominant (non-engrafting) unit
Figure 5. Chimerism patterns after haplo-dCBT in patients with no myeloid bridge and sustained CB engraftment (Group 3, n = 21).
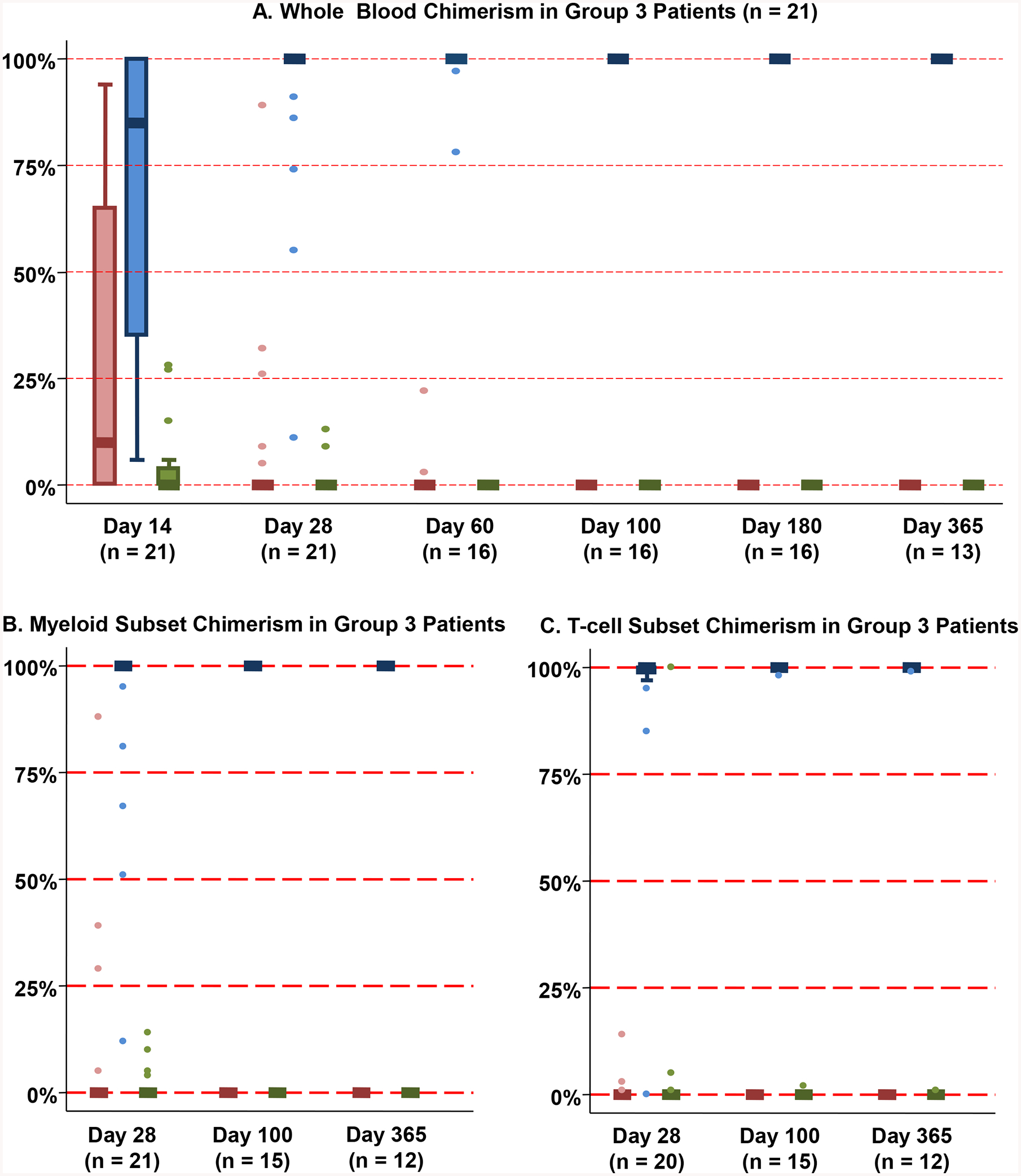
Whole blood chimerism analysis (Figure 5A) revealed that the majority of Group 3 patients had either no or minimal haplo-identical donor engraftment (day 14 median chimerism 10%, range 0–94%) and the dominant CB unit pre-dominated thereafter. The dominant CB unit was either the only or greatest contributor to myeloid (Figure 5B) and T-cell (Figure 5C) lineages at day 28 and beyond.
*Footnote: Red: Haplo-identical donor; Blue: Dominant (engrafting) CB unit; Green: Non-dominant (non-engrafting) unit
Group 1 patients (34/77, 44%) had early sustained myeloid recovery at a median of 12 days (range 10–14) post-transplant (Figure 2A). This rapid recovery was almost always mediated by the haplo-identical graft with subsequent transition to CB-derived hematopoiesis. These patients had a high median day 14 haplo-identical graft whole blood chimerism of 95% (range 24–100) (Figure 3A). Subsequently, they had increasing dominant CB unit chimerism. By day 180, the haplo-identical graft was detected in only a minority (5/28, 18%) of patients (median haplo-identical donor chimerism 7%, range 2–68). By 1 year, haplo-identical cells were only detected in 3 patients (contributions 3%, 12% and 52%) with 2 having since converted to 100% dominant CB unit. In white cell subset analyses, the majority of myeloid cells were derived from the haplo-identical donor at day 28 (Figure 3B). In contrast, T-cells were derived from the dominant CB unit (Figure 3C) with a progressive increase in dominant CB unit-derived chimerism in all lineages thereafter. In Group 1 patients, a higher infused haplo-CD34+ dose was associated with faster neutrophil recovery (r = −0.74, p < 0.001). Platelet recovery was also enhanced in these patients (Table 2).
Group 2 patients (20/77, 26%) had initial myeloid recovery (defined as a neutrophil count ≥ 0.5 k/mcL for ≥ 1 day) followed by a second nadir (< 0.5 k/mcL for ≥ 2 consecutive days) preceding sustained engraftment (Figure 2B–C). The median times to first and second neutrophil recoveries were 12 days (range 11–18) and 26.5 days (range 20–46), respectively. Short-lived haplo-CD34+ engraftment accounted for the transient myeloid bridge [median day 14 haplo-identical donor chimerism 82% (range 0–100)], although in 2 patients the haplo-identical donor was undetectable by 2 weeks post-transplant (Figure 4A). This was followed by sustained CB-derived hematopoiesis (median whole blood chimerism 100% dominant CB unit by day 60 and beyond). Additionally, myeloid and T-cell lineages were primarily derived from the dominant CB unit as early as day 28 (Figures 4B–C). Notably, a higher dominant CB unit infused viable CD3+ dose correlated with a slower time to the first (haplo-derived) neutrophil recovery (r = 0.43, p = 0.058) whereas a higher dominant CB unit infused viable CD34+ cell dose correlated with a faster time to the second (CB-derived) neutrophil recovery (r = −0.44, p = 0.052), although statistical significance at the 0.05 level was not reached.
Figure 4. Chimerism patterns after haplo-dCBT in patients with a transient myeloid bridge (Group 2, n = 20).
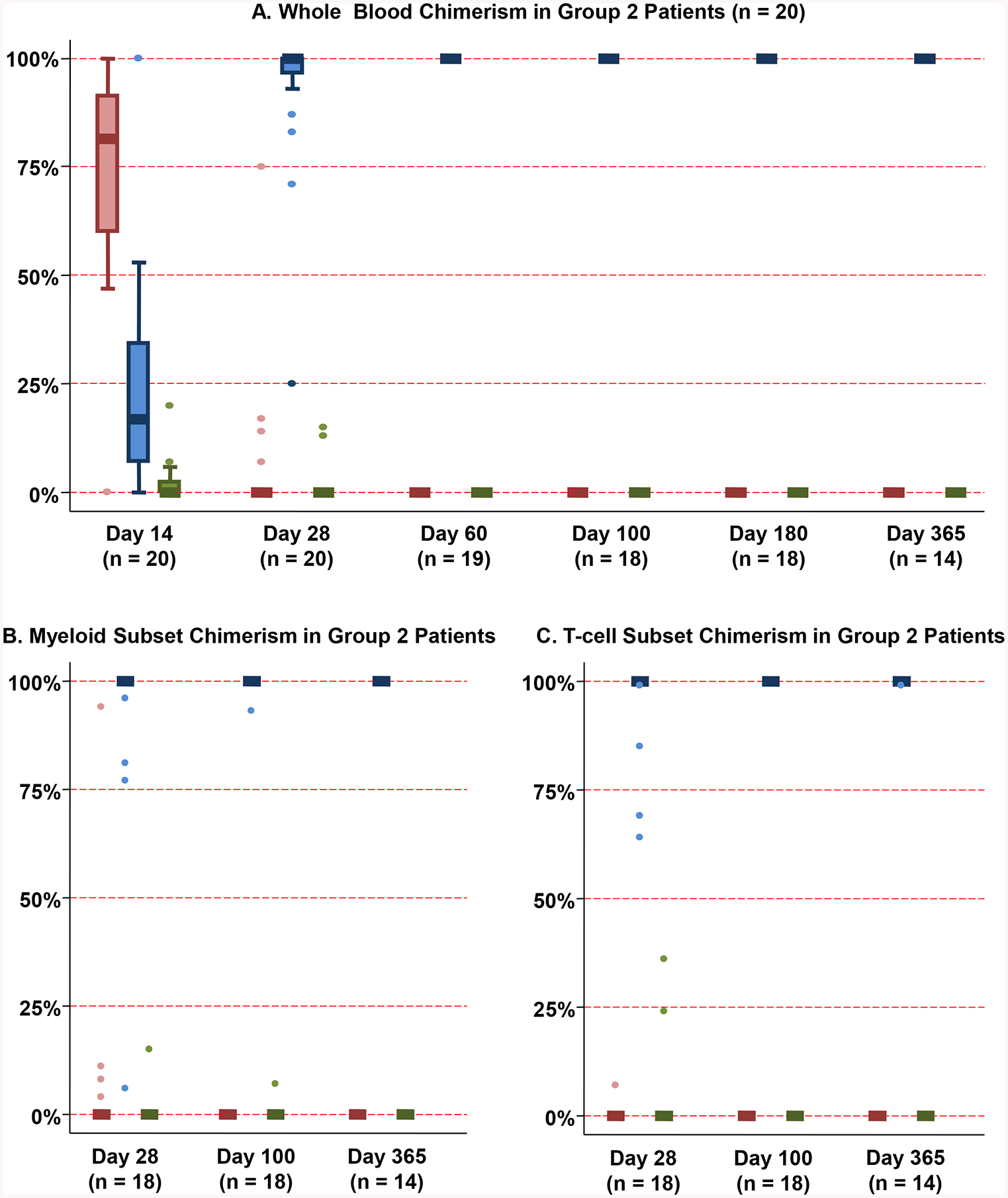
Whole blood chimerism analysis (Figure 4A) revealed short-lived haplo-identical donor engraftment (median day 14 haplo-identical donor chimerism 82%, range 0–100%) followed by sustained CB-derived hematopoiesis. Both myeloid (Figure 4B) and T-cell (Figure 4C) lineages were primarily derived from the dominant CB unit as early as day 28.
*Footnote: Red: Haplo-identical donor; Blue: Dominant (engrafting) CB unit; Green: Non-dominant (non-engrafting) unit
Group 3 patients (21/77, 27%) had delayed neutrophil recovery (median 25 days, range 15–33) (Figure 2D). At day 14 post-transplant, the majority had either no or minimal haplo-identical donor chimerism [median 10% (range 0–94)] in whole blood (Figure 5A), and no haplo-identical donor was ever detected in 9 patients. Additionally, 7 patients had no myeloid bridge despite a day 14 haplo-identical donor contribution > 50%. By day 60, all but two patients were 100% donor with the dominant CB unit, and this unit accounted for hematopoiesis in all patients subsequently. Moreover, in subset analysis, the dominant CB unit was either the only or greatest contributor to all lineages as of day 28 and beyond (Figure 5B–C). In Group 3 patients, a higher infused viable CD34+ cell dose of the dominant CB unit was associated with faster neutrophil recovery, although this correlation was not significant at the 0.05 level (r = −0.42, p = 0.06).
The 2 remaining evaluable patients (Group 4, 3%) had graft failure (Table 2). One, a prior allograft recipient, with DSA against the haplo-identical donor and both CB units had failure of haplo-CD34+ and CB engraftment. The second had transient haplo-identical donor-derived neutrophil recovery followed by failure of CB engraftment. CB graft failure was likely due to the very low infused viable CD34+ cell doses of each unit (0.22 × 105/kg and 0.35 × 105/kg, respectively). Both patients were successfully re-transplanted with single CB units.
Factors Associated with an Optimal Myeloid Bridge
Next, we investigated the association between graft characteristics and the likelihood of achieving an optimal myeloid bridge (Table 3). This was defined as early, haplo-identical donor-derived, sustained neutrophil recovery by 2 weeks post-transplant (i.e. without a second nadir) prior to CB engraftment. In multivariate analysis, a higher haplo-CD34+ dose [OR: 1.2 (95%CI: 1.01–1.47), p = 0.047] significantly improved the odds of achieving an optimal myeloid bridge. While there was no haplo-identical CD34+ dose threshold that could guarantee optimal bridging, none of the 8 patients who received a haplo-CD34+ cell dose < 3 × 106/kg had a bridge. Furthermore, a ≥ 3/8 HLA-match of the dominant CB unit to the haplo-identical donor was also associated with higher odds of optimal bridging [OR: 3.49 (95%CI: 1.27–10.42), p = 0.019].
Table 3.
Factors associated with an optimal haplo-identical donor-derived myeloid bridge after haplo-dCBT (n=75)*.
| Variables | N | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| Haplo-CD34+ characteristics | ||||||
| Cryopreserved haplo-CD34+ graft | Yes No |
11 64 |
reference 1.54 (0.42–6.38) |
0.52 | - | |
| CD34+ cell dose/kg | continuous | - | 1.17 (1–1.38) | 0.059 | 1.20 (1.01–1.47) | 0.047 |
| CD3+ cell dose/kg# | continuous | - | 1.28 (0.68–2.43) | 0.445 | - | |
| Haplo-recipient 8-allele HLA-match | 4/8 5–7/8 |
59 16 |
reference 3.4 (1.1–12.1) |
0.04 | reference 3.11 (0.91–11.92) |
0.079 |
| DSA against haplo-identical donor | No Yes |
65 10 |
reference 3.28 (0.83–16.31) |
0.11 | ||
| Dominant CB unit characteristics | ||||||
| TNC dose/kg | continuous | - | 1.02 (0.51–2.02) |
0.953 | - | |
| CD34+ cell dose/kg | continuous | - | 1.0 (0.5–2.2) | 0.967 | - | |
| CD3+ cell dose/kg | continuous | - | 1.1 (0.8–1.5) | 0.677 | - | |
| Unit-recipient 8-allele HLA-match | < 5/8 ≥ 5/8 |
28 47 |
reference 0.74 (0.3–1.9) |
0.531 | - | |
| Unit-haplo 8-allele HLA-match | < 3/8 ≥ 3/8 |
35 40 |
reference 2.95 (1.16–7.84) |
0.026 | reference 3.49 (1.27–10.42) |
0.019 |
| Non-dominant CB unit characteristics | ||||||
| TNC dose/kg | continuous | - | 1.18 (0.69–2.06) | 0.546 | - | |
| CD34+ cell dose/kg | continuous | - | 0.78 (0.37–1.55) | 0.482 | - | |
| CD3+ cell dose/kg | continuous | - | 0.98 (0.68–1.42) | 0.931 | - | |
|
Unit-recipient 8-allele HLA-match |
< 5/8 ≥ 5/8 |
30 45 |
reference 0.91 (0.36–2.32) |
0.85 | - | |
|
Unit-haplo 8-allele HLA-match |
< 3/8 ≥ 3/8 |
35 40 |
reference 0.78 (0.31–1.95) |
0.598 | - | |
Success of optimal myeloid bridge was evaluated only in patients who achieved sustained CB engraftment (n = 75)
log-transformed due to skewness
A ≥ 5/8 HLA-match of the haplo-identical donor to the recipient was associated with optimal myeloid bridging in the univariate but not the multivariate model. Presence of DSA against the haplo-identical graft had no impact. Cell dose and HLA-match of the non-dominant CB unit were also not associated with the likelihood of optimal bridging.
Association of Optimal Myeloid Bridging with Duration of Hospitalization and Day 100 TRM
Of 70 engrafted patients discharged from their initial hospitalization, Group 1 patients with sustained myeloid bridge were discharged earlier [median 28 days (range 20–60)] than the Group 2–3 patients with transient or no bridging [median 36 days (range 28–98)]. However, the day 100 TRM in Group 1 patients was not different than that of Group 2–3 patients [9% (95%CI: 2–21) vs. 15% (95%CI: 6–27), p = 0.388]. Additionally, optimal bridging in Group 1 patients was not associated with improved immune recovery (Supplemental Figure 1).
Determinants of CB Unit Dominance
CB unit dominance was associated with a higher infused viable CD3+ cell dose [median dose 3.3 (range: 0.9 – 8.0) versus median 2.6 (range: 0.3 – 6.0) × 106/kg, p < 0.001]. In contrast, CB infused TNC dose, infused viable CD34+ cell dose and 8-allele CB unit-recipient HLA-match were not significant (data not shown).
DISCUSSION
The addition of haplo-identical CD34+ cells to CB grafts to enhance myeloid recovery by providing an early myeloid bridge has been investigated as a potential alternative to ex vivo expansion. This approach has the advantages that most patients have a suitable haplo-identical donor, CD34+ cell selection is feasible in many centers, and complex CB graft manipulation is not required. Multiple groups have demonstrated that neutrophil recovery is enhanced in most recipients of ATG-based haplo-CBT(8, 12, 14). Many unanswered questions remain, however, as the influence of the conditioning, immunosuppression and ATG omission on the likelihood of haplo-identical myeloid bridging has not been investigated.
We report for the first time the efficacy of the addition of haplo-identical CD34+ cells to CB grafts as a strategy to enhance myeloid recovery in a serotherapy-free platform. Investigation of this approach in the absence of ATG is critical given it is well established that use of ATG in adult CBT is associated with increased mortality(15, 23–27). We acknowledge that the cost of a dCB graft supplemented with haplo-identical CD34+ cells would be prohibitive for most centers. However, in this single center trial, a dCB graft was used to ensure patient safety in case the haplo-identical graft was rejected but a single unit was inadequate to facilitate sustained hematopoiesis. Additionally, given only a single variable was changed (the addition of haplo-CD34+ cells), this approach enables direct comparison with our dCBT only controls who have had a median neutrophil recovery of 24 days post-transplant(5).
We demonstrate that haplo-dCBT recipients who achieved an optimal myeloid bridge had faster neutrophil and platelet recovery compared to either haplo-dCBT patients who did not bridge or dCBT only historic controls(5). Optimal myeloid bridging was also associated with earlier hospital discharge. However, bridging was observed in less than half of the ATG-free haplo-dCBT recipients and it was not associated with an improvement in early TRM or immune recovery (Supplemental Figure 1).
Notably, we found long-term hematopoiesis was provided by a single dominant CB unit in all engrafted patients (including those with minimal early CB-derived hematopoiesis), and CB graft failure was very rare. Furthermore, dominant CB unit T-cells were observed as early as day +28 in almost all patients. High day +28 dominant unit T-cell chimerism was associated with loss of the haplo-identical graft and conversion to dominant CB unit-derived hematopoiesis. This finding suggests that in haplo-dCBT, dominant CB unit T-cells are able to reject the non-engrafting unit (as with dCBT alone) and also the haplo-identical graft even despite its higher CD34+ cell dose. Moreover, haplo-identical donor-derived myeloid bridging was more likely with a higher degree of HLA-match between the haplo-identical graft and the dominant CB unit. This novel finding suggests that better HLA-match slows the speed of haplo-identical graft rejection and is consistent with the observation that, in dCBT, closer unit-unit HLA-match is associated with initial co-engraftment of both units(33).
There were multiple additional findings of interest. As expected(34), early neutrophil recovery was haplo-derived but a high haplo-identical donor chimerism early post-transplant did not guarantee myeloid bridging. Also, a higher dominant CB unit infused viable CD3+ dose was associated with delayed time to first (haplo-derived) neutrophil recovery in patients with a transient bridge. These observations could be explained by dominant CB unit T-cell mediated inhibition of haplo-identical hematopoiesis. It was also notable that, like dCBT alone(4, 33, 35, 36), a higher infused CD3+ cell dose was associated with CB unit dominance. Moreover, in patients with either a transient or no bridge a higher dominant unit CD34+ cell dose was associated with faster sustained neutrophil recovery(5).
Our findings are in marked contrast to ATG-based haplo-CBT series which have been characterized by accelerated haplo-identical donor-derived neutrophil recovery in the majority of patients(11, 12), slower transition to CB-derived hematopoeisis(12), and higher rates of CB graft failure(34, 37),(38). In addition, unlike our findings, low CB chimerism early post-transplant predicted for CB graft failure in these series(34, 37). Also, a higher haplo-identical cell dose(37, 39) and a better haplo-identical donor-recipient HLA-match(39) have been associated with failure of CB engraftment in ATG-based haplo-CBT. Conversely, these graft characteristics were associated with an increased likelihood of bridging and did not lead to CB graft failure in our study. Finally, as with ATG-free CBT(19, 20, 40) and in contrast to ATG-based haplo-CBT(18), T-cell recovery after haplo-dCBT was prompt regardless of bridging(40).
These differences have important implications, considering that ATG omission in adult CBT is associated with reduced mortality(23–27) and improved immune reconstitution(15–17, 21, 40). Most critical is that myeloid bridging is not guaranteed after serotherapy-free haplo-dCBT and the engrafting CB unit will mediate sustained engraftment. Consequently, the CB graft characteristics are paramount and it should not be assumed that such a strategy could be used to safely transplant small CB units that would otherwise be inadequate for transplantation(11, 12, 14, 39). It is likely that these differences would also apply in ATG-free haplo-CBT using single units, although it is possible that the higher T-cell dose and unit-unit interactions of a double unit graft could augment the speed of haplo-identical graft rejection.
While we have not performed formal cost and resource utilization analyses in comparison to dCBT historic controls, our results suggest that haplo-dCBT is not cost-effective considering the high cost of graft acquisition and the limited efficacy. They also support pursuit of alternative strategies to improve CB myeloid recovery including enriching the CB inventory with high dose units(41, 42), optimization of unit selection incorporating unit quality and CD34+ cell dose(30), and investigation of ex vivo expansion or augmentation of stem cell homing(43–50). Ongoing investigation of such strategies is warranted given that multiple series have demonstrated high survival after CBT(2, 3) and that some patients, especially those of African ancestry, do not have suitable haplo-donors(51).
Our findings also have important implications for strategies that combine unmanipulated CB with any third-party or ex vivo expanded T-cell depleted product, as well as the design of future clinical trials aiming to enhance engraftment post-CBT. They suggest that a better HLA-match of a third-party product to the unmanipulated CB unit, and a higher dose of third-party CD34+ cells, could improve the likelihood of bridging. Notably, however, CB graft supplementation with a high dose CB-derived myeloid product that was not HLA-matched did not enhance engraftment in the trial of Milano et al(52), likely due to early product rejection by the unmanipulated CB graft. Therefore, the requirement for at least partial HLA-matching cannot be obviated. This greatly limits haplo-identical cell supplementation, as purposeful HLA-matching of the haplo-identical donor to a CB graft is not feasible. Therefore, these observations are now forming the basis of a novel clinical trial at our center in which we will investigate engraftment after transplantation of a single unmanipulated CB unit supplemented by a second, CD34+ selected, ex vivo expanded unit specifically chosen to match the first. The aim of this trial will be to enhance the potential for a myeloid bridge by close HLA-matching of expanded T-cell depleted CB cells to a T-cell replete single unit CB graft.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health (NIH) Grant P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict-of-Interest disclosure: I.P. has received research funding from Merck and serves on a Data and Safety Monitoring Board (DSMB) for ExCellThera. A.S. serves on a DSMB for ExcellThera and is the medical director of the New York Blood Center/ National Cord Blood Program. S.T.A. has received honoraria from Abbott Laboratories. S.A.G. has served as a consultant for Amgen, Actinium, Celgene, Johnson & Johnson, Jazz pharmaceutical, Takeda, Novartis, Kite, Spectrum Pharma and has received research funding from Amgen, Actinium, Celgene, Johnson & Johnson, Miltenyi, Takeda. M.A.P. has received honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda; serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune; and has received research support for clinical trials from Incyte, Kite (Gilead) and Miltenyi Biotec. C.S.S. has served as a paid consultant on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite, a Gilead Company, Celgene, Gamida Cell, Pfizer, and GSK, and has received research funding for clinical trials from Juno Therapeutics, Celgene, Precision Biosciences and Sanofi-Genzyme. R.J.O.R. receives royalties from Atara Biotherapeutics. J.N.B. has received research funding from Angiocrine Bioscience, Gamida Cell and Merck. The authors have no other relevant conflicts of interest to declare.
REFERENCES
- 1.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med. 2016;375(10):944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponce DM, Hilden P, Devlin SM, Maloy M, Lubin M, Castro-Malaspina H, et al. High Disease-Free Survival with Enhanced Protection against Relapse after Double-Unit Cord Blood Transplantation When Compared with T Cell-Depleted Unrelated Donor Transplantation in Patients with Acute Leukemia and Chronic Myelogenous Leukemia. Biol Blood Marrow Transplant. 2015;21(11):1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–7. [DOI] [PubMed] [Google Scholar]
- 5.Purtill D, Smith K, Devlin S, Meagher R, Tonon J, Lubin M, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124(19):2905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler C, Stevenson K, Kim HT, Brown J, McDonough S, Herrera M, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46(5):659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez MN, Regidor C, Cabrera R, Garcia-Marco JA, Fores R, Sanjuan I, et al. Unrelated umbilical cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol. 2003;31(6):535–44. [DOI] [PubMed] [Google Scholar]
- 9.Magro E, Regidor C, Cabrera R, Sanjuan I, Fores R, Garcia-Marco JA, et al. Early hematopoietic recovery after single unit unrelated cord blood transplantation in adults supported by co-infusion of mobilized stem cells from a third party donor. Haematologica. 2006;91(5):640–8. [PubMed] [Google Scholar]
- 10.Bautista G, Cabrera JR, Regidor C, Fores R, Garcia-Marco JA, Ojeda E, et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009;43(5):365–73. [DOI] [PubMed] [Google Scholar]
- 11.Kwon M, Bautista G, Balsalobre P, Sanchez-Ortega I, Serrano D, Anguita J, et al. Haplo-cord transplantation using CD34+ cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2014;20(12):2015–22. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118(24):6438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindemans CA, Te Boome LC, Admiraal R, Jol-van der Zijde EC, Wensing AM, Versluijs AB, et al. Sufficient Immunosuppression with Thymoglobulin Is Essential for a Successful Haplo-Myeloid Bridge in Haploidentical-Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(10):1839–45. [DOI] [PubMed] [Google Scholar]
- 14.van Besien K, Childs R. Haploidentical cord transplantation-The best of both worlds. Semin Hematol. 2016;53(4):257–66. [DOI] [PubMed] [Google Scholar]
- 15.Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108(8):2874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110(13):4543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(4):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain N, Liu H, Artz AS, Anastasi J, Odenike O, Godley LA, et al. Immune reconstitution after combined haploidentical and umbilical cord blood transplant. Leuk Lymphoma. 2013;54(6):1242–9. [DOI] [PubMed] [Google Scholar]
- 19.Lindemans CA, Chiesa R, Amrolia PJ, Rao K, Nikolajeva O, de Wildt A, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123(1):126–32. [DOI] [PubMed] [Google Scholar]
- 20.Admiraal R, Lindemans CA, van Kesteren C, Bierings MB, Versluijs AB, Nierkens S, et al. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood. 2016;128(23):2734–41. [DOI] [PubMed] [Google Scholar]
- 21.Castillo N, Garcia-Cadenas I, Barba P, Canals C, Diaz-Heredia C, Martino R, et al. Early and Long-Term Impaired T Lymphocyte Immune Reconstitution after Cord Blood Transplantation with Antithymocyte Globulin. Biol Blood Marrow Transplant. 2017;23(3):491–7. [DOI] [PubMed] [Google Scholar]
- 22.Pascal L, Mohty M, Ruggeri A, Tucunduva L, Milpied N, Chevallier P, et al. Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant. 2015;50(1):45–50. [DOI] [PubMed] [Google Scholar]
- 23.Pascal L, Tucunduva L, Ruggeri A, Blaise D, Ceballos P, Chevallier P, et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood. 2015;126(8):1027–32. [DOI] [PubMed] [Google Scholar]
- 24.Shouval R, Ruggeri A, Labopin M, Mohty M, Sanz G, Michel G, et al. An Integrative Scoring System for Survival Prediction Following Umbilical Cord Blood Transplantation in Acute Leukemia. Clin Cancer Res. 2017;23(21):6478–86. [DOI] [PubMed] [Google Scholar]
- 25.Tozatto-Maio K, Giannotti F, Labopin M, Ruggeri A, Volt F, Paviglianiti A, et al. Cord Blood Unit Dominance Analysis and Effect of the Winning Unit on Outcomes after Double-Unit Umbilical Cord Blood Transplantation in Adults with Acute Leukemia: A Retrospective Study on Behalf of Eurocord, the Cord Blood Committee of Cellular Therapy, Immunobiology Working Party, and the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24(8):1657–63. [DOI] [PubMed] [Google Scholar]
- 26.Wakamatsu M, Terakura S, Ohashi K, Fukuda T, Ozawa Y, Kanamori H, et al. Impacts of thymoglobulin in patients with acute leukemia in remission undergoing allogeneic HSCT from different donors. Blood Adv. 2019;3(2):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballen K, Logan BR, Chitphakdithai P, Kuxhausen M, Spellman SR, Adams A, et al. Unlicensed Umbilical Cord Blood Units Provide a Safe and Effective Graft Source for a Diverse Population: A Study of 2456 Umbilical Cord Blood Recipients. Biol Blood Marrow Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahi PB, Barone J, Devlin SM, Byam C, Lubin M, Ponce DM, et al. Sustained donor engraftment in recipients of double-unit cord blood transplantation is possible despite donor-specific human leukoctye antigen antibodies. Biol Blood Marrow Transplant. 2014;20(5):735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117(8):2332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barker JN, Kurtzberg J, Ballen K, Boo M, Brunstein C, Cutler C, et al. Optimal Practices in Unrelated Donor Cord Blood Transplantation for Hematologic Malignancies. Biol Blood Marrow Transplant. 2017;23(6):882–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponce DM, Sauter C, Devlin S, Lubin M, Gonzales AM, Kernan NA, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(5):799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avery S, Shi W, Lubin M, Gonzales AM, Heller G, Castro-Malaspina H, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117(12):3277–85; quiz 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon M, Martinez-Laperche C, Balsalobre P, Serrano D, Anguita J, Gayoso J, et al. Early peripheral blood and T-cell chimerism dynamics after umbilical cord blood transplantation supported with haploidentical cells. Bone Marrow Transplant. 2014;49(2):212–8. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez P, Wagner JE, DeFor TE, Blazar BR, Verneris MR, Miller JS, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant. 2012;47(6):799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scaradavou A, Smith KM, Hawke R, Schaible A, Abboud M, Kernan NA, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010;16(4):500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai SB, Liu H, Shore T, Fan Y, Bishop M, Cushing MM, et al. Frequency and Risk Factors Associated with Cord Graft Failure after Transplant with Single-Unit Umbilical Cord Cells Supplemented by Haploidentical Cells with Reduced-Intensity Conditioning. Biol Blood Marrow Transplant. 2016;22(6):1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Besien K, Koshy N, Gergis U, Mayer S, Cushing M, Rennert H, et al. Cord blood chimerism and relapse after haplo-cord transplantation. Leuk Lymphoma. 2017;58(2):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Besien K, Koshy N, Gergis U, Mayer S, Cushing M, Rennert H, et al. Haplo-cord transplant: HLA-matching determines graft dominance. Leuk Lymphoma. 2017;58(6):1512–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Politikos I, Lavery JA, Hilden P, Cho C, Borrill T, Maloy MA, et al. Robust CD4+ T-cell recovery in adults transplanted with cord blood and no antithymocyte globulin. Blood Adv. 2020;4(1):191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stritesky G, Wadsworth K, Duffy M, Buck K, Dehn J. Evaluation of the impact of banking umbilical cord blood units with high cell dose for ethnically diverse patients. Transfusion. 2018;58(2):345–51. [DOI] [PubMed] [Google Scholar]
- 42.Magalon J, Maiers M, Kurtzberg J, Navarrete C, Rubinstein P, Brown C, et al. Banking or Bankrupting: Strategies for Sustaining the Economic Future of Public Cord Blood Banks. PLoS One. 2015;10(12):e0143440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, Rafii S. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120(6):1344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345(6203):1509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popat U, Mehta RS, Rezvani K, Fox P, Kondo K, Marin D, et al. Enforced fucosylation of cord blood hematopoietic cells accelerates neutrophil and platelet engraftment after transplantation. Blood. 2015;125(19):2885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner JE Jr., Brunstein CG, Boitano AE, DeFor TE, McKenna D, Sumstad D, et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell. 2016;18(1):144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horwitz ME, Wease S, Blackwell B, Valcarcel D, Frassoni F, Boelens JJ, et al. Phase I/II Study of Stem-Cell Transplantation Using a Single Cord Blood Unit Expanded Ex Vivo With Nicotinamide. J Clin Oncol. 2018:JCO1800053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen S, Roy J, Lachance S, Delisle JS, Marinier A, Busque L, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1–2 safety and feasibility study. Lancet Haematol. 2019. [DOI] [PubMed] [Google Scholar]
- 51.Kosuri S, Wolff T, Devlin SM, Byam C, Mazis CM, Naputo K, et al. Prospective Evaluation of Unrelated Donor Cord Blood and Haploidentical Donor Access Reveals Graft Availability Varies by Patient Ancestry: Practical Implications for Donor Selection. Biol Blood Marrow Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milano F, Rezvani AR, Kurtzberg J, Karanes C, Gutman JA, Duncan C, et al. No Engraftment Advantage after Single or Double Umbilical Cord Blood Transplant (CBT) with the Addition of a Non-HLA Matched Off-the-Shelf Expanded Cord Blood Unit Compared to Conventional CBT: Results of a Randomized Trial. Blood. 2019;134(Supplement_1):146–. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


