Abstract
Lipoproteins (LPs) are circulating heterogeneous nanoparticles produced by the liver and intestines. LPs play a major role in the transport of dietary and endogenous lipids to target cells through cell membrane receptors or cell surface-bound lipoprotein lipase. The stability, biocompatibility, and selective transport of LPs make them promising delivery vehicles for various therapeutic and imaging agents. This review discusses isolation, manufacturing, and drug loading techniques used for LP-based drug delivery, as well as recent applications for diagnosis and treatment of cancer, atherosclerosis, and other life-threatening diseases.
Keywords: Biocompatible nanoparticles, chemotherapy, chylomicrons, drug delivery, high-density lipoproteins, low-density lipoproteins, lymphatic transport, purification, synthetic lipoproteins, very low-density lipoproteins
Graphical abstract
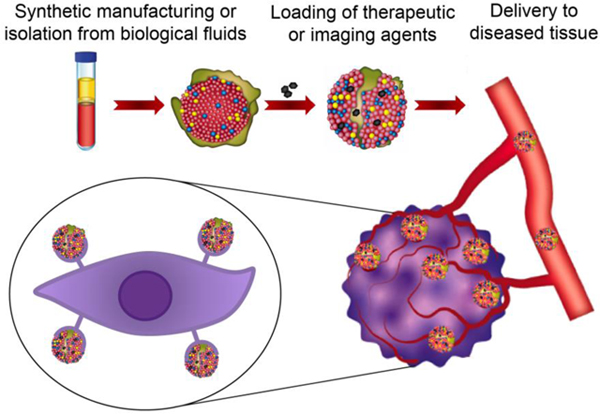
1. Introduction
Lipoproteins (LPs) are a heterogeneous class of biogenic, intrinsically stable, and non-immunogenic nanoparticles produced by the liver and intestines [1]. LPs cover a broad range of sizes and densities, as each subclass is composed of various lipid to protein ratios [2]. The types of lipids (triglycerides and cholesterol) and proteins (apolipoprotein) also differ among LP subclasses and vary throughout the lifecycle of these nanoparticles, resulting in dynamic transitions between subclasses [2, 3]. LPs usually have a sphere-like shape [4] but can be discoidal during synthesis and hepatic catabolism [3]. LPs facilitate complex metabolism of lipids, particularly enabling their transport in aqueous environments, such as the blood circulation. LPs can either mediate lipid transfer from the intestines to other tissues for storage or transport lipids from tissues to the liver for catabolism [3]. Low-density lipoproteins (LDLs) and high-density lipoproteins (HDLs) interact with specific cell types involved in lipid metabolism by binding to receptors, including the LDL receptor (LDL-R) [5], scavenger receptor class B type 1 (SRB1) [6, 7], and adenosine triphosphate-binding cassette transporter subfamily G member 1 (ABCG1) [7]. Notably, some of these receptors are also overexpressed on cancer cells and atherosclerotic plaques, making LPs, particularly HDLs and LDLs, attractive for drug delivery purposes [8, 9]. In addition to receptor binding, lipoproteins, such as chylomicrons, release lipids to target tissues upon exposure to lipoprotein lipase [10], which is thought to be bound to endothelial cell surfaces through electrostatic interactions [11].
In addition to being isolated from biological fluids, primarily plasma, LPs can be synthetically manufactured by mixing protein subunits with lipids. The association of these two components can generate nanoparticles that mimic the delivery properties of natural LPs, while also retaining biocompatibility when administered in vivo [12, 13]. In the past few years, various applications of natural and synthetic LPs have been extensively explored in nanomedicine, revealing promising opportunities for future clinical translation as delivery vehicles [13]. Obtaining a favorable biodistribution represents a considerable challenge for clinically approved drugs. Specifically, side effects of therapeutic agents can be attributed primarily to limited site-specific delivery, i.e., ~0.01–0.1% and ~1% of systemically administered doses for small molecule drugs and nanodrugs, respectively [14–16]. Preclinical studies have demonstrated that molecular targeting approaches can increase the efficacy of synthetic nanodrugs [17–19], although such approaches have consistently failed in clinical trials [14, 20], due in part to the complexity of ligand-receptor binding (e.g., interference by plasma proteins [21]). The use of LPs is a promising alternative strategy, as these nanoparticles have evolutionary selected molecular targeting mechanisms. Nevertheless, it is worth noting that side effects are still likely to occur due to LP receptor expression in other tissues, e.g., atherosclerosis plaques in the case of cancer drug delivery. There are also many limitations hampering clinical application of LPs, such as poor scalability due to challenges in purification from biological fluids and costly synthetic manufacturing.
This review discusses LP biogenesis, isolation, synthesis, and medical applications in the context of drug delivery. Additionally, limitations hindering clinical translation of LPs and potential solutions to overcome such challenges are presented [22].
2. Lipoprotein biogenesis
LPs can be categorized into the following five major subclasses based on density, size, and lipid/apolipoprotein composition: chylomicrons, very low-density lipoproteins (VLDLs), LDLs, intermediate-density lipoproteins (IDLs), and HDLs (Table 1) [3]. Each LP subclass has an important role in either endogenous or exogenous lipid metabolism. The endogenous pathway involves lipid synthesis by hepatic tissue and subsequent VLDL, LDL, or HDL-mediated transport throughout the body, while the exogenous pathway encompasses intestinal uptake of lipids from dietary sources and subsequent chylomicron-dependent transport into the lymphatic system [3].
Table 1.
Physical characteristics of lipoproteins.
| Lipoprotein subclass | Density (g/cm3) | Size (nm) | Enriche d apo | Origin | Primary function | Major lipids | Ref |
|---|---|---|---|---|---|---|---|
| Chylomicrons | < 0.950 | 80-1000 | apoB-48, apoC-II, apoA-I, apoA-II, apoA-IV, apoE | Intestines | Transport of dietary triglycerides to non-hepatic tissues | Triglycerides | [3, 10, 3] |
| VLDL | 0.930-1.006 | 30-80 | apoB-100, apoC, apoE | Liver, intestines | Transport of endogenous triglycerides to non-hepatic tissues | Triglycerides | [3, 26] |
| IDL | 1.006-1.019 | ~25 | apoB-100, apoC, apoE | Catabolism of VLDL | Intermediate of VLDL and LDL | Cholesterol | [3, 26] |
| LDL | 1.019-1.063 | ~20 | apoB-100 | Catabolism of IDL | Transport of the majority of cholesterol to non-hepatic tissues | Cholesterol | [3, 27] |
| HDL | 1.063-1.210 | 5–10 | apoA-I, apoA-II, apoC, apoE | Liver, intestines, other | Transport of cholesterol from non-hepatic tissues to the liver | Cholesterol, phospholipids | [3] |
Apo, apolipoprotein; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; Ref, reference; VLDL, very low-density lipoprotein.
Chylomicrons have the lowest density of all LPs (below 0.950 g/cm3) and a broad size range (80–1000 nm), which is dependent on the amount of ingested dietary lipids [3, 23]. Chylomicrons are synthesized in the digestive tract by enterocytes, which are cells specialized in biomolecular absorption of numerous small molecules (e.g., ions, water, sugars, peptides, amino acids, lipids, and vitamins). Food-derived triglycerides, phospholipids, and cholesterol are coupled with apolipoproteins, mainly apolipoprotein B-48 (apoB-48), to form chylomicrons. Newly synthesized chylomicrons enter the intestinal lymph and are subsequently transferred into the systemic circulation through the thoracic duct [10]. After entering the bloodstream, nascent chylomicrons acquire apolipoproteins, such as apoC-II, from HDLs. ApoC-II is a co-enzyme that activates peripheral lipoprotein lipase, enabling the release of lipids from chylomicrons to muscle and adipose tissue [24]. Namely, lipoprotein lipases lining the capillary endothelium cause hydrolysis of the chylomicron triglyceride core, liberating free fatty acids that are then stored in adipocytes or oxidized by skeletal muscle cells [11]. After hydrolysis, the remnant chylomicrons have a high content of cholesterol esters and various apolipoproteins, including apoC-III, a lipoprotein lipase inhibitor, and HDL-recruited apoE, which is capable of binding to hepatic receptors inducing the clearance of these remnants [24]. Under normal physiological conditions, chylomicrons are present in the blood for approximately 1–5 hours post-prandium and are usually completely cleared from circulation within 12 hours of fasting [25].
VLDLs are synthesized by liver hepatocytes and have a smaller size (30–80 nm) and higher density (0.930–1.006 g/cm3) than chylomicrons [3]. VLDLs are primarily composed of apoB-100, and also include apoC, apoE, and triglycerides, of which the latter is synthesized de novo or delivered to the liver from the intestines [26]. After synthesis, VLDLs are released into the systemic circulation, where a transitory exchange of apoC-II occurs with HDLs. In the capillaries, interactions between VLDLs and lipoprotein lipase trigger the release of free fatty acids to tissues for energy storage or immediate adenosine triphosphate (ATP) synthesis [3, 26].
IDLs are transitory LPs derived from VLDLs following triglyceride depletion by lipoprotein lipase in the capillaries. IDLs contain apoB-100 and have an intermediate size (about 25 nm) and density (1.006–1.019 g/cm3) compared to other LPs [3, 26]. IDLs are either cleared by the liver through apoE binding or release apoE and bind cholesterol esters, becoming circulating LDLs [3].
LDLs are enriched in apoB-100 and are derived from IDLs that bind to cholesterol, accounting for ~75% of all cholesterol in human blood [25]. These LPs are small (~ 20 nm) and have a low density (1.019–1.063 g/cm3) compared to other LPs [3, 27]. The main role of LDLs is to supply cholesterol to non-hepatic tissues expressing LDL receptors, in particular, adrenal glands, skeletal muscle, lymphocytes, gonads, and kidneys [27]. Although cholesterol is important for membrane and hormone synthesis, high amounts of circulating LDLs can trigger pathological processes, such as hyperlipidemia and atherosclerotic plaque formation, by increasing the supply of cholesterol to peripheral tissues [27].
HDLs are the smallest subclass of LPs (5–10 nm) and have the highest density (1.063–1.210 g/cm3) [3]. HDLs are synthesized by the liver and contain high amounts of apoA-I, apoA-II, apoC, and apoE compared to lipid amounts (cholesterol and phospholipids) [3]. These LPs are predominantly responsible for reverse lipid transport, a preventative mechanism against cholesterol accumulation in the body. In particular, HDLs carry cholesterol from non-hepatic tissues to the liver for eventual removal [7]. Therefore, HDLs are thought to be protective of chronic and acute cardiovascular events, such as the formation and rupture of atherosclerotic plaques [3, 7].
3. Isolation of lipoproteins from biological fluids
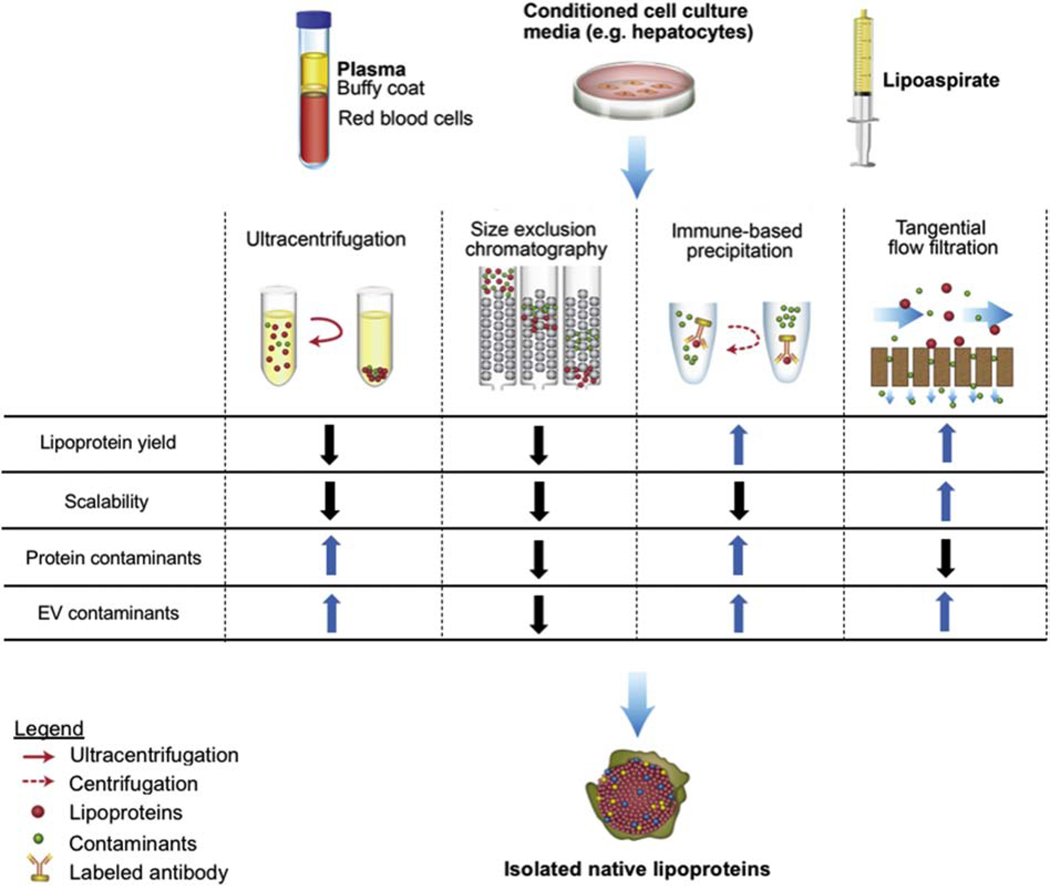
LPs are naturally occurring nanoparticles present in biological fluids [28], such as blood [25], cerebrospinal fluid [29], lipoaspirate [30], and conditioned media from, e.g., hepatocyte cell lines [31]. LPs can be separated from other biological components based on differences in size and density [32] or by using immunocapture assays that rely on interactions between antibodies or probes and surface proteins [33] (Fig. 1). However, biological fluids contain other nanoparticles, such as extracellular vesicles (EVs), which overlap with LPs in regard to size and density, making separation of these two components challenging [28, 34]. Accurate and efficient LP isolation and subsequent quantification usually require time-consuming and expensive protocols that are poorly scalable and difficult to standardize [28]. Despite these challenges, several techniques have been implemented in both research laboratories and clinical settings for LP isolation, including ultracentrifugation, size-exclusion chromatography (SEC), tangential flow filtration, density gradient assays, and immune-based assays.
Fig. 1. Examples of lipoprotein (LP) isolation methods.
LPs have been isolated from plasma, conditioned cell culture media (e.g., hepatocytes), and lipoaspirate using ultracentrifugation, size-exclusion chromatography, immune-based precipitation, or tangential flow filtration. EV, extracellular vesicle.
A tangential flow filtration (TFF)-based method [35] was utilized for sterile, quick, efficient, and scalable separation of biological nanoparticles (EVs and LPs) from larger and smaller components in human lipoaspirate [30]. This filtration technique, which can process large volumes (> 1 L) in a few hours, leads to isolation of higher quantities of biological nanoparticles with almost no albumin contaminants compared to conventional ultracentrifugation [35]. Lipoaspirate-derived biological nanoparticles isolated with TFF were shown to suppress toll-like receptor 4 (TLR4)-induced expression of inflammatory cytokines in macrophages [30]. An additional SEC separation step was used to further separate EVs from apoE-containing LPs, revealing that both the mixed and EV-enriched populations displayed similar TLR4-suppressive effects when normalized for nanoparticle amount [30]. These findings indicate that both lipoaspirate-derived EVs and LPs may have anti-inflammatory properties. Although SEC provided mechanistic understanding of subcomponent effects, this additional separation step makes the method less scalable due to volume limitations [30].
In another study, biological nanoparticles were isolated from human platelet-free plasma, and platelet concentrates collected from pre and post-prandial donors using multiple size-based protocols, namely dead-end filtration, differential centrifugation, and ultracentrifugation. Some samples were further processed with density gradient-based ultracentrifugation [32]. The presence of LPs (apoB-100 and apoB-48) and EVs (cluster of differentiation 9, cluster of differentiation 41, cluster of differentiation 63, and annexin V) was evaluated by flow cytometry and Western blot [32]. Platelet concentrates were expected to contain a more homogeneous nanoparticle population compared to whole plasma, whereas plasma from post-prandial donors was expected to contain higher levels of LPs containing apoB. However, all samples isolated through size-based techniques, regardless of platelet content or prandial status, contained high levels of LPs and low levels of EVs, with the latter being slightly increased in samples from post-prandial donors [32]. The density-gradient ultracentrifugation step was able to further eliminate all chylomicrons (apoB-48 positive) and reduce the presence of apoB-100 containing LPs [32]. Notably, this study also revealed that LDLs attach to the surface of EVs, indicating that these biological nanoparticles interact, further complicating separation attempts [32].
It is possible that more efficient separation of EVs and LPs could be achieved through the optimization of such protocols, as the aforementioned study used standard gradient conditions and centrifugation settings. In fact, EVs and LPs from mouse serum and conditioned cell culture media were efficiently separated using a differential centrifugation protocol entailing both ultracentrifugation and sucrose cushions [29]. Furthermore, an additional isolation step consisting of the ultracentrifugation of a discontinuous potassium bromide gradient was able to further separate LDLs from HDLs [29]. Other studies have also demonstrated a successful separation of HDLs from other apoB-containing LPs. For example, mouse plasma was filtered and processed using three in-series SEC columns and applying a fast protein liquid chromatography protocol followed by a size-based filtration and concentration step [36]. Each eluted fraction was analyzed for total protein, cholesterol, and triglyceride content in order to identify HDLs and apoB-containing LPs based on protein to lipid ratios and lipid content. The isolation protocol resulted in the collection of separate fractions containing either HDLs or a mixture of VLDLs and LDLs [36]. The small non-coding RNA (sRNA) content of each fraction was identified, as LPs are known to carry both host and non-host sRNAs [36]. Both HDLs and VLDLs/LDLs displayed a sRNAs profile that differed from that of the host liver, suggesting an active sorting mechanism. Specifically, the two LP subclasses were associated with distinct subsets of non-host sRNAs, mainly bacterial, to a greater extent than other samples, such as host liver, bile, and urine. Further studies are necessary to determine the function of non-host sRNA enrichment on LPs [36].
In addition to size and density-based separation methods, immunocapture assays have been used to specifically isolate LP subclasses. For example, HDLs were efficiently isolated from human plasma using immunocapture columns functionalized with anti-HDL immunoglobulin (Ig) Y and a sandwich-enzyme-linked immunosorbent assay [33]. Although HDLs are generally known to exert anti-inflammatory effects, this separation protocol revealed that rheumatoid arthritis patients had higher levels of HDL compared to healthy controls. Proteomic analysis demonstrated that HDLs in rheumatoid arthritis patients were significantly enriched with 12 acute phase proteins [33], which could provide a possible explanation for the association of HDL with this inflammatory disease.
In conclusion, the separation of LPs from other components in biological fluids has been achieved. However, LP purification requires multi-step protocols that are time-consuming and poorly scalable, facing similar challenges as EV-based therapeutics [37–39]. Therefore, such protocols may not be ideal for clinical drug delivery applications that require mass production. Moreover, batch-to-batch consistency and high reproducibility could be compromised due to donor-dependent variations in biological fluid composition. However, the aforementioned methods may be suitable for biomarker-based applications that require much smaller processing volumes.
4. Synthetic lipoproteins
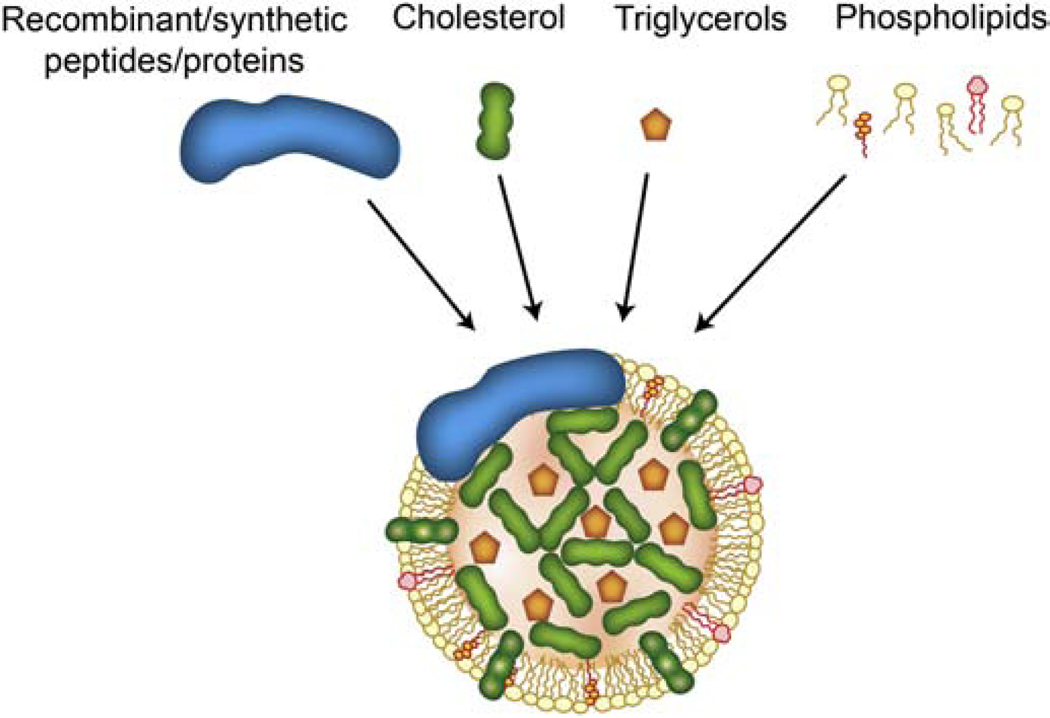
Manufacturing of synthetic (s) LPs, which have been shown to mimic the structure, biocompatibility, stability, targeting, and immunoevasion of natural ones, is an alternative to LP isolation from biological fluids [12, 40]. Assembly of sLPs is achieved by combining protein/peptide components (recombinant or synthetic origin) with synthetic lipids or other hydrophobic compounds in solution (Fig. 2) [41]. Protocols for sLP synthesis vary significantly in terms of equipment, technical complexity, cost, and time. For example, lipid emulsions/liposomes can be mixed with synthetic peptides through vortexing in the absence [42] or presence of detergents (e.g., cholate) [43], while other protocols rely on and microfluidic-based reconstitution [44]. sLPs can be separated from excess reagents using dialysis or density gradient-based ultracentrifugation [13, 40, 42, 43]. Compared to sHDLs, sLDLs usually require more complex synthesis due to the large size of ApoB [45]. The size and shape of sLPs can be customized by adjusting lipid types and stoichiometric lipid-to-protein ratios [41, 46]. For example, the use of long saturated lipids and high lipid-to-protein ratios results in large discoidal sLPs, while unsaturated lipids and low lipid-to-protein ratios lead to the formation of small discoidal sLPs [41]. Studies have shown that sLPs reconstituted from lipids and apolipoproteins display shape, size, and density ranges comparable to native counterparts [41].
Fig. 2. Example structure of synthetic LPs.
LPs can be synthesized in a laboratory setting combining synthetic/recombinant proteins, cholesterol, phospholipids, cholesterol esters, and triacylglycerols.
In one study, sHDLs were synthesized from various lipids and six different types of recombinant human apolipoproteins using the ethanol injection method and cholate dialysis [47]. These sHDLs triggered cholesterol efflux in cell culture and decreased the concentrations of cholesterol, triglycerides, and LDLs in the blood of mice fed a high-fat diet. Additionally, the sHDLs regulated prostaglandin 2 and thromboxane 2 levels in a similar manner as natural HDLs, and reduced liver steatosis and inflammation [47]. Overall, these results suggest that sHDLs can have comparable protective and anti-inflammatory effects to native counterparts. Other studies have synthesized sHDLs using fewer apolipoproteins [48]. For example, sHDLs have been synthesized using various ratios of apoA-I and apoA-V and a fixed quantity of synthetic lipids, revealing that apoA-V has an inverse relation with lipoprotein lipase activity, suppressing uptake and clearance of VLDLs in hepatocyte cell culture and in vivo [49]. This study highlights the importance of apolipoprotein type and ratio in sHDL synthesis in order to obtain LPs that correctly mimic the native physiological role of HDLs [49]. Similarly, sLDLs synthesized from various lipids and a synthetic apoB peptide using a solvent evaporation method followed by homogenization have been used as a cell culture supplement for cell lines that are unable to perform de novo cholesterol synthesis [50]. These sLDLs were shown to increase cell viability and proliferation in a dose-dependent fashion [50]. Cell proliferation correlated with both the cholesterol ester and peptide concentration in sLDLs, suggesting an important role of receptor-mediated uptake [50].
In conclusion, the characteristics of sLPs can be controlled and adjusted to mimic natural LPs, representing a valuable alternative to the isolation of LPs from biological fluids. However, a major drawback is the requirement of specific protocols and equipment to ensure proper assembly of lipids with proteins [13]. Chemical engineering approaches, such as solid-phase synthesis [51, 52], enable the production of tens-of-kilograms of peptides [53]. Microorganisms (e.g., bacteria and yeasts) genetically modified to secrete soluble recombinant apolipoproteins can also be used for sLP synthesis and production [13]. However, these techniques are often more expensive than solid-phase synthesis for quantities under 100 kg [53]. Additionally, it is likely that clinical-grade manufacturing of sLPs will face similar challenges in regard standardization and batch-to-batch consistency as synthetic nanoparticles [54].
5. Lipoprotein-based drug delivery
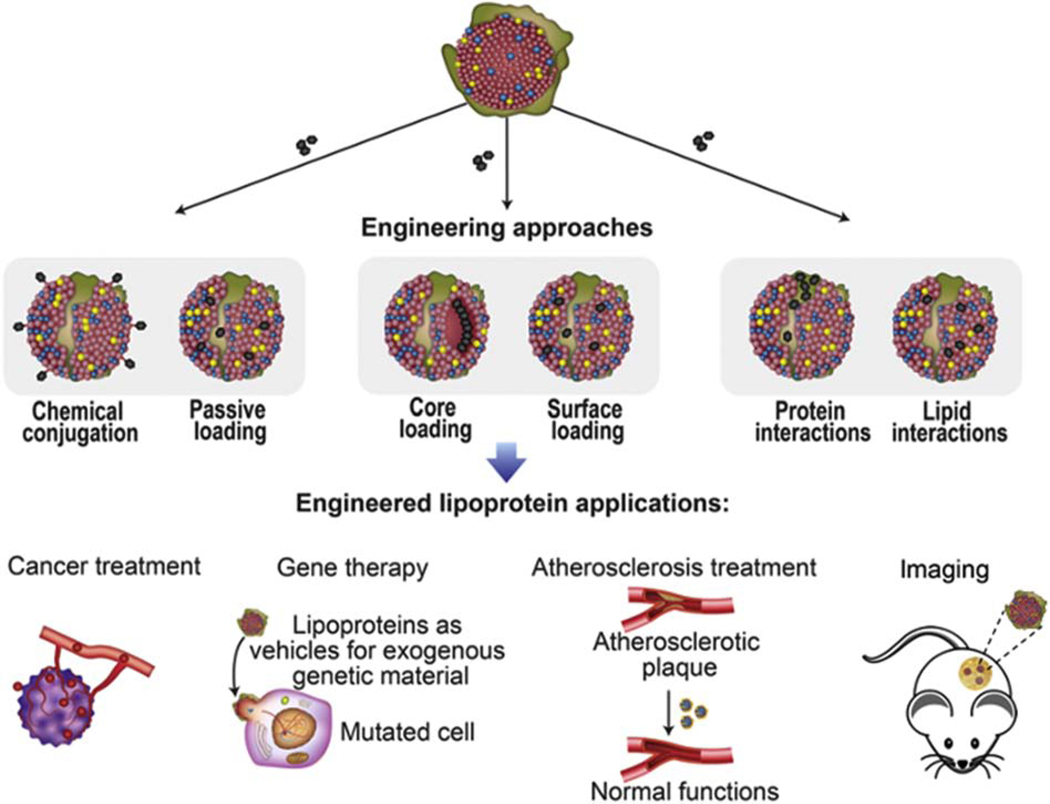
Blood-based LP assays are commonly used in the clinic for diagnostic purposes. However, alternative uses of LPs for applications, such as drug delivery, are primarily in preclinical development. On the contrary, synthetic liposomes have been used in the clinic since the 1990s for the delivery of therapeutic agents for fungal infections and cancer [55, 56]. The major ways in which liposomes differ from LPs are the presence of an aqueous core and the lack of apolipoproteins. LPs represent a valuable alternative to liposomes, as the latter has short circulations times unless coated with polyethylene glycol (PEG), which can activate the immune system upon repeated administration, leading to rapid clearance and potentially adverse effect [14, 57]. The heterogeneous structure of LPs enables the loading of a broad range of molecules. For example, small lipophilic molecules can be passively loaded in LPs through simple mixing protocols [58], while hydrophilic molecules can be chemically conjugated to lipid or protein components [59]. Payloads can be incorporated into the LP core or associated with lipid or protein components on the LP surface (Fig. 3) [45, 60]. For example, lipid core reconstitution is a common method entailing the replacement of LP core lipids with the therapeutic agent through a chemical process [61]. Among the various LPs, LDLs and HDLs have primarily been used for drug delivery due to their small size (< 25 nm), which facilitates tissue penetration and receptor-mediated uptake (e.g., LDLR and SRB1), enabling selective transport to target tissues [45, 60]. Natural and synthetic chylomicrons have also been proposed as delivery vehicles for lymphatic transport [62, 63]. LP safety profiles and examples of engineering strategies for drug delivery applications (Table 2) are discussed in the following section.
Fig. 3. Examples of LP drug loading.
Various approaches can be used to load therapeutic or imaging agents in LPs, including passive loading or chemical conjugation, core loading or surface loading, and interactions with protein or lipids. In preclinical studies, engineered LPs have been used for various applications, including cancer treatment, gene therapy, atherosclerosis treatment, and imaging.
Table 2.
Lipoprotein-based drug delivery systems.
| Chemotherapy | ||||
|---|---|---|---|---|
| Molecule | Lipoprotein subclass | Pathology | Experiments | Ref |
| 5-fluorouracil, 5-iododeoxyuridine, doxorubicin, and vindesine | Natural VLDLs, LDLs, and HDLs | Cervical cancer and breast cancer | In vitro Human cervical cancer (HeLa) and breast cancer (MCF-7) | [58] |
| Paclitaxel oleate | sLDLs | GBM | In vitro Human cervical cancer (HeLa) and GBM (SF-767, U-251, SF-763) | [42] |
| Paclitaxel-alpha linolenic acid | sLDLs | GBM and hepatocarcinoma |
In vitro Human GBM (U-87 MG) and hepatocarcinoma (HepG2) In vivo BALB/c nude mice bearing subcutaneous tumors (U-87 MG) |
[64] |
| Paclitaxel | sHDLs | Breast cancer, prostate cancer, and ovarian cancer |
In vitro Human breast cancer (MCF7), prostate cancer (DU145), and ovarian cancer (OVCAR-3 OV1063) In vivo C57BL6 mice |
[65] |
| Withalongolide A 4,19,27-triacetate | sHDLs | Adrenocortical carcinoma |
In vitro Human adrenocortical carcinoma (H295R, SW13). In vivo Athymic nude mice Foxn1nu bearing subcutaneous tumors (H295R). |
[66] |
| Sorafenib and dihydroartemisinin | sLDLs | Liver cancer |
In vitro Human liver cancer (HepG2) In vivoBALB/c nude mice bearing subcutaneous tumors (HepG2) |
[67] |
| Toll-like receptor 9 agonist and tumor-specific neoantigens | sHDLs | Gliomas | In vivo C57BL/6 mice bearing subcutaneous tumors (GL261) and CD4−/− and CD8−/− knockout (KO) mice bearing orthotopic tumors (GL261) | [68] |
| Antigen peptides and CpG | sHDLs | Colon adenocarcinoma | In vivo C57BL/6 mice bearing subcutaneous tumors (MC-38) | [69] |
| Nucleic acids | ||||
| Molecule | Lipoprotein subclass | Pathology | Experiments | Ref # |
| Androgen receptor siRNA | sHDLs | Prostate cancer |
In vivo Human prostate cancer (LNCaP), melanoma (A375), breast cancer (MDA-MB-231), and ovarian cancer (OvCar3) In vivo Athymic nude mice bearing subcutaneous tumors (LNCaP) |
[70] |
| ApoB siRN | sHDLs sLDLs | Atherosclerosis |
In vitro Mouse primary hepatocytes In vivo C57BL6 double transgenic mice expressing human apoB and cholesterol ester transfer protein |
[71] |
| Activating transcription factor-5 siRNA | sHDLs | Glioblastoma |
In vitro Rat glioblastoma cells (C6) In vivo NOD/SCID mice bearing subcutaneous tumors (C6) |
[72] |
| Atherosclerosis treatments | ||||
| Molecule | Lipoprotein subclass | Pathology | Experiments | Ref # |
| Anti-inflammatory liver X receptor agonist | sHDLs | Atherosclerotic plaques |
In vitro Mouse macrophages (J774A.1) In vivo C57BL/6 J mice and apoE-deficient mice (B6.129P2-Apoetm1Unc/J) |
[73] |
| Lovastatin | sHDLs | Hypercholesterole mia |
In vitro Mouse macrophages (RAW 264.7) In vivo ApoE knockout mice (B6.129P2-Apoetm1Unc/J) |
[74] |
| Simvastatin | Hyaluronan-anchored core-shell sHDL | Atherosclerotic plaques |
In vitro Human umbilical vein endothelial cells (HUVECs) and mouse macrophages (RAW 264.7) In vivo Atherosclerotic New Zealand White (NZW) rabbits |
[75] |
| Simvastatin | sHDL | Atherosclerotic plaques |
In vitro Mouse macrophages (J774A.1), mouse endothelial cells (MS1), mouse aortic smooth muscle cells (MOVAS) and mouse liver cells (Hepa-1c1c7) In vivo ApoE-knockout mice (B6.129P2-Apoetm1Unc/J) |
[76] |
| β-cyclodextrin and simvastatin | sHDL | Atherosclerotic plaques | In vitro Mouse macrophages (RAW 264.7) | [77] |
| Imaging molecules | ||||
| Molecule | Lipoprotein subclass | Pathology | Experiments | Ref # |
| Amphiphilic gadolinium-diethylenetriaminepenta acetic acid chelates | LDLs | Liver cancer |
In vitro Human liver cancer (HepG2) and Chinese hamster ovary cells (ldlA7). In vivo Nude mice bearing subcutaneous tumors (HepG2) |
[78] |
| Silicon tetra-tert-butyl-phthalocyanine | sLDLs | Liver cancer | In vitro Human liver cancer (HepG2) | [79] |
| Nanocrystals as imaging molecules | sHDLs | Atherosclerosis | In vivo Wild type and apoE knockout mice | [80] |
| Valrubicin | sHDLs | Prostate cancer | In vitro Human prostate cancer (PC3) | [81] |
| 99mTc- hydrazinonicotinic acid -N-dodecylamide | sHDLs | Prostate cancer |
In vitro Human prostate cancer (PC3) In vivo In vivo subcutaneous tumor (PC3) |
[82] |
| Triphenylphosphonium cations and quantum dots | sHDLs | Atherosclerotic plaques |
In vitro Mouse macrophages (RAW 264.7) In vivo Sprague Dawley rats |
[83] |
| Anti-virals | ||||
| Molecule | Lipoprotein subclass | Pathology | Experiments | Ref # |
| Lopinavir and Ritonavir | sChylomicrons | Human immunodeficiency virus |
In vitro Human colorectal adenocarcinoma cells (Caco-2). In vivo Sprague Dawley rats |
[62] |
| Hepatitis B antigens | sChylomicrons | Hepatitis B |
In vitro Human bone marrow-derived dendritic cells and monocyte-derived dendritic cells In vivo Sprague Dawley rats |
[84] |
| His-tagged West Nile virus envelope protein | Nickel-chelating nanolipoprotein particles | West Nile encephalitis | In vivo Swiss Webster mice | [85] |
| Alzheimer’s disease | ||||
| ApoE3 | sHDL | Alzheimer’s disease | In vivo Senescence-accelerated prone mouse (SAMP8) and senescence-accelerated mouse resistant R1 (SAMR1) | [86] |
| Tissue regeneration | ||||
| Rapamycin | sHDL | Tissue regeneration |
In vitro Human monocyte cell line (THP-1) In vivo Zebrafish larvae |
[87] |
Apo, apolipoprotein; DHL, dihydroartemisinin; Foxn1, forkhead Box N1; GBM, glioblastoma multiforme; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NOD, non-obese diabetic; nu, nude; Ref, reference; s, synthetic; SCID, severe combined immune deficiency; siRNA, small interfering RNA; VLDL, very low-density lipoprotein.
5.1. Stability and safety
The natural origin and function of LPs provide many benefits for drug delivery, including stability in circulation, safety, limited interactions with the mononuclear phagocyte system, targeting ability, and biodegradation [13, 22, 40, 45, 48, 60, 70, 88, 89]. In particular, the ability of delivery vehicles to evade the immune system is beneficial as prolonged circulation times usually cause increased interactions with target tissues [66, 90].
A wide variety of therapeutic agents have been delivered by LDL carriers, including chemotherapy, photosensitizers, nucleotides, anti-human immunodeficiency virus (HIV) drugs, and imaging agents [22]. Studies have revealed that the stability of LDL-based delivery systems is highly dependent on the loading strategy [48]. For example, an imaging study found that gadolinium probes containing a hydrophobic moiety that bound to the LDL surface following a passive mixing protocol were transferred to cell membranes upon in vivo administration [91]. However, probe detachment from LDLs did not prevent magnetic resonance visualization of subcutaneous tumors in mice [91]. In addition to loading methods, the shape of the LP carrier has been found to impact stability. For example, disc-shaped HDLs were found to be unstable in plasma [92], which is not entirely unexpected as a shape-based transition from discoidal to spherical occurs during HDL maturation. It is also critical to assess how drug loading impacts LP transport and functional properties. Radiolabeling of tyrosine side chains of apoB-100 using three different radioiodination techniques (iodogen tyrosine radiolabeling, chloramine tyrosine radiolabeling, and monochloride tyrosine radiolabeling) was found to change the properties of LDLs [59]. For example, compared to native LDLs the presence of radioactive iodine isotopes reduced the antioxidative potential [59], which could potentially impact the circulation time due to increased uptake by phagocytic cells, as demonstrated for oxidized LDLs [93, 94].
It is conceivable that changes to native LP properties as a consequence of drug loading could result in safety concerns, such as immunological reactions. It is also essential to address other potential safety concerns, including the presence of infectious agents in LP samples obtained from biological fluids [95, 96]. In fact, viruses are in the same size range and density as LPs, which may lead to potential co-isolation [95, 96]. Moreover, it is possible that the administration of drug-loaded LPs may have unfavorable effects on lipid metabolism; however, such effects are likely to be highly dependent on dosing. Storage-induced aggregation of LDLs could also be a potential safety concern for drug delivery applications [22]. Additionally, it is foreseeable that excessive quantities of intravenously injected LPs could cause infusion reactions or vascular obstructions, as it the case for systemically injected synthetic nanoparticles [14].
Despite the aforementioned safety concerns, LPs have the potential to substantially improve the safety profile of cytotoxic agents. For example, daunorubicin-loaded LDL carriers were more than twice as cytotoxic in acute leukemia blast cells compared to healthy bone marrow cells, whereas free daunorubicin was equally cytotoxic in both cell lines [97]. It remains to be seen whether such improvements in safety would outperform clinically approved synthetic nanoparticles that in, many cases, have gained approval due to decreased toxicity with equivalent therapeutic efficacy compared to free drug counterparts [16, 98].
5.2. Cancer
Conventional chemotherapy has many on-target (suppression of cell division) and off-target (e.g., generation of reactive oxygen species) effects, which also damage healthy tissues, causing side effects and limiting therapeutic efficacy due to dose restrictions [99, 100]. Therefore, research efforts have focused on developing drug delivery strategies for chemotherapeutic agents to reduce exposure to healthy organs [101, 102]. LP-based drug delivery systems can facilitate the delivery of chemotherapeutic drugs to cancer cells, as LP receptors are overexpressed in some tumors (Fig. 3) [88, 103, 104]. In fact, the high metabolic rate of cancer cells requires accelerated membrane synthesis that relies on the uptake of cholesterol carried by endogenous LPs [12, 105, 106]. Additionally, macrophages and monocytes in the tumor microenvironment frequently promote tumor progression [107–110] and could be targeted by LP-based therapy due to high expression of scavenger receptors, which internalize modified LDL [111, 112].
Many chemotherapeutic agents display hydrophobic interactions with LPs, which enables loading through simple mixing [58]. The loading efficiencies of four anticancer molecules, doxorubicin (Dox), vindesine (Vin), 5-iododeoxyuridine, and 5-fluorouracil, in VLDLs, LDLs, and HDLs were shown to be directly proportional to drug hydrophobicity and molecular weight [58]. Specifically, Vin, which has the highest molecular weight (750 g/mol) and mean partition coefficient (3.62), displayed the highest loading efficiency, followed by Dox (546 g/mol and mean partition coefficient of 1.34) and 5-iododeoxyuridine (354.1 g/mol and mean partition coefficient of −0.68), and lastly 5-fluorouracil (130.8 g/mol and a mean partition coefficient of −2.10) [58]. Notably, all four chemotherapeutic agents were more efficiently incorporated in HDLs compared to other LP subtypes [58]. The morphology and structural integrity of LPs were not affected, except for Dox and Vin-loaded LDLs, which exhibited decreased thermal stability [58]. In general, drug-loaded HDLs and LDLs displayed comparable and dose-dependent therapeutic effects in cervical and breast cancer cells, outperforming free drugs, and drug-loaded VLDLs [58]. However, in some cell lines, Dox-loaded HDLs were more efficient than LDL counterparts [58].
In addition to passive loading of chemotherapeutic agents in LPs, several studies have also relied on chemical conjugation strategies. For example, sLDL have been chemically conjugated to paclitaxel (PTX) through oleate (fatty acid), leading to improved therapeutic effects in glioblastoma multiforme cell lines [42]. These effects were more pronounced in cells with higher expression of LDLR [42]. In a similar study, sLDL-PTX displayed enhanced cytotoxicity in glioblastoma cells compared to hepatocarcinoma cells, which was attributed to higher LDLR expression in the former cell line [64]. In mice studies, the highest levels of sLDL-PTX were observed in subcutaneous glioblastoma xenografts 36 hours after intravenous administration [64]. Daily administration of sLDL-PTX for ten days caused a substantial decrease in tumor volume (3.6-fold) compared to clinically approved solvent-based PTX (Taxol) (2.1-fold), lipid conjugated PTX (2-fold), and PTX in lipid emulsion (2.4-fold) [64]. In addition to sLDLs, PTX has also been loaded in HDLs, which significantly decreased the half-maximal inhibitory concentration dose (IC50) compared to free PTX in ovarian, prostate, and breast cancer cell lines [65]. Moreover, the maximum tolerated PTX dose and mean body weight were higher in mice treated with sHDL-PTX compared to clinically approved solvent-based (Taxol) or protein-based (Abraxane) PTX, indicating the LP-based delivery may be more tolerable than currently available clinical alternatives [65].
The aforementioned examples of drug delivery for cancer-based applications relied on the use of spherical LPs. However, discoidal LPs have also been used for delivery of anticancer agents despite displaying stability issues in other studies [92]. For example, disc-shaped sHDLs were loaded with a novel hydrophobic compound (withalongolide A 4,19,27-triacetate). These drug-loaded sHDLs displayed a significantly increased IC50 compared to the free drug in adrenocortical carcinoma (ACC) cells overexpressing SRB1 [66]. In mice bearing subcutaneous ACCs, intravenous or intraperitoneal administration of sHDLs resulted in high hepatic and intratumoral accumulation. Compared to the free drug, drug-loaded sHDLs caused a more substantial suppression of tumor growth [66].
In conclusion, both natural and synthetic LPs have been successfully used in preclinical studies for delivery of chemotherapeutic agents, displaying improved therapeutic efficacy and safety compared to free drug counterparts. In certain cases where LPs were compared to clinically approved drug delivery systems (i.e., Abraxane), the former was superior in regard to safety.
5.3. Gene silencing
Chemically unmodified freely circulating nucleic acids are susceptible to rapid degradation by nucleases [113–115]. Naturally occurring nanoparticles in the blood, mainly EVs [116] and LPs (LDLs and HDLs) [117], act as vehicles for sRNAs that are transferred between cells in the body. These biological nanoparticles also represent promising drug delivery vehicles for therapeutic nucleotides [13, 70, 71].
In particular, HDLs have been proposed as promising carriers for micro RNAs (miRNAs) and small interfering RNAs (siRNAs), which are short oligonucleotides that act post-transcriptionally to alter cell function [70, 118]. For example, mixing of sHDLs, composed of a gold nanoparticle core covered with apoA-I, phospholipids, and cholesterol, with siRNAs complexed with cationic lipids, led to self-assembly of sHDL-siRNA [70]. sHDL-siRNA caused a selective and lasting suppression of target gene expression in prostate cancer cells (up to 96 hours) [70]. Tumor accumulation and therapeutic efficacy were also demonstrated in mice bearing prostate tumor xenografts upon repeated intravenous administration of sHDL-siRNA against the androgen receptor (AR) [70]. Administration of sHDL-siRNA also caused a substantial reduction in circulating blood cells, particularly white blood cells and neutrophils, which is expected following systemic AR knockdown. Other parameters measured in the blood and liver, as well as animal body weights, were not significantly affected, indicating negligible systemic toxicity [70].
In another study, apoE or apoA-I proteins were assembled with phospholipids and cholesterol conjugated siRNAs against apoB [71]. Following intravenous administration in mice, the selective knockdown of apoB in the liver (50%) with sHDLs (apoA-I) siRNA delivery was similar to that of siRNA delivered with endogenous HDLs isolated from mouse plasma [71]. However, apoE-sLPs caused a dose-dependent silencing of apoB up to 80%, causing a one-third reduction in serum LDL cholesterol levels, substantially outperforming sHDLs. ApoE is an LDLR ligand that is responsible for the uptake of LP remnants by the liver [71]. Therefore, the role of ApoE in LP catabolism may explain the superior performance of apoE-sLPs compared to sHDLs [71]. Collectively, these studies suggest that LPs represent a promising delivery system for sRNAs in vitro and in vivo, in particular for applications involving cells that express high levels of LP receptors.
5.4. Atherosclerosis
HDLs play a major role in reverse cholesterol transport (removal of excess cholesterol from non-hepatic tissues) and are generally protective against adverse cardiac events and blood hereditary hyperlipidemia [119]. Therefore, the use of HDL is a promising strategy for concurrent stimulation of reverse cholesterol transport and delivery of therapeutic agents to atherosclerotic plaques [73, 74, 119, 120] (Fig. 3).
Various therapeutic agents with anti-inflammatory and/or cholesterol-lowering properties have been loaded in HDLs for treatment of atherosclerosis. For example, sHDLs were loaded with a liver X receptor (LXR) agonist [73]. LXR is known to regulate ABC transporters expressed on the cell surface of, e.g., hepatocytes and macrophages, the latter being a fundamental component of atherosclerotic plaques. sHDL-LXR caused cholesterol efflux in cultured macrophages after 48 hours of exposure without affecting cell viability [73]. Both the LXR agonist and sHDL-LXR agonist increased messenger RNA (mRNA) and protein expression of ABC transporter sub-family A member 1 (Abca1) and Abcg1 in mouse macrophages. Treatment of these macrophages with sHDL alone decreased Abca1 and Abcg1 expression in mouse macrophages, which was likely due to low intracellular levels of cholesterol [73]. Intraperitoneal injection of fluorescent sHDLs-LXR agonist into apoE deficient mice fed a high-fat diet resulted in the accumulation of the delivery system in the aorta [73].
Moreover, aorta tissue analysis showed increased expression of Abca1 and Abcg1 transporter mRNAs in response to the free LXR agonist or sHDL-LXR agonist, thus corroborating the in vitro findings [73]. Treatment with the sHDL-LXR agonist for six weeks reduced the aortic lesion and plaque area to a greater extent than that of free LXR and sHDLs. Notably, systemic administration of the free LXR agonist caused various side effects, such as liver steatosis and increased triglyceride levels. These side effects occurred due to activation of the hepatic sterol regulatory element-binding protein 1 (SREBP-1) pathway that causes increased expression of LXR-target genes in the liver. Notably, delivery of the LXR agonist in sHDL did not activate the SREBP1 pathway, thus avoiding the aforementioned side effects [73]. A lack of side effects was not due to protective effects of sHDL, as combination treatment with sHDL and free LXR failed to prevent liver toxicity [73]. Potential reasons for the lack of sHDL-LXR side-effects despite liver accumulation of sHDL include alterations in LXR pharmacokinetics, which is expected upon nanoparticle encapsulation of small molecules and is known to impact safety profiles [121, 122]. For example, HDL circulates for many hours [123] and may slowly release LXR in extracellular and intracellular compartments, thereby, causing negligible toxicity, as drug exposure is spread out over time, as previously demonstrated for synthetic nanoparticles [124]. In fact, it is unknown whether drug internalization by hepatocytes occurred with HDL delivery, as intrahepatic biodistribution was not studied. It is possible that the drug remained in the hepatic extracellular matrix or was internalized by other cells in the liver, such as Kupffer cells.
In another study, sHDL was used for the delivery of lovastatin (LOV), a cholesterol-lowering molecule that also exhibits potential anti-inflammatory effects [74]. sHDL-based delivery of LOV for atherosclerosis is promising, as prior use of free LOV for this condition has been limited by low intra-aortal accumulation [74]. LOV was incorporated in sHDL through a film dispersion-apoA-I incubation method. In cultured macrophages, free LOV, sHDL-LOV, and sHDL caused impaired oxidate LDL (oxLDL) uptake and consequent suppression of cluster of differentiation 36 and class A scavenger receptors, which mediate oxLDL uptake. Notably, sHDL-LOV caused the most substantial decrease in receptor expression, indicating that the combination therapy was superior to either treatment alone [74]. Fluorescently labeled sHDL administered to apoE knockout mice fed a high-fat diet accumulated in the aorta, and treatment with sHDL-LOV lead to a substantial decrease in atherosclerotic plaque area along with an inhibition of metalloproteinase activity [74]. Notably, therapeutic doses of sHDL-LOV did not cause systemic toxicity or changes in cell viability [74].
In another study, discoidal sHDLs were loaded with simvastatin (sHDLsim) and co-administered with β-cyclodextrin to target foam cells, a type of macrophage in atherosclerotic plaques that has limited permeability to drugs due to abnormal cholesterol accumulation [77]. This combination therapy could simultaneously promote cholesterol efflux from foam cells (β-cyclodextrin), cholesterol uptake and transport to hepatic tissue (sHDLs), and inhibition of cholesterol synthesis (simvastatin) [77]. The authors demonstrated that combination therapy with β-cyclodextrin and sHDLsim was superior to monotherapy in terms of cholesterol efflux, decreased membrane cholesterol content, reduced intracellular lipid accumulation, decreased secretion of proinflammatory cytokines, reversion of foam cell phenotype, and improved simvastatin delivery [77].
In conclusion, sHDLs have shown promise for combination therapy, delivery of therapeutic agents, and improved safety profiles for treatment of atherosclerotic plaques.
5.5. Imaging
In addition to delivery of therapeutic agents, LDLs and HDLs are promising carriers for imaging agents (Fig. 3). For example, an LDL-loaded magnetic resonance imaging (MRI) contrast agent was used in preclinical studies for tumor imaging [78]. Specifically, amphiphilic gadolinium (Gd)-diethylenetriaminepentaacetic acid chelates were incorporated into LDLs isolated from human plasma and intravenously administered to mice with subcutaneous hepatoblastoma G2 xenografts [78]. Selective delivery to tissues expressing LDLR was observed within five hours for the liver and 24 hours for the tumor. A similar study investigated the use of the photosensitizer, tetra-t-butyl silicon phthalocyanine bisoleate (SiPcBOA), reconstituted into the lipid core of LDLs isolated from human plasma [79]. Laser scanning confocal microscopy was used to visualize SiPcBOA-LDL uptake by human hepatoblastoma G2 (HepG2) tumor cells via the LDLR pathway, and a clonogenic assay revealed that SiPcBOA-LDL-based photodynamic therapy was more efficient than free SiPcBOA in HepG2 cells [79].
In addition to LDLs, HDLs are also promising for imaging purposes, mainly due to the small size of these LPs, which enables improved tissue penetration [66]. Various types of HDLs have been used in imaging studies, including radiolabeled HDLs [59] and nanocrystal HDLs [80]. The latter involves the reconstitution of the HDL core with nanocrystals of various materials depending on the imaging modality [80]. For example, gold nanocrystals allow visualization by computerized tomography, iron oxide by MRI, and quantum dots by optical imaging. All three of these nanocrystals were shown to be taken up by macrophages during in vitro experiments. In addition to modifying the core composition, the HDLs were further modified to carry additional contrast agents, thus creating multifunctional LPs [80].
In another study, sHDL were designed to detect healthy versus apoptotic macrophages, the latter being major components of atherosclerotic plaques characterized by a high risk of disruption [83]. Triphenyl phosphonium (TPP), a hydrophobic cation able to accumulate into active mitochondria, was included as an sHDL constituent during sHDL synthesis. Additionally, the sHDL core was loaded with quantum dots to allow for sHDL imaging both in vitro and in vivo, as identification of unstable atherosclerotic plaques is important for prevention of thrombosis and other acute cardiac events [83]. In vitro studies assessed the ability of TPP-sHDL to target the mitochondria matrix through a mechanism dependent on the mitochondrial membrane potential, characteristic of healthy macrophages. Accordingly, TPP-sHDL exhibited decreased accumulation in apoptotic macrophage mitochondria compared to the viable counterpart, corroborating sHDL ability to sense cell status [83].
Another study compared the optical properties of free valrubicin, an anticancer drug, to sHDL enclosed valrubicin, demonstrating that the latter has the potential to be a valuable theranostic agent [81]. In particular, sHDL delivery overcomes previous limitations of valrubicin use due to hydrophobicity and enables SR-B1-mediated targeted delivery. Notably, the quantum yield and lifetime of fluorescence increased six-fold and two-fold, respectively, upon valrubicin enclosure within sHDL particles [81]. Overall, LP-based delivery vehicles are promising multifunctional platforms that enable theranostic approaches to disease management.
5.6. Vaccines
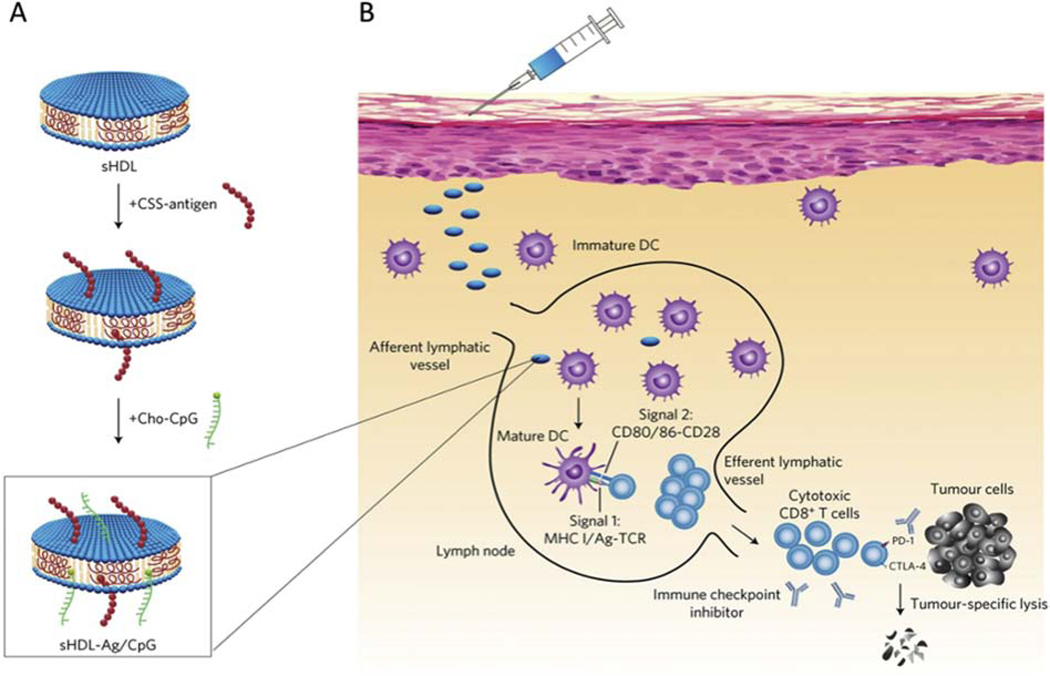
Synthetic LPs have shown potential as a vaccine delivery platform for a variety of diseases. Notably, sHDL has shown promise as a carrier for peptide-based cancer vaccines. A preclinical study developed an sHDL discoidal nanocarrier conjugated with tumor antigen peptides and adjuvants for personalized cancer immunotherapy (Fig. 4a) [69]. Utilization of sHDL nanodiscs helps to address the issue of the poor immunogenicity of antigen peptides by allowing co-delivery of an immunostimulatory adjuvant (Fig. 4b) [69, 125]. Subcutaneous administration of these sHDL nanodiscs into mice resulted in significantly stronger CD8+ T cell lymphocyte (CTL) responses compared to free antigen peptide and water-in-oil Montanide (a commonly used peptide adjuvant system for peptide-based cancer vaccines in clinical trials) with a 47-fold and a 31-fold greater CTL response frequency, respectively [69]. Upon challenge with administration of melanoma cells, mice immunized with the sHDL vaccine were tumor-free up to 28 days, while the mice of the free peptide and Montanide vaccine groups all succumbed to tumors [69]. Similar results were observed in another study, in which the same sHDL nanodiscs were modified for glioma vaccination and resulted in a strong CTL response and glioma inoculation in mice compared to the free peptide [68]. Synthetic lipoprotein carriers have also been used for peptide-based vaccines for infectious diseases, including those caused by West Nile virus, Streptococcus pyogenes, and Clostridium difficile [85, 126, 127].
Fig. 4. sHDL nanodisc-based cancer vaccine.
(A) Synthetic high-density lipoprotein (sHDL) nanodiscs are conjugated with antigen (Ag) peptides and cholesterol-modified immunostimulatory molecules (Cho-CpG) adjuvant. Ag peptides were modified with a cysteine-serine-serine (CSS) linker. (B) Following subcutaneous administration, the sHDL nanodiscs deliver Ag peptides and CpG to draining lymph nodes, promoting antigen presentation and dendritic cell (DC) maturation. This activates CD8+ T cell responses that target and kill cancer cells in peripheral tissues. Adapted from Kuai et al. [69] with permission. CD, cluster of differentiation. CTLA-4, cytotoxic T-lymphocyte-associated protein 4. MHC, major histocompatibility complex. PD-1, programmed cell death protein 1. TCR, T-cell receptor.
Additionally, chylomicrons have been used for vaccine delivery in preclinical studies. For example, an oral vaccine against hepatitis B virus was developed by loading antigens into nanoparticles enriched in polyunsaturated lipids that were further encapsulated in an enteric capsule [84]. Orally administered medications absorbed by the intestines are usually subjected to a hepatic first-pass effect, entailing rapid metabolization [62]. In contrast, dietary lipids, mainly long-chain fatty acids absorbed by the intestines, are assembled into chylomicrons and directly released into the lymphatic system, avoiding the first-pass effect and improving drug pharmacokinetics [62]. In the hepatitis B vaccine study, administration of the chylomicron-based vaccine in rats resulted in IgA and IgG antibody production, and similar immunization levels as those achieved using a commercial intramuscular hepatitis B virus vaccine [84]. Overall, chylomicron-mediated oral delivery represents a promising strategy for improving drug pharmacokinetics and targeting the lymphatic system.
In addition to vaccines, chylomicron-based drug delivery is especially suitable for treating autoimmune diseases, malignancies, and infections that involve lymphoid organs, as conventionally administered therapeutic agents, display low accumulation in the lymphatic system [62, 84]. For example, in a preclinical study, diglyceride-covered mesoporous silica nanoparticles were loaded with the anti-HIV drug, lopinavir, as a strategy to reach virus reservoirs in the drug-privileged lymphatic system [62]. Following oral administration in rats, the nanoparticles were able to enter enteric epithelial cells through various endocytic pathways and were recycled as constituents of chylomicrons that were transported to the lymphatic circulation [62]. Notably, nanoparticle-based delivery substantially improved the pharmacokinetics of lopinavir compared to free drug administration [62]. In another study, treatment of Caco2 colon epithelial cells with unsaturated micelles loaded with the lipophilic antioxidant compound, 5-demethylnobiletin, triggered secretion of chylomicrons carrying the therapeutic agent [128].
5.7. Clinical studies
Drug-encapsulated LPs have not yet entered clinical studies despite promising preclinical results. However, several HDL products without additional imaging or therapeutic agents have been evaluated in clinical trials for atherosclerosis and metabolic disorders. For example, apoA-I Milano, an HDL mimetic consisting of recombinant mutant apoA-I (mutation is associated with cardioprotective effects) combined with phospholipids, displayed initial promising results in patients with coronary artery disease [129]. However, manufacturing difficulties and contamination from host-derived components necessitated the development of a non-contaminated version, which was named MDCO-216 [130]. In 2016, development of MDCO-216 was halted due to lack of efficacy [130, 131]. Another HDL mimetic (pre-β HDL), CER-001 (recombinant apoA-I combined with phospholipids), was shown to be well tolerated and induce reverse lipid transport in healthy subjects in a phase I clinical trial [132]. However, a lack of therapeutic efficacy was observed in a clinical trial of statin-treated patients with acute coronary syndrome [133].
In addition to HDL products based on recombinant apoA-1, plasma-derived ApoA-1 has also been used in clinical studies. For example, administration of autologous HDL that had undergone delipidation led to a non-significant trend in the reduction of carotid atheroma volume in patients with acute coronary syndrome [134]. Additionally, CSL-111 and CSL-112 are HDL mimetics consisting of apoA-I purified from human plasma and reconstituted with phospholipids [129]. Administration of CSL-111 [135, 136] or CSL-112 [129] in clinical studies caused a two-fold and more than 30-fold increase in apoA-1 and pre-ßHDL content in plasma, respectively [129], indicating the ability of these products to impact cholesterol efflux and elimination. ApoA-1 in these studies exhibited a relatively short duration of elevation with a range of approximately 48 to 72 hours [129]. CSL-111 demonstrated altered plaque morphology in initial clinical trials; however, high infusion concentrations led to liver toxicity [129]. Toxic effects were attributed to formulation excipients, and the product was reformulated as CSL-112. Phase I study results of CSL-112 demonstrated a more favorable safety profile [129]. Phase II clinical trials of CSL-112 in a total of 1,258 patients with acute myocardial infarction demonstrated that four weekly infusions were well tolerated and did not influence liver or kidney function [137]. Furthermore, CSL-112 acutely augmented cholesterol efflux [137]. Currently, there is an ongoing phase III clinical trial with CSL-112 (e.g., NCT03473223) in patients with acute coronary syndrome with an estimated enrollment of 17,400 participants [138].
Future considerations for clinical use of HDL-based products include assessment of long-term effects, as well as optimization of treatment dosage and duration. Overall, HDL-based therapies are well tolerated in patients, which is promising for future clinical evaluation of LP-based drug delivery vehicles. Incorporation of therapeutic agents in HDL products has the potential to improve treatment, especially as several clinical trials have shown limited efficacy for treatment of atherosclerosis and metabolic disorders.
6. Conclusions
LPs are a class of biological nanoparticles secreted by the intestines and liver. LPs are heterogeneous in size, density, and transport properties, serving as endogenous carriers for various lipids in circulation. LP-mediated transport of cargo to target cells relies on cell membrane receptor binding, followed by endocytosis or enzymatic digestion through lipases tethered to the cell surface. Such selective transport properties make LPs promising for drug delivery applications, mainly to cells and tissues expressing high levels of LP receptors such as LDL-R and SRB1. For example, several LP receptors are overexpressed on cancer cells and atherosclerotic plaques. Additionally, chylomicron-mediated oral drug delivery represents a promising strategy to avoid the hepatic first-pass effect and to target lymph organs for the treatment of autoimmune diseases, lymphatic malignancies, and infectious diseases. In addition to selective transport, other advantages of LPs as delivery vehicles include stability and biocompatibility in physiological environments.
LPs can be isolated from biological fluids or synthetically manufactured by combining lipids and synthetic peptides or recombinant apolipoproteins. In fact, a major advantage of LPs compared to other biological nanoparticles, such as EVs, is that the former can be synthetically manufactured in a controlled manner, displaying similar properties as natural LPs. Therapeutic and imaging agents can be loaded in LPs using a variety of methods, including passive loading, chemical conjugation, and lipid core reconstitution. Notably, both hydrophilic and lipophilic small molecules, as well as RNA-based therapeutic agents have successfully been delivered using LPs. Recent preclinical studies have demonstrated that LPs show promise for the diagnosis and treatment of cancer, atherosclerosis, and other life-threatening diseases by improving therapeutic efficacy and reducing side effects compared to free drug counterparts. Notably, in a few animal studies where comparisons were made to clinically approved synthetic nanoparticles, LPs were shown to be superior in regard to reducing side effects. Despite promising potential, LPs will require further evaluation in additional animal models (e.g., mice are poorly representative of human atherosclerosis) and in future clinical studies.
Other challenges in the field of LP-based drug delivery include poor scalability due to challenges in purification from biological fluids and costly synthetic manufacturing. In particular, a major challenge has been the efficient separation of natural LPs from other biological nanoparticles, such as EVs, as well as foreseeable issues maintaining batch-to-batch consistency due to donor-dependent variations in biological fluid composition. However, several LP-based therapies have been evaluated in clinical trials for atherosclerosis and metabolic disorders, such as diabetes or hypercholesterolemia [139–141]. Notably, such HDL mimetics are not used for drug delivery purposes, i.e., they do not include additional imaging or therapeutic agents beyond HDL. Phase III clinical trials with HDL mimetics involving thousands of patients [138] indicate the potential feasibility of scalable clinical-grade manufacturing of LPs for drug delivery in the future. The development of cost-efficient and large-scale drug loading methods combined with isolation techniques and synthetic manufacturing protocols that are already in clinical use is likely to pave the way for clinical translation of LP-based drug delivery.
Highlights.
Lipoproteins are circulating endogenous nanoparticles targeted to specific tissues
Lipoproteins can be isolated from biological fluids or synthetically manufactured
Therapeutic and imaging molecules can be loaded in lipoproteins
Lipoprotein-based drug delivery is promising for e.g. cancer and atherosclerosis
Challenges include cost-efficient, scalable, and standardized manufacturing
Acknowledgements
Conceptualization J.W., and S.B.; writing and editing. A.P., G.W., J.B., J.W., N.N., M.T., S.B., and S.W.; figure design, S.B.; approval of the final version, all the authors; supervision, J.W.; funding acquisition, J.W. This work was supported by the Mayo Clinic Center for Regenerative Medicine Florida Award (JW), Mayo Clinic Florida Focused Research Team Program (JW), and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, United States under award number R21AI152318 (JW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Abbreviations
- ATP
adenosine triphosphate
- ABC
ATP-binding cassette transporter
- ABCA1
ATP-binding cassette transporter sub-family A member 1
- ABCG1
ATP-binding cassette transporter sub-family G member 1
- ACC
adrenocortical carcinoma apolipoprotein Apo
- AR
androgen receptor
- Dox
doxorubicin
- EV
extracellular vesicle
- HDLs
high-density lipoproteins
- HepG2
hepatoblastoma G2 cells
- HIV
human immunodeficiency virus
- IC50
half maximal inhibitory concentration
- IDLs
intermediate-density lipoproteins
- Ig
immunoglobulin
- LDLs
low-density lipoproteins
- LDL-R
low-density lipoprotein receptor
- LP
lipoprotein
- LOV
Lovastatin
- LXR
liver X receptor
- mRNA
messenger
- miRNA
micro RNA
- MRI
magnetic resonance imaging
- oxLDL
oxidate LDL
- PTX
paclitaxel
- S
synthetic
- SEC
size exclusion chromatography
- SiPcBOA
tetra-t-butyl silicon phthalocyanine bisoleate
- siRNA
small interfering RNA
- SRB1
scavenger receptor class B type 1
- sRNA
small non-coding RNA
- TFF
tangential flow filtration
- Vin
vindesine
- VLDLs
very low-density lipoproteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tsimikas S, A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies, J Am Coll Cardiol 69(6) (2017) 692–711. [DOI] [PubMed] [Google Scholar]
- [2].Ramasamy I, Recent advances in physiological lipoprotein metabolism, Clin Chem Lab Med 52(12) (2014) 1695–727. [DOI] [PubMed] [Google Scholar]
- [3].Feingold KR, Grunfeld C, Introduction to Lipids and Lipoproteins, in: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP (Eds.), Endotext, South Dartmouth (MA), 2000. [Google Scholar]
- [4].Kumar V, Butcher SJ, Oorni K, Engelhardt P, Heikkonen J, Kaski K, Ala-Korpela M, Kovanen PT, Three-dimensional cryoEM reconstruction of native LDL particles to 16A resolution at physiological body temperature, PLoS One 6(5) (2011) e18841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ellsworth JL, Kraemer FB, Cooper AD, Transport of beta-very low density lipoproteins and chylomicron remnants by macrophages is mediated by the low density lipoprotein receptor pathway, J Biol Chem 262(5) (1987) 2316–25. [PubMed] [Google Scholar]
- [6].Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O, Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein, J Lipid Res 42(9) (2001) 1474–82. [PubMed] [Google Scholar]
- [7].Yvan-Charvet L, Wang N, Tall AR, Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses, Arterioscler Thromb Vasc Biol 30(2) (2010) 139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mooberry LK, Sabnis NA, Panchoo M, Nagarajan B, Lacko AG, Targeting the SR-B1 Receptor as a Gateway for Cancer Therapy and Imaging, Front Pharmacol 7 (2016) 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gallagher EJ, Zelenko Z, Neel BA, Antoniou IM, Rajan L, Kase N, LeRoith D, Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia, Oncogene 36(46) (2017) 6462–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hussain MM, Intestinal lipid absorption and lipoprotein formation, Curr Opin Lipidol 25(3) (2014) 200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ory DS, Chylomicrons and lipoprotein lipase at the endothelial surface: bound and GAG-ged?, Cell Metab 5(4) (2007) 229–31. [DOI] [PubMed] [Google Scholar]
- [12].Corbin IR, Zheng G, Mimicking nature’s nanocarrier: synthetic low-density lipoprotein-like nanoparticles for cancer-drug delivery, Nanomedicine (Lond) 2(3) (2007) 375–80. [DOI] [PubMed] [Google Scholar]
- [13].Huang H, Cruz W, Chen J, Zheng G, Learning from biology: synthetic lipoproteins for drug delivery, Wiley Interdiscip Rev Nanomed Nanobiotechnol 7(3) (2015) 298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wolfram J, Ferrari M, Clinical cancer nanomedicine, Nano Today 25 (2019) 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Khalid A, Persano S, Shen H, Zhao Y, Blanco E, Ferrari M, Wolfram J, Strategies for improving drug delivery: nanocarriers and microenvironmental priming, Expert opinion on drug delivery (2016) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, Fresta M, Nie G, Chen C, Shen H, Ferrari M, Zhao Y, Safety of Nanoparticles in Medicine, Curr. Drug Targets 16(14) (2015) 1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu H, Mai J, Shen J, Wolfram J, Li Z, Zhang G, Xu R, Li Y, Mu C, Zu Y, Li X, Lokesh GL, Thiviyanathan V, Volk DE, Gorenstein DG, Ferrari M, Hu Z, Shen H, A Novel DNA Aptamer for Dual Targeting of Polymorphonuclear Myeloid-derived Suppressor Cells and Tumor Cells, Theranostics 8(1) (2018) 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Busatto S, Pham A, Suh A, Shapiro S, Wolfram J, Organotropic drug delivery: Synthetic nanoparticles and extracellular vesicles, Biomed. Microdevices 21(2) (2019) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paolino D, Cosco D, Gaspari M, Celano M, Wolfram J, Voce P, Puxeddu E, Filetti S, Celia C, Ferrari M, Russo D, Fresta M, Targeting the thyroid gland with thyroid stimulating hormone (TSH)-nanoliposomes Biomaterials 35(25) (2014) 7101–7109. [DOI] [PubMed] [Google Scholar]
- [20].Rosenblum D, Joshi N, Tao W, Karp JM, Peer D, Progress and challenges towards targeted delivery of cancer therapeutics, Nature communications 9(1) (2018) 1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wolfram J, Yang Y, Shen J, Moten A, Chen C, Shen H, Ferrari M, Zhao Y, The nano-plasma interface: Implications of the protein corona, Colloids Surf. B. Biointerfaces 124 (2014) 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sabnis N, Lacko AG, Drug delivery via lipoprotein-based carriers: answering the challenges in systemic therapeutics, Ther Deliv 3(5) (2012) 599–608. [DOI] [PubMed] [Google Scholar]
- [23].Redgrave TG, Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons, J Clin Invest 49(3) (1970) 465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Julve J, Martin-Campos JM, Escola-Gil JC, Blanco-Vaca F, Chylomicrons: Advances in biology, pathology, laboratory testing, and therapeutics, Clin Chim Acta 455 (2016) 134–48. [DOI] [PubMed] [Google Scholar]
- [25].Cox RA, Garcia-Palmieri MR, Cholesterol, Triglycerides, and Associated Lipoproteins, in: Walker rd H.K., Hall WD, Hurst JW (Eds.), Clinical Methods: The History, Physical, and Laboratory Examinations, Boston, 1990. [Google Scholar]
- [26].Packard CJ, Demant T, Stewart JP, Bedford D, Caslake MJ, Schwertfeger G, Bedynek A, Shepherd J, Seidel D, Apolipoprotein B metabolism and the distribution of VLDL and LDL subfractions, J Lipid Res 41(2) (2000) 305–18. [PubMed] [Google Scholar]
- [27].Murtola T, Vuorela TA, Hyvönen MT, Marrink S-J, Karttunen M, and Vattulainen I, Low density lipoprotein: structure, dynamics, and interactions of apoB-100 with lipids, Soft Matter 7 (2011) 8135–8141. [Google Scholar]
- [28].Busatto S, Zendrini A, Radeghieri A, Paolini L, Romano M, Presta M, Bergese P, The nanostructured secretome, Biomater Sci 8(1) (2019) 39–63. [DOI] [PubMed] [Google Scholar]
- [29].Kaiser K, Gyllborg D, Prochazka J, Salasova A, Kompanikova P, Molina FL, Laguna-Goya R, Radaszkiewicz T, Harnos J, Prochazkova M, Potesil D, Barker RA, Casado AG, Zdrahal Z, Sedlacek R, Arenas E, Villaescusa JC, Bryja V, WNT5A is transported via lipoprotein particles in the cerebrospinal fluid to regulate hindbrain morphogenesis, Nat Commun 10(1) (2019) 1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tian M, Ticer T, Wang Q, Walker S, Pham A, Suh A, Busatto S, Davidovich I, Al-Kharboosh R, Lewis-Tuffin L, Ji B, Quinones-Hinojosa A, Talmon Y, Shapiro S, Ruckert F, Wolfram J, Adipose-Derived Biogenic Nanoparticles for Suppression of Inflammation, Small 16(10) (2020) e1904064. [DOI] [PubMed] [Google Scholar]
- [31].Wang SR, Infante J, Catala D, Petit D, Bonnefis MT, Infante R, Lipid and lipoprotein synthesis in isolated and cultured hepatocytes from lean and obese Zucker rats, Biochim Biophys Acta 1002(3) (1989) 302–11. [DOI] [PubMed] [Google Scholar]
- [32].Sodar BW, Kittel A, Paloczi K, Vukman KV, Osteikoetxea X, Szabo-Taylor K, Nemeth A, Sperlagh B, Baranyai T, Giricz Z, Wiener Z, Turiak L, Drahos L, Pallinger E, Vekey K, Ferdinandy P, Falus A, Buzas EI, Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection, Sci Rep 6 (2016) 24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, Lee TD, Reddy ST, Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis, Arthritis Rheum 64(6) (2012) 1828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Karimi N, Cvjetkovic A, Jang SC, Crescitelli R, Hosseinpour Feizi MA, Nieuwland R, Lotvall J, Lasser C, Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins, Cell Mol Life Sci 75(15) (2018) 2873–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, Bergese P, Wolfram J, Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid, Cells 7(12) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Allen RM, Zhao S, Ramirez Solano MA, Zhu W, Michell DL, Wang Y, Shyr Y, Sethupathy P, Linton MF, Graf GA, Sheng Q, Vickers KC, Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins, J Extracell Vesicles 7(1) (2018) 1506198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Borrelli DA, Yankson K, Shukla N, Vilanilam G, Ticer T, Wolfram J, Extracellular vesicle therapeutics for liver disease, J. Control. Release 273 (2018) 86–98. [DOI] [PubMed] [Google Scholar]
- [38].Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, Quintero A, Lafrence M, Malik H, Santana MX, Wolfram J, Extracellular vesicle-based drug delivery systems for cancer treatment, Theranostics 9(26) (2019) 8001–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Suh A, Pham A, Cress MJ, Pincelli T, TerKonda SP, Bruce AJ, Zubair AC, Wolfram J, Shapiro SA, Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation, Ageing Res Rev 54 (2019) 100933. [DOI] [PubMed] [Google Scholar]
- [40].Thaxton CS, Rink JS, Naha PC, Cormode DP, Lipoproteins and lipoprotein mimetics for imaging and drug delivery, Adv Drug Deliv Rev 106(Pt A) (2016) 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bricarello DA, Smilowitz JT, Zivkovic AM, German JB, Parikh AN, Reconstituted lipoprotein: a versatile class of biologically-inspired nanostructures, ACS Nano 5(1) (2011) 42–57. [DOI] [PubMed] [Google Scholar]
- [42].Nikanjam M, Gibbs AR, Hunt CA, Budinger TF, Forte TM, Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme, J Control Release 124(3) (2007) 163–71. [DOI] [PubMed] [Google Scholar]
- [43].Arrese EL, Rivera L, Hamada M, Mirza S, Hartson SD, Weintraub S, Soulages JL, Function and structure of lipid storage droplet protein 1 studied in lipoprotein complexes, Arch Biochem Biophys 473(1) (2008) 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim Y, Fay F, Cormode DP, Sanchez-Gaytan BL, Tang J, Hennessy EJ, Ma M, Moore K, Farokhzad OC, Fisher EA, Mulder WJ, Langer R, Fayad ZA, Single step reconstitution of multifunctional high-density lipoprotein-derived nanomaterials using microfluidics, ACS Nano 7(11) (2013) 9975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang X, Huang G, Synthetic lipoprotein as nano-material vehicle in the targeted drug delivery, Drug Deliv 24(sup1) (2017) 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Foit L, Giles FJ, Gordon LI, Thaxton CS, Synthetic high-density lipoprotein-like nanoparticles for cancer therapy, Expert Rev Anticancer Ther 15(1) (2015) 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Su M, Chang W, Shi K, Wang D, Wang M, Xu T, Yan W, Preparation and activity analysis of recombinant human high-density lipoprotein, Assay Drug Dev Technol 10(5) (2012) 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang Y, Sun T, Jiang C, Biomacromolecules as carriers in drug delivery and tissue engineering, Acta Pharm Sin B 8(1) (2018) 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang X, Chen B, Synthesis of recombinant high density lipoprotein with apolipoprotein A-I and apolipoprotein A-V, Biol Chem 392(5) (2011) 423–9. [DOI] [PubMed] [Google Scholar]
- [50].Hayavi S, Halbert GW, Synthetic low-density lipoprotein, a novel biomimetic lipid supplement for serum-free tissue culture, Biotechnol Prog 21(4) (2005) 1262–8. [DOI] [PubMed] [Google Scholar]
- [51].Fairwell T, Hospattankar AV, Brewer HB Jr., Khan SA, Human plasma apolipoprotein C-II: total solid-phase synthesis and chemical and biological characterization, Proc Natl Acad Sci U S A 84(14) (1987) 4796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Reddy ST, Navab M, Anantharamaiah GM, Fogelman AM, Apolipoprotein A-I mimetics, Curr Opin Lipidol 25(4) (2014) 304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chandrudu S, Simerska P, Toth I, Chemical methods for peptide and protein production, Molecules 18(4) (2013) 4373–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Leong HS, Butler KS, Brinker CJ, Azzawi M, Conlan S, Dufes C, Owen A, Rannard S, Scott C, Chen C, Dobrovolskaia MA, Kozlov SV, Prina-Mello A, Schmid R, Wick P, Caputo F, Boisseau P, Crist RM, McNeil SE, Fadeel B, Tran L, Hansen SF, Hartmann NB, Clausen LPW, Skjolding LM, Baun A, Agerstrand M, Gu Z, Lamprou DA, Hoskins C, Huang L, Song W, Cao H, Liu X, Jandt KD, Jiang W, Kim BYS, Wheeler KE, Chetwynd AJ, Lynch I, Moghimi SM, Nel A, Xia T, Weiss PS, Sarmento B, das Neves J, Santos HA, Santos L, Mitragotri S, Little S, Peer D, Amiji MM, Alonso MJ, Petri-Fink A, Balog S, Lee A, Drasler B, Rothen-Rutishauser B, Wilhelm S, Acar H, Harrison RG, Mao C, Mukherjee P, Ramesh R, McNally LR, Busatto S, Wolfram J, Bergese P, Ferrari M, Fang RH, Zhang L, Zheng J, Peng C, Du B, Yu M, Charron DM, Zheng G, Pastore C, On the issue of transparency and reproducibility in nanomedicine, Nature nanotechnology 14(7) (2019) 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gentile E, Cilurzo F, Di Marzio L, Carafa M, Ventura CA, Wolfram J, Paolino D, Celia C, Liposomal chemotherapeutics, Future Oncology 9 (2013) 1849–1859. [DOI] [PubMed] [Google Scholar]
- [56].Meyerhoff A, U.S. Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis, Clin. Infect. Dis. 28(1) (1999) 42–8; discussion 49–51. [DOI] [PubMed] [Google Scholar]
- [57].Samuelsson E, Shen H, Blanco E, Ferrari M, Wolfram J, Contribution of Kupffer cells to liposome accumulation in the liver, Colloids Surf. B. Biointerfaces 158 (2017) 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kader A, Pater A, Loading anticancer drugs into HDL as well as LDL has little affect on properties of complexes and enhances cytotoxicity to human carcinoma cells, J Control Release 80(1–3) (2002) 29–44. [DOI] [PubMed] [Google Scholar]
- [59].Sobal G, Resch U, Sinzinger H, Modification of low-density lipoprotein by different radioiodination methods, Nucl Med Biol 31(3) (2004) 381–8. [DOI] [PubMed] [Google Scholar]
- [60].Ma X, Song Q, Gao X, Reconstituted high-density lipoproteins: novel biomimetic nanocarriers for drug delivery, Acta Pharm Sin B 8(1) (2018) 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Krieger M, Reconstitution of the hydrophobic core of low-density lipoprotein, Methods Enzymol 128 (1986) 608–13. [DOI] [PubMed] [Google Scholar]
- [62].Mao Y, Feng S, Li S, Zhao Q, Di D, Liu Y, Wang S, Chylomicron-pretended nano-bio self-assembling vehicle to promote lymphatic transport and GALTs target of oral drugs, Biomaterials 188 (2019) 173–186. [DOI] [PubMed] [Google Scholar]
- [63].Murakami M, Nishina K, Watanabe C, Yoshida-Tanaka K, Piao W, Kuwahara H, Horikiri Y, Miyata K, Nishiyama N, Kataoka K, Yoshida M, Mizusawa H, Yokota T, Enteral siRNA delivery technique for therapeutic gene silencing in the liver via the lymphatic route, Sci Rep 5 (2015) 17035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Su HT, Li X, Liang DS, Qi XR, Synthetic low-density lipoprotein (sLDL) selectively delivers paclitaxel to tumor with low systemic toxicity, Oncotarget 7(32) (2016) 51535–51552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].McConathy WJ, Nair MP, Paranjape S, Mooberry L, Lacko AG, Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel, Anticancer Drugs 19(2) (2008) 183–8. [DOI] [PubMed] [Google Scholar]
- [66].Kuai R, Subramanian C, White PT, Timmermann BN, Moon JJ, Cohen MS, Schwendeman A, Synthetic high-density lipoprotein nanodisks for targeted withalongolide delivery to adrenocortical carcinoma, Int J Nanomedicine 12 (2017) 6581–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang Z, Duan X, Lv Y, Zhao Y, Low density lipoprotein receptor (LDLR)-targeted lipid nanoparticles for the delivery of sorafenib and Dihydroartemisinin in liver cancers, Life Sci 239 (2019) 117013. [DOI] [PubMed] [Google Scholar]
- [68].Scheetz L, Kadiyala P, Sun X, Son S, Najafabadi AH, Aikins M, Lowenstein PR, Schwendeman A, Castro MG, Moon JJ, Synthetic high-density lipoprotein nanodiscs for personalized immunotherapy against gliomas, Clin Cancer Res (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ, Designer vaccine nanodiscs for personalized cancer immunotherapy, Nat Mater 16(4) (2017) 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].McMahon KM, Plebanek MP, Thaxton CS, Properties of Native High-Density Lipoproteins Inspire Synthesis of Actively Targeted In Vivo siRNA Delivery Vehicles, Adv Funct Mater 26(43) (2016) 7824–7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Nakayama T, Butler JS, Sehgal A, Severgnini M, Racie T, Sharman J, Ding F, Morskaya SS, Brodsky J, Tchangov L, Kosovrasti V, Meys M, Nechev L, Wang G, Peng CG, Fang Y, Maier M, Rajeev KG, Li R, Hettinger J, Barros S, Clausen V, Zhang X, Wang Q, Hutabarat R, Dokholyan NV, Wolfrum C, Manoharan M, Kotelianski V, Stoffel M, Sah DW, Harnessing a physiologic mechanism for siRNA delivery with mimetic lipoprotein particles, Mol Ther 20(8) (2012) 1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Huang JL, Jiang G, Song QX, Gu X, Hu M, Wang XL, Song HH, Chen LP, Lin YY, Jiang D, Chen J, Feng JF, Qiu YM, Jiang JY, Jiang XG, Chen HZ, Gao XL, Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis, Nat Commun 8 (2017) 15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Guo Y, Yuan W, Yu B, Kuai R, Hu W, Morin EE, Garcia-Barrio MT, Zhang J, Moon JJ, Schwendeman A, Eugene Chen Y, Synthetic High-Density Lipoprotein-Mediated Targeted Delivery of Liver X Receptors Agonist Promotes Atherosclerosis Regression, EBioMedicine 28 (2018) 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jiang C, Qi Z, Tang Y, Jia H, Li Z, Zhang W, Liu J, Rational Design of Lovastatin-Loaded Spherical Reconstituted High Density Lipoprotein for Efficient and Safe Anti-Atherosclerotic Therapy, Mol Pharm 16(7) (2019) 3284–3291. [DOI] [PubMed] [Google Scholar]
- [75].Zhang M, He J, Jiang C, Zhang W, Yang Y, Wang Z, Liu J, Plaque-hyaluronidase-responsive high-density-lipoprotein-mimetic nanoparticles for multistage intimal-macrophage-targeted drug delivery and enhanced anti-atherosclerotic therapy, Int J Nanomedicine 12 (2017) 533–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, Priem B, Kuan EL, Martel C, Hewing B, Sager H, Nahrendorf M, Randolph GJ, Stroes ES, Fuster V, Fisher EA, Fayad ZA, Mulder WJ, A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation, Nat Commun 5 (2014) 3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].He J, Yang Y, Zhou X, Zhang W, Liu J, Shuttle/sink model composed of beta-cyclodextrin and simvastatin-loaded discoidal reconstituted high-density lipoprotein for enhanced cholesterol efflux and drug uptake in macrophage/foam cells, J Mater Chem B 8(7) (2020) 1496–1506. [DOI] [PubMed] [Google Scholar]
- [78].Corbin IR, Li H, Chen J, Lund-Katz S, Zhou R, Glickson JD, Zheng G, Low-density lipoprotein nanoparticles as magnetic resonance imaging contrast agents, Neoplasia 8(6) (2006) 488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li H, Marotta DE, Kim S, Busch TM, Wileyto EP, Zheng G, High payload delivery of optical imaging and photodynamic therapy agents to tumors using phthalocyanine-reconstituted low-density lipoprotein nanoparticles, J Biomed Opt 10(4) (2005) 41203. [DOI] [PubMed] [Google Scholar]
- [80].Skajaa T, Cormode DP, Falk E, Mulder WJ, Fisher EA, Fayad ZA, High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis, Arterioscler Thromb Vasc Biol 30(2) (2010) 169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shah S, Chib R, Raut S, Bermudez J, Sabnis N, Duggal D, Kimball JD, Lacko AG, Gryczynski Z, Gryczynski I, Photophysical characterization of anticancer drug valrubicin in rHDL nanoparticles and its use as an imaging agent, J Photochem Photobiol B 155 (2016) 60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Isaac-Olive K, Ocampo-Garcia BE, Aranda-Lara L, Santos-Cuevas CL, Jimenez-Mancilla NP, Luna-Gutierrez MA, Medina LA, Nagarajan B, Sabnis N, Raut S, Prokai L, Lacko AG, [(99m)Tc-HYNIC-N-dodecylamide]: a new hydrophobic tracer for labelling reconstituted high-density lipoproteins (rHDL) for radioimaging, Nanoscale 11(2) (2019) 541–551. [DOI] [PubMed] [Google Scholar]
- [83].Marrache S, Dhar S, Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis, Proc Natl Acad Sci U S A 110(23) (2013) 9445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sahu KK, Kaurav M, Pandey RS, Chylomicron mimicking solid lipid nanoemulsions encapsulated enteric minicapsules targeted to colon for immunization against hepatitis B, Int Immunopharmacol 66 (2019) 317–329. [DOI] [PubMed] [Google Scholar]
- [85].Fischer NO, Infante E, Ishikawa T, Blanchette CD, Bourne N, Hoeprich PD, Mason PW, Conjugation to nickel-chelating nanolipoprotein particles increases the potency and efficacy of subunit vaccines to prevent West Nile encephalitis, Bioconjug Chem 21(6) (2010) 1018–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Song Q, Huang M, Yao L, Wang X, Gu X, Chen J, Chen J, Huang J, Hu Q, Kang T, Rong Z, Qi H, Zheng G, Chen H, Gao X, Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer’s disease by accelerating the clearance of amyloid-beta, ACS Nano 8(3) (2014) 2345–59. [DOI] [PubMed] [Google Scholar]
- [87].Cho KH, Enhanced delivery of rapamycin by V156K-apoA-I high-density lipoprotein inhibits cellular proatherogenic effects and senescence and promotes tissue regeneration, J Gerontol A Biol Sci Med Sci 66(12) (2011) 1274–85. [DOI] [PubMed] [Google Scholar]
- [88].Mahmoudian M, Salatin S, Khosroushahi AY, Natural low- and high-density lipoproteins as mighty bio-nanocarriers for anticancer drug delivery, Cancer Chemother Pharmacol 82(3) (2018) 371–382. [DOI] [PubMed] [Google Scholar]
- [89].Raut S, Mooberry L, Sabnis N, Garud A, Dossou AS, Lacko A, Reconstituted HDL: Drug Delivery Platform for Overcoming Biological Barriers to Cancer Therapy, Front Pharmacol 9 (2018) 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Moghimi SM, Hunter AC, Murray JC, Long-circulating and target-specific nanoparticles: theory to practice, Pharmacol Rev 53(2) (2001) 283–318. [PubMed] [Google Scholar]
- [91].Crich SG, Lanzardo S, Alberti D, Belfiore S, Ciampa A, Giovenzana GB, Lovazzano C, Pagliarin R, Aime S, Magnetic resonance imaging detection of tumor cells by targeting low-density lipoprotein receptors with Gd-loaded low-density lipoprotein particles, Neoplasia 9(12) (2007) 1046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Denisov IG, Grinkova YV, Lazarides AA, Sligar SG, Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size, J Am Chem Soc 126(11) (2004) 3477–87. [DOI] [PubMed] [Google Scholar]
- [93].Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA, CD36 is a receptor for oxidized low density lipoprotein, J Biol Chem 268(16) (1993) 11811–6. [PubMed] [Google Scholar]
- [94].Levitan I, Volkov S, Subbaiah PV, Oxidized LDL: diversity, patterns of recognition, and pathophysiology, Antioxid Redox Signal 13(1) (2010) 39–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lacko AG, Sabnis NA, Nagarajan B, McConathy WJ, HDL as a drug and nucleic acid delivery vehicle, Front Pharmacol 6 (2015) 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M Jr., Ye J, Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins, Proc Natl Acad Sci U S A 104(14) (2007) 5848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dorlhiac-Llacer PE, Marquezini MV, Toffoletto O, Carneiro RC, Maranhao RC, Chamone DA, In vitro cytotoxicity of the LDE: daunorubicin complex in acute myelogenous leukemia blast cells, Braz J Med Biol Res 34(10) (2001) 1257–63. [DOI] [PubMed] [Google Scholar]
- [98].Martins JP, das Neves J, de la Fuente M, Celia C, Florindo H, Gunday-Tureli N, Popat A, Santos JL, Sousa F, Schmid R, Wolfram J, Sarmento B, Santos HA, The solid progress of nanomedicine, Drug Deliv Transl Res (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Razga F, Nemethova V, Selective Therapeutic Intervention: A Challenge against Off-Target Effects, Trends Mol Med 23(8) (2017) 671–674. [DOI] [PubMed] [Google Scholar]
- [100].Ray-Coquard I, Le Cesne A, Rubio MT, Mermet J, Maugard C, Ravaud A, Chevreau C, Sebban C, Bachelot T, Biron P, Blay JY, Risk model for severe anemia requiring red blood cell transfusion after cytotoxic conventional chemotherapy regimens. The Elypse 1 Study Group, J Clin Oncol 17(9) (1999) 2840–6. [DOI] [PubMed] [Google Scholar]
- [101].Sehouli J, Stengel D, Elling D, Ortmann O, Blohmer J, Riess H, Lichtenegger W, Ovarian O Cancer Study Group of the Nordostdeutsche Gesellschaft fur Gynakologische, First-line chemotherapy with weekly paclitaxel and carboplatin for advanced ovarian cancer: a phase I study, Gynecol Oncol 85(2) (2002) 321–6. [DOI] [PubMed] [Google Scholar]
- [102].Liang XJ, Chen C, Zhao Y, Wang PC, Circumventing tumor resistance to chemotherapy by nanotechnology, Methods Mol. Biol. 596 (2010) 467–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Acton SL, Scherer PE, Lodish HF, Krieger M, Expression cloning of SR-BI, a CD36-related class B scavenger receptor, J Biol Chem 269(33) (1994) 21003–9. [PubMed] [Google Scholar]
- [104].Salatin S, Maleki Dizaj S, Yari Khosroushahi A, Effect of the surface modification, size, and shape on cellular uptake of nanoparticles, Cell Biol Int 39(8) (2015) 881–90. [DOI] [PubMed] [Google Scholar]
- [105].Sag D, Cekic C, Wu R, Linden J, Hedrick CC, The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity, Nat Commun 6 (2015) 6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR, Crawford S, Fujii H, Georgakilas AG, Guha G, Halicka D, Helferich WG, Heneberg P, Honoki K, Keith WN, Kerkar SP, Mohammed SI, Niccolai E, Nowsheen S, Vasantha Rupasinghe HP, Samadi A, Singh N, Talib WH, Venkateswaran V, Whelan RL, Yang X, Felsher DW, Cancer prevention and therapy through the modulation of the tumor microenvironment, Semin Cancer Biol 35 Suppl (2015) S199–S223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW, CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis, Nature 475(7355) (2011) 222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kitamura T, Doughty-Shenton D, Cassetta L, Fragkogianni S, Brownlie D, Kato Y, Carragher N, Pollard JW, Monocytes Differentiate to Immune Suppressive Precursors of Metastasis-Associated Macrophages in Mouse Models of Metastatic Breast Cancer, Front. Immunol. 8 (2017) 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Gil-Bernabe AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, Watson K, Hill SA, Amirkhosravi A, Francis JL, Pollard JW, Ruf W, Muschel RJ, Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice, Blood 119(13) (2012) 3164–75. [DOI] [PubMed] [Google Scholar]
- [110].Khalid A, Wolfram J, Ferrari I, Mu C, Mai J, Yang Z, Zhao Y, Ferrari M, Ma X, Shen H, Recent Advances in Discovering the Role of CCL5 in Metastatic Breast Cancer, Mini Rev. Med. Chem. 15(13) (2015) 1063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Crucet M, Wust SJ, Spielmann P, Luscher TF, Wenger RH, Matter CM, Hypoxia enhances lipid uptake in macrophages: role of the scavenger receptors Lox1, SRA, and CD36, Atherosclerosis 229(1) (2013) 110–7. [DOI] [PubMed] [Google Scholar]
- [112].Lu M, Gursky O, Aggregation and fusion of low-density lipoproteins in vivo and in vitro, Biomol Concepts 4(5) (2013) 501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhang Y, Wang Z, Gemeinhart RA, Progress in microRNA delivery, J Control Release 172(3) (2013) 962–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Shen J, Liu H, Mu C, Wolfram J, Zhang W, Kim HC, Zhu G, Hu Z, Ji LN, Liu X, Ferrari M, Mao ZW, Shen H , Multi-step encapsulation of chemotherapy and gene silencing agents in functionalized mesoporous silica nanoparticles, Nanoscale 9(16) (2017) 5329–5341. [DOI] [PubMed] [Google Scholar]
- [115].Shen J, Wu X, Lee Y, Wolfram J, Mao Z, Ferrari M, Shen H, Porous silicon microparticles for delivery of siRNA therapeutics, J Vis Exp. 15(95) (2015) 52075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Luz I, Cooks T, Extracellular vesicles: what secrets do they hold inside?, Cell Death Dis. 10(6) (2019) 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Li K, Wong DK, Luk FS, Kim RY, Raffai RL, Isolation of Plasma Lipoproteins as a Source of Extracellular RNA, Methods Mol Biol 1740 (2018) 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].McMahon KM, Thaxton CS, High-density lipoproteins for the systemic delivery of short interfering RNA, Expert Opin Drug Deliv 11(2) (2014) 231–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mahdy Ali K, Wonnerth A, Huber K, Wojta J, Cardiovascular disease risk reduction by raising HDL cholesterol--current therapies and future opportunities, Br J Pharmacol 167(6) (2012) 1177–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lerch PG, Fortsch V, Hodler G, Bolli R, Production and characterization of a reconstituted high density lipoprotein for therapeutic applications, Vox Sang 71(3) (1996) 155–64. [DOI] [PubMed] [Google Scholar]
- [121].Venuta A, Wolfram J, Shen H, Ferrari M, Post-nano strategies for drug delivery: multistage porous silicon microvectors, Journal of Materials Chemistry B 5(2) (2017) 207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shen J, Wolfram J, Ferrari M, Shen H, Taking the vehicle out of drug delivery, Mater Today (Kidlington) 20(3) (2017) 95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kuai R, Li D, Chen YE, Moon JJ, Schwendeman A, High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles, ACS nano 10(3) (2016) 3015–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Scavo MP, Gentile E, Wolfram J, Gu J, Barone M, Evangelopoulos M, Martinez JO, Liu X, Celia C, Tasciotti E, Vilar E, Shen H, Multistage vector delivery of sulindac and silymarin for prevention of colon cancer, Colloids Surf. B. Biointerfaces 136 (2015) 694–703. [DOI] [PubMed] [Google Scholar]
- [125].Melief CJ, van der Burg SH, Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines, Nat Rev Cancer 8(5) (2008) 351–60. [DOI] [PubMed] [Google Scholar]
- [126].Moyle PM, Dai W, Zhang Y, Batzloff MR, Good MF, Toth I, Site-specific incorporation of three toll-like receptor 2 targeting adjuvants into semisynthetic, molecularly defined nanoparticles: application to group a streptococcal vaccines, Bioconjug Chem 25(5) (2014) 965–78. [DOI] [PubMed] [Google Scholar]
- [127].Huang JH, Wu CW, Lien SP, Leng CH, Hsiao KN, Liu SJ, Chen HW, Siu LK, Chong P, Recombinant lipoprotein-based vaccine candidates against C. difficile infections, J Biomed Sci 22(1) (2015) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Yao D.J.M. Mingfei, Zhao Faqing, Craig Roger W., Xiao Hang, Controlling the gastrointestinal fate of nutraceutical and pharmaceutical-enriched lipid nanoparticles: From mixed micelles to chylomicrons, NanoImpact 5 (2017) 13–21. [Google Scholar]
- [129].Kingwell BA, Chapman MJ, Future of high-density lipoprotein infusion therapies: potential for clinical management of vascular disease, Circulation 128(10) (2013) 1112–21. [DOI] [PubMed] [Google Scholar]
- [130].Kosmas CE, Martinez I, Sourlas A, Bouza KV, Campos FN, Torres V, Montan PD, Guzman E, High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease, Drugs Context 7 (2018) 212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Nicholls SJ, Puri R, Ballantyne CM, Jukema JW, Kastelein JJP, Koenig W, Wright RS, Kallend D, Wijngaard P, Borgman M, Wolski K, Nissen SE, Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial, JAMA Cardiol 3(9) (2018) 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Keyserling CH, Barbaras R, Benghozi R, Dasseux JL, Development of CER-001: Preclinical Dose Selection Through to Phase I Clinical Findings, Clin. Drug Investig. 37(5) (2017) 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Nicholls SJ, Andrews J, Kastelein JJP, Merkely B, Nissen SE, Ray KK, Schwartz GG, Worthley SG, Keyserling C, Dasseux JL, Griffith L, Kim SW, Janssan A, Di Giovanni G, Pisaniello AD, Scherer DJ, Psaltis PJ, Butters J, Effect of Serial Infusions of CER-001, a Pre-beta High-Density Lipoprotein Mimetic, on Coronary Atherosclerosis in Patients Following Acute Coronary Syndromes in the CER-001 Atherosclerosis Regression Acute Coronary Syndrome Trial: A Randomized Clinical Trial, JAMA Cardiol 3(9) (2018) 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, Fitzgerald PJ, Brewer HB Jr., A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome, J Am Coll Cardiol 55(24) (2010) 2727–35. [DOI] [PubMed] [Google Scholar]
- [135].Nanjee MN, Doran JE, Lerch PG, Miller NE, Acute effects of intravenous infusion of ApoA1/phosphatidylcholine discs on plasma lipoproteins in humans, Arterioscler Thromb Vasc Biol 19(4) (1999) 979–89. [DOI] [PubMed] [Google Scholar]
- [136].Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA, Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes, J Am Coll Cardiol 53(11) (2009) 962–71. [DOI] [PubMed] [Google Scholar]
- [137].Michael Gibson C, Korjian S, Tricoci P, Daaboul Y, Yee M, Jain P, Alexander JH, Steg PG, Lincoff AM, Kastelein JJ, Mehran R, D’Andrea DM, Deckelbaum LI, Merkely B, Zarebinski M, Ophuis TO, Harrington RA, Safety and Tolerability of CSL112, a Reconstituted, Infusible, Plasma-Derived Apolipoprotein A-I, After Acute Myocardial Infarction: The AEGIS-I Trial (ApoA-I Event Reducing in Ischemic Syndromes I), Circulation 134(24) (2016) 1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Study to Investigate CSL112 in Subjects With Acute Coronary Syndrome (AEGIS-II), 2020. https://clinicaltrials.gov/ct2/show/NCT03473223.
- [139].Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R, Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial, JAMA 290(17) (2003) 2292–300. [DOI] [PubMed] [Google Scholar]
- [140].Calkin AC, Drew BG, Ono A, Duffy SJ, Gordon MV, Schoenwaelder SM, Sviridov D, Cooper ME, Kingwell BA, Jackson SP, Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux, Circulation 120(21) (2009) 2095–104. [DOI] [PubMed] [Google Scholar]
- [141].Spieker LE, Sudano I, Hurlimann D, Lerch PG, Lang MG, Binggeli C, Corti R, Ruschitzka F, Luscher TF, Noll G, High-density lipoprotein restores endothelial function in hypercholesterolemic men, Circulation 105(12) (2002) 1399–402. [DOI] [PubMed] [Google Scholar]