Abstract
Several randomized clinical trials (RCTs) have investigated the effect of dietary advanced glycation end products (AGE) on obesity factors and related hormones in adults; results were conflicting. Therefore, a study was performed to assess the effect of low advanced glycation end products diet on obesity and related hormones. A comprehensive literature search without any limitation on language was conducted using the following bibliographical databases: Web of Science, Scopus, Ovid MEDLINE, Cochrane, and Embase up to October, 2019. From the eligible trials, 13 articles were selected for the systematic review and meta-analysis. Our systematic reviews and meta-analyses have shown a significant decrease in BMI (WMD: − 0.3 kg/m2; 95% CI: − 0.52, − 0.09, p = 0.005; I2 = 55.8%), weight (WMD: − 0.83 kg; 95% CI: − 1.55, − 0.10, p = 0.026; I2 = 67.0%), and leptin (WMD: − 19.85 ng/ml; 95% CI: − 29.88, − 9.82, p < 0.001; I2 = 81.8%) and an increase in adiponectin (WMD: 5.50 µg/ml; 95% CI: 1.33, 9.67, p = 0.010; I2 = 90.6%) levels after consumption of the low AGE diets compared to the high AGE diets. Also, the effect of intake of low AGE compared to high AGE diets was more pronounced in subgroup with duration > 8 weeks for the BMI and weight. Overall, according to our results, although low AGE diets appeared to be statistically significant in reducing the prevalence of obesity and chronic diseases compared to high consumption of dietary AGEs. But, no clinical significance was observed. Therefore, to confirm these results clinically, further prospective studies should be conducted in this regard. The study protocol was registered in the in International prospective register of systematic reviews (PROSPERO) database as CRD42020203734.
Subject terms: Diseases, Gastroenterology, Nutrition
Introduction
Overeating and concomitant obesity is a serious public health problem worldwide with close links to chronic health conditions such as diabetes mellitus and cardiovascular diseases1. Obesity is associated with Inflammation and oxidative stress, which in turn lead to the genesis of non-communicable chronic diseases2,3. Lifestyle modification, with a special focus on dietary pattern, plays an important role in the prevention and control of obesity and its related complication. Recent studies have demonstrated that the consumption of highly processed foods that are extremely high in fat, sugar, salt and potentially toxic compounds known as advanced glycation end-products (AGEs)4,5 are associated with increased risk of chronic diseases6,7. AGEs are a group of sugar modifications which are formed through the non-enzymatic reaction of sugars with free amino groups of proteins, lipids or nucleic acids5,8. AGEs have noticeable pro-inflammatory and prooxidant impacts9. Furthermore a positive association between AGEs intake and serum level of AGEs, visceral fat and insulin resistance has been detected, which suggests a casual role of dietary AGEs in metabolic syndrome independent from energy balance10,11. When that AGE production binding to receptors AGEs (RAGE), AGEs trigger generation of reactive oxygen species (ROS) and initiate a downstream pro-inflammatory signaling cascade including activation of RAGE/TLR4 (Toll-like receptors 4)-NF-κB-ROS pathways12 and contribute to both obesity and related inflammatory diseases13. Since diet derived AGEs increase the risk of obesity and related inflammatory diseases, reduced intake of AGEs is thought to be beneficial, independently from the intake of a standard calorie restricted diet7. Several studies have shown that consumption of low dietary AGEs is associated with reduced circulating and urinary levels of AGE markers and improved anthropometric, glycemic, cardio metabolic and inflammatory indices in individual with overweight and obese14–17. However, the exact mechanism of action of AGEs in obesity-related complications remains unknown. Also there is no established recommendation surrounding the intake of foods with high amount of AGEs such as heat treated cereal or powdered milk which may be considered contributing to a healthy diet. Therefore, this meta-analysis was carried out to analyze the effects of diet-derived AGEs on obesity and related hormones, as well as to discuss the molecular mechanisms of action of these compounds on the development of chronic diseases.
Results
Search results
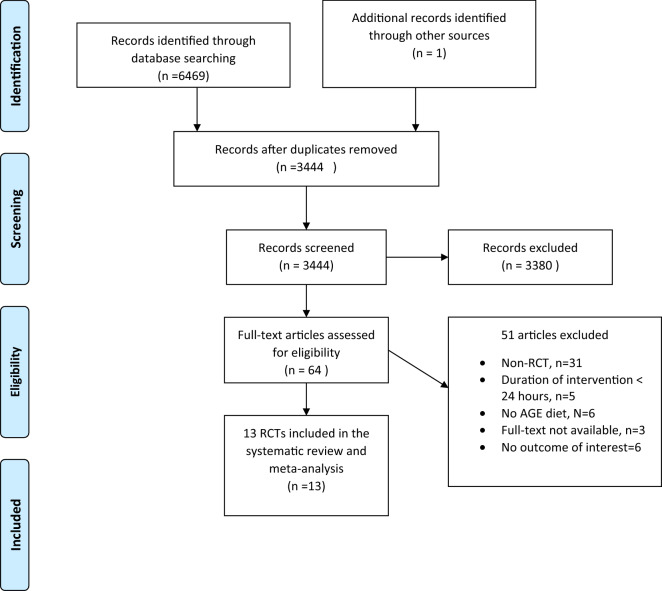
The flow diagram of the process of study selection is shown in Fig. 1. In total, we identified 6469 through database searching and 2 studies identified through other sources. After excluding of duplicates, 3444 papers remained for additional screening based on title and abstract. 3380 one studies were discarded leaving after screening based on title and abstract. Finally, 64 potentially eligible studies for further evaluation that 51 article excluded due to; non-RCT (n = 31), duration of intervention < 24 h (n = 5), no AGE diet (n = 6), full-text not available (n = 3) and no outcome of interest (n = 6). Finally, 13 RCT studies included for this systematic review and meta-analysis.
Figure 1.
Flow chart of study selection process.
Study characteristics
The detailed characteristics of all included studies are described in Table 1. Nine studies used parallel design6,14,15,18–23 and four used cross-over design16,17,24,25. The intervention duration varied from 216,17,24 to 48 weeks6. Four studies have investigated the effects of AGE diet in patients with T2DM18–21, six studies in Obese or overweight people6,14–17,24, two studies in healthy individuals22,23 and one study in women with PCOS25. Overall, ten RCTs reported changes in BMI6,14,15,17,19,20,22–25, seven weight6,14–18,21, three WC6,14,15 , three leptin6,18,23, and tree adiponectin6,18,23, following AGE diet consumption.
Table 1.
Included randomized controlled trial study characteristics by population.
| Study ID | Study design | Participants | Sample size | Intervention diet | Control diet | Duration | dAGE content | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Harcourt 2011 | Cross over | Overweight males aged 18–50 years and BMI 26–39 kg/m2) | 11 | LAGE diet | HAGE diet | 2 weeks | HAGE = 14,090, LAGE = 3302 kU AGE/day | BMI |
| Uribarri 2011 | Parallel | Type 2 diabetic patients | 18 randomised, 18 assessed: 12 LAGE & 6 HAGE | LAGE diet | HAGE diet | 4 months | HAGE = > 20, LAGE = < 10 AGE Eq/day | Weight Leptin adiponectin |
| de Courten 2016 | Cross over | Healthy but overweight individuals | 28 randomised, 20 assessed | LAGE diet | HAGE diet | 2 weeks | HAGE = 59, LAGE = 49 mg CML/day | Weight |
| Mark 2014 | Parallel | Overweight women aged 20–50 years | 99 randomised, 74 assessed: 36 LAGE & 37 HAGE diet | LAGE diet | HAGE diet | 4 weeks | HAGE = 24.6, LAGE = 10.7 mg CML/day | BMI Weight WC |
| Macías-Cervantes 2015 | Parallel | Overweight men (BMI > 25 kg/m2) aged 30 to 55 years | 75 randomised, 45 assessed: 15 in the diet plus exercise group, 14 in the exercise group, and 14 in the diet group | LAGE diet | HAGE diet | 12 weeks | HAGE = 13,284 + 4983, LAGE = 13,019 + 4526 kU AGE/day | BMI Weight WC |
| Tantalaki 2014 | Cross over | Polycystic ovary syndrome (PCOS) age range: 18–40 years | 34 randomised, 23 assessed | LAGE diet | HAGE diet | 8 weeks | HAGE = 1869.6 ± 114.6, LAGE = 1869.6 ± 114.6 kU AGE/day | BMI |
| Di Pino 2016 | Parallel | Adults with prediabetes age range between 35 and 65 years; body mass index (BMI) between 18.5 and 40 kg/ m2 |
62 randomised, 57 assessed: 29 LAGE & 28 HAGE diet |
LAGE diet | HAGE diet | 24 weeks | N/A | BMI |
| Vlassara 2016 | Parallel | Obese subjects with metabolic syndrome aged 50 years or above | 138 randomised, 100 assessed: 51 in LAGE & 49 HAGE | LAGE diet | HAGE diet | 48 weeks | HAGE = 20 + 11, LAGE = 7 + 6 AGE Eq/day | BMI Weight WC Leptin adiponectin |
| Cai 2004 | Parallel | Diabetic patients with normal lipid profile & renal function | 24 randomised: 11 HAGE & 13 LAGE | LAGE diet | HAGE diet | 6 weeks | HAGE = 5 times higher than LAGE | BMI |
| Vlassara 2002 | Parallel | Diabetic subjects with good glycemic control and normal renal function | 11 for cross over and 13 for parallel trials (6 in high and 7 in low AGE diet) | LAGE diet | HAGE diet | 6 weeks | HAGE = 5 times higher than LAGE | weight |
| Baye 2017 | cross over | Overweight and obese otherwise healthy adults | 204 randomised: 11 HAGE & 13 LAGE | LAGE diet | HAGE diet | 2 weeks | HAGE = 3 times higher than LAGE | BMI weight |
| Yacoub2017 | Parallel | Healthy adults aged 50–69 years | 20 randomised, 20 assessed: 10 in each group | LAGE diet | HAGE diet | 6 weeks | HAGE = 26.96 , LAGE = 26.18 AGE Eq/day | BMI |
| Uribarri 2014 | Parallel | Healthy participants over the age of 60 | 18 randomised, 18 assessed: 10 LAGE & 6 HAGE | LAGE diet | HAGE diet | 16 weeks | HAGE = > 15, LAGE = < 10 AGE Eq/day | BMI Leptin adiponectin |
WC; Waist circumference; BMI; Body mass index.
Risk of bias assessment
As shown in Table 2, six studies were assessed as unclear risk in random sequence generation, cause they did not explicitly mention the random sequence generation methods14,18,20,21,23,24 and one as high risk25. Two studies were assessed as low risk in allocation concealment15,16 and one as high risk25, while the other ten studies as unclear. There was one study which was double-blinded, thus considered as low risk of bias for blinding of participants and personnel17. Four of the trials provided a clear description of blinding of outcome assessment6,15–17. Six studies were clear on providing incomplete outcome data and then were considered as low risk of bias6,14,16–19. Six studies were assessed as unclear risk of bias in selective reporting17,20–24, and the other seven studies were assessed as low risk of bias. Except for one study that was considered as high risk in quality25, the other twelve studies were considered as unclear risk of bias, cause we found at least one of their six key domains of quality as unclear.
Table 2.
Risk of bias assessment according to the Cochrane collaboration’s risk of bias assessment tool.
| Study, Year (reference) | Random sequence generation | Allocation concealment | Blinding of participant and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Overall assessment of risk of bias |
|---|---|---|---|---|---|---|---|
| Harcourt 2011 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Uribarri 2011 | Low | Unclear | Unclear | Low | Low | Low | Unclear |
| de Courten 2016 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Mark 2014 | Low | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Macías-Cervantes 2015 | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Tantalaki 2014 | Low | Low | Unclear | Low | Low | Low | Unclear |
| Di Pino 2016 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Vlassara 201(28) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Cai 2004(13) | Low | Low | Unclear | Low | Unclear | Low | Unclear |
| Vlassara 2002 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Baye 2017 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Yacoub 2017 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Uribarri 2014 | High | High | Unclear | Unclear | Unclear | Low | High |
Meta-analysis
Effects of low dietary AGEs on BMI
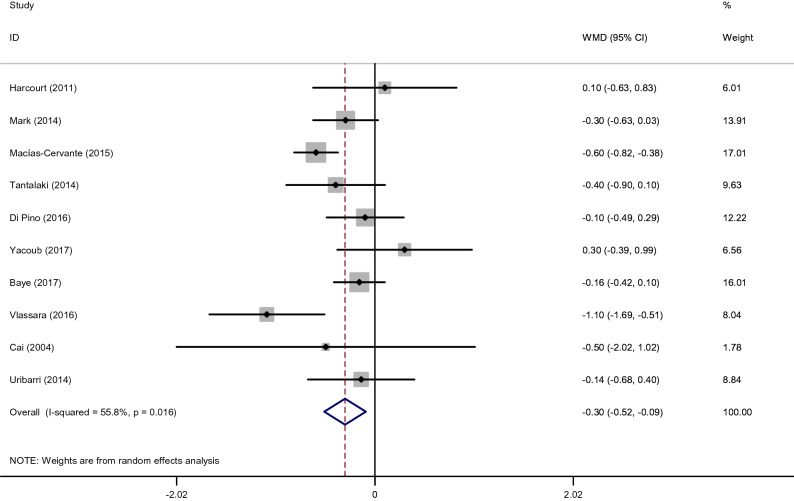
The quantitative analysis of value indicated a significant reduction of BMI after consumption of the low AGE diets compared to the high AGE diets (WMD: − 0.3 kg/m2; 95% CI: − 0.52, − 0.09, p = 0.005; I2 = 55.8%) (Fig. 2). Our findings for the subgroup analyses showed that low AGE diets reduced BMI regardless of length of follow up. These analyses displayed that length of follow up ≥ 8 weeks (WMD: − 0.47 kg/m2, 95% CI: − 0.85, − 0.09) more effectively reduced BMI compared with length of follow up > 8 weeks. Also, the findings from the subgroup analyses showed that BMI levels were significantly reduced in patients with overweight or obese (WMD: − 0.41 kg/m2, 95% CI: − 0.72, − 0.11) (Supplemental Fig. 1).
Figure 2.
Forest plot of randomized controlled trials investigating the effects of low dietary AGEs on BMI.
Effects of low dietary AGEs on Weight
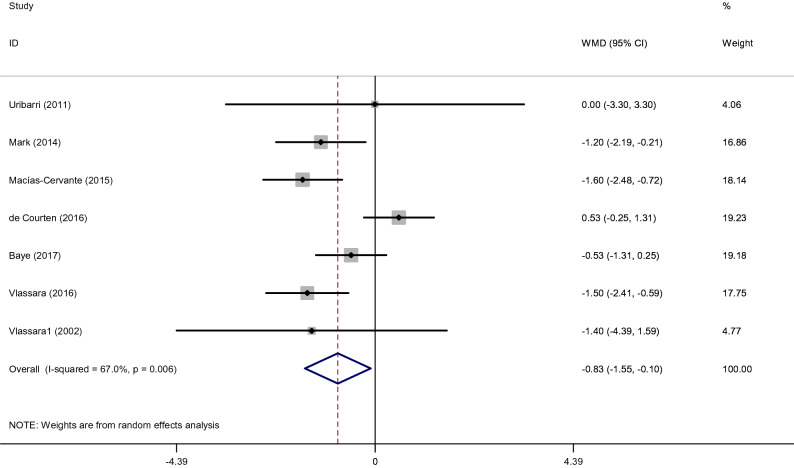
After intake of low AGE compared to high AGE diets, Pooled results from the random-effects model showed a significant decrease in Weight(WMD: − 0.83 kg; 95% CI: − 1.55, − 0.10, p = 0.026; I2 = 67.0%) (Fig. 3). The duration of intervention was considered as a heterogeneity factor on overall effect size. When studies were categorized based on length of follow-up, the effect of intake of low AGE compared to high AGE diets was more pronounced in subgroup with duration > 8 weeks (WMD: − 1.50 kg, 95% CI: − 2.12, − 0.88). In addition, low AGE diets were associated with a significant reduction in weight regardless of the participants’ with overweight or obese (WMD: − 0.84 kg, 95% CI: − 1.65, − 0.02) (Supplemental Fig. 2).
Figure 3.
Forest plot of randomized controlled trials investigating the effects of low dietary AGEs on weight.
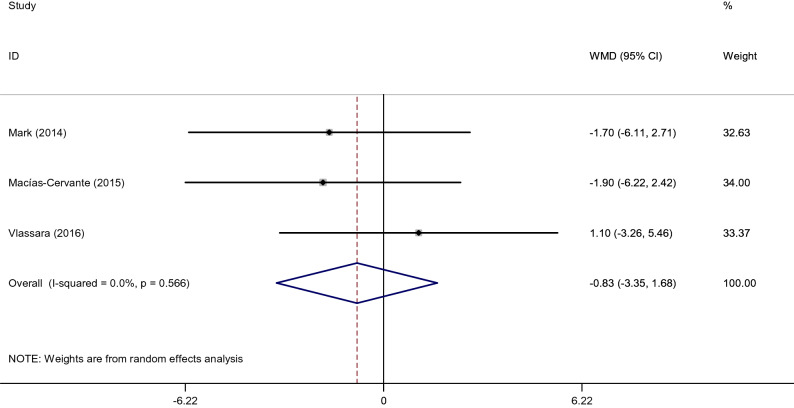
Effects of low dietary AGEs on waist circumference
Among the included studies, three clinical article examined WC. After pooling effect sizes, no significant difference between low and high AGE diets with regards to WC (WMD: − 0.81 cm; 95% CI: − 2.80, 1.18, p = 0.43; I2 = 93.2%) (Fig. 4). Also, subgroup analysis for WC was not possible as there were no enough studies in each group.
Figure 4.
Forest plot of randomized controlled trials investigating the effects of low dietary AGEs on WC.
Effects of low dietary AGEs on leptin and adiponectin
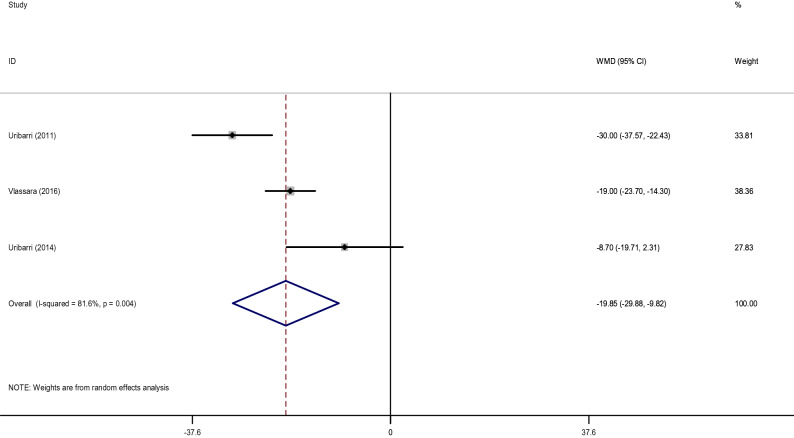
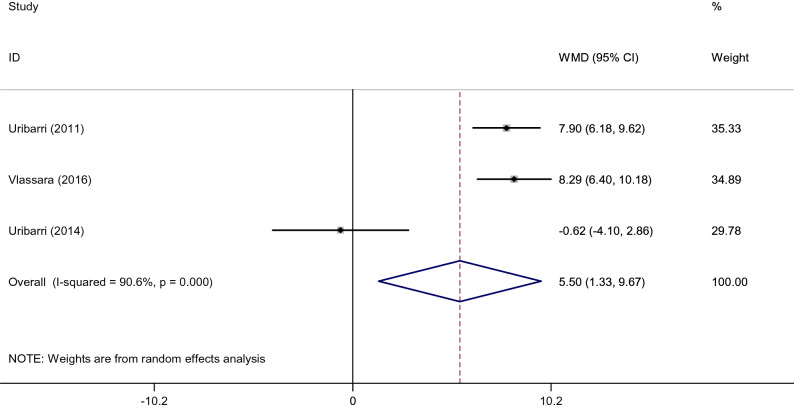
The pooled WMD of 3 effect sizes showed a significant decrease in leptin (WMD: − 19.85 ng/ml; 95% CI: − 29.88, − 9.82, p < 0.001; I2 = 81.8%) (Fig. 5) and an increase in adiponectin levels (WMD: 5.50 µg/ml; 95% CI: 1.33, 9.67, p = 0.010; I2 = 90.6%) (Fig. 6) after consumption of the low AGE diets compared to the high AGE diets. It was not possible to conduct subgroup analysis for leptin and adiponectin with regards to length of follow-up and health status, due to the low number of studies.
Figure 5.
Forest plot of randomized controlled trials investigating the effects of low dietary AGEs on leptin level.
Figure 6.
Forest plot of randomized controlled trials investigating the effects of low dietary AGEs on adiponectin level.
Meta-regression results
We performed a meta‐regression analysis to examine the variation in treatment effect of low AGE diets based on duration of intervention and mean age of participants. The duration of intervention did not demonstrate any significant changes in BMI (p = 0.07, coef: 0.445) and weight (p = 0.43, coef: 0.321). Also, meta‐ regression analysis of mean age of intervention showed no significant effect on BMI (p = 0.86, coef: 0.630) and weight (p = 0.39, coef: 0.673) (supplemental Figs. 3, 4). Due to the limited number of studies on WC, leptin, and adiponectin, we could not perform the meta‐regression analysis.
Sensitivity analysis
We removed each trial from the analysis, step by step, in order to discover the impact of each single study on the combine effect size. We observed no significant effect of any individual study on the combine effect sizes of BMI and Weight (supplemental Fig. 5). Due to the limited number of studies on WC, leptin, and adiponectin, we could not perform the analysis.
Publication bias
A review of the funnel plots used to assess the publication bias is shown in Supplementary Fig. 6. Funnel plot showed no publication bias for BMI and Weight that were confirmed with Egger's regression test (BMI: p = 0.53) and (weight: p = 0.45).
Discussion
This systematic review and meta-analysis was conducted to assess the effects of consumption of low dietary AGEs on obesity and its related hormone. Our findings regarding obesity indices showed that low dietary AGEs could reduce BMI and body weight but had no noticeable impact on waist circumference. Regarding obesity related hormones our results revealed a significant reduction in leptin and rise in adiponectin levels following consumption of low diet derived AGEs. These findings agree with the previous literature reporting that higher dietary consmption of AGEs was linked to increased body weight26,27.The evidently high amounts of AGEs in the diet especially in heat processed foods might be considered as one of the major potential factors contributting to energy over intake26. In a randomized controlled clinical trial, consumption of ultraprocessed foods resulted in markedly excessive energy intake and weight gain in comparision with to an unprocessed diet which caused reduction in body weight28. AGEs may be considered as important dietary compounds that can result in energy imbalance and subseqently increased body weight. However, the underlying mechanism by which higher AGEs content of diet may increase the risk of weight gain is not fully understood. Suggestive evidence obtained from experimental and human studies have demonstrated that higher dietary AGEs intake can induce or aggravate insulin resistance16,29,30. A systemaic review conduced by Clarke and colleagues demonstrated that insulin sensitivity was improved following intake of low dietary AGEs in healthy individuals and patients with type 2 diabetes mellitus. However no change in fasting glucose and HbA1c was observed31.
High-normal insulin levels appear to prevent lipolysis and stimulate lipogenesis in adipocytes32. Also an association between AGEs, insulin resistance, and weight gain has been reported in an in vivo study in Drosophila where increased methylglyoxal stimulated insulin resistance and weight gain30. It seems that hypothalamic inflammation is another pathway whereby higher dietary AGEs intake can increase weight. In an animal study over intake of fat and sugar stimulated hypothalamic inflammatory responses33. In hypothalamic inflammatory state, the signaling pathway of insulin and leptin is impaired which can lead to an adaptive increase of food consumption relative to energy expenditure that advocates weight gain34. However in Harcourt's study, body weight and BMI were not significantly changed after a 2 weeks either isoenergetic low or high dietary AGEs. Matching of energy intake and the short duration of the intervention might explain the null effects on BMI and body weight24. In case of waist circumferences, although the results of two studies14,15 showed beneficial effects of GAEs on WC, analysis the results of all studies6,14,15 showed no overall significant effects. The number of studied assessing the effects of low dietary AGEs on WC was too small. Therefore non-significant effects observed in our analysis might be related to this issue. The duration of intervention is an important factor to obtain real conclusion regarding the effects of nutritional intervention on health outcomes7. The length of interventions varied among studies included in this meta-analysis and the results of subgroup analysis revealed that reduction effects of low AGE diets on body weight and BMI were more pronounced in groups with length of follow up ≥ 8 weeks. Also BMI were significantly reduced in individuals with overweight or obese after intake of a low AGEs diet. Overweight and obesity are considered as the two main risk factors for the development of inflammatory process and insulin resistance35,36. Several Studies have demonstrated that consumption of low amount of dietary AGEs was associated with improved insulin resistance, reduced inflammatory markers and oxidative stress in overweight individuals18,37. Therefore the observed reduction effects of low AGEs diet on BMI might be attributed to its beneficial impacts on inflammation and insulin resistance which is more noticeable in overweight and obesity. However the results of this meta-analysis revealed that the reduction impacts of low dietary AGEs on body weigh were not significantly different between weight subgroups. This finding may be due to the variation in methodology of the studies included in subgroup analysis, differences in the type of prescribed diet or prepared meals in each study and the length of follow up. Regarding adiponectin and leptin, our finding showed that a diet with low AGEs content could significantly increase adiponectin and decrease leptin levels, two important markers of insulin resistance, suggesting that diet derived AGEs also have effects on insulin sensitive tissues38–41. These results are consistent with previous meta-analysis findings showing the same improvement effects of the low AGEs diet on adiponectin and leptin levels42. Another meta-analysis conducted by Kellow et al., has also showed that consumption of a low AGEs diet significantly decreased TNFα and 8-isoprostanes in healthy individuals11. SIRT1 which is a gene encoding protein belonging to the sirtuin family, is considered as a major regulator of inflammatory processes and also adiponectin levels23. Several studies have reported the suppression impacts of oxidant AGEs on gene expression of SIRT118,23. Therefore it is thought that improving impacts of the AGE-restricted diet on insulin resistance be related to its reduction impacts on inflammatory processes, oxidative stress and leptin levels along with an increased in adiponectin and sirtuin-143,44. Also, AGEs storage in adipose tissue by binding to receptors AGEs (RAGE unregulated the production of adipokines, such as adiponectin, leptin, monocyte chemotherapy protein (MCP-1), and plasminogen activator inhibitor type I (PAI-1), which Recent studies have shown that these compound poses a potential risk for cancer and other immune-related diseases through a variety of factors, such as suppressing the immune system and disrupting the regulation of monocytes, basophil, T lymphocytes, and NK cells45,46. AGEs-derived adipokines also appear to increase the production of reactive oxygen species and initiate anti-inflammatory signaling, which in turn further impairs the immune system12.
This systematic review and meta-analysis has a number of strengths. In this study, we performed a systematic review and meta-analysis on a wide range of obesity related factors including values of BMI, WC, Weight, leptin, and adiponectin that have not been reported before. Meta-analysis was also conducted according to subgroups to further detect the results of each risk factor. In addition, publication bias was checked for all of the obesity related factors. Despite the above strengths, some limitations should be considered when interpreting the results. Approximately half of the studies included in this review had poor methodological quality and length of intervention < 24 h. Therefore, we were unable to conduct a meta-analysis for these studies. In addition, differences in characteristic of prescribed diets or meals such as baseline dietary AGE levels, reliability of methods used for food preparation amongst studies must also be taken into account a confounding factor. Also, in most studies, the food was not prepared for the participants (a small number of them, but most of them only provided dietary advice on how to reduce the AGEs in their usual diet), This problem may cause a not match in the total energy received between the groups and the results may be at risk of bias.
In conclusion, our systematic reviews and meta-analyses have shown a significant decrease in BMI, weight, and leptin and an increase in adiponectin levels after consumption of the low AGE diets compared to the high AGE diets. Also, the effect of intake of low AGE compared to high AGE diets was more pronounced in subgroup with duration > 8 weeks for the BMI and weight. According to our study, low AGE diet can be effective in reducing the incidence of obesity and chronic diseases associated with high consumption of dietary AGEs. Therefore, reducing the consumption of processed foods that have high AGE content and changing food preparation methods are good strategies to promote health. Further prospective studies should be conducted in this regard.
Methods
Protocol
The present systematic review and meta-analysis was performed based on the principals of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement47.
Search strategy
A systematic literature search was conducted in four electronic databases: Ovid MEDLINE, ISI Web of Science, Scopus, the Cochrane Central Register of Controlled Trials, and, Embase up to October, 2019. The following MeSH and text keywords were applied to identify relevant articles: (“advanced glycation end products” OR “glycation end products, advanced” OR “maillard reaction” OR “dietary advanced glycation end products” OR “circulating advanced glycation end products”) AND (obesity OR overweight OR adiponectin OR leptin) NOT review*. No language or date restrictions were imposed in the search. The detailed search strategy is provided in the Supplementary Appendix. To find relevant studies, 2 authors (Mh, S and AL) independently screened the titles, abstracts, and full texts of the retrieved articles. The references of the included reviews were also hand-searched to identify further related papers. The study protocol was previously registered with the International prospective register of systematic reviews (PROSPERO) database as CRD42020203734.
Study selection
All adult clinical trials examining the impacts of low dietary intake of AGEs on obesity and related hormone were included. We excluded studies if they were (1) conducted on children; (2) assessed single food item rather than whole diet; (3) lasted < 24 h; 4) not reported sufficient data on targeted outcomes. Reviews, comments, abstracts, letters, case reports and unpublished articles were also excluded from the study.
Data extraction
All studies were stored and managed by Endnote. After reading the selected articles all data were extracted and their integrity and reliability were assessed by two independent reviewers (Mh, S and SF) which were double checked by other authors (FSH and AL). Differences in decisions about the selected studies were resolved by consensus. Extracted information regarding each included study was as follows: title, author name(s), year of study publication, study aim, population's characteristics, sample size, study design, type of intervention (low/high AGE consumption), duration of the study duration, and, means and standard deviations of weight, BMI, WC, leptin, and adiponectin levels at baseline, post treatment and/or changes between baseline and post treatment. . Data regarding obesity related hormones and anthropometric indices were also extracted. The detailed characteristics of all included studies are described in Table 1.
Assessment of risk of bias
The Cochrane Risk of Bias Tool for Randomized Controlled Trials48 was used by two authors to identify potential risks of bias. The quality assessment tool encompasses the following items: adequacy of random sequence generation, allocation concealment, blinding, the detection of incomplete outcome data as well as selective outcome reporting, and other potential sources of bias. Based on the recommendations of the Cochrane Handbook, judgment of each domain was recorded as “Low”, “High”, or “Unclear” risk of bias. Any disagreement in the data extraction and the risk of bias assessment was solved by a third reviewer.
Data analyses
All studies were reviewed based on their main characteristic and results concerning obesity related factors. The primary outcome was Body weight, BMI and waist circumference. The serum levels of leptin and adiponectin were considered as secondary outcomes of interest.
Data synthesis and statistical analysis
Data were combined, if there were ≥ 3 trials within a single grouping using the generic inverse variance approach with random effects model and reported as weighted mean differences (WMDs). The random effects model and reported as weighted mean differences (WMDs) were used because included studies were performed on different populations. The statistical analysis was done using RevMan V.5.3 software and STATA version 12.0 (Stata Corp, College Station, TX, USA). If data were expressed in a different format, standard calculations were executed to obtain the mean and SDs48,49. For instance, if the SDs of the change were not stated in the trials, we derived it using the following formula: SD changes = square root [(SD baseline 2 + SD final 2) − (2 × R × SD baseline × SD final)]. Also, for trials that only reported standard error of the mean (SEM), SDs were obtained using the following formula: SD = SEM × √n, where “n” is the number of subjects in each group. Heterogeneity was examined using the I-squared (I2) statistic, in which source of heterogeneity was determined if the I2 value was > 50%, or if there in the case of inconsistency across RCTs data50. In order to identify potential sources of heterogeneity, a pre-defined subgroup analysis based on amount of low AGE, duration of intervention, and health status of subjects was performed. Meta‐regression was used to determine effect of duration of intervention and mean age of participants on outcomes. A sensitivity analysis was applied to assess the contribution of each study to the overall mean difference. We assessed the presence of publication bias using the formal Egger’s test51.
Supplementary Information
Acknowledgements
This review was supported by Iran University of Medical Sciences, Tehran, Iran
Author contributions
M.H.S and A.L. contributed to design and data extraction. S.F. and F.S. carried out the data analysis. M.H.S, E.S. prepared the manuscript. F.S. and M.H.S. performed the critical review. The manuscript has been revised and approved by all authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-79216-y.
References
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N. Engl. J. Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Sánchez A, et al. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012;68:701–711. doi: 10.1007/s13105-012-0154-2. [DOI] [PubMed] [Google Scholar]
- 4.Henle T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids. 2005;29:313–322. doi: 10.1007/s00726-005-0200-2. [DOI] [PubMed] [Google Scholar]
- 5.Uribarri J, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010;110:911–916. e912. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlassara H, et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2016;59:2181–2192. doi: 10.1007/s00125-016-4053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro PV, Tavares JF, Costa MA, Mattar JB, Alfenas RC. Effect of reducing dietary advanced glycation end products on obesity-associated complications: a systematic review. Nutr. Rev. 2019;77:725–734. doi: 10.1093/nutrit/nuz034. [DOI] [PubMed] [Google Scholar]
- 8.Poulsen MW, et al. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Negrean M, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2007;85:1236–1243. doi: 10.1093/ajcn/85.5.1236. [DOI] [PubMed] [Google Scholar]
- 10.Uribarri J, et al. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity? J. Clin. Endocrinol. Metab. 2015;100:1957–1966. doi: 10.1210/jc.2014-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kellow NJ, Savige GS. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic review. Eur. J. Clin. Nutr. 2013;67:239–248. doi: 10.1038/ejcn.2012.220. [DOI] [PubMed] [Google Scholar]
- 12.Ott C, et al. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajith T, Vinodkumar P. Advanced glycation end products: association with the pathogenesis of diseases and the current therapeutic advances. Curr. Clin. Pharmacol. 2016;11:118–127. doi: 10.2174/1574884711666160511150028. [DOI] [PubMed] [Google Scholar]
- 14.Mark AB, et al. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care. 2014;37:88–95. doi: 10.2337/dc13-0842. [DOI] [PubMed] [Google Scholar]
- 15.Macías-Cervantes MH, et al. Effect of an advanced glycation end product-restricted diet and exercise on metabolic parameters in adult overweight men. Nutrition. 2015;31:446–451. doi: 10.1016/j.nut.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 16.de Courten B, et al. Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial. Am. J. Clin. Nutr. 2016;103:1426–1433. doi: 10.3945/ajcn.115.125427. [DOI] [PubMed] [Google Scholar]
- 17.Baye E, et al. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: a randomised crossover trial. Sci. Rep. 2017;7:1–6. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uribarri J, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care. 2011;34:1610–1616. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Pino A, et al. Low advanced glycation end product diet improves the lipid and inflammatory profiles of prediabetic subjects. J. Clin. Lipidol. 2016;10:1098–1108. doi: 10.1016/j.jacl.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Cai W, et al. High levels of dietary advanced glycation end products transform low-dersity lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation. 2004;110:285–291. doi: 10.1161/01.CIR.0000135587.92455.0D. [DOI] [PubMed] [Google Scholar]
- 21.Vlassara H, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc. Natl. Acad. Sci. 2002;99:15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yacoub R, et al. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS ONE. 2017;12:e0184789. doi: 10.1371/journal.pone.0184789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uribarri J, et al. Suppression of native defense mechanisms, SIRT1 and PPARγ, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids. 2014;46:301–309. doi: 10.1007/s00726-013-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harcourt BE, et al. Targeted reduction of advanced glycation improves renal function in obesity. Kidney Int. 2011;80:190–198. doi: 10.1038/ki.2011.57. [DOI] [PubMed] [Google Scholar]
- 25.Tantalaki E, et al. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS) Hormones. 2014;13:65–73. doi: 10.1007/BF03401321. [DOI] [PubMed] [Google Scholar]
- 26.Cordova R, et al. Dietary intake of advanced glycation end products (AGEs) and changes in body weight in European adults. Eur. J. Nutr. 2019;59:2893–2904. doi: 10.1007/s00394-019-02129-8. [DOI] [PubMed] [Google Scholar]
- 27.Angoorani P, Ejtahed HS, Mirmiran P, Mirzaei S, Azizi F. Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int. J. Food Sci. Nutr. 2016;67:170–176. doi: 10.3109/09637486.2015.1137889. [DOI] [PubMed] [Google Scholar]
- 28.Hall KD, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30:67–77. e63. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birlouez-Aragon I, et al. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am. J. Clin. Nutr. 2010;91:1220–1226. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- 30.Moraru A, et al. Elevated levels of the reactive metabolite methylglyoxal recapitulate progression of type 2 diabetes. Cell Metab. 2018;27:926–934. e928. doi: 10.1016/j.cmet.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT. Dietary advanced glycation end products and risk factors for chronic disease: a systematic review of randomised controlled trials. Nutrients. 2016;8:125. doi: 10.3390/nu8030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018;16:232. doi: 10.1186/s12916-018-1225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Y, et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol. Metab. 2017;6:897–908. doi: 10.1016/j.molmet.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisse BE, Schwartz MW. Does hypothalamic inflammation cause obesity? Cell Metab. 2009;10:241–242. doi: 10.1016/j.cmet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee A, Sharma D, Trivedi R, Singh J. Treatment of insulin resistance in obesity-associated type 2 diabetes mellitus through adiponectin gene therapy. Int. J. Pharm. 2020 doi: 10.1016/j.ijpharm.2020.119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aleidi S, Issa A, Bustanji H, Khalil M, Bustanji Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm. J. 2015;23:250–256. doi: 10.1016/j.jsps.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luévano-Contreras C, Garay-Sevilla ME, Wrobel K, Malacara JM, Wrobel K. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J. Clin. Biochem. Nutr. 2012;52:22–20. doi: 10.3164/jcbn.12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat. Rev. Endocrinol. 2009;5:367. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 39.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 40.de Kreutzenberg SV, et al. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2010;59:1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardellini M, et al. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009;58:2396–2401. doi: 10.2337/db09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baye E, Kiriakova V, Uribarri J, Moran LJ, De Courten B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein α transcriptional complex. J. Biol. Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 44.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Gaens KH, Stehouwer CD, Schalkwijk CG. The Nε-(carboxymethyl) lysine–RAGE axis: putative implications for the pathogenesis of obesity-related complications. Expert Rev. Endocrinol. Metab. 2010;5:839–854. doi: 10.1586/eem.10.68. [DOI] [PubMed] [Google Scholar]
- 46.Gaens KH, et al. Nε-(carboxymethyl) lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2014;34:1199–1208. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 47.Shamseer L, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 48.Higgins, J. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. www.cochrane-handbook.org (2011).
- 49.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.