ABSTRACT
Background: Humans have an evolutionary need for a well-preserved internal ‘clock’, adjusted to the 24-hour rotation period of our planet. This intrinsic circadian timing system enables the temporal organization of numerous physiologic processes, from gene expression to behaviour. The human circadian system is tightly and bidirectionally interconnected to the human stress system, as both systems regulate each other’s activity along the anticipated diurnal challenges. The understanding of the temporal relationship between stressors and stress responses is critical in the molecular pathophysiology of stress-and trauma-related diseases, such as posttraumatic stress disorder (PTSD).
Objectives/Methods: In this narrative review, we present the functional components of the stress and circadian system and their multilevel interactions and discuss how traumatic stress can affect the harmonious interplay between the two systems.
Results: Circadian dysregulation after trauma exposure (posttraumatic chronodisruption) may represent a core feature of trauma-related disorders mediating enduring neurobiological correlates of traumatic stress through a loss of the temporal order at different organizational levels. Posttraumatic chronodisruption may, thus, affect fundamental properties of neuroendocrine, immune and autonomic systems, leading to a breakdown of biobehavioral adaptive mechanisms with increased stress sensitivity and vulnerability. Given that many traumatic events occur in the late evening or night hours, we also describe how the time of day of trauma exposure can differentially affect the stress system and, finally, discuss potential chronotherapeutic interventions.
Conclusion: Understanding the stress-related mechanisms susceptible to chronodisruption and their role in PTSD could deliver new insights into stress pathophysiology, provide better psychochronobiological treatment alternatives and enhance preventive strategies in stress-exposed populations.
KEYWORDS: Circadian system, stress, trauma, posttraumatic stress disorder (PTSD), HPA axis, autonomic nervous system, cortisol, glucocorticoids, sleep
HIGHLIGHTS
• The human circadian and stress system are both essential for biobehavioural regulation with numerous reciprocal interaction. • Posttraumatic chronodisruption (i.e., circadian dysregulation after trauma) represents a core feature of PTSD, mediating neurobiological correlates of trauma through multilevel temporal order loss.
Abstract
Antecedentes: Los seres humanos tenemos una necesidad evolutiva por un ‘reloj’ interno bien preservado, ajustado al periodo de rotación de 24 horas de nuestro planeta. Este sistema de sincronización circadiano intrínseco permite la organización temporal de numerosos procesos psicólogicos, desde la expresión génica al comportamiento. El sistema circadiano humano está estrecha y bidireccionalmente interconectado al sistema humano de estrés, dado que cada uno de ellos regula la actividad del otro a lo largo de los desafíos diurnos esperados. La comprensión de la relación temporal entre estresores y respuestas a estrés es crítica en la fisiopatología molecular de las enfermedades relacionadas al estrés y al trauma, como el trastorno de estrés postraumático (TEPT).
Objetivos/Métodos: En esta revsión narrativa, presentamos los componentes funcionales de los sistemas circadiano y de estrés junto a sus interacciones multinivel, y discutimos cómo el estrés traumático puede afectar a la interacción armoniosa entre los dos sistemas.
Resultados: La disregulación circadiana luego de la exposición al trauma (cronodisrupción postraumática) puede representar una característica central de los trastornos relacionados al trauma, mediando correlatos neurobiológicos duraderos del estrés traumático a través de una pérdida del órden temporal a diferentes niveles organizacionales. La cronodisrupción postraumática puede, por lo tanto, afectar a propiedades fundamentales de los sistemas neuroendocrino, inmune y autonómico, llevando a un quiebre de los mecanismos adaptativos biocomportamentales con un incremento de la sensibilidad y vulnerabilidad al estrés. Dado que muchos eventos traumáticos ocurren en altas horas de la tarde o la noche, describimos también cómo la hora de la exposición al trauma puede afectar diferentemente al sistema de estrés y, finalmente, discutimos potenciales intrevenciones cronoterapéuticas.
Conclusiones: La comprensión de los mecanismos relacionados al estrés suceptibles de cronodisrupción y su rol en el TEPT podría ofrecer nuevas perspectivas a la fisiopatología del estrés, entregar mejores alternativas de tratamiento psicocronobiológicas y mejorar la estrategias preventivas en las poblaciones expuestas a estrés.
Palabras clave: Sistema Circadiano, estrés, trauma, trastorno de estrés postraumático (TEPT), Eje HHA, sistema nervioso autónomo, cortisol, glucocorticoides, sueño
Abstract
背景: 人类对保存完好, 适应我们星球24小时旋转周期的内部‘时钟’有进化的需求。这种内在的昼夜节律计时系统使从基因表达到行为的许多生理过程得到时间上的组织。人类昼夜节律系统与人类应激系统紧密且双向互连, 因为这两个系统都将根据预期的日间挑战来调节彼此的活动。了解应激源和应激反应之间的时间关系对于应激和如创伤后应激障碍 (PTSD) 的创伤相关疾病的分子病理生理学至关重要。
目的/方法: 在本篇叙事性综述中, 我们介绍了应激和昼夜节律系统的功能构成以及它们之间的多水平交互作用, 并讨论了创伤性应激如何影响两个系统之间的协调交互作用。
结果: 创伤暴露后的昼夜节律失调 (创伤后计时失调) 可能代表了创伤相关疾病的一个核心特征, 通过在不同组织水平上时间顺序的丢失来调节创伤性应激相关神经生物学机制的维持。因此, 创伤后计时失调可能会影响神经内分泌, 免疫和自主神经系统的基本特性, 从而增强应激敏感性和易感性, 导致生物行为适应机制的崩溃。鉴于许多创伤事件发生在傍晚或夜间, 我们还描述了一天中创伤暴露的时间如何可以不同地影响应激系统, 最后讨论了潜在的计时疗法。
结论: 了解计时失调易感的应激相关机制及其在PTSD中的作用可以为应激病理生理学带来新见解, 提供更好的时间性生物心理治疗替代方法, 并提高在创伤暴露人群中的预防策略。
关键词: 昼夜节律, 应激, 创伤, 创伤后应激障碍 (PTSD), HPA轴, 自主神经系统, 皮质醇, 糖皮质, 激素, 睡眠
1. Introduction
The earth’s rotation around its own axis has created a geophysical evolutionary need for internal adjustment to the dramatic energy demand fluctuations between night and day across phylogeny. This need resulted in a highly conserved and sophisticated internal time keeping system, creating a strict temporal organization and an endogenous rhythmicity adjusted to the earth’s 24-hour rotation (Ko & Takahashi, 2006; Panda, Hogenesch, & Kay, 2002; Paranjpe & Sharma, 2005). This intrinsic circadian (lat. circa diem – around a day) timing system, creates an internal representation of the external Zeitraum (germ. time-space) in order to synchronize homoeostatic mechanisms (Hastings, Maywood, & Brancaccio, 2018; Hastings, O’Neill, & Maywood, 2007) and create a dynamic ‘internal milieu’ that oscillates with a 24-h rhythm. The circadian system, thus prepares living organisms for the expected cyclic challenges, from gene expression to behaviour (Dibner, Schibler, & Albrecht, 2010; Moore, 2013; Saper, 2013; Takahashi et al., 2008).
Thereby, the interaction of the circadian system with another fundamental system, the stress response system, is vital. The stress response system has per se a baseline, circadian activity (Buijs, van Eden, Goncharuk, & Kalsbeek, 2003), which, however, is affected by numerous cognitive, emotional, neurosensory, humoral, immune, blood-borne, digestive, thermostatic, limbic and peripheral somatic signals through different pathways. When stressors exceed a certain severity or temporal threshold, stressor-related information initiates a complex stress response to induce remarkably consistent acute, normally adaptive and time-limited micro-, meso- and macrophysiologic compensatory responses, redirecting energy according to the current needs (Chrousos, 2009; Elenkov & Chrousos, 2006; Nicolaides, Kyratzi, Lamprokostopoulou, Chrousos, & Charmandari, 2015; Ulrich-Lai & Herman, 2009). Together, these responses through different stress effector tissues produce an orchestrated ‘symphony’ enabling a fine-tuned response to challenge (Joels & Baram, 2009).
Thus, the circadian and the stress response system are both essential for survival and regulate each other’s activity, through multiple reciprocal interactions (Morris, Aeschbach, & Scheer, 2012a; Nader, Chrousos, & Kino, 2010). An intact communication between the circadian and the stress system is a vital premise for preserving homeodynamic balance and enhancing environmental adaptation (i.e., resilience) (Buijs, Escobar, & Swaab, 2013, Koch, Leinweber, Drengberg, Blaum, & Oster, 2017; Tsang, Barclay, & Oster, 2014). Investigating the interactions between the two systems is critical for the understanding of pathophysiological trajectories mediating risk for disease (Helfrich-Forster, 2017). However, during the last decades, technological advances and new social norms have cultivated a new, round-the-clock lifestyle, which enhances a temporal misalignment between internal and geophysical/social circadian cycles and may lead to an altered homeodynamic state (dyshomeostasis/allostasis, or more accurately, cacostasis) with accumulated allostatic load (cacostatic load) with higher stress sensitivity and vulnerability for stress-related disorders. Understanding the pathways susceptible to circadian dysregulation following stress and their role in stress-related disorders could deliver new insights into pathophysiology of associated disease mechanisms (Germain, Buysse, & Nofzinger, 2008; Roenneberg & Merrow, 2003; Takahashi, Shimomura, & Kumar, 2008).
In this review, following a general overview of the human circadian and stress system and their multilevel interactions, we discuss how excessive (i.e., traumatic) stress can affect this interplay and lead to a reversible or sustained circadian dysregulation in the paradigm of posttraumatic stress disorder (PTSD). At the final part of this review we also address possible chronotherapeutic interventions for the prevention and treatment of PTSD.
2. The human stress and circadian system
2.1. The human stress system
The human stress system is crucial for adaptive responses to external and internal stressors and comprises of central and peripheral components (Charmandari, Tsigos, & Chrousos, 2005; Chrousos, 2009; Chrousos & Gold, 1992; Tsigos & Chrousos, 2002; Ulrich-Lai & Herman, 2009) (cf. Figure S1). The peripheral components of the human stress system include: a) the hypothalamic-pituitary-adrenal (HPA) axis and b) the limbs of the autonomic nervous system (ANS) i.e., i) the sympathetic nervous (SNS) and sympatho-adrenomedullary (SAM) system and ii) the parasympathetic nervous system (PNS), which all exert complementary actions throughout the body (Agorastos et al., 2020). The principal peripheral effector molecules are catecholamines (CAs; norepinephrine and epinephrine) for the SAM, acetylcholine (Ach) for the PNS and glucocorticoids (GCs; i.e., cortisol in humans) for the HPA axis. GCs influence countless physiologic actions through genomic, nongenomic and mitochondrial actions of the intracellular cognate glucocorticoid and mineralocorticoid receptors (GR, MR) and are essential for activating, maintaining and downregulating the stress response (Chrousos, Charmandari, & Kino, 2004; Chrousos & Kino, 2009, Nicolaides, Galata, Kino, Chrousos, & Charmandari, 2010; Nicolaides et al., 2015). Upon ligand-binding, the receptors translocate to the nucleus and bind to specific DNA response elements in the regulatory regions of responsive genes, leading to their transactivation or transrepression (Chrousos & Kino, 2009; Nicolaides et al., 2010; Oakley & Cidlowski, 2013). Altered GC-signalling, through dysregulations at different levels of the HPA axis, may have deleterious effects for the organism (Chrousos & Kino, 2009; Rohleder, Wolf, & Wolf, 2010).
2.2. The human circadian system
The mammalian circadian system represents an extensive network of time-keeping machineries that generate and preserve a cellular and systemic rhythmicity, through temporal organization and coordination of countless transcriptional oscillating processes throughout all structural levels in the organism (i.e., hormonal fluctuations, sleep/wakefulness, immune activity, thermoregulation, energy household, gene expression) (Dibner et al., 2010; Hastings et al., 2018; Moore, 2013; Saper, 2013). The circadian system is organized in a hierarchical manner with a central, pacemaking “master clock“ in the central nervous system (CNS) and a peripheral, subordinated and adaptive multi-oscillator system (‘slave clocks’) (cf. Figure S2). The suprachiasmatic nucleus (SCN) is the integrative ‘master clock’ of the organism with a distinct intrinsic molecular pacemaker activity based on a main and an auxiliary transcriptional/translational feedback loop (TTFL) of a core set of clock genes (e.g., circadian locomotor output cycle kaput, CLOCK; brain-muscle-ARNT-like protein 1, BMAL-1; period 1–3, PER1-3; cryptochrome 1–2, CRY1-2; reverse viral erythroblastosis oncogene product α/β, REV-ERBα/β; retinoic acid receptor-related orphan receptorα, RORα) maintaining an approximately 24-hour oscillation (cf. Figure S3). All peripheral ‘slave clocks’ show also a tissue-specific and cell-autonomous molecular rhythm generation, which, however, is kept synchronized by the main integrative SCN rhythm via different pathways (cf. Figure S2). This orchestration of all diverging tissue-specific peripheral oscillations into a main rhythmic symphony is of vital importance for the promotion of homoeostasis and adaptation in higher organisms.
2.3. Circadian properties of the stress system
The circadian system upregulates the stress system before the organism’s active phase and downregulates it again for the resting and restorative phase. (Gamble, Berry, Frank, & Young, 2014). HPA axis and ANS activity both show a distinct circadian pattern at rest. Circulating GCs (i.e., cortisol, CORT) levels exhibit a robust diurnal fluctuation, with a sharp rise in the middle of the biological night, peaking in the early morning, and a nadir preceding the habitual inactive phase (Dickmeis, 2009; Gan & Quinton, 2010; Nader et al., 2010; Qian, Droste, Lightman, Reul, & Linthorst, 2012). Similarly, major human autonomic markers, such as heart rate, blood pressure, baroreflex, heart rate variability, plasma epinephrine and norepinephrine levels also show robust circadian variations with a distinct peak of SNS activity and nadir of PNS activity in the morning hours (Portaluppi et al., 2012; Scheer, Van Doornen, & Buijs, 2004; Vandewalle et al., 2007). By doing so, HPA axis and ANS prepare the organism for the higher energetic demand of the waking phase (Gamble et al., 2014).
The circadian system orchestrates the circadian activity and reactivity of the HPA axis through both hormonal and neuronal pathways (cf. Figure 2). Apart from the negative feedback regulation of adrenocorticotropic hormone (ACTH) release through GC systemic levels (Jacobson, 2005), there are another five main pathways of circadian influence on the HPA axis, all five involved in the steroidogenic pathway and the ACTH-dependent transduction cascade in the zona glomerulosa and zona fasciculata of the adrenal gland: i) direct influence on HPA axis through neuronal SCN projections at the hypothalamic level (i.e., subparaventricular area, subPMV; dorsomedial nucleus of the hypothalamus, DMH) modulating corticotropin releasing hormone and arginine-vasopressin (CRH/AVP) secretion from parvocellular paraventricular nucleus (PVN) neurons, ii) direct SCN influence on the adrenal glands through multisynaptic autonomic innervation of the adrenal medulla and from there through CAs of the cortex, modulating diurnal ACTH sensitivity of the cortex and stimulating the GC circadian release through an HPA axis-independent manner, iii) intrinsic peripheral rhythms of local adrenal clocks gene expression, iv) direct melatonergic influence of adrenal GC production and release through ACTH adrenal response prevention and the melatonin (MLT) receptor 1 (MT1)-related inhibition of CORT production through the 3β-hydroxysteroid dehydrogenase (3β -HSD) enzyme and v) direct melatonergic modulation of GR acetylation rhythm, GR translocation to the nuclei and GR-related transcriptional activity through MTs (Agorastos et al., 2020). Jointly, this illustrates a multi-level circadian ‘gating’ control on the physiological GC secretion rhythm through SCN, HPA axis and ANS activity, GC and MLT levels and the robust intrinsic rhythm of the adrenal gland itself, involving clock gene expression in the metabolism and secretion of GCs (Buijs et al., 2003; Dickmeis, 2009; Oster et al., 2006).
Figure 2.
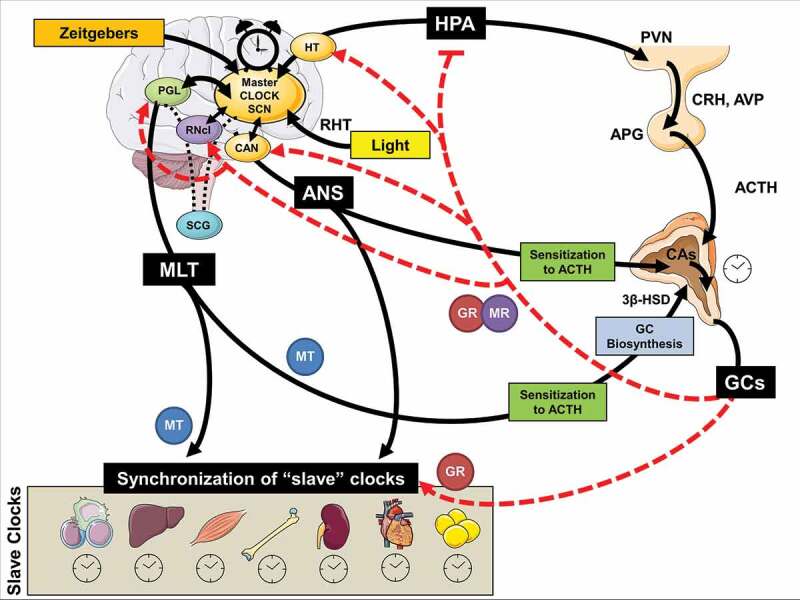
Schematic model of multilevel interactions between the human stress and circadian system
3β-HSD: 3β-Hydroxysteroid dehydrogenase; ANS: autonomic nervous system; ACTH: adrenocorticotropic hormone; APG: anterior pituitary gland; AVP: arginine vasopressin; CA: catecholamines; CAN: central autonomic network; CRH: corticotropin releasing hormone; HPA: hypothalamus-pituitary-adrenal axis; HT: hypothalamus; GC: glucocorticoids; GR: glucocorticoid receptor; MLT: melatonin; MT: MLT receptor; MR: mineralocorticoid receptor; PGL: pineal gland; PVN: paraventricular nucleus; RHT: retinohypothalamic tract; RNcl: raphe nucleus; SCG: superior cervical ganglia; SCN: suprachiasmatic nucleus.
These neurohumoral circadian influences on the stress system have further molecular underpinnings at the cellular level, with the GR playing a fundamental role. For example, CLOCK/BMAL1 heterodimer behaves as a reverse-phase negative regulator of GR reducing GR’s affinity to its cognate GC response elements (GREs) and decreasing GR-induced transcriptional activity of GC-responsive genes through acetylation at multiple lysine residues in the ligand-binding domain of the GR (Charmandari et al., 2011; Han, Lee, Kim, Kim, & Cho, 2014, Kino & Chrousos, 2011; Nader, Chrousos, & Kino, 2009). This leads to a circadian fluctuation in the GR transactivational activity in reverse phase with CLOCK/BMAL1 mRNA expression (Nader et al., 2009) with higher GR acetylation and decreased tissue GC sensitivity in the morning, mirroring the circadian pattern of serum GC concentrations (Charmandari et al., 2011).
3. Stress and circadian dysregulation
3.1. Defining chronodisruption
Biologically relevant disruption of circadian rhythms (chronodisturbance) are likely to occur over time depending on several intrinsic and extrinsic factors, but this process is being constantly physiologically compensated by the organism. However, when chronodisturbance exceeds a certain homoeostatic threshold to chronicity, phase or amplitude, the misalignment of the circadian rhythm leads to a critical loss of the harmonious biological temporal order throughout different organizational levels and is defined as chronodisruption (Erren & Reiter, 2009; Zelinski, Deibel, & McDonald, 2014). Chronodisruption, represents a breakdown and dissociation of mutual entrainment and temporal relationship among different oscillatory subsystems and may gradually change the fundamental properties of brain systems regulating neuroendocrine, immune metabolic and autonomic function, as well as restorative processes (Smolensky, Hermida, Reinberg, Sackett-Lundeen, & Portaluppi, 2016). Hereby, chronodisruption may, thus, alter biobehavioral adaptations to stressors, including gene expression, with increased stress sensitivity and vulnerability to stress-related disorders through various pathways (see above and Figure 2) (McEwen & Karatsoreos, 2015; Meerlo, Sgoifo, & Suchecki, 2008; Morris, Aeschbach, & Scheer, 2012b). Chronodisruption-related cacostatic load with short- and long-term pathophysiologic and epigenetic consequences (Orozco-Solis & Sassone-Corsi, 2014; Zelinski et al., 2014) can lead to a wide range of biological consequences in the organism (Karatsoreos, 2011; McEwen & Karatsoreos, 2015; Scheer, Hilton, Mantzoros, & Shea, 2009; Zelinski et al., 2014).
3.2. Sleep and circadian system
In clinical human research, the relationship between circadian system and sleep regulatory processes is of particular importance as both greatly influence a plethora of central and downstream physiologic processes in diurnal fashion (Panda et al., 2002). For example, the sleep/wake cycle is often considered the most overt manifestation of the circadian system (Paranjpe & Sharma, 2005). Indeed, despite some findings suggesting an independence of sleep and circadian regulatory processes (Ko & Takahashi, 2006), mounting evidence supports rather a very close, interdependent and reciprocal interaction of both systems throughout several functional levels (Hastings et al., 2018, 2007; Paranjpe & Sharma, 2005), regularly relating to brain areas also involved in the neurocircuitry of the central stress system (Dibner et al., 2010; Hastings et al., 2018; Takahashi et al., 2008).
Sleep is regulated by both a homoeostatic and a circadian process (Paranjpe & Sharma, 2005). These two processes jointly influence most properties of soporific and alerting homoeostatic neurocircuitry. This two-process model posits that the main aspects of sleep regulation rely on the interaction of a homoeostatic process (process S – defined by the prior amount of sleep and waking), with a circadian process controlled by the SCN (process C) (Buijs et al., 2003; Moore, 2013; Saper, 2013). Although during the years, additional sleep regulation models emerged, the concept of two processes influencing sleep physiology and behaviour is generally accepted.
Arousal and wakefulness are maintained through a dorsal cholinergic pathway from the pontine tegmentum to the thalamus and a ventral aminergic pathway from the locus coeruleus and raphe nuclei to the lateral hypothalamus (LH) and cerebral cortex, where orexin plays a major role (Hastings et al., 2018). On the other hand, sleep promotion and maintenance is sustained by neurons of the ventrolateral preoptic area (VLPOA) affecting hypothalamic and brainstem arousal centres through γ-aminobutyric acid (GABA)-ergic and galanininergic transmission (Hastings et al., 2018). The central circadian system is able to influence those sleep and wake pathways through indirect connections of the SCN to several hypothalamic nuclei and the VLPO, and vice versa (Paranjpe & Sharma, 2005; Ulrich-Lai & Herman, 2009). In addition, MLT, the core hormonal output of the circadian system, is considered to be greatly implicated in sleep regulation and have sleep-gating and soporific properties by muting wakefulness mechanisms through MT1 and MT2 receptors and, thus, mediate a closer interaction between the circadian system and sleep/wake neurocircuitry (Chrousos, 2009; Elenkov & Chrousos, 2006; Nicolaides et al., 2015).
Sleep deprivation and forced desynchrony experiments in the 1990s offered strong in-vivo evidence of objective and subjective measures being influenced by both endogenous clock phase and duration of prior sleep and waking (for a review, please see (Paranjpe & Sharma, 2005)). In particular, those experiments confirmed a strong influence of several objective and subjective sleep parameters on clock functioning, as well as the fact that circadian clock phase is less susceptible phase change during high sleep pressure (Paranjpe & Sharma, 2005). Interestingly, solid evidence of genetic studies confirms that clock genes play a major role in sleep homoeostasis (Joels & Baram, 2009).
Respectively, SD and circadian dysregulation are very hard to functionally separate and regularly co-occur in clinical populations (Buijs et al., 2013; Helfrich-Forster, 2017; Koch et al., 2017; Morris et al., 2012a; Nader et al., 2010; Paranjpe & Sharma, 2005; Tsang et al., 2014). Circadian disruption is closely associated with sleep pattern changes and vice versa (Buijs et al., 2013; Helfrich-Forster, 2017; Koch et al., 2017; Morris et al., 2012a; Tsang et al., 2014). Interestingly, even circadian clock polymorphisms and clock gene expression regularly manifest behaviourally as SD (Roenneberg & Merrow, 2003; Takahashi et al., 2008), while conversely, SD can alter clock gene expression (Charmandari et al., 2005; Germain et al., 2008). Thus, the terms chronodisruption and SD in human clinical research are closely associated and usually find use interchangeably on the practical level for the assessment of circadian rhythmicity and the description of the diurnal timing phenomenology especially in clinical populations (e.g., affective, neurodegenerative, neurodevelopmental disorders) (Agorastos et al., 2020; Buijs et al., 2013; Chrousos & Gold, 1992; Helfrich-Forster, 2017; Nicolaides et al., 2010; Paranjpe & Sharma, 2005; Tsigos & Chrousos, 2002).
3.3. Chronodisruption-related findings in clinical human research
Chronodisruption is a disturbance of biological timing, which can occur at different organizational levels and/or between different organizational levels, ranging from molecular rhythms in individual cells to misalignment of behavioural cycles with environmental changes and not restricted to the central circadian rhythm (circadian misalignment/desynchrony). Assessing the endogenous circadian rhythm in humans, requires elaborated experimental protocols in specialized facilities (e.g. forced desynchrony protocols) and are therefore labour-intensive, time-consuming, costly and non-comfortable for participants, while they do not take usual environmental factors into consideration nor the adaptive and integrative function of the circadian system. Applied, in-vivo human research therefore rather uses other objective proxy parameters for the assessment of the circadian system activity (e.g., sleep/wake timing, sleep stages, diurnal endocrine measures – especially melatonin and cortisol, physiological monitoring, locomotor activity, core body temperature) in semi-controlled or normal-life conditions (Bedrosian, Fonken, & Nelson, 2016; Novakova & Sumova, 2014; Smolensky et al., 2016; Vetter, 2020). These parameters are considered valid assessments of circadian output in humans (Germain & Kupfer, 2008; Walker et al., 2020). Chronodisruption, thus, represents a broader term, which includes more specific pathophysiological phenomena like, for example, sleep disturbances (SD), disrupted endocrine, activity and social rhythms (Walker et al., 2020).
Various SD in humans have been associated with alterations of the physiologic oscillations of clock gene expression (Ackermann et al., 2013; Moller-Levet et al., 2013), HPA axis dysregulation (e.g., flattened CORT rhythm amplitude, blunted CORT awakening response (CAR), increased but also decreased diurnal CORT levels, higher CRH levels, attenuated pituitary ACTH response, increased adrenocortical ACTH sensitivity) (Backhaus, Junghanns, & Hohagen, 2004; Buckley & Schatzberg, 2005; Meerlo et al., 2008; Rodenbeck & Hajak, 2001, Vgontzas et al., 2001; Wright, Valdimarsdottir, Erblich, & Bovbjerg, 2007) and altered autonomic regulation (i.e., increased SAM and reduced PNS activity and blunting of cardiovascular autonomic rhythmicity and responsiveness) (Meerlo et al., 2008; Mullington, Haack, Toth, Serrador, & Meier-Ewert, 2009, Ruger & Scheer, 2009). Accordingly, SD in humans has been also associated with increased risk for cardiovascular morbidity, metabolic consequences, inflammation, immune dysregulation, psychiatric disorders and even elevated cancer risk (Albrecht, 2017; Fujino et al., 2006; Savvidis & Koutsilieris, 2012; Sookoian et al., 2007; Stevens et al., 2011). The particular association between SD/chronodisruption and psychopathology was first officially noted by Emil Kreapelin in 1883 (Kraeplin, 1883) and has evolved since then through the years by numerous biological findings (Wulff, Gatti, Wettstein, & Foster, 2010). In the last years, a close association between specific clock gene polymorphisms, circadian phase and vulnerability to chronodisruption has emerged (Chellappa et al., 2014; Maire et al., 2014; Reichert et al., 2014), suggesting that clock gene polymorphisms and circadian gene expression may play a crucial role in the individual risk for stress-related disorders (Liberman, Halitjaha, Ay, & Ingram, 2018).
3.4. Posttraumatic chronodisruption
Apart from other Zeitgebers that can influence and potentially dysregulate circadian rhythms (e.g., SD, nutrition, light), physical, psychological, inflammatory or metabolic stress can also lead to acute/reversible or sustained chronodisruption. These stress-related effects on biological rhythms have enhanced a recent research interest on the potential causal role of SD and chronodisruption in the pathophysiology of trauma-related disorders, suggesting that dysregulation of circadian rhythmicity may be implicated in the acute pathophysiology and development of these disorders (Agorastos et al., 2020, 2018; Agorastos, Kellner, Baker, & Otte, 2014; Germain, 2013, Germain et al., 2008; Mellman & Hipolito, 2006). Traumatic stress exposure may cause both immediate and long-lasting SD/chronodisruption (cf. Figure 1) (Lavie, 2001; Philbert et al., 2011), which may represent a core, rather than a secondary pathway mediating the long-term effects and enduring neurobiological correlates of trauma (Germain et al., 2008; Lavie, 2001; Mellman, Bustamante, Fins, Pigeon, & Nolan, 2002; Mellman & Hipolito, 2006, Mellman, Knorr, Pigeon, Leiter, & Akay, 2004, Spoormaker & Montgomery, 2008). Accordingly, several human cohort studies have associated early-life traumatic stress exposure with adult SD years later (Agorastos et al., 2019, Baiden, Fallon, den Dunnen, & Boateng, 2015, Greenfield, Lee, Friedman, & Springer, 2011, Kajeepeta, Gelaye, Jackson, & Williams, 2015, Lind, Aggen, Kendler, York, & Amstadter, 2016). Posttraumatic chronodisruption could negatively influence the traumatic memory encoding and consolidation (Henckens, Hermans, Pu, Joels, & Fernandez, 2009, Sopp, Brueckner, Schäfer, Lass-Hennemann, & Michael, 2019) and at the same time enhance maladaptive neuroendocrine, immune, metabolic and autonomic stress regulation, resulting in the extensive symptomatology and comorbidity of trauma-related disorders (Agorastos et al., 2020, 2018, 2019; Agorastos, 2017; Agorastos et al., 2014, 2014; Boscarino, 2004; Entringer et al., 2012; Pervanidou, Agorastos, Kolaitis, & Chrousos, 2017).
Figure 1.
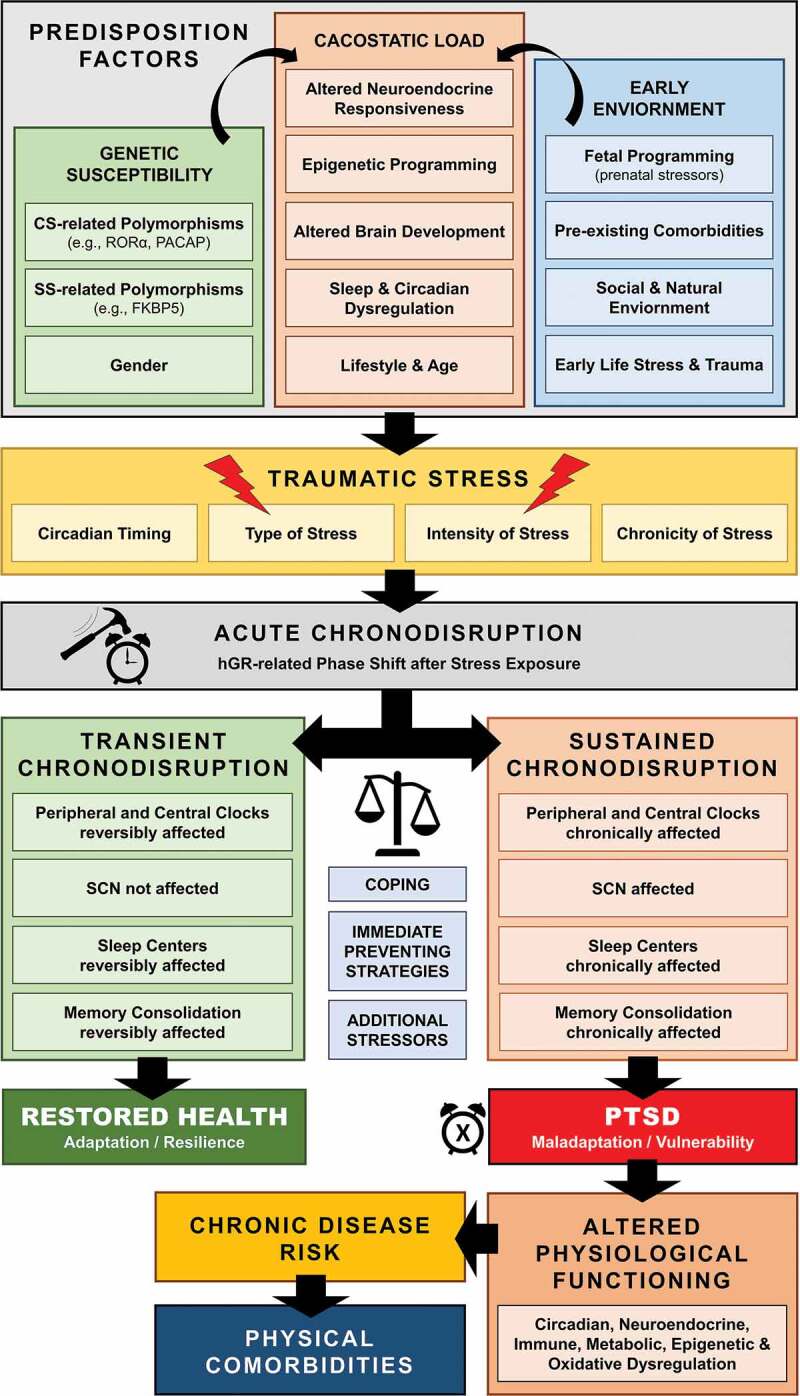
Schematic model of posttraumatic chronodisruption as underlying biological pathway leading to PTSD
The close and probably causal association between traumatic stress and chronodisruption becomes more obvious in posttraumatic stress disorder (PTSD), the model disorder following traumatic stress (American Psychiatric Association, 2013). Even though there is very limited research using laboratory methodology to assess circadian rhythms, mounting evidence of circadian dysregulation in PTSD mostly originates indirectly from sleep, physiological/activity monitoring and neuroendocrine research findings.
According to DSM-5, SD represents prominent clinical feature of the disorder with very high prevalence (American Psychiatric Association, 2013; Germain et al., 2008; Spoormaker & Montgomery, 2008), and is often closely related to severity of overall PTSD symptoms (Clum, Nishith, & Resick, 2001; Nishith, Resick, & Mueser, 2001) and resistant to first-line treatments (Belleville, Guay, & Marchand, 2011; Schoenfeld, Deviva, & Manber, 2012; Zayfert & DeViva, 2004). SD observed in PTSD are associated with sleep-related arousal regulation (Mellman, 1997) and include insomnia, nightmares, hyperarousal states, sleep terrors and nocturnal anxiety attacks, body-movement and breathing-related sleep disorders (Harvey, Jones, & Schmidt, 2003; Maher, Rego, & Asnis, 2006; Mellman & Hipolito, 2006; Pillar, Malhotra, & Lavie, 2000; Spoormaker & Montgomery, 2008; Westermeyer et al., 2010), with heightened sympathovagal tone during rapid-eye-movement (REM) sleep, fragmented REM sleep patterns, reduced REM theta activity (Cowdin, Kobayashi, & Mellman, 2014; Germain, 2013; Germain et al., 2008; Kobayashi, Boarts, & Delahanty, 2007; Lamarche & De Koninck, 2007; Mellman et al., 2002; Mellman & Hipolito, 2006) and altered EEG spectral topology (de Boer et al., 2019). Interestingly, SD (e.g., disrupted REM sleep, self-reported insomnia and general sleep quality problems) immediately after (Koren, Arnon, Lavie, & Klein, 2002; Luik, Iyadurai, Gebhardt, & Holmes, 2019; Mellman et al., 2002, Mellman & Hipolito, 2006), as well as prior to trauma exposure could both increase the risk of PTSD development (Acheson et al., 2019, Bryant, Creamer, O’Donnell, Silove, & McFarlane, 2010, Koffel, Polusny, Arbisi, & Erbes, 2013). Self-reported SD prior to trauma, in particular, has been associated with a 2.5-fold increased risk of PTSD 3 months later in both general population or deployed military troops (Bryant et al., 2010; Koffel et al., 2013). SD and chronodisruption after trauma, thus, represent core rather than secondary features of PTSD (Germain et al., 2008; Lavie, 2001; Mellman & Hipolito, 2006; Spoormaker & Montgomery, 2008) and may be both a precipitating and perpetuating factor of the disorder (Ticlea, Bajor, & Osser, 2013; van Liempt, 2012). Similarly, disrupted MLT levels in the first 48 h after trauma have been associated with a higher future PTSD risk (McFarlane, Barton, Briggs, & Kennaway, 2010).
Further indirect evidence of chronodisruption in PTSD originates from findings on blunted neuroendocrine, autonomic and immune rhythmicity. Neuroendocrine PTSD findings consistently demonstrate altered HPA axis reactivity with enhanced negative feedback inhibition and blunted circadian CORT rhythm and CAR, while some studies – but not all – have shown decreased circulating concentrations of CORT (Heim & Nemeroff, 2009; Pitman et al., 2012; Raison & Miller, 2003; Thomas et al., 2012; van Liempt et al., 2013; Yehuda, 2002; Yehuda, Golier, & Kaufman, 2005). Interestingly, a recent study showed also blunted nocturnal MLT secretion profiles in military-related PTSD (Paul et al., 2019). These findings are similar to many human SD studies, suggesting that the HPA axis- and GC-signalling-specific alterations in PTSD may be partially mediated by sleep and circadian disruption (Chrousos & Kino, 2009; Germain et al., 2008; Otte et al., 2005). Similarly, PTSD patients show increased autonomic reactivity, elevated central and peripheral norepinephrine concentrations, higher basal heart rate, increased sympathovagal balance, blunted salivary alpha-amylase awakening response and, most importantly, blunted autonomic differences between day and night-time measures (Agorastos et al., 2013; Pole, 2007; Thoma, Joksimovic, Kirschbaum, Wolf, & Rohleder, 2012; Ulmer, Calhoun, Bosworth, Dennis, & Beckham, 2013; van Liempt et al., 2013, Woodward et al., 2009), suggesting central neuroautonomic dysregulation, very similar to human SD studies (Meerlo et al., 2008; Ruger & Scheer, 2009). Additionally, as immune system activity tightly follows circadian rhythms imposed by the circadian system and sleep synchronization (Bryant, Trinder, & Curtis, 2004; Cermakian et al., 2013; Coogan & Wyse, 2008; Irwin, 2002; Lorton et al., 2006), the recent first report on the loss of the typical peripheral biphasic rhythm of IL-6 in combat stress exposed individuals (Agorastos et al., 2018) is of particular importance. Recently, candidate-gene and genome-wide association studies have involved two core clock genes (adenylate cyclase-activating polypeptide, PACAP and retinoid-related orphan receptor alpha, RORA-α, respectively) as candidate risk genes for PTSD, although these findings have not been replicated. PACAP is involved in phase resetting in response to light (Dias & Ressler, 2013; Ressler et al., 2011), while RORA-α regulates BMAL activity (Amstadter et al., 2013; Logue et al., 2013).
4. Neurobiology of stress-related chronodisruption
4.1. Physiological stress system influence on circadian rhythms
The stress system is unquestionably at the heart of circadian biology. In addition to other Zeitgebers, the diurnal stress system activity and, in particular, the peripheral GC level fluctuations also exert a dynamic and crucial synchronizing effect on the central and peripheral circadian system (cf. Figure 2) (Koch et al., 2017; Nader et al., 2010; Stratmann & Schibler, 2006). Diurnally circulating GCs’ synchronizing effects mainly involve peripheral GR-related phase adjustment of clock gene expression (Balsalobre, 2000; Nader et al., 2010; Pezuk, Mohawk, Wang, & Menaker, 2012). Activated GRs in peripheral clocks translocate into the nucleus and bind to the functional GREs in the promoter regions of several clock genes (e.g., PER1/2), thus modulating their transcriptional activity, while transrepressing genes expressing transcription factors of the auxiliary TTFL (e.g., Rev-ERBα, RORα) (cf. Figure S3) (Cheon, Park, Cho, & Kim, 2013, Conway-Campbell et al., 2010; Landgraf, McCarthy, & Welsh, 2014; So, Bernal, Pillsbury, Yamamoto, & Feldman, 2009; Surjit et al., 2011; Torra et al., 2000). A genetical, functional (e.g., adrenalectomy) or pharmacological (i.e. externally administered corticosteroids) attenuation of GC rhythmicity leads also to abolished or shifted clock gene (e.g., PER1/2) expression in several peripheral tissues (e.g., liver, preadipocytes, kidney, bronchial epithelial cells, pancreas, bone tissue, cornea, fibroblasts cardiac muscle tissue), despite the presence of an intact molecular oscillator (Balsalobre, 2000; Fujihara, Kondo, Noguchi, & Togari, 2014; Koyanagi et al., 2006; Pezuk et al., 2012). However, GC signalling also modulates periodic clock gene expression in vital CNS regions (apart from the SCN) (Lamont, Robinson, Stewart, & Amir, 2005), such as PER2 expression in the amygdala (Segall, Milet, Tronche, & Amir, 2009, Segall, Perrin, Walker, Stewart, & Amir, 2006). On the other hand, GC level alterations related with adrenalectomy are shown to increase PER gene expression in the PMV, bed nucleus of stria terminalis (BNST) and other limbic areas (Amir, 2004; Conway-Campbell et al., 2010; Su et al., 2015; Takahashi et al., 2001). Interestingly, GC can be also indirectly involved in the crucial SCN entrainment (Buijs & Escobar, 2007), as serotonergic projections of the raphe nucleus to the SCN responsible for light entrainment (Sage et al., 2004) show a GC-dependent circadian transcription of tryptophan hydroxylase-2 (TH-2), an enzyme involved in serotonin synthesis (Malek, Sage, Pévet, & Raison, 2007). GCs are, thus, not just a downstream hormonal output of the circadian system, but can also influence the circadian system itself and interact with peripheral clocks towards a circadian symphony (Balsalobre, 2000; Dickmeis, 2009). Even externally applied GCs can alter circadian gene oscillation in peripheral clocks (Kamagata et al., 2017; Pezuk et al., 2012) and are able to even speed up or slow down circadian adaptation to a new extrenal nyctohemeral rhythm (Kiessling, Eichele, & Oster, 2010). GC rhythm alterations can, thus, affect the central and peripheral circadian system and vice versa (Koyanagi et al., 2006; Son et al., 2008).
4.2. How stress overrides the circadian system
As stated above, the stress system efficiently adjusts the central and peripheral circadian activity to appropriately respond to stressors, providing stress resilience and counteracting uncoordinated circadian shifts (Koch et al., 2017). Normally, sub-acute and time-restricted stress system activation can transiently override peripheral circadian rhythms through a GR-related phase shift of clock-gene expression, creating a temporary uncoupling of the central and peripheral circadian system (Balsalobre, 2000; Kiessling et al., 2010; Le Minh, 2001; Nader et al., 2009; Nicolaides, Charmandari, Kino, & Chrousos, 2017; So et al., 2009; Tahara, Aoyama, & Shibata, 2017; Torra et al., 2000; Yamamoto et al., 2005). However, the ‘master clock’ in the is able to SCN can maintain its central pacemaker rhythm, as it does not express GRs (Balsalobre, 2000; Bartlang & Lundkvist, 2017). The SCN can, therefore, and restore the regular rhythm in the periphery after termination of the stress system activation (Bartlang et al., 2014; Meerlo, van den Hoofdakker, Koolhaas, & Daan, 1997; Tahara et al., 2017, 2015).
On the other hand, extensive acute or chronic stress exposure can also affect the SCN ‘master clock’ stability and lead to sustained chronodisruption (cf. Figure 1) (Helfrich-Forster, 2017). For example, a social defeat animal research model showed that single stress exposure advances only the adrenal peripheral clock, while chronic stress affects also the central circadian system (Razzoli, Karsten, Yoder, Bartolomucci, & Engeland, 2014). Further animal research repeatedly shows that chronic stress disrupts circadian gene expression not only in several peripheral (Razzoli et al., 2014; Takahashi et al., 2013), but also in vital CNS tissues (e.g., hippocampus, amygdala, PFC) (Koresh et al., 2012; Logan et al., 2015; Tahara et al., 2015, Weber, Johnson, Yamamoto, & Gudelsky, 2014) including the SCN (Bartlang et al., 2014; Jiang et al., 2011; Kinoshita, Miyazaki, & Ishida, 2012; Tahara et al., 2017). Moreover, numerous human and animal findings show that severe acute or chronic stress can both affect key sleep centres in the CNS and alter sleep physiology, leading to both immediate and long-lasting SD and chronodisruption (Couto-Moraes, Palermo-Neto, & Markus, 2009; Koresh et al., 2012; Lavie, 2001; Philbert et al., 2011; Touma et al., 2009; Weber et al., 2014). As the CNS does not express GR, such affection of the ‘master clock’ in the SCN through extensive acute or chronic stress must implicate alternative indirect pathways within the CNS. For example, serotonergic projections of the raphe nucleus to the SCN involved in light entrainment (Sage et al., 2004) show circadian activity (Malek et al., 2007). In addition, GCs and CRH are suggested to directly influence PGL activity and stimulate MLT synthesis, interfering in the daily adjustment of the light/dark cycle (Campino, 2011; Couto-Moraes et al., 2009; Kellner et al., 1997; Mazzoccoli et al., 2011; Steiger, 2007). Subsequently, a sharp GC rise through acute stress exposure or exogenous GC application has been shown to enhance AVP and vasoactive intestinal peptide (VIP) mRNA expression as well as upregulation of Per1 and Per2 protein expression in the SCN (Engelmann, Ebner, Landgraf, & Wotjak, 1998; Koresh et al., 2012; Larsen et al., 1994).
4.3. Timing of stress effects on circadian system
Occupational, social and recreational routines follow temporal patterns, as does the onset of certain acute medical diseases and injuries. Time of day is one of the factors that shows the highest variation in motor vehicle accident trauma incidence (Pape-Kohler, Simanski, Nienaber, & Lefering, 2014), but also in ambulance demand and severe trauma admissions in hospitals (Cantwell, 2013; Stonko et al., 2018), with injuries between midnight and early morning exhibiting higher level of severity compared to those occurring at other times of day (Chen et al., 2016). Likewise, there is a similar clear pattern in intentional injury and violence victim admissions, with a greater proportion of intentional injuries occur during the night, while unintentional injury peaks late in the afternoon (Schuurman et al., 2015). Accordingly, sexual assaults (i.e., rape, etc.), the trauma type with the most distinct impact on humans, also show an epidemiological peak between midnight and early morning (Perkins et al., 1996). Thus, given that acute traumatic events in humans usually take place between late evening early morning hours (e.g., bar fights, sexual violence, car accidents, accidents or stressors during shift work) (Pape-Kohler et al., 2014; Schuurman et al., 2015; Stonko et al., 2018), the time of day of traumatic exposure could be of vital importance for the stress system responsiveness and psychological sequel of trauma.
Apart from stress system activity, stress system reactivity also follows diurnal sensitivity through connectivity variations between the SCN and other stress system-related CNS areas (Atkinson, Wood, Kershaw, Bate, & Lightman, 2006; Kalsbeek et al., 2006, 2012). Accordingly, stress exposure at different time zones can differentially affect circadian rhythmicity (cf. Figure 3). Acute psychological stress, involving higher brain areas and the limbic system, as well as acute physical external stress (i.e., restraint/immobilization, foot shock, shaking stress) exert the largest stress response during the rest phase (Bernatova, Key, Lucot, & Morris, 2002, Cohen et al., 2015; Gattermann & Weinandy, 1996), when the HPA axis is less responsive. Inversely, acute physiological internal stress (i.e., oxidative stress, hypoglycaemia, haemorrhage), relayed to the PVN and brainstem, exert the largest stress response at the beginning of the activity phase (Antoch, Kondratov, & Takahashi, 2005; Fanjul-Moles & Lopez-Riquelme, 2016), when the HPA axis is most sensitive to stimulation (Jacobson, 2005). This appears reasonable, as internal physiological stress represents a greater threat during the active phase of animals, while acute external physical stress (e.g., predator attack) during the inactive phase, when animals sleep. Interestingly, additional animal research findings suggest that repeated external stress exposure (i.e., chronic physical stress) is more likely to lead to chronodisruption when applied during the inactive phase, (Aslani, 2014; Bartlang, Oster, & Helfrich-Forster, 2015; Bartlang et al., 2014; Fonken et al., 2016; Rybkin et al., 1997), while chronic psychosocial stress (i.e. social-defeat paradigm) during the active phase (Bartlang et al., 2015; Koch, 2016). These results jointly suggest that the effect of a stressor depends not only on the circadian phase of exposure, but also on the interaction of the circadian phase with the stressor type, as well as with the chronicity of the stressor (Helfrich-Forster, 2017; Kalsbeek, Ruiter, la Fleur, Van Heijningen, & Buijs, 2003). For example, both physical and psychological stress at the beginning of the light phase leads to a phase advance, while at the beginning of the dark phase to a phase delay of PER2 expression in mice (Tahara et al., 2015).
Figure 3.
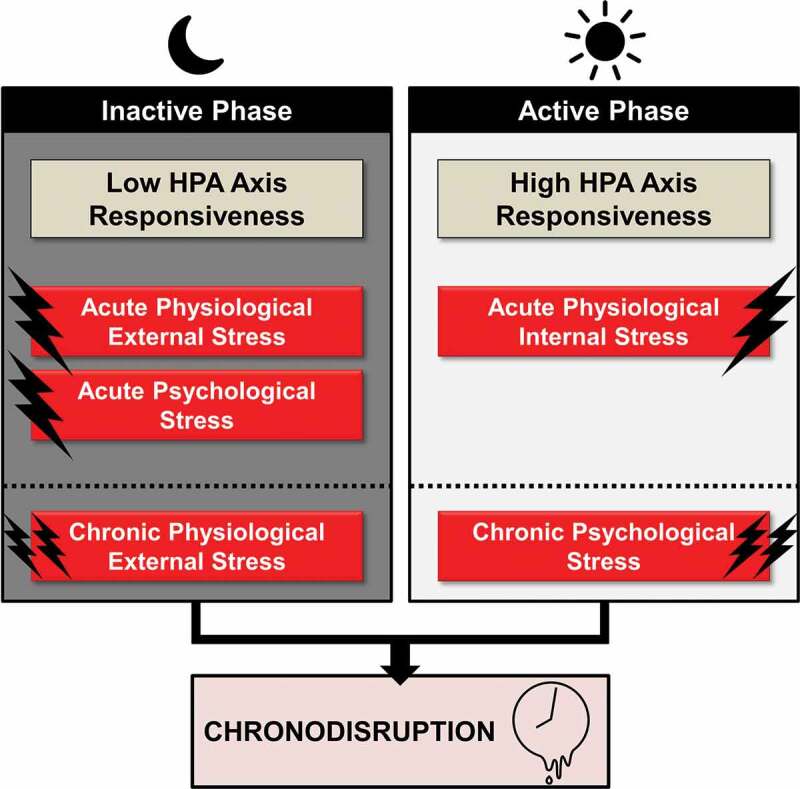
Acute and chronic time-of-day dependent effects of stress on circadian rhythmicity
5. Chronotherapeutic implications for posttraumatic chronodisruption
Current state of knowledge suggests that SD and chronodisruption play a causal role in PTSD pathophysiology (Agorastos et al., 2014), while their effective treatment can lead to substantial improvement of overall PTSD symptom severity (Germain, 2007; Germain et al., 2008; Krakow et al., 2001; Raskind et al., 2003). Although research has offered substantial findings on the importance of SD in PTSD, this issue is yet still often assessed and treated as secondary PTSD symptom only in everyday clinical routine practice. Careful assessment and management of SD and chronodisruption should therefore be fundamental in PTSD treatment and prevention (Bajor, Ticlea, & Osser, 2011; Lamarche & De Koninck, 2007; Schoenfeld et al., 2012; Ticlea et al., 2013; van Liempt, 2012).
5.1. Pharmacological interventions
Specific pharmacological treatments for PTSD-related SD have emerged through the years. For example, the α1 adrenoreceptor antagonist prazosin and α2 adrenoreceptor agonist clonidine and the multi-modal antidepressant trazodone (i.e., serotonin-reuptake inhibitor, 5-HT2A receptor agonist, histamine H1 receptor antagonist, α1 and α2 adrenoreceptor antagonist) have all been shown to be effective pharmacological approaches for PTSD-related sleep disturbances, with prazosin targeting specifically PTSD- and trauma-related nightmares (Alao, Selvarajah, & Razi, 2012; Aurora et al., 2010; Raskind et al., 2013; Warner, Dorn, & Peabody, 2001). Interestingly, also eszopiclone, a positive allosteric modulator of GABAA receptors, used as sleep medication in insomnia, has been found not only to improve sleep quality, but also overall PTSD symptom severity in a randomized, placebo-controlled clinical trial (Pollack, 2011). However, symptomatic pharmacological PTSD sleep management may effectively address sleep quality and quantity, but often fails to improve daytime functioning and sufficiently restore circadian rhythms (Pollack, 2011; Zisapel, 2007).
Hence, there is a need for the introduction and clinical investigation of novel chronobiological interventions, capable of effectively restoring posttraumatic chronodisruption by influencing the interplay between stress and circadian system and herethrough counteracting sustained, PTSD-related CNS neurocircuitry changes (Comai & Gobbi, 2014; Marshall & Garakani, 2002; Mendlewicz, 2009; Pilorz et al., 2014). Newest findings implicate serotonergic, melatonergic, opioidergic, γ-amino butyric acid (GABA)-ergic, cannabinoidergic and glucocorticoid signalling, as well as 3,4-Methylenedioxymethamphetamine (MDMA) as potential new treatment strategies (cf. Chapter S1).
5.2. Non-pharmacological interventions
Cognitive-behavioural sleep management constitutes a widely acknowledge, acceptable and durably effective treatment option in PTSD (Epstein, Babcock-Parziale, Haynes, & Herb, 2012; Margolies et al., 2013; Schoenfeld et al., 2012; Talbot et al., 2014). In addition, cognitive-behavioural social rhythm group therapy have been also found to have positive effects on SD in veterans with PTSD (Haynes et al., 2016), supporting the importance of circadian synchronization in the treatment of the disorder. Interestingly, a recent placebo-controlled pilot study assessing morning bright light treatment in PTSD through a wearable device has also demonstrated efficacy in reducing PTSD symptoms (Zalta, Bravo, Valdespino‐Hayden, Pollack, & Burgess, 2019). Finally, there are some reports that posttraumatic sleep deprivation in the immediate aftermath (i.e., first night) of the trauma, can lead to reduced stress responses in the future, possibly by re-synchronizing the circadian and stress system (Cohen, Kaplan, Zohar, & Cohen, 2017, Cohen et al., 2012).
5.3. Prevention strategies
Given that stress exposure at different time zones (i.e., rest vs. activity phase) can differentially affect circadian rhythmicity due to circadian differences in HPA axis responsivity, sleep and circadian regulation through forced intrinsic or extrinsic circadian entrainment in the time before or immediately after traumatic stress exposure (e.g., behavioural circadian adaptation, sleep hygiene, pharmacologic treatment) could influence the long-term effects of traumatic stress and play a central role in the prevention and of PTSD. For example, as SD prior to traumatic stress exposure can result in an up to 2.5-fold increased risk of fulfiling PTSD criteria 3 months after a trauma, proper assessment and treatment of SD in advance and avoidance of night-time activities, might positively affect adaptive mechanisms and be very important in specific populations (e.g., military personnel). Similarly, acute interventions in the first night after traumatic stress exposure targeting REM sleep disruption associated with sympathetic activation and impaired trauma memory consolidation, might be able to moderate traumatic impact and the course of PTSD development.
6. Conclusion
Posttraumatic chronodisruption fundamentally affects the neuroendocrine, immune and autonomic system and may play a causal role in the development of stress-related disorders and PTSD in particular. Understanding the pathways susceptible to chronodisruption following traumatic stress and their role in a chronically chronodisrupted neurobiology in stress-related disorders could deliver new insights into pathophysiology of associated disorders. Novel state-of-the-art methods of chronodisruption assessment and treatment are needed to bridge the gap between clinical significance and limited understanding of the relationship between traumatic stress, sleep and circadian system.
Supplementary Material
Funding Statement
None of the authors received funding for this paper.
Author contributions statement
AA managed all literature searches and wrote the first draft of the paper. MO contributed with significant text passages and revised the draft for important intellectual content. All authors have contributed to, read and approved the final version of the manuscript.
Disclosure statement
All authors declare no biomedical competing financial interests or potential conflicts of interest.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Acheson, D. T., Kwan, B., Maihofer, A. X., Risbrough, V. B., Nievergelt, C. M., Clark, J. W., & Baker, D. G. (2019). Sleep disturbance at pre-deployment is a significant predictor of post-deployment re-experiencing symptoms. European Journal of Psychotraumatology, 10(1), 1679964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann, K., Plomp, R., Lao, O., Middleton, B., Revell, V. L., Skene, D. J., & Kayser, M. (2013). Effect of sleep deprivation on rhythms of clock gene expression and melatonin in humans. Chronobiology International, 30(7), 901–20. [DOI] [PubMed] [Google Scholar]
- Agorastos, A. (2017). Pathophysiological trajectories and biological consequences of early life trauma. European Journal of Psychotraumatology, 8, (suppl. 4), 1351159-2. [Google Scholar]
- Agorastos, A., Pervanidou, P., Chrousos, G. P., & Kolaitis, G. (2018). Early life stress and trauma: Developmental neuroendocrine aspects of prolonged stress system dysregulation. Hormones (Athens), 4, 507–520. [DOI] [PubMed] [Google Scholar]
- Agorastos, A., Pervanidou, P., Chrousos, G. P., & Baker, D. G. (2019). Developmental trajectories of early life stress and trauma: A narrative review on neurobiological aspects beyond stress system dysregulation. Frontiers In Psychiatry / Frontiers Research Foundation, 10, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos, A., Nicolaides, N. C., Bozikas, V. P., Chrousos, G. P., & Pervanidou, P. (2020). Multilevel interactions of stress and circadian system: Implications for traumatic stress. Frontiers In Psychiatry / Frontiers Research Foundation, 10, 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos, A., Boel, J. A., Heppner, P. S., Hager, T., Moeller-Bertram, T., Haji, U., … Stiedl, O. (2013). Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress, 16(3), 300–310. [DOI] [PubMed] [Google Scholar]
- Agorastos, A., Hauger, R. L., Barkauskas, D. A., Lerman, I. R., Moeller-Bertram, T., Snijders, C., & Baker, D. G. (2018). Relations of combat stress and posttraumatic stress disorder to 24-h plasma and cerebrospinal fluid interleukin-6 levels and circadian rhythmicity. Psychoneuroendocrinology, 100, 237–245. [DOI] [PubMed] [Google Scholar]
- Agorastos, A., Kellner, M., Baker, D. G., & Otte, C. (2014). When time stands still. An integrative review on the role of chronodisruption in PTSD. Current Opinion in Psychiatry, 27, 385–392. [DOI] [PubMed] [Google Scholar]
- Agorastos, A., Pittman, J. O. E., Angkaw, A. C., Nievergelt, C. M., Hansen, C. J., Aversa, L. H., & Baker, D. G. (2014). The cumulative effect of different childhood trauma types on self-reported symptoms of adult male depression and PTSD, substance abuse and health-related quality of life in a large active-duty military cohort. Journal of Psychiatric Research, 58, 46–54. [DOI] [PubMed] [Google Scholar]
- Alao, A., Selvarajah, J., & Razi, S. (2012). The use of clonidine in the treatment of nightmares among patients with co-morbid PTSD and traumatic brain injury. The International Journal of Psychiatry in Medicine, 44(2), 165–169. [DOI] [PubMed] [Google Scholar]
- Albrecht, U. (2017). Molecular mechanisms in mood regulation involving the Circadian clock. Frontiers in Neurology, 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Publishing, Inc. [Google Scholar]
- Amir, S. (2004). A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. Journal of Neuroscience, 24(4), 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter, A. B., Sumner, J. A., Acierno, R., Ruggiero, K. J., Koenen, K. C., Kilpatrick, D. G., … Gelernter, J. (2013). Support for association of RORA variant and post traumatic stress symptoms in a population-based study of hurricane exposed adults. Molecular Psychiatry, 18(11), 1148–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoch, M. P., Kondratov, R. V., & Takahashi, J. S. (2005). Circadian clock genes as modulators of sensitivity to genotoxic stress. Cell Cycle, 4(7), 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslani, S. (2014). Day and night: Diurnal phase influences the response to chronic mild stress. Frontiers in Behavioral Neuroscience, 8, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, H. C., Wood, S. A., Kershaw, Y. M., Bate, E., & Lightman, S. L. (2006). Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. Journal of Neuroendocrinology, 18(7), 526–533. [DOI] [PubMed] [Google Scholar]
- Aurora, R. N., Zak, R. S., Auerbach, S. H., Casey, K. R., Chowdhuri, S., Karippot, A., & Morgenthaler, T. I. (2010). Best practice guide for the treatment of nightmare disorder in adults. Journal of Clinical Sleep Medicine, 6(4), 389–401. [PMC free article] [PubMed] [Google Scholar]
- Backhaus, J., Junghanns, K., & Hohagen, F. (2004). Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology, 29(9), 1184–1191. [DOI] [PubMed] [Google Scholar]
- Baiden, P., Fallon, B., den Dunnen, W., & Boateng, G. O. (2015). The enduring effects of early-childhood adversities and troubled sleep among Canadian adults: A population-based study. Sleep Medicine, 16(6), 760–767. [DOI] [PubMed] [Google Scholar]
- Bajor, L. A., Ticlea, A. N., & Osser, D. N. (2011). The psychopharmacology algorithm project at the harvard south shore program: An update on posttraumatic stress disorder. Harvard Review of Psychiatry, 19(5), 240–258. [DOI] [PubMed] [Google Scholar]
- Balsalobre, A. (2000). Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science, 289(5488), 2344–2347. [DOI] [PubMed] [Google Scholar]
- Bartlang, M. S., & Lundkvist, G. B. (2017). Stress and the central circadian clock, in Stress: Neuroendocrinology and neurobiology (pp. 385–393). G. Fink, Editor. San Diego, CA: Academic Press. [Google Scholar]
- Bartlang, M. S., Oster, H., & Helfrich-Forster, C. (2015). Repeated psychosocial stress at night affects the circadian activity rhythm of male mice. Journal of Biological Rhythms, 30(3), 228–241. [DOI] [PubMed] [Google Scholar]
- Bartlang, M. S., Savelyev, S. A., Johansson, A.-S., Reber, S. O., Helfrich-Förster, C., & Lundkvist, G. B. S. (2014). Repeated psychosocial stress at night, but not day, affects the central molecular clock. Chronobiology International, 31(9), 996–1007. [DOI] [PubMed] [Google Scholar]
- Bedrosian, T. A., Fonken, L. K., & Nelson, R. J. (2016). Endocrine Effects of Circadian Disruption. Annual Review of Physiology, 78, 109–131. [DOI] [PubMed] [Google Scholar]
- Belleville, G., Guay, S., & Marchand, A. (2011). Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. Journal of Psychosomatic Research, 70(4), 318–327. [DOI] [PubMed] [Google Scholar]
- Bernatova, I., Key, M. P., Lucot, J. B., & Morris, M. (2002). Circadian differences in stress-induced pressor reactivity in mice. Hypertension, 40(5), 768–773. [DOI] [PubMed] [Google Scholar]
- Boscarino, J. A. (2004). Posttraumatic stress disorder and physical illness: Results from clinical and epidemiologic studies. Annals of the New York Academy of Sciences, 1032, 141–153. [DOI] [PubMed] [Google Scholar]
- Bryant, P. A., Trinder, J., & Curtis, N. (2004). Sick and tired: Does sleep have a vital role in the immune system? Nature Reviews Immunology, 4(6), 457–467. [DOI] [PubMed] [Google Scholar]
- Bryant, R. A., Creamer, M., O’Donnell, M., Silove, D., & McFarlane, A. C. (2010). Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep, 33(1), 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, T. M., & Schatzberg, A. F. (2005). On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. The Journal of Clinical Endocrinology & Metabolism, 90(5), 3106–3114. [DOI] [PubMed] [Google Scholar]
- Buijs, R. M., & Escobar, C. (2007). Corticosterone and activity: The long arms of the clock talk back. Endocrinology, 148(11), 5162–5164. [DOI] [PubMed] [Google Scholar]
- Buijs, R. M., Escobar, C., & Swaab, D. F. (2013). The circadian system and the balance of the autonomic nervous system. Handbook of Clinical Neurology, 117, 173–191. [DOI] [PubMed] [Google Scholar]
- Buijs, R. M., van Eden, C. G., Goncharuk, V. D., & Kalsbeek, A. (2003). The biological clock tunes the organs of the body: Timing by hormones and the autonomic nervous system. Journal of Endocrinology, 177(1), 17–26. [DOI] [PubMed] [Google Scholar]
- Campino, C. (2011). Melatonin exerts direct inhibitory actions on ACTH responses in the human adrenal gland. Hormone and Metabolic Research, 43(5), 337–342. [DOI] [PubMed] [Google Scholar]
- Cantwell, K. (2013). Ambulance demand: Random events or predicable patterns? Emergency Medicine Journal, 30(11), 883–887. [DOI] [PubMed] [Google Scholar]
- Cermakian, N., Lange, T., Golombek, D., Sarkar, D., Nakao, A., Shibata, S., & Mazzoccoli, G. (2013). Crosstalk between the circadian clock circuitry and the immune system. Chronobiology International, 30(7), 870–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari, E., Chrousos, G. P., Lambrou, G. I., Pavlaki, A., Koide, H., Ng, S. S., & Kino, T. (2011). Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS One, 6(9), e25612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari, E., Tsigos, C., & Chrousos, G. (2005). Endocrinology of the stress response. Annual Review of Physiology, 67, 259–284. [DOI] [PubMed] [Google Scholar]
- Chellappa, S. L., Viola, A. U., Schmidt, C., Bachmann, V., Gabel, V., Maire, M., … Cajochen, C. (2014). Light modulation of human sleep depends on a polymorphism in the clock gene Period3. Behavioural Brain Research, 271, 23–29. [DOI] [PubMed] [Google Scholar]
- Chen, H., Chen, Q., Chen, L., & Zhang, G. (2016). Analysis of risk factors affecting driver injury and crash injury with drivers under the influence of alcohol (DUI) and non-DUI. Traffic Injury Prevention, 17(8), 796–802. [DOI] [PubMed] [Google Scholar]
- Cheon, S., Park, N., Cho, S., & Kim, K. (2013). Glucocorticoid-mediated Period2 induction delays the phase of circadian rhythm. Nucleic Acids Research, 41(12), 6161–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos, G. P. (2009). Stress and disorders of the stress system. Nature Reviews Endocrinology, 5(7), 374–381. [DOI] [PubMed] [Google Scholar]
- Chrousos, G. P., Charmandari, E., & Kino, T. (2004). Glucocorticoid action networks–an introduction to systems biology. The Journal of Clinical Endocrinology & Metabolism, 89(2), 563–564. [DOI] [PubMed] [Google Scholar]
- Chrousos, G. P., & Gold, P. W. (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA, 267(9), 1244–1252. [PubMed] [Google Scholar]
- Chrousos, G. P., & Kino, T. (2009). Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Annals of the New York Academy of Sciences, 1179, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clum, G. A., Nishith, P., & Resick, P. A. (2001). Trauma-related sleep disturbance and self-reported physical health symptoms in treatment-seeking female rape victims. The Journal of Nervous and Mental Disease, 189(9), 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S., Kaplan, Z., Zohar, J., & Cohen, H. (2017). Preventing sleep on the first resting phase following a traumatic event attenuates anxiety-related responses. Behavioural Brain Research, 320, 450–456. [DOI] [PubMed] [Google Scholar]
- Cohen, S., Kozlovsky, N., Matar, M. A., Kaplan, Z., Zohar, J., & Cohen, H. (2012). Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacology, 37(11), 2388–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S., Vainer, E., Matar, M. A., Kozlovsky, N., Kaplan, Z., Zohar, J., … Cohen, H. (2015). Diurnal fluctuations in HPA and neuropeptide Y-ergic systems underlie differences in vulnerability to traumatic stress responses at different zeitgeber times. Neuropsychopharmacology, 40(3), 774–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai, S., & Gobbi, G. (2014). Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: A novel target in psychopharmacology. Journal of Psychiatry & Neuroscience, 39(1), 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway-Campbell, B. L., Sarabdjitsingh, R. A., McKenna, M. A., Pooley, J. R., Kershaw, Y. M., Meijer, O. C., … , Lightman, S. (2010). Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. Journal of Neuroendocrinology, 22(10), 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan, A. N., & Wyse, C. A. (2008). Neuroimmunology of the circadian clock. Brain Research, 1232, 104–112. [DOI] [PubMed] [Google Scholar]
- Couto-Moraes, R., Palermo-Neto, J., & Markus, R. P. (2009). The immune-pineal axis: Stress as a modulator of pineal gland function. Annals of the New York Academy of Sciences, 1153, 193–202. [DOI] [PubMed] [Google Scholar]
- Cowdin, N., Kobayashi, I., & Mellman, T. A. (2014). Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Experimental Brain Research, 232(5), 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer, M., Nijdam, M. J., Jongedijk, R. A., Bangel, K. A., Olff, M., Hofman, W. F., & Talamini, L. M. (2019). The spectral fingerprint of sleep problems in post-traumatic stress disorder. Sleep, 43(4), zsz269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, B. G., & Ressler, K. J. (2013). PACAP and the PAC1 receptor in post-traumatic stress disorder. Neuropsychopharmacology, 38(1), 245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner, C., Schibler, U., & Albrecht, U. (2010). The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annual Review of Physiology, 72, 517–549. [DOI] [PubMed] [Google Scholar]
- Dickmeis, T. (2009). Glucocorticoids and the circadian clock. The Journal of Endocrinology, 200(1), 3–22. [DOI] [PubMed] [Google Scholar]
- Elenkov, I. J., & Chrousos, G. P. (2006). Stress system–organization, physiology and immunoregulation. Neuroimmunomodulation, 13(5–6), 257–267. [DOI] [PubMed] [Google Scholar]
- Engelmann, M., Ebner, K., Landgraf, R., & Wotjak, C. T. (1998). Swim stress triggers the release of vasopressin within the suprachiasmatic nucleus of male rats. Brain Research, 792(2), 343–347. [DOI] [PubMed] [Google Scholar]
- Entringer, S., Epel, E. S., Lin, J., Buss, C., Blackburn, E. H., Simhan, H. N., & Wadhwa, P. D. (2012). Prenatal programming of newborn and infant telomere length. European Journal of Psychotraumatology, 3(1), 19555. [Google Scholar]
- Epstein, D. R., Babcock-Parziale, J. L., Haynes, P. L., & Herb, C. A. (2012). Insomnia treatment acceptability and preferences of male Iraq and Afghanistan combat veterans and their healthcare providers. The Journal of Rehabilitation Research and Development, 49(6), 867–878. [DOI] [PubMed] [Google Scholar]
- Erren, T. C., & Reiter, R. J. (2009). Defining chronodisruption. Journal of Pineal Research, 46(3), 245–247. [DOI] [PubMed] [Google Scholar]
- Fanjul-Moles, M. L., & Lopez-Riquelme, G. O. (2016). Relationship between Oxidative Stress, Circadian Rhythms, and AMD. Oxidative Medicine and Cellular Longevity, 2016, 7420637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken, L. K., Weber, M. D., Daut, R. A., Kitt, M. M., Frank, M. G., Watkins, L. R., & Maier, S. F. (2016). Stress-induced neuroinflammatory priming is time of day dependent. Psychoneuroendocrinology, 66, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara, Y., Kondo, H., Noguchi, T., & Togari, A. (2014). Glucocorticoids mediate circadian timing in peripheral osteoclasts resulting in the circadian expression rhythm of osteoclast-related genes. Bone, 61, 1–9. [DOI] [PubMed] [Google Scholar]
- Fujino, Y., Iso, H., Tamakoshi, A., Inaba, Y., Koizumi, A., Kubo, T., & Yoshimura, T. (2006). A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. American Journal of Epidemiology, 164(2), 128–135. [DOI] [PubMed] [Google Scholar]
- Gamble, K. L., Berry, R., Frank, S. J., & Young, M. E. (2014). Circadian clock control of endocrine factors. Nature Reviews Endocrinology, 10(8), 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, E. H., & Quinton, R. (2010). Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Progress in Brain Research, 181, 111–126. [DOI] [PubMed] [Google Scholar]
- Gattermann, R., & Weinandy, R. (1996). Time of day and stress response to different stressors in experimental animals. Part I: Golden hamster (Mesocricetus auratus Waterhouse, 1839). Journal of Experimental Animal Science, 38(2), 66–76. [PubMed] [Google Scholar]
- Germain, A. (2007). Effects of a brief behavioral treatment for PTSD-related sleep disturbances: A pilot study. Behaviour Research and Therapy, 45(3), 627–632. [DOI] [PubMed] [Google Scholar]
- Germain, A. (2013). Sleep disturbances as the hallmark of PTSD: Where are we now? American Journal of Psychiatry, 170(4), 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain, A., Buysse, D. J., & Nofzinger, E. (2008). Sleep-specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Medicine Reviews, 12(3), 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain, A., & Kupfer, D. J. (2008). Circadian rhythm disturbances in depression. Human Psychopharmacology: Clinical and Experimental, 23(7), 571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield, E. A., Lee, C., Friedman, E. L., & Springer, K. W. (2011). Childhood abuse as a risk factor for sleep problems in adulthood: Evidence from a U.S. national study. Annals of Behavioral Medicine, 42(2), 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D.-H., Lee, Y.-J., Kim, K., Kim, C.-J., & Cho, S. (2014). Modulation of glucocorticoid receptor induction properties by core circadian clock proteins. Molecular and Cellular Endocrinology, 383(1–2), 170–180. [DOI] [PubMed] [Google Scholar]
- Harvey, A. G., Jones, C., & Schmidt, D. A. (2003). Sleep and posttraumatic stress disorder: A review. Clinical Psychology Review, 23(3), 377–407. [DOI] [PubMed] [Google Scholar]
- Hastings, M., O’Neill, J. S., & Maywood, E. S. (2007). Circadian clocks: Regulators of endocrine and metabolic rhythms. Journal of Endocrinology, 195(2), 187–198. [DOI] [PubMed] [Google Scholar]
- Hastings, M. H., Maywood, E. S., & Brancaccio, M. (2018). Generation of circadian rhythms in the suprachiasmatic nucleus. Nature Reviews Neuroscience, 19(8), 453–469. [DOI] [PubMed] [Google Scholar]
- Haynes, P. L., Kelly, M., Warner, L., Quan, S. F., Krakow, B., & Bootzin, R. R. (2016). Cognitive Behavioral Social Rhythm Group Therapy for Veterans with posttraumatic stress disorder, depression, and sleep disturbance: Results from an open trial. Journal of Affective Disorders, 192, 234–243. [DOI] [PubMed] [Google Scholar]
- Heim, C., & Nemeroff, C. B. (2009). Neurobiology of posttraumatic stress disorder. CNS Spectrums, 14(1), 13–24. [PubMed] [Google Scholar]
- Helfrich-Forster, C. (2017). Interactions between psychosocial stress and the circadian endogenous clock. PsyCh Journal, 6(4), 277–289. [DOI] [PubMed] [Google Scholar]
- Henckens, M. J., Hermans, E. J., Pu, Z., Joels, M., & Fernandez, G. (2009). Stressed memories: How acute stress affects memory formation in humans. Journal of Neuroscience, 29(32), 10111–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin, M. (2002). Effects of sleep and sleep loss on immunity and cytokines. Brain, Behavior, and Immunity, 16(5), 503–512. [DOI] [PubMed] [Google Scholar]
- Jacobson, L. (2005). Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinology and Metabolism Clinics of North America, 34(2), 271–92, vii. [DOI] [PubMed] [Google Scholar]
- Jiang, W.-G., Li, S.-X., Zhou, S.-J., Sun, Y., Shi, J., & Lu, L. (2011). Chronic unpredictable stress induces a reversible change of PER2 rhythm in the suprachiasmatic nucleus. Brain Research, 1399, 25–32. [DOI] [PubMed] [Google Scholar]
- Joels, M., & Baram, T. Z. (2009). The neuro-symphony of stress. Nature Reviews Neuroscience, 10(6), 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajeepeta, S., Gelaye, B., Jackson, C. L., & Williams, M. A. (2015). Adverse childhood experiences are associated with adult sleep disorders: A systematic review. Sleep Medicine, 16(3), 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek, A., Palm, I. F., La Fleur, S. E., Scheer, F. A. J. L., Perreau-Lenz, S., Ruiter, M., … , Buijs, R.M. (2006). SCN outputs and the hypothalamic balance of life. Current Psychiatry Reports, 21(6), 458–469. [DOI] [PubMed] [Google Scholar]
- Kalsbeek, A., Ruiter, M., la Fleur, S. E., Van Heijningen, C., & Buijs, R. M. (2003). The diurnal modulation of hormonal responses in the rat varies with different stimuli. Journal of Neuroendocrinology, 15(12), 1144–1155. [DOI] [PubMed] [Google Scholar]
- Kalsbeek, A., van der Spek, R., Lei, J., Endert, E., Buijs, R. M., & Fliers, E. (2012). Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Molecular and Cellular Endocrinology, 349(1), 20–29. [DOI] [PubMed] [Google Scholar]
- Kamagata, M., Ikeda, Y., Sasaki, H., Hattori, Y., Yasuda, S., Iwami, S., … Shibata, S. (2017). Potent synchronization of peripheral circadian clocks by glucocorticoid injections in PER2::LUC- Clock/Clock mice. Chronobiology International, 34(8), 1067–1082. [DOI] [PubMed] [Google Scholar]
- Karatsoreos, I. N. (2011). Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proceedings of the National Academy of Sciences, 108(4), 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner, M., Yassouridis, A., Manz, B., Steiger, A., Holsboer, F., & Wiedemann, K. (1997). Corticotropin-releasing hormone inhibits melatonin secretion in healthy volunteers–a potential link to low-melatonin syndrome in depression? Neuroendocrinology, 65(4), 284–290. [DOI] [PubMed] [Google Scholar]
- Kiessling, S., Eichele, G., & Oster, H. (2010). Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. Journal of Clinical Investigation, 120(7), 2600–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino, T., & Chrousos, G. P. (2011). Circadian CLOCK-mediated regulation of target-tissue sensitivity to glucocorticoids: Implications for cardiometabolic diseases. Endocrine Development, 20, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, C., Miyazaki, K., & Ishida, N. (2012). Chronic stress affects PERIOD2 expression through glycogen synthase kinase-3beta phosphorylation in the central clock. Neuroreport, 23(2), 98–102. [DOI] [PubMed] [Google Scholar]
- Ko, C. H., & Takahashi, J. S. (2006). Molecular components of the mammalian circadian clock. Human Molecular Genetics, 15(2), R271–7. [DOI] [PubMed] [Google Scholar]
- Kobayashi, I., Boarts, J. M., & Delahanty, D. L. (2007). Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology, 44(4), 660–669. [DOI] [PubMed] [Google Scholar]
- Koch, C. E. (2016). Time-of-day-dependent adaptation of the HPA axis to predictable social defeat stress. Journal of Endocrinology, 231(3), 209–221. [DOI] [PubMed] [Google Scholar]
- Koch, C. E., Leinweber, B., Drengberg, B. C., Blaum, C., & Oster, H. (2017). Interaction between circadian rhythms and stress. Neurobiology of Stress, 6, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel, E., Polusny, M. A., Arbisi, P. A., & Erbes, C. R. (2013). Pre-deployment daytime and nighttime sleep complaints as predictors of post-deployment PTSD and depression in National Guard troops. Journal of Anxiety Disorders, 27(5), 512–519. [DOI] [PubMed] [Google Scholar]
- Koren, D., Arnon, I., Lavie, P., & Klein, E. (2002). Sleep complaints as early predictors of posttraumatic stress disorder: A 1-year prospective study of injured survivors of motor vehicle accidents. American Journal of Psychiatry, 159(5), 855–857. [DOI] [PubMed] [Google Scholar]
- Koresh, O., Kozlovsky, N., Kaplan, Z., Zohar, J., Matar, M. A., & Cohen, H. (2012). The long-term abnormalities in circadian expression of Period 1 and Period 2 genes in response to stress is normalized by agomelatine administered immediately after exposure. European Neuropsychopharmacology, 22(3), 205–221. [DOI] [PubMed] [Google Scholar]
- Koyanagi, S., Okazawa, S., Kuramoto, Y., Ushijima, K., Shimeno, H., Soeda, S., … Ohdo, S. (2006). Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Molecular Endocrinology, 20(3), 573–583. [DOI] [PubMed] [Google Scholar]
- Kraeplin, E. (1883). Compendium der Psychiatrie zum Gebrauch fuer Studierende und Aerzte. Leipzig: Abel Verlag. [Google Scholar]
- Krakow, B., Hollifield, M., Johnston, L., Koss, M., Schrader, R., Warner, T. D., … Prince, H. (2001). Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: A randomized controlled trial. JAMA, 286(5), 537–545. [DOI] [PubMed] [Google Scholar]
- Lamarche, L. J., & De Koninck, J. (2007). Sleep disturbance in adults with posttraumatic stress disorder: A review. The Journal of Clinical Psychiatry, 68(8), 1257–1270. [DOI] [PubMed] [Google Scholar]
- Lamont, E. W., Robinson, B., Stewart, J., & Amir, S. (2005). The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proceedings of the National Academy of Sciences, 102(11), 4180–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf, D., McCarthy, M. J., & Welsh, D. K. (2014). Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Current Psychiatry Reports, 16(10), 483. [DOI] [PubMed] [Google Scholar]
- Larsen, P. J., Vrang, N., Møller, M., Jessop, D. S., Lightman, S. L., Chowdrey, H. S., & Mikkelsen, J. D. (1994). The diurnal expression of genes encoding vasopressin and vasoactive intestinal peptide within the rat suprachiasmatic nucleus is influenced by circulating glucocorticoids. Molecular Brain Research, 27(2), 342–346. [DOI] [PubMed] [Google Scholar]
- Lavie, P. (2001). Sleep disturbances in the wake of traumatic events. New England Journal of Medicine, 345(25), 1825–1832. [DOI] [PubMed] [Google Scholar]
- Le Minh, N. (2001). Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. The EMBO Journal, 20(24), 7128–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman, A. R., Halitjaha, L., Ay, A., & Ingram, K. K. (2018). Modeling strengthens molecular link between Circadian polymorphisms and major mood disorders. Journal of Biological Rhythms, 33(3), 318–336. [DOI] [PubMed] [Google Scholar]
- Lind, M. J., Aggen, S. H., Kendler, K. S., York, T. P., & Amstadter, A. B. (2016). An epidemiologic study of childhood sexual abuse and adult sleep disturbances. Psychological Trauma: Theory, Research, Practice, and Policy, 8(2), 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, R. W., Edgar, N., Gillman, A. G., Hoffman, D., Zhu, X., & McClung, C. A. (2015). Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in Mice. Biological Psychiatry, 78(4), 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue, M. W., Baldwin, C., Guffanti, G., Melista, E., Wolf, E. J., Reardon, A. F., & Miller, M. W. (2013). A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Molecular Psychiatry, 18(8), 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorton, D., Lubahn, C. L., Estus, C., Millar, B. A., Carter, J. L., Wood, C. A., & Bellinger, D. L. (2006). Bidirectional communication between the brain and the immune system: Implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation, 13(5–6), 357–374. [DOI] [PubMed] [Google Scholar]
- Luik, A. I., Iyadurai, L., Gebhardt, I., & Holmes, E. A. (2019). Sleep disturbance and intrusive memories after presenting to the emergency department following a traumatic motor vehicle accident: An exploratory analysis. European Journal of Psychotraumatology, 10(1), 1556550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher, M. J., Rego, S. A., & Asnis, G. M. (2006). Sleep disturbances in patients with post-traumatic stress disorder: Epidemiology, impact and approaches to management. CNS Drugs, 20(7), 567–590. [DOI] [PubMed] [Google Scholar]
- Maire, M., Reichert, C. F., Gabel, V., Viola, A. U., Strobel, W., Krebs, J., … Schmidt, C. (2014). Sleep ability mediates individual differences in the vulnerability to sleep loss: Evidence from a PER3 polymorphism. Cortex, 52, 47–59. [DOI] [PubMed] [Google Scholar]
- Malek, Z. S., Sage, D., Pévet, P., & Raison, S. (2007). Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology, 148(11), 5165–5172. [DOI] [PubMed] [Google Scholar]
- Margolies, S. O., Rybarczyk, B., Vrana, S. R., Leszczyszyn, D. J., & Lynch, J. (2013). Efficacy of a cognitive-behavioral treatment for insomnia and nightmares in Afghanistan and Iraq veterans with PTSD. Journal of Clinical Psychology, 69(10), 1026–1042. [DOI] [PubMed] [Google Scholar]
- Marshall, R. D., & Garakani, A. (2002). Psychobiology of the acute stress response and its relationship to the psychobiology of post-traumatic stress disorder. Psychiatric Clinics of North America, 25(2), 385–395. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli, G., Carughi, S., Sperandeo, M., Pazienza, V., Giuliani, F., & Tarquini, R. (2011). Neuro-endocrine correlations of hypothalamic-pituitary-thyroid axis in healthy humans. Journal of Biological Regulators and Homeostatic Agents, 25(2), 249–257. [PubMed] [Google Scholar]
- McEwen, B. S., & Karatsoreos, I. N. (2015). Sleep deprivation and circadian disruption: Stress, allostasis, and allostatic load. Sleep Medicine Clinics, 10(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane, A. C., Barton, C. A., Briggs, N., & Kennaway, D. J. (2010). The relationship between urinary melatonin metabolite excretion and posttraumatic symptoms following traumatic injury. Journal of Affective Disorders, 127(1–3), 365–369. [DOI] [PubMed] [Google Scholar]
- Meerlo, P., Sgoifo, A., & Suchecki, D. (2008). Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Medicine Reviews, 12(3), 197–210. [DOI] [PubMed] [Google Scholar]
- Meerlo, P., van den Hoofdakker, R. H., Koolhaas, J. M., & Daan, S. (1997). Stress-induced changes in circadian rhythms of body temperature and activity in rats are not caused by pacemaker changes. Journal of Biological Rhythms, 12(1), 80–92. [DOI] [PubMed] [Google Scholar]
- Mellman, T. A. (1997). Psychobiology of sleep disturbances in posttraumatic stress disorder. Annals of the New York Academy of Sciences, 821, 142–149. [DOI] [PubMed] [Google Scholar]
- Mellman, T. A., Bustamante, V., Fins, A. I., Pigeon, W. R., & Nolan, B. (2002). REM sleep and the early development of posttraumatic stress disorder. American Journal of Psychiatry, 159(10), 1696–1701. [DOI] [PubMed] [Google Scholar]
- Mellman, T. A., & Hipolito, M. M. (2006). Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectrums, 11(8), 611–615. [DOI] [PubMed] [Google Scholar]
- Mellman, T. A., Knorr, B. R., Pigeon, W. R., Leiter, J. C., & Akay, M. (2004). Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biological Psychiatry, 55(9), 953–956. [DOI] [PubMed] [Google Scholar]
- Mendlewicz, J. (2009). Disruption of the circadian timing systems: Molecular mechanisms in mood disorders. CNS Drugs, 23(Suppl 2), 15–26. [DOI] [PubMed] [Google Scholar]
- Moller-Levet, C. S., Archer, S. N., Bucca, G., Laing, E. E., Slak, A., Kabiljo, R., … Dijk, D.-J. (2013). Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proceedings of the National Academy of Sciences, 110(12), E1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, R. Y. (2013). The suprachiasmatic nucleus and the circadian timing system. Progress in Molecular Biology and Translational Science, 119, 1–28. [DOI] [PubMed] [Google Scholar]
- Morris, C. J., Aeschbach, D., & Scheer, F. A. (2012a). Circadian system, sleep and endocrinology. Molecular and Cellular Endocrinology, 349(1), 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, C. J., Aeschbach, D., & Scheer, F. A. J. L. (2012b). Circadian system, sleep and endocrinology. Molecular and Cellular Endocrinology, 349(1), 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington, J. M., Haack, M., Toth, M., Serrador, J. M., & Meier-Ewert, H. K. (2009). Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Progress in Cardiovascular Diseases, 51(4), 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader, N., Chrousos, G. P., & Kino, T. (2009). Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. The FASEB Journal, 23(5), 1572–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader, N., Chrousos, G. P., & Kino, T. (2010). Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology & Metabolism, 21(5), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides, N. C., Charmandari, E., Kino, T., & Chrousos, G. P. (2017). Stress-related and circadian secretion and target tissue actions of glucocorticoids: Impact on health. Frontiers in Endocrinology, 8, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides, N. C., Galata, Z., Kino, T., Chrousos, G. P., & Charmandari, E. (2010). The human glucocorticoid receptor: Molecular basis of biologic function. Steroids, 75(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides, N. C., Kyratzi, E., Lamprokostopoulou, A., Chrousos, G. P., & Charmandari, E. (2015). Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation, 22(1–2), 6–19. [DOI] [PubMed] [Google Scholar]
- Nishith, P., Resick, P. A., & Mueser, K. T. (2001). Sleep difficulties and alcohol use motives in female rape victims with posttraumatic stress disorder. Journal of Traumatic Stress, 14(3), 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova, M., & Sumova, A. (2014). New methods to assess circadian clocks in humans. Indian Journal of Experimental Biology, 52(5), 404–412. [PubMed] [Google Scholar]
- Oakley, R. H., & Cidlowski, J. A. (2013). The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. Journal of Allergy and Clinical Immunology, 132(5), 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Solis, R., & Sassone-Corsi, P. (2014). Epigenetic control and the circadian clock: Linking metabolism to neuronal responses. Neuroscience, 264, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster, H., Damerow, S., Kiessling, S., Jakubcakova, V., Abraham, D., Tian, J., … Eichele, G. (2006). The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metabolism, 4(2), 163–173. [DOI] [PubMed] [Google Scholar]
- Otte, C., Lenoci, M., Metzler, T., Yehuda, R., Marmar, C. R., & Neylan, T. C. (2005). Hypothalamic-pituitary-adrenal axis activity and sleep in posttraumatic stress disorder. Neuropsychopharmacology, 30(6), 1173–1180. [DOI] [PubMed] [Google Scholar]
- Panda, S., Hogenesch, J. B., & Kay, S. A. (2002). Circadian rhythms from flies to human. Nature, 417(6886), 329–335. [DOI] [PubMed] [Google Scholar]
- Pape-Kohler, C. I., Simanski, C., Nienaber, U., & Lefering, R. (2014). External factors and the incidence of severe trauma: Time, date, season and moon. Injury, 45(Suppl 3), S93–9. [DOI] [PubMed] [Google Scholar]
- Paranjpe, D. A., & Sharma, V. K. (2005). Evolution of temporal order in living organisms. Journal of Circadian Rhythms, 3(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, M. A., Love, R. J., Jetly, R., Richardson, J. D., Lanius, R. A., Miller, J. C., & Rhind, S. G. (2019). Blunted nocturnal salivary melatonin secretion profiles in military-related posttraumatic stress disorder. Frontiers in Psychiatry, 10, 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, C. A., Klaus, P. A., Bastian, L. D., & Cohen, R. L. (1996). Criminal victimization in the USA, 1993 - A national crime victimization survey report. Rockville, MD: U.S. Department of Justice - Bureau of Justice Statistics. [Google Scholar]
- Pervanidou, P., Agorastos, A., Kolaitis, G., & Chrousos, G. P. (2017). Neuroendocrine responses to early life stress and trauma and susceptibility to disease. European Journal of Psychotraumatology, 8, 1351218. [Google Scholar]
- Pezuk, P., Mohawk, J. A., Wang, L. A., & Menaker, M. (2012). Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology, 153(10), 4775–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbert, J., Pichat, P., Beeské, S., Decobert, M., Belzung, C., & Griebel, G. (2011). Acute inescapable stress exposure induces long-term sleep disturbances and avoidance behavior: A mouse model of post-traumatic stress disorder (PTSD). Behavioural Brain Research, 221(1), 149–154. [DOI] [PubMed] [Google Scholar]
- Pillar, G., Malhotra, A., & Lavie, P. (2000). Post-traumatic stress disorder and sleep-what a nightmare! Sleep Medicine Reviews, 4(2), 183–200. [DOI] [PubMed] [Google Scholar]
- Pilorz, V., Cunningham, P., Jackson, A., West, A., Wager, T., Loudon, A. I., & Bechtold, D. (2014). A novel mechanism controlling resetting speed of the Circadian clock to environmental stimuli. Current Biology, 24(7), 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shin, L. M., Orr, S. P., Gilbertson, M. W., … , Liberzon, I. (2012). Biological studies of post-traumatic stress disorder. Nature Reviews. Neuroscience, 13(11), 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole, N. (2007). The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin, 133(5), 725–746. [DOI] [PubMed] [Google Scholar]
- Pollack, M. H. (2011). Eszopiclone for the treatment of posttraumatic stress disorder and associated insomnia: A randomized, double-blind, placebo-controlled trial. The Journal of Clinical Psychiatry, 72(7), 892–897. [DOI] [PubMed] [Google Scholar]
- Portaluppi, F., Tiseo, R., Smolensky, M. H., Hermida, R. C., Ayala, D. E., & Fabbian, F. (2012). Circadian rhythms and cardiovascular health. Sleep Medicine Reviews, 16(2), 151–166. [DOI] [PubMed] [Google Scholar]
- Qian, X., Droste, S. K., Lightman, S. L., Reul, J. M. H. M., & Linthorst, A. C. E. (2012). Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology, 153(9), 4346–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison, C. L., & Miller, A. H. (2003). When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry, 160(9), 1554–1565. [DOI] [PubMed] [Google Scholar]
- Raskind, M. A., Peskind, E. R., Kanter, E. D., Petrie, E. C., Radant, A., Thompson, C. E., & McFall, M. M. (2003). Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. American Journal of Psychiatry, 160(2), 371–373. [DOI] [PubMed] [Google Scholar]
- Raskind, M. A., Peterson, K., Williams, T., Hoff, D. J., Hart, K., Holmes, H., & Peskind, E. R. (2013). A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. American Journal of Psychiatry, 170(9), 1003–1010. [DOI] [PubMed] [Google Scholar]
- Razzoli, M., Karsten, C., Yoder, J. M., Bartolomucci, A., & Engeland, W. C. (2014). Chronic subordination stress phase advances adrenal and anterior pituitary clock gene rhythms. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 307(2), R198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert, C. F., Maire, M., Gabel, V., Viola, A. U., Kolodyazhniy, V., Strobel, W., … Schmidt, C. (2014). Insights into behavioral vulnerability to differential sleep pressure and Circadian phase from a functional ADA polymorphism. Journal of Biological Rhythms, 29(2), 119–130. [DOI] [PubMed] [Google Scholar]
- Ressler, K. J., Mercer, K. B., Bradley, B., Jovanovic, T., Mahan, A., Kerley, K., … May, V. (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature, 470(7335), 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenbeck, A., & Hajak, G. (2001). Neuroendocrine dysregulation in primary insomnia. Revue neurologique, 157(11 Pt 2), S57–61. [PubMed] [Google Scholar]
- Roenneberg, T., & Merrow, M. (2003). The network of time: Understanding the molecular circadian system. Current Biology, 13(5), R198–207. [DOI] [PubMed] [Google Scholar]
- Rohleder, N., Wolf, J. M., & Wolf, O. T. (2010). Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews, 35(1), 104–114. [DOI] [PubMed] [Google Scholar]
- Ruger, M., & Scheer, F. A. (2009). Effects of circadian disruption on the cardiometabolic system. Reviews in Endocrine and Metabolic Disorders, 10(4), 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybkin, I. I., Zhou, Y., Volaufova, J., Smagin, G. N., Ryan, D. H., & Harris, R. B. (1997). Effect of restraint stress on food intake and body weight is determined by time of day. The American Journal of Physiology, 273(5 Pt 2), R1612–22. [DOI] [PubMed] [Google Scholar]
- Sage, D., Ganem, J., Guillaumond, F., Laforge-Anglade, G., François-Bellan, A.-M., Bosler, O., & Becquet, D. (2004). Influence of the corticosterone rhythm on photic entrainment of locomotor activity in rats. Journal of Biological Rhythms, 19(2), 144–156. [DOI] [PubMed] [Google Scholar]
- Saper, C. B. (2013). The central circadian timing system. Current Opinion in Neurobiology, 23(5), 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savvidis, C., & Koutsilieris, M. (2012). Circadian rhythm disruption in cancer biology. Molecular Medicine (Cambridge, Mass.), 18, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer, F. A., Hilton, M. F., Mantzoros, C. S., & Shea, S. A. (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences, 106(11), 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer, F. A., Van Doornen, L. J., & Buijs, R. M. (2004). Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock. Autonomic Neuroscience, 110(1), 44–48. [DOI] [PubMed] [Google Scholar]
- Schoenfeld, F. B., Deviva, J. C., & Manber, R. (2012). Treatment of sleep disturbances in posttraumatic stress disorder: A review. The Journal of Rehabilitation Research and Development, 49(5), 729–752. [DOI] [PubMed] [Google Scholar]
- Schuurman, N., Cinnamon, J., Walker, B. B., Fawcett, V., Nicol, A., Hameed, S. M., & Matzopoulos, R. (2015). Intentional injury and violence in Cape Town, South Africa: An epidemiological analysis of trauma admissions data. Global Health Action, 8, 27016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall, L. A., Milet, A., Tronche, F., & Amir, S. (2009). Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neuroscience Letters, 457(1), 58–60. [DOI] [PubMed] [Google Scholar]
- Segall, L. A., Perrin, J. S., Walker, C.-D., Stewart, J., & Amir, S. (2006). Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience, 140(3), 753–757. [DOI] [PubMed] [Google Scholar]
- Smolensky, M. H., Hermida, R. C., Reinberg, A., Sackett-Lundeen, L., & Portaluppi, F. (2016). Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiology International, 33(8), 1101–1119. [DOI] [PubMed] [Google Scholar]
- So, A. Y., Bernal, T. U., Pillsbury, M. L., Yamamoto, K. R., & Feldman, B. J. (2009). Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences, 106(41), 17582–17587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son, G. H., Chung, S., Choe, H. K., Kim, H.-D., Baik, S.-M., Lee, H., … Kim, K. (2008). Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proceedings of the National Academy of Sciences, 105(52), 20970–20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian, S., Gemma, C., Fernández Gianotti, T., Burgueño, A., Alvarez, A., González, C. D., & Pirola, C. J. (2007). Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. Journal of Internal Medicine, 261(3), 285–292. [DOI] [PubMed] [Google Scholar]
- Sopp, M. R., Brueckner, A. H., Schäfer, S. K., Lass-Hennemann, J., & Michael, T. (2019). Differential effects of sleep on explicit and implicit memory for potential trauma reminders: Findings from an analogue study. European Journal of Psychotraumatology, 10(1), 1644128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker, V. I., & Montgomery, P. (2008). Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Medicine Reviews, 12(3), 169–184. [DOI] [PubMed] [Google Scholar]
- Steiger, A. (2007). Neurochemical regulation of sleep. Journal of Psychiatric Research, 41(7), 537–552. [DOI] [PubMed] [Google Scholar]
- Stevens, R. G., Hansen, J., Costa, G., Haus, E., Kauppinen, T., Aronson, K. J., … Straif, K. (2011). Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occupational and Environmental Medicine, 68(2), 154–162. [DOI] [PubMed] [Google Scholar]
- Stonko, D. P., Dennis, B. M., Callcut, R. A., Betzold, R. D., Smith, M. C., Medvecz, A. J., & Guillamondegui, O. D. (2018). Identifying temporal patterns in trauma admissions: Informing resource allocation. PLoS One, 13(12), e0207766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann, M., & Schibler, U. (2006). Properties, entrainment, and physiological functions of mammalian peripheral oscillators. Journal of Biological Rhythms, 21(6), 494–506. [DOI] [PubMed] [Google Scholar]
- Su, Y., van der Spek, R., Foppen, E., Kwakkel, J., Fliers, E., & Kalsbeek, A. (2015). Effects of adrenalectomy on daily gene expression rhythms in the rat suprachiasmatic and paraventricular hypothalamic nuclei and in white adipose tissue. Chronobiology International, 32(2), 211–224. [DOI] [PubMed] [Google Scholar]
- Surjit, M., Ganti, K., Mukherji, A., Ye, T., Hua, G., Metzger, D., … Chambon, P. (2011). Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell, 145(2), 224–241. [DOI] [PubMed] [Google Scholar]
- Tahara, Y., Aoyama, S., & Shibata, S. (2017). The mammalian circadian clock and its entrainment by stress and exercise. The Journal of Physiological Sciences, 67(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara, Y., Shiraishi, T., Kikuchi, Y., Haraguchi, A., Kuriki, D., Sasaki, H., … Shibata, S. (2015). Entrainment of the mouse circadian clock by sub-acute physical and psychological stress. Scientific Reports, 5, 11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, J. S., Hong, H. K., Ko, C. H., & McDearmon, E. L. (2008). The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nature Reviews Genetics, 9(10), 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, J. S., Shimomura, K., & Kumar, V. (2008). Searching for genes underlying behavior: Lessons from circadian rhythms. Science, 322(5903), 909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., Yamada, T., Tsukita, S., Kaneko, K., Shirai, Y., Munakata, Y., … Katagiri, H. (2013). Chronic mild stress alters circadian expressions of molecular clock genes in the liver. American Journal of Physiology-Endocrinology and Metabolism, 304(3), E301–9. [DOI] [PubMed] [Google Scholar]
- Takahashi, S., Yokota, S.-I., Hara, R., Kobayashi, T., Akiyama, M., Moriya, T., & Shibata, S. (2001). Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology, 142(11), 4910–4917. [DOI] [PubMed] [Google Scholar]
- Talbot, L. S., Maguen, S., Metzler, T. J., Schmitz, M., McCaslin, S. E., Richards, A., & Neylan, T. C. (2014). Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: A randomized controlled trial. Sleep, 37(2), 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma, M. V., Joksimovic, L., Kirschbaum, C., Wolf, J. M., & Rohleder, N. (2012). Altered salivary alpha-amylase awakening response in Bosnian War refugees with posttraumatic stress disorder. Psychoneuroendocrinology, 37(6), 810–817. [DOI] [PubMed] [Google Scholar]
- Thomas, K. S., Bower, J. E., Williamson, T. J., Hoyt, M. A., Wellisch, D., Stanton, A. L., & Irwin, M. (2012). Post-traumatic disorder symptoms and blunted diurnal cortisol production in partners of prostate cancer patients. Psychoneuroendocrinology, 37(8), 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticlea, A. N., Bajor, L. A., & Osser, D. N. (2013). Addressing sleep impairment in treatment guidelines for PTSD. American Journal of Psychiatry, 170(9), 1059. [DOI] [PubMed] [Google Scholar]
- Torra, I. P., Tsibulsky, V., Delaunay, F., Saladin, R., Laudet, V., Fruchart, J.-C., … Staels, B. (2000). Circadian and Glucocorticoid Regulation of Rev-erbα Expression in Liver 1. Endocrinology, 141(10), 3799–3806. [DOI] [PubMed] [Google Scholar]
- Touma, C., Fenzl, T., Ruschel, J., Palme, R., Holsboer, F., Kimura, M., … Baune, B. (2009). Rhythmicity in mice selected for extremes in stress reactivity: Behavioural, endocrine and sleep changes resembling endophenotypes of major depression. PLoS One, 4(1), e4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, A. H., Barclay, J. L., & Oster, H. (2014). Interactions between endocrine and circadian systems. Journal of Molecular Endocrinology, 52(1), R1–16. [DOI] [PubMed] [Google Scholar]
- Tsigos, C., & Chrousos, G. P. (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53(4), 865–871. [DOI] [PubMed] [Google Scholar]
- Ulmer, C. S., Calhoun, P. S., Bosworth, H. B., Dennis, M. F., & Beckham, J. C. (2013). Nocturnal blood pressure non-dipping, posttraumatic stress disorder, and sleep quality in women. Behavioral Medicine, 39(4), 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai, Y. M., & Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Liempt, S. (2012). Sleep disturbances and PTSD: A perpetual circle? European Journal of Psychotraumatology, 3. doi: 10.3402/ejpt.v3i0.19142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Liempt, S., Arends, J., Cluitmans, P. J. M., Westenberg, H. G. M., Kahn, R. S., & Vermetten, E. (2013). Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: A study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology, 38(1), 155–165. [DOI] [PubMed] [Google Scholar]
- Vandewalle, G., Middleton, B., Rajaratnam, S. M. W., Stone, B. M., Thorleifsdottir, B., Arendt, J., & DIJK, D.-J. (2007). Robust circadian rhythm in heart rate and its variability: Influence of exogenous melatonin and photoperiod. Journal of Sleep Research, 16(2), 148–155. [DOI] [PubMed] [Google Scholar]
- Vetter, C. (2020). Circadian disruption: What do we actually mean? European Journal of Neuroscience, 51(1), 531–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas, A. N., Bixler, E. O., Lin, H.-M., Prolo, P., Mastorakos, G., Vela-Bueno, A., & Chrousos, G. P. (2001). Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: Clinical implications. The Journal of Clinical Endocrinology & Metabolism, 86(8), 3787–3794. [DOI] [PubMed] [Google Scholar]
- Walker, W. H., 2nd, Walton, J. C., DeVries, A. C., & Nelson, R. J. (2020). Circadian rhythm disruption and mental health. Translational Psychiatry, 10(1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner, M. D., Dorn, M. R., & Peabody, C. A. (2001). Survey on the usefulness of trazodone in patients with PTSD with insomnia or nightmares. Pharmacopsychiatry, 34(4), 128–131. [DOI] [PubMed] [Google Scholar]
- Weber, G. F., Johnson, B. N., Yamamoto, B. K., & Gudelsky, G. A. (2014). Effects of Stress and MDMA on Hippocampal Gene Expression. BioMed Research International, 2014, 141396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermeyer, J., Khawaja, I. S., Freerks, M., Sutherland, R. J., Engle, K., Johnson, D., … , Hurwitz, T. (2010). Quality of sleep in patients with posttraumatic stress disorder. Psychiatry (Edgmont), 7(9), 21–27. [PMC free article] [PubMed] [Google Scholar]
- Woodward, S. H., Arsenault, N. J., Voelker, K., Nguyen, T., Lynch, J., Skultety, K., & Sheikh, J. I. (2009). Autonomic activation during sleep in posttraumatic stress disorder and panic: A mattress actigraphic study. Biological Psychiatry, 66(1), 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, C. E., Valdimarsdottir, H. B., Erblich, J., & Bovbjerg, D. H. (2007). Poor sleep the night before an experimental stress task is associated with reduced cortisol reactivity in healthy women. Biological Psychology, 74(3), 319–327. [DOI] [PubMed] [Google Scholar]
- Wulff, K., Gatti, S., Wettstein, J. G., & Foster, R. G. (2010). Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nature Reviews Neuroscience, 11(8), 589–599. [DOI] [PubMed] [Google Scholar]
- Yamamoto, T., Nakahata, Y., Tanaka, M., Yoshida, M., Soma, H., Shinohara, K., … Takumi, T. (2005). Acute physical stress elevates mouse Period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. Journal of Biological Chemistry, 280(51), 42036–42043. [DOI] [PubMed] [Google Scholar]
- Yehuda, R. (2002). Current status of cortisol findings in post-traumatic stress disorder. Psychiatric Clinics of North America, 25(2), 341–68, vii. [DOI] [PubMed] [Google Scholar]
- Yehuda, R., Golier, J. A., & Kaufman, S. (2005). Circadian rhythm of salivary cortisol in holocaust survivors with and without PTSD. American Journal of Psychiatry, 162(5), 998–U5. [DOI] [PubMed] [Google Scholar]
- Zalta, A. K., Bravo, K., Valdespino‐Hayden, Z., Pollack, M. H., & Burgess, H. J. (2019). A placebo-controlled pilot study of a wearable morning bright light treatment for probable PTSD. Depression and Anxiety, 36(7), 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayfert, C., & DeViva, J. C. (2004). Residual insomnia following cognitive behavioral therapy for PTSD. Journal of Traumatic Stress, 17(1), 69–73. [DOI] [PubMed] [Google Scholar]
- Zelinski, E. L., Deibel, S. H., & McDonald, R. J. (2014). The trouble with circadian clock dysfunction: Multiple deleterious effects on the brain and body. Neuroscience & Biobehavioral Reviews, 24(40C), 80–101. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zisapel, N. (2007). Sleep and sleep disturbances: Biological basis and clinical implications. Cellular and Molecular Life Sciences, 64(10), 1174–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


