Abstract
Plant-associated microbes are critical for plant growth and survival under natural environmental conditions. To date, most plant microbiome studies involving high-throughput amplicon sequencing have focused on the relative abundance of microbial taxa. However, this technique does not assess the total microbial load and the abundance of individual microbes relative to the amount of host plant tissues. Here, we report the development of a host-associated quantitative abundance profiling (HA-QAP) method that can accurately examine total microbial load and colonization of individual root microbiome members relative to host plants by the copy-number ratio of microbial marker gene to plant genome. We validate the HA-QAP method using mock experiments, perturbation experiments, and metagenomic sequencing. The HA-QAP method eliminates the generation of spurious outputs in the classical method based on microbial relative abundance, and reveals the load of root microbiome to host plants. Using the HA-QAP method, we found that the copy-number ratios of microbial marker genes to plant genome range from 1.07 to 6.61 for bacterial 16S rRNA genes and from 0.40 to 2.26 for fungal internal transcribed spacers in the root microbiome samples from healthy rice and wheat. Furthermore, using HA-QAP we found that an increase in total microbial load represents a key feature of changes in root microbiome of rice plants exposed to drought stress and of wheat plants with root rot disease, which significantly influences patterns of differential taxa and species interaction networks. Given its accuracy and technical feasibility, HA-QAP would facilitate our understanding of genuine interactions between root microbiome and plants.
Key words: microbial load, host-associated quantitative abundance profiling, root microbiome
This study established a host-associated quantitative abundance profiling (HA-QAP) method that can accurately examine the total microbial load and colonization of individual root microbiome members relative to host plants by the copy-number ratio of the microbial marker gene to plant genome. Furthermore, this study found that the total microbial load represents a key feature of changes in root microbiome of plants exposed to abiotic and biotic stress conditions.
Introduction
Roots of healthy plants are colonized by complex, diverse microbial communities (the microbiome), which play crucial roles in plant nutrient acquisition, stress tolerance, and disease resistance (Van Wees et al., 2008, Hacquard et al., 2015, Franco et al., 2017). Indeed, the rhizosphere has 10–100 times more microbes compared with unplanted soil (Lynch, 1982). High-throughput amplicon sequencing of the bacterial 16S rRNA genes or the fungal internal transcribed spacers (ITSs) has been used in several model and crop species (Bulgarelli et al., 2012, Lundberg et al., 2012, Edwards et al., 2015, Zgadzaj et al., 2016, Niu et al., 2017, Duran et al., 2018) to detect root microbiome composition and variation based on relative microbial abundance, revealing that root microbiome composition is mainly determined by geographical location, soil type, abiotic and biotic stresses, and host genotype (Edwards et al., 2015, Muller et al., 2016). In light of the increasing focus on root microbiome–host plant interactions (Hiruma et al., 2016, Castrillo et al., 2017), there is a growing need to investigate microbial load of root microbiome relative to host plants.
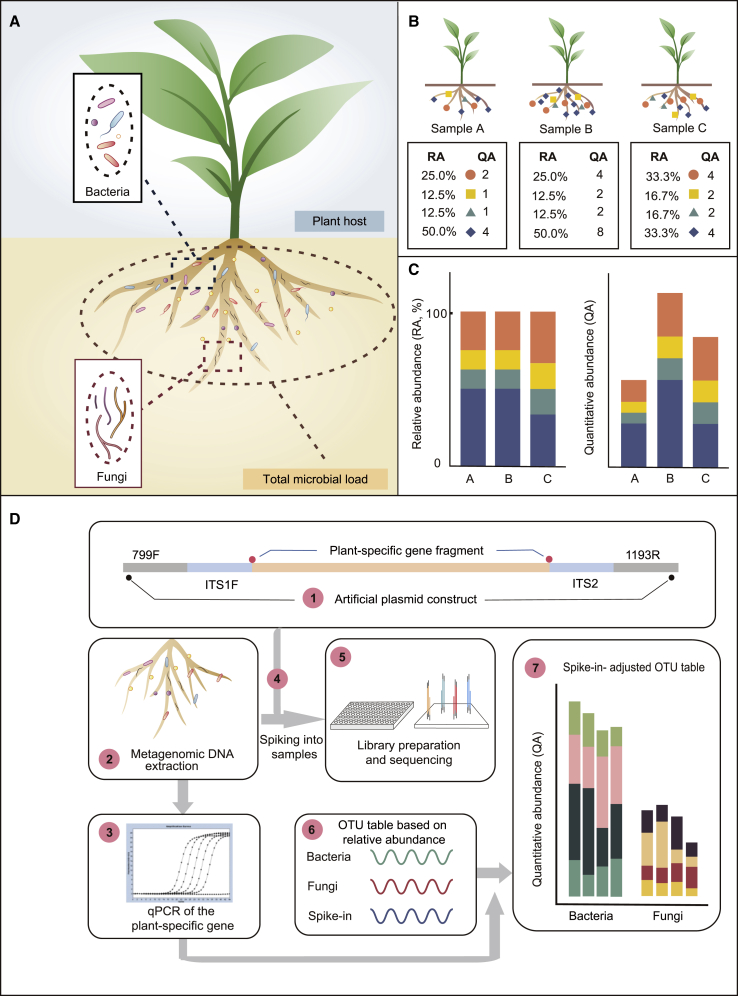
To date, the commonly used microbiome profiling methods are basically based on the relative abundance of bacteria or fungi (referred as the classical profiling method; see below) and fail to reveal the actual microbial load relative to the amount of host plant tissue (Gloor et al., 2017, Almeida and Shao, 2018) (Figure 1A). Without knowing total microbial load, it is impossible to determine whether an enrichment in certain species resulted from an increase in their absolute abundance or a decrease in the abundances of other dominant taxa (Stammler et al., 2016). Accordingly, the classical profiling method sometimes generates spurious outputs, since it is based on determining relative abundance (Figure 1B and 1C). For example, plant root B contains microbes with the same proportional composition as those in root A, but with twice the total microbial load. The classical profiling method detects the same microbiome (with identical microbial composition) in roots A and B when it actually has changed substantially in terms of its microbial load (Figure 1B and 1C). In another typical example, the level of a single microbe (blue diamond) is unchanged, while the levels of other microbes are dramatically higher, in root C versus root A. The classical profiling method detects a higher relative abundance of those microbes with increased levels, but also reports a reduction in the level of this specific microbe, even though it did not actually change. This is because the sum of the relative abundances of all microbes is 100%. When the relative abundances of some microbes significantly increase, the relative abundances of other microbes necessarily appear to decrease (Figure 1B and 1C). Therefore, the main problem with the classical profiling method is that it is unable to detect changes in microbial load relative to the host plant tissue.
Figure 1.
Advantages and Experimental Procedure for Quantitative Abundance Profiling of the Microbiome in Plant Roots.
(A) The major limitation of the current root microbiome profiling technique based on relative abundance is the lack of a method for quantitatively assessing the microbial load per unit amount of plant root tissue.
(B) Three simulated root microbiomes associated with the same amounts of root tissue (upper panel) and the corresponding microbial profiling based on relative or quantitative abundance (lower panel). RA, relative abundance; QA, quantitative abundance, representing the copy-number ratio of microbial marker genes relative to plant genome.
(C) Bar plots showing a comparison of relative (left panel) versus quantitative (right panel) microbial profiling of simulated root microbiome samples in (B).
(D) Schematic diagram showing the workflow of HA-QAP. (1) The artificial plasmid is designed and constructed as a spike-in control, containing conserved 16S rRNA, ITS primer regions (799F/1193R and ITS1F/ITS2), and a plant-specific gene sequence (the RID1 gene in this study). (2) DNA is extracted from the root microbiome, including plants and microbes. (3) A selected plant marker gene found in the total DNA is quantified by qPCR; this value is used to calibrate microbial relative abundance to quantitative abundance in step (7). (4) The predefined amount of spike-in plasmid is added to the DNA of root microbiome samples based on experience. (5 and 6) PCR amplicon libraries (bacteria and fungi, respectively) are prepared, sequenced (5), and analyzed (6). (7) The bacterial and fungal reads are calibrated based on the read counts of the spike-in and the amount of the plant marker gene quantified by qPCR in step (3) to determine the quantitative abundance relative to the host plant tissue.
Various methods have been proposed to assess the microbial load in the microbiome relative to the host. In the human gut, 16S ribosomal RNA (rRNA) sequencing was combined with flow cytometry-based microbial cell counting or microbial quantitative PCR (qPCR) to quantify absolute microbial abundances in fecal samples (Vandeputte et al., 2017). However, neither of these two methods is suitable for studying the microbial load associated with host plant tissues, such as the root-associated microbiome. Flow cytometry cannot precisely count microbial and plant cells in living or disrupted root samples, and universal 16S rRNA or ITS primers do not discriminate sequences from microbes versus plant plastids (chloroplasts and mitochondria), which prevents the accurate detection of microbial DNA by qPCR. Furthermore, staining methods such as CARD-FISH (fluorescence in situ hybridization coupled with catalyzed reporter deposition) (Schmidt and Eickhorst, 2014) also are not suitable for detection and taxonomic classification of individual microbiome members associated with plant roots in a high-throughput manner. The analysis of DNA from plant plastids has been proposed as a solution (Edwards et al., 2015, Lebeis et al., 2015), but it is not an ideal internal control due to the high amount and variable copy number of plastids per cell (Cole, 2016). Plastid sequences typically account for >80% of the 16S rRNA sequences in root microbiome datasets and are experimentally removed during library preparation protocols (Bulgarelli et al., 2012, Lundberg et al., 2012, Lundberg et al., 2013). By contrast, metagenome-based sequencing can be used to detect root microbial load in plant tissues by classifying reads as belonging to microbes and plants, but cannot be applied to numerous samples due to the high cost (Karasov et al., 2018).
The utilization of spiking internal control is a prospective strategy for solving the problem about quantifying microbial load. Synthetic spikes have been established in the field of RNA sequencing (Risso et al., 2014), proteomics (Geiger et al., 2011), and metagenomics (Hardwick et al., 2018). Recently, spiking known sequences (Smets et al., 2016, Lin et al., 2019) or cultures that do not exist in the community (Stammler et al., 2016), or using synthetic spikes of 16S (Tourlousse et al., 2017) and a combination of 16S, 18S, and ITS synthetic spikes (Tkacz et al., 2018), were developed for quantitative microbiome analyses. However, these spike-in-based microbiome profiling methods were only used for microbiome samples without the host. In addition, universal microbial genes, such as 16S rRNA gene or ITS, have high sequence similarity with sequences in plant genomes, making it impossible to examine microbiome load by comparing genes in microbes and plants. Therefore, quantitative detection of microbiome abundance to host tissues, such as plant roots, remains a major challenge. In this study, we developed a simple, cost-effective, and modular host-associated quantitative abundance profiling (HA-QAP) method that allows detecting microbial load and colonization of individual root microbiome members relative to host plants by the copy-number ratio of the microbial marker genes (16S rRNA gene or ITS) to plant genome. This high-throughput technique detects the colonization of an individual microbe and accurately assesses variations in root-associated microbiomes and microbe–microbe interactions. Using mock experiments, perturbation experiments, and metagenomic sequencing, we validated the accuracy and reproducibility of the HA-QAP. Moreover, using HA-QAP we found that an increase in total microbial load is a key feature of the changes that occur in the microbiomes of rice plants under drought stress and in wheat with root rot disease.
Results
The Spike-In Plasmid and Principle of HA-QAP
We generated an artificial spike-in plasmid as an internal control to quantitatively detect the load and abundance profiles of root microbiome associated with host plants. The spike-in plasmid we designed contains a plant-specific gene fragment flanked by conserved regions of the bacterial 16S rRNA gene and fungal ITS (see Methods). The plant gene fragment is the RID1 gene, encoding a master regulator of flowering in rice (Wu et al., 2008), which is not present in microbial genomes. The gene fragment can be amplified and integrated into the classical PCR-based bacterial and fungal profiling (Figure 1D). Because the amplified plant-specific sequence does not exist in the natural microbial community, the spike-in sequences could be easily identified during data analysis. We added a defined amount of the spike-in plasmid to the DNA samples of root microbiome (containing both plant and microbial DNA) before PCR amplification, measured plant DNA amount by qPCR with the plant marker gene (e.g., RID1 in the rice genome), and determined the relationship between the amounts of spike-in and plant DNA (see Methods). Based on this relationship, the copy number of microbial marker genes (16S rRNA gene or ITS) can then be normalized to the copy number of plant genome, which reflects the quantitative microbial colonization on host plant roots (Figure 1D; see also Supplemental Figure 1 and Methods).
The Spike-In Plasmid Is Suitable for Quantitative Microbiome Profiling
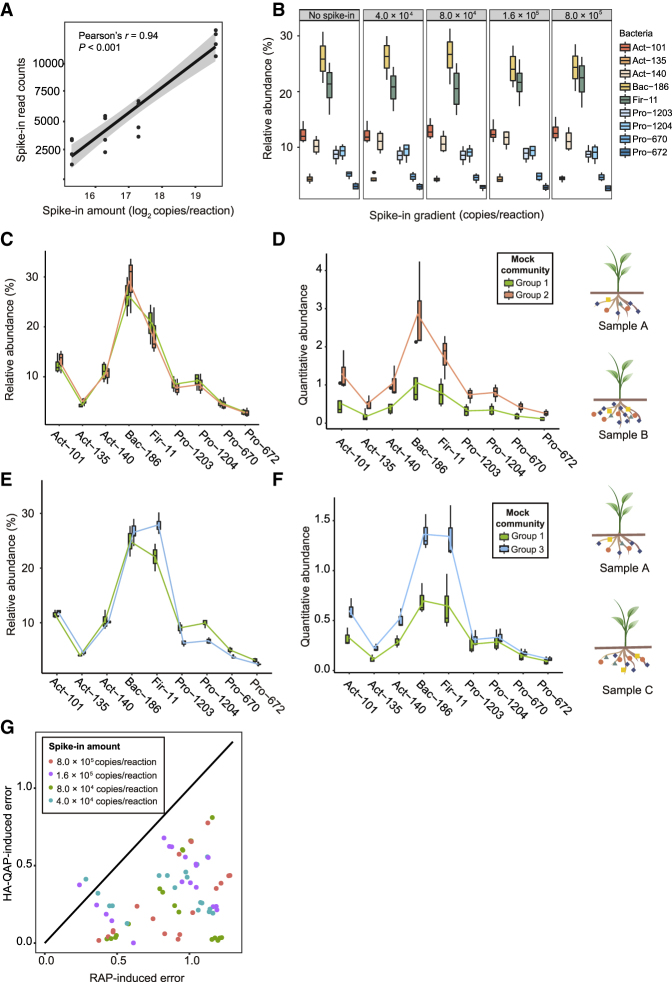
Because a correlation between the counts of spike-in reads in the profiling results and the amount of spike-in plasmid in the DNA template is critical for spike-in-based quantification (Jiang et al., 2011), we tested this correlation using different amounts of the spike-in plasmid. After adjustment for uneven sequencing depth by rarefaction, we found that read counts of the spike-in plasmid in the sequencing data increased with increasing amounts of spike-in in the input, following a Poisson general linear regression model (Pearson's r = 0.94, P < 0.001, Figure 2A). This result indicates that quantitative detection of the spike-in plasmid was reliable across a dynamic range of 4.0 × 104 to 8.0 × 105 copies per PCR, in which we could expect a linear response of spike-in reads in relation to input amount (see also Supplemental Table 1).
Figure 2.
HA-QAP Is More Accurate than Classical Profiling Based on Relative Abundance in the Bacterial Mock Experiments.
(A) Dose–response curves for the linear correlation between read counts of spike-in plasmid (BI12-4) obtained by Illumina sequencing and amount of spike-in plasmid in the DNA samples, indicating that bacterial reads of the spike-in plasmid in the sequencing data reflect the amount of spike-in plasmid in the initial DNA samples. The gray region indicates 95% confidence intervals (CIs).
(B) Box plots representing the relative abundance of the nine bacteria in the same mock experiment with a gradient of spike-in levels of 0–8.0 × 105 copies per reaction. Wilcoxon rank-sum test showed no significant differences in bacterial relative abundance between the control group without spike-in and groups with different spike-in levels (4.0 × 104, 8.0 × 104, 1.6 × 105, and 8.0 × 105 copies per reaction, P > 0.05).
(C and D) The HA-QAP method revealed the significant increase in bacterial load (two-sided t-test, P < 0.05), which could not be detected by the classical method based on relative abundance. Box plots showing a comparison of bacterial profiles in mock experiments between groups 1 and 2 using the classical method based on relative abundance (RAP) (C) and HA-QAP (D). Quantitative abundance represents the copy-number ratio of bacterial 16S rRNA genes relative to plant genome.
(E and F) The HA-QAP method improved the detection of the increases in the levels of Actinobacteria, Bacteroidetes, and Firmicutes (two-sided t-test, P < 0.05) and revealed that the quantitative abundance of Proteobacteria did not change (two-sided t-test, P > 0.05) when the amounts of other bacteria increased. Box plots showing a comparison of bacterial profiles in mock experiments between groups 1 and 3 using RAP (E) and HA-QAP (F). Quantitative abundance represents the copy-number ratio of bacterial 16S rRNA genes relative to plant genome. Notably, the RAP method showed a spurious reduction in Proteobacteria levels, but HA-QAP did not.
(G) Scatter plot showing the ratio of errors between HA-QAP and RAP. Dots on the line with fixed slope = 1 represent errors from HA-QAP equivalent to those from RAP. Most dots fall below the line with slope = 1, demonstrating that the HA-QAP method presents more real data. Data are based on comparisons (group 1 versus group 2; group 1 versus group 3) at different spike-in levels. Three groups of mock experiments were designed; n = 3, 4, and 5 for groups 1, 2, and 3, respectively.
All data shown in (C) to (F) are from samples with 4.0 × 104 copies of spike-in per reaction. The trend was consistent using other amounts of spike-in. Act, Actinobacteria; Bac, Bacteroidetes; Fir, Firmicutes; Pro, Proteobacteria. See also Supplemental Figures 2 and 3.
To determine whether the spike-in plasmid affects the quantification of microbial relative abundance, we determined the relative abundance of individual bacteria using DNA from the bacterial mock experiments in the presence of a gradient concentration of the spike-in plasmid (0, 4.0 × 104, 8.0 × 104, 1.6 × 105, and 8.0 × 105 copies per PCR). We then investigated the distribution of the relative abundance of each reference bacterial strain after filtering out the spike-in sequences. The relative abundances of individual bacterial strains in the samples containing the spike-in plasmid remained consistent with those of the control samples without the spike-in plasmid (Figure 2B; Wilcoxon rank-sum test, P > 0.05; see also Supplemental Table 2). Our results demonstrate that the spike-in plasmid did not affect bacterial PCR amplification and detection, indicating that the spike-in plasmid is suitable for quantitative microbiome profiling.
Design and Rationale to Detect Microbial Load in Mock Experiments
To verify the reliability of HA-QAP, we compared it with the classical method based on relative abundance profiling (RAP) using three groups of premixed DNAs from cultivated microbes and germ-free plants (mock experiment, Figure 2C–2F). These three groups of mock samples represent typical samples that produce spurious results (shown in Figure 1B) using the RAP method. Group 1 contained mixed DNA from germ-free plants, nine bacteria, and three fungi at a fixed ratio, including strains of four bacterial phyla (Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria) and two fungal phyla (Ascomycota and Basidiomycota). Group 2 contained an amount of DNA from germ-free plants equal to that in group 1 but with twice the amount of microbial DNA with the same interspecies ratio. Group 3 contained the same amounts of DNA from germ-free plants, Proteobacteria, and Ascomycota as those of group 1, but with twice the amount of microbial DNA from the remaining microbes with the same interspecies ratio (see also Supplemental Table 3). To determine the appropriate concentration of spike-in to use for accurate analysis, we performed quantitative profiling of each group using a gradient of spike-in plasmid (0, 4.0 × 104, 8.0 × 104, 1.6 × 105, and 8.0 × 105 copies per PCR). As we knew the exact differences between groups 1 and 2 and between groups 1 and 3 in the mock experiments, we evaluated the accuracy of the HA-QAP and classical RAP methods, respectively.
HA-QAP Detects the Microbial Load of Root Microbiome in Mock Experiments
The HA-QAP method detected the shift in total bacterial load relative to plant root DNA in the mock experiments (Figure 2C and 2D). Since classical 16S rRNA gene profiling can only detect the relative abundance of bacterial members of the microbiome, it does not reflect the changes in the bacterial load relative to plant roots (samples A and B in Figures 1B, 1C, and 2C). We compared the accuracy of the HA-QAP and RAP methods in groups 1 and 2, as mentioned above. The classical RAP method revealed the same relative abundances of each strain within groups 1 and 2 (two-tailed Student's t-test, P > 0.05), which did not reflect the bacterial load and abundance relative to roots (Figure 2C; see also Supplemental Table 4). By contrast, the HA-QAP method detected an average of 2.4-fold change (expected ratio of 2-fold) in bacterial abundance relative to plant root DNA between groups 1 and 2 at a spike-in concentration of 4.0 × 104 copies per PCR (two-tailed Student's t-test, P < 0.05; Figure 2D; see also Supplemental Table 4). The same trends were observed in three other experiments with different amounts of spike-in plasmid (8.0 × 104, 1.6 × 105, and 8.0 × 105 copies per PCR) (see also Supplemental Figure 2 and Supplemental Table 4).
HA-QAP Accurately Detects the Changes of Individual Root Microbiome Members in Mock Experiments
The HA-QAP method detected the quantitative abundance of individual bacteria relative to root DNA when there were dramatic changes of highly abundant bacteria in mock experiments (Figure 2E and 2F). The classical RAP method (based on relative abundance) often generates spurious results when the levels of highly abundant bacteria increase or decrease. We investigated the microbial communities in two mock experiments (groups 1 and 3) using the HA-QAP and RAP methods. The classical RAP method detected a reduction (two-tailed Student's t-test, P < 0.05) in the levels of Proteobacteria (Pro-1203, Pro-1204, and Pro-670) from groups 1 to 3 when their abundance relative to plant roots did not change (Figure 2E; see also Supplemental Table 5). Notably, the HA-QAP method detected the true changes in bacterial communities from groups 1 to 3, with increases in the abundances of Actinobacteria, Bacteroidetes, and Firmicutes (two-tailed Student's t-test, P < 0.05; average fold change, 1.9; see also Supplemental Table 6) and no significant difference in the levels of Proteobacteria strains when the spike-in concentration was 4.0 × 104 copies per PCR (two-tailed Student's t-test, P > 0.05; Figure 2F; see also Supplemental Table 5). These trends were also observed in experiments using three other gradients of spike-in plasmid (see also Supplemental Figure 3; Supplemental Tables 5 and 6).
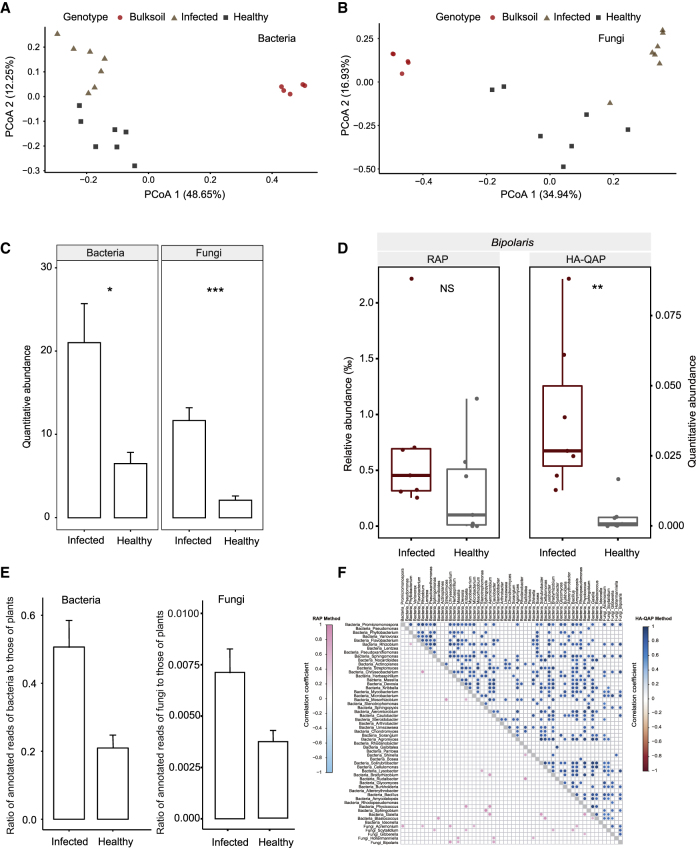
Figure 6.
HA-QAP Reveals Microbial Load Increase in Wheat Plant with Root Rot Disease.
(A and B) Principal coordinates analysis of Bray–Curtis distances showing that infected and healthy roots formed distinct clusters in the bacterial (A) or fungal (B) microbiome. Each point corresponds to a sample: infected root samples, triangles; healthy root samples, squares; bulk soil samples, circles.
(C) Infected root samples had significantly higher bacterial and fungal load than healthy roots. Quantitative abundance represents the copy-number ratio of bacterial 16S rRNA genes and fungal ITS relative to plant genome.
(D) Relative versus quantitative abundance of the potential disease-associated pathogenic genus Bipolaris in healthy and infected samples with root rot disease assessed by RAP and HA-QAP. Quantitative abundance represents the copy-number ratio of fungal ITS relative to plant genome.
(E) Metagenomic data showing higher ratios of bacterial (left) and fungal (right) reads to host plant reads in infected root samples (infected samples, n = 4; healthy samples, n = 4).
(F) Genus co-occurrence patterns within the top 55 most abundant genera and the potential disease-associated pathogenic genus Bipolaris detected by RAP and HA-QAP. Pairwise correlations between taxon abundances were calculated using RAP (lower triangle) and HA-QAP (upper triangle). Significant correlations (two-sided adjusted test, false discovery rate < 0.05) are represented by circles; the color of each circle represents the correlation coefficient (Spearman's ρ). The taxa are firstly ranked according to the bacterial and fungal domain and then ordered by the individual's quantitative abundance within the same domain. The leftmost/top represents the most highly abundant.
For all analyses (except E), the number of biological replicates is as follows: infected root samples, n = 7; healthy root samples, n = 7; bulk soil samples, n = 5. Error bars represent SD calculated from replicates. Statistical significance was determined by Wilcoxon rank-sum test. Asterisks indicate statistically significant differences (*P < 0.05; **P < 0.01). NS, not significant.
To assess the RAP and HA-QAP methods, we calculated the errors between the expected values and measured values in the microbial mock experiments using the two methods (Figure 2G). We plotted the ratio between HA-QAP-induced errors and RAP-induced errors (Figure 2G), where the line with a slope = 1 would represent that the HA-QAP-induced error is equivalent to the RAP-induced error. Most ratios between HA-QAP-induced errors and RAP-induced errors were below this line, demonstrating that the HA-QAP method presented more real data. Taken together, the HA-QAP method overcame the limitations of the RAP method, revealing the changes in bacterial load and quantitative abundance of individual bacterial members relative to plant roots.
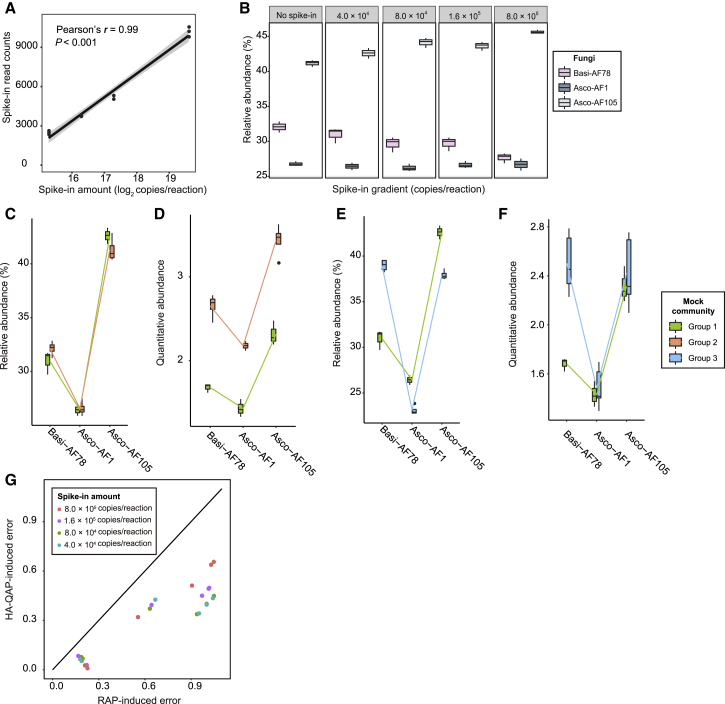
Similar to the results observed in the bacterial mock experiments, HA-QAP also detected the total fungal load and quantitative abundance of individual fungal strains relative to plant roots. Spike-in read counts and input amounts followed a Poisson general linear regression model (Pearson's r = 0.98, P < 0.001; Figure 3A), indicating that quantitative detection of the spike-in plasmid was reliable across a dynamic range of 4.0 × 104 to 8.0 × 105 copies per PCR (see also Supplemental Table 7). As shown in Figure 3B, the spike-in plasmid did not affect the trends of relative abundance of each fungus (Wilcoxon rank-sum test, P > 0.05; see also Supplemental Table 8). As expected, the classical RAP method did not detect differences in the total fungal loads between groups 1 and 2 (two-tailed Student's t-test, P > 0.05; Figure 3C; see also Supplemental Figure 4A and Supplemental Table 9), whereas HA-QAP revealed the increase in fungal load relative to plant roots from groups 1 and 2 (two-tailed Student's t-test, P < 0.05; Figure 3D; see also Supplemental Figure 4B and Supplemental Table 9). Moreover, the HA-QAP method detected the specific increase in abundance of the Basi-AF78 fungus relative to plant roots (two-tailed Student's t-test, P < 0.05; Figure 3E and 3F). By contrast, this increase leads to a spurious reduction in abundance of the two Ascomycota isolates (Asco-AF1 and Asco-AF105) in the RAP method (two-tailed Student's t-test, P < 0.05; Figure 3E), which is not visible with the HA-QAP method (two-tailed Student's t-test, P > 0.05; Figure 3E and 3F; see also Supplemental Figure 5A and 5B; Supplemental Table 10). Finally, we calculated the ratio between HA-QAP-induced error and RAP-induced error and plotted the values (Figure 3G). For the fungal mock experiments, all dots fell below the line with a slope = 1, demonstrating that the HA-QAP method presented more real data, as observed for bacteria.
Figure 3.
HA-QAP Is More Accurate than Classical Profiling Based on Relative Abundance in the Fungal Mock Experiments.
(A) Dose–response curves for linear correlation between read counts of spike-in plasmid (BI12-4) obtained by Illumina sequencing and amount of spike-in plasmid in the DNA samples, indicating that fungal reads of the spike-in plasmid in the sequencing data reflect the amount of spike-in plasmid in the initial DNA samples. The gray region indicates 95% CIs.
(B) Box plots representing the relative abundance of reads assigned to the three fungi in the same mock experiment with a gradient of spike-in levels from 0 to 8.0 × 105 copies per reaction. Wilcoxon rank-sum test showed no significant differences in fungal relative abundance between the control group without spike-in and groups with different spike-in levels (4.0 × 104, 8.0 × 104, 1.6 × 105, and 8.0 × 105 copies per reaction, P > 0.05).
(C and D) The HA-QAP method revealed the significant increase in fungal load (two-sided t-test, P < 0.05), which could not be detected by the classical method based on relative abundance. Box plots showing a comparison of fungal profiles in mock experiments between group 1 and group 2 samples using RAP (C) and HA-QAP (D). Quantitative abundance represents the copy-number ratio of fungal ITS relative to plant genome.
(E and F) The HA-QAP method more accurately detected the increase in Basidiomycota levels (two-sided t-test, P < 0.05) and revealed that the quantitative abundance of Ascomycota did not change (two-sided t-test, P > 0.05) when the levels of the other fungal isolates increased. Box plots showing a comparison of fungal profiles in mock experiments between groups 1 and 3 using RAP (E) and HA-QAP (F). Quantitative abundance represents the copy-number ratio of fungal ITS relative to plant genome. Notably, RAP showed a spurious reduction in Ascomycota levels (Asco-AF1 and Asco-AF105), but HA-QAP did not.
(G) Scatter plot showing the ratio of errors between HA-QAP and RAP. Dots on the line with fixed slope = 1 represent errors from HA-QAP equivalent to those from RAP. All dots fall below the line with slope = 1, demonstrating that the HA-QAP method presents more real data. Data are based on comparisons (group 1 versus group 2; group 1 versus group 3) at different spike-in levels. Three groups of mock experiments were designed; n = 4, 3, and 5 for groups 1, 2, and 3, respectively.
All data shown in (C) to (F) are from samples with 4.0 × 104 copies of spike-in per reaction. The trend was consistent when using other spike-in levels. Asco, Ascomycota; Basi, Basidiomycota. See also Supplemental Figures 4 and 5.
HA-QAP Detects Changes in Microbial Load in Natural Root Samples
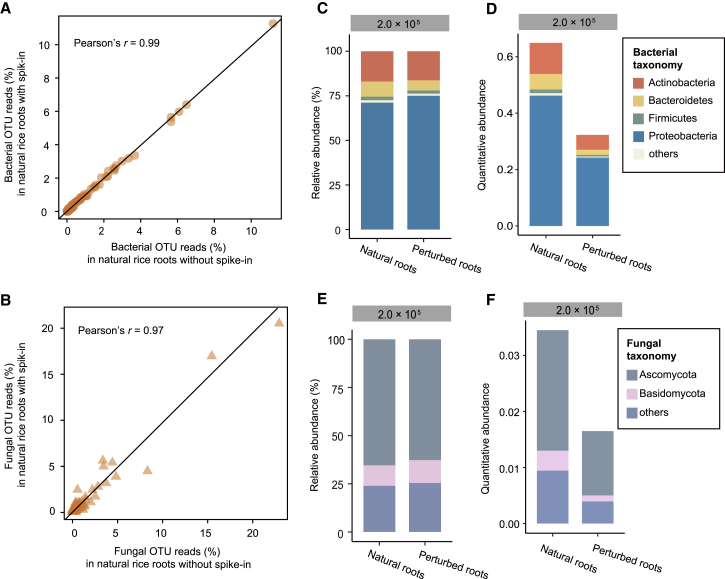
Since the HA-QAP method worked well in the mock experiments, we further validated the utility of this plasmid in natural root samples. The spike-in plasmid did not alter the relative abundance of bacteria or fungi in natural root samples. The relative abundances of both bacterial and fungal operational taxonomic units (OTUs) between natural root samples with and without the spike-in plasmid were highly comparable (for bacteria, Pearson's r = 0.99, Figure 4A; for fungi, Pearson's r = 0.97, Figure 4B; see also Supplemental Figure 6 for other spike-in concentrations). The Shannon index also showed no significant difference between control and spiked samples (Wilcoxon rank-sum test, P > 0.05; see also Supplemental Figure 7).
Figure 4.
HA-QAP Reveals a Reduction in Microbial Load in Perturbed Natural Root Samples.
(A and B) Scatter plot showing the correlation between the relative abundance of bacterial OTUs (A) and fungal OTUs (B) in natural rice roots with spike-in (spike-in levels: 2.0 × 105 copies per reaction, in y axis) versus rice roots without spike-in (x axis), demonstrating that spike-in did not influence the relative abundance of bacterial or fungal OTUs in natural root samples.
(C and D) Bacterial profile based on relative abundance (C) and quantitative abundance (D) in natural rice roots and rice roots with 55% microbial load compared with the original natural samples by RAP and HA-QAP, respectively. Quantitative abundance represents the copy-number ratio of bacterial 16S rRNA genes relative to plant genome. Note that the HA-QAP method revealed the reduction in bacterial load in perturbed natural rice roots.
(E and F) Fungal profile based on relative abundance (E) and quantitative abundance (F) in natural rice roots and rice roots with 55% microbial load compared with the original natural samples by RAP and HA-QAP, respectively. Quantitative abundance represents the copy-number ratio of fungal ITS relative to plant genome. Note that the HA-QAP method revealed the reduction in fungal load in perturbed natural rice roots.
Data in (C) to (F) represent three technical replicates.
To test the performance of the HA-QAP method in natural root samples, we perturbed the microbiome samples of natural roots with defined amounts of DNA from the roots of germ-free rice to artificially reduce the microbial load of samples to 55% compared with the original samples. The HA-QAP method detected a decrease in bacterial and fungal microbial load relative to plant root tissue in the perturbed samples (Figure 4D and 4F), while the classical RAP method did not (Figure 4C and 4E), since the classical RAP method only detected the relative abundance of microbes. The HA-QAP method revealed decreases in total bacterial and fungal loads of 50% and 52%, respectively, at a spike-in concentration of 2.0 × 105 copies per reaction (Figure 4D and 4F). Similar trends were observed in other experiments using different concentrations of spike-in plasmid (see also Supplemental Figure 8). Taken together, the HA-QAP method successfully detected the microbial load relative to root tissue in both mock and natural samples, and thus can be used to investigate quantitative interactions between the microbiome and roots.
Microbial Load Is a Key Feature of the Changes in Root Microbiome Response to Drought Stress in Rice
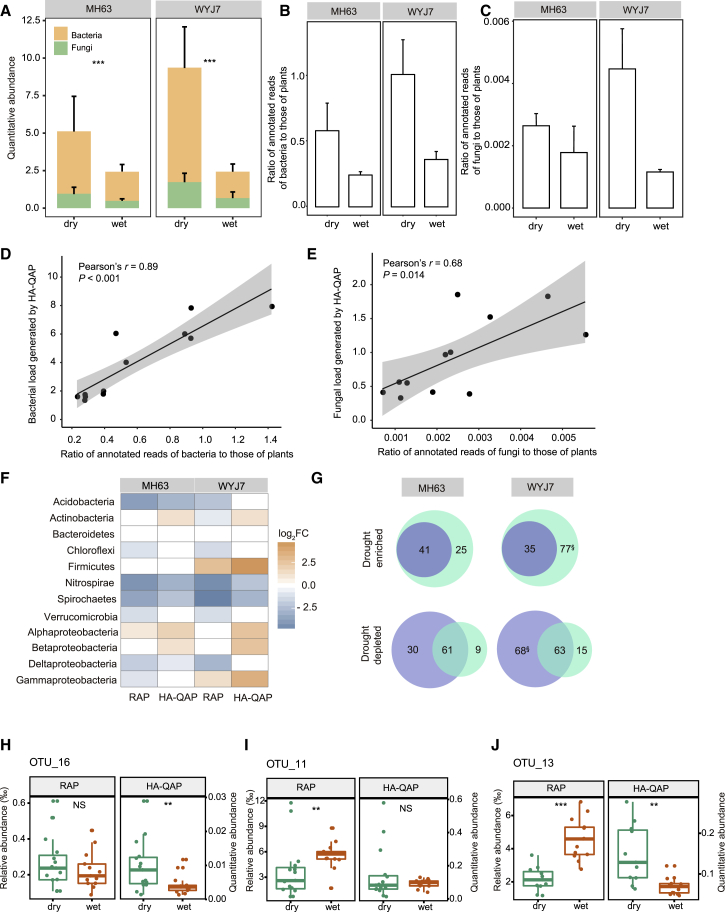
Drought stress is one of the most significant obstacles to agricultural productivity, resulting in billions of dollars in worldwide losses annually (Lesk et al., 2016). Efforts to ensure global food security are increasingly focusing on investigating the root microbiome in crop plants under drought conditions (Santos-Medellin et al., 2017, Xu et al., 2018b). Drought significantly alters root microbiome composition across several plant species, including wheat, barley, sorghum, and rice (Naylor et al., 2017, Santos-Medellin et al., 2017, Xu et al., 2018b). However, the changes in root microbial load under drought stress are not known. To address this issue, we grew two rice varieties, MH63 and WYJ7, under drought and wet conditions at both the Hainan Farm and the Anhui Farm in China.
Consistent with a previous report (Santos-Medellin et al., 2017), we found that drought treatment influenced rice root-associated microbial composition based on microbial relative abundance (see also Supplemental Figure 9). Using the HA-QAP method, we found that the bacterial and fungal loads of the rice root microbiome significantly increased under drought conditions at the Hainan Farm (Figure 5A). The total microbial load of the root microbiome relative to host plant tissue increased 1.1- and 2.8-fold in MH63 and WYJ7, respectively, under drought conditions (Wilcoxon rank-sum test, P < 0.05, Figure 5A). To validate the increase in microbial load detected by the HA-QAP method in plant roots under drought stress, we performed metagenomic sequencing of the same samples and evaluated the microbial load by assessing the proportion of reads that can be mapped to microbes and plants in the publicly available NCBI-NR database (Benson et al., 2006). The metagenomic data revealed the similar trend of increases in the microbial load that correlated with those detected by the HA-QAP method (Figure 5B–5E). Moreover, the trend of microbial load increase in roots of MH63 and WYJ7 varieties under drought stress was also detected at the Anhui Farm, which is located 1650 km away from the Hainan Farm (see also Supplemental Figure 10A). Thus, our data suggest that drought-mediated increase in microbial load in plant roots represents an intrinsic feature of plant microbiome response to drought stress.
Figure 5.
HA-QAP Reveals Microbial Load Increase in Rice Roots under Drought Stress.
(A) The HA-QAP method showing the significant increases in bacterial and fungal loads in the root microbiome of two rice varieties (MH63 and WYJ7) under drought-stress conditions. Quantitative abundance represents the copy-number ratio of bacterial 16S rRNA genes and fungal ITS relative to plant genome.
(B and C) Metagenomic data showing higher ratios of annotated reads of bacteria (B) and fungi (C) to those of plants under drought conditions in rice varieties MH63 and WYJ7 grown in Hainan (dry: n = 3; wet: n = 3).
(D and E) Scatter plots showing the linear correlation between the ratio of annotated reads of bacteria (D) and fungi (E) to those of plants detected by metagenomic sequencing (x axis) versus the microbial load detected by the HA-QAP method (y axis). Gray region indicates 95% CIs.
(F) Heatmap showing the log2 fold change in abundance of drought-responsive bacterial phyla with respect to control treatment (wet) in rice varieties MH63 and WYJ7 detected by HA-QAP and RAP. Orange boxes indicate an increase while blue boxes indicate a decrease. White boxes indicate no significant fold change. Note that for the root microbiome of both varieties, the HA-QAP method revealed a shift in drought-responsive phyla, with an enhanced enrichment and reduced depletion under drought-stress conditions compared with wet conditions due to the increase in root bacterial load detected by HA-QAP.
(G) Venn diagram showing the overlap and differences in drought-responsive OTUs detected by the RAP and HA-QAP methods. The purple and green ellipses represent drought-responsive OTUs detected by the RAP and HA-QAP method, respectively. § indicates the five OTUs detected as depleted OTUs using RAP but identified as enriched OTUs using HA-QAP, suggesting that the microbial load value is essential for tracking the changes in the abundances of these bacteria.
(H–J) Examples showing the changes in OTUs in rice roots under drought and wet conditions using RAP and HA-QAP. Quantitative abundance represents the copy-number ratio of bacterial 16S rRNA genes relative to plant genome. The responses of three OTUs to drought-stress conditions differed depending on the method: OTU_16 (H) was detected as an enriched group responsive to drought only using HA-QAP. Conversely, OTU_11 (I) was detected as significantly depleted in dry root samples only using RAP and not HA-QAP. OTU_13 (J) was found to be significantly depleted in dry root samples using RAP but enriched using HA-QAP.
Note that all data from Hainan are consistent with those from Anhui (see also Supplemental Figure 9 and 10). For all analyses (except for B and C), the number of biological replicates was as follows: MH63 (dry: n = 15; wet: n = 13); WYJ7 (dry: n = 11, wet: n = 13); bulk soil (dry: n = 6; wet: n = 6). Error bars represent SD calculated from replicates. Statistical significance was determined by Wilcoxon rank-sum test. Asterisks indicate statistically significant differences (**P < 0.01; ***P < 0.001). NS, not significant.
To assess the effects of microbial load on the patterns of differential taxa in the root microbiome of rice plants grown under drought and wet conditions, we compared the enrichment and depletion profiles based on the RAP and HA-QAP methods. We found that the phyla and OTUs detected as enriched in rice roots under drought conditions using the RAP method were more significantly enriched using HA-QAP. By contrast, those depleted under drought stress using the RAP method showed less pronounced depletion when HA-QAP was used. These trends were observed in both rice varieties and locations (Hainan and Anhui Farms; Figure 5F and 5G; see also Supplemental Figure 10B and 10C). The increase of Actinobacteria in MH63 roots under drought conditions is not revealed by RAP, whereas the HA-QAP method revealed a 0.2-fold increase relative to rice roots (Figure 5F).
The influence of HA-QAP on differential bacteria was further illustrated at the OTU level. As shown in Figure 5H, OTU_16 (Actinobacteria) was enriched in response to drought only when using the HA-QAP method. For the depleted OTUs under drought conditions detected by RAP, the difference was less pronounced or reversed with HA-QAP analysis (Figure 5I and 5J). OTU_11 (Proteobacteria) was significantly depleted in response to drought when examined by RAP but not HA-QAP (Figure 5I). OTU_13 (Proteobacteria) was significantly enriched in drought-stressed root samples using RAP but was depleted using HA-QAP (Figure 5J). Together, the aforementioned different results obtained from RAP versus HA-QAP methods rely on accurate assessment of microbial load in plant tissues. The HA-QAP method is potentially a more useful tool for identifying beneficial, agriculturally important microbes that improve drought tolerance in rice.
HA-QAP Reveals Absolute Increases in Total Microbial Load in Wheat Roots Infected with Root Rot Disease
Common root rot is a disease of winter wheat and causes yield losses every year (Kumar et al., 2002, Xu et al., 2018a). Symptoms include dark-brown lesions on roots, subcrown internodes, and stem bases. Little is known regarding the influence of root rot disease on root microbiome composition and microbial load in wheat. Using the HA-QAP method, we investigated the root microbiome in healthy wheat plants and wheat plants exhibiting common root rot under field conditions (Triticum aestivum cv. “Lumai21”; see also Supplemental Figure 11).
We found that plants with root rot disease showed differential root microbiome composition and increased microbial load compared with healthy plants. First, principal coordinates analysis (PCoA) based on microbial composition revealed that the root microbiomes of healthy and infected wheat plants separated in the first and second coordinates (Figure 6A and 6B). For the bacterial microbiome, a clear separation of root samples distinguished by health status was observed on the PCoA2 coordinate, explaining 22.4% of the variance (PERMANOVA, P < 0.001). For fungal communities, healthy samples and infected samples were obviously separated on the first two ordination axes, explaining 23.2% of the total variance (PERMANOVA, P < 0.001). Second, the HA-QAP method revealed that the bacterial and fungal loads of the root microbiome significantly increased in infected samples (Wilcoxon rank-sum test, P < 0.05, Figure 6C). The total bacterial load increased by 2.2-fold in samples with root rot disease, while the fungal load increased by 4.5-fold. Notably, the abundance of potential disease-associated genus Bipolaris significantly increased relative to plant roots when considering the total microbial load (Wilcoxon rank-sum test, P < 0.005), whereas the important microbial signal was lost when using the RAP method (P > 0.05) (Figure 6D). The increase in bacterial and fungal load was further validated by metagenomic sequencing (Figure 6E). Therefore, although RAP allowed us to discriminate between healthy and infected wheat plants, only HA-QAP analysis identified increased microbial abundance as a key feature of the changes in the microbiome associated with common root rot disease.
Microorganisms do not exist in isolation, instead forming complex ecological interactions (Faust and Raes, 2012). Co-occurrence networks are widely used to infer microbial interactions through correlations of microbial relative abundance (van der Heijden and Hartmann, 2016, de Vries et al., 2018, Duran et al., 2018). Nevertheless, the results of such analyses should be interpreted with caution, as they are susceptible to compositionality effects based on relative abundances (Faust and Raes, 2012, Morton et al., 2017, Vandeputte et al., 2017). To evaluate the influence of microbial load on co-occurrence patterns, we constructed a genus co-occurrence network based on abundance information from the top 55 most abundant genera and the potential disease-associated genus Bipolaris using the RAP and HA-QAP methods. As shown in Figure 6F, the HA-QAP method (upper triangle) detected more co-varying genus pairs than RAP, including 16 genera correlating with Bipolaris (bacteria, 13; fungi, 3; P < 0.05). In contrast to the HA-QAP method, the RAP method (lower triangle) only detected three Bipolaris-associated trade-offs. Taken together, the HA-QAP method identified more potential disease-associated microbiome signals driven by the increase in microbial load than the RAP method, which may facilitate the study of microbe–microbe interactions and microbe-associated pathogenesis involved in common root rot disease.
Discussion
Environmental forces, nutritional elements, and disease can affect the colonization of the host by microorganisms (Stammler et al., 2016, Castrillo et al., 2017, Santos-Medellin et al., 2017, Vandeputte et al., 2017, Xu et al., 2018b). By integrating spike-in DNA into root microbiome samples, we established a straightforward, high-throughput method to detect the total microbial load of the root microbiome relative to host plant tissue. This method, HA-QAP, links the microbiome with the host plant through a predefined proportional relationship of spike-in plasmid and plant DNA. We validated the HA-QAP methods via mock experiments (Figures 2 and 3), perturbation experiments (Figure 4), and metagenomic sequencing of natural samples (Figures 5B–5E and 6E). By applying HA-QAP to the root microbiome of rice plants under wet and drought conditions, we demonstrated that the microbial load of the rice root microbiome increased under drought conditions in two rice varieties grown in two locations (Figure 5 and Supplemental Figure 10). Moreover, using HA-QAP, we detected an increase in total microbial load in wheat roots infected with root rot disease (Figure 6). These results suggest that changes in the microbial load are a key feature of the root microbiome in plants under abiotic and biotic stress conditions.
Since the HA-QAP method is based on microbial load, it reveals the quantitative abundance of individual microbes relative to host plant tissue, avoiding the spurious results obtained from the classical method based on relative abundance (RAP). Applying the HA-QAP method to mock experiments, we demonstrated that HA-QAP detected increases in the levels of individual microbes relative to root tissue when the relative microbial composition was the same across samples (see Figures 2C, 2D, 3C, and 3D). As the quantitative abundance of an individual microbe is not influenced by that of other microbes, the HA-QAP method avoids the false detection of changes in unaltered microbiome members resulting from increases in the levels of highly abundant microbes (see Figures 2E, 2F, 3E, and 3F). Therefore, the HA-QAP method provides accurate information about changes in the directionality and extent of individual microbiome members.
Plants live under abiotic and biotic stress conditions, which significantly affect the root microbiome (Lebeis et al., 2015, Castrillo et al., 2017, Santos-Medellin et al., 2017). HA-QAP allows us to identify beneficial or detrimental microbes associated with plant roots. For example, Actinobacteria, the most prominent phylum whose population increases under drought conditions (Naylor and Coleman-Derr, 2017, Naylor et al., 2017, Santos-Medellin et al., 2017, Xu et al., 2018b), were significantly enriched in response to drought in our test fields when measured using HA-QAP but not RAP (Figure 5). In addition to abiotic stress, various biotic stresses have profound effects on the plant-associated microbiome, which is thought to represent a microbial mechanism for disease suppression (Muller et al., 2016, Busby et al., 2017, Kwak et al., 2018). Similar to Tkacz et al. (2018), the spike-in plasmid designed for this study also supports cross-domain comparison. Unlike adding a combination of 16S and ITS synthetic spikes, the spike-in plasmid designed in this study simultaneously contains universal primer binding sites specific for bacteria and fungi (Figure 1D), enabling us to use a universal plasmid to spike root microbiome samples to normalize both bacterial and fungal reads and then construct a co-occurrence network for observing the synergistic effects of microbiome members on the host. Owing using the same plasmid for bacterial and fungal library construction, this might reduce the bias from PCR amplification, sequencing, and subsequent data normalization. As shown in Figure 6F, HA-QAP detected more microbes correlated with the potential disease-associated fungus Bipolaris compared with RAP. Such information is particularly helpful, providing guidance for further investigating plant–bacteria–fungi interactions.
Determination of the number of bacteria on roots is critical for plant–bacteria interaction studies. Previous studies on quantification of root-associated bacteria mainly include the plate counting and various types of microscopy associated with FISH, GFP tagging, β-glucuronidase staining, and fluorogenic dye staining, which can calculate the colony-forming units per unit mass or volume of roots (Kandel et al., 2017). The HA-QAP developed in this study estimates the copy-number ratio of bacterial 16S rRNA gene to plant genome, approximately 1.07–1.94 for rice and 6.61 for wheat under the normal field conditions. The ratio of bacteria to rice is similar to the ratio of bacteria to human cells (around 1:1) (Sender et al., 2016). Knowing the ratio of bacteria in plant tissues can be an important indicator in understanding microbiome variation among plants.
As this method relies on the number of genome copies in a specific sample being representative of the amount of plant tissue collected, a few factors are relative to the output of HA-QAP. First, endoreduplication, which affects the genome copy/cell ratio, needs to be considered (John and Qi, 2008). In many plants, cell expansion accompanied by endoreduplication is closely related to the plant development (Breuer et al., 2010, De Veylder et al., 2011). Second, the section of host tissue being sampled should be consistent. Root tips are going to be more cell-dense than root samples in the elongation and maturation zones, which may also have a big effect on the amount of DNA per amount of root tissue collected. Therefore, sampling needs to be taken by using a standardized procedure at the same stage and section of root tissue, which will make the result more reliable. Furthermore, we suggest using a coverage of spike-in at 10%–60% in a library. Low levels of spike-in (<5%) might increase the instability and variability of calibrated results.
Given its accuracy and technical feasibility, the HA-QAP method expands classical microbiome analysis based on relative abundance, may further be explored as tools to survey the root-associated microbiome dynamics over time, or be associated with hosts under various environmental conditions (both biotic and abiotic stress), linking microbiome features to host-associated quantitative data, including phenotype data, physiological parameters or plant exudates, and metabolite concentrations. This method should help reveal important biological mechanisms underlying the role of the microbiome in plant health and production in the future. With slight modifications, HA-QAP could be utilized in any type of amplicon profiling study of host–microbiome interactions.
Methods
Design and Construction of the Spike-In Plasmids
The spike-in plasmid BI12-4 sequence was designed to comprise specific plant gene RID1 fragments and conserved 16S rRNA gene (799F/1193R) and ITS (ITS1F/ITS2) primer regions. Considering the different amplicon sizes between bacteria and fungi, the ITS primer conserved regions were designed to be adjacent to the plant gene fragment, and the 16S rRNA primer regions were designed in the two sides of the ITS primer fragments. Design of the synthetic spike-in was based on the following two criteria: (1) the plant gene is present in plants and not normally found in environmental microbiome; (2) the amplicon length targeting the bacteria and fungi was close to the most common size of the real microorganism. For spike-in plasmid BI12-4, the amplicon fragment is 378 bp for 16S sequencing and 336 bp for ITS sequencing.
Assessment of spike-in BI12-4 sequence designed in this work by BLAST search against a range of NCBI databases verified that the artificial spike-in shared no identity with known microorganisms. The conserved primer regions were added into two sides of the selected plant marker gene fragment via PCR amplification, and the resulting spike-in was checked by gel electrophoresis and Sanger sequencing. The artificial spike-in DNA fragment was cloned into a pGEM-T Easy Vector by using pGEM-T Easy Vector System I (Promega, Madison, WI, USA) and was transformed into Escherichia coli DH5α competent cell. Plasmid DNA was extracted from overnight liquid cultures using the Wizard Plus SV Minipreps DNA Purification System (Promega) and linearized using ScaI (New England BioLabs, Hitchin, UK). Linearized plasmid DNA was purified using the Wizard SV Gel and PCR Clean-Up System (Promega), and its integrity was confirmed by amplifying 799F/1193R and ITS1F/ITS2. Spike-in DNA concentrations were measured with a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen Life Technologies, Grand Island, NY, USA), and plasmid copy numbers were calculated according to Lee et al. (2006). The spike-in plasmid was stored at −80°C for further processing.
Mock and Perturbation Experiments Preparation
To verify the utility of the spike-in plasmid, we used two experiments to mimic the taxonomic diversity of the root microbiome in natural environments. (1) Mock experiments consisting of genomic DNA of germ-free rice roots and 12 cultivated microbes. Germ-free plant DNA was extracted from sterilized rice Zhonghua 11 roots. Nine different bacteria belonged to four typical phyla, which were dominantly found in the root microbiomes, contributing to Bacteroidetes (Bac-186), Firmicutes (Fir-11), Actinobacteria (Act-101, Act-135, Act-140), and Proteobacteria (Pro-1203, Pro-1204, Pro-670, Pro-672), respectively. Three fungal isolates mainly belonged to Ascomycota (AF1, AF105) and Basidiomycota (AF78). All the DNA concentrations of microbes and plants were quantified through a PicoGreen dsDNA Assay Kit (Invitrogen) and subsequently diluted to 3.5 ng μl−1 for subsequent mixing. Details on the designed mock DNA mixtures are provided in Supplemental Table 3. The linearized spike-in plasmid BI12-4 was spiked into samples with five different concentrations: 0, 4.0 × 104, 8.0 × 104, 1.6 × 105, and 8.0 × 105 copies per PCR reaction. (2) Perturbation experiments comprising natural wild rice Zhonghua 11 root DNA with four different concentrations of spike-in: 0, 9.8 × 103, 9.8 × 104, and 2.0 × 105 copies per PCR reaction. These samples were then split into two aliquots: one was used as a normal natural sample and the other was diluted by a defined amount of germ-free rice DNA, responding to the total microbial load of the perturbed samples decreasing to 55% of the original samples. For all defined DNA mixtures, we examined three independent preparations.
Natural Root Samples Collection, Processing, and DNA Extraction
Two rice (Oryza sativa) cultivars, MH63 (subsp. indica) and WYJ7 (subsp. japonica), were grown in two geographically distant locations in China: an agricultural farm in Hainan (18°31′29.17″N, 110°01′4.76″E) and a farm in Anhui (31°54′3.29″N, 117°06′40.24″E). Both farms have only been used for rice cultivation for several years. Dehulled seeds were surface sterilized and germinated on Murashige and Skoog (MS) agar media. After germination, 2-week-old rice seedlings in MS agar were transferred to farms. The drought treatment was imposed during the period from tillering emergence (40 days after the seedlings were transplanted). Water was drained until the soil cracked. At this point, water was added to the field every other day, which kept the rice alive but still under drought stress. For control groups, plots were irrigated every other day to keep the soil under submergence. Fifteen days after water withdrawal, sample collection and processing were done following Zhang et al. (2018). DNA was extracted using a FastDNA SPIN Kit for Soil (MP Biomedicals, USA) according to the manufacturer's instructions. Extracted DNA was stored in nuclease-free H2O at −80°C and subsequently diluted to 3.5 ng μl−1 for subsequent mixing. According to the experimental design, the spike-in plasmid BI12-4 with predefined amounts (5.85 × 104 copies/PCR reaction) was added into each rice root DNA extract.
Healthy and infected wheat root samples from Chinese cultivar Lumai21 were collected in an agricultural field in Shandong province, China (36°40′53.58″N, 117°25′4.14″E). Processing of roots and extraction of DNA were the same as described above. According to the experimental design, spike-in plasmid BI12-4 with predefined amounts (3.5 × 104 copies/PCR reaction) was added into each wheat root DNA extract.
Amplicon Library and Metagenomic Library Construction, and Illumina Sequencing
Samples were split into two aliquots and amplified with either 799F/1193R for bacteria (Bai et al., 2015) or ITS1F/ITS2 for fungi (Santos-Medellin et al., 2017). PCR was performed in triplicate in 30-μl reaction mixtures containing 6 μl of 5× PrimeSTAR Buffer (Mg2+ Plus), 2.4 μl of dNTP Mixture (2.5 mM each), 0.75 μl of each primer (final concentration 0.2 μM), 0.75 U of PrimeSTAR HS DNA Polymerase (Takara Bio), and 3 μl of DNA template. For 16S-sequencing libraries, the V5–V7 region was amplified according to a two-step PCR program: The first PCR program used was carried out over 98°C for 30 s followed by 25 cycles (98°C for 10 s, 55°C for 15 s, and 72°C for 60 s) and a final elongation step of 5 min at 72°C. The first PCR products were cleaned with AmPure magnetic beads (Beckman Coulter) and diluted to 10 ng μl−1 as templates for the second step of PCR. All samples were amplified in triplicate for eight cycles under conditions identical to those of the first step of PCR. 16S PCR products were extracted by a 2% (w/v) agarose gel and then purified with the Wizard SV Gel and PCR Clean-Up System (Promega), quantified, and pooled in equimolar concentrations. For ITS libraries, the ITS1 region was amplified according to a modified two-step PCR program: after an initial hot start step at 98°C for 30 s, the targeted region was amplified by 30 cycles of 98°C for 10 s, 50°C for 15 s, and 72°C for 60 s, followed by a final elongation step of 5 min at 72°C. The first PCR products were cleaned with AmPure magnetic beads and diluted to 10 ng μl−1 as templates for the second step of PCR, in which samples were amplified in triplicate for 10 cycles under conditions identical to those of the first step of PCR. ITS PCR products were verified by a 1.2% (w/v) agarose gel and purified with AmPure magnetic beads, quantified, and pooled in equimolar concentrations. Final pooled libraries were purified with AmPure magnetic beads, concentrated, and sent for sequencing by an Illumina HiSeq 2500 platform (BGI-Shenzhen, Shenzhen, China). To confirm the accuracy of the HA-QAP method, we sent 12 drought-stress-associated rice root DNA samples and eight root rot disease-associated wheat root DNA samples to Novegene (Beijing, China) for metagenomic sequencing by an Illumina HiSeq X Ten platform.
Quantification of Plant Marker Gene by Quantitative Real-Time PCR
To calibrate the quantitative data, we selected RID1 gene and Pinb gene to represent the marker gene of rice and wheat, respectively. Their copy numbers in each root sample were determined by absolute qPCR on a LightCycler 480 II Instrument (Roche). The root genomic DNA concentrations were adjusted to 3.5 ng μl−1 for the subsequent qPCR. Primers were used by Wu et al. (2008) and Gasparis et al. (2011). PCR assay mixtures consisted of 10 μl of SYBR Green I Master Mix (Roche), 1 μl of each primer (10 nM), 6 μl of nuclease-free water, and 2 μl of template DNA. qPCR conditions were as follows: 5 min at 95°C, then 45 cycles of 10 s at 95°C, 10 s at 60°C, and 10 s at 72°C. The standard curve was generated using 10-fold dilution of a plasmid containing the PCR fragments of the RID1 gene and Pinb gene. The copy number of marker gene per nanogram of root genomic DNA was calculated based on Ct values and standard curves.
Illumina Sequencing Data Processing and Analysis for 16S rRNA
The 16S rRNA gene sequences were processed using QIIME 1.9.1 (Caporaso et al., 2010), USEARCH10 (Edgar, 2013), vsearch 2.7.1 (Rognes et al., 2016), and custom scripts. Quality evaluation on raw sequence data was provided by FastQC (available at http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Paired Illumina reads were subsequently joined (usearch -fastq_mergepairs) and quality-filtered (usearch -fastq_filter). Barcodes and primer sequences (usearch -fastx_truncate) were removed. For mock experiments, reads were mapped into reference sequences (map_reads_to_reference.py) with perfect match. For natural root samples, reads were dereplicated with minuniquesize threshold of 30 to remove low-abundance reads (usearch -fastx_uniques). The unique reads with abundance were clustered into OTUs by usearch -cluster_otu at 97% sequence identity. Chimeric sequences were identified against the SILVA50 database and removed (usearch -uchime2_ref); taxonomy was then assigned with usearch --sintax method to filter the mitochondria, chloroplast, or eukaryote sequence (DeSantis et al., 2006, Quast et al., 2013, Stoddard et al., 2015, Edgar, 2016) related to host plant. Finally, the spike-in sequences were added into the OTUs, and the OTU table was obtained by vsearch --usearch_global.
Sequence Processing and Analysis for ITS
As mentioned earlier, ITS sequences were subjected to quality filtering, demultiplexing, dereplication, and OTU clustering. No errors were allowed in the barcodes. Sequences below 170 nt (after trimming) were subsequently removed. Retained high-quality sequences were subsequently mapped into reference sequences with perfect match (map_reads_to_reference.py) in mock experiments, or OTUs were defined at 97% sequence identity through UPARSE pipeline for natural root samples, respectively. Taxonomy assignment was performed with the QIIME (assign_taxonomy.py) using BLAST (Altschul et al., 1990) against version 7 of the UNITE database (Koljalg et al., 2013). OTUs not classified as fungi at the kingdom level were removed from the OTU table before downstream analysis. Spike-in sequences were added into the OTUs and the OTU table was obtained.
Metagenomic Data Analysis
Raw paired-end Illumina reads of metagenome data were subjected to trimming adaptor and filtering of low-quality reads by Trimmomatics (version 0.38) (Bolger et al., 2014) with a minimum quality threshold of 20 and length threshold of 50 when the sliding window was set to 4 (SLIDINGWINDOW:4:20 MINLEN:50). To calculate the ratio of reads of host-originated contamination with total microbiome, we mapped high-quality trimmed reads to the manually aggregated rice genome sequence (Oryza sativa indica ASM465v1 and O. sativa japonica IRGSP-1.0 from EnsemblPlants release 41) and wheat genome (Triticum aestivum IWGSC from EnsemblPlants release 41) using bowtie2 (--very-sensitive --dovetail) (Langmead and Salzberg, 2012), and mapped reads as host were subsequently removed from the dataset. The remaining sequences were assigned taxonomic labels with Kraken2 classification (--use-names --use-mpa-style --report-zero-counts for output readable result) which utilizes exact alignments of k-mers by achieving the less sensitive but ultrafast speed (Wood and Salzberg, 2014).
Quantitative Abundance Calculation
To detect the quantitative profile of root microbiome, we used the amounts of spike-in to build a relationship between plant DNA and OTUs. The Illumina reads number of individual bacterial or fungal OTU was denoted as Rmicro and the reads number of spike-in was denoted as Rspike-in. The copy number of spike-in per reaction was denoted as Cspike-in. The copy number of plant root marker gene per PCR reaction, which represents plant tissue, can be measured by qPCR and denoted as Cplant.
Based on the above, quantitative abundance (QA) representing the copy-number ratio of microbial marker genes (referred as 16S rRNA gene and ITS in this study) relative to the plant genome was
Statistical Analysis
All statistical analyses were conducted in the R Environment (v3.4.3) (R Core Team, 2017). Statistical analyses and plotting were performed in Rstudio server (R Core Team., 2016) using the dplyr (v0.7.4) (Wickham et al., 2017), pheatmap (Kolde, 2015), tidyr (Wickham and Henry, 2018), ggplot2 (v2.2.1) (Wickham, 2009), ggpubr (Kassambara, 2017), and vegan (v2.5–2) (Oksanen et al., 2016) packages. All statistical tests used were two-sided.
Dose–response curves for linear correlation between spike-in sequencing reads and spike-in amounts were fitted with a Poisson generalized linear model, using the function “glm()” of the R package stats. For alpha diversity, samples were first rarefied at minimal sequences (of all the sample sequence sizes) using the function “rrarefy().” Shannon alpha index was performed with the function “diversity()” in R. For beta diversity measurements, Bray–Curtis distance matrices were calculated based on the rarefied OTU counts. Unconstrained PCoA was performed using the function “capscale()” from the vegan package. Differential abundance analyses were performed using Wilcoxon rank-sum tests at phyla and OTU levels. Differences between multiple groups were performed using non-parametric Wilcoxon rank-sum tests or two-tailed Student's t-test for all pairs of comparisons. Corrections for multiple testing were performed with Benjamini–Hochberg adjusted P value (Benjamini and Hochberg, 1995). Heatmaps were generated using the R package “pheatmap().” Genus–genus associations were defined using RAP and QAP matrices by Spearman pairwise correlations of taxa with multiple testing corrections. Taxa were prefiltered to exclude taxa unclassified at the genus level and taxa that were present in less than 70% of the samples. A documented R-script and the data needed to reproduce figures and analyses have been deposited in the GitHub repository (https://github.com/TankMermaid/spike-in or https://github.com/microbiota/spike-in).
Funding
This work is financially supported by the National Natural Science Foundation of China (grant nos. 31772400, 31761143017), the National Natural Science Foundation for Young Scientists of China (grant no. 31701997), the Key Research Program of the Chinese Academy of Sciences (grant nos. KFZD-SW-112-02-02 and KFZD-SW-219), and the Key Research Program of Frontier Sciences, CAS (grant no. QYZDB-SSW-SMC021).
Author Contributions
X.G. and Y.B. designed the research; X.G. performed the experiments, tested and validated the method; X.Z. and Y.Q. conducted bioinformatics analyses and visual plotting; Y.-X.L. analyzed the metagenomic data and offered help with bioinformatics analyses; J.Z. and N.Z. collected drought-stress-associated rice root samples and provided the DNA extracts; K.W. provided seeds for MH63 and WYJ7, and offered help in the drought-stress-associated field experiment; B.Q., Z.H., S.H., and X.W. were involved in many discussions; X.Z. provided wheat root samples; X.F. and Y.B. oversaw and designed the field experiment about rice plants with drought stress; X.G., X.Z., S.H., and Y.B. wrote the manuscript.
Acknowledgments
No conflict of interest declared.
Published: September 3, 2019
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and IPPE, CAS.
Supplemental Information is available at Plant Communications Online.
Contributor Information
Xiangdong Fu, Email: xdfu@genetics.ac.cn.
Yang Bai, Email: ybai@genetics.ac.cn.
Accession Numbers
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Wang et al., 2017) BIG Data Center (BIG Data Center Members, 2018), Beijing Institute of Genomics (BIG), and Chinese Academy of Sciences under accession numbers CRA001526 and CRA001527, publicly accessible at https://bigd.big.ac.cn/gsa/.
Supplemental Information
References
- Almeida A., Shao Y. Genome watch: keeping tally in the microbiome. Nat. Rev. Microbiol. 2018;16:124. doi: 10.1038/nrmicro.2018.13. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bai Y., Muller D.B., Srinivas G., Garrido-Oter R., Potthoff E., Rott M., Dombrowski N., Munch P.C., Spaepen S., Remus-Emsermann M. Functional overlap of the Arabidopsis leaf and root microbiota. Nature. 2015;528:364–369. doi: 10.1038/nature16192. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- Benson D.A., Karsch-Mizrachi I., Lipman D.J., Ostell J., Wheeler D.L. GenBank. Nucleic Acids Res. 2006;34:D16–D20. doi: 10.1093/nar/gkj157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIG Data Center Members Database resources of the BIG Data Center in 2018. Nucleic Acids Res. 2018;46:D14–D20. doi: 10.1093/nar/gkx897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer C., Ishida T., Sugimoto K. Developmental control of endocycles and cell growth in plants. Curr. Opin. Plant Biol. 2010;13:654–660. doi: 10.1016/j.pbi.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Bulgarelli D., Rott M., Schlaeppi K., Ver Loren van Themaat E., Ahmadinejad N., Assenza F., Rauf P., Huettel B., Reinhardt R., Schmelzer E. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012;488:91–95. doi: 10.1038/nature11336. [DOI] [PubMed] [Google Scholar]
- Busby P.E., Soman C., Wagner M.R., Friesen M.L., Kremer J., Bennett A., Morsy M., Eisen J.A., Leach J.E., Dangl J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017;15:e2001793. doi: 10.1371/journal.pbio.2001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo G., Teixeira P.J., Paredes S.H., Law T.F., de Lorenzo L., Feltcher M.E., Finkel O.M., Breakfield N.W., Mieczkowski P., Jones C.D. Root microbiota drive direct integration of phosphate stress and immunity. Nature. 2017;543:513–518. doi: 10.1038/nature21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole L.W. The evolution of per-cell organelle number. Front. Cell Dev. Biol. 2016;4:85. doi: 10.3389/fcell.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran P., Thiergart T., Garrido-Oter R., Agler M., Kemen E., Schulze-Lefert P., Hacquard S. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell. 2018;175:973–983. doi: 10.1016/j.cell.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv. 2016 doi: 10.1101/074161. [DOI] [Google Scholar]
- Edwards J., Johnson C., Santos-Medellin C., Lurie E., Podishetty N.K., Bhatnagar S., Eisen J.A., Sundaresan V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. U S A. 2015;112:911–920. doi: 10.1073/pnas.1414592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust K., Raes J. Microbial interactions: from networks to models. Nat. Rev. Microbiol. 2012;10:538–550. doi: 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- Franco F.P., Moura D.S., Vivanco J.M., Silva-Filho M.C. Plant-insect-pathogen interactions: a naturally complex ménage à trois. Curr. Opin. Microbiol. 2017;37:54–60. doi: 10.1016/j.mib.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Gasparis S., Orczyk W., Zalewski W., Nadolska-Orczyk A. The RNA-mediated silencing of one of the Pin genes in allohexaploid wheat simultaneously decreases the expression of the other, and increases grain hardness. J. Exp. Bot. 2011;62:4025–4036. doi: 10.1093/jxb/err103. [DOI] [PubMed] [Google Scholar]
- Geiger T., Wisniewski J.R., Cox J., Zanivan S., Kruger M., Ishihama Y., Mann M. Use of stable isotope labeling by amino acids in cell culture as a spike-in standard in quantitative proteomics. Nat. Protoc. 2011;6:147–157. doi: 10.1038/nprot.2010.192. [DOI] [PubMed] [Google Scholar]
- Gloor G.B., Macklaim J.M., Pawlowsky-Glahn V., Egozcue J.J. Microbiome datasets are compositional: and this is not optional. Front. Microbiol. 2017;8:2224. doi: 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacquard S., Garrido-Oter R., Gonzalez A., Spaepen S., Ackermann G., Lebeis S., McHardy A.C., Dangl J.L., Knight R., Ley R. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe. 2015;17:603–616. doi: 10.1016/j.chom.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Hardwick S.A., Chen W.Y., Wong T., Kanakamedala B.S., Deveson I.W., Ongley S.E., Santini N.S., Marcellin E., Smith M.A., Nielsen L.K. Synthetic microbe communities provide internal reference standards for metagenome sequencing and analysis. Nat. Commun. 2018;9:3096. doi: 10.1038/s41467-018-05555-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden M.G., Hartmann M. Networking in the plant microbiome. PLoS Biol. 2016;14:e1002378. doi: 10.1371/journal.pbio.1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K., Gerlach N., Sacristan S., Nakano R.T., Hacquard S., Kracher B., Neumann U., Ramirez D., Bucher M., O'Connell R.J. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell. 2016;165:464–474. doi: 10.1016/j.cell.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Schlesinger F., Davis C.A., Zhang Y., Li R., Salit M., Gingeras T.R., Oliver B. Synthetic spike-in standards for RNA-seq experiments. Genome Res. 2011;21:1543–1551. doi: 10.1101/gr.121095.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John P.C., Qi R. Cell division and endoreduplication: doubtful engines of vegetative growth. Trends Plant Sci. 2008;13:121–127. doi: 10.1016/j.tplants.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Kandel S.L., Joubert P.M., Doty S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms. 2017;5:E77. doi: 10.3390/microorganisms5040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov T.L., Almario J., Friedemann C., Ding W., Giolai M., Heavens D., Kersten S., Lundberg D.S., Neumann M., Regalado J. Arabidopsis thaliana and Pseudomonas pathogens exhibit stable associations over evolutionary timescales. Cell Host Microbe. 2018;24:168–179. doi: 10.1016/j.chom.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A. ggpubr: 'ggplot2' based publication ready plots. R package version 0.1.6. 2017. https://CRAN.R-project.org/package=ggpubr
- Kolde R. pheatmap: pretty heatmaps. R package version 1.0.8. 2015. https://CRAN.R-project.org/package=pheatmap
- Koljalg U., Nilsson R.H., Abarenkov K., Tedersoo L., Taylor A.F., Bahram M., Bates S.T., Bruns T.D., Bengtsson-Palme J., Callaghan T.M. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013;22:5271–5277. doi: 10.1111/mec.12481. [DOI] [PubMed] [Google Scholar]
- Kumar J., Schafer P., Huckelhoven R., Langen G., Baltruschat H., Stein E., Nagarajan S., Kogel K.H. Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Mol. Plant Pathol. 2002;3:185–195. doi: 10.1046/j.1364-3703.2002.00120.x. [DOI] [PubMed] [Google Scholar]
- Kwak M.J., Kong H.G., Choi K., Kwon S.K., Song J.Y., Lee J., Lee P.A., Choi S.Y., Seo M., Lee H.J. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018;36:1100–1109. doi: 10.1038/nbt.4232. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeis S.L., Paredes S.H., Lundberg D.S., Breakfield N., Gehring J., McDonald M., Malfatti S., Glavina del Rio T., Jones C.D., Tringe S.G. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349:860–864. doi: 10.1126/science.aaa8764. [DOI] [PubMed] [Google Scholar]
- Lee C., Kim J., Shin S.G., Hwang S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 2006;123:273–280. doi: 10.1016/j.jbiotec.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Lesk C., Rowhani P., Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016;529:84–87. doi: 10.1038/nature16467. [DOI] [PubMed] [Google Scholar]
- Lin Y., Gifford S., Ducklow H., Schofield O., Cassar N. Towards quantitative microbiome community profiling using internal standards. Appl. Environ. Microbiol. 2019;85:e02634-18. doi: 10.1128/AEM.02634-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg D.S., Lebeis S.L., Paredes S.H., Yourstone S., Gehring J., Malfatti S., Tremblay J., Engelbrektson A., Kunin V., Del Rio T.G. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg D.S., Yourstone S., Mieczkowski P., Jones C.D., Dangl J.L. Practical innovations for high-throughput amplicon sequencing. Nat. Methods. 2013;10:999–1002. doi: 10.1038/nmeth.2634. [DOI] [PubMed] [Google Scholar]
- Lynch J.M. Limits to microbial growth in soil. Microbiology. 1982;128:405–410. [Google Scholar]
- Morton J.T., Sanders J., Quinn R.A., McDonald D., Gonzalez A., Vazquez-Baeza Y., Navas-Molina J.A., Song S.J., Metcalf J.L., Hyde E.R. Balance trees reveal microbial niche differentiation. mSystems. 2017;2:e00162-16. doi: 10.1128/mSystems.00162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D.B., Vogel C., Bai Y., Vorholt J.A. The plant microbiota: systems-level insights and perspectives. Annu. Rev. Genet. 2016;50:211–234. doi: 10.1146/annurev-genet-120215-034952. [DOI] [PubMed] [Google Scholar]
- Naylor D., Coleman-Derr D. Drought stress and root-associated bacterial communities. Front. Plant Sci. 2017;8:2223. doi: 10.3389/fpls.2017.02223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor D., DeGraaf S., Purdom E., Coleman-Derr D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017;11:2691–2704. doi: 10.1038/ismej.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu B., Paulson J.N., Zheng X., Kolter R. Simplified and representative bacterial community of maize roots. Proc. Natl. Acad. Sci. U S A. 2017;114:E2450–E2459. doi: 10.1073/pnas.1616148114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F.G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P.R., O'Hara R.B., Simpson G.L., Solymos P. Vegan: community ecology package. R package version 2.3-5. 2016. http://CRAN.R-project.org/package=vegan
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . RStudio, Inc.; Boston, MA: 2016. RStudio: Integrated Development Environment for R.http://www.rstudio.com [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2017. R: A Language and Environment for Statistical Computing.https://www.R-project.org [Google Scholar]
- Risso D., Ngai J., Speed T.P., Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014;32:896–902. doi: 10.1038/nbt.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognes T., Flouri T., Nichols B., Quince C., Mahe F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Medellin C., Edwards J., Liechty Z., Nguyen B., Sundaresan V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio. 2017;8:e00764-17. doi: 10.1128/mBio.00764-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H., Eickhorst T. Detection and quantification of native microbial populations on soil-grown rice roots by catalyzed reporter deposition-fluorescence in situ hybridization. FEMS Microbiol. Ecol. 2014;87:390–402. doi: 10.1111/1574-6941.12232. [DOI] [PubMed] [Google Scholar]
- Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets W., Leff J.M., Bradford M.A., McCulley R.L., Lebeer S., Fierer N. A method for simultaneous measurement of soil bacterial abundances and community composition via 16S rRNA gene sequencing. Soil Biol. Biochem. 2016;96:145–151. [Google Scholar]
- Stammler F., Glasner J., Hiergeist A., Holler E., Weber D., Oefner P.J., Gessner A., Spang R. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome. 2016;4:28. doi: 10.1186/s40168-016-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard S.F., Smith B.J., Hein R., Roller B.R., Schmidt T.M. rrnDB: improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res. 2015;43:593–598. doi: 10.1093/nar/gku1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz A., Hortala M., Poole P.S. Absolute quantitation of microbiota abundance in environmental samples. Microbiome. 2018;6:110. doi: 10.1186/s40168-018-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourlousse D.M., Yoshiike S., Ohashi A., Matsukura S., Noda N., Sekiguchi Y. Synthetic spike-in standards for high-throughput 16S rRNA gene amplicon sequencing. Nucleic Acids Res. 2017;45:23. doi: 10.1093/nar/gkw984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte D., Kathagen G., D'Hoe K., Vieira-Silva S., Valles-Colomer M., Sabino J., Wang J., Tito R.Y., De Commer L., Darzi Y. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- De Veylder L., Larkin J.C., Schnittger A. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci. 2011;16:624–634. doi: 10.1016/j.tplants.2011.07.001. [DOI] [PubMed] [Google Scholar]
- de Vries F.T., Griffiths R.I., Bailey M., Craig H., Girlanda M., Gweon H.S., Hallin S., Kaisermann A., Keith A.M., Kretzschmar M. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018;9:3033. doi: 10.1038/s41467-018-05516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Song F., Zhu J., Zhang S., Yang Y., Chen T., Tang B., Dong L., Ding N., Zhang Q. GSA: genome sequence archive. Genomics Proteomics Bioinformatics. 2017;15:14–18. doi: 10.1016/j.gpb.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wees S.C., Van der Ent S., Pieterse C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008;11:443–448. doi: 10.1016/j.pbi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Wickham H. Springer-Verlag; NY: 2009. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Wickham, H., and Henry, L. (2018). tidyr: easily tidy data with 'spread' and 'gather'Functions. R package version 0.8.0, https://CRAN.R-project.org/package=tidyr.
- Wickham H., Francois R., Henry L., Müller K. dplyr: a Grammar of Data Manipulation. R package version 0.7.4. 2017. https://CRAN.R-project.org/package=dplyr
- Wood D.E., Salzberg S.L. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., You C., Li C., Long T., Chen G., Byrne M.E., Zhang Q. RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc. Natl. Acad. Sci. U S A. 2008;105:12915–12920. doi: 10.1073/pnas.0806019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Yang G., Wang J., Song Y., Liu L., Zhao K., Li Y., Han Z. Spatial distribution of root and crown rot fungi associated with winter wheat in the north China plain and its relationship with climate variables. Front. Microbiol. 2018;9:1054. doi: 10.3389/fmicb.2018.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Naylor D., Dong Z., Simmons T., Pierroz G., Hixson K.K., Kim Y.M., Zink E.M., Engbrecht K.M., Wang Y. Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. Proc. Natl. Acad. Sci. U S A. 2018;115:4284–4293. doi: 10.1073/pnas.1717308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgadzaj R., Garrido-Oter R., Jensen D.B., Koprivova A., Schulze-Lefert P., Radutoiu S. Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc. Natl. Acad. Sci. U S A. 2016;113:7996–8005. doi: 10.1073/pnas.1616564113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhang N., Liu Y.X., Zhang X., Hu B., Qin Y., Xu H., Wang H., Guo X., Qian J. Root microbiota shift in rice correlates with resident time in the field and developmental stage. Science China. Life Sci. 2018;61:613–621. doi: 10.1007/s11427-018-9284-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.