Abstract
Food allergy and allergen management are important global public health issues. In 2011, the first iteration of our allergen threshold database (ATDB) was established based on individual NOAELs and LOAELs from oral food challenge in roughly 1750 allergic individuals. Population minimal eliciting dose (EDp) distributions based on this dataset were published for 11 allergenic foods in 2014. Systematic data collection has continued (2011–2018) and the dataset now contains over 3400 data points. The current study provides new and updated EDp values for 14 allergenic foods and incorporates a newly developed Stacked Model Averaging statistical method for interval-censored data. ED01 and ED05 values, the doses at which 1%, and respectively 5%, of the respective allergic population would be predicted to experience any objective allergic reaction were determined. The 14 allergenic foods were cashew, celery, egg, fish, hazelnut, lupine, milk, mustard, peanut, sesame, shrimp (for crustacean shellfish), soy, walnut, and wheat. Updated ED01 estimates ranged between 0.03 mg for walnut protein and 26.2 mg for shrimp protein. ED05 estimates ranged between 0.4 mg for mustard protein and 280 mg for shrimp protein. The ED01 and ED05 values presented here are valuable in the risk assessment and subsequent risk management of allergenic foods.
Keywords: Allergy, Food, Threshold, Risk assessment, Labeling, Model averaging
1. Introduction
Food allergy and allergen management are significant global public health issues. Food-allergic individuals must adhere to specific avoidance diets to prevent the occurrence of allergic reactions (Muraro et al., 2014; Boyce et al., 2010) and as such, a number of countries and regulatory bodies who recognized this importance have enacted critical laws, regulations or standards for labeling of “priority allergens” to enable avoidance (Gendel, 2012; Yeung and Robert, 2018). However, allergen control in the processing facility and throughout the ingredient supply chain is a challenging venture for manufacturers in a globalized economy (Yeung and Robert, 2018). When ingredients and technologies are sourced worldwide from multiple business partners, complexity rises, which can increase the chance for the unintended presence of allergens in food, leading to potential harm as demonstrated by the number of large scale recalls that have affected a large number of food companies (Garber et al., 2016; Gendel, 2018; Sayers et al., 2016; Walker et al., 2016). Fear of causing unintended harm to an allergic consumer, coupled with the lack of regulation regarding the procedures or warnings on labels surrounding unintentional allergen presence in foods has led to the proliferation of voluntary precautionary allergen labels on packaged foods (Allen and Taylor, 2018).
Depending on the level of sensitivity of an allergic individual (minimal eliciting dose of an individual), exposures from the unintended presence of residues of allergenic foods that are not declared on product labels (undeclared allergens) can pose a risk to food-allergic individuals (Blom et al., 2018; Remington et al., 2015; Zurzolo et al., 2019). In general, precautionary (advisory) allergen labeling (i.e. may contain) is used to inform consumers of products with a potential unintended allergen presence (UAP). However, it is now well-recognized that excessive use of precautionary labeling results in unintended consequences, in particular a decline in trust of the label and ignoring of the warnings which negates the original intent of the labeling (Allen and Taylor, 2018). The implementation of risk assessment approaches as a basis for the establishment of action levels for precautionary (advisory) allergen labeling is needed to quantify the level of risk. By quantifying the risk of UAP, it is possible to allow for risk management strategies that would sufficiently protect food-allergic consumers. PAL would be the final risk management option and using quantitative risk assessment as a support for the risk management decision making process would prevent PAL from being overly burdensome which has been shown to contribute to a reduction in the quality of life of food-allergic individuals.
In 2007, the Allergen Bureau of Australia & New Zealand (ABA) released their VITAL (Voluntary Incidental Trace Allergen Labeling) allergen management program with the goal of limiting precautionary allergen labeling related to the presence of an unintended allergen. As part of the VITAL 2.0 updated guidance in 2011, the VITAL Scientific Expert Panel identified doses of allergens where 1% and/or 5% of the respective allergic-population would be predicted to experience any objective allergic reaction (the ED01 and the ED05) and recommended Reference Doses (based on the ED01 and/or the 95% lower confidence interval of the ED05) to guide the risk management and the application of precautionary labels on food products (Allen et al., 2014; Taylor et al., 2014).
Since publication of the updated VITAL 2.0 Reference Doses, a number of stakeholders and national agencies have begun to adopt the use of Reference Doses (FAVV SciCom, 2017; NVWA BuRO, 2016; Sjögren Bolin, 2015; Waiblinger and Schulze, 2018). However, wide variations exist regarding approaches to the implementation of the data and models underpinning Reference Doses. Additional scientific improvements, in both the amount of data for individual eliciting doses from oral food challenges and the development of more advanced statistical modeling approaches, have also become available and their use could encourage the harmonization of risk assessment or risk management approaches and applications.
Previously, three parametric models (log normal, log logistic, Weibull) were used with Interval-Censoring Survival Analysis (ICSA) to fit results from individual oral food challenge data and to estimate the dose of an allergen (EDp) at which a proportion (p) of the allergic population would be likely to react (Allen et al., 2014; Ballmer-Weber et al., 2015; Taylor et al., 2014, 2010, 2009). However, limitations to this approach do exist. First and foremost, each of the parametric models provides a different EDp estimate. No biological basis exists for selecting between the different models. This limitation encourages multiple possible interpretations of the same data. Additionally, these previously available ICSA models were not able to incorporate random effects into their calculations. Random effects enter the models through study-to-study heterogeneity (i.e. differing protocols, participant recruitment, dosing schemes, possible regional genetic or environmental differences, etc). As a consequence, estimation of a reference dose using any single model may result in bias and estimates whose parameters do not fully reflect the true uncertainties. In other fields of risk assessment, especially regarding benchmark dose (BMD) approaches for chemical risk assessment, single model selection and rejection has been acknowledged as a suboptimal approach and a more advanced, preferred method for BMD calculation is model averaging (EFSA Scientific Committee, 2017). Bayesian model averaging has also been incorporated into the EPA’s Benchmark Dose Software v3.1.1 (BMDS 3.1.1) application in an effort to incorporate available state-of-the-art methods in the publicly available BMD software (US EPA et al., 2019). However, model averaging techniques for interval-censored data were not available.
To fill this need, the National Institute for Occupational Safety and Health (NIOSH), the Netherlands Organisation for Applied Scientific Research (TNO) and the University of Nebraska-Lincoln, developed a Bayesian “Stacked Model Averaging” approach for interval-censored data (Wheeler et al., 2019). This approach combines parametric survival estimates from multiple models into a single EDp estimation based upon a weighted average of survival estimates designed to estimate the true survival curve and the EDp estimations from food allergen minimal eliciting dose distribution data. Furthermore, the approach allows for the inclusion of additional models not previously available; these distributions might better fit the food allergen dose distribution data. The Stacked Model Averaging approach also allows inclusion of random effects for representation of the study-to-study heterogeneity of the allergen dose distribution curve (Wheeler et al., 2019). This state-of-the-art, flexible method allows for more robust EDp estimations than previously available ICSA methods when using interval-censored data from oral food challenges.
In 2011, the first iteration of our allergen threshold database (ATDB), containing roughly 1750 individual data points, was established. On the basis of this dataset, the first population eliciting dose (EDp) results were published in 2014 (Allen et al., 2014; Taylor et al., 2014). The systematic collection of oral food challenge data has continued from 2011 until the fall of 2018 in a similar fashion and the dataset now contains over 3400 data points. The current study provides updated EDp values using stacked model averaging for the previously reported allergenic foods, as well as several new foods based on the expanded database where data have recently become available.
2. Methods
Publications were selected based upon the criteria outlined previously (Taylor et al., 2009), in particular focusing on results from low-dose oral challenges. Additionally, unpublished data were obtained from clinical records where possible. Data from double-blind, placebo-controlled food challenges (DBPCFCs) were preferred, except in the case of data from infants and very young children where blinding was not considered necessary. In the selected available studies, subjects were challenged with increasing doses of allergenic materials at intervals until a response was observed; the data were collected and assessed in terms of discrete dose and cumulative dose datasets.
Clinical oral challenge protocols are conducted with short (15–30 min) intervals between doses (Sampson et al., 2012), and it is not possible for patients to fully assimilate one dose before the next dose is administered. As a consequence, an argument can be made for using the cumulative dose administered for EDp assessment (Blumchen et al., 2014; Niggemann et al., 2012). At the same time, it is known that allergic symptoms may develop within minutes and it cannot be excluded that an allergic response is due to a discrete dose. Therefore, both discrete and cumulative EDp values have been assessed and presented. The first objective symptoms of an allergic response occurring in an individual were used as the basis for the individual lowest observed adverse effect level (LOAELi) with the individual no observed adverse effect level (NOAELi) set at the previous dose in the clinical protocol as is detailed in (Westerhout et al., 2019); individual LOAELi’s and NOAELi’s for objective symptoms were recorded in terms of mg of total protein from the allergenic food as previously detailed (Taylor et al., 2014). For each subject, the true individual eliciting dose lies, by definition, in the interval between the individual NOAELi and LOAELi. Individuals were left-censored if they reacted to the first challenge dose, while individuals were right-censored if they failed to respond to the uppermost challenge dose, but did have clear histories of allergic reaction upon consumption of the offending food (Taylor et al., 2009).
Individual studies were combined per allergen and analyzed with Bayesian Stacked Parametric Survival methods with Frailty Components and Interval Censored Failure Times as described (Wheeler et al., 2019), which for the remainder of this paper we will refer to as “Stacked Model Averaging.” Briefly, a Bayesian approach to analyze interval-censored data combining multiple parametric survival estimates into a single survival estimate through weighted averaging was developed (Wheeler et al., 2019). Weights are formed to maximize the predictive accuracy of the calculated EDp values for future observations. The approach utilizes five parametric survival distributions including the Weibull distribution, Log-Gaussian (Log-Normal), Log-Logistic, Generalized Pareto and Log-Laplace (Log-Double-Exponential) to give a range of survival functions that are broadly applicable.
For our analysis, the five individual distributions were fit to the data, including accounting for random effects through frailty components which enter the model through study-to-study heterogeneity (differing protocols, differing participant recruitment, differing cumulative dosing designs, differing symptom interpretations by clinicians and nurses, and an array of possible regional genetic or environmental differences present in each study’s population) (Wheeler et al., 2019), and the EDp was estimated. The Stacked Model Averaging method itself relies on a Markov chain Monte Carlo (MCMC) methodology, which is a random sampling procedure, and this can lead to small variations in the final EDp estimate (e.g. sampling error). To minimize this variability, the stacked model averaging estimation procedure was repeated independently 10 times and the mean of these 10 EDp estimates was used as a central estimate. Population EDs were determined based on both a discrete dosing and cumulative dosing, with EDp values derived where 1% or 5% of the respective allergic-population would be predicted to experience an allergic reaction (ED01, ED05). For all analyses, the R software (https://www.r-project.org/) and publicly available Stackedsurv package (http://doi.org/10.5281/zenodo.3401471) were used.
3. Results
From 2011 until August 2018, over 2516 titles and abstracts were identified from PubMed and Scopus and were screened for further review, 570 peer-reviewed articles were kept for full PDF review and 47 were identified as containing quantitative data in a useable format for the current study (Supplementary Table 1). Further data were added from unpublished clinical datasets if available (~25% of total data available). Already in 2011, sufficient data were available for the derivation of the ED01, ED05 and corresponding 95% confidence intervals for egg, hazelnut, lupine, milk, mustard, peanut, sesame, shrimp (for crustacean shellfish), soy flour, and wheat; with provisional data available for cashew nut (provisional due to limited numbers from only a single hospital and lack of data on adults). The current study obtained additional data to supplement these previously available data where possible for analysis with Stacked Model Averaging. Moreover, additional data were obtained for buckwheat, celery, fish, and walnut. Previous research demonstrated that the bias and accuracy of EDp estimations improved the most with increasing sample size N = 20 to N = 60. For larger N, and thereafter the marginal gains in bias and accuracy declined, suggesting that a sample size of N = 60 or larger is recommended for obtaining stable EDp estimates (Klein Entink et al., 2014). In the current analysis, only buckwheat, lupine, mustard and sesame contain less than 60 individual data points. All allergens, except for buckwheat, contained datasets sufficient for EDp derivation, when considering both number of subjects available and data points in the lower end of the distribution. No data points were obtained for buckwheat from the available clinical data in the lower range of the distribution (below the ED15). Thus the distribution for buckwheat was not extrapolated outside of the data range and the buckwheat dataset was deemed insufficient for ED01 or ED05 derivation. Insufficient data or no data from low-dose clinical challenges were available for other tree nuts (almond, pecan, pistachio, Brazil nut, macadamia nut), other crustacean shellfish (crab, lobster), or any molluscan shellfish. Supplementary Table 1 provides the total number of data points for each of the allergenic foods along with the number of right- or left-censored subjects, geographic location, first mg protein dose in food challenge protocol and age groups where known. The number of subjects ranged from 1306 subjects for peanut to 25 subjects for lupine. The largest datasets existed for peanut, milk, egg, and hazelnut with data points for more than 400 subjects; followed by cashew with 245 subjects (ATDB registered at the Benelux Office for Intellectual Property, i-DEPOT number 117608; https://www.boip.int/).
The age of each individual person was not always able to be gleaned from the available publications. However, when available the populations tended to be skewed towards children (< 18 years of age). Similar to the previous iteration of our ATDB (Allen et al., 2014; Taylor et al., 2014), children were the predominant data source for egg, milk, and wheat. Peanut also contains a larger percentage of children but the size of the dataset still ensures that over 150 adults are included. Celery, fish and shrimp were exceptions as these datasets had more adults than children. Cashew remains an exception in that only data from children are available, as was the case in 2011, but data is now available for 245 children from multiple hospitals instead of 31 children from a single hospital.
The Stacked Model Averaging technique was applied to yield estimates of the ED01, ED05 and their respective 95% confidence intervals for cashew, celery, egg, fish, hazelnut, lupine, milk, mustard, peanut, sesame, shrimp, soy, walnut, and wheat. Table 1 provides these ED estimates for each of the allergenic foods on the basis of both discrete and cumulative dosing schemes. A visual example of the Stacked Model Averaging results and fitted dose distribution can be seen in Fig. 1. The individual distributions given the most weight in the Stacked Model Averaging results varied per allergen and the most heavily weighted distribution was unpredictable prior to analysis, thus supporting the use of the Stacked Model Averaging method for EDp derivations (Supplementary Table 2). As expected, once individual distribution weights were established for each food, the weighting results were relatively stable and reproducible per food across 10 different runs (example shown in Supplementary Table 3).
Table 1.
Food-allergic population Eliciting Doses (EDs) for 14 allergenic foods based on “Model averaged interval-censored survival analysis” with inclusion of random effects to account for study-to-study heterogeneity. Population EDs were estimated where 1% or 5% of the respective allergic-population would be predicted to experience an allergic reaction (ED01 and the ED05 with their respective 95% confidence intervals, calculated using both discrete and cumulative dosing schemes).
| Allergen | No. of individuals | Discretea ED01 (95% CI) |
Cumulativea ED01 (95% CI) |
Discrete ED05 (95% CI) |
Cumulative ED05 (95% CI) |
|---|---|---|---|---|---|
| Cashew | 245 | 0.05 (0.02, 0.3) | 0.09 (0.04, 0.5) | 0.8 (0.2, 5.0) | 1.6 (0.4, 9.4) |
| Celery | 82 | 0.07 (0.02, 1.9) | 0.05 (0.02, 0.5) | 1.5 (0.3, 11.8) | 1.3 (0.2, 7.9) |
| Egg | 431 | 0.2 (0.1, 0.5) | 0.2 (0.1, 0.5) | 2.3 (1.2, 4.7) | 2.4 (1.3, 5.3) |
| Fish | 82 | 2.6 (1.0, 12.0) | 1.3 (0.4, 12.7) | 12.1 (4.5, 43.9) | 15.6 (4.6, 102) |
| Hazelnut | 411 | 0.1 (0.07, 0.6) | 0.2 (0.09, 0.7) | 3.5 (1.3, 12.1) | 4.7 (1.7, 15.7) |
| Lupine | 25 | 2.9 (1.3, 9.1) | 2.6 (0.5, 14.8) | 15.3 (6.7, 47.0) | 16.8 (4.7, 70.0) |
| Milk | 450 | 0.2 (0.1, 0.5) | 0.3 (0.2, 0.6) | 2.4 (1.3, 5.0) | 3.1 (1.6, 6.6) |
| Mustard | 33 | 0.07 (0.009, 1.1) | 0.05 (0.006, 0.9) | 0.4 (0.1, 3.6) | 0.5 (0.09, 3.9) |
| Peanut | 1306 | 0.2 (0.1, 0.4) | 0.7 (0.5, 1.3) | 2.1 (1.2, 4.6) | 3.9 (2.8, 7.1) |
| Sesame | 40 | 0.1 (0.03, 2.7) | 0.2 (0.04, 4.8) | 2.7 (0.4, 33.6) | 4.2 (0.6, 57.7) |
| Shrimp | 75 | 26.2 (2.7, 166) | 30.8 (3.4, 326) | 280 (69.3, 880) | 429 (94.0, 1854) |
| Soy | 87 | 0.5 (0.2, 3.5) | 0.7 (0.3, 4.5) | 10.0 (2.2, 54.6) | 14.1 (3.1, 76.2) |
| Walnut | 74 | 0.03 (0.01, 0.5) | 0.04 (0.02, 0.6) | 0.8 (0.1, 8.9) | 1.2 (0.1, 13.0) |
| Wheat | 99 | 0.7 (0.3, 2.5) | 1.1 (0.4, 3.8) | 6.1 (2.6, 15.6) | 9.3 (3.9, 24.9) |
Discrete dosing schemes are reported as the mg protein amount of each separate dose within a food challenge when determining the individual NOAELi and LOAELi. Cumulative dosing schemes are reported as the cumulative sum of all prior doses within a food challenge when calculating the individual NOAELi and LOAELi.
Fig. 1.
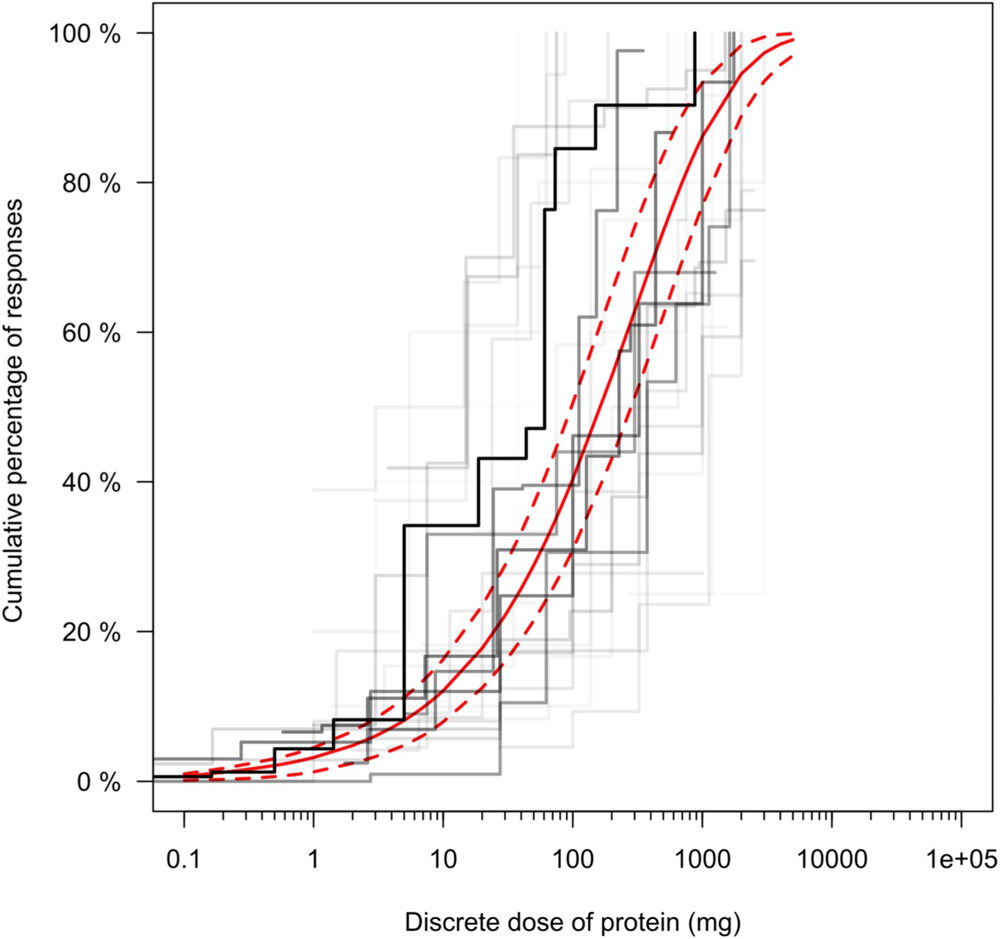
Dose distribution modelling for peanut (expressed as discrete dose of mg peanut protein) utilizing Bayesian Stacked Parametric Survival methods with Frailty Components and Interval Censored Failure Times as described (Wheeler et al., 2019). In this Stacked Model Averaging technique, five different parametric distributions are modelled, weighted and combined into a single dose distribution estimate in order to maximize the predictive accuracy of the calculated ED values. The predicted Stacked Model Averaging distribution estimate (red line) is presented with its corresponding 95% posterior predicted failure times (dashed red lines). The Kaplan-Meier curves for each individual study in the dataset are also presented (black lines, darker indicates study with more observations).
4. Discussion
In this current study, data from over 3400 low-dose oral clinical challenges were used to determine the ED01 (estimated protection levels of 99%) and the ED05 (estimated protection levels of 95%) with the respective 95% confidence intervals through the Stacked Model Averaging for 14 different allergens.
For risk management of unintended allergen presence (UAP) in food, zero risk in food allergy management cannot be achieved similar to other risk factors encountered in everyday life in society (Turner et al., 2017). Allergic individuals continue to report reactions in daily life despite efforts to follow a strict avoidance diet (Gupta et al., 2018; Michelsen-Huisman et al., 2018; Zurzolo et al., 2019). Zero risk is also not operationally achievable in the food industry given the complexities in the supply chain and in processing facilities that handle multiple allergenic foods, often on shared processing equipment. The lack of established regulatory reference doses or action levels results in case-by-case decisions by regulators and the food industry which can lead to inconsistencies in the use of PAL and a lack of transparency in the use of PAL, thus leading to risk-taking behavior by consumers (DunnGalvin et al., 2015; Hattersley et al., 2014; Madsen et al., 2010). While many companies in the food industry have committed substantial resources into establishment of good manufacturing practices and allergen management (Gupta et al., 2017), ensuring that 100% of all allergen residue is removed from shared equipment, thus ensuring zero risk, is not operationally achievable despite best efforts in controlling allergen cross-contact (Crevel et al., 2014). Since zero risk is not feasible from a risk management perspective for allergic individuals or the food industry, consensus on an accepted level of risk is needed (Madsen et al., 2010). Thus, when possible, the aim should be for the protection of a certain proportion of the population, e.g. to protect 99% of the allergic population from any objective reaction (DunnGalvin et al., 2015, 2019; Madsen et al., 2010; Taylor et al., 2014; Zurzolo et al., 2016). Previously, Taylor et al. (2014) selected either the ED01 or lower 95% confidence interval of the ED05 to derive Reference Doses, based upon the number of individual NOAELi and LOAELi data points available for modeling. These Reference Doses (mg protein) were meant to serve as a basis for calculating action levels (concentrations, mg protein/kg) for precautionary allergen labeling. This approach afforded a possibly acceptable public health outcome by striking a balance between the statistical certainty of the estimate, level of consumer protection, ability to analytically verify & enforce, and hopeful enhancement of consumer observance & avoidance of food products with precautionary labeling. In using the ED01 or lower 95% confidence interval of the ED05 as a basis for Reference Doses, these ED doses were assumed to be sufficiently safe. A recent study has confirmed that a dose equivalent to the ED05 for peanut minimized the probability of occurrence of anything more serious than a mild to moderate reaction (Hourihane et al., 2017). In this single-dose challenge study using the ED05 for peanut, exposure to the ED05 for peanut was safe for more than 95% of unselected peanut allergic patients and mild, transitory reactions according to the pre-defined study criteria were observed in only 2.1% of participants (Hourihane et al., 2017). Further ongoing single dose studies with low doses of specific allergenic foods (milk, egg, hazelnut (iFAAM, 2018):) can provide further confirmation of model estimates and will further document the nature of symptoms occurring at defined low doses. Notably, since the publication of EDs and Reference Doses for risk management purposes by Taylor et al. (2014), a number of stakeholders and national agencies have begun to adopt the use of reference doses in their risk assessment practices or analytical guidances (FAVV SciCom, 2017; NVWA BuRO, 2016; Sjögren Bolin, 2015; Waiblinger and Schulze, 2018).
Previously, researchers have applied three different dose-distribution models (log normal, log logistic, Weibull) when modelling food challenge data and, as no biological rationale exists to select one model over another, EDp values have been reported from multiple distributions in the same publication (Ballmer-Weber et al., 2015; Taylor et al., 2014). Until recently, no better statistical option was available to further inform risk assessment and risk management decision making. However, Wheeler et al. (2019) have recently developed a Stacked Model Averaging method for interval-censored data with the incorporation of random effects for study-to-study heterogeneity; a methodology with great potential for clarifying discussions regarding appropriate reference doses during the decision making process. One major benefit of Stacked Model Averaging is the simplification of the outcome as a weighted combination of multiple parametric distributions results in one single EDp value. This simplification should enable clearer, focused conversations among all stakeholders regarding the doses that comply with a specified level of protection within allergen and allergy management programs. One example demonstrating a more transparent decision making process through the use of Stacked Model Averaging in the current analysis regards the EDp reporting for egg. Previously, Taylor et al. (2014) reported ED01 results from a Weibull distribution of 0.03–0.045 mg egg protein, from a loglogistic distribution of 0.12–0.13 mg egg protein and from a lognormal distribution of 0.2–0.21 mg egg protein. At that time the use of “expert judgement” determined that the most conservative model should be used for risk management purposes and a Reference Dose for egg was conservatively selected as 0.03 mg egg protein based on the ED01 results from a single distribution. The most conservative model was used despite the fact that all three statistical models in Taylor et al. (2014) fit the egg data quite well and there was no reason to exclude any of the models. Additionally, single dose studies with egg were administered to 75 children but none reacted to the ED05 (iFAAM, 2018), indicating that the Taylor et al. (2014) results for egg were potentially too conservative for egg. In contrast, Stacked Model Averaging automatically accounts for model differences and removes a layer of expert judgement from the end results to provide a simpler, more transparent decision making process for EDp derivation and reporting. The current analysis with Stacked Model Averaging utilized 5 statistical distributions (Weibull distribution, Log-Gaussian (Log-Normal), Log-Logistic, Generalized Pareto and Log-Laplace (Log-Double-Exponential)) and reported a single averaged ED01 of 0.2 mg egg protein for both the discrete and cumulative dosing schemes.
Conversely, one limitation of the Stacked Model Averaging technique is that it is unable to utilize single data points from individual case studies. While this limitation is not significant for allergens with abundant data, some foods only have very limited data supported by sporadic individual case reports. For allergens with such limited data (e.g. in the case of some tree nuts), other risk assessment and risk management methods should be discussed as a generic placeholder until more specific data are obtained (e.g. placeholders inferred from related foods with abundant data, or an agreed upon generic placeholder across foods with limited data).
The Stacked Model Averaging distributions were fit to the food challenge data, including random effects modelling to account for study-to-study heterogeneity (Wheeler et al., 2019). Prior attempts to study possible differences between age groups or differences arising from variations in geographic location or challenge material have found no difference between the studies, or have been confounded by patient selection bias and differing study protocols/dosing schemes and no true differences could be observed (Allen et al., 2014; Remington et al., 2017). Thus, the current study combines all available individual studies per food into a single model which accounts for random effects and study-to-study heterogeneity providing the most robust statistical estimate for EDp values available. Additionally, the adverse reactions experienced by patients in these low-dose oral clinical challenges were generally mild to moderate (especially in the extreme low-dose range of interest) and single-dose studies with the ED05 have produced less frequent reactions than predicted with only mild to moderate symptoms (Hourihane et al., 2017). Further uncertainty factors were not applied to the EDp estimations as data were available from the appropriate sensitive sub-population of humans. It should be noted that EDp values presented in this study reflect the population of allergic consumers who are clinically well managed with respect to medications, illnesses and influencing factors. There may be individual co-factors such as exercising, drinking alcohol, sleep deprivation, or menstruation, that may not be fully covered by these EDp values, and may need to exhibit more dietary caution on an individual level (Dua et al., 2019; Dubois et al., 2018; Turner et al., 2016). While modest differences occurred in individual minimal eliciting doses for peanut from sleep deprivation and exercising (Dua et al., 2019), the ED01 and ED05 reference doses would remain protective for the vast majority of food-allergic individuals. The difference between allergen and allergy management should be emphasized in this respect. It is recommended to account for individual co-factors as part of the allergy management advice, as discussed between an allergic individual and their physician, not within allergen risk assessment and allergen risk management on a population level.
While previous research indicated that the variation in the forms of food has limited impact on the individual NOAELi/LOAELi during food challenges for milk, egg and peanut (Allen et al., 2014; Remington et al., 2017), soy merits further discussion in part due to the wide variety of processing techniques applied to it within the food industry and differing clinical response profiles to these soy-based products. Some soy-allergic patients react to certain brands of soy milk but can safely ingest soy flour and other forms of soy (Mittag et al., 2004). The reasons for the differing susceptibility to specific forms of soy is unknown. However, soy proteins can be commercially subjected to proteolysis, fermentation, and chemical modification processes during production of different product types. The resulting differences in soy protein structures could make a difference in the tolerability of various soy products but this is not yet well studied. Thus, while it is understood that these two materials are likely not equivalent after processing, these soy milk allergic patients were included along with soy flour allergic patients in the analyses for the current study from a precautionary point of view.
It should be noted that the EDp results listed in Table 1 of this study relate specifically to IgE-mediated food allergies and not to other, non-IgE-mediated forms of food allergy/sensitivity that might be associated with any of these foods. However, wheat is a unique food as a gluten-free specialty food labeling guideline of < 20 ppm (mg/kg) has been established by the Codex Alimentarius Commission (and adopted by various legislatures) for specially labelled foods intended for individuals with celiac disease. While not specifically intended for them, just as previously highlighted by Taylor et al. (2014), individuals with IgE-mediated wheat allergy could possibly be protected when selecting gluten-free products manufactured in conformity to Codex guidance as both the ED01 of 0.7 mg of wheat protein (discrete) and ED05 of 6.1 mg wheat protein (discrete) indicate that products containing < 20 ppm of gluten consumed in limited amounts could lead to exposures of wheat protein less than these EDp estimates. However, caution should be exercised as mg wheat protein does not equate to ppm (mg/kg) for gluten-free labelling. For example, a food product containing for instance 14 ppm wheat protein complies with the gluten-free criterion of < 20 ppm, but consumption of 200 gr of such food would lead to an intake of wheat protein of 2.8 mg, which is a factor of 4 in excess of the ED01. Additionally, multiple studies have indicated a range of “gluten-free” product categories with levels of gluten higher than 20 ppm present (Do et al., 2018). It is recommended that individuals with IgE-mediated wheat allergy discuss with their physician before consuming “gluten-free” labelled products.
Previously, Taylor et al. (2014) utilized both the discrete dose and the cumulative dose for the elaboration of the ED01 or (the lower 95% confidence interval of) the ED05 but only small differences were observed between the mg protein amounts for discrete or cumulative EDp values generated by any of the respective parametric models. Similar results were seen in the current study, with the discrete and cumulative estimates for ED01 or ED05 being relatively similar to each other. For risk management purposes it would seem intuitive that EDp values calculated using discrete dosing schemes are always more sensitive than the cumulative EDp values, however the ED01 and ED05 estimates in this study illustrate that is not always the case (Table 1). Thus it is recommended that the data be analyzed in both the discrete and cumulative manner to ensure a complete overview of the results is obtained and the most sensitive of EDp of the two could potentially be used in further applications, and as a result, serve as a safety factor.
Finally, it is envisioned that the ED values listed in Table 1 could be utilized to provide updated mg protein amounts for Reference Doses in a risk management system, such as VITAL, for guidance surrounding the use of precautionary allergen labelling on food products. However recommending specific Reference Doses for food allergen risk management is a multi-stakeholder process; due to necessary stakeholder agreement when determining the acceptable risks (e.g. ED01 vs ED05 for any objective symptom) and consideration of Other Legitimate Factors.
In the absence of legislation regarding appropriate Reference Doses, the voluntary use of precautionary allergen labeling on packaged foods has proliferated in many countries to the point where nearly 50% of the allergic consumers ignore precautionary warnings, in part due to the absence of a relationship between the precautionary label and the actual risk (Blom et al., 2018; DunnGalvin et al., 2015; Michelsen-Huisman et al., 2018; Turner et al., 2011; Zurzolo et al., 2013). The success of any risk assessment and risk management program for precautionary labeling, such as VITAL, is predicated upon the protection of the vast majority of the food-allergic population while allowing access to the widest possible variety of packaged foods. However, the allergic consumer currently has no way to identify if a product without a precautionary statement has been deemed safe after a comprehensive risk assessment or if no risk assessment was done; similar situations also arise for products with precautionary statements which together leads to frustration and ignoring of labels (Zurzolo et al., 2016). Thus, as recommended by multiple stakeholder groups, science-based thresholds with standardized, identifiable labelling for products that have undergone a risk assessment should be a priority for both regulatory authorities and the food industry (DunnGalvin et al., 2019; National Academies of Sciences Engineering and Medicine, 2017; Yeung and Robert, 2018).
Moving forward, the previously described single-dose approach was shown to be quick, safe and highly cost-effective in identifying and managing the most sensitive subgroup of the peanut-allergic population (Hourihane et al., 2017; Shaker and Greenhawt, 2018). Adaptation of a single-dose approach to establish tolerance to the ED05 for a specific food, in combination with regulatory establishment of Reference Doses and industry-wide application of precautionary labeling according to risk management programs using the same ED05 or the ED01 could have significant clinical advantages, open up food choices for allergic consumers, reduce frustrations with precautionary labelling, and significantly improve the quality-of-life for food allergic individuals.
Supplementary Material
Acknowledgements
The authors would like to thank Jamie Kabourek, M.S., R.D. for her contributions to enable a smooth literature review process through her role as the Resource Manager for the Food Allergy Research and Resource Program at the University of Nebraska. The Food Allergy Research & Resource Program is a food industry-funded consortium with more than 100 member food companies. This research was party financially supported through Dutch Governmental TNO Research Cooperation Funds.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fct.2020.111259.
References
- Allen KJ, Remington BC, Baumert JL, Crevel RWR, Houben GF, Brooke-Taylor S, Kruizinga AG, Taylor SL, 2014. Allergen reference doses for precautionary labeling (VITAL 2.0): clinical implications. J. Allergy Clin. Immunol 133, 156–164. 10.1016/j.jaci.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Allen KJ, Taylor SL, 2018. The consequences of precautionary allergen labeling: safe haven or unjustifiable burden? J. Allergy Clin. Immunol. Pract 6, 400–407. 10.1016/j.jaip.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Ballmer-Weber BK, Fernandez-Rivas M, Beyer K, Defernez M, Sperrin M, Mackie AR, Salt LJ, Hourihane JOB, Asero R, Belohlavkova S, Kowalski M, De Blay F, Papadopoulos NG, Clausen M, Knulst AC, Roberts G, Popov T, Sprikkelman AB, Dubakiene R, Vieths S, Van Ree R, Crevel R, Mills ENC, 2015. How much is too much? Threshold dose distributions for 5 food allergens. J. Allergy Clin. Immunol 135, 964–971. 10.1016/j.jaci.2014.10.047. [DOI] [PubMed] [Google Scholar]
- Blom WM, Michelsen-Huisman AD, van Os-Medendorp H, van Duijn G, de Zeeuw-Brouwer M, Versluis A, Castenmiller JJM, Noteborn HPJM, Kruizinga AG, Knulst AC, Houben GF, 2018. Accidental food allergy reactions: products and undeclared ingredients. J. Allergy Clin. Immunol 142, 865–875. 10.1016/j.jaci.2018.04.041. [DOI] [PubMed] [Google Scholar]
- Blumchen K, Beder A, Beschorner J, Ahrens F, Gruebl A, Hamelmann E, Hansen G, Heinzmann A, Nemat K, Niggemann B, Wahn U, Beyer K, 2014. Modified oral food challenge used with sensitization biomarkers provides more real-life clinical thresholds for peanut allergy. J. Allergy Clin. Immunol 134, 390–398. 10.1016/j.jaci.2014.03.035. e4. [DOI] [PubMed] [Google Scholar]
- Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA Jr., Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM, NIAID-Sponsored Expert Panel, 2010. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol 126, S1–58. 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevel RWR, Taylor SL, Pfaff S, Alldrick A, 2014. Chapter nine - managing food allergens: case histories and how they were handled. In: Madsen CB, Crevel RWR, Mills C, Taylor SL (Eds.), Risk Management for Food Allergy. Academic Press, San Diego, pp. 167–188. 10.1016/B978-0-12-381988-8.00009-9. [DOI] [Google Scholar]
- Do AB, Khuda SE, Sharma GM, 2018. Undeclared food allergens and gluten in commercial food products analyzed by ELISA. J. AOAC Int 101, 1–13. 10.5740/jaoacint.17-0384. [DOI] [PubMed] [Google Scholar]
- Dua S, Ruiz-Garcia M, Bond S, Durham SR, Kimber I, Mills C, Roberts G, Skypala I, Wason J, Ewan P, Boyle R, Clark A, 2019. Effect of sleep deprivation and exercise on reaction threshold in adults with peanut allergy: a randomized controlled study. J. Allergy Clin. Immunol 144, 1584–1594. 10.1016/j.jaci.2019.06.038. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois AEJ, Turner PJ, Hourihane J, Ballmer-Weber B, Beyer K, Chan C-H, Gowland MH, O’Hagan S, Regent L, Remington B, Schnadt S, Stroheker T, Crevel RWR, 2018. How does dose impact on the severity of food-induced allergic reactions, and can this improve risk assessment for allergenic foods? Allergy 73, 1383–1392. 10.1111/all.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DunnGalvin A, Chan C-H, Crevel R, Grimshaw K, Poms R, Schnadt S, Taylor SL, Turner P, Allen KJ, Austin M, Baka A, Baumert JL, Baumgartner S, Beyer K, Bucchini L, Fernández-Rivas M, Grinter K, Houben GF, Hourihane J, Kenna F, Kruizinga AG, Lack G, Madsen CB, Clare Mills EN, Papadopoulos NG, Alldrick A, Regent L, Sherlock R, Wal J-M, Roberts G, 2015. Precautionary allergen labelling: perspectives from key stakeholder groups. Allergy 70, 1039–1051. 10.1111/all.12614. [DOI] [PubMed] [Google Scholar]
- DunnGalvin A, Roberts G, Schnadt S, Astley S, Austin M, Blom WM, Baumert J, Chan C-H, Crevel RWR, Grimshaw KEC, Kruizinga AG, Regent L, Taylor S, Walker M, Mills ENC, 2019. Evidence‐based approaches to the application of precautionary allergen labelling: report from two iFAAM workshops. Clin. Exp. Allergy 49, 1191–1200. 10.1111/cea.13464. [DOI] [PubMed] [Google Scholar]
- EFSA Scientific Committee, 2017. Update: use of the benchmark dose approach in risk assessment. EFSA J 15, 1–41. 10.2903/j.efsa.2017.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVV SciCom, 2017. ADVIES 24–2017 Betreft: Referentiedosissen voor de allergenen die zijn opgenomen in bijlage II van de Verordening. ( EU ) nr. 1169/2011 van 25 oktober 2011.
- Garber EAE, Parker CH, Handy SM, Cho CY, Panda R, Samadpour M, Reynaud DH, Ziobro GC, 2016. Presence of undeclared food allergens in cumin: the need for multiplex methods. J. Agric. Food Chem 64, 1202–1211. 10.1021/acs.jafc.5b05497. [DOI] [PubMed] [Google Scholar]
- Gendel SM, 2018. Food allergen recalls: the past as prologue. In: Fu T-J, Jackson LS, Krishnamurthy K, Bedale W (Eds.), Food Allergens: Best Practices for Assessing, Managing and Communicating the Risks. Springer International Publishing, Cham, pp. 95–102. 10.1007/978-3-319-66586-3_5. [DOI] [Google Scholar]
- Gendel SM, 2012. Comparison of international food allergen labeling regulations. Regul. Toxicol. Pharmacol 63, 279–285. 10.1016/j.yrtph.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Taylor SL, Baumert JL, Kao LM, Schuster E, Smith BM, 2017. Economic factors impacting food allergen Management : perspectives from the food industry. J. Food Protect 80, 1719–1725. 10.4315/0362-028X.JFP-17-060. [DOI] [PubMed] [Google Scholar]
- Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, Nadeau KC, 2018. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics 142 10.1542/peds.2018-1235. e20181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley S, Ward R, Baka A, Crevel RWR, 2014. Advances in the risk management of unintended presence of allergenic foods in manufactured food products - an overview. Food Chem. Toxicol 10.1016/j.fct.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Hourihane JOB, Allen KJ, Shreffler WG, Dunngalvin G, Nordlee JA, Zurzolo GA, Dunngalvin A, Gurrin LC, Baumert JL, Taylor SL, 2017. Peanut Allergen Threshold Study (PATS): novel single-dose oral food challenge study to validate eliciting doses in children with peanut allergy. J. Allergy Clin. Immunol 139, 1583–1590. 10.1016/j.jaci.2017.01.030. [DOI] [PubMed] [Google Scholar]
- iFAAM, 2018. Project final report iFAAM GA 312147. [WWW Document]. ETICA EU Proj URL. https://cordis.europa.eu/project/id/312147/reporting accessed 1.30.20.
- Klein Entink RH, Remington BC, Blom WM, Rubingh CM, Kruizinga AG, Baumert JL, Taylor SL, Houben GF, 2014. Food allergy population thresholds: an evaluation of the number of oral food challenges and dosing schemes on the accuracy of threshold dose distribution modeling. Food Chem. Toxicol 70, 134–143. 10.1016/j.fct.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Madsen CB, Crevel R, Chan C, Dubois AEJ, Dunngalvin A, Flokstra-de Blok BMJ, Gowland MH, Hattersley S, Hourihane JOB, Nørhede P, Pfaff S, Rowe G, Schnadt S, Vlieg-boerstra BJ, 2010. Food allergy : stakeholder perspectives on acceptable risk. Regul. Toxicol. Pharmacol 10.1016/j.yrtph.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Michelsen-Huisman AD, van Os-Medendorp H, Blom WM, Versluis A, Castenmiller JJM, Noteborn HPJM, Kruizinga AG, Houben GF, Knulst AC, 2018. Accidental allergic reactions in food allergy: causes related to products and patient’s management. Allergy 73, 2377–2381. 10.1111/all.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag D, Vieths S, Vogel L, Becker WM, Rihs HP, Helbling A, Wüthrich B, Ballmer-Weber BK, Wuthrich B, Ballmer-Weber BK, Wüthrich B, Ballmer-Weber BK, Wuthrich B, Ballmer-Weber BK, 2004. Soybean allergy in patients allergic to birch pollen: clinical investigation and molecular characterization of allergens. J. Allergy Clin. Immunol 113, 148–154. 10.1016/j.jaci.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, Cardona V, Dubois A, Dutoit G, Eigenmann P, Fernandez Rivas M, Halken S, Hickstein L, Høst A, Knol E, Lack G, Marchisotto MJ, Niggemann B, Nwaru BI, Papadopoulos NG, Poulsen LK, Santos AF, Skypala I, Schoepfer A, Van Ree R, Venter C, Worm M, Vlieg-Boerstra B, Panesar S, De Silva D, Soares-Weiser K, Sheikh A, Ballmer-Weber BK, Nilsson C, De Jong NW, Akdis CA, 2014. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy Eur. J. Allergy Clin. Immunol 69, Food and Chemical Toxicology 139 (2020) 111259 1008–1025. 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering and Medicine, 2017. Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. The National Academies Press, Washington DC, United States: 10.17226/23658. [DOI] [PubMed] [Google Scholar]
- Niggemann B, Lange L, Finger A, Ziegert M, Müller V, Beyer K, 2012. Accurate oral food challenge requires a cumulative dose on a subsequent day. J. Allergy Clin. Immunol 130, 261–263. 10.1016/j.jaci.2012.03.021. [DOI] [PubMed] [Google Scholar]
- NVWA BuRO, 2016. Advice on Preliminary Reference Doses for Food Allergens. (Utrecht). https://english.nvwa.nl/documents/consumers/food/safety/documents/advice-of-buro-on-preliminary-reference-doses-for-food-allergens, Accessed date: 30 January 2020.
- Remington BC, Baumert JL, Blom WM, Houben GF, Taylor SL, Kruizinga AG, 2015. Unintended allergens in precautionary labelled and unlabelled products pose significant risks to UK allergic consumers. Allergy 70, 813–819. 10.1111/all.12625. [DOI] [PubMed] [Google Scholar]
- Remington BC, Westerhout J, Campbell DE, Turner PJ, 2017. Minimal impact of extensive heating of hen’s egg and cow’s milk in a food matrix on threshold dose-distribution curves. Allergy 72, 1816–1819. 10.1111/all.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson HA, Gerth Van Wijk R, Bindslev-jensen C, Sicherer S, Teuber SS, Dubois AEJJ, Beyer K, Eigenmann PA, Spergel JM, Werfel T, Chinchilli VM, Wijk G. Van, Bindslev-jensen C, Sicherer S, Gerth Van Wijk R, Bindslev-jensen C, Sicherer S, Teuber SS, Burks a.W., Dubois AEJJ, Beyer K, Eigenmann PA, Spergel JM, Werfel T, Chinchilli VM, Wijk G. Van, Bindslev-jensen C, Sicherer S, 2012. Standardizing double-blind, placebo-controlled oral food challenges: American academy of allergy, asthma & immunology-European academy of allergy and clinical immunology PRACTALL consensus report. J. Allergy Clin. Immunol 130, 1260–1274. 10.1016/j.jaci.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Sayers RL, Gethings L, Wallace A, Semic-Jusufgic A, Simpson A, Barran P, Gilbert J, Senyuva H, Rodgers A, Bromley M, Walker M, Brown H, Mills ENC, 2016. How much of a problem is peanut in ground cumin for individuals with peanut allergy? J. Allergy Clin. Immunol 137, AB142 10.1016/j.jaci.2015.12.597. [DOI] [Google Scholar]
- Shaker M, Greenhawt M, 2018. The health and economic outcomes of peanut allergy management practices. J. Allergy Clin. Immunol. Pract 6, 2073–2080. 10.1016/j.jaip.2018.04.036. [DOI] [PubMed] [Google Scholar]
- Sjögren Bolin Y, 2015. Undeclared Milk, Peanut, Hazelnut or Egg - Guide on How to Assess the Risk of Allergic Reactions in the Population. Livsmedelsverkets Rapportserie Nr 17/2015. Livsmedelsverket Natl. Food Agency https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2015/rapport-17-riskvarderingsguide-allergener.pdf, Accessed date: 30 January 2020.
- Taylor SL, Baumert JL, Kruizinga AG, Remington BC, Crevel RWR, Brooke-Taylor S, Allen KJ, The Allergen Bureau of Australia & New Zealand, Houben G, 2014. Establishment of reference doses for residues of allergenic foods: report of the VITAL expert panel. Food Chem. Toxicol 63, 9–17. 10.1016/j.fct.2013.10.032. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Crevel RWR, Sheffield D, Kabourek J, Baumert J, 2009. Threshold dose for peanut: risk characterization based upon published results from challenges of peanut-allergic individuals. Food Chem. Toxicol 47, 1198–1204. 10.1016/j.fct.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Moneret-Vautrin DA, Crevel RWR, Sheffield D, Morisset M, Dumont P, Remington BC, Baumert JL, 2010. Threshold dose for peanut: risk characterization based upon diagnostic oral challenge of a series of 286 peanut-allergic individuals. Food Chem. Toxicol 48, 814–819. 10.1016/j.fct.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Turner PJ, Baumert JL, Beyer K, Boyle RJ, Chan C-H, Clark AT, Crevel RWR, Dunngalvin A, Fernández-Rivas M, Gowland MH, Grabenhenrich L, Hardy S, Houben GF, O’B Hourihane J, Muraro A, Poulsen LK, Pyrz K, Remington BC, Schnadt S, van Ree R, Venter C, Worm M, Mills ENC, Roberts G, Ballmer-Weber BK, 2016. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy 71, 1241–1255. 10.1111/all.12924. [DOI] [PubMed] [Google Scholar]
- Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ, 2017. Fatal anaphylaxis: mortality rate and risk factors. J. Allergy Clin. Immunol. Pract 5, 1169–1178. 10.1016/j.jaip.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PJ, Kemp AS, Campbell DE, 2011. Advisory food labels: consumers with allergies need more than “traces” of information. BMJ 343 10.1136/bmj.d6180. [DOI] [PubMed] [Google Scholar]
- US EPA, Gift J, Davis JA, Blessinger T, Wheeler M, Olszyk L, Simmons C, Allen B, Brown ME, 2019. Benchmark dose software (BMDS 3.0) user manual (EPA/600/ R-18/310). https://www.epa.gov/sites/production/files/2018-09/documents/bmds_3.0_user_guide.pdf. https://www.epa.gov/bmds/benchmark-dose-software-bmds-version-30.
- Waiblinger H-U, Schulze G, 2018. Action levels for food allergens: an approach for official food control in Germany. J. AOAC Int 101, 17–22. 10.5740/jaoacint.17-0383. [DOI] [PubMed] [Google Scholar]
- Walker MJ, Burns DT, Elliott CT, Gowland MH, Mills ENCC, 2016. Is food allergen analysis flawed? Health and supply chain risks and a proposed framework to address urgent analytical needs. Analyst 141, 24–35. 10.1039/c5an01457c. [DOI] [PubMed] [Google Scholar]
- Westerhout J, Baumert JL, Blom WM, Allen KJ, Ballmer-Weber B, Crevel RWR, Dubois AEJ, Fernández-Rivas M, Greenhawt MJ, Hourihane JO, Koplin JJ, Kruizinga AG, Le T-M, Sampson HA, Shreffler WG, Turner PJ, Taylor SL, Houben GF, Remington BC, 2019. Deriving individual threshold doses from clinical food challenge data for population risk assessment of food allergens. J. Allergy Clin. Immunol 144, 1290–1309. 10.1016/j.jaci.2019.07.046. [DOI] [PubMed] [Google Scholar]
- Wheeler MW, Westerhout J, Baumert JL, Remington BC, 2019. Bayesian Stacked Parametric Survival with Frailty Components and Interval Censored Failure Times. arXiv:1908.11334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J, Robert MC, 2018. Challenges and path forward on mandatory allergen labeling and voluntary precautionary allergen labeling for a global company. J. AOAC Int 101, 70–76. 10.5740/jaoacint.17-0391. [DOI] [PubMed] [Google Scholar]
- Zurzolo GA, Allen KJ, Peters RL, Dharmage SC, Tang MLK, Said M, Field MJ, de Courten M, Mathai ML, Campbell DE, 2019. Self-reported anaphylaxis to packaged foods in Australia. J. Allergy Clin. Immunol. Pract 7, 687–689. 10.1016/j.jaip.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Zurzolo GA, Koplin JJ, Mathai ML, Tang MKLL, Allen KJ, 2013. Perceptions of precautionary labelling among parents of children with food allergy and anaphylaxis. Med. J. Aust 198, 621–623. 10.5694/mja12.11669. [DOI] [PubMed] [Google Scholar]
- Zurzolo GA, Koplin JJ, Ponsonby AL, McWilliam V, Dharmage S, Heine RG, Tang MLK, Prescott S, Campbell DE, Loh R, Rueter K, Netting M, Frith K, Norton W, Said M, Gold M, Lee NA, Mathai M, de Courten M, Allen KJ, 2016. Consensus of stakeholders on precautionary allergen labelling: a report from the Centre for Food and Allergy Research. J. Paediatr. Child Health 52, 797–801. 10.1111/jpc.13202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


