Abstract
Purpose of Review:
‘Broadly neutralizing antibodies’ (bNAbs), are rare HIV-specific antibodies which exhibit the atypical ability to potently neutralize diverse viral isolates. While efforts to elicit bNAbs through vaccination have yet to succeed, recent years have seen remarkable pre-clinical and clinical advancements of passive immunization approaches targeting both HIV prevention and cure. We focus here on the potential to build upon this success by moving beyond neutralization to additionally harness the diverse effector functionalities available to antibodies via Fc-effector functions.
Recent Findings:
Recent studies have leveraged the ability to engineer bNAb Fc domains to either enhance or abrogate particular effector functions to demonstrate that activities such as antibody dependent cell-mediated cytotoxicity contribute substantially to in vivo antiviral activity. Intriguingly, recent studies in both non-human primates and in humans have suggested that passive bNAb infusion can lead to durable immunity by enhancing virus-specific T-cell responses through a ‘vaccinal effect’.
Summary:
The combination of antibody engineering strategies designed to enhance effector functions, with the broad and potent antigen recognition profile of bNAbs, has the potential to give rise to powerful new therapeutics for HIV. We aim to provide a timely review of recent advances to catalyze this development.
Keywords: HIV, broadly neutralizing antibodies, Fc effector function, immunotherapy, vaccinal effect
Introduction
Human immunodeficiency virus (HIV)-specific antibody (Ab) responses against the viral Envelope (gp120, gp41) are typically detectable within a few weeks of infection, and increase over the course of disease (1–3). However, the majority of these antibodies are non-neutralizing (4). Broadly neutralizing antibodies (bNAbs) are defined as Abs with the ability to neutralize highly variable viral pathogens. In the case of HIV, although the natural emergence of bNAbs is rare, substantial efforts have led to the isolation of an array of bNAbs which achieve varying neutralization potencies and breadths across the vast diversity of HIV clades by binding to vulnerable sites on the Envelope glycoprotein (5). The development of vaccine approaches capable of eliciting bNAbs remains a ‘holy grail’ in the field, towards which progress continues. In the absence of a vaccine, however, the passive infusion of bNAbs holds potential both for the prevention and treatment of HIV, through direct neutralization of the HIV virions and by boosting different components of the immune system to control or eliminate infection (5). Building off of success in non-human primate simian-human immunodeficiency virus (SHIV) models of HIV infection (6, 7), the passive transfer of several different bNAbs to HIV-infected individuals has been shown to transiently suppress viremia (8, 9**). Beyond neutralization, a critical aspect of Abs in general – which extends to bNAbs - is their ability to exert diverse effector functionalities by virtue of interactions mediated through their ‘Fragment crystallizable’ (Fc) domains (10). These Fc domains of bNAbs mediate the opsonization of virions or infected cells by complement components, which leads directly to lysis (11, 12). By engaging with Fc receptors on effector cells, bNAbs can also enhance the killing of infected CD4+ T cells by natural killer (NK) cells, and increase the phagocytosis of infected cells by macrophages and neutrophils (13, 14). Here, we summarize the current knowledge about Fc-mediated functions of bNAbs, and technical advancements that have led to improved efficacy of these antibodies by modifying their Fc domains.
Antibody-dependent complement-mediated lysis (ADCML)
The classical pathway of the complement system activates by the attachment of C1q to the Fc domain of antibodies that have bound to pathogens or to infected cells. C1q activates C1r and C1s serine proteases that initiate the recruitment of other complement factors. This proteolytic cascade leads to the formation of membrane attack complex (MAC) and lysis of the pathogen or infected cell (Figure 1A) (15). IgG1 and IgG3 subclasses are the most efficient activators of complement (16). Mujib et al tested a large panel of bNAbs and concluded that anti- V1/V2/glycan bNAbs such as PG9, PG16, and PGT145 bound to HIV Envelope on the surface of primary HIV-infected cells and induced ADCML (17). 2F5, 4E10, 2G12, VRC01, and 3BNC117 were not able to enhance the clearance of virus-infected cells by ADCML in their experiments (17). Complement activation and its lysis effect can be prevented by specific surface molecules that are called regulators of complement activation (RCA) (18, 19). RCAs like CD46, CD55, and D59 prevent the generation of MAC, which is the final step of all three pathways of complement activation (18, 19). RCAs can be transferred to virions from infected cells, and therefore, be present on both the cell-free virus and the CD4+ infected cells (20, 21). Saifuddin et al showed that HIV virions hijacked and expressed human CD46, CD55, and CD59 on their surfaces to escaped ADCML (20). Functional blockade of CD59 significantly enhanced the ADCML activity of 2F5, 4E10, and 2G12 bNAbs against three HIV laboratory strains (R5, X4, and R5/X4), six primary isolates, and provirus-activated ACH-2 cells (21). Similarly, Hu et al reported that a recombinant protein of the bacterial toxin intermedilysin inhibited human CD59 function, enabling efficient ADCML of virions and infected cells (22). In this context, blockade of RCAs by anti-RCA-antibodies, inhibitory peptides, or other potential small molecules may boost the function of bNAbs and facilitate the neutralization of the cell-free virions or infected cells by ADCML.
Figure 1: Antibody-dependent killing of infected CD4+ T cells is mediated by bNAbs.
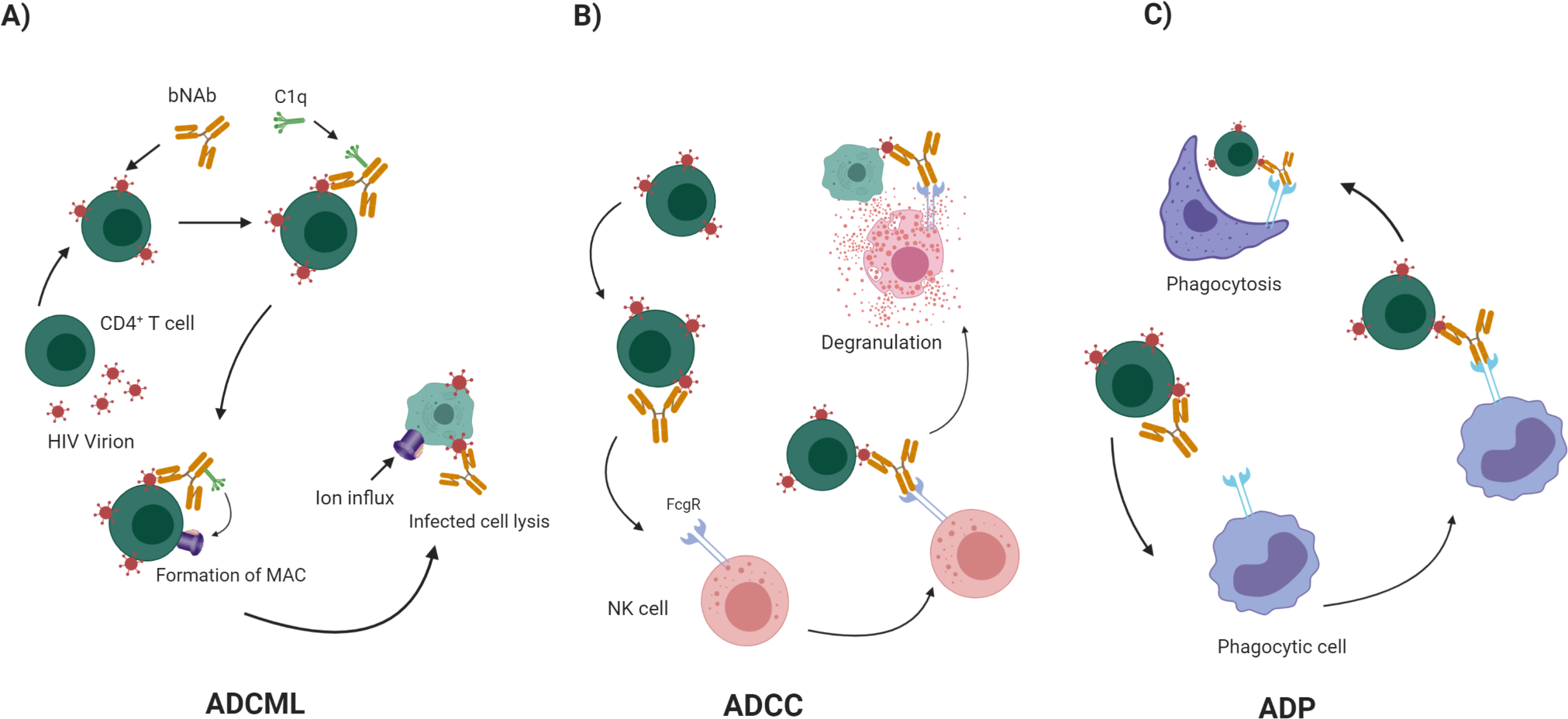
bNAbs initiate several effector functions through their Fc domain. A) Attachment of C1q to the Fc domain of bNAbs that have bound to HIV infected cells initiates the complement cascade, which leads to formation of MAC and lysis of the infected cells by ADCML. B) bNAbs bind to the Env glycoprotein on the surface of HIV-infected CD4+ T cells. NK cells recognize infected cells and bind to Fc domain of the Ab through their FcγR. This binding allows their activation and degranulation, which leads to ADCC killing of the infected cells. B) Professional phagocytes express a diverse set of FcγRs on their surfaces that can bind to bNAb-coated infected cells, deriving them to eliminate infected cells by ADP.
Antibody-dependent cellular cytotoxicity (ADCC)
NK cells bind to antibody-coated infected cells by their Fc receptors and induce them to undergo apoptosis, or other mechanisms of death, in a process called ADCC (Figure 1B). Disease progression has been reported to be slower in HIV-infected individuals that exhibit potent ADCC activity (23–28). NK cells use FcγRIIIA (CD16), a low affinity receptor, for binding to clustered IgG molecules on the surface of coated cells. Upon binding, NK cells release perforin and granzymes that trigger the target cell to die (13, 25–27, 29). There are thus two critical, and separable components of Ab function that determine ADCC potency: i) degree of binding to infected cells – determined by the Fab Ab region, and ii) degree of binding to CD16 on NK cells – determined by the Fc Ab region.
With respect to Fab activity, the relationship between the ability of an Ab to neutralize virus, and its ability to bind to infected cells correlate only in a non-reciprocal way. That is, Abs that exhibit potent virus neutralization generally are also effective at binding to infected cells while, on the other hand, it is common to identify antibodies that are able to bind to infected cells but cannot neutralize virus. This discrepancy arises from the fact that neutralization of a viral particle specifically requires binding to the functional viral trimers (or “spikes”) that mediate binding to the CD4 receptor and CCR5 or CXCR4 co-receptor, and subsequent fusion to infected cells. While such functional trimers will be present at some level on infected cells, these cells will also be decorated by a variety of non-functional forms of Envelope, including gp41 ‘stumps’, gp120/gp41 monomers, and other species – which commonly expose epitopes that are not present on functional trimers. Antibodies that target such non-functional forms of Envelope can therefore bind to infected cells, and target these cells for ADCC, but do not neutralize viral particles. When Env is in open conformation, antibodies against conserved CD4-induced (CD4i) epitopes can be generated. These anti-CD4i Abs have shown to be strong inducers of ADCC (29–32), however both the Vpu and Nef proteins of HIV have been shown to attenuate ADCC by anti-CD4i Abs (24, 33, 34).
While it is therefore possible to use non-neutralizing antibodies in therapeutic strategies aimed at inducing ADCC, there are three main advantages for utilizing bNAbs. First, there is extensive clinical experience with several bNAbs from passive infusion studies. Second, there are advantages to harnessing neutralizing activity alongside ADCC in an antibody therapeutic. Third, the neutralizing activity against a broad array of viral isolates that defines a bNAb is likely to translate into equally broad ADCC activity. Supporting this, we recently demonstrated, using a panel of clade B virus reactivated from latent reservoirs, that the ability to neutralize a given virus generally correlated with the ability to bind to corresponding infected cells – a critical pre-requisite for ADCC (35*).
Several other studies have assessed and compared ADCC activity across different bNAbs. Bruel et al investigated the CD16 signaling and the killing activity of NK cells, using a panel of ten bNAbs and found that the most active bNAbs were those that induced the strongest CD16 stimulation (36). NIH45–46 and 3BNC117 antibodies, which target the CD4bs, anti-glycan/V3 antibodies 10–1074 and PGT121, and 10E8 anti-gp41 antibody induced strong ADCC, while anti-glycan/V3 antibody PG16 and anti-CD4bs VRC01 were less active (36). In contrast, the anti-CD4bs antibody 12A12, anti-gp120/gp41 interface antibody 8ANC195, and anti-MPER antibody 4E10 were not observed to induce ADCC (36).
For bNAbs that bind equivalently to infected cells, the second factor that will determine ADCC potency is the degree to which these bind to CD16 on NK cells. Of the subclasses of IgG, the affinity for CD16 is highest for IgG3 - followed by IgG1, IgG4 and IgG2. Fc glycosylation variations, and polymorphic differences between individuals, can also affect the binding strength of IgG molecules to CD16 (37*). It has been shown that afucosylated IgG1 at asparagine (N)-297 position increased the binding affinity of IgG1 to CD16 on NK cells by 50 folds, which led to enhanced ADCC activity (38). Fucosylation of N-297 has been suggested to limit the conformational flexibility of IgG1 through its steric interaction with N162 on CD16, as truncation of N162 reverses the decreased binding affinity (39). A number of mutations have been discovered which enhance ADCC activity by augmenting CD16 binding, as reviewed below.
Antibody-dependent phagocytosis (ADP)
Granulocytes, monocytes, and macrophages are professional phagocytes express which express a diverse set of FcγRs Fc receptors on their surfaces, which mediate elimination of infected cells through ADP (Figure 1C) (40). Neutrophils are abundant in the circulation and rapidly migrate to sites of infection and perform effector functions (41). However, they have mainly been considered as anti-bacterial and anti-fungal immune cells, and their protective role in HIV infection is not well characterized (42). Saitoh et al reported that neutrophil extracellular trap (NETs) capture HIV by first detecting virus via TLR7 and TLR8, and then eliminating the virus through myeloperoxidase and α-defensin, but did not study the effect of anti-HIV antibodies and the roles of Fc receptors (43). Sips et al reported evidence supporting that in certain mucosal sites, ADP may be the dominant mode by which Abs engage effector cells. Contrary to low expression of CD16 on NK cells and their weak engagement in ADCC, macrophages and neutrophils expressed high levels of CD16 and CD32 on their surface and showed effective phagocytic clearance of immune complexes. Fc-engineered variants of the bNAb VRC01 bearing each the following sets of mutations all enhanced macrophage phagocytosis: S267E/H268F/S324T (SEHFST), S239D/I332E/G236A (SDIEGA), S239D/I332E (SDIE) and S239D/I332E/A330L (SDIEAL), with all except SEHFST increasing neutrophil phagocytosis as well (14). In addition to FcγRs, mucosal macrophages and monocytes express FcαR that may be involved in IgA2-mediated phagocytosis (14). The length of the hinge between the Fab and Fc may play an important role in increasing the magnitude of ADP (44–46). Chu et al used THP-1 monocytic cell line and constructed hinge variants of HIV-specific antibody subclasses. They found that native IgG3 subclasses of VRC01 (anti-CD4bs) and 447–52D (anti-loop 3) bNAbs had higher phagocytic activity compared to their corresponding native IgG1 subclasses. They also showed that IgG1, and IgG3 variants of both bNAbs with extended hinges elicited stronger phagocytic activity compared to their native forms (47*). Although there are many studies about the biology of macrophages in response to HIV, details of their roles in ADP clearance of HIV are not well known. The interaction of granulocytes with HIV, including their potential to reduce HIV reservoirs through ADP (ex. mediated by bNAbs) also requires further study.
HIV bNAb isotypes and subclasses
The majority of bNAbs which have been isolated thus far possess the IgG isotype Fc domain, and many of these are of the IgG1 subclass, however this may not be optimal for the engagement of effector functionalities. Richardson et al demonstrated that an IgG3 bNAb that is specific for V2 loop of the Envelope (VRC026), mediated enhanced ADCC and neutralization potency compared to the corresponding IgG1 variant (48). Of note, the in vivo half-life of IgG3 is shorter than IgG1, which might necessitate shorter intervals between administration in a therapeutic setting. Hypothetically, the elicitation or infusion of bNAbs in a prophylactic setting may benefit from a combination of anti-HIV IgG and IgA isotypes, to achieve protective mucosal immunity (49). IgG has multiple advantages in terms of engaging effector functions: it is the main isotype involved in complement fixation and thus a potent driver of ADCML virions (17), IgG is also a potent inducer of ADCC by NK cells to induce cell death in infected cells, as well as of engulfment of infected cells through ADP by monocytes, macrophages and neutrophils (49–51). IgA, however, is the dominant isotype in mucosal secretions, and the mucosa is the natural site of most HIV transmissions. Thus, IgA may be more effective in blocking transcytosis of HIV, preventing the infection of CD4+ T cells at mucosal surfaces (52–56), and protecting DCs from infection, which is of great importance as well (57, 58), whereas IgG may complement these functions by enabling clearance of cells that do become infected. In two studies, anti-gp41 IgA protected HIV transmission in IgG seronegative individuals that were highly exposed to HIV (56–59). Tudor et al have investigated the role of CH1 domain by constructing a 2F5 IgA2 bNAb and comparing it with 2F5 IgG1. They demonstrated that 2F5 IgA2 bound to gp41 with a higher affinity and blocked CCR5-tropic HIV transcytosis across epithelial cells more efficiently (60). It is interesting to note that the dominant subtype of IgA in mucosal tissues is IgA2, whereas IgA1 is more prevalent in the periphery (46, 60), where its role in HIV-inhibition is not well studied. A distinct potential advantage of IgA1 with respect to harnessing bNAbs for HIV cure, is its ability to engage the IgA1 receptor, FcαRI (CD89) which is expressed on monocytes, macrophages and granulocytes (61), and can drive potent elimination of HIV infected cells by ADP (62). Thus, whereas all antibodies currently in the clinic or in clinical trials – for any indication, ex. rituximab for lymphoma – have been IgG, exciting opportunities exist to explore alternative isotypes of bNAbs.
While bNAbs can hypothetically be produced as a variety of different Ig isotypes and subclasses, with the goal of leveraging the various advantages of each – either alone, or in combinations – the possibility for negative synergies should also be considered. This can be illustrated using the RV144 HIV vaccine trial, in which both: i) IgG antibodies that bound to the variable regions 1 and 2 of Envelope and ii) ADCC activity correlated inversely with the rate of infection amongst vaccines, whereas levels of binding of plasma IgA antibodies to these same sites correlated directly with the rate of infection (63, 64). This has been suggested to indicate that ADCC mediated through IgG may have offered protection, which was inhibited by competition with IgA – an effect that could be observed in vitro (65). In moving forwards to explore alternative isotypes of bNAbs, investigators should be mindful for the potential for such competitive interactions.
Engineered Neutralizing antibodies
The density of HIV Envelope on the surfaces of virions and infected cells is sufficiently low that two such molecules are rarely in close enough proximity to be recognized by the Fabs of a single antibody, potentially limiting ADCC (66, 67). To overcome this limitation, Ramadoss et al constructed a bispecific bNAb antibody, using VCR01 as the backbone that had a high affinity single chain variable fragment (scFv) to target CD16 on NK cells. The affinity of this recombinant bNAb to gp41 was equal to original VCR01, and its affinity to CD16 was increased. This recombinant bNAb bound to infected primary CD4+ T cells and boosted NK cell killing activity (68*). The scarcity of Envelope glycoprotein may also be addressed by making bi- or tri-specific antibodies, which can increase both avidity and the neutralization breath (69). It has been suggested that increased avidity of multivalent bNAbs also enhances other functionalities such as ADCML, ADCC, and ADP, but this needs to be investigated in more detail (10, 70, 71). Bourzanos et al generated Fc domain variants of 3BNC117, 10–1074 and PG16 IgG1 bNAbs including G236A/S239D/A330L/I332E (GASDALIE) and G236R/L328R (GRLR) and compared these with wild type bNAbs. While no differences in binding to gp120 and neutralization of viruses were observed, GASDALIE versions of these bNAbs showed increased binding to FcγRs and enhanced antiviral activity in vivo, while binding was abrogated for GRLR variants (72). Next, they developed a bi-specific anti-Envelope bNAbs of IgG1 subclass and generated a modified version of this antibody as well. A hinge domain of IgG3 was added to the Fc domain of the modified version to increase Fab domain flexibility. They observed increased neutralization activity of the modified version compared to the parental Ab in vitro and in HIV infected humanized mice (73). Improving antibody half-life is another aspect of bNAb engineering, as LS mutant (M428L and N434S) of VRC01 showed enhanced binding to neonatal Fc receptor (FcRn), which led to increased half-life, but did not affect its ADCC activity in a cynomolgus macaque model of SHIV (74). The LS mutant of VRC01 was shown to be safe in a phase one clinical trial, and exhibited a 4-fold longer half-life than the parental Ab (75). As FcRn-enhancing mutations may decrease ADCC activity, one study investigated the combinational effect of QL, LS, A, AAA and YTE mutations that increase FcRn binding, with DE and DLE mutations that increase ADCC. While the gp120 binding affinities of all combinations were similar to the original VRC01, only DE-LS and DLE-LS mutants enhanced both epithelial transcytosis as an indication of half-life, and ADCC in vitro (76). Recently, Kerwin et al replaced potential destabilizing residues in the variable region of 10–1074 IgG1 bNAb. They replaced the heavy chain residue T108 with R108 at the base of the CDR3 loop which allowed for the formation of a nascent salt bridge with heavy chain residue D137. They also induced three additional mutations to increase conformational stability (77). We also have shown in a novel humanized mouse model that Fc domain of 10–1074 antibody was essential for viral control – where treatment of these mice with 10–1074 bNAb significantly reduced the viral load compared to 10–1074-FcRnull (78*). As is evident in the above, there is tremendous potential to employ protein engineering approaches to enhance multiple aspects of bNAbs in order to fully leverage the potential of these as therapeutics.
Vaccinal effect of bNAbs
In the use of tumor-targeting Abs to treat cancer, there have been multiple reports of anti-tumor activity has persisted after Abs have cleared. This has led to the postulation of a ‘vaccinal effect’ whereby transient treatment with these Abs is thought to have primed adaptive immune responses (79, 80). How might such a vaccinal effect arise, and could this be of benefit in the HIV setting?
Immature DCs sense pathogens through pattern recognition receptors and initiate a proinflammatory immune response by secretion of cytokines. They develop to mature DCs after the phagocytosis of pathogens and become professional antigen presenting cells upon migration to secondary lymphoid tissues (81). DCs can uptake HIV virions and infect CD4+ T cells through infectious synapse, and therefore contribute to pathogenesis of HIV. Interestingly, some bNAbs like 10–1074 and PGT121 have been shown to accumulate at virological synapses and block the transfer of viral material to uninfected T cells (82). However, the main role of DCs is to prime both cellular and humoral immunity. The use of new generation of bNAbs that show increased neutralization breath, and the presence of FcγRs on DCs, may contribute to efficient uptake of neutralized virus that is not infectious for DCs anymore. This may allow for the development of a similar vaccinal effect that has been proposed in the cancer field, which needs to be investigated in detail. It has been shown that treatment of SHIV-infected cynomolgus macaques with 3BNC117 and 10–1074 bNAbs for two weeks led to undetectable viral loads that were maintained for two to six months. Almost half of the animals behaved as controllers with undetectable viral loads, and the rest maintained a very low level of viremia for two years. Infusion of an anti-CD8β depleting antibody to controller animals resulted in specific decline of CD8+ T cells and subsequent viral rebound, suggesting the existence of a vaccinal effect by these two potent bNAbs (83**). In line with this study, HIV-infected individuals with undetectable viral load were treated with 3BNC117 and 10–1074 bNAbs after analytical antiretroviral (ART) interruption (ATI). All participants developed enhanced Gag-specific CD8+ T cell responses, and eight of nine participants had increased specific CD4+ T cell responses (84**). The mechanism of generation of the adjuvant effect of these bNAbs at the presence of natural antigen and its effectiveness in long term control of HIV infection needs be investigated in more details, including the consideration of whether this can be modulated through the use of alternative Fc domains.
Conclusion
The absence of an effective vaccine has made bNAbs highly appealing in the field of HIV, as these antibodies can be used prophylactically to prevent infection. Several studies indicate that bnAbs may be effective as a prophylactic treatment but there is not yet a licensed product to this effect. The effect of a single dose of these antibodies can last for several months, which makes them a potentially attractive alternative to daily ART. bNAbs also hold potential to contribute to curing HIV infection, or achieving ART-free remission, as a result of targeting various effector cells to eliminate reservoir-harboring cells - an outcome that depends on the binding activities of both the Fab and Fc domains. New advances have allowed for the modification of Fab to generate multivalent antibodies, and the modification of Fc domains to substantially increase the binding of these antibodies to their corresponding Fc receptors. The use of one highly potent bNAb alone, will lead to temporary viral control and then viral escape mutation. Similar to the excellent effectiveness of combinational ART, the use of multiple engineered antibodies might be a new path towards the eradication of the HIV reservoir or durable suppression of viral replication.
Key points.
The role of bNAbs in HIV reservoir control is not limited to neutralization as their Fc domains are involved in diverse effector functionalities.
The use of alternative isotypes, or engineered Fc domains can enhance multiple functional aspects of bNAbs toward their potential application as prophylactic or therapeutic agents.
The potential for passive administration of bNAbs to enhance virus-specific T-cell responses through a vaccinal effect holds promise as a strategy to achieve HIV remission.
Acknowledgements:
This work was supported by the NIH funded R33 grant AI122391 (to RBJ). It was also supported in part by the NIH funded R01 grants AI31798 and AI147845 (RBJ) and the Martin Delaney ‘BELIEVE’ Collaboratory (NIH grant 1UM1AI26617), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, and OAR. We gratefully acknowledge the contributions of the study participants, without whom the work reviewed here would not be possible. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest:
The authors report that no conflicts of interest exist.
References and Recommended Readings
Papers of interest have been highlighted as:
* of special interest
** of outstanding interest
- 1.Aasa-Chapman MM, Hayman A, Newton P, Cornforth D, Williams I, Borrow P, et al. Development of the antibody response in acute HIV-1 infection. AIDS. 2004;18(3):371–81. [DOI] [PubMed] [Google Scholar]
- 2.Pellegrin I, Legrand E, Neau D, Bonot P, Masquelier B, Pellegrin JL, et al. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1996;11(5):438–47. [DOI] [PubMed] [Google Scholar]
- 3.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):4144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Dietrich U. The role of neutralizing antibodies in HIV infection. AIDS reviews. 2006;8(2):51–9. [PubMed] [Google Scholar]
- 5.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS pathogens. 2009;5(5):e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.**.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nature medicine. 2017;23(2):185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows for the first time that passive transfer of 10–1074 bNAb transiently controls HIV infection in humans.
- 10.Bournazos S, Ravetch JV. Anti-retroviral antibody FcgammaR-mediated effector functions. Immunological reviews. 2017;275(1):285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillay Y, Moodley J, Naicker T. The role of the complement system in HIV infection and preeclampsia. Inflammation research : official journal of the European Histamine Research Society [et al. ]. 2019;68(6):459–69. [DOI] [PubMed] [Google Scholar]
- 12.Su B, Dispinseri S, Iannone V, Zhang T, Wu H, Carapito R, et al. Update on Fc-Mediated Antibody Functions Against HIV-1 Beyond Neutralization. Frontiers in immunology. 2019;10:2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mielke D, Bandawe G, Pollara J, Abrahams MR, Nyanhete T, Moore PL, et al. Antibody-Dependent Cellular Cytotoxicity (ADCC)-Mediating Antibodies Constrain Neutralizing Antibody Escape Pathway. Frontiers in immunology. 2019;10:2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sips M, Krykbaeva M, Diefenbach TJ, Ghebremichael M, Bowman BA, Dugast AS, et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal immunology. 2016;9(6):1584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayilyan KR. Complement genetics, deficiencies, and disease associations. Protein & cell. 2012;3(7):487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valenzuela NM, Schaub S. The Biology of IgG Subclasses and Their Clinical Relevance to Transplantation. Transplantation. 2018;102(1S Suppl 1):S7–S13. [DOI] [PubMed] [Google Scholar]
- 17.Mujib S, Liu J, Rahman A, Schwartz JA, Bonner P, Yue FY, et al. Comprehensive Cross-Clade Characterization of Antibody-Mediated Recognition, Complement-Mediated Lysis, and Cell-Mediated Cytotoxicity of HIV-1 Envelope-Specific Antibodies toward Eradication of the HIV-1 Reservoir. Journal of virology. 2017;91(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelderman KA, Blok VT, Fleuren GJ, Gorter A. The inhibitory effect of CD46, CD55, and CD59 on complement activation after immunotherapeutic treatment of cervical carcinoma cells with monoclonal antibodies or bispecific monoclonal antibodies. Laboratory investigation; a journal of technical methods and pathology. 2002;82(4):483–93. [DOI] [PubMed] [Google Scholar]
- 19.Mamidi S, Cinci M, Hasmann M, Fehring V, Kirschfink M. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Molecular oncology. 2013;7(3):580–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saifuddin M, Hedayati T, Atkinson JP, Holguin MH, Parker CJ, Spear GT. Human immunodeficiency virus type 1 incorporates both glycosyl phosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. The Journal of general virology. 1997;78 (Pt 8):1907–11. [DOI] [PubMed] [Google Scholar]
- 21.Yang K, Lan J, Shepherd N, Hu N, Xing Y, Byrd D, et al. Blockage of CD59 Function Restores Activities of Neutralizing and Nonneutralizing Antibodies in Triggering Antibody-Dependent Complement-Mediated Lysis of HIV-1 Virions and Provirus-Activated Latently Infected Cells. Journal of virology. 2015;89(18):9393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu W, Yu Q, Hu N, Byrd D, Amet T, Shikuma C, et al. A high-affinity inhibitor of human CD59 enhances complement-mediated virolysis of HIV-1: implications for treatment of HIV-1/AIDS. J Immunol. 2010;184(1):359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349(6245):320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Euler Z, Alter G. Exploring the potential of monoclonal antibody therapeutics for HIV-1 eradication. AIDS research and human retroviruses. 2015;31(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramski M, Stratov I, Kent SJ. The role of HIV-specific antibody-dependent cellular cytotoxicity in HIV prevention and the influence of the HIV-1 Vpu protein. AIDS. 2015;29(2):137–44. [DOI] [PubMed] [Google Scholar]
- 26.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23(8):897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee WS, Parsons MS, Kent SJ, Lichtfuss M. Can HIV-1-Specific ADCC Assist the Clearance of Reactivated Latently Infected Cells? Frontiers in immunology. 2015;6:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko SY, et al. Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. Journal of virology. 2012;86(16):8672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard J, Prevost J, Baxter AE, von Bredow B, Ding S, Medjahed H, et al. Uninfected Bystander Cells Impact the Measurement of HIV-Specific Antibody-Dependent Cellular Cytotoxicity Responses. mBio. 2018;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, et al. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(1):E69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veillette M, Coutu M, Richard J, Batraville LA, Dagher O, Bernard N, et al. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. Journal of virology. 2015;89(1):545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veillette M, Desormeaux A, Medjahed H, Gharsallah NE, Coutu M, Baalwa J, et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. Journal of virology. 2014;88(5):2633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, et al. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. Journal of virology. 2014;88(11):6031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, et al. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(17):6425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.*.Ren Y, Korom M, Truong R, Chan D, Huang SH, Kovacs CC, et al. Susceptibility to Neutralization by Broadly Neutralizing Antibodies Generally Correlates with Infected Cell Binding for a Panel of Clade B HIV Reactivated from Latent Reservoirs. Journal of virology. 2018;92(23). [DOI] [PMC free article] [PubMed] [Google Scholar]; In this paper we show that neutralization of a given virus generally correlated with the ability to bind to corresponding infected cells, which is a critical pre-requisite for ADCC.
- 36.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nature communications. 2016;7:10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.*.de Taeye SW, Bentlage AEH, Mebius MM, Meesters JI, Lissenberg-Thunnissen S, Falck D, et al. FcgammaR Binding and ADCC Activity of Human IgG Allotypes. Frontiers in immunology. 2020;11:740. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the effcets of Fc glycosylation variations and polymorphic differences on binding strength of IgG molecules to CD16. These variations can decrease or increase bNAb functions such as ADCC.
- 38.Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. The Journal of biological chemistry. 2002;277(30):26733–40. [DOI] [PubMed] [Google Scholar]
- 39.Falconer DJ, Subedi GP, Marcella AM, Barb AW. Antibody Fucosylation Lowers the FcgammaRIIIa/CD16a Affinity by Limiting the Conformations Sampled by the N162-Glycan. ACS chemical biology. 2018;13(8):2179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual review of immunology. 1999;17:593–623. [DOI] [PubMed] [Google Scholar]
- 41.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews Immunology. 2013;13(3):159–75. [DOI] [PubMed] [Google Scholar]
- 42.Mocsai A Diverse novel functions of neutrophils in immunity, inflammation, and beyond. The Journal of experimental medicine. 2013;210(7):1283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell host & microbe. 2012;12(1):109–16. [DOI] [PubMed] [Google Scholar]
- 44.Boesch AW, Kappel JH, Mahan AE, Chu TH, Crowley AR, Osei-Owusu NY, et al. Enrichment of high affinity subclasses and glycoforms from serum-derived IgG using FcgammaRs as affinity ligands. Biotechnology and bioengineering. 2018;115(5):1265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musich T, Li L, Liu L, Zolla-Pazner S, Robert-Guroff M, Gorny MK. Monoclonal Antibodies Specific for the V2, V3, CD4-Binding Site, and gp41 of HIV-1 Mediate Phagocytosis in a Dose-Dependent Manner. Journal of virology. 2017;91(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tay MZ, Liu P, Williams LD, McRaven MD, Sawant S, Gurley TC, et al. Antibody-Mediated Internalization of Infectious HIV-1 Virions Differs among Antibody Isotypes and Subclasses. PLoS pathogens. 2016;12(8):e1005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.*.Chu TH, Crowley AR, Backes I, Chang C, Tay M, Broge T, et al. Hinge length contributes to the phagocytic activity of HIV-specific IgG1 and IgG3 antibodies. PLoS pathogens. 2020;16(2):e1008083. [DOI] [PMC free article] [PubMed] [Google Scholar]; Compared to ADDC, the role of Ab hinge is less studied in ADP. The authors found that native IgG3 subclasses of VRC01 and 447–52D bNAbs had higher phagocytic activity than that of native IgG1 subclasses. They also showed that variants of both subclasses of these Abs with extended hinge had stronger phagocytic activity.
- 48.Richardson SI, Lambson BE, Crowley AR, Bashirova A, Scheepers C, Garrett N, et al. IgG3 enhances neutralization potency and Fc effector function of an HIV V2-specific broadly neutralizing antibody. PLoS pathogens. 2019;15(12):e1008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34(2):269–80. [DOI] [PubMed] [Google Scholar]
- 50.Klein JS, Webster A, Gnanapragasam PN, Galimidi RP, Bjorkman PJ. A dimeric form of the HIV-1 antibody 2G12 elicits potent antibody-dependent cellular cytotoxicity. AIDS. 2010;24(11):1633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tudor D, Bomsel M. The broadly neutralizing HIV-1 IgG 2F5 elicits gp41-specific antibody-dependent cell cytotoxicity in a FcgammaRI-dependent manner. AIDS. 2011;25(6):751–9. [DOI] [PubMed] [Google Scholar]
- 52.Alfsen A, Iniguez P, Bouguyon E, Bomsel M. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J Immunol. 2001;166(10):6257–65. [DOI] [PubMed] [Google Scholar]
- 53.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9(2):277–87. [DOI] [PubMed] [Google Scholar]
- 54.Devito C, Hinkula J, Kaul R, Kimani J, Kiama P, Lopalco L, et al. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J Acquir Immune Defic Syndr. 2002;30(4):413–20. [DOI] [PubMed] [Google Scholar]
- 55.Miyazawa M, Lopalco L, Mazzotta F, Lo Caputo S, Veas F, Clerici M. The ‘immunologic advantage’ of HIV-exposed seronegative individuals. AIDS. 2009;23(2):161–75. [DOI] [PubMed] [Google Scholar]
- 56.Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, et al. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal immunology. 2009;2(5):412–26. [DOI] [PubMed] [Google Scholar]
- 57.Ganesh L, Leung K, Lore K, Levin R, Panet A, Schwartz O, et al. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. Journal of virology. 2004;78(21):11980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magerus-Chatinet A, Yu H, Garcia S, Ducloux E, Terris B, Bomsel M. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology. 2007;362(1):67–74. [DOI] [PubMed] [Google Scholar]
- 59.Mazzoli S, Trabattoni D, Lo Caputo S, Piconi S, Ble C, Meacci F, et al. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nature medicine. 1997;3(11):1250–7. [DOI] [PubMed] [Google Scholar]
- 60.Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, et al. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(31):12680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brandsma AM, Bondza S, Evers M, Koutstaal R, Nederend M, Jansen JHM, et al. Potent Fc Receptor Signaling by IgA Leads to Superior Killing of Cancer Cells by Neutrophils Compared to IgG. Frontiers in immunology. 2019;10:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wills S, Hwang KK, Liu P, Dennison SM, Tay MZ, Shen X, et al. HIV-1-Specific IgA Monoclonal Antibodies from an HIV-1 Vaccinee Mediate Galactosylceramide Blocking and Phagocytosis. Journal of virology. 2018;92(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, et al. Dissecting Polyclonal Vaccine-Induced Humoral Immunity against HIV Using Systems Serology. Cell. 2015;163(4):988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 2012;366(14):1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):9019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu P, Chertova E, Bess J Jr., Lifson JD, Arthur LO, Liu J, et al. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu P, Liu J, Bess J Jr., Chertova E, Lifson JD, Grise H, et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441(7095):847–52. [DOI] [PubMed] [Google Scholar]
- 68.*.Ramadoss NS, Zhao NQ, Richardson BA, Grant PM, Kim PS, Blish CA. Enhancing natural killer cell function with gp41-targeting bispecific antibodies to combat HIV infection. AIDS. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors in this work show that VRC01 bispecific bNAb with specificity for CD16 had similar affinity to gp41 compared to original VRC01, and its affinity to CD16 was increased, which enhanced ADCC activity of NK cells and subsequent killing of infected primary CD4+ T cells.
- 69.Karuna ST, Corey L. Broadly Neutralizing Antibodies for HIV Prevention. Annual review of medicine. 2020;71:329–46. [DOI] [PubMed] [Google Scholar]
- 70.Hua CK, Ackerman ME. Increasing the Clinical Potential and Applications of Anti-HIV Antibodies. Frontiers in immunology. 2017;8:1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lambour J, Naranjo-Gomez M, Piechaczyk M, Pelegrin M. Converting monoclonal antibody-based immunotherapies from passive to active: bringing immune complexes into play. Emerging microbes & infections. 2016;5(8):e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. Bispecific Anti-HIV-1 Antibodies with Enhanced Breadth and Potency. Cell. 2016;165(7):1609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS medicine. 2018;15(1):e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sung-Youl Ko TK, Richard Blumberg, Gary Nabel. Modification of VRC01 for enhancing half-life and ADCC activity. The Journal of Immunology. 2011;186(1):155. [Google Scholar]
- 77.Kerwin BA, Bennett C, Brodsky Y, Clark R, Floyd JA, Gillespie A, et al. Framework Mutations of the 10–1074 bnAb Increase Conformational Stability, Manufacturability, and Stability While Preserving Full Neutralization Activity. Journal of pharmaceutical sciences. 2020;109(1):233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.*.Flerin NC, Bardhi A, Zheng JH, Korom M, Folkvord J, Kovacs C, et al. Establishment of a Novel Humanized Mouse Model To Investigate In Vivo Activation and Depletion of Patient-Derived HIV Latent Reservoirs. Journal of virology. 2019;93(6). [DOI] [PMC free article] [PubMed] [Google Scholar]; In this work we show that Fc domain of 10–1074 antibody was essential for viral control in a novel humanized mouse model as was compared to 10–1074-FcRnull.
- 79.Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104(9):2635–42. [DOI] [PubMed] [Google Scholar]
- 80.DiLillo DJ, Ravetch JV. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell. 2015;161(5):1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Veglia F, Gabrilovich DI. Dendritic cells in cancer: the role revisited. Current opinion in immunology. 2017;45:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. The Journal of experimental medicine. 2013;210(13):2813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.**.Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017;543(7646):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]; The vaccinal effect of two potent bNAbs (3BNC117 and 10–1074) was studied in a non-human primate model of HIV infection (SHIV-infected cynomolgus macaques). transient treatment of animals with these bNAbs led to relatively long-term control of viremia that was mediated by specific CD8+ T cells. This study paved the way for evaluation of the vaccinal effect of these bNAbs in HIV-infected individuals by Niessl et al.
- 84.**.Niessl J, Baxter AE, Mendoza P, Jankovic M, Cohen YZ, Butler AL, et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nature medicine. 2020;26(2):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; For the first time in this study, the vaccinal effect of transient bNAb therapy was explored in HIV-infected inindividuls that were subject to ATI. participants received 3BNC117 and 10–1074 bNAbs and developed enhanced Gag-specific CD8+ T cell and specific CD4+ T cell responses. this study shows the probable role of bNAbs as an adjuvant for natural antigen to behave as a vaccine.


