Abstract
Accumulating evidence suggests that the use of cannabis and nicotine and tobacco-related products (NTPs) during the adolescent years has harmful effects on the developing brain. Yet, few studies have focused on the developing brain as it relates to the co-administration of cannabis and NTPs, despite the high prevalence rates of co-use in adolescence. The review aimed to synthesize the existing literature on the neurocognitive, structural, and functional outcomes associated with cannabis and NTP co-use. A systematic search of peer-reviewed articles resulted in a pool of 1,107 articles. Inclusion criteria were: 1) data-based, 2) age range of 13-35 or, for preclinical studies, non-adult subjects, 3) cannabis and NTP group jointly considered, and 4) neurocognitive, structural neuroimaging, or functional neuroimaging as an outcome measure. A total of 12 studies met inclusion criteria. Consistent with the literature, cannabis and nicotine were found to have independent effects on cognition. The available research on the co-use of cannabis and NTPs demonstrates a potential nicotine-related masking effect on cognitive deficits associated with cannabis use, yet there is little research on co-use and associations with neuroimaging indices. Of the neuroimaging studies, there is preliminary evidence for hippocampal volume differences in co-users and a lack of evidence for co-use differences related to nucleus accumbens activity during reward processing. Notably, no structural neuroimaging studies were found to examine the combined effects of nicotine and cannabis in adolescent-only populations. Further research, including longitudinal studies, is warranted to investigate the influence of cannabis and NTP co-use on maturation.
Keywords: Adolescent, Cannabis use, Nicotine use, Brain development, Cognition, Neuroimaging
INTRODUCTION
Cannabis and nicotine and tobacco products (NTPs) are two of the most commonly used substances among adolescents in the United States, second to alcohol (1). In 2019, the lifetime prevalence rates for cannabis and tobacco cigarettes in 12th graders were 44% and 24%, respectively (2). Understanding the effects of adolescent substance use on brain physiology, neurocognition, and human behavior is an ongoing, multifaceted, and broad research area with significant clinical and practical implications. Cannabis use during adolescence may affect brain development and result in impaired cognition, including altered gray and white matter tissue integrity and functional brain activation patterns (3–23). Similarly, evidence suggests that NTPs, particularly at a younger age, may have neurotoxic effects on the developing brain (24–35). The relationships between substance use and lasting brain effects are likely complex, and patterns of use (e.g., frequency, age of onset, use of multiple substances) and other shared biological and psychosocial risk factors (e.g., genetics, education, socioeconomic status) may account for and/or interact with cannabis and/or NTPs use to contribute to neurobehavioral outcomes (36, 37).
The interactive relationship between cannabis and NTPs remains understudied despite substantial co-use prevalence rates (18-52%) in young adult tobacco users (38–41) and biochemical interactions between delta-9-tetrahydrocannabinol (THC), the principal psychoactive component of cannabis, and nicotine (42). THC exerts effects by binding to endocannabinoid receptors such as cannabinoid receptor 1 (CB1) in the brain (43). Regarding nicotine, the psychoactive effects occur through activation of the nicotinic acetylcholine receptors (nAChRs) (44). Overlap of CB1 receptors and nAChRs in cortico-limbic brain reward regions and growing preclinical evidence of bidirectional crosstalk between these two systems suggests the endocannabinoid system may modulate the cholinergic system and the highly rewarding and reinforcing effects of nicotine (42).
Many reviews have examined the independent effect of cannabis and NTP use on the developing brain (13, 36, 44–49), and one systematic review has examined the correlates, consequences, interventions, and use patterns of nicotine and cannabis co-use (50). However, no known reviews have focused on their interactive effects in regard to neurobiological outcomes nor taken into account preclinical studies. Thus, this brief overview aims to synthesize the existing literature on cannabis and nicotine co-use, as it relates to neurodevelopment in the domains of neurocognition and structural and functional neural development. We systematically review and describe the literature on animal and human adolescent and young adult studies (ages 13-35) that have utilized neurocognitive testing and structural and functional magnetic resonance imaging (fMRI) modalities (e.g., blood-oxygen-level-dependent (BOLD) signal, cerebral blood flow) to probe the impact of co-use of cannabis and NTPs on development. The findings presented in this review will mold future questions and aid in better understanding the complex relationship between cannabis and nicotine co-use and the developing brain.
METHOD
A systematic review was carried out following the recommendations of The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (see Figure 1) (51). All studies with a scientific aim of examining both cannabis and nicotine use on neurocognitive, structural neuroimaging, or functional neuroimaging outcome measures were deemed eligible based on the selection criteria detailed in the legend of Figure 1. Data extraction from each study included participant demographics (age, sex), sample size, group conditions, abstinence period before testing, covariates, measures used, and co-use results (see Table 1). The primary results of interest for the qualitative synthesis of cannabis and nicotine co-use were findings associated with the neurocognitive and neuroimaging outcomes. All included studies were rated for risk of bias using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) scale (52, 53). Overall, most studies were rated as low (54–57) to moderate quality (58–61), with one human study (62) and the preclinical studies being high quality (63–65). Evidence for outcomes of interest (clinical cognition, preclinical cognition, structural imaging, and functional imaging) was rated as moderate, high, low, and moderate, respectively. Full details regarding the search strategy, data extraction, and risk of bias ratings are included in the online supplement.
Figure 1.
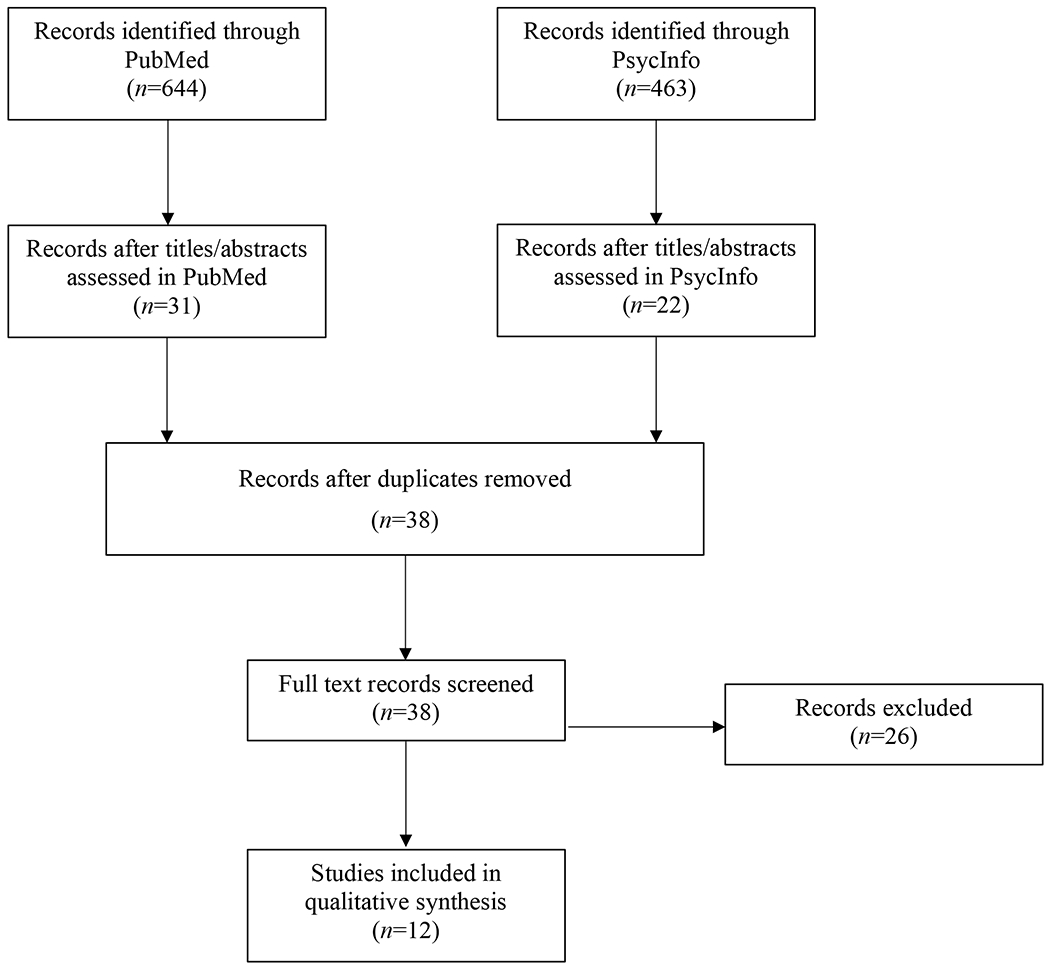
PRISMA (Preferred Reporting Items for Systematic Meta-Analyses) flow diagram of included and excluded studies of adolescent cannabis and nicotine co-use. Inclusion criteria were as follows: 1) the study had to report on neurocognitive outcomes, structural neuroimaging (e.g., MRI, diffusion tensor imaging[DTI]), or functional neuroimaging (e.g., blood-oxygen-level-dependent signal[BOLD]) finding as an outcome measure; 2) have a user group where cannabis/cannabinoids and nicotine/tobacco are jointly considered; 3) the study had to have an age range of 13-35 or, for preclinical studies, non-adult subjects; and 4) the study had to be data-based as review articles were not included. All studies with a scientific aim of examining both cannabis and nicotine use on outcomes measures were deemed eligible based on the present selection criteria, and thus there was no minimal level of substance use required for inclusion. PUBMED and PSYCINFO pulled AUGUST 27-31, 2020
Table 1.
Summary Characteristics and Findings of Studies Examining Cannabis and Nicotine Co-use in Adolescents + Young Adults
| Study | Mean Age±SD % Female | Group/Conditions | Abstinence | Measure | Covariate | Summary | |
|---|---|---|---|---|---|---|---|
| Neurocognitive | |||||||
| Episodic Memory | Jacobsen et al., 2007 (54) | 13-18 73.3% |
Cannabis: n=20 Tobacco: n=25 |
NTP: Both ad libitum and 24 hours | HVLT-R | Parental education, symptoms of depression | During abstinence period CAN users: ↓ delayed recall |
| Schuster et al., 2015 (58) | 20.81±1.82 35.9% |
Sporadic NTP: n=34 Consistent NTP users: n=30 |
NTP: None CAN: 24 hours |
HVLT-R | Race,education, BIS score, past year ALC use | In sporadic NTP users: ↑ CAN use = ↓ initial acquisition, ↓ immediate recall, ↓ delayed recall CAN users: ↓ episodic memory |
|
| Hindocha et al.,2017 (62) | 24.46±3.96 50% |
Conditions Administered: CAN-TOB CAN TOB PLACEBO n=24 | NTP, CAN, ALC, + other drugs: ≥12 hours | Rivermead Behavioral Memory Test–Prose Recall | N/A | CAN+NTP > CAN: ↑ Delayed Recall CAN users: ↓ episodic memory |
|
| Working Memory | Hindocha et al., 2017 (62) | 24.46±3.96 50% |
Conditions Administered: CAN-TOB CAN TOB PLACEBO n=24 |
NTP, CAN, ALC, + other drugs: ≥12 hours | Spatial N-BACK | N/A | No CAN+NTP findings CAN: ↓ working memory NTP: ↑ working memory |
| Schuster et al.,2016 (55) | 21.3±0.8 54% |
Variables in regression: CAN TOB TOB+CAN ALC CAN+ALC TOB+ALC n=287 |
N/A | Brief working memory task following Ecological Momentary Assessment (EMA) | Gender, GPA,Momentary Level factors (e.g., day/time, affect, substance use) | CAN+NTP = CON CAN: ↓ working memory NTP: ↑ working memory |
|
| Structural Neuroimaging | |||||||
| Hippocampal volume + Working Memory | Filbey et al., 2015 (56) | CON (26.88±6.89) NTP (29.63±11.31) CAN (24.92±8.78) CAN+NTP (23.26±7.32) |
CON: n=16 NTP: n=19 CAN: n=36 CAN+NTP: n=19 |
CAN: 3 days NTP: 3 hours |
Hippocampal volume WMS-III Logical Memory |
Age, IQ, gender, alcohol frequency, ADHD symptoms Nicotine intensity |
CAN and CAN+NTP< NTP and CON groups: right hippocampal volume CAN+NTP: ↓ hippocampal volume = ↑ memory scores |
| Functional Neuroimaging | |||||||
| Working Memory | Jacobsen et al., 2007 (54) | 13-18 73.3% |
Cannabis: n=15 Tobacco: n=18 |
NTP: Both ad libitum and 24 hours | Auditory n-back fMRI task | Parental education, symptoms of depression | During abstinence period CAN users: ↑ activity in frontocortical regions and ↓ frontoparietal connectivity during higher working memory load tasks |
| Reward Responsivity | Karoly et al., 2015 (57) | 14-18 34% |
CAN: n=14 NTP: n=34 ALC: n=12 CAN+NTP: n=17 CAN+NTP+ALC: n=17 CON: n=38 |
All substances including NTP: ≥3 hours |
Monetary incentive delay task-Reward Anticipation | N/A | No CAN+NTP findings NTP> CON, ALC, CAN+NTP, and CAN+NTP+ALC: ↓ in NAcc activation |
| NAcc Activation | Martz et al., 2016 (59) | 20.1±1.4 at time 1 36.1% |
Cross-lagged model n=109 | Self-reported 48-hour CAN abstinence before scan | Monetary incentive delay task-Reward Anticipation | Sex, age at time 1, parental history of SUD, previous CAN use, previous binge drinking, previous + past yr CIG use | No CAN+NTP findings ↑ past-year CAN use: ↓ activation in NAcc |
| Resting State Cerebral Blood Flow | Courtney et al., 2020 (60) | 16-22 33.3% |
NTP: n=37 Non-NTP: n=26 |
NTP: None CAN, ALC, + other drugs = 12 hours |
Optimized pseudo-continuous arterial spin labeling | Cannabis and nicotine recency | ↑ past-year CAN use: ↑ white matter CBF in non-NTP group only |
| Cue Reactivity | Kuhns et. al., 2020 (61) | 18-25 51.5% |
CAN: n=16 NTP: n=18 CAN+NTP: n= 14 CON: n=18 |
NTP: None CAN, ALC, + other drugs = 24 hours |
Cannabis cue reactivity task | AUDIT score | No CAN+NTP findings NTP>CAN>CAN+NTP>CON-: heightened activation in regions of interest |
| Pre-Clinical | |||||||
| Memory | Mateos et al., 2011 (63) | Postnatal day 28-43 51% |
NTP Males: n=8 NTP Females: n=8 CP Males: n=12 CP Females: n=12 NTP+CP Males: n=12 NTP+CP Females: n=12 Control Males: n=7 Control Females: n=8 |
N/A | Spontaneous alternation task, object location task, novel object test | N/A | ↑ discrimination index: CP males > control males After 1 month abstinence: ↓ discrimination index in females; no difference in males ↓ recognition in CP males; ↓ recognition in NTP females |
| Memory | Pekala et al., 2018 (64) | 2-months old 0% |
12 group combinations: stressed/unstressed; chronic nicotine; acute nicotine; acute cannabinoids | N/A | Operant Learning Passive Avoidance Task |
N/A | Stressed>Unstressed: ↑ memory when administered CAN+NTP acutely or chronically |
| Cognitive Flexibility | Pushkin et al., 2019 (65) | Postnatal day 38–49 (~13-17 years) | NTP WIN NTP+WIN Control Male mice: n= 9-10 per group Female mice: n=7-9 per group |
N/A | Operant Learning and Reversal Task | N/A | No CAN+NTP findings Males>Females: ↑ cog flexibility when administered mod dose of WIN |
Notes: Summary results focus on notable co-use results; cited manuscripts should be reviewed for full results. CAN = Cannabis; NTP = Nicotine tobacco product; ALC = Alcohol; HVLT-R = Hopkins Verbal Learning Task – Revised; BIS = Barratt Impulsiveness Scale-11; SUD = Substance use disorder; NAcc = Nucleus accumbens; WIN = Cannabinoid agonist WIN55-212,2; CP = cannabinoid receptor agonist CP 55,940.
RESULTS
Neurocognition
Episodic Memory.
Studies examining the neurocognitive outcomes of cannabis and NTP co-use among adolescents are remarkably limited. One of the first studies to look at co-use examined the cognitive effects of nicotine withdrawal among daily tobacco smokers, with either at least 60 or less than 40 lifetime episodes of cannabis use (54). The authors found a significant interaction between group status and smoking conditions (ad libitum smoking v. 24-hour abstinence). Specifically, cannabis users reporting more than 60-lifetime episodes recalled fewer words than those reporting less than 40 episodes during the abstinence period. Group differences, however, were not observed during the ad libitum smoking condition nor immediate recall.
In another study, Schuster and colleagues found significant interactions between cannabis and tobacco cigarette use on episodic memory performance (58). To examine the effects of tobacco, participants were stratified based on past-year cigarette use (<100 vs. > 100 tobacco cigarettes). The amount of past-year cannabis use was negatively associated with initial learning acquisition, total immediate recall/learning, and delayed recall among sporadic cigarette users, but not among consistent cigarette users. That is, the amount of past-year cannabis use interacted with tobacco cigarette use in relation to episodic memory among sporadic cigarette smokers.
To date, only one human subject drug administration study has investigated the interactive effects of cannabis and tobacco on cognition (62). Relative to placebo, participants demonstrated poorer performance when recalling the second story, but not the first following cannabis-only administration. When administered cannabis and tobacco (versus cannabis only), participants exhibited no difference on immediate recall but better performance on delayed recall. Taken together, the extant literature on episodic memory among co-users suggests nicotine use may compensate for cannabis-related impairment on learning and memory. However, more research is needed to determine how frequency and consistency of use influence these potential relationships.
Working Memory.
Hindocha and colleagues (62) also examined working memory performance in their acute drug administration study described above. To examine spatial working memory, participants were shown visual stimuli on a computer and then asked to recall the positioning of a stimulus based on a pre-defined location (zero-back), the stimulus shown one trial before (1-back), and the stimulus shown two trials before (2-back). Cannabis impaired spatial N-back performance during 1- and 2-back conditions, relative to placebo, and tobacco was associated with improved N-back performance relative to placebo. However, there was no interaction or effect of co-use on working memory performance.
In another study, Schuster and colleagues examined co-use effects during a 7-day ecological momentary assessment (EMA) monitoring period (55). Consistent with Hindocha and colleagues (62), mixed effect regression results suggest cannabis had a negative, independent impact on working memory performance while tobacco had a positive, independent effect. Similarly, there was no cannabis by tobacco interaction, suggesting co-use performance was more similar to non-use performance. This suggests that tobacco use may compensate for cannabis-related decrements in working memory performance, similarly to episodic memory, given that cannabis did not lead to poorer memory with cannabis and NTP co-use. Notably, however, when alcohol was added to the model, working memory performance decreased, and the cannabis and tobacco main effects were no longer present.
Structural Neuroimaging
Structural imaging studies have only focused on younger adult samples, rather than adolescents, and suggest unique relationships with structural brain volume and cannabis and nicotine co-use compared to single substance users in subcortical gray matter volume (56). In a study by Filbey and colleagues (56), differences in (1) hippocampal volumes, (2) working memory performance, and (3) hippocampal volumes and working memory relationships were investigated. The cannabis and cannabis+nicotine groups exhibited reduced right hippocampal volume, compared to the control and nicotine-only groups. Although memory performance was lower in cannabis and cannabis+nicotine groups, groups were not significantly different. Additionally, an inverse interaction was found between group status and hippocampal volume on immediate and delayed recall performance. That is, whereby larger hippocampi were associated with better memory scores in controls, larger bilateral hippocampal volumes were associated with worse working performance in the cannabis+nicotine group. Lastly, follow-up analyses showed a significant hippocampal volume by nicotine use intensity interaction on immediate memory, suggesting heavy nicotine use (i.e., using 3+ tobacco cigarettes per day) may have contributed to the inverse volumetric-cognition findings for co-users. Surprisingly, no structural imaging studies were found to examine the combined effects of nicotine and cannabis in adolescent-only populations. Therefore, more work in this area is warranted.
Functional Neuroimaging
BOLD Signal Correlates of Working Memory.
Among adolescents, preliminary evidence also shows unique functional neurobiological characteristics among those who tend toward single-substance tobacco use versus co-use as compared to their non-substance-using peers (54, 57). In the study conducted by Jacobsen et al. (54) described above, participants also completed an auditory n-back fMRI task assessing during both tobacco conditions. Abstinent cannabis users, under the smoking abstinence condition, demonstrated increased activity in frontocortical regions with corresponding decreased frontoparietal connectivity during higher working memory load tasks. Group differences, however, were not observed during the libitum smoking condition. Together, results from this study provide preliminary evidence of a compensatory relationship between cannabis and nicotine use on functional connectivity, which is diminished after a 24-hr nicotine abstinence period.
BOLD Signal Correlates of Reward Responsivity.
In a cross-sectional study, participants completed a BOLD functional magnetic resonance monetary incentive delay (MID) task to probe neural activation to anticipation and responsiveness to reward within the nucleus accumbens (NAcc) (57). The NAcc is a region of the ventral striatum that plays a vital role in reward processing and addiction via dopaminergic signaling in response to reinforcing stimuli (66, 67). When examining group differences, the tobacco-only group showed less bilateral NAcc activation than the control group during reward trials. Less activation in the bilateral NAcc was observed in the tobacco-only group than the cannabis+tobacco group across both low and high reward trials, suggesting that adolescents engaging in tobacco use may demonstrate different brain activity patterns related to reward activation.
In a longitudinal study examining NAcc activation, previous and past-year tobacco cigarette use was not found to influence the relationship of cannabis and NAcc response to a modified MID task (59). Participants completed three consecutive fMRI scans at approximately 2-year intervals. Primary findings showed a significant dose-dependent cannabis relationship with later blunted activation in the NAcc across time during monetary reward anticipation (reward minus neutral trials). The effect of past cigarette use or co-use was nonsignificant.
Cue Reactivity.
Recently, Kuhns and colleagues (61) examined the relationship between cigarette and cannabis use on cannabis cue reactivity among a sample of 66 heavy- and non-cannabis users using two subtraction contrast parameters. Co-users did not show increased cue reactivity compared to cigarette users; however, a positive correlation was found between ventral tegmental area (VTA) activity and amount of cannabis consumption, showing higher cannabis consumption to be associated with heightened VTA activity. Additionally, both region of interest (ROI; amygdala, striatum, anterior cingulate cortex, and VTA) and exploratory whole brain analyses showed higher activity among cigarette users than co-users and non-using control groups.
Resting State Cerebral Blood Flow.
In a recent study, Courtney and colleagues (60) investigated the effects of nicotine and cannabis co-use on white matter cerebral blood flow (CBF) in adolescents and young adults. Positive correlations between five white matter CBF clusters and past-year cannabis use was observed for the non-NTP group only, controlling for nicotine and cannabis use recency. Greater CBF was also found in frontal cortical association fiber tracts with poorer structural integrity among the cannabis users. These results suggest a potential compensatory relationship between cannabis and nicotine use on white matter CBF.
Preclinical Studies
To our knowledge, three studies using animal models have examined the effects of cannabis and nicotine during adolescence (63–65). In a study investigating the long-term effects of nicotine and/or synthetic cannabinoid receptor agonist (CP) on adolescent rats, Mateos and colleagues (63) found gender-dependent memory impairments for both single-substance nicotine and cannabis use and co-use. When assessing working memory, the authors found (1) a significantly higher discrimination index (DI; a metric of memory) in control females compared to control males and (2) the administration of CP to significantly elevate DI in males in comparison to the control males. In the spatial memory task (after one month of withdrawal), substance-exposed females exhibited significant decreases in DI regardless of substance group, while males showed no difference. Regarding recognition memory (after one month of withdrawal), CP was found to have a harmful effect on males compared to the control males, whereas NIC was found to have a similar effect in females. Taken together, findings from this study suggest females may be more vulnerable to the effects of nicotine and their combination on different aspects of memory.
Similarly, Pushkin et al. (65) found gender-specific effects when investigating the relationship between the cannabinoid agonist WIN55-212,2 (WIN) and nicotine (NIC) on affective behaviors and cognitive flexibility in wildtype mice. Males in the WIN and NIC/WIN groups demonstrated increased lever-pressing behavior under higher cognitive demand conditions compared to the Control and NIC groups. In contrast, this difference was not found among the female groups. In a follow-up experiment, however, no significant differences were found for males or females in the number of active lever presses using a lower dose of WIN. Together, this study provides evidence for a gender by drug dose interaction, as male adolescent mice exhibited increased cognitive flexibility, compared to females, only when WIN was administered at moderate doses but not at lower doses. Further, this study did not show an additive effect of co-use, as the NIC and NIC/WIN groups did not significantly differ in either of the two experiments.
In a study investigating the cognitive effects of acute and subchronic nicotine exposure with cannabinoid receptor ligands during stress on male Swiss mice, Pekala and colleagues (64) found better memory following nicotine and cannabinoid administration after chronic stress. The acute administration of nicotine and the combined administration of acute nicotine and CB1 and CB2 antagonists improved memory performance in stressed mice, compared to the stressed saline-treated controls. The subchronic administration of nicotine, on the other hand, decreased memory performance in stressed mice, in comparison to the unstressed, saline-treated group; and improved in comparison to those that received the acute administration of nicotine or saline injections. Additionally, the combination of subchronic nicotine administration and acute cannabinoid receptor antagonists improved memory performance in stressed mice compared with both the stressed subchronically nicotine- and saline-treated groups. Overall findings from this study suggest a decrease in memory problems among stressed mice after acute and subchronic nicotine administration combined with acute administration of cannabinoids.
DISCUSSION
Despite high rates of co-use of cannabis and NTP use in adolescents and young adults, studies focused on the effects of combined use on neural outcomes are limited. Results from the present systematic review reveal a complex pattern of substance-related deleterious effects unique to cannabis and nicotine and, at times, potential compensatory effects associated with co-use—all of which tend to be domain, if not study, specific. With only nine human studies, at a reasonably broad age range (13–35), and three preclinical studies included, firm conclusions cannot be made. On balance, preliminary evidence does suggest notable patterns that warrant further prospective investigation and replication in this important domain of research.
In reviewing the literature, the cognitive effects of co-use may depend on the proximal timing of last nicotine use. This perhaps makes sense in light of nicotinergic receptors which, may increase cognitive function acutely (68). To that end, better cognitive performance was found in studies of co-users who were permitted to use nicotine ad libitum compared to during withdrawal (54) or when using both cannabis and nicotine (55, 62). Interestingly, however, even studies investigating more chronic effects of co-use sometimes suggest co-use may facilitate better cognitive performance than cannabis use alone (58). Importantly, dosing is likely a significant factor (e.g., sporadic vs. frequent) in considering individuals who may exhibit NTP-associated cognitive improvement, as regular users likely become dependent on nicotine to maintain normal cognitive functioning (69). Given the limited nature of these findings, however, much more research is needed to assess patterns and frequencies of co-use in relation to cognition.
Similarly, while several studies examining the independent effects of cannabis (5, 70–76) and nicotine (24, 25, 28, 30–35, 77) suggest deleterious effects of these substances on neural health compared to non-users, the combination in the neuroimaging literature remains understudied. Surprisingly, there have been no structural neuroimaging studies examining the effects of cannabis and NTP co-use on adolescent-only populations’ brain development. The only known morphometric structural MRI study, including both adolescents and younger adults, found reduced right hippocampal volume among co-users, but an inverse interaction between cannabis and NTP co-use depicting larger hippocampal volume associated with worse memory scores among co-users, but not among other groups (56). Recent work among older adolescents and emerging adults found increased cerebral blood flow in white matter tracts associated with poorer structural integrity in cannabis users, but not among co-users (60). However, co-use does not likely come without a neural cost. In a study of adults up through middle age, Leroy and colleagues (78) discovered decreased dopamine binding levels in subcortical reward regions among co-users and tobacco only users, suggesting a mechanism by which nicotine dependence may change brain structure. Thus, it appears that there are times when co-use may negate decrements observed among single-substance users, but this does not necessarily suggest an overall improvement in health or functioning, nor is it clear the exact parameters of when this relative benefit may occur or dissipate with time.
Reward responsivity is associated with substance use onset and its consequences (e.g. craving, dependence, and cognitive decline) (79–81). While there are mixed findings as to whether co-use is associated with altered reward-related activation (57, 59, 61), it does appear that cannabis is related to aberrant processing of rewarding stimuli, as evidenced by dose-dependent relationships (59, 61). This is similar to the broader literature on cannabis, wherein studies have found cannabis users (often controlling for other substance use) have increased activity in reward regions (e.g, ventral striatum, orbitofrontal cortex, cingulate gyrus, and ventral tegmental area) in response to reward processing paradigms (82). Additionally, while two studies focused on reward anticipation in the NAcc among co-users, one included only adolescent participants, while the other was a slightly older cohort (~age 20). In the adolescent-only sample, the tobacco-only group showed altered bilateral NAcc activation during reward trials; whereas among emerging adults cumulative cannabis use was correlated with BOLD signal reward anticipation. Differences by age, even within an emerging adult cohort, may then be important for revealing different brain-behavior relationships impacted by cannabis and nicotine use patterns. In sum, given the nascent state of this research and preliminary evidence of disrupted reward processing related to both nicotine and cannabis use (62, 64), more research is warranted before firm conclusions can be drawn.
A possible explanation for improved cognitive performance and limited neural differences related to co-use (56–58, 62, 64) might be that concurrent cannabis and NTP use have a compensatory effect on functioning. That is, as endocannabinoid receptors and nAChRs overlap in the cortico-limbic brain reward regions, the endocannabinoid and cholinergic systems likely interact (42). In some instances, this interaction of endocannabinoid and cholinergic systems may lead to attenuation or dampening of the adverse effects of independent substance use (56, 58, 62, 64), though other evidence indicates the interaction between the systems does not influence larger brain-behavior outcomes (55, 59, 65). Previous studies evaluating the effects of nicotinic agonists on cognition have improved the efficiency of information transfer in local and global regions (68). Specifically, nicotine may ‘stimulate’ cognitive functions by increasing the integration of information within the brain’s limbic and paralimbic areas through interneurons (68). The synergistic effect of cannabis and nicotine co-stimulation may also potentiate signaling in dopamine-innervated brain regions (e.g., limbic and cortical structures) and neurodevelopmental processes (83, 84), which, during adolescence, undergoes dynamic changes in tissue organization that underlies higher-level adaptive functioning. Yet, the possibility remains that this process may also increase vulnerability for future addiction-related problems. Thus, during adolescence, repeated nicotine exposure is associated with increased dopamine levels in the NAcc and may lead to altered reward responses, such as tolerance and/or neurodevelopmental trajectories that may facilitate addiction in youth (67).
To date, however, there still remains limited evidence for nicotine and cannabis synergistic effects on neurocognition and neural integrity among adolescent and young adult populations, despite evidence suggesting co-users are more likely to continue using substances in mid-life, show increased addictions severity symptom, poorer behavioral and educational outcomes, and increased risk for heavier subsequent use (50, 85–91). As suggested by the preclinical literature, other factors (e.g., sex, environmental stress) are also important determinants of co-use relationships (63–65). Longitudinal studies of co-use are greatly needed in this area of research to clarify causal relationships between co-use and related outcomes. Therefore, a better understanding of the neural consequences related to co-use has relevance to a better understanding of behavioral outcomes among adolescent substance users.
Limitations and Future Considerations
The differential outcomes presented in this review may also be the result of different methodologies, use of other substances, frequency and magnitude of use, drug product choice (e.g., high potency cannabis; nicotine vs. tobacco product), and mode of substance use. One drawback of the data reviewed is that studies have not independently investigated vaping of nicotine or cannabinoid products or high potency cannabis products. Therefore, a more comprehensive investigation should include vaping behaviors among youth (90, 92, 93). Not only were there only 12 studies that met inclusion criteria, reducing the sample size of this review, but studies included often consisted of small samples (e.g., n=24). Larger studies are needed to be able to better assess real effects and reduce type II error. Of note, two preclinical studies found a gender by drug dose interaction, suggesting gender differences may also play a role in different outcomes (63, 65). This finding, while preliminary, suggests sex-dependent relationships in cognition may exist among co-users. Further work exploring NTP and cannabis potency relationships on cognition in adolescents, is therefore required. Lastly, several studies used group-based analyses (55, 56, 58, 62), and limited investigations examined dose-dependent relationships (58, 59).
Notably, other complicating factors in assessing the additive effects of NTPs during cannabis co-use have to do with the potential lack of effects when alcohol is considered (55) and chronic vs. acute use effects. Polysubstance use and chronic heavy use are important use patterns that likely differentially impact neural circuitry, considering the different pharmacological mechanisms involved in single vs. polysubstance use and repeated exposure (67). Lastly, the required abstinence period among the studies reviewed ranged from a minimum of 3 hours to a maximum of 3 days. Thus, differences in required abstinence periods may lead to different brain-behavior relationships.
CONCLUSIONS
The limited research on the effects of nicotine and cannabis co-administration on neurodevelopment is remarkable, particularly given the high prevalence rates of these substances in the past several years (2, 85). While there is evidence suggesting that NTP use may compensate for neurocognitive alterations related to cannabis use among youth, very little is known about how co-use patterns influence brain morphometry, neurocircuitry, and neurobiological maturation, and neurobehavioral outcomes. Most importantly, the longer-term consequences as related to the unique synergistic effects of recurrent co-stimulation of the endocannabinoid and nicotinic cholinergic system during the adolescent period of development remain understudied (94). More prospective studies are needed that examine co-use, both simultaneous and concurrent, as well as the pattern of use, with neurodevelopmental indices. Factors to consider in future research to better understand discrepant findings involve taking into account, as much as possible, the association of biological, environmental, and behavioral factors with the emergence of substance use in adolescence (95).
Supplementary Material
Acknowledgments
This work was supported by T32 AA013525 (PI: Riley/Tapert to Wade and Hernandez Mejia), U01DA041089 (PI: Tapert/Jacobus), NIH/NIDA R21 DA047953 (PI: Jacobus), and California Tobacco-Related Disease Research Grants Program Office of the University of California Grant 580264 (PI: Jacobus). The content is solely the view of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME, et al. (2019): Monitoring the Future National Survey Results on Drug Use, 1975-2018: Overview, Key Findings on Adolescent Drug Use. Institute for Social Research. [Google Scholar]
- 2.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME (2020): Monitoring the Future national survey results on drug use 1975-2019: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan. [Google Scholar]
- 3.Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M (2015): Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cogn Neurosci. 16:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker MP, Collins PF, Schultz A, Urosevic S, Schmaling B, Luciana M (2018): Longitudinal changes in cognition in young adult cannabis users. J Clin Exp Neuropsychol. 40:529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, et al. (2014): Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 39:2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Infante MA, Courtney KE, Castro N, Squeglia LM, Jacobus J (2018): Adolescent Brain Surface Area Pre- and Post-Cannabis and Alcohol Initiation. Journal of Studies on Alcohol and Drugs. 79:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenders L, Cousijn J, Vingerhoets WA, van den Brink W, Wiers RW, Meijer CJ, et al. (2016): Grey Matter Changes Associated with Heavy Cannabis Use: A Longitudinal sMRI Study. PLoS One. 11:e0152482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. (2012): Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 109:E2657–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meier MH, Caspi A, Danese A, Fisher HL, Houts R, Arseneault L, et al. (2018): Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction. 113:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. (2011): Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl). 216:131–144. [DOI] [PubMed] [Google Scholar]
- 11.Scott JC, Wolf DH, Calkins ME, Bach EC, Weidner J, Ruparel K, et al. (2017): Cognitive functioning of adolescent and young adult cannabis users in the Philadelphia Neurodevelopmental Cohort. Psychol Addict Behav. 31:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wade NE, Wallace AL, Swartz AM, Lisdahl KM (2019): Aerobic Fitness Level Moderates the Association Between Cannabis Use and Executive Functioning and Psychomotor Speed Following Abstinence in Adolescents and Young Adults. J Int Neuropsychol Soc. 25:134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez R, Pacheco-Colon I, Duperrouzel JC, Hawes SW (2017): Does Cannabis Use Cause Declines in Neuropsychological Functioning? A Review of Longitudinal Studies. J Int Neuropsychol Soc. 23:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R (2013): Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev. 23:117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross JM, Ellingson JM, Rhee SH, Hewitt JK, Corley RP, Lessem JM, et al. (2020): Investigating the causal effect of cannabis use on cognitive function with a quasi-experimental co-twin design. Drug Alcohol Depend. 206:107712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobus J, Tapert S,F. (2014): Effects of Cannabis on the Adolescent Brain. Current Pharmaceutical Design. 20:2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blest-Hopley G, Giampietro V, Bhattacharyya S (2018): Residual effects of cannabis use in adolescent and adult brains - A meta-analysis of fMRI studies. Neurosci Biobehav Rev. 88:26–41. [DOI] [PubMed] [Google Scholar]
- 18.Blest-Hopley G, Giampietro V, Bhattacharyya S (2019): Regular cannabis use is associated with altered activation of central executive and default mode networks even after prolonged abstinence in adolescent users: Results from a complementary meta-analysis. Neurosci Biobehav Rev. 96:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobus J, Courtney KE, Hodgdon EA, Baca R (2019): Cannabis and the developing brain: What does the evidence say? Birth Defects Res. 111:1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jager G, Block RI, Luijten M, Ramsey NF (2010): Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 49:561–572, 572.e561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzetti V, Alonso-Lana S, Youssef GJ, Verdejo-Garcia A, Suo C, Cousijn J, et al. (2016): Adolescent Cannabis Use: What is the Evidence for Functional Brain Alteration? Curr Pharm Des. 22:6353–6365. [DOI] [PubMed] [Google Scholar]
- 22.Tervo-Clemmens B, Simmonds D, Calabro FJ, Montez DF, Lekht JA, Day NL, et al. (2018): Early Cannabis Use and Neurocognitive Risk: A Prospective Functional Neuroimaging Study. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wade NE, Bagot KS, Cota CI, Fotros A, Squeglia LM, Meredith LR, et al. (2019): Orbitofrontal cortex volume prospectively predicts cannabis and other substance use onset in adolescents. J Psychopharmacol. 33:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR (2005): Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 57:56–66. [DOI] [PubMed] [Google Scholar]
- 25.Treur JL, Willemsen G, Bartels M, Geels LM, van Beek JH, Huppertz C, et al. (2015): Smoking During Adolescence as a Risk Factor for Attention Problems. Biol Psychiatry. 78:656–663. [DOI] [PubMed] [Google Scholar]
- 26.Mashhoon Y, Betts J, Farmer SL, Lukas SE (2018): Early onset tobacco cigarette smokers exhibit deficits in response inhibition and sustained attention. Drug Alcohol Depend. 184:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R (2005): Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 113:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Yuan K, Cai C, Feng D, Yin J, Bi Y, et al. (2015): Reduced frontal cortical thickness and increased caudate volume within fronto-striatal circuits in young adult smokers. Drug Alcohol Depend. 151:211–219. [DOI] [PubMed] [Google Scholar]
- 29.Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, et al. (2016): Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol. 21:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akkermans SEA, van Rooij D, Rommelse N, Hartman CA, Hoekstra PJ, Franke B, et al. (2017): Effect of tobacco smoking on frontal cortical thickness development: A longitudinal study in a mixed cohort of ADHD-affected and -unaffected youth. Eur Neuropsychopharmacol. 27:1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaarani B, Kan K-J, Mackey S, Spechler PA, Potter A, Orr C, et al. (2019): Low Smoking Exposure, the Adolescent Brain, and the Modulating Role of CHRNA5 Polymorphisms. Biological Psychiatry Cognitive Neuroscience And Neuroimaging. 4:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, et al. (2007): Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci. 27:13491–13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Ewijk H, Groenman AP, Zwiers MP, Heslenfeld DJ, Faraone SV, Hartman CA, et al. (2015): Smoking and the developing brain: altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum Brain Mapp. 36:1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D, Yuan K, Zhang B, Liu J, Dong M, Jin C, et al. (2016): White matter integrity in young smokers: a tract-based spatial statistics study. Addict Biol. 21:679–687. [DOI] [PubMed] [Google Scholar]
- 35.Kangiser MM, Thomas AM, Kaiver CM, Lisdahl KM (2019): Nicotine Effects on White Matter Microstructure in Young Adults. Arch Clin Neuropsychol. 35:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, Gur RC (2018): Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry. 75:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco C, Florez-Salamanca L, Secades-Villa R, Wang S, Hasin DS (2018): Predictors of initiation of nicotine, alcohol, cannabis, and cocaine use: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Am J Addict. 27:477–484. [DOI] [PubMed] [Google Scholar]
- 38.Ramo DE, Prochaska JJ (2012): Prevalence and co-use of marijuana among young adult cigarette smokers: An anonymous online national survey. Addict Sci Clin Pract. 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy SM, Caraballo RS, Rolle IV, Rock VJ (2016): Not Just Cigarettes: A More Comprehensive Look at Marijuana and Tobacco Use Among African American and White Youth and Young Adults. Nicotine Tob Res. 18 Suppl 1:S65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M (2015): Assessing the overlap between tobacco and marijuana: Trends in patterns of co-use of tobacco and marijuana in adults from 2003-2012. Addict Behav. 49:26–32. [DOI] [PubMed] [Google Scholar]
- 41.Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M (2016): Differences in Tobacco Product Use Among Past Month Adult Marijuana Users and Nonusers: Findings From the 2003-2012 National Survey on Drug Use and Health. Nicotine Tob Res. 18:281–288. [DOI] [PubMed] [Google Scholar]
- 42.Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R (2002): Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 135:564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atakan Z (2012): Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan M, Cross SJ, Loughlin SE, Leslie FM (2015): Nicotine and the adolescent brain. J Physiol. 593:3397–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorenzetti V, Chye Y, Silva P, Solowij N, Roberts CA (2019): Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. Eur Arch Psychiatry Clin Neurosci. 269:59–71. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. (2016): Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry. 73:292–297. [DOI] [PubMed] [Google Scholar]
- 47.Levine A, Clemenza K, Rynn M, Lieberman J (2017): Evidence for the Risks and Consequences of Adolescent Cannabis Exposure. J Am Acad Child Adolesc Psychiatry. 56:214–225. [DOI] [PubMed] [Google Scholar]
- 48.Lydon DM, Wilson SJ, Child A, Geier CF (2014): Adolescent brain maturation and smoking: what we know and where we’re headed. Neurosci Biobehav Rev. 45:323–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF (2009): Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 92:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramo DE, Liu H, Prochaska JJ (2012): Tobacco and marijuana use among adolescents and young adults: a systematic review of their co-use. Clin Psychol Rev. 32:105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. (2015): Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. (2008): GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldet G, Howick J (2013): Understanding GRADE: an introduction. J Evid Based Med. 6:50–54. [DOI] [PubMed] [Google Scholar]
- 54.Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE (2007): Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 61:31–40. [DOI] [PubMed] [Google Scholar]
- 55.Schuster RM, Mermelstein RJ, Hedeker D (2016): Ecological momentary assessment of working memory under conditions of simultaneous marijuana and tobacco use. Addiction. 111:1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filbey FM, McQueeny T, Kadamangudi S, Bice C, Ketcherside A (2015): Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav Brain Res. 293:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW (2015): Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci. 16:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuster RM, Crane NA, Mermelstein R, Gonzalez R (2015): Tobacco may mask poorer episodic memory among young adult cannabis users. Neuropsychology. 29:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, et al. (2016): Association of Marijuana Use With Blunted Nucleus Accumbens Response to Reward Anticipation. JAMA Psychiatry. 73:838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Courtney KE, Baca R, Doran N, Jacobson A, Liu TT, Jacobus J (2020): The effects of nicotine and cannabis co-use during adolescence and young adulthood on white matter cerebral blood flow estimates. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhns L, Kroon E, Filbey F, Cousijn J (2020): Unraveling the role of cigarette use in neural cannabis cue reactivity in heavy cannabis users. Addict Biol.e12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hindocha C, Freeman TP, Xia JX, Shaban NDC, Curran HV (2017): Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: a placebo-controlled trial. Psychol Med. 47:2708–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, et al. (2011): Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors. J Psychopharmacol. 25:1676–1690. [DOI] [PubMed] [Google Scholar]
- 64.Pekala K, Michalak A, Kruk-Slomka M, Budzynska B, Biala G (2018): Impacts of cannabinoid receptor ligands on nicotine- and chronic mild stress-induced cognitive and depression-like effects in mice. Behav Brain Res. 347:167–174. [DOI] [PubMed] [Google Scholar]
- 65.Pushkin AN, Eugene AJ, Lallai V, Torres-Mendoza A, Fowler JP, Chen E, et al. (2019): Cannabinoid and nicotine exposure during adolescence induces sex-specific effects on anxiety- and reward-related behaviors during adulthood. PLoS One. 14:e0211346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dafny N, Rosenfeld GC (2017): Neurobiology of Drugs of Abuse. Conn’s Translational Neuroscience, pp 715–722. [Google Scholar]
- 67.Volkow ND, Morales M (2015): The Brain on Drugs: From Reward to Addiction. Cell. 162:712–725. [DOI] [PubMed] [Google Scholar]
- 68.Wylie KP, Rojas DC, Tanabe J, Martin LF, Tregellas JR (2012): Nicotine increases brain functional network efficiency. Neuroimage. 63:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prochaska JJ, Benowitz NL (2019): Current advances in research in treatment and recovery: Nicotine addiction. Sci Adv. 5:eaay9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA (2010): Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol. 1:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orr C, Spechler P, Cao Z, Albaugh M, Chaarani B, Mackey S, et al. (2019): Grey Matter Volume Differences Associated with Extremely Low Levels of Cannabis Use in Adolescence. J Neurosci. 39:1817–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mashhoon Y, Sava S, Sneider JT, Nickerson LD, Silveri MM (2015): Cortical thinness and volume differences associated with marijuana abuse in emerging adults. Drug Alcohol Depend. 155:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE (2012): Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 59:3845–3851. [DOI] [PubMed] [Google Scholar]
- 74.Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. (2014): Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A. 111:16913–16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilman JM, Kuster JK, Lee S, Lee MJ, Kim BW, Makris N, et al. (2014): Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 34:5529–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Filbey FM, McQueeny T, DeWitt SJ, Mishra V (2015): Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci. 16:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin F, Han X, Wang Y, Ding W, Sun Y, Zhou Y, et al. (2020): Sex-specific effects of cigarette smoking on caudate and amygdala volume and resting-state functional connectivity. Brain Imaging Behav. [DOI] [PubMed] [Google Scholar]
- 78.Leroy C, Karila L, Martinot JL, Lukasiewicz M, Duchesnay E, Comtat C, et al. (2012): Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study. Addict Biol. 17:981–990. [DOI] [PubMed] [Google Scholar]
- 79.Wetherill R, Tapert SF (2013): Adolescent brain development, substance use, and psychotherapeutic change. Psychol Addict Behav. 27:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF (2012): Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J Stud Alcohol Drugs. 73:749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, et al. (2014): Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend. 141:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fatima H, Howlett AC, Whitlow CT (2019): Reward, Control & Decision-Making in Cannabis Use Disorder: Insights from Functional MRI. Br J Radiol. 92:20190165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu JJ, Mohila CA, Gong Y, Govindarajan N, Onn SP (2005): Chronic nicotine exposure during adolescence differentially influences calcium-binding proteins in rat anterior cingulate cortex. Eur J Neurosci. 22:2462–2474. [DOI] [PubMed] [Google Scholar]
- 84.Garrido R, King-Pospisil K, Son KW, Hennig B, Toborek M (2003): Nicotine upregulates nerve growth factor expression and prevents apoptosis of cultured spinal cord neurons. Neuroscience Research. 47:349–355. [DOI] [PubMed] [Google Scholar]
- 85.Hublet A, Bendtsen P, de Looze ME, Fotiou A, Donnelly P, Vilhjalmsson R, et al. (2015): Trends in the co-occurrence of tobacco and cannabis use in 15-year-olds from 2002 to 2010 in 28 countries of Europe and North America. Eur J Public Health. 25 Suppl 2:73–75. [DOI] [PubMed] [Google Scholar]
- 86.Ream GL, Benoit E, Johnson BD, Dunlap E (2008): Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 95:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tucker JS, Pedersen ER, Seelam R, Dunbar MS, Shih RA, D’Amico EJ (2019): Types of cannabis and tobacco/nicotine co-use and associated outcomes in young adulthood. Psychol Addict Behav. 33:401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stiby AI, Hickman M, Munafo MR, Heron J, Yip VL, Macleod J (2015): Adolescent cannabis and tobacco use and educational outcomes at age 16: birth cohort study. Addiction. 110:658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dai H, Hao J (2017): Electronic cigarette and marijuana use among youth in the United States. Addict Behav. 66:48–54. [DOI] [PubMed] [Google Scholar]
- 90.Chadi N, Schroeder R, Jensen JW, Levy S (2019): Association Between Electronic Cigarette Use and Marijuana Use Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr.e192574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peters EN, Budney AJ, Carroll KM (2012): Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 107:1404–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zare S, Nemati M, Zheng Y (2018): A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS One. 13:e0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldenson NI, Leventhal AM, Simpson KA, Barrington-Trimis JL (2019): A Review of the Use and Appeal of Flavored Electronic Cigarettes. Curr Addict Rep. 6:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scherma M, Muntoni AL, Melis M, Fattore L, Fadda P, Fratta W, et al. (2016): Interactions between the endocannabinoid and nicotinic cholinergic systems: preclinical evidence and therapeutic perspectives. Psychopharmacology (Berl). 233:1765–1777. [DOI] [PubMed] [Google Scholar]
- 95.Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. (2018): The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev Cogn Neurosci. 32:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


