Abstract
Three potential rhizobacteria namely Burkholderia gladioli (MTCC 10216), Pseudomonas sp. (MTCC 9002) and Bacillus subtilis (MTCC 8528) procured from IMTECH, Chandigarh (India) were evaluated individually and as consortia for its phosphate (P) solubilizing ability and effect of growth of fenugreek (Trigonella foenum-graecum L.) and tomato (Lycopersicon esculentum L.). Phosphate solubilizing ability of these strains individually and as consortia was tested on Pikovskayas agar medium, Phosphate solubilizing agar medium and National Botanical Research Institute phosphate agar medium containing six different sources of insoluble inorganic phosphate such as tri-calcium phosphate (TCP), di-calcium phosphate (DCP), zinc phosphate (ZP), ferric phosphate (FP), sodium di-hydrogen phosphate (SP), and aluminum phosphate (AP), and two organic P such as calcium and sodium phytate. The maximum P solubilizing ability was recorded in consortium-4 having all three potential bacterial strains. Phosphate solubilization after 7th day of incubation was 37.9 mg/100 ml of TCP, 40.01 mg/100 ml of DCP, 15.79 mg/100 ml of FP, 43.02 mg/100 ml of SP, no solubilization of ZP and AP, 39.75 mg/100 ml of calcium phytate and 24.01mg/100 ml of sodium phytate. Seed germination and the other plant parameters such as plant height and weight significantly increased in fenugreek and tomato seeds, bio-primed with consortium-4 followed by consortium-3. After bio-priming of seeds in pot assay, the level of phosphorus in soil got increased by 54% in consortium-4 treated soil followed by consortium-3 (47%) over untreated control soil. Based on these findings, consoritium-4 could be recommended as a good bio-inoculant for fenugreek, tomato and other crops in comparison to individual strains and other consortia.
Keywords: Bioinformatics, Biotechnology, Ecology, Microbiology, Plant biology, Agricultural soil science, Organic farming, Pesticide, Phosphate solubilization, Consortia, Fenugreek and tomato
Bioinformatics; Biotechnology; Ecology; Microbiology; Plant biology; Agricultural soil science; Organic farming; Pesticide; Phosphate solubilization; Consortia; Fenugreek and tomato.
1. Introduction
The present scenario of soil engineering is totally based on synthetic chemicals which are responsible for several problems of human health and ecological disturbance [1]. The application of potential plant growth promoting rhizobacteria (PGPR) as bioinoculants is the only strategy to address these problems [2, 3]. The world population is increasing rapidly, but the sufficient and healthy food is not being produced as per demand [4]. Therefore to address these concerns, we must move towards organic agriculture. The rhizosphere is a zone of predominantly commensal and mutualistic interactions between plant and microbes and influenced by root system [5]. The rhizosphere region is rich in nutrients as compared to the bulk soil due to the accumulation of various root exudates like organic acids, amino acids, sugars, etc. released by the root system affecting biological activities [6].
Phosphorus (P) is an essential element for plant, but normally not available directly for plants because of its non-bioavailability form in soil. Phosphate solubilizing rhizobacteria (PSR) solubilize the insoluble soil P and help in utilization by plants for their various metabolic activities [7]. The insoluble P in soil is available as an inorganic mineral for example, apatite, tri-calcium phosphate (TCP), di-calcium phosphate (DCP), hydroxyapatite, zinc phosphate (ZP), sodium di-hydrogen phosphate (SP), aluminium phosphate (AP), ferric phosphate (FP) and rock phosphate (RP), besides these inorganic phosphate several other organic forms including inositol phosphate (soil phytate), phosphomonoesters, calcium phytate, sodium phytate and phosphotriesters are also available [8, 9]. Among these phosphates, the solubilization of inorganic P takes place due to low molecular weight microbial organic acids (OA), such as gluconic acid, iso-valeric acid, iso-vandic acid, α-ketoglutaric acid and citric acid [10, 11]. These organic acids produced by numerous PSR in the natural surrounding conditions or under in vitro condition chelate the cationic partners of phosphate or decrease the pH to make P free (soluble) in solution [12]. The acidification of microbial cells and its surrounding results in the discharge of P-ions from the P mineral by H+ cation replacement [13, 14]. However, the effectiveness of solubilization relies on the types and concentration of organic acid released in the medium [15].
On the other hand, organic phosphorus mineralization takes place through the synthesis of various phosphatases (phosphohydrolase), catalyzing the hydrolysis of phosphoric esters and releasing phosphorus from organic phosphate [9]. Some other types of enzymes like phytase, phosphonatases and C–P lyases are also involved in mineralization of organic P. The PSR stimulate plant growth either directly by synthesizing the hormones such as indole-3-acetic acid or by supporting nutrition, such as P solubilization or more generally by accelerating process of mineralization [16, 17], indirectly they can also boost the development of plant by acting as bio-control agents against soil-borne phytopathogens [18, 19].
Most of the soil phosphorus is fixed, and just a little portion is accessible to plants. About 0.05% phosphorus available in Indian soils which constitutes approximately 0.2% of the plant dry weight. The cell may take several forms of phosphorus, but most of them are absorbed in the form of hydrogen P (HPO4−2) or dihydrogen P (H2PO4−2) [8]. Phosphorus deficiency brings about hindered development, dull leaves, and hindrance of blooming and root framework development [20]. One conceivable approach to relieve the phosphorus deficiency under soil-plant-microbe framework through eco-friendly use of PSR by seeds bio-priming, just as soil bio-priming procedure i.e. seed covering with any beneficial microorganisms for example, Bacillus, Pseudomonas and Rhizobium species etc. which were effectively connected under greenhouse nursery and field conditions with multi-cropping system. These rhizospheric microorganisms alone or in combination have multifunctional sway on soil-plant framework, for example, improved nutrient use proficiency, expanding nutrient uptake, plant development advancement, nodulation, and plant resistance to abiotic and biotic stress, reduced environmental contamination and expanding agrarian sustainability [21, 22, 23, 24, 25, 26].
Bio-priming helps seeds to germinate uniformly, even under adverse conditions [27]. Fenugreek (Trigonella foenum-graecum L.) an annual plant belonging to the family Fabaceae is commonly known as “methi” in India. It is a multifunctional crop cultivated during the winter season in Northern India. Each part of this plant is used as a leafy vegetable, forage and condiment [28]. Its seeds are a good source of protein, vitamins, alkaloids tri-gonellin, and essential oil and have enormous medicinal value especially against digestive disorders [29]. It contains a variety of bioactive compounds such as alkaloids, glycosides, polyphenols, steroids, amino acids, and volatiles, and so on. It is also used as anti-diabetic, anti-fertility, anti-microbial, anti-parasitic and hypocholesterolaemic, anti-epileptic, anti-bronchitis, carminative, aphrodisiac, analgesic, anti-pyretic, anti-cancer, anti-oxidant, immunomodulator, phlegm disorders and recently in blood glucose balancing.
Tomato (Lycopersicon esculentum L.) is the member of family Solanaceae. Its fruits are a rich source of minerals, vitamins and organic acids and have 3–4% total sugar, 4–7% total solids, 15–30 mg/100g of ascorbic acid, 7.5–10 mg/100 ml titratable acidity and 20–50 mg/100 g fruit weight of lycopene.
The present study was aimed to assess the effect of single and composite inoculations of PSR on P solubilization from different P-minerals and their effects on growth promotion of fenugreek and tomato plants.
2. Materials and methods
2.1. Microbial strains
In this study, three PSR strains, such as Burkholderia gladioli (MTCC 10216), Pseudomonas sp. (MTCC 9002), and Bacillus subtilis (MTCC 8528), were procured from the Institute of Microbial Technology (IMTECH), Chandigarh (www.imtech.res.in), India. For further studies, all strains were maintained on the slants containing nutrient agar medium (NAM) at 4 °C.
2.2. In vitro interaction among the PSR strains to prepare consortia
All the three PSR strains were evaluated for their antagonistic/synergistic activities against each other following the methods of Pierson and Weller [30] to prepare consortia. B. gladioli (MTCC 10216), Pseudomonas sp. (MTCC 9002) and B. subtilis (MTCC 8528) were separately inoculated in NAM broth and incubated in shaker at 28 °C for 24 h. 5 μl of each culture was spot inoculated on NAM plates (1.5 cm from the edge) and the plates were incubated at 28 °C for 24 h. Further, the plates were sprayed with a 24 h old culture of single strain using a chromatography sprayer and again incubated at 28 °C for 24 h to measure zones of inhibition (if present around each test strain on plates); each treatment was replicated thrice.
2.3. Qualitative estimation of phosphate solubilization
The P solubilization activities of the PSR were investigated on Pikovskayas agar medium, P solubilizing agar medium (PSM) and National Botanical Research Institute Phosphate (NBRIP) agar medium containing 6 different insoluble phosphate sources such as TCP, DCP, ZP, FP, SP, and AP, separately, as source of insoluble inorganic P along with bromophenol blue as a pH indicator. Plates were incubated at 28 °C for 3 days to observe clearing zone around the colonies. The P solubilizing index (PSI) and P solubilizing efficiency (PSE) were calculated using Eqs. (1) and (2):
| eq. 1 |
| eq. 2 |
2.4. Quantitative estimation of phosphate solubilization
Further, the same experiment was repeated with NBRIP medium [31]. The individual PSR strains and their consortia were evaluated for quantitative estimation of water extractable free inorganic P (Pi) as per method mentioned by Dubey and Maheshwari [32]. Briefly, NBRIP (pH 7.2) broth was seeded with respective young cultures and incubated at 28 °C and 150 rpm. After every 24 h, 10 ml of broth was aseptically withdrawn and centrifuged at 7,500 rpm. Culture supernatant was filtered through 0.45 μm Millipore filter and 1g activated carbon was added to it, repeatedly centrifuged again at 10,000 rpm for 10–15 min and the culture was filtered to get a clear solution. Sterile distilled water (SDW) was added in this clear solution to make-up a final volume of 50 ml. Aliquot of 10 ml of this freshly prepared solution in a flask and 25 ml of Barton's reagent was added. Sterile distilled water was added in this solution to make the volume to 50 ml. This mixture was incubated at room temperature for 10 min and optical density (OD) was measured at 430 nm with UV-VIS spectrophotometer (Lasany International, Haryana, India). Amount of free P released was then estimated by plotting absorbance against standard curve of potassium hydrogen P (K2HPO4) (mg/ml). The pH of centrifuged product was recorded to measure free inorganic P.
2.5. Phytase activity
Phytase activity of each PSR strains was investigated by spot inoculation of log phase culture on phytase screening media having calcium and sodium phytate as sole source of organic P [33]. Plates were incubated at 28 °C for 3 days to observe clearing zone around the colonies.
2.6. Available phosphate in soil
For calculation of available P in soil, a mixture of 2.5 g of soil, 50 ml of 0.5M NaHCO3 (pH 8.5) and 0.5 ml of 5N H2SO4 was prepared and shaken till CO2 evolution disappeared. 4 ml of ascorbic acid was added and made up the volume 100 ml with distilled water. After 10 min incubation, the intensity of blue color was measured at 760 nm wavelength using spectrophotometer. Blank reading was taken in the same manner without soil [34].
2.7. Seed bio-priming
Healthy seeds of fenugreek and tomato were selected from locally purchased seeds. Fenugreek and tomato seeds (each 10 seeds per pot) were sterilized and bio-primed with bacterial strains and their consortia. The cultures of PSR strains and their consortia were mixed with 1% carboxy methyl cellulose (CMC) solution separately to form slurry and coated on the surface of sterile seeds of both crops.
2.8. Pot assay and seed germination study
Sterilized garden soil was transferred to experimental pot. Bio-primed seeds (10 seeds per pots) were transferred in pots for fenugreek and tomato. Phosphate solubilizing rhizobacterial cultures and their consortia were applied into their respective pots. Sterile water was slowly added over the top soil in each pot to maintain water holding capacity. After 21 days of sowing, the plants were uprooted for measurement of vegetative parameters such as root length, shoot length, root and shoot weight (fresh and dry). Treatments of seeds was as follows: T1; seeds bio-primed with B. gladioli, T2; seeds bio-primed with Pseudomonas sp., T3; seeds bio-primed with B. subtilis, T4; seeds bio-primed with B. gladioli + Pseudomonas sp., T5; seeds bio-primed with B. gladioli + B. subtilis, T6; seeds bio-primed with Pseudomonas sp. + B. subtilis, T7; seeds bio-primed with B. gladioli + Pseudomonas sp. + B. subtilis, and T8; seeds coated with 1% CMC as control (no any biological agent). Bio-primed seeds were also used for plate assay to measure germination percentage following the standard procedure.
2.9. Statistical analysis
The data were analyzed by applying Analysis of Variance (ANOVA) by using SPSS 20.0 software.
3. Results
3.1. In vitro interaction study to prepare consotia
Three different PGPR strains viz., B. gladioli (MTCC 10216), Pseudomonas sp. (MTCC 9002), and B. subtilis (MTCC 8528) were selected for consortia development. All the strains were exposed to interact with each other on plate. All three strains B. gladioli, Pseudomonas sp. and B. subtilis were able to grow simultaneously, i.e. they did not inhibit the growth of each other. Hence, we selected these individual strains for development of consortia (Table 1).
Table 1.
Individual strains and its consortia composition.
| Strains and its consortia | Notations |
|---|---|
| Burkholderia gladioli (MTCC 10216) | S1 |
| Pseudomonas sp. (MTCC 9002) | S2 |
| Bacillus subtilis (MTCC 8528) | S3 |
| Burkholderia gladioli + Pseudomonas sp. | S1 + S2 = C1 |
| Burkholderia gladioli + Bacillus subtilis | S1 + S3 = C2 |
| Pseudomonas sp. + Bacillus subtilis | S2 + S3 = C3 |
| Burkholderia gladioli + Pseudomonas sp. + Bacillus subtilis | S1+S2+S3 = C4 |
Abbreviation: S, Strain; C, Consortium.
3.2. Phosphate solubilization
All the individual strains and their consortia formed clear halos zone around colonies by solubilizing TCP on Pikovskayas agar, NBRIP agar and PSM. Pikovskayas agar having bromothymol blue changes its color from blue to yellow due to decrease in pH. The same experiment was carried out by replacing TCP in Pikovskayas agar, NBRIP agar and PSM with DCP, ZP, FP, SP and AP. None of the strains solubilized ZP on Pikovskayas agar, NBRIP agar and PSM, while almost all strains solubilized TCP, DCP, FP and SP except Pseudomonas sp. (MTCC 9002), B. subtilis (MTCC 8528) and consortium-3 on Pikovskayas agar.
Since Pikovskayas agar plate based assay is well known for screening of PSR which gives variable results, therefore, to further confirm the results for phosphate solubilization, NBRIP agar and PSM were used separately. Results on NBRIP agar were almost similar to Pikovskayas agar. But on PSM, almost all strains and their consortia were found to solubilize TCP, DCP and SP while none of them solubilized ZP, FP and AP.
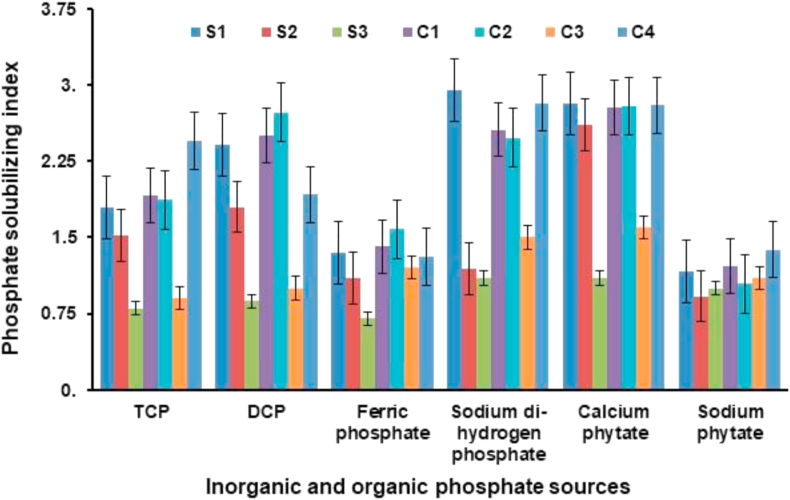
This technique of testing for P solubilization activities has yielded relatively fast outcomes than the agar plate assay of Pikovskayas as the pH shift and zone were visible overnight, i.e. after 12–14 h, while it took 48 h to several days in Pikovskayas agar plate assay. In NBRIP (TCP as sole source of insoluble inorganic P), all strains and their consortia solubilized P except B. subtilis (MTCC 8528). When DCP was used in NBRIP as the sole source of insoluble inorganic P, all strains and consortia solubilized P except B. subtilis (MTCC 8528) and consortium-3. When FP was used in NBRIP as the sole source of insoluble inorganic P, only B. gladioli (MTCC 10216), consortium-1, 2 and 4 solubilized P. When SP was used in NBRIP as the sole source of insoluble inorganic P, all individual strains and consortia solubilized P except B. subtilis (MTCC 8528). When ZP and AP were used as sole source of inorganic P, none of the strain and consortia solubilized P. In PSM (TCP as inorganic P), all strains and their consortia solubilized P except B. subtilis (MTCC 8528). When DCP used (as inorganic P in PSM) all strains and consortia solubilized P except B. subtilis (MTCC 8528) and consortium-3. When SP was used as inorganic P all strains and their consortia solubilized P. When FP, ZP and AP (used as inorganic P) none of the strains and consortia solubilized P. Maximum PSI of 2.82 cm was obtained from consortium-4 with solubilization zone as wide as the colony diameter in PSM having sodium di-hydrogen P (Figure 1).
Figure 1.
Phosphate solubilizing index (PSI) of bacteria and its consortia in different inorganic and organic sources (S, Strain; C, Consortium).
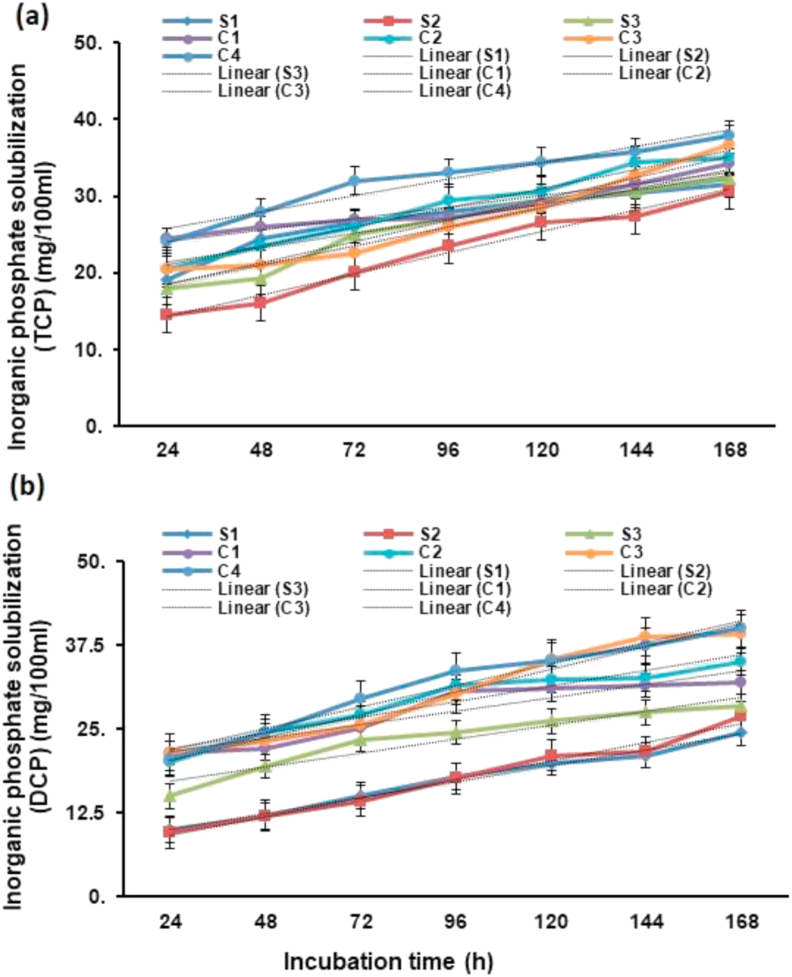
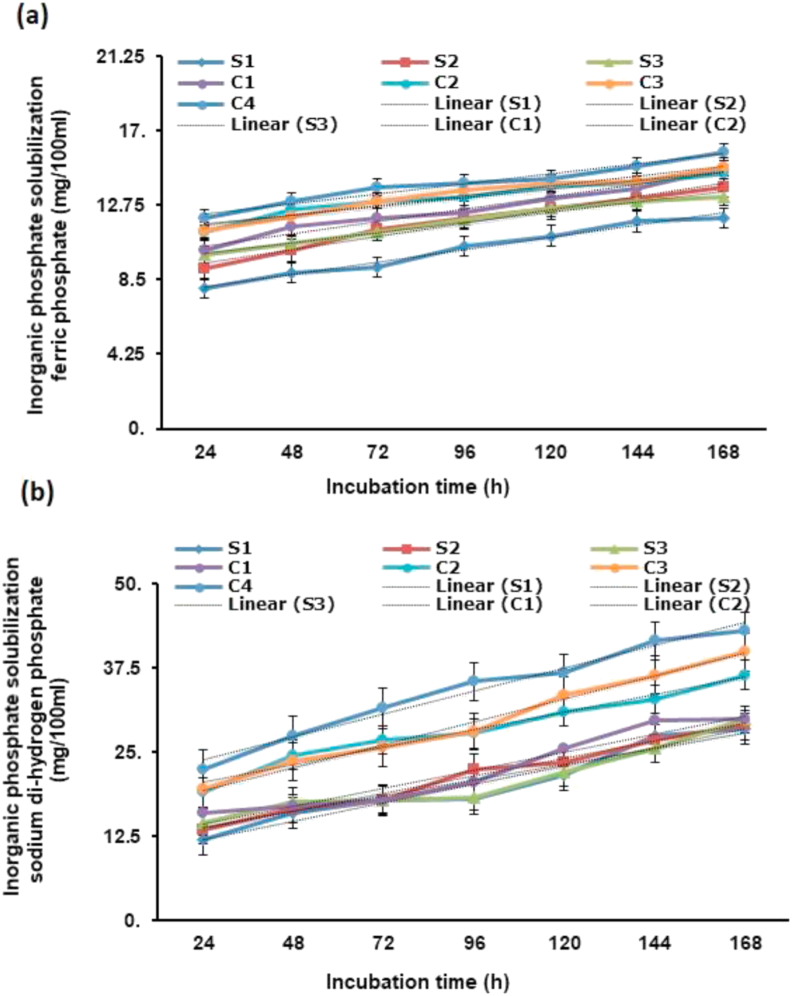
The P solubilization production profile was estimated using NBRIP broth with distinct inorganic P substrates having B. gladioli, Pseudomonas sp., B. subtilis and their consortia. When TCP was used, P solubilization was noted after 14–16 h and it was the maximum after 7th day of incubation. The P solubilization ability of strains was noted to be time-dependent and improved with a reduction in broth pH corresponding to the incubation time. The peak free P was recorded in consortium-4 (37.9 mg/100 ml) followed by consortium-3 (36.78 mg/100 ml) and consortium-2 (34.9 mg/100ml) after 7th day of incubation when TCP was used as in insoluble inorganic P substrate (Figure 2a). When DCP was used the peak free P was recorded in consortium-4 (40.01 mg/100 ml) followed by consortium-3 (39.2 mg/100 ml) and consortium-2 (35.00 mg/100 ml) after 7th day of incubation (Figure 2b). Similarly, when FP was used the peak free P was found in consortium-4 (15.79 mg/100 ml) followed by consortium-3 (14.85 mg/100 ml) and consortium-2 (14.54 mg/100 ml) after 7th day of incubation (Figure 3a). When SP was used the peak free P was found in consortium-4 (43.02 mg/100 ml) followed by consortium-3 (39.89 mg/100 ml) and consortium-2 (36.50 mg/100 ml) after 7th day of incubation (Figure 3b).
Figure 2.
Solubilization of inorganic phosphate (Pi) by bacteria individually and by its consortia in NBRIP with incubation time by using (a) tri-calcium phosphate (TCP) and (b) di-calcium phosphate (DCP) as inorganic phosphate sources.
Figure 3.
Solubilization of inorganic phosphate (Pi) by bacteria and its consortia in NBRIP with incubation time by using (a) ferric phosphate and (b) sodium di-hydrogen phosphate as inorganic phosphate sources.
3.3. Phytase production
All the three strains were screened on the phytase screening medium with two distinct organic P sources such as calcium and sodium phytate for their solubilizing capacity of insoluble organic P. All the strains and consortia solubilized calcium and sodium phytate as verified by the development of halo zone around the spots indicating release of free P.
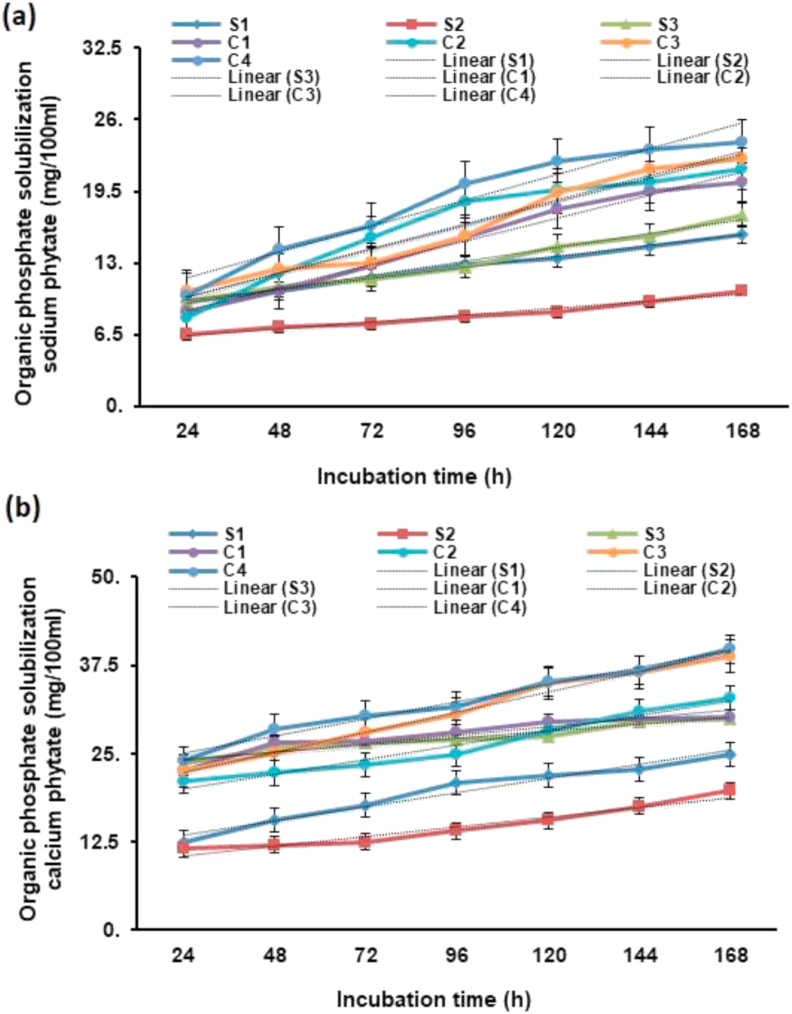
Production profile of P (organic) solubilization was also evaluated with both organic phosphate in phytase screening broth having B. gladioli (MTCC 10216), Pseudomonas sp. (MTCC 9002), B. subtilis (MTCC 8528), and their consortia. The solubilization of P started after 20–24 h and was the maximum after 7th day of incubation; it was time dependent and enhanced corresponding to time of incubation. When calcium phytate was used as substrate of insoluble organic P, the maximum P solubilization was recorded in consortium-4 (39.75 mg/100 ml) followed by consortium-3 (38.89 mg/100 ml) and consortium-2 (32.87 mg/100 ml) after 7th day of incubation (Figure 4a). When sodium phytate was used as a substrate of insoluble organic P, the maximum P solubilization was recorded in consortium-4 (24.01 mg/100 ml) followed by consortium-3 (21.42 mg/100 ml) and consortium-2 (21.54 mg/100 ml) after 7th day of incubation (Figure 4b).
Figure 4.
Solubilization of organic phosphate (Po) by bacteria and its consortia in PSM with incubation time by using (a) sodium phytate (b) calcium phytate as organic phosphate sources.
3.4. Estimation of phosphorus in soil before and after inoculation of bacterial cultures and their consortia
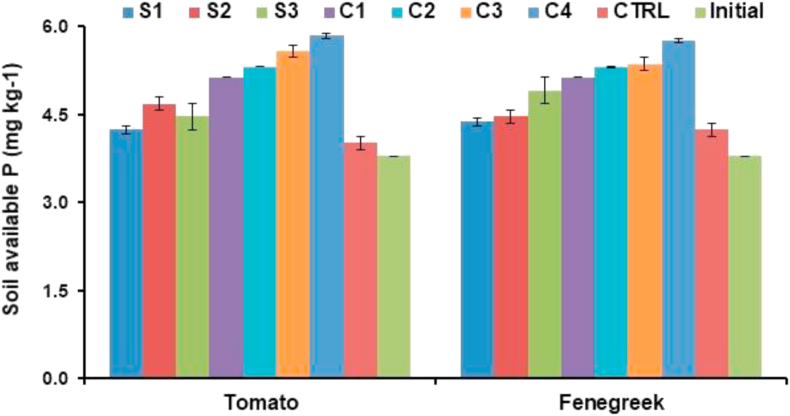
The level of phosphorus in soil was estimated, which was 3.79 mg/kg before inoculation of soil with bacterial cultures and their consortia. After inoculation of bacterial cultures and their consortia, the levels of phosphorus increased in each treatment, which were (5.84 mg/kg) in consortium-4 followed by consortium-3 (5.58 mg/kg) and consortium-2 (5.31 mg/kg) (Figure 5).
Figure 5.
Effect of PSR and its consortia with crops on rhizospheric soils available phosphate (P) at 21 days after inoculation.
3.5. Plate assay
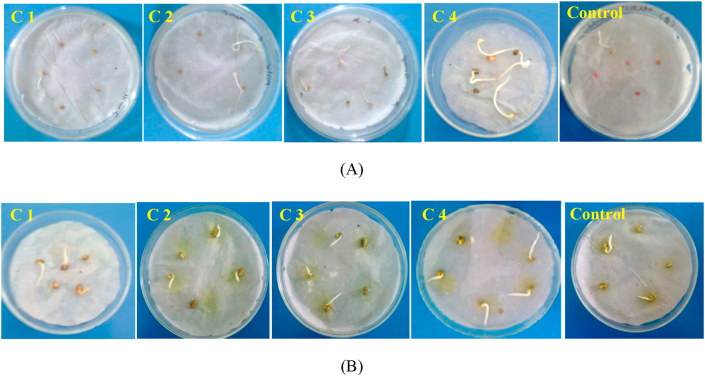
Tomato seeds bio-primed with B. gladioli, Pseudomonas sp., B. subtilis and their consortia enhanced seed germination in tomato plate. Seed germination of tomato in consortium-1, consortium-2, consortium-3, and consortium-4 was 79.9%, 80.1%, 83.3% and 95.8%, respectively. In the control seed germination was 56.6% (Figure 6A).
Figure 6.
Plate assay germination study of inoculated tomato (A) and fenugreek (B) seeds with consortium-1 (B. gladioli + Pseudomonas sp.), consortium-2 (B. gladioli + B. subtilis), consortium-3 (Pseudomonas sp.+ B. subtilis), consortium-4 (B. gladioli + Pseudomonas sp.+B. subtilis), and Control (without inoculation).
Fenugreek seeds bio-primed with B. gladioli, Pseudomonas sp., B. subtilis and their consortia enhanced seed germination of fenugreek in plates. Seed germination of fenugreek in consortium-1, consortium-2, consortium-3 and consortium-4 was 78.9%, 80.1%, 80.0% and 99.75%, respectively. In the control seed germination was 61.52% (Figure 6B).
3.6. Pot assay
Tomato seeds bio-primed with B. gladioli, Pseudomonas sp., B. subtilis individually and with their consortia also increased seed germination in pots as compared to control after 10 DAS. Consortium-1, consortium-2, consortium-3, and consortium-4 treated seeds showed 68.4%, 70.1%, 73.3%, and 80.8% seed germination, respectively, that was 27.47%, 29.24%, 32.33%, and 38.60% greater than that of control (49.61%). Single inoculation, co-inoculation and consortium preparations applied to seeds demonstrated increased germination of seeds and showed improved plant height, plant weight and dry weight in comparison to control. It was noticed that consortium-4 treated seeds showed maximum plant growth (43.94%) as compared to single and co-inoculation after 21 days of sowing.
The fenugreek seeds bio-primed with B. gladioli, Pseudomonas sp., B. subtilis and their consortia also promoted seed germination in pots as compared to control after 10 days of showing. Consortium-1, consortium-2, consortium-3, and consortium-4 treated seeds showed 73.6%, 75.5%, 76.56%, and 90.7% seed germination, respectively, that was 24.55% 26.45%, 27.48%, and 38.78% higher than that of control (55.53). It was observed that consortium-4 treated seeds showed maximum plant growth (45.94%) as compared to single and co-inoculation after 21 days of sowing.
In plate assay the seed germination percentage of both crops was found better in comparison to pot assay because of controlled condition. Consortium coated tomato seeds showed a significant (p > 0.01) increase in seed germination by 95.8% in T7 followed by 83.3% in T6 and 56.6% in control. Consortium coated fenugreek seeds showed a significant increase in percentage of seed germination which was 99.7% in T7 followed by 80% in T6 and 61.52% in control. In both fenugreek and tomato, the maximum number of plant, maximum shoot and root length, fresh and dry plant weight were noted with T7 (B. gladioli, Pseudomonas sp. and B. subtilis) followed by T6 (Pseudomonas sp. and B. subtilis) and T5 (Burkholderia gladioli and B. subtilis) (Table 2). All the data was statistically significant at 1% level of LCD (Table 3).
Table 2.
Effect of PSR and its consortia on seed germination and vegetative growth of Lycopersicon esculentum L.
| PSR strains | Seed germination (%) | Root length (cm) | Shoot length (cm) | Fresh weight (g) |
Dry weight (g) |
||
|---|---|---|---|---|---|---|---|
| Root wt. | Shoot wt. | Root wt. | Shoot wt. | ||||
| S1 | 60.9 | 1.533∗ | 4.266∗ | 0.0050∗ | 0.013ns | 0.0036ns | 0.0050∗ |
| S2 | 65.6 | 1.166ns | 7.10∗∗ | 0.0173∗∗ | 0.086∗∗ | 0.0070∗∗ | 0.0076∗∗ |
| S3 | 70.0 | 1.633∗∗ | 7.00∗∗ | 0.0103∗ | 0.070∗ | 0.0040ns | 0.0070∗∗ |
| C1 | 79.9 | 1.433∗ | 7.10∗∗ | 0.0076∗ | 0.077∗ | 0.0052∗ | 0.0060∗∗ |
| C2 | 80.1 | 1.366∗ | 8.00∗∗ | 0.0163∗∗ | 0.082∗∗ | 0.0070∗∗ | 0.0060∗∗ |
| C3 | 83.3 | 1.466∗ | 6.06∗ | 0.0146∗∗ | 0.112∗∗ | 0.0043ns | 0.0080∗∗ |
| C4 | 95.8 | 1.366∗ | 6.66∗∗ | 0.0046ns | 0.045ns | 0.0016ns | 0.0036∗ |
| Control | 56.6 | 0.600 | 2.700 | 0.0023 | 0.031 | 0.0010 | 0.0016 |
| CD at 1% | 1.028 | 1.915 | 0.0052 | 0.387 | 0.0058 | 0.0231 | |
| CD at 5% | 0.650 | 1.380 | 0.0037 | 0.0279 | 0.0042 | 0.0026 | |
Abbreviations: S1, S2, S3, C1, C2, C3, C4 (as described in Table 1), Control = Without any biological agent; CD = Critical Difference, Value are mean of 3 randomly selected plants from each set. ٭٭ significant at 1%, ٭significant at 5 %; ns = non-significant. as compared to control (non-bacterized seeds).
Table 3.
Effect of PSR strains and its consortia on seed germination and vegetative growth of Trigonella foenum-graecum L.
| PSR strains | Seed germination (%) | Root length (cm) | Shoot length (cm) | Fresh weight (g) |
Dry weight (g) |
||
|---|---|---|---|---|---|---|---|
| Root wt. | Shoot wt. | Root wt. | Shoot wt. | ||||
| S1 | 76.87 | 2.03∗ | 4.26ns | 0.0050ns | 0.013ns | 0.003∗ | 0.0050ns |
| S2 | 74.00 | 2.06∗ | 6.10∗ | 0.017∗∗ | 0.086∗ | 0.007∗∗ | 0.0076∗∗ |
| S3 | 75.00 | 2.13∗ | 6.00∗ | 0.0103∗∗ | 0.080∗ | 0.004∗ | 0.0070∗∗ |
| C1 | 78.90 | 1.23ns | 7.10∗ | 0.056∗ | 0.077∗ | 0.005∗ | 0.0060∗ |
| C2 | 80.10 | 2.23∗ | 8.00∗∗ | 0.063∗∗ | 0.082∗ | 0.007∗ | 0.0060∗ |
| C3 | 80.00 | 2.33∗ | 8.63∗∗ | 0.066∗ | 0.112∗∗ | 0.004∗ | 0.0080∗∗ |
| C4 | 99.75 | 2.43∗∗ | 8.66∗∗ | 0.076∗ | 0.078∗ | 0.0016ns | 0.0056∗ |
| Control | 61.52 | 0.700 | 5.033 | 0.0036 | 0.0443 | 0.0013 | 0.0026 |
| CD at 1% | 2.234 | 2.214 | 0.0053 | 0.0446 | 0.0038 | 0.0035 | |
| CD at 5% | 1.610 | 1.596 | 0.0038 | 0.0321 | 0.0021 | 0.0025 | |
Abbreviations S1, S2, S3, C1, C2, C3, C4 (as described in Table 1), Control = Without any biological agent; CD = Critical Difference, Value are mean of 3 randomly selected plants from each set. ٭٭ significant at 1%, ٭significant at 5 %; ns = non-significant. as compared to control (non-bacterized seeds).
4. Discussion
Qualitative and quantitative analyses of inorganic and organic P solubilization by three potential PSR (B. gladioli, Pseudomonas sp. and B. subtilis) on various culture media revealed that they are very effective phosphate solubilizers as evidenced by the data. In a study, it has been reported that Pseudomonas spp. (PF 23) and Rhizobacteria (RH 24) solubilize insoluble TCP and size of solubilization zone was 22 mm and 11.5 mm respectively on Pikovskayas agar medium [35]. Several PSR (Agrobacterium sp., Bacillus sp., Burkholderia cepacia, Enterobacter sp., Mesorhizobium sp., Pseudomonas sp., Rhizobium sp. etc.) of maize and other plant rhizosphere formed halo zone ranging from 10 to 19 mm on Pikovskayas medium with TCP [36, 37, 38]. Kumar et al. [39] also observed solubilization of TCP, DCP, ZP on Pikovskayas agar, PSM and NBRIP media by Bacillus sp., Pseudomonas sp. and Rhizobium leguminosarum. The highest PSI ranged from 1.13-2.50 by Bacillus sp. PSM-1, Burkholderia cepacia, Pseudomonas sp. PSM-2, Pantoea sp. S32 on TCP and other media were recorded by several groups [36, 40, 41, 42].
During production profile study of P solubilization by potential three PSR and their consortia in NBRIP broth, the PSR liberated phosphorus by decreasing pH of the medium due to production of several organic acids. The least pH values were recorded during the growth phase on 7th days of inoculation. Zhao et al. [36] noticed that amount of solubilized P increases with pH drop of media by organic acid produced by Burkholderia cepacia SCAVK0330. They recorded the amount of solubilized P up to 452 μg/ml and pH of the medium 3.12 on 5th days of inoculation. Kurabachew and Wydra [43] noticed that nine among thirteen isolates efficiently solubilized the insoluble inorganic P which is accompanied by a decline in pH of broth, suggesting production of organic acids by PSR. According to Walpola and Yoon [44] inoculation of individual strain (Pseudomonas agglomerans PSB-1 and Burkholderia anthina PSB-2) or co-inoculation increase soil phosphorus content and decrease soil pH in comparison to un-inoculated soil. Such type of pH drop has also been reported by other author and stated that production of organic and inorganic acid was critical for solubilization of Ca–P complex [45].
All the three potential strains efficiently solubilized CP and SP and produced halo zone around spot inoculation indicating the release of free P. Plant growth promoting rhizobacteria such as Bacillus sp., Burkholderia sp., Enterobacter sp., Pseudomonas sp., and Staphylococcus sp. are the most prominent phytate solublizers [46, 47, 48]. Kumar et al. [39] found in a study that Bacillus sp. Pseudomonas sp., and R. leguminosarum solubilized CP and SP by releasing the free P. Mineralization of these organic P is carried out by several enzymes. Similarly, Ramesh et al. [49] found that B. aryabhattai MDSR7 and MDSR14 significantly solubilized organic phosphate by their phosphatase and phytase activities. Recently, You et al. [50] also observed similar results in maize.
The level of phosphorus in soil was estimated and recorded 3.79 mg/kg before inoculation of soil with bacterial culture and their consortia. After inoculation of bacterial culture and their consortia the level of phosphorus increased in each treatment which was maximum in consortium-4 (5.84 mg/kg) treated soil. A another study in which inoculation of PSR (P. synxantha) and their consortium increased the phosphorus content of the soil and recorded more phosphorus content in consortium treated soil than individual PSR [51]. A good amount (25.29 kg/ha) of phosphorous uptake by grain was also recorded in co-inoculated seed by Bacillus and Rhizobium followed by Bacillus inoculation [52].
After 21 days of sowing of seeds of fenugreek and tomato, the plant parameters like root and shoot length and root and shoot weight enhanced due to individual strains and consortia in comparison to control. The nodulation, root and shoot biomass, straw and grain yield as well as phosphorous and nitrogen level of cowpea improved by PSR Burkholderia sp. [53]. Walpola and Yoon [42] recorded higher plant height and weight in tomato inoculated singly with P. agglomerans and Burkhoderia anthina or co-inoculated with both strains compared to un-inoculated plants. Similar finding were also recorded by Korir et al [23] that co-inoculation of rhizobia with other PGPR enhanced nodulation, plant weight of common bean over the control. Akhtar et al. [52] found that inoculation of Rhizobium sp. and Bacillus sp. improved the grain yield up to 17.5% followed by single inoculation of Bacillus sp. (7.7%) over control.
5. Conclusion
Based on above findings, it might be concluded that the bacterial strains of B. gladioli, Pseudomonas sp. and B. subtilis with their P solubilization ability will attract more attention in the field of bio-fertilization. Present investigation revealed the ability of B. gladioli, B. subtilis and Pseudomonas sp. and their consortia to solubilize insoluble inorganic and organic P into absorbable form for plants, resulting in better growth of crop plants. Therefore, B. gladioli, Pseudomonas sp., B. subtilis and their consortia can be used as bio-inoculants for tomato, fenugreek and other crops.
Declarations
Author contribution statement
Pankaj Kumar: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Abhinav Aeron: Analyzed and interpreted the data.
Niru Shaw: Performed the experiments; Analyzed and interpreted the data.
Ajay Singh: Contributed reagents, materials, analysis tools or data.
V. K. Bajpai: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shailja Pant: Analyzed and interpreted the data; Wrote the paper.
Ramesh Chandra Dubey: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank the Dolphin (PG) College of Science and Agriculture, Chandigarh (Punjab) for providing laboratory facilities.
References
- 1.Savci S. Investigation of effect of chemical fertilizers on environment. APCBEE Procedia. 2012;1:287–292. [Google Scholar]
- 2.Bhardwaj D., Ansari M.W., Sahoo R.K. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories. 2014;13:e66. doi: 10.1186/1475-2859-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youssef M.M.A., Eissa M.F.M. Biofertilizers and their role in management of plant parasitic nematodes: a review. E3 J. Biotechnol. Pharma Res. 2014;5(1):1–6. [Google Scholar]
- 4.Godfray H.C.J., Garnett T. Food security and sustainable intensification. Philos. Trans. Royal Soc. B. 2014;369 doi: 10.1098/rstb.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobbelaere S., Vanderleyden J., Okon Y. Plant growth promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 2003;22:107–149. [Google Scholar]
- 6.Gray E.J., Smith D.L. Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005;37:395–412. [Google Scholar]
- 7.Vessey K.J. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. [Google Scholar]
- 8.Beever R.E., Burns D.J.W. Phosphorus uptake, storage and utilization by fungi. Adv. Bot. Res. 1980;8:127–219. [Google Scholar]
- 9.Glick B.R. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaidi A., Khan M.S., Ahamed A. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung. 2009;56:263–284. doi: 10.1556/AMicr.56.2009.3.6. [DOI] [PubMed] [Google Scholar]
- 11.Castaneda S.M.C., Godoy M.A.M., Ortiz J.P.H. Solubilization of phosphorus from phosphate rocks with Acidithiobacillus thioxidans following a growing-then-recovery process. World J. Microbiol. Biotechnol. 2018;34:e17. doi: 10.1007/s11274-017-2390-7. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan N., Shukla L.B. Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr. J. Biotechnol. 2005;5:850–854. [Google Scholar]
- 13.Mullen M. Phosphorus in soils: biological interactions. Encyclopedia Soils Environ. 2005;3:210–215. [Google Scholar]
- 14.Trivedi P., Sa T. Pseudomonas corrugate (NRRL B-30409) mutants increased phosphate solubilization, organic acid production, and plant growth at lower temperatures. Curr. Microbiol. 2008;56:140–144. doi: 10.1007/s00284-007-9058-8. [DOI] [PubMed] [Google Scholar]
- 15.Prabhu N., Borkar S., Garg S. Phosphate solubilization by microorganisms: overview, mechanisms, applications and advances. In: Meena S.N., Naik M.M., editors. Advances in Biological Science Research (A Practical Approach) Academic Press, U.S.A.; 2019. pp. 161–176. [Google Scholar]
- 16.Baniaghil N., Arzanesh M.H., Ghorbanli M. The effect of plant growth promoting rhizobacteria on growth parameters, antioxidant enzymes and microelements of canola under salt stress. J. Appl. Environ. Biol. Sci. 2013;3(1):17–27. [Google Scholar]
- 17.Verma J.P., Yadav J., Tiwari K.N. Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol. Eng. 2013;51:282–286. [Google Scholar]
- 18.Abbasi M.K., Sharif S., Kazmi M. Isolation of plant growth promoting rhizobacteria from wheat rhizosphere and their effect on improving growth, yield and nutrient uptake of plants. Plant Biosyst. 2011;145:159–168. [Google Scholar]
- 19.Bhattacharyya P., Jha D. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 2011;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- 20.Khan M.S., Zaidi A., Ahmad E. Mechanism of phosphate solubilization and physiological functions of phosphate-Solubilizing microorganisms. In: Khan M.S., Zaidi A., Musarrat M., editors. Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology. Springer International Publishing Switzerland; 2014. pp. 31–62. [Google Scholar]
- 21.Ma Y., Rajkumar M., Luo Y. Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. J. Hazard Mater. 2011;195:230–237. doi: 10.1016/j.jhazmat.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Yanni Y.G., Dazzo F.B. Enhancement of rice production using endophytic strains of Rhizobium leguminosarum bv. Trifolii in extensive field inoculation trials within the Egypt Nile delta. Plant Soil. 2010;336:129–142. [Google Scholar]
- 23.Korir H., Mungai N.W., Thuita M. Co-inoculation effect of Rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front. Plant Sci. 2017;8:e141. doi: 10.3389/fpls.2017.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olanrewaju O.S., Babalola O.O. Bacterial consortium for improved maize (Zea mays L.) Production. Microorganisms. 2019;1(11):519. doi: 10.3390/microorganisms7110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L.N., Wang D.C., Hu Q. Consortium of plant growth promoting rhizobacteria strains suppresses sweet pepper disease by altering the rhizosphere microbiota. Front. Microbiol. 2019;23(10):1668. doi: 10.3389/fmicb.2019.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi B., Chaudhary A., Singh H. Plant Soil; 2020. Prospective Evaluation of Individual and Consortia Plant Growth Promoting Rhizobacteria for Drought Stress Amelioration in rice (Oryza Sativa L.) [Google Scholar]
- 27.Contreras-Cornejo H.A., Macha-Rodrhguez L., Herrera-Estrella A. The 4-phosphopantetheinyl transferees of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant Soil. 2014;379:261–274. [Google Scholar]
- 28.Khiriya K.D., Singh B.P. Effect of phosphorus and farmyard manure on yield, yield attributes and nitrogen, phosphorus and potassium uptake of fenugreek (Trigonella foenum-graecum) Indian J. Agron. 2003;48(1):62–65. [Google Scholar]
- 29.Bhunia S.R., Chauhan R.P.S., Yadav B.S. Effect of phosphorus, irrigation and Rhizobium on productivity, water use and nutrient uptake in fenugreek (Trigonella foenum-graecum L) Indian J. Agron. 2006;51:239–241. [Google Scholar]
- 30.Pierson E.A., Weller D.M. Use of mixtures of fluorescent pseudomonads to suppress take all and improve the growth of wheat. Phytopathology. 1994;84:940–947. [Google Scholar]
- 31.Nautiyal C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 32.Dubey R.C., Maheshwari D.K. S Chand and Co; New Delhi, India: 2012. Practical Microbiology. [Google Scholar]
- 33.Idriss E.E., Makarewicz O., Farouk A. Extracellular phytase activity of Bacillus amyloliquefaciens FZ45 contributes to its plant growth promoting effect. Microbiology. 2002;148:2097–2109. doi: 10.1099/00221287-148-7-2097. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe F.S., Olsen S.R. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 1965;29:677–678. [Google Scholar]
- 35.Singh R., Arora N.K., Gautam P., Lal S. Enhancement of plant growth of Trigonella foenumgraecum by co-inoculation of Pseudomonas fluorescence and Rhizobium for the sustainability of agriculture. Asian J. Plant Sci. Res. 2013;3(3):74–79. [Google Scholar]
- 36.Zhao K., Penttinen P., Zhang X. Maize rhizosphere in Sinchuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 2014;169:76–82. doi: 10.1016/j.micres.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Chawngthu L., Hnamte R., Lalfakzuala R. Isolation and characterization of rhizospheric phosphate solubilizing bacteria from wetland paddy field of Mizoram, India. Geomicrobiol. J. 2020;37(4):366–375. [Google Scholar]
- 38.Mendoza-Arroyo G.E., Chan-Bacab M.J., Aguila-Ramírez R.N. Inorganic phosphate solubilization by a novel isolated bacterial strain Enterobacter sp. ITCB-09 and its application potential as biofertilizer. Agriculture. 2020;10:383. [Google Scholar]
- 39.Kumar P., Pandey P., Dubey R.C. Bacteria consortium optimization improves nutrient uptake, nodulation, disease suppression and growth of the common bean (Phaseolus vulgaris) in both pot and field studies. Rhizosphere. 2016;2:13–23. [Google Scholar]
- 40.Karpagam T., Nagalakshmi P.K. Isolation and characterization of phosphate solubilizing microbes from agriculture soil. Int. J. Curr. Microbiol. Appl. Sci. 2014;3(3):601–614. [Google Scholar]
- 41.Chen Q., Liu S. Identification and characterization of the phosphate-solubilizing bacterium Pantoea sp. s32 in reclamation soil in Shanxi, China. Front. Microbiol. 2019;19(10):2171. doi: 10.3389/fmicb.2019.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chouyia F.E., Romano I., Fechtali T. P-Solubilizing Streptomyces roseocinereus MS1B15 with multiple plant growth-promoting traits enhance barley development and regulate rhizosphere microbial population. Front. Plant Sci. 2020;7(11):1137. doi: 10.3389/fpls.2020.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurabachew H., Wydra K. Characterization of plant growth promoting rhizobacteria and their potential as bioprotectant against tomato bacterial wilt caused by Ralstonia solanacereum. Biol. Cont. 2013;67:75–83. [Google Scholar]
- 44.Walpola B.C., Yoon M.H. Isolation and characterization of phosphate solubilizing bacteria and their co-inoculation efficiency on tomato plant growth and phosphorous uptake. Afr. J. Microbiol. Res. 2013;7(3):266–275. [Google Scholar]
- 45.Whitelaw M.A. Growth promotion of plants inoculated with phosphate solubilizing fungi. Adv. Agron. 2000;69:99–151. [Google Scholar]
- 46.Hussin A.S.M., Farouk A.E., Greiner R. Phytate-degrading enzyme production by bacteria isolated from Malaysian soil. World J. Microbiol. Biotechnol. 2007;23(12):1653–1660. doi: 10.1007/s11274-007-9412-9. [DOI] [PubMed] [Google Scholar]
- 47.Alori E.T., Glick B.R., Babalola O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017;8:971. doi: 10.3389/fmicb.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan W., Qin Y., Wu H. Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 2020;23(11):752. doi: 10.3389/fmicb.2020.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramesh A., Sharma S.K., Yadav N. Phosphate mobilization from native soil P-pool upon inoculation with phytate-mineralizing and phosphate solubilizing Bacillus aryabhattai isolates for improved P-acquisition and growth of soybean and wheat crops in microcosm conditions. Agric. Res. 2014;3(2):118–127. [Google Scholar]
- 50.You M., Fang S., MacDonald J. Isolation and characterization of Burkholderia cenocepacia CR318, a phosphate solubilizing bacterium promoting corn growth. Microbiol. Res. 2020;233:126395. doi: 10.1016/j.micres.2019.126395. [DOI] [PubMed] [Google Scholar]
- 51.Gupta M., Kiran S., Gulati A. Isolation and identification of phosphate solubilizing bacteria able to enhance the growth and aloin-A biosynthesis of Aleo barbadensis Miller. Microbiol. Res. 2012;167:358–363. doi: 10.1016/j.micres.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Akhtar N., Mujeeb F., Qureshi M.A. Phosphate solubilizing potential of Rhizobium and Bacillus species for enhancing yield and available phosphorus in maize crop (Zea mays) Int. J. Agric. Res. 2014;4(1):58–66. [Google Scholar]
- 53.Linu M.S., Stephen J., Jisha M.S. Phosphate solubilizing Gluconacetobacter sp. (MTCC 8368) and Burkholderia sp. (MTCC 8369) and their potential interaction with cowpea (Vigna unguiculata L. Walp) Int. J. Agric. Res. 2009;4(2):79–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article.