This diagnostic/prognostic study describes the use of cell-free transcriptomics, urine metabolomics, and plasma proteomics for identifying the biological measurements associated with preterm birth.
Key Points
Question
What maternal biological modalities are associated with preterm birth (PTB)?
Findings
In this diagnostic/prognostic study of 81 pregnant women from 5 birth cohorts in low- and middle-income countries, several correlates of preterm birth in urine and blood were found to be associated with PTB. Although cohort-specific signatures were present, a machine learning algorithm was able to generate a model that was capable of predicting PTB across the cohorts.
Meaning
Results of this study suggest that most PTBs can be predicted using blood and urine samples collected early in the pregnancy, providing opportunities for interventions.
Abstract
Importance
Worldwide, preterm birth (PTB) is the single largest cause of deaths in the perinatal and neonatal period and is associated with increased morbidity in young children. The cause of PTB is multifactorial, and the development of generalizable biological models may enable early detection and guide therapeutic studies.
Objective
To investigate the ability of transcriptomics and proteomics profiling of plasma and metabolomics analysis of urine to identify early biological measurements associated with PTB.
Design, Setting, and Participants
This diagnostic/prognostic study analyzed plasma and urine samples collected from May 2014 to June 2017 from pregnant women in 5 biorepository cohorts in low- and middle-income countries (LMICs; ie, Matlab, Bangladesh; Lusaka, Zambia; Sylhet, Bangladesh; Karachi, Pakistan; and Pemba, Tanzania). These cohorts were established to study maternal and fetal outcomes and were supported by the Alliance for Maternal and Newborn Health Improvement and the Global Alliance to Prevent Prematurity and Stillbirth biorepositories. Data were analyzed from December 2018 to July 2019.
Exposures
Blood and urine specimens that were collected early during pregnancy (median sampling time of 13.6 weeks of gestation, according to ultrasonography) were processed, stored, and shipped to the laboratories under uniform protocols. Plasma samples were assayed for targeted measurement of proteins and untargeted cell-free ribonucleic acid profiling; urine samples were assayed for metabolites.
Main Outcomes and Measures
The PTB phenotype was defined as the delivery of a live infant before completing 37 weeks of gestation.
Results
Of the 81 pregnant women included in this study, 39 had PTBs (48.1%) and 42 had term pregnancies (51.9%) (mean [SD] age of 24.8 [5.3] years). Univariate analysis demonstrated functional biological differences across the 5 cohorts. A cohort-adjusted machine learning algorithm was applied to each biological data set, and then a higher-level machine learning modeling combined the results into a final integrative model. The integrated model was more accurate, with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% CI, 0.72-0.91) compared with the models derived for each independent biological modality (transcriptomics AUROC, 0.73 [95% CI, 0.61-0.83]; metabolomics AUROC, 0.59 [95% CI, 0.47-0.72]; and proteomics AUROC, 0.75 [95% CI, 0.64-0.85]). Primary features associated with PTB included an inflammatory module as well as a metabolomic module measured in urine associated with the glutamine and glutamate metabolism and valine, leucine, and isoleucine biosynthesis pathways.
Conclusions and Relevance
This study found that, in LMICs and high PTB settings, major biological adaptations during term pregnancy follow a generalizable model and the predictive accuracy for PTB was augmented by combining various omics data sets, suggesting that PTB is a condition that manifests within multiple biological systems. These data sets, with machine learning partnerships, may be a key step in developing valuable predictive tests and intervention candidates for preventing PTB.
Introduction
Preterm birth (PTB) is defined by the World Health Organization as the delivery of a live infant before the completion of 37 weeks of gestation.1,2 The worldwide rate of PTB in 2014 was estimated to be 10.6% (uncertainty interval, 9.0%-12.0%), with 80% of all cases occurring in South Asia and sub-Saharan Africa.2 Many risk factors for PTB have been highlighted in previous studies and include obstetrical (eg, previous PTB and multiple gestation), medical (eg, maternal obesity, diabetes, and chronodisruption), and external (eg, smoking and maternal stress) conditions.3,4,5,6,7,8,9 For example, a meta-analysis of individual- and population-level attributes among 4.1 million births concluded that “unknown factors requiring further research to act upon account for ~2/3 of the preterm birth rate.”10(p13) Unveiling and elucidating the role of early biological antecedents of PTB has been deemed a necessary step toward developing new diagnostic tests and therapeutic interventions.11,12,13 Biological investigations into the mechanisms of PTB are complicated, as indicated by accumulating evidence that distinct patient subpopulations follow divergent biological trajectories.14,15 Given this heterogeneity, simultaneously studying diverse cohorts is critical for identification of generalizable biological pathways.16
Recent technological advances have enabled the characterization of a broad range of biological changes during pregnancy. Biological layers explored include single-cell profiling of signaling pathways,17 measurements of plasma cell-free ribonucleic acid (cfRNA),18 proteome19,20 and metabolome21 characterization of the microbiome,14,22 and detailed genomics analysis.23 In addition, a recent multiomics investigation demonstrated that biological changes during normal pregnancy involve a number of intricate interactions of biological processes, which can be measured using a coordinated set of assays.24 The integration of the large, multidimensional data sets generated in a multiomics setting requires complex machine learning pipelines that will remain robust in the face of the inconsistent intrinsic properties of these high-throughput assays and cohort-specific variations.15
To our knowledge, this is the first multiomics analysis of term and preterm pregnancies from multiple cohorts in low- and middle-income countries (LMICs). These cohorts were established using biorepositories of samples and phenotypic data for studying maternal and fetal outcomes collected and stored from diverse populations of South Asia and sub-Saharan Africa. The study aimed to investigate the ability of transcriptomics and proteomics profiling of blood plasma and metabolomics analysis of urine to identify early biological measurements associated with PTB.
Methods
Approval was obtained from the Stanford University Institutional Review Board, and ethical exemptions were sought and obtained independently from the respective country by each birth cohort supported by the Alliance for Maternal and Newborn Health Improvement (AMANHI) and the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) biorepositories. Written informed patient consent was obtained from each participant in the original cohorts and extends to the present study. We followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline. This study analyzed plasma and urine samples collected from May 2014 to June 2017, and data were analyzed from December 2018 to July 2019.
Participants and Study Design
The study population comprised pregnant women selected from 5 biorepository-supported cohorts in Matlab, Bangladesh; Lusaka, Zambia; Sylhet, Bangladesh; Karachi, Pakistan; and Pemba, Tanzania. No compensation or incentives were provided for participating in this study.
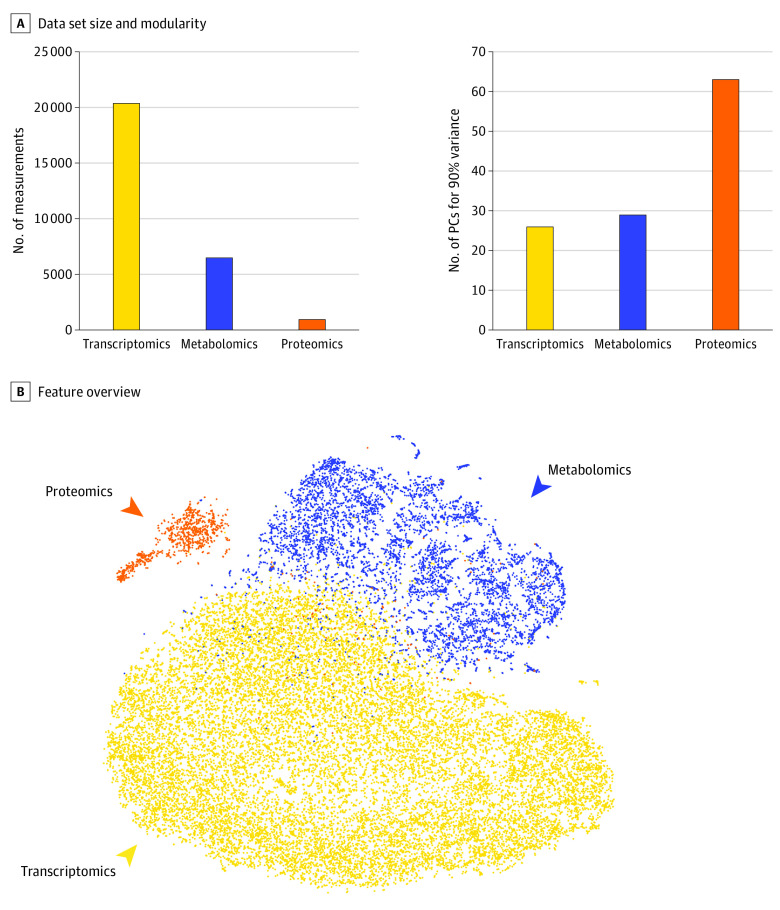
Plasma samples were assayed to measure targeted proteins and cfRNA, and urine samples were analyzed for metabolites. The cfRNA analysis resulted in 20 659 measurements, the targeted proteomics assay measured 1002 proteins in plasma, and 6630 metabolites were measured in urine. The number of measurements of these assays did not correlate with their modularity, as indicated by the number of principal components needed to account for 90% of the total variance (Figure 1A). This result highlighted the need for a 2-layer metadimensional integrative approach to prevent the assays with more measurements to bias the predictive models (eMethods in the Supplement). An overview of the entire data set was produced by first calculating a correlation network of all available measurements and then producing a 2-dimensional layout for visualization using the t-SNE25 algorithm (Figure 1B).
Figure 1. Study Overview.
A, The 3 data sets (plasma cell-free ribonucleic acid [cfRNA] or transcriptomics, metabolomics, and proteomics) produced a number of different features and had a range of correlations among the measured features. The internal correlation between features from each data set was quantified using the number of principal components (PCs) needed to capture 90% variance (eg, the cf-RNA data set had the most features but was highly correlated internally; therefore, fewer PCs were needed). B, A 2-dimensional representation of all measurements demonstrates the correlation between subsets of urine metabolites and cfRNA detected in plasma as well as a limited number of plasma proteins.
Biological Assays
From all AMANHI and GAPPS cohorts, trained phlebotomists collected blood samples for centrifugation and aliquoting of serum, plasma, and buffy coat for storage and future analyses. In addition, maternal urine was collected in parallel. Collection and processing of all sample types were performed according to harmonized operating procedures at all study cohorts. The eMethods in the Supplement provides details on the biological assays.
Statistical Analysis
Data were analyzed from December 2018 to July 2019. All analyses were performed with R, version 3.6.1 (R Foundation for Statistical Computing). All multivariate modeling was performed with a 2-layer cross-validation strategy to prevent overfitting of the data and to ensure generalizability. Mixed-effect models were used to account for cohort-specific variations (eMethods in the Supplement). The analysis is independently reproducible. The measured features from all 3 omics data sets (transcriptomics, metabolomics, and proteomics); the algorithms and source codes for reproduction of the results; and an interactive website for visualizing the entire data set, the feature evaluation scores for PTB and gestational age (GA) at sampling, and the pathway enrichment analysis are available online (https://nalab.stanford.edu/multiomicsmulticohortpreterm/).
We used linear discriminant analysis and principal component analysis (PCA), respectively, to create a 2-dimensional representation of the entire cohort with cohort labels as the supervised guide and without supervised information. To confirm the presence of cohort-specific signatures, we used random forest analysis. We created models for each patient to estimate GA at the time of sample collection. To simultaneously optimize the integrative model and test the performance of the model on previously unseen patients, we applied a cross-validation strategy. To predict PTB (GA at delivery <37 weeks), we used a leave-one-out cross-validation procedure to test the models on blinded participants.
Results
Of the 81 pregnant women included in this study, 39 had PTBs (48.1%) and 42 had term pregnancies (51.9%). The mean (SD) maternal age was 24.8 (5.3) years. The median sampling time was 13.6 weeks of gestation, according to ultrasonography (Figure 1A).
Data Quality Assessment
To investigate cohort-specific data signatures, PCA was used to create a 2-dimensional representation of the entire cohort for each biological modality and all modalities combined (eFigure 1A in the Supplement). The PCA demonstrated that the largest source of variation in the data was not driven by fundamental differences between the cohorts. Supervised linear discriminant analysis26 confirmed the existence of more subtle cohort-specific signatures that were not statistically significant enough to be visualized in an unsupervised PCA (eFigure 1B in the Supplement). The presence of cohort-specific signatures was confirmed using random forest analysis27 that underwent cross-validation to predict the cohort from which the patient was selected exclusively on the basis of each biological modality (eFigure 1C in the Supplement).
The impact of sample storage time was quantified with random forest analysis that underwent cross-validation in which the number of days between sample collection and laboratory analyses was used as a continuous prediction target. The results were statistically significant (thresholds of P = 1.25762 × 10−01 for transcriptomics, P = 8.83433 × 10−06 for metabolomics, and P = 5.56758 × 10−02 for proteomics) only in the case of the urine metabolomics data set, indicating the potential for sample degradation over time (eFigure 1D in the Supplement). However, this result did not confound the design of this study as GA at delivery did not correlate with storage time (r = –0.092; P > .41).
Predictive Modeling of Chronicity of Pregnancy
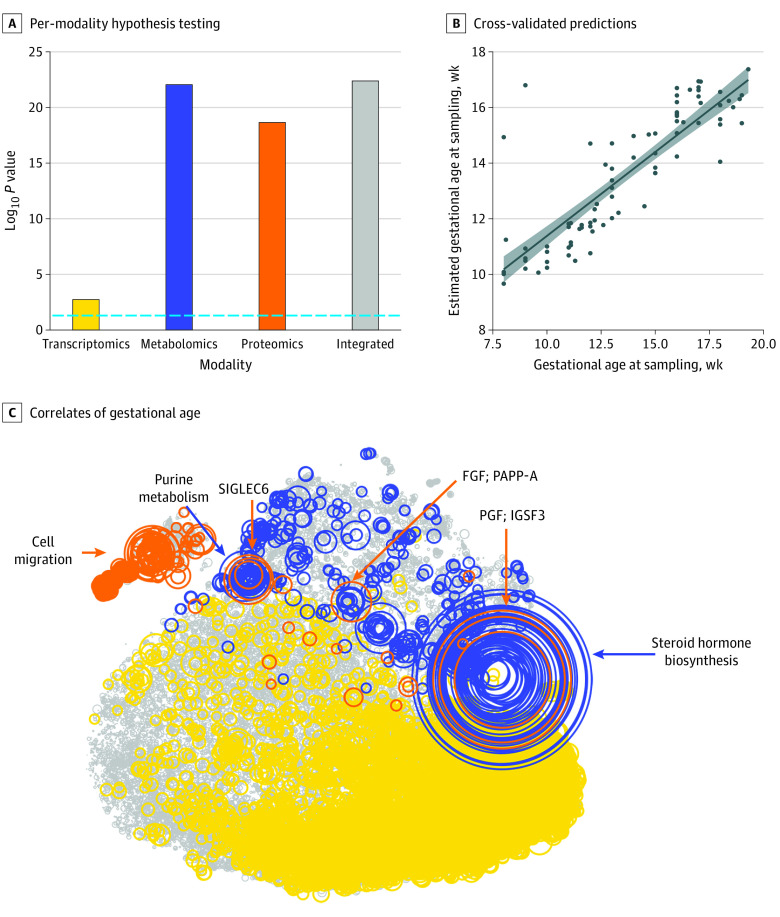
We built models to estimate GA at the time of sample collection (as a surrogate for the chronicity of pregnancy) for each patient. A cross-validation strategy was used to simultaneously optimize the integrative model and test the performance of the model on previously unseen patients. Models built on all 3 modalities (transcriptomics, metabolomics, and proteomics) as well as the integrated model were statistically significantly correlated with GA at the time of sample collection (transcriptomics: 1.736089 × 10−03; metabolomics: 8.936983 × 10−23; proteomics: 2.227379 × 10−19; and integrated model: 8.990768 × 10−22; Bonferroni-adjusted Spearman correlation P < .05) (Figure 2A and B). The features that most correlated with the progression of pregnancy (Spearman correlation P < .05) are color-coded in Figure 2C. A cluster of highly correlated metabolomics and proteomics features was identified that included the trophoblast-derived placental growth factor (PGF). Previous studies have demonstrated that PGF plays a substantial role in the pathogenesis of preeclampsia but has not been associated with spontaneous PTB.28,29 Pathway analysis30 of the metabolites in this module indicated the enrichment of the steroid hormone biosynthesis pathway (Fisher test for pathway enrichment analysis P < 1.2 × 10−12). The purine metabolism pathway was enriched in an additional module of metabolites (Fisher test for pathway enrichment analysis P < 1.7 × 10−5). Other proteins that were included in the model and close to this cluster were PAPP-A (pregnancy-associated plasma protein A), MMP-7 (matrix metallopeptidase 7), FGF and FGFBP1 (fibroblast growth factors), and SIGLEC6 (sialic acid binding Ig-like lectin 6), all of which play important roles in placental development.31,32,33,34 An additional cluster of proteins associated with cell migration and localization was identified by gene ontology analysis (Protein Analysis Through Evolutionary Relationships overrepresentation P < 10 × 10−7).
Figure 2. Prediction of Gestational Age (GA) at the Time of Sample Collection.
A, A cross-validation strategy was used to simultaneously optimize the integrated model and test the performance of the model on previously unseen patients. Models built on all 3 modalities (transcriptomics, metabolomics, and proteomics) and the integrated model were statistically significantly correlated with GA at the time of sample collection (Bonferroni-adjusted Spearman correlation P < .05). B, The correlation between GA at the time of sample collection and the estimated values on the blinded samples are shown. The shaded area represents the 95% CI. C, The features correlated with the progression of pregnancy (Spearman correlation P < .05) are color-coded according to biological modality. FGF indicates fibroblast growth factor; IGSF3, immunoglobulin superfamily member 3; PAPP-A, pregnancy-associated plasma protein A; PGF, placental growth factor; and SIGLEC6, sialic acid binding Ig-like lectin 6.
To further highlight the interplay between plasma proteins and urine metabolites, we developed a random forest model to estimate the PGF levels of each patient using only the urine metabolomics data set (eFigure 2 in the Supplement). Overall, this analysis highlighted the potential for biological profiling for estimating GA during pregnancy (a substantial challenge in LMICs) and the use of urine-based metabolite biomarkers as low-cost surrogates for models developed through multiomics analysis.
Predictive Modeling of PTB
For prediction of PTB (GA at delivery <37 weeks), we used a leave-one-out cross-validation procedure to test the models on blinded participants. Before training the model using the entire data set, the feature space was limited to the top features in the cohort that corresponded to the blinded sample based on univariate testing. Overall, the models relied on a subset of all available features. The median number of features used by the models during cross-validation was 36 for transcriptomics, 35 for metabolomics, and 9 for proteomics. To combine predictions from each model, we developed an additional integration layer to produce the final weighted probabilities for statistical testing. The integrated model was more accurate than the model for each independent modality (Figure 3A). The mean area under the receiver operating characteristic curve (AUROC) and 95% CI for each modality were as follows: transcriptomics (AUROC, 0.73; 95% CI, 0.61-0.83), metabolomics (AUROC, 0.59; 95% CI, 0.47-0.72), proteomics (AUROC, 0.75; 95% CI, 0.64-0.85), and integrated (AUROC, 0.83; 95% CI, 0.72-0.91) (Figure 3A). eFigure 3 in the Supplement provides a comparison against other machine learning strategies applied to the same data set (support vector regression AUROC, 0.57; random forest AUROC, 0.66; lasso AUROC, 0.68; Gaussian process AUROC, 0.71; supervised learning cohort-adjusted model AUROC, 0.83; merging AUROC, 0.71; stacked generalization AUROC, 0.76; data integration cohort-adjusted model AUROC, 0.83). In an independent analysis, this same pipeline was used to model participants who were randomly assigned to case and control groups, confirming that the findings presented in Figure 3 did not result from model overfitting (transcriptomics AUROC, 0.54; metabolomics AUROC, 0.50; proteomics AUROC, 0.50; integrated AUROC, 0.50) (eFigure 4 in the Supplement).
Figure 3. Predictive Modeling of Preterm Birth (PTB).
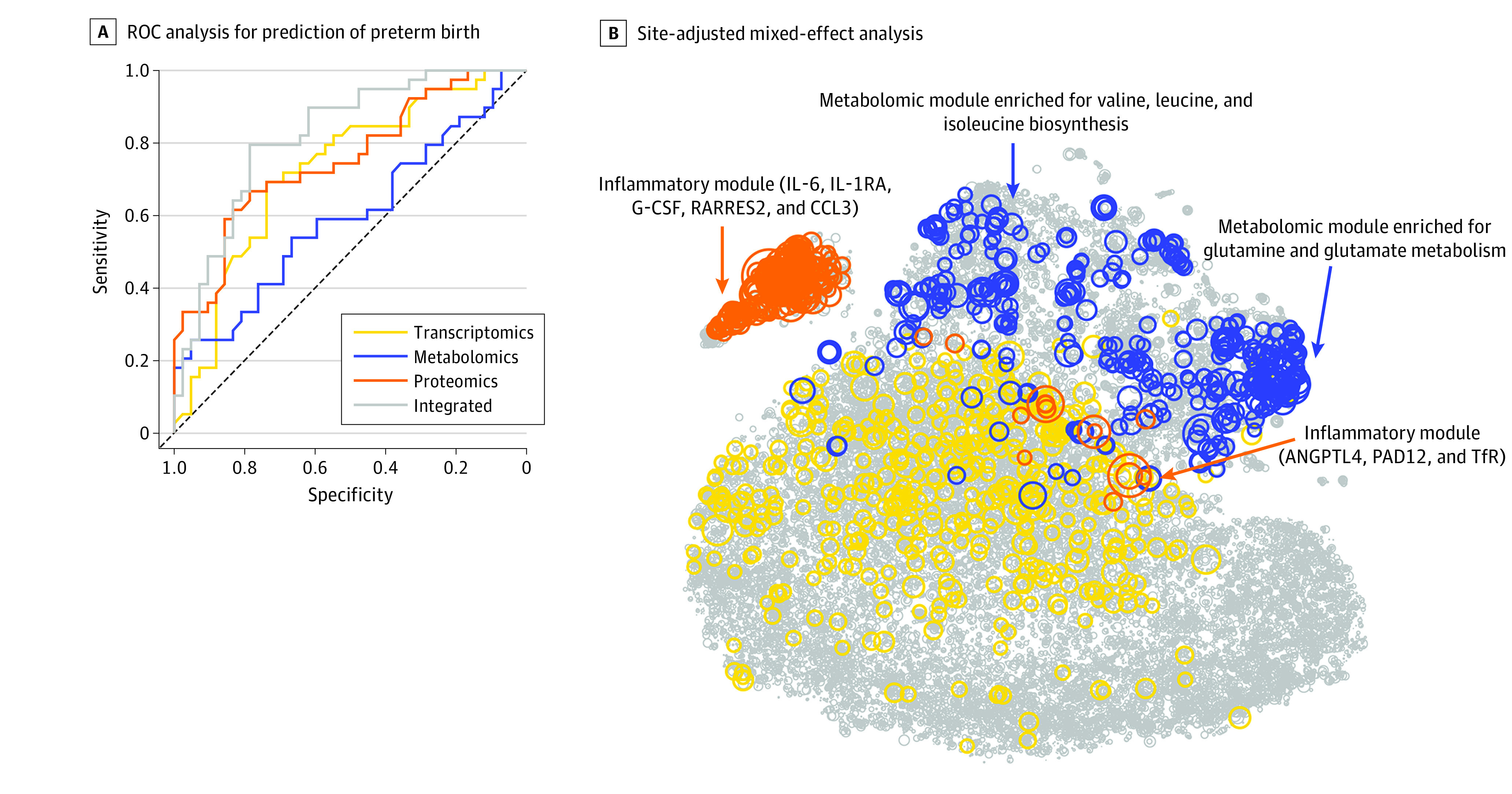
A, This receiver operating characteristic (ROC) curve analysis used each biological modality and the integrated approach. The mean area under the ROC curve and 95% CI for each modality were as follows: transcriptomics (AUROC, 0.73; 95% CI, 0.61-0.83), metabolomics (AUROC, 0.59; 95% CI, 0.47-0.72), proteomics (AUROC, 0.75; 95% CI, 0.64-0.85), and integrated (AUROC, 0.83; 95% CI, 0.72-0.91). B, Circle size is proportional to −log10 (Wilcoxon) P value for discrimination between term pregnancies and PTBs. Top features included an inflammatory module (which included interleukin 6 [IL-6]; IL-1 receptor antagonist [IL-1RA], a regulatory member of the IL-1 family whose expression is induced IL-1β under inflammatory conditions; granulocyte colony-stimulating factor [G-CSF]; retinoic acid receptor responder protein 2 [RARRES2]; chemokine ligand 3 [CCL3]; angiopoietin-like 4 [ANGPTL4]; protein-arginine deiminase type II [PADI2]; and transferrin receptor [TfR]) and a metabolomic module (which was enriched for glutamine and glutamate metabolism [Fisher test for pathway enrichment analysis P < 4.4 × 10−9] and valine, leucine, and isoleucine biosynthesis pathways [P < 7.3 × 10−6]).
Field workers were trained to collect detailed phenotypic and demographic data from the women and their families through scheduled household visits during pregnancy and postpartum. Clinical covariates were manually harmonized across all 5 cohorts. Of all the variables collected, only the weight of the baby and GA at delivery were statistically significantly correlated with the final outcome of the model predicting PTB (Spearman correlation = 0.73). (eFigure 5 and eTable in the Supplement). This finding confirmed that the model was not confounded by the other measured clinical covariates.
Given the statistically significant differences observed across various cohorts, we used mixed-effect models (with each cohort encoded as a random effect) to compare the distribution of each measurement between term pregnancies and PTBs (Figure 3B). Top features were contained within 2 correlated modules: (1) an inflammatory module, which included interleukin 6 (IL-6), IL-1 receptor antagonist (IL-1RA, a regulatory member of the IL-1 family whose expression is induced IL-1β under inflammatory conditions35,36), granulocyte colony-stimulating factor (G-CSF), retinoic acid receptor responder 2 (RARRES2), and chemokine ligand 3 (CCL3), and (2) a metabolomic module, which primarily consisted of urine metabolites enriched for glutamine and glutamate metabolism (Fisher test for pathway enrichment analysis P < 4.4 × 10−9)30 and valine, leucine, and isoleucine biosynthesis pathways (P < 7.3 × 10−6).37
The presence of inflammatory mediators among the features correlated with PTB is consistent with finding in previous studies that suggested dysfunctional immune adaptations during pregnancy was central to the pathogenesis of PTB.38,39 However, the predictive model also highlighted a set of proteomic features with no known inflammatory properties that were correlated with features from the inflammatory module. These proteins included protein-arginine deiminase type II (PADI2), a peptidylarginine deiminase that is responsible for protein citrullination and implicated in parturition and sensing infections40,41; transferrin receptor (TfR), which is implicated in iron transport; angiopoietin-like 4 (ANGPTL4), which regulates glucose homeostasis and lipid metabolism42; and RARRES2, an adipokine that is increased in metabolic syndrome and gestational diabetes.43,44
To ascertain whether observed correlations between these proteins and the inflammatory module reflected biologically relevant inflammatory properties, we examined the capacity of each of these factors to stimulate human peripheral blood leukocytes using an ex vivo mass cytometry assay.45 The activity of major intracellular signaling responses previously17 implicated in maternal immune adaptations during pregnancy was assessed at baseline and after a 15-minute stimulation in major innate and adaptive immune cell types (eMethods in the Supplement). As expected, robust and cell-specific signaling responses along the JAK/STAT and MyD88 signaling pathways were observed in classical monocytes (CMC) after stimulation with known proinflammatory cytokines, including IL-6 (mean [SD] pSTAT3 ArcSinh ratio over endogenous signal, 2.64 [0.22]; false discovery rate [FDR]–adjusted vs unstimulated P < 1.0 × 10−6), G-CSF (mean [SD] pSTAT5 ArcSinh ratio over endogenous signal, 0.42 [0.12]; P = .007), and CCL3 (mean [SD] pCREB ArcSinh ratio over endogenous signal, 0.35 [0.09]; P < 1.0 × 10−6) (eFigures 6 and 7 and the eMethods in the Supplement). Stimulation with PADI2 activated the key elements of the MyD88 pathway, including P38 (mean [SD] ArcSinh ratio over endogenous signal, 0.91 [0.52]; FDR-adjusted vs unstimulated P = .007), MK2 (mean [SD] ArcSinh ratio over endogenous signal, 0.38 [0.10]; P = .002), and NFkB (mean [SD] ArcSinh ratio over endogenous signal, 0.14 [0.03]; P = .009), in monocytes, although little or no signaling responses were observed after stimulation with ANGPTL4, TfR, or RARRES2.
We also tested whether stimulation with the most informative proteomic features of the predictive model of PTB would alter the effector function of circulating immune cells. To this end, we quantified the intracellular expression of select cytokines in circulating immune cells that were stimulated with the target proteins for 4 hours. In addition to the expected cytokine responses after exposure to CCL3, IL-6, and G-CSF, the results show that PADI2 and ANGPTL4 stimulated proinflammatory cytokine production in CMC (mean [SD] frequency of PADI2-stimulated IL-1β + CMC: 18.66 [1.93], FDR-adjusted vs unstimulated P < 1.0 × 10−6; mean [SD] frequency of PADI2-stimulated IL-6 + CMC: 8.01 [1.47], P = 1.0 × 10−6; mean [SD] frequency of PADI2-stimulated TNF + CMC: 7.43 [1.44], P = 1.0 × 10−6) (eFigure 8 and eMethods in the Supplement).
In contrast, stimulation with RARRES2 or TfR elicited little intracellular cytokine responses (mean [SD] frequency of RARRES2-stimulated IL-1β + CMC: 5.63 [0.25], FDR-adjusted vs unstimulated P < 1.0 × 10−6; mean [SD] frequency of TfR-stimulated IL-1β + CMC: 2.25 [0.66], P = .16). These results provide evidence of the potential communication between different biological systems and add new elements to the complex pathogenesis of preterm birth. Furthermore, the results suggest that PADI2, in conjunction with other inflammatory cytokines (such as IL-1β), may exacerbate proinflammatory innate immune responses during PTBs, thereby playing a role in the early onset of labor.
Discussion
To our knowledge, this study is the first multicohort and multiomics analyses of term and preterm birth conducted in LMICs through use of biorepository samples from relevant geographies in a harmonized fashion. The plasma and urine samples were collected, processed, stored, and shipped to the laboratories under uniform protocols. In this proof-of-concept study, a machine learning approach was implemented for quality control, analysis of the timing of pregnancy, and prediction of PTB. Cohort-specific signatures were observed in all cohorts, and data quality was consistent across all modalities.
The prediction of GA at the time of sample collection was driven by an internally correlated module of placenta-related plasma proteins and urine metabolites. Correlations within this module provided an excellent example of leveraging multiomics data for identification of low-cost surrogates in an accessible biological sample (in this case, urine) for an otherwise complex plasma-based measurement with direct applications in LMICs. Accurate prediction of GA through laboratory testing of blood or urine, if validated in larger and more diverse cohorts, has the potential for widespread implementation in settings in which ultrasonography-based GA dating is not available or is impractical.
Prediction of PTB using a multiomics model adjusted for each cohort resulted in an AUROC of 0.83. The sparse nature of the developed methods indicated the possibility of developing simplified models in a validation cohort for scalable analysis of larger cohorts. Mixed-effect modeling revealed several features of interest. The top-ranked features, including IL-1RA, pointed to promising anti-inflammatory therapy candidates that were under active development.46 Although the prediction of GA at the time of sample collection was consistent across all 5 cohorts, models for prediction of PTB required cohort-specific adjustments. This finding is consistent with that in previous publications that indicated that, although the normal chronicity of pregnancy may be shared across populations, pathological pregnancies are likely to be population-specific.47,48
Each multiomics data set differed not only across the subcohorts but also in terms of their size and internal complexities. Therefore, we used a 2-step machine learning strategy in which a model was first built on each omics data set and then combined for final predictions. This approach prevented large untargeted data sets from overwhelming small yet carefully targeted assays that could have a similar or even more discriminatory information content. This approach resulted in an increase in predictive power and improved interpretability of the results.
In the present study, the predictive accuracy for PTB was augmented by combining various omics data sets, which was consistent with previous studies suggesting that PTB was a condition manifesting within multiple biological systems.18,49,50,51,52 Observed differences between cohorts also highlighted that the causes of PTB may be associated with varying environmental and socioeconomic factors.53 From a biological standpoint, examination of individual components of the multiomics model emphasized the role of inflammation in the pathobiological features of PTB. As such, inflammatory cytokines previously shown to be elevated in PTBs, including IL-6 and IL-1RA (often considered as a surrogate marker of IL-1β expression54) were among the most informative features of the multiomics model.55 These cytokines were integrated within a broader inflammatory module that revealed novel factors associated with preterm labor with previously unsuspected properties (eg, PADI2). In neutrophils, citrullination of histones by PADI2 is an important step in the formation of neutrophil extracellular traps, a defensive immunity tool that allows neutrophils to trap and kill bacteria.56,57,58,59,60 Increased soluble PADI2 observed in PTBs may potentially reflect heightened inflammatory responses to a bacterial pathogen, consistent with an infectious cause for PTB. We show that soluble PADI2 can also directly activate proinflammatory signaling pathways and cytokine production in classical monocytes, highlighting a synergistic mechanism that may further enhance the inflammatory state of PTB.
Strengths and Limitations
This study had several strengths. First, the AMANHI and GAPPS biorepositories used accurate early trimester ultrasonography scans for GA dating. Second, urine and plasma specimens were collected, processed, and transported in a harmonized manner. All samples underwent a single freeze-thaw cycle only at Stanford University before final processing and analysis. Third, the machine learning strategy used was able to detect patterns that were generalizable across cohorts.
This study also had several limitations. First, it used a small sample size compared with the number of measurements (which we accounted for through a rigorous 2-step cross-validation process). Therefore, reproduction of these results in larger and more diverse cohorts remains a major priority for our future efforts. For reproduction of these results to be successful, the validation of a reduced model with increased scalability will be a key step. Second, given the exploratory nature of this study, the cohort was clinically homogeneous (eTable and eFigure 2 in the Supplement), which limits the generalizability of the results to real-world heterogeneous populations. Therefore, a future area of investigation is the direct integration of clinical covariates into the predictive models61 to increase the generalizability in data sets with diverse phenotypes.
Conclusions
This diagnostic/prognostic study found that, in LMICs and high PTB settings, major biological adaptations during pregnancy may follow a generalizable model, but the biological signals that correlate with or are potentially associated with PTB can be detected using robust machine learning algorithms. In addition, this study demonstrated that a multiomics approach has the potential to both improve and help identify low-cost predictive surrogates in accessible biological samples for LMICs. Research to expand this analysis to a larger patient population and to broader cohorts and omics platforms are already under way. The data sets, together with state-of-the-art machine learning partnerships,62 will be a key step in developing valuable predictive tests and intervention candidates to tackle the long-term clinical challenge of preventing PTB.
eMethods.
eFigure 1. Data Quality Assessment
eFigure 2. Urine Metabolites as a Surrogate for PGF in Plasma
eFigure 3. Empirical Algorithm Comparison
eFigure 4. A Lower Bound for the Analysis Pipeline Using a Negative Example
eFigure 5. Analysis of Clinical Covariates
eTable. Table of Clinical Covariates Harmonized Across All Cohorts
eFigure 6. Comprehensive Visualization of Single-Cell-Level Intracellular Signaling in Response to Selected Plasma Proteins
eFigure 7. Top Proteomics Features Activate Intracellular Signaling Pathways in Peripheral Blood Classical Monocytes
eFigure 8. Top Proteomics Features Activate Cytokine Production in Peripheral Blood Classical Monocytes
eReferences
References
- 1.Howson CP, Kinney MV, McDougall L, Lawn JE; Born Too Soon Preterm Birth Action Group . Born too soon: preterm birth matters. Reprod Health. 2013;10(suppl 1):S1. doi: 10.1186/1742-4755-10-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, Vogel JP, Moller A-B, et al. . Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37-e46. doi: 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Baer RJ, Berghella V, et al. . Recurrence of preterm birth and early term birth. Obstet Gynecol. 2016;128(2):364-372. doi: 10.1097/AOG.0000000000001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lengyel CS, Ehrlich S, Iams JD, Muglia LJ, DeFranco EA. Effect of modifiable risk factors on preterm birth: a population based-cohort. Matern Child Health J. 2017;21(4):777-785. doi: 10.1007/s10995-016-2169-8 [DOI] [PubMed] [Google Scholar]
- 5.DeFranco EA, Hall ES, Muglia LJ. Racial disparity in previable birth. Am J Obstet Gynecol. 2016;214(3):394.e1-e7. doi: 10.1016/j.ajog.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 6.Modi BP, Parikh HI, Teves ME, et al. . Discovery of rare ancestry-specific variants in the fetal genome that confer risk of preterm premature rupture of membranes (PPROM) and preterm birth. BMC Med Genet. 2018;19(1):181. doi: 10.1186/s12881-018-0696-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Ananth CV, Li Z, Smulian JC. Maternal anaemia and preterm birth: a prospective cohort study. Int J Epidemiol. 2009;38(5):1380-1389. doi: 10.1093/ije/dyp243 [DOI] [PubMed] [Google Scholar]
- 8.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19(12):773-782. doi: 10.1080/14767050600965882 [DOI] [PubMed] [Google Scholar]
- 9.Reschke L, McCarthy R, Herzog ED, Fay JC, Jungheim ES, England SK. Chronodisruption: an untimely cause of preterm birth? Best Pract Res Clin Obstet Gynaecol. 2018;52:60-67. doi: 10.1016/j.bpobgyn.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 10.Ferrero DM, Larson J, Jacobsson B, et al. . Cross-country individual participant analysis of 4.1 million singleton births in 5 countries with very high human development index confirms known associations but provides no biologic explanation for 2/3 of all preterm births. PLoS One. 2016;11(9):e0162506. doi: 10.1371/journal.pone.0162506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vora B, Wang A, Kosti I, et al. . Meta-analysis of maternal and fetal transcriptomic data elucidates the role of adaptive and innate immunity in preterm birth. Front Immunol. 2018;9:993. doi: 10.3389/fimmu.2018.00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang B, Faucette AN, Pawlitz MD, et al. . Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat Med. 2017;23(1):128-135. doi: 10.1038/nm.4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wegorzewska M, Nijagal A, Wong CM, et al. . Fetal intervention increases maternal T cell awareness of the foreign conceptus and can lead to immune-mediated fetal demise. J Immunol. 2014;192(4):1938-1945. doi: 10.4049/jimmunol.1302403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan BJ, DiGiulio DB, Goltsman DSA, et al. . Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc Natl Acad Sci U S A. 2017;114(37):9966-9971. doi: 10.1073/pnas.1705899114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugawara J, Ochi D, Yamashita R, et al. . Maternity log study: a longitudinal lifelog monitoring and multiomics analysis for the early prediction of complicated pregnancy. BMJ Open. 2019;9(2):e025939. doi: 10.1136/bmjopen-2018-025939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paquette AG, Hood L, Price ND, Sadovsky Y. Deep phenotyping during pregnancy for predictive and preventive medicine. Sci Transl Med. 2020;12(527):eaay1059. doi: 10.1126/scitranslmed.aay1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aghaeepour N, Ganio EA, Mcilwain D, et al. . An immune clock of human pregnancy. Sci Immunol. 2017;2(15):eaan2946. doi: 10.1126/sciimmunol.aan2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngo TTM, Moufarrej MN, Rasmussen MH, et al. . Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science. 2018;360(6393):1133-1136. doi: 10.1126/science.aar3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghaeepour N, Lehallier B, Baca Q, et al. . A proteomic clock of human pregnancy. Am J Obstet Gynecol. 2018;218(3):347.e1-347.e14. doi: 10.1016/j.ajog.2017.12.208 [DOI] [PubMed] [Google Scholar]
- 20.Romero R, Erez O, Maymon E, et al. . The maternal plasma proteome changes as a function of gestational age in normal pregnancy: a longitudinal study. Am J Obstet Gynecol. 2017;217(1):67.e1-67.e21. doi: 10.1016/j.ajog.2017.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R, Mazaki-Tovi S, Vaisbuch E, et al. . Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. J Matern Fetal Neonatal Med. 2010;23(12):1344-1359. doi: 10.3109/14767058.2010.482618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koren O, Goodrich JK, Cullender TC, et al. . Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470-480. doi: 10.1016/j.cell.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, Feenstra B, Bacelis J, et al. . Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. 2017;377(12):1156-1167. doi: 10.1056/NEJMoa1612665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghaemi MS, DiGiulio DB, Contrepois K, et al. . Multiomics modeling of the immunome, transcriptome, microbiome, proteome and metabolome adaptations during human pregnancy. Bioinformatics. 2019;35(1):95-103. doi: 10.1093/bioinformatics/bty537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Maaten L, Hinton G. Visualizing data using t-SNE. J Machine Learn Res. 2008;9(86):2579-2605. [Google Scholar]
- 26.Lachenbruch PA, Goldstein M. Discriminant analysis. Biometrics. 1979;35(1):69. doi: 10.2307/25299376080201 [DOI] [Google Scholar]
- 27.Breiman L. Random forests. Machine Learning. 2001;45(1):5-32. doi: 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 28.Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol. 2003;101(6):1266-1274. [DOI] [PubMed] [Google Scholar]
- 29.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188(1):177-182. doi: 10.1067/mob.2003.111 [DOI] [PubMed] [Google Scholar]
- 30.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res. 2015;43(W1):W251-257. doi: 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rumer KK, Uyenishi J, Hoffman MC, Fisher BM, Winn VD. Siglec-6 expression is increased in placentas from pregnancies complicated by preterm preeclampsia. Reprod Sci. 2013;20(6):646-653. doi: 10.1177/1933719112461185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conover CA, Bale LK, Overgaard MT, et al. . Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131(5):1187-1194. doi: 10.1242/dev.00997 [DOI] [PubMed] [Google Scholar]
- 33.Ocón-Grove OM, Cooke FNT, Alvarez IM, Johnson SE, Ott TL, Ealy AD. Ovine endometrial expression of fibroblast growth factor (FGF) 2 and conceptus expression of FGF receptors during early pregnancy. Domest Anim Endocrinol. 2008;34(2):135-145. doi: 10.1016/j.domaniend.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 34.Harris LK. Review: trophoblast-vascular cell interactions in early pregnancy: how to remodel a vessel. Placenta. 2010;31(suppl):S93-S98. doi: 10.1016/j.placenta.2009.12.012 [DOI] [PubMed] [Google Scholar]
- 35.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27-55. doi: 10.1146/annurev.immunol.16.1.27 [DOI] [PubMed] [Google Scholar]
- 36.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89-102. doi: 10.1038/nri2691 [DOI] [PubMed] [Google Scholar]
- 37.Chong J, Soufan O, Li C, et al. . MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486-W494. doi: 10.1093/nar/gky310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760-765. doi: 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. 2013;19(5):548-556. doi: 10.1038/nm.3160 [DOI] [PubMed] [Google Scholar]
- 40.Lange S. Peptidylarginine deiminases as drug targets in neonatal hypoxic-ischemic encephalopathy. Front Neurol. 2016;7:22. doi: 10.3389/fneur.2016.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arai T, Kusubata M, Kohsaka T, Shiraiwa M, Sugawara K, Takahara H. Mouse uterus peptidylarginine deiminase is expressed in decidual cells during pregnancy. J Cell Biochem. 1995;58(3):269-278. doi: 10.1002/jcb.240580302 [DOI] [PubMed] [Google Scholar]
- 42.Ortega-Senovilla H, van Poppel MNM, Desoye G, Herrera E. Angiopoietin-like protein 4 (ANGPTL4) is related to gestational weight gain in pregnant women with obesity. Sci Rep. 2018;8(1):12428. doi: 10.1038/s41598-018-29731-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Poppel MNM, Zeck W, Ulrich D, et al. . Cord blood chemerin: differential effects of gestational diabetes mellitus and maternal obesity. Clin Endocrinol (Oxf). 2014;80(1):65-72. doi: 10.1111/cen.12140 [DOI] [PubMed] [Google Scholar]
- 44.Zhou Z, Chen H, Ju H, Sun M. Circulating chemerin levels and gestational diabetes mellitus: a systematic review and meta-analysis. Lipids Health Dis. 2018;17(1):169. doi: 10.1186/s12944-018-0826-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bendall SC, Simonds EF, Qiu P, et al. . Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332(6030):687-696. doi: 10.1126/science.1198704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quiniou C, Sapieha P, Lahaie I, et al. . Development of a novel noncompetitive antagonist of IL-1 receptor. J Immunol. 2008;180(10):6977-6987. doi: 10.4049/jimmunol.180.10.6977 [DOI] [PubMed] [Google Scholar]
- 47.Villar J, Giuliani F, Barros F, et al. . Monitoring the postnatal growth of preterm infants: a paradigm change. Pediatrics. 2018;141(2):e20172467. doi: 10.1542/peds.2017-2467 [DOI] [PubMed] [Google Scholar]
- 48.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. ; International Fetal and Newborn Growth Consortium for the 21(st) Century (INTERGROWTH-21(st)) . The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol. 2018;218(2S):S630-S640. doi: 10.1016/j.ajog.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 49.Knijnenburg TA, Vockley JG, Chambwe N, et al. . Genomic and molecular characterization of preterm birth. Proc Natl Acad Sci U S A. 2019;116(12):5819-5827. doi: 10.1073/pnas.1716314116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghartey J, Bastek JA, Brown AG, Anglim L, Elovitz MA. Women with preterm birth have a distinct cervicovaginal metabolome. Am J Obstet Gynecol. 2015;212(6):776.e1-776.e12. doi: 10.1016/j.ajog.2015.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirota M, Thomas CG, Liu R, et al. ; March of Dimes Prematurity Research Centers . Enabling precision medicine in neonatology, an integrated repository for preterm birth research. Sci Data. 2018;5:180219. doi: 10.1038/sdata.2018.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menon R, Dixon CL, Sheller-Miller S, et al. . Quantitative proteomics by SWATH-MS of maternal plasma exosomes determine pathways associated with term and preterm birth. Endocrinology. 2019;160(3):639-650. doi: 10.1210/en.2018-00820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burris HH, Wright CJ, Kirpalani H, et al. . The promise and pitfalls of precision medicine to resolve black-white racial disparities in preterm birth. Pediatr Res. 2020;87(2):221-226. doi: 10.1038/s41390-019-0528-z [DOI] [PubMed] [Google Scholar]
- 54.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13(4-5):323-340. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz RJ, Jallo N, Murphey C, Marti CN, Godbold E, Pickler RH. Second trimester maternal plasma levels of cytokines IL-1Ra, Il-6 and IL-10 and preterm birth. J Perinatol. 2012;32(7):483-490. doi: 10.1038/jp.2011.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giaglis S, Stoikou M, Sur Chowdhury C, et al. . Multimodal regulation of NET formation in pregnancy: progesterone antagonizes the pro-NETotic effect of estrogen and G-CSF. Front Immunol. 2016;7:565. doi: 10.3389/fimmu.2016.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134-147. doi: 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 58.Bawadekar M, Shim D, Johnson CJ, et al. . Peptidylarginine deiminase 2 is required for tumor necrosis factor alpha-induced citrullination and arthritis, but not neutrophil extracellular trap formation. J Autoimmun. 2017;80:39-47. doi: 10.1016/j.jaut.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Lightfoot YL, Seto N, et al. . Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus. JCI Insight. 2018;3(23):124729. doi: 10.1172/jci.insight.124729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tong M, Potter JA, Mor G, Abrahams VM. Lipopolysaccharide-stimulated human fetal membranes induce neutrophil activation and release of vital neutrophil extracellular traps. J Immunol. 2019;203(2):500-510. doi: 10.4049/jimmunol.1900262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tibshirani R, Friedman J. A pliable lasso. arXiv. Published 2017. Preprint posted online January 10, 2018. doi: 10.1080/10618600.2019.1648271 [DOI]
- 62.Ewing AD, Houlahan KE, Hu Y, et al. ; ICGC-TCGA DREAM Somatic Mutation Calling Challenge participants . Combining tumor genome simulation with crowdsourcing to benchmark somatic single-nucleotide-variant detection. Nat Methods. 2015;12(7):623-630. doi: 10.1038/nmeth.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Data Quality Assessment
eFigure 2. Urine Metabolites as a Surrogate for PGF in Plasma
eFigure 3. Empirical Algorithm Comparison
eFigure 4. A Lower Bound for the Analysis Pipeline Using a Negative Example
eFigure 5. Analysis of Clinical Covariates
eTable. Table of Clinical Covariates Harmonized Across All Cohorts
eFigure 6. Comprehensive Visualization of Single-Cell-Level Intracellular Signaling in Response to Selected Plasma Proteins
eFigure 7. Top Proteomics Features Activate Intracellular Signaling Pathways in Peripheral Blood Classical Monocytes
eFigure 8. Top Proteomics Features Activate Cytokine Production in Peripheral Blood Classical Monocytes
eReferences