Abstract
Background
Quantification of skeletal muscle using computed tomography (CT) is accessible using cancer patients' standard oncologic images. Reduced muscle mass may be related to reduced respiratory muscle strength; however, the impact of this on lung functional parameters is not characterized in adult allogeneic haematopoietic stem cell transplant (alloHCT) recipients.
Methods
A consecutive retrospective series (n = 296) of patients who had alloHCT at a comprehensive cancer centre between March 2005 and April 2015 were included. Pre‐transplant CT scans were used to quantify skeletal muscle and adipose tissue at the fourth thoracic (T4) and/or third lumbar (L3) level. Tumour and patient characteristics were recorded, including forced expiratory volume in 1 second (FEV1) by spirometry. Regression models were created to characterize predictive relationships.
Results
A total of 296 patients (♂n = 161; ♀n = 135) were included, all of whom had chest CT as part of standard care; a subset of these (n = 215, 72.6%) also had abdominal CT. Diagnoses were non‐Hodgkins lymphoma (n = 165), acute myeloid leukaemia (n = 66), Hodgkin's disease (n = 14), acute lymphocytic leukaemia (n = 14), myelodysplastic syndromes (n = 18), and other (n = 19). In multivariable linear regression adjusted for sex (P < 0.0001), age (P < 0.0001), haematopoietic cell transplantation‐specific co‐morbidity index (P = 0.010), and parameters of pulmonary function testing (defined by spirometry, P < 0.0001), both T4 muscle index [β 0.127 (95% confidence interval 0.019; 0.252), P < 0.0001] and T4 muscle radiodensity [β 0.132 (95% confidence interval 0.087; 0.505), P = 0.006] were independently associated with FEV1; disease risk index (P = 0.877) and Karnofsky performance status (P = 0.548) were not associated with FEV1. Similar conclusions were obtained when L3 muscle index and radiodensity were considered. Unlike T4, L3 muscle index values can be compared with published cut‐off values for sarcopenia. Overall rates of sarcopenia were uniformly higher in the HCT population than in age‐matched and sex‐matched patients with solid tumours [alloHCT ♂64.7% vs. solid tumour ♂56.6% (P < 0.001); alloHCT ♀57.6% vs. solid tumour ♀36.0% (P < 0.001)]. Significant but moderate correlations (P < 0.001) were found for muscle area and radiodensity between L3 and T4, for both men and women; adipose tissue quantity also correlated significantly (P < 0.001) between L3 and T4 for both men and women.
Conclusions
Lumbar or thoracic CT images are useful for body composition assessment in this population and reveal high rates of sarcopenia, similar to those reported in very elderly patients. Reduced muscle mass and radiodensity associate with impaired FEV1 even after adjustment for clinical covariables including co‐morbidities, performance status, disease risk, and mild intrinsic pulmonary disease (chronic obstructive pulmonary disease) defined by spirometry.
Keywords: Sarcopenia, Allogeneic haematopoietic transplantation, Muscle radiodensity, Forced expiratory volume
Introduction
Healthy haematopoietic stem cell transplantation from an allogeneic donor (alloHCT) is a treatment option to improve outcomes for otherwise incurable diseases. With the development of safer transplant conditioning regimens and supportive care measures, transplant can now be offered as a potential curative option to a wider population that include older, frail patients with co‐morbidities. However, the potential benefits of alloHCT can be offset by potential treatment‐related mortality. Candidate selection includes evaluation of a patient's diagnosis, stage, and remission status, but additionally patient tolerability of the procedure is influenced by co‐morbidity burden, performance status, and chronological age. 1 Given the limitations of currently utilized performance status measures and co‐morbidity indices, additional efforts to find alternative supplemental tools have been made in hopes to better ascertain functional performance status pre‐transplantation. 2 , 3
Sarcopenia, severe depletion of muscle mass [indicated by low skeletal muscle index (SMI)], is characterized by impairments in strength, functional limitations, and physical disability. 4 , 5 , 6 This can be, and is, readily identified via radiologic imaging in cancer patients, including those with haematologic malignancies, and is further associated with poor clinical outcomes. 7 , 8 , 9 Reduced skeletal muscle radiodensity (SMR) is distinct characteristic of muscle, indicative of fatty infiltration, and this has also been related to a growing number of adverse clinical outcomes in recent years. 10 , 11 , 12 , 13 SMI and SMR have been related to physical functioning, but this has not usually included pulmonary function(s). Pulmonary complications and dysfunction after alloHCT have been described to a significant extent over the years as being a primary cause of early morbidity and mortality, particularly for their association to graft‐vs.‐host disease. 14 , 15 , 16 , 17 Thus, it is common practice to carry out functional pulmonary assessment prior to and after HCT, by measuring pulmonary function including forced expiratory volume in 1 second (FEV1). Many muscles are recruited for forceful expiration including, primarily, the internal intercostals, intercostalis intimi, and subcostals, while accessory expiratory muscles include the rectus abdominis, external oblique, internal oblique, and transversus abdominis. This degree of involvement would suggest that persons affected by substantial overall muscle wasting may have impaired forced expiration.
While the research on each of these three parameters (SMI, SMR, and FEV1) independently is extensive, until this point, literature that aims to associate FEV1 to SMI/SMR in cancer patients has been limited and absent in alloHCT. In hepatocellular carcinoma, a significant relationship between preoperative FEV1 and psoas muscle index was found, although the use of only the psoas muscle limits the strength of this postulation as it is not involved in respiration. 18 Outside of cancer, multiple studies of chronic obstructive pulmonary disease (COPD) have posited a relationship between fat‐free mass index and intercostal muscle cross‐sectional area with FEV1 and COPD severity; however, the body composition of these patients compared with those of alloHCT likely differs significantly. 19 , 20 Lastly, in a large‐scale study of healthy elderly adults, regression analysis showed that computed tomography (CT)‐defined thigh muscle area was indeed an independent predictor of FEV1. 21 In sum, recent publications have certainly suggested a potential relationship between body composition and FEV1, though until this point, not in HCT patients.
We hypothesized that recipients of alloHCT were likely to have sarcopenia given that those patients are often heavily pretreated with chemotherapy prior to the procedure. Thus, we aimed to evaluate patients about to receive alloHCT to determine the incidence of sarcopenia in this population. Further, we aimed to characterize how sarcopenia, as well as reduced SMR, would relate to pre‐HCT FEV1. Additionally, we sought to demonstrate the relationship between CT image parameters analysed at chest level (T4) vs. lumbar (L3) level.
Population and methods
Study population for this analysis included adults ≥18 years of age who had received alloHCT for haematologic malignancies at the H. Lee Moffitt Cancer Center, the single regional site for stem cell transplantation serving the greater Tampa Bay area (pop. 2.7 million). Consecutive patients were included with a diagnosis of lymphoma (March 2005 to April 2015) and all diagnoses (November 2013 to November 2014). Ethical approval was granted by Advarra Institutional Research Board (protocol numbers Pro00015786 and Pro00014686). Eligible subjects required analysable CT imaging of the lumbar or thoracic region within 60 days prior to transplantation. If multiple CT scans were available, CT scan closest to transplant date was analysed. Patient demographics obtained from medical records included age, sex, body mass index (BMI), diagnosis, performance status, previous chemotherapy regimens, length of stay, albumin, and co‐morbidities. Pulmonary function was characterized by FEV1 (normalized for age, ethnicity, height, and sex), forced vital capacity (FVC), and the diffusing capacity of the lungs for carbon monoxide (DLCO). FEV1 and FVC were captured by spirometry performed within 30 days prior to transplantation, and COPD was defined and graded using FEV1 and FVC as per the criteria established by the COPD Foundation. 22 DLCO was measured by ERS/ATS standards and adjusted for anaemia per guidelines. 23 , 24 Co‐morbidities collected were extensive, in accordance with the haematopoietic cell transplantation‐specific co‐morbidity index (HCT‐CI), which is standard for patients who are being considered for alloHCT. 25 Disease risk index (DRI), developed for the purpose of evaluating patients together across diseases, was utilized to stratify patients according to their level of risk based on diagnosis and staging information. 26
Computed tomography assessment
Pre‐transplantation CT images were acquired as part of institutional standard practice and were retrieved from the institutional Picture Archiving Communication System. CT chest (unenhanced) is standard for all haematological malignancies. CT abdomen (contrast‐enhanced) is additionally performed in patients with lymphoma to obtain evidence of remission or disease progression (e.g. review of spleen size, liver, and retroperitoneal lymphadenopathy). Vertebral land‐marking was performed by a board‐certified radiologist. For thoracic series, we identified a single axial CT image landmarked at the fourth thoracic vertebra (T4); for lumbar series, a secondary single axial image at the third lumbar vertebra was selected. Vertebral bodies were selected at the level of the pedicles. Selected images were from the closest date prior to transplant (within 60 days). All images were of 3 mm slice thickness, with a peak kilovoltage of 120 and current varying by patient within the standard algorithms within the SIEMENS CT scanners at this site.
We quantified tissue cross‐sectional areas with SliceOmatic® (TomoVision, Magog, Quebec, Canada). Predetermined Hounsfield unit (HU) thresholds for muscle were −29 to +150 HU, −50 to −150 HU for visceral adipose tissue (VAT), and −30 to −190 HU for subcutaneous adipose tissue (SAT). Skeletal muscle area (SMA), mean SMR, and areas of VAT and SAT were reported. Adipose tissue outside of the abdominal wall but inside the muscle fascia was included with SAT. Areas of VAT and SAT were summed to obtain the area for total adipose tissue (TAT) at the L3 level (VAT is absent at T4). Example images are present in Figure 1 (T4, panel a; L3, panel b). SMA was normalized for height2 and reported as SMI (cm2/m2). For the purpose of comparing the incidence of sarcopenia with reports in the literature, sarcopenia was defined by the widely used lumbar SMI cut‐offs of 52.4 cm2/m2 for men and 38.5 cm2/m2 for women. 27
Figure 1.
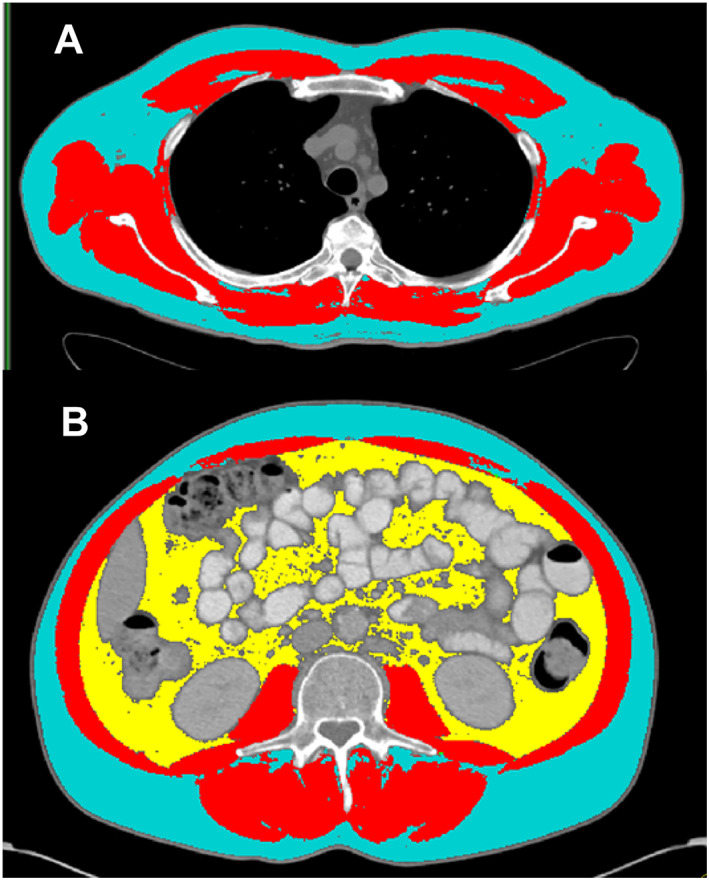
Example thoracic (T4) and lumbar (L3) computed tomography scans with tissue quantification. At the thoracic level (A), skeletal muscle is displayed in red, and subcutaneous adipose tissue is displayed in blue. At the lumbar level (B), skeletal muscle is displayed in red, subcutaneous adipose tissue in blue, and visceral adipose tissue in yellow.
Statistics
Frequencies and summary statistics are reported. Comparisons were assessed with parametric tests [independent t‐tests,χ 2 test, or Fisher's exact test (post hoc Bonferroni corrections)]. Correlations were evaluated with Pearson correlation coefficients. To evaluate predictive relationships between our demographic, body composition, and functional variables, we created multivariable linear regression models. For these models, we included both conventional covariates well known to be related to alloHCT outcomes [Karnofsky performance status (KPS), HCT‐CI, and DRI] 26 , 28 as well as those related to body composition with previously uncharacterized relationships in this context (SMI and SMR). COPD defined through spirometry was included in predictive models for FEV1 as a co‐morbidity that is generally mild in this population and thus is not comprehensively captured by the HCT‐CI. Ethnicity/race was not evaluated as it has not been shown to impact alloHCT outcomes to this point in US‐based populations. 29 Analyses were completed using IBM SPSS Statistics for Windows version 23.0 (SPSS, Chicago, IL), and results were considered significant at the P < 0.05 level.
Results
Demographics
A total of 299 consecutively transplanted patients were identified, of which n = 296 (99%) had CT images of acceptable quality within 60 days prior to transplant and were included for this analysis. Patient characteristics are detailed in Table 1. There were 161 men (54%) and 135 (46%) women, with an overall mean age of 52.4 ± 12.1 years. Diagnoses spanned indications for alloHCT in haematologic malignancies including non‐Hodgkins lymphoma (n = 165), acute myeloid leukaemia (n = 66), Hodgkin's disease (n = 14), acute lymphocytic leukaemia (n = 14), myelodysplastic syndromes (n = 18), and other (n = 19). Scans were taken a median of 26 (SD 11.5) days prior to stem cell infusion from allogeneic donor, with a majority of scans (69.6%) taken within 30 days of transplantation. Forty‐eight% (n = 143) of patients had high co‐morbidity burden (score ≥3) based on pre‐transplant HCT‐CI. Pulmonary decompensation, using defined metrics of COPD, 22 had a low prevalence—only 10.5% of patients had Stage 1 COPD and 14.5% of patients had Stage U (undefined), as expected in this population. The mean KPS of 90.2 was consistent with ability to carry on normal activity with minor signs or symptoms of the underlying malignancy. The majority of patients (52.7%) were also classified as having an intermediate DRI, whereas the classifications of low risk (22.6%) and high or very high risk (24.7%) were less common. It was also most common that a patient received a myeloablative conditioning regimen (70.6% overall), compared with 29.4% for reduced intensity/non‐myeloablative conditioning. All 296 included subjects had thoracic CT performed during pre‐transplant evaluation capturing the T4 region as is standard in alloHCT. A subset of these (72.6%, n = 215) also had an abdominal CT capturing L3. For purposes of consistency with the literature, we performed analyses on the data retrieved from both levels. However for the reason of intrinsic variation of muscle radiodensity at different vertebral levels, 30 and for the reason that in standard haematological oncology thoracic images are not contrast enhanced but abdominal images are, we have separately analysed thoracic and lumbar regions in all of our analysis.
Table 1.
Patient demographic characteristics
| Characteristic | Overall (n = 296) | Male (n = 161) | Female (n = 135) | P‐value |
|---|---|---|---|---|
| Age (years) | 52.4 ± 12.1 | 52.9 ± 11.5 | 51.9 ± 12.9 | 0.477 |
| HCT type | ||||
| Allogeneic | 296 (100%) | 161 (100%) | 135 (100%) | |
| Days CT to transplant | 26 (11.5) | 27 (13) | 25 (11.2) | 0.608 c |
| Diagnosis, n (%) | ||||
| Non‐Hodgkins lymphoma | 165 (55.7%) | 98 (60.9%) | 67 (49.6%) | 0.394 a |
| Acute lymphocytic leukaemia | 14 (4.7%) | 7 (4.3%) | 7 (5.2%) | |
| Acute myeloid leukaemia | 66 (22.3%) | 34 (21.1%) | 32 (23.7%) | |
| Hodgkins disease | 14 (4.7%) | 7 (4.3%) | 7 (5.2%) | |
| Myelodysplastic syndrome | 18 (6.1%) | 8 (5.0%) | 10 (7.4%) | |
| Other | 19 (6.4%) | 7 (4.3%) | 12 (8.9%) | |
| HCT‐CI score, n (%) | 0.597 a | |||
| 0 | 63 (21.3%) | 32 (19.9%) | 31 (23.0%) | |
| 1 | 23 (7.8%) | 15 (9.3%) | 8 (5.9%) | |
| 2 | 67 (22.6%) | 40 (24.8%) | 27 (20.0%) | |
| 3 | 69 (23.3%) | 37 (23.0%) | 32 (23.7%) | |
| 4+ | 74 (25.0%) | 37 (23.0%) | 37 (27.4%) | |
| Disease risk index, n (%) | 0.940 a | |||
| Low | 67 (22.6%) | 36 (22.4%) | 31 (23.0%) | |
| Intermediate | 156 (52.7%) | 84 (52.2%) | 72 (53.3%) | |
| High/very high | 73 (24.7%) | 41 (25.5%) | 32 (23.7%) | |
| Regimen intensity | 0.798 b | |||
| Myeloablative | 209 (70.6%) | 115 (71.4%) | 94 (69.6%) | |
| Reduced intensity/non‐myeloablative | 87 (29.4%) | 46 (28.6%) | 41 (30.4%) | |
| Previous chemotherapy regimens | 3.27 ± 1.8 | 3.28 ± 1.8 | 3.25 ± 1.9 | 0.722 |
| KPS | 90.2 ± 8.1 | 90.5 ± 8.0 | 89.8 ± 8.0 | 0.447 |
| Albumin | 4.08 ± 0.4 | 4.09 ± 0.4 | 4.07 ± 0.3 | 0.702 |
| Prior HCT, n (%) | 21 (7.1%) | 11 (6.8%) | 10 (7.4%) | 1.0 b |
| FEV1 (%) | 92.0 ± 15.3 | 89.9 ± 14.8 | 94.6 ± 15.6 | 0.009 |
| DLCO | 74.5 ± 14.6 | 76.5 ± 15.6 | 72.1 ± 12.9 | 0.009 |
| COPD | 0.113 a | |||
| Stage 0 (no COPD) | 222 (75.0%) | 113 (70.2%) | 109 (80.7%) | |
| Stage 1 (mild) | 31 (10.5%) | 20 (12.4%) | 11 (8.2%) | |
| Stage 2–3 (moderate–severe) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Stage U (undefined) | 43 (14.5%) | 28 (17.4%) | 15 (11.1%) | |
| Lumbar CT Available, n (%) | 215 (72.6%) | 116 (72.0%) | 99 (73.3%) | 0.896 |
| Length of Stay | 24.0 (6) | 23 (5) | 24 (6.5) | 0.140 c |
| T4 body composition | ||||
| Skeletal muscle (cm2) | 183.9 ± 50.7 | 218.2 ± 40.0 | 142.9 ± 25.4 | <0.001 |
| Subcutaneous adipose tissue (cm2) | 212.9 ± 103.9 | 194.9 ± 93.5 | 234.3 ± 111.7 | 0.001 |
| Skeletal muscle radiodensity (HU) | 38.5 ± 6.8 | 38.9 ± 7.0 | 37.9 ± 6.6 | 0.211 |
| L3 body composition d | ||||
| Skeletal muscle (cm2) | 128.2 ± 34.7 | 152.1 ± 26.7 | 100.1 ± 18.0 | <0.001 |
| Subcutaneous adipose tissue (cm2) | 198.8 ± 103.6 | 192.4 ± 102.7 | 206.2 ± 104.5 | 0.331 |
| Visceral adipose tissue (cm2) | 131.3 ± 96.3 | 170.2 ± 100.3 | 85.8 ± 67.5 | <0.001 |
| Skeletal muscle radiodensity (HU) | 35.5 ± 7.3 | 35.9 ± 7.2 | 35.2 ± 7.4 | 0.487 |
| Sarcopenia, n (%) | 132 (61.4%) | 75 (64.7%) | 57 (57.6%) | 0.326 b |
| BMI category, n (%) | 0.005 a | |||
| Underweight | 4 (1.4%) | 1 (0.6%) | 3 (2.2%) | |
| Normal weight | 89 (30.1%) | 36 (22.4%) | 53 (39.3%) | |
| Overweight | 116 (39.2%) | 68 (42.2%) | 48 (35.6%) | |
| Obese | 87 (29.4%) | 56 (34.8%) | 31 (23.0%) | |
Variables are presented as mean ± standard deviation, or median (interquartile range). P‐values are Student's t‐tests unless otherwise specified. BMI, body mass index; COPD, chronic obstructive pulmonary disease defined by spirometry 22 ; CT, computed tomography; DLCO, diffusing capacity of the lung for carbon monoxide, adjusted for haemoglobin; FEV1, forced expiratory volume in 1 second; HCT, haematopoietic cell transplantation; HCT‐CI, haematopoietic cell transplantation‐specific co‐morbidity index; HU, Hounsfield unit; KPS, Karnofsky performance status.
χ 2 test.
Fisher's exact test.
Mann–Whitney U test.
Variables of L3 body composition, including sarcopenia prevalence, have a sample size of 215 (n = 99 female, n = 116 male).
Linear regression analysis: FEV1
In multivariable linear regression with FEV1 as the dependent variable, when we adjusted our model for clinically relevant covariates, age (P < 0.0001), sex (P = 0.001), HCT‐CI (P = 0.010) and COPD grading (P < 0.0001), T4 SMI (P = 0.023), and T4 SMR (P = 0.006) remained as independent predictors, whereas DRI (P = 0.877) and KPS (P = 0.548) did not (Table 2). When evaluating the lumbar region with the same model, age (P < 0.0001), sex (P = 0.002), HCT‐CI (P < 0.0001), COPD (P < 0.0001), and L3 SMI (P = 0.016) remained as independent predictors. L3 SMR (P = 0.106) was no longer an independent predictor, though retained a β coefficient (β 0.093) only slightly below that of the β coefficient (β 0.132) founded in the T4 model of greater sample size. KPS (P = 0.954) and DRI (P = 0.309) were again not associated with FEV1. Conditioning regimen and days between CT transplant both were not associated with FEV1 in all models.
Table 2.
Linear regression models for prediction of forced expiratory volume in 1 s (FEV1) prior to haematopoietic cell transplantation using thoracic (T4) or lumbar (L3) data
| Linear Regression Models | B | SE B | β | t | P‐value |
|---|---|---|---|---|---|
| FEV1 | |||||
| Thoracic (n = 296) | |||||
| Intercept | 81.706 | 9.742 | 8.387 | 0.000 | |
| Sex (male vs. female) | −5.424 | 1.639 | −0.177 | −3.310 | 0.001 |
| Age | 0.204 | 0.057 | 0.162 | 3.594 | 0.000 |
| Disease risk index | −0.149 | 0.965 | −0.007 | −0.155 | 0.877 |
| HCT‐CI | −0.936 | 0.360 | −0.117 | −2.602 | 0.010 |
| KPS | −0.049 | 0.082 | −0.026 | −0.602 | 0.548 |
| Skeletal muscle index | 0.135 | 0.059 | 0.127 | 2.282 | 0.023 |
| Skeletal muscle radiodensity | 0.296 | 0.106 | 0.132 | 2.786 | 0.006 |
| COPD | −12.010 | 0.942 | −0.573 | −12.751 | 0.000 |
| R 2 | 0.492 | ||||
| Lumbar (n = 215) | |||||
| Intercept | 74.739 | 11.237 | 6.651 | 0.000 | |
| Sex (male vs. female) | −6.064 | 1.928 | −0.195 | −3.145 | 0.002 |
| Age | 0.285 | 0.072 | 0.218 | 3.950 | 0.000 |
| Disease risk index | 1.191 | 1.169 | 0.051 | 1.019 | 0.309 |
| HCT‐CI | −1.748 | 0.431 | −0.208 | −4.052 | 0.000 |
| KPS | 0.005 | 0.094 | 0.003 | 0.058 | 0.954 |
| Skeletal muscle index | 0.244 | 0.101 | 0.153 | 2.423 | 0.016 |
| Skeletal muscle radiodensity | 0.199 | 0.123 | 0.093 | 1.621 | 0.106 |
| COPD | −11.989 | 1.188 | −0.536 | −10.094 | 0.000 |
| R 2 | 0.516 |
COPD, chronic obstructive pulmonary disease, defined by spirometry 22 ; HCT‐CI, haematopoietic cell transplantation‐specific co‐morbidity index; KPS, Karnofsky performance status; SE, standard error.
Linear regression analysis: skeletal muscle index and skeletal muscle radiodensity
For part two of our regression analysis, predicting either SMI or SMR, we created homologous models with sex, age, BMI, DRI, and HCT‐CI as covariates. There were four models—SMI at T4/L3 and SMR at T4/L3 (Table 3). For T4 SMI, independent predictors were age (P = 0.005), sex (P < 0.0001), and BMI (P < 0.0001) (Table 3). HCT‐CI score (P = 0.059) and DRI (P = 0.131) were not significant in this model. For L3 SMI, sex (P < 0.0001) and BMI (P < 0.0001) were independent predictors, while age (P = 0.093) fell out of the model. Interestingly, DRI did act as an independent predictor in this instance (P = 0.012), while HCT‐CI remained insignificant. At the thoracic level for SMR (T4 SMR), all of our covariates were independent predictors—sex (P = 0.009), age (P < 0.0001), BMI (P < 0.0001), DRI (0.013), and HCT‐CI (P = 0.011) significance. At the L3 level, sex (P = 0.081) and HCT‐CI (P = 0.225) did not achieve significance, while age (P < 0.0001), BMI (P < 0.0001), and DRI (P = 0.019) performed like their counterparts at the T4 level. KPS was not related to either SMI or SMR (data not shown).
Table 3.
Linear regression models for prediction of pre‐transplant skeletal muscle index and radiodensity using thoracic (T4) or lumbar (L3) data
| Linear regression models | B | SE B | β | t | P‐value |
|---|---|---|---|---|---|
| Skeletal muscle index | |||||
| Thoracic (n = 296) | |||||
| Intercept | 31.279 | 4.568 | 6.847 | 0.000 | |
| Sex (male vs. female) | 15.239 | 1.270 | 0.530 | 12.002 | 0.000 |
| Age | −0.147 | 0.052 | −0.125 | −2.842 | 0.005 |
| BMI | 0.707 | 0.104 | 0.302 | 6.821 | 0.000 |
| Disease risk index | −1.402 | 0.925 | −0.067 | −1.514 | 0.131 |
| HCT‐CI | −0.634 | 0.334 | −0.085 | −1.889 | 0.059 |
| R 2 | 0.451 | ||||
| Lumbar (n = 215) | |||||
| Intercept | 10.762 | 3.372 | 3.191 | 0.002 | |
| Sex (male vs. female) | 10.103 | 0.879 | 0.519 | 11.491 | 0.000 |
| Age | −0.062 | 0.037 | −0.076 | −1.688 | 0.093 |
| BMI | 0.900 | 0.086 | 0.473 | 10.407 | 0.000 |
| Disease risk index | −1.655 | 0.656 | −0.114 | −2.522 | 0.012 |
| HCT‐CI | −0.229 | 0.240 | −0.044 | −0.955 | 0.341 |
| R 2 | 0.585 | ||||
| Skeletal muscle radiodensity | |||||
| Thoracic (n = 296) | |||||
| Intercept | 58.578 | 2.533 | 23.124 | 0.000 | |
| Sex (male vs. female) | 1.838 | 0.704 | 0.135 | 2.611 | 0.009 |
| Age | −0.174 | 0.029 | −0.310 | −6.043 | 0.000 |
| BMI | −0.360 | 0.057 | −0.324 | −6.272 | 0.000 |
| Disease risk index | −1.281 | 0.513 | −0.129 | −2.495 | 0.013 |
| HCT‐CI | −0.473 | 0.185 | −0.133 | −2.559 | 0.011 |
| R 2 | 0.252 | ||||
| Lumbar (n = 215) | |||||
| Intercept | 59.719 | 3.340 | 17.880 | 0.000 | |
| Sex (male vs. female) | 1.529 | 0.871 | 0.105 | 1.756 | 0.081 |
| Age | −0.253 | 0.036 | −0.411 | −6.933 | 0.000 |
| BMI | −0.347 | 0.086 | −0.243 | −4.050 | 0.000 |
| Disease risk index | −1.541 | 0.650 | −0.142 | −2.370 | 0.019 |
| HCT‐CI | −0.289 | 0.238 | −0.073 | −1.216 | 0.225 |
| R 2 | 0.274 | ||||
BMI, body mass index; HCT‐CI, haematopoietic cell transplantation‐specific co‐morbidity index; SE, standard error.
Sarcopenia prevalence
Weight and body composition features are provided in Table 1. Mean BMI was high for both men and women, demonstrating an overweight population—69% of subjects were overweight (BMI 25–29.9) or obese (BMI 30.0+). The majority of men and women met criteria for sarcopenia (♂64.7%; ♀57.6%) based on published cut‐offs at the L3 level. 27 These overall rates of sarcopenia were markedly higher (P < 0.001) in alloHCT recipients than patients with locally advanced or metastatic malignancies of the lung or gastrointestinal tract (♂56.6%; ♀36.0%). 31 As seen in Table 4, the difference in sarcopenia prevalence is most notable when comparing across age‐matched and sex‐matched cohorts. When comparing with those with solid tumours, alloHCT patients in our study had a significantly higher prevalence of sarcopenia. This included men (P = 0.012) and women (P = 0.014) aged 50–60 and was even more prominent in men (P = 0.004) and women (P = 0.002) aged 60–70. These rates of sarcopenia founded in alloHCT patients were systematically higher than expected for their chronological age; for example, male alloHCT recipients aged 50–60 years were found to have similar rates of sarcopenia (74.4%) as seen in men 20 years their senior (70–80) with metastatic solid tumours. As another point of reference, we have included a population of healthy community‐dwelling young adults in Table 4 (from reference 30).
Table 4.
Comparison of sarcopenia prevalence in patients having haematopoietic cell transplantation versus advanced solid tumour patients (from reference 31 ), versus healthy young adults (from reference 30 )
| Population | n | SMI | Sarcopenia prevalence (%) | P‐value |
|---|---|---|---|---|
| Healthy young | ||||
| Male mean age = 30.9 y | 317 | 60.9 ± 7.8 | n/a | |
| Female mean age = 31.2 y | 410 | 47.5 ± 6.6 | n/a | |
| Cancer | ||||
| Age group: 50–60 y | ||||
| Solid tumour | ||||
| Male | 178 | 52.6 ± 8.4 | 52.2 | |
| Female | 135 | 41.9 ± 6.6 | 34.1 | |
| alloHCT | ||||
| Male | 39 | 48.7 ± 7.4 | 74.4 | 0.012 * |
| Female | 37 | 37.8 ± 5.4 | 56.8 | 0.014 * |
| Age group: 60–70 y | ||||
| Solid tumour | ||||
| Male | 260 | 52.6 ± 9.6 | 48.1 | |
| Female | 200 | 41.9 ± 7.3 | 32.0 | |
| alloHCT | ||||
| Male | 35 | 46.6 ± 9.1 | 74.3 | 0.004 * |
| Female | 30 | 37.0 ± 8.6 | 63.3 | 0.002 * |
Prevalence of sarcopenia according to the criteria of Prado et al. 27 alloHCT, allogeneic haematopoietic stem cell transplant; n/a, not applicable; SMI, skeletal muscle index.
P‐values calculated by Fisher's exact test, between the age‐matched and sex‐matched tumour groups.
Sarcopenia prevalence is not easily compared with data in the literature as different methods are used to quantify muscle and different sarcopenia thresholds are used; however, prevalence of sarcopenia in community‐dwelling American men ≥80 years of age is about 50%, 32 much lower than our findings in alloHCT patients.
Fourth thoracic vs. third lumbar comparison
When comparing T4 and L3 regions of the body within patients, men and women were both more muscular at T4 than at L3. Correlative analyses performed for T4 vs. L3 demonstrated moderate correlation for skeletal muscle parameters SMA (women: r 2 = 0.33, P < 0.001; men: r 2 = 0.47, P < 0.001, Figure 2B and 2C) and SMR (women: r 2 = 0.58, P < 0.001; men: r 2 = 0.63, P < 0.001, Figure 2E and 2F). Subcutaneous adipose tissues at the T4 vs. L3 level had higher degrees of correlation, with women having an r 2 value of 0.69 (P < 0.001) and men having a value of 0.71 (P < 0.001) (Figure 3B and 3C). Lastly, the highest correlation coefficients we observed were in comparing TAT at the L3 level (consisting of SAT + VAT) with SAT at the T4 level (which is considered TAT, as the only adipose tissue constituent present at this level), an r 2 value of 0.79 for women (P < 0.001) and 0.77 for men (P < 0.001) (Figure 3E and 3F).
Figure 2.
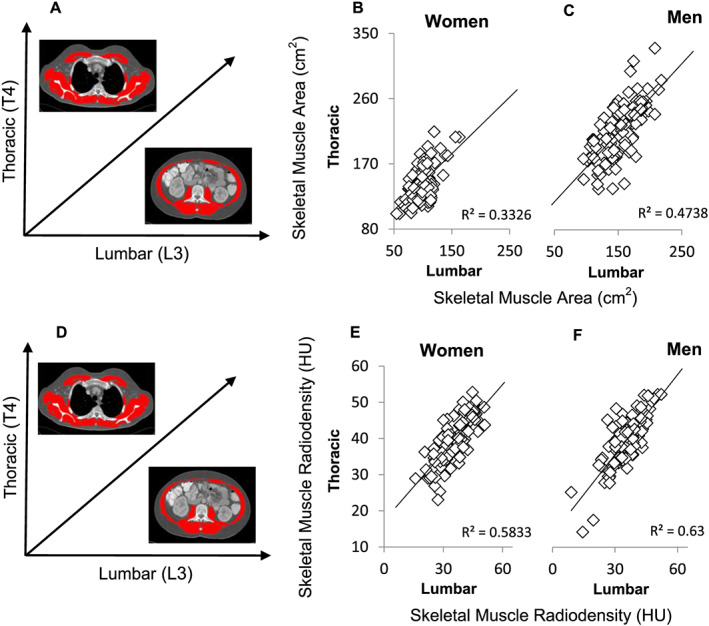
T4 vs. L3 correlations for muscle characteristics (skeletal muscle area/skeletal muscle radiodensity). Panel (A) illustrates the vertebral landmark and tissue on the axes of panels (B) and (C); panel (D) does the same for (E) and (F). Correlations were assessed between skeletal muscle area and skeletal muscle radiodensity at two vertebral levels and are presented for male (n = 116; panels C/F, respectively) and female (n = 99; panels B/E, respectively) patients. HU, Hounsfield unit.
Figure 3.
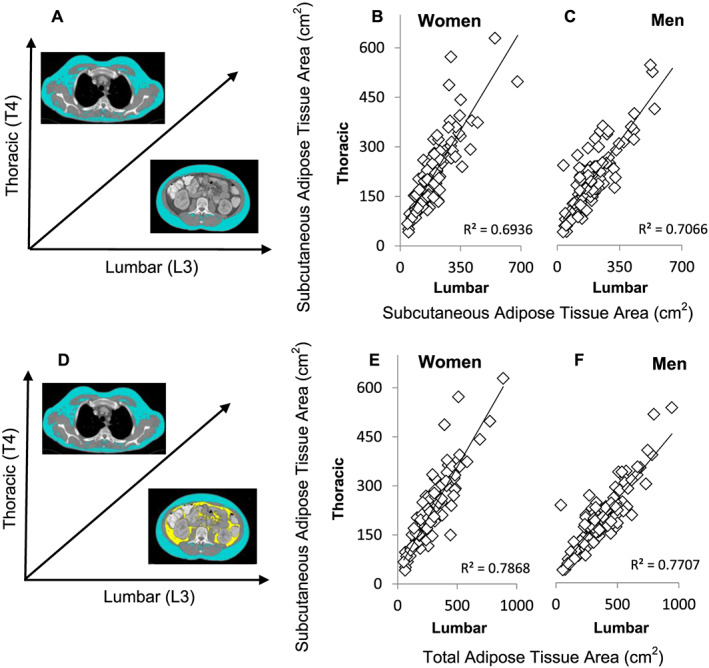
T4 vs. L3 correlations for characteristics of adiposity (subcutaneous adipose tissue/total adipose tissue). Panel (A) illustrates the vertebral landmark and tissue on the axes of panels (B) and (C); panel (D) does the same for (E) and (F). Correlations were assessed between subcutaneous adipose tissue at two vertebral levels and of T4 subcutaneous adipose tissue vs. total adipose tissue at the L3 level, which consists additionally of visceral adipose tissue. Values are presented for male (n = 116; panels C/F, respectively) and female (n = 99; panels B/E, respectively) patients.
Discussion
Herein, we describe a population‐based cohort of allogeneic HCT recipients evaluated for sarcopenia. Of the many techniques currently available to assess both muscle mass and adipose tissue, CT scan analysis allows for precise and specific examination of SMI and radiodensity and is considered to be ‘gold standard’ for estimating sarcopenia. 33 , 34 This report displays the feasibility of evaluating body composition via CT imaging in an HCT recipient cohort, given the frequency of CT scans performed for disease assessment or infection monitoring purposes, as is performed routinely during transplant candidacy evaluation. This approach has been adopted in recent studies 35 , 36 ; however, pulmonary function was not a focus of those works. Moreover, we demonstrate the high prevalence of sarcopenia amongst alloHCT recipients, with the majority of recipients having evidence of sarcopenia prior to HCT; although not studied here, it may be expected that the extremely high dose chemotherapy used to ablate the bone marrow would induce even further large magnitude muscle loss. 14 , 37 Notably, while sarcopenia was identified in the majority of our cohort, currently utilized traditional measurements of performance status (KPS) did not predict deficits in muscle mass, radiodensity, or FEV1 within this group. The mean KPS in our cohort was 90.2 ± 8.1, consistent with ability to carry on normal activity, and only 4.1% of patients had KPS <80. Our results suggest that KPS, as assessed by the medical team, has limited association with muscle mass and may need to be supplemented by sarcopenia measurement.
The importance of assessment of SMI and radiodensity in HCT recipients has been previously underscored by physical functional decline after transplantation as assessed by the Medical Outcomes Study Short‐Form 36 questionnaire 38 , 39 with full physical recovery often requiring several years after allograft 38 , 40 , 41 and predisposing patients to ongoing medical risk. 41 Our report suggests that a high proportion of alloHCT recipients could potentially be predisposed to sequelae of sarcopenia such as transplant‐related morbidity or mortality that would otherwise not be identified as such without CT quantification of the characteristics of their musculature. An investigation of mortality in our population will be the subject of a future report. The high prevalence of sarcopenia in this curative intent population is particularly striking when comparing across age‐matched and sex‐matched solid tumour patients receiving palliative chemotherapy. 31 Additionally, the mean age in our cohort was 52.4 ± 12.1; the degree of sarcopenia in this population was comparable with solid tumour patients approximately 20 years their senior and above that of community‐dwelling seniors in their 80s.
Current transplant physician attitude towards patient tolerance of alloHCT procedure is influenced by the assessment of co‐morbidity burden, performance status, and chronological age 1 due to their association with inferior outcomes. 25 , 28 , 42 , 43 , 44 However, assessment of ‘biological’ or ‘functional’ age vs. chronological age remains a challenge. Our study demonstrates the association of SMI and SMR with FEV1 by pulmonary function testing, an objective measurement associated with functionality, mortality, and potential long‐term morbidity in allograft recipients. 14 , 15 , 16 , 17 , 25 , 44 , 45 Age and sex are known factors associated with FEV1 as also seen in our cohort; however, both skeletal muscle quantity and fatty infiltration as measured by SMI and SMR, respectively, are independent predictors of FEV1 even when the models are heavily adjusted for clinical covariables including mild intrinsic pulmonary disease (COPD). Pulmonary involvement by the cancer itself is rarely seen in either AML or MDS 46 as they are diseases of the bone marrow micro‐environment, and while the other diseases could have pulmonary involvement, the vast majority of patients are in complete remission at time of transplant and do not have any discernible disease in the lungs. Acceptable pulmonary function is an eligibility criterion for transplantation as this treatment entails risk of pulmonary complications, so the FEV1 is uniformly high; however, this does not necessarily indicate ideal pulmonary function as some transplant eligible patients in our population had low grade pulmonary decompensation. Inconsistency of FEV1 and pulmonary symptoms has also been reported, and patients with preserved pulmonary function remain at risk for pulmonary complication and activity limitations. 47
Body composition evaluation can be assessed in both the thoracic and lumbar regions; here, we present the first study in alloHCT that includes full analysis at both vertebral levels. This is significant when considering sarcopenia evaluations, and only a certain radiographic field is available; our analyses demonstrate that many of the same results can be produced by data retrieved from T4 vs. L3, with some variation. While there are differences in quantitative amounts of skeletal muscle and adipose mass as expected between chest and abdomen, they are however correlated. Transplant‐related factors are predictive of SMI in both the chest and abdomen. Interestingly, in both the thoracic and abdominal regions, HCT‐CI was not associated with sarcopenia demonstrating the independent value of body composition not otherwise captured in a co‐morbidity evaluation.
Limitations
This study had the inherent limitations of a retrospective design. Future studies are required to look at the association between SMI/SMR and morbidity/mortality post‐transplantation.
In summary, body composition assessments in alloHCT recipients are feasible. Similar conclusions based on thoracic and lumbar CT imaging help to broaden the evaluable patient population, as use can be made of thoracic or lumbar regions. Evaluation of sarcopenia may further be valuable in risk‐stratifying patients beyond currently known paradigms and may be a composite evaluation of biological age including actual years, co‐morbidities, sex, and body mass.
Conflict of interest
A.M., K.D.B., M.E., R.F., K.T., J.A.P., and V.E. B. declare that they have no conflict of interest.
Acknowledgements
We acknowledge the following funding support: American Cancer Society's Institutional Research Grant (to A.M.) and Moffitt Cancer Center Support Grant P30 CA076292. V.E.B. receives financial support from the Canadian Institutes of Health Research and the Alberta Cancer Foundation. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 48
Mishra A., Bigam K. D., Extermann M., Faramand R., Thomas K., Pidala J. A., and Baracos V. E. (2020) Sarcopenia and low muscle radiodensity associate with impaired FEV1 in allogeneic haematopoietic stem cell transplant recipients, Journal of Cachexia, Sarcopenia and Muscle, 11, 1570–1579, 10.1002/jcsm.12604
References
- 1. Artz AS. From biology to clinical practice: aging and hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012;18:S40–S45. [DOI] [PubMed] [Google Scholar]
- 2. Muffly LS, Boulukos M, Swanson K, Kocherginsky M, Cerro P, Schroeder L, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant 2013;19:429–434. [DOI] [PubMed] [Google Scholar]
- 3. Swanson K, van Besien K, Rich E, Stock W, Larson R, Artz A. Geriatric assessment (GA) may identify vulnerable older allogeneic hematopoietic cell transplantation (HCT) recipients. Biol Blood Marrow Transplant 2009;15:S102–S103. [Google Scholar]
- 4. Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross‐sectional study of muscle strength and mass in 45‐ to 78‐yr‐old men and women. J Appl Physiol 1991;71:644–650. [DOI] [PubMed] [Google Scholar]
- 5. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 6. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. New Engl J Med 1995;332:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prado CMM, Lieffers JR, Bowthorpe L, Baracos VE, Mourtzakis M, McCargar LJ. Sarcopenia and physical function in overweight patients with advanced cancer. Can J Diet Pract Res 2013;74:69–74. [DOI] [PubMed] [Google Scholar]
- 8. Lanic H, Kraut‐Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Leuk Lymphoma 2014;55:817–823. [DOI] [PubMed] [Google Scholar]
- 9. Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A, et al. Skeletal muscle density is an independent predictor of diffuse large B‐cell lymphoma outcomes treated with rituximab‐based chemoimmunotherapy. J Cachexia Sarcopenia Muscle 2017;8:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vrieling A, Kampman E, Knijnenburg NC, Mulders PF, Sedelaar JPM, Baracos VE, et al. Body composition in relation to clinical outcomes in renal cell cancer: a systematic review and meta‐analysis. Eur Urol Focus 2018;4:420–434. [DOI] [PubMed] [Google Scholar]
- 11. Chu MP, Lieffers J, Ghosh S, Belch AR, Chua NS, Fontaine A, et al. Skeletal muscle radio‐density is an independent predictor of response and outcomes in follicular lymphoma treated with chemoimmunotherapy. PLoS ONE 2015;10:e0127589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee J, Lin J, Wu M, Jan Y, Chang C, Huang C, et al. Muscle radiodensity loss during cancer therapy is predictive for poor survival in advanced endometrial cancer. J Cachexia Sarcopenia Muscle 2019;10:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin L, Hopkins J, Malietzis G, Jenkins JT, Sawyer MB, Brisebois R, et al. Assessment of computed tomography (CT)‐defined muscle and adipose tissue features in relation to short‐term outcomes after elective surgery for colorectal cancer: a multicenter approach. Ann Surg Oncol 2018;25:2669–2680. [DOI] [PubMed] [Google Scholar]
- 14. Sorror ML, Logan BR, Zhu X, Rizzo JD, Cooke KR, McCarthy PL, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant 2015;21:1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chow EJ, Cushing‐Haugen KL, Cheng G, Boeckh M, Khera N, Lee SJ, et al. Morbidity and mortality differences between hematopoietic cell transplantation survivors and other cancer survivors. J Clin Oncol 2017;35:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carpenter PA, Kitko CL, Elad S, Flowers MED, Gea‐Banacloche JC, Halter JP, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: V. The 2014 ancillary therapy and supportive care working group report. Biol Blood Marrow Transplant 2015;21:1167–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chien JW, Madtes DK, Clark JG. Pulmonary function testing prior to hematopoietic stem cell transplantation. Bone Marrow Transplant 2005;35:429–435. [DOI] [PubMed] [Google Scholar]
- 18. Shirai H, Kaido T, Hamaguchi Y, Kobayashi A, Okumura S, Yao S, et al. Preoperative low muscle mass and low muscle quality negatively impact on pulmonary function in patients undergoing hepatectomy for hepatocellular carcinoma. Liver Cancer 2018;7:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ju S, Lee SJ, Park MJ, Cho YJ, Jeong YY, Jeon KN, et al. Clinical importance of cross‐sectional area of intercostal muscles in patients with chronic obstructive pulmonary disease. Clin Respir J 2018;12:939–947. [DOI] [PubMed] [Google Scholar]
- 20. Pothirat C, Chaiwong W, Phetsuk N, Liwsrisakun C, Bumroongkit C, Deesomchok A, et al. The relationship between body composition and clinical parameters in chronic obstructive pulmonary disease. J Med Assoc Thailand 2016;99:386–393. [PubMed] [Google Scholar]
- 21. Rossi AP, Watson NL, Newman AB, Harris TB, Kritchevsky SB, Bauer DC, et al. Effects of body composition and adipose tissue distribution on respiratory function in elderly men and women: The health, aging, and body composition study. J Gerontol Ser A Biol Sci Med Sci 2011;66A:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yawn BB, Thomashaw B, Mannino DM, et al. The 2017 update to the COPD Foundation COPD Pocket Consultant Guide. Chronic Obstr Pulm Dis 2017;4:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacIntyre N, Crapo RO, Viegi G, Cooper BG, Jensen R, Kendrick A, et al. Standardisation of the single‐breath determination of carbon monoxide uptake in the lung. Rev Mal Respir 2007;24:2S65–2S82. [DOI] [PubMed] [Google Scholar]
- 24. Graham BL, Brusasco V, Burgos F, et al. Executive summary: 2017 ERS/ATS standards for single‐breath carbon monoxide uptake in the lung. Eur Respir J 2017;49:1600016. [DOI] [PubMed] [Google Scholar]
- 25. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)‐specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 2012;120:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 28. Sorror M, Storer B, Sandmaier BM, Maloney DG, Chauncey TR, Langston A, et al. Hematopoietic cell transplantation‐comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer 2008;112:1992–2001. [DOI] [PubMed] [Google Scholar]
- 29. Hamilton BK, Rybicki L, Sekeres M, et al. Racial differences in allogeneic hematopoietic cell transplantation outcomes among African Americans and whites. Bone Marrow Transplant 2015;50:834–839. [DOI] [PubMed] [Google Scholar]
- 30. Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 2018;8:11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kazemi‐Bajestani SMR, Mazurak VC, Baracos V. Computed tomography‐defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 2016;54:2–10. [DOI] [PubMed] [Google Scholar]
- 32. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 2012;3: JUL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging 2009;13:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeFilipp Z, Troschel FM, Qualls DA, Li S, Kuklinski MW, Kempner ME, et al. Evolution of body composition following autologous and allogeneic hematopoietic cell transplantation: incidence of sarcopenia and association with clinical outcomes. Biol Blood Marrow Transplant 2018;24:1741–1747. [DOI] [PubMed] [Google Scholar]
- 36. Jabbour J, Manana B, Zahreddine A, Saade C, Charafeddine M, Bazarbachi A, et al. Sarcopenic obesity derived from PET/CT predicts mortality in lymphoma patients undergoing hematopoietic stem cell transplantation. Curr Res Transl Medicine 2019;67:93–99. [DOI] [PubMed] [Google Scholar]
- 37. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2018. Available at https://www.cibmtr.org. Accessed 06/04/2019.
- 38. Bevans MF, Marden S, Leidy NK, Soeken K, Cusack G, Rivera P, et al. Health‐related quality of life in patients receiving reduced‐intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2006;38:101–109. [DOI] [PubMed] [Google Scholar]
- 39. Altmaier EM, Ewell M, McQuellon R, Geller N, Carter SL, Henslee‐Downey J, et al. The effect of unrelated donor marrow transplantation on health‐related quality of life: a report of the unrelated donor marrow transplantation trial (T‐cell depletion trial). Biol Blood Marrow Transplant 2006;12:648–655. [DOI] [PubMed] [Google Scholar]
- 40. Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, et al. Long‐term health‐related quality of life, growth, and spiritual well‐being after hematopoietic stem‐cell transplantation. J Clin Oncol 2005;23:599–608. [DOI] [PubMed] [Google Scholar]
- 41. Bush NE, Donaldson GW, Haberman MH, Dacanay R, Sullivan KM. Conditional and unconditional estimation of multidimensional quality of life after hematopoietic stem cell transplantation: a longitudinal follow‐up of 415 patients. Biol Blood Marrow Transplant 2000;6:576–591. [DOI] [PubMed] [Google Scholar]
- 42. Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. EUR J CANCER PART A 1996;32:1135–1141. [DOI] [PubMed] [Google Scholar]
- 43. Goldberg SL, Klumpp TR, Magdalinski AJ, Mangan KF. Value of the pretransplant evaluation in predicting toxic day‐100 mortality among blood stem‐cell and bone marrow transplant recipients. J Clin Oncol 1998;16:3796–3802. [DOI] [PubMed] [Google Scholar]
- 44. Raimondi R, Tosetto A, Oneto R, Cavazzina R, Rodeghiero F, Bacigalupo A, et al. Validation of the hematopoietic cell transplantation‐specific comorbidity index: a prospective, multicenter GITMO study. Blood 2012;120:1327–1333. [DOI] [PubMed] [Google Scholar]
- 45. Cheng G, Storer B, Chien JW, Jagasia M, Hubbard JJ, Burns L, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc 2016;13:1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ganzel C, Manola J, Douer D, et al. Extramedullary disease in adult acute myeloid leukemia is common but lacks independent significance: analysis of patients in ECOG‐ACRIN cancer research group trials, 1980–2008. J Clin Oncol 2016;34:3544–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. New Engl J Med 2016;374:1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


