Abstract
Peri-implant bone formation depends on the ability of mesenchymal stem cells (MSCs) to colonize implant surfaces and differentiate into osteoblasts, but the precise mechanisms controlling this process remain unclear. In vitro, MSCs undergo osteoblastic differentiation on microstructured titanium (Ti) surfaces in the absence of exogenous media supplements and produce factors that promote osteogenesis while regulating osteoclast activity, including semaphorins. The goal of this study was to evaluate the role of semaphorin 3A (Sema3A) on surface-mediated osteoblastic differentiation and determine the hierarchy of this signaling cascade. Human MSCs were cultured on 15mm grade 2 smooth (pretreatment, PT), hydrophobic-microrough (sand blasted/acid etched, SLA), hydrophilic-microrough Ti (mSLA) (Institut Straumann AG, Basel, Switzerland), or tissue culture polystyrene (TCPS). Expression of SEMA3A family proteins increased after 7 days of culture, and the increased expression in response to microstructured Ti was dependent on recognition of the surface by integrin α2β1. Exogenous Sema3A increased differentiation whereas differentiation was decreased in cells treated with a Sema3A antibody. Furthermore, Sema3A influenced the production of osteoprotegerin and osteopontin suggesting it as an important local regulator of bone remodeling. Inhibition of Wnt3A and Wnt5A revealed that activation of Sema3A occurs downstream of Wnt5A and may facilitate the translocation of β-catenin bypassing the canonical Wnt3A initiating signal associated with osteoblastic differentiation. Furthermore, chemical inhibition of calmodulin (CaM), Ca2+/calmodulin-dependent protein kinase (CaMKII), phospholipase A2 (PLA2), protein kinase C (PKC), and BMP receptors suggest that Sema3A could serve as a feedback mechanism for both Wnt5A and BMP2. Here, we show novel roles for Sema3A family proteins in the surface-dependent modulation of MSCs as well as important interactions with pathways known to be associated with osteoblastic differentiation. Moreover, their effects on bone remodeling markers have significant implications for peri-implant bone remodeling and downstream modulation of osteoclastic activity. These results suggest that Sema3A aids in peri-implant bone formation through regulation on multiple stages of osseointegration, making it a potential target to promote osseointegration in patients with compromised bone remodeling.
Keywords: Mesenchymal Stem Cell, Osteoblast, Titanium, Semaphorin3A, Surface Topography, Implant
1. Introduction
Osseointegration of dental and orthopaedic implants is a complex biological process involving the communication among osteoprogenitor cells, osteoblasts, osteocytes, and osteoclasts. This requires a versatile system of communication that highlights the importance of locally generated and regulated factors capable of controlling cell differentiation, extracellular matrix synthesis, mineral deposition, and the balance between bone formation and bone resorption (i.e. bone remodeling). Several molecules have been identified as important mediators of osteoblastic differentiation in vitro that correlate with preclinical studies in animal models and with human clinical studies [1,2]. Over the last few decades, research has made it clear that production of these molecules is mediated by topographical and chemical modifications to the surface of titanium (Ti) and Ti-based alloy implants [3–9].
In vitro studies using different models of osteoblast cultures demonstrate a more differentiated phenotype on hydrophobic or hydrophilic microtextured implant surfaces compared to cultures grown on smooth Ti or tissue culture polystyrene (TCPS) [10–12]. Moreover, the mechanisms associated with this enhanced differentiation are very different than those on TCPS. On TCPS, osteoblastic cells primarily express the α5β1 integrin complex, and differentiation occurs through the canonical Wnt3A pathway, causing an accumulation of β-catenin in the cytoplasm and its translocation into the nucleus to serve as a transcriptional activator [7,13]. When grown on microstructured substrates, cells shift to the α1β1 and α2β1 integrin complexes, downregulating genes associated with the Wnt3A pathway while upregulating genes of the Wnt11 and non-canonical calcium dependent Wnt5A pathway [11,13,14].
Local regulatory factor production has also been shown to be sensitive to microstructured Ti implants. In addition to the high production of negative regulators of osteoclast activity, cells produce high levels of osteocalcin and osteopontin [10,15,16]. Osteocalcin has been shown to promote the chemotaxis, adhesion, and spreading of osteoclasts [17,18] while osteopontin plays a role in anchoring osteoclasts to the mineralized bone matrix [19]. The sequential production of these factors suggests microstructured Ti facilitates the production of a cascade of factors by MSCs and osteoblasts to mediate osteoclast recruitment while preventing their premature fusion and activity. Transforming growth factor beta-1 (TGFβ1) enhances the proliferation of MSCs and osteoblasts and stimulates production of extracellular matrix production [20]. TGFβ1 also acts on osteoclasts downregulating their activity, in part by regulating production of osteoprotegerin by MSCs and osteoblasts [20]. Osteoprotegerin regulates osteoclasts by binding serving as a RANKL decoy receptor [21]. Excess osteoprotegerin prevents RANKL from binding to RANK thus preventing the osteoclast formation [21]. Autocrine and paracrine actions are also observed in bone morphogenetic proteins (BMPs) [22]. Among these proteins, BMPs 2, 4, and 7 have shown to be the most important in bone formation and healing [23,24]. Modulation of these osteogenic factors by implant surface topography provides molecular evidence of the increased osteoblastogenesis seen in vitro and decreased healing times seen clinically. MSCs cultured on microstructured Ti substrates, display temporal upregulation of TGFβ1, osteoprotegerin, and BMPs occurring as early as 4 days, suggesting that they are early regulators of surface-mediated osteogenesis [12,20]. Their presence could also serve as the impetus for the commitment of MSCs to the osteoblast lineage when cultured on microstructured Ti, as they are known to regulate embryonic skeletal development [25].
Recently, semaphorin signaling through their receptors, neuropilins (NRPs) and plexins (PLXNs) has been identified as a potential important regulator of bone homeostasis [26–28]. Semaphorins were originally identified as axon guidance molecules but have also been implicated in various other biological process including cell motility, proliferation, differentiation, angiogenesis, cardiogenesis, tumor growth, tissue development, immune response, and, more recently, the coupling of osteoblasts and osteoclasts during bone remodeling [29–35]. Of the eight classes of semaphorin family proteins categorized by structure and sequence similarities, the class III semaphorin, Sema3A, has shown particularly convincing and intriguing control over osteoblastic differentiation while also regulating the activity of osteoclasts [36]. Sema3A is a secreted protein that exerts its biological activity through interactions with NRP1, which subsequently complexes with PLXNA1, increasing osteoblastic bone formation [27,37]. The binding of Sema3A to NRP1 also disrupts the interaction between PLXNA1 and triggering receptor expressed by myeloid cells 2 (TREM-2) [27]. The binding of PLXNA1 to TREM-2 is favored during osteoclastogenesis. These findings suggested that Sema3A secreted by osteoblast lineage cells synchronously affects both osteoclasts and osteoblasts in paracrine and autocrine manners, respectively.
Sema3A signaling shares significant crosstalk with the previously mentioned signaling pathways. PLXN-mediated signaling is important in the inhibition of integrin-mediated cellular adhesion and cytoskeletal remodeling [38]. Interestingly, some receptors for semaphorins are integrin complexes such as α1β1 [39–41]. Semaphorin signaling also bares striking resemblances to BMP signaling as they are both capable of multidirectional signaling, involved in embryonic development, and activate similar downstream pathways [42,43]. Furthermore, Sema3A is known to activate Rac1 in osteoblasts facilitating the translocation of β-catenin into the nucleus bypassing the canonical Wnt3A initiating signal [27,44,45]. Current efforts to elucidate the effects of Sema3A and its associated pathways have only been conducted on plastic, and it is unknown whether the effects of Sema3A on osteoblasts is altered by microstructured hydrophobic or hydrophilic surfaces. The goal of this study was to evaluate the role of Sema3A on surface-mediated osteoblastic differentiation and determine the hierarchy of this signaling cascade.
2. Materials and Methods
2.1. Ti Disk Preparation
15mm diameter disks were punched from 1mm thick sheets of grade 2 Ti (Institut Straumann AG, Basel, Switzerland). Disks were prepared as described previously and sterilized with 25kGy gamma irradiation prior to use. Briefly, disks were degreased in acetone and processed for 30s in in a 55°C 2% ammonium fluoride/2% hydrofluoric acid/10% nitric acid solution to produce pretreatment Ti disks (PT; [SA=0.59μm; Specific surface area (SSA)=0.51mm2/mg; θCA=93.6°; %C=31]). SLA [SA=3.58μm; (SSA)=0.56 mm2/mg; θCA=120.9°; %C=35] substrates were prepared by subjecting PT substrates to sand blasting (250 – 500μm corundum) and acid etching (HCl/H2SO4). Disks were cleaned in HNO3, rinsed in deionized water, air dried, and packed in aluminum foil. mSLA [SA=3.64μm; (SSA)=0.56 mm2/mg; θCA=0°; %C=17] substrates were produced using the same sandblasting and acid etching procedure as used for SLA, but subsequent steps took place under nitrogen gas to prevent exposure to air. mSLA disks were rinsed and stored in 0.9% NaCl solution. Detailed methods and characterization of the disks have been previously published [12,46].
2.2. Cell Culture
Human bone marrow-derived MSCs (Institute for Regenerative Medicine, Texas A&M Health Science Center, Bryan, TX) were cultured in Complete Culture Medium (CCM) containing Alpha Modified Eagle Medium (αMEM, Life Technologies, Carlsbad, CA) supplemented with 16.5% fetal bovine serum (FBS, Life Technologies), 2mM L-glutamine (Life Technologies), and 1% penicillin-streptomycin (Life Technologies). MSCs were cultured on tissue culture polystyrene (TCPS), PT, SLA, or mSLA at a density of 10,000 cells/cm2 at 37°C, 5% CO2 and 100% humidity for all experiments. Media were changed 24h after plating and every 48h thereafter for 7d. At 7d, cells were incubated with fresh media for either 12h or 24h. Conditioned media and cell lysates were harvested and assayed as described below.
2.3. ITGA2 and ITGB1 Silencing
MISSION® shRNA lentiviral transduction particles (Sigma-Aldrich) were used to silence human MSCs for either ITGA2 (SHCLNV-NM_002203) or ITGB1 (SHCLNV-NM_002211). MSCs were plated at 20,000 cells/cm2 and cultured overnight at 37°C in 5% CO2 and 100% humidity. Particles were added to the cells at a multiplicity of infection of 2 in culture media supplemented with 8μg/ml hexadimethrine bromide for 18h. Transduced cells were selected using CCM supplemented with 0.5μg/ml of puromycin. Silencing of ITGB1 and ITGA2 were confirmed using real-time qPCR. A 70% reduction in mRNA levels compared to wild-type (WT) controls was considered the minimum acceptable decrease. Prior to their use in subsequent experiments, shITGB1 MSCs were 93.1% silenced and shITGA2 were 86.9% silenced compared to WT controls (Supplementary Fig.1). After 7d of culture, MSCs remained at least 70% silenced on TCPS (ITGB1: 79.4%; ITGA2: 91.6%;) and mSLA (ITGB1: 96.3%; ITGA2: 87.8%;) indicating a maintenance of silencing over the period of culture.
2.4. Gene Expression
To quantify mRNA, cells were plated as described above (Section 2.2). At 7d, cells were incubated with fresh media for 12h. mRNA was isolated from cells using a TRIzol® (Invitrogen, Carlsbad, CA) extraction method. RNA was quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and then reverse transcribed into 250ng/μl cDNA. Real-time PCR was performed using a fluorescent dye (Power SYBR Green, Applied Biosystems, Foster City, CA) to quantify starting mRNA levels using gene specific primers (Table 1). Levels of mRNA are normalized to GAPDH.
Table 1.
Human Primers Used for Real-Time PCR Analysis
| Name | Gene | Primer Sequence | |
|---|---|---|---|
| Runt-related transcription factor 2 | RUNX2 | F | GTCTCACTGCCTCTCACTTG |
| R | CACACATCTCCTCCCTTCTG | ||
| Osteocalcin | BGLAP | F | CTCACACTCCTCGCCCTATT |
| R | GGGTCTCTTCACTACCTCGC | ||
| Integrin α2 | CD49b | F | CCCGAGGGCATTGAAAACAC |
| R | CGGATAGTGCCCTGATGACC | ||
| Integrin β1 | ITGB1 | F | ATTACTCAGATCCAACCAC |
| R | TCCTCCTCATTTCATTCATC | ||
| Semaphorin 3A | SEMA3A | F | AGGAGAAAGGAGGAGAGGTGT |
| R | CTGTGCCAAGGATGCGAATG | ||
| Neuropilin 1 | NRP1 | F | GCGGACTTTTCCAGCTCTCT |
| R | CAGTTGGCCTGGTCGTCAT | ||
| Neuropilin 2 | NRP2 | F | TCGGCTTTTGCAGTGGACAT |
| R | GCTCCAGTCCACCTCGTATT | ||
| Plexin A1 | PLXNA1 | F | TGTGGAGAAGTCGCTGACAC |
| R | GCCTGTGGCGTATTCCATCT | ||
| Plexin A2 | PLXNA2 | F | AGAGAGACCCCGAGAGCTT |
| R | CTCCGGTTCGTTCACAGTCC | ||
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | F | GCTCTCCAGAACATCATCC |
| R | TGCTTCACCACCTTCTTG |
2.5. Autocrine and Paracrine Effects of Sema3A
To test the effect of exogenous Sema3A on our model system, cultures were treated with 1μg/mL recombinant human Sema3A (R&D Systems, Minneapolis, MN). To test the effect of endogenous Sema3A, cultures were treated with either 1:200 or 1:500 dilutions of a polyclonal anti-Sema3A antibody (R&D Systems). Cells were plated as described above (Section 2.2), and media were changed every 48h and cells treated with either proteins or antibodies to Sema3A for 7d. At 7d, cells were incubated with fresh CCM without treatment for 24h. The conditioned media were collected, and ELISAs were used to measure levels of osteocalcin (Thermo Fisher Scientific, Waltham, MA), BMP2 (R&D Systems), OPG (R&D Systems), OPN (R&D Systems), Wnt3A (Biomatik, Cambridge, Ontario), and Wnt5A (Biomatik). The quantity of Sema3A was also measured in the conditioned media from untreated control cultures by ELISA (Lifespan Biosciences, Seattle, WA). Cell monolayers were washed twice with 0.2ml phosphate-buffered saline (PBS), lysed in 0.05% Triton X-100, and homogenized by sonication at 40A using a Vibra-Cell ultrasonicator (Sonics & Materials Inc., Newtown, CT). DNA content in the cell lysate was measured with PicoGreen (Promega, Madison, WI) using a Synergy H1 Hybrid Reader fluorescence detector (BioTek, Winooski, VT) at an excitation of 504nm and emission of 531nm. Immunoassay data were normalized to DNA content. Cell types were treated with 1:200 and 1:500 dilutions of IgG; results were not significantly different from untreated cells (data not shown).
2.6. Wnt Pathway Analysis
To determine whether Sema3A interacts with either the Wnt3A or Wnt5A pathways shown to be associated with surface mediated osteoblastic differentiation of MSCs, cultures were treated with different chemical inhibitors to various pathway components. Cultures supplemented with or without 1μg/mL Sema3A were treated with either 5μM or 10μM IWR-1 (Sigma-Aldrich, St. Louis, MO) or IWP-2 (Sigma-Aldrich) to inhibit Wnt3A signaling or a 1:200 dilution of a polyclonal anti-Wnt5A antibody (R&D Systems) to inhibit Wnt5A signaling. Downstream activation of calmodulin (CaM), Ca2+/calmodulin-dependent protein kinase (CaMKII), and phospholipase A2 (PLA2) were inhibited by treating cultures with 10μM W7 (Tocris Bioscience, Bristol, United Kingdom), 10μM KN93 (Tocris Bioscience), or 10μM AACOCF3 (Tocris Bioscience) respectively with or without the addition of 1μg/mL Sema3A. Protein kinase C (PKC) was inhibited using either 1μM chelerythrine chloride (Tocris Bioscience) or 1μM GF109203X (Tocris Bioscience) with or without the addition of 1μg/mL Sema3A. Cells were plated as described above (Section 2.2), and media were changed every 48h with treatment for 7d. At 7d, cells were incubated with fresh CCM without treatment for 24h and assayed as described previously (Section 2.5).
2.7. BMP2 Pathway Analysis
To determine whether Sema3A interacts with the BMP2 pathway, cultures were treated with chemicals known to inhibit receptors of BMPs. 0.1μM or 1μM of two chemicals known to inhibit ALK2, ALK3, and ALK6 [DMH-2 and dorsomorphin dihydrochloride (DDCl); Tocris Bioscience]; or LDN 193189 dihydrochloride (ALK2 and ALK3 inhibitor; Tocris Bioscience) were used to treat surface cultured MSCs. 0.1μM, 1μM, or 10μM DMH-1 (ALK2 inhibitor; Tocris Bioscience) with or without the addition of 1μg/mL Sema3A was also used to treat surface cultured MSCs. The production of Sema3A after the addition of 40ng/mL recombinant human BMP2 (R&D Systems) was also measured. Cells were plated as described above (Section 2.2), and media were changed every 48h with treatment for 7d. At 7d, cells were incubated with fresh CCM without treatment for 24h and assayed as described previously (Section 2.5).
2.8. Statistical Analysis
Data are presented as the mean ± standard error (SE) of n=6 cultures per variable. All experiments were repeated at least three times to ensure validity of the results. Data shown in the figures are from representative experiments. A two-tailed t-test was used to determine significant differences for experiments with only two groups. Experiments with more than two groups were subjected to a one–way analysis of variance with a two-tailed Tukey correction to adjust for multiple comparisons to maintain an experiment-wise error rate (α) of 0.05. All statistical analyses were performed using JMP statistical software (SAS Institute Inc., Cary, North Carolina).
3. Results
3.1. Effect of Surface Topography and Hydrophilicity on Expression of Sema3A and Its Receptors
After 7d of culture, RUNX2 (Fig.1A), OCN (Fig.1B), ITGA2 (Fig.1C), and ITGB1 (Fig.1D) were first measured to verify the effect of different surfaces on MSC differentiation and integrin receptor expression. mRNA levels of RUNX2, ITGA2, and ITGB1 increased in a surface dependent manner with the highest expression observed on the microrough/hydrophilic mSLA substrate. Although no differences between TCPS and PT cultures were detected, OCN expression was increased in SLA cultures compared to TCPS with further increases seen in mSLA cultures compared to SLA cultures.
Figure 1. Effect of surface microstructured and hydrophilicity on MSC semaphorin expression and production.
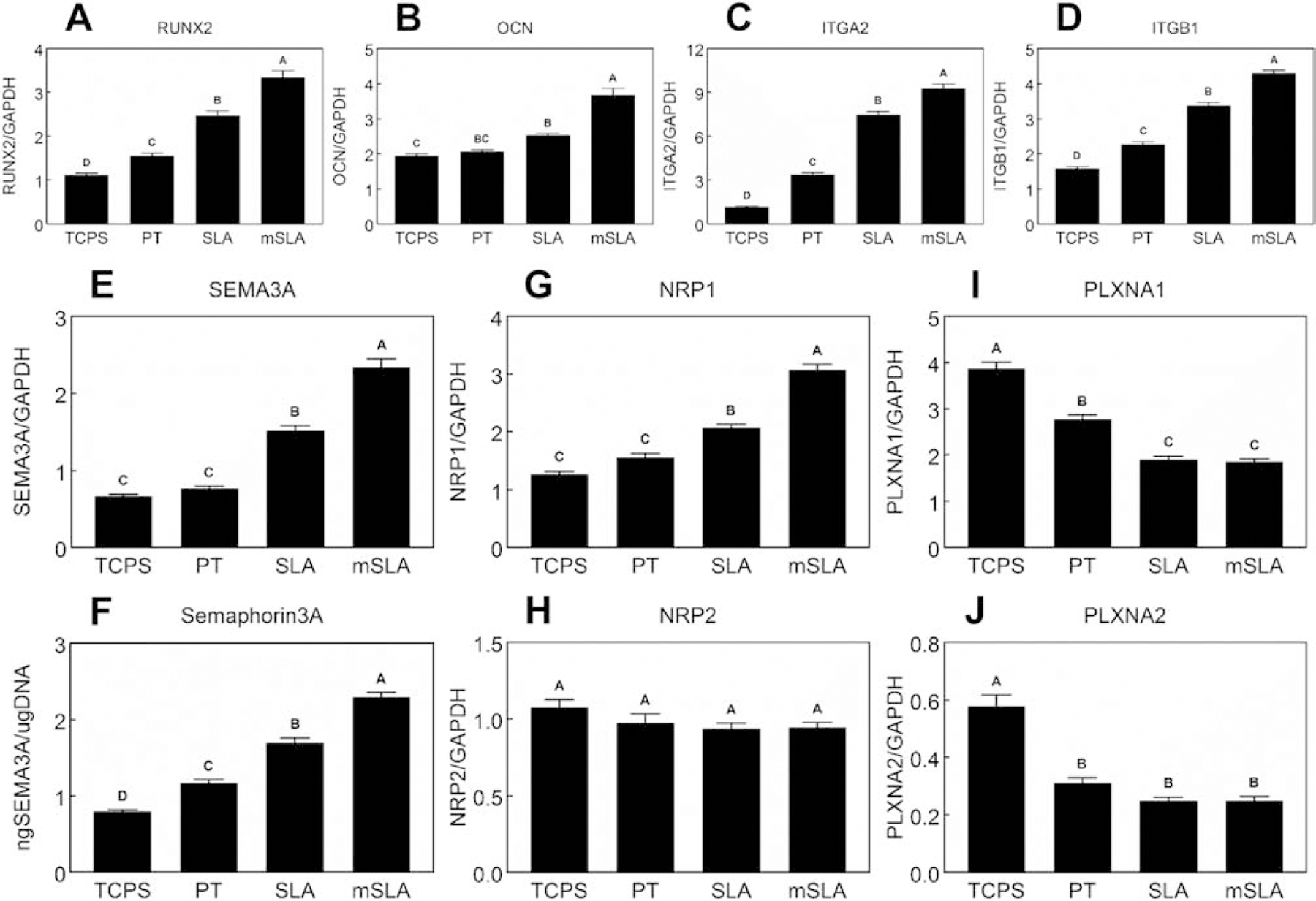
MSCs were cultured on TCPS or Ti substrates. After 7d, mRNA levels of RUNX2 (A), OCN (B), ITGA2 (C), ITGB1 (D), SEMA3A (E) as well as Semaphorin3A protein levels (F), NRP1 (G), NRP2 (H), PLXNA1 (I), and PLXNA2 (J) were measured. Data shown are the mean ± standard error (SE) of six independent samples. Groups not sharing a letter are statistically significant at α=0.05.
Expression of SEMA3A mRNA levels (Fig.1E) were increased on SLA compared to TCPS and PT with the highest levels observed on mSLA. MSC protein production of Sema3A (Fig.1F) increased on PT compared to TCPS cultures. A greater production of Sema3A was observed in SLA cultures compared to TCPS and PT with the highest production observed in mSLA cultures.
No differences in the expression of the SEMA3A receptor, NRP1, was observed between TCPS and PT cultures (Fig.1G). Microroughness increased NRP1 expression while the combination of microroughness and hydrophilicity further enhanced its expression. No differences in NRP2 mRNA levels (Fig.1H) were detected. PLXNA1 (Fig.1I) and PLXNA2 (Fig.1J) expression decreased on Ti surfaces. A further decrease in PLXNA1 expression was seen in SLA and mSLA cultures, although this was not observed for PLXNA2 expression.
Compared to the scramble control on TCPS cultures, a 77.97% reduction in ITGB1 (Fig.2A) expression was achieved in ITGB1-silenced MSCs (shITGB1) and a 70.04% reduction in ITGA2 (Fig.2B) expression was achieved in ITGA2-silenced MSCs (shITGA2). Reductions in expression were also observed in Ti surface cultures. shITGB1 and shITGA2 cells had reduced expression of RUNX2 (Fig.2C) in Ti surface cultures. shITGA2 cells had lower SEMA3A expression (Fig.2D) compared to controls within the same substrate culture and lower expression compared to shITGB1 cells in TCPS, PT, and SLA substrate cultures. Reduced SEMA3A mRNA levels were also observed in shITGB1 cells but only for those cultured on rough Ti. The reductions in SEM3A expression translated to a reduced production of Sema3A protein (Fig.2E) into the conditioned media by cells cultured on Ti surfaces. mRNA levels for NRP1 (Fig.2F) were reduced in shITGB1 cells when cultured on rough Ti substrates. NRP2 mRNA levels (Fig.2G) were abrogated in all shITGB1 substrate cultures while PLXNA1 (Fig.2H) was reduced in shITGB1 Ti substrate cultures. No differences in NRP1, NRP2, and PLXNA1 were observed between controls and shITGA2 cells when cultured on Ti substrates. Expression of PLXNA2 (Fig.2I) was only affected by shITGB1 and shITA2 in TCPS cultures.
Figure 2. Effect of integrin signaling on MSC semaphorin expression and production.
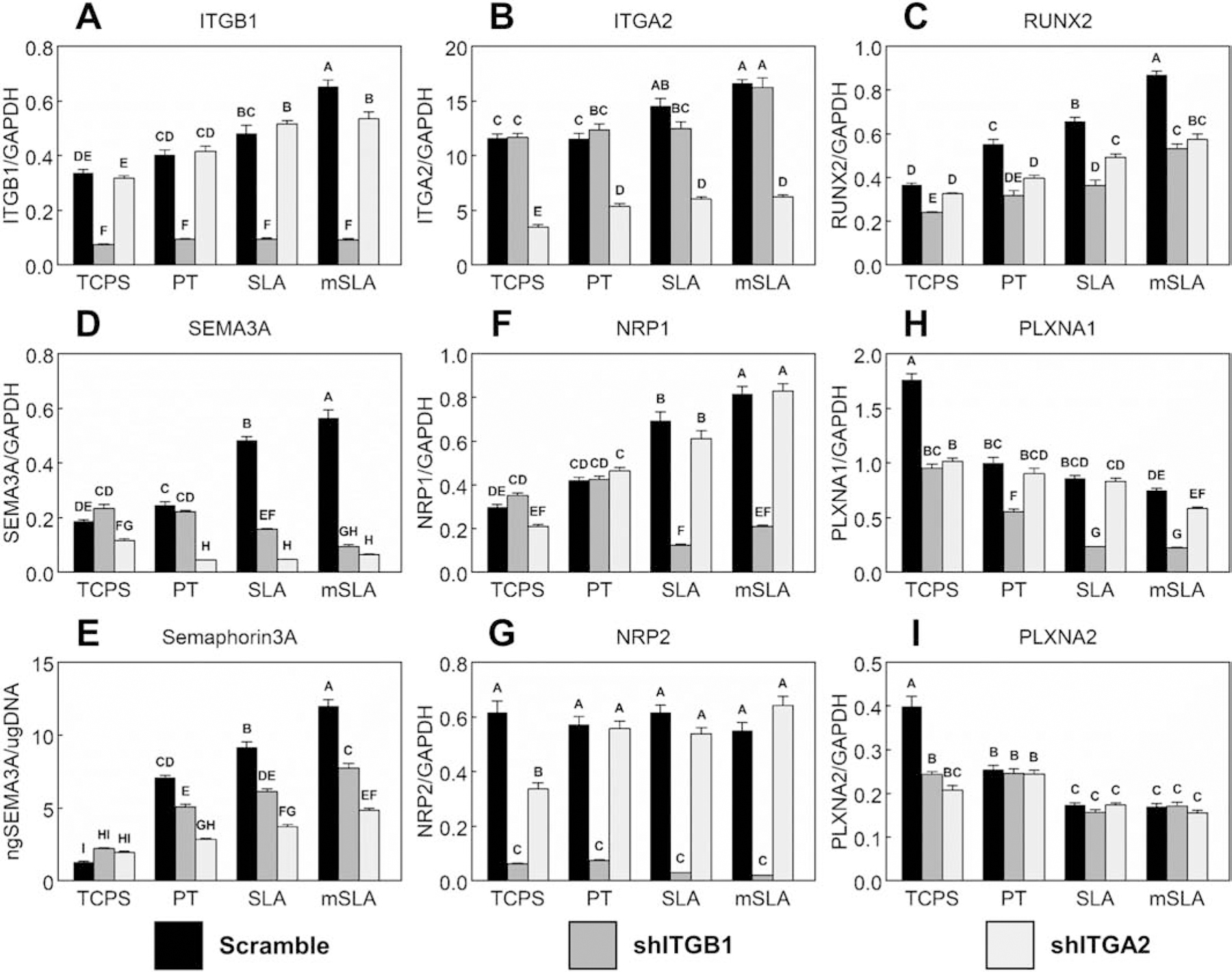
MSCs (WT scramble), MSCs with silenced ITGB1 (shITGB1), and MSCs with silenced ITGA2 (shITGA2) were plated on TCPS or Ti substrates. After 7d, mRNA levels of RUNX2 (A), OCN (B), ITGA2 (C), ITGB1 (D), SEMA3A (E) as well as Semaphorin3A protein levels (F), NRP1 (G), NRP2 (H), PLXNA1 (I), and PLXNA2 (J) were measured. Data shown are the mean ± standard error (SE) of six independent samples. Groups not sharing a letter are statistically significant at α=0.05.
3.2. Effect of Sema3A on MSC Differentiation
In order to optimize the quantity of exogenous Sema3A for in vitro cultures, a dosing study was conducted using MSCs cultured on mSLA (Supplementary Fig.2). Cells were plated on mSLA as described previously (Section 2.2.2) and treated with different concentrations of Sema3A (0.0, 0.0156, 0.0625, 0.25, or 1.0μg/mL) every 48h. High concentrations of Sema3A (0.25μg/mL and 1.0μg/mL) increased osteocalcin, BMP2, osteoprotegerin, and IL-10 compared to other treatments. Production of OPN was reduced with these high concentrations of Sema3A. 1.0μg/mL of Sema3A was chosen for subsequent experiments as further increases in osteocalcin, BMP2, and IL-10 were observed compared to 0.25μg/mL.
Cultures on Ti surfaces decreased DNA content with an additional decrease due to hydrophilicity (Fig.3A), but no additional effect on DNA content was observed with Sema3A treatment. Production of osteocalcin (Fig.3B), BMP2 (Fig.3C), OPN (Fig.3D), OPG (Fig.3E), WNT3A (Fig.3F), and WNT5A (Fig.3G) increased on Ti surfaces with the largest quantities detected on mSLA. Treatment with Sema3A facilitated increased production of osteocalcin, BMP2, OPG, and WNT5A on Ti surface cultures as well as TCPS cultures; however, OPN levels were decreased in Ti surface cultures. Addition of Sema3A increased WNT3A production only in TCPS and PT cultures. Since the effects were most robust on mSLA surfaces subsequent experiments were performed using only this substrate. Blocking endogenous Sema3A with AbSema3A treatment did not affect DNA content (Fig.3H). Osteocalcin (Fig.3I) was unaffected by treatment with low doses (1:500) of AbSema3A; however, high doses (1:200) of AbSema3A reduced osteocalcin production compared to the vehicle control. A dose dependent effect was observed for BMP2 (Fig.3J) production by cells cultured on mSLA. Both doses of AbSema3A increased levels of OPN (Fig.3K). Like BMP2, a dose dependent reduction in OPG (Fig.3L) production was observed in cells cultured on mSLA. AbSema3A reduced levels of WNT3A (Fig.3M) while Wnt5A (Fig.3N) production remained unchanged.
Figure 3. Autocrine and paracrine effects of Sema3A on MSC response to surface microstructure and hydrophilicity.
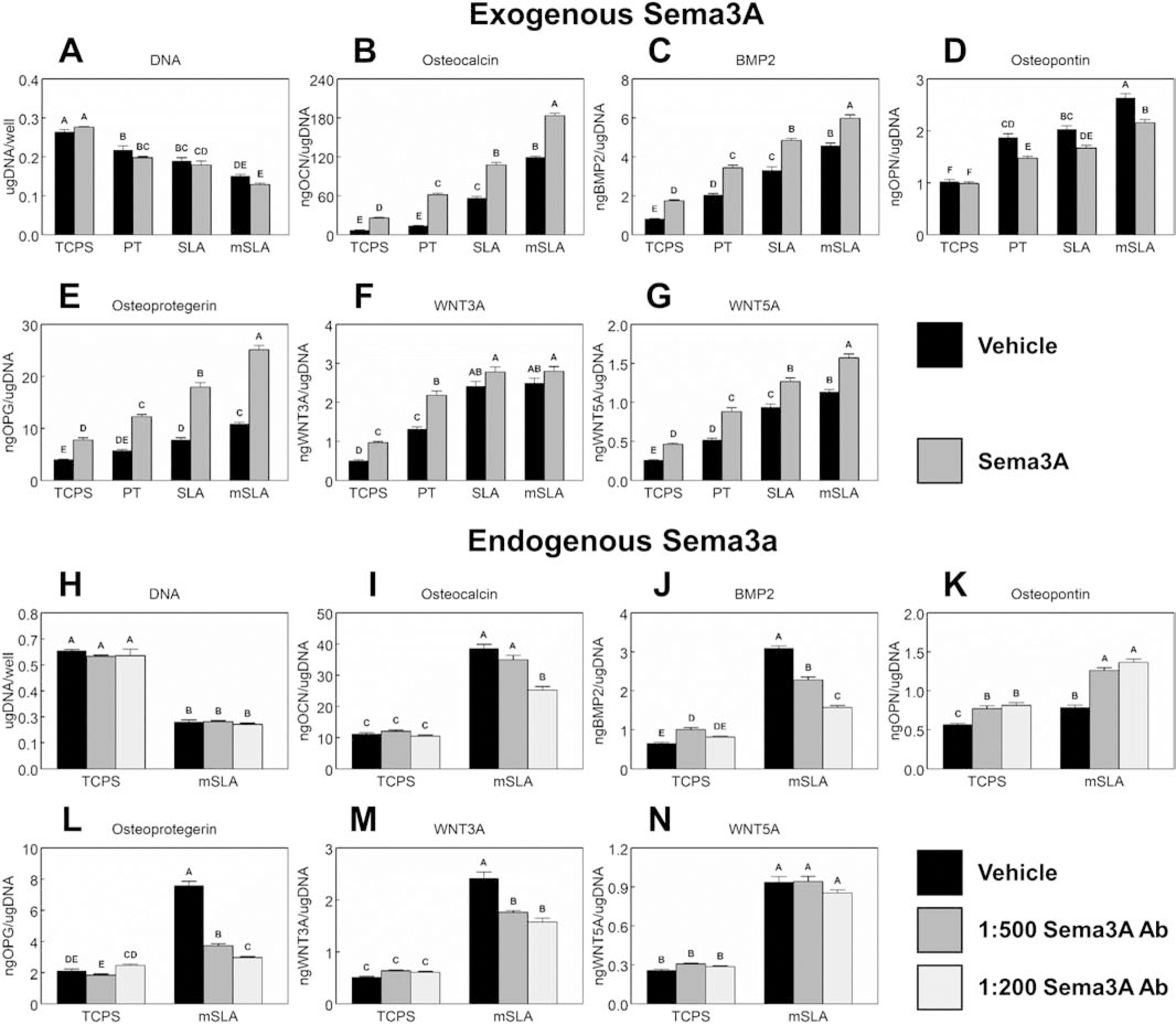
MSCs plated on TCPS, PT, SLA, or mSLA surfaces were cultured with 1μg/ml Sema3A (A – G) or 1:500 or 1:200 AbSema3A (H – N) for 7d. Cells were then treated with fresh media for 24h. After 24h, media were collected, and cell lysates were assayed for DNA content (A, H). Media were assayed for osteocalcin (B, I), BMP2 (C, J), osteopontin (D, K), osteoprotegerin (E, L), WNT3A (F, M), and WNT5A (G, N). Data shown are the mean ± standard error (SE) of six independent samples. Groups not sharing a letter are statistically significant at α=0.05.
3.3. Effect of Sema3A on Wnt3A Signaling
Figure 4 shows the effects of Wnt3A inhibition with and without the addition of Sema3A on cells cultured only on mSLA. Sema3A and IWR-1 treatment individually reduced DNA content (Fig.4A) and their combination was able to further reduce these levels. Compared to untreated controls, IWR-1 treatment enhanced the production of osteocalcin (Fig.4B), BMP2 (Fig.4C), OPN (Fig.4D), and OPG (Fig.4E). Inhibition of Wnt3A with IWR-1 did not affect Sema3A production (Fig.4F). A dose dependent effect was observed for BMP2 and OPG production while high doses of IWR-1 increased osteocalcin production and both doses of IWR-1 enhanced OPN to similar levels. The combination of Sema3A and IWR-1 further enhanced production of these proteins to levels greater than individual treatments. Except for osteocalcin production, no differences were detected between either dose of IWR-1 in combination with Sema3A. Similar results were observed with IWP-2 (Supplementary Fig.3).
Figure 4. Effect of Wnt signaling and Sema3A on MSC response to microstructured and hydrophilic surfaces.
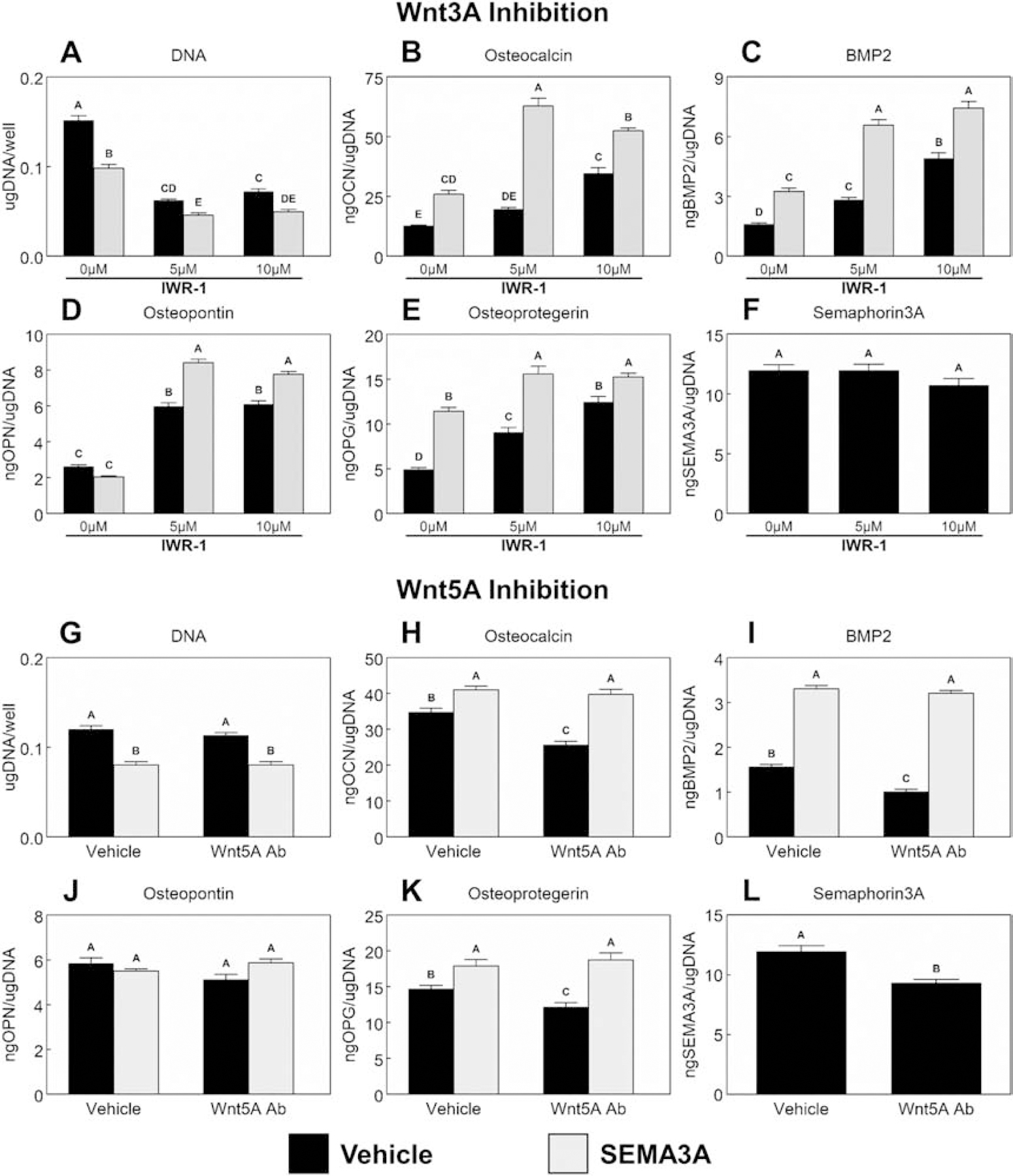
MSCs were cultured on TCPS or Ti substrates. Cultures supplemented with or without 1μg/mL Sema3A were treated with either 5μM or 10μM IWR-1 (A – F) to inhibit Wnt3A signaling or a 1:200 dilution of a polyclonal anti-Wnt5A antibody (G – L) to inhibit Wnt5A signaling for 7d. Cells were then treated with fresh media for 24h. After 24h, media were collected, and cell lysates were assayed for DNA content (A, G). Media were assayed for osteocalcin (B, H), BMP2 (C, I), osteopontin (D, J), osteoprotegerin (E, K) and Semaphorin3A (F, L). Data shown are the mean ± standard error (SE) of six independent samples. Groups not sharing a letter are statistically significant at α=0.05.
3.4. Effect of Sema3A on Wnt5A Signaling
Blocking endogenous Wnt5A with AbWnt5A reduced DNA content (Fig.4G), osteocalcin (Fig.4H), and BMP2 (Fig.4I). OPN production (Fig.4J) was unaffected by any treatment, while production of OPG (Fig.4K) and Sema3A (Fig.4L) was reduced by AbWnt5A. When Sema3A was added to cultures, all parameters except OPN were enhanced. No effect was observed when AbWnt5A was added to cultures receiving Sema3A.
Figure 5 shows the effects of the inhibition of the downstream components of the Wnt5A pathway with and without the addition of Sema3A on cells cultured only on mSLA. The calmodulin (CaM) antagonist, W7, increased DNA content (Fig.5A) and the addition of Sema3A to W7 treated cultures reduced the DNA content to levels similar to both untreated controls and those cultures receiving Sema3A. Osteocalcin (Fig.5B), BMP2 (Fig.5C), OPG (Fig.5D) and Sema3A (Fig.5E) production were reduced with W7 treatment. Addition of Sema3A alongside W7 recovered the production of osteocalcin and BMP2 to levels similar to untreated controls; however, OPG remained undetected with W7 and Sema3A treatment. Inhibition of CamKII with KN93 reduced DNA content (Fig.5F) to similar levels as individual treatment with Sema3A. Further reductions in DNA content were observed in cultures treated with both KN93 and Sema3A. KN93 had no effects on osteocalcin production (Fig.5G) and, likewise, the combination of KN93 and Sema3A were not different from cultures receiving only Sema3A. BMP2 production (Fig.5H) production was inhibited by KN93 treatment while OPG (Fig.5I) was not affected. Addition of Sema3A rescued BMP2 production to levels similar to untreated controls, while their combination decreased OPG production. Sema3A production (Fig.5J) was inhibited by KN93 treatment. The PLA2 inhibitor, AACOCF3, increased DNA content (Fig.5K) compared to untreated controls. Combined treatment of Sema3A and AACOCF3 reduced DNA content to similar levels. Inhibition of PLA2 reduced the production of osteocalcin (Fig.5L), BMP2 (Fig.5M), OPG (Fig.5N), and Sema3A (Fig.5O). Addition of Sema3A recovered the impaired BMP2 production caused by AACOCF3 and enhanced osteocalcin production compared to Sema3A treatment alone. OPG levels were slightly increased compared to AACOCF3 treatment alone with the addition of Sema3A. PKC inhibition by two different chemicals is shown in Fig.5P – Y. Both chemicals had no effect on DNA content (Fig.5P, U). Inhibition of PKC decreased osteocalcin (Fig.5Q, V) production but enhanced BMP2 (Fig.5R, W) production as well as Sema3A (Fig.5T, Y) production. Addition of Sema3A in combination with PKC inhibition increased osteocalcin production but levels were lower than Sema3A treatment alone. BMP2 production was similar between cultures receiving Sema3A alone and those receiving the combination of Sema3A and PKC inhibition. PKC inhibition by Chelerythrine Chloride (Fig.5S) had no effect on OPG while inhibition via GF109203X (Fig.5X) increased OPG production. The addition of Sema3A in combination with chelerythrine chloride increased OPG production to levels comparable to Sema3A treatment alone, while Sema3A in combination with GF109203X remained unchanged compared to GF109203X treatment alone.
Figure 5. Effect of CaM, CaMKII, PLA2, and PKC inhibition in addition to Sema3A addition on MSC response to microstructured and hydrophilic surfaces.
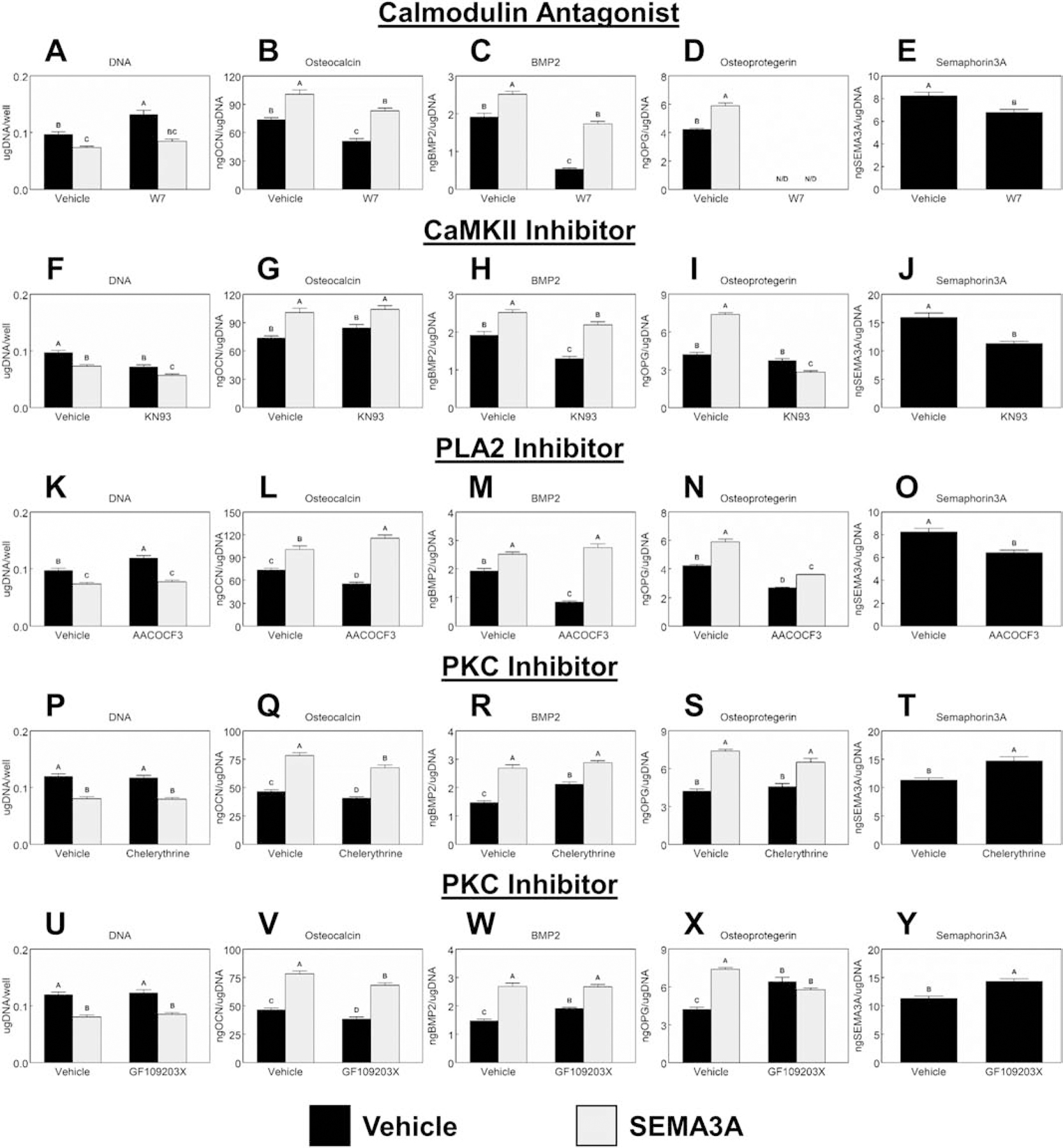
Downstream activation of calmodulin (CaM), Ca2+/calmodulin-dependent protein kinase (CaMKII), and phospholipase A2 (PLA2) were inhibited by treating cultures with 10μM W7 (A – E), 10μM KN93 (F – J), or 10μM AACOCF3 (K – O) respectively with or without the addition of 1μg/mL Sema3A. Protein kinase C (PKC) was inhibited using either 1μM chelerythrine chloride (P – T) or 1μM GF109203X (U – Y) with or without the addition of 1μg/mL Sema3A for 7d. Cells were then treated with fresh media for 24h. After 24h, media were collected, and cell lysates were assayed for DNA content. Media were assayed for osteocalcin, BMP2, osteoprotegerin, and Semaphorin3A. Data shown are the mean ± standard error (SE) of six independent samples. Groups not sharing a letter are statistically significant at α=0.05.
3.5. Effect of Sema3A on BMP2 Signaling
The effects of BMP receptor inhibition on Sema3A production on cells cultured only on mSLA is shown in Figure 6. Osteocalcin production (Fig.6A–D) was enhanced through inhibition of different BMP receptors. A dose dependent increase of osteocalcin was seen in cultures treated with DMH-1 (Fig.6A) and LDN (Fig.6D) while both doses of DMH-2 (Fig.6B) enhanced osteocalcin production to similar levels. 1μM DDCl enhanced osteocalcin production compared to 0μM and 0.1μM doses (Fig.6C). 1μM doses of DMH-1 (Fig.6E) and DMH-2 (Fig.6F) facilitated higher BMP2 production compared to 0μM and 0.1μM, while DDCl (Fig.6G) and LDN (Fig.6H) had a dose-dependent increase in BMP2 production. Sema3A production was similarly enhanced after inhibition of BMP receptors (Fig.6I–J). Both doses of DMH-1 (Fig.6I), DMH-2 (Fig.6J), and LDN (Fig.6L) enhanced Sema3A production compared to untreated controls, while the low dose of DDCl (Fig.6K) increased Sema3A production. Since similar results were observed with each chemical, we selected DMH-1 as the best candidate to assess in combination with Sema3A as it also blocks only ALK2 receptors. Both DMH-1, Sema3A, and their combination reduced DNA content (Fig.6M) to similar levels compared to the untreated controls. Osteocalcin (Fig.6N) production and BMP2 (Fig.6N) production were enhanced to similar levels after treatment with Sema3A or DMH-1. The combination of Sema3A and DMH-1 further increased the production of the two proteins. Treatment of surface cultured MSCs with 40ng/mL recombinant human BMP2 decreased the production of Sema3A (Fig.6P).
Figure 6. Effect of BMP2 signaling and Sema3A on MSC response to microstructured and hydrophilic surfaces.
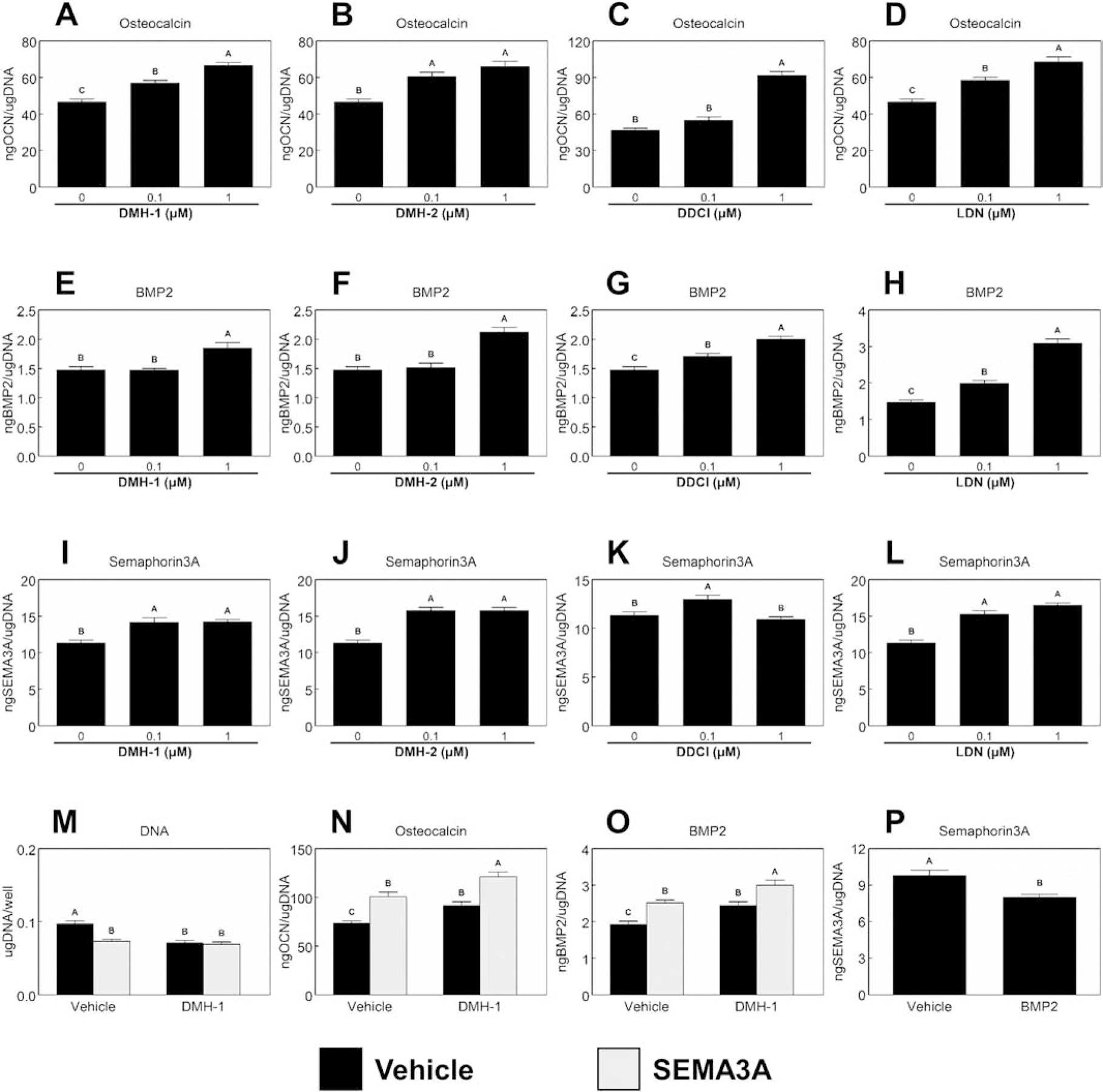
0.1μM or 1μM of two chemicals known to inhibit ALK2, ALK3, and ALK6 [DMH-2 and dorsomorphin dihydrochloride (DDCl)]; LDN 193189 dihydrochloride (ALK2 and ALK3 inhibitor) or DMH-1 (ALK2 inhibitor) were used to treat surface cultured MSCs. After 7d, cells were treated with fresh media for 24h. After 24h, media were collected and assayed for osteocalcin (A – D), BMP2 (E – H), and Semaphorin3A (I – L). MSCs cultured on mSLA were also treated with 10μM DMH-1 with or without the addition of 1μg/mL Sema3A for 7d. Cultures were then treated for 24h with fresh media. After 24h, media were collected, and cell lysates were assayed for DNA content (M). Media were assayed for osteocalcin (N) and BMP2 (O). The production of Sema3A after the addition of 40ng/mL recombinant human BMP2 was also measured (P). Data shown are the mean ± standard error (SE) of six independent samples. Groups not sharing a letter are statistically significant at α=0.05.
4. Discussion
Microstructured and hydrophilic Ti surfaces increase MSC differentiation and peri-implant bone formation. Our lab has previously demonstrated that in the absence of exogenous growth factors or osteogenic media are enough to induce osteoblastic differentiation and maturation. However, the precise molecular mechanisms controlling this process remain unclear. Here we demonstrate novel roles for Sema3A family proteins in the surface-dependent modulation of MSCs. Containing significant crosstalk with other major pathways that have been associated with surface mediated osteoblastic differentiation, which have been summarized graphically in figure 7. Moreover, their effects on bone remodeling markers have significant implications for peri-implant bone remodeling and downstream modulation of osteoclastic activity.
Figure 7.
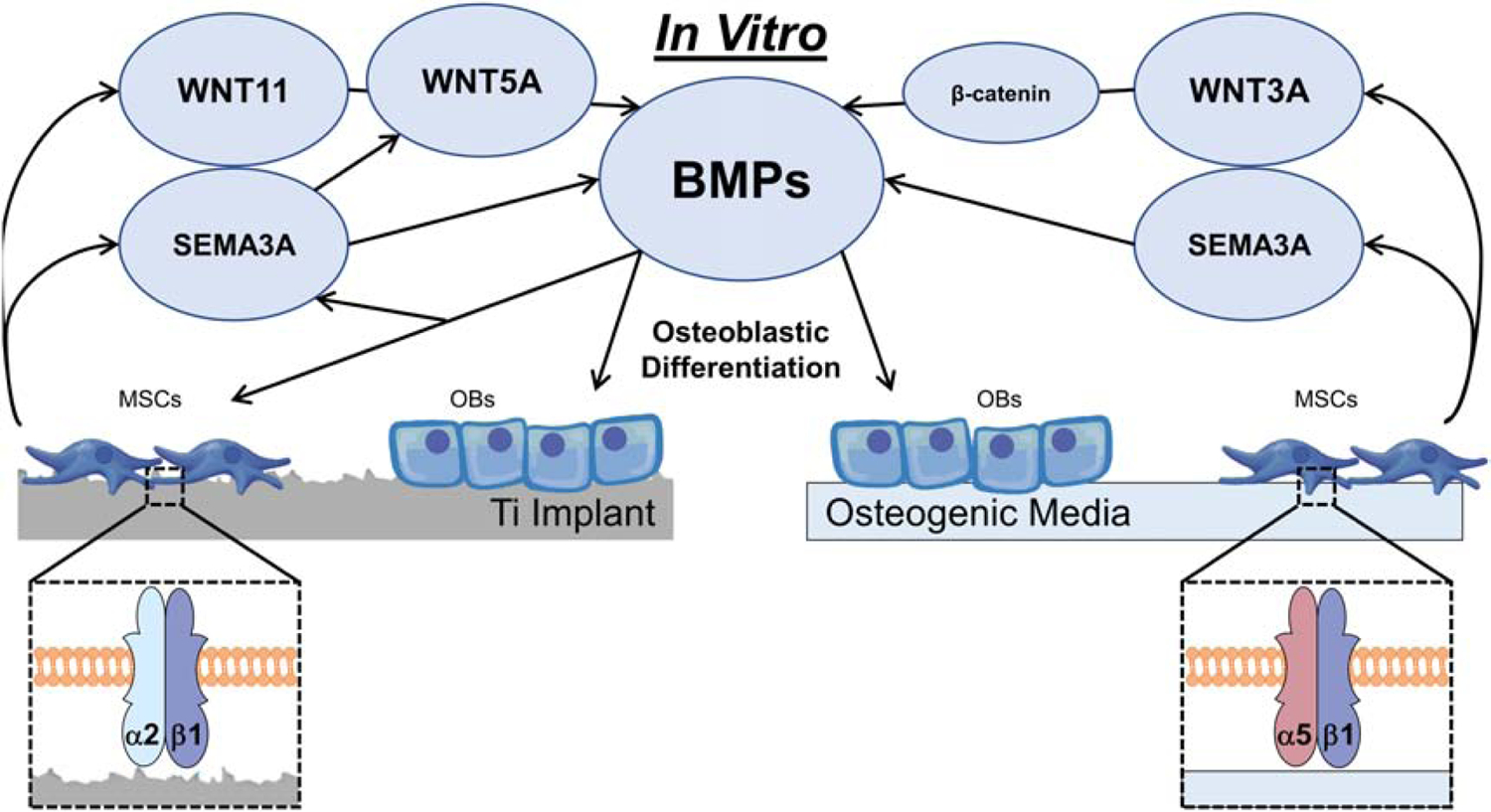
Proposed role of Sema3A on the osteoblastic differentiation of mesenchymal stem cells cultured on microstructured Ti surfaces or in osteogenic media.
Implant surfaces were able to upregulate the expression and production of Sema3A. Sema3A has shown to play an osteoprotective role by reducing osteoclastic bone resorption and by increasing osteoblastic bone formation [27,47,48]. This pro-osteogenic signaling is mediated by the formation of the NRP1 – PLXNA1 receptor complex [48,49]. Interestingly, microstructured implant surfaces upregulated NRP1 expression in MSCs while downregulating PLXNA1 expression. This can be explained by the dual role PLXNA1 plays in regulating bone remodeling. When NRP1 expression is low, binding of PLXNA1 to TREM-2 is favored which stimulates osteoclastogenesis. Alternatively, in the presence of SEMA3A, PLXNA1 will complex with NRP1, which stimulates osteogenesis. NRP1 has also shown to be more important for the osteogenic phenotype compared to PLXNA1 [37]. Although both receptors are present on osteoblasts, NRP1 deficiency results in an osteoporotic phenotype characterized by a marked decrease in osteoblast number and activity [26,27]. In addition, PLXNA1 deficient mice have an osteopetrotic phenotype associated with a decreased osteoclast number and decreased osteoclast activity, whereas osteoblast number and activity are not altered [50]. The NRP1 – PLXNA1 complex may be required only at early time points to stimulate osteoblastic differentiation, and the lack of reciprocal communication from osteoclasts may lead to decreased PLXNA1 expression over time.
We previously reported that integrin subunits α2 and β1 play an important role in surface-mediated osteoblastic differentiation [7,8,51,52]. When integrin signaling is inhibited either by silencing the α2 or β1 subunit, osteoblast differentiation is inhibited. Each subunit has also been shown to recognize a specific characteristic of microstructured and hydrophilic surfaces. Studies suggests that the α2 subunit is response for surface chemistry recognition while the β1 subunit is involved in roughness recognition [7,8,51,52]. Silencing of either the α2 or β1 subunit resulted in decreased SEMA3A expression and production, while receptor expression decreased when only β1 was silenced suggesting unique roles for these surface features in their regulation of semaphorin family proteins.
It has been suggested that Sema3A treatment stimulates the maturation of osteoblasts but does not promote the osteoblastic differentiation of MSCs [27]. Our results demonstrate that the addition of Sema3A to surface cultured MSCs increases markers of osteoblastic differentiation compared cultures not supplemented with Sema3A. This effect is more robust with the combination of microstructured surfaces and exogenous Sema3A and decreased when endogenous Sema3A is blocked. These results indicate an increase in osteoblastic differentiation on rough surfaces, which is enhanced by exogenous Sema3A. Interestingly, exogenous Sema3A decreased osteopontin production and increased osteoprotegerin production. Osteopontin plays a role in anchoring osteoclasts to the mineralized bone matrix [19] while osteoprotegerin serves as a decoy receptor for RANKL preventing the formation of osteoclasts [21]. Alternatively, blocking endogenous Sema3A enhanced osteopontin production while reducing osteoprotegerin. Taken together these data further support the unique role of Sema3A as a coupling factor capable of regulating bone remodeling.
Microstructured surfaces and Sema3A have both been shown to regulate osteoblastic differentiation and maturation through the Wnt pathway. On TCPS, MSC differentiation occurs through external factors like BMPs, which activate the canonical Wnt3A pathway, causing an accumulation of β-catenin in the cytoplasm and its translocation into the nucleus to serve as a transcriptional activator. Previous studies have demonstrated surface mediated MSC differentiation downregulates genes associated with the Wnt3A pathway while upregulating genes of the Wnt11 and non-canonical, calcium dependent Wnt5a pathway [11,12,14]. The Sema3A pathway has significant crossover with the canonical Wnt3A pathway. In Sema3A−/− calvarial cells, the activation of Rac1 in response to Wnt3A treatment was significantly decreased [27]. In these same mice, Sema3A treatment was able to induce the nuclear translocation of β-catenin after activation of Rac1 through FARP2 [27]. In the present study, exogenous Sema3A increased Wnt3A on smooth Ti, but not microrough and hydrophilic Ti substrates. Furthermore, blocking endogenous Sema3A reduced the production of Wnt3A on the microrough/hydrophilic mSLA surface. Decreased expression of Sema3A has been observed when the canonical Wnt3A pathway was activated using BIO and LiCl [53]. Surprisingly, inhibition of the Wnt3A pathway using IWR-1 did not alter the production of Sema3A; however, when Sema3A was added to cultures receiving IWR-1, further increases of osteogenic markers were seen compared to IWR-1 treatments alone. It is possible that the canonical Wnt pathway is important for the stimulation of osteoblastic differentiation on microstructured surfaces. However, instead of Wnt3A, the nuclear translocation of β-catenin is mediated by Sema3A bypassing the need for any Wnt3A initiating signals. Future studies are needed to validate this hypothesis.
The non-canonical, calcium dependent Wnt5A pathway has been shown to be the primary driver of osteoblastic differentiation on microstructured Ti substrates [11,12,14]. Few studies, however, has been done on any crosstalk between Wnt5A and Sema3A. In the present study, exogenous Sema3A increased Wnt5A on all surfaces while blocking endogenous Sema3A had no effect on Wnt5A production. AbWnt5A was able to suppress the production of osteogenic factors by MSCs cultured on microrough and hydrophilic implant surfaces. Sema3A was able to recover the production of osteogenic factors when Wnt5A was inhibited; however, no synergistic effects were observed like those seen with Wnt3A inhibition. Moreover, AbWnt5A decreased Sema3A production. These data suggest that Wnt5A remains the primary driver of osteoblastic differentiation on microstructured Ti substrates, but when Wnt5A levels are low, Sema3A is able to drive this process instead.
In order to further elucidate this crosstalk, the interaction of Sema3A and downstream components of the Wnt5A pathway were examined. It has been shown that CaM, CaMKII, and PLA2 are critical for Wnt5A stimulated activation of [54]. Additionally, binding of Wnt5A to its receptor complex can activate the planar cell polarity pathway which is important for osteoblast differentiation [55]. In the present study, CaM inhibition by W7, CamKII inhibition by KN93, and PLA2 inhibition by AACOCF3 reduced the production of osteogenic factors by MSCS which was similar to blocking endogenous Wnt5A. The production of Sema3A was also reduced with these inhibitors. Interestingly inhibition of PKC by either Chelerythrine chloride or GF109203X increases the production Sema3A. Together this suggests that that the regulation of Sema3A by Wnt5A comes after activation of PLA2. Additionally, the increased production of Sema3A that occurs after PKC inhibition could serve as a feedback mechanism for Wnt5A, which also supports the fact that Sema3A increased the production of Wnt5A on all surfaces.
MSCs cultured on microstructured Ti substrates, display temporal upregulation of BMPs with increases of BMP2 and BMP4 occurring as early as 4 days, suggesting that they are early regulators of surface-mediated osteogenesis [22]. Inhibition of PKC also led to increased BMP2 production on the microstructured/hydrophilic mSLA surface. As such, the interaction between the BMP and Sema3A signaling pathways were investigated by using inhibitors of BMP receptors. Inhibition of the type I BMP receptors enhanced the production of osteogenic factors, including the production of BMP2 itself. Furthermore, these inhibitors also increased the production of BMP2. When ALK2 was inhibited, Sema3A was able to increase the production of osteocalcin and BMP2 to levels greater than Sema3A treatment alone. Interestingly, the addition of BMP2 reduced the production of Sema3A. Together this suggests Sema3A has a similar feedback effect on BMP2 as it does to Wnt5A.
5. Conclusions
In conclusion, this study investigated the role of SEMA3A on surface-mediated osteoblastic differentiation. Furthermore, the hierarchy of this signaling cascade was also elucidated. We found that Sema3A stimulates increased productions of osteogenic factors in MSCs cultured on microstructured Ti substrates with significant crosstalk with other major pathways associated with osteoblastic differentiation. The production of Sema3A is dependent on the recognition of the implant surface by the α2 and β1integrin subunits. A feedback loop exists between the Sema3A pathway and both the Wnt5A pathway as well as the BMP pathway. Moreover, Sema3A could facilitate the nuclear translocation of β-catenin without the Wnt3A initiating signal. The Sema3A system plays an important role around implants and its pathways may be tightly regulated for successful osteoblast differentiation, maturation, and eventual peri-implant bone formation. Moreover, its effects on bone remodeling markers have significant implications for peri-implant bone remodeling and downstream modulation of osteoclastic activity. These results suggest that Sema3A aids in peri-implant bone formation through regulation on multiple stages of osseointegration making it a potential target to promote osseointegration in patients with compromised bone remodeling.
Supplementary Material
Highlights.
Mesenchymal stem cell production of Sema3A is modulated by titanium substrates
Sema3A production is dependent on titanium surface recognition through integrins
Sem3A works alongside Wnt5A and BMP pathways to induce osteoblastic differentiation
Sema3A could facilitate the nuclear translocation of β-catenin independent of Wnt3A
Sema3A has potential to regulate multiple stages of osseointegration
Acknowledgements
Institut Straumann AG (Basel, Switzerland) provided the surfaces and support for this study. Additional support was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01AR052102 and R01AR072500. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mellis DJ, Itzstein C, Helfrich MH, Crockett JC, The skeleton: A multi-functional complex organ. The role of key signalling pathways in osteoclast differentiation and in bone resorption, J. Endocrinol (2011). 10.1530/JOE-11-0212. [DOI] [PubMed]
- [2].Cochran DL, A comparison of endosseous dental implant surfaces, J. Periodontol 70 (1999) 1523–1539. 10.1902/jop.1999.70.12.1523. [DOI] [PubMed] [Google Scholar]
- [3].Gittens RA, Olivares-Navarrete R, Schwartz Z, Boyan BD, Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants, Acta Biomater 10 (2014) 3363–3371. 10.1016/j.actbio.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, Schwartz Z, Boyan BD, A review on the wettability of dental implant surfaces II: Biological and clinical aspects, Acta Biomater 10 (2014) 2907–2918. 10.1016/j.actbio.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boyan BD, Lotz EM, Schwartz Z, Roughness and hydrophilicity as osteogenic biomimetic surface properties, Tissue Eng. Part A 23 (2017) 1479–1489. 10.1089/ten.TEA.2017.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Keselowsky BG, Wang L, Schwartz Z, Garcí AJ, Boyan BD, Integrin α5 controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner, J. Biomed. Mater. Res. Part A 80 (2007) 700–710. 10.1002/jbm.a.30898. [DOI] [PubMed] [Google Scholar]
- [7].Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z, Integrin α2β1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates, Proc. Natl. Acad. Sci 105 (2008) 15767–15772. 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang L, Zhao G, Olivares-Navarrete R, Bell BF, Wieland M, Cochran DL, Schwartz Z, Boyan BD, Integrin β1 silencing in osteoblasts alters substrate-dependent responses to 1,25-dihydroxy vitamin D3, Biomater 27 (2006) 3716–3725. 10.1016/j.biomaterials.2006.02.022. [DOI] [PubMed] [Google Scholar]
- [9].Park JH, Wasilewski CE, Almodovar N, Olivares-Navarrete R, Boyan BD, Tannenbaum R, Schwartz Z, The responses to surface wettability gradients induced by chitosan nanofilms on microtextured titanium mediated by specific integrin receptors, Biomater 33 (2012) 7386–7393. 10.1016/j.biomaterials.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lotz EM, Olivares-Navarrete R, Berner S, Boyan BD, Schwartz Z, Osteogenic response of human MSCs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces, J. Biomed. Mater. Res. - Part A 104 (2016) 3137–3148. 10.1002/jbm.a.35852. [DOI] [PubMed] [Google Scholar]
- [11].Olivares-Navarrete R, Hyzy SL, Park JH, Dunn GR, Haithcock DA, Wasilewski CE, Boyan BD, Schwartz Z, Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop, Biomater 32 (2011) 6399–6411. 10.1016/j.biomaterials.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, Schwartz Z, Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage, Biomater 31 (2010) 2728–2735. 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Olivares-Navarrete R, Hyzy SL, Hutton DL, Dunn GR, Appert C, Boyan BD, Schwartz Z, Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces, Acta Biomater 7 (2011) 2740–2750. 10.1016/j.actbio.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boyan BD, Olivares-Navarrete R, Berger MB, Hyzy SL, Schwartz Z, Role of Wnt11 during osteogenic differentiation of human mesenchymal stem cells on microstructured titanium surfaces, Sci. Rep 8 (2018) 1–11. 10.1038/s41598-018-26901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lotz EM, Cohen DJ, Ellis RA, Wayne JS, Schwartz Z, Boyan BD, Ibandronate treatment before and after implant insertion impairs osseointegration in aged rats with ovariectomy induced osteoporosis, JBMR Plus 3 (2019) 1–14. 10.1002/jbm4.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lotz EM, Olivares-Navarrete R, Hyzy SL, Berner S, Schwartz Z, Boyan BD, Comparable responses of osteoblast lineage cells to microstructured hydrophilic titanium–zirconium and microstructured hydrophilic titanium, Clin. Oral Implants Res 28 (2017) e51–e59. 10.1111/clr.12855. [DOI] [PubMed] [Google Scholar]
- [17].Glowacki J, Rey C, Glimcher MJ, Cox KA, Lian J, A role for osteocalcin in osteoclast differentiation, J. Cell. Biochem 45 (1991) 292–302. 10.1002/jcb.240450312. [DOI] [PubMed] [Google Scholar]
- [18].Ritter NM, Farach-Carson MC, Butler WT, Farach-Carson MC, Butler WT, Evidence for the formation of a complex between osteopontin and osteocalcin, J. Bone Miner. Res 7 (2009) 877–885. 10.1002/jbmr.5650070804. [DOI] [PubMed] [Google Scholar]
- [19].Reinholt FP, Hultenby K, Oldberg A, Heinegard D, Osteopontin--a possible anchor of osteoclasts to bone., Proc. Natl. Acad. Sci 87 (1990) 4473–4475. 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schwartz Z, Olivares-Navarrete R, Wieland M, Cochran DL, Boyan BD, Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces, Biomater 30 (2009) 3390–3396. 10.1016/j.biomaterials.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boyce BF, Xing L, The RANKL/RANK/OPG pathway, Curr. Osteoporos. Rep 5 (2007) 98–104. 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- [22].Olivares-Navarrete R, Hyzy SL, Haithcock DA, Cundiff CA, Schwartz Z, Boyan BD, Coordinated regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by endogenous bone morphogenetic proteins, Bone 73 (2015) 208–216. 10.1016/j.bone.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wall A, Board T, Bone morphogenetic protein, Class. Pap. Orthop 362 (2014) 465–467. 10.1007/978-1-4471-5451-8_118. [DOI] [Google Scholar]
- [24].Gilboa L, Nohe A, Geissendorfer T, Sebald W, Henis YI, Knaus P, Bone morphogenetic protein receptor complexes on the surface of live cells: A new oligomerization mode for serine/threonine kinase receptors, Mol. Biol. Cell 11 (2013) 1023–1035. 10.1091/mbc.11.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wan M, Cao X, BMP signaling in skeletal development, Biochem. Biophys. Res. Commun 328 (2005) 651–657. 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- [26].Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, Ma C, Xu C, Bando W, Fujita K, Shinomiya K, Hirai T, Asou Y, Enomoto M, Okano H, Okawa A, Itoh H, Sema3A regulates bone-mass accrual through sensory innervations, Nature 497 (2013) 490–493. 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- [27].Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H, Osteoprotection by semaphorin 3A, Nature 485 (2012) 69–74. 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- [28].Kim BJ, Koh JM, Coupling factors involved in preserving bone balance, Cell. Mol. Life Sci (2019). 10.1007/s00018-018-2981-y. [DOI] [PMC free article] [PubMed]
- [29].Arese M, Serini G, Bussolino F, Nervous vascular parallels: Axon guidance and beyond, Int. J. Dev. Biol (2011). 10.1387/ijdb.103242ma. [DOI] [PubMed]
- [30].Suzuki K, Kumanogoh A, Kikutani H, Semaphorins and their receptors in immune cell interactions, Nat. Immunol (2008). 10.1038/ni1553. [DOI] [PubMed]
- [31].Pasquale EB, Eph receptor signalling casts a wide net on cell behaviour, Nat. Rev. Mol. Cell Biol 6 (2005) 462–475. 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- [32].Castellani V, Rougon G, Control of semaphorin signaling, Curr. Opin. Neurobiol (2002). 10.1016/S0959-4388(02)00357-4. [DOI] [PubMed]
- [33].Epstein JA, Aghajanian H, Singh MK, Semaphorin signaling in cardiovascular development, Cell Metab (2015). 10.1016/j.cmet.2014.12.015. [DOI] [PMC free article] [PubMed]
- [34].Jongbloets BC, Pasterkamp RJ, Semaphorin signalling during development, Development 141 (2014) 3292–3297. 10.1242/dev.105544. [DOI] [PubMed] [Google Scholar]
- [35].Takamatsu H, Okuno T, Kumanogoh A, Regulation of immune cell responses by semaphorins and their receptors, Cell. Mol. Immunol (2010). 10.1038/cmi.2009.111. [DOI] [PMC free article] [PubMed]
- [36].Verlinden L, Vanderschueren D, Verstuyf A, Semaphorin signaling in bone, Mol. Cell. Endocrinol 432 (2016) 66–74. 10.1016/j.mce.2015.09.009. [DOI] [PubMed] [Google Scholar]
- [37].Gomez C, Burt-Pichat B, Mallein-Gerin F, Merle B, Delmas PD, Skerry TM, Vico L, Malaval L, Chenu C, Expression of semaphorin-3A and its receptors in endochondral ossification: Potential role in skeletal development and innervation, Dev. Dyn 234 (2005) 393–403. 10.1002/dvdy.20512. [DOI] [PubMed] [Google Scholar]
- [38].Kruger RP, Aurandt J, Guan KL, Semaphorins command cells to move, Nat. Rev. Mol. Cell Biol (2005). 10.1038/nrm1740. [DOI] [PubMed]
- [39].Delorme G, Saltel F, Bonnelye E, Jurdic P, Machuca-Gayet I, Expression and function of semaphorin 7A in bone cells, Biol. Cell (2005). 10.1042/bc20040103. [DOI] [PubMed]
- [40].Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J, Rennert PD, Kolodkin AL, Kumanogoh A, Kikutani H, Semaphorin 7A initiates T-cell-mediated inflammatory responses through α1β1 integrin, Nature (2007). 10.1038/nature05652. [DOI] [PubMed]
- [41].Xie J, Wang H, Semaphorin 7A as a potential immune regulator and promising therapeutic target in rheumatoid arthritis, Arthritis Res. Ther 19 (2017) 1–12. 10.1186/s13075-016-1217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kumanogoh A, Kikutani H, Immunological functions of the neuropilins and plexins as receptors for semaphorins, Nat. Rev. Immunol 13 (2013) 802–814. 10.1038/nri3545. [DOI] [PubMed] [Google Scholar]
- [43].Kang S, Kumanogoh A, Semaphorins in bone development, homeostasis, and disease, Semin. Cell Dev. Biol 24 (2013) 163–171. 10.1016/j.semcdb.2012.09.008. [DOI] [PubMed] [Google Scholar]
- [44].Liu F, Shen W, Qiu H, Hu X, Zhang C, Chu T, Prostate cancer cells induce osteoblastic differentiation via semaphorin 3A, Prostate (2015). 10.1002/pros.22923. [DOI] [PubMed]
- [45].Shen WW, Chen WG, Liu FZ, Hu X, Wang HK, Zhang Y, Chu TW, Breast cancer cells promote osteoblastic differentiation via sema 3A signaling pathway in vitro, Int. J. Clin. Exp. Pathol (2015). [PMC free article] [PubMed]
- [46].Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares-Navarrete R, Titanium surface characteristics, including topography and wettability, alter macrophage activation, Acta Biomater 31 (2016) 425–434. 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xu R, Semaphorin 3A a new player in bone remodeling, Cell Adhes. Migr 8 (2014) 5–10. 10.4161/cam.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li Z, Hao J, Duan X, Wu N, Zhou Z, Yang F, Li J, Zhao Z, Huang S, The role of semaphorin 3A in bone remodeling, Front. Cell. Neurosci 11 (2017) 1–8. 10.3389/fncel.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kular J, Tickner J, Chim SM, Xu J, An overview of the regulation of bone remodeling at the cellular level, Clin. Biochem 45 (2012) 863–873. 10.1016/j.clinbiochem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- [50].Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DVR, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H, Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis, Nat. Cell Biol (2006). 10.1038/ncb1416. [DOI] [PubMed]
- [51].Lai M, Hermann CD, Cheng A, Olivares-Navarrete R, Gittens RA, Bird MM, Walker M, Cai Y, Cai K, Sandhage KH, Schwartz Z, Boyan BD, Role of α2β1 integrins in mediating cell shape on microtextured titanium surfaces, J. Biomed. Mater. Res. - Part A 103 (2014) 564–573. 10.1002/jbm.a.35185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Olivares-Navarrete R, Rodil SE, Hyzy SL, Dunn GR, Almaguer-Flores A, Schwartz Z, Boyan BD, Role of integrin subunits in mesenchymal stem cell differentiation and osteoblast maturation on graphitic carbon-coated microstructured surfaces, Biomater 51 (2015) 69–79. 10.1016/j.biomaterials.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hughes A, Kleine-Albers J, Helfrich MH, Ralston SH, Rogers MJ, A Class III semaphorin (Sema3e) inhibits mouse osteoblast migration and decreases osteoclast formation in vitro, Calcif. Tissue Int (2012). 10.1007/s00223-011-9560-7. [DOI] [PMC free article] [PubMed]
- [54].Doroudi M, Olivares-Navarrete R, Hyzy SL, Boyan BD, Schwartz Z, Signaling components of the 1α,25(OH)2D3-dependent Pdia3 receptor complex are required for Wnt5a calcium-dependent signaling, Biochim. Biophys. Acta - Mol. Cell Res 1843 (2014) 2365–2375. 10.1016/j.bbamcr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kikuchi A, Yamamoto H, Sato A, Matsumoto S, Wnt5a: Its signalling, functions and implication in diseases, Acta Physiol (2012). 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


