Abstract
Background
: magnetic resonance imaging (MRI) has been increasingly used to study breast cancer for screening high-risk cases, pre-operative staging, and problem-solving because of its high sensitivity. However, its cost-effectiveness is still debated. Thus, the concept of abbreviated MRI (ABB-MRI) protocols was proposed as a possible solution for reducing MRI costs
Purpose
: to investigate the role of the abbreviated MRI protocols in detecting and staging breast cancer
Methods
: a systematic search of the literature was carried out in the bibliographic databases: Scopus, PubMed, Medline, and Science Direct.
Results
: forty-one articles were included, which described results of the assessment of fifty-three abbreviated protocols for screening, staging, recurrence assessing, and problem-solving or clarification.
Conclusions
: the use of ABB-MRI protocols allows reducing the acquisition and reading times, maintaining a high concordance with the final interpretation, in comparison to a complete protocol. However, larger prospective and multicentre trials are necessary to validate the performance in specific clinical environments
Keywords: Breast cancer, Magnetic resonance imaging, Abbreviated protocols, Screening, Staging
1. Introduction
Breast cancer is one of the leading causes of death in the world and the leading cause of death by cancer among women around the world [1]. In 2018, the World Health Organisation (WHO) reported more than 2 million new cases and 626,679 deaths by this disease [2]. Early detection continues to be the best strategy to improve the prognosis of breast cancer, and mammography remains the standard screening method for detection in women over 40 years, with a sensitivity of 70% and a specificity of 92% in the general population [3]. Different studies have shown that screening with mammography has an effect on mortality by breast cancer [4].
Despite this, mammography has shown poor performance in the detection of cancer in patients with dense breasts [5], [3]. It is also not indicated in young patients because of the possible effect of radiation exposure. To counteract this problem, other imaging modalities have been proposed, such as tomosynthesis, a variation of mammography that generates three-dimensional images but whose performance is not significantly greater; breast ultrasound, which is useful as a complementary study, but with a low-positive predictive value [6], and magnetic resonance imaging (MRI), and specifically the Dynamic Contrast-Enhanced MRI, which is currently considered the most sensitive method for detecting breast cancer without the use of ionizing radiation and is proposed as an effective screening alternative in high-risk population [7].
The usefulness of breast magnetic resonance imaging not only includes an initial diagnosis and detection of breast cancer, but it is also recommended for preoperative staging, problem-solving, follow-up of treatment response, among others. A great benefit of MRI is its high detection sensitivity of breast cancer and its usefulness for finding subtle tumors in mammograms, ultrasounds, and physical examination [8]. Particularly, breast cancer screening with MRI alone or combined with ultrasound or mammography has shown variable cost-effectiveness regarding the objective population. Studies have shown that MRI is cost-effective for high-risk patients [9], [10]. In fact, in the last years has been a generalised agreement among the main European and American radiology associations (the European Society of Breast Cancer Specialists - EUSOMA, European Society of Breast Imaging-EUSOBI, Society of Breast Imaging-SBI, American College of Radiology-ACR, and American Cancer Society -ACS) regarding to recommendations for annual screening with MRI and mammography for women with a risk of [11], [12], [13], [14], [15], [16], which includes women with BRCA1 or BRCA2 mutation, women with relatives of first degree of consanguinity with a BRCA mutation, clinical history of thoracic radiation, and Li-Fraumeni, Cowden or Bannayan-Riley-Ruvalcaba syndrome [9]. However, its use as a screening method in the general population or with an intermediate or lower risk of life is hindered due to the cost associated with the study acquisition and the radiology time interpretation. Thus, although recent studies have estimated that screening with MRI alone in women whose only risk factor is dense breasts is economically feasible [17], [18], none of the current guidelines recommend MRI as a screening imaging technique in those cases.
In 2014, Cristiane Kuhl [19] introduced the concept of an abbreviated protocol for breast MRI (ABB-MRI) for breast cancer screening, which required the acquisition of only two sequences, i.e., T1-weighted, acquired before and immediately after the application of gadolinium; from them, two derived images, i.e., the First contrast-enhanced Acquisition SubTracted (FAST) and maximum-intensity projection (MIP) were used for interpretation. This study, which included women with increased risk of developing the disease, allowed establishing the absence of cancer with a negative predictive value of and a diagnostic accuracy equivalent to that obtained by a complete protocol but reducing the acquisition and interpretation times to 3 minutes and fewer than 30 seconds, respectively. From this study, the research into the use of abbreviated protocols for the detection, characterization, and staging of lesions in breast magnetic resonance has become a relevant topic due to the feasibility of substantially reducing the image acquisition and reading times.
Other review articles have described some of the abbreviated protocols published in the literature [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]; however, none of them performed a comprehensive systematic review. Recently, Baxter et al [31] presented a systematic review and meta-analysis of published ABB-MRI studies but focused on screening only cohorts and enriched cohorts separately. Additionally, the search was carried out in August 2019, which implies the exclusion of recent works. Similarly, Geach et al. [32] presented a systematic review and meta-analysis of ABB-MRI for breast cancer screening that included the FAST sequence; the search was performed in November 2019.
This document presents the results of a comprehensive systematic review of literature on abbreviated protocols for breast cancer study, including screening, follow-up, staging, among other applications. We analyze the configuration of the proposed protocols, its performance, and the effect produced by the acquisition and interpretation times.
2. Materials and methods
2.1. Sources consulted and search strategy
A search of published documents was carried out using the following equation: (MRI AND “breast cancer” AND (abbreviated OR accelerated OR fast)), in the databases PubMed (134), Medline (124), Science Direct (27) and Scopus (253). The search was restricted to metadata title, abstract, and keywords. It was also limited to the period between January 1, 2013, and July 31, 2020.
2.2. Eligibility criteria
For this review, full-text availability articles published in a peer-reviewed journal (abstracts and conference proceedings were excluded) and written in English were considered. Studies that include a reduced number of MRI sequences for the detection, staging, and/or follow-up of breast cancer were evaluated. A comparison concerning a referenced standard (complete protocol interpretation, biopsy, or imaging follow-up) was also required.
2.3. Data extraction
Data extraction was performed by one of three reviewers and confirmed by two other reviewers. Study details (imaging use, study design, number of cases, age range, ABB-MRI protocol, acquisition and reading times, summary findings) were extracted from the included full-text articles and recorded on a database (Excel, Microsoft, Redmond WA, USA).
3. Results and discussion
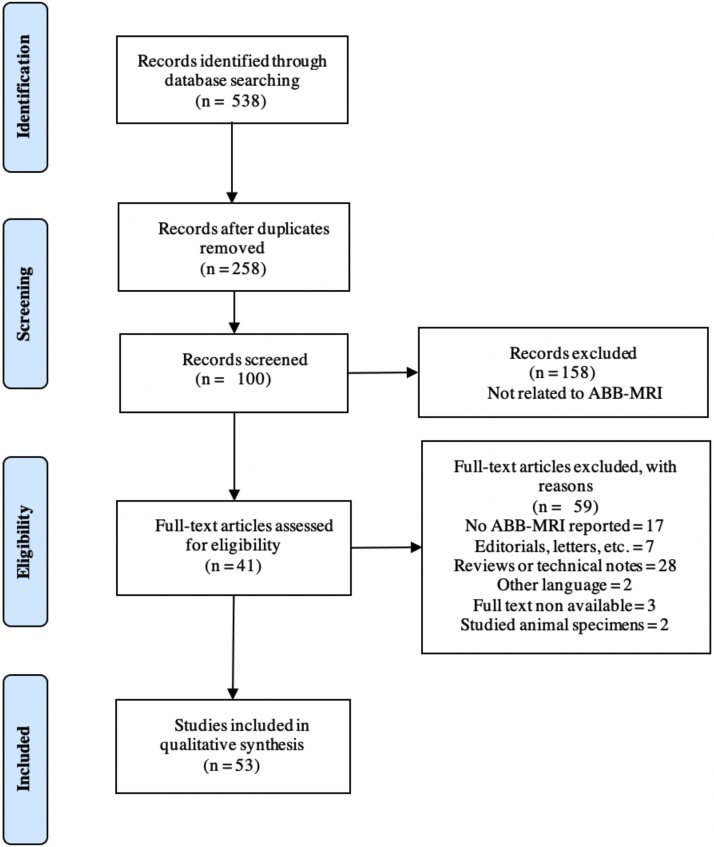
Fig. 1 illustrates the flow of searching, selection, and inclusion/exclusion performed in this systematic literature review [33]. The initial search yielded 538 documents, with 280 duplicated documents, which implied a final recovery of 258 documents. Each of these articles was independently reviewed by one of the three of the researchers, based on the reading of the title and abstract, in order to establish if its content did or did not describe the use of abbreviated protocols for the study of breast cancer, discarding articles that did not include this aspect. Then, a more detailed review determined if the articles that were maintained in the previous step presented evaluations for the implementation of ABB-MRI, according to the criteria defined above. Three researchers carried out this review, and in case of disagreement, some articles were included for full-text review. Therefore, documents were excluded, of them because not reporting abbreviated protocols, for being reviews or technical notes, either of general aspects of the use of magnetic resonance in the diagnosis of breast cancer or specifically on abbreviated protocols for breast cancer [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [32], [31], seven documents that corresponded to editorials, letters or erratum, two documents because the full text was not available in the English language, three more there was no full-text availability, and two documents that studied animal specimens. After the previous process, articles were maintained for full-text review, which reported breast ABB-MRI protocols.
Fig. 1.
Flow diagram for study searching, selection and inclusion. A total of 41 articles that reported 53 ABB-MRI protocols were included.
The main technical specifications of the studies included in this review are presented in Table 1. A total of articles reviewed in full text, were published between and , and were published between and , demonstrating a growing interest in this subject area the last years. In studies, patients were retrospectively identified from consecutive breast MRI studies performed for many clinical indications such as preoperative staging for patients with known malignant lesions, screening for high-risk patients, problem-solving, among others, which entails a potential selection bias. At the same time, in the other articles, all data were prospectively acquired, the largest being the multicentre, cross-sectional EA1141 randomized trial [34], which was conducted at institutions in the United States and one institution in Germany. This study compared the diagnostic performance of ABB-MRI and Digital Breast Tomosynthesis (DBT) for screening average-risk women with dense breasts. Study results showed a significantly higher rate of invasive breast cancer detection.
Table 1.
Technical specifications of included ABB-MRI studies.
| Study | Year | Purpose | Design | Field strength | Population | Subjects | Age range | MRI protocol | Acquisition time (minutes) | Reading time (minutes) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kuhl et al. [19] | Screening: detection | Prospective | Women at low to moderate risk |
|
T1W 1st post-contrast subtracted + MIP | seg | ||||
| Grimm et al. [35] | Screening: detection | Retrospective | Women at high risk of breast cancer |
|
T2W FS T1W pre and 1st post-contrast |
|
|
|||
| T2W FS, Pre, 1st and 2nd post-contrast. | ||||||||||
| Mango et al. [45] | Screening: detection | Retrospective | Women with biopsy proven unicentric breast carcinoma visualized on MRI |
|
T1W pre and 1st post-contrast subtraction and MIP |
|||||
| Harvey et al. [46] | Screening: detection | Retrospective | Women at high risk of breast cancer |
|
Axial T1W-FS pre and 1st post-contrast Subtraction + MIP |
|||||
| Heacock et al. [47] | Screening: detection | Retrospective | Women who had undergone breast MRI |
|
T1W post-contrast |
|
|
|||
| T1W post-contrast with prior imaging |
|
|
||||||||
| T2W, T1W post-contrast with prior imaging | ||||||||||
| Moschetta et al. [36] | Screening: detection | Retrospective | Women with a family history of cancer and dense glandular structure |
|
STIR - T2W - THRIVE pre and 1st post-contrast THRIVE |
|||||
| Bickelhaupt et al. [69] | Screening clarification | Retrospective | Women with suspicious breast lesions detected in x-ray screening (BIRADS 4-5) |
|
T2W, DWI with background suppression | |||||
| Bickelhaupt et al. [70] | Screening clarification | Retrospective | Asymptomatic women with suspicious breast lesions detected in x-ray screening (BIRADS 4) |
|
T2W, maximum intensity breast diffusion (MIBD) | |||||
| Chen et al. [48] | Screening with dense breast tissue | Retrospective | Women with dense breast tissue and negative previous results who had undergone routine breast MRI |
|
1st post-contrast subtracted + MIP |
|||||
| Chen et al. [49] | Screening of dense breast tissue | Retrospective | Women with dense breast tissue who had undergone routine breast MRI |
|
1st post-contrast subtracted + MIP |
|
|
|||
| 1st post-contrast 1st subtraction + MIP, DWI |
||||||||||
| Jain et al. [37] | Screening: detection | Retrospective | Women with a personal or family history of cancer |
|
T1W pre and 1st post-contrast, 1st subtraction | |||||
| Kang et al. [71] | Screening: detection | Retrospective | Women with a personal history of breast cancer |
|
T1W-FS and Non FS, DWI 1st and 5th post-contrast |
|||||
| Machida et al. [64] | Screening: detection | Retrospective | Women who had undergone breast MRI |
|
Coronal VIBE pre-contrast and coronal TWIST post-contrast | |||||
| Oldrini et al. [38] | Screening: detection and characterization | Retrospective | Women who had undergone breast MRI |
|
1st T1W post-contrast + MIP |
|
|
|||
| 1st T1W post-contrast, subtraction and T2W |
|
|
||||||||
| TRICKS + MIP |
|
|
||||||||
| 1st T1W post-contrast, subtraction, T2W and TRICKS + MIP | ||||||||||
| Panigrahi et al. [50] | Screening: detection | Prospective | Women at high risk of breast cancer |
|
T1W-FS 1st subtraction + MIP |
|||||
| Petrillo et al. [51] | Screening: detection and characterization | Retrospective | Women who had undergone breast MRI |
|
T1W 1st subtraction + MIP | |||||
| Romeo et al. [39] | Screening: detection and characterization | Retrospective | Women at high risk of breast cancer with previous screening |
|
T1W FS pre-contrast, 1st, 2nd, 3th subtractions | |||||
| Strahle et al. [40] | Screening | Prospective | Asymptomatic women with a negative mammogram |
|
T1W pre and 1st post-contrast, T2, 6th post-contrast | |||||
| Choi et al. [52] | Recurrent breast cancer diagnosis | Prospective | Women with a history of breast cancer surgery |
|
Sagittal T2W-FS, sagittal T1W pre and 1st post-contrast, subtraction + MIP | |||||
| Dogan et al. [63] | Screening (Quality image evaluation) | Prospective | Patients at increased breast cancer risk |
|
T2W FSE Triple-Echo Dixon T2W, 3D Dual-Echo FSPGR Dixon (DCE) | |||||
| Oldrini et al. [41] | Screening: detection, time | Retrospective | Women who had undergone breast MRI |
|
Sagittal T1W pre and 1st post-contrast subtraction | |||||
| Seppala et al. [53] | Screening: detection | Retrospective | Women at high risk of breast cancer |
|
T1W sagittal pre and first post-contrast subtraction + MIP | |||||
| Zelst et al. [65] | Screening: detection | Prospective | Women at high risk |
|
TWIST | |||||
| Yamada et al. [54] | Screening: detection | Retrospective | Women who had undergone breast MRI with breast cancer cm | T2W FS,DWI, MIP-DWI |
|
|
||||
| T2W FS, 2nd subtraction + MIP | NR | |||||||||
| Borthakur et al. [42] | Comparing time to Screening | Retrospective | Women who had undergone breast MRI as part of their scheduled clinical examinations | Axial T2W STIR, Axial T1W pre and 1st post-contrast | ||||||
| Dialani et al. [55] | Screening: detection | Retrospective | Women at high risk who had a negative mammogram in the previous year |
|
T2W MIP post-contrast |
|
|
|||
| T2W MIP post-contrast, T1W pre and 1st post-contrast |
|
|
||||||||
| T2W MIP post-contrast, T1W Pre and 1st postcontrast, T2W | ||||||||||
| Girometti et al. [74] | Detection of additional disease in breast cancer staging. | Retrospective | Women with a histological diagnosis of breast cancer |
|
FLASH T pre-contrast, 1st post-contrast + MIP | |||||
| Goto et al. [66] | Screening: detection and classification | Prospective | Women with a history of chemotherapy |
|
TWIST-VIBE | |||||
| Ha et al. [43] | Screening: detection | Retrospective | Women with a history of breast cancer | T2W-FS T1W-FS 1st post-contrast | ||||||
| Jones et al. [56] | Screening performed by mammogram readers | Prospective | Women at high risk |
|
1st post-contrast-subtracted images+MIP | |||||
| Lee-Felker et al. [57] | Estimating extent of disease in diagnosed breast cancer | Retrospective | Women with breast cancer newly diagnosed |
|
T1W FS pre and 1st post-contrast, subtraction + MIP | to | ||||
| Milon et al. [27] | Screening: detection and characterization | Retrospective | Women who had undergone breast MRI |
|
T1W, T2W, T1W-FS 1st post-contrast |
|
|
|||
| T1W, T2W, T1W-FS 1st post-contrast, HTR DCE | ||||||||||
| An et al. [58] | Screening: detection | Retrospective | Women with a personal history of cancer and post-surgical mammography and ultrasound testing |
|
T2W, T1W pre- and 1st post-contrast, subtraction and MIP. | |||||
| Comstock et al. [34] | Screening of dense breast tissue | Prospective | Women with heterogeneously dense or extremely dense breasts |
|
T2W and T1W pre and post-contrast | |||||
| Choudhery et al. [67] | Comparing kinetic parameters of the RAMP MRI protocol Vs. DCE | Retrospective | Women with tissue diagnoses of suspicious MRI lesions | Rapid Abridged Multiphase (RAMP) | ||||||
| Kwon et al. [44] | Screening: detection | Retrospective | Women with previously treated cancer |
|
T2, 1st and 2nd post-contrast | |||||
| Marquina et al. [59] | Screening: detection | Retrospective | Women at high risk of breast cancer | T1W pre and 1st post-contrast + MIP | ||||||
| Mori et al. [68] | Comparing kinetic parameters of the Ultrafast MRI protocol Vs. DCE | Retrospective | Patients with histologic diagnosis of NMEs made by core biopsy or clinically confirmed benign NME. |
|
T1W pre-contrast, Ultrafast (8 series) post-contrast | |||||
| Park et al. [60] | Recurrent breast cancer diagnosis | Retrospective | Women with a personal history of breast cancer | T2W, T1W pre, 1st and 2nd post-contrast, subtractions, Axial and Sagital MIP | ||||||
| Shiraishi et al. [61] | Estimating extent of disease in diagnosed breast cancer | Retrospective | Patients with pathologically proven DCIS who underwent preoperative breast MRI |
|
T1W pre and 1st post-contrast + MIP | |||||
| Scoggins et al. [62] | Screening: detection and categoriztion | Prospective | Women at high risk of breast cancer |
|
Subtraction MIPs |
|
|
|||
| Non-subtraction and subtraction DCE |
|
|
||||||||
| Non subtraction, subtraction DCE + MIP, T2W-FSE Dixon, 3D dual-echo Dixon |
Another aspect that stands out from the included studies is the variation in the field strength of the scanners. Thus, studies acquired images using a scanner of 1.5T, used scanners of 3T, and in studies, both 1.5T and 3T scanners were used, respectively.
3.1. Abbreviated protocols specification
Varying versions of the abbreviated protocols have been reported in the literature. The selection of the sequences that make them up is apparently heuristic, with a general tendency using a non-contrast T1-weighted (T1W) or T2-weighted (T2W) acquisition plus at least one contrast-enhanced sequence [35], [36], [37], [38], [39], [40], [41], [42], [43], [27], [34], [44]. This is to improve the specificity, including information that allows greater discrimination between benign and malignant lesions and make it possible to evaluate the tumor uptake. Other studies explicitly incorporate the use of maximum intensity projection (MIP) images of post-contrast examinations as part of the reading protocol [19], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]. This derivated image highlights maximum enhancement areas such as tumor or lymph nodes, and allows for the assessment of the relationship among arteries, veins, and blood vessels.
Some studies have focused on determining the effect by including the T2W sequence in an ABB-protocol [47], [40]. Strahle et al. [40] developed an evaluation of the conspicuity of lesions in each sequence to determine those that could contribute better to the detection of suspicious lesions of malignancy. In this study, the authors request each radiologist to assign a conspicuous value for each observed lesion, that is, the intensity relative to the surrounding tissue, on the hypothesis that acquisitions that present greater conspicuity in the intensity of the signal improves the morphological characterization of the lesions, and consequently the ability to differentiate benign from malignant lesions. As a result, the proposed protocol contained the sequences T1W pre and post-contrast T2W post-contrast (taken at one and a half minutes), and T1 postcontrast at minute 6. This protocol has not been evaluated with respect to a complete protocol, and neither follow-up period was part of the study, which does not allow for calculation of sensitivity, specificity, and negative predictive values. The assessment of conspicuity has also been included as a variable of analysis in the study of Heacock et al. [47]; the authors demonstrated that the T2W sequence does not affect the performance detection of lesions using an abbreviated protocol and is better than the T1W sequence. Thus, T2W sequence has been incorporated in several ABB-MRI protocols such as [35], [36], [38], [52], [63], [54], [42], [55], [43], [27], [58], [34], [44], [60], [62]. Dixon sequence has also been proposed in [63] and [62] to form up an ABB-MRI protocol for screening patients with high and increased risk. These sequences generate both water-only and fat-only series in a single acquisition, reducing the study acquisition time significantly while minimizes false-positive rates with no impact on cancer detection.
Incorporating ultrafast DCE MRI sequences has also been proposed as an alternative for reducing the acquisition time on dynamic contrast enhanced studies. Ultrafast contrast-enhanced sequence refers to a new technique that allows capturing early contrast material wash-in at high temporal resolution, obtaining a series of images that describe the path of the contrast bolus within the first 2 minutes after contrast injection. Time-resolved angiography with interleaved stochastic trajectories (TWIST) by Siemens, Time-Resolved Imaging of Contrast Kinetics (TRICKS) and Differential Subsampling with Cartesian Ordering (DISCO) by GE, are the sequences used in the included studies [64], [38], [65], [66], [27]. In particular, Zelst et al. [65] proposed to use TWIST as a single-sequence in an ultrafast breast MRI protocol (UBMP) with an acquisition time of 1 minute and 42 seconds. Reported results indicated that this protocol is at least as accurate as a complete protocol. Additionally, Choudhery et al. [67] found no significant difference between the rapid abridged multiphase (RAMP) ACR-accredited breast MRI protocol and one standard DCE-MRI for discriminating benign from malignant lesions, based on delayed-phase percentage washout, predominant curve type, or worst curve type. Similar results were found by Mori et al. [68] but only for non-mass enhancement lesions. Unfortunately, specific MRI coil and sequence requirements are needed to achieve diagnostic spatial resolution at the high temporal resolution; it is because ultrafast imaging is not readily feasible with all available scanners.
On the other hand, there is a growing interest in the development of contrast-agent free protocols by using the acquisition of diffusion-weighted images (DWI) and their respective Apparent diffusion coefficient (ADC) maps [69], [70], [49], [71], [72], [54], especially because gadolinium deposition in the brain has been described recently [73]. Bickelhaupt et al. [69], [70] proposed to use diffusion-weighted imaging with background suppression (DWIBS) for defining an ABB-MRI protocol and clarify mammogram based screening findings. The proposed protocol is composed of the DWIBS, TW2, and the fusion of both images. With this protocol, the false-positive rate of non-invasive measures decreased from 64% to 19%, whereas preserving the sensitivity. In a similar way, Kang et al. [71] proposed an abbreviated protocol consisted of fused DWI and unenhanced T1W images, and the DWI-MIP for screening patients with a personal history of breast cancer, which diagnostic performance was similar to a complete protocol. Chen et al. [49] showed that adding the diffusion to a protocol based on the first post-contrast sequence and MIP, significantly improves the performance of the protocol. Likewise, in 2018, Kul et al. [72] conducted a study that seeks to establish the viability of using the acquisition of diffusion-weighted images and ADC map for describing the cellularity of masses detected in the breasts and to establish the contribution of this evaluation for its characterization. From the reported results, the diffusion images showed a high to moderate concordance during breast masses description respect to dynamic contrast-enhanced (DCE) study. In addition, the diagnostic accuracy achieved with the morphological evaluation was slightly lower in comparison with the DCE. Furthermore, this study showed that the combination of DWI and ADC acquisitions could achieve even better performance than the DCE. Chen et al. [49] showed that adding the diffusion imaging to a protocol based on the first post-contrast sequence and MIP significantly improves the performance of the protocol. Moreover, Yamada et al. [54] reported that the detectability of the unenhanced abbreviated protocol based on DWI would be comparable to that abbreviated protocol based on postcontrast. According to the above, abbreviated unenhanced MRI based on DWI seems to have the potential for screening breast MRI.
3.2. Study purpose and population
Several included articles varied in both the study purpose and population, respectively. articles studied the ABB-MRI protocols in breast cancer screening from both screening only and enriched cohorts with known cancers. In two others, the evaluation of recurrency was addressed [52], [60]. Two of the articles evaluating the use of ABB-MRI for the extent of the disease assessing [57], [61], and the other one for detecting additional lesions in confirmed breast cancer patients [74].
A total of breast MRI studies were evaluated in the includes studies, of which were acquired prospectively. In four articles, MRI studies corresponded to subjects reported in another study; therefore, they were counted only once. The total number of subjects by study vary between and in retrospective studies; and between and in the prospective studies. Age ranges were between 14 and 92 years, with a mean of .
From the set of retrospective studies, included women who had undergone breast MRI by currently accepted indications, such as preoperative breast staging, problem-solving, follow-up for previous non-surgical breast intervention, and probably benign findings detected on previous studies, among others [47], [48], [49], [64], [38], [51], [41], [54], [42], [27]; two of them were specifically oriented to the detection of lesions in women with heterogeneously or extremely dense breasts [48], [49], according to ACR categorization, and another one in to detect lesions in pathologically proven breast cancer studies [45]. Six aimed to evaluate the feasibility of using abbreviated protocols to screening high risk women [35], [46], [59], [39], [53], [55]; and others, to women with a personal or family history of breast cancer [71], [36], [43], [37], [58], [44], [60]. Additionally, studies established as inclusion criteria to present lesions previously identified by another imaging modality [69], [70], [67]; and the other , focused on pathologically proven breast cancer studies [74], [57], [68], [61].
On the other hand, from the subset of articles that studied subjects prospectively enrolled: studied feasibility of ABB-AP for screening patients at high-risk of breast cancer [50], [65], [56], [62]; two analyzed studies of women who carried a mildly or moderately increased risk, either because of dense breast tissue, mild or moderate family history, or personal history of breast cancer [19], [63]; and two more, women with a personal history of breast cancer [52], [66]. Another one, studied asymptomatic women with a negative mammogram, which did not have a personal history of breast cancer or prior chest radiation therapy [40]. Additionally, as was depicted above, Comstock et al. [34] studied clinically asymptomatic women with heterogeneously dense or extremely dense breasts.
From the foregoing, although the abbreviated magnetic resonance protocols are presented to the scientific and healthcare community as an alternative for screening of breast cancer, few studies are oriented to assess their feasibility in the general population or in women with moderate risk. Additionally, given the advantages of magnetic resonance imaging, there does not seem to be any restriction to include women in a wide range of ages. Thus, the inclusion of young women with a moderate or high risk of developing the disease could be one of the main advantages of this type of studies.
3.3. Acquisition and interpretation times
From the studies that reported acquisition and interpretation times, we can conclude that there is evidence of a substantial reduction in an abbreviated protocol compared to the conventional complete MRI protocol. The average acquisition time of the includes studies was minutes ( minutes - minutes), and the interpretation time of minutes ( seconds - minutes). It should be noted that the times to review the medical history, the previous studies (mammograms or comparison ultrasounds), time to load images in the PACS system, or the time to report the findings, which consume an important part of the interpretation process, were not included. In this regard, Borthakur et al. [42] analyzed the activity times from ABB-MRI and complete protocols for the examination, scan, and technologist activity, included both scan-related and non-scan-related activities. They found that the scan-related activities, such as creating post-processing images and injecting contrast, are performed during the long and idle times, which are more frequently available during the longer scan sequences of the complete protocol. That is to say, those times are absorbed by the longer imaging acquisition times during the complete protocol, offsetting some of the gains from performing the ABB-MRI protocol. Consequently, the implementation of an ABB-MRI increased only at the patient flow rate, which was less than the that was anticipated based on expected scan times.
3.4. Diagnostic performance
Despite the variability of the protocol designs, the included results consistently report non-significant differences in the diagnostic performance between Abbreviated and complete breast MRI protocols. Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 summarize the main performance measures reported in the included studies: Sensitivity (Sens.), Specificity (Spec.), Predictive Positive Value (PPV), Predictive Negative Value (PNV), and Inter-observer agreement (Agree). Overall ABB-MRI protocols used biopsy or follow-up imaging results as standard of reference. Additionally, the varied inclusion/exclusion criteria for each study makes the enrolled subject cohorts differ greatly regarding cancer frequency and alter the diagnostic performance. Thus, articles were grouped according to their purpose and study population for readability. No larger differences were found in performance measures reported for each subset, although, as was expected, PPV was higher in studies with a larger prevalence of cancer.
Table 2.
Performance of ABB-MRI protocols in screening settings to women who had undergone breast MRI for varied indications.
| Study | Lesions | Cancers/ Subjects | Reference | Readers | Exper. (years) | Sens. | Spec. | PPV | PNV | Agree. |
|---|---|---|---|---|---|---|---|---|---|---|
| Mango et al. [45] | Biopsy | |||||||||
| Heacock et al. [47] | Biopsy |
|
|
|
|
|
||||
|
|
|
|
|
|
||||||
| Chen et al. [48] | Biopsy/ follow-up | |||||||||
| Chen et al. [49] | Biopsy/ follow-up |
|
|
|
|
|
||||
| Machida et al. [64] | Biopsy/ follow-up | |||||||||
| Oldrini et al. [38] | Biopsy |
|
|
|
|
|
||||
|
|
|
|
|
|
||||||
|
|
|
|
|
|
||||||
| Petrillo et al. [51] | Biopsy/ follow-up | |||||||||
| Oldrini et al. [41] | Biopsy/ follow-up | |||||||||
| Yamada et al. [54] | Biopsy |
|
|
|
|
|
||||
| Milon et al. [27] | Biopsy |
|
|
|
|
|
||||
Table 3.
Performance of ABB-MRI protocols in screening settings for women at high-risk of breast cancer.
| Study | Lesions | Cancers/ Subjects | Reference | Readers | Exper. (years) | Sens. | Spec. | PPV | PNV | Agree. |
|---|---|---|---|---|---|---|---|---|---|---|
| Grimm et al. [35] | Biopsy/ follow-up | |||||||||
| Harvey et al. [46] | Biopsy | |||||||||
| Panigrahi et al. [50] | Biopsy | |||||||||
| Romeo et al. [39] | Biopsy/ follow-up | |||||||||
| Seppala et al. [53] | Biopsy | |||||||||
| Zelst et al. [65] | Biopsy/ follow-up | |||||||||
| Dialani et al. [55] | Biopsy/ follow-up |
|
|
|
|
|
||||
|
|
|
|
|
|
||||||
| Jones et al. [56] | Biopsy/ follow-up | |||||||||
| Marquina et al. [59] | Biopsy/ follow-up | |||||||||
| Scoggins et al. [62] | Biopsy/ follow-up |
|
|
|
|
|
||||
|
|
|
|
|
|
||||||
Table 4.
Performance of ABB-MRI protocols in screening settings for women at middle or moderate risk of breast cancer because of personal or familiar history.
| Study | Lesions | Cancers/ Subjects | Reference | Readers | Exper. (years) | Sens. | Spec. | PPV | PNV | Agree. |
|---|---|---|---|---|---|---|---|---|---|---|
| Kuhl et al. [19] | Biopsy/ follow-up | |||||||||
| Moschetta et al. [36] | Biopsy | |||||||||
| Jain et al. [37] | Biopsy | |||||||||
| Kang et al. [71] | Biopsy | |||||||||
| Choi et al. [52] | Biopsy | |||||||||
| Goto et al. [66] | Biopsy | |||||||||
| Ha et al. [43] | Biopsy | |||||||||
| An et al. [58] | Biopsy/ follow-up | |||||||||
| Kwon et al. [44] | Biopsy |
Table 5.
Performance of ABB-MRI protocols in screening clarification settings.
Table 6.
Performance of ABB-MRI protocols in screening settings for asymptomatic or health women.
Table 7.
Performance of ABB-MRI protocols in recurrence, disease extension and staging settings.
Although Girometti et al. [74] reported that less experienced radiologists induced more false-positive findings than experienced ones, no large differences were reported between them for interpreting the ABB-MRI protocols. However, most studies were performed by experienced readers, which could bias the interobserver agreement that was reported between moderate and perfect in most of the cases when this measure was computed.
Some articles were excluded from these tables because they non focused on diagnostic or categorization tasks [63], [67], [68], [61]. However, the reported results are mostly consistent with the feasibility of using abbreviated protocols for breast cancer. In [42], activity times of an ABB-MRI were compared with the complete-protocol examination, but no diagnostic comparison was made. Dogan et al. [63] showed that an abbreviated protocol comprising a T2W and a 3D dual-echo fast spoiled gradient echo two-point Dixon sequences, not shown significantly image quality differences to that of a standard-of-care MRI protocol. Choudhery et al. [67] demonstrates comparable kinetic characteristics for discriminating benign from malignant lesions between the ACR-accredited rapid abridged multiphase (RAMP) MRI protocol and a complete DCE MRI protocol. Likewise, the kinetic characteristics of ultrafast DCE-MRI allow differentiating benign from malignant non-mass enhancements as a standard DCE MRI protocol [68].
Finally, it is important to mention the results reported by Shiraishi et al. [61], who evaluated the feasibility of using an ABB-MRI protocol for preoperative assessment of the tumor extent in patients with pure DCIS. The ABB-MRI protocol was comprised of a pre-contrast and 60 seg post-contrast T1W images with MIP.
The performance was assessed by the rate of concordance between pathology and the extent estimated by image interpretation. In this case, the rate of concordance for the complete protocol was higher than for the ABB-MRI. However, variation in measurements between readers tended to be slightly lower for the ABB-MRI than the complete protocol.
3.5. Cost-effectiveness
The potential cost and time savings of the abbreviated protocols have been indicated in all of the studies included in this review; however, none of them evaluated the cost-effectiveness of abbreviated breast MRI relative to other imaging techniques. These analyzes are required to advance in the incorporation of the use of these protocols for screening low and intermediate risk patients in both current guidelines and clinical settings. For cost-effectiveness analyses of abbreviated MRI protocols, it is important to realize that time saving must consider both scan-related and non-scan-related activities, in order to compute the real value of an abbreviated MRI study.
4. Conclusions
Several studies in the literature have proposed the use of abbreviated MRI protocols for breast cancer. Most of them were assessed for screening purposes; however, the most recent publications have considered other diagnostic tasks, such as recurrence, staging, screening clarification, and disease extension. Overall, the abbreviated protocols have diagnostic performances comparable to that of the standard breast MRI protocol, except for the tumor extent estimation, when the concordance rate between the extent estimated by image interpretation and pathological results was higher for the complete protocol than ABB-MRI. Moreover, abbreviated MRI has also been shown to have superior cancer yield to that of digital breast tomosynthesis (DBT).
Abbreviated protocols were initially comprised of a subset of sequences of the standard complete protocol. However, at last times, the use of ultrafast o high temporal resolution (HTR) sequences has been introduced in both standalone and added to abbreviated MRI schemes. Ultrafast MRI sequences seem to be a really viable alternative since they allow a kinetic analysis of the contrast enhancement uptake of the lesions. On the other hand, unenhanced or free contrast MRI techniques such as diffusion-weighted imaging (DWI) are also being investigated because gadolinium deposition in the brain recently described. These have shown a larger cancer detectability of mammography but slightly inferior to DCE based MRI protocols.
In summary, shorter imaging times achieved with the abbreviated MRI protocols have the potential to increase efficiency in breast imaging practice, which would be expected to improve their cost-effectively even for the screening of increased-risk women such as women with dense breast tissue. However, further studies about the effect on clinical outcomes, physicians and patients acceptability, and cost-effectiveness compared with other technologies such as ABUS or CES are also required.
Author contributions
This work was developed with the substantial contribution of all the authors. G.D. and M.L.H. conceived and designed the study and searching strategy. S.O., K.F. and A.O carried out the searching and selection of articles and the information extraction, which was confirmed by G.D. and M.L.H. All authors have contributed to the preparation of the paper, the discussing of the searching results and the reviewing of the content of this article, and have approved the manuscript.
Ethical statement
Authors confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied authorship criteria. Authors further confirm that all have approved the order of authors listed in the manuscript. Additionally, authors declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
Funding
This work was funded by MinCiencias (Colombia), Instituto Tecnológico Metropolitano, and Ayudas Diagnósticas Sura. Project RC 740-2017.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Abbreviated Magnetic Resonance Imaging in Breast Cancer.
Contributor Information
María Liliana Hernández, Email: mlhernandezp@sura.com.co.
Santiago Osorio, Email: saosorior@uces.edu.co.
Katherine Florez, Email: florezl.maria@uces.edu.co.
Alejandra Ospino, Email: aospino@sura.com.co.
Gloria M. Díaz, Email: gloriadiaz@itm.edu.co.
References
- 1.Wild C.P., Widerpass E., Stewart B.W. World Health Organization, international agency for research on cancer; 2015. World Cancer Report 2014. Technical Report. [Google Scholar]
- 2.International Agency for Research on Cancer; 2018. Breast Fact Sheet. Technical Report. [Google Scholar]
- 3.Morrow M., Waters J., Morris E. MRI for breast cancer screening, diagnosis, and treatment. The Lancet. 2011;378:1804–1811. doi: 10.1016/S0140-6736(11)61350-0. [DOI] [PubMed] [Google Scholar]
- 4.Gøtzsche P.C., Nielsen M. Screening for breast cancer with mammography. In: Gøtzsche P.C., editor. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; Chichester, UK: 2011. [DOI] [Google Scholar]
- 5.Ciatto S., Visioli C., Paci E., Zappa M. Breast density as a determinant of interval cancer at mammographic screening. Br. J. Cancer. 2004;90:393–396. doi: 10.1038/sj.bjc.6601548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg W.A., Blume J.D., Cormack J.B., Mendelson E.B., Lehrer D., B“ohm-Vélez M., Pisano E.D., Jong R.A., Evans W.P., Morton M.J., Mahoney M.C., Larsen L.H., Barr R.G., Farria D.M., Marques H.S., Boparai K. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwood H.I., Dodelzon K., Katzen J.T. Impact of advancing technology on diagnosis and treatment of breast cancer. Surg. Clin. North Am. 2018;98:703–724. doi: 10.1016/j.suc.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Demartini W., Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Top. Magn. Reson. Imaging. 2008;19:143–150. doi: 10.1097/RMR.0b013e31818a40a5. [DOI] [PubMed] [Google Scholar]
- 9.Taneja C., Edelsberg J., Weycker D., Guo A., Oster G., Weinreb J. Cost effectiveness of breast cancer screening with contrast-enhanced MRI in high-risk women. J. Am. Coll. Radiol. 2009;6:171–179. doi: 10.1016/j.jacr.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Saadatmand S., Tilanus-Linthorst M.M.A., Rutgers E.J.T., Hoogerbrugge N., Oosterwijk J.C., Tollenaar R.A.E.M., Hooning M., Loo C.E., Obdeijn I.-M., Heijnsdijk E.A.M., de Koning H.J. Cost-effectiveness of screening women with familial risk for breast cancer with magnetic resonance imaging. JNCI: J. Natl. Cancer Inst. 2013;105:1314–1321. doi: 10.1093/jnci/djt203. [DOI] [PubMed] [Google Scholar]
- 11.Saslow D., Boetes C., Burke W., Harms S., Leach M.O., Lehman C.D., Morris E., Pisano E., Schnall M., Sener S., Smith R.A., Warner E., Yaffe M., Andrews K.S., Russell C.A., American Cancer Society Breast Cancer Advisory Group American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA. Cancer J. Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 12.Lee C.H., Dershaw D.D., Kopans D., Evans P., Monsees B., Monticciolo D., Brenner R.J., Bassett L., Berg W., Feig S., Hendrick E., Mendelson E., D’Orsi C., Sickles E., Burhenne L.W. Breast cancer screening with imaging: recommendations from the society of breast imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J. Am. Coll. Radiol. 2010;7:18–27. doi: 10.1016/j.jacr.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Mann R.M., Balleyguier C., Baltzer P.A., Bick U., Colin C., Cornford E., Evans A., Fallenberg E., Forrai G., Fuchsj”ager M.H. Breast mri: eusobi recommendations for women's information. Eur. Radiol. 2015;25:3669–3678. doi: 10.1007/s00330-015-3807-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sardanelli F., Boetes C., Borisch B., Decker T., Federico M., Gilbert F.J., Helbich T., Heywang-K“obrunner S.H., Kaiser W.A., Kerin M.J. Magnetic resonance imaging of the breast: recommendations from the eusoma working group. Eur. J. Cancer. 2010;46:1296–1316. doi: 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D., Wender R.C. Cancer screening in the united states, 2019: a review of current american cancer society guidelines and current issues in cancer screening. CA. Cancer J. Clin. 2019;69:184–210. doi: 10.3322/caac.21557. [DOI] [PubMed] [Google Scholar]
- 16.AB D.L. Screening in patients with increased risk of breast cancer (part 1): pros and cons of mri screening. Radiologia. 2020;62:252–265. doi: 10.1016/j.rxeng.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser C.G., Dietzel M., Vag T., Froelich M.F. Cost-effectiveness of MR-mammography vs. conventional mammography in screening patients at intermediate risk of breast cancer - A model-based economic evaluation. Eur. J. Radiol. 2020:109355. doi: 10.1016/j.ejrad.2020.109355. [DOI] [PubMed] [Google Scholar]
- 18.Froelich M.F., Kaiser C.G. Cost-effectiveness of MR-mammography as a solitary imaging technique in women with dense breasts: an economic evaluation of the prospective TK-Study. Eur. Radiol. 2020 doi: 10.1007/s00330-020-07129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhl C.K., Schrading S., Strobel K., Schild H.H., Hilgers R.-D., Bieling H.B. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J. Clin. Oncol. 2014;32:2304–2310. doi: 10.1200/JCO.2013.52.5386. [DOI] [PubMed] [Google Scholar]
- 20.Chhor C.M., Mercado C.L. Abbreviated MRI protocols: wave of the future for breast cancer screening. Am. J. Roentgenol. 2017;208:284–289. doi: 10.2214/AJR.16.17205. [DOI] [PubMed] [Google Scholar]
- 21.Leithner D., Moy L., Morris E.A., Marino M.A., Helbich T.H., Pinker K. Abbreviated MRI of the breast: does it provide value? J. Magn. Reson. Imaging. 2018 doi: 10.1002/jmri.26291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheth D., Abe H. Abbreviated MRI and accelerated MRI for screening and diagnosis of breast cancer. Top. Magn. Reson. Imaging. 2017;26:183–189. doi: 10.1097/RMR.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 23.Deike-Hofmann K., Koenig F., Paech D., Dreher C., Delorme S., Schlemmer H.-P., Bickelhaupt S. Abbreviated MRI protocols in breast cancer diagnostics. J. Magn. Reson. Imaging. 2019;49:647–658. doi: 10.1002/jmri.26525. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood H.I. Abbreviated protocol breast MRI: the past, present, and future. Clin. Imaging. 2019;53:169–173. doi: 10.1016/j.clinimag.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Ko E.S., Morris E.A. Abbreviated magnetic resonance imaging for breast cancer screening: concept, early results, and considerations. Korean J. Radiol. 2019;20:533–541. doi: 10.3348/kjr.2018.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhl C.K. Abbreviated magnetic resonance imaging (MRI) for breast cancer screening: rationale, concept, and transfer to clinical practice. Annu. Rev. Med. 2019;70:501–519. doi: 10.1146/annurev-med-121417-100403. [DOI] [PubMed] [Google Scholar]
- 27.Milon A., Vande Perre S., Poujol J., Trop I., Kermarrec E., Bekhouche A., Thomassin-Naggara I. Abbreviated breast MRI combining FAST protocol and high temporal resolution (HTR) dynamic contrast enhanced (DCE) sequence. Eur. J. Radiol. 2019;117:199–208. doi: 10.1016/j.ejrad.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Mann R.M., van Zelst J.C.M., Vreemann S., Mus R.D.M. Is ultrafast or abbreviated breast MRI ready for prime time? Curr. Breast Cancer Rep. 2019;11:9–16. doi: 10.1007/s12609-019-0300-8. [DOI] [Google Scholar]
- 29.Mootz A.R., Madhuranthakam A.J., Dogan B. Changing paradigms in breast cancer screening: abbreviated breast MRI. Eur. J. Breast Health. 2019;15:1–6. doi: 10.5152/ejbh.2018.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y., Heller S.L. Abbreviated and ultrafast breast MRI in clinical practice. Radiographics. 2020;40:1507–1527. doi: 10.1148/rg.2020200006. [DOI] [PubMed] [Google Scholar]
- 31.Baxter G.C., Selamoglu A., Mackay J.W., Bond S., Gray E., Gilbert F.J. A meta-analysis comparing the diagnostic performance of abbreviated mri (abb-mri) and a full diagnostic protocol (fdp-mri) in breast cancer. Clin. Radiol. 2020;266:4–5. doi: 10.1016/j.crad.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Geach R., Jones L.I., Harding S.A., Marshall A., Taylor-Phillips S., McKeown-Keegan S., Dunn J.A., Kuhl C., Vinnicombe S., O’Flynn E., Wookey J., Rose J., Foy C., Taylor V., Valencia A., Gifford J., Gray R., Jones T.W., Litton K., Lloyd S., Kutt E., Pocklington A., Mahatma A., Massey H., Clark G., McLachlan C., Beckett G., Alison C., Barta M., Betancourt C., Bramwell J., Bright N., Burt H., Cann L., Ceney J., Cornford E., Dalgliesh D., Doyle S., Fearn S., Godden D., Goldthorpe Z., Hobson L., Hynam P., Jackson E., Jenkin M., Kingsnorth B., Klimczak K., Moody A., Perrin S., Peters A., Preston E., Ratsey A., Sidebottom R., Steel J., Stephenson L., Taylor M., Toth E., Vincent F., Watkin S., Widdison S., Williams J., Wilmot K., Elangovan P., Halling-Brown M., Ghiasvand H., Hulme C., Singamaneni S., Friedrich Z., Robson J., Mankelow A. The potential utility of abbreviated breast MRI (FAST MRI) as a tool for breast cancer screening: a systematic review and meta-analysis. Clin. Radiol. 2020 doi: 10.1016/j.crad.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comstock C.E., Gatsonis C., Newstead G.M., Snyder B.S., Gareen I.F., Bergin J.T., Rahbar H., Sung J.S., Jacobs C., Harvey J.A., Nicholson M.H., Ward R.C., Holt J., Prather A., Miller K.D., Schnall M.D., Kuhl C.K. comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746. doi: 10.1001/jama.2020.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm L.J., Soo M.S., Yoon S., Kim C., Ghate S.V., Johnson K.S. Abbreviated screening protocol for breast MRI. a feasibility study. Acad. Radiol. 2015;22:1157–1162. doi: 10.1016/j.acra.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Moschetta M., Telegrafo M., Rella L., Stabile Ianora A.A., Angelelli G., Ianora A.A.S., Angelelli G., Stabile Ianora A.A., Angelelli G. Abbreviated combined MR protocol: a new faster strategy for characterizing breast lesions. Clin. Breast Cancer. 2016;16:207–211. doi: 10.1016/j.clbc.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Jain M., Jain A., Hyzy M.D., Werth G. FAST MRI breast screening revisited. J. Med. Imaging Radiat. Oncol. 2017;61:24–28. doi: 10.1111/1754-9485.12502. [DOI] [PubMed] [Google Scholar]
- 38.Oldrini G., Fedida B., Poujol J., Felblinger J., Trop I., Henrot P., Darai E., Thomassin-Naggara I. Abbreviated breast magnetic resonance protocol: value of high-resolution temporal dynamic sequence to improve lesion characterization. Eur. J. Radiol. 2017;95:177–185. doi: 10.1016/j.ejrad.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 39.Romeo V., Cuocolo R., Liuzzi R., Riccardi A., Accurso A., Acquaviva A., Buonocore R., Imbriaco M. Preliminary results of a simplified breast mri protocol to characterize breast lesions: comparison with a full diagnostic protocol and a review of the current literature. Acad. Radiol. 2017;24:1387–1394. doi: 10.1016/j.acra.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Strahle D.A., Pathak D.R., Sierra A., Saha S., Strahle C., Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res. Treat. 2017;162:283–295. doi: 10.1007/s10549-017-4112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldrini G., Derraz I., Salleron J., Marchal F., Henrot P. Impact of an abbreviated protocol for breast mri in diagnostic accuracy. Diagn. Interv. Radiol. 2018;24:12–16. doi: 10.5152/dir.2018.16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borthakur A., Weinstein S.P., Schnall M.D., Conant E.F. Comparison of study activity times for ”Full“ versus ”Fast MRI” for breast cancer screening. J. Am. Coll. Radiol. 2019;16:1046–1051. doi: 10.1016/j.jacr.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Ha T., Jung Y., Kim J.Y., Park S.Y., Kang D.K., Kim T.H. Comparison of the diagnostic performance of abbreviated MRI and full diagnostic MRI using a computer-aided diagnosis (CAD) system in patients with a personal history of breast cancer: the effect of CAD-generated kinetic features on reader performance. Clin. Radiol. 2019;74 doi: 10.1016/j.crad.2019.06.025. 817.e15-817.e21. [DOI] [PubMed] [Google Scholar]
- 44.Kwon M.-r., Ko E.Y., Han B.-K., Ko E.S., Choi J.S., Park K.W. Diagnostic performance of abbreviated breast MRI for screening of women with previously treated breast cancer. Medicine (Baltimore). 2020;99:e19676. doi: 10.1097/MD.0000000000019676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mango V.L., Morris E.A., Dershaw D.D., Abramson A., Fry C., Moskowitz C.S., Hughes M., Kaplan J., Jochelson M.S., David Dershaw D., Abramson A., Fry C., Moskowitz C.S., Hughes M., Kaplan J., Jochelson M.S., Dershaw D.D., Abramson A., Fry C., Moskowitz C.S., Hughes M., Kaplan J., Jochelson M.S., David Dershaw D., Abramson A., Fry C., Moskowitz C.S., Hughes M., Kaplan J., Jochelson M.S. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur. J. Radiol. 2015;84:65–70. doi: 10.1016/j.ejrad.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Harvey S.C., Di Carlo P.A., Lee B., Obadina E., Sippo D., Mullen L. An Abbreviated protocol for high-risk screening breast mri saves time and resources. J. Am. Coll. Radiol. 2016;13:374–380. doi: 10.1016/j.jacr.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Heacock L., Melsaether A.N., Heller S.L., Gao Y., Pysarenko K.M., Babb J.S., Kim S.G., Moy L. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur. J. Radiol. 2016;85:815–823. doi: 10.1016/j.ejrad.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Chen S.-Q., Huang M., Shen Y.-Y., Liu C.-L., Xu C.-X. Application of abbreviated protocol of magnetic resonance imaging for breast cancer screening in dense breast tissue. Acad. Radiol. 2017;24:316–320. doi: 10.1016/j.acra.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Chen S.-Q.S.-Q., Huang M., Shen Y.-Y., Liu C.-L.C.-L., Xu C.-X.C.-X. Abbreviated MRI protocols for detecting breast cancer in women with dense breasts. Korean J. Radiol. 2017;18:470–475. doi: 10.3348/kjr.2017.18.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panigrahi B.B.B.B., Mullen L., Falomo E., Panigrahi B.B.B.B., Harvey S. An abbreviated protocol for high-risk screening breast magnetic resonance imaging: impact on performance metrics and BI-RADS assessment. Acad. Radiol. 2017;24:1132–1138. doi: 10.1016/j.acra.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Petrillo A., Fusco R., Sansone M., Cerbone M., Filice S., Porto A., Rubulotta M.R., D’Aiuto M., Avino F., Di Bonito M., Botti G., D’Aiuto M., Avino F., Di Bonito M., Botti G., D’Aiuto M., Avino F., Di Bonito M., Botti G. Abbreviated breast dynamic contrast-enhanced MR imaging for lesion detection and characterization: the experience of an Italian oncologic center. Breast Cancer Res. Treat. 2017;164:401–410. doi: 10.1007/s10549-017-4264-y. [DOI] [PubMed] [Google Scholar]
- 52.Choi B.H., Choi N., Kim M.Y., Yang J.-H.J.-H., Yoo Y.B., Jung H.K. Usefulness of abbreviated breast MRI screening for women with a history of breast cancer surgery. Breast Cancer Res. Treat. 2018;167:495–502. doi: 10.1007/s10549-017-4530-z. [DOI] [PubMed] [Google Scholar]
- 53.Seppala N., Fallah Rastegar R., Richmond L., Betel C., Hack K., Skarpathiotakis M., Jong R., Thornhill R., Curpen B. Rapid MRI of the breast in evaluating lesions discovered on screening. Breast J. 2018 doi: 10.1111/tbj.13109. [DOI] [PubMed] [Google Scholar]
- 54.Yamada T., Kanemaki Y., Okamoto S., Nakajima Y. Comparison of detectability of breast cancer by abbreviated breast MRI based on diffusion-weighted images and postcontrast MRI. Jpn. J. Radiol. 2018;36:331–339. doi: 10.1007/s11604-018-0731-6. [DOI] [PubMed] [Google Scholar]
- 55.Dialani V., Tseng I., Slanetz P.J., Fein-Zachary V., Phillips J., Karimova E., Brook A., Mehta T.S. Potential role of abbreviated MRI for breast cancer screening in an academic medical center. Breast J. 2019;25:604–611. doi: 10.1111/tbj.13297. [DOI] [PubMed] [Google Scholar]
- 56.Jones L.I., Geach R., Harding S.A., Foy C., Taylor V., Marshall A., Taylor-Phillips S., Dunn J.A. Can mammogram readers swiftly and effectively learn to interpret first post-contrast acquisition subtracted (FAST) MRI, a type of abbreviated breast MRI?.: a single centre data-interpretation study. Brit. J. Radiol. 2019;92:20190663. doi: 10.1259/bjr.20190663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee-Felker S., Joines M., Storer L., Li B., DeBruhl N., Sayre J., Hoyt A. Abbreviated breast MRI for estimating extent of disease in newly diagnosed breast cancer. J. Breast Imaging. 2020;2:43–49. doi: 10.1093/jbi/wbz071. [DOI] [PubMed] [Google Scholar]
- 58.An Y.Y., Kim S.H., Kang B.J., Suh Y.J., Jeon Y.W. Feasibility of abbreviated magnetic resonance imaging (AB-MRI) screening in women with a personal history (PH) of breast cancer. PLOS ONE. 2020;15:e0230347. doi: 10.1371/journal.pone.0230347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marquina D., Cruz C S., García B A., Suñén A I., García M C. Value of an abbreviated protocol of breast magnetic resonance imaging for screening high-risk patients. Radiología (English Edition) 2020;62:198–204. doi: 10.1016/j.rxeng.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Park K.W., Han S.B., Han B.-K.K., Ko E.S.Y.S., Choi J.S., Rhee S.J., Ko E.S.Y.S. MRI surveillance for women with a personal history of breast cancer: comparison between abbreviated and full diagnostic protocol. Brit. J. Radiol. 2020;93:20190733. doi: 10.1259/bjr.20190733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiraishi M., Igarashi T., Terayama T., Watanabe K., Ashida H., Ojiri H. Breast magnetic resonance imaging for estimation of the tumour extent in patients with pure ductal carcinoma in situ: comparison between full diagnostic and abbreviated protocols. Eur. J. Radiol. 2020;123:108788. doi: 10.1016/j.ejrad.2019.108788. [DOI] [PubMed] [Google Scholar]
- 62.Scoggins M.E., Arun B.K., Candelaria R.P., Dryden M.J., Wei W., Son J.B., Ma J., Dogan B.E. Should abbreviated breast MRI be compliant with American College of Radiology requirements for MRI accreditation? Magn. Reson. Imaging. 2020;72:87–94. doi: 10.1016/j.mri.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dogan B.E., Scoggins M.E., Son J.B., Wei W., Candelaria R., Yang W.T., Ma J. American college of radiology-compliant short protocol breast MRI for high-risk breast cancer screening: a prospective feasibility study. Am. J. Roentgenol. 2018;210:214–221. doi: 10.2214/AJR.17.18267. [DOI] [PubMed] [Google Scholar]
- 64.Machida Y., Shimauchi A., Kanemaki Y., Igarashi T., Harada M., Fukuma E. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast cancer. 2017;24:411–419. doi: 10.1007/s12282-016-0718-z. [DOI] [PubMed] [Google Scholar]
- 65.van Zelst J.C., Vreemann S., Witt H.-J., Gubern-Merida A., Dorrius M.D., Duvivier K., Lardenoije-Broker S., Lobbes M.B., Loo C., Veldhuis W., Veltman J., Drieling D., Karssemeijer N., Mann R.M. Multireader study on the diagnostic accuracy of ultrafast breast magnetic resonance imaging for breast cancer screening. Invest. Radiol. 2018:1. doi: 10.1097/RLI.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 66.Goto M., Sakai K., Yokota H., Kiba M., Yoshida M., Imai H., Weiland E., Yokota I., Yamada K. Diagnostic performance of initial enhancement analysis using ultra-fast dynamic contrast-enhanced MRI for breast lesions. Eur. Radiol. 2019;29:1164–1174. doi: 10.1007/s00330-018-5643-4. [DOI] [PubMed] [Google Scholar]
- 67.Choudhery S., Chou S.-H.S., Chang K., Kalpathy-Cramer J., Lehman C.D. Kinetic analysis of lesions identified on a rapid abridged multiphase (RAMP) breast MRI protocol. Acad. Radiol. 2020;27:672–681. doi: 10.1016/j.acra.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mori N., Sheth D., Abe H. Nonmass enhancement breast lesions: Diagnostic performance of kinetic assessment on ultrafast and standard dynamic contrast-enhanced MRI in comparison with morphologic evaluation. Am. J. Roentgenol. 2020;215:511–518. doi: 10.2214/AJR.19.21920. [DOI] [PubMed] [Google Scholar]
- 69.Bickelhaupt S., Tesdorff J., Laun F.B., Kuder T.A., Lederer W., Teiner S., Maier-Hein K., Daniel H., Stieber A., Delorme S., Schlemmer H.-P. Independent value of image fusion in unenhanced breast MRI using diffusion-weighted and morphological T2-weighted images for lesion characterization in patients with recently detected BI-RADS 4/5 x-ray mammography findings. Eur. Radiol. 2017;27:562–569. doi: 10.1007/s00330-016-4400-9. [DOI] [PubMed] [Google Scholar]
- 70.Bickelhaupt S., Paech D., Laun F.B., Steudle F., Kuder T.A., Mlynarska A., Bach M., Lederer W., Teiner S., Schneider S., Ladd M.E., Daniel H., Stieber A., Kopp-Schneider A., Delorme S., Schlemmer H.-P.H.-P. Maximum intensity breast diffusion MRI for BI-RADS 4 lesions detected on X-ray mammography. Clin. Radiol. 2017;72 doi: 10.1016/j.crad.2017.05.017. 900.e1 - 900.e8. [DOI] [PubMed] [Google Scholar]
- 71.Kang J.W., Shin H.J., Shin K.C., Chae E.Y., Choi W.J., Cha J.H., Kim H.H. Unenhanced magnetic resonance screening using fused diffusion-weighted imaging and maximum-intensity projection in patients with a personal history of breast cancer: role of fused DWI for postoperative screening. Breast Cancer Res. Treat. 2017;165:119–128. doi: 10.1007/s10549-017-4322-5. [DOI] [PubMed] [Google Scholar]
- 72.Kul S., Metin Y., Kul M., Metin N., Eyuboglu I., Ozdemir O. Assessment of breast mass morphology with diffusion-weighted MRI: beyond apparent diffusion coefficient. J. Magn. Reson. Imaging. 2018 doi: 10.1002/jmri.26175. [DOI] [PubMed] [Google Scholar]
- 73.Kanda T., Oba H., Toyoda K., Kitajima K., Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn. J. Radiol. 2016;34:3–9. doi: 10.1007/s11604-015-0503-5. [DOI] [PubMed] [Google Scholar]
- 74.Girometti R., Nitti A., Lorenzon M., Greco F., Londero V., Zuiani C. Comparison between an abbreviated and full MRI protocol for detecting additional disease when doing breast cancer staging. J. Magn. Reson. Imaging. 2019;49:e222–e230. doi: 10.1002/jmri.26339. [DOI] [PubMed] [Google Scholar]