Abstract
Resting metabolic rate (RMR) tends to decline with aging. The age-trajectory of decline in RMR is similar to changes that occur in muscle mass, muscle strength, and fitness, but while the decline in these phenotypes has been related to changes of mitochondrial function and oxidative capacity, whether lower RMR is associated with poorer mitochondrial oxidative capacity is unknown. In 619 participants of the Baltimore Longitudinal Study of Aging, we analyzed the cross-sectional association between RMR (kcal/day), assessed by indirect calorimetry, and skeletal muscle maximal oxidative phosphorylation capacity, assessed as postexercise phosphocreatine recovery time constant (τ PCr), by phosphorous magnetic resonance spectroscopy. Linear regression models were used to evaluate the relationship between τ PCr and RMR, adjusting for potential confounders. Independent of age, sex, lean body mass, muscle density, and fat mass, higher RMR was significantly associated with shorter τ PCr, indicating greater mitochondrial oxidative capacity. Higher RMR is associated with a higher mitochondrial oxidative capacity in skeletal muscle. This association may reflect a relationship between better muscle quality and greater mitochondrial health.
Keywords: Metabolism, Mitochondria, Muscles, Biology of aging
Mitochondrial dysfunction is considered a major hallmark of aging (1). Mitochondria provide the majority of energy for biological processes in the form of adenosine triphosphate (ATP), which can be dephosphorylated causing release of free energy. In skeletal muscle, mitochondrial density, O2 consumption at peak exercise, and tricarboxylic acid cycle enzyme activity decline with aging (2). Such decline is associated with dysfunction of the electron transport chain and decreased ATP production, leading to energetic deficits (3,4). This age-related reduction of oxidative capacity in skeletal muscle, due in large part to mitochondrial dysfunction, is considered an important factor driving muscle aging and sarcopenia (2,5). In vivo studies utilizing phosphorus magnetic resonance spectroscopy (31P-MRS), a technique that measures postexercise mitochondrial oxidative capacity in skeletal muscle, have generally reported a downward trend in mitochondrial function with aging, although substantial variability across individuals of the same age also has been observed (6–9). Lower skeletal muscle oxidative capacity is associated with slower gait speed (10), lower muscle strength and muscle quality (11), and insulin resistance (12).
Resting metabolic rate (RMR) represents the amount of energy expended under resting conditions and accounts for 60%–70% of daily energy expenditure, while physical activity represents 15%–30%, and diet-induced thermogenesis about 10% (13). RMR is largely determined by the most metabolically active tissues, that is, skeletal muscle, heart, brain, kidney, and liver, but in obese individuals, adipose tissue also plays an important role (14,15). Because of its size, skeletal muscle mass is the major correlate of RMR and, therefore, measures of RMR need to be referenced to specific body composition parameters (16,17). RMR is also consistently lower in women than men independent of body composition (18). RMR steadily declines with aging, more steeply in the first three decades of life and more gradually throughout adulthood until death (18,19). This age-related decline is partially but not completely explained by changes in body composition, reduction of lean mass in particular (20,21). In persons with impaired health, the relationship between RMR and age is more complex, as decline due to muscle loss may be offset by increased metabolic demands associated with homeostatic dysregulation (22). Specifically, independent of age, sex, body composition, and physical activity, higher RMR has been associated with a greater number of chronic diseases (23,24), frailty (25), and higher mortality (26,27). Thus, elevated RMR relative to age, sex, body composition, and physical activity may be a global marker of poor health (28).
However, while the level of physical activity was used as a potential confounder in evaluating RMR as a biomarker of health, the relationship between mitochondrial function and RMR in vivo has not been fully investigated. Better mitochondrial function requires higher mitochondrial mass and number, as well as greater metabolic rate for conversion of substrates to ATP, which likely results in greater demand for oxygen and substrate even at rest, translating into higher RMR. Conversely, better mitochondrial health/function is likely associated with better oxidative capacity, namely higher ATP production for each unit of oxygen consumed, possibly because of lower production of reactive oxygen species (ROS) and a consequent lower need for oxidative phosphorylation uncoupling. However, whether one of the two mechanisms is more important than the other in tilting the balance toward a positive or a negative relationship between RMR and mitochondrial function has not been fully investigated. To begin to address mitochondrial function and oxidative capacity, we characterized the association between RMR, as assessed by indirect calorimetry, and skeletal muscle mitochondrial oxidative capacity, as measured by 31P-MRS, in participants of the Baltimore Longitudinal Study of Aging, a well-characterized cohort of community-dwelling adults. Furthermore, we determined the presence of chronic diseases and evaluated whether they affected the association between RMR and oxidative capacity.
Materials and Methods
Participants
The study participants included 619 men and women aged 24–97 years, from the Baltimore Longitudinal Study of Aging (BLSA), who were seen between April 2013 and June 2019. The BLSA is a prospective open cohort study of community-dwelling volunteers primarily from the Baltimore, MD and Washington, DC area. Since 1958, the study has been continuously enrolling participants, who were free of major chronic conditions and functional impairments at enrollment, and following them for life regardless of changes in health and functional status, as described in detail elsewhere (29).
This study characterized the cross-sectional association of mitochondrial oxidative capacity with RMR. Assessments were performed at the Clinical Research Unit of the Intramural Research Program of the National Institute on Aging during an on-unit clinic visit that consisted of 2.5–3.5 days of medical, physiological, and psychological exams. Certified nurse practitioners and certified technicians conducted all assessments according to standardized procedures. The study protocol was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences (NIH, North Carolina) and was conducted in accordance with the 1964 Helsinki Declaration. At every visit, after receiving a detailed description of the scope, procedures, and related risk of participating in the study, all participants consented to participate in the study.
Demographic and health characteristics of the population were determined according to self-reported questionnaires or using standard criteria and algorithms (30). Physical activity was determined through the administration of a standardized questionnaire about activities of different intensities performed during a regular week, and then transformed into an estimate of energy expenditure (kcal/week) using standard conversion factors (31).
Chronic Diseases
Participants underwent an in-depth clinical evaluation, and the presence versus absence of major chronic conditions was established based on standard clinical, laboratory, and anamnestic criteria. A range of 15 conditions that occur with higher frequency and are associated with high disability and mortality risk in the aging population was selected “a priori” and included: chronic heart failure, myocardial infarction, cerebrovascular accidents, hypertension, Type 2 diabetes mellitus, anemia, peripheral artery disease (PAD), cognitive impairment, depression, Parkinson’s disease, chronic kidney disease (CKD), cataract, chronic obstructive pulmonary disease (COPD), cancer, and osteoarthritis. Multimorbidity was defined as the co-occurrence of multiple diseases and computed as the sum of all diseases present at the same visit (multimorbidity index: range 1–15).
Resting Metabolic Rate
RMR was determined using indirect calorimetry (Cosmed K4b2, Rome, Italy) (32,33). RMR was assessed first thing in the morning, after an overnight stay in the clinic, in a quiet, thermoneutral environment for 16 minutes. Participants were in a fasting, resting state. Before testing, the analyzer was warmed-up for 20 minutes and calibrated using a 3.0-L flow syringe and gases of known concentrations. The analyzer collects gas-exchange data on a breath-by-breath basis averaged over 30-second intervals to reduce variability. RMR was collected in milliliters per minute and then transformed into kilocalories per day using the Weir equation (1949) (34). The first 5 minutes of data were discarded to allow adaptation to the testing procedures, and the remaining 11 minutes were averaged to obtain a single measure of RMR (35).
Body Composition
Total body dual-energy x-ray absorptiometry (DXA) was performed using the Prodigy Scanner (General Electric, Madison, WI) and analyzed with version 10.51.006 software. DXA uses tissue absorption of x-ray beams to identify different components of the human body (bone mineral content, lean body mass, and fat mass) and to provide quantitative data on body composition (36,37).
Muscle density was assessed by computed tomography (CT; Somatom Sensation 10; Siemens, Malvern, PA) in a 10-mm thick slice of the thigh acquired at mid-femur, and was quantified by customized software with manual checking for quality control (GEANIE software, version 2.1; BonAnalyse, Jyvaskyla, Finland). The muscle outline was traced manually, excluding subcutaneous fat, intermuscular fat macroscopically detectable, and bone, by a trained and certified study coordinator. The mean attenuation coefficient values of muscle within the regions outlined on the images were determined by averaging the CT number (pixel intensity) in Hounsfield units (HU).
Phosphorus Magnetic Resonance Spectroscopy
In vivo 31P-MRS measurements of the concentrations of the phosphorus-containing metabolites phosphocreatine (PCr), inorganic phosphate (Pi), and ATP, were obtained from the vastus lateralis muscle of the left thigh using a 3T MR scanner (Achieva, Philips Healthcare, Andover, MA), following a standardized protocol described previously (10,38). Briefly, spectra of phosphorous-containing metabolites were acquired before, during, and after a rapid ballistic knee extension exercise performed by the participants for an average duration of 30 seconds. Before, during, and after the exercise, a series of pulse-acquire 31P spectra were obtained with a repetition time of 1.5 seconds, with a 10-cm 31P-tuned surface coil (PulseTeq, Surrey, UK) fastened above the middle of the left thigh. The muscle group of interest was the same previously described as the focus of CT assessments. Signals were averaged over four successive acquisitions for signal-to-noise ratio enhancement, so the data consisted of 75 spectra obtained with a temporal resolution of 6 seconds. To standardize the measure of oxidative function across different subjects, the duration of exercise was carefully optimized by consistently requiring a reduction in PCr peak height of 33%–67% compared with initial baseline values, and avoiding intramuscular acidosis, defined as intracellular pH lower than 6.8 (39). The pH was determined according to the chemical shift of Pi relative to PCr (40). Spectra were processed with jMRUI software (MRUI Consortium, version 5.2), and metabolite concentrations were calculated by nonlinear least squares fitting implemented through AMARES (41,42).
Postexercise PCr recovery rate was calculated by fitting time-dependent changes in PCr peak area to the monoexponential recovery function:
where PCr(0) is the end-of-exercise PCr signal area (ie, the PCr signal area at the beginning of the recovery period), ΔPCr is the decrease in signal area from its pre-exercise baseline value to PCr(0) resulting from in-magnet exercise, and τ PCr is the PCr exponential recovery time constant, measured in seconds (10). This time constant is inversely proportional to the maximum in vivo oxidative capacity of skeletal muscle. Hence, a longer τ PCr reflects slower recovery and hence lower oxidative capacity, while a shorter τ PCr is a sign of a more rapid capacity for PCr resynthesis (43). There are minimal energy demands during postexercise PCr resynthesis and, thus, 1/τ PCr reflects the maximum mitochondrial ATP production rate (8,10,44).
Statistical Analysis
Variables were described as mean values and standard deviations or proportions as appropriate. The cross-sectional association between τ PCr and RMR was assessed using bivariate and multivariable linear regression analysis. Covariates considered in the model (Model 1) were sex (encoded as 1 = male, 0 = female), age, total body fat mass, total body lean mass, and self-reported physical activity, as all these variables are known to contribute to resting energy metabolism. A second set of covariates that included the multimorbidity index was included in Model 2. The analyses were performed using RStudio version 1.1.453. p < .05 was considered statistically significant.
Results
The demographic and health characteristics of the 619 study participants are given in Table 1. The majority were Caucasian (66.7%), and slightly more than half were women (55.6%). Nearly all participants had completed high school. Only 10 participants (1.6%) were active smokers. Besides the high prevalence of hypertension (34.6%), the prevalence of chronic diseases in this study population was moderately low, with a mean multimorbidity score of 2.14.
Table 1.
Demographic and Health Characteristics of 619 Adults From the Baltimore Longitudinal Study of Aging
| Characteristic | Mean (SD) or % |
|---|---|
| Age, years | 67.7 (14.8) |
| Race, % | |
| White | 66.7 |
| Black | 24.1 |
| Other | 9.2 |
| Sex, % female | 55.6 |
| Education <12 years, % | 0.81 |
| Smoking status, % | |
| Never | 67.7 |
| Former | 30.7 |
| Current | 1.6 |
| Physical activity, kcal/week | 8,192 (6,358) |
| Body mass index, kg/m2 | 26.92 (4.23) |
| Lean mass, kg | 46.17 (10.09) |
| Fat mass, kg | 26.06 (9.95) |
| Muscle density, Hounsfield units | 50.02 (4.48) |
| τ-PCr, s | 50.28 (11.62) |
| Resting metabolic rate, kcal/d | 1,226 (319) |
| Multimorbidity index (1–15) | 2.14 (1.78) |
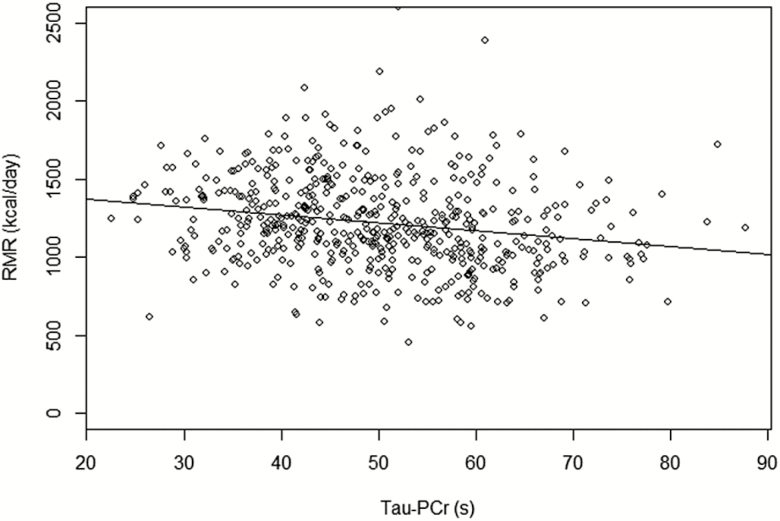
A scatterplot of the relationship between τ PCr and RMR is shown in Figure 1. Univariate relationships of RMR with other characteristics are reported in Table 2. Higher RMR was associated with younger age (p < .0001), male sex (p < .0001), higher lean body mass (p < .0001), higher fat mass (p < .0001), higher physical activity (p < .0001), and shorter τ PCr (p < .0001).
Figure 1.
Scatterplot of τ PCr (s) versus resting metabolic rate (kcal/day) with the regression line.
Table 2.
Relationship of Demographic and Other Characteristics With Resting Metabolic Rate in 619 Adults From the Baltimore Longitudinal Study of Aging, Using Univariate Linear Regression Models
| Variable | Beta Coefficient | Standard Error | p Value |
|---|---|---|---|
| Age, years | −5.29 | 0.85 | <.0001 |
| Sex (male = 1, female = 0) | 327.5 | 22.24 | <.0001 |
| Body mass index, kg/m2 | 20.73 | 2.78 | <.0001 |
| Lean mass, kg | 20.69 | 0.97 | <.0001 |
| Fat mass, kg | 5.22 | 1.28 | <.0001 |
| Physical activity, kcal/week | 0.008 | 0.002 | <.0001 |
| τ PCr | −4.64 | 1.09 | <.0001 |
The independent relationship between skeletal muscle mitochondrial oxidative capacity (τ PCr) and RMR was estimated from the multivariable linear regression model given in Table 3 (Model 1). τ PCr was inversely and significantly associated with RMR, after adjusting for age and sex, physical activity, and body composition. In additional analyses, we included interaction terms for “age*τ PCr” and “sex*τ PCr” in the models predicting RMR, but neither interaction term was significant (p = .58 and p = .84, respectively) and did not improve the model fit. Since DXA cannot discriminate the metabolically active, contractile component of muscle mass of detect infiltration of small lipid droplets, in additional analysis, we adjusted for muscle density, as estimated from the CT scan of the middle thigh. In this regression model, muscle density was not a significant predictor of RMR (p = .37) and the other parameters in the model remained substantially unchanged (Supplementary Table 1). In Model 2, Table 4, we evaluated whether the presence of multiple chronic pathological conditions, as measured by the multimorbidity index, was affecting the association between τ PCr and RMR. Multimorbidity contributed significantly to the model (p = .0002, β = 26.22), but the association of RMR with τ PCr persisted (p = .03).
Table 3.
Multivariable Linear Regression Model of the Relationship of τ PCr (s) With RMR (kcal/day) After Adjusting for Covariates (Model 1)
| Variable | Beta Coefficient | Standard Error | p Value |
|---|---|---|---|
| τ PCr | −2.26 | 0.97 | .019 |
| Age | −2.80 | 0.85 | .001 |
| Sex | 38.99 | 40.94 | .34 |
| Lean body mass (kg) | 17.78 | 2.09 | <.0001 |
| Fat body mass (kg) | 3.04 | 1.12 | .007 |
| Physical activity (kcal/week) | −0.004 | 0.002 | .035 |
Table 4.
Multivariable Linear Regression Model of the Relationship of τ PCr (s) With RMR (kcal/day) After Adjusting for Covariates, Including Multimorbidity (Model 2)
| Variable | Beta Coefficient | Standard Error | p Value |
|---|---|---|---|
| τ PCr (s) | −2.04 | 0.99 | .03 |
| Age | −7.38 | 0.89 | <.0001 |
| Sex (male = 1, female = 0) | 237.6 | 30.15 | <.0001 |
| Lean body mass (kg) | 0.002 | 1.27 | <.0001 |
| Fat body mass (kg) | 1.25 | 1.10 | .26 |
| Physical activity (kcal/week) | −0.002 | 0.002 | .24 |
| Multimorbidity | 26.22 | 7.05 | .0002 |
Discussion
In a relatively healthy community resident cohort of largely older adults, we found postexercise muscle phosphocreatine recovery rate, an indirect measure of mitochondrial maximal oxidative capacity, to be associated with RMR independent of age, sex, lean body mass, and self-reported level of physical activity. This is in line with previous work that reported similar results in a smaller population (45).
In highly energetically demanding tissues, such as muscle, heart, and brain, energy in the form of ATP is accumulated and released through the phosphocreatine shuttle system. ATP generated by mitochondria is used to bind a high-energy phosphate to a creatine molecule, therefore producing phosphocreatine. The high energy phosphate can be rapidly mobilized and anaerobically donated to ADP, to form new ATP and provide energy for contraction as well as for many other metabolic activities occurring in muscle fibers. The possibility of performing rapid ATP resynthesis as the demand for energy rises is critically important, since the pool of ATP readily available as a source of energy in the cytoplasm of myocytes is relatively small and, in the absence of this system, a rapid drop in ATP concentrations with increasing energy demand would be unavoidable. Both during exercise and, especially, at rest, ATP produced by mitochondria “recharges” the phosphocreatine. In fact, the phosphocreatine recovery rate after exercise is considered a good biomarker of mitochondrial oxidative capacity (8,44).
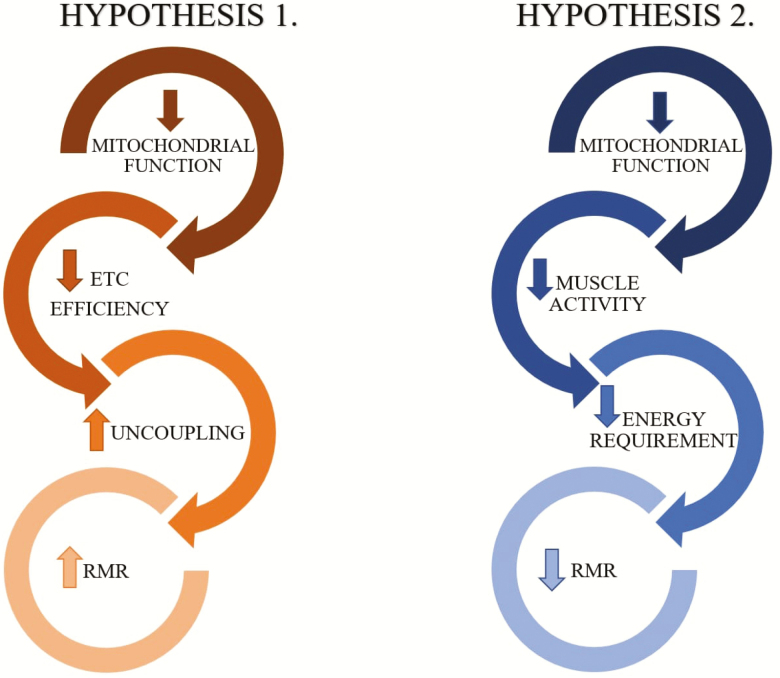
In resting muscle, energy demand is correlated with the metabolic activity of myocytes, with the greatest energy required by the sodium–potassium pump that maintains the outward sodium and inner potassium transmembrane gradients necessary to maintain cell function and excitability (46,47). As mentioned previously, the production of ATP is tightly regulated to maintain a stable intracellular concentration and depends mostly on cellular metabolic activity rather than mitochondrial quantity or function. However, there are at least two mechanisms by which RMR and mitochondrial function may be correlated, directly or inversely (Figure 2): (i) More efficient mitochondrial function is likely associated with better mitochondrial health, more efficient oxidative phosphorylation and lower production of ROS. When mitochondria become dysfunctional, the flow of electrons between complexes is slowed, leading to the production of ROS. In this situation, mitochondria offset the production of ROS by “leaking” a portion of the protons accumulated in the intermembrane space to the mitochondrial matrix. Since these electrons do not pass through the complex V ATPase, the amount of ATP produced per electron moved through the electron transport chain is lower. Thus, lower mitochondrial function could hypothetically cause both slower PCr recovery after exercise and higher RMR, as part of the oxygen consumption would not be used to generate ATP. (ii) Alternatively, healthy mitochondria are likely associated with high quality muscle tissue, which is characterized by higher cellularity and smaller or absent deposition of fat, collagen, or other noncontractile proteins. It is conceivable that such muscle requires more energy even at rest, which translates into higher RMR. Of note, although our analysis was adjusted for muscle mass, residual confounding is likely present because DXA cannot discriminate differences in muscle composition that may imply different energetic demands. Muscle density measured by CT may contribute to a better approximation of the true mass of metabolically active tissue. However, adjusting for muscle density did not change the results of our analysis. It is also possible that the larger number of mitochondria in healthy, fit individuals may require higher oxidative capacity at rest, simply for maintaining their background metabolism, contributing to higher RMR in fit individuals.
Figure 2.
Hypothesized pathways leading from impaired mitochondrial function to alterations in RMR. Hypothesis 1: decreased mitochondrial oxidative capacity determines a dissipation of the proton gradient with production of heat, which contributes to an increase in RMR. Hypothesis 2: impaired mitochondrial function causes decreased muscle activation and consequently reduced energy metabolism. ETC = electron transport chain; RMR = resting metabolic rate.
The measurements adopted in this study do not allow to definitely distinguish between a higher RMR due to mitochondrial inefficiency and a higher RMR due to better muscle quality, a differentiation that would have required invasive biopsy sampling and assessment of ETC function and mitochondrial mass and number.
Although we cannot exclude that both these mechanisms may be at play, by showing a direct correlation between mitochondrial oxidative capacity and RMR, our study suggests that the second mechanism, namely better mitochondrial oxidative capacity associated with high-quality muscle tissue, prevails over the first one in this cross-sectional analysis, performed in a relatively old but healthy population. However, it is plausible that the balance between the two hypothesized mechanisms may shift with aging and with the development of chronic morbidity. Specifically, declining health and progressive mitochondrial impairment may cause higher ROS production compensated by uncoupling, which results in higher RMR. This would contribute to the lack of decline of RMR with aging observed in subjects whose health declines (23,24). According to this hypothesis, the relationship between mitochondrial function and RMR would change over time, as people age and their health deteriorates. Our cross-sectional study as reported here provides strong evidence of a relationship between mitochondrial function and RMR, strongly motivating further longitudinal studies of the potentially dynamic, age-dependent, nature of that relationship.
Our data suggest that individuals with high mitochondrial function, likely those with better fitness, have higher RMR. Thus, improvement of fitness and muscle oxidative capacity is followed by increased needs for energy expenditure at rest.
This study has limitations. First, because of the exceptional healthiness of the BLSA participants included in this study, our findings may not directly apply to a less healthy population. Second, the ethnic distribution of the study population does not reflect that of the general U.S. population, and racial differences in mitochondrial function and energy expenditure have been previously described (48).
Moreover, while our measure of mitochondrial function targets a specific muscle group, RMR is a summary of the RMR of multiple body tissues, among which highly metabolically active tissues such as brain, liver, or other muscle groups. It is also possible that mitochondrial function in skeletal muscle is different from mitochondrial function in other tissues. In addition, RMR and τ-PCr are different measurements obtained during two very different conditions: the former is assessed at rest and reported as a 24-hours value, while the latter follows an intense exercise and is expressed in seconds.
Finally, our measure of muscle mass was based on DXA, which cannot discriminate the contractile, metabolically active component of muscle tissue as opposed to other molecules, mainly of connective tissue, that tend to accumulate with aging. Utilization of other, more precise and reliable techniques, such as dilution of deuterated creatine, should be considered in future studies (49).
Further longitudinal research is needed to better understand the relationship between energy metabolism at rest and mitochondrial function in the aging population. Evaluating this association in a longitudinal study would provide substantial further insight into potential mechanisms and trajectories.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Supplementary Table S1. Multivariable Linear Regression Model of the Relationship of τPCr (s) With RMR (kcal/day) After Adjusting for Covariates, Including Muscle Density
Funding
This work was supported by the Intramural Research Program of the National Institute on Aging and National Institutes of Health (R01 AG027012 and R01 AG057723).
Conflict of Interest
None reported.
References
- 1. López-Otín C, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marzetti E, Calvani R, Cesari M, et al. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45:2288–2301. doi: 10.1016/j.biocel.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson ML, Robinson MM, Nair KS. Skeletal muscle aging and the mitochondrion. Trends Endocrinol Metab. 2013;24:247–256. doi: 10.1016/j.tem.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adelnia F, Urbanek J, Osawa Y, et al. Moderate-to-vigorous physical activity is associated with higher muscle oxidative capacity in older adults. J Am Geriatr Soc. 2019;67:1695–1699. doi: 10.1111/jgs.15991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez-Freire M, Scalzo P, D’Agostino J, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: the Baltimore Longitudinal Study of Aging. Aging Cell. 2018;17(2):e12725. doi: 10.1111/acel.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526 Pt 1:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Porter C, Hurren NM, Cotter MV, et al. Mitochondrial respiratory capacity and coupling control decline with age in human skeletal muscle. Am J Physiol Endocrinol Metab. 2015;309:E224–E232. doi: 10.1152/ajpendo.00125.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi S, Reiter DA, Shardell M, et al. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2016;71:1638–1645. doi: 10.1093/gerona/glw059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zane AC, Reiter DA, Shardell M, et al. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell. 2017;16:461–468. doi: 10.1111/acel.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fabbri E, Chia CW, Spencer RG, et al. Insulin resistance is associated with reduced mitochondrial oxidative capacity measured by 31P-magnetic resonance spectroscopy in participants without diabetes from the Baltimore Longitudinal Study of Aging. Diabetes. 2017;66:170–176. doi: 10.2337/db16-0754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. doi: 10.1097/00005768-200009001-00009 [DOI] [PubMed] [Google Scholar]
- 14. Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN, eds. Energy Metabolism: Tissue Determinants and Cellular Corollaries. New York: Raven Press; 1992:61–79. [Google Scholar]
- 15. Müller MJ, et al. Advances in the understanding of specific metabolic rates of major organs and tissues in humans. Curr Opin Clin Nutr Metab Care. 2013;16(5):501–508. doi: 10.1097/MCO.0b013e328363bdf9 [DOI] [PubMed] [Google Scholar]
- 16. Herbert BM, Neuh M. Effects of fat mass and body fat distribution on resting metabolic rate in the elderly. Metab Clin Exp. 2001;50(8):972–975. doi: 10.1053/meta.2001.24871 [DOI] [PubMed] [Google Scholar]
- 17. Tzankoff S, Norris A. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol. 1977;43(6):1001–1006. doi: 10.1152/jappl.1977.43.6.1001 [DOI] [PubMed] [Google Scholar]
- 18. Henry C. Mechanisms of changes in basal metabolism during ageing. Eur J Clin Nutr. 2000;54(S3):S77. doi: 10.1038/sj.ejcn.1601029 [DOI] [PubMed] [Google Scholar]
- 19. Lührmann P, et al. Longitudinal changes in energy expenditure in an elderly German population: a 12-year follow-up. Eur J Clin Nutr. 2009;63(8):986. doi: 10.1038/ejcn.2009.1 [DOI] [PubMed] [Google Scholar]
- 20. Alfonzo-González G, Doucet E, Bouchard C, Tremblay A. Greater than predicted decrease in resting energy expenditure with age: cross-sectional and longitudinal evidence. Eur J Clin Nutr. 2006;60:18–24. doi: 10.1038/sj.ejcn.1602262 [DOI] [PubMed] [Google Scholar]
- 21. St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–155. doi: 10.1016/j.nut.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruggiero C, Ferrucci L. The endeavor of high maintenance homeostasis: resting metabolic rate and the legacy of longevity. J Gerontol A Biol Sci Med Sci. 2006;61:466–471. doi: 10.1093/gerona/61.5.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fabbri E, An Y, Schrack JA, et al. Energy metabolism and the burden of multimorbidity in older adults: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2015;70:1297–1303. doi: 10.1093/gerona/glu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagel A, Jungert A, Spinneker A, Neuhäuser-Berthold M. The Impact of Multimorbidity on resting metabolic rate in community-dwelling women over a ten-year period: a cross-sectional and longitudinal study. J Nutr Health Aging. 2017;21:781–786. doi: 10.1007/s12603-016-0840-9 [DOI] [PubMed] [Google Scholar]
- 25. Kim S, Welsh DA, Ravussin E, et al. An elevation of resting metabolic rate with declining health in nonagenarians may be associated with decreased muscle mass and function in women and men, respectively. J Gerontol A Biol Sci Med Sci. 2014;69:650–656. doi: 10.1093/gerona/glt150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruggiero C, Metter EJ, Melenovsky V, et al. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2008;63:698–706. doi: 10.1093/gerona/63.7.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jumpertz R, Hanson RL, Sievers ML, Bennett PH, Nelson RG, Krakoff J. Higher energy expenditure in humans predicts natural mortality. J Clin Endocrinol Metab. 2011;96:E972–E976. doi: 10.1210/jc.2010-2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. “IDEAL” aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:667–672. doi: 10.1111/jgs.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore longitudinal study. J Gerontol. 1966;21:575–580. doi: 10.1093/geronj/21.4.575 [DOI] [PubMed] [Google Scholar]
- 30. Guralnik JM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women With Disability. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 31. Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB; Health, Aging and Body Composition Study Research Group The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x [DOI] [PubMed] [Google Scholar]
- 32. Rumpler WV, Seale JL, Conway JM, Moe PW. Repeatability of 24-h energy expenditure measurements in humans by indirect calorimetry. Am J Clin Nutr. 1990;51:147–152. doi: 10.1093/ajcn/51.2.147 [DOI] [PubMed] [Google Scholar]
- 33. Schrack JA, Simonsick EM, Ferrucci L. Comparison of the Cosmed K4b(2) portable metabolic system in measuring steady-state walking energy expenditure. PLoS One. 2010;5:e9292. doi: 10.1371/journal.pone.0009292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 3rd ed. Philadelphia, PA: Lea and Febiger; 1991. [Google Scholar]
- 35. Compher C, Frankenfield D, Keim N, Roth-Yousey L; Evidence Analysis Working Group Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106:881–903. doi: 10.1016/j.jada.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 36. Van ML, Mayclin P. Body composition assessment: dual-energy X-ray absorptiometry (DEXA) compared to reference methods. Eur J Clin Nutr. 1992;46(2):125–130. [PubMed] [Google Scholar]
- 37. Pritchard JE, Nowson CA, Strauss BJ, Carlson JS, Kaymakci B, Wark JD. Evaluation of dual energy X-ray absorptiometry as a method of measurement of body fat. Eur J Clin Nutr. 1993;47:216–228. [PubMed] [Google Scholar]
- 38. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455. doi: 10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paganini AT, Foley JM, Meyer RA. Linear dependence of muscle phosphocreatine kinetics on oxidative capacity. Am J Physiol. 1997;272(2 Pt 1):C501–C510. doi: 10.1152/ajpcell.1997.272.2.C501 [DOI] [PubMed] [Google Scholar]
- 40. Taylor DJ, Styles P, Matthews PM, et al. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med. 1986;3:44–54. doi: 10.1002/mrm.1910030107 [DOI] [PubMed] [Google Scholar]
- 41. Vanhamme L, Van Huffel S, Van Hecke P, van Ormondt D. Time-domain quantification of series of biomedical magnetic resonance spectroscopy signals. J Magn Reson. 1999;140:120–130. doi: 10.1006/jmre.1999.1835 [DOI] [PubMed] [Google Scholar]
- 42. Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–286. doi: 10.1016/s0010-4825(01)00006-3 [DOI] [PubMed] [Google Scholar]
- 43. Prompers JJ, Wessels B, Kemp GJ, Nicolay K. MITOCHONDRIA: investigation of in vivo muscle mitochondrial function by 31P magnetic resonance spectroscopy. Int J Biochem Cell Biol. 2014;50:67–72. doi: 10.1016/j.biocel.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 44. Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson Med. 1984;1:307–315. doi: 10.1002/mrm.1910010303 [DOI] [PubMed] [Google Scholar]
- 45. Edwards LM, Kemp GJ, Dwyer RM, et al. Integrating muscle cell biochemistry and whole-body physiology in humans:(31)P-MRS data from the InSight trial. Sci Rep. 2013;3:1182. doi: 10.1038/srep01182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Milligan LP, McBride BW. Energy costs of ion pumping by animal tissues. J Nutr. 1985;115:1374–1382. doi: 10.1093/jn/115.10.1374 [DOI] [PubMed] [Google Scholar]
- 47. Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic Biol Med. 2000;29:946–968. doi: 10.1016/s0891-5849(00)00417-2 [DOI] [PubMed] [Google Scholar]
- 48. Toledo FGS, Dubé JJ, Goodpaster BH, Stefanovic-Racic M, Coen PM, DeLany JP. Mitochondrial respiration is associated with lower energy expenditure and lower aerobic capacity in African American women. Obesity (Silver Spring). 2018;26:903–909. doi: 10.1002/oby.22163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3-creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.