Abstract
Background
Respiratory syncytial virus (RSV) is a leading cause of respiratory tract illness in young children and a major cause of hospital admissions globally.
Methods
Here we fit age-structured transmission models with immunity propagation to data from the Netherlands (2012–2017). Data included nationwide hospitalizations with confirmed RSV, general practitioner (GP) data on attendance for care from acute respiratory infection, and virological testing of acute respiratory infections at the GP. The transmission models, equipped with key parameter estimates, were used to predict the impact of maternal and pediatric vaccination.
Results
Estimates of the basic reproduction number were generally high (R0 > 10 in scenarios with high statistical support), while susceptibility was estimated to be low in nonelderly adults (<10% in persons 20–64 years) and was higher in older adults (≥65 years). Scenario analyses predicted that maternal vaccination reduces the incidence of infection in vulnerable infants (<1 year) and shifts the age of first infection from infants to young children.
Conclusions
Pediatric vaccination is expected to reduce the incidence of infection in infants and young children (0–5 years), slightly increase incidence in 5 to 9-year-old children, and have minor indirect benefits.
Keywords: respiratory syncytial virus, hospital data, GP consultations, transmission model, evidence synthesis, vaccination
Respiratory syncytial virus (RSV) infection results in a substantial disease burden in young children and older adults in all parts of the world [1–4]. For instance, it has been estimated that approximately 3 million hospitalizations and up to 150 000 deaths may occur globally in children younger than 5 years [4], and that total disease burden in adults over 65 years may be even larger [3]. Unsuccessful attempts to develop a vaccine in the 1960s [5] have given way to optimism that a vaccine or long-acting monoclonal antibodies will become available in the foreseeable future. Vaccines under development contain of a wide range of vaccine formulations and are aimed at different target groups. Vaccination strategies include maternal vaccination using particle-based and subunit vaccines, pediatric vaccination with live attenuated vaccines, and vaccination of older adults using particle-based, subunit, and vector-based vaccines [6–10].
Even though several vaccine candidates are homing in on the final stages of clinical trials, many unknowns remain, in particular on vaccine efficacy and duration of protection. In addition, there is at present still only a partial quantitative understanding of the transmission dynamics of RSV [11–16]. These studies show that incidence is highest in infants and young children (0–2 years), that children (1–10 years) are drivers of the yearly epidemics, while infant infections are commonly caused by siblings in the household, and that the disease burden in older adults is nonnegligible. Transmission models to date have built on this information, and have either used small data sets or have not included formal parameter inference, focusing on household models in selected low-income countries, on a variety of maternal and pediatric vaccination strategies, and even on including the impact of current and future climatic drivers [6, 17–22].
Here we fit age-structured epidemic models to population data from the Netherlands (2012–2017), in particular RSV coded hospitalizations (n = 12 038), general practitioner (GP) consultations for acute respiratory infection (ARI; n = 877 752), and virological testing of ARI at the GP (n = 4514). We integrated the data in a Bayesian evidence synthesis [23] while adopting a multiyear approach in which susceptibility from one epidemic to the next increases by demographic turnover and waning of immunity. Quantifying the gains of immunity during an epidemic together with interepidemic losses of immunity is crucial for proper evaluations of the impact of vaccination with vaccines that do not provide permanent immunity [24–27]. We provide a comparative assessment of high-coverage maternal and pediatric vaccination, illustrating how the analyses can give insights into the expected impact of vaccination.
METHODS
Data
Transmission models were fitted to 3 data sets. The first contains weekly age-stratified hospitalizations with confirmed RSV in the Netherlands for the years 2013–2017. Details, including ICD10 codes used and numbers of cases are given in the Supplementary Material. Second, we obtained GP consultations for ARIs from the Nivel Primary Care Database from 2012 to 2017. For each of the age classes and weeks, the number of cases and size of the catchment populations are available (covering approximately 7% of the Dutch population). Third, patient age-specific virological data are available from RIVM/Nivel sentinel surveillance of influenza-like illness and ARI [28]. These data are obtained from a small subset of approximately 40 GP practices representing 0.8% of the population in the Netherlands. Available specimens were tested for influenza virus, RSV, rhinovirus, and enterovirus using reverse transcription polymerase chain reaction (RT-PCR). Finally, age-specific contact rates are hard-wired into the model and were obtained from a contact survey carried out in the Netherlands in 2006–2007 [29]. Data are available in our repository (www.github.com/mvboven/RSV) and details are given in the Supplementary Material.
Transmission Model
At the core are age-structured SIR transmission models describing transmission of RSV in the population during an epidemic. In the models, individuals are classified as susceptible (S), infected and infectious (I), or removed (ie, immune, R). Throughout, we consider 7 age classes, that is [0,1) years (abbreviated as 0 year), [1,5) years (1–4 years), [5,10) years (5–9 years), [10,20) years (10–19 years), [20,45) years (20–44 years), [45,65) years (45–64 years), and [65,100) years (65+ years). These age groups correspond to a natural grouping of individuals by similarity of contact patterns while taking into account that only limited case data are available for nonelderly adults [29].
If we let the vectors x(t), y(t), and z(t) contain the age-specific relative frequencies of S, I, and R in different age groups, then z(t) = 1 − x(t) − y(t) represent the age-specific frequencies of removed individuals. The model dynamics are governed by the following ordinary differential equations:
| (1) |
where β, D, and C are the transmission rate parameter, infectious period, and contact matrix. As is common practice, the contact matrix is hard-wired into the model using Dutch person-to-person contact rates and the demographic composition of the Netherlands in 2014.
Details and additional results are given in the Supplementary Material. Briefly, we mention that if the probability that an infected persons reports at the GP or hospital is proportional to infectiousness of the individual, then the seemingly simple model in Equation (1) has broad applicability, and in particular can describe a scenario with variable severity, variable infectiousness, and variable probability of reporting for infections [30].
Immunity Propagation and Demographic Transitions
Next, we include demographic transitions and immunity propagation into the model [24–27]. To this end, we separate the epidemic occurring in winter from demographic turnover and immunity losses occurring throughout the year. Starting from initial conditions in a given year describing the fraction of the population that is immune in different age classes, the epidemic is modelled by the ordinary differential equations given in Equation (1). At the end of the epidemic, susceptibility in the population will have decreased and immunity will have increased. Subsequently, losses of immunity and demographic transitions between seasons are modelled with a discrete mapping, yielding the susceptibility (initial conditions) for the next epidemic. Details and additional scenarios are presented in the Supplementary Material.
Observation Model
Next, we specify the observation model. Throughout, each incident infection in age group a has an age-specific probability to be reported as an ARI case at the GP, and an (independent) age-specific probability to be reported as a confirmed RSV case in the hospital. Not all ARIs at the GP are caused by RSV, however, and we add a function for all other causes of ARI at the GP. These other sources include influenza, rhinovirus, enterovirus, and others, and can be highly variable between years, within a year, and between age groups. To accommodate this variability, we fit a flexible cubic spline to each epidemic season and age group. Details are given in the Supplementary Material.
Parameter Estimation
Parameters are estimated in a Bayesian framework using Hamiltonian Monte Carlo, implemented in Stan [31]. Main parameters are the basic reproduction number (R0), the infectious period (D), the reporting probabilities for hospitalization and GP consultation in different age groups ( and ), the age-specific probabilities that immunity is retained from one epidemic to the next (), and the spline weights. Because data were scarce in adults, we have lumped reporting probabilities in the age groups 5–9 years, 10–19 years, 20–44 years, and 45–64 years. In addition, we estimated the rate parameter of the gamma prior distribution for the B-splines generating the background ARI. Prior distributions and other details are given in the Supplementary Material.
Vaccination
As an illustration, we provide 2 vaccination scenarios based on the model that had been fitted to the data. In both scenarios we assumed an effective vaccination coverage of 50%. The first scenario mimics maternal vaccination, by removing part of infant age group (0 year) from the susceptible compartment to a temporarily protected vaccinated compartment. Here we assumed, quite arbitrarily in the absence of information from clinical studies, that maternal vaccination provides protection for 6 months, so that half of the infants born from maternally vaccinated mothers in the first age group are protected (those under 6 months of age). For a vaccination coverage of 50%, we thus move 0.5 × 50% = 25% of infants to the protected compartment. The second scenario is loosely based on a pediatric vaccination program. Here we assumed that vaccination is administered in the first half year of life in a diphtheria, tetanus, and pertussis (DTaP-IPV)-like program, so that 25% of infants in the first year of life are protected (those 6–12 months of age). We assumed that immunity lasts as long as individuals are in the 0 year and 1 to 4-year age classes, and is lost thereafter. We also assumed that susceptibility at the start of epidemics is determined fully by the estimated rates at which immunity is lost, neglecting potential year-to-year variations (eg, caused by variable evolution of the virus). Using 1000 samples from the posterior distribution we performed 1000 vaccination simulations for both scenarios. We ran the model for 20 years without vaccination, and then for an additional 20 years with vaccination. The impact of vaccination is calculated as 1 minus the ratio of the infection attack rates after 20 years with and 20 years without vaccination.
RESULTS
Estimation
Supplementary Table 1 shows the parameter estimates (posterior medians and 95% credible intervals [CrI]). Estimates of the reproduction number were high (21.9; 95% CrI, 20.1–25.7), and the infectious period was estimated at more than 2 weeks (2.5 weeks; 95% CrI, 2.2–2.8). The probability of hospitalization was estimated with some precision, and was highest in infants (0.014; 95% CrI, 0.013–0.015), approximately an order of magnitude lower in young children and older adults (1–4 years and 65+ years), and much lower in children and adults (5–64 year). For the probability of GP consultation, estimates were slightly less precise and differences between age groups were less pronounced. The fractions of the population that retained their immunity was high in most age groups (>0.9), and lower in older adults (65+ years, 0.85; 95% CrI, 0.64–0.96), and highly uncertain in young infants (0.47; 95% CrI, 0.02–0.97).
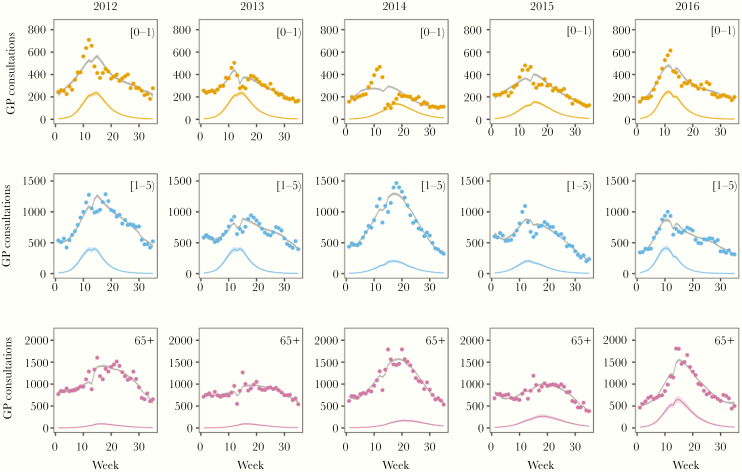
Figure 1 (GP consultations) and Supplementary Figures 2 and 3 (hospitalizations and virological data) show the data with model fit. Overall, the hospitalization data, GP consultations, and virological data are largely in agreement, both with respect to timing of the epidemics and age groups affected. Incidence, in terms of the proportion within the groups that is infected, was by far highest in infants, lower in young children, and much lower in all other age groups (see the repository for full results). Interestingly, while in the youngest age groups epidemics were very similar in size, there was substantial variation in the yearly attack rates in older age groups. In fact, in infants the estimated yearly attack rates were 0.73 (95% CrI, 0.69–0.78) in 2012/2013, 0.69 (95% CrI, 0.64–0.73) in 2013/2014, 0.67 (95% CrI, 0.62–0.73) in 2014/2015, 0.70 (95% CrI, 0.66–0.74) in 2015/2016, and 0.79 (95% CrI, 0.75–0.84) in 2016/2017 (Supplementary Table 4). In young children (1–5 years), the corresponding estimated attack rates were also similar between epidemics, with median infection attack rates ranging from 0.19 to 0.30. In older adults (older than 65 years), on the other hand, estimates were more variable, ranging from less than 0.03 in 2012/2013 and 2013/204 to more than 0.17 in 2016/2017 (Supplementary Table 4). In fact, in older adults RSV incidence seems to be increasing over time, and was highest in the 2016/2017 epidemic. This increase is visible in all 3 data sets: unusually high incidence of RSV confirmed hospitalizations and high incidence of GP consultations in 2016/2017, and high positive rates in virological data in 2016/2017.
Figure 1.
Weekly age-stratified number of GP consultations for ARI in the Netherlands (dots) with model fit (lines) for age groups 0–1, 1–5, and 65+ years. Bold lines correspond to posterior medians, and shaded areas represent 95% credible ranges of the posterior distribution. Gray lines represent the total weekly numbers of ARI and colored lines represent ARI that was attributed to RSV. The sudden changes at the end of calendar years were caused by changes in the number of participating GPs, leading to changes in the size of catchment populations. Abbreviations: ARI, acute respiratory infection; GP, general practitioner; RSV, respiratory syncytial virus.
The analyses also provide estimates of the fraction of ARI reported at the GP that can be attributed to RSV (calculated as the ratio of the ARI caused by RSV and ARI caused by RSV and other causes). In the years analyzed here, the RSV epidemic invariably peaked in the period between December and early February. During this period and in infants, the probability that ARI consultations at the GP are due to RSV were high, ranging from 20% to more than 50%. To a lesser extent this also applied to young children (1–5 years). In older children and adults, on the other hand, RSV infection was a very unlikely cause of GP consultation. Exceptions to this rule were GP visits in older adults at the peak of the 2015/2016 and 2016/2017 RSV epidemics. In these years, it is estimated that at the peak of the epidemic up to 50% of GP consultations with ARI in older adults may have been caused by RSV. As a result, in 2016/2017 more than 500 weekly consultations in older adults were caused by RSV infection at the epidemic peak, which is substantially more than the number of GP consultations in infants caused by RSV (Figure 1).
In the default scenario there are strong correlations between the basic reproduction number, the infectious period, and the reporting probabilities. Typically, a high reproduction number is associated with a long infectious period (to get the right shape and duration of epidemics), and with higher incidence and lower reporting probabilities (Supplementary Figure 5). In a sensitivity analysis we therefore fixed the expected duration of the infectious period at 1 week. The results show that estimates of the reproduction number strongly decreased (5.7; 95% CrI, 5.5–6.0), and that estimates of the reporting probabilities strongly increased (Supplementary Table 5). Comparison of the 2 scenarios using the Widely applicable Bayesian Information Criterion (WBIC) indicates that there is strong statistical evidence in favor of the default model scenario (ΔWBIC = 109). More importantly, in the variant scenario the estimated infection attack rates in infants were much lower than in the default scenario (Supplementary Table 6; 0.29 in 2012–2013, 0.27 in 2013–2014, 0.27 in 2014–2015, 0.27 in 2015–2016, and 0.33 in 2016–2017). This is biologically implausible in view of analyses of serological data from the Netherlands, which show that a majority of infants are infected at the age of 1 year [32]. We have further explored a scenario with low reproduction number and more frequent reinfection in adults. This scenario also had similarly low statistical support (not shown). We conclude that in the absence of evidence of substantial circulation of RSV in children and adults in our data (5–64 years), the analyses point to high values of the basic reproduction number.
Vaccination
The transmission model was used to gauge the impact of vaccination. In both the scenario with maternal vaccination and the scenario with pediatric vaccination we assumed an effective vaccination coverage of 50%. In both scenarios the attack rates in the targeted groups were most strongly reduced during a 2 to 4-year honeymoon period after the start of the vaccination campaign (not shown) [33]. After this period, a stable pattern emerged with regular yearly epidemics. Supplementary Table 2 shows the results 20 years into the vaccination campaign. Without vaccination, the infection attack rates were very high in infants, high in 1 to 4-year-old children, and much lower in all other age groups. Maternal vaccination reduced the attack rates in infants (27% decrease), but lead to an increase in 1 to 4-year-old children (10% increase). The impact on the attack rates in older adults was negligible. For infant vaccination, the reduction in the attack rates in infants was comparable (30% decrease). In addition, pediatric vaccination also reduced the attack rates in children (1–4 years, 24% decrease; 5–9 years, 8% decrease), and had negligible impact in adults.
DISCUSSION
Our analyses provide estimates of transmission parameters of RSV (reproduction number, infectious period) together with elements of the reporting pyramid (probabilities of hospitalization and GP consultation) while taking into account losses of immunity by demographic turnover and waning of immunity during interepidemic periods. The results indicate that RSV is among the most transmissible infectious diseases, and yield reasonable estimates of the probabilities of hospitalization and GP consultation (Supplementary Table 1). Importantly, estimates of the rates at which natural immunity is lost were low in most age groups (approximately 6% per year), uncertain in young infants, and substantially higher in older adults (approximately 15% per year). This is true not only in our default model scenario with high estimated reproduction number but also in the scenario with an infectious period with fixed expected duration. This implies that a substantial proportion of older adults, up to 15% in our default scenario, may be susceptible to infection.
Overall, the different data sources are in remarkable agreement, and point to high infection attack rates per epidemic in infants (67%–79%), followed by young children (1–4 years; 19%–32%), and older adults (65+ years; 3%–18%). In other age groups, estimated attack rates were low (<5%) owing to the paucity of hospitalizations with confirmed RSV and virological positives in these age groups. Comparing the different RSV seasons, we found that in infants and children the attack rates were broadly similar, while in older adults there was a clear increase in infection attack rates in the 2016/2017 epidemic. The increase is visible both in the hospitalizations and virological data. There is evidence that the increase in older adults may be caused by specific antibody epitope escape mutations in the G protein of the circulating RSV-A virus, in particular the K216N mutation [34]. It would be interesting to try to use (real-time) sequencing data not only for post hoc comparisons but also for predictive purposes (by predicting which strains are antigenically most advanced), as has been attempted for the influenza A H3N2 subtype [35].
The results presented here indicate that RSV may be substantially more transmissible than suggested by previous studies [13, 18, 36]. The main reason is that infants are infected at a very young age (mean age at first infection is <1 year), while our estimates imply that infections are rare in older children and adults (Supplementary Table 4). Part of the discrepancy may be due to the fact that previous studies have often focused on low- and middle-income countries, and that the epidemiology in these countries differs from the situation in the Netherlands. Ultimately, direct observations using large cohorts that are densely sampled over time may be needed to provide more definitive answers to the question of the true level of circulation of RSV in older children and nonelderly adults.
We used the model to analyze the impact of maternal and pediatric vaccination that takes herd effects into account (see [37] for an alternative attempt without herd effects). Assuming a vaccination coverage of 50% and perfect vaccine efficacy, we found that both maternal vaccination and pediatric vaccination were able to reduce the attack rate in infants. By shifting the ages at infection upward, however, maternal vaccination is expected to increase the infection attack rates in children. The indirect benefits in other age groups were small due to the high transmissibility of RSV. Of course, while maternal vaccination mostly prevents infection in the first half year of life, pediatric vaccination reduces infection in the second half of the first year of life (assuming a DTaP-IPV–like vaccination program), and this will in practice be crucial when comparing these strategies. Thus, while we believe that the results on vaccination are qualitatively robust, there remains a need for transmission model analyses that not only take broad effects across all age groups into account, but also include submodels for the first years of life (eg, maternal immunity, date of birth relative to the RSV epidemic). This will be a main challenge for future studies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
RESCEU investigators. Rachel M. Reeves, You Li, Harry Campbell, Harish Nair (University of Edinburgh, UK); Maarten van Wijhe, Thea Kølsen Fischer, Lone Simonsen, Ramona Trebbien (Statens Serum Institut, Denmark); Sabine Tong, Scott Gallichan, Mathieu Bangert, Clarisse Demont (Sanofi Pasteur); Toni Lehtonen (Finnish Institute for Health and Welfare, Turku University Hospital, Finland); Terho Heikkinen (Turku University Hospital, Finland); Anne Teirlinck, Michiel van Boven, Wim van der Hoek, Nicoline van der Maas, Adam Meijer (National Institute for Public Health and the Environment, Netherlands); Liliana Vazquez Fernandez, Håkon Bøas, Terese Bekkevold, Elmira Flem (Norwegian Institute of Public Health, Norway); Luca Stona, Irene Speltra, Carlo Giaquinto (Penta, Italy); Arnaud Cheret (Janssen); Amanda Leach, Sonia Stoszek (GlaxoSmithKline); Philippe Beutels (University of Antwerp, Belgium); Louis Bont (University Medical Centre, Utrecht, Netherlands); Andrew Pollard (University of Oxford, UK); Peter Openshaw (Imperial College, UK); Michael Abram (AstraZeneca); Kena Swanson (Pfizer); Brian Rosen (Novavax, Rockville, MD, USA); Eva Molero (Synapse Research Management Partners SL).
Acknowledgments. Louis Bont, Jaap van Dissel, Susan van den Hof, and an anonymous reviewer are gratefully acknowledged for valuable input.
Financial support. This work was supported by the Respiratory Syncytial Virus Consortium in Europe (RESCEU). RESCEU has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (grant number 116019), which is supported by the EU Horizon 2020 Research and Innovation Programme and European Federation of Pharmaceutical Industries and Associations. Contribution of C. H. v. D. was under the auspices of the US Department of Energy (contract number 89233218CNA000001) and supported by the National Institutes of Health (grants numbers P01-AI131365 and R01-OD011095).
Supplement sponsorship. This supplement is sponsored by RESCEU (REspiratory Syncytial Virus Consortium in EUrope).
Potential conflicts of interest. H. C. reports grants and personal fees from Bill and Melinda Gates Foundation, World Health Organization, and Sanofi, all outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
RESCEU Investigators:
Rachel M Reeves, You Li, Harry Campbell, Harish Nair, Maarten van Wijhe, Thea Kølsen Fischer, Lone Simonsen, Ramona Trebbien, Sabine Tong, Scott Gallichan, Mathieu Bangert, Clarisse Demont, Toni Lehtonen, Terho Heikkinen, Anne Teirlinck, Michiel van Boven, Wim van der Hoek, Nicoline van der Maas, Adam Meijer, Liliana Vazquez Fernandez, Håkon Bøas, Terese Bekkevold, Elmira Flem, Luca Stona, Irene Speltra, Carlo Giaquinto, Arnaud Cheret, Amanda Leach, Sonia Stoszek, Philippe Beutels, Louis Bont, Andrew Pollard, Peter Openshaw, Michael Abram, Kena Swanson, Brian Rosen, and Eva Molero
References
- 1. Li Y, Reeves RM, Wang X, et al. ; RSV Global Epidemiology Network; RESCEU Investigators Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019; 7:e1031–45. [DOI] [PubMed] [Google Scholar]
- 2. Scheltema NM, Gentile A, Lucion F, et al. ; PERCH Study Group Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health 2017; 5:e984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis [published online ahead of print 18 March 2019]. J Infect Dis doi: 10.1093/infdis/jiz059. [DOI] [PubMed] [Google Scholar]
- 4. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mazur NI, Higgins D, Nunes MC, et al. ; Respiratory Syncytial Virus Network (ReSViNET) Foundation The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18:e295–311. [DOI] [PubMed] [Google Scholar]
- 6. Brand SPC, Munywoki P, Walumbe D, Keeling MJ, Nokes DJ. Reducing RSV hospitalisation in a lower-income country by vaccinating mothers-to-be and their households [published online ahead of print 27 March 2020]. Elife doi: 10.7554/eLife.47003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham BS. Vaccine development for respiratory syncytial virus. Curr Opin Virol 2017; 23:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graham BS. Immunological goals for respiratory syncytial virus vaccine development. Curr Opin Immunol 2019; 59:57–64. [DOI] [PubMed] [Google Scholar]
- 9. Jorquera PA, Tripp RA. Respiratory syncytial virus: prospects for new and emerging therapeutics. Expert Rev Respir Med 2017; 11:609–15. [DOI] [PubMed] [Google Scholar]
- 10. Noor A, Krilov LR. Respiratory syncytial virus vaccine: where are we now and what comes next? Expert Opin Biol Ther 2018; 18:1247–56. [DOI] [PubMed] [Google Scholar]
- 11. Bont L, Checchia PA, Fauroux B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther 2016; 5:271–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldstein E, Finelli L, O’Halloran A, et al. Hospitalizations associated with respiratory syncytial virus and influenza in children, including children diagnosed with asthma. Epidemiology 2019; 30:918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstein E, Nguyen HH, Liu P, et al. On the relative role of different age groups during epidemics associated with respiratory syncytial virus. J Infect Dis 2017; 217:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munywoki PK, Koech DC, Agoti CN, et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis 2014; 209:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reeves RM, Hardelid P, Panagiotopoulos N, Minaji M, Warburton F, Pebody R. Burden of hospital admissions caused by respiratory syncytial virus (RSV) in infants in England: a data linkage modelling study. J Infect 2019; 78:468–75. [DOI] [PubMed] [Google Scholar]
- 16. Taylor S, Taylor RJ, Lustig RL, et al. Modelling estimates of the burden of respiratory syncytial virus infection in children in the UK. BMJ Open 2016; 6:e009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker RE, Mahmud AS, Wagner CE, et al. Epidemic dynamics of respiratory syncytial virus in current and future climates. Nat Commun 2019; 10:5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hogan AB, Glass K, Moore HC, Anderssen RS. Exploring the dynamics of respiratory syncytial virus (RSV) transmission in children. Theor Popul Biol 2016; 110:78–85. [DOI] [PubMed] [Google Scholar]
- 19. Kinyanjui TM, House TA, Kiti MC, Cane PA, Nokes DJ, Medley GF. Vaccine induced herd immunity for control of respiratory syncytial virus disease in a low-income country setting. PLoS One 2015; 10:e0138018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahikul W, White LJ, Poovorawan K, et al. Modeling household dynamics on respiratory syncytial virus (RSV). PLoS One 2019; 14:e0219323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poletti P, Merler S, Ajelli M, et al. Evaluating vaccination strategies for reducing infant respiratory syncytial virus infection in low-income settings. BMC Med 2015; 13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamin D, Jones FK, DeVincenzo JP, et al. Vaccination strategies against respiratory syncytial virus. Proc Natl Acad Sci U S A 2016; 113:13239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Angelis D, Presanis AM, Birrell PJ, Tomba GS, House T. Four key challenges in infectious disease modelling using data from multiple sources. Epidemics 2015; 10:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Backer JA, van Boven M, van der Hoek W, Wallinga J. Vaccinating children against influenza increases variability in epidemic size. Epidemics 2019; 26:95–103. [DOI] [PubMed] [Google Scholar]
- 25. Backer JA, Wallinga J, Meijer A, Donker GA, van der Hoek W, van Boven M. The impact of influenza vaccination on infection, hospitalisation and mortality in the Netherlands between 2003 and 2015. Epidemics 2019; 26:77–85. [DOI] [PubMed] [Google Scholar]
- 26. Hill EM, Petrou S, de Lusignan S, Yonova I, Keeling MJ. Seasonal influenza: modelling approaches to capture immunity propagation. PLoS Comput Biol 2019; 15:e1007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Woolthuis RG, Wallinga J, van Boven M. Variation in loss of immunity shapes influenza epidemics and the impact of vaccination. BMC Infect Dis 2017; 17:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vos LM, Teirlinck AC, Lozano JE, et al. Use of the moving epidemic method (MEM) to assess national surveillance data for respiratory syncytial virus (RSV) in the Netherlands, 2005 to 2017. Euro Surveill 2019; 24:1800469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van de Kassteele J, van Eijkeren J, Wallinga J. Efficient estimation of age-specific social contact rates between men and women. Ann Appl Stat 2017; 11:320–39. [Google Scholar]
- 30. van Dorp CH, Woolthuis RG, Yu JHC, de Boer RJ, van Boven M. Estimation of age-specific susceptibility to influenza in the Netherlands and its relation to loss of CD8+ T-cell memory. bioRxiv [Preprint]. 5. February 2018. [cited 27 July 2020]. Available from: 10.1101/259614. [DOI] [Google Scholar]
- 31. Carpenter B, Gelman A, Hoffman M, et al. Stan: a probabilistic programming language. J Stat Softw 2017; 76:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berbers G, Mollema L, van der Klis F, den Hartog G, Schepp S. Antibody responses to Respiratory Syncytial Virus: a cross-sectional serosurveillance study in the Dutch population with emphasis on infants up to 2 years and COPD patients. J Infect Dis. doi: 10.1093/infdis/jiaa483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLean AR, Anderson RM. Measles in developing countries. Part II. The predicted impact of mass vaccination. Epidemiol Infect 1988; 100:419–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vos LM, Oosterheert JJ, Kuil SD, et al. High epidemic burden of RSV disease coinciding with genetic alterations causing amino acid substitutions in the RSV G-protein during the 2016/2017 season in the Netherlands. J Clin Virol 2019; 112:20–6. [DOI] [PubMed] [Google Scholar]
- 35. Neher RA, Bedford T, Daniels RS, Russell CA, Shraiman BI. Prediction, dynamics, and visualization of antigenic phenotypes of seasonal influenza viruses. Proc Natl Acad Sci USA 2016; 113:E1701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Otomaru H, Kamigaki T, Tamaki R, et al. Transmission of respiratory syncytial virus among children under 5 years in households of rural communities, the Philippines. Open Forum Infect Dis 2019; 6:ofz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scheltema NM, Kavelaars XM, Thorburn K, et al. Potential impact of maternal vaccination on life-threatening respiratory syncytial virus infection during infancy. Vaccine 2018; 36:4693–700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.