Abstract
Background
Type 2 diabetes mellitus (T2DM) considered as one of the cardiovascular disorders (CVD) principle risk factor as diabetes is associated with abnormal levels of endothelial function, inflammatory and adipocytokines.
Objective
The aim of this study was to measure the impact of weight reducing on inflammatory cytokines, adipocytokines and endothelial function biomarkers among obese T2DM patients.
Methods
One-hundred T2DM patients enrolled in the present study; the age range was 35–55 year. Participants shared in this study were enrolled in group (A) received diet control and aerobic exercise on treadmill, while, group (B) had no intervention for 3 months.
Results
The mean values of body mass index (BMI), tumor necrosis factor -alpha (TNF-α), interleukin-6 (IL-6), leptin, inter-cellular adhesion molecule (ICAM-1), vascular cell adhesion molecule (VCAM-1), E-selectin and plasminogen activator inhibitor-1 activity (PAI-1 activity) were significantly decreased and adiponectin was increased significantly in the training group, however the results of the control group were not significant. Also, there were significant differences between both groups at the end of the study.
Conclusion
Weight reducing program modulates inflammatory cytokines, adipocytokines and endothelial function biomarkers among obese T2DM patients.
Keywords: Diabetes, endothelial dysfunction biomarkers, cytokines, adipocytokines, weight reduction
Introduction
Type 2 diabetes mellitus (T2DM) is one of the cardiovascular disorders (CVD) principle risk factor 1–3, the risk of coronary artery disease is 2–4 times among patients with diabetes than non-diabetic subjects and peripheral vascular diseases risk is 10 times higher in diabetics than non-diabetics 4–5. Impaired endothelial function is an early predictor of CVD 6–10.
Endothelial dysfunction means loss of endothelial ability in regulation of vascular homeostasis through control of vasoconstriction, inflammatory and thrombotic markers 11. However, abnormal levels of endothelial function considered a predictor for CVD12.
Non-insulin dependent diabetes usually associated with several CVD and other body systems which are related to impaired endothelial function 13–17. Increased level of systemic inflammation markers and decreased plasma adiponectin promote endothelial dysfunction which could be considered as an important pathogenic factors and potential triggers for cardiovascular disorders, insulin resistance and atherosclerosis in T2DM patients18–19. However, weight reducing program composed of diet control and exercises associated with good prognosis as it modulated atherosclerosis and inflammatory biomarkers in T2DM patients 20–22.
As there is inconclusive data regarding the impact of weight reduction upon the inflammatory cytokines, adipokines and endothelial function in obese T2DM patients. Therefore, the study aimed to determine the impact of 12 weeks of weight reducing program on inflammatory cytokines, adipokines and endothelial function in obese T2DM patients.
Patients and methods
Subjects
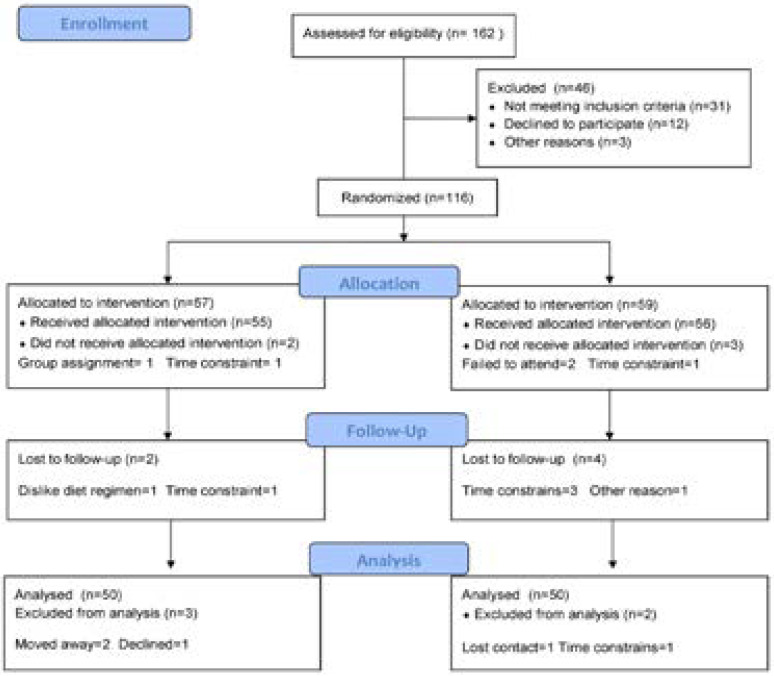
One-hundred T2DM subjects who were out patients of the Internal Medicine Department at King Abdul Aziz University Hospital enrolled in the present study; the age range was 35–55 year. Hypertension, musculoskeletal disorders, smoking, congestive heart failure and intake of medicine that affect the endothelial function were the exclusion criteri. Participants shared in this study were enrolled in group (A) received diet control and aerobic exercise on treadmill, while, group (B) had no intervention (figure 1).
Figure 1.
Subjects screening and recruitment CONSORT diagram.
Measurements
A. Endothelial function biomarkers: Enzyme-linked immunosorbent assays (ELISAs) was used to measure inter-cellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1). While the level of PAI-1 activity was determined using a commercial kit (Hyphen BioMed for PAI-1, France).
B. Inflammatory cytokines and adipokines: Overnight fasting venous blood sample using ELISA was used to measure levels of Interleukin-6 (IL-6) and tumor necrosis factor- alpha (TNF-α) “Immulite 2000” immunassay analyzer (Siemens Healthcare Diagnostics, Deerfield, USA). Also, level of adiponectin and Leptin was measured with K2EDTA in plasma sample “Hitachi 7170 Autoanalyser, Tokyo, Japan”.
Procedures
participants shared in the following groups:
1. Group (A) : Fifty type 2 diabetic patients received 3 months of aerobic treadmill exercise training conducted according to of American College of Sports Medicine recommendations 23. Exercise program included warming -up for five minutes as range motion and stretching exercises, thirty minutes of aerobic exercise training (60–70% of maximum heart rate) and cooling down for ten minutes (on treadmill with low speed and without inclination). Participants received three sessions /week for three months. Also, diet control supervised by a dietician and to prescribe the balanced low caloric diet that provide 1200 Kilocalories/day for 12 weeks
2. Group (B): Fifty type 2 diabetic patients of both sexes conducted their usual life style without intervention.
Statistical analysis
Paired “t” test was used to detect significance level of the investigated parameters measured before and after the study in both groups. While, independent “t” test was used in comparing parameters between the two groups (P<0.05).
Results
One hundred patients with T2DM shared in the present study. However, basic criteria of all participants are shown in table (1). The majority of participants (66%) were men. However, there were no significant differences related to baseline criteria between the two groups.
Table 1.
Mean value of baseline characteristics of subjects for participants in both groups
| Group (A) | Group (B) | Significance | |
| Age (year) | 41.53 ± 5.18 | 42.17 ± 4.85 | P>0.05 |
| Gender (male/female) | 34/16 | 32/18 | P>0.05 |
| BMI (kg/m2) | 31.16 ± 2.89 | 30.58 ± 2.76 | P>0.05 |
| Duration of diabetes (years) | 10.19 ± 4.15 | 9.76 ± 3.47 | P>0.05 |
| Waist circumference (cm) | 101.32 ± 8.61 | 99.74 ± 9.39 | P>0.05 |
|
Systolic blood pressure (mmHg) |
141.75 ± 11.13 | 138.22 ± 10.68 | P>0.05 |
|
Diastolic blood pressure (mmHg) |
80.14 ± 6.51 | 78.96 ± 7.05 | P>0.05 |
| HBA1c (%) | 8.26 ± 1.71 | 8.13 ± 1.58 | P>0.05 |
BMI= Body Mass Index; HBA1c = glycosylated hemoglobin.
The mean values of BMI, TNF-α, IL-6, Leptin, ICAM-1, VCAM-1, E-selectin and PAI-1 activity were significantly decreased and adiponectin was increased significantly in the training group (table 2), however the results of the control group were not significant (table 3). Also, there were significant differences between both groups at the end of the study (table 4).
Table (2).
Mean value and significance of BMI, TNF-α, IL-6, Leptin, Adiponectin, ICAM-1, VCAM-1, E-selectin and PAI-1 activity in group (A) before and after treatment
| Mean +SD | T- value |
Significance | ||
| Pre | Post | |||
| BMI (kg/m2) | 31.16 ± 2.89* | 26.81 ± 2.27 | 7.86 | P<0.05 |
| TNF-α (pg/mL) | 4.62 ± 1.44* | 3.21 ± 1.19 | 6.92 | P<0.05 |
| IL-6 (pg/mL) | 2.48± 0.91* | 1.76 ± 0.82 | 5.87 | P<0.05 |
| Leptin (Ng/ml) | 32.11 ± 5.23* | 25.74 ± 4.19 | 7.93 | P<0.05 |
| Adiponectin (µg/mL) | 11.24 ± 2.26* | 15.16 ± 2.73 | 6.76 | P<0.05 |
| ICAM-1 (ng/ml) | 92.18 ± 6.22* | 83.14 ± 5.71 | 8.23 | P<0.05 |
| VCAM-1 (ng /ml) | 819.70 ± 32.21* | 743.25 ± 27.95 | 9.18 | P<0.05 |
| E-selectin (ng/ml) | 15.03 ± 2.91* | 9.13 ± 2.34 | 7.65 | P<0.05 |
|
PAI-1 activity (ng/ml) |
0.57 ±0.21* | 0.42 ±0.16 | 4.15 | P<0.05 |
BMI= Body Mass Index; TNF-α = Tumor necrosis factor –alpha; IL-6= Interleukin-6; ICAM-1 = Inter-Cellular Adhesion Molecule; VCAM-1 = Vascular Cell Adhesion Molecule; PAI-1: Ac = Plasminogen Activator Inhibitor-1 Activity
indicates a significant difference, P < 0.05.
Table (3).
Mean value and significance of BMI, TNF-α, IL-6, Leptin, Adiponectin, ICAM-1, VCAM-1, E-selectin and PAI-1 activity in group (B) before and after treatment
| Mean +SD | T-value | Significance | ||
| Pre | Post | |||
| BMI (kg/m2) | 30.58 ± 2.76 | 32.23 ± 2.01 | 1.19 | P>0.05 |
| TNF-α (pg/mL) | 4.13 ± 1.54 | 4.26 ± 1.43 | 0.75 | P>0.05 |
| IL-6 (pg/mL) | 2.18 ± 0.92 | 2.31 ± 0.86 | 0.58 | P>0.05 |
| Leptin (Ng/ml) | 30.74 ± 5.11 | 31.12 ± 5.15 | 1.04 | P>0.05 |
| Adiponectin (µg/mL) | 11.15 ± 2.78 | 11.03 ± 2.75 | 0.87 | P>0.05 |
| ICAM-1 (ng/ml) | 92.66 ± 6.43 | 94.05 ± 6.14 | 0.65 | P>0.05 |
| VCAM-1 (ng /ml) | 822.12 ± 29.13 | 825.10± 29.21 | 0.98 | P>0.05 |
| E-selectin (ng/ml) | 15.11 ± 3.91 | 15.86 ± 3.87 | 0.83 | P>0.05 |
|
PAI-1 activity (ng/ml) |
0.55 ±0.14 | 0.57 ±0.16 | 0.62 | P>0.05 |
BMI= Body Mass Index; TNF-α = Tumor necrosis factor –alpha; IL-6= Interleukin-6; ICAM-1 = Inter-Cellular Adhesion Molecule; VCAM-1 = Vascular Cell Adhesion Molecule; PAI-1: Ac = Plasminogen Activator Inhibitor-1 Activity; (*) indicates a significant difference, P < 0.05.
Table (4).
Mean value and significance of BMI, TNF-α, IL-6, Leptin, Adiponectin, ICAM-1, VCAM-1, E-selectin and PAI-1 activity in group (A) and group (B) at the end of the study
| Mean +SD | T-value | Significance | ||
| Group (A) | Group (B) | |||
| BMI (kg/m2) | 26.81 ± 2.27* | 32.23 ± 2.01 | 6.32 | P <0.05 |
| TNF-α (pg/mL) | 3.21 ± 1.19* | 4.26 ± 1.43 | 5.48 | P <0.05 |
| IL-6 (pg/mL) | 1.76 ± 0.82* | 2.31 ± 0.86 | 4.76 | P <0.05 |
| Leptin (Ng/ml) | 25.74 ± 4.19* | 31.12 ± 5.15 | 6.54 | P <0.05 |
| Adiponectin (µg/mL) | 15.16 ± 2.73* | 11.03 ± 2.75 | 5.23 | P <0.05 |
| ICAM-1 (ng/ml) | 83.14 ± 5.71* | 94.05 ± 6.14 | 7.41 | P <0.05 |
| VCAM-1 (ng /ml) | 743.25 ± 27.95* | 825.10± 29.21 | 8.32 | P <0.05 |
| E-selectin (ng/ml) | 9.13 ± 2.34* | 15.86 ± 3.87 | 6.19 | P <0.05 |
|
PAI-1 activity (ng/ml) |
0.42 ±0.16* | 0.57 ±0.17 | 3.75 | P <0.05 |
BMI= Body Mass Index; TNF-α = Tumor necrosis factor –alpha; IL-6= Interleukin-6; ICAM-1 = Inter-Cellular Adhesion Molecule; VCAM-1 = Vascular Cell Adhesion Molecule; PAI-1: Ac = Plasminogen Activator Inhibitor-1 Activity
indicates a significant difference, P < 0.05.
Discussion
Vascular disorders are common among T2DM patients 24, 25 which are related to impaired endothelial function and systemic inflammation 26. Obesity is usually associated with abnormal levels of inflammatory cytokines that induce endothelial dysfunction 27–29. Level of markers of endothelial function is predictor for CVD future events 30. Weight reducing program is the key for management of obesity 31–33.
The principle results of the present study proved that weight loss ameliorated inflammatory cytokines (TNF-α, IL-6 and leptin) and endothelial function markers (ICAM-1 VCAM-1, E-selectin and PAI-1 activity) as well as improvement in adiponectin in T2DM patients, these findings agreed with Cotie et al. found that four months of weight reduction program modulated endothelial function and IL-6 34. Lang et al. proved that weight reducing program for 2 months ameliorated inflammatory cytokines and abnormal blood lipid profile. 35. Choo et al stated that long term weight reduction program reduced atherosclerosis and CVD risk factors 36. Several studies proved that weight loss associated with improvement of inflammatory cytokines and increase level of adiponectin levels 37–50.
Concerning endothelial function, results of the present study proved that weight reducing program resulted reduced levels of VCAM-1, ICAM-1 and PAI-1. These findings agreed with several studies 51–56. While, Sharman and Volek found that 6-weeks of weight reducing program resulted in reduction in TNF-α, IL-6 and ICAM-157. Forsythe et al. stated that three months of diet control resulted in weight loss and reduced TNF-α, IL-6, E-selectin, ICAM and plasminogen activator inhibitor-1 (PAI-1) 58. Thomson et al mentioned that five months of diet control significantly reduced levels of VCAM-1, and ICAM-1 and PAI-1 among with polycystic ovary syndrome (PCOS) obese women 59.
The current study has important strengths and limitations. The major strength is the supervised nature of the study. Supervising food intake and physical activity removes the need to question compliance or to rely on food and activity questionnaires. Further, all exercise sessions were supervised and adherence to the diet and activities was essentially 100%. Moreover, the study was randomized; hence, we can extrapolate adherence to the general population. In the other hand, the major limitations is measuring selected cytokines (i.e. TNF-α and IL-6) even obesity does not only increase the cytokines selected by the authors, but also IL-5, IL-10, IL-12, IL-13 and IFN-γ 60, in addition small sample size in both groups may limit the possibility of generalization of the findings in the present study.
Conclusion
Weight reducing program modulates inflammatory cytokines, adipocytokines and endothelial function biomarkers among obese T2DM patients.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G-262-142-40). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Conflict of interest
None declared.
References
- 1.Tak WW, Wong, Ling S, Yu TX, Yu H. Endothelial Dysfunction: The Common Consequence in Diabetes and Hypertension. J Cardiovasc Pharmacol. 2010;55:300–307. doi: 10.1097/fjc.0b013e3181d7671c. PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Andreassi MG, Barale R, Iozzo P, Picano E. The association of micronucleus frequency with obesity, diabetes and cardiovascular disease. Mutagenesis. 2011;26(1):77–83. doi: 10.1093/mutage/geq077. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation, author. Diabetes: a major risk fac¬tor. [17 October 2012]. http://www.cvd.idf.org.
- 4.Karasu C. Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: effects of antioxidant treatment. Open Cardiovasc Med J. 2010;4:240–256. doi: 10.2174/1874192401004010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding H, Triggle CR. Endothelial cell dysfunction and the vascular complications associated with type 2 diabetes: assessing the health of the endothelium. Vasc Health Risk Manag. 2005;1(1):55–71. doi: 10.2147/vhrm.1.1.55.58939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23) Suppl 1:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Olson NC, Callas PW, Hanley AJ, Festa A, Haffner SM, Wagenknecht LE, Tracy RP. Circulating levels of TNF-alpha are associated with impaired glucose tolerance, increased insulin resistance, and ethnicity: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2012;97:1032–1040. doi: 10.1210/jc.2011-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bose KS, Gupta SK, Vyas P. Adipocytokine levels in genetically high risk for type 2 diabetes in the Indian population: a cross-sectional study. Exp Diabetes Res. 2012;2012:386524. doi: 10.1155/2012/386524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelaye B, Revilla L, Lopez T, Suarez L, Sanchez SE, Hevner K, Fitzpatrick AL, Williams MA. Association between insulin resistance and c-reactive protein among Peruvian adults. Diabetol Metab Syndr. 2010;2:30. doi: 10.1186/1758-5996-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhu SV. Endothelial Dysfunction and Diabetes. JAPI. 58:475–476. [PubMed] [Google Scholar]
- 11.Xu J, Zou M-H. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabit C, Chung W, Hamburg N, et al. Endothelial dysfunc¬tion in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, Lemaitre RN. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord. 2010;11(1):53–59. doi: 10.1007/s11154-010-9133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grauslund J, Green A, Kawasaki R, Hodgson L, Sjølie AK, Wong TY. Retinal vascular fractals and microvascular and macrovascular complications in type 1 diabetes. Ophthalmology. 2010;117(7):1400–1405. doi: 10.1016/j.ophtha.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Petrofsky J, Lee S. The effects of type 2 diabetes and aging on vascular endothelial and autonomic function. Med Sci Monit. 2005;11(6):CR247–CR254. PubMed. [PubMed] [Google Scholar]
- 16.Okon EB, Szado T, Laher I, McManus B, van Breemen C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res. 2003;40(6):520–530. doi: 10.1159/000075238. [DOI] [PubMed] [Google Scholar]
- 17.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 18.Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, Christopher T, Lopez B, Chan L, Goldstein B, Ma XL. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413–419. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity (Silver Spring) 2011;19:1131–1136. doi: 10.1038/oby.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010;2010:520258. doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorge ML, de Oliveira VN, Resende NM, Paraiso LF, Calixto A, Diniz AL. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60:1244–1252. doi: 10.1016/j.metabol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 23.American College of Sports Medicine Guidelines for graded exercise testing and exercise prescription. Philadelphia: Lea & Febiger; 2005. [Google Scholar]
- 24.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care. 2011;34(Suppl 2):S285–S290. doi: 10.2337/dc11-s239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch. 2010;459:977–994. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng KT, Chang CY, Chang LF, Nesaretnam K. Modulation of obesity-induced inflammation by dietary fats: mechanisms and clinical evidence. Nutr J. 2014 Jan 29;13:12. doi: 10.1186/1475-2891-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97(2):3A–11A. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103(5):398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial function. J Am Coll Cardiol. 2003;42(7):1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 31.Balagopal P, George D, Patton N, Yarandi H, Roberts WL, et al. Lifestyle-only intervention attenuates the inflammatory state associated with obesity: a randomized controlled study in adolescents. J Pediatr. 2005;146:342–348. doi: 10.1016/j.jpeds.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 32.Roth CL, Kratz M, Ralston MM, Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism. 2011;60:445–452. doi: 10.1016/j.metabol.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Roberts CK, Chen AK, Barnard RJ. Effect of a short-term diet and exercise intervention in youth on atherosclerotic risk factors. Atherosclerosis. 2007;191:98–106. doi: 10.1016/j.atherosclerosis.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Cotie LM, Josse AR, Phillips SM, MacDonald MJ. Endothelial function increases after a 16-week diet and exercise intervention in overweight and obese young women. Biomed Res Int. 2014;2014:327395. doi: 10.1155/2014/327395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang HF, Chou CY, Sheu WH, Lin JY. Weight loss increased serum adiponectin but decreased lipid levels in obese subjects whose body mass index was lower than 30 kg/m2. Nutr Res. 2011 May;31(5):378–386. doi: 10.1016/j.nutres.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Choo J, Lee J, Cho JH, Burke LE, Sekikawa A, Jae SY. Effects of weight management by exercise modes on markers of subclinical atherosclerosis and cardiometabolic profile among women with abdominal obesity: a randomized controlled trial. BMC Cardiovasc Disord. 2014 Jul 10;14:82. doi: 10.1186/1471-2261-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madsen EL, Rissanen A, Bruun JM, et al. Weight loss larger than 10% is needed for general improvement of levels of circulating adiponectin and markers of inflammation in obese subjects: a 3-year weight loss study. Eur J Endocrinol. 2008;158(2):179–187. doi: 10.1530/EJE-07-0721. [DOI] [PubMed] [Google Scholar]
- 38.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. PubMed. [DOI] [PubMed] [Google Scholar]
- 39.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27:1066–1071. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 40.Nicklas BJ, You T, Pahor M. Behavioral treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–1209. doi: 10.1503/cmaj.1040769. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loria-Kohen V, Fernández-Fernández C, Bermejo LM, Morencos E, Romero-Moraleda B, Gómez-Candela C. Effect of different exercise modalities plus a hypocaloric diet on inflammation markers in overweight patients: a randomised trial. Clin Nutr. 2013 Aug;32(4):511–518. doi: 10.1016/j.clnu.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Sheu WH, Chang TM, Lee WJ, Ou HC, Wu CM, Tseng LN, Lang HF, Wu CS, Wan CJ, Lee IT. Effect of weight loss on proinflammatory state of mononuclear cells in obese women. Obesity (Silver Spring) 2008 May;16(5):1033–1038. doi: 10.1038/oby.2008.37. [DOI] [PubMed] [Google Scholar]
- 43.Rokling-Andersen MH, Reseland JE, Veierød MB, Anderssen SA, Jacobs DR, Jr, Urdal P, Jansson JO, Drevon CA. Effects of long-term exercise and diet intervention on plasma adipokine concentrations. Am J Clin Nutr. 2007 Nov;86(5):1293–1301. doi: 10.1093/ajcn/86.5.1293. [DOI] [PubMed] [Google Scholar]
- 44.Garanty-Bogacka B, Rać M, Syrenicz M, Gębala A, Walczak M, Syrenicz A. Changes in Serum Adipocytokines and Inflammatory Biomarkers Following One-Year of Exercise Training in Obese Adolescents. J Diabetes Metab. 2012;3(7):212–217. [Google Scholar]
- 45.Habib P, Scrocco JD, Terek M, Vanek V, Mikolich JR. Effects of bariatric surgery on inflammatory, functional and structural markers of coronary atherosclerosis. Am J Cardiol. 2009;104:1251–1255. doi: 10.1016/j.amjcard.2009.06.042. PubMed. [DOI] [PubMed] [Google Scholar]
- 46.Sledzinski T, Sledzinski M, Smolenski RT, Swierczynski J. Increased serum nitric oxide concentration after bariatric surgery--a potential mechanism for cardiovascular benefit. Obes Surg. 2010;20:204–210. doi: 10.1007/s11695-009-0041-2. PubMed. [DOI] [PubMed] [Google Scholar]
- 47.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, Kaser S, Kaser A, Tilg H. Anti-inflammatory effects of excessive weight loss: Potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. PubMed. [DOI] [PubMed] [Google Scholar]
- 48.Laimer M, Ebenbichler CF, Kaser S, Sandhofer A, Weiss H, Nehoda H, Aigner F, Patsch JR. Markers of chronic inflammation and obesity: A prospective study on the reversibility of this association in middle-aged women undergoing weight loss by surgical intervention. Int J Obes Relat Metab Disord. 2002;26:659–662. doi: 10.1038/sj.ijo.0801970. [DOI] [PubMed] [Google Scholar]
- 49.Garcia de la Torre N, Rubio MA, Bordiu E, Cabrerizo L, Aparicio E, Hernandez C, Sanchez-Pernaute A, Diez-Valladares L, Torres AJ, Puente M, Charro AL. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-a, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008;93:4276–4281. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 50.Marfella R, Esposito K, Siniscalchi M, et al. Effect of weight loss on cardiac synchronization and proinflammatory cytokines in premenopausal obese women. Diabetes Care. 2004;27:47–52. doi: 10.2337/diacare.27.1.47. PubMed. [DOI] [PubMed] [Google Scholar]
- 51.Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Caselli A, Caballero AE, Economides PA, et al. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care. 2003;26:2119–2125. doi: 10.2337/diacare.26.7.2119. PubMed. [DOI] [PubMed] [Google Scholar]
- 52.Murakami T, Horigome H, Tanaka K, Nakata Y, Ohkawara K, Katayama Y, Matsui A. Impact of weight reduction on production of platelet-derived microparticles and fibrinolytic parameters in obesity. Thromb Res. 2007;119:45–53. doi: 10.1016/j.thromres.2005.12.013. PubMed. [DOI] [PubMed] [Google Scholar]
- 53.Meckling KA, O'Sullivan C, Saari D. Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endocrinol Metab. 2004;89:2717–2723. doi: 10.1210/jc.2003-031606. [DOI] [PubMed] [Google Scholar]
- 54.Keogh JB, Brinkworth GD, Noakes M, Belobrajdic DP, Buckley JD, Clifton PM. Effects of weight loss from a very-low-carbohydrate diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am J Clin Nutr. 2008;87:567–576. doi: 10.1093/ajcn/87.3.567. PubMed. [DOI] [PubMed] [Google Scholar]
- 55.Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism. 2004;53:377–381. doi: 10.1016/j.metabol.2003.10.016. PubMed. [DOI] [PubMed] [Google Scholar]
- 56.Rector RS, Turk JR, Sun GY, Guilford BL, Toedebusch BW, McClanahan MW, Thomas TR. Shortterm lifestyle modification alters circulating biomarkers of endothelial health in sedentary, overweight adults. Appl Physiol Nutr Metab. 2006;31:512–517. doi: 10.1139/h06-040. [DOI] [PubMed] [Google Scholar]
- 57.Sharman MJ, Volek JS. Weight loss leads to reductions in inflammatory biomarkers after a very-low-carbohydrate diet and a low-fat diet in overweight men. Clin Sci (Lond) 2004;107:365–369. doi: 10.1042/CS20040111. PubMed. [DOI] [PubMed] [Google Scholar]
- 58.Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43:65–77. doi: 10.1007/s11745-007-3132-7. PubMed. [DOI] [PubMed] [Google Scholar]
- 59.Thomson RL, Brinkworth GD, Noakes M, Clifton PM, Norman RJ, Buckley JD. The effect of diet and exercise on markers of endothelial function in overweight and obese women with polycystic ovary syndrome. Hum Reprod. 2012;27(7):2169–2176. doi: 10.1093/humrep/des138. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T, Mergl R, Kirkby KC, Faβhauer M, Stumvoll M, Holdt LM, Teupser D, Hegerl U, Himmerich H. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS One. 2015;10(3):e0121971. doi: 10.1371/journal.pone.0121971. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]